User login
In the United States, candida now accounts for between 8% and 12% of all catheter‐associated blood stream infections (BSIs).1 Additionally, crude mortality rates in candidemia exceed 40%, and a recent systematic review demonstrated that the attributable mortality of candidemia ranges from 5% to 71%.2 Candidal BSIs also affect resource utilization. These infections independently increase length of stay and result in substantial excess costs.3 Most cases of candidemia arise in noncritically ill patients and thus may be managed by hospitalists.
Historically, the majority of candidal BSIs were caused by C. albicans. Presently, C. albicans accounts for only half of all yeast BSIs, and approximately 20% of these infections are caused by organisms such as C. glabrata and C. krusei.4 These 2 organisms have either variable or no susceptibility to agents, such as fluconazole, empirically employed against yeast. Parallel with the evolution in microbiology of candidemia has been recognition that inappropriate treatment of these infections independently increases mortality.5 These factors underscore the need for the clinician to treat suspected candidal BSIs aggressively in order to avoid the risks associated with inappropriate treatment.
Efforts to enhance rates of initial appropriate therapy for bacterial infections have encompassed the realization that health care‐associated infections (HAIs) represent a distinct syndrome.6, 7 Traditionally, infections were considered either community‐acquired or nosocomial in origin. However, with the spread of health care delivery beyond the hospital, multiple studies indicate that patients may now present to the emergency department with infections caused by pathogens such as Methicillin‐resistant Staphylococcus aureus (MRSA) and P. aeruginosaorganisms that were previously thought limited to hospital‐acquired processes.69 Furthermore, hospitalists often encounter subjects presenting to the hospital with suspected BSIs who have an active and ongoing interaction with the healthcare system.
The importance of candida as a health care‐associated pathogen in BSI remains unclear. We hypothesized that health care‐associated candidemia (HCAC) represented a distinct clinical entity. In order to confirm our theory, we conducted a retrospective analysis of all cases of candidal BSI at our institution over a 3‐year period.
Methods
We reviewed the records of all patients diagnosed with candidemia at our hospital between January 1, 2004 and December 31, 2006. Our institutional review board approved this study. We included adult patients diagnosed with candidemia. The diagnosis of candidemia was based on the isolation of yeast from the blood in at least one blood culture. We employ the BACTEC 9240 blood Culture System (Becton Dickinson Microbiology Systems, Sparks, MD). We excluded subjects who were admitted to the hospital within one month of a known diagnosis of candidemia.
We defined a nosocomial candidal BSI as the diagnosis of candidemia based on cultures drawn after the patient had been hospitalized for >48 hours. We considered HCAC to be present based on previously employed criteria for identifying HAI.69 Specifically, for patients with candidemia based on blood cultures obtained within 48 hours of hospitalization, a patient had to meet at least 1 of the following criteria: (1) receipt of intravenous therapy outside the hospital, (2) end stage renal disease necessitating hemodialysis (ESRD requiring HD), (3) hospitalization within previous 30 days, (4) residence in a nursing home or long term care facility, or (5) underwent an invasive procedure as an outpatient within 30 days of presentation. Community‐acquired candidemia was restricted to patients whose index culture was drawn within 48 hours of admission but who failed to meet the definition for HCAC.
The prevalence of the various forms of candidemia served as our primary endpoint. In addition, we compared patients with respect to demographic factors, comorbidities, and severity of illness. Severity of illness was calculated based on the Acute Physiology and Chronic Health Evaluation (APACHE) II score. We further noted rates of immune suppression in the cohort and defined this as treatment with corticosteroids (10 mg of prednisone or equivalent daily for more than 30 consecutive days), other immunosuppressants (eg, methotrexate), or chemotherapy. Those with acquired immune deficiency syndrome (AIDS) or another immunodeficiency syndrome were defined as immunosuppressed as well. We examined the distribution of yeast species across the 3 forms of candidemia. Finally, we assessed the prevalence of fluconazole resistance. Fluconazole susceptibilities were determined based on Etest (AB BIODISK, Solna, Sweden). An isolate was considered resistant to fluconazole if the minimum inhibitory concentration was >64 g/mL.
We compared categorical variables with the Fisher's exact test. Continuous variables were analyzed with either the Student's t‐test or a Mann‐Whitney test, as appropriate. All tests were 2 tailed and a P value of <0.05 was assumed to represent statistical significance. Analyses were performed with Stata 9.1 (Stata Corp., College Station, TX).
Results
The final cohort included 223 subjects. The mean age of the patients was 59.6 15.7 years and 49% were male. Nearly one quarter (n = 55) fulfilled our criteria for HCAC. The remainder met the definition for nosocomial candidemia. We observed no cases of community‐acquired candidemia. Most (n = 33) patients with HCAC had exposure to more than 1 health care‐related source and many were initially admitted to the medicine/hospitalist service as opposed to the intensive care unit (ICU). The most common criteria leading to categorization as HCAC was recent hospitalization (n = 30, 54.5% of all HCAC). The median time from recent hospitalization to admission was 17 days (Range: 5‐28 days). Other common reasons for classification as HCAC included ESRD requiring HD (30.9%), residence in a nursing home (25.5%), and undergoing an invasive outpatient procedure (16.4%). More than 75% of subjects with HCAC (n = 42) had central venous catheters in place at presentation. Between 2004 and 2006, the proportion of all candidemia due to HCAC increased from 20.9% to 26.9%, but this difference was not statistically significant.
