User login
The adult female patient presenting with severe acne vulgaris may raise special diagnostic concerns, including consideration of an underlying hormonal disorder. Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in women of reproductive age with an estimated prevalence as high as 12%.1 Many women with undiagnosed PCOS may be referred to dermatologists for evaluation of its cutaneous manifestations of hyperandrogenism including acne, hirsutism, and androgenic alopecia.2 Given the prevalence of PCOS and its long-term health implications, dermatologists can play an important role in the initial evaluation of these patients. Acne and androgenic alopecia, however, are quite common, and in the absence of red flags such as menstrual irregularities, virilization, visual field deficits, or signs of Cushing syndrome,3 clinicians must decide when to pursue limited versus comprehensive evaluation.
Despite being common in patients with PCOS, a recent study suggests that acne is an unreliable marker of biochemical hyperandrogenism, and specific features of acne (ie, lesion counts, lesional types, distribution) cannot reliably discriminate women who meet PCOS diagnostic criteria from those who do not.4 Similarly, the study found that androgenic alopecia was not associated with biochemical hyperandrogenism and was no more common in women with PCOS than women of similar age in a high-risk population. Unlike acne and androgenic alopecia, however, the study identified hirsutism, especially truncal hirsutism, as a reliable indicator of hyperandrogenemia and PCOS. Hirsutism also is associated with metabolic sequelae of PCOS. These findings suggest that hirsutism, but not acne or androgenic alopecia, in a female of reproductive age warrants a workup for PCOS.4 This report is consistent with a recommendation from the Androgen Excess and Polycystic Ovary Syndrome Society (AE-PCOS) to pursue a diagnostic evaluation in any woman presenting with hirsutism.5 Acanthosis nigricans also was found to be a reliable indicator of hyperandrogenemia, PCOS, and associated metabolic derangement. Thus, although recent evidence indicates that acne as an isolated cutaneous finding does not warrant further diagnostic evaluation, acne in the setting of hirsutism, acanthosis nigricans, menstrual irregularities, or additional specific signs of endocrine dysregulation should prompt focused workup.4
Multiple clinical practice guidelines for the evaluation of hirsutism and PCOS based on literature review and expert opinion have been proposed5-8; however, these guidelines vary in recommendations for routine diagnostic steps to exclude mimickers of PCOS such as prolactinoma/pituitary adenoma and congenital adrenal hyperplasia (CAH)(Table). In 2009, an AE-PCOS task force suggested that routine testing of thyroid function and serum prolactin in the absence of additional clinical signs may not be necessary based on the low prevalence of thyroid disorders and hyperprolactinemia in patients presenting with hyperandrogenism.6 In 2013, the Endocrine Society’s (ENDO) clinical guideline, however, recommended routine measurement of serum thyroid-stimulating hormone (TSH) to exclude thyroid disease and serum prolactin to exclude hyperprolactinemia in all women before making a diagnosis of PCOS.7 In 2015, the AE-PCOS collaborated with the American Association of Clinical Endocrinologists (AACE) and American College of Endocrinology to publish an updated guideline for best practices, which was consistent with the prior AE-PCOS recommendation in 2009 for routine screening including to test 17-hydroxyprogesterone to exclude nonclassical CAH.8
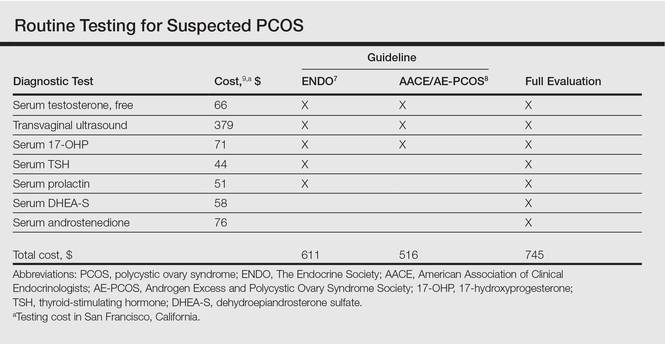
Importantly, these recommendations for routine testing for mimickers of PCOS are based on the rare prevalence of these etiologies in multiple studies of women presenting for hyperandrogenism. One study included 873 women presenting to an academic reproductive endocrine clinic for evaluation of symptoms potentially related to androgen excess. In addition to cutaneous manifestations of hirsutism, acne, and alopecia, the study also included women presenting with oligomenorrhea/amenorrhea, ovulatory dysfunction, and even virilization.10 A second study included 950 women presenting to academic endocrine departments with hirsutism, acne, or androgenic alopecia.11 Both studies defined hirsutism as having a modified Ferriman-Gallwey score of 6 or greater. Both studies also only measured serum prolactin or TSH when clinically indicated (ie, patients with ovulatory dysfunction).10,11
The diagnostic yield of tests for mimickers of PCOS was exceedingly low in both studies. For example, of the patients evaluated, only 0.4% to 0.7% had thyroid dysfunction, 0% to 0.3% had hyperprolactinemia, 0.2% had androgen-secreting neoplasms, 2.1% to 4.3% had nonclassical CAH, 0.7% had CAH, and 3.8% had HAIR-AN (hyperandrogenism, insulin resistance, and acanthosis nigricans) syndrome.10,11 Because patients in both studies were only tested for hyperprolactinemia and thyroid dysfunction when clinically indicated, it is probable that routine screening without clinical indication would result in even lower yields.
Given the increasing importance of high-value, cost-conscious care,12 clinicians must consider the costs associated with testing in the face of low pretest probability. Although some studies have examined the cost-effectiveness of fertility treatments in PCOS,13,14 no studies have examined the cost-effectiveness of diagnostic strategies for PCOS. Cost-effectiveness studies are emerging to provide important guidance on high-value, cost-conscious diagnostic evaluation and monitoring15 and are much needed in dermatology.16,17
In the case of PCOS, the costs of some diagnostic tests are relatively low. For example, based on estimates from Healthcare Bluebook,9 serum TSH and prolactin tests in San Francisco, California, are $44 and $51, respectively. However, the cumulative costs for even the most stringent routine workup for PCOS recommended in the AACE/AE-PCOS guideline consisting of a free testosterone measurement, 17-hydroxyprogesterone, and transvaginal ultrasound would still cost a total of $516. Additional TSH and prolactin tests recommended by ENDO would increase the cost of PCOS testing by approximately 18%. Routine testing for additional serum androgens—dehydroepiandrosterone sulfate (DHEA-S) and androstenedione—would further increase this amount by an additional $134 to a total cost of $745. The ENDO guideline only recommends DHEA-S testing to assist in the diagnosis of an androgen-secreting tumor when signs of virilization are present, while the AACE/AE-PCOS guideline discourages routine testing for DHEA-S and androstenedione based on the low frequency of cases in which these androgens are elevated in isolation.7,8
Although the selection of tests influences total cost, the setting of tests (ie, hospitals, physician offices, independent test settings) also can contribute to wide variations in cost. For example, Healthcare Bluebook’s estimates for transvaginal ultrasound in Chicago, Illinois, range from $236 to more than $740.9 When the separate physician visit fees are included, the total cost of a routine diagnostic evaluation of a patient with acne or hirsutism concerning for PCOS is not trivial.
Large national clinical registries and formal cost-effectiveness analyses are necessary to shed light on this issue, but it is clear that clinicians should rely on their clinical judgment when ordering laboratory tests in the evaluation for PCOS given the apparent low yield of routine screening for PCOS mimickers in the absence of clinical indications. For example, a TSH would not be warranted in a patient without evidence of thyroid dysfunction (ie, weight gain, fatigue, constipation, menstrual irregularities). Similarly, clinicians should routinely consider the principle of high-value care: whether the results of a test will change management of the patient. For example, a woman with amenorrhea and severe acne who already meets diagnostic criteria for PCOS would benefit from a combined oral contraceptive for both acne and endometrial protection. An ovarian ultrasound may not be needed to confirm the diagnosis unless there is suspicion for an ovarian condition other than PCOS causing the symptoms.
Finally, clinicians should discuss testing options and involve patients in decisions around testing. Although PCOS treatments generally target individual symptoms rather than the syndrome as a whole, confirmation of a PCOS diagnosis importantly informs women of their risk for cardiovascular and metabolic disease. The ENDO recommends screening for impaired glucose tolerance, type 2 diabetes mellitus, obesity, family history of early cardiovascular disease, tobacco use, hypertension, dyslipidemia, and obstructive sleep apnea in all women with PCOS, including nonobese patients.7 Ongoing efforts to gain and understand evidence to support high-value, cost-conscious care should be prioritized and kept in balance with shared decision-making in individual patients suspected of having PCOS.
- March WA, Moore VM, Willson KJ, et al. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25:544-551.
- Sivayoganathan D, Maruthini D, Glanville JM, et al. Full investigation of patients with polycystic ovary syndrome (PCOS) presenting to four different clinical specialties reveals significant differences and undiagnosed morbidity. Hum Fertil. 2011;14:261-265.
- Schmidt TH, Shinkai K. Evidence-based approach to cutaneous hyperandrogenism in women. J Am Acad Dermatol. 2015;73:672-690.
- Schmidt TH, Khanijow K, Cedars MI, et al. Cutaneous findings and systemic associations in women with polycystic ovary syndrome. JAMA Dermatol. 2015;152:391-398.
- Escobar-Morreale HF, Carmina E, Dewailly D, et al. Epidemiology, diagnosis and management of hirsutism: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update. 2012;18:146-170.
- Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456-488.
- Legro RS, Arslanian SA, Ehermann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline [published online October 22, 2013]. J Clin Endocrinol Metab. 2013;98:4565-4592.
- Goodman NF, Cobin RH, Futterweit W, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 1. Endocr Pract. 2015;21:1291-1300.
- Healthcare Bluebook. https://healthcarebluebook.com. Accessed June 13, 2016.
- Azziz R, Sanchez LA, Knochenhauer ES, et al. Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab. 2004;89:453-462.
- Carmina E, Rosato F, Jannì A, et al. Relative prevalence of different androgen excess disorders in 950 women referred because of clinical hyperandrogenism [published online November 1, 2005]. J Clin Endocrinol Metab. 2006;91:2-6.
- Owens DK, Qaseem A, Chou R, et al. High-value, cost-conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med. 2011;154:174-180.
- Nahuis MJ, Oude Lohuis E, Kose N, et al. Long-term follow-up of laparoscopic electrocautery of the ovaries versus ovulation induction with recombinant FSH in clomiphene citrate-resistant women with polycystic ovary syndrome: an economic evaluation. Hum Reprod Oxf Engl. 2012;27:3577-3582.
- Moolenaar LM, Nahuis MJ, Hompes PG, et al. Cost-effectiveness of treatment strategies in women with PCOS who do not conceive after six cycles of clomiphene citrate. Reprod Biomed Online. 2014;28:606-613.
- Chogle A, Saps M. Yield and cost of performing screening tests for constipation in children. Can J Gastroenterol. 2013;27:E35-E38.
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory monitoring during isotretinoin therapy for acne: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:35-44.
- Shinkai K, McMichael A, Linos E. Isotretinoin laboratory test monitoring—a call to decrease testing in an era of high-value, cost-conscious care. JAMA Dermatol. 2016;152:17-19.
The adult female patient presenting with severe acne vulgaris may raise special diagnostic concerns, including consideration of an underlying hormonal disorder. Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in women of reproductive age with an estimated prevalence as high as 12%.1 Many women with undiagnosed PCOS may be referred to dermatologists for evaluation of its cutaneous manifestations of hyperandrogenism including acne, hirsutism, and androgenic alopecia.2 Given the prevalence of PCOS and its long-term health implications, dermatologists can play an important role in the initial evaluation of these patients. Acne and androgenic alopecia, however, are quite common, and in the absence of red flags such as menstrual irregularities, virilization, visual field deficits, or signs of Cushing syndrome,3 clinicians must decide when to pursue limited versus comprehensive evaluation.
Despite being common in patients with PCOS, a recent study suggests that acne is an unreliable marker of biochemical hyperandrogenism, and specific features of acne (ie, lesion counts, lesional types, distribution) cannot reliably discriminate women who meet PCOS diagnostic criteria from those who do not.4 Similarly, the study found that androgenic alopecia was not associated with biochemical hyperandrogenism and was no more common in women with PCOS than women of similar age in a high-risk population. Unlike acne and androgenic alopecia, however, the study identified hirsutism, especially truncal hirsutism, as a reliable indicator of hyperandrogenemia and PCOS. Hirsutism also is associated with metabolic sequelae of PCOS. These findings suggest that hirsutism, but not acne or androgenic alopecia, in a female of reproductive age warrants a workup for PCOS.4 This report is consistent with a recommendation from the Androgen Excess and Polycystic Ovary Syndrome Society (AE-PCOS) to pursue a diagnostic evaluation in any woman presenting with hirsutism.5 Acanthosis nigricans also was found to be a reliable indicator of hyperandrogenemia, PCOS, and associated metabolic derangement. Thus, although recent evidence indicates that acne as an isolated cutaneous finding does not warrant further diagnostic evaluation, acne in the setting of hirsutism, acanthosis nigricans, menstrual irregularities, or additional specific signs of endocrine dysregulation should prompt focused workup.4
Multiple clinical practice guidelines for the evaluation of hirsutism and PCOS based on literature review and expert opinion have been proposed5-8; however, these guidelines vary in recommendations for routine diagnostic steps to exclude mimickers of PCOS such as prolactinoma/pituitary adenoma and congenital adrenal hyperplasia (CAH)(Table). In 2009, an AE-PCOS task force suggested that routine testing of thyroid function and serum prolactin in the absence of additional clinical signs may not be necessary based on the low prevalence of thyroid disorders and hyperprolactinemia in patients presenting with hyperandrogenism.6 In 2013, the Endocrine Society’s (ENDO) clinical guideline, however, recommended routine measurement of serum thyroid-stimulating hormone (TSH) to exclude thyroid disease and serum prolactin to exclude hyperprolactinemia in all women before making a diagnosis of PCOS.7 In 2015, the AE-PCOS collaborated with the American Association of Clinical Endocrinologists (AACE) and American College of Endocrinology to publish an updated guideline for best practices, which was consistent with the prior AE-PCOS recommendation in 2009 for routine screening including to test 17-hydroxyprogesterone to exclude nonclassical CAH.8

Importantly, these recommendations for routine testing for mimickers of PCOS are based on the rare prevalence of these etiologies in multiple studies of women presenting for hyperandrogenism. One study included 873 women presenting to an academic reproductive endocrine clinic for evaluation of symptoms potentially related to androgen excess. In addition to cutaneous manifestations of hirsutism, acne, and alopecia, the study also included women presenting with oligomenorrhea/amenorrhea, ovulatory dysfunction, and even virilization.10 A second study included 950 women presenting to academic endocrine departments with hirsutism, acne, or androgenic alopecia.11 Both studies defined hirsutism as having a modified Ferriman-Gallwey score of 6 or greater. Both studies also only measured serum prolactin or TSH when clinically indicated (ie, patients with ovulatory dysfunction).10,11
The diagnostic yield of tests for mimickers of PCOS was exceedingly low in both studies. For example, of the patients evaluated, only 0.4% to 0.7% had thyroid dysfunction, 0% to 0.3% had hyperprolactinemia, 0.2% had androgen-secreting neoplasms, 2.1% to 4.3% had nonclassical CAH, 0.7% had CAH, and 3.8% had HAIR-AN (hyperandrogenism, insulin resistance, and acanthosis nigricans) syndrome.10,11 Because patients in both studies were only tested for hyperprolactinemia and thyroid dysfunction when clinically indicated, it is probable that routine screening without clinical indication would result in even lower yields.
Given the increasing importance of high-value, cost-conscious care,12 clinicians must consider the costs associated with testing in the face of low pretest probability. Although some studies have examined the cost-effectiveness of fertility treatments in PCOS,13,14 no studies have examined the cost-effectiveness of diagnostic strategies for PCOS. Cost-effectiveness studies are emerging to provide important guidance on high-value, cost-conscious diagnostic evaluation and monitoring15 and are much needed in dermatology.16,17
In the case of PCOS, the costs of some diagnostic tests are relatively low. For example, based on estimates from Healthcare Bluebook,9 serum TSH and prolactin tests in San Francisco, California, are $44 and $51, respectively. However, the cumulative costs for even the most stringent routine workup for PCOS recommended in the AACE/AE-PCOS guideline consisting of a free testosterone measurement, 17-hydroxyprogesterone, and transvaginal ultrasound would still cost a total of $516. Additional TSH and prolactin tests recommended by ENDO would increase the cost of PCOS testing by approximately 18%. Routine testing for additional serum androgens—dehydroepiandrosterone sulfate (DHEA-S) and androstenedione—would further increase this amount by an additional $134 to a total cost of $745. The ENDO guideline only recommends DHEA-S testing to assist in the diagnosis of an androgen-secreting tumor when signs of virilization are present, while the AACE/AE-PCOS guideline discourages routine testing for DHEA-S and androstenedione based on the low frequency of cases in which these androgens are elevated in isolation.7,8
Although the selection of tests influences total cost, the setting of tests (ie, hospitals, physician offices, independent test settings) also can contribute to wide variations in cost. For example, Healthcare Bluebook’s estimates for transvaginal ultrasound in Chicago, Illinois, range from $236 to more than $740.9 When the separate physician visit fees are included, the total cost of a routine diagnostic evaluation of a patient with acne or hirsutism concerning for PCOS is not trivial.
Large national clinical registries and formal cost-effectiveness analyses are necessary to shed light on this issue, but it is clear that clinicians should rely on their clinical judgment when ordering laboratory tests in the evaluation for PCOS given the apparent low yield of routine screening for PCOS mimickers in the absence of clinical indications. For example, a TSH would not be warranted in a patient without evidence of thyroid dysfunction (ie, weight gain, fatigue, constipation, menstrual irregularities). Similarly, clinicians should routinely consider the principle of high-value care: whether the results of a test will change management of the patient. For example, a woman with amenorrhea and severe acne who already meets diagnostic criteria for PCOS would benefit from a combined oral contraceptive for both acne and endometrial protection. An ovarian ultrasound may not be needed to confirm the diagnosis unless there is suspicion for an ovarian condition other than PCOS causing the symptoms.
Finally, clinicians should discuss testing options and involve patients in decisions around testing. Although PCOS treatments generally target individual symptoms rather than the syndrome as a whole, confirmation of a PCOS diagnosis importantly informs women of their risk for cardiovascular and metabolic disease. The ENDO recommends screening for impaired glucose tolerance, type 2 diabetes mellitus, obesity, family history of early cardiovascular disease, tobacco use, hypertension, dyslipidemia, and obstructive sleep apnea in all women with PCOS, including nonobese patients.7 Ongoing efforts to gain and understand evidence to support high-value, cost-conscious care should be prioritized and kept in balance with shared decision-making in individual patients suspected of having PCOS.
The adult female patient presenting with severe acne vulgaris may raise special diagnostic concerns, including consideration of an underlying hormonal disorder. Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in women of reproductive age with an estimated prevalence as high as 12%.1 Many women with undiagnosed PCOS may be referred to dermatologists for evaluation of its cutaneous manifestations of hyperandrogenism including acne, hirsutism, and androgenic alopecia.2 Given the prevalence of PCOS and its long-term health implications, dermatologists can play an important role in the initial evaluation of these patients. Acne and androgenic alopecia, however, are quite common, and in the absence of red flags such as menstrual irregularities, virilization, visual field deficits, or signs of Cushing syndrome,3 clinicians must decide when to pursue limited versus comprehensive evaluation.
Despite being common in patients with PCOS, a recent study suggests that acne is an unreliable marker of biochemical hyperandrogenism, and specific features of acne (ie, lesion counts, lesional types, distribution) cannot reliably discriminate women who meet PCOS diagnostic criteria from those who do not.4 Similarly, the study found that androgenic alopecia was not associated with biochemical hyperandrogenism and was no more common in women with PCOS than women of similar age in a high-risk population. Unlike acne and androgenic alopecia, however, the study identified hirsutism, especially truncal hirsutism, as a reliable indicator of hyperandrogenemia and PCOS. Hirsutism also is associated with metabolic sequelae of PCOS. These findings suggest that hirsutism, but not acne or androgenic alopecia, in a female of reproductive age warrants a workup for PCOS.4 This report is consistent with a recommendation from the Androgen Excess and Polycystic Ovary Syndrome Society (AE-PCOS) to pursue a diagnostic evaluation in any woman presenting with hirsutism.5 Acanthosis nigricans also was found to be a reliable indicator of hyperandrogenemia, PCOS, and associated metabolic derangement. Thus, although recent evidence indicates that acne as an isolated cutaneous finding does not warrant further diagnostic evaluation, acne in the setting of hirsutism, acanthosis nigricans, menstrual irregularities, or additional specific signs of endocrine dysregulation should prompt focused workup.4
Multiple clinical practice guidelines for the evaluation of hirsutism and PCOS based on literature review and expert opinion have been proposed5-8; however, these guidelines vary in recommendations for routine diagnostic steps to exclude mimickers of PCOS such as prolactinoma/pituitary adenoma and congenital adrenal hyperplasia (CAH)(Table). In 2009, an AE-PCOS task force suggested that routine testing of thyroid function and serum prolactin in the absence of additional clinical signs may not be necessary based on the low prevalence of thyroid disorders and hyperprolactinemia in patients presenting with hyperandrogenism.6 In 2013, the Endocrine Society’s (ENDO) clinical guideline, however, recommended routine measurement of serum thyroid-stimulating hormone (TSH) to exclude thyroid disease and serum prolactin to exclude hyperprolactinemia in all women before making a diagnosis of PCOS.7 In 2015, the AE-PCOS collaborated with the American Association of Clinical Endocrinologists (AACE) and American College of Endocrinology to publish an updated guideline for best practices, which was consistent with the prior AE-PCOS recommendation in 2009 for routine screening including to test 17-hydroxyprogesterone to exclude nonclassical CAH.8

Importantly, these recommendations for routine testing for mimickers of PCOS are based on the rare prevalence of these etiologies in multiple studies of women presenting for hyperandrogenism. One study included 873 women presenting to an academic reproductive endocrine clinic for evaluation of symptoms potentially related to androgen excess. In addition to cutaneous manifestations of hirsutism, acne, and alopecia, the study also included women presenting with oligomenorrhea/amenorrhea, ovulatory dysfunction, and even virilization.10 A second study included 950 women presenting to academic endocrine departments with hirsutism, acne, or androgenic alopecia.11 Both studies defined hirsutism as having a modified Ferriman-Gallwey score of 6 or greater. Both studies also only measured serum prolactin or TSH when clinically indicated (ie, patients with ovulatory dysfunction).10,11
The diagnostic yield of tests for mimickers of PCOS was exceedingly low in both studies. For example, of the patients evaluated, only 0.4% to 0.7% had thyroid dysfunction, 0% to 0.3% had hyperprolactinemia, 0.2% had androgen-secreting neoplasms, 2.1% to 4.3% had nonclassical CAH, 0.7% had CAH, and 3.8% had HAIR-AN (hyperandrogenism, insulin resistance, and acanthosis nigricans) syndrome.10,11 Because patients in both studies were only tested for hyperprolactinemia and thyroid dysfunction when clinically indicated, it is probable that routine screening without clinical indication would result in even lower yields.
Given the increasing importance of high-value, cost-conscious care,12 clinicians must consider the costs associated with testing in the face of low pretest probability. Although some studies have examined the cost-effectiveness of fertility treatments in PCOS,13,14 no studies have examined the cost-effectiveness of diagnostic strategies for PCOS. Cost-effectiveness studies are emerging to provide important guidance on high-value, cost-conscious diagnostic evaluation and monitoring15 and are much needed in dermatology.16,17
In the case of PCOS, the costs of some diagnostic tests are relatively low. For example, based on estimates from Healthcare Bluebook,9 serum TSH and prolactin tests in San Francisco, California, are $44 and $51, respectively. However, the cumulative costs for even the most stringent routine workup for PCOS recommended in the AACE/AE-PCOS guideline consisting of a free testosterone measurement, 17-hydroxyprogesterone, and transvaginal ultrasound would still cost a total of $516. Additional TSH and prolactin tests recommended by ENDO would increase the cost of PCOS testing by approximately 18%. Routine testing for additional serum androgens—dehydroepiandrosterone sulfate (DHEA-S) and androstenedione—would further increase this amount by an additional $134 to a total cost of $745. The ENDO guideline only recommends DHEA-S testing to assist in the diagnosis of an androgen-secreting tumor when signs of virilization are present, while the AACE/AE-PCOS guideline discourages routine testing for DHEA-S and androstenedione based on the low frequency of cases in which these androgens are elevated in isolation.7,8
Although the selection of tests influences total cost, the setting of tests (ie, hospitals, physician offices, independent test settings) also can contribute to wide variations in cost. For example, Healthcare Bluebook’s estimates for transvaginal ultrasound in Chicago, Illinois, range from $236 to more than $740.9 When the separate physician visit fees are included, the total cost of a routine diagnostic evaluation of a patient with acne or hirsutism concerning for PCOS is not trivial.
Large national clinical registries and formal cost-effectiveness analyses are necessary to shed light on this issue, but it is clear that clinicians should rely on their clinical judgment when ordering laboratory tests in the evaluation for PCOS given the apparent low yield of routine screening for PCOS mimickers in the absence of clinical indications. For example, a TSH would not be warranted in a patient without evidence of thyroid dysfunction (ie, weight gain, fatigue, constipation, menstrual irregularities). Similarly, clinicians should routinely consider the principle of high-value care: whether the results of a test will change management of the patient. For example, a woman with amenorrhea and severe acne who already meets diagnostic criteria for PCOS would benefit from a combined oral contraceptive for both acne and endometrial protection. An ovarian ultrasound may not be needed to confirm the diagnosis unless there is suspicion for an ovarian condition other than PCOS causing the symptoms.
Finally, clinicians should discuss testing options and involve patients in decisions around testing. Although PCOS treatments generally target individual symptoms rather than the syndrome as a whole, confirmation of a PCOS diagnosis importantly informs women of their risk for cardiovascular and metabolic disease. The ENDO recommends screening for impaired glucose tolerance, type 2 diabetes mellitus, obesity, family history of early cardiovascular disease, tobacco use, hypertension, dyslipidemia, and obstructive sleep apnea in all women with PCOS, including nonobese patients.7 Ongoing efforts to gain and understand evidence to support high-value, cost-conscious care should be prioritized and kept in balance with shared decision-making in individual patients suspected of having PCOS.
- March WA, Moore VM, Willson KJ, et al. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25:544-551.
- Sivayoganathan D, Maruthini D, Glanville JM, et al. Full investigation of patients with polycystic ovary syndrome (PCOS) presenting to four different clinical specialties reveals significant differences and undiagnosed morbidity. Hum Fertil. 2011;14:261-265.
- Schmidt TH, Shinkai K. Evidence-based approach to cutaneous hyperandrogenism in women. J Am Acad Dermatol. 2015;73:672-690.
- Schmidt TH, Khanijow K, Cedars MI, et al. Cutaneous findings and systemic associations in women with polycystic ovary syndrome. JAMA Dermatol. 2015;152:391-398.
- Escobar-Morreale HF, Carmina E, Dewailly D, et al. Epidemiology, diagnosis and management of hirsutism: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update. 2012;18:146-170.
- Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456-488.
- Legro RS, Arslanian SA, Ehermann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline [published online October 22, 2013]. J Clin Endocrinol Metab. 2013;98:4565-4592.
- Goodman NF, Cobin RH, Futterweit W, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 1. Endocr Pract. 2015;21:1291-1300.
- Healthcare Bluebook. https://healthcarebluebook.com. Accessed June 13, 2016.
- Azziz R, Sanchez LA, Knochenhauer ES, et al. Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab. 2004;89:453-462.
- Carmina E, Rosato F, Jannì A, et al. Relative prevalence of different androgen excess disorders in 950 women referred because of clinical hyperandrogenism [published online November 1, 2005]. J Clin Endocrinol Metab. 2006;91:2-6.
- Owens DK, Qaseem A, Chou R, et al. High-value, cost-conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med. 2011;154:174-180.
- Nahuis MJ, Oude Lohuis E, Kose N, et al. Long-term follow-up of laparoscopic electrocautery of the ovaries versus ovulation induction with recombinant FSH in clomiphene citrate-resistant women with polycystic ovary syndrome: an economic evaluation. Hum Reprod Oxf Engl. 2012;27:3577-3582.
- Moolenaar LM, Nahuis MJ, Hompes PG, et al. Cost-effectiveness of treatment strategies in women with PCOS who do not conceive after six cycles of clomiphene citrate. Reprod Biomed Online. 2014;28:606-613.
- Chogle A, Saps M. Yield and cost of performing screening tests for constipation in children. Can J Gastroenterol. 2013;27:E35-E38.
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory monitoring during isotretinoin therapy for acne: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:35-44.
- Shinkai K, McMichael A, Linos E. Isotretinoin laboratory test monitoring—a call to decrease testing in an era of high-value, cost-conscious care. JAMA Dermatol. 2016;152:17-19.
- March WA, Moore VM, Willson KJ, et al. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25:544-551.
- Sivayoganathan D, Maruthini D, Glanville JM, et al. Full investigation of patients with polycystic ovary syndrome (PCOS) presenting to four different clinical specialties reveals significant differences and undiagnosed morbidity. Hum Fertil. 2011;14:261-265.
- Schmidt TH, Shinkai K. Evidence-based approach to cutaneous hyperandrogenism in women. J Am Acad Dermatol. 2015;73:672-690.
- Schmidt TH, Khanijow K, Cedars MI, et al. Cutaneous findings and systemic associations in women with polycystic ovary syndrome. JAMA Dermatol. 2015;152:391-398.
- Escobar-Morreale HF, Carmina E, Dewailly D, et al. Epidemiology, diagnosis and management of hirsutism: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update. 2012;18:146-170.
- Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456-488.
- Legro RS, Arslanian SA, Ehermann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline [published online October 22, 2013]. J Clin Endocrinol Metab. 2013;98:4565-4592.
- Goodman NF, Cobin RH, Futterweit W, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 1. Endocr Pract. 2015;21:1291-1300.
- Healthcare Bluebook. https://healthcarebluebook.com. Accessed June 13, 2016.
- Azziz R, Sanchez LA, Knochenhauer ES, et al. Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab. 2004;89:453-462.
- Carmina E, Rosato F, Jannì A, et al. Relative prevalence of different androgen excess disorders in 950 women referred because of clinical hyperandrogenism [published online November 1, 2005]. J Clin Endocrinol Metab. 2006;91:2-6.
- Owens DK, Qaseem A, Chou R, et al. High-value, cost-conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med. 2011;154:174-180.
- Nahuis MJ, Oude Lohuis E, Kose N, et al. Long-term follow-up of laparoscopic electrocautery of the ovaries versus ovulation induction with recombinant FSH in clomiphene citrate-resistant women with polycystic ovary syndrome: an economic evaluation. Hum Reprod Oxf Engl. 2012;27:3577-3582.
- Moolenaar LM, Nahuis MJ, Hompes PG, et al. Cost-effectiveness of treatment strategies in women with PCOS who do not conceive after six cycles of clomiphene citrate. Reprod Biomed Online. 2014;28:606-613.
- Chogle A, Saps M. Yield and cost of performing screening tests for constipation in children. Can J Gastroenterol. 2013;27:E35-E38.
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory monitoring during isotretinoin therapy for acne: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:35-44.
- Shinkai K, McMichael A, Linos E. Isotretinoin laboratory test monitoring—a call to decrease testing in an era of high-value, cost-conscious care. JAMA Dermatol. 2016;152:17-19.
