User login
A 62-year-old man presented to our skin clinic with multiple pruritic, tense, bullous lesions that manifested on his arms, abdomen, back, and upper thighs over a 1-month period. There were no lesions in his oral cavity or around his eyes, nose, or penile region. He denied dysphagia.
The patient had multiple comorbidities, including diabetes, hypertension, recent stroke, and end-stage renal disease. He was being prepared for dialysis. His medications included torsemide, warfarin, amiodarone, metoprolol, pantoprozole, atorvastatin, and nifedipine. About 3 months prior to this presentation, he was started on oral linaglipton 5 mg/d, an oral antihyperglycemic medication. He had no history of skin disease or cancer, and his family history was not significant.
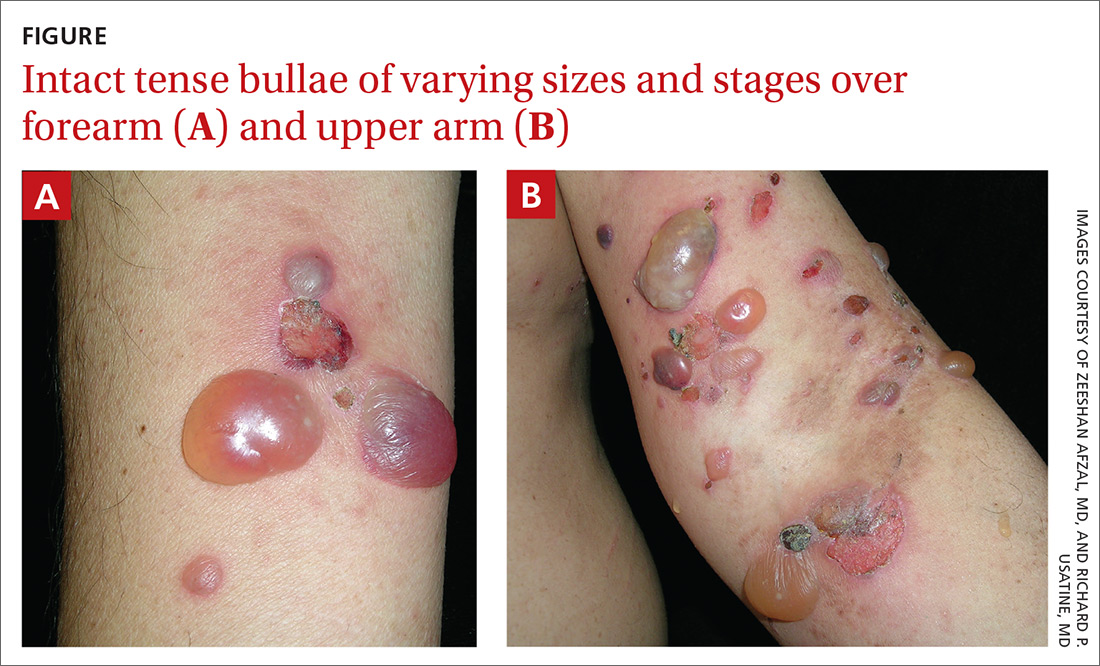
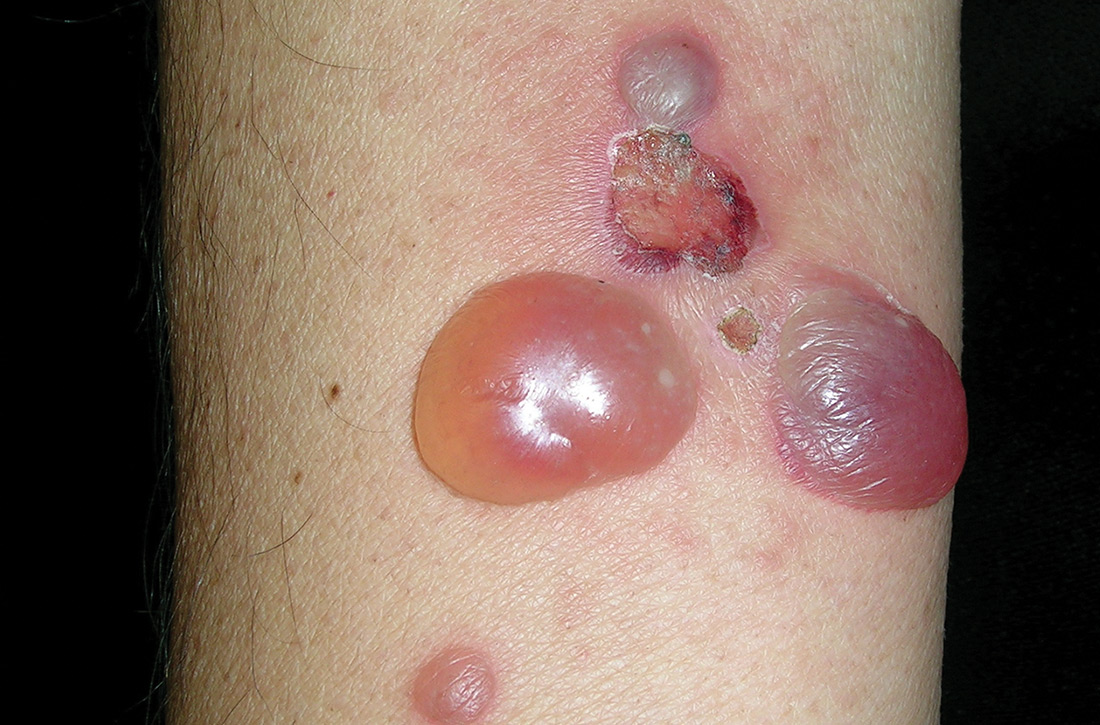
Physical examination showed multiple 5-mm to 2-cm blisters and bullae on the flexural surface of both of his arms (FIGURE), back, lower abdomen, and upper thighs. His palms and soles were not involved. The lesions were nontender, tense, and filled with clear fluid. Some were intact and others were rupturing. There was no mucocutaneous involvement. Nikolsky sign was negative. There were no signs of bleeding.
The family physician (FP) obtained a 4-mm punch biopsy at the edge of a 6-mm blister for light microscopy and a 3-mm perilesional punch biopsy for direct immunofluorescence (DIF) microscopy.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Bullous pemphigoid secondary to linagliptin use
DIF of the biopsy sample demonstrated linear deposition of complement 3 (C3) and immunoglobulin (Ig) G along the basement membrane zone. Indirect immunofluorescence on salt-split skin demonstrated linear deposition of IgG and C3 on both the roof and floor of the induced blisters. These findings and the patient’s clinical presentation met the criteria for bullous pemphigoid (BP), which is the most common autoimmune skin-blistering disease.1
