User login
› Screen for lower
extremity complications at every visit for all patients with a suspected or confirmed diagnosis of diabetes. A
› Consider implementing a risk-based referral system to connect primary screening with a specialist's care. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Foot ulcers and other lower-limb complications secondary to diabetes are common, complex, costly, and associated with increased morbidity and mortality.1-6 Unfortunately, patients often have difficulty recognizing the heightened risk status that accompanies the diagnosis of diabetes, particularly the substantial risk for lower limb complications.7 In addition, loss of protective sensation (LOPS) can render patients unable to recognize damage to their lower extremities, thus creating a cycle of tissue damage and other foot complications. Strong evidence suggests that consistent provision of foot-care services and preventive care can reduce amputations among patients with diabetes.7-9 However, routine foot examination and rapid risk stratification is often difficult to incorporate into busy primary care settings. Data suggest that the diabetic foot is adequately evaluated only 12% to 20% of the time.10
In response to the need for more consistent foot exams, an American Diabetes Association (ADA) task force lead by 2 of the authors of this article (AB and DA) created the Comprehensive Foot Examination and Risk Assessment.5 This set the standard for the detailed investigation of lower limb pathology by a specialist, but was not well suited for other practice settings, including primary care. One reason is that it would be difficult to complete the comprehensive examination during a typical 15-minute primary care office visit. In addition, certain examination parameters require the use of neurologic and vascular assessment equipment and training not available in all health care settings.11
With these thoughts in mind, we set out to develop an exam that could be done by a wide range of health care providers—one that takes substantially less time to complete than a comprehensive exam and eliminates common barriers to frequent assessment. The exam, which we’ll describe here, consists of 3 components: taking a patient history, performing a physical exam, and providing patient education. And best of all, it should only take 3 minutes.
The patient history (1 minute)
Patients may present with concerns about their feet, but may not be able to differentiate between benign and threatening symptoms. A thorough medical history can identify factors that may increase patients’ risk of developing lower-limb complications. Reviewing the patient’s medical history also can help guide the physical exam.
Review the patient’s diabetic history, blood glucose control, and previous diabetic complications. Ask patients about their history of peripheral vascular disease, quality of peripheral protective sensation, and previous lower-limb interventions and operations (TABLE 15,12). Patients with diabetes and suboptimal glycemic control have an increased risk for LOPS, chronic and recalcitrant ulcers, and wound infections.2 Additionally, patients with diabetes and a previous lower extremity amputation are at high risk for reulceration.5,12 Lastly, nicotine use and smoking are common pathogenic risk factors that contribute to peripheral artery disease (PAD).13
Physical examination (1 minute)
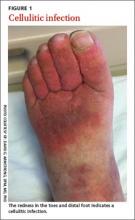
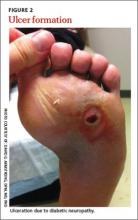
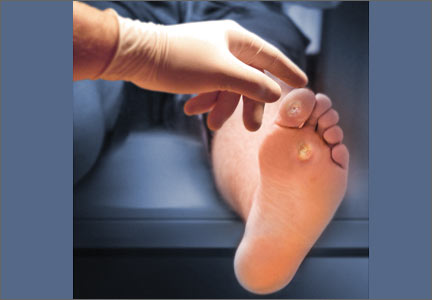
Careful inspection of the feet should be performed at every visit for patients with confirmed or suspected diabetes. Because up to 50% of patients with significant sensory loss due to neuropathy may be completely asymptomatic,14 failing to search for early signs of infection (FIGURE 1), skin breakdown, ulcer formation (FIGURE 2), skin temperature changes, and inadequate vascular perfusion may allow complications to develop.5 TABLE 25,15,16 outlines the essential components—dermatologic, neurologic, musculoskeletal, and vascular—of a rapid lower limb physical exam.
The dermatologic exam. This serves as a barometer for early intervention, and often results in a limb-saving referral to a specialist. It should begin with a global inspection for discolorations, calluses, wounds, fissures, macerations, nail dystrophy, or paronychia.5 Skin discoloration or loss of hair growth may be the first signs of vascular insufficiency, while calluses and hypertrophic skin often are precursors to ulcers.5,17-19 Inspection of the toes should include a search for fungal, ingrown, or elongated nails. Carefully examine the areas between the toes, where deeper lesions may go unnoticed.5
The neurologic exam. Without protective sensation, patients with neuropathy are at a heightened risk of unrecognized injury and are unlikely to mention their deformities to medical staff.20-23 Consequently, skin deterioration may unknowingly progress to ulceration that requires extensive medical intervention or amputation.
Neuropathic LOPS is easily detectable, yet it is linked to at least 75% of all nontraumatic diabetic amputations.20-23 Adiminished vibratory perception threshold (VPT) is one of the earliest indicators of neuropathic LOPS and is the best predictor of long-term lower extremity complications.1,24,25 However, VPT devices are expensive and time-consuming to operate, and they require training to ensure proper use. The Semmes-Weinstein monofilament is a well-documented alternative to VPT for predicting ulcer risk26-28 and has long been advocated as an essential component of a thorough foot exam.5 The 128 Hz tuning fork is another regularly used alternative.5 However, physicians would need to purchase one of these devices and receive training on how to use it, and, in the case of the monofilament, to regularly stock replacements to maintain accurate results.16
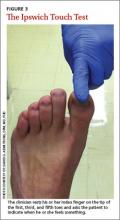
The Ipswich Touch Test (IpTT) is an alternative neurologic test that requires only the physician’s index finger. During the IpTT, the physician instructs the patient to close his or her eyes while the physician lightly rests his or her finger on each of the patient’s first, third, and fifth toes for 1 to 2 seconds (FIGURE 3). Patients are instructed to respond with a “yes” when they feel the physician’s touch. In a head-to-head trial, diagnostic results of the IpTT directly paralleled those of the monofilament in detecting LOPS; IpTT was also equally sensitive and specific (k=.88, indicating almost perfect agreement; P<.0001).29 The IpTT’s use of only 6 palpation points, constant availability, and accuracy make it a first-line neurologic test for rapidly screening the feet of a patient with diabetes.