Patients with HCAC were similar to those with nosocomial candidemia (Table 1). There was no difference in either severity of illness or the frequency of neutropenia. The prevalence of most comorbidities did not differ between those with nosocomial candidemia and persons with HCAC. However, immunosuppression was more prevalent among patients with HCAC (prevalence ratio, 1.67; 95% CI, 1.13‐3.08; P = 0.004). In part this finding is expected given that our definition of HCAC includes exposure to agents which may lead to immunosuppression, such as chemotherapy. Of patients with HCAC, the majority (n = 38, 69.1%) were initially admitted to the general medicine service and not to the ICU.
| Characteristic | Healthcare‐Associated Candidemia (n = 55) | Nosocomial Candidemia (n = 168) | P |
|---|---|---|---|
| |||
| Demographics | |||
| Age, mean SD | 61.0 12.9 | 59.1 16.6 | 0.45 |
| Male, % | 60.0 | 45.8 | 0.08 |
| Severity of illness | |||
| APACHE II score, mean SD | 15.9 6.8 | 14.6 6.3 | 0.21 |
| Co‐morbid illnesses | |||
| Diabetes mellitus, % | 36.4 | 32.7 | 0.87 |
| Malignancy, % | 36.4 | 22.6 | 0.04 |
| ESRD on HD, % | 30.9 | 23.2 | 0.25 |
| AIDS, % | 7.2 | 6.0 | 0.73 |
| Immunosupressed, % | 54.5 | 32.7 | 0.004 |
| White cell status | |||
| ANC, 1000/mm3, mean SD | 10.7 7.2 | 12.3 8.0 | 0.20 |
| Neutropenic, % | 2.0 | 2.2 | 0.91 |
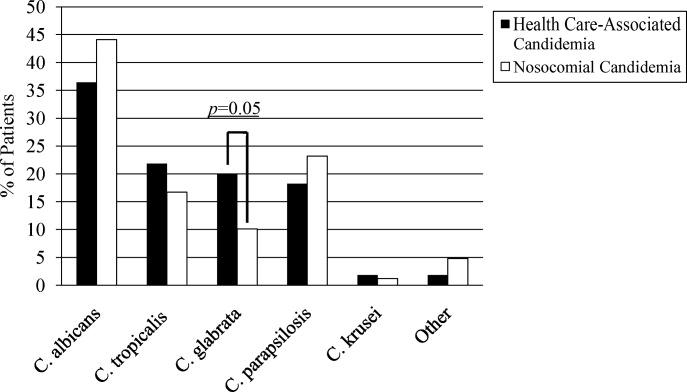
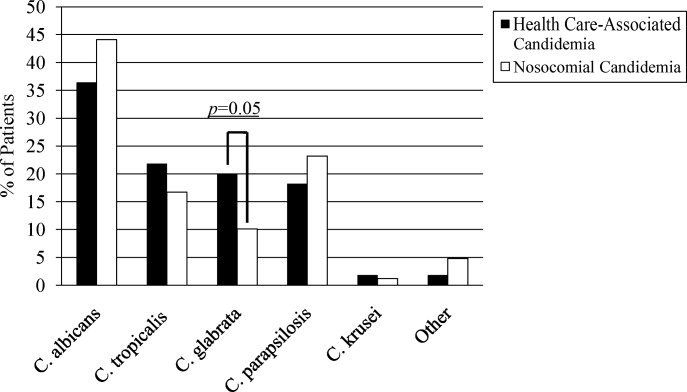
A multitude of various yeast species were recovered (Figure 1). Overall, nonalbicans candida were responsible for nearly 60% of all infections. Nonalbicans yeast were as likely to be recovered in HCAC as in nosocomial yeast infection. Among both types of Candidemia, C. krusei was a rare culprit accounting for fewer than 2% of infections. C. glabrata, however, occurred more often in HCAC. Specifically, C. glabrata represented 1 in 5 cases of HCAC as opposed to approximately 10% of all nosocomial yeast BSIs (P = 0.05). In part reflecting this, fluconazole resistance was noted more often in HCAC (18.2% of patients vs. 7.7% among nosocomial candidemia, P = 0.036). There was no difference in the eventual diagnosis of deep‐seeded yeast infections (ie, endocarditis, endopthlamitis, or osteomyelitis) between those with HCAC and persons with nosocomial candidemia (3 cases in each group).
Discussion
This analysis demonstrates that HCAC accounts for approximately a quarter of all candidemia. Our findings underscore that candidemia can present to the emergency department as an HAI and may potentially be initially cared for by a hospitalist. In addition, patients with HCAC and nosocomial candidemia share many attributes. Furthermore, nonalbicans yeast are as prevalent in HCAC as in nosocomial candidal infection. Nonetheless, there appear to be important differences in these syndromes. Immunosuppression appears to be more common in HCAC as does infection due to C. glabrata.