BP is associated with subepidermal blistering, which can occur in reaction to a variety of triggers. Pathogenesis of this condition involves IgG anti-basement membrane autoantibody complex formation with the hemidesmosomal antigens BP230 and BP180—a process that activates C3 and the release of proteases that can be destructive to tissue along the dermo-epidermal junction.1
Growing incidence. BP usually occurs in patients > 60 years, with no racial or gender preference.1 The incidence rate of BP ranges from 2.4 to 21.7 new cases per 1 million individuals among various worldwide populations.2 The incidence appears to have increased 1.9- to 4.3-fold over the past 2 decades.2
What you’ll see, who’s at risk
Symptoms of BP include localized areas of erythema or pruritic urticarial plaques that gradually become more extensive. A patient may have pruritis alone for an extended period prior to developing blisters and bullae. The bullae are tense and normally 1 to 7 cm in size.1 Eruption is generalized, mostly affecting the lower abdomen, as well as the flexural parts of the extremities. The palms and soles also can be affected.
FPs should be aware of the atypical clinical variants of BP. In a review by Kridin and Ludwig, variants can be prurigo-like, eczema-like, urticaria-like, dyshidrosiform type, erosive type, and erythema annulare centrifugum–like type.2 At-risk populations, such as elderly patients (> 70 years), whose pruritis manifests with or without bullous formation, should be screened for BP.3,4
Continue to: Risk factors for BP
Risk factors for BP. Certain conditions linked to developing BP include neurologic disorders (dementia and Parkinson disease) and psychiatric disorders (unipolar and bipolar disorder).4 Further, it is important to note any medications that could be the cause of a patient’s BP, including dipeptidyl peptidase-4 (DPP-4) inhibitors, psychotropic medications, spironolactone, furosemide, beta-blockers, and antibiotics.3 This patient was taking a beta-blocker (metoprolol) and a DPP-4 inhibitor (linagliptin). Because he was most recently started on linagliptin, we suspected it may have had a causal role in the development of BP.
