User login
Metronidazole is no longer recommended
Case
A 45-year-old woman on omeprazole for gastroesophageal reflux disease and recent treatment with ciprofloxacin for a urinary tract infection (UTI), who also has had several days of frequent watery stools, is admitted. She does not appear ill, and her abdominal exam is benign. She has normal renal function and white blood cell count. How should she be evaluated and treated for Clostridium difficile–associated disease (CDAD)?
Brief overview
C. difficile, a gram-positive anaerobic bacillus that exists in vegetative and spore forms, is a leading cause of hospital-associated diarrhea. C. difficile has a variety of presentations, ranging from asymptomatic colonization to CDAD, including severe diarrhea, ileus, and megacolon, and may be associated with a fatal outcome on rare occasions. The incidence of CDAD has been rising since the emergence of a hypervirulent strain (NAP1/BI/027) in the early 2000s and, not surprisingly, the number of deaths attributed to CDAD has also increased.1
CDAD requires acquisition of C. difficile as well as alteration in the colonic microbiota, often precipitated by antibiotics. The vegetative form of C. difficile can produce up to three toxins that are responsible for a cascade of reactions beginning with intestinal epithelial cell death followed by a significant inflammatory response and migration of neutrophils that eventually lead to the formation of the characteristic pseudomembranes.2
Until recently, the mainstay treatment for CDAD consisted of metronidazole and oral preparations of vancomycin. Recent results from randomized controlled trials and the increasing popularity of fecal microbiota transplant (FMT), however, have changed the therapeutic landscape of CDAD dramatically. Not surprisingly, the 2017 Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America joint guidelines for CDAD represent a significant change to the treatment of CDAD, compared with previous guidelines.3
Overview of data
The hallmark of CDAD is a watery, nonbloody diarrhea. Given many other causes of diarrhea in hospitalized patients (e.g., direct effect of antibiotics, laxative use, tube feeding, etc.), hospitalists should focus on testing those patients who have three or more episodes of diarrhea in 24 hours and risk factors for CDAD (See Table 1).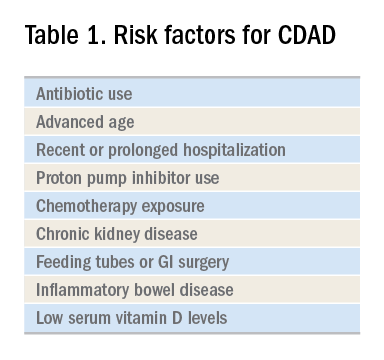
Exposure to antibiotics remains the greatest risk factor. It’s important to note that, while most patients develop CDAD within the first month after receiving systemic antibiotics, many patients remain at risk for up to 3 months.4 Although exposure to antibiotics, particularly in health care settings, is a significant risk factor for CDAD, up to 30%-40% of community-associated cases may not have a substantial antibiotic or health care facility exposure.5
Hospitalists should also not overlook the association between proton pump inhibitor (PPI) use and the development of CDAD.3 Although the IDSA/SHEA guidelines do not recommend discontinuation of PPIs solely for treatment or prevention of CDAD, at the minimum, the indication for their continued use in patients with CDAD should be revisited.
Testing for CDAD ranges from immunoassays that detect an enzyme common to all strains of C. difficile, glutamate dehydrogenase antigen (GDH), or toxins to nucleic acid amplification tests (NAATs), such as polymerase chain reaction [PCR]).1,6 GDH tests have high sensitivity but poor specificity, while testing for the toxin has high specificity but lower sensitivity (40%-80%) for CDAD.1 Although NAATs are highly sensitive and specific, they often have a poor positive predictive value in low-risk populations (e.g., those who do not have true diarrhea or whose diarrhea resolves before test results return). In these patients, a positive NAAT test may reflect colonization with toxigenic C. difficile, not necessarily CDAD. Except in rare instances, laboratories should only accept unformed stools for testing. Since the choice of testing for C. difficile varies by institution, hospitalists should understand the algorithm used by their respective hospitals and familiarize themselves with the sensitivity and specificity of each test.