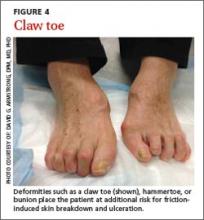
Neuromuscular/musculoskeletal exam. Neuromuscular disturbances, such as a reduction in the strength of dorsiflexion and plantar flexion, may indicate a complicated neurologic compromise.5 In addition to being aesthetically problematic, musculoskeletal deformities such as a hammer toe, claw toe (FIGURE 4), or bunion can cause significant pain and/or gait disturbance, and can increase patients’ risk for ulceration.30 These deformities also may compromise patients’ general health and grossly escalate their risk of falls and resultant injuries.5,31 Therefore, patients who present with previously unreported musculoskeletal deformities should be referred to a specialist.31
Also screen patients for Charcot neuroarthropathy (FIGURE 5), a devastating complication that classically presents as a hot, red, swollen foot; the redness resolves upon elevation.32 Charcot neuroarthropathy is hypothesized to be a dysregulation of normal bone metabolism typically occurring secondary to diabetic neuropathy and repetitive minor trauma.33,34 This dysregulation leads to joint instability and disorganization of normal midfoot bone architecture.31,32 Charcot neuroarthropathy is an urgent pathology that requires management by a foot specialist.35
Vascular exam. PAD is particularly common in patients with diabetes and contributes to the development of impaired healing in up to half of foot ulcers.13,18,36-39 Bilateral femoral, popliteal, posterior tibial, or dorsalis pedis pulses should be assessed by palpation; a diminished or absent pulse is a key indicator of vascular compromise.40,41 An integrated care approach between foot specialists and vascular surgeons results in optimal treatment.
Patient education (1 minute)
It is imperative to include patients in their treatment process to reduce the likelihood of complications and, ultimately, decrease the incidence of amputations.12,42 Patient education improves patients’ self-reported home care behaviors, even at the most fundamental levels.43,44 TABLE 35,15,45 lists topics to cover during patient education.
Patients’ lack of understanding about self-care for diabetes is a common barrier to prevention.23 El-Nahas et al46 found a lack of appropriate education regarding diabetes was a factor in more than 90% of recurrent ulcers, which emphasizes the need for repeated education for at-risk patients.47,48 Involve all levels of medical staff in the effort to educate patients on the importance of foot screenings, both at home and in-office. Even with proper patient education, many patients may be in various stages of coping with this all-consuming yet frequently asymptomatic condition, which makes the need for repeated patient education even more critical.
Who to refer, and when
After completing the 3-minute foot exam, create a treatment and follow-up plan, focusing on the need for referral to a specialist. TABLE 4 outlines suggested indications, priorities, and timelines for referral based on ADA guidelines.5 It incorporates the ADA’s patient risk categories (very low, low, moderate, and high risk) and also provides a recommended frequency for patient follow-ups.
Care for patients with lower extremity complications of diabetes mellitus is time-consuming and expensive. The brief exam described here can help you to rapidly identify patients at risk for these complications and prompt you to provide timely referrals to appropriate specialists.
CORRESPONDENCE
David G. Armstrong, DPM, MD, PhD, Professor, Department of Surgery, Director, Southern Arizona Limb Salvage Alliance (SALSA), 1501 N. Campbell Avenue, Tucson, AZ 85724-5072; armstrong@usa.net
1. Shearer A, Scuffham P, Gordois A, et al. Predicted costs and outcomes from reduced vibration detection in people with diabetes in the U.S. Diabetes Care. 2003;26:2305-2310.
2. Apelqvist J, Larsson J. What is the most effective way to reduce incidence of amputation in the diabetic foot? Diabetes Metab Res Rev. 2000;16 suppl 1:S75-S83.
3. Armstrong DG, Kanda VA, Lavery LA, et al. Mind the gap: disparity between research funding and costs of care for diabetic foot ulcers. Diabetes Care. 2013;36:1815-1817.
4. Driver VR, Fabbi M, Lavery LA, et al. The costs of diabetic foot: the economic case for the limb salvage team. J Vasc Surg. 2010;52(3 suppl):17S-22S.
5. Boulton AJ, Armstrong DG, Albert SF, et al; American Diabetes Association; American Association of Clinical Endocrinologists. Comprehensive foot examination and risk assessment: a report of the Task Force of the Foot Care Interest Group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31:1679-1685.
6. American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37 suppl 1:S14-S80.
7. Sloan FA, Feinglos MN, Grossman DS. Receipt of care and reduction of lower extremity amputations in a nationally representative sample of U.S. Elderly. Health Serv Res. 2010;45(6 pt 1):1740-1762.
8. Carls GS, Gibson TB, Driver VR, et al. The economic value of specialized lower-extremity medical care by podiatric physicians in the treatment of diabetic foot ulcers. J Am Podiatr Med Assoc. 2011;101:93-115.
9. McCabe CJ, Stevenson RC, Dolan AM. Evaluation of a diabetic foot screening and protection programme. Diabet Med. 1998;15:80-84.
10. Bailey TS, Yu HM, Rayfield EJ. Patterns of foot examination in a diabetes clinic. Am J Med. 1985;78:371-374.
11. Chin MH, Cook S, Jin L, et al. Barriers to providing diabetes care in community health centers. Diabetes Care. 2001;24:268-274.
12. Abbott CA, Carrington AL, Ashe H, et al; North-West Diabetes Foot Care Study. The North-West Diabetes Foot Care Study: incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohort. Diabet Med. 2002;19:377-384.
13. Fowkes FG, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329-1340.
14. Boulton A, Vinik AI, Arezzo JC, et al; American Diabetes Association. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes. 2005;28:956-962.
15. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217-228.
16. Pham H, Armstrong DG, Harvey C, et al. Screening techniques to identify people at high risk for diabetic foot ulceration: a prospective multicenter trial. Diabetes Care. 2000;23:606-611.
17. Marso SP, Hiatt WR. Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol. 2006;47:921-929.
18. American Diabetes Association. Peripheral arterial disease in people with diabetes. JAPMA. 2005;95:309-319.
19. Pataky Z, Golay A, Faravel L, et al. The impact of callosities on the magnitude and duration of plantar pressure in patients with diabetes mellitus. A callus may cause 18,600 kilograms of excess plantar pressure per day. Diabetes Metab. 2002;28: 356-361.
20. Holzer SE, Camerota A, Martens L, et al. Costs and duration of care for lower extremity ulcers in patients with diabetes. Clin Ther. 1998;20:169-181.
21. Boulton AJ, Gries FA, Jervell JA. Guidelines for the diagnosis and outpatient management of diabetic peripheral neuropathy. Diabet Med. 1998;15:508-514.
22. Malay DS, Margolis DJ, Hoffstad OJ, et al. The incidence and risks of failure to heal after lower extremity amputation for the treatment of diabetic neuropathic foot ulcer. J Foot Ankle Surg. 2006;45:366-374.