The association of DPP-4 inhibitors and BP
FPs are increasingly using DPP-4 inhibitors—including sitagliptin, vildagliptin, and linagliptin—as oral antihyperglycemic agents for type 2 diabetes mellitus. Therefore, it’s important to recognize this medication class’s association with BP.5 In a case-control study of 165 patients with BP, Benzaquen et al reported that 28 patients who were taking DPP-4 inhibitors had an associated increased risk for BP (adjusted odds ratio = 2.64; 95% confidence interval [CI], 1.19-5.85).3
The pathophysiology of BP associated with DPP-4 inhibitors remains unclear, but mechanisms have been proposed. The DPP-4 enzyme is expressed on many cells, including keratinocytes, T cells, and endothelial cells.3 It is possible that DPP-4 inhibition at these cells could stimulate activity of inflammatory cytokines, which can lead to enhanced local eosinophil activation and trigger bullous formation. DPP-4 enzymes are also involved in forming plasmin, which is a protease that cleaves BP180.3 Inhibition of this process can affect proper cleavage of BP180, impacting its function and antigenicity.3,6
Other conditions that also exhibit blisters
There are some skin conditions with similar presentations that need to be ruled out in the work-up.
Bullous diabeticorum is a rare, spontaneous, noninflammatory condition found in patients with diabetes.1 Blisters usually manifest as large, tense, asymmetrical, mildly tender lesions that commonly affect the feet and lower legs but can involve the trunk. These usually develop overnight without preceding trauma. Biopsy would show both intra-epidermal and subepidermal bulla with normal DIF findings.1 This condition usually has an excellent prognosis.
Continue to: Pemphigus vulgaris
Pemphigus vulgaris is characterized by nonpruritic, flaccid, painful blisters. This condition usually begins with manifestation of painful oral lesions that evolve into skin blisters. Some patients can develop mucocutaneous lesions.1 Nikolsky sign is positive in these cases. Light microscopy would show intra-epidermal bullae.
Dermatitis herpetiformis. This condition—usually affecting middle-age patients—is associated with severe pruritis and burning. It may start with a few pruritic papules or vesicles that later evolve into urticarial papules, vesicles, or bullae. Dermatitis herpetiformis can resemble herpes simplex virus. It can also be associated with gluten-sensitive enteropathy and small bowel lymphoma.1 DIF of a biopsy sample would show granular deposition of IgA within the tips of the dermal papillae and along the basement membrane of perilesional skin.1
Epidermolysis bullosa acquisita is a rare, severe, chronic condition with subepidermal mucocutaneous blistering.1 It is associated with skin fragility and spontaneous trauma-induced blisters that heal with scar formation and milia. IgG autoantibodies reacting to proteins in the basement membrane zone can cause the disease. It is also associated with Crohn disease.1 DIF findings are similar in BP, but they are differentiated by location of IgG deposits; they can be found on the dermal side of separation in epidermolysis bullosa acquisita, as compared with the epidermal side in BP.1
How to make the Dx in 3 steps
To effectively diagnose and classify BP, use the following 3-step method:
- Establish the presence of 3 of 4 clinical characteristics: patient’s age > 60 years, absence of atrophic scars, absence of mucosal involvement, and absence of bullous lesions on the head and neck.
- Order light microscopy. Findings should be consistent with eosinophils and neutrophils containing subepidermal bullae.
- Order a punch biopsy to obtain a perilesional specimen. DIF of the biopsy findings should feature linear deposits of IgG with or without C3 along the dermo-epidermal junction. This step is essential for an accurate diagnosis.
There also is benefit in ordering supplemental studies, such as an enzyme-linked immunosorbent assay for the detection of anti-BP180 or anti-BP230 IgG autoantibodies.7 However, for this patient, we did not order this study.