Once a patient is diagnosed with CDAD, the hospitalist should assess the severity of the disease. The IDSA/SHEA guidelines still use leukocytosis and creatinine to separate mild from severe cases; the presence of fever and hypoalbuminemia also points to a more complicated course.3
The treatment of CDAD involves a strategy of withdrawing the putative culprit antibiotic(s) whenever possible and initiating of antibiotics effective against C. difficile. Following the publication of two randomized controlled trials demonstrating the inferiority of metronidazole to vancomycin in clinical cure of CDAD,2,7 the IDSA/SHEA guidelines no longer recommend metronidazole for the treatment of CDAD. Instead, a 10-day course of oral vancomycin or fidaxomicin has been recommended.2 Although fidaxomicin is associated with lower rates of recurrence of CDAD, it is also substantially more expensive than oral vancomycin, with a 10-day course often costing over $3,000.8 When choosing oral vancomycin for completion of therapy following discharge, hospitalists should also consider whether the dispensing outpatient pharmacy can provide the less-expensive liquid preparation of vancomycin. In resource-poor settings, consideration can still be given to metronidazole, an inexpensive drug, compared with both oral vancomycin and fidaxomicin. “Test of cure” with follow-up stool testing is not recommended.
For patients who require systemic antibiotics that precipitated their CDAD, it is common practice to extend CDAD treatment by providing a “tail” coverage with an agent effective against CDAD for 7-10 days following the completion of the inciting antibiotic. A common clinical question relates to the management of patients with prior history of CDAD but in need of a new round of systemic antibiotic therapy. In these patients, concurrent prophylactic doses of oral vancomycin have been found to be effective in preventing recurrence.9 The IDSA/SHEA guidelines conclude that “it may be prudent to administer low doses of vancomycin or fidaxomicin (e.g., 125 mg or 200 mg, respectively, once daily) while systemic antibiotics are administered.”3
For patients whose presentation extends beyond diarrhea, the IDSA/SHEA guidelines have changed the nomenclature for CDAD from “severe, complicated” to “fulminant.” Although there are no strict definitions, the IDSA/SHEA guidelines suggest that fulminant CDAD is characterized by “hypotension or shock, ileus, or megacolon.” In these patients, surgical intervention can be life saving, though mortality rates may remain over 50%.10 Hospitalists whose patients with CDAD are experiencing an acute abdomen or concern for colonic perforation, megacolon, shock, or organ system failure should obtain prompt surgical consultation. Antibiotic treatment should consist of a combination of higher doses of oral vancomycin and intravenous metronidazole (See Table 2).
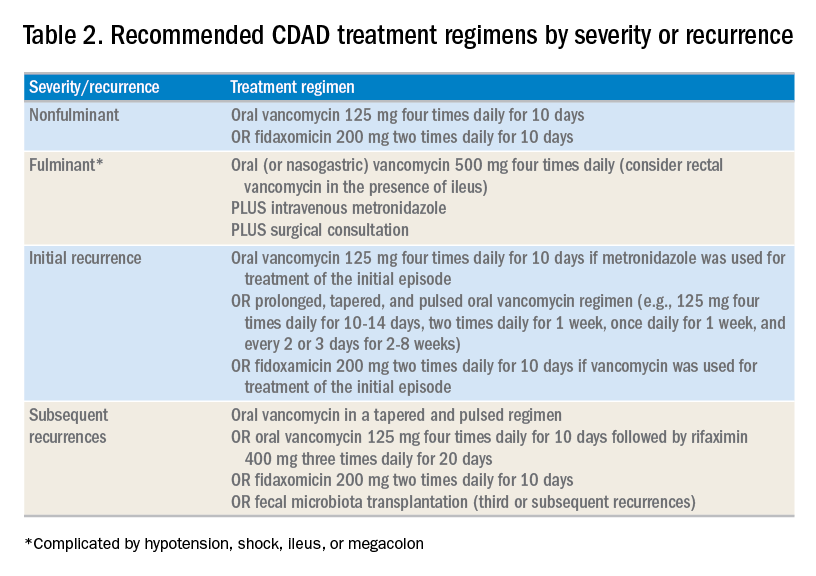
In addition to occasional treatment failures, a vexing characteristic of CDAD is its frequent recurrence rate, which may range from 15% to 30% or higher.11 The approach to recurrences is twofold: treatment of the C. difficile itself, and attempts to restore the colonic microbiome. The antibiotic treatment of the first recurrence of CDAD consists of either a 10-day course of fidaxomicin or a tapered, pulsed dose of vancomycin, which may be more effective than a repeat 10-day course of oral vancomycin.12 Although the treatment is unchanged for subsequent recurrences, the guidelines suggest consideration of rifaximin after a course of vancomycin (See Table 2).
