Article
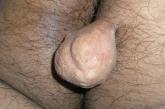
Pedunculated gluteal mass
- Author:
- Zeeshan Afzal, MD
- Richard P. Usatine, MD
Forceful compression made a small papule grow into this 3.5 × 4.5–cm mass.
Article
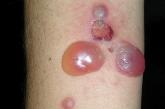
Generalized pruritic blisters and bullous lesions
- Author:
- Zeeshan Afzal, MD
- Richard P. Usatine, MD
In a patient with no history of skin disease, a recent change provided a clue to his condition.
Article
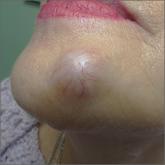
Painful, slow-growing recurrent nodules
- Author:
- Zeeshan Afzal, MD
- Sunand Kallumadanda, MD
- Oscar Sotelo, MD
The patient’s mother and son had similar lesions. Histopathology revealed an uncommon diagnosis.