Continue to: Management focuses on steroids
Management focuses on steroids
The offending agent should be discontinued immediately. Depending on the severity of disease, treatment can include the use of potent topical corticosteroids alone or in combination with systemic corticosteroids and anti-inflammatory antibiotics (eg, doxycycline, minocycline, erythromycin).1,7 For patients with resistant or refractory disease, consider azathioprine, methotrexate, dapsone, and chlorambucil.1,7 Exceptional cases may benefit from the use of mycophenolate mofetil, intravenous immunoglobulin, or plasmapheresis.1,7
For this patient, initial treatment included discontinuation of linagliption and introduction of topical clobetasol 0.05% and oral prednisone 40 mg/d for 7 days, followed by prednisone 20 mg for 7 days. He was also started on oral doxycycline 100 mg bid and oral nicotinamide 500 mg bid.
1. Habif TP. Vesicular and bullous diseases. In: Habif TP, ed. Clinical Dermatology: a Color Guide to Diagnosis and Therapy. 6th ed. Elsevier; 2016:635-666.
2. Kridin K, Ludwig RJ. The growing incidence of bullous pemphigoid: overview and potential explanations. Front Med (Lausanne). 2018;5:220.
3. Benzaquen M, Borradori L, Berbis P, et al. Dipeptidyl peptidase IV inhibitors, a risk factor for bullous pemphigoid: retrospective multicenter case-control study from France and Switzerland. J Am Acad Dermatol. 2017;78:1090-1096.
4. Bastuji-Garin S, Joly P, Lemordant P, et al. Risk factors for bullous pemphigoid in the elderly: a prospective case-control study. J Invest Dermatol. 2011;131:637-643.
5. Kridin K, Bergman R. Association of bullous pemphigoid with dipeptidyl-peptidase 4 inhibitors in patients with diabetes: estimating the risk of the new agents and characterizing the patients. JAMA Dermatol. 2018;154:1152-1158.
6. Haber R, Fayad AM, Stephan F, et al. Bullous pemphigoid associated with linagliptin treatment. JAMA Dermatol. 2016;152:224-226.Management of bullous pemphigoid: the European Dermatology Forum consensus in collaboration with the European Academy of Dermatology and Venereology. Br J Dermatol. 2015;172:867-877.
A 62-year-old man presented to our skin clinic with multiple pruritic, tense, bullous lesions that manifested on his arms, abdomen, back, and upper thighs over a 1-month period. There were no lesions in his oral cavity or around his eyes, nose, or penile region. He denied dysphagia.
The patient had multiple comorbidities, including diabetes, hypertension, recent stroke, and end-stage renal disease. He was being prepared for dialysis. His medications included torsemide, warfarin, amiodarone, metoprolol, pantoprozole, atorvastatin, and nifedipine. About 3 months prior to this presentation, he was started on oral linaglipton 5 mg/d, an oral antihyperglycemic medication. He had no history of skin disease or cancer, and his family history was not significant.
Physical examination showed multiple 5-mm to 2-cm blisters and bullae on the flexural surface of both of his arms (FIGURE), back, lower abdomen, and upper thighs. His palms and soles were not involved. The lesions were nontender, tense, and filled with clear fluid. Some were intact and others were rupturing. There was no mucocutaneous involvement. Nikolsky sign was negative. There were no signs of bleeding.
The family physician (FP) obtained a 4-mm punch biopsy at the edge of a 6-mm blister for light microscopy and a 3-mm perilesional punch biopsy for direct immunofluorescence (DIF) microscopy.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Bullous pemphigoid secondary to linagliptin use
DIF of the biopsy sample demonstrated linear deposition of complement 3 (C3) and immunoglobulin (Ig) G along the basement membrane zone. Indirect immunofluorescence on salt-split skin demonstrated linear deposition of IgG and C3 on both the roof and floor of the induced blisters. These findings and the patient’s clinical presentation met the criteria for bullous pemphigoid (BP), which is the most common autoimmune skin-blistering disease.1
BP is associated with subepidermal blistering, which can occur in reaction to a variety of triggers. Pathogenesis of this condition involves IgG anti-basement membrane autoantibody complex formation with the hemidesmosomal antigens BP230 and BP180—a process that activates C3 and the release of proteases that can be destructive to tissue along the dermo-epidermal junction.1
Growing incidence. BP usually occurs in patients > 60 years, with no racial or gender preference.1 The incidence rate of BP ranges from 2.4 to 21.7 new cases per 1 million individuals among various worldwide populations.2 The incidence appears to have increased 1.9- to 4.3-fold over the past 2 decades.2
What you’ll see, who’s at risk
Symptoms of BP include localized areas of erythema or pruritic urticarial plaques that gradually become more extensive. A patient may have pruritis alone for an extended period prior to developing blisters and bullae. The bullae are tense and normally 1 to 7 cm in size.1 Eruption is generalized, mostly affecting the lower abdomen, as well as the flexural parts of the extremities. The palms and soles also can be affected.