Probiotics have been investigated as a means of restoring the colonic microbiome. Use of probiotics for both primary and secondary prevention of CDAD has resulted in conflicting data, with pooled analyses showing some benefit, while randomized controlled trials demonstrate less benefit.13 In addition, reports of bloodstream infections with Lactobacillus in frail patients and Saccharomyces in immunocompromised patients and those with central venous catheters raise doubts regarding their safety in certain patient populations.13 The IDSA/SHEA guidelines make no recommendations about the use of probiotics for the prevention of CDAD at this time.
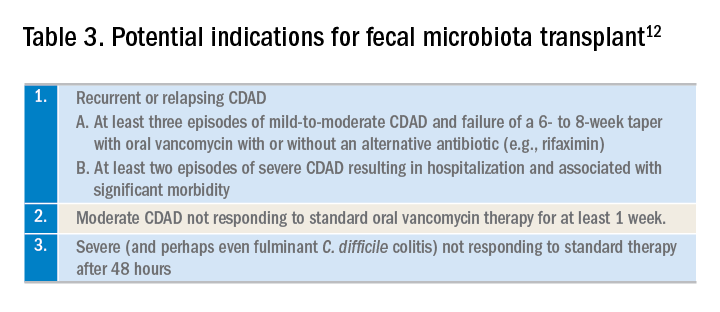
Fecal microbiota transplant (FMT), however, does appear to be effective, especially in comparison to antibiotics alone in patients with multiple recurrences of CDAD.13 The IDSA/SHEA guidelines recommend consideration for FMT after the second recurrence of CDAD. The Fecal Microbiota Transplantation Workgroup has also proposed a set of guidelines for consideration of FMT when available (See Table 3).
Application of data
The recent IDSA/SHEA guidelines have revised the treatment paradigm for CDAD. Most notably, metronidazole is no longer recommended for treatment of either initial or subsequent episodes of mild to severe CDAD, except when the cost of treatment may preclude the use of more effective therapies.
Initial episodes of mild to severe infection should be treated with either oral vancomycin or fidaxomicin. Recurrent episodes of CDAD should be treated with an agent different from that used for the initial episode, or with a pulsed, tapered regimen of oral vancomycin. FMT, where available, should be considered with multiple recurrences, or with moderate to severe infection not responding to standard therapy.
Fulminant CDAD, characterized by hypotension, shock, severe ileus, or megacolon, is a life-threatening medical emergency with a high mortality rate. Treatment should include high-dose oral vancomycin and intravenous metronidazole, with consideration of rectal vancomycin in patients with ileus. Immediate surgical consultation should be obtained to weigh the benefits of colectomy.
Back to our case
Our patient was treated with a 10-day course of vancomycin because this was uncomplicated CDAD and was her initial episode. Were she to develop a recurrence, she could be treated with a pulsed, tapered vancomycin regimen or fidaxomicin.
Bottom line
Vancomycin and fidaxomicin are recommended for the initial episode as well as recurrent CDAD. FMT should be considered for patients with multiple episodes of CDAD or treatment failures.
Dr. Roberts, Dr. Hillman, and Dr. Manian are hospitalists at Massachusetts General Hospital in Boston.
References
1. Louie TJ et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011 Feb 3;364:422-31. doi: 10.1056/NEJMoa0910812.
2. Burnham CA et al. Diagnosis of Clostridium difficile infection: an ongoing conundrum for clinicians and for clinical laboratories. Clin Microbiol Rev. 2013 Jul;26:604-30. doi: 10.1128/CMR.00016-13.
3. McDonald LC et al. Clinical Practice Guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018 Mar 19;66:987-94. doi: 10.1093/cid/ciy149.
4. Hensgens MP et al. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J Antimicrob Chemother. 2012 Mar;67:742-8. doi: 10.1093/jac/dkr508. Epub 2011 Dec 6.
5. Chitnis AS et al. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med. 2013 Jul 22;173:1359-67. doi: 10.1001/jamainternmed.2013.7056.
6. Solomon DA et al. ID learning unit: Understanding and interpreting testing for Clostridium difficile. Open Forum Infectious Diseases. 2014 Mar;1(1);ofu007. doi: 10.1093/ofid/ofu007.
7. Johnson S et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014 Aug 1;59(3):345-54. doi: 10.1093/cid/ciu313. Epub 2014 May 5.
8. https://m.goodrx.com/fidaxomicin, accessed June 24, 2018.
9. Van Hise NW et al. Efficacy of oral vancomycin in preventing recurrent Clostridium difficile infection in patients treated with systemic antimicrobial agents. Clin Infect Dis. 2016 Sep 1;63:651-3. doi: 10.1093/cid/ciw401. Epub 2016 Jun 17.