23. van Houtum WH. Barriers to implementing foot care. Diabetes Metab Res Rev. 2012;28 suppl 1:112-115.
24. Jayaprakash P, Bhansali A, Bhansali S, et al. Validation of bedside methods in evaluation of diabetic peripheral neuropathy. Indian J Med Res. 2011;133:645-649.
25. Young MJ, Breddy JL, Veves A, et al. The prediction of diabetic neuropathic foot ulceration using vibration perception thresholds. A prospective study. Diabetes Care. 1994;17:557-560.
26. Leese GP, Reid F, Green V, et al. Stratification of foot ulcer risk in patients with diabetes: a population-based study. Int J Clin Pract. 2006;60:541-545.
27. Adler AI, Boyko EJ, Ahroni JH, et al. Risk factors for diabetic peripheral sensory neuropathy. Results of the Seattle Prospective Diabetic Foot Study. Diabetes Care. 1997;20:1162-1167.
28. Armstrong DG, Lavery LA, Vela SA, et al. Choosing a practical screening instrument to identify patients at risk for diabetic foot ulceration. Arch Intern Med. 1998;158:289-292.
29. Rayman G, Vas PR, Baker N, et al. The Ipswich Touch Test: a simple and novel method to identify inpatients with diabetes at risk of foot ulceration. Diabetes Care. 2011;34:1517-1518.
30. Lavery LA, Armstrong DG, Vela SA, et al. Practical criteria for screening patients at high risk for diabetic foot ulceration. Arch Intern Med. 1998;158:157-162.
31. Frykberg RG, Zgonis T, Armstrong DG, et al; American College of Foot and Ankle Surgeons. Diabetic foot disorders. A clinical practice guideline (2006 revision). J Foot Ankle Surg. 2006;45(5 suppl):S1-S66.
32. Nielson DL, Armstrong DG. The natural history of Charcot’s neuroarthropathy. Clin Podiatr Med Surg. 2008;25:53-62,vi.
33. Jeffcoate W, Lima J, Nobrega L. The Charcot foot. Diabet Med. 2000;17:253-258.
34. Blume PA, Sumpio B, Schmidt B, et al. Charcot neuroarthropathy of the foot and ankle: diagnosis and management strategies. Clin Podiatr Med Surg. 2014;31:151-172.
35. Petrova NL, Edmonds ME. Medical management of Charcot arthropathy. Diabetes Obes Metab. 2012;15:193-197.
36. Prompers L, Huijberts M, Apelqvist J, et al. Delivery of care to diabetic patients with foot ulcers in daily practice: results of the Eurodiale Study, a prospective cohort study. Diabet Med. 2008;25:700-707.
37. Armstrong DG, Bharara M, White M, et al. The impact and outcomes of establishing an integrated interdisciplinary surgical team to care for the diabetic foot. Diabetes Metab Res Rev. 2012;28:514-518.
38. Rogers LC, Andros G, Caporusso J, et al. Toe and flow: essential components and structure of the amputation prevention team. J Vasc Surg. 2010;52:23S-27S.
39. Mills JL Sr, Conte MS, Armstrong DG, et al; Society for Vascular Surgery Lower Extremity Guidelines Committee. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg. 2014;59:220-34.e1-2.
40. Khan NA, Rahim SA, Anand SS, et al. Does the clinical examination predict lower extremity peripheral arterial disease? JAMA. 2006;295:536-546.
41. Sumpio BE, Lee T, Blume PA. Vascular evaluation and arterial reconstruction of the diabetic foot. Clin Podiatr Med Surg. 2003;20:689-708.
42. Dorresteijn JAN, Valk GD. Patient education for preventing diabetic foot ulceration. Diabetes Metab Res Rev. 2012;28 Suppl 1:101-106.
43. Lincoln NB, Radford KA, Game FL, et al. Education for secondary prevention of foot ulcers in people with diabetes: a randomised controlled trial. Diabetologia. 2008;51:1954-1961.
44. McMurray SD, Johnson G, Davis S, et al. Diabetes education and care management significantly improve patient outcomes in the dialysis unit. Am J Kidney Dis. 2002;40:566-575.
45. Armstrong DG, Lavery LA. Diabetic foot ulcers: prevention, diagnosis and classification. Am Fam Physician. 1998;57:1325-1332,1337-1338.
46. El-Nahas MR, Gawish HMS, Tarshoby MM, et al. The prevalence of risk factors for foot ulceration in Egyptian diabetic patients. Practical Diabetes Int. 2008;25:362-366.
47. Hämäläinen H, Rönnemaa T, Toikka T, et al. Long-term effects of one year of intensified podiatric activities on foot-care knowledge and self-care habits in patients with diabetes. Diabetes Educ. 1998;24:734-740.
48. Rönnemaa T, Hämäläinen H, Toikka T, et al. Evaluation of the impact of podiatrist care in the primary prevention of foot problems in diabetic subjects. Diabetes Care. 1997;20:1833-1837.
› Screen for lower
extremity complications at every visit for all patients with a suspected or confirmed diagnosis of diabetes. A
› Consider implementing a risk-based referral system to connect primary screening with a specialist's care. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Foot ulcers and other lower-limb complications secondary to diabetes are common, complex, costly, and associated with increased morbidity and mortality.1-6 Unfortunately, patients often have difficulty recognizing the heightened risk status that accompanies the diagnosis of diabetes, particularly the substantial risk for lower limb complications.7 In addition, loss of protective sensation (LOPS) can render patients unable to recognize damage to their lower extremities, thus creating a cycle of tissue damage and other foot complications. Strong evidence suggests that consistent provision of foot-care services and preventive care can reduce amputations among patients with diabetes.7-9 However, routine foot examination and rapid risk stratification is often difficult to incorporate into busy primary care settings. Data suggest that the diabetic foot is adequately evaluated only 12% to 20% of the time.10
In response to the need for more consistent foot exams, an American Diabetes Association (ADA) task force lead by 2 of the authors of this article (AB and DA) created the Comprehensive Foot Examination and Risk Assessment.5 This set the standard for the detailed investigation of lower limb pathology by a specialist, but was not well suited for other practice settings, including primary care. One reason is that it would be difficult to complete the comprehensive examination during a typical 15-minute primary care office visit. In addition, certain examination parameters require the use of neurologic and vascular assessment equipment and training not available in all health care settings.11
With these thoughts in mind, we set out to develop an exam that could be done by a wide range of health care providers—one that takes substantially less time to complete than a comprehensive exam and eliminates common barriers to frequent assessment. The exam, which we’ll describe here, consists of 3 components: taking a patient history, performing a physical exam, and providing patient education. And best of all, it should only take 3 minutes.
The patient history (1 minute)
Patients may present with concerns about their feet, but may not be able to differentiate between benign and threatening symptoms. A thorough medical history can identify factors that may increase patients’ risk of developing lower-limb complications. Reviewing the patient’s medical history also can help guide the physical exam.
Review the patient’s diabetic history, blood glucose control, and previous diabetic complications. Ask patients about their history of peripheral vascular disease, quality of peripheral protective sensation, and previous lower-limb interventions and operations (TABLE 15,12). Patients with diabetes and suboptimal glycemic control have an increased risk for LOPS, chronic and recalcitrant ulcers, and wound infections.2 Additionally, patients with diabetes and a previous lower extremity amputation are at high risk for reulceration.5,12 Lastly, nicotine use and smoking are common pathogenic risk factors that contribute to peripheral artery disease (PAD).13
Physical examination (1 minute)
Careful inspection of the feet should be performed at every visit for patients with confirmed or suspected diabetes. Because up to 50% of patients with significant sensory loss due to neuropathy may be completely asymptomatic,14 failing to search for early signs of infection (FIGURE 1), skin breakdown, ulcer formation (FIGURE 2), skin temperature changes, and inadequate vascular perfusion may allow complications to develop.5 TABLE 25,15,16 outlines the essential components—dermatologic, neurologic, musculoskeletal, and vascular—of a rapid lower limb physical exam.
The dermatologic exam. This serves as a barometer for early intervention, and often results in a limb-saving referral to a specialist. It should begin with a global inspection for discolorations, calluses, wounds, fissures, macerations, nail dystrophy, or paronychia.5 Skin discoloration or loss of hair growth may be the first signs of vascular insufficiency, while calluses and hypertrophic skin often are precursors to ulcers.5,17-19 Inspection of the toes should include a search for fungal, ingrown, or elongated nails. Carefully examine the areas between the toes, where deeper lesions may go unnoticed.5
The neurologic exam. Without protective sensation, patients with neuropathy are at a heightened risk of unrecognized injury and are unlikely to mention their deformities to medical staff.20-23 Consequently, skin deterioration may unknowingly progress to ulceration that requires extensive medical intervention or amputation.
Neuropathic LOPS is easily detectable, yet it is linked to at least 75% of all nontraumatic diabetic amputations.20-23 Adiminished vibratory perception threshold (VPT) is one of the earliest indicators of neuropathic LOPS and is the best predictor of long-term lower extremity complications.1,24,25 However, VPT devices are expensive and time-consuming to operate, and they require training to ensure proper use. The Semmes-Weinstein monofilament is a well-documented alternative to VPT for predicting ulcer risk26-28 and has long been advocated as an essential component of a thorough foot exam.5 The 128 Hz tuning fork is another regularly used alternative.5 However, physicians would need to purchase one of these devices and receive training on how to use it, and, in the case of the monofilament, to regularly stock replacements to maintain accurate results.16
The Ipswich Touch Test (IpTT) is an alternative neurologic test that requires only the physician’s index finger. During the IpTT, the physician instructs the patient to close his or her eyes while the physician lightly rests his or her finger on each of the patient’s first, third, and fifth toes for 1 to 2 seconds (FIGURE 3). Patients are instructed to respond with a “yes” when they feel the physician’s touch. In a head-to-head trial, diagnostic results of the IpTT directly paralleled those of the monofilament in detecting LOPS; IpTT was also equally sensitive and specific (k=.88, indicating almost perfect agreement; P<.0001).29 The IpTT’s use of only 6 palpation points, constant availability, and accuracy make it a first-line neurologic test for rapidly screening the feet of a patient with diabetes.
Neuromuscular/musculoskeletal exam. Neuromuscular disturbances, such as a reduction in the strength of dorsiflexion and plantar flexion, may indicate a complicated neurologic compromise.5 In addition to being aesthetically problematic, musculoskeletal deformities such as a hammer toe, claw toe (FIGURE 4), or bunion can cause significant pain and/or gait disturbance, and can increase patients’ risk for ulceration.30 These deformities also may compromise patients’ general health and grossly escalate their risk of falls and resultant injuries.5,31 Therefore, patients who present with previously unreported musculoskeletal deformities should be referred to a specialist.31
Also screen patients for Charcot neuroarthropathy (FIGURE 5), a devastating complication that classically presents as a hot, red, swollen foot; the redness resolves upon elevation.32 Charcot neuroarthropathy is hypothesized to be a dysregulation of normal bone metabolism typically occurring secondary to diabetic neuropathy and repetitive minor trauma.33,34 This dysregulation leads to joint instability and disorganization of normal midfoot bone architecture.31,32 Charcot neuroarthropathy is an urgent pathology that requires management by a foot specialist.35
Vascular exam. PAD is particularly common in patients with diabetes and contributes to the development of impaired healing in up to half of foot ulcers.13,18,36-39 Bilateral femoral, popliteal, posterior tibial, or dorsalis pedis pulses should be assessed by palpation; a diminished or absent pulse is a key indicator of vascular compromise.40,41 An integrated care approach between foot specialists and vascular surgeons results in optimal treatment.
Patient education (1 minute)
It is imperative to include patients in their treatment process to reduce the likelihood of complications and, ultimately, decrease the incidence of amputations.12,42 Patient education improves patients’ self-reported home care behaviors, even at the most fundamental levels.43,44 TABLE 35,15,45 lists topics to cover during patient education.
Patients’ lack of understanding about self-care for diabetes is a common barrier to prevention.23 El-Nahas et al46 found a lack of appropriate education regarding diabetes was a factor in more than 90% of recurrent ulcers, which emphasizes the need for repeated education for at-risk patients.47,48 Involve all levels of medical staff in the effort to educate patients on the importance of foot screenings, both at home and in-office. Even with proper patient education, many patients may be in various stages of coping with this all-consuming yet frequently asymptomatic condition, which makes the need for repeated patient education even more critical.
Who to refer, and when
After completing the 3-minute foot exam, create a treatment and follow-up plan, focusing on the need for referral to a specialist. TABLE 4 outlines suggested indications, priorities, and timelines for referral based on ADA guidelines.5 It incorporates the ADA’s patient risk categories (very low, low, moderate, and high risk) and also provides a recommended frequency for patient follow-ups.