FPs should be aware of the atypical clinical variants of BP. In a review by Kridin and Ludwig, variants can be prurigo-like, eczema-like, urticaria-like, dyshidrosiform type, erosive type, and erythema annulare centrifugum–like type.2 At-risk populations, such as elderly patients (> 70 years), whose pruritis manifests with or without bullous formation, should be screened for BP.3,4
Continue to: Risk factors for BP
Risk factors for BP. Certain conditions linked to developing BP include neurologic disorders (dementia and Parkinson disease) and psychiatric disorders (unipolar and bipolar disorder).4 Further, it is important to note any medications that could be the cause of a patient’s BP, including dipeptidyl peptidase-4 (DPP-4) inhibitors, psychotropic medications, spironolactone, furosemide, beta-blockers, and antibiotics.3 This patient was taking a beta-blocker (metoprolol) and a DPP-4 inhibitor (linagliptin). Because he was most recently started on linagliptin, we suspected it may have had a causal role in the development of BP.
The association of DPP-4 inhibitors and BP
FPs are increasingly using DPP-4 inhibitors—including sitagliptin, vildagliptin, and linagliptin—as oral antihyperglycemic agents for type 2 diabetes mellitus. Therefore, it’s important to recognize this medication class’s association with BP.5 In a case-control study of 165 patients with BP, Benzaquen et al reported that 28 patients who were taking DPP-4 inhibitors had an associated increased risk for BP (adjusted odds ratio = 2.64; 95% confidence interval [CI], 1.19-5.85).3
The pathophysiology of BP associated with DPP-4 inhibitors remains unclear, but mechanisms have been proposed. The DPP-4 enzyme is expressed on many cells, including keratinocytes, T cells, and endothelial cells.3 It is possible that DPP-4 inhibition at these cells could stimulate activity of inflammatory cytokines, which can lead to enhanced local eosinophil activation and trigger bullous formation. DPP-4 enzymes are also involved in forming plasmin, which is a protease that cleaves BP180.3 Inhibition of this process can affect proper cleavage of BP180, impacting its function and antigenicity.3,6
Other conditions that also exhibit blisters
There are some skin conditions with similar presentations that need to be ruled out in the work-up.
Bullous diabeticorum is a rare, spontaneous, noninflammatory condition found in patients with diabetes.1 Blisters usually manifest as large, tense, asymmetrical, mildly tender lesions that commonly affect the feet and lower legs but can involve the trunk. These usually develop overnight without preceding trauma. Biopsy would show both intra-epidermal and subepidermal bulla with normal DIF findings.1 This condition usually has an excellent prognosis.
Continue to: Pemphigus vulgaris
Pemphigus vulgaris is characterized by nonpruritic, flaccid, painful blisters. This condition usually begins with manifestation of painful oral lesions that evolve into skin blisters. Some patients can develop mucocutaneous lesions.1 Nikolsky sign is positive in these cases. Light microscopy would show intra-epidermal bullae.
Dermatitis herpetiformis. This condition—usually affecting middle-age patients—is associated with severe pruritis and burning. It may start with a few pruritic papules or vesicles that later evolve into urticarial papules, vesicles, or bullae. Dermatitis herpetiformis can resemble herpes simplex virus. It can also be associated with gluten-sensitive enteropathy and small bowel lymphoma.1 DIF of a biopsy sample would show granular deposition of IgA within the tips of the dermal papillae and along the basement membrane of perilesional skin.1
Epidermolysis bullosa acquisita is a rare, severe, chronic condition with subepidermal mucocutaneous blistering.1 It is associated with skin fragility and spontaneous trauma-induced blisters that heal with scar formation and milia. IgG autoantibodies reacting to proteins in the basement membrane zone can cause the disease. It is also associated with Crohn disease.1 DIF findings are similar in BP, but they are differentiated by location of IgG deposits; they can be found on the dermal side of separation in epidermolysis bullosa acquisita, as compared with the epidermal side in BP.1
How to make the Dx in 3 steps
To effectively diagnose and classify BP, use the following 3-step method:
- Establish the presence of 3 of 4 clinical characteristics: patient’s age > 60 years, absence of atrophic scars, absence of mucosal involvement, and absence of bullous lesions on the head and neck.