10. Sailhamer EA et al. Fulminant Clostridium difficile colitis: Patterns of care and predictors of mortality. Arch Surg. 2009;144:433-9. doi: 10.1001/archsurg.2009.51.
11. Zar FA et al. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302-7. doi: 10.1086/519265. Epub 2007 Jun 19.
12. Bakken JS et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011;9:1044-9. doi: 10.1016/j.cgh.2011.08.014. Epub 2011 Aug 24.
13. Crow JR et al. Probiotics and fecal microbiota transplant for primary and secondary prevention of Clostridium difficile infection. Pharmacotherapy. 2015 Nov;35:1016-25. doi: 10.1002/phar.1644. Epub 2015 Nov 2.
Additional reading
1. McDonald LC et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018 Mar 19;66:987-94. doi: 10.1093/cid/ciy149.
2. Burnham CA et al. Diagnosis of Clostridium difficile infection: an ongoing conundrum for clinicians and for clinical laboratories. Clin Microbiol Rev. 2013 Jul;26:604-30. doi: 10.1128/CMR.00016-13.
3. Crow JR, Davis SL, Chaykosky DM, Smith TT, Smith JM. Probiotics and fecal microbiota transplant for primary and secondary prevention of Clostridium difficile infection. Pharmacotherapy. 2015 Nov; 35:1016-25. doi: 10.1002/phar.1644. Epub 2015 Nov 2. Review.
Key points
1. Metronidazole is inferior to oral vancomycin and fidaxomicin for clinical cure of CDAD. The IDSA/SHEA guidelines now recommend a 10-day course of oral vancomycin or fidaxomicin for nonfulminant cases of CDAD.
2. For fulminant CDAD, the IDSA/SHEA guidelines suggest an increased dose of vancomycin and the addition of IV metronidazole. In such cases, surgical consultation should also be obtained.
3. After the second recurrence of Clostridium difficile infection, hospitalists should consider referral for FMT where available.
Quiz
The recent IDSA/SHEA guidelines no longer recommend metronidazole in the treatment of CDAD, except for which of the following scenarios (best answer)?
A. Treatment of a first episode of nonfulminant CDAD.
B. Treatment of recurrent CDAD following an initial course of oral vancomycin.
C. Treatment of fulminant infection with IV metronidazole in addition to oral or rectal vancomycin.
D. For prophylaxis following fecal microbiota transplant.
Answer: C. In fulminant infection, concurrent ileus may interfere with appropriate delivery of oral vancomycin to the colon. Adding intravenous metronidazole can allow this antibiotic to reach the bowel. Adding intravenous metronidazole to oral vancomycin is also recommended by IDSA/SHEA guidelines in cases of fulminant CDAD. Evidence from high-quality randomized controlled trials has shown that vancomycin is superior to oral metronidazole for treatment of initial and recurrent episodes of CDAD. There is no evidence to support the use of metronidazole for recurrent CDAD following an initial course of oral vancomycin or for prophylaxis following FMT.
Metronidazole is no longer recommended
Metronidazole is no longer recommended
Case
A 45-year-old woman on omeprazole for gastroesophageal reflux disease and recent treatment with ciprofloxacin for a urinary tract infection (UTI), who also has had several days of frequent watery stools, is admitted. She does not appear ill, and her abdominal exam is benign. She has normal renal function and white blood cell count. How should she be evaluated and treated for Clostridium difficile–associated disease (CDAD)?
Brief overview
C. difficile, a gram-positive anaerobic bacillus that exists in vegetative and spore forms, is a leading cause of hospital-associated diarrhea. C. difficile has a variety of presentations, ranging from asymptomatic colonization to CDAD, including severe diarrhea, ileus, and megacolon, and may be associated with a fatal outcome on rare occasions. The incidence of CDAD has been rising since the emergence of a hypervirulent strain (NAP1/BI/027) in the early 2000s and, not surprisingly, the number of deaths attributed to CDAD has also increased.1
CDAD requires acquisition of C. difficile as well as alteration in the colonic microbiota, often precipitated by antibiotics. The vegetative form of C. difficile can produce up to three toxins that are responsible for a cascade of reactions beginning with intestinal epithelial cell death followed by a significant inflammatory response and migration of neutrophils that eventually lead to the formation of the characteristic pseudomembranes.2
Until recently, the mainstay treatment for CDAD consisted of metronidazole and oral preparations of vancomycin. Recent results from randomized controlled trials and the increasing popularity of fecal microbiota transplant (FMT), however, have changed the therapeutic landscape of CDAD dramatically. Not surprisingly, the 2017 Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America joint guidelines for CDAD represent a significant change to the treatment of CDAD, compared with previous guidelines.3
Overview of data
The hallmark of CDAD is a watery, nonbloody diarrhea. Given many other causes of diarrhea in hospitalized patients (e.g., direct effect of antibiotics, laxative use, tube feeding, etc.), hospitalists should focus on testing those patients who have three or more episodes of diarrhea in 24 hours and risk factors for CDAD (See Table 1).