Others have explored the concept of HCAC. Kung et al.10 described community‐onset candidemia at a single center over a 10‐year period. They described 56 patients and noted that the majority had been recently hospitalized or had ongoing interaction with the healthcare system. Sofair et al.11 followed subjects presenting to emergency departments with candidemia. Overall, more than one‐third met criteria for community‐onset infection. In this analysis, though, Sofair et al.11 did not distinguish between community‐acquired processes and HCAC. From a population perspective, Chen et al.12 explored candidemia in Australia. Among over 1000 patients, the noted that 11.6% represented HCAC and, as we note, that select nonalbicans yeast occurred more often in HCAC than in nosocomial candidemia. Our project builds on and adds to these earlier efforts. First, we confirm the general observation that candidemia is no longer solely a nosocomial pathogen. Second, unlike several of these earlier reports we examined a larger cohort of candidemia. Third, beyond the observations of Chen et al.,12 we note that currently, the proportion of Candidal BSI classified as HACA relative to nosocomial candidemia seems larger than reported in the past. Finally, a unique aspect of our report is that we employed express criteria to define HAI.
Our findings have several implications. First, hospitalists and emergency department physicians, along with others, must remain vigilant when approaching patients presenting to the hospital with signs and symptoms of BSI and multiple risk factors for candidal BSI. The fact that the patient has not been hospitalized should not preclude consideration of and treatment for candidemia. The current evidence does not support broad, empiric use of antifungal agents, as this would lead to excessive costs and potentially expose many patients to unnecessary antifungal coverage. On the other hand, given the association between delayed antifungal therapy and the risk for death in candidemia, failure to consider this infection in at‐risk subjects may have adverse consequences. Second, our observations emphasize the need for clinical risk stratification schemes and rapid diagnostic modalities. Such tools are urgently needed if physicians hope to target antifungal therapies more appropriately. Third, if the clinician opts to initiate therapy for possible HCAC, reliance on fluconazole alone may prove inadequate. As the generalizability of our conclusions is necessarily limited, we recommend that infection control practitioners review local epidemiologic data regarding HCAC so that physicians can have the best available guidance.
Our study has several important limitations. Its retrospective nature exposes it to several forms of bias. The single center design limits the generalizability of our findings. Prospective, multicenter studies are needed to validate our results. Additionally, no universally accepted criteria exist to define HAI syndromes. Nonetheless, the criteria we employed have been used by others. We also lacked data on exposure to recent broad spectrum antimicrobials. Selection pressure via exposure to such agents is a risk factor for candidemia and without this data we cannot gauge the impact of this on our findings. Finally, we cannot control for the possibility that some patients were miscategorized. This could have arisen because of: (1) either limitations inherent in the definition of HCAC or (2) because the clinician delayed the decision to obtain blood cultures. Some patients classified as nosocomial may actually have had HCAC or community‐acquired diseasebut for some reason blood cultures were not drawn at time of admission but were deferred until later. Although a difficult issue to address in any study of the epidemiology of infection, the significance of this misclassification bias must be considered a significant concern.
In summary, Candidemia can be the cause of BSI presenting to the hospital. Moreover, HCAC represents a significant proportion of all Candidemia. Although patients with HCAC and nosocomial candidemia share select characteristics, there appear to be some differences in the microbiology of these syndromes.
- CDC.National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1990‐‐May 1999, issued June 1999.Am J Infect Control.1999;27:520–532.
- ,,.Attributable mortality of candidemia: a systematic review of matched cohort and case‐control studies.Eur J Clin Microbiol Infect Dis.2006;25:419–425.
- ,,, et al.Excess mortality, hospital stay, and cost due to candidemia: a case‐control study using data from population‐based candidemia surveillance.Infect Control Hosp Epidemiol.2005;26:540–547.
- .Shifting patterns in the epidemiology of nosocomial Candida infections.Chest.2003;123:500S–503S.
- ,,.Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality.Antimicrob Agents Chemother.2005;49:3640–3645.
- ,,, et al.Healthcare‐associated bloodstream infection: A distinct entity? Insights from a large U.S. database.Crit Care Med.2006;34:2588–2595.
- ,,, et al.Health care‐‐associated bloodstream infections in adults: a reason to change the accepted definition of community‐acquired infections.Ann Intern Med.2002;137:791–797.
- ,.Epidemiology of healthcare‐associated pneumonia (HCAP).Semin Respir Crit Care Med.2009;30:10–15.
- ,,, et al.Health care associated pneumonia and community‐acquired pneumonia: a single‐center experience.Antimicrob Agents Chemother.2007;51:3568–3573.
- ,,, et al.Communtiy‐onset candidemia at a university hospital, 1995‐2005.J Microbiol Immunol Infect.2007;40:355–363.
- ,,, et al.Epidemiology of community‐onset candidemia in Connecticut and Maryland.Clin Infect Dis.2006;43:32–39.
- ,,, et al.Active surveillance for candidemia, Australia.Emerg Infect Dis.2006;12:1508–1516.
In the United States, candida now accounts for between 8% and 12% of all catheter‐associated blood stream infections (BSIs).1 Additionally, crude mortality rates in candidemia exceed 40%, and a recent systematic review demonstrated that the attributable mortality of candidemia ranges from 5% to 71%.2 Candidal BSIs also affect resource utilization. These infections independently increase length of stay and result in substantial excess costs.3 Most cases of candidemia arise in noncritically ill patients and thus may be managed by hospitalists.
Historically, the majority of candidal BSIs were caused by C. albicans. Presently, C. albicans accounts for only half of all yeast BSIs, and approximately 20% of these infections are caused by organisms such as C. glabrata and C. krusei.4 These 2 organisms have either variable or no susceptibility to agents, such as fluconazole, empirically employed against yeast. Parallel with the evolution in microbiology of candidemia has been recognition that inappropriate treatment of these infections independently increases mortality.5 These factors underscore the need for the clinician to treat suspected candidal BSIs aggressively in order to avoid the risks associated with inappropriate treatment.
Efforts to enhance rates of initial appropriate therapy for bacterial infections have encompassed the realization that health care‐associated infections (HAIs) represent a distinct syndrome.6, 7 Traditionally, infections were considered either community‐acquired or nosocomial in origin. However, with the spread of health care delivery beyond the hospital, multiple studies indicate that patients may now present to the emergency department with infections caused by pathogens such as Methicillin‐resistant Staphylococcus aureus (MRSA) and P. aeruginosaorganisms that were previously thought limited to hospital‐acquired processes.69 Furthermore, hospitalists often encounter subjects presenting to the hospital with suspected BSIs who have an active and ongoing interaction with the healthcare system.
The importance of candida as a health care‐associated pathogen in BSI remains unclear. We hypothesized that health care‐associated candidemia (HCAC) represented a distinct clinical entity. In order to confirm our theory, we conducted a retrospective analysis of all cases of candidal BSI at our institution over a 3‐year period.
Methods
We reviewed the records of all patients diagnosed with candidemia at our hospital between January 1, 2004 and December 31, 2006. Our institutional review board approved this study. We included adult patients diagnosed with candidemia. The diagnosis of candidemia was based on the isolation of yeast from the blood in at least one blood culture. We employ the BACTEC 9240 blood Culture System (Becton Dickinson Microbiology Systems, Sparks, MD). We excluded subjects who were admitted to the hospital within one month of a known diagnosis of candidemia.
We defined a nosocomial candidal BSI as the diagnosis of candidemia based on cultures drawn after the patient had been hospitalized for >48 hours. We considered HCAC to be present based on previously employed criteria for identifying HAI.69 Specifically, for patients with candidemia based on blood cultures obtained within 48 hours of hospitalization, a patient had to meet at least 1 of the following criteria: (1) receipt of intravenous therapy outside the hospital, (2) end stage renal disease necessitating hemodialysis (ESRD requiring HD), (3) hospitalization within previous 30 days, (4) residence in a nursing home or long term care facility, or (5) underwent an invasive procedure as an outpatient within 30 days of presentation. Community‐acquired candidemia was restricted to patients whose index culture was drawn within 48 hours of admission but who failed to meet the definition for HCAC.
The prevalence of the various forms of candidemia served as our primary endpoint. In addition, we compared patients with respect to demographic factors, comorbidities, and severity of illness. Severity of illness was calculated based on the Acute Physiology and Chronic Health Evaluation (APACHE) II score. We further noted rates of immune suppression in the cohort and defined this as treatment with corticosteroids (10 mg of prednisone or equivalent daily for more than 30 consecutive days), other immunosuppressants (eg, methotrexate), or chemotherapy. Those with acquired immune deficiency syndrome (AIDS) or another immunodeficiency syndrome were defined as immunosuppressed as well. We examined the distribution of yeast species across the 3 forms of candidemia. Finally, we assessed the prevalence of fluconazole resistance. Fluconazole susceptibilities were determined based on Etest (AB BIODISK, Solna, Sweden). An isolate was considered resistant to fluconazole if the minimum inhibitory concentration was >64 g/mL.
We compared categorical variables with the Fisher's exact test. Continuous variables were analyzed with either the Student's t‐test or a Mann‐Whitney test, as appropriate. All tests were 2 tailed and a P value of <0.05 was assumed to represent statistical significance. Analyses were performed with Stata 9.1 (Stata Corp., College Station, TX).
Results
The final cohort included 223 subjects. The mean age of the patients was 59.6 15.7 years and 49% were male. Nearly one quarter (n = 55) fulfilled our criteria for HCAC. The remainder met the definition for nosocomial candidemia. We observed no cases of community‐acquired candidemia. Most (n = 33) patients with HCAC had exposure to more than 1 health care‐related source and many were initially admitted to the medicine/hospitalist service as opposed to the intensive care unit (ICU). The most common criteria leading to categorization as HCAC was recent hospitalization (n = 30, 54.5% of all HCAC). The median time from recent hospitalization to admission was 17 days (Range: 5‐28 days). Other common reasons for classification as HCAC included ESRD requiring HD (30.9%), residence in a nursing home (25.5%), and undergoing an invasive outpatient procedure (16.4%). More than 75% of subjects with HCAC (n = 42) had central venous catheters in place at presentation. Between 2004 and 2006, the proportion of all candidemia due to HCAC increased from 20.9% to 26.9%, but this difference was not statistically significant.
Patients with HCAC were similar to those with nosocomial candidemia (Table 1). There was no difference in either severity of illness or the frequency of neutropenia. The prevalence of most comorbidities did not differ between those with nosocomial candidemia and persons with HCAC. However, immunosuppression was more prevalent among patients with HCAC (prevalence ratio, 1.67; 95% CI, 1.13‐3.08; P = 0.004). In part this finding is expected given that our definition of HCAC includes exposure to agents which may lead to immunosuppression, such as chemotherapy. Of patients with HCAC, the majority (n = 38, 69.1%) were initially admitted to the general medicine service and not to the ICU.
| Characteristic | Healthcare‐Associated Candidemia (n = 55) | Nosocomial Candidemia (n = 168) | P |
|---|---|---|---|
| |||
| Demographics | |||
| Age, mean SD | 61.0 12.9 | 59.1 16.6 | 0.45 |
| Male, % | 60.0 | 45.8 | 0.08 |
| Severity of illness | |||
| APACHE II score, mean SD | 15.9 6.8 | 14.6 6.3 | 0.21 |
| Co‐morbid illnesses | |||
| Diabetes mellitus, % | 36.4 | 32.7 | 0.87 |
| Malignancy, % | 36.4 | 22.6 | 0.04 |
| ESRD on HD, % | 30.9 | 23.2 | 0.25 |
| AIDS, % | 7.2 | 6.0 | 0.73 |
| Immunosupressed, % | 54.5 | 32.7 | 0.004 |
| White cell status | |||
| ANC, 1000/mm3, mean SD | 10.7 7.2 | 12.3 8.0 | 0.20 |
| Neutropenic, % | 2.0 | 2.2 | 0.91 |
A multitude of various yeast species were recovered (Figure 1). Overall, nonalbicans candida were responsible for nearly 60% of all infections. Nonalbicans yeast were as likely to be recovered in HCAC as in nosocomial yeast infection. Among both types of Candidemia, C. krusei was a rare culprit accounting for fewer than 2% of infections. C. glabrata, however, occurred more often in HCAC. Specifically, C. glabrata represented 1 in 5 cases of HCAC as opposed to approximately 10% of all nosocomial yeast BSIs (P = 0.05). In part reflecting this, fluconazole resistance was noted more often in HCAC (18.2% of patients vs. 7.7% among nosocomial candidemia, P = 0.036). There was no difference in the eventual diagnosis of deep‐seeded yeast infections (ie, endocarditis, endopthlamitis, or osteomyelitis) between those with HCAC and persons with nosocomial candidemia (3 cases in each group).

Discussion
This analysis demonstrates that HCAC accounts for approximately a quarter of all candidemia. Our findings underscore that candidemia can present to the emergency department as an HAI and may potentially be initially cared for by a hospitalist. In addition, patients with HCAC and nosocomial candidemia share many attributes. Furthermore, nonalbicans yeast are as prevalent in HCAC as in nosocomial candidal infection. Nonetheless, there appear to be important differences in these syndromes. Immunosuppression appears to be more common in HCAC as does infection due to C. glabrata.
Others have explored the concept of HCAC. Kung et al.10 described community‐onset candidemia at a single center over a 10‐year period. They described 56 patients and noted that the majority had been recently hospitalized or had ongoing interaction with the healthcare system. Sofair et al.11 followed subjects presenting to emergency departments with candidemia. Overall, more than one‐third met criteria for community‐onset infection. In this analysis, though, Sofair et al.11 did not distinguish between community‐acquired processes and HCAC. From a population perspective, Chen et al.12 explored candidemia in Australia. Among over 1000 patients, the noted that 11.6% represented HCAC and, as we note, that select nonalbicans yeast occurred more often in HCAC than in nosocomial candidemia. Our project builds on and adds to these earlier efforts. First, we confirm the general observation that candidemia is no longer solely a nosocomial pathogen. Second, unlike several of these earlier reports we examined a larger cohort of candidemia. Third, beyond the observations of Chen et al.,12 we note that currently, the proportion of Candidal BSI classified as HACA relative to nosocomial candidemia seems larger than reported in the past. Finally, a unique aspect of our report is that we employed express criteria to define HAI.
Our findings have several implications. First, hospitalists and emergency department physicians, along with others, must remain vigilant when approaching patients presenting to the hospital with signs and symptoms of BSI and multiple risk factors for candidal BSI. The fact that the patient has not been hospitalized should not preclude consideration of and treatment for candidemia. The current evidence does not support broad, empiric use of antifungal agents, as this would lead to excessive costs and potentially expose many patients to unnecessary antifungal coverage. On the other hand, given the association between delayed antifungal therapy and the risk for death in candidemia, failure to consider this infection in at‐risk subjects may have adverse consequences. Second, our observations emphasize the need for clinical risk stratification schemes and rapid diagnostic modalities. Such tools are urgently needed if physicians hope to target antifungal therapies more appropriately. Third, if the clinician opts to initiate therapy for possible HCAC, reliance on fluconazole alone may prove inadequate. As the generalizability of our conclusions is necessarily limited, we recommend that infection control practitioners review local epidemiologic data regarding HCAC so that physicians can have the best available guidance.
Our study has several important limitations. Its retrospective nature exposes it to several forms of bias. The single center design limits the generalizability of our findings. Prospective, multicenter studies are needed to validate our results. Additionally, no universally accepted criteria exist to define HAI syndromes. Nonetheless, the criteria we employed have been used by others. We also lacked data on exposure to recent broad spectrum antimicrobials. Selection pressure via exposure to such agents is a risk factor for candidemia and without this data we cannot gauge the impact of this on our findings. Finally, we cannot control for the possibility that some patients were miscategorized. This could have arisen because of: (1) either limitations inherent in the definition of HCAC or (2) because the clinician delayed the decision to obtain blood cultures. Some patients classified as nosocomial may actually have had HCAC or community‐acquired diseasebut for some reason blood cultures were not drawn at time of admission but were deferred until later. Although a difficult issue to address in any study of the epidemiology of infection, the significance of this misclassification bias must be considered a significant concern.
In summary, Candidemia can be the cause of BSI presenting to the hospital. Moreover, HCAC represents a significant proportion of all Candidemia. Although patients with HCAC and nosocomial candidemia share select characteristics, there appear to be some differences in the microbiology of these syndromes.
In the United States, candida now accounts for between 8% and 12% of all catheter‐associated blood stream infections (BSIs).1 Additionally, crude mortality rates in candidemia exceed 40%, and a recent systematic review demonstrated that the attributable mortality of candidemia ranges from 5% to 71%.2 Candidal BSIs also affect resource utilization. These infections independently increase length of stay and result in substantial excess costs.3 Most cases of candidemia arise in noncritically ill patients and thus may be managed by hospitalists.
Historically, the majority of candidal BSIs were caused by C. albicans. Presently, C. albicans accounts for only half of all yeast BSIs, and approximately 20% of these infections are caused by organisms such as C. glabrata and C. krusei.4 These 2 organisms have either variable or no susceptibility to agents, such as fluconazole, empirically employed against yeast. Parallel with the evolution in microbiology of candidemia has been recognition that inappropriate treatment of these infections independently increases mortality.5 These factors underscore the need for the clinician to treat suspected candidal BSIs aggressively in order to avoid the risks associated with inappropriate treatment.
Efforts to enhance rates of initial appropriate therapy for bacterial infections have encompassed the realization that health care‐associated infections (HAIs) represent a distinct syndrome.6, 7 Traditionally, infections were considered either community‐acquired or nosocomial in origin. However, with the spread of health care delivery beyond the hospital, multiple studies indicate that patients may now present to the emergency department with infections caused by pathogens such as Methicillin‐resistant Staphylococcus aureus (MRSA) and P. aeruginosaorganisms that were previously thought limited to hospital‐acquired processes.69 Furthermore, hospitalists often encounter subjects presenting to the hospital with suspected BSIs who have an active and ongoing interaction with the healthcare system.
The importance of candida as a health care‐associated pathogen in BSI remains unclear. We hypothesized that health care‐associated candidemia (HCAC) represented a distinct clinical entity. In order to confirm our theory, we conducted a retrospective analysis of all cases of candidal BSI at our institution over a 3‐year period.
Methods
We reviewed the records of all patients diagnosed with candidemia at our hospital between January 1, 2004 and December 31, 2006. Our institutional review board approved this study. We included adult patients diagnosed with candidemia. The diagnosis of candidemia was based on the isolation of yeast from the blood in at least one blood culture. We employ the BACTEC 9240 blood Culture System (Becton Dickinson Microbiology Systems, Sparks, MD). We excluded subjects who were admitted to the hospital within one month of a known diagnosis of candidemia.
We defined a nosocomial candidal BSI as the diagnosis of candidemia based on cultures drawn after the patient had been hospitalized for >48 hours. We considered HCAC to be present based on previously employed criteria for identifying HAI.69 Specifically, for patients with candidemia based on blood cultures obtained within 48 hours of hospitalization, a patient had to meet at least 1 of the following criteria: (1) receipt of intravenous therapy outside the hospital, (2) end stage renal disease necessitating hemodialysis (ESRD requiring HD), (3) hospitalization within previous 30 days, (4) residence in a nursing home or long term care facility, or (5) underwent an invasive procedure as an outpatient within 30 days of presentation. Community‐acquired candidemia was restricted to patients whose index culture was drawn within 48 hours of admission but who failed to meet the definition for HCAC.
The prevalence of the various forms of candidemia served as our primary endpoint. In addition, we compared patients with respect to demographic factors, comorbidities, and severity of illness. Severity of illness was calculated based on the Acute Physiology and Chronic Health Evaluation (APACHE) II score. We further noted rates of immune suppression in the cohort and defined this as treatment with corticosteroids (10 mg of prednisone or equivalent daily for more than 30 consecutive days), other immunosuppressants (eg, methotrexate), or chemotherapy. Those with acquired immune deficiency syndrome (AIDS) or another immunodeficiency syndrome were defined as immunosuppressed as well. We examined the distribution of yeast species across the 3 forms of candidemia. Finally, we assessed the prevalence of fluconazole resistance. Fluconazole susceptibilities were determined based on Etest (AB BIODISK, Solna, Sweden). An isolate was considered resistant to fluconazole if the minimum inhibitory concentration was >64 g/mL.
We compared categorical variables with the Fisher's exact test. Continuous variables were analyzed with either the Student's t‐test or a Mann‐Whitney test, as appropriate. All tests were 2 tailed and a P value of <0.05 was assumed to represent statistical significance. Analyses were performed with Stata 9.1 (Stata Corp., College Station, TX).
Results
The final cohort included 223 subjects. The mean age of the patients was 59.6 15.7 years and 49% were male. Nearly one quarter (n = 55) fulfilled our criteria for HCAC. The remainder met the definition for nosocomial candidemia. We observed no cases of community‐acquired candidemia. Most (n = 33) patients with HCAC had exposure to more than 1 health care‐related source and many were initially admitted to the medicine/hospitalist service as opposed to the intensive care unit (ICU). The most common criteria leading to categorization as HCAC was recent hospitalization (n = 30, 54.5% of all HCAC). The median time from recent hospitalization to admission was 17 days (Range: 5‐28 days). Other common reasons for classification as HCAC included ESRD requiring HD (30.9%), residence in a nursing home (25.5%), and undergoing an invasive outpatient procedure (16.4%). More than 75% of subjects with HCAC (n = 42) had central venous catheters in place at presentation. Between 2004 and 2006, the proportion of all candidemia due to HCAC increased from 20.9% to 26.9%, but this difference was not statistically significant.
Patients with HCAC were similar to those with nosocomial candidemia (Table 1). There was no difference in either severity of illness or the frequency of neutropenia. The prevalence of most comorbidities did not differ between those with nosocomial candidemia and persons with HCAC. However, immunosuppression was more prevalent among patients with HCAC (prevalence ratio, 1.67; 95% CI, 1.13‐3.08; P = 0.004). In part this finding is expected given that our definition of HCAC includes exposure to agents which may lead to immunosuppression, such as chemotherapy. Of patients with HCAC, the majority (n = 38, 69.1%) were initially admitted to the general medicine service and not to the ICU.
| Characteristic | Healthcare‐Associated Candidemia (n = 55) | Nosocomial Candidemia (n = 168) | P |
|---|---|---|---|
| |||
| Demographics | |||
| Age, mean SD | 61.0 12.9 | 59.1 16.6 | 0.45 |
| Male, % | 60.0 | 45.8 | 0.08 |
| Severity of illness | |||
| APACHE II score, mean SD | 15.9 6.8 | 14.6 6.3 | 0.21 |
| Co‐morbid illnesses | |||
| Diabetes mellitus, % | 36.4 | 32.7 | 0.87 |
| Malignancy, % | 36.4 | 22.6 | 0.04 |
| ESRD on HD, % | 30.9 | 23.2 | 0.25 |
| AIDS, % | 7.2 | 6.0 | 0.73 |
| Immunosupressed, % | 54.5 | 32.7 | 0.004 |
| White cell status | |||
| ANC, 1000/mm3, mean SD | 10.7 7.2 | 12.3 8.0 | 0.20 |
| Neutropenic, % | 2.0 | 2.2 | 0.91 |
A multitude of various yeast species were recovered (Figure 1). Overall, nonalbicans candida were responsible for nearly 60% of all infections. Nonalbicans yeast were as likely to be recovered in HCAC as in nosocomial yeast infection. Among both types of Candidemia, C. krusei was a rare culprit accounting for fewer than 2% of infections. C. glabrata, however, occurred more often in HCAC. Specifically, C. glabrata represented 1 in 5 cases of HCAC as opposed to approximately 10% of all nosocomial yeast BSIs (P = 0.05). In part reflecting this, fluconazole resistance was noted more often in HCAC (18.2% of patients vs. 7.7% among nosocomial candidemia, P = 0.036). There was no difference in the eventual diagnosis of deep‐seeded yeast infections (ie, endocarditis, endopthlamitis, or osteomyelitis) between those with HCAC and persons with nosocomial candidemia (3 cases in each group).

Discussion
This analysis demonstrates that HCAC accounts for approximately a quarter of all candidemia. Our findings underscore that candidemia can present to the emergency department as an HAI and may potentially be initially cared for by a hospitalist. In addition, patients with HCAC and nosocomial candidemia share many attributes. Furthermore, nonalbicans yeast are as prevalent in HCAC as in nosocomial candidal infection. Nonetheless, there appear to be important differences in these syndromes. Immunosuppression appears to be more common in HCAC as does infection due to C. glabrata.
Others have explored the concept of HCAC. Kung et al.10 described community‐onset candidemia at a single center over a 10‐year period. They described 56 patients and noted that the majority had been recently hospitalized or had ongoing interaction with the healthcare system. Sofair et al.11 followed subjects presenting to emergency departments with candidemia. Overall, more than one‐third met criteria for community‐onset infection. In this analysis, though, Sofair et al.11 did not distinguish between community‐acquired processes and HCAC. From a population perspective, Chen et al.12 explored candidemia in Australia. Among over 1000 patients, the noted that 11.6% represented HCAC and, as we note, that select nonalbicans yeast occurred more often in HCAC than in nosocomial candidemia. Our project builds on and adds to these earlier efforts. First, we confirm the general observation that candidemia is no longer solely a nosocomial pathogen. Second, unlike several of these earlier reports we examined a larger cohort of candidemia. Third, beyond the observations of Chen et al.,12 we note that currently, the proportion of Candidal BSI classified as HACA relative to nosocomial candidemia seems larger than reported in the past. Finally, a unique aspect of our report is that we employed express criteria to define HAI.
Our findings have several implications. First, hospitalists and emergency department physicians, along with others, must remain vigilant when approaching patients presenting to the hospital with signs and symptoms of BSI and multiple risk factors for candidal BSI. The fact that the patient has not been hospitalized should not preclude consideration of and treatment for candidemia. The current evidence does not support broad, empiric use of antifungal agents, as this would lead to excessive costs and potentially expose many patients to unnecessary antifungal coverage. On the other hand, given the association between delayed antifungal therapy and the risk for death in candidemia, failure to consider this infection in at‐risk subjects may have adverse consequences. Second, our observations emphasize the need for clinical risk stratification schemes and rapid diagnostic modalities. Such tools are urgently needed if physicians hope to target antifungal therapies more appropriately. Third, if the clinician opts to initiate therapy for possible HCAC, reliance on fluconazole alone may prove inadequate. As the generalizability of our conclusions is necessarily limited, we recommend that infection control practitioners review local epidemiologic data regarding HCAC so that physicians can have the best available guidance.
Our study has several important limitations. Its retrospective nature exposes it to several forms of bias. The single center design limits the generalizability of our findings. Prospective, multicenter studies are needed to validate our results. Additionally, no universally accepted criteria exist to define HAI syndromes. Nonetheless, the criteria we employed have been used by others. We also lacked data on exposure to recent broad spectrum antimicrobials. Selection pressure via exposure to such agents is a risk factor for candidemia and without this data we cannot gauge the impact of this on our findings. Finally, we cannot control for the possibility that some patients were miscategorized. This could have arisen because of: (1) either limitations inherent in the definition of HCAC or (2) because the clinician delayed the decision to obtain blood cultures. Some patients classified as nosocomial may actually have had HCAC or community‐acquired diseasebut for some reason blood cultures were not drawn at time of admission but were deferred until later. Although a difficult issue to address in any study of the epidemiology of infection, the significance of this misclassification bias must be considered a significant concern.
In summary, Candidemia can be the cause of BSI presenting to the hospital. Moreover, HCAC represents a significant proportion of all Candidemia. Although patients with HCAC and nosocomial candidemia share select characteristics, there appear to be some differences in the microbiology of these syndromes.
- CDC.National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1990‐‐May 1999, issued June 1999.Am J Infect Control.1999;27:520–532.
- ,,.Attributable mortality of candidemia: a systematic review of matched cohort and case‐control studies.Eur J Clin Microbiol Infect Dis.2006;25:419–425.
- ,,, et al.Excess mortality, hospital stay, and cost due to candidemia: a case‐control study using data from population‐based candidemia surveillance.Infect Control Hosp Epidemiol.2005;26:540–547.
- .Shifting patterns in the epidemiology of nosocomial Candida infections.Chest.2003;123:500S–503S.
- ,,.Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality.Antimicrob Agents Chemother.2005;49:3640–3645.
- ,,, et al.Healthcare‐associated bloodstream infection: A distinct entity? Insights from a large U.S. database.Crit Care Med.2006;34:2588–2595.
- ,,, et al.Health care‐‐associated bloodstream infections in adults: a reason to change the accepted definition of community‐acquired infections.Ann Intern Med.2002;137:791–797.
- ,.Epidemiology of healthcare‐associated pneumonia (HCAP).Semin Respir Crit Care Med.2009;30:10–15.
- ,,, et al.Health care associated pneumonia and community‐acquired pneumonia: a single‐center experience.Antimicrob Agents Chemother.2007;51:3568–3573.
- ,,, et al.Communtiy‐onset candidemia at a university hospital, 1995‐2005.J Microbiol Immunol Infect.2007;40:355–363.
- ,,, et al.Epidemiology of community‐onset candidemia in Connecticut and Maryland.Clin Infect Dis.2006;43:32–39.
- ,,, et al.Active surveillance for candidemia, Australia.Emerg Infect Dis.2006;12:1508–1516.
- CDC.National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1990‐‐May 1999, issued June 1999.Am J Infect Control.1999;27:520–532.
- ,,.Attributable mortality of candidemia: a systematic review of matched cohort and case‐control studies.Eur J Clin Microbiol Infect Dis.2006;25:419–425.
- ,,, et al.Excess mortality, hospital stay, and cost due to candidemia: a case‐control study using data from population‐based candidemia surveillance.Infect Control Hosp Epidemiol.2005;26:540–547.
- .Shifting patterns in the epidemiology of nosocomial Candida infections.Chest.2003;123:500S–503S.
- ,,.Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality.Antimicrob Agents Chemother.2005;49:3640–3645.
- ,,, et al.Healthcare‐associated bloodstream infection: A distinct entity? Insights from a large U.S. database.Crit Care Med.2006;34:2588–2595.
- ,,, et al.Health care‐‐associated bloodstream infections in adults: a reason to change the accepted definition of community‐acquired infections.Ann Intern Med.2002;137:791–797.
- ,.Epidemiology of healthcare‐associated pneumonia (HCAP).Semin Respir Crit Care Med.2009;30:10–15.
- ,,, et al.Health care associated pneumonia and community‐acquired pneumonia: a single‐center experience.Antimicrob Agents Chemother.2007;51:3568–3573.
- ,,, et al.Communtiy‐onset candidemia at a university hospital, 1995‐2005.J Microbiol Immunol Infect.2007;40:355–363.
- ,,, et al.Epidemiology of community‐onset candidemia in Connecticut and Maryland.Clin Infect Dis.2006;43:32–39.
- ,,, et al.Active surveillance for candidemia, Australia.Emerg Infect Dis.2006;12:1508–1516.