- Order light microscopy. Findings should be consistent with eosinophils and neutrophils containing subepidermal bullae.
- Order a punch biopsy to obtain a perilesional specimen. DIF of the biopsy findings should feature linear deposits of IgG with or without C3 along the dermo-epidermal junction. This step is essential for an accurate diagnosis.
There also is benefit in ordering supplemental studies, such as an enzyme-linked immunosorbent assay for the detection of anti-BP180 or anti-BP230 IgG autoantibodies.7 However, for this patient, we did not order this study.
Continue to: Management focuses on steroids
Management focuses on steroids
The offending agent should be discontinued immediately. Depending on the severity of disease, treatment can include the use of potent topical corticosteroids alone or in combination with systemic corticosteroids and anti-inflammatory antibiotics (eg, doxycycline, minocycline, erythromycin).1,7 For patients with resistant or refractory disease, consider azathioprine, methotrexate, dapsone, and chlorambucil.1,7 Exceptional cases may benefit from the use of mycophenolate mofetil, intravenous immunoglobulin, or plasmapheresis.1,7
For this patient, initial treatment included discontinuation of linagliption and introduction of topical clobetasol 0.05% and oral prednisone 40 mg/d for 7 days, followed by prednisone 20 mg for 7 days. He was also started on oral doxycycline 100 mg bid and oral nicotinamide 500 mg bid.
A 62-year-old man presented to our skin clinic with multiple pruritic, tense, bullous lesions that manifested on his arms, abdomen, back, and upper thighs over a 1-month period. There were no lesions in his oral cavity or around his eyes, nose, or penile region. He denied dysphagia.
The patient had multiple comorbidities, including diabetes, hypertension, recent stroke, and end-stage renal disease. He was being prepared for dialysis. His medications included torsemide, warfarin, amiodarone, metoprolol, pantoprozole, atorvastatin, and nifedipine. About 3 months prior to this presentation, he was started on oral linaglipton 5 mg/d, an oral antihyperglycemic medication. He had no history of skin disease or cancer, and his family history was not significant.
Physical examination showed multiple 5-mm to 2-cm blisters and bullae on the flexural surface of both of his arms (FIGURE), back, lower abdomen, and upper thighs. His palms and soles were not involved. The lesions were nontender, tense, and filled with clear fluid. Some were intact and others were rupturing. There was no mucocutaneous involvement. Nikolsky sign was negative. There were no signs of bleeding.
The family physician (FP) obtained a 4-mm punch biopsy at the edge of a 6-mm blister for light microscopy and a 3-mm perilesional punch biopsy for direct immunofluorescence (DIF) microscopy.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Bullous pemphigoid secondary to linagliptin use
DIF of the biopsy sample demonstrated linear deposition of complement 3 (C3) and immunoglobulin (Ig) G along the basement membrane zone. Indirect immunofluorescence on salt-split skin demonstrated linear deposition of IgG and C3 on both the roof and floor of the induced blisters. These findings and the patient’s clinical presentation met the criteria for bullous pemphigoid (BP), which is the most common autoimmune skin-blistering disease.1
BP is associated with subepidermal blistering, which can occur in reaction to a variety of triggers. Pathogenesis of this condition involves IgG anti-basement membrane autoantibody complex formation with the hemidesmosomal antigens BP230 and BP180—a process that activates C3 and the release of proteases that can be destructive to tissue along the dermo-epidermal junction.1
Growing incidence. BP usually occurs in patients > 60 years, with no racial or gender preference.1 The incidence rate of BP ranges from 2.4 to 21.7 new cases per 1 million individuals among various worldwide populations.2 The incidence appears to have increased 1.9- to 4.3-fold over the past 2 decades.2
What you’ll see, who’s at risk
Symptoms of BP include localized areas of erythema or pruritic urticarial plaques that gradually become more extensive. A patient may have pruritis alone for an extended period prior to developing blisters and bullae. The bullae are tense and normally 1 to 7 cm in size.1 Eruption is generalized, mostly affecting the lower abdomen, as well as the flexural parts of the extremities. The palms and soles also can be affected.