Exposure to antibiotics remains the greatest risk factor. It’s important to note that, while most patients develop CDAD within the first month after receiving systemic antibiotics, many patients remain at risk for up to 3 months.4 Although exposure to antibiotics, particularly in health care settings, is a significant risk factor for CDAD, up to 30%-40% of community-associated cases may not have a substantial antibiotic or health care facility exposure.5
Hospitalists should also not overlook the association between proton pump inhibitor (PPI) use and the development of CDAD.3 Although the IDSA/SHEA guidelines do not recommend discontinuation of PPIs solely for treatment or prevention of CDAD, at the minimum, the indication for their continued use in patients with CDAD should be revisited.
Testing for CDAD ranges from immunoassays that detect an enzyme common to all strains of C. difficile, glutamate dehydrogenase antigen (GDH), or toxins to nucleic acid amplification tests (NAATs), such as polymerase chain reaction [PCR]).1,6 GDH tests have high sensitivity but poor specificity, while testing for the toxin has high specificity but lower sensitivity (40%-80%) for CDAD.1 Although NAATs are highly sensitive and specific, they often have a poor positive predictive value in low-risk populations (e.g., those who do not have true diarrhea or whose diarrhea resolves before test results return). In these patients, a positive NAAT test may reflect colonization with toxigenic C. difficile, not necessarily CDAD. Except in rare instances, laboratories should only accept unformed stools for testing. Since the choice of testing for C. difficile varies by institution, hospitalists should understand the algorithm used by their respective hospitals and familiarize themselves with the sensitivity and specificity of each test.
Once a patient is diagnosed with CDAD, the hospitalist should assess the severity of the disease. The IDSA/SHEA guidelines still use leukocytosis and creatinine to separate mild from severe cases; the presence of fever and hypoalbuminemia also points to a more complicated course.3
The treatment of CDAD involves a strategy of withdrawing the putative culprit antibiotic(s) whenever possible and initiating of antibiotics effective against C. difficile. Following the publication of two randomized controlled trials demonstrating the inferiority of metronidazole to vancomycin in clinical cure of CDAD,2,7 the IDSA/SHEA guidelines no longer recommend metronidazole for the treatment of CDAD. Instead, a 10-day course of oral vancomycin or fidaxomicin has been recommended.2 Although fidaxomicin is associated with lower rates of recurrence of CDAD, it is also substantially more expensive than oral vancomycin, with a 10-day course often costing over $3,000.8 When choosing oral vancomycin for completion of therapy following discharge, hospitalists should also consider whether the dispensing outpatient pharmacy can provide the less-expensive liquid preparation of vancomycin. In resource-poor settings, consideration can still be given to metronidazole, an inexpensive drug, compared with both oral vancomycin and fidaxomicin. “Test of cure” with follow-up stool testing is not recommended.
For patients who require systemic antibiotics that precipitated their CDAD, it is common practice to extend CDAD treatment by providing a “tail” coverage with an agent effective against CDAD for 7-10 days following the completion of the inciting antibiotic. A common clinical question relates to the management of patients with prior history of CDAD but in need of a new round of systemic antibiotic therapy. In these patients, concurrent prophylactic doses of oral vancomycin have been found to be effective in preventing recurrence.9 The IDSA/SHEA guidelines conclude that “it may be prudent to administer low doses of vancomycin or fidaxomicin (e.g., 125 mg or 200 mg, respectively, once daily) while systemic antibiotics are administered.”3
For patients whose presentation extends beyond diarrhea, the IDSA/SHEA guidelines have changed the nomenclature for CDAD from “severe, complicated” to “fulminant.” Although there are no strict definitions, the IDSA/SHEA guidelines suggest that fulminant CDAD is characterized by “hypotension or shock, ileus, or megacolon.” In these patients, surgical intervention can be life saving, though mortality rates may remain over 50%.10 Hospitalists whose patients with CDAD are experiencing an acute abdomen or concern for colonic perforation, megacolon, shock, or organ system failure should obtain prompt surgical consultation. Antibiotic treatment should consist of a combination of higher doses of oral vancomycin and intravenous metronidazole (See Table 2).