Care for patients with lower extremity complications of diabetes mellitus is time-consuming and expensive. The brief exam described here can help you to rapidly identify patients at risk for these complications and prompt you to provide timely referrals to appropriate specialists.
CORRESPONDENCE
David G. Armstrong, DPM, MD, PhD, Professor, Department of Surgery, Director, Southern Arizona Limb Salvage Alliance (SALSA), 1501 N. Campbell Avenue, Tucson, AZ 85724-5072; armstrong@usa.net
› Screen for lower
extremity complications at every visit for all patients with a suspected or confirmed diagnosis of diabetes. A
› Consider implementing a risk-based referral system to connect primary screening with a specialist's care. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Foot ulcers and other lower-limb complications secondary to diabetes are common, complex, costly, and associated with increased morbidity and mortality.1-6 Unfortunately, patients often have difficulty recognizing the heightened risk status that accompanies the diagnosis of diabetes, particularly the substantial risk for lower limb complications.7 In addition, loss of protective sensation (LOPS) can render patients unable to recognize damage to their lower extremities, thus creating a cycle of tissue damage and other foot complications. Strong evidence suggests that consistent provision of foot-care services and preventive care can reduce amputations among patients with diabetes.7-9 However, routine foot examination and rapid risk stratification is often difficult to incorporate into busy primary care settings. Data suggest that the diabetic foot is adequately evaluated only 12% to 20% of the time.10
In response to the need for more consistent foot exams, an American Diabetes Association (ADA) task force lead by 2 of the authors of this article (AB and DA) created the Comprehensive Foot Examination and Risk Assessment.5 This set the standard for the detailed investigation of lower limb pathology by a specialist, but was not well suited for other practice settings, including primary care. One reason is that it would be difficult to complete the comprehensive examination during a typical 15-minute primary care office visit. In addition, certain examination parameters require the use of neurologic and vascular assessment equipment and training not available in all health care settings.11
With these thoughts in mind, we set out to develop an exam that could be done by a wide range of health care providers—one that takes substantially less time to complete than a comprehensive exam and eliminates common barriers to frequent assessment. The exam, which we’ll describe here, consists of 3 components: taking a patient history, performing a physical exam, and providing patient education. And best of all, it should only take 3 minutes.
The patient history (1 minute)
Patients may present with concerns about their feet, but may not be able to differentiate between benign and threatening symptoms. A thorough medical history can identify factors that may increase patients’ risk of developing lower-limb complications. Reviewing the patient’s medical history also can help guide the physical exam.
Review the patient’s diabetic history, blood glucose control, and previous diabetic complications. Ask patients about their history of peripheral vascular disease, quality of peripheral protective sensation, and previous lower-limb interventions and operations (TABLE 15,12). Patients with diabetes and suboptimal glycemic control have an increased risk for LOPS, chronic and recalcitrant ulcers, and wound infections.2 Additionally, patients with diabetes and a previous lower extremity amputation are at high risk for reulceration.5,12 Lastly, nicotine use and smoking are common pathogenic risk factors that contribute to peripheral artery disease (PAD).13
Physical examination (1 minute)
Careful inspection of the feet should be performed at every visit for patients with confirmed or suspected diabetes. Because up to 50% of patients with significant sensory loss due to neuropathy may be completely asymptomatic,14 failing to search for early signs of infection (FIGURE 1), skin breakdown, ulcer formation (FIGURE 2), skin temperature changes, and inadequate vascular perfusion may allow complications to develop.5 TABLE 25,15,16 outlines the essential components—dermatologic, neurologic, musculoskeletal, and vascular—of a rapid lower limb physical exam.
The dermatologic exam. This serves as a barometer for early intervention, and often results in a limb-saving referral to a specialist. It should begin with a global inspection for discolorations, calluses, wounds, fissures, macerations, nail dystrophy, or paronychia.5 Skin discoloration or loss of hair growth may be the first signs of vascular insufficiency, while calluses and hypertrophic skin often are precursors to ulcers.5,17-19 Inspection of the toes should include a search for fungal, ingrown, or elongated nails. Carefully examine the areas between the toes, where deeper lesions may go unnoticed.5
The neurologic exam. Without protective sensation, patients with neuropathy are at a heightened risk of unrecognized injury and are unlikely to mention their deformities to medical staff.20-23 Consequently, skin deterioration may unknowingly progress to ulceration that requires extensive medical intervention or amputation.
Neuropathic LOPS is easily detectable, yet it is linked to at least 75% of all nontraumatic diabetic amputations.20-23 Adiminished vibratory perception threshold (VPT) is one of the earliest indicators of neuropathic LOPS and is the best predictor of long-term lower extremity complications.1,24,25 However, VPT devices are expensive and time-consuming to operate, and they require training to ensure proper use. The Semmes-Weinstein monofilament is a well-documented alternative to VPT for predicting ulcer risk26-28 and has long been advocated as an essential component of a thorough foot exam.5 The 128 Hz tuning fork is another regularly used alternative.5 However, physicians would need to purchase one of these devices and receive training on how to use it, and, in the case of the monofilament, to regularly stock replacements to maintain accurate results.16
The Ipswich Touch Test (IpTT) is an alternative neurologic test that requires only the physician’s index finger. During the IpTT, the physician instructs the patient to close his or her eyes while the physician lightly rests his or her finger on each of the patient’s first, third, and fifth toes for 1 to 2 seconds (FIGURE 3). Patients are instructed to respond with a “yes” when they feel the physician’s touch. In a head-to-head trial, diagnostic results of the IpTT directly paralleled those of the monofilament in detecting LOPS; IpTT was also equally sensitive and specific (k=.88, indicating almost perfect agreement; P<.0001).29 The IpTT’s use of only 6 palpation points, constant availability, and accuracy make it a first-line neurologic test for rapidly screening the feet of a patient with diabetes.
Neuromuscular/musculoskeletal exam. Neuromuscular disturbances, such as a reduction in the strength of dorsiflexion and plantar flexion, may indicate a complicated neurologic compromise.5 In addition to being aesthetically problematic, musculoskeletal deformities such as a hammer toe, claw toe (FIGURE 4), or bunion can cause significant pain and/or gait disturbance, and can increase patients’ risk for ulceration.30 These deformities also may compromise patients’ general health and grossly escalate their risk of falls and resultant injuries.5,31 Therefore, patients who present with previously unreported musculoskeletal deformities should be referred to a specialist.31
Also screen patients for Charcot neuroarthropathy (FIGURE 5), a devastating complication that classically presents as a hot, red, swollen foot; the redness resolves upon elevation.32 Charcot neuroarthropathy is hypothesized to be a dysregulation of normal bone metabolism typically occurring secondary to diabetic neuropathy and repetitive minor trauma.33,34 This dysregulation leads to joint instability and disorganization of normal midfoot bone architecture.31,32 Charcot neuroarthropathy is an urgent pathology that requires management by a foot specialist.35
Vascular exam. PAD is particularly common in patients with diabetes and contributes to the development of impaired healing in up to half of foot ulcers.13,18,36-39 Bilateral femoral, popliteal, posterior tibial, or dorsalis pedis pulses should be assessed by palpation; a diminished or absent pulse is a key indicator of vascular compromise.40,41 An integrated care approach between foot specialists and vascular surgeons results in optimal treatment.
Patient education (1 minute)
It is imperative to include patients in their treatment process to reduce the likelihood of complications and, ultimately, decrease the incidence of amputations.12,42 Patient education improves patients’ self-reported home care behaviors, even at the most fundamental levels.43,44 TABLE 35,15,45 lists topics to cover during patient education.
Patients’ lack of understanding about self-care for diabetes is a common barrier to prevention.23 El-Nahas et al46 found a lack of appropriate education regarding diabetes was a factor in more than 90% of recurrent ulcers, which emphasizes the need for repeated education for at-risk patients.47,48 Involve all levels of medical staff in the effort to educate patients on the importance of foot screenings, both at home and in-office. Even with proper patient education, many patients may be in various stages of coping with this all-consuming yet frequently asymptomatic condition, which makes the need for repeated patient education even more critical.
Who to refer, and when
After completing the 3-minute foot exam, create a treatment and follow-up plan, focusing on the need for referral to a specialist. TABLE 4 outlines suggested indications, priorities, and timelines for referral based on ADA guidelines.5 It incorporates the ADA’s patient risk categories (very low, low, moderate, and high risk) and also provides a recommended frequency for patient follow-ups.
Care for patients with lower extremity complications of diabetes mellitus is time-consuming and expensive. The brief exam described here can help you to rapidly identify patients at risk for these complications and prompt you to provide timely referrals to appropriate specialists.
CORRESPONDENCE
David G. Armstrong, DPM, MD, PhD, Professor, Department of Surgery, Director, Southern Arizona Limb Salvage Alliance (SALSA), 1501 N. Campbell Avenue, Tucson, AZ 85724-5072; armstrong@usa.net
1. Shearer A, Scuffham P, Gordois A, et al. Predicted costs and outcomes from reduced vibration detection in people with diabetes in the U.S. Diabetes Care. 2003;26:2305-2310.
2. Apelqvist J, Larsson J. What is the most effective way to reduce incidence of amputation in the diabetic foot? Diabetes Metab Res Rev. 2000;16 suppl 1:S75-S83.
3. Armstrong DG, Kanda VA, Lavery LA, et al. Mind the gap: disparity between research funding and costs of care for diabetic foot ulcers. Diabetes Care. 2013;36:1815-1817.
4. Driver VR, Fabbi M, Lavery LA, et al. The costs of diabetic foot: the economic case for the limb salvage team. J Vasc Surg. 2010;52(3 suppl):17S-22S.
5. Boulton AJ, Armstrong DG, Albert SF, et al; American Diabetes Association; American Association of Clinical Endocrinologists. Comprehensive foot examination and risk assessment: a report of the Task Force of the Foot Care Interest Group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31:1679-1685.
6. American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37 suppl 1:S14-S80.
7. Sloan FA, Feinglos MN, Grossman DS. Receipt of care and reduction of lower extremity amputations in a nationally representative sample of U.S. Elderly. Health Serv Res. 2010;45(6 pt 1):1740-1762.
8. Carls GS, Gibson TB, Driver VR, et al. The economic value of specialized lower-extremity medical care by podiatric physicians in the treatment of diabetic foot ulcers. J Am Podiatr Med Assoc. 2011;101:93-115.
9. McCabe CJ, Stevenson RC, Dolan AM. Evaluation of a diabetic foot screening and protection programme. Diabet Med. 1998;15:80-84.
10. Bailey TS, Yu HM, Rayfield EJ. Patterns of foot examination in a diabetes clinic. Am J Med. 1985;78:371-374.
11. Chin MH, Cook S, Jin L, et al. Barriers to providing diabetes care in community health centers. Diabetes Care. 2001;24:268-274.
12. Abbott CA, Carrington AL, Ashe H, et al; North-West Diabetes Foot Care Study. The North-West Diabetes Foot Care Study: incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohort. Diabet Med. 2002;19:377-384.
13. Fowkes FG, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329-1340.
14. Boulton A, Vinik AI, Arezzo JC, et al; American Diabetes Association. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes. 2005;28:956-962.
15. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217-228.
16. Pham H, Armstrong DG, Harvey C, et al. Screening techniques to identify people at high risk for diabetic foot ulceration: a prospective multicenter trial. Diabetes Care. 2000;23:606-611.
17. Marso SP, Hiatt WR. Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol. 2006;47:921-929.
18. American Diabetes Association. Peripheral arterial disease in people with diabetes. JAPMA. 2005;95:309-319.
19. Pataky Z, Golay A, Faravel L, et al. The impact of callosities on the magnitude and duration of plantar pressure in patients with diabetes mellitus. A callus may cause 18,600 kilograms of excess plantar pressure per day. Diabetes Metab. 2002;28: 356-361.
20. Holzer SE, Camerota A, Martens L, et al. Costs and duration of care for lower extremity ulcers in patients with diabetes. Clin Ther. 1998;20:169-181.
21. Boulton AJ, Gries FA, Jervell JA. Guidelines for the diagnosis and outpatient management of diabetic peripheral neuropathy. Diabet Med. 1998;15:508-514.
22. Malay DS, Margolis DJ, Hoffstad OJ, et al. The incidence and risks of failure to heal after lower extremity amputation for the treatment of diabetic neuropathic foot ulcer. J Foot Ankle Surg. 2006;45:366-374.
23. van Houtum WH. Barriers to implementing foot care. Diabetes Metab Res Rev. 2012;28 suppl 1:112-115.
24. Jayaprakash P, Bhansali A, Bhansali S, et al. Validation of bedside methods in evaluation of diabetic peripheral neuropathy. Indian J Med Res. 2011;133:645-649.
25. Young MJ, Breddy JL, Veves A, et al. The prediction of diabetic neuropathic foot ulceration using vibration perception thresholds. A prospective study. Diabetes Care. 1994;17:557-560.
26. Leese GP, Reid F, Green V, et al. Stratification of foot ulcer risk in patients with diabetes: a population-based study. Int J Clin Pract. 2006;60:541-545.
27. Adler AI, Boyko EJ, Ahroni JH, et al. Risk factors for diabetic peripheral sensory neuropathy. Results of the Seattle Prospective Diabetic Foot Study. Diabetes Care. 1997;20:1162-1167.
28. Armstrong DG, Lavery LA, Vela SA, et al. Choosing a practical screening instrument to identify patients at risk for diabetic foot ulceration. Arch Intern Med. 1998;158:289-292.
29. Rayman G, Vas PR, Baker N, et al. The Ipswich Touch Test: a simple and novel method to identify inpatients with diabetes at risk of foot ulceration. Diabetes Care. 2011;34:1517-1518.
30. Lavery LA, Armstrong DG, Vela SA, et al. Practical criteria for screening patients at high risk for diabetic foot ulceration. Arch Intern Med. 1998;158:157-162.
31. Frykberg RG, Zgonis T, Armstrong DG, et al; American College of Foot and Ankle Surgeons. Diabetic foot disorders. A clinical practice guideline (2006 revision). J Foot Ankle Surg. 2006;45(5 suppl):S1-S66.
32. Nielson DL, Armstrong DG. The natural history of Charcot’s neuroarthropathy. Clin Podiatr Med Surg. 2008;25:53-62,vi.
33. Jeffcoate W, Lima J, Nobrega L. The Charcot foot. Diabet Med. 2000;17:253-258.
34. Blume PA, Sumpio B, Schmidt B, et al. Charcot neuroarthropathy of the foot and ankle: diagnosis and management strategies. Clin Podiatr Med Surg. 2014;31:151-172.
35. Petrova NL, Edmonds ME. Medical management of Charcot arthropathy. Diabetes Obes Metab. 2012;15:193-197.
36. Prompers L, Huijberts M, Apelqvist J, et al. Delivery of care to diabetic patients with foot ulcers in daily practice: results of the Eurodiale Study, a prospective cohort study. Diabet Med. 2008;25:700-707.
37. Armstrong DG, Bharara M, White M, et al. The impact and outcomes of establishing an integrated interdisciplinary surgical team to care for the diabetic foot. Diabetes Metab Res Rev. 2012;28:514-518.
38. Rogers LC, Andros G, Caporusso J, et al. Toe and flow: essential components and structure of the amputation prevention team. J Vasc Surg. 2010;52:23S-27S.
39. Mills JL Sr, Conte MS, Armstrong DG, et al; Society for Vascular Surgery Lower Extremity Guidelines Committee. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg. 2014;59:220-34.e1-2.
40. Khan NA, Rahim SA, Anand SS, et al. Does the clinical examination predict lower extremity peripheral arterial disease? JAMA. 2006;295:536-546.
41. Sumpio BE, Lee T, Blume PA. Vascular evaluation and arterial reconstruction of the diabetic foot. Clin Podiatr Med Surg. 2003;20:689-708.
42. Dorresteijn JAN, Valk GD. Patient education for preventing diabetic foot ulceration. Diabetes Metab Res Rev. 2012;28 Suppl 1:101-106.
43. Lincoln NB, Radford KA, Game FL, et al. Education for secondary prevention of foot ulcers in people with diabetes: a randomised controlled trial. Diabetologia. 2008;51:1954-1961.
44. McMurray SD, Johnson G, Davis S, et al. Diabetes education and care management significantly improve patient outcomes in the dialysis unit. Am J Kidney Dis. 2002;40:566-575.
45. Armstrong DG, Lavery LA. Diabetic foot ulcers: prevention, diagnosis and classification. Am Fam Physician. 1998;57:1325-1332,1337-1338.
46. El-Nahas MR, Gawish HMS, Tarshoby MM, et al. The prevalence of risk factors for foot ulceration in Egyptian diabetic patients. Practical Diabetes Int. 2008;25:362-366.
47. Hämäläinen H, Rönnemaa T, Toikka T, et al. Long-term effects of one year of intensified podiatric activities on foot-care knowledge and self-care habits in patients with diabetes. Diabetes Educ. 1998;24:734-740.
48. Rönnemaa T, Hämäläinen H, Toikka T, et al. Evaluation of the impact of podiatrist care in the primary prevention of foot problems in diabetic subjects. Diabetes Care. 1997;20:1833-1837.
1. Shearer A, Scuffham P, Gordois A, et al. Predicted costs and outcomes from reduced vibration detection in people with diabetes in the U.S. Diabetes Care. 2003;26:2305-2310.
2. Apelqvist J, Larsson J. What is the most effective way to reduce incidence of amputation in the diabetic foot? Diabetes Metab Res Rev. 2000;16 suppl 1:S75-S83.
3. Armstrong DG, Kanda VA, Lavery LA, et al. Mind the gap: disparity between research funding and costs of care for diabetic foot ulcers. Diabetes Care. 2013;36:1815-1817.
4. Driver VR, Fabbi M, Lavery LA, et al. The costs of diabetic foot: the economic case for the limb salvage team. J Vasc Surg. 2010;52(3 suppl):17S-22S.
5. Boulton AJ, Armstrong DG, Albert SF, et al; American Diabetes Association; American Association of Clinical Endocrinologists. Comprehensive foot examination and risk assessment: a report of the Task Force of the Foot Care Interest Group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31:1679-1685.
6. American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37 suppl 1:S14-S80.
7. Sloan FA, Feinglos MN, Grossman DS. Receipt of care and reduction of lower extremity amputations in a nationally representative sample of U.S. Elderly. Health Serv Res. 2010;45(6 pt 1):1740-1762.
8. Carls GS, Gibson TB, Driver VR, et al. The economic value of specialized lower-extremity medical care by podiatric physicians in the treatment of diabetic foot ulcers. J Am Podiatr Med Assoc. 2011;101:93-115.
9. McCabe CJ, Stevenson RC, Dolan AM. Evaluation of a diabetic foot screening and protection programme. Diabet Med. 1998;15:80-84.
10. Bailey TS, Yu HM, Rayfield EJ. Patterns of foot examination in a diabetes clinic. Am J Med. 1985;78:371-374.
11. Chin MH, Cook S, Jin L, et al. Barriers to providing diabetes care in community health centers. Diabetes Care. 2001;24:268-274.
12. Abbott CA, Carrington AL, Ashe H, et al; North-West Diabetes Foot Care Study. The North-West Diabetes Foot Care Study: incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohort. Diabet Med. 2002;19:377-384.
13. Fowkes FG, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329-1340.
14. Boulton A, Vinik AI, Arezzo JC, et al; American Diabetes Association. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes. 2005;28:956-962.
15. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217-228.
16. Pham H, Armstrong DG, Harvey C, et al. Screening techniques to identify people at high risk for diabetic foot ulceration: a prospective multicenter trial. Diabetes Care. 2000;23:606-611.
17. Marso SP, Hiatt WR. Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol. 2006;47:921-929.
18. American Diabetes Association. Peripheral arterial disease in people with diabetes. JAPMA. 2005;95:309-319.
19. Pataky Z, Golay A, Faravel L, et al. The impact of callosities on the magnitude and duration of plantar pressure in patients with diabetes mellitus. A callus may cause 18,600 kilograms of excess plantar pressure per day. Diabetes Metab. 2002;28: 356-361.
20. Holzer SE, Camerota A, Martens L, et al. Costs and duration of care for lower extremity ulcers in patients with diabetes. Clin Ther. 1998;20:169-181.
21. Boulton AJ, Gries FA, Jervell JA. Guidelines for the diagnosis and outpatient management of diabetic peripheral neuropathy. Diabet Med. 1998;15:508-514.
22. Malay DS, Margolis DJ, Hoffstad OJ, et al. The incidence and risks of failure to heal after lower extremity amputation for the treatment of diabetic neuropathic foot ulcer. J Foot Ankle Surg. 2006;45:366-374.
23. van Houtum WH. Barriers to implementing foot care. Diabetes Metab Res Rev. 2012;28 suppl 1:112-115.
24. Jayaprakash P, Bhansali A, Bhansali S, et al. Validation of bedside methods in evaluation of diabetic peripheral neuropathy. Indian J Med Res. 2011;133:645-649.
25. Young MJ, Breddy JL, Veves A, et al. The prediction of diabetic neuropathic foot ulceration using vibration perception thresholds. A prospective study. Diabetes Care. 1994;17:557-560.
26. Leese GP, Reid F, Green V, et al. Stratification of foot ulcer risk in patients with diabetes: a population-based study. Int J Clin Pract. 2006;60:541-545.
27. Adler AI, Boyko EJ, Ahroni JH, et al. Risk factors for diabetic peripheral sensory neuropathy. Results of the Seattle Prospective Diabetic Foot Study. Diabetes Care. 1997;20:1162-1167.
28. Armstrong DG, Lavery LA, Vela SA, et al. Choosing a practical screening instrument to identify patients at risk for diabetic foot ulceration. Arch Intern Med. 1998;158:289-292.
29. Rayman G, Vas PR, Baker N, et al. The Ipswich Touch Test: a simple and novel method to identify inpatients with diabetes at risk of foot ulceration. Diabetes Care. 2011;34:1517-1518.
30. Lavery LA, Armstrong DG, Vela SA, et al. Practical criteria for screening patients at high risk for diabetic foot ulceration. Arch Intern Med. 1998;158:157-162.
31. Frykberg RG, Zgonis T, Armstrong DG, et al; American College of Foot and Ankle Surgeons. Diabetic foot disorders. A clinical practice guideline (2006 revision). J Foot Ankle Surg. 2006;45(5 suppl):S1-S66.
32. Nielson DL, Armstrong DG. The natural history of Charcot’s neuroarthropathy. Clin Podiatr Med Surg. 2008;25:53-62,vi.
33. Jeffcoate W, Lima J, Nobrega L. The Charcot foot. Diabet Med. 2000;17:253-258.
34. Blume PA, Sumpio B, Schmidt B, et al. Charcot neuroarthropathy of the foot and ankle: diagnosis and management strategies. Clin Podiatr Med Surg. 2014;31:151-172.
35. Petrova NL, Edmonds ME. Medical management of Charcot arthropathy. Diabetes Obes Metab. 2012;15:193-197.
36. Prompers L, Huijberts M, Apelqvist J, et al. Delivery of care to diabetic patients with foot ulcers in daily practice: results of the Eurodiale Study, a prospective cohort study. Diabet Med. 2008;25:700-707.
37. Armstrong DG, Bharara M, White M, et al. The impact and outcomes of establishing an integrated interdisciplinary surgical team to care for the diabetic foot. Diabetes Metab Res Rev. 2012;28:514-518.
38. Rogers LC, Andros G, Caporusso J, et al. Toe and flow: essential components and structure of the amputation prevention team. J Vasc Surg. 2010;52:23S-27S.
39. Mills JL Sr, Conte MS, Armstrong DG, et al; Society for Vascular Surgery Lower Extremity Guidelines Committee. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg. 2014;59:220-34.e1-2.
40. Khan NA, Rahim SA, Anand SS, et al. Does the clinical examination predict lower extremity peripheral arterial disease? JAMA. 2006;295:536-546.
41. Sumpio BE, Lee T, Blume PA. Vascular evaluation and arterial reconstruction of the diabetic foot. Clin Podiatr Med Surg. 2003;20:689-708.
42. Dorresteijn JAN, Valk GD. Patient education for preventing diabetic foot ulceration. Diabetes Metab Res Rev. 2012;28 Suppl 1:101-106.
43. Lincoln NB, Radford KA, Game FL, et al. Education for secondary prevention of foot ulcers in people with diabetes: a randomised controlled trial. Diabetologia. 2008;51:1954-1961.
44. McMurray SD, Johnson G, Davis S, et al. Diabetes education and care management significantly improve patient outcomes in the dialysis unit. Am J Kidney Dis. 2002;40:566-575.
45. Armstrong DG, Lavery LA. Diabetic foot ulcers: prevention, diagnosis and classification. Am Fam Physician. 1998;57:1325-1332,1337-1338.
46. El-Nahas MR, Gawish HMS, Tarshoby MM, et al. The prevalence of risk factors for foot ulceration in Egyptian diabetic patients. Practical Diabetes Int. 2008;25:362-366.
47. Hämäläinen H, Rönnemaa T, Toikka T, et al. Long-term effects of one year of intensified podiatric activities on foot-care knowledge and self-care habits in patients with diabetes. Diabetes Educ. 1998;24:734-740.
48. Rönnemaa T, Hämäläinen H, Toikka T, et al. Evaluation of the impact of podiatrist care in the primary prevention of foot problems in diabetic subjects. Diabetes Care. 1997;20:1833-1837.