FPs should be aware of the atypical clinical variants of BP. In a review by Kridin and Ludwig, variants can be prurigo-like, eczema-like, urticaria-like, dyshidrosiform type, erosive type, and erythema annulare centrifugum–like type.2 At-risk populations, such as elderly patients (> 70 years), whose pruritis manifests with or without bullous formation, should be screened for BP.3,4
Continue to: Risk factors for BP
Risk factors for BP. Certain conditions linked to developing BP include neurologic disorders (dementia and Parkinson disease) and psychiatric disorders (unipolar and bipolar disorder).4 Further, it is important to note any medications that could be the cause of a patient’s BP, including dipeptidyl peptidase-4 (DPP-4) inhibitors, psychotropic medications, spironolactone, furosemide, beta-blockers, and antibiotics.3 This patient was taking a beta-blocker (metoprolol) and a DPP-4 inhibitor (linagliptin). Because he was most recently started on linagliptin, we suspected it may have had a causal role in the development of BP.
The association of DPP-4 inhibitors and BP
FPs are increasingly using DPP-4 inhibitors—including sitagliptin, vildagliptin, and linagliptin—as oral antihyperglycemic agents for type 2 diabetes mellitus. Therefore, it’s important to recognize this medication class’s association with BP.5 In a case-control study of 165 patients with BP, Benzaquen et al reported that 28 patients who were taking DPP-4 inhibitors had an associated increased risk for BP (adjusted odds ratio = 2.64; 95% confidence interval [CI], 1.19-5.85).3
The pathophysiology of BP associated with DPP-4 inhibitors remains unclear, but mechanisms have been proposed. The DPP-4 enzyme is expressed on many cells, including keratinocytes, T cells, and endothelial cells.3 It is possible that DPP-4 inhibition at these cells could stimulate activity of inflammatory cytokines, which can lead to enhanced local eosinophil activation and trigger bullous formation. DPP-4 enzymes are also involved in forming plasmin, which is a protease that cleaves BP180.3 Inhibition of this process can affect proper cleavage of BP180, impacting its function and antigenicity.3,6
Other conditions that also exhibit blisters
There are some skin conditions with similar presentations that need to be ruled out in the work-up.
Bullous diabeticorum is a rare, spontaneous, noninflammatory condition found in patients with diabetes.1 Blisters usually manifest as large, tense, asymmetrical, mildly tender lesions that commonly affect the feet and lower legs but can involve the trunk. These usually develop overnight without preceding trauma. Biopsy would show both intra-epidermal and subepidermal bulla with normal DIF findings.1 This condition usually has an excellent prognosis.
Continue to: Pemphigus vulgaris
Pemphigus vulgaris is characterized by nonpruritic, flaccid, painful blisters. This condition usually begins with manifestation of painful oral lesions that evolve into skin blisters. Some patients can develop mucocutaneous lesions.1 Nikolsky sign is positive in these cases. Light microscopy would show intra-epidermal bullae.
Dermatitis herpetiformis. This condition—usually affecting middle-age patients—is associated with severe pruritis and burning. It may start with a few pruritic papules or vesicles that later evolve into urticarial papules, vesicles, or bullae. Dermatitis herpetiformis can resemble herpes simplex virus. It can also be associated with gluten-sensitive enteropathy and small bowel lymphoma.1 DIF of a biopsy sample would show granular deposition of IgA within the tips of the dermal papillae and along the basement membrane of perilesional skin.1
Epidermolysis bullosa acquisita is a rare, severe, chronic condition with subepidermal mucocutaneous blistering.1 It is associated with skin fragility and spontaneous trauma-induced blisters that heal with scar formation and milia. IgG autoantibodies reacting to proteins in the basement membrane zone can cause the disease. It is also associated with Crohn disease.1 DIF findings are similar in BP, but they are differentiated by location of IgG deposits; they can be found on the dermal side of separation in epidermolysis bullosa acquisita, as compared with the epidermal side in BP.1
How to make the Dx in 3 steps
To effectively diagnose and classify BP, use the following 3-step method:
- Establish the presence of 3 of 4 clinical characteristics: patient’s age > 60 years, absence of atrophic scars, absence of mucosal involvement, and absence of bullous lesions on the head and neck.
- Order light microscopy. Findings should be consistent with eosinophils and neutrophils containing subepidermal bullae.
- Order a punch biopsy to obtain a perilesional specimen. DIF of the biopsy findings should feature linear deposits of IgG with or without C3 along the dermo-epidermal junction. This step is essential for an accurate diagnosis.
There also is benefit in ordering supplemental studies, such as an enzyme-linked immunosorbent assay for the detection of anti-BP180 or anti-BP230 IgG autoantibodies.7 However, for this patient, we did not order this study.
Continue to: Management focuses on steroids
Management focuses on steroids
The offending agent should be discontinued immediately. Depending on the severity of disease, treatment can include the use of potent topical corticosteroids alone or in combination with systemic corticosteroids and anti-inflammatory antibiotics (eg, doxycycline, minocycline, erythromycin).1,7 For patients with resistant or refractory disease, consider azathioprine, methotrexate, dapsone, and chlorambucil.1,7 Exceptional cases may benefit from the use of mycophenolate mofetil, intravenous immunoglobulin, or plasmapheresis.1,7
For this patient, initial treatment included discontinuation of linagliption and introduction of topical clobetasol 0.05% and oral prednisone 40 mg/d for 7 days, followed by prednisone 20 mg for 7 days. He was also started on oral doxycycline 100 mg bid and oral nicotinamide 500 mg bid.
1. Habif TP. Vesicular and bullous diseases. In: Habif TP, ed. Clinical Dermatology: a Color Guide to Diagnosis and Therapy. 6th ed. Elsevier; 2016:635-666.
2. Kridin K, Ludwig RJ. The growing incidence of bullous pemphigoid: overview and potential explanations. Front Med (Lausanne). 2018;5:220.
3. Benzaquen M, Borradori L, Berbis P, et al. Dipeptidyl peptidase IV inhibitors, a risk factor for bullous pemphigoid: retrospective multicenter case-control study from France and Switzerland. J Am Acad Dermatol. 2017;78:1090-1096.
4. Bastuji-Garin S, Joly P, Lemordant P, et al. Risk factors for bullous pemphigoid in the elderly: a prospective case-control study. J Invest Dermatol. 2011;131:637-643.
5. Kridin K, Bergman R. Association of bullous pemphigoid with dipeptidyl-peptidase 4 inhibitors in patients with diabetes: estimating the risk of the new agents and characterizing the patients. JAMA Dermatol. 2018;154:1152-1158.
6. Haber R, Fayad AM, Stephan F, et al. Bullous pemphigoid associated with linagliptin treatment. JAMA Dermatol. 2016;152:224-226.Management of bullous pemphigoid: the European Dermatology Forum consensus in collaboration with the European Academy of Dermatology and Venereology. Br J Dermatol. 2015;172:867-877.
1. Habif TP. Vesicular and bullous diseases. In: Habif TP, ed. Clinical Dermatology: a Color Guide to Diagnosis and Therapy. 6th ed. Elsevier; 2016:635-666.
2. Kridin K, Ludwig RJ. The growing incidence of bullous pemphigoid: overview and potential explanations. Front Med (Lausanne). 2018;5:220.
3. Benzaquen M, Borradori L, Berbis P, et al. Dipeptidyl peptidase IV inhibitors, a risk factor for bullous pemphigoid: retrospective multicenter case-control study from France and Switzerland. J Am Acad Dermatol. 2017;78:1090-1096.
4. Bastuji-Garin S, Joly P, Lemordant P, et al. Risk factors for bullous pemphigoid in the elderly: a prospective case-control study. J Invest Dermatol. 2011;131:637-643.
5. Kridin K, Bergman R. Association of bullous pemphigoid with dipeptidyl-peptidase 4 inhibitors in patients with diabetes: estimating the risk of the new agents and characterizing the patients. JAMA Dermatol. 2018;154:1152-1158.
6. Haber R, Fayad AM, Stephan F, et al. Bullous pemphigoid associated with linagliptin treatment. JAMA Dermatol. 2016;152:224-226.Management of bullous pemphigoid: the European Dermatology Forum consensus in collaboration with the European Academy of Dermatology and Venereology. Br J Dermatol. 2015;172:867-877.