In addition to occasional treatment failures, a vexing characteristic of CDAD is its frequent recurrence rate, which may range from 15% to 30% or higher.11 The approach to recurrences is twofold: treatment of the C. difficile itself, and attempts to restore the colonic microbiome. The antibiotic treatment of the first recurrence of CDAD consists of either a 10-day course of fidaxomicin or a tapered, pulsed dose of vancomycin, which may be more effective than a repeat 10-day course of oral vancomycin.12 Although the treatment is unchanged for subsequent recurrences, the guidelines suggest consideration of rifaximin after a course of vancomycin (See Table 2).
Probiotics have been investigated as a means of restoring the colonic microbiome. Use of probiotics for both primary and secondary prevention of CDAD has resulted in conflicting data, with pooled analyses showing some benefit, while randomized controlled trials demonstrate less benefit.13 In addition, reports of bloodstream infections with Lactobacillus in frail patients and Saccharomyces in immunocompromised patients and those with central venous catheters raise doubts regarding their safety in certain patient populations.13 The IDSA/SHEA guidelines make no recommendations about the use of probiotics for the prevention of CDAD at this time.
Fecal microbiota transplant (FMT), however, does appear to be effective, especially in comparison to antibiotics alone in patients with multiple recurrences of CDAD.13 The IDSA/SHEA guidelines recommend consideration for FMT after the second recurrence of CDAD. The Fecal Microbiota Transplantation Workgroup has also proposed a set of guidelines for consideration of FMT when available (See Table 3).

Application of data
The recent IDSA/SHEA guidelines have revised the treatment paradigm for CDAD. Most notably, metronidazole is no longer recommended for treatment of either initial or subsequent episodes of mild to severe CDAD, except when the cost of treatment may preclude the use of more effective therapies.
Initial episodes of mild to severe infection should be treated with either oral vancomycin or fidaxomicin. Recurrent episodes of CDAD should be treated with an agent different from that used for the initial episode, or with a pulsed, tapered regimen of oral vancomycin. FMT, where available, should be considered with multiple recurrences, or with moderate to severe infection not responding to standard therapy.
Fulminant CDAD, characterized by hypotension, shock, severe ileus, or megacolon, is a life-threatening medical emergency with a high mortality rate. Treatment should include high-dose oral vancomycin and intravenous metronidazole, with consideration of rectal vancomycin in patients with ileus. Immediate surgical consultation should be obtained to weigh the benefits of colectomy.
Back to our case
Our patient was treated with a 10-day course of vancomycin because this was uncomplicated CDAD and was her initial episode. Were she to develop a recurrence, she could be treated with a pulsed, tapered vancomycin regimen or fidaxomicin.
Bottom line
Vancomycin and fidaxomicin are recommended for the initial episode as well as recurrent CDAD. FMT should be considered for patients with multiple episodes of CDAD or treatment failures.
Dr. Roberts, Dr. Hillman, and Dr. Manian are hospitalists at Massachusetts General Hospital in Boston.
References
1. Louie TJ et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011 Feb 3;364:422-31. doi: 10.1056/NEJMoa0910812.
2. Burnham CA et al. Diagnosis of Clostridium difficile infection: an ongoing conundrum for clinicians and for clinical laboratories. Clin Microbiol Rev. 2013 Jul;26:604-30. doi: 10.1128/CMR.00016-13.
3. McDonald LC et al. Clinical Practice Guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018 Mar 19;66:987-94. doi: 10.1093/cid/ciy149.
4. Hensgens MP et al. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J Antimicrob Chemother. 2012 Mar;67:742-8. doi: 10.1093/jac/dkr508. Epub 2011 Dec 6.
5. Chitnis AS et al. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med. 2013 Jul 22;173:1359-67. doi: 10.1001/jamainternmed.2013.7056.
6. Solomon DA et al. ID learning unit: Understanding and interpreting testing for Clostridium difficile. Open Forum Infectious Diseases. 2014 Mar;1(1);ofu007. doi: 10.1093/ofid/ofu007.
7. Johnson S et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014 Aug 1;59(3):345-54. doi: 10.1093/cid/ciu313. Epub 2014 May 5.
8. https://m.goodrx.com/fidaxomicin, accessed June 24, 2018.
9. Van Hise NW et al. Efficacy of oral vancomycin in preventing recurrent Clostridium difficile infection in patients treated with systemic antimicrobial agents. Clin Infect Dis. 2016 Sep 1;63:651-3. doi: 10.1093/cid/ciw401. Epub 2016 Jun 17.
10. Sailhamer EA et al. Fulminant Clostridium difficile colitis: Patterns of care and predictors of mortality. Arch Surg. 2009;144:433-9. doi: 10.1001/archsurg.2009.51.
11. Zar FA et al. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302-7. doi: 10.1086/519265. Epub 2007 Jun 19.
12. Bakken JS et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011;9:1044-9. doi: 10.1016/j.cgh.2011.08.014. Epub 2011 Aug 24.
13. Crow JR et al. Probiotics and fecal microbiota transplant for primary and secondary prevention of Clostridium difficile infection. Pharmacotherapy. 2015 Nov;35:1016-25. doi: 10.1002/phar.1644. Epub 2015 Nov 2.
Additional reading
1. McDonald LC et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018 Mar 19;66:987-94. doi: 10.1093/cid/ciy149.
2. Burnham CA et al. Diagnosis of Clostridium difficile infection: an ongoing conundrum for clinicians and for clinical laboratories. Clin Microbiol Rev. 2013 Jul;26:604-30. doi: 10.1128/CMR.00016-13.
3. Crow JR, Davis SL, Chaykosky DM, Smith TT, Smith JM. Probiotics and fecal microbiota transplant for primary and secondary prevention of Clostridium difficile infection. Pharmacotherapy. 2015 Nov; 35:1016-25. doi: 10.1002/phar.1644. Epub 2015 Nov 2. Review.
Key points
1. Metronidazole is inferior to oral vancomycin and fidaxomicin for clinical cure of CDAD. The IDSA/SHEA guidelines now recommend a 10-day course of oral vancomycin or fidaxomicin for nonfulminant cases of CDAD.
2. For fulminant CDAD, the IDSA/SHEA guidelines suggest an increased dose of vancomycin and the addition of IV metronidazole. In such cases, surgical consultation should also be obtained.
3. After the second recurrence of Clostridium difficile infection, hospitalists should consider referral for FMT where available.
Quiz
The recent IDSA/SHEA guidelines no longer recommend metronidazole in the treatment of CDAD, except for which of the following scenarios (best answer)?
A. Treatment of a first episode of nonfulminant CDAD.
B. Treatment of recurrent CDAD following an initial course of oral vancomycin.
C. Treatment of fulminant infection with IV metronidazole in addition to oral or rectal vancomycin.
D. For prophylaxis following fecal microbiota transplant.
Answer: C. In fulminant infection, concurrent ileus may interfere with appropriate delivery of oral vancomycin to the colon. Adding intravenous metronidazole can allow this antibiotic to reach the bowel. Adding intravenous metronidazole to oral vancomycin is also recommended by IDSA/SHEA guidelines in cases of fulminant CDAD. Evidence from high-quality randomized controlled trials has shown that vancomycin is superior to oral metronidazole for treatment of initial and recurrent episodes of CDAD. There is no evidence to support the use of metronidazole for recurrent CDAD following an initial course of oral vancomycin or for prophylaxis following FMT.
Case
A 45-year-old woman on omeprazole for gastroesophageal reflux disease and recent treatment with ciprofloxacin for a urinary tract infection (UTI), who also has had several days of frequent watery stools, is admitted. She does not appear ill, and her abdominal exam is benign. She has normal renal function and white blood cell count. How should she be evaluated and treated for Clostridium difficile–associated disease (CDAD)?
Brief overview
C. difficile, a gram-positive anaerobic bacillus that exists in vegetative and spore forms, is a leading cause of hospital-associated diarrhea. C. difficile has a variety of presentations, ranging from asymptomatic colonization to CDAD, including severe diarrhea, ileus, and megacolon, and may be associated with a fatal outcome on rare occasions. The incidence of CDAD has been rising since the emergence of a hypervirulent strain (NAP1/BI/027) in the early 2000s and, not surprisingly, the number of deaths attributed to CDAD has also increased.1
CDAD requires acquisition of C. difficile as well as alteration in the colonic microbiota, often precipitated by antibiotics. The vegetative form of C. difficile can produce up to three toxins that are responsible for a cascade of reactions beginning with intestinal epithelial cell death followed by a significant inflammatory response and migration of neutrophils that eventually lead to the formation of the characteristic pseudomembranes.2
Until recently, the mainstay treatment for CDAD consisted of metronidazole and oral preparations of vancomycin. Recent results from randomized controlled trials and the increasing popularity of fecal microbiota transplant (FMT), however, have changed the therapeutic landscape of CDAD dramatically. Not surprisingly, the 2017 Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America joint guidelines for CDAD represent a significant change to the treatment of CDAD, compared with previous guidelines.3
Overview of data
The hallmark of CDAD is a watery, nonbloody diarrhea. Given many other causes of diarrhea in hospitalized patients (e.g., direct effect of antibiotics, laxative use, tube feeding, etc.), hospitalists should focus on testing those patients who have three or more episodes of diarrhea in 24 hours and risk factors for CDAD (See Table 1).
Exposure to antibiotics remains the greatest risk factor. It’s important to note that, while most patients develop CDAD within the first month after receiving systemic antibiotics, many patients remain at risk for up to 3 months.4 Although exposure to antibiotics, particularly in health care settings, is a significant risk factor for CDAD, up to 30%-40% of community-associated cases may not have a substantial antibiotic or health care facility exposure.5
Hospitalists should also not overlook the association between proton pump inhibitor (PPI) use and the development of CDAD.3 Although the IDSA/SHEA guidelines do not recommend discontinuation of PPIs solely for treatment or prevention of CDAD, at the minimum, the indication for their continued use in patients with CDAD should be revisited.
Testing for CDAD ranges from immunoassays that detect an enzyme common to all strains of C. difficile, glutamate dehydrogenase antigen (GDH), or toxins to nucleic acid amplification tests (NAATs), such as polymerase chain reaction [PCR]).1,6 GDH tests have high sensitivity but poor specificity, while testing for the toxin has high specificity but lower sensitivity (40%-80%) for CDAD.1 Although NAATs are highly sensitive and specific, they often have a poor positive predictive value in low-risk populations (e.g., those who do not have true diarrhea or whose diarrhea resolves before test results return). In these patients, a positive NAAT test may reflect colonization with toxigenic C. difficile, not necessarily CDAD. Except in rare instances, laboratories should only accept unformed stools for testing. Since the choice of testing for C. difficile varies by institution, hospitalists should understand the algorithm used by their respective hospitals and familiarize themselves with the sensitivity and specificity of each test.
Once a patient is diagnosed with CDAD, the hospitalist should assess the severity of the disease. The IDSA/SHEA guidelines still use leukocytosis and creatinine to separate mild from severe cases; the presence of fever and hypoalbuminemia also points to a more complicated course.3
The treatment of CDAD involves a strategy of withdrawing the putative culprit antibiotic(s) whenever possible and initiating of antibiotics effective against C. difficile. Following the publication of two randomized controlled trials demonstrating the inferiority of metronidazole to vancomycin in clinical cure of CDAD,2,7 the IDSA/SHEA guidelines no longer recommend metronidazole for the treatment of CDAD. Instead, a 10-day course of oral vancomycin or fidaxomicin has been recommended.2 Although fidaxomicin is associated with lower rates of recurrence of CDAD, it is also substantially more expensive than oral vancomycin, with a 10-day course often costing over $3,000.8 When choosing oral vancomycin for completion of therapy following discharge, hospitalists should also consider whether the dispensing outpatient pharmacy can provide the less-expensive liquid preparation of vancomycin. In resource-poor settings, consideration can still be given to metronidazole, an inexpensive drug, compared with both oral vancomycin and fidaxomicin. “Test of cure” with follow-up stool testing is not recommended.
For patients who require systemic antibiotics that precipitated their CDAD, it is common practice to extend CDAD treatment by providing a “tail” coverage with an agent effective against CDAD for 7-10 days following the completion of the inciting antibiotic. A common clinical question relates to the management of patients with prior history of CDAD but in need of a new round of systemic antibiotic therapy. In these patients, concurrent prophylactic doses of oral vancomycin have been found to be effective in preventing recurrence.9 The IDSA/SHEA guidelines conclude that “it may be prudent to administer low doses of vancomycin or fidaxomicin (e.g., 125 mg or 200 mg, respectively, once daily) while systemic antibiotics are administered.”3
For patients whose presentation extends beyond diarrhea, the IDSA/SHEA guidelines have changed the nomenclature for CDAD from “severe, complicated” to “fulminant.” Although there are no strict definitions, the IDSA/SHEA guidelines suggest that fulminant CDAD is characterized by “hypotension or shock, ileus, or megacolon.” In these patients, surgical intervention can be life saving, though mortality rates may remain over 50%.10 Hospitalists whose patients with CDAD are experiencing an acute abdomen or concern for colonic perforation, megