User login
The Leaky Pipeline: A Narrative Review of Diversity in Dermatology
With a majority-minority population expected in the United States by 2044, improving diversity and cultural competency in the dermatology workforce is now more important than ever. A more diverse workforce increases the cultural competence of all providers, provides greater opportunities for mentorship and sponsorship of underrepresented minority (URM) trainees, establishes a more inclusive environment for learners, and enhances the knowledge and productivity of the workforce.1-3 Additionally, it is imperative to address clinical care disparities seen in minority patients in dermatology, including treatment of skin cancer, psoriasis, acne, atopic dermatitis, and other diseases.4-7
Despite the attention that has been devoted to improving diversity in medicine,8-10 dermatology remains one of the least diverse specialties, prompting additional calls to action within the field.11 Why does the lack of diversity still exist in dermatology, and what is the path to correcting this problem? In this article, we review the evidence of diversity barriers at different stages of medical education training that may impede academic advancement for minority learners pursuing careers in dermatology.
Undergraduate Medical Education
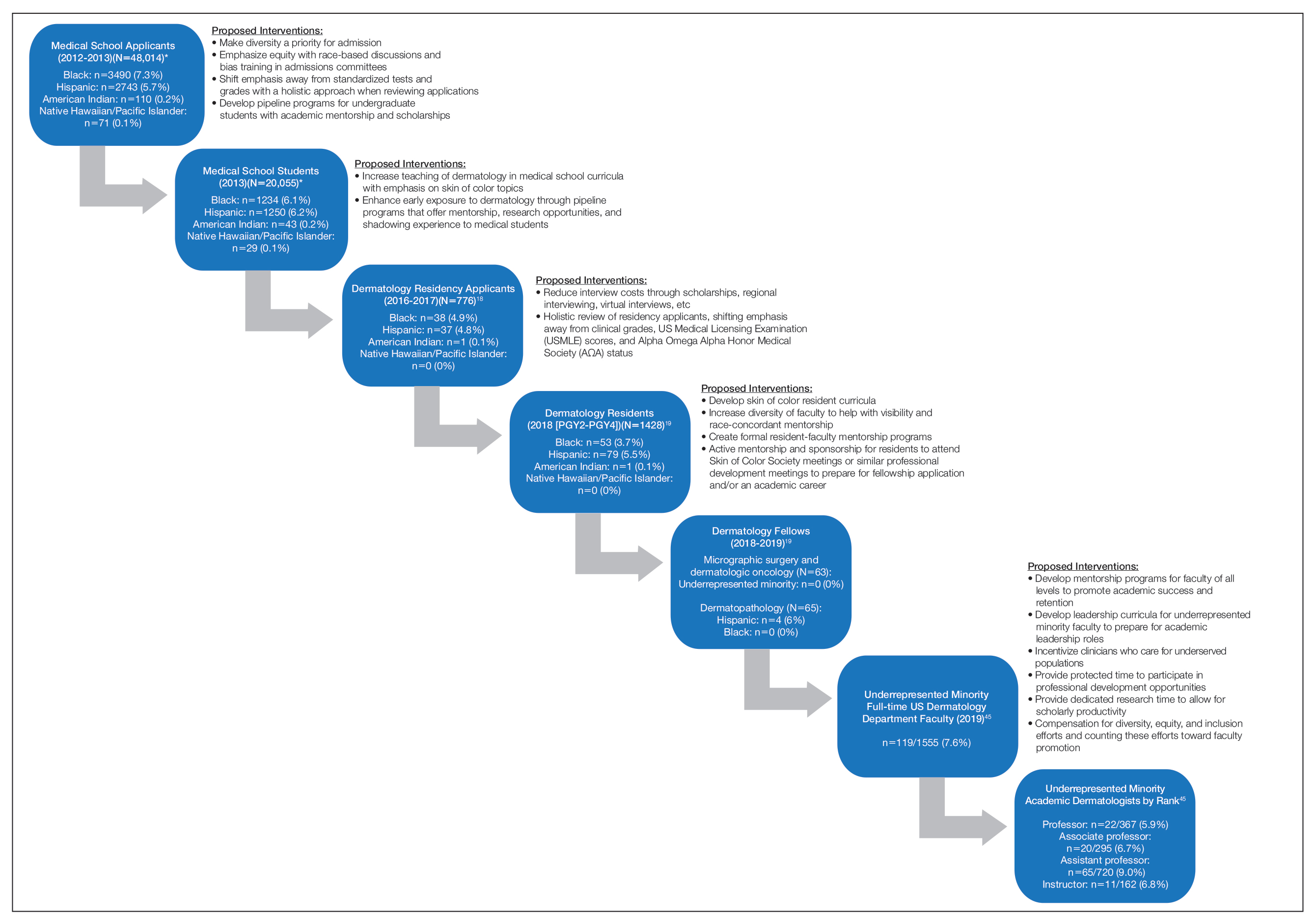
The term leaky pipeline refers to the progressive decline in the number of URMs along a given career path, including in dermatology. The Association of American Medical Colleges defines URMs as racial/ethnic populations that are “underrepresented in the medical profession relative to their numbers in the general population.”9 The first leak in the pipeline is that URMs are not applying to medical school. From 2002 and 2017, rates of both application and matriculation to medical school were lower by 30% to 70% in URM groups compared to White students, including Hispanic, Black, and American Indian/Alaska Native students.12,13 The decision not to apply to medical school was greater in URM undergraduate students irrespective of scholastic ability as measured by SAT scores.14
A striking statistic is that the number of Black men matriculating into medical school in 2014 was less than it was in 1978 despite the increase in the number of US medical schools and efforts to recruit more diverse student populations. The Association of American Medical Colleges identified potential reasons for this decline, including poor early education, lack of mentorship, negative perceptions of Black men due to racial stereotypes, and lack of financial and academic resources to support the application process.8,13,15-17 Implicit racial bias by admission committees also may play a role.
Medical School Matriculation and Applying to Dermatology Residency
There is greater representation of URM students in medical school than in dermatology residency, which means URM students are either not applying to dermatology programs or they are not matching into the specialty. In the Electronic Residency Application Service’s 2016-2017 application cycle (N=776), there were 76 (9.8%) URM dermatology residency applicants.18 In 2018, there was a notable decline in representation of Black students among residency applicants (4.9%) to matched residents (3.7%), and there were only 133 (9.3%) URM dermatology residents in total (PGY2-PGY4 classes).19 The lack of exposure to medical subspecialties and the recommendation by medical schools for URM medical students to pursue careers in primary care have been cited as reasons that these students may not apply to residency programs in specialty care.20,21 The presence of an Accreditation Council for Graduate Medical Education dermatology residency program, fellowships, and dermatology interest groups at their medical schools correlated with higher proportions of URM students applying to dermatology programs.20
Underrepresented minority students face critical challenges during medical school, including receiving lower grades in both standardized and school-designated assessments and clerkship grades.21,22 A 2019 National Board of Medical Examiners study found that Hispanic and Black test takers scored 12.1 and 16.6 points lower than White men, respectively, on the
A recent cross-sectional study showed that lack of equitable resources, lack of support, financial constrictions, and lack of group identity were 4 barriers to URM students matching into dermatology.26 Dermatology is a competitive specialty with the highest median Electronic Residency Application Service applications submitted per US applicant (n=90)27 and an approximate total cost per US applicant of $10,781.28,29 Disadvantaged URM applicants noted relying on loans while non-URM applicants cited family financial support as being beneficial.26 In addition, an increasing number of applicants take gap years for research, which pose additional costs for finances and resources. In contrast, mentorship and participation in pipeline/enrichment programs were factors associated with URM students matching into dermatology.26
Dermatology Residency and the Transition to Advanced Dermatology Fellowships
Similar to the transition from medical school into dermatology residency, URM dermatology residents are either not applying to fellowships or are not getting in. In the 2018-2019 academic year, there were no Black, Hispanic, Native Hawaiian/Pacific Islander, or American Indian/Alaska Native Mohs micrographic surgery and dermatologic oncology fellows.19 Similarly, there were no Black, Native Hawaiian/Pacific Islander, or American Indian/Alaska Native dermatopathology fellows. There were 4 (6%) Hispanic dermatopathology fellows.19
There also is marked underrepresentation of minority groups—and minimal growth over time—in the dermatology procedural subspecialty. Whereas the percentage of female Mohs surgeons increased considerably from 1985 to 2005 (12.7% to 40.9%, respectively), the percentage of URM Mohs surgeons remained steady from 4.2% to 4.6%, respectively, and remained at 4.5% in 2014.30
There are no available data on the race/ethnicity of fellowship applicants, as these demographic data for the application process have not been consistently or traditionally collected. The reasons why there are so few URM dermatology fellows is not known; whether this is due to a lack of mentorship or whether other factors lead to residents not applying for advanced training needs further study. Financial factors related to prolonged training, which include lower salaries and delayed loan repayment, may present barriers to applying to fellowships.
Lack of URM Academic Faculty in Dermatology
At the academic faculty level, URM representation continues to worsen. Lett et al31 found that there is declining racial and ethnic representation in clinical academic medicine relative to US census data for 16 US medical specialties, including dermatology, with growing underrepresentation of Black and Hispanic faculty at the associate professor and full professor levels and underrepresentation in all faculty ranks. From 1970 to 2018, URM faculty in dermatology only increased from 4.8% to 7.4%, respectively. Non-URM female and male faculty members increased by 13.8 and 10.8 faculty members per year, respectively, while URM female and male faculty members increased by 1.2 and 0.8 faculty members per year, respectively.32
Underrepresentation of minorities seen in dermatology faculty may result from clinical demands, minority taxation (defined as the extensive service requirements uniquely experienced by URM faculty to disproportionately serve as representatives on academic committees and to mentor URM students), and barriers to academic promotion, which are challenges uniquely encountered by URMs in academic dermatology.33 Increased clinical demand may result from the fact that URM physicians are more likely to care for underserved populations, those of lower socioeconomic status, non-English–speaking patients, those on Medicaid, and those who are uninsured, which may impact renumeration. Minority tax experienced by URM faculty includes mentoring URM medical students, providing cultural expertise to departments and institutions, and participating in community service projects and outreach programs. Specifically, many institutional committees require the participation of a URM member, resulting in URM faculty members experiencing higher committee service burden. Many, if not all, of these responsibilities often are not compensated through salary or academic promotion.
A Call to Action
There are several steps that can be taken to create a pathway to dermatology that is inclusive, flexible, and supportive of URMs.
• Increase early exposure to dermatology in medical school. Early exposure and mentorship opportunities are associated with higher rates of students pursuing specialty field careers.34 Increased early opportunities allow for URM students to consider and explore a career in dermatology; receive mentorship; and ensure that dermatology, including topics related to skin of color (SOC), is incorporated into their learning. The American Academy of Dermatology has contributed to these efforts by its presence at every national meeting of the Student National Medical Association and Latino Medical Student Association, as well as its involvement with Nth Dimensions, which offers various educational opportunities for URM medical students.
• Implement equitable grading and holistic review processes in medical school. Racial/ethnic differences in clinical grading and standardized test scores in medical school demonstrate why holistic review of dermatology residency applicants is needed and why other metrics such as USMLE scores and AΩA status should be de-emphasized or eliminated when evaluating candidates. To support equity, many medical schools have eliminated honors grading, and some schools have eliminated AΩA distinction.
• Increase diversity of dermatology residents and residency programs. Implicit bias training for a medical school admissions committee has been shown to increase diversity in medical school enrollment.35 Whether implicit bias training and other diversity training may benefit dermatology residency selection must be examined, including study of unintended consequences, such as reduced diversity, increased microaggressions toward minority colleagues, and the illusion of fairness.36-39 Increasing representation is not sufficient—creating inclusive residency training environments is a critical parallel aim. Prioritizing diversity in dermatology residency recruitment is imperative. Creating dermatology residency positions specifically for URM residents may be an important option, as done at the University of Pennsylvania (Philadelphia, Pennsylvania) and Duke University (Durham, North Carolina).
• Create effective programs for URM mentorship. Due to the competitive nature of dermatology residency, the need for mentors in dermatology is critically vital for URM medical students, especially those without a home dermatology program at their medical school. Further development of formal mentorship and pipeline programs is essential at both the local and national levels. Some national examples of these initiatives include diversity mentorship programs offered by the American Academy of Dermatology, Skin of Color Society, Women’s Dermatologic Society, and Student National Medical Association. Many institutional programs also offer invaluable opportunities, such as the summer research fellowship at the University of California, San Francisco (UCSF); visiting clerkship grants for URMs at the University of Pennsylvania (Philadelphia, Pennsylvania) and Johns Hopkins University (Baltimore, Maryland); and integrated programs, such as the Visiting Elective Scholarship Program at UCSF, which provides funding and faculty mentorship for URM students completing an away rotation at UCSF.
• Establish longitudinal skin-of-color curricula and increased opportunities for research. More robust SOC training may lead to an increasingly diverse workforce. It is important that medical student and dermatology resident and fellow education include training on SOC to ensure high-quality care to diverse patient populations, which also may enhance the knowledge of trainees, encourage clinical and research interest in this field, and reduce health care disparities. Increasing research opportunities and offering formalized longitudinal training in SOC as well as incorporating more diverse images in medical school education may foster greater interest in this field at a time when trainees are establishing their career interests. At present, there is considerable room for improvement. Nijhawan et al40 surveyed 63 dermatology chief residents and 41 program directors and found only 14.3% and 14.6%, respectively, reported having an expert who conducts clinic specializing in SOC. Only 52.4% and 65.9% reported having didactic sessions or lectures focused on SOC diseases, and 30.2% and 12.2% reported having a dedicated rotation for residents to gain experience in SOC.40 A more recent study showed that when faculty were asked to incorporate more SOC content into lectures, the most commonly identified barrier to implementation was a lack of SOC images.41 Additionally, there remains a paucity of published research on this topic, with SOC articles representing only 2.7% of the literature.42 These numbers demonstrate the continued need for a more inclusive and comprehensive curriculum in dermatology residency programs and more robust funding for SOC research.
• Recruit and support URM faculty. Increasing diversity in dermatology residency programs likely will increase the number of potential URMs pursuing additional fellowship training and academic dermatology with active career mentorship and support. In addition, promoting faculty retention by combatting the progressive loss of URMs at all faculty levels is paramount. Mentorship for URM physicians has been shown to play a key role in the decision to pursue academic medicine as well as academic productivity and job satisfaction.43,44 The visibility, cultural competency, clinical work, academic productivity, and mentorship efforts that URM faculty provide are essential to enhancing patient care, teaching diverse groups of learners, and recruiting more diverse trainees. Protected time to participate in professional development opportunities has been shown to improve recruitment and retention of URM faculty and offer additional opportunities for junior faculty to find mentors.35,36 Incentivizing clinical care of underserved populations also may augment financial stability for URM physicians who choose to care for these patients. Finally, diversity work and community service should be legitimized and count toward faculty promotion.
Conclusion
There are numerous factors that contribute to the leaky pipeline in dermatology (eFigure). Many challenges that are unique to the URM population disadvantage these students from entering medical school, applying to dermatology residency, matching into dermatology fellowships, pursuing and staying in faculty positions, and achieving faculty advancement into leadership positions. With each progressive step along this trajectory, there is less minority representation. All dermatologists, regardless of race/ethnicity, need to play an active role and must prioritize diversity, equity, and inclusion efforts at all levels of education and training for the betterment of the specialty.
- Dixon G, Kind T, Wright J, et al. Factors that influence the choice of academic pediatrics by underrepresented minorities. Pediatrics. 2019;144:E20182759. doi:10.1542/peds.2018-2759
- Yehia BR, Cronholm PF, Wilson N, et al. Mentorship and pursuit of academic medicine careers: a mixed methods study of residents from diverse backgrounds. BMC Med Educ. 2014:14:2-26. doi:10.1186/1472-6920-14-26
- Saha S, Guiton G, Wimmers PF, et al. Student body racial and ethnic composition and diversity-related outcomes in US medical schools. JAMA. 2008;300:1135-1145. doi:10.1001/jama.300.10.1135
- Hsu DY, Gordon K, Silverberg JI. The patient burden of psoriasis in the United States. J Am Acad Dermatol. 2016;75:33-41. doi:10.1016/j.jaad.2016.03.048
- Silverberg JI. Racial and ethnic disparities in atopic dermatitis. Curr Dermatol Rep. 2015;4:44-48.
- Buster KJ, Sevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59. doi:10.1016/j.det.2011.08.002
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Smedley BD, Stith AY, Colburn L, et al. The Right Thing To Do, The Smart Thing to Do: Enhancing Diversity in the Health Professions. National Academies Press; 2001.
- Association of American Medical Colleges. Minorities in medical education: fact and figures 2019. Accessed December 9, 2021. https://www.aamc.org/datareports/workforce/report/diversity-medicine-facts-and-figures-2019
- Liaison Committee on Medical Education (LCME) standards on diversity. University of South Florida Health website. Accessed December 9, 2021. https://health.usf.edu/~/media/Files/Medicine/MD%20Program/Diversity/LCMEStandardsonDiversity1.ashx?la=en
- Granstein RD, Cornelius L, Shinkai K. Diversity in dermatology—a call for action. JAMA Dermatol. 2017;153:499-500. doi:10.1001/jamadermatol.2017.0296
- Lett LA, Murdock HM, Orji W, et al. Trends in racial/ethnic representation among US medical students. JAMA Netw Open. 2019;2:e1910490. doi:10.1001/jamanetworkopen.2019.10490
- Association of American Medical Colleges. Altering the course: Black males in medicine. Published 2015. Accessed December 8, 2021. https://store.aamc.org/downloadable/download/sample/sample_id/84/
- Barr DA, Gonzalez ME, Wanat SF. The leaky pipeline: factors associated with early decline in interest in premedical studies among underrepresented minority undergraduate students. Acad Med. 2008;83:5:503-511. doi:10.1097/ACM.0b013e31816bda16
- Flores RL. The rising gap between rich and poor: a look at the persistence of educational disparities in the United States and why we should worry. Cogent Soc Sci. 2017;3:1323698.
- Jackson D. Why am I behind? an examination of low income and minority students’ preparedness for college. McNair Sch J. 2012;13:121-138.
- Rothstein R. The racial achievement gap, segregated schools, andsegregated neighborhoods: a constitutional insult. Race Soc Probl. 2015;7:21-30.
- Association of American Medical Colleges. Residency Applicants From US MD Granting Medical Schools to ACGME-Accredited Programs by Specialty and Race/Ethnicity. Association of American Medical Colleges; 2017.
- Brotherton SE, Etzel SL. Graduate medical education, 2018-2019. JAMA. 2019;322:996-1016. doi:10.1001/jama.2019.10155
- Barnes LA, Bae GH, Nambudiri V. Sex and racial/ethnic diversity of US medical students and their exposure to dermatology programs. JAMA Dermatol. 2019;155:490-491. doi:10.1001/jamadermatol.2018.5025
- Soliman YS, Rzepecki AK, Guzman AK. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254. doi:10.1001/jamadermatol.2018.4813
- Low D, Pollack SW, Liao Z, et al. Racial/ethnic disparities in clinical grading in medical school. Teach Learn Med. 2019;31:487-496. doi:10.1080/10401334.2019.1597724
- Rubright JD, Jodoin M, Barone MA. Examining demographics, prior academic performance and United States medical licensing examination scores. Acad Med. 2019;94;364-370. doi:10.1097/ACM.0000000000002366
- Boatright D, Ross D, O’Connor P, et al. Racial disparities in medical student membership in the alpha omega honor society. JAMA Intern Med. 2017;177:659-665. doi:10.1001/jamainternmed.2016.9623
- Gorouhi F, Alikhan A, Rezaei A, et al. Dermatology residency selection criteria with an emphasis on program characteristics: a national program director survey [published online March 17, 2014]. Dermatol Res Pract. doi:10.1155/2014/692760
- Vasquez R, Jeong H, Florez-Pollack S, et al. What are the barriers faced by underrepresented minorities applying to dermatology? a qualitative cross-sectional study of applicants applying to a large dermatology residency program. J Am Acad Dermatol. 2020;83:1770-1773. doi:10.1016/j.jaad.2020.03.067
- Results of the 2019 NRMP applicant survey by preferred specialty and applicant type. National Resident Matching Program website. Published July 2019. Accessed December 8, 2021. https://www.nrmp.org/wp-content/uploads/2019/06/Applicant-Survey-Report-2019.pdf
- Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756. doi:10.1016/j.jaad.2015.10.049
- Polacco MA, Lally J, Walls A, et al. Digging into debt: the financial burden associated with the otolaryngology match. Otolaryngol Head Neck Surg. 2017;12:1091-1096. doi:10.1177/0194599816686538
- Feng H, Feng PW, Geronemus RG. Diversity in the US Mohs micrographic surgery workforce. Dermatol Surg. 2020:46:1451-1455. doi:10.1097/DSS.0000000000002080
- Lett LA, Orji WU, Sebro R. Declining racial and ethnic representation in clinical academic medicine: a longitudinal study of 16 US medical specialties. PLoS ONE. 2018;13:e0207274. doi:10.1371/journal.pone.020727432. Xierali IM, Nivet MA, Pandya AG. US Dermatology department faculty diversity trends by sex and underrepresented-in-medicine status, 1970-2018. JAMA Dermatol. 2020;156:280-287. doi:10.1001/jamadermatol.2019.4297
- Okoye GA. Supporting underrepresented minority women in academic dermatology. Intl J Womens Dermatol. 2020;6:57-60. doi:10.1016/j.ijwd.2019.09.009
- Bernstein J, Dicaprio MR, Mehta S. The relationship between required medical school instruction in musculoskeletal medicine and application rates to orthopaedic surgery residency programs. J Bone Joint Surg Am. 2004;86:2335-2338. doi:10.2106/00004623-200410000-00031
- Capers Q, Clinchot D, McDougle L, et al. Implicit racial bias in medical school admissions. Acad Med. 2017;92:365-369. doi:10.1097/ACM.0000000000001388
- Dobbin F, Kalev A. Why diversity programs fail. Harvard Business Rev. 2016;52-60. Accessed December 8, 2021. https://hbr.org/2016/07/why-diversity-programs-fail
- Kalev A, Dobbin F, Kelly E. Best practices or best guesses? assessing the efficacy of corporate affirmative action and diversity policies. Am Sociol Rev. 2006;71:589-617.
- Sanchez JI, Medkik N. The effects of diversity awareness training on differential treatment. Group Organ Manag. 2004;29:517-536.
- Kaiser CR, Major B, Jurcevic I, et al. Presumed fair: ironic effects of organizational diversity structures. J Pers Soc Psychol. 2013;104:504-519. doi:10.1037/a0030838
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-617.
- Jia JL, Gordon JS, Lester JC, et al. Integrating skin of color and sexual and gender minority content into dermatology residency curricula: a prospective program initiative [published online April 16, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.04.018
- Amuzie AU, Lia JL, Taylor SC, et al. Skin of color article representation in dermatology literature 2009-2019: higher citation counts and opportunities for inclusion [published online March 24, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.063
- Beech BM, Calles-Escandon J, Hairston KC, et al. Mentoring programs for underrepresented minority faculty in academic medical center: a systematic review of the literature. Acad Med. 2013;88:541-549. doi:10.1097/ACM.0b013e31828589e3
- Daley S, Wingard DL, Reznik V. Improving the retention of underrepresented minority faculty in academic medicine. J Natl Med Assoc. 2006;98:1435-1440. doi:10.1016/s0027-9684(15)31449-8
- Association of American Medical Colleges. US medical school faculty by sex, race/ethnicity, rank, and department, 2019. Published December 31, 2019. Accessed December 20, 2021. https://www.aamc.org/media/8476/download?attachment
With a majority-minority population expected in the United States by 2044, improving diversity and cultural competency in the dermatology workforce is now more important than ever. A more diverse workforce increases the cultural competence of all providers, provides greater opportunities for mentorship and sponsorship of underrepresented minority (URM) trainees, establishes a more inclusive environment for learners, and enhances the knowledge and productivity of the workforce.1-3 Additionally, it is imperative to address clinical care disparities seen in minority patients in dermatology, including treatment of skin cancer, psoriasis, acne, atopic dermatitis, and other diseases.4-7
Despite the attention that has been devoted to improving diversity in medicine,8-10 dermatology remains one of the least diverse specialties, prompting additional calls to action within the field.11 Why does the lack of diversity still exist in dermatology, and what is the path to correcting this problem? In this article, we review the evidence of diversity barriers at different stages of medical education training that may impede academic advancement for minority learners pursuing careers in dermatology.
Undergraduate Medical Education
The term leaky pipeline refers to the progressive decline in the number of URMs along a given career path, including in dermatology. The Association of American Medical Colleges defines URMs as racial/ethnic populations that are “underrepresented in the medical profession relative to their numbers in the general population.”9 The first leak in the pipeline is that URMs are not applying to medical school. From 2002 and 2017, rates of both application and matriculation to medical school were lower by 30% to 70% in URM groups compared to White students, including Hispanic, Black, and American Indian/Alaska Native students.12,13 The decision not to apply to medical school was greater in URM undergraduate students irrespective of scholastic ability as measured by SAT scores.14
A striking statistic is that the number of Black men matriculating into medical school in 2014 was less than it was in 1978 despite the increase in the number of US medical schools and efforts to recruit more diverse student populations. The Association of American Medical Colleges identified potential reasons for this decline, including poor early education, lack of mentorship, negative perceptions of Black men due to racial stereotypes, and lack of financial and academic resources to support the application process.8,13,15-17 Implicit racial bias by admission committees also may play a role.
Medical School Matriculation and Applying to Dermatology Residency
There is greater representation of URM students in medical school than in dermatology residency, which means URM students are either not applying to dermatology programs or they are not matching into the specialty. In the Electronic Residency Application Service’s 2016-2017 application cycle (N=776), there were 76 (9.8%) URM dermatology residency applicants.18 In 2018, there was a notable decline in representation of Black students among residency applicants (4.9%) to matched residents (3.7%), and there were only 133 (9.3%) URM dermatology residents in total (PGY2-PGY4 classes).19 The lack of exposure to medical subspecialties and the recommendation by medical schools for URM medical students to pursue careers in primary care have been cited as reasons that these students may not apply to residency programs in specialty care.20,21 The presence of an Accreditation Council for Graduate Medical Education dermatology residency program, fellowships, and dermatology interest groups at their medical schools correlated with higher proportions of URM students applying to dermatology programs.20
Underrepresented minority students face critical challenges during medical school, including receiving lower grades in both standardized and school-designated assessments and clerkship grades.21,22 A 2019 National Board of Medical Examiners study found that Hispanic and Black test takers scored 12.1 and 16.6 points lower than White men, respectively, on the
A recent cross-sectional study showed that lack of equitable resources, lack of support, financial constrictions, and lack of group identity were 4 barriers to URM students matching into dermatology.26 Dermatology is a competitive specialty with the highest median Electronic Residency Application Service applications submitted per US applicant (n=90)27 and an approximate total cost per US applicant of $10,781.28,29 Disadvantaged URM applicants noted relying on loans while non-URM applicants cited family financial support as being beneficial.26 In addition, an increasing number of applicants take gap years for research, which pose additional costs for finances and resources. In contrast, mentorship and participation in pipeline/enrichment programs were factors associated with URM students matching into dermatology.26
Dermatology Residency and the Transition to Advanced Dermatology Fellowships
Similar to the transition from medical school into dermatology residency, URM dermatology residents are either not applying to fellowships or are not getting in. In the 2018-2019 academic year, there were no Black, Hispanic, Native Hawaiian/Pacific Islander, or American Indian/Alaska Native Mohs micrographic surgery and dermatologic oncology fellows.19 Similarly, there were no Black, Native Hawaiian/Pacific Islander, or American Indian/Alaska Native dermatopathology fellows. There were 4 (6%) Hispanic dermatopathology fellows.19
There also is marked underrepresentation of minority groups—and minimal growth over time—in the dermatology procedural subspecialty. Whereas the percentage of female Mohs surgeons increased considerably from 1985 to 2005 (12.7% to 40.9%, respectively), the percentage of URM Mohs surgeons remained steady from 4.2% to 4.6%, respectively, and remained at 4.5% in 2014.30
There are no available data on the race/ethnicity of fellowship applicants, as these demographic data for the application process have not been consistently or traditionally collected. The reasons why there are so few URM dermatology fellows is not known; whether this is due to a lack of mentorship or whether other factors lead to residents not applying for advanced training needs further study. Financial factors related to prolonged training, which include lower salaries and delayed loan repayment, may present barriers to applying to fellowships.
Lack of URM Academic Faculty in Dermatology
At the academic faculty level, URM representation continues to worsen. Lett et al31 found that there is declining racial and ethnic representation in clinical academic medicine relative to US census data for 16 US medical specialties, including dermatology, with growing underrepresentation of Black and Hispanic faculty at the associate professor and full professor levels and underrepresentation in all faculty ranks. From 1970 to 2018, URM faculty in dermatology only increased from 4.8% to 7.4%, respectively. Non-URM female and male faculty members increased by 13.8 and 10.8 faculty members per year, respectively, while URM female and male faculty members increased by 1.2 and 0.8 faculty members per year, respectively.32
Underrepresentation of minorities seen in dermatology faculty may result from clinical demands, minority taxation (defined as the extensive service requirements uniquely experienced by URM faculty to disproportionately serve as representatives on academic committees and to mentor URM students), and barriers to academic promotion, which are challenges uniquely encountered by URMs in academic dermatology.33 Increased clinical demand may result from the fact that URM physicians are more likely to care for underserved populations, those of lower socioeconomic status, non-English–speaking patients, those on Medicaid, and those who are uninsured, which may impact renumeration. Minority tax experienced by URM faculty includes mentoring URM medical students, providing cultural expertise to departments and institutions, and participating in community service projects and outreach programs. Specifically, many institutional committees require the participation of a URM member, resulting in URM faculty members experiencing higher committee service burden. Many, if not all, of these responsibilities often are not compensated through salary or academic promotion.
A Call to Action
There are several steps that can be taken to create a pathway to dermatology that is inclusive, flexible, and supportive of URMs.
• Increase early exposure to dermatology in medical school. Early exposure and mentorship opportunities are associated with higher rates of students pursuing specialty field careers.34 Increased early opportunities allow for URM students to consider and explore a career in dermatology; receive mentorship; and ensure that dermatology, including topics related to skin of color (SOC), is incorporated into their learning. The American Academy of Dermatology has contributed to these efforts by its presence at every national meeting of the Student National Medical Association and Latino Medical Student Association, as well as its involvement with Nth Dimensions, which offers various educational opportunities for URM medical students.
• Implement equitable grading and holistic review processes in medical school. Racial/ethnic differences in clinical grading and standardized test scores in medical school demonstrate why holistic review of dermatology residency applicants is needed and why other metrics such as USMLE scores and AΩA status should be de-emphasized or eliminated when evaluating candidates. To support equity, many medical schools have eliminated honors grading, and some schools have eliminated AΩA distinction.
• Increase diversity of dermatology residents and residency programs. Implicit bias training for a medical school admissions committee has been shown to increase diversity in medical school enrollment.35 Whether implicit bias training and other diversity training may benefit dermatology residency selection must be examined, including study of unintended consequences, such as reduced diversity, increased microaggressions toward minority colleagues, and the illusion of fairness.36-39 Increasing representation is not sufficient—creating inclusive residency training environments is a critical parallel aim. Prioritizing diversity in dermatology residency recruitment is imperative. Creating dermatology residency positions specifically for URM residents may be an important option, as done at the University of Pennsylvania (Philadelphia, Pennsylvania) and Duke University (Durham, North Carolina).
• Create effective programs for URM mentorship. Due to the competitive nature of dermatology residency, the need for mentors in dermatology is critically vital for URM medical students, especially those without a home dermatology program at their medical school. Further development of formal mentorship and pipeline programs is essential at both the local and national levels. Some national examples of these initiatives include diversity mentorship programs offered by the American Academy of Dermatology, Skin of Color Society, Women’s Dermatologic Society, and Student National Medical Association. Many institutional programs also offer invaluable opportunities, such as the summer research fellowship at the University of California, San Francisco (UCSF); visiting clerkship grants for URMs at the University of Pennsylvania (Philadelphia, Pennsylvania) and Johns Hopkins University (Baltimore, Maryland); and integrated programs, such as the Visiting Elective Scholarship Program at UCSF, which provides funding and faculty mentorship for URM students completing an away rotation at UCSF.
• Establish longitudinal skin-of-color curricula and increased opportunities for research. More robust SOC training may lead to an increasingly diverse workforce. It is important that medical student and dermatology resident and fellow education include training on SOC to ensure high-quality care to diverse patient populations, which also may enhance the knowledge of trainees, encourage clinical and research interest in this field, and reduce health care disparities. Increasing research opportunities and offering formalized longitudinal training in SOC as well as incorporating more diverse images in medical school education may foster greater interest in this field at a time when trainees are establishing their career interests. At present, there is considerable room for improvement. Nijhawan et al40 surveyed 63 dermatology chief residents and 41 program directors and found only 14.3% and 14.6%, respectively, reported having an expert who conducts clinic specializing in SOC. Only 52.4% and 65.9% reported having didactic sessions or lectures focused on SOC diseases, and 30.2% and 12.2% reported having a dedicated rotation for residents to gain experience in SOC.40 A more recent study showed that when faculty were asked to incorporate more SOC content into lectures, the most commonly identified barrier to implementation was a lack of SOC images.41 Additionally, there remains a paucity of published research on this topic, with SOC articles representing only 2.7% of the literature.42 These numbers demonstrate the continued need for a more inclusive and comprehensive curriculum in dermatology residency programs and more robust funding for SOC research.
• Recruit and support URM faculty. Increasing diversity in dermatology residency programs likely will increase the number of potential URMs pursuing additional fellowship training and academic dermatology with active career mentorship and support. In addition, promoting faculty retention by combatting the progressive loss of URMs at all faculty levels is paramount. Mentorship for URM physicians has been shown to play a key role in the decision to pursue academic medicine as well as academic productivity and job satisfaction.43,44 The visibility, cultural competency, clinical work, academic productivity, and mentorship efforts that URM faculty provide are essential to enhancing patient care, teaching diverse groups of learners, and recruiting more diverse trainees. Protected time to participate in professional development opportunities has been shown to improve recruitment and retention of URM faculty and offer additional opportunities for junior faculty to find mentors.35,36 Incentivizing clinical care of underserved populations also may augment financial stability for URM physicians who choose to care for these patients. Finally, diversity work and community service should be legitimized and count toward faculty promotion.
Conclusion
There are numerous factors that contribute to the leaky pipeline in dermatology (eFigure). Many challenges that are unique to the URM population disadvantage these students from entering medical school, applying to dermatology residency, matching into dermatology fellowships, pursuing and staying in faculty positions, and achieving faculty advancement into leadership positions. With each progressive step along this trajectory, there is less minority representation. All dermatologists, regardless of race/ethnicity, need to play an active role and must prioritize diversity, equity, and inclusion efforts at all levels of education and training for the betterment of the specialty.
With a majority-minority population expected in the United States by 2044, improving diversity and cultural competency in the dermatology workforce is now more important than ever. A more diverse workforce increases the cultural competence of all providers, provides greater opportunities for mentorship and sponsorship of underrepresented minority (URM) trainees, establishes a more inclusive environment for learners, and enhances the knowledge and productivity of the workforce.1-3 Additionally, it is imperative to address clinical care disparities seen in minority patients in dermatology, including treatment of skin cancer, psoriasis, acne, atopic dermatitis, and other diseases.4-7
Despite the attention that has been devoted to improving diversity in medicine,8-10 dermatology remains one of the least diverse specialties, prompting additional calls to action within the field.11 Why does the lack of diversity still exist in dermatology, and what is the path to correcting this problem? In this article, we review the evidence of diversity barriers at different stages of medical education training that may impede academic advancement for minority learners pursuing careers in dermatology.
Undergraduate Medical Education
The term leaky pipeline refers to the progressive decline in the number of URMs along a given career path, including in dermatology. The Association of American Medical Colleges defines URMs as racial/ethnic populations that are “underrepresented in the medical profession relative to their numbers in the general population.”9 The first leak in the pipeline is that URMs are not applying to medical school. From 2002 and 2017, rates of both application and matriculation to medical school were lower by 30% to 70% in URM groups compared to White students, including Hispanic, Black, and American Indian/Alaska Native students.12,13 The decision not to apply to medical school was greater in URM undergraduate students irrespective of scholastic ability as measured by SAT scores.14
A striking statistic is that the number of Black men matriculating into medical school in 2014 was less than it was in 1978 despite the increase in the number of US medical schools and efforts to recruit more diverse student populations. The Association of American Medical Colleges identified potential reasons for this decline, including poor early education, lack of mentorship, negative perceptions of Black men due to racial stereotypes, and lack of financial and academic resources to support the application process.8,13,15-17 Implicit racial bias by admission committees also may play a role.
Medical School Matriculation and Applying to Dermatology Residency
There is greater representation of URM students in medical school than in dermatology residency, which means URM students are either not applying to dermatology programs or they are not matching into the specialty. In the Electronic Residency Application Service’s 2016-2017 application cycle (N=776), there were 76 (9.8%) URM dermatology residency applicants.18 In 2018, there was a notable decline in representation of Black students among residency applicants (4.9%) to matched residents (3.7%), and there were only 133 (9.3%) URM dermatology residents in total (PGY2-PGY4 classes).19 The lack of exposure to medical subspecialties and the recommendation by medical schools for URM medical students to pursue careers in primary care have been cited as reasons that these students may not apply to residency programs in specialty care.20,21 The presence of an Accreditation Council for Graduate Medical Education dermatology residency program, fellowships, and dermatology interest groups at their medical schools correlated with higher proportions of URM students applying to dermatology programs.20
Underrepresented minority students face critical challenges during medical school, including receiving lower grades in both standardized and school-designated assessments and clerkship grades.21,22 A 2019 National Board of Medical Examiners study found that Hispanic and Black test takers scored 12.1 and 16.6 points lower than White men, respectively, on the
A recent cross-sectional study showed that lack of equitable resources, lack of support, financial constrictions, and lack of group identity were 4 barriers to URM students matching into dermatology.26 Dermatology is a competitive specialty with the highest median Electronic Residency Application Service applications submitted per US applicant (n=90)27 and an approximate total cost per US applicant of $10,781.28,29 Disadvantaged URM applicants noted relying on loans while non-URM applicants cited family financial support as being beneficial.26 In addition, an increasing number of applicants take gap years for research, which pose additional costs for finances and resources. In contrast, mentorship and participation in pipeline/enrichment programs were factors associated with URM students matching into dermatology.26
Dermatology Residency and the Transition to Advanced Dermatology Fellowships
Similar to the transition from medical school into dermatology residency, URM dermatology residents are either not applying to fellowships or are not getting in. In the 2018-2019 academic year, there were no Black, Hispanic, Native Hawaiian/Pacific Islander, or American Indian/Alaska Native Mohs micrographic surgery and dermatologic oncology fellows.19 Similarly, there were no Black, Native Hawaiian/Pacific Islander, or American Indian/Alaska Native dermatopathology fellows. There were 4 (6%) Hispanic dermatopathology fellows.19
There also is marked underrepresentation of minority groups—and minimal growth over time—in the dermatology procedural subspecialty. Whereas the percentage of female Mohs surgeons increased considerably from 1985 to 2005 (12.7% to 40.9%, respectively), the percentage of URM Mohs surgeons remained steady from 4.2% to 4.6%, respectively, and remained at 4.5% in 2014.30
There are no available data on the race/ethnicity of fellowship applicants, as these demographic data for the application process have not been consistently or traditionally collected. The reasons why there are so few URM dermatology fellows is not known; whether this is due to a lack of mentorship or whether other factors lead to residents not applying for advanced training needs further study. Financial factors related to prolonged training, which include lower salaries and delayed loan repayment, may present barriers to applying to fellowships.
Lack of URM Academic Faculty in Dermatology
At the academic faculty level, URM representation continues to worsen. Lett et al31 found that there is declining racial and ethnic representation in clinical academic medicine relative to US census data for 16 US medical specialties, including dermatology, with growing underrepresentation of Black and Hispanic faculty at the associate professor and full professor levels and underrepresentation in all faculty ranks. From 1970 to 2018, URM faculty in dermatology only increased from 4.8% to 7.4%, respectively. Non-URM female and male faculty members increased by 13.8 and 10.8 faculty members per year, respectively, while URM female and male faculty members increased by 1.2 and 0.8 faculty members per year, respectively.32
Underrepresentation of minorities seen in dermatology faculty may result from clinical demands, minority taxation (defined as the extensive service requirements uniquely experienced by URM faculty to disproportionately serve as representatives on academic committees and to mentor URM students), and barriers to academic promotion, which are challenges uniquely encountered by URMs in academic dermatology.33 Increased clinical demand may result from the fact that URM physicians are more likely to care for underserved populations, those of lower socioeconomic status, non-English–speaking patients, those on Medicaid, and those who are uninsured, which may impact renumeration. Minority tax experienced by URM faculty includes mentoring URM medical students, providing cultural expertise to departments and institutions, and participating in community service projects and outreach programs. Specifically, many institutional committees require the participation of a URM member, resulting in URM faculty members experiencing higher committee service burden. Many, if not all, of these responsibilities often are not compensated through salary or academic promotion.
A Call to Action
There are several steps that can be taken to create a pathway to dermatology that is inclusive, flexible, and supportive of URMs.
• Increase early exposure to dermatology in medical school. Early exposure and mentorship opportunities are associated with higher rates of students pursuing specialty field careers.34 Increased early opportunities allow for URM students to consider and explore a career in dermatology; receive mentorship; and ensure that dermatology, including topics related to skin of color (SOC), is incorporated into their learning. The American Academy of Dermatology has contributed to these efforts by its presence at every national meeting of the Student National Medical Association and Latino Medical Student Association, as well as its involvement with Nth Dimensions, which offers various educational opportunities for URM medical students.
• Implement equitable grading and holistic review processes in medical school. Racial/ethnic differences in clinical grading and standardized test scores in medical school demonstrate why holistic review of dermatology residency applicants is needed and why other metrics such as USMLE scores and AΩA status should be de-emphasized or eliminated when evaluating candidates. To support equity, many medical schools have eliminated honors grading, and some schools have eliminated AΩA distinction.
• Increase diversity of dermatology residents and residency programs. Implicit bias training for a medical school admissions committee has been shown to increase diversity in medical school enrollment.35 Whether implicit bias training and other diversity training may benefit dermatology residency selection must be examined, including study of unintended consequences, such as reduced diversity, increased microaggressions toward minority colleagues, and the illusion of fairness.36-39 Increasing representation is not sufficient—creating inclusive residency training environments is a critical parallel aim. Prioritizing diversity in dermatology residency recruitment is imperative. Creating dermatology residency positions specifically for URM residents may be an important option, as done at the University of Pennsylvania (Philadelphia, Pennsylvania) and Duke University (Durham, North Carolina).
• Create effective programs for URM mentorship. Due to the competitive nature of dermatology residency, the need for mentors in dermatology is critically vital for URM medical students, especially those without a home dermatology program at their medical school. Further development of formal mentorship and pipeline programs is essential at both the local and national levels. Some national examples of these initiatives include diversity mentorship programs offered by the American Academy of Dermatology, Skin of Color Society, Women’s Dermatologic Society, and Student National Medical Association. Many institutional programs also offer invaluable opportunities, such as the summer research fellowship at the University of California, San Francisco (UCSF); visiting clerkship grants for URMs at the University of Pennsylvania (Philadelphia, Pennsylvania) and Johns Hopkins University (Baltimore, Maryland); and integrated programs, such as the Visiting Elective Scholarship Program at UCSF, which provides funding and faculty mentorship for URM students completing an away rotation at UCSF.
• Establish longitudinal skin-of-color curricula and increased opportunities for research. More robust SOC training may lead to an increasingly diverse workforce. It is important that medical student and dermatology resident and fellow education include training on SOC to ensure high-quality care to diverse patient populations, which also may enhance the knowledge of trainees, encourage clinical and research interest in this field, and reduce health care disparities. Increasing research opportunities and offering formalized longitudinal training in SOC as well as incorporating more diverse images in medical school education may foster greater interest in this field at a time when trainees are establishing their career interests. At present, there is considerable room for improvement. Nijhawan et al40 surveyed 63 dermatology chief residents and 41 program directors and found only 14.3% and 14.6%, respectively, reported having an expert who conducts clinic specializing in SOC. Only 52.4% and 65.9% reported having didactic sessions or lectures focused on SOC diseases, and 30.2% and 12.2% reported having a dedicated rotation for residents to gain experience in SOC.40 A more recent study showed that when faculty were asked to incorporate more SOC content into lectures, the most commonly identified barrier to implementation was a lack of SOC images.41 Additionally, there remains a paucity of published research on this topic, with SOC articles representing only 2.7% of the literature.42 These numbers demonstrate the continued need for a more inclusive and comprehensive curriculum in dermatology residency programs and more robust funding for SOC research.
• Recruit and support URM faculty. Increasing diversity in dermatology residency programs likely will increase the number of potential URMs pursuing additional fellowship training and academic dermatology with active career mentorship and support. In addition, promoting faculty retention by combatting the progressive loss of URMs at all faculty levels is paramount. Mentorship for URM physicians has been shown to play a key role in the decision to pursue academic medicine as well as academic productivity and job satisfaction.43,44 The visibility, cultural competency, clinical work, academic productivity, and mentorship efforts that URM faculty provide are essential to enhancing patient care, teaching diverse groups of learners, and recruiting more diverse trainees. Protected time to participate in professional development opportunities has been shown to improve recruitment and retention of URM faculty and offer additional opportunities for junior faculty to find mentors.35,36 Incentivizing clinical care of underserved populations also may augment financial stability for URM physicians who choose to care for these patients. Finally, diversity work and community service should be legitimized and count toward faculty promotion.
Conclusion
There are numerous factors that contribute to the leaky pipeline in dermatology (eFigure). Many challenges that are unique to the URM population disadvantage these students from entering medical school, applying to dermatology residency, matching into dermatology fellowships, pursuing and staying in faculty positions, and achieving faculty advancement into leadership positions. With each progressive step along this trajectory, there is less minority representation. All dermatologists, regardless of race/ethnicity, need to play an active role and must prioritize diversity, equity, and inclusion efforts at all levels of education and training for the betterment of the specialty.
- Dixon G, Kind T, Wright J, et al. Factors that influence the choice of academic pediatrics by underrepresented minorities. Pediatrics. 2019;144:E20182759. doi:10.1542/peds.2018-2759
- Yehia BR, Cronholm PF, Wilson N, et al. Mentorship and pursuit of academic medicine careers: a mixed methods study of residents from diverse backgrounds. BMC Med Educ. 2014:14:2-26. doi:10.1186/1472-6920-14-26
- Saha S, Guiton G, Wimmers PF, et al. Student body racial and ethnic composition and diversity-related outcomes in US medical schools. JAMA. 2008;300:1135-1145. doi:10.1001/jama.300.10.1135
- Hsu DY, Gordon K, Silverberg JI. The patient burden of psoriasis in the United States. J Am Acad Dermatol. 2016;75:33-41. doi:10.1016/j.jaad.2016.03.048
- Silverberg JI. Racial and ethnic disparities in atopic dermatitis. Curr Dermatol Rep. 2015;4:44-48.
- Buster KJ, Sevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59. doi:10.1016/j.det.2011.08.002
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Smedley BD, Stith AY, Colburn L, et al. The Right Thing To Do, The Smart Thing to Do: Enhancing Diversity in the Health Professions. National Academies Press; 2001.
- Association of American Medical Colleges. Minorities in medical education: fact and figures 2019. Accessed December 9, 2021. https://www.aamc.org/datareports/workforce/report/diversity-medicine-facts-and-figures-2019
- Liaison Committee on Medical Education (LCME) standards on diversity. University of South Florida Health website. Accessed December 9, 2021. https://health.usf.edu/~/media/Files/Medicine/MD%20Program/Diversity/LCMEStandardsonDiversity1.ashx?la=en
- Granstein RD, Cornelius L, Shinkai K. Diversity in dermatology—a call for action. JAMA Dermatol. 2017;153:499-500. doi:10.1001/jamadermatol.2017.0296
- Lett LA, Murdock HM, Orji W, et al. Trends in racial/ethnic representation among US medical students. JAMA Netw Open. 2019;2:e1910490. doi:10.1001/jamanetworkopen.2019.10490
- Association of American Medical Colleges. Altering the course: Black males in medicine. Published 2015. Accessed December 8, 2021. https://store.aamc.org/downloadable/download/sample/sample_id/84/
- Barr DA, Gonzalez ME, Wanat SF. The leaky pipeline: factors associated with early decline in interest in premedical studies among underrepresented minority undergraduate students. Acad Med. 2008;83:5:503-511. doi:10.1097/ACM.0b013e31816bda16
- Flores RL. The rising gap between rich and poor: a look at the persistence of educational disparities in the United States and why we should worry. Cogent Soc Sci. 2017;3:1323698.
- Jackson D. Why am I behind? an examination of low income and minority students’ preparedness for college. McNair Sch J. 2012;13:121-138.
- Rothstein R. The racial achievement gap, segregated schools, andsegregated neighborhoods: a constitutional insult. Race Soc Probl. 2015;7:21-30.
- Association of American Medical Colleges. Residency Applicants From US MD Granting Medical Schools to ACGME-Accredited Programs by Specialty and Race/Ethnicity. Association of American Medical Colleges; 2017.
- Brotherton SE, Etzel SL. Graduate medical education, 2018-2019. JAMA. 2019;322:996-1016. doi:10.1001/jama.2019.10155
- Barnes LA, Bae GH, Nambudiri V. Sex and racial/ethnic diversity of US medical students and their exposure to dermatology programs. JAMA Dermatol. 2019;155:490-491. doi:10.1001/jamadermatol.2018.5025
- Soliman YS, Rzepecki AK, Guzman AK. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254. doi:10.1001/jamadermatol.2018.4813
- Low D, Pollack SW, Liao Z, et al. Racial/ethnic disparities in clinical grading in medical school. Teach Learn Med. 2019;31:487-496. doi:10.1080/10401334.2019.1597724
- Rubright JD, Jodoin M, Barone MA. Examining demographics, prior academic performance and United States medical licensing examination scores. Acad Med. 2019;94;364-370. doi:10.1097/ACM.0000000000002366
- Boatright D, Ross D, O’Connor P, et al. Racial disparities in medical student membership in the alpha omega honor society. JAMA Intern Med. 2017;177:659-665. doi:10.1001/jamainternmed.2016.9623
- Gorouhi F, Alikhan A, Rezaei A, et al. Dermatology residency selection criteria with an emphasis on program characteristics: a national program director survey [published online March 17, 2014]. Dermatol Res Pract. doi:10.1155/2014/692760
- Vasquez R, Jeong H, Florez-Pollack S, et al. What are the barriers faced by underrepresented minorities applying to dermatology? a qualitative cross-sectional study of applicants applying to a large dermatology residency program. J Am Acad Dermatol. 2020;83:1770-1773. doi:10.1016/j.jaad.2020.03.067
- Results of the 2019 NRMP applicant survey by preferred specialty and applicant type. National Resident Matching Program website. Published July 2019. Accessed December 8, 2021. https://www.nrmp.org/wp-content/uploads/2019/06/Applicant-Survey-Report-2019.pdf
- Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756. doi:10.1016/j.jaad.2015.10.049
- Polacco MA, Lally J, Walls A, et al. Digging into debt: the financial burden associated with the otolaryngology match. Otolaryngol Head Neck Surg. 2017;12:1091-1096. doi:10.1177/0194599816686538
- Feng H, Feng PW, Geronemus RG. Diversity in the US Mohs micrographic surgery workforce. Dermatol Surg. 2020:46:1451-1455. doi:10.1097/DSS.0000000000002080
- Lett LA, Orji WU, Sebro R. Declining racial and ethnic representation in clinical academic medicine: a longitudinal study of 16 US medical specialties. PLoS ONE. 2018;13:e0207274. doi:10.1371/journal.pone.020727432. Xierali IM, Nivet MA, Pandya AG. US Dermatology department faculty diversity trends by sex and underrepresented-in-medicine status, 1970-2018. JAMA Dermatol. 2020;156:280-287. doi:10.1001/jamadermatol.2019.4297
- Okoye GA. Supporting underrepresented minority women in academic dermatology. Intl J Womens Dermatol. 2020;6:57-60. doi:10.1016/j.ijwd.2019.09.009
- Bernstein J, Dicaprio MR, Mehta S. The relationship between required medical school instruction in musculoskeletal medicine and application rates to orthopaedic surgery residency programs. J Bone Joint Surg Am. 2004;86:2335-2338. doi:10.2106/00004623-200410000-00031
- Capers Q, Clinchot D, McDougle L, et al. Implicit racial bias in medical school admissions. Acad Med. 2017;92:365-369. doi:10.1097/ACM.0000000000001388
- Dobbin F, Kalev A. Why diversity programs fail. Harvard Business Rev. 2016;52-60. Accessed December 8, 2021. https://hbr.org/2016/07/why-diversity-programs-fail
- Kalev A, Dobbin F, Kelly E. Best practices or best guesses? assessing the efficacy of corporate affirmative action and diversity policies. Am Sociol Rev. 2006;71:589-617.
- Sanchez JI, Medkik N. The effects of diversity awareness training on differential treatment. Group Organ Manag. 2004;29:517-536.
- Kaiser CR, Major B, Jurcevic I, et al. Presumed fair: ironic effects of organizational diversity structures. J Pers Soc Psychol. 2013;104:504-519. doi:10.1037/a0030838
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-617.
- Jia JL, Gordon JS, Lester JC, et al. Integrating skin of color and sexual and gender minority content into dermatology residency curricula: a prospective program initiative [published online April 16, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.04.018
- Amuzie AU, Lia JL, Taylor SC, et al. Skin of color article representation in dermatology literature 2009-2019: higher citation counts and opportunities for inclusion [published online March 24, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.063
- Beech BM, Calles-Escandon J, Hairston KC, et al. Mentoring programs for underrepresented minority faculty in academic medical center: a systematic review of the literature. Acad Med. 2013;88:541-549. doi:10.1097/ACM.0b013e31828589e3
- Daley S, Wingard DL, Reznik V. Improving the retention of underrepresented minority faculty in academic medicine. J Natl Med Assoc. 2006;98:1435-1440. doi:10.1016/s0027-9684(15)31449-8
- Association of American Medical Colleges. US medical school faculty by sex, race/ethnicity, rank, and department, 2019. Published December 31, 2019. Accessed December 20, 2021. https://www.aamc.org/media/8476/download?attachment
- Dixon G, Kind T, Wright J, et al. Factors that influence the choice of academic pediatrics by underrepresented minorities. Pediatrics. 2019;144:E20182759. doi:10.1542/peds.2018-2759
- Yehia BR, Cronholm PF, Wilson N, et al. Mentorship and pursuit of academic medicine careers: a mixed methods study of residents from diverse backgrounds. BMC Med Educ. 2014:14:2-26. doi:10.1186/1472-6920-14-26
- Saha S, Guiton G, Wimmers PF, et al. Student body racial and ethnic composition and diversity-related outcomes in US medical schools. JAMA. 2008;300:1135-1145. doi:10.1001/jama.300.10.1135
- Hsu DY, Gordon K, Silverberg JI. The patient burden of psoriasis in the United States. J Am Acad Dermatol. 2016;75:33-41. doi:10.1016/j.jaad.2016.03.048
- Silverberg JI. Racial and ethnic disparities in atopic dermatitis. Curr Dermatol Rep. 2015;4:44-48.
- Buster KJ, Sevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59. doi:10.1016/j.det.2011.08.002
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Smedley BD, Stith AY, Colburn L, et al. The Right Thing To Do, The Smart Thing to Do: Enhancing Diversity in the Health Professions. National Academies Press; 2001.
- Association of American Medical Colleges. Minorities in medical education: fact and figures 2019. Accessed December 9, 2021. https://www.aamc.org/datareports/workforce/report/diversity-medicine-facts-and-figures-2019
- Liaison Committee on Medical Education (LCME) standards on diversity. University of South Florida Health website. Accessed December 9, 2021. https://health.usf.edu/~/media/Files/Medicine/MD%20Program/Diversity/LCMEStandardsonDiversity1.ashx?la=en
- Granstein RD, Cornelius L, Shinkai K. Diversity in dermatology—a call for action. JAMA Dermatol. 2017;153:499-500. doi:10.1001/jamadermatol.2017.0296
- Lett LA, Murdock HM, Orji W, et al. Trends in racial/ethnic representation among US medical students. JAMA Netw Open. 2019;2:e1910490. doi:10.1001/jamanetworkopen.2019.10490
- Association of American Medical Colleges. Altering the course: Black males in medicine. Published 2015. Accessed December 8, 2021. https://store.aamc.org/downloadable/download/sample/sample_id/84/
- Barr DA, Gonzalez ME, Wanat SF. The leaky pipeline: factors associated with early decline in interest in premedical studies among underrepresented minority undergraduate students. Acad Med. 2008;83:5:503-511. doi:10.1097/ACM.0b013e31816bda16
- Flores RL. The rising gap between rich and poor: a look at the persistence of educational disparities in the United States and why we should worry. Cogent Soc Sci. 2017;3:1323698.
- Jackson D. Why am I behind? an examination of low income and minority students’ preparedness for college. McNair Sch J. 2012;13:121-138.
- Rothstein R. The racial achievement gap, segregated schools, andsegregated neighborhoods: a constitutional insult. Race Soc Probl. 2015;7:21-30.
- Association of American Medical Colleges. Residency Applicants From US MD Granting Medical Schools to ACGME-Accredited Programs by Specialty and Race/Ethnicity. Association of American Medical Colleges; 2017.
- Brotherton SE, Etzel SL. Graduate medical education, 2018-2019. JAMA. 2019;322:996-1016. doi:10.1001/jama.2019.10155
- Barnes LA, Bae GH, Nambudiri V. Sex and racial/ethnic diversity of US medical students and their exposure to dermatology programs. JAMA Dermatol. 2019;155:490-491. doi:10.1001/jamadermatol.2018.5025
- Soliman YS, Rzepecki AK, Guzman AK. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254. doi:10.1001/jamadermatol.2018.4813
- Low D, Pollack SW, Liao Z, et al. Racial/ethnic disparities in clinical grading in medical school. Teach Learn Med. 2019;31:487-496. doi:10.1080/10401334.2019.1597724
- Rubright JD, Jodoin M, Barone MA. Examining demographics, prior academic performance and United States medical licensing examination scores. Acad Med. 2019;94;364-370. doi:10.1097/ACM.0000000000002366
- Boatright D, Ross D, O’Connor P, et al. Racial disparities in medical student membership in the alpha omega honor society. JAMA Intern Med. 2017;177:659-665. doi:10.1001/jamainternmed.2016.9623
- Gorouhi F, Alikhan A, Rezaei A, et al. Dermatology residency selection criteria with an emphasis on program characteristics: a national program director survey [published online March 17, 2014]. Dermatol Res Pract. doi:10.1155/2014/692760
- Vasquez R, Jeong H, Florez-Pollack S, et al. What are the barriers faced by underrepresented minorities applying to dermatology? a qualitative cross-sectional study of applicants applying to a large dermatology residency program. J Am Acad Dermatol. 2020;83:1770-1773. doi:10.1016/j.jaad.2020.03.067
- Results of the 2019 NRMP applicant survey by preferred specialty and applicant type. National Resident Matching Program website. Published July 2019. Accessed December 8, 2021. https://www.nrmp.org/wp-content/uploads/2019/06/Applicant-Survey-Report-2019.pdf
- Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756. doi:10.1016/j.jaad.2015.10.049
- Polacco MA, Lally J, Walls A, et al. Digging into debt: the financial burden associated with the otolaryngology match. Otolaryngol Head Neck Surg. 2017;12:1091-1096. doi:10.1177/0194599816686538
- Feng H, Feng PW, Geronemus RG. Diversity in the US Mohs micrographic surgery workforce. Dermatol Surg. 2020:46:1451-1455. doi:10.1097/DSS.0000000000002080
- Lett LA, Orji WU, Sebro R. Declining racial and ethnic representation in clinical academic medicine: a longitudinal study of 16 US medical specialties. PLoS ONE. 2018;13:e0207274. doi:10.1371/journal.pone.020727432. Xierali IM, Nivet MA, Pandya AG. US Dermatology department faculty diversity trends by sex and underrepresented-in-medicine status, 1970-2018. JAMA Dermatol. 2020;156:280-287. doi:10.1001/jamadermatol.2019.4297
- Okoye GA. Supporting underrepresented minority women in academic dermatology. Intl J Womens Dermatol. 2020;6:57-60. doi:10.1016/j.ijwd.2019.09.009
- Bernstein J, Dicaprio MR, Mehta S. The relationship between required medical school instruction in musculoskeletal medicine and application rates to orthopaedic surgery residency programs. J Bone Joint Surg Am. 2004;86:2335-2338. doi:10.2106/00004623-200410000-00031
- Capers Q, Clinchot D, McDougle L, et al. Implicit racial bias in medical school admissions. Acad Med. 2017;92:365-369. doi:10.1097/ACM.0000000000001388
- Dobbin F, Kalev A. Why diversity programs fail. Harvard Business Rev. 2016;52-60. Accessed December 8, 2021. https://hbr.org/2016/07/why-diversity-programs-fail
- Kalev A, Dobbin F, Kelly E. Best practices or best guesses? assessing the efficacy of corporate affirmative action and diversity policies. Am Sociol Rev. 2006;71:589-617.
- Sanchez JI, Medkik N. The effects of diversity awareness training on differential treatment. Group Organ Manag. 2004;29:517-536.
- Kaiser CR, Major B, Jurcevic I, et al. Presumed fair: ironic effects of organizational diversity structures. J Pers Soc Psychol. 2013;104:504-519. doi:10.1037/a0030838
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-617.
- Jia JL, Gordon JS, Lester JC, et al. Integrating skin of color and sexual and gender minority content into dermatology residency curricula: a prospective program initiative [published online April 16, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.04.018
- Amuzie AU, Lia JL, Taylor SC, et al. Skin of color article representation in dermatology literature 2009-2019: higher citation counts and opportunities for inclusion [published online March 24, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.063
- Beech BM, Calles-Escandon J, Hairston KC, et al. Mentoring programs for underrepresented minority faculty in academic medical center: a systematic review of the literature. Acad Med. 2013;88:541-549. doi:10.1097/ACM.0b013e31828589e3
- Daley S, Wingard DL, Reznik V. Improving the retention of underrepresented minority faculty in academic medicine. J Natl Med Assoc. 2006;98:1435-1440. doi:10.1016/s0027-9684(15)31449-8
- Association of American Medical Colleges. US medical school faculty by sex, race/ethnicity, rank, and department, 2019. Published December 31, 2019. Accessed December 20, 2021. https://www.aamc.org/media/8476/download?attachment
Practice Points
- Dermatology remains the second least diverse specialty in medicine, which has important implications for the workforce and clinical excellence of the specialty.
- Barriers presenting at different stages of medical education and training result in the loss of underrepresented minority (URM) learners pursuing or advancing careers in dermatology.
- Understanding these barriers is the first step to creating and implementing important structural changes to the way we mentor, teach, and support URM students in the specialty.
Management of Classic Ulcerative Pyoderma Gangrenosum
Pyoderma gangrenosum (PG) is a rare, chronic, ulcerative, neutrophilic dermatosis of unclear etiology. Large, multicentered, randomized controlled trials (RCTs) are challenging due to the rarity of PG and the lack of a diagnostic confirmatory test; therefore, evidence-based guidelines for diagnosis and treatment are not well established. Current management of PG primarily is guided by case series, small clinical trials, and expert opinion.1-4 We conducted a survey of expert medical dermatologists to highlight best practices in diagnostic and therapeutic approaches to PG.
Methods
The Society of Dermatology Hospitalists (SDH) Scientific Task Force gathered expert opinions from members of the SDH and Rheumatologic Dermatology Society (RDS) regarding PG workup and treatment through an online survey of 15 items (eTable 1). Subscribers of the SDH and RDS LISTSERVs were invited via email to participate in the survey from January 2016 to February 2016. Anonymous survey responses were collected and collated using SurveyMonkey. The survey results identified expert recommendations for evaluation, diagnosis, and treatment of PG and are reported as the sum of the percentage of respondents who answered always (almost 100% of the time) or often (more than half the time) following a particular course of action. A subanalysis was performed defining 2 groups of respondents based on the number of cases of PG treated per year (≥10 vs <10). Survey responses between each group were compared using χ2 analysis with statistical significance set at P=.05.

Results
Fifty-one respondents completed the survey out of 140 surveyed (36% response rate). All respondents were dermatologists, and 96% (49/51) were affiliated with an academic institution. Among the respondents, the number of PG cases managed per year ranged from 2 to 35.
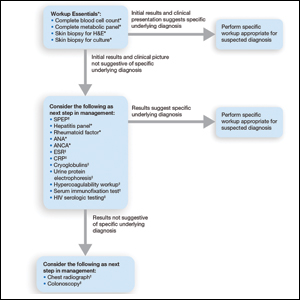
Respondents consistently ordered skin biopsies (92% [47/51]) and tissue cultures (90% [46/51]), as well as certain ancillary tests, including complete blood cell count (96% [49/51]), complete metabolic panel (86% [44/51]), serum protein electrophoresis (76% [39/51]), and hepatitis panel (71% [36/51]). Other frequently ordered studies were rheumatoid factor (69% [35/51]), antinuclear antibodies (67% [34/51]), and antineutrophilic antibodies (65% [33/51]). Respondents frequently ordered erythrocyte sedimentation rate (59% [30/51]), C-reactive protein (55% [28/51]), cryoglobulins (53% [27/51]), urine protein electrophoresis (53% [27/51]), hypercoagulability workup (49% [25/51]), and serum immunofixation test (49% [25/51]). Human immunodeficiency virus testing (43% [22/51]), chest radiograph (41% [21/51]), colonoscopy (41% [21/51]) and referral to other specialties for workup—gastroenterology (38% [19/51]), hematology/oncology (14% [7/51]), and rheumatology (10% [5/51])—were less frequently ordered (eTable 2).
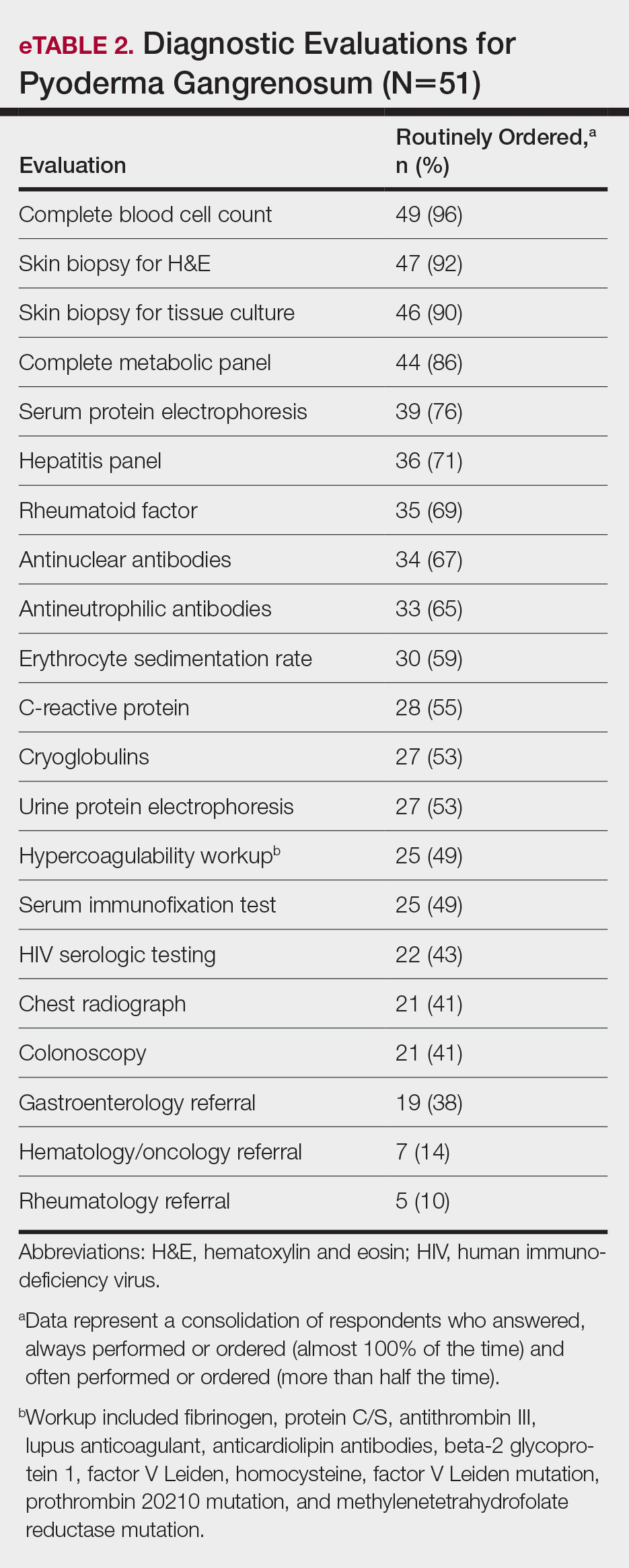
Systemic corticosteroids were reported as first-line therapy by most respondents (94% [48/51]), followed by topical immunomodulatory therapies (63% [32/51]). Topical corticosteroids (75% [38/51]) were the most common first-line topical agents. Thirty-nine percent of respondents (20/51) prescribed topical calcineurin inhibitors as first-line topical therapy. Additional therapies frequently used included systemic cyclosporine (47% [24/51]), antineutrophilic agents (41% [21/51]), and biologic agents (37% [19/51]). Fifty-seven percent of respondents (29/51) supported using combination topical and systemic therapy (Table).
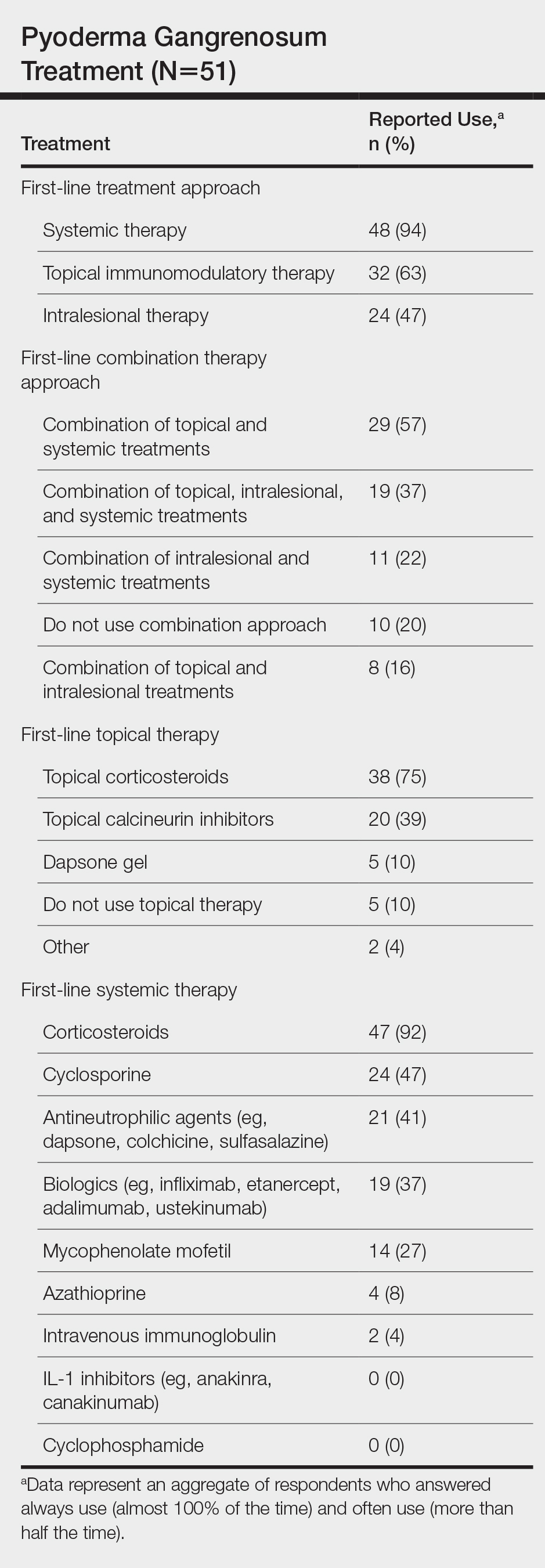
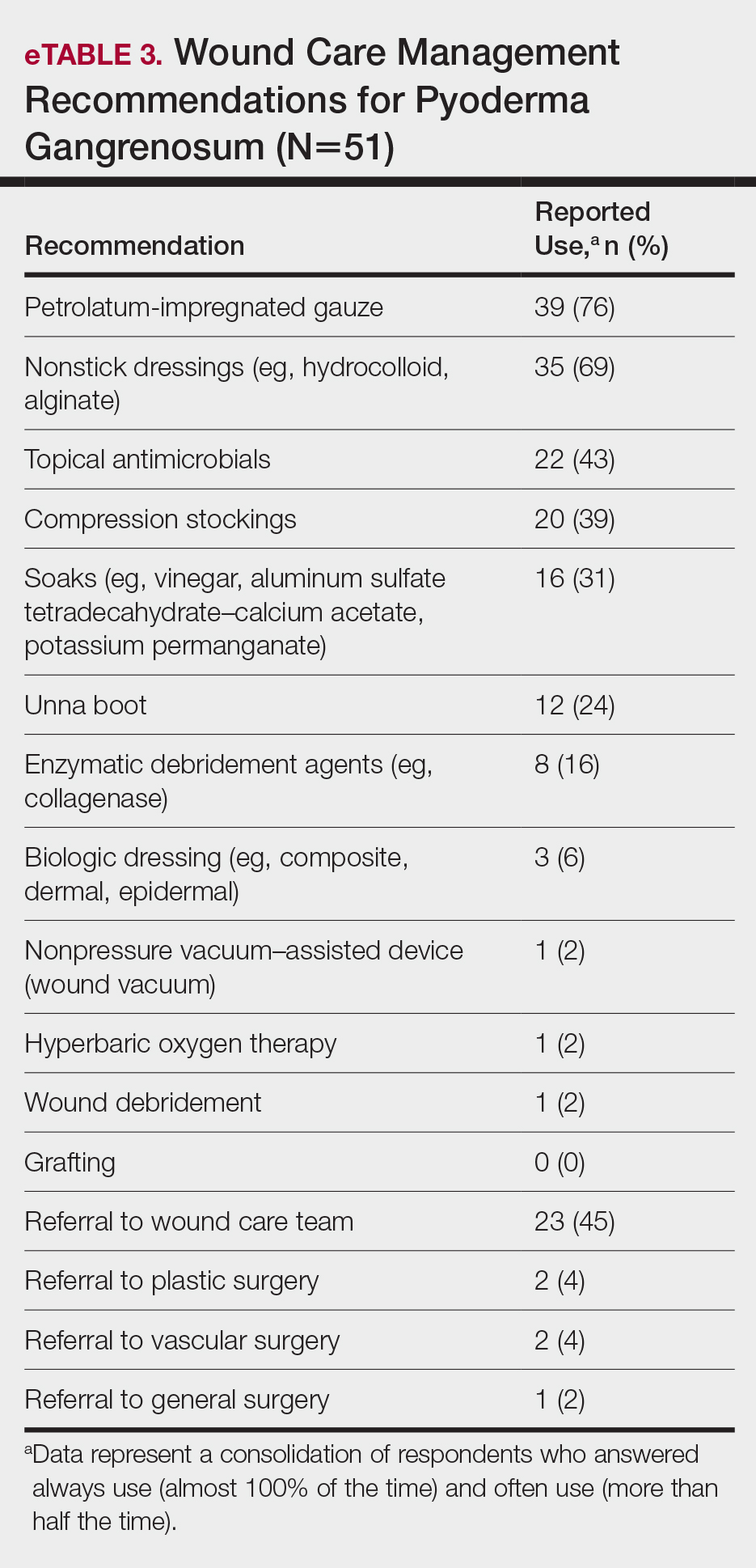
A wide variety of wound care practices were reported in the management of PG. Seventy-six percent of respondents (39/51) favored petroleum-impregnated gauze, 69% (35/51) used nonadhesive dressings, and 43% (22/51) added antimicrobial therapy for PG wound care (eTable 3). In the subanalysis, there were no significant differences in the majority of answer responses in patients treating 10 or more PG cases per year vs fewer than 10 PG cases, except with regard to the practice of combination therapy. Those treating more than 10 cases of PG per year more frequently reported use of combination therapies compared to respondents treating fewer than 10 cases (P=.04).
Comment
Skin biopsies and tissue cultures were strongly recommended (>90% survey respondents) for the initial evaluation of lesions suspected to be PG to evaluate for typical histopathologic changes that appear early in the disease, to rule out PG mimickers such as infectious or vascular causes, and to prevent the detrimental effects of inappropriate treatment and delayed diagnosis.5
Suspected PG warrants a reasonable search for related conditions because more than 50% of PG cases are associated with comorbidities such as rheumatoid arthritis, inflammatory bowel disease, and hematologic disease/malignancy.6,7 A complete blood cell count and comprehensive metabolic panel were recommended by most respondents, aiding in the preliminary screening for hematologic and infectious causes as well as detecting liver and kidney dysfunction associated with systemic conditions. Additionally, exclusion of infection or malignancy may be particularly important if the patient will undergo systemic immunosuppression. In challenging PG cases when initial findings are inconclusive and the clinical presentation does not direct workup (eg, colonoscopy to evaluate gastrointestinal tract symptoms), serum protein electrophoresis, hepatitis panel, rheumatoid factor, antinuclear antibodies, and antineutrophilic antibody tests also were frequently ordered by respondents to further evaluate for underlying or associated conditions.
This consensus regarding skin biopsies and certain ancillary tests is consistent with the proposed diagnostic criteria for classic ulcerative PG in which the absence or exclusion of other relevant causes of cutaneous ulcers is required based on the criteria.8 The importance of ensuring an accurate diagnosis is paramount, as a 10% misdiagnosis rate has been documented in the literature.5
Importantly, a stepwise diagnostic workup for PG is proposed based on survey results, which may limit unnecessary testing and the associated costs to the health care system (Figure 1). Selection of additional testing is guided by initial test results and features of the patient’s clinical presentation, including age, review of systems, and associated comorbidities. Available data suggest that underlying inflammatory bowel disease is more frequent in PG patients who are younger than 65 years, whereas those who are 65 years and older are more likely to have inflammatory arthritis, cancer, or an underlying hematologic disorder.9
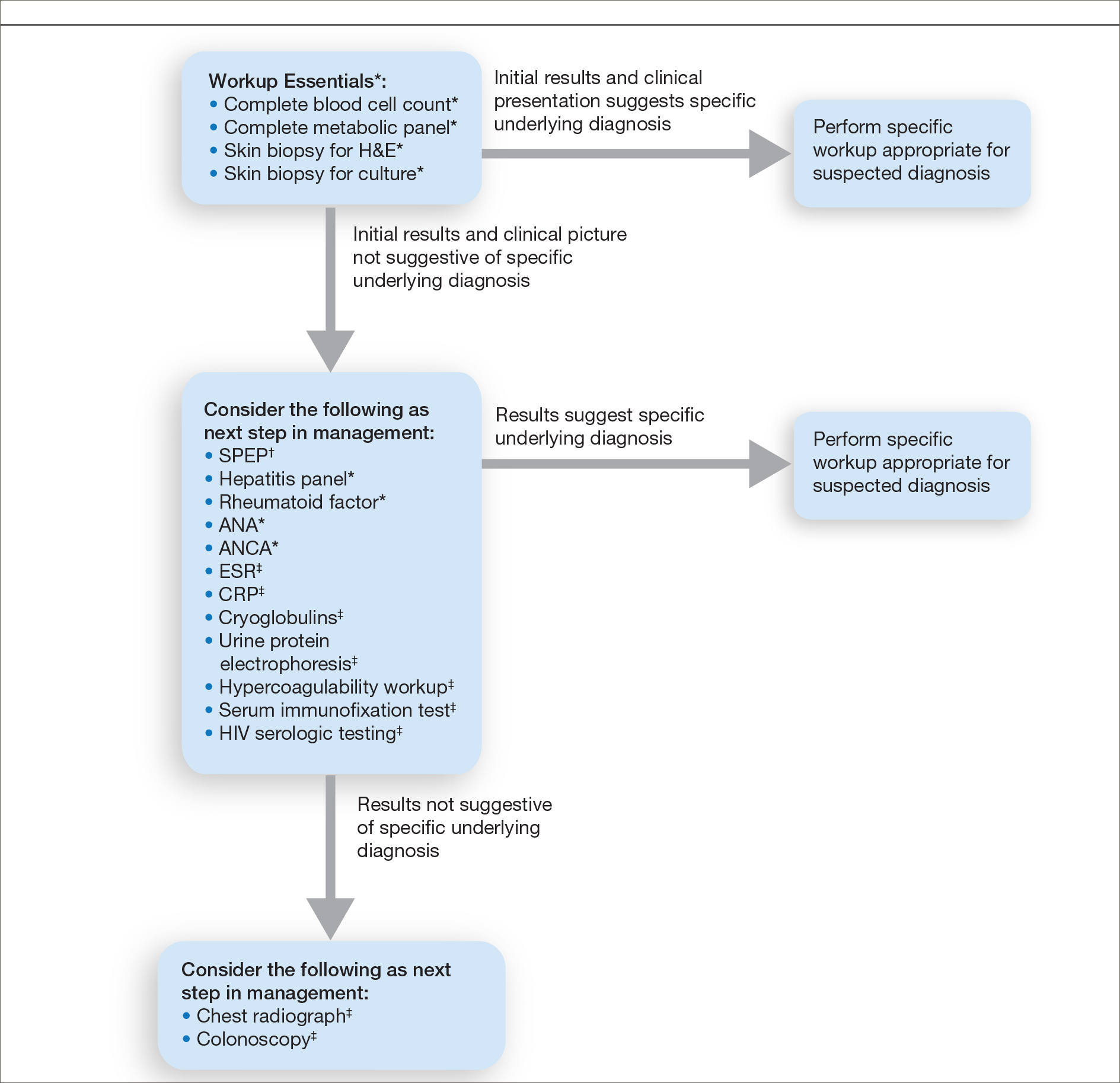
Treatment of PG should address both the inflammatory and wound components of the disease (Figure 2).7 In our survey results, systemic corticosteroids were identified as an important first-line therapy supported by reasonable evidence and were favored for their rapid response and minimal cost.1,10,11 Many respondents endorsed the use of systemic therapy in combination with topical steroids or calcineurin inhibitors. Combination therapy may provide more immediate control of rapidly progressing disease while minimizing adverse effects of long-term systemic corticosteroid use. A survey of German wound experts similarly endorsed frequent use of topical calcineurin inhibitors and combination systemic and topical glucocorticoid therapy as common therapeutic approaches.1
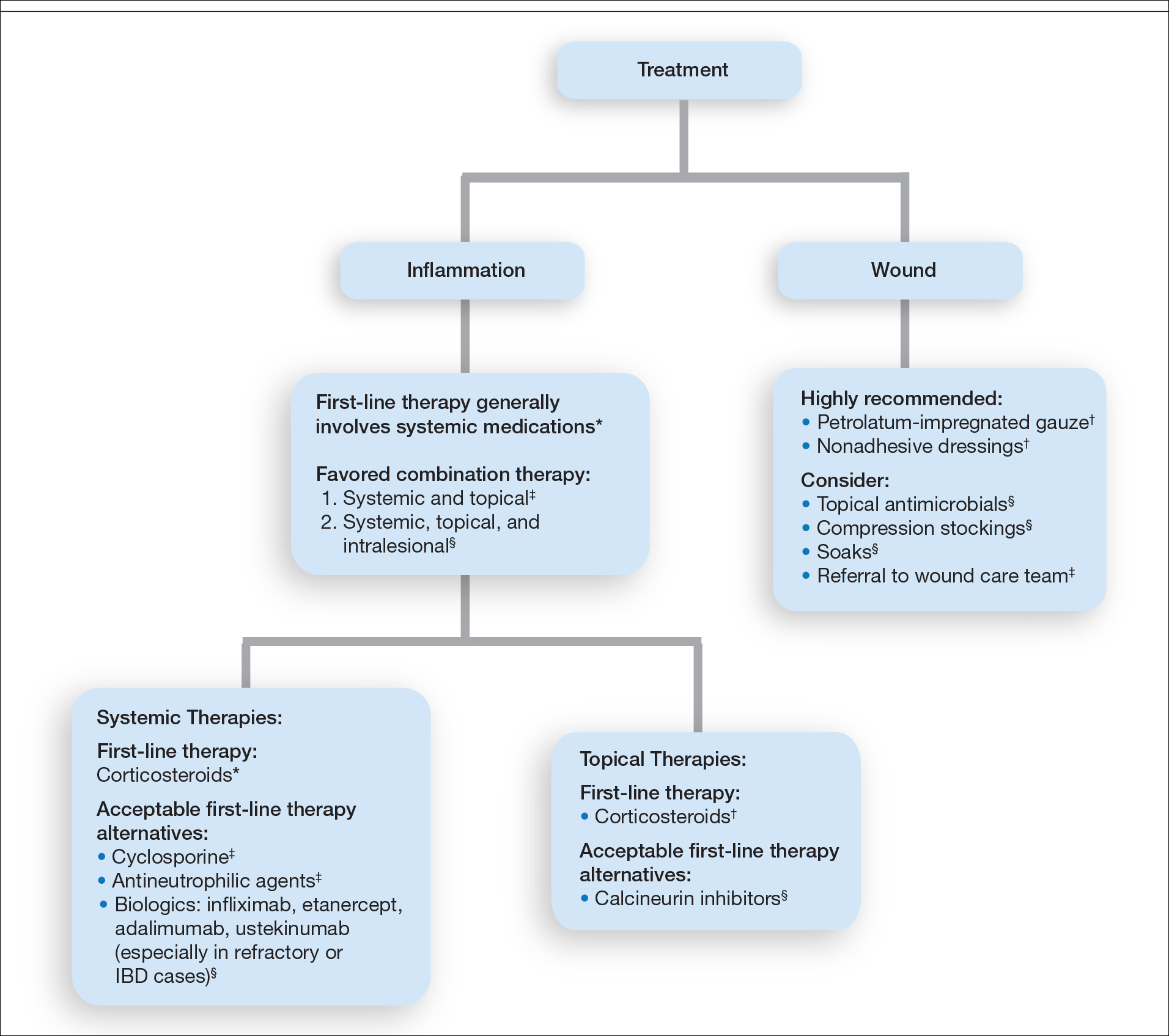
Importantly, treatments may vary depending on patient characteristics, comorbidities, and underlying disease, which underscores the need for individualized treatment approaches. Alternative first-line systemic treatments favored by respondents were cyclosporine, biologic medications, and antineutrophilic agents such as dapsone. Cyclosporine has demonstrated comparable efficacy to systemic glucocorticoids in one RCT and is considered an important steroid-sparing alternative for PG treatment.2 Biologic agents, especially tumor necrosis factor inhibitors, may be effective in treating cases of refractory PG or for concomitant inflammatory bowel disease management, as demonstrated by a small RCT documenting improvement of PG following infliximab infusion.3
Respondents strongly recommended petrolatum-impregnated gauze and other nonadhesive dressings, including alginate and hydrocolloid dressings, as part of PG wound care. Topical antimicrobials and compression stockings also were recommended by respondents. These practices aim to promote moist environments for healing, avoid maceration, prevent superinfection, optimize wound healing, and minimize damage from adhesive injury.12 Wound debridement and grafting generally were not recommended. However, pathergy is not a universal phenomenon in PG, and wounds that are no longer in the inflammatory phase may benefit from gentle debridement of necrotic tissue and/or grafting in select cases.10
Conclusion
An approach to modifying PG management based on clinical presentation and the practice of combination therapy with multiple systemic agents in refractory PG cases was not addressed in our survey. The low response rate is a limitation; however, the opinions of 51 medical dermatologist experts who regularly manage PG (in contrast to papers based on individualized clinical experience) can provide important clinical guidance until more scientific evidence is established.
Acknowledgments
We would like to thank the SDH and RDS membership for their participation in this survey. We especially acknowledge the other members of the SDH Scientific Task Force for their feedback: Misha Rosenbach, MD (Philadelphia, Pennsylvania); Robert G. Micheletti, MD (Philadelphia, Pennsylvania); Karolyn Wanat, MD (Milwaukee, Wisconsin); Amy Chen, MD (Cromwell, Connecticut); and A. Rambi Cardones, MD (Durham, North Carolina).
- Al Ghazal P, Dissemond J. Therapy of pyoderma gangrenosum in Germany: results of a survey among wound experts. J Dtsch Dermatol Ges . 2015;13:317-324.
- Ormerod AD, Thomas KS, Craig FE, et al. Comparison of the two most commonly used treatments for pyoderma gangrenosum: results of the STOP GAP randomised controlled trial. BMJ. 2015;350:h2958.
- Brooklyn TN, Dunnill MG, Shetty A, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut. 2006;55:505-509.
- Al Ghazal P, Klode J, Dissemond J. Diagnostic criteria for pyoderma gangrenosum: results of a survey among dermatologic wound experts in Germany. J Dtsch Dermatol Ges. 2014;12:1129-1131.
- Weenig RH, Davis MD, Dahl PR, et al. Skin ulcers misdiagnosed as pyoderma gangrenosum. N Engl J Med. 2002;347:1412-1418.
- Powell FC, Su WP, Perry HO. Pyoderma gangrenosum: classification and management. J Am Acad Dermatol. 1996;34:395-409.
- Bennett ML, Jackson JM, Jorizzo JL, et al. Pyoderma gangrenosum: a comparison of typical and atypical forms with an emphasis on time to remission. case review of 86 patients from 2 institutions. Medicine. 2000;79:37-46.
- Su WP, Davis MD, Weening RH, et al. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol. 2004;43:790-800.
- Aschyan H, Butler DC, Nelson CA, et al. The association of age with clinical presentation and comorbidities of pyoderma gangrenosum. JAMA Dermatol. 2018;154:409-413.
- Binus AM, Qureshi AA, Li VW, et al. Pyoderma gangrenosum: a retrospective review of patient characteristics, comorbidities and therapy in 103 patients. Br J Dermatol. 2011;165:1244-1250.
- Reichrath J, Bens G, Bonowitz A, et al. Treatment recommendations for pyoderma gangrenosum: an evidence-based review of the literature based on more than 350 patients. J Am Acad Dermatol. 2005;53:273-283.
- Miller J, Yentzer BA, Clark A, et al. Pyoderma gangrenosum: a review and update on new therapies. J Am Acad Dermatol. 2010;62:646-654.
Pyoderma gangrenosum (PG) is a rare, chronic, ulcerative, neutrophilic dermatosis of unclear etiology. Large, multicentered, randomized controlled trials (RCTs) are challenging due to the rarity of PG and the lack of a diagnostic confirmatory test; therefore, evidence-based guidelines for diagnosis and treatment are not well established. Current management of PG primarily is guided by case series, small clinical trials, and expert opinion.1-4 We conducted a survey of expert medical dermatologists to highlight best practices in diagnostic and therapeutic approaches to PG.
Methods
The Society of Dermatology Hospitalists (SDH) Scientific Task Force gathered expert opinions from members of the SDH and Rheumatologic Dermatology Society (RDS) regarding PG workup and treatment through an online survey of 15 items (eTable 1). Subscribers of the SDH and RDS LISTSERVs were invited via email to participate in the survey from January 2016 to February 2016. Anonymous survey responses were collected and collated using SurveyMonkey. The survey results identified expert recommendations for evaluation, diagnosis, and treatment of PG and are reported as the sum of the percentage of respondents who answered always (almost 100% of the time) or often (more than half the time) following a particular course of action. A subanalysis was performed defining 2 groups of respondents based on the number of cases of PG treated per year (≥10 vs <10). Survey responses between each group were compared using χ2 analysis with statistical significance set at P=.05.

Results
Fifty-one respondents completed the survey out of 140 surveyed (36% response rate). All respondents were dermatologists, and 96% (49/51) were affiliated with an academic institution. Among the respondents, the number of PG cases managed per year ranged from 2 to 35.
Respondents consistently ordered skin biopsies (92% [47/51]) and tissue cultures (90% [46/51]), as well as certain ancillary tests, including complete blood cell count (96% [49/51]), complete metabolic panel (86% [44/51]), serum protein electrophoresis (76% [39/51]), and hepatitis panel (71% [36/51]). Other frequently ordered studies were rheumatoid factor (69% [35/51]), antinuclear antibodies (67% [34/51]), and antineutrophilic antibodies (65% [33/51]). Respondents frequently ordered erythrocyte sedimentation rate (59% [30/51]), C-reactive protein (55% [28/51]), cryoglobulins (53% [27/51]), urine protein electrophoresis (53% [27/51]), hypercoagulability workup (49% [25/51]), and serum immunofixation test (49% [25/51]). Human immunodeficiency virus testing (43% [22/51]), chest radiograph (41% [21/51]), colonoscopy (41% [21/51]) and referral to other specialties for workup—gastroenterology (38% [19/51]), hematology/oncology (14% [7/51]), and rheumatology (10% [5/51])—were less frequently ordered (eTable 2).

Systemic corticosteroids were reported as first-line therapy by most respondents (94% [48/51]), followed by topical immunomodulatory therapies (63% [32/51]). Topical corticosteroids (75% [38/51]) were the most common first-line topical agents. Thirty-nine percent of respondents (20/51) prescribed topical calcineurin inhibitors as first-line topical therapy. Additional therapies frequently used included systemic cyclosporine (47% [24/51]), antineutrophilic agents (41% [21/51]), and biologic agents (37% [19/51]). Fifty-seven percent of respondents (29/51) supported using combination topical and systemic therapy (Table).

A wide variety of wound care practices were reported in the management of PG. Seventy-six percent of respondents (39/51) favored petroleum-impregnated gauze, 69% (35/51) used nonadhesive dressings, and 43% (22/51) added antimicrobial therapy for PG wound care (eTable 3). In the subanalysis, there were no significant differences in the majority of answer responses in patients treating 10 or more PG cases per year vs fewer than 10 PG cases, except with regard to the practice of combination therapy. Those treating more than 10 cases of PG per year more frequently reported use of combination therapies compared to respondents treating fewer than 10 cases (P=.04).

Comment
Skin biopsies and tissue cultures were strongly recommended (>90% survey respondents) for the initial evaluation of lesions suspected to be PG to evaluate for typical histopathologic changes that appear early in the disease, to rule out PG mimickers such as infectious or vascular causes, and to prevent the detrimental effects of inappropriate treatment and delayed diagnosis.5
Suspected PG warrants a reasonable search for related conditions because more than 50% of PG cases are associated with comorbidities such as rheumatoid arthritis, inflammatory bowel disease, and hematologic disease/malignancy.6,7 A complete blood cell count and comprehensive metabolic panel were recommended by most respondents, aiding in the preliminary screening for hematologic and infectious causes as well as detecting liver and kidney dysfunction associated with systemic conditions. Additionally, exclusion of infection or malignancy may be particularly important if the patient will undergo systemic immunosuppression. In challenging PG cases when initial findings are inconclusive and the clinical presentation does not direct workup (eg, colonoscopy to evaluate gastrointestinal tract symptoms), serum protein electrophoresis, hepatitis panel, rheumatoid factor, antinuclear antibodies, and antineutrophilic antibody tests also were frequently ordered by respondents to further evaluate for underlying or associated conditions.
This consensus regarding skin biopsies and certain ancillary tests is consistent with the proposed diagnostic criteria for classic ulcerative PG in which the absence or exclusion of other relevant causes of cutaneous ulcers is required based on the criteria.8 The importance of ensuring an accurate diagnosis is paramount, as a 10% misdiagnosis rate has been documented in the literature.5
Importantly, a stepwise diagnostic workup for PG is proposed based on survey results, which may limit unnecessary testing and the associated costs to the health care system (Figure 1). Selection of additional testing is guided by initial test results and features of the patient’s clinical presentation, including age, review of systems, and associated comorbidities. Available data suggest that underlying inflammatory bowel disease is more frequent in PG patients who are younger than 65 years, whereas those who are 65 years and older are more likely to have inflammatory arthritis, cancer, or an underlying hematologic disorder.9

Treatment of PG should address both the inflammatory and wound components of the disease (Figure 2).7 In our survey results, systemic corticosteroids were identified as an important first-line therapy supported by reasonable evidence and were favored for their rapid response and minimal cost.1,10,11 Many respondents endorsed the use of systemic therapy in combination with topical steroids or calcineurin inhibitors. Combination therapy may provide more immediate control of rapidly progressing disease while minimizing adverse effects of long-term systemic corticosteroid use. A survey of German wound experts similarly endorsed frequent use of topical calcineurin inhibitors and combination systemic and topical glucocorticoid therapy as common therapeutic approaches.1

Importantly, treatments may vary depending on patient characteristics, comorbidities, and underlying disease, which underscores the need for individualized treatment approaches. Alternative first-line systemic treatments favored by respondents were cyclosporine, biologic medications, and antineutrophilic agents such as dapsone. Cyclosporine has demonstrated comparable efficacy to systemic glucocorticoids in one RCT and is considered an important steroid-sparing alternative for PG treatment.2 Biologic agents, especially tumor necrosis factor inhibitors, may be effective in treating cases of refractory PG or for concomitant inflammatory bowel disease management, as demonstrated by a small RCT documenting improvement of PG following infliximab infusion.3
Respondents strongly recommended petrolatum-impregnated gauze and other nonadhesive dressings, including alginate and hydrocolloid dressings, as part of PG wound care. Topical antimicrobials and compression stockings also were recommended by respondents. These practices aim to promote moist environments for healing, avoid maceration, prevent superinfection, optimize wound healing, and minimize damage from adhesive injury.12 Wound debridement and grafting generally were not recommended. However, pathergy is not a universal phenomenon in PG, and wounds that are no longer in the inflammatory phase may benefit from gentle debridement of necrotic tissue and/or grafting in select cases.10
Conclusion
An approach to modifying PG management based on clinical presentation and the practice of combination therapy with multiple systemic agents in refractory PG cases was not addressed in our survey. The low response rate is a limitation; however, the opinions of 51 medical dermatologist experts who regularly manage PG (in contrast to papers based on individualized clinical experience) can provide important clinical guidance until more scientific evidence is established.
Acknowledgments
We would like to thank the SDH and RDS membership for their participation in this survey. We especially acknowledge the other members of the SDH Scientific Task Force for their feedback: Misha Rosenbach, MD (Philadelphia, Pennsylvania); Robert G. Micheletti, MD (Philadelphia, Pennsylvania); Karolyn Wanat, MD (Milwaukee, Wisconsin); Amy Chen, MD (Cromwell, Connecticut); and A. Rambi Cardones, MD (Durham, North Carolina).
Pyoderma gangrenosum (PG) is a rare, chronic, ulcerative, neutrophilic dermatosis of unclear etiology. Large, multicentered, randomized controlled trials (RCTs) are challenging due to the rarity of PG and the lack of a diagnostic confirmatory test; therefore, evidence-based guidelines for diagnosis and treatment are not well established. Current management of PG primarily is guided by case series, small clinical trials, and expert opinion.1-4 We conducted a survey of expert medical dermatologists to highlight best practices in diagnostic and therapeutic approaches to PG.
Methods
The Society of Dermatology Hospitalists (SDH) Scientific Task Force gathered expert opinions from members of the SDH and Rheumatologic Dermatology Society (RDS) regarding PG workup and treatment through an online survey of 15 items (eTable 1). Subscribers of the SDH and RDS LISTSERVs were invited via email to participate in the survey from January 2016 to February 2016. Anonymous survey responses were collected and collated using SurveyMonkey. The survey results identified expert recommendations for evaluation, diagnosis, and treatment of PG and are reported as the sum of the percentage of respondents who answered always (almost 100% of the time) or often (more than half the time) following a particular course of action. A subanalysis was performed defining 2 groups of respondents based on the number of cases of PG treated per year (≥10 vs <10). Survey responses between each group were compared using χ2 analysis with statistical significance set at P=.05.

Results
Fifty-one respondents completed the survey out of 140 surveyed (36% response rate). All respondents were dermatologists, and 96% (49/51) were affiliated with an academic institution. Among the respondents, the number of PG cases managed per year ranged from 2 to 35.
Respondents consistently ordered skin biopsies (92% [47/51]) and tissue cultures (90% [46/51]), as well as certain ancillary tests, including complete blood cell count (96% [49/51]), complete metabolic panel (86% [44/51]), serum protein electrophoresis (76% [39/51]), and hepatitis panel (71% [36/51]). Other frequently ordered studies were rheumatoid factor (69% [35/51]), antinuclear antibodies (67% [34/51]), and antineutrophilic antibodies (65% [33/51]). Respondents frequently ordered erythrocyte sedimentation rate (59% [30/51]), C-reactive protein (55% [28/51]), cryoglobulins (53% [27/51]), urine protein electrophoresis (53% [27/51]), hypercoagulability workup (49% [25/51]), and serum immunofixation test (49% [25/51]). Human immunodeficiency virus testing (43% [22/51]), chest radiograph (41% [21/51]), colonoscopy (41% [21/51]) and referral to other specialties for workup—gastroenterology (38% [19/51]), hematology/oncology (14% [7/51]), and rheumatology (10% [5/51])—were less frequently ordered (eTable 2).

Systemic corticosteroids were reported as first-line therapy by most respondents (94% [48/51]), followed by topical immunomodulatory therapies (63% [32/51]). Topical corticosteroids (75% [38/51]) were the most common first-line topical agents. Thirty-nine percent of respondents (20/51) prescribed topical calcineurin inhibitors as first-line topical therapy. Additional therapies frequently used included systemic cyclosporine (47% [24/51]), antineutrophilic agents (41% [21/51]), and biologic agents (37% [19/51]). Fifty-seven percent of respondents (29/51) supported using combination topical and systemic therapy (Table).

A wide variety of wound care practices were reported in the management of PG. Seventy-six percent of respondents (39/51) favored petroleum-impregnated gauze, 69% (35/51) used nonadhesive dressings, and 43% (22/51) added antimicrobial therapy for PG wound care (eTable 3). In the subanalysis, there were no significant differences in the majority of answer responses in patients treating 10 or more PG cases per year vs fewer than 10 PG cases, except with regard to the practice of combination therapy. Those treating more than 10 cases of PG per year more frequently reported use of combination therapies compared to respondents treating fewer than 10 cases (P=.04).

Comment
Skin biopsies and tissue cultures were strongly recommended (>90% survey respondents) for the initial evaluation of lesions suspected to be PG to evaluate for typical histopathologic changes that appear early in the disease, to rule out PG mimickers such as infectious or vascular causes, and to prevent the detrimental effects of inappropriate treatment and delayed diagnosis.5
Suspected PG warrants a reasonable search for related conditions because more than 50% of PG cases are associated with comorbidities such as rheumatoid arthritis, inflammatory bowel disease, and hematologic disease/malignancy.6,7 A complete blood cell count and comprehensive metabolic panel were recommended by most respondents, aiding in the preliminary screening for hematologic and infectious causes as well as detecting liver and kidney dysfunction associated with systemic conditions. Additionally, exclusion of infection or malignancy may be particularly important if the patient will undergo systemic immunosuppression. In challenging PG cases when initial findings are inconclusive and the clinical presentation does not direct workup (eg, colonoscopy to evaluate gastrointestinal tract symptoms), serum protein electrophoresis, hepatitis panel, rheumatoid factor, antinuclear antibodies, and antineutrophilic antibody tests also were frequently ordered by respondents to further evaluate for underlying or associated conditions.
This consensus regarding skin biopsies and certain ancillary tests is consistent with the proposed diagnostic criteria for classic ulcerative PG in which the absence or exclusion of other relevant causes of cutaneous ulcers is required based on the criteria.8 The importance of ensuring an accurate diagnosis is paramount, as a 10% misdiagnosis rate has been documented in the literature.5
Importantly, a stepwise diagnostic workup for PG is proposed based on survey results, which may limit unnecessary testing and the associated costs to the health care system (Figure 1). Selection of additional testing is guided by initial test results and features of the patient’s clinical presentation, including age, review of systems, and associated comorbidities. Available data suggest that underlying inflammatory bowel disease is more frequent in PG patients who are younger than 65 years, whereas those who are 65 years and older are more likely to have inflammatory arthritis, cancer, or an underlying hematologic disorder.9

Treatment of PG should address both the inflammatory and wound components of the disease (Figure 2).7 In our survey results, systemic corticosteroids were identified as an important first-line therapy supported by reasonable evidence and were favored for their rapid response and minimal cost.1,10,11 Many respondents endorsed the use of systemic therapy in combination with topical steroids or calcineurin inhibitors. Combination therapy may provide more immediate control of rapidly progressing disease while minimizing adverse effects of long-term systemic corticosteroid use. A survey of German wound experts similarly endorsed frequent use of topical calcineurin inhibitors and combination systemic and topical glucocorticoid therapy as common therapeutic approaches.1

Importantly, treatments may vary depending on patient characteristics, comorbidities, and underlying disease, which underscores the need for individualized treatment approaches. Alternative first-line systemic treatments favored by respondents were cyclosporine, biologic medications, and antineutrophilic agents such as dapsone. Cyclosporine has demonstrated comparable efficacy to systemic glucocorticoids in one RCT and is considered an important steroid-sparing alternative for PG treatment.2 Biologic agents, especially tumor necrosis factor inhibitors, may be effective in treating cases of refractory PG or for concomitant inflammatory bowel disease management, as demonstrated by a small RCT documenting improvement of PG following infliximab infusion.3
Respondents strongly recommended petrolatum-impregnated gauze and other nonadhesive dressings, including alginate and hydrocolloid dressings, as part of PG wound care. Topical antimicrobials and compression stockings also were recommended by respondents. These practices aim to promote moist environments for healing, avoid maceration, prevent superinfection, optimize wound healing, and minimize damage from adhesive injury.12 Wound debridement and grafting generally were not recommended. However, pathergy is not a universal phenomenon in PG, and wounds that are no longer in the inflammatory phase may benefit from gentle debridement of necrotic tissue and/or grafting in select cases.10
Conclusion
An approach to modifying PG management based on clinical presentation and the practice of combination therapy with multiple systemic agents in refractory PG cases was not addressed in our survey. The low response rate is a limitation; however, the opinions of 51 medical dermatologist experts who regularly manage PG (in contrast to papers based on individualized clinical experience) can provide important clinical guidance until more scientific evidence is established.
Acknowledgments
We would like to thank the SDH and RDS membership for their participation in this survey. We especially acknowledge the other members of the SDH Scientific Task Force for their feedback: Misha Rosenbach, MD (Philadelphia, Pennsylvania); Robert G. Micheletti, MD (Philadelphia, Pennsylvania); Karolyn Wanat, MD (Milwaukee, Wisconsin); Amy Chen, MD (Cromwell, Connecticut); and A. Rambi Cardones, MD (Durham, North Carolina).
- Al Ghazal P, Dissemond J. Therapy of pyoderma gangrenosum in Germany: results of a survey among wound experts. J Dtsch Dermatol Ges . 2015;13:317-324.
- Ormerod AD, Thomas KS, Craig FE, et al. Comparison of the two most commonly used treatments for pyoderma gangrenosum: results of the STOP GAP randomised controlled trial. BMJ. 2015;350:h2958.
- Brooklyn TN, Dunnill MG, Shetty A, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut. 2006;55:505-509.
- Al Ghazal P, Klode J, Dissemond J. Diagnostic criteria for pyoderma gangrenosum: results of a survey among dermatologic wound experts in Germany. J Dtsch Dermatol Ges. 2014;12:1129-1131.
- Weenig RH, Davis MD, Dahl PR, et al. Skin ulcers misdiagnosed as pyoderma gangrenosum. N Engl J Med. 2002;347:1412-1418.
- Powell FC, Su WP, Perry HO. Pyoderma gangrenosum: classification and management. J Am Acad Dermatol. 1996;34:395-409.
- Bennett ML, Jackson JM, Jorizzo JL, et al. Pyoderma gangrenosum: a comparison of typical and atypical forms with an emphasis on time to remission. case review of 86 patients from 2 institutions. Medicine. 2000;79:37-46.
- Su WP, Davis MD, Weening RH, et al. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol. 2004;43:790-800.
- Aschyan H, Butler DC, Nelson CA, et al. The association of age with clinical presentation and comorbidities of pyoderma gangrenosum. JAMA Dermatol. 2018;154:409-413.
- Binus AM, Qureshi AA, Li VW, et al. Pyoderma gangrenosum: a retrospective review of patient characteristics, comorbidities and therapy in 103 patients. Br J Dermatol. 2011;165:1244-1250.
- Reichrath J, Bens G, Bonowitz A, et al. Treatment recommendations for pyoderma gangrenosum: an evidence-based review of the literature based on more than 350 patients. J Am Acad Dermatol. 2005;53:273-283.
- Miller J, Yentzer BA, Clark A, et al. Pyoderma gangrenosum: a review and update on new therapies. J Am Acad Dermatol. 2010;62:646-654.
- Al Ghazal P, Dissemond J. Therapy of pyoderma gangrenosum in Germany: results of a survey among wound experts. J Dtsch Dermatol Ges . 2015;13:317-324.
- Ormerod AD, Thomas KS, Craig FE, et al. Comparison of the two most commonly used treatments for pyoderma gangrenosum: results of the STOP GAP randomised controlled trial. BMJ. 2015;350:h2958.
- Brooklyn TN, Dunnill MG, Shetty A, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut. 2006;55:505-509.
- Al Ghazal P, Klode J, Dissemond J. Diagnostic criteria for pyoderma gangrenosum: results of a survey among dermatologic wound experts in Germany. J Dtsch Dermatol Ges. 2014;12:1129-1131.
- Weenig RH, Davis MD, Dahl PR, et al. Skin ulcers misdiagnosed as pyoderma gangrenosum. N Engl J Med. 2002;347:1412-1418.
- Powell FC, Su WP, Perry HO. Pyoderma gangrenosum: classification and management. J Am Acad Dermatol. 1996;34:395-409.
- Bennett ML, Jackson JM, Jorizzo JL, et al. Pyoderma gangrenosum: a comparison of typical and atypical forms with an emphasis on time to remission. case review of 86 patients from 2 institutions. Medicine. 2000;79:37-46.
- Su WP, Davis MD, Weening RH, et al. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol. 2004;43:790-800.
- Aschyan H, Butler DC, Nelson CA, et al. The association of age with clinical presentation and comorbidities of pyoderma gangrenosum. JAMA Dermatol. 2018;154:409-413.
- Binus AM, Qureshi AA, Li VW, et al. Pyoderma gangrenosum: a retrospective review of patient characteristics, comorbidities and therapy in 103 patients. Br J Dermatol. 2011;165:1244-1250.
- Reichrath J, Bens G, Bonowitz A, et al. Treatment recommendations for pyoderma gangrenosum: an evidence-based review of the literature based on more than 350 patients. J Am Acad Dermatol. 2005;53:273-283.
- Miller J, Yentzer BA, Clark A, et al. Pyoderma gangrenosum: a review and update on new therapies. J Am Acad Dermatol. 2010;62:646-654.
Practice Points
- The diagnosis of pyoderma gangrenosum (PG) poses a challenge in clinical practice that could be minimized by following a stepwise algorithm based on initial test results (including skin biopsies) and features of the patient’s clinical presentation.
- As there is no US Food and Drug Administration–approved treatment for PG, a stepwise algorithm approach in combination with the clinical experience addressing inflammation and wound care is essential to reach control and remission of PG.
Diversity and Inclusivity Are Essential to the Future of Dermatology
Over the last 5 years, there has been an important dialogue among dermatologists about diversity in our specialty that has shifted the mind-set of the dermatology community and highlighted an intent to build a diverse workforce. It is important to reflect on this effort and acknowledge the progress that has been made. Additionally, it also is important to envision what our ideal specialty will look like 10 years from now and to discuss specific ways that we can achieve that vision for the future of dermatology.
At the 2015 Annual Meeting of the American Academy of Dermatology (AAD), Bruce E. Wintroub, MD, highlighted the importance of diversity in dermatology when he presented the Clarence S. Livingood lecture.1 His discussion was followed by a call to action from Pandya et al2 in 2016, which described the lack of diversity in our specialty (the second least diverse specialty in medicine) and proposed specific steps that can be taken by individuals and organizations to address the issue. In line with this effort, the AAD’s Diversity Task Force, Diversity Mentorship Program,3 and Diversity Champion Initiative were created. The latter program enlisted dermatology residency programs across the country to select a diversity champion who would lead efforts to increase diversity in each participating department, including mentorship of underrepresented-in-medicine college and medical students. The AAD’s 2019 Diversity Champion Workshop4 (September 12–13, 2019) will be held for the first time prior to the Association of Professors of Dermatology Annual Meeting (September 13–14, 2019) in an attempt to scale up the Diversity Champion Initiative. This workshop has galvanized widespread support and will be collaboratively hosted by the AAD, Association of Professors of Dermatology, Skin of Color Society, Society for Investigative Dermatology, and Women’s Dermatologic Society.
Current diversity efforts have largely focused on increasing representation in the dermatology workforce. A publication in 2017 challenged the tenets of dermatology resident selection and advocated for holistic review of residency program applicants as one way to address the lack of diversity in dermatology.5 This viewpoint highlighted that dermatology’s traditional focus on US Medical Licensing Examination scores and Alpha Omega Alpha Honor Medical Society membership leads to bias6-8; the viewpoint proposed several ways to change the resident selection process to enhance diversity.5 A recent proposal to eliminate numerical scores on the US Medical Licensing Examination Step 1 and move to a pass/fail grading system aligns well with this viewpoint.9 Defining best practices to perform holistic reviews is an ongoing effort and challenge for many programs, one that will be discussed at the AAD’s 2019 Diversity Champion Workshop. Implementing best practices will require individual residency programs to develop review processes tailored to departmental resources and strengths. Achieving increased representation must be an active process starting with an explicit commitment to improving diversity.
Through these efforts, we are poised to improve our specialty; however, it is critical to recognize that simply increasing the number of underrepresented dermatologists is not enough to improve diversity in dermatology. What does meaningful change look like? In 10 years, we hope that, in addition to a more inclusive workforce, we will see expanded diversity efforts beyond race and ethnicity; improved cultural competence within dermatology departments and organizations that creates more inclusive places to work, learn, and practice medicine; intentional broader representation in dermatology leadership; high-quality, evidence-based, inclusive, and culturally competent education, patient care, and research; and equal and improved outcomes for all of our patients, particularly those who traditionally experience health care disparities. To this end, ensuring diversity in research and publications is paramount. Academic journals should be actively working to include articles in the literature that help us better understand health care differences, including research that examines the presentations of skin disease in a broad spectrum of study populations, as well as to spotlight and solicit content from diverse voices. Inclusion of a diverse range of participants in research based on human subjects should be a requirement for publication, which would ensure more generalizable data. Diversity in clinical trials is improving,10 but more effort should be devoted to further increasing diversity in medical research. In particular, we need to broaden the inclusivity of dermatology research efforts and outcomes data to include more patients with skin of color as well as other underrepresented groups, thus helping to improve our understanding of the differential effects of certain interventions.
We also must educate trainees and practicing dermatologists to better understand the diagnosis and management of skin diseases in all populations; to this end, it is essential to develop a culturally competent curriculum and continuing medical education on diseases of the skin and hair that affect patients with skin of color as well as cutaneous conditions that present in groups such as sexual and gender minorities.11,12 All dermatologists—not just the experts in academic skin of color and other specialty clinics—should have expertise in the dermatologic care of diverse patients.
We have made notable and important strides with regard to diversity in dermatology by beginning this conversation, identifying problems, coming up with solutions, and implementing them.13 This progress has been made relatively quickly and is commendable; however, we have more work to do before our specialty is inclusive of underrepresented-in-medicine physicians and provides excellent care to all patients.
- Wintroub BE. Dermatology: insuring the future for the patients we serve. Presented at: 73rd Annual Meeting of the American Academy of Dermatology; March 20-24, 2015; San Francisco, California.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Diversity Mentorship Program: current mentors. American Academy of Dermatology website. https://www.aad.org/members/leadership-institute/mentoring/diversity-mentorship-program-current-mentors. Accessed July 17, 2019.
- Diversity Champion Workshop. American Academy of Dermatology website. https://www.aad.org/meetings/diversity-champion-workshop. Accessed July 17, 2019.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260.
- McGaghie WC, Cohen ER, Wayne DB. Are United States Medical Licensing Exam Step 1 and 2 scores valid measures for postgraduate medical residency selection decisions? Acad Med. 2011;86:48-52.
- Edmond MB, Deschenes JL, Eckler M, et al. Racial bias in using USMLE step 1 scores to grant internal medicine residency interviews. Acad Med. 2001;76:1253-1256.
- Boatright D, Ross D, O’Connor P, et al. Racial disparities in medical student membership in the Alpha Omega Alpha Honor Society. JAMA Intern Med. 2017;177:659-665.
- The conversation continues: exploring possible changes to USMLE score reporting. US Medical Licensing Examination website. https://www.usmle.org/usmlescoring/. Accessed July 17, 2019.
- Charrow A, Xia FD, Joyce C, et al. Diversity in dermatology clinical trials: a systematic review. JAMA Dermatol. 2017;153:193-198.
- Vashi NA, Patzelt N, Wirya S, et al. Dermatoses caused by cultural practices: therapeutic cultural practices. J Am Acad Dermatol. 2018;79:1-16.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
Over the last 5 years, there has been an important dialogue among dermatologists about diversity in our specialty that has shifted the mind-set of the dermatology community and highlighted an intent to build a diverse workforce. It is important to reflect on this effort and acknowledge the progress that has been made. Additionally, it also is important to envision what our ideal specialty will look like 10 years from now and to discuss specific ways that we can achieve that vision for the future of dermatology.
At the 2015 Annual Meeting of the American Academy of Dermatology (AAD), Bruce E. Wintroub, MD, highlighted the importance of diversity in dermatology when he presented the Clarence S. Livingood lecture.1 His discussion was followed by a call to action from Pandya et al2 in 2016, which described the lack of diversity in our specialty (the second least diverse specialty in medicine) and proposed specific steps that can be taken by individuals and organizations to address the issue. In line with this effort, the AAD’s Diversity Task Force, Diversity Mentorship Program,3 and Diversity Champion Initiative were created. The latter program enlisted dermatology residency programs across the country to select a diversity champion who would lead efforts to increase diversity in each participating department, including mentorship of underrepresented-in-medicine college and medical students. The AAD’s 2019 Diversity Champion Workshop4 (September 12–13, 2019) will be held for the first time prior to the Association of Professors of Dermatology Annual Meeting (September 13–14, 2019) in an attempt to scale up the Diversity Champion Initiative. This workshop has galvanized widespread support and will be collaboratively hosted by the AAD, Association of Professors of Dermatology, Skin of Color Society, Society for Investigative Dermatology, and Women’s Dermatologic Society.
Current diversity efforts have largely focused on increasing representation in the dermatology workforce. A publication in 2017 challenged the tenets of dermatology resident selection and advocated for holistic review of residency program applicants as one way to address the lack of diversity in dermatology.5 This viewpoint highlighted that dermatology’s traditional focus on US Medical Licensing Examination scores and Alpha Omega Alpha Honor Medical Society membership leads to bias6-8; the viewpoint proposed several ways to change the resident selection process to enhance diversity.5 A recent proposal to eliminate numerical scores on the US Medical Licensing Examination Step 1 and move to a pass/fail grading system aligns well with this viewpoint.9 Defining best practices to perform holistic reviews is an ongoing effort and challenge for many programs, one that will be discussed at the AAD’s 2019 Diversity Champion Workshop. Implementing best practices will require individual residency programs to develop review processes tailored to departmental resources and strengths. Achieving increased representation must be an active process starting with an explicit commitment to improving diversity.
Through these efforts, we are poised to improve our specialty; however, it is critical to recognize that simply increasing the number of underrepresented dermatologists is not enough to improve diversity in dermatology. What does meaningful change look like? In 10 years, we hope that, in addition to a more inclusive workforce, we will see expanded diversity efforts beyond race and ethnicity; improved cultural competence within dermatology departments and organizations that creates more inclusive places to work, learn, and practice medicine; intentional broader representation in dermatology leadership; high-quality, evidence-based, inclusive, and culturally competent education, patient care, and research; and equal and improved outcomes for all of our patients, particularly those who traditionally experience health care disparities. To this end, ensuring diversity in research and publications is paramount. Academic journals should be actively working to include articles in the literature that help us better understand health care differences, including research that examines the presentations of skin disease in a broad spectrum of study populations, as well as to spotlight and solicit content from diverse voices. Inclusion of a diverse range of participants in research based on human subjects should be a requirement for publication, which would ensure more generalizable data. Diversity in clinical trials is improving,10 but more effort should be devoted to further increasing diversity in medical research. In particular, we need to broaden the inclusivity of dermatology research efforts and outcomes data to include more patients with skin of color as well as other underrepresented groups, thus helping to improve our understanding of the differential effects of certain interventions.
We also must educate trainees and practicing dermatologists to better understand the diagnosis and management of skin diseases in all populations; to this end, it is essential to develop a culturally competent curriculum and continuing medical education on diseases of the skin and hair that affect patients with skin of color as well as cutaneous conditions that present in groups such as sexual and gender minorities.11,12 All dermatologists—not just the experts in academic skin of color and other specialty clinics—should have expertise in the dermatologic care of diverse patients.
We have made notable and important strides with regard to diversity in dermatology by beginning this conversation, identifying problems, coming up with solutions, and implementing them.13 This progress has been made relatively quickly and is commendable; however, we have more work to do before our specialty is inclusive of underrepresented-in-medicine physicians and provides excellent care to all patients.
Over the last 5 years, there has been an important dialogue among dermatologists about diversity in our specialty that has shifted the mind-set of the dermatology community and highlighted an intent to build a diverse workforce. It is important to reflect on this effort and acknowledge the progress that has been made. Additionally, it also is important to envision what our ideal specialty will look like 10 years from now and to discuss specific ways that we can achieve that vision for the future of dermatology.
At the 2015 Annual Meeting of the American Academy of Dermatology (AAD), Bruce E. Wintroub, MD, highlighted the importance of diversity in dermatology when he presented the Clarence S. Livingood lecture.1 His discussion was followed by a call to action from Pandya et al2 in 2016, which described the lack of diversity in our specialty (the second least diverse specialty in medicine) and proposed specific steps that can be taken by individuals and organizations to address the issue. In line with this effort, the AAD’s Diversity Task Force, Diversity Mentorship Program,3 and Diversity Champion Initiative were created. The latter program enlisted dermatology residency programs across the country to select a diversity champion who would lead efforts to increase diversity in each participating department, including mentorship of underrepresented-in-medicine college and medical students. The AAD’s 2019 Diversity Champion Workshop4 (September 12–13, 2019) will be held for the first time prior to the Association of Professors of Dermatology Annual Meeting (September 13–14, 2019) in an attempt to scale up the Diversity Champion Initiative. This workshop has galvanized widespread support and will be collaboratively hosted by the AAD, Association of Professors of Dermatology, Skin of Color Society, Society for Investigative Dermatology, and Women’s Dermatologic Society.
Current diversity efforts have largely focused on increasing representation in the dermatology workforce. A publication in 2017 challenged the tenets of dermatology resident selection and advocated for holistic review of residency program applicants as one way to address the lack of diversity in dermatology.5 This viewpoint highlighted that dermatology’s traditional focus on US Medical Licensing Examination scores and Alpha Omega Alpha Honor Medical Society membership leads to bias6-8; the viewpoint proposed several ways to change the resident selection process to enhance diversity.5 A recent proposal to eliminate numerical scores on the US Medical Licensing Examination Step 1 and move to a pass/fail grading system aligns well with this viewpoint.9 Defining best practices to perform holistic reviews is an ongoing effort and challenge for many programs, one that will be discussed at the AAD’s 2019 Diversity Champion Workshop. Implementing best practices will require individual residency programs to develop review processes tailored to departmental resources and strengths. Achieving increased representation must be an active process starting with an explicit commitment to improving diversity.
Through these efforts, we are poised to improve our specialty; however, it is critical to recognize that simply increasing the number of underrepresented dermatologists is not enough to improve diversity in dermatology. What does meaningful change look like? In 10 years, we hope that, in addition to a more inclusive workforce, we will see expanded diversity efforts beyond race and ethnicity; improved cultural competence within dermatology departments and organizations that creates more inclusive places to work, learn, and practice medicine; intentional broader representation in dermatology leadership; high-quality, evidence-based, inclusive, and culturally competent education, patient care, and research; and equal and improved outcomes for all of our patients, particularly those who traditionally experience health care disparities. To this end, ensuring diversity in research and publications is paramount. Academic journals should be actively working to include articles in the literature that help us better understand health care differences, including research that examines the presentations of skin disease in a broad spectrum of study populations, as well as to spotlight and solicit content from diverse voices. Inclusion of a diverse range of participants in research based on human subjects should be a requirement for publication, which would ensure more generalizable data. Diversity in clinical trials is improving,10 but more effort should be devoted to further increasing diversity in medical research. In particular, we need to broaden the inclusivity of dermatology research efforts and outcomes data to include more patients with skin of color as well as other underrepresented groups, thus helping to improve our understanding of the differential effects of certain interventions.
We also must educate trainees and practicing dermatologists to better understand the diagnosis and management of skin diseases in all populations; to this end, it is essential to develop a culturally competent curriculum and continuing medical education on diseases of the skin and hair that affect patients with skin of color as well as cutaneous conditions that present in groups such as sexual and gender minorities.11,12 All dermatologists—not just the experts in academic skin of color and other specialty clinics—should have expertise in the dermatologic care of diverse patients.
We have made notable and important strides with regard to diversity in dermatology by beginning this conversation, identifying problems, coming up with solutions, and implementing them.13 This progress has been made relatively quickly and is commendable; however, we have more work to do before our specialty is inclusive of underrepresented-in-medicine physicians and provides excellent care to all patients.
- Wintroub BE. Dermatology: insuring the future for the patients we serve. Presented at: 73rd Annual Meeting of the American Academy of Dermatology; March 20-24, 2015; San Francisco, California.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Diversity Mentorship Program: current mentors. American Academy of Dermatology website. https://www.aad.org/members/leadership-institute/mentoring/diversity-mentorship-program-current-mentors. Accessed July 17, 2019.
- Diversity Champion Workshop. American Academy of Dermatology website. https://www.aad.org/meetings/diversity-champion-workshop. Accessed July 17, 2019.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260.
- McGaghie WC, Cohen ER, Wayne DB. Are United States Medical Licensing Exam Step 1 and 2 scores valid measures for postgraduate medical residency selection decisions? Acad Med. 2011;86:48-52.
- Edmond MB, Deschenes JL, Eckler M, et al. Racial bias in using USMLE step 1 scores to grant internal medicine residency interviews. Acad Med. 2001;76:1253-1256.
- Boatright D, Ross D, O’Connor P, et al. Racial disparities in medical student membership in the Alpha Omega Alpha Honor Society. JAMA Intern Med. 2017;177:659-665.
- The conversation continues: exploring possible changes to USMLE score reporting. US Medical Licensing Examination website. https://www.usmle.org/usmlescoring/. Accessed July 17, 2019.
- Charrow A, Xia FD, Joyce C, et al. Diversity in dermatology clinical trials: a systematic review. JAMA Dermatol. 2017;153:193-198.
- Vashi NA, Patzelt N, Wirya S, et al. Dermatoses caused by cultural practices: therapeutic cultural practices. J Am Acad Dermatol. 2018;79:1-16.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Wintroub BE. Dermatology: insuring the future for the patients we serve. Presented at: 73rd Annual Meeting of the American Academy of Dermatology; March 20-24, 2015; San Francisco, California.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Diversity Mentorship Program: current mentors. American Academy of Dermatology website. https://www.aad.org/members/leadership-institute/mentoring/diversity-mentorship-program-current-mentors. Accessed July 17, 2019.
- Diversity Champion Workshop. American Academy of Dermatology website. https://www.aad.org/meetings/diversity-champion-workshop. Accessed July 17, 2019.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260.
- McGaghie WC, Cohen ER, Wayne DB. Are United States Medical Licensing Exam Step 1 and 2 scores valid measures for postgraduate medical residency selection decisions? Acad Med. 2011;86:48-52.
- Edmond MB, Deschenes JL, Eckler M, et al. Racial bias in using USMLE step 1 scores to grant internal medicine residency interviews. Acad Med. 2001;76:1253-1256.
- Boatright D, Ross D, O’Connor P, et al. Racial disparities in medical student membership in the Alpha Omega Alpha Honor Society. JAMA Intern Med. 2017;177:659-665.
- The conversation continues: exploring possible changes to USMLE score reporting. US Medical Licensing Examination website. https://www.usmle.org/usmlescoring/. Accessed July 17, 2019.
- Charrow A, Xia FD, Joyce C, et al. Diversity in dermatology clinical trials: a systematic review. JAMA Dermatol. 2017;153:193-198.
- Vashi NA, Patzelt N, Wirya S, et al. Dermatoses caused by cultural practices: therapeutic cultural practices. J Am Acad Dermatol. 2018;79:1-16.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
Linear Vulvar Lesions
The Diagnosis: Vestibular Papillomatosis
Vestibular papillomatosis (VP), the female equivalent of pearly penile papules, is characterized by multiple papules in a linear array on the labia minora and is considered a normal anatomic variant. It typically presents as monomorphous, soft, flesh-colored, filiform papules that are distributed in a symmetric fashion. In women, the papules present as linear arrays on the inner aspects of the labia minora, whereas in men, they present in a circumferential array along the sulcus of the glans penis.1 Lesions often are asymptomatic but may cause itching, burning, and dyspareunia.2 Previously believed to be associated with human papillomavirus infection,3 VP is now considered a noninfectious condition. Biopsy reveals parakeratosis and perinuclear vacuolization in the absence of true koilocytes.4,5 Dermoscopy and reflectance confocal microscopy have been used to differentiate VP from clinically similar lesions (eg, condyloma acuminatum).6,7 The prevalence of this condition is not well established; however, one study found VP in 1% of women attending genitourinary medicine clinics.3
Condyloma acuminatum, known colloquially as genital warts, is a human papillomavirus infection. Lesions tend to be painless and firm and are distributed asymmetrically with a cauliflowerlike appearance.1 Condyloma latum, found in secondary syphilis, is characterized by papules that are pale, smooth, flat topped, and moist.8 Molluscum contagiosum is an infection caused by a poxvirus presenting with flesh-colored, dome-shaped papules with central umbilication.9 The lesions of papulosquamous lichen planus are violaceous polygonal papules that affect the clitoral hood and labia minora and may cause pruritus. The cause of lichen planus is unknown; however, clinically similar lesions may occur in a lichenoid drug eruption due to certain medications.
Vestibular papillomatosis typically does not require treatment, except in symptomatic cases. To date, limited studies have reported variable treatment success utilizing destructive techniques such as CO2 laser or topical application of 5-fluorouracil or trichloroacetic acid.10
The lesions on our patient's left medial labia minora were successfully treated with low-voltage (3.0 V) electrodesiccation. Following local anesthesia with 1% lidocaine, each papule was gently electrodesiccated utilizing a standard hyfrecation electrode tip to a light gray discoloration. Postprocedural care involved only twice-daily cleansing with a gentle soap and application of petrolatum. The patient tolerated the procedure well and was satisfied with the cosmetic and functional results. She subsequently underwent treatment of the lesions on the right labia minora with equivalent treatment success.
- Moyal-Barracco M, Leibowitch M, Orth G. Vestibular papillae of the vulva. lack of evidence for human papillomavirus etiology. Arch Dermatol. 1990;126:1594-1598.
- Strand A, Wilander E, Zehbe I, et al. Vulvar papillomatosis, aceto-white lesions, and normal-looking vulvar mucosa evaluated by microscopy and human papillomavirus analysis. Gynecol Obstet Invest. 1995;40:265-270.
- Welch JM, Nayagam M, Parry G, et al. What is vestibular papillomatosis? a study of its prevalence, aetiology and natural history. Br J Obstet Gynaecol. 1993;100:939-942.
- Wilkinson EJ, Guerrero E, Daniel R, et al. Vulvar vestibulitis is rarely associated with human papillomavirus infection types 6, 11, 16, or 18. Int J Gynecol Pathol. 1993;12:344-349.
- Beznos G, Coates V, Focchi J, et al. Biomolecular study of the correlation between papillomatosis of the vulvar vestibule in adolescents and human papillomavirus. ScientificWorldJournal. 2006;6:628-636.
- Kim SH, Seo SH, Ko HC, et al. The use of dermatoscopy to differentiate vestibular papillae, a normal variant of the female external genitalia, from condyloma acuminata. J Am Acad Dermatol. 2009;60:353-355.
- Ozkur E, Falay T, Turgut Erdemir AV, et al. Vestibular papillomatosis: an important differential diagnosis of vulvar papillomas. Dermatol Online J. 2016;22. pii:13030/qt7933q377
- Chang GJ, Welton ML. Human papillomavirus, condylomata acuminata, and anal neoplasia. Clin Colon Rectal Surg. 2004;17:221-230.
- Lynch PJ, Moyal-Barracco M, Bogliatto F, et al. 2006 ISSVD classification of vulvar dermatoses: pathologic subsets and their clinical correlates. J Reprod Med. 2007;52:3-9.
- Bergeron C, Ferenczy A, Richart RM, et al. Micropapillomatosis labialis appears unrelated to human papillomavirus. Obstet Gynecol. 1990;76:281-286.
The Diagnosis: Vestibular Papillomatosis
Vestibular papillomatosis (VP), the female equivalent of pearly penile papules, is characterized by multiple papules in a linear array on the labia minora and is considered a normal anatomic variant. It typically presents as monomorphous, soft, flesh-colored, filiform papules that are distributed in a symmetric fashion. In women, the papules present as linear arrays on the inner aspects of the labia minora, whereas in men, they present in a circumferential array along the sulcus of the glans penis.1 Lesions often are asymptomatic but may cause itching, burning, and dyspareunia.2 Previously believed to be associated with human papillomavirus infection,3 VP is now considered a noninfectious condition. Biopsy reveals parakeratosis and perinuclear vacuolization in the absence of true koilocytes.4,5 Dermoscopy and reflectance confocal microscopy have been used to differentiate VP from clinically similar lesions (eg, condyloma acuminatum).6,7 The prevalence of this condition is not well established; however, one study found VP in 1% of women attending genitourinary medicine clinics.3
Condyloma acuminatum, known colloquially as genital warts, is a human papillomavirus infection. Lesions tend to be painless and firm and are distributed asymmetrically with a cauliflowerlike appearance.1 Condyloma latum, found in secondary syphilis, is characterized by papules that are pale, smooth, flat topped, and moist.8 Molluscum contagiosum is an infection caused by a poxvirus presenting with flesh-colored, dome-shaped papules with central umbilication.9 The lesions of papulosquamous lichen planus are violaceous polygonal papules that affect the clitoral hood and labia minora and may cause pruritus. The cause of lichen planus is unknown; however, clinically similar lesions may occur in a lichenoid drug eruption due to certain medications.
Vestibular papillomatosis typically does not require treatment, except in symptomatic cases. To date, limited studies have reported variable treatment success utilizing destructive techniques such as CO2 laser or topical application of 5-fluorouracil or trichloroacetic acid.10
The lesions on our patient's left medial labia minora were successfully treated with low-voltage (3.0 V) electrodesiccation. Following local anesthesia with 1% lidocaine, each papule was gently electrodesiccated utilizing a standard hyfrecation electrode tip to a light gray discoloration. Postprocedural care involved only twice-daily cleansing with a gentle soap and application of petrolatum. The patient tolerated the procedure well and was satisfied with the cosmetic and functional results. She subsequently underwent treatment of the lesions on the right labia minora with equivalent treatment success.
The Diagnosis: Vestibular Papillomatosis
Vestibular papillomatosis (VP), the female equivalent of pearly penile papules, is characterized by multiple papules in a linear array on the labia minora and is considered a normal anatomic variant. It typically presents as monomorphous, soft, flesh-colored, filiform papules that are distributed in a symmetric fashion. In women, the papules present as linear arrays on the inner aspects of the labia minora, whereas in men, they present in a circumferential array along the sulcus of the glans penis.1 Lesions often are asymptomatic but may cause itching, burning, and dyspareunia.2 Previously believed to be associated with human papillomavirus infection,3 VP is now considered a noninfectious condition. Biopsy reveals parakeratosis and perinuclear vacuolization in the absence of true koilocytes.4,5 Dermoscopy and reflectance confocal microscopy have been used to differentiate VP from clinically similar lesions (eg, condyloma acuminatum).6,7 The prevalence of this condition is not well established; however, one study found VP in 1% of women attending genitourinary medicine clinics.3
Condyloma acuminatum, known colloquially as genital warts, is a human papillomavirus infection. Lesions tend to be painless and firm and are distributed asymmetrically with a cauliflowerlike appearance.1 Condyloma latum, found in secondary syphilis, is characterized by papules that are pale, smooth, flat topped, and moist.8 Molluscum contagiosum is an infection caused by a poxvirus presenting with flesh-colored, dome-shaped papules with central umbilication.9 The lesions of papulosquamous lichen planus are violaceous polygonal papules that affect the clitoral hood and labia minora and may cause pruritus. The cause of lichen planus is unknown; however, clinically similar lesions may occur in a lichenoid drug eruption due to certain medications.
Vestibular papillomatosis typically does not require treatment, except in symptomatic cases. To date, limited studies have reported variable treatment success utilizing destructive techniques such as CO2 laser or topical application of 5-fluorouracil or trichloroacetic acid.10
The lesions on our patient's left medial labia minora were successfully treated with low-voltage (3.0 V) electrodesiccation. Following local anesthesia with 1% lidocaine, each papule was gently electrodesiccated utilizing a standard hyfrecation electrode tip to a light gray discoloration. Postprocedural care involved only twice-daily cleansing with a gentle soap and application of petrolatum. The patient tolerated the procedure well and was satisfied with the cosmetic and functional results. She subsequently underwent treatment of the lesions on the right labia minora with equivalent treatment success.
- Moyal-Barracco M, Leibowitch M, Orth G. Vestibular papillae of the vulva. lack of evidence for human papillomavirus etiology. Arch Dermatol. 1990;126:1594-1598.
- Strand A, Wilander E, Zehbe I, et al. Vulvar papillomatosis, aceto-white lesions, and normal-looking vulvar mucosa evaluated by microscopy and human papillomavirus analysis. Gynecol Obstet Invest. 1995;40:265-270.
- Welch JM, Nayagam M, Parry G, et al. What is vestibular papillomatosis? a study of its prevalence, aetiology and natural history. Br J Obstet Gynaecol. 1993;100:939-942.
- Wilkinson EJ, Guerrero E, Daniel R, et al. Vulvar vestibulitis is rarely associated with human papillomavirus infection types 6, 11, 16, or 18. Int J Gynecol Pathol. 1993;12:344-349.
- Beznos G, Coates V, Focchi J, et al. Biomolecular study of the correlation between papillomatosis of the vulvar vestibule in adolescents and human papillomavirus. ScientificWorldJournal. 2006;6:628-636.
- Kim SH, Seo SH, Ko HC, et al. The use of dermatoscopy to differentiate vestibular papillae, a normal variant of the female external genitalia, from condyloma acuminata. J Am Acad Dermatol. 2009;60:353-355.
- Ozkur E, Falay T, Turgut Erdemir AV, et al. Vestibular papillomatosis: an important differential diagnosis of vulvar papillomas. Dermatol Online J. 2016;22. pii:13030/qt7933q377
- Chang GJ, Welton ML. Human papillomavirus, condylomata acuminata, and anal neoplasia. Clin Colon Rectal Surg. 2004;17:221-230.
- Lynch PJ, Moyal-Barracco M, Bogliatto F, et al. 2006 ISSVD classification of vulvar dermatoses: pathologic subsets and their clinical correlates. J Reprod Med. 2007;52:3-9.
- Bergeron C, Ferenczy A, Richart RM, et al. Micropapillomatosis labialis appears unrelated to human papillomavirus. Obstet Gynecol. 1990;76:281-286.
- Moyal-Barracco M, Leibowitch M, Orth G. Vestibular papillae of the vulva. lack of evidence for human papillomavirus etiology. Arch Dermatol. 1990;126:1594-1598.
- Strand A, Wilander E, Zehbe I, et al. Vulvar papillomatosis, aceto-white lesions, and normal-looking vulvar mucosa evaluated by microscopy and human papillomavirus analysis. Gynecol Obstet Invest. 1995;40:265-270.
- Welch JM, Nayagam M, Parry G, et al. What is vestibular papillomatosis? a study of its prevalence, aetiology and natural history. Br J Obstet Gynaecol. 1993;100:939-942.
- Wilkinson EJ, Guerrero E, Daniel R, et al. Vulvar vestibulitis is rarely associated with human papillomavirus infection types 6, 11, 16, or 18. Int J Gynecol Pathol. 1993;12:344-349.
- Beznos G, Coates V, Focchi J, et al. Biomolecular study of the correlation between papillomatosis of the vulvar vestibule in adolescents and human papillomavirus. ScientificWorldJournal. 2006;6:628-636.
- Kim SH, Seo SH, Ko HC, et al. The use of dermatoscopy to differentiate vestibular papillae, a normal variant of the female external genitalia, from condyloma acuminata. J Am Acad Dermatol. 2009;60:353-355.
- Ozkur E, Falay T, Turgut Erdemir AV, et al. Vestibular papillomatosis: an important differential diagnosis of vulvar papillomas. Dermatol Online J. 2016;22. pii:13030/qt7933q377
- Chang GJ, Welton ML. Human papillomavirus, condylomata acuminata, and anal neoplasia. Clin Colon Rectal Surg. 2004;17:221-230.
- Lynch PJ, Moyal-Barracco M, Bogliatto F, et al. 2006 ISSVD classification of vulvar dermatoses: pathologic subsets and their clinical correlates. J Reprod Med. 2007;52:3-9.
- Bergeron C, Ferenczy A, Richart RM, et al. Micropapillomatosis labialis appears unrelated to human papillomavirus. Obstet Gynecol. 1990;76:281-286.
A 30-year-old woman with congenital absence of the uterus presented to dermatology for a second opinion of vulvar lesions that were first noted during adolescence. The patient reported that the lesions had not changed and were painful during sexual intercourse. The lesions were otherwise asymptomatic, and she had no additional relevant medical history or family history of similar lesions. She denied any history of sexually transmitted infections. Physical examination revealed multiple, soft, flesh-colored, 1- to 2-mm, discrete and coalescing, filiform papules distributed symmetrically in a linear array on the inner aspect of the bilateral medial labia minora. The rest of the mucocutaneous examination was normal.
The lesions on the left medial labia minora were treated with low-voltage (3.0 V) electrodesiccation following local anesthesia with 1% lidocaine (red arrow), while the lesions on the right medial labia minora were left untreated (black arrow). The clinical image shows the left labia minora approximately 1 month after treatment; the papules on the right labia minora were unchanged from the prior examination.
Concurrent Anticytokine Biologics for the Management of Severe Hidradenitis Suppurativa: Are They Safe and Effective?
Dysregulated immune responses including elevations in the inflammatory cytokines tumor necrosis factor (TNF),1-4 IL- 1 β ,3 and IL-12/235-7 have been identified in hidradenitis suppurativa (HS). Targeted biologic agents may offer an opportunity to intervene in specific aberrant inflammatory pathways to effectively treat HS while minimizing a dverse effects (AEs). There is growing evidence, however, that treatment of HS with a single biologic agent is not effective in all patients.6,8-17 The TNF antagonist adalimumab has been shown to achieve clinical response in approximately 50% of patients (N = 633). 18
The administration of concurrent biologics may offer the potential for improved disease control through synergistic targeting of multiple inflammatory pathways, particularly for severe and recalcitrant HS. This approach may be effective given insights from mechanistic studies suggesting the involvement of multiple inflammatory pathways in the disease pathogenesis.3,21 Concurrent anticytokine biologics have been used safely and effectively in other inflammatory diseases; for example, combination therapy with TNF and IL-12/23 antagonists have resulted in near-complete to complete resolution of severe psoriatic skin and joint disease without AEs.22-24
An increased risk for infection without increased efficacy associated with the use of concurrent anticytokine biologics for treatment of rheumatoid arthritis (RA) has raised concerns about the safety of this therapeutic approach. In a study of concurrent etanercept and anakinra therapy for RA (N=244), the combined therapy was not more efficacious than etanercept alone (American College of Rheumatology 50% response at week 24: etanercept 25 mg twice weekly, 41%; etanercept 25 mg twice weekly plus anakinra 100 mg once daily, 31%; etanercept 25 mg once weekly plus anakinra 100 mg once daily, 39% [P=.914]).25 Combination therapy also was associated with a higher overall incidence of serious AEs, serious infections requiring antibiotics or hospitalizations, and serious infections leading to study withdrawal. Reported infections included pneumonia, cellulitis, herpes zoster, pneumonitis, and pyelonephritis, but no opportunistic infections or tuberculosis were reported. A single case of lymphoma was reported in the full-dose etanercept plus anakinra group; however, the association with therapy is unclear, as RA itself is associated with an increased risk of malignancy.25
Although these results are notable, caution must be exercised in extrapolating safety and efficacy data for treatment with concurrent biologics from the RA literature for management of HS for several reasons. First, RA is an autoimmune disease that is associated with an increased risk for genitourinary and bronchopulmonary infections and septic arthritis, even in the absence of treatment with steroids and immunomodulatory drugs.26,27 Increased risk for development of lymphoma, lung cancer, and nonmelanoma skin cancer also has been associated with RA.28,29 The exact etiology of this increased risk is unknown, but it is thought to relate to immunologic disturbances and chronic systemic inflammation associated with RA.29 Furthermore, RA disease characteristics and comorbidities that may contribute to an increased risk for infection and malignancy include advanced age as well as a history of leukopenia, chronic lung disease, diabetes mellitus, alcoholism, and/or smoking.30 Infection and malignancy risk in RA also may be compounded by immunomodulatory therapies.31,32
Conversely, although microbes are believed to play an important role in HS initiation and progression, HS is neither considered an infectious disease nor associated with an increased risk for infection.33 Increased malignancy risk generally is not reported with HS, and systematic therapeutic trials of biologic therapies for HS have been notable for an absence of infectious or malignant AEs compared to placebo.12,14,16,18,19 From a mechanistic standpoint, data suggest that HS may be fundamentally distinct from RA and other autoimmune diseases; therefore, it may not be appropriate to extrapolate safety data from the latter to guide therapeutic strategies for the former.
The concept that different inflammatory diseases harbor distinct risks for comorbidities and AEs associated with medications is further supported by data from patients with PAPA syndrome (pyogenic arthritis, pyoderma gangrenosum, and acne), a monogenic autoinflammatory disease characterized by inflammasome activation and subsequent increased signaling via IL-1.34
We have safely and effectively treated 2 patients with severe HS with extended courses of concurrent TNF and IL-1 antagonists. Both patients had previously failed treatment with multiple therapeutic interventions, including topical and systemic antibiotics, disease-modifying antirheumatic drugs, hormonal therapy, biologic monotherapy with several targeted agents, and wide local excision. In the setting of concurrent certolizumab plus anakinra in the first patient and adalimumab plus anakinra in the second, both patients reported reduced drainage, pain, and number of disease flares. Both patients also were maintained on extended treatment courses (11 months and 2 years, respectively) without evidence of infection or malignancy.
Concurrent biologics may be safe and effective in managing recalcitrant HS; however, large prospective studies are needed to confirm these anecdotal findings. As our understanding of HS pathogenesis expands, novel and more effective therapeutic options will be developed. Until then, concurrent biologics may be a potential option for patients with severe recalcitrant HS.
- Jemec GB. Predicting response to anti-TNF-alpha treatment in hidradenitis suppurativa. Br J Dermatol. 2013;168:233.
- Sbidian E, Hotz C, Seneschal J, et al. Antitumour necrosis factor-α therapy for hidradenitis suppurativa: results from a national cohort study between 2000 and 2013 [published online December 22, 2015]. Br J Dermatol. 2016;174:667-670.
- van der Zee HH, de Ruiter L, van den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-α and IL-1β [published online May 17, 2011]. Br J Dermatol. 2011;164:1292-1298.
- van Rappard DC, Limpens J, Mekkes JR. The off-label treatment of severe hidradenitis suppurativa with TNF-alpha inhibitors: a systematic review. J Dermatolog Treat. 2013;24:392-404.
- Baerveldt EM, Kappen JH, Thio HB, et al. Successful long-term triple disease control by ustekinumab in a patient with Behcet’s disease, psoriasis and hidradenitis suppurativa. Ann Rheum Dis. 2013;72:626-627.
- Gulliver WP, Jemec GB, Baker KA. Experience with ustekinumab for the treatment of moderate to severe hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2012;26:911-914.
- Santos-Peréz MI, García-Rodicio S, Del Olmo-Revuelto MA, et al. Ustekinumab for hidradenitis suppurativa: a case report [published online December 3, 2013]. Actas Dermosifiliogr. 2014;105:720-722.
- Amano M, Grant A, Kerdel FA. A prospective open-label clinical trial of adalimumab for the treatment of hidradenitis suppurativa. Int J Dermatol. 2010;49:950-955.
- Blanco R, Gonzalez-Lopez MA, Gonzalez-Vela MC, et al. Disparate results in studies of adalimumab in the treatment of hidradenitis suppurativa: comment on the article by Amano et al. Int J Dermatol. 2013;52:380-381.
- Fardet L, Dupuy A, Kerob D, et al. Infliximab for severe hidradenitis suppurativa: transient clinical efficacy in 7 consecutive patients. J Am Acad Dermatol. 2007;56:624-628.
- Grant A, Gonzalez T, Montgomery MO, et al. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double-blind, placebo-controlled crossover trial. J Am Acad Dermatol. 2010;62:205-217.
- Kimball AB, Kerdel F, Adams D, et al. Adalimumab for the treatment of moderate to severe hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med. 2012;157:846-855.
- Usmani N, Clayton TH, Everett S, et al. Variable response of hidradenitis suppurativa to infliximab in four patients. Clin Exp Dermatol. 2007;32:204-205.
- Leslie KS, Tripathi SV, Nguyen TV, et al. An open-label study of anakinra for the treatment of moderate to severe hidradenitis suppurativa. J Am Acad Dermatol. 2014;70:243-251.
- Menis D, Maronas-Jimenez L, Delgado-Marquez AM, et al. Two cases of severe hidradenitis suppurativa with failure of anakinra therapy [published online January 22, 2015]. Br J Dermatol. 2015;172:810-811.
- Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. 2016;152:52-59.
- Zarchi K, Dufour DN, Jemec GB. Successful treatment of severe hidradenitis suppurativa with anakinra. JAMA Dermatol. 2013;149:1192-1194.
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434.
- Blok JL, Li K, Brodmerkel C, et al. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol. 2016;174:839-846.
- Hoffman LK, Ghias MH, Garg A, et al. Major gaps in understanding and treatment of hidradenitis suppurativa. Semin Cutan Med Surg. 2017;36:86-92.
- Schlapbach C, Hanni T, Yawalkar N, et al. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2011;65:790-798.
- Torre KM, Payette MJ. Combination biologic therapy for the treatment of severe palmoplantar pustulosis. JAAD Case Rep. 2017;3:240-242.
- Babalola O, Lakdawala N, Strober BE. Combined biologic therapy for the treatment of psoriasis and psoriatic arthritis: a case report. JAAD Case Rep. 2015;1:3-4.
- Cuchacovich R, Garcia-Valladares I, Espinoza LR. Combination biologic treatment of refractory psoriasis and psoriatic arthritis. J Rheumatol. 2012;39:187-193.
- Genovese MC, Cohen S, Moreland L, et al. Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis Rheum. 2004;50:1412-1419.
- Baum J. Infection in rheumatoid arthritis. Arthritis Rheum. 1971;14:135-137.
- Doran MF, Crowson CS, Pond GR, et al. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2287-2293.
- Askling J, Fored CM, Baecklund E, et al. Haematopoietic malignancies in rheumatoid arthritis: lymphoma risk and characteristics after exposure to tumour necrosis factor antagonists. Ann Rheum Dis. 2005;64:1414-1420.
- Smitten AL, Simon TA, Hochberg MC, et al. A meta-analysis of the incidence of malignancy in adult patients with rheumatoid arthritis [published online April 23, 2008]. Arthritis Res Ther. 2008;10:R45.
- Doran MF, Crowson CS, Pond GR, et al. Predictors of infection inrheumatoid arthritis. Arthritis Rheum. 2002;46:2294-2300.
- Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56:2886-2895.
- Raaschou P, Simard JF, Asker Hagelberg C, et al. Rheumatoid arthritis, anti-tumour necrosis factor treatment, and risk of squamous cell and basal cell skin cancer: cohort study based on nationwide prospectively recorded data from Sweden. BMJ. 2016;352:i262.
- Ring HC, Riis Mikkelsen P, Miller IM, et al. The bacteriology of hidradenitis suppurativa: a systematic review. Exp Dermatol. 2015;24:727-731.
- Smith EJ, Allantaz F, Bennett L, et al. Clinical, molecular, and genetic characteristics of PAPA syndrome: a review. Curr Genomics. 2010;11:519-527.
Dysregulated immune responses including elevations in the inflammatory cytokines tumor necrosis factor (TNF),1-4 IL- 1 β ,3 and IL-12/235-7 have been identified in hidradenitis suppurativa (HS). Targeted biologic agents may offer an opportunity to intervene in specific aberrant inflammatory pathways to effectively treat HS while minimizing a dverse effects (AEs). There is growing evidence, however, that treatment of HS with a single biologic agent is not effective in all patients.6,8-17 The TNF antagonist adalimumab has been shown to achieve clinical response in approximately 50% of patients (N = 633). 18
The administration of concurrent biologics may offer the potential for improved disease control through synergistic targeting of multiple inflammatory pathways, particularly for severe and recalcitrant HS. This approach may be effective given insights from mechanistic studies suggesting the involvement of multiple inflammatory pathways in the disease pathogenesis.3,21 Concurrent anticytokine biologics have been used safely and effectively in other inflammatory diseases; for example, combination therapy with TNF and IL-12/23 antagonists have resulted in near-complete to complete resolution of severe psoriatic skin and joint disease without AEs.22-24
An increased risk for infection without increased efficacy associated with the use of concurrent anticytokine biologics for treatment of rheumatoid arthritis (RA) has raised concerns about the safety of this therapeutic approach. In a study of concurrent etanercept and anakinra therapy for RA (N=244), the combined therapy was not more efficacious than etanercept alone (American College of Rheumatology 50% response at week 24: etanercept 25 mg twice weekly, 41%; etanercept 25 mg twice weekly plus anakinra 100 mg once daily, 31%; etanercept 25 mg once weekly plus anakinra 100 mg once daily, 39% [P=.914]).25 Combination therapy also was associated with a higher overall incidence of serious AEs, serious infections requiring antibiotics or hospitalizations, and serious infections leading to study withdrawal. Reported infections included pneumonia, cellulitis, herpes zoster, pneumonitis, and pyelonephritis, but no opportunistic infections or tuberculosis were reported. A single case of lymphoma was reported in the full-dose etanercept plus anakinra group; however, the association with therapy is unclear, as RA itself is associated with an increased risk of malignancy.25
Although these results are notable, caution must be exercised in extrapolating safety and efficacy data for treatment with concurrent biologics from the RA literature for management of HS for several reasons. First, RA is an autoimmune disease that is associated with an increased risk for genitourinary and bronchopulmonary infections and septic arthritis, even in the absence of treatment with steroids and immunomodulatory drugs.26,27 Increased risk for development of lymphoma, lung cancer, and nonmelanoma skin cancer also has been associated with RA.28,29 The exact etiology of this increased risk is unknown, but it is thought to relate to immunologic disturbances and chronic systemic inflammation associated with RA.29 Furthermore, RA disease characteristics and comorbidities that may contribute to an increased risk for infection and malignancy include advanced age as well as a history of leukopenia, chronic lung disease, diabetes mellitus, alcoholism, and/or smoking.30 Infection and malignancy risk in RA also may be compounded by immunomodulatory therapies.31,32
Conversely, although microbes are believed to play an important role in HS initiation and progression, HS is neither considered an infectious disease nor associated with an increased risk for infection.33 Increased malignancy risk generally is not reported with HS, and systematic therapeutic trials of biologic therapies for HS have been notable for an absence of infectious or malignant AEs compared to placebo.12,14,16,18,19 From a mechanistic standpoint, data suggest that HS may be fundamentally distinct from RA and other autoimmune diseases; therefore, it may not be appropriate to extrapolate safety data from the latter to guide therapeutic strategies for the former.
The concept that different inflammatory diseases harbor distinct risks for comorbidities and AEs associated with medications is further supported by data from patients with PAPA syndrome (pyogenic arthritis, pyoderma gangrenosum, and acne), a monogenic autoinflammatory disease characterized by inflammasome activation and subsequent increased signaling via IL-1.34
We have safely and effectively treated 2 patients with severe HS with extended courses of concurrent TNF and IL-1 antagonists. Both patients had previously failed treatment with multiple therapeutic interventions, including topical and systemic antibiotics, disease-modifying antirheumatic drugs, hormonal therapy, biologic monotherapy with several targeted agents, and wide local excision. In the setting of concurrent certolizumab plus anakinra in the first patient and adalimumab plus anakinra in the second, both patients reported reduced drainage, pain, and number of disease flares. Both patients also were maintained on extended treatment courses (11 months and 2 years, respectively) without evidence of infection or malignancy.
Concurrent biologics may be safe and effective in managing recalcitrant HS; however, large prospective studies are needed to confirm these anecdotal findings. As our understanding of HS pathogenesis expands, novel and more effective therapeutic options will be developed. Until then, concurrent biologics may be a potential option for patients with severe recalcitrant HS.
Dysregulated immune responses including elevations in the inflammatory cytokines tumor necrosis factor (TNF),1-4 IL- 1 β ,3 and IL-12/235-7 have been identified in hidradenitis suppurativa (HS). Targeted biologic agents may offer an opportunity to intervene in specific aberrant inflammatory pathways to effectively treat HS while minimizing a dverse effects (AEs). There is growing evidence, however, that treatment of HS with a single biologic agent is not effective in all patients.6,8-17 The TNF antagonist adalimumab has been shown to achieve clinical response in approximately 50% of patients (N = 633). 18
The administration of concurrent biologics may offer the potential for improved disease control through synergistic targeting of multiple inflammatory pathways, particularly for severe and recalcitrant HS. This approach may be effective given insights from mechanistic studies suggesting the involvement of multiple inflammatory pathways in the disease pathogenesis.3,21 Concurrent anticytokine biologics have been used safely and effectively in other inflammatory diseases; for example, combination therapy with TNF and IL-12/23 antagonists have resulted in near-complete to complete resolution of severe psoriatic skin and joint disease without AEs.22-24
An increased risk for infection without increased efficacy associated with the use of concurrent anticytokine biologics for treatment of rheumatoid arthritis (RA) has raised concerns about the safety of this therapeutic approach. In a study of concurrent etanercept and anakinra therapy for RA (N=244), the combined therapy was not more efficacious than etanercept alone (American College of Rheumatology 50% response at week 24: etanercept 25 mg twice weekly, 41%; etanercept 25 mg twice weekly plus anakinra 100 mg once daily, 31%; etanercept 25 mg once weekly plus anakinra 100 mg once daily, 39% [P=.914]).25 Combination therapy also was associated with a higher overall incidence of serious AEs, serious infections requiring antibiotics or hospitalizations, and serious infections leading to study withdrawal. Reported infections included pneumonia, cellulitis, herpes zoster, pneumonitis, and pyelonephritis, but no opportunistic infections or tuberculosis were reported. A single case of lymphoma was reported in the full-dose etanercept plus anakinra group; however, the association with therapy is unclear, as RA itself is associated with an increased risk of malignancy.25
Although these results are notable, caution must be exercised in extrapolating safety and efficacy data for treatment with concurrent biologics from the RA literature for management of HS for several reasons. First, RA is an autoimmune disease that is associated with an increased risk for genitourinary and bronchopulmonary infections and septic arthritis, even in the absence of treatment with steroids and immunomodulatory drugs.26,27 Increased risk for development of lymphoma, lung cancer, and nonmelanoma skin cancer also has been associated with RA.28,29 The exact etiology of this increased risk is unknown, but it is thought to relate to immunologic disturbances and chronic systemic inflammation associated with RA.29 Furthermore, RA disease characteristics and comorbidities that may contribute to an increased risk for infection and malignancy include advanced age as well as a history of leukopenia, chronic lung disease, diabetes mellitus, alcoholism, and/or smoking.30 Infection and malignancy risk in RA also may be compounded by immunomodulatory therapies.31,32
Conversely, although microbes are believed to play an important role in HS initiation and progression, HS is neither considered an infectious disease nor associated with an increased risk for infection.33 Increased malignancy risk generally is not reported with HS, and systematic therapeutic trials of biologic therapies for HS have been notable for an absence of infectious or malignant AEs compared to placebo.12,14,16,18,19 From a mechanistic standpoint, data suggest that HS may be fundamentally distinct from RA and other autoimmune diseases; therefore, it may not be appropriate to extrapolate safety data from the latter to guide therapeutic strategies for the former.
The concept that different inflammatory diseases harbor distinct risks for comorbidities and AEs associated with medications is further supported by data from patients with PAPA syndrome (pyogenic arthritis, pyoderma gangrenosum, and acne), a monogenic autoinflammatory disease characterized by inflammasome activation and subsequent increased signaling via IL-1.34
We have safely and effectively treated 2 patients with severe HS with extended courses of concurrent TNF and IL-1 antagonists. Both patients had previously failed treatment with multiple therapeutic interventions, including topical and systemic antibiotics, disease-modifying antirheumatic drugs, hormonal therapy, biologic monotherapy with several targeted agents, and wide local excision. In the setting of concurrent certolizumab plus anakinra in the first patient and adalimumab plus anakinra in the second, both patients reported reduced drainage, pain, and number of disease flares. Both patients also were maintained on extended treatment courses (11 months and 2 years, respectively) without evidence of infection or malignancy.
Concurrent biologics may be safe and effective in managing recalcitrant HS; however, large prospective studies are needed to confirm these anecdotal findings. As our understanding of HS pathogenesis expands, novel and more effective therapeutic options will be developed. Until then, concurrent biologics may be a potential option for patients with severe recalcitrant HS.
- Jemec GB. Predicting response to anti-TNF-alpha treatment in hidradenitis suppurativa. Br J Dermatol. 2013;168:233.
- Sbidian E, Hotz C, Seneschal J, et al. Antitumour necrosis factor-α therapy for hidradenitis suppurativa: results from a national cohort study between 2000 and 2013 [published online December 22, 2015]. Br J Dermatol. 2016;174:667-670.
- van der Zee HH, de Ruiter L, van den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-α and IL-1β [published online May 17, 2011]. Br J Dermatol. 2011;164:1292-1298.
- van Rappard DC, Limpens J, Mekkes JR. The off-label treatment of severe hidradenitis suppurativa with TNF-alpha inhibitors: a systematic review. J Dermatolog Treat. 2013;24:392-404.
- Baerveldt EM, Kappen JH, Thio HB, et al. Successful long-term triple disease control by ustekinumab in a patient with Behcet’s disease, psoriasis and hidradenitis suppurativa. Ann Rheum Dis. 2013;72:626-627.
- Gulliver WP, Jemec GB, Baker KA. Experience with ustekinumab for the treatment of moderate to severe hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2012;26:911-914.
- Santos-Peréz MI, García-Rodicio S, Del Olmo-Revuelto MA, et al. Ustekinumab for hidradenitis suppurativa: a case report [published online December 3, 2013]. Actas Dermosifiliogr. 2014;105:720-722.
- Amano M, Grant A, Kerdel FA. A prospective open-label clinical trial of adalimumab for the treatment of hidradenitis suppurativa. Int J Dermatol. 2010;49:950-955.
- Blanco R, Gonzalez-Lopez MA, Gonzalez-Vela MC, et al. Disparate results in studies of adalimumab in the treatment of hidradenitis suppurativa: comment on the article by Amano et al. Int J Dermatol. 2013;52:380-381.
- Fardet L, Dupuy A, Kerob D, et al. Infliximab for severe hidradenitis suppurativa: transient clinical efficacy in 7 consecutive patients. J Am Acad Dermatol. 2007;56:624-628.
- Grant A, Gonzalez T, Montgomery MO, et al. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double-blind, placebo-controlled crossover trial. J Am Acad Dermatol. 2010;62:205-217.
- Kimball AB, Kerdel F, Adams D, et al. Adalimumab for the treatment of moderate to severe hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med. 2012;157:846-855.
- Usmani N, Clayton TH, Everett S, et al. Variable response of hidradenitis suppurativa to infliximab in four patients. Clin Exp Dermatol. 2007;32:204-205.
- Leslie KS, Tripathi SV, Nguyen TV, et al. An open-label study of anakinra for the treatment of moderate to severe hidradenitis suppurativa. J Am Acad Dermatol. 2014;70:243-251.
- Menis D, Maronas-Jimenez L, Delgado-Marquez AM, et al. Two cases of severe hidradenitis suppurativa with failure of anakinra therapy [published online January 22, 2015]. Br J Dermatol. 2015;172:810-811.
- Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. 2016;152:52-59.
- Zarchi K, Dufour DN, Jemec GB. Successful treatment of severe hidradenitis suppurativa with anakinra. JAMA Dermatol. 2013;149:1192-1194.
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434.
- Blok JL, Li K, Brodmerkel C, et al. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol. 2016;174:839-846.
- Hoffman LK, Ghias MH, Garg A, et al. Major gaps in understanding and treatment of hidradenitis suppurativa. Semin Cutan Med Surg. 2017;36:86-92.
- Schlapbach C, Hanni T, Yawalkar N, et al. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2011;65:790-798.
- Torre KM, Payette MJ. Combination biologic therapy for the treatment of severe palmoplantar pustulosis. JAAD Case Rep. 2017;3:240-242.
- Babalola O, Lakdawala N, Strober BE. Combined biologic therapy for the treatment of psoriasis and psoriatic arthritis: a case report. JAAD Case Rep. 2015;1:3-4.
- Cuchacovich R, Garcia-Valladares I, Espinoza LR. Combination biologic treatment of refractory psoriasis and psoriatic arthritis. J Rheumatol. 2012;39:187-193.
- Genovese MC, Cohen S, Moreland L, et al. Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis Rheum. 2004;50:1412-1419.
- Baum J. Infection in rheumatoid arthritis. Arthritis Rheum. 1971;14:135-137.
- Doran MF, Crowson CS, Pond GR, et al. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2287-2293.
- Askling J, Fored CM, Baecklund E, et al. Haematopoietic malignancies in rheumatoid arthritis: lymphoma risk and characteristics after exposure to tumour necrosis factor antagonists. Ann Rheum Dis. 2005;64:1414-1420.
- Smitten AL, Simon TA, Hochberg MC, et al. A meta-analysis of the incidence of malignancy in adult patients with rheumatoid arthritis [published online April 23, 2008]. Arthritis Res Ther. 2008;10:R45.
- Doran MF, Crowson CS, Pond GR, et al. Predictors of infection inrheumatoid arthritis. Arthritis Rheum. 2002;46:2294-2300.
- Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56:2886-2895.
- Raaschou P, Simard JF, Asker Hagelberg C, et al. Rheumatoid arthritis, anti-tumour necrosis factor treatment, and risk of squamous cell and basal cell skin cancer: cohort study based on nationwide prospectively recorded data from Sweden. BMJ. 2016;352:i262.
- Ring HC, Riis Mikkelsen P, Miller IM, et al. The bacteriology of hidradenitis suppurativa: a systematic review. Exp Dermatol. 2015;24:727-731.
- Smith EJ, Allantaz F, Bennett L, et al. Clinical, molecular, and genetic characteristics of PAPA syndrome: a review. Curr Genomics. 2010;11:519-527.
- Jemec GB. Predicting response to anti-TNF-alpha treatment in hidradenitis suppurativa. Br J Dermatol. 2013;168:233.
- Sbidian E, Hotz C, Seneschal J, et al. Antitumour necrosis factor-α therapy for hidradenitis suppurativa: results from a national cohort study between 2000 and 2013 [published online December 22, 2015]. Br J Dermatol. 2016;174:667-670.
- van der Zee HH, de Ruiter L, van den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-α and IL-1β [published online May 17, 2011]. Br J Dermatol. 2011;164:1292-1298.
- van Rappard DC, Limpens J, Mekkes JR. The off-label treatment of severe hidradenitis suppurativa with TNF-alpha inhibitors: a systematic review. J Dermatolog Treat. 2013;24:392-404.
- Baerveldt EM, Kappen JH, Thio HB, et al. Successful long-term triple disease control by ustekinumab in a patient with Behcet’s disease, psoriasis and hidradenitis suppurativa. Ann Rheum Dis. 2013;72:626-627.
- Gulliver WP, Jemec GB, Baker KA. Experience with ustekinumab for the treatment of moderate to severe hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2012;26:911-914.
- Santos-Peréz MI, García-Rodicio S, Del Olmo-Revuelto MA, et al. Ustekinumab for hidradenitis suppurativa: a case report [published online December 3, 2013]. Actas Dermosifiliogr. 2014;105:720-722.
- Amano M, Grant A, Kerdel FA. A prospective open-label clinical trial of adalimumab for the treatment of hidradenitis suppurativa. Int J Dermatol. 2010;49:950-955.
- Blanco R, Gonzalez-Lopez MA, Gonzalez-Vela MC, et al. Disparate results in studies of adalimumab in the treatment of hidradenitis suppurativa: comment on the article by Amano et al. Int J Dermatol. 2013;52:380-381.
- Fardet L, Dupuy A, Kerob D, et al. Infliximab for severe hidradenitis suppurativa: transient clinical efficacy in 7 consecutive patients. J Am Acad Dermatol. 2007;56:624-628.
- Grant A, Gonzalez T, Montgomery MO, et al. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double-blind, placebo-controlled crossover trial. J Am Acad Dermatol. 2010;62:205-217.
- Kimball AB, Kerdel F, Adams D, et al. Adalimumab for the treatment of moderate to severe hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med. 2012;157:846-855.
- Usmani N, Clayton TH, Everett S, et al. Variable response of hidradenitis suppurativa to infliximab in four patients. Clin Exp Dermatol. 2007;32:204-205.
- Leslie KS, Tripathi SV, Nguyen TV, et al. An open-label study of anakinra for the treatment of moderate to severe hidradenitis suppurativa. J Am Acad Dermatol. 2014;70:243-251.
- Menis D, Maronas-Jimenez L, Delgado-Marquez AM, et al. Two cases of severe hidradenitis suppurativa with failure of anakinra therapy [published online January 22, 2015]. Br J Dermatol. 2015;172:810-811.
- Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. 2016;152:52-59.
- Zarchi K, Dufour DN, Jemec GB. Successful treatment of severe hidradenitis suppurativa with anakinra. JAMA Dermatol. 2013;149:1192-1194.
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434.
- Blok JL, Li K, Brodmerkel C, et al. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol. 2016;174:839-846.
- Hoffman LK, Ghias MH, Garg A, et al. Major gaps in understanding and treatment of hidradenitis suppurativa. Semin Cutan Med Surg. 2017;36:86-92.
- Schlapbach C, Hanni T, Yawalkar N, et al. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2011;65:790-798.
- Torre KM, Payette MJ. Combination biologic therapy for the treatment of severe palmoplantar pustulosis. JAAD Case Rep. 2017;3:240-242.
- Babalola O, Lakdawala N, Strober BE. Combined biologic therapy for the treatment of psoriasis and psoriatic arthritis: a case report. JAAD Case Rep. 2015;1:3-4.
- Cuchacovich R, Garcia-Valladares I, Espinoza LR. Combination biologic treatment of refractory psoriasis and psoriatic arthritis. J Rheumatol. 2012;39:187-193.
- Genovese MC, Cohen S, Moreland L, et al. Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis Rheum. 2004;50:1412-1419.
- Baum J. Infection in rheumatoid arthritis. Arthritis Rheum. 1971;14:135-137.
- Doran MF, Crowson CS, Pond GR, et al. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2287-2293.
- Askling J, Fored CM, Baecklund E, et al. Haematopoietic malignancies in rheumatoid arthritis: lymphoma risk and characteristics after exposure to tumour necrosis factor antagonists. Ann Rheum Dis. 2005;64:1414-1420.
- Smitten AL, Simon TA, Hochberg MC, et al. A meta-analysis of the incidence of malignancy in adult patients with rheumatoid arthritis [published online April 23, 2008]. Arthritis Res Ther. 2008;10:R45.
- Doran MF, Crowson CS, Pond GR, et al. Predictors of infection inrheumatoid arthritis. Arthritis Rheum. 2002;46:2294-2300.
- Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56:2886-2895.
- Raaschou P, Simard JF, Asker Hagelberg C, et al. Rheumatoid arthritis, anti-tumour necrosis factor treatment, and risk of squamous cell and basal cell skin cancer: cohort study based on nationwide prospectively recorded data from Sweden. BMJ. 2016;352:i262.
- Ring HC, Riis Mikkelsen P, Miller IM, et al. The bacteriology of hidradenitis suppurativa: a systematic review. Exp Dermatol. 2015;24:727-731.
- Smith EJ, Allantaz F, Bennett L, et al. Clinical, molecular, and genetic characteristics of PAPA syndrome: a review. Curr Genomics. 2010;11:519-527.
Diversity in the Dermatology Workforce: 2017 Status Update
Physician diversity benefits patient care: Patients are more satisfied during race-concordant visits, report their physicians as more engaged and responsive to their needs, and experience notably longer visits.1,2 Nonwhite physicians (ie, races and ethnicities that are underrepresented in medicine [URM] with respect to the general population) are more likely to care for underserved communities. Furthermore, increased diversity in the learning environment supports preparedness of all trainees to serve diverse patients.3 For these reasons, a more diverse physician workforce can contribute to better access to care in all communities, thus addressing health disparities.1,4
Increasing diversity in the dermatology workforce has been identified as an emerging priority.5 Dermatology is one of the least diverse specialties,5 and the representation of URM dermatologists is lower compared to other medical specialties and the general US population. The proportion of specialty leaders from underrepresented backgrounds may be even smaller. The lack of diversity in academic dermatology has negative consequences for patients and communities. Increasing the diversity of resident trainees is the only way to improve the diversity gap within the dermatology workforce.6
Recent commentary on this topic has highlighted several priorities for addressing the dermatology diversity gap,6-11 including the following: (1) making diversity an explicit goal in dermatology; (2) ensuring early exposure to dermatology in medical school; (3) supporting mentorship programs for minority medical students; (4) increasing medical student diversity; (5) encouraging that all dermatology program directors and leaders train in implicit bias; and (6) reviewing residency admission criteria to ensure they are objective and equitable, not biased against any applicants.
The process of reviewing residency selection criteria has begun. In 2017, Chen and Shinkai7 called for our specialty to rethink the selection process. The authors argued that emphasis on test scores, grades, and publications systematically disadvantages underrepresented minorities and students from lower socioeconomic statuses. The authors proposed several solutions: (1) make diversity an explicit goal of the selection process, (2) shift away from test scores for all applicants, (3) change the interview format, (4) prioritize other competencies such as observation skills, and (5) recruit and retain faculty who support URM trainees.7
Several dermatology leadership groups have taken action to promote programs that aim to improve diversity within dermatology. The Dermatology Diversity Champions initiative includes 6 US dermatology residency programs that are committed to increasing diversity and collaborate to evaluate pilot approaches. The American Academy of Dermatology President’s Conference on Diversity in Dermatology in Chicago, Illinois, in August 2017, as well as the focus on diversity in residency training programs at the Annual Meeting of the Association of Professors of Dermatology in Chicago, Illinois, in October 2017, are strong indicators that our specialty as a whole is aware and eager to embrace diversity as a priority. The American Academy of Dermatology President’s Conference, which was comprised of representatives from many leadership organizations and interest groups within dermatology, identified 3 action items: (1) increase the pipeline of URM students into medical school, (2) increase interest in dermatology among URM medical students, and (3) increase URM representation in residency training programs.
There are many strengths, weaknesses, opportunities, and threats/barriers (SWOT) to attaining this goal. Current strengths include strong support from dermatology leaders and activities that build on existing mentorship and diversity efforts by leaders within our specialty. SWOT analysis highlights several key opportunities of this mission, including connecting with the House of Medicine in shared efforts to improve diversity, as well as increased understanding of skin of color, health disparities, and implicit bias among physicians. Although faculty development will require time and financial investment, it will lead to tremendous benefits and opportunities for all dermatologists, including URM physicians. Other weaknesses and threats/barriers are outlined in the Figure.
Final Thoughts
We are far from reaching our goal of a diverse dermatology workforce, and the road ahead is long. We have a start and we have momentum. We can move forward by spreading the word that all types of diversity are a priority for our specialty. Making a true difference will require commitment and sustained efforts. Dermatology can lead the way as all of American medicine strives to attain workforce diversity.
- Saha S. Taking diversity seriously: the merits of increasing minority representation in medicine. JAMA Intern Med. 2014;174:291-292.
- Cooper LA, Roter DL, Johnson RL, et al. Patient-centered communication, ratings of care, and concordance of patient and physician race. Ann Intern Med. 2003;139:907-915.
- Saha S, Guiton G, Wimmers PF, et al. Student body racial and ethnic composition and diversity-related outcomes in US medical schools. JAMA. 2008;300:1135-1145.
- Marrast LM, Zallman L, Woolhandler S, et al. Minority physicians’ role in the care of underserved patients: diversifying the physician workforce may be key in addressing health disparities. JAMA Intern Med. 2014;174:289-291.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Lester J, Wintroub B, Linos E. Disparities in academic dermatology. JAMA Dermatol. 2016;152:878-879.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260.
- Granstein RD, Cornelius L, Shinkai K. Diversity in dermatology—a call for action. JAMA Dermatol. 2017;153:499-500.
- McKesey J, Berger TG, Lim HW, et al. Cultural competence for the 21st century dermatologist practicing in the United States. J Am Acad Dermatol. 2017;77:1159-1169.
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49.
- Imadojemu S, James WD. Increasing African American representation in dermatology. JAMA Dermatol. 2016;152:15-16.
Physician diversity benefits patient care: Patients are more satisfied during race-concordant visits, report their physicians as more engaged and responsive to their needs, and experience notably longer visits.1,2 Nonwhite physicians (ie, races and ethnicities that are underrepresented in medicine [URM] with respect to the general population) are more likely to care for underserved communities. Furthermore, increased diversity in the learning environment supports preparedness of all trainees to serve diverse patients.3 For these reasons, a more diverse physician workforce can contribute to better access to care in all communities, thus addressing health disparities.1,4
Increasing diversity in the dermatology workforce has been identified as an emerging priority.5 Dermatology is one of the least diverse specialties,5 and the representation of URM dermatologists is lower compared to other medical specialties and the general US population. The proportion of specialty leaders from underrepresented backgrounds may be even smaller. The lack of diversity in academic dermatology has negative consequences for patients and communities. Increasing the diversity of resident trainees is the only way to improve the diversity gap within the dermatology workforce.6
Recent commentary on this topic has highlighted several priorities for addressing the dermatology diversity gap,6-11 including the following: (1) making diversity an explicit goal in dermatology; (2) ensuring early exposure to dermatology in medical school; (3) supporting mentorship programs for minority medical students; (4) increasing medical student diversity; (5) encouraging that all dermatology program directors and leaders train in implicit bias; and (6) reviewing residency admission criteria to ensure they are objective and equitable, not biased against any applicants.
The process of reviewing residency selection criteria has begun. In 2017, Chen and Shinkai7 called for our specialty to rethink the selection process. The authors argued that emphasis on test scores, grades, and publications systematically disadvantages underrepresented minorities and students from lower socioeconomic statuses. The authors proposed several solutions: (1) make diversity an explicit goal of the selection process, (2) shift away from test scores for all applicants, (3) change the interview format, (4) prioritize other competencies such as observation skills, and (5) recruit and retain faculty who support URM trainees.7
Several dermatology leadership groups have taken action to promote programs that aim to improve diversity within dermatology. The Dermatology Diversity Champions initiative includes 6 US dermatology residency programs that are committed to increasing diversity and collaborate to evaluate pilot approaches. The American Academy of Dermatology President’s Conference on Diversity in Dermatology in Chicago, Illinois, in August 2017, as well as the focus on diversity in residency training programs at the Annual Meeting of the Association of Professors of Dermatology in Chicago, Illinois, in October 2017, are strong indicators that our specialty as a whole is aware and eager to embrace diversity as a priority. The American Academy of Dermatology President’s Conference, which was comprised of representatives from many leadership organizations and interest groups within dermatology, identified 3 action items: (1) increase the pipeline of URM students into medical school, (2) increase interest in dermatology among URM medical students, and (3) increase URM representation in residency training programs.
There are many strengths, weaknesses, opportunities, and threats/barriers (SWOT) to attaining this goal. Current strengths include strong support from dermatology leaders and activities that build on existing mentorship and diversity efforts by leaders within our specialty. SWOT analysis highlights several key opportunities of this mission, including connecting with the House of Medicine in shared efforts to improve diversity, as well as increased understanding of skin of color, health disparities, and implicit bias among physicians. Although faculty development will require time and financial investment, it will lead to tremendous benefits and opportunities for all dermatologists, including URM physicians. Other weaknesses and threats/barriers are outlined in the Figure.
Final Thoughts
We are far from reaching our goal of a diverse dermatology workforce, and the road ahead is long. We have a start and we have momentum. We can move forward by spreading the word that all types of diversity are a priority for our specialty. Making a true difference will require commitment and sustained efforts. Dermatology can lead the way as all of American medicine strives to attain workforce diversity.
Physician diversity benefits patient care: Patients are more satisfied during race-concordant visits, report their physicians as more engaged and responsive to their needs, and experience notably longer visits.1,2 Nonwhite physicians (ie, races and ethnicities that are underrepresented in medicine [URM] with respect to the general population) are more likely to care for underserved communities. Furthermore, increased diversity in the learning environment supports preparedness of all trainees to serve diverse patients.3 For these reasons, a more diverse physician workforce can contribute to better access to care in all communities, thus addressing health disparities.1,4
Increasing diversity in the dermatology workforce has been identified as an emerging priority.5 Dermatology is one of the least diverse specialties,5 and the representation of URM dermatologists is lower compared to other medical specialties and the general US population. The proportion of specialty leaders from underrepresented backgrounds may be even smaller. The lack of diversity in academic dermatology has negative consequences for patients and communities. Increasing the diversity of resident trainees is the only way to improve the diversity gap within the dermatology workforce.6
Recent commentary on this topic has highlighted several priorities for addressing the dermatology diversity gap,6-11 including the following: (1) making diversity an explicit goal in dermatology; (2) ensuring early exposure to dermatology in medical school; (3) supporting mentorship programs for minority medical students; (4) increasing medical student diversity; (5) encouraging that all dermatology program directors and leaders train in implicit bias; and (6) reviewing residency admission criteria to ensure they are objective and equitable, not biased against any applicants.
The process of reviewing residency selection criteria has begun. In 2017, Chen and Shinkai7 called for our specialty to rethink the selection process. The authors argued that emphasis on test scores, grades, and publications systematically disadvantages underrepresented minorities and students from lower socioeconomic statuses. The authors proposed several solutions: (1) make diversity an explicit goal of the selection process, (2) shift away from test scores for all applicants, (3) change the interview format, (4) prioritize other competencies such as observation skills, and (5) recruit and retain faculty who support URM trainees.7
Several dermatology leadership groups have taken action to promote programs that aim to improve diversity within dermatology. The Dermatology Diversity Champions initiative includes 6 US dermatology residency programs that are committed to increasing diversity and collaborate to evaluate pilot approaches. The American Academy of Dermatology President’s Conference on Diversity in Dermatology in Chicago, Illinois, in August 2017, as well as the focus on diversity in residency training programs at the Annual Meeting of the Association of Professors of Dermatology in Chicago, Illinois, in October 2017, are strong indicators that our specialty as a whole is aware and eager to embrace diversity as a priority. The American Academy of Dermatology President’s Conference, which was comprised of representatives from many leadership organizations and interest groups within dermatology, identified 3 action items: (1) increase the pipeline of URM students into medical school, (2) increase interest in dermatology among URM medical students, and (3) increase URM representation in residency training programs.
There are many strengths, weaknesses, opportunities, and threats/barriers (SWOT) to attaining this goal. Current strengths include strong support from dermatology leaders and activities that build on existing mentorship and diversity efforts by leaders within our specialty. SWOT analysis highlights several key opportunities of this mission, including connecting with the House of Medicine in shared efforts to improve diversity, as well as increased understanding of skin of color, health disparities, and implicit bias among physicians. Although faculty development will require time and financial investment, it will lead to tremendous benefits and opportunities for all dermatologists, including URM physicians. Other weaknesses and threats/barriers are outlined in the Figure.
Final Thoughts
We are far from reaching our goal of a diverse dermatology workforce, and the road ahead is long. We have a start and we have momentum. We can move forward by spreading the word that all types of diversity are a priority for our specialty. Making a true difference will require commitment and sustained efforts. Dermatology can lead the way as all of American medicine strives to attain workforce diversity.
- Saha S. Taking diversity seriously: the merits of increasing minority representation in medicine. JAMA Intern Med. 2014;174:291-292.
- Cooper LA, Roter DL, Johnson RL, et al. Patient-centered communication, ratings of care, and concordance of patient and physician race. Ann Intern Med. 2003;139:907-915.
- Saha S, Guiton G, Wimmers PF, et al. Student body racial and ethnic composition and diversity-related outcomes in US medical schools. JAMA. 2008;300:1135-1145.
- Marrast LM, Zallman L, Woolhandler S, et al. Minority physicians’ role in the care of underserved patients: diversifying the physician workforce may be key in addressing health disparities. JAMA Intern Med. 2014;174:289-291.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Lester J, Wintroub B, Linos E. Disparities in academic dermatology. JAMA Dermatol. 2016;152:878-879.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260.
- Granstein RD, Cornelius L, Shinkai K. Diversity in dermatology—a call for action. JAMA Dermatol. 2017;153:499-500.
- McKesey J, Berger TG, Lim HW, et al. Cultural competence for the 21st century dermatologist practicing in the United States. J Am Acad Dermatol. 2017;77:1159-1169.
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49.
- Imadojemu S, James WD. Increasing African American representation in dermatology. JAMA Dermatol. 2016;152:15-16.
- Saha S. Taking diversity seriously: the merits of increasing minority representation in medicine. JAMA Intern Med. 2014;174:291-292.
- Cooper LA, Roter DL, Johnson RL, et al. Patient-centered communication, ratings of care, and concordance of patient and physician race. Ann Intern Med. 2003;139:907-915.
- Saha S, Guiton G, Wimmers PF, et al. Student body racial and ethnic composition and diversity-related outcomes in US medical schools. JAMA. 2008;300:1135-1145.
- Marrast LM, Zallman L, Woolhandler S, et al. Minority physicians’ role in the care of underserved patients: diversifying the physician workforce may be key in addressing health disparities. JAMA Intern Med. 2014;174:289-291.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Lester J, Wintroub B, Linos E. Disparities in academic dermatology. JAMA Dermatol. 2016;152:878-879.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260.
- Granstein RD, Cornelius L, Shinkai K. Diversity in dermatology—a call for action. JAMA Dermatol. 2017;153:499-500.
- McKesey J, Berger TG, Lim HW, et al. Cultural competence for the 21st century dermatologist practicing in the United States. J Am Acad Dermatol. 2017;77:1159-1169.
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49.
- Imadojemu S, James WD. Increasing African American representation in dermatology. JAMA Dermatol. 2016;152:15-16.
High-Value, Cost-Conscious Evaluation for PCOS: Which Tests Should Be Routinely Ordered in Acne Patients?
The adult female patient presenting with severe acne vulgaris may raise special diagnostic concerns, including consideration of an underlying hormonal disorder. Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in women of reproductive age with an estimated prevalence as high as 12%.1 Many women with undiagnosed PCOS may be referred to dermatologists for evaluation of its cutaneous manifestations of hyperandrogenism including acne, hirsutism, and androgenic alopecia.2 Given the prevalence of PCOS and its long-term health implications, dermatologists can play an important role in the initial evaluation of these patients. Acne and androgenic alopecia, however, are quite common, and in the absence of red flags such as menstrual irregularities, virilization, visual field deficits, or signs of Cushing syndrome,3 clinicians must decide when to pursue limited versus comprehensive evaluation.
Despite being common in patients with PCOS, a recent study suggests that acne is an unreliable marker of biochemical hyperandrogenism, and specific features of acne (ie, lesion counts, lesional types, distribution) cannot reliably discriminate women who meet PCOS diagnostic criteria from those who do not.4 Similarly, the study found that androgenic alopecia was not associated with biochemical hyperandrogenism and was no more common in women with PCOS than women of similar age in a high-risk population. Unlike acne and androgenic alopecia, however, the study identified hirsutism, especially truncal hirsutism, as a reliable indicator of hyperandrogenemia and PCOS. Hirsutism also is associated with metabolic sequelae of PCOS. These findings suggest that hirsutism, but not acne or androgenic alopecia, in a female of reproductive age warrants a workup for PCOS.4 This report is consistent with a recommendation from the Androgen Excess and Polycystic Ovary Syndrome Society (AE-PCOS) to pursue a diagnostic evaluation in any woman presenting with hirsutism.5 Acanthosis nigricans also was found to be a reliable indicator of hyperandrogenemia, PCOS, and associated metabolic derangement. Thus, although recent evidence indicates that acne as an isolated cutaneous finding does not warrant further diagnostic evaluation, acne in the setting of hirsutism, acanthosis nigricans, menstrual irregularities, or additional specific signs of endocrine dysregulation should prompt focused workup.4
Multiple clinical practice guidelines for the evaluation of hirsutism and PCOS based on literature review and expert opinion have been proposed5-8; however, these guidelines vary in recommendations for routine diagnostic steps to exclude mimickers of PCOS such as prolactinoma/pituitary adenoma and congenital adrenal hyperplasia (CAH)(Table). In 2009, an AE-PCOS task force suggested that routine testing of thyroid function and serum prolactin in the absence of additional clinical signs may not be necessary based on the low prevalence of thyroid disorders and hyperprolactinemia in patients presenting with hyperandrogenism.6 In 2013, the Endocrine Society’s (ENDO) clinical guideline, however, recommended routine measurement of serum thyroid-stimulating hormone (TSH) to exclude thyroid disease and serum prolactin to exclude hyperprolactinemia in all women before making a diagnosis of PCOS.7 In 2015, the AE-PCOS collaborated with the American Association of Clinical Endocrinologists (AACE) and American College of Endocrinology to publish an updated guideline for best practices, which was consistent with the prior AE-PCOS recommendation in 2009 for routine screening including to test 17-hydroxyprogesterone to exclude nonclassical CAH.8
Importantly, these recommendations for routine testing for mimickers of PCOS are based on the rare prevalence of these etiologies in multiple studies of women presenting for hyperandrogenism. One study included 873 women presenting to an academic reproductive endocrine clinic for evaluation of symptoms potentially related to androgen excess. In addition to cutaneous manifestations of hirsutism, acne, and alopecia, the study also included women presenting with oligomenorrhea/amenorrhea, ovulatory dysfunction, and even virilization.10 A second study included 950 women presenting to academic endocrine departments with hirsutism, acne, or androgenic alopecia.11 Both studies defined hirsutism as having a modified Ferriman-Gallwey score of 6 or greater. Both studies also only measured serum prolactin or TSH when clinically indicated (ie, patients with ovulatory dysfunction).10,11
The diagnostic yield of tests for mimickers of PCOS was exceedingly low in both studies. For example, of the patients evaluated, only 0.4% to 0.7% had thyroid dysfunction, 0% to 0.3% had hyperprolactinemia, 0.2% had androgen-secreting neoplasms, 2.1% to 4.3% had nonclassical CAH, 0.7% had CAH, and 3.8% had HAIR-AN (hyperandrogenism, insulin resistance, and acanthosis nigricans) syndrome.10,11 Because patients in both studies were only tested for hyperprolactinemia and thyroid dysfunction when clinically indicated, it is probable that routine screening without clinical indication would result in even lower yields.
Given the increasing importance of high-value, cost-conscious care,12 clinicians must consider the costs associated with testing in the face of low pretest probability. Although some studies have examined the cost-effectiveness of fertility treatments in PCOS,13,14 no studies have examined the cost-effectiveness of diagnostic strategies for PCOS. Cost-effectiveness studies are emerging to provide important guidance on high-value, cost-conscious diagnostic evaluation and monitoring15 and are much needed in dermatology.16,17
In the case of PCOS, the costs of some diagnostic tests are relatively low. For example, based on estimates from Healthcare Bluebook,9 serum TSH and prolactin tests in San Francisco, California, are $44 and $51, respectively. However, the cumulative costs for even the most stringent routine workup for PCOS recommended in the AACE/AE-PCOS guideline consisting of a free testosterone measurement, 17-hydroxyprogesterone, and transvaginal ultrasound would still cost a total of $516. Additional TSH and prolactin tests recommended by ENDO would increase the cost of PCOS testing by approximately 18%. Routine testing for additional serum androgens—dehydroepiandrosterone sulfate (DHEA-S) and androstenedione—would further increase this amount by an additional $134 to a total cost of $745. The ENDO guideline only recommends DHEA-S testing to assist in the diagnosis of an androgen-secreting tumor when signs of virilization are present, while the AACE/AE-PCOS guideline discourages routine testing for DHEA-S and androstenedione based on the low frequency of cases in which these androgens are elevated in isolation.7,8
Although the selection of tests influences total cost, the setting of tests (ie, hospitals, physician offices, independent test settings) also can contribute to wide variations in cost. For example, Healthcare Bluebook’s estimates for transvaginal ultrasound in Chicago, Illinois, range from $236 to more than $740.9 When the separate physician visit fees are included, the total cost of a routine diagnostic evaluation of a patient with acne or hirsutism concerning for PCOS is not trivial.
Large national clinical registries and formal cost-effectiveness analyses are necessary to shed light on this issue, but it is clear that clinicians should rely on their clinical judgment when ordering laboratory tests in the evaluation for PCOS given the apparent low yield of routine screening for PCOS mimickers in the absence of clinical indications. For example, a TSH would not be warranted in a patient without evidence of thyroid dysfunction (ie, weight gain, fatigue, constipation, menstrual irregularities). Similarly, clinicians should routinely consider the principle of high-value care: whether the results of a test will change management of the patient. For example, a woman with amenorrhea and severe acne who already meets diagnostic criteria for PCOS would benefit from a combined oral contraceptive for both acne and endometrial protection. An ovarian ultrasound may not be needed to confirm the diagnosis unless there is suspicion for an ovarian condition other than PCOS causing the symptoms.
Finally, clinicians should discuss testing options and involve patients in decisions around testing. Although PCOS treatments generally target individual symptoms rather than the syndrome as a whole, confirmation of a PCOS diagnosis importantly informs women of their risk for cardiovascular and metabolic disease. The ENDO recommends screening for impaired glucose tolerance, type 2 diabetes mellitus, obesity, family history of early cardiovascular disease, tobacco use, hypertension, dyslipidemia, and obstructive sleep apnea in all women with PCOS, including nonobese patients.7 Ongoing efforts to gain and understand evidence to support high-value, cost-conscious care should be prioritized and kept in balance with shared decision-making in individual patients suspected of having PCOS.
- March WA, Moore VM, Willson KJ, et al. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25:544-551.
- Sivayoganathan D, Maruthini D, Glanville JM, et al. Full investigation of patients with polycystic ovary syndrome (PCOS) presenting to four different clinical specialties reveals significant differences and undiagnosed morbidity. Hum Fertil. 2011;14:261-265.
- Schmidt TH, Shinkai K. Evidence-based approach to cutaneous hyperandrogenism in women. J Am Acad Dermatol. 2015;73:672-690.
- Schmidt TH, Khanijow K, Cedars MI, et al. Cutaneous findings and systemic associations in women with polycystic ovary syndrome. JAMA Dermatol. 2015;152:391-398.
- Escobar-Morreale HF, Carmina E, Dewailly D, et al. Epidemiology, diagnosis and management of hirsutism: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update. 2012;18:146-170.
- Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456-488.
- Legro RS, Arslanian SA, Ehermann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline [published online October 22, 2013]. J Clin Endocrinol Metab. 2013;98:4565-4592.
- Goodman NF, Cobin RH, Futterweit W, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 1. Endocr Pract. 2015;21:1291-1300.
- Healthcare Bluebook. https://healthcarebluebook.com. Accessed June 13, 2016.
- Azziz R, Sanchez LA, Knochenhauer ES, et al. Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab. 2004;89:453-462.
- Carmina E, Rosato F, Jannì A, et al. Relative prevalence of different androgen excess disorders in 950 women referred because of clinical hyperandrogenism [published online November 1, 2005]. J Clin Endocrinol Metab. 2006;91:2-6.
- Owens DK, Qaseem A, Chou R, et al. High-value, cost-conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med. 2011;154:174-180.
- Nahuis MJ, Oude Lohuis E, Kose N, et al. Long-term follow-up of laparoscopic electrocautery of the ovaries versus ovulation induction with recombinant FSH in clomiphene citrate-resistant women with polycystic ovary syndrome: an economic evaluation. Hum Reprod Oxf Engl. 2012;27:3577-3582.
- Moolenaar LM, Nahuis MJ, Hompes PG, et al. Cost-effectiveness of treatment strategies in women with PCOS who do not conceive after six cycles of clomiphene citrate. Reprod Biomed Online. 2014;28:606-613.
- Chogle A, Saps M. Yield and cost of performing screening tests for constipation in children. Can J Gastroenterol. 2013;27:E35-E38.
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory monitoring during isotretinoin therapy for acne: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:35-44.
- Shinkai K, McMichael A, Linos E. Isotretinoin laboratory test monitoring—a call to decrease testing in an era of high-value, cost-conscious care. JAMA Dermatol. 2016;152:17-19.
The adult female patient presenting with severe acne vulgaris may raise special diagnostic concerns, including consideration of an underlying hormonal disorder. Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in women of reproductive age with an estimated prevalence as high as 12%.1 Many women with undiagnosed PCOS may be referred to dermatologists for evaluation of its cutaneous manifestations of hyperandrogenism including acne, hirsutism, and androgenic alopecia.2 Given the prevalence of PCOS and its long-term health implications, dermatologists can play an important role in the initial evaluation of these patients. Acne and androgenic alopecia, however, are quite common, and in the absence of red flags such as menstrual irregularities, virilization, visual field deficits, or signs of Cushing syndrome,3 clinicians must decide when to pursue limited versus comprehensive evaluation.
Despite being common in patients with PCOS, a recent study suggests that acne is an unreliable marker of biochemical hyperandrogenism, and specific features of acne (ie, lesion counts, lesional types, distribution) cannot reliably discriminate women who meet PCOS diagnostic criteria from those who do not.4 Similarly, the study found that androgenic alopecia was not associated with biochemical hyperandrogenism and was no more common in women with PCOS than women of similar age in a high-risk population. Unlike acne and androgenic alopecia, however, the study identified hirsutism, especially truncal hirsutism, as a reliable indicator of hyperandrogenemia and PCOS. Hirsutism also is associated with metabolic sequelae of PCOS. These findings suggest that hirsutism, but not acne or androgenic alopecia, in a female of reproductive age warrants a workup for PCOS.4 This report is consistent with a recommendation from the Androgen Excess and Polycystic Ovary Syndrome Society (AE-PCOS) to pursue a diagnostic evaluation in any woman presenting with hirsutism.5 Acanthosis nigricans also was found to be a reliable indicator of hyperandrogenemia, PCOS, and associated metabolic derangement. Thus, although recent evidence indicates that acne as an isolated cutaneous finding does not warrant further diagnostic evaluation, acne in the setting of hirsutism, acanthosis nigricans, menstrual irregularities, or additional specific signs of endocrine dysregulation should prompt focused workup.4
Multiple clinical practice guidelines for the evaluation of hirsutism and PCOS based on literature review and expert opinion have been proposed5-8; however, these guidelines vary in recommendations for routine diagnostic steps to exclude mimickers of PCOS such as prolactinoma/pituitary adenoma and congenital adrenal hyperplasia (CAH)(Table). In 2009, an AE-PCOS task force suggested that routine testing of thyroid function and serum prolactin in the absence of additional clinical signs may not be necessary based on the low prevalence of thyroid disorders and hyperprolactinemia in patients presenting with hyperandrogenism.6 In 2013, the Endocrine Society’s (ENDO) clinical guideline, however, recommended routine measurement of serum thyroid-stimulating hormone (TSH) to exclude thyroid disease and serum prolactin to exclude hyperprolactinemia in all women before making a diagnosis of PCOS.7 In 2015, the AE-PCOS collaborated with the American Association of Clinical Endocrinologists (AACE) and American College of Endocrinology to publish an updated guideline for best practices, which was consistent with the prior AE-PCOS recommendation in 2009 for routine screening including to test 17-hydroxyprogesterone to exclude nonclassical CAH.8

Importantly, these recommendations for routine testing for mimickers of PCOS are based on the rare prevalence of these etiologies in multiple studies of women presenting for hyperandrogenism. One study included 873 women presenting to an academic reproductive endocrine clinic for evaluation of symptoms potentially related to androgen excess. In addition to cutaneous manifestations of hirsutism, acne, and alopecia, the study also included women presenting with oligomenorrhea/amenorrhea, ovulatory dysfunction, and even virilization.10 A second study included 950 women presenting to academic endocrine departments with hirsutism, acne, or androgenic alopecia.11 Both studies defined hirsutism as having a modified Ferriman-Gallwey score of 6 or greater. Both studies also only measured serum prolactin or TSH when clinically indicated (ie, patients with ovulatory dysfunction).10,11
The diagnostic yield of tests for mimickers of PCOS was exceedingly low in both studies. For example, of the patients evaluated, only 0.4% to 0.7% had thyroid dysfunction, 0% to 0.3% had hyperprolactinemia, 0.2% had androgen-secreting neoplasms, 2.1% to 4.3% had nonclassical CAH, 0.7% had CAH, and 3.8% had HAIR-AN (hyperandrogenism, insulin resistance, and acanthosis nigricans) syndrome.10,11 Because patients in both studies were only tested for hyperprolactinemia and thyroid dysfunction when clinically indicated, it is probable that routine screening without clinical indication would result in even lower yields.
Given the increasing importance of high-value, cost-conscious care,12 clinicians must consider the costs associated with testing in the face of low pretest probability. Although some studies have examined the cost-effectiveness of fertility treatments in PCOS,13,14 no studies have examined the cost-effectiveness of diagnostic strategies for PCOS. Cost-effectiveness studies are emerging to provide important guidance on high-value, cost-conscious diagnostic evaluation and monitoring15 and are much needed in dermatology.16,17
In the case of PCOS, the costs of some diagnostic tests are relatively low. For example, based on estimates from Healthcare Bluebook,9 serum TSH and prolactin tests in San Francisco, California, are $44 and $51, respectively. However, the cumulative costs for even the most stringent routine workup for PCOS recommended in the AACE/AE-PCOS guideline consisting of a free testosterone measurement, 17-hydroxyprogesterone, and transvaginal ultrasound would still cost a total of $516. Additional TSH and prolactin tests recommended by ENDO would increase the cost of PCOS testing by approximately 18%. Routine testing for additional serum androgens—dehydroepiandrosterone sulfate (DHEA-S) and androstenedione—would further increase this amount by an additional $134 to a total cost of $745. The ENDO guideline only recommends DHEA-S testing to assist in the diagnosis of an androgen-secreting tumor when signs of virilization are present, while the AACE/AE-PCOS guideline discourages routine testing for DHEA-S and androstenedione based on the low frequency of cases in which these androgens are elevated in isolation.7,8
Although the selection of tests influences total cost, the setting of tests (ie, hospitals, physician offices, independent test settings) also can contribute to wide variations in cost. For example, Healthcare Bluebook’s estimates for transvaginal ultrasound in Chicago, Illinois, range from $236 to more than $740.9 When the separate physician visit fees are included, the total cost of a routine diagnostic evaluation of a patient with acne or hirsutism concerning for PCOS is not trivial.
Large national clinical registries and formal cost-effectiveness analyses are necessary to shed light on this issue, but it is clear that clinicians should rely on their clinical judgment when ordering laboratory tests in the evaluation for PCOS given the apparent low yield of routine screening for PCOS mimickers in the absence of clinical indications. For example, a TSH would not be warranted in a patient without evidence of thyroid dysfunction (ie, weight gain, fatigue, constipation, menstrual irregularities). Similarly, clinicians should routinely consider the principle of high-value care: whether the results of a test will change management of the patient. For example, a woman with amenorrhea and severe acne who already meets diagnostic criteria for PCOS would benefit from a combined oral contraceptive for both acne and endometrial protection. An ovarian ultrasound may not be needed to confirm the diagnosis unless there is suspicion for an ovarian condition other than PCOS causing the symptoms.
Finally, clinicians should discuss testing options and involve patients in decisions around testing. Although PCOS treatments generally target individual symptoms rather than the syndrome as a whole, confirmation of a PCOS diagnosis importantly informs women of their risk for cardiovascular and metabolic disease. The ENDO recommends screening for impaired glucose tolerance, type 2 diabetes mellitus, obesity, family history of early cardiovascular disease, tobacco use, hypertension, dyslipidemia, and obstructive sleep apnea in all women with PCOS, including nonobese patients.7 Ongoing efforts to gain and understand evidence to support high-value, cost-conscious care should be prioritized and kept in balance with shared decision-making in individual patients suspected of having PCOS.
The adult female patient presenting with severe acne vulgaris may raise special diagnostic concerns, including consideration of an underlying hormonal disorder. Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in women of reproductive age with an estimated prevalence as high as 12%.1 Many women with undiagnosed PCOS may be referred to dermatologists for evaluation of its cutaneous manifestations of hyperandrogenism including acne, hirsutism, and androgenic alopecia.2 Given the prevalence of PCOS and its long-term health implications, dermatologists can play an important role in the initial evaluation of these patients. Acne and androgenic alopecia, however, are quite common, and in the absence of red flags such as menstrual irregularities, virilization, visual field deficits, or signs of Cushing syndrome,3 clinicians must decide when to pursue limited versus comprehensive evaluation.
Despite being common in patients with PCOS, a recent study suggests that acne is an unreliable marker of biochemical hyperandrogenism, and specific features of acne (ie, lesion counts, lesional types, distribution) cannot reliably discriminate women who meet PCOS diagnostic criteria from those who do not.4 Similarly, the study found that androgenic alopecia was not associated with biochemical hyperandrogenism and was no more common in women with PCOS than women of similar age in a high-risk population. Unlike acne and androgenic alopecia, however, the study identified hirsutism, especially truncal hirsutism, as a reliable indicator of hyperandrogenemia and PCOS. Hirsutism also is associated with metabolic sequelae of PCOS. These findings suggest that hirsutism, but not acne or androgenic alopecia, in a female of reproductive age warrants a workup for PCOS.4 This report is consistent with a recommendation from the Androgen Excess and Polycystic Ovary Syndrome Society (AE-PCOS) to pursue a diagnostic evaluation in any woman presenting with hirsutism.5 Acanthosis nigricans also was found to be a reliable indicator of hyperandrogenemia, PCOS, and associated metabolic derangement. Thus, although recent evidence indicates that acne as an isolated cutaneous finding does not warrant further diagnostic evaluation, acne in the setting of hirsutism, acanthosis nigricans, menstrual irregularities, or additional specific signs of endocrine dysregulation should prompt focused workup.4
Multiple clinical practice guidelines for the evaluation of hirsutism and PCOS based on literature review and expert opinion have been proposed5-8; however, these guidelines vary in recommendations for routine diagnostic steps to exclude mimickers of PCOS such as prolactinoma/pituitary adenoma and congenital adrenal hyperplasia (CAH)(Table). In 2009, an AE-PCOS task force suggested that routine testing of thyroid function and serum prolactin in the absence of additional clinical signs may not be necessary based on the low prevalence of thyroid disorders and hyperprolactinemia in patients presenting with hyperandrogenism.6 In 2013, the Endocrine Society’s (ENDO) clinical guideline, however, recommended routine measurement of serum thyroid-stimulating hormone (TSH) to exclude thyroid disease and serum prolactin to exclude hyperprolactinemia in all women before making a diagnosis of PCOS.7 In 2015, the AE-PCOS collaborated with the American Association of Clinical Endocrinologists (AACE) and American College of Endocrinology to publish an updated guideline for best practices, which was consistent with the prior AE-PCOS recommendation in 2009 for routine screening including to test 17-hydroxyprogesterone to exclude nonclassical CAH.8

Importantly, these recommendations for routine testing for mimickers of PCOS are based on the rare prevalence of these etiologies in multiple studies of women presenting for hyperandrogenism. One study included 873 women presenting to an academic reproductive endocrine clinic for evaluation of symptoms potentially related to androgen excess. In addition to cutaneous manifestations of hirsutism, acne, and alopecia, the study also included women presenting with oligomenorrhea/amenorrhea, ovulatory dysfunction, and even virilization.10 A second study included 950 women presenting to academic endocrine departments with hirsutism, acne, or androgenic alopecia.11 Both studies defined hirsutism as having a modified Ferriman-Gallwey score of 6 or greater. Both studies also only measured serum prolactin or TSH when clinically indicated (ie, patients with ovulatory dysfunction).10,11
The diagnostic yield of tests for mimickers of PCOS was exceedingly low in both studies. For example, of the patients evaluated, only 0.4% to 0.7% had thyroid dysfunction, 0% to 0.3% had hyperprolactinemia, 0.2% had androgen-secreting neoplasms, 2.1% to 4.3% had nonclassical CAH, 0.7% had CAH, and 3.8% had HAIR-AN (hyperandrogenism, insulin resistance, and acanthosis nigricans) syndrome.10,11 Because patients in both studies were only tested for hyperprolactinemia and thyroid dysfunction when clinically indicated, it is probable that routine screening without clinical indication would result in even lower yields.
Given the increasing importance of high-value, cost-conscious care,12 clinicians must consider the costs associated with testing in the face of low pretest probability. Although some studies have examined the cost-effectiveness of fertility treatments in PCOS,13,14 no studies have examined the cost-effectiveness of diagnostic strategies for PCOS. Cost-effectiveness studies are emerging to provide important guidance on high-value, cost-conscious diagnostic evaluation and monitoring15 and are much needed in dermatology.16,17
In the case of PCOS, the costs of some diagnostic tests are relatively low. For example, based on estimates from Healthcare Bluebook,9 serum TSH and prolactin tests in San Francisco, California, are $44 and $51, respectively. However, the cumulative costs for even the most stringent routine workup for PCOS recommended in the AACE/AE-PCOS guideline consisting of a free testosterone measurement, 17-hydroxyprogesterone, and transvaginal ultrasound would still cost a total of $516. Additional TSH and prolactin tests recommended by ENDO would increase the cost of PCOS testing by approximately 18%. Routine testing for additional serum androgens—dehydroepiandrosterone sulfate (DHEA-S) and androstenedione—would further increase this amount by an additional $134 to a total cost of $745. The ENDO guideline only recommends DHEA-S testing to assist in the diagnosis of an androgen-secreting tumor when signs of virilization are present, while the AACE/AE-PCOS guideline discourages routine testing for DHEA-S and androstenedione based on the low frequency of cases in which these androgens are elevated in isolation.7,8
Although the selection of tests influences total cost, the setting of tests (ie, hospitals, physician offices, independent test settings) also can contribute to wide variations in cost. For example, Healthcare Bluebook’s estimates for transvaginal ultrasound in Chicago, Illinois, range from $236 to more than $740.9 When the separate physician visit fees are included, the total cost of a routine diagnostic evaluation of a patient with acne or hirsutism concerning for PCOS is not trivial.
Large national clinical registries and formal cost-effectiveness analyses are necessary to shed light on this issue, but it is clear that clinicians should rely on their clinical judgment when ordering laboratory tests in the evaluation for PCOS given the apparent low yield of routine screening for PCOS mimickers in the absence of clinical indications. For example, a TSH would not be warranted in a patient without evidence of thyroid dysfunction (ie, weight gain, fatigue, constipation, menstrual irregularities). Similarly, clinicians should routinely consider the principle of high-value care: whether the results of a test will change management of the patient. For example, a woman with amenorrhea and severe acne who already meets diagnostic criteria for PCOS would benefit from a combined oral contraceptive for both acne and endometrial protection. An ovarian ultrasound may not be needed to confirm the diagnosis unless there is suspicion for an ovarian condition other than PCOS causing the symptoms.
Finally, clinicians should discuss testing options and involve patients in decisions around testing. Although PCOS treatments generally target individual symptoms rather than the syndrome as a whole, confirmation of a PCOS diagnosis importantly informs women of their risk for cardiovascular and metabolic disease. The ENDO recommends screening for impaired glucose tolerance, type 2 diabetes mellitus, obesity, family history of early cardiovascular disease, tobacco use, hypertension, dyslipidemia, and obstructive sleep apnea in all women with PCOS, including nonobese patients.7 Ongoing efforts to gain and understand evidence to support high-value, cost-conscious care should be prioritized and kept in balance with shared decision-making in individual patients suspected of having PCOS.
- March WA, Moore VM, Willson KJ, et al. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25:544-551.
- Sivayoganathan D, Maruthini D, Glanville JM, et al. Full investigation of patients with polycystic ovary syndrome (PCOS) presenting to four different clinical specialties reveals significant differences and undiagnosed morbidity. Hum Fertil. 2011;14:261-265.
- Schmidt TH, Shinkai K. Evidence-based approach to cutaneous hyperandrogenism in women. J Am Acad Dermatol. 2015;73:672-690.
- Schmidt TH, Khanijow K, Cedars MI, et al. Cutaneous findings and systemic associations in women with polycystic ovary syndrome. JAMA Dermatol. 2015;152:391-398.
- Escobar-Morreale HF, Carmina E, Dewailly D, et al. Epidemiology, diagnosis and management of hirsutism: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update. 2012;18:146-170.
- Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456-488.
- Legro RS, Arslanian SA, Ehermann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline [published online October 22, 2013]. J Clin Endocrinol Metab. 2013;98:4565-4592.
- Goodman NF, Cobin RH, Futterweit W, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 1. Endocr Pract. 2015;21:1291-1300.
- Healthcare Bluebook. https://healthcarebluebook.com. Accessed June 13, 2016.
- Azziz R, Sanchez LA, Knochenhauer ES, et al. Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab. 2004;89:453-462.
- Carmina E, Rosato F, Jannì A, et al. Relative prevalence of different androgen excess disorders in 950 women referred because of clinical hyperandrogenism [published online November 1, 2005]. J Clin Endocrinol Metab. 2006;91:2-6.
- Owens DK, Qaseem A, Chou R, et al. High-value, cost-conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med. 2011;154:174-180.
- Nahuis MJ, Oude Lohuis E, Kose N, et al. Long-term follow-up of laparoscopic electrocautery of the ovaries versus ovulation induction with recombinant FSH in clomiphene citrate-resistant women with polycystic ovary syndrome: an economic evaluation. Hum Reprod Oxf Engl. 2012;27:3577-3582.
- Moolenaar LM, Nahuis MJ, Hompes PG, et al. Cost-effectiveness of treatment strategies in women with PCOS who do not conceive after six cycles of clomiphene citrate. Reprod Biomed Online. 2014;28:606-613.
- Chogle A, Saps M. Yield and cost of performing screening tests for constipation in children. Can J Gastroenterol. 2013;27:E35-E38.
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory monitoring during isotretinoin therapy for acne: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:35-44.
- Shinkai K, McMichael A, Linos E. Isotretinoin laboratory test monitoring—a call to decrease testing in an era of high-value, cost-conscious care. JAMA Dermatol. 2016;152:17-19.
- March WA, Moore VM, Willson KJ, et al. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25:544-551.
- Sivayoganathan D, Maruthini D, Glanville JM, et al. Full investigation of patients with polycystic ovary syndrome (PCOS) presenting to four different clinical specialties reveals significant differences and undiagnosed morbidity. Hum Fertil. 2011;14:261-265.
- Schmidt TH, Shinkai K. Evidence-based approach to cutaneous hyperandrogenism in women. J Am Acad Dermatol. 2015;73:672-690.
- Schmidt TH, Khanijow K, Cedars MI, et al. Cutaneous findings and systemic associations in women with polycystic ovary syndrome. JAMA Dermatol. 2015;152:391-398.
- Escobar-Morreale HF, Carmina E, Dewailly D, et al. Epidemiology, diagnosis and management of hirsutism: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update. 2012;18:146-170.
- Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456-488.
- Legro RS, Arslanian SA, Ehermann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline [published online October 22, 2013]. J Clin Endocrinol Metab. 2013;98:4565-4592.
- Goodman NF, Cobin RH, Futterweit W, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 1. Endocr Pract. 2015;21:1291-1300.
- Healthcare Bluebook. https://healthcarebluebook.com. Accessed June 13, 2016.
- Azziz R, Sanchez LA, Knochenhauer ES, et al. Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab. 2004;89:453-462.
- Carmina E, Rosato F, Jannì A, et al. Relative prevalence of different androgen excess disorders in 950 women referred because of clinical hyperandrogenism [published online November 1, 2005]. J Clin Endocrinol Metab. 2006;91:2-6.
- Owens DK, Qaseem A, Chou R, et al. High-value, cost-conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med. 2011;154:174-180.
- Nahuis MJ, Oude Lohuis E, Kose N, et al. Long-term follow-up of laparoscopic electrocautery of the ovaries versus ovulation induction with recombinant FSH in clomiphene citrate-resistant women with polycystic ovary syndrome: an economic evaluation. Hum Reprod Oxf Engl. 2012;27:3577-3582.
- Moolenaar LM, Nahuis MJ, Hompes PG, et al. Cost-effectiveness of treatment strategies in women with PCOS who do not conceive after six cycles of clomiphene citrate. Reprod Biomed Online. 2014;28:606-613.
- Chogle A, Saps M. Yield and cost of performing screening tests for constipation in children. Can J Gastroenterol. 2013;27:E35-E38.
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory monitoring during isotretinoin therapy for acne: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:35-44.
- Shinkai K, McMichael A, Linos E. Isotretinoin laboratory test monitoring—a call to decrease testing in an era of high-value, cost-conscious care. JAMA Dermatol. 2016;152:17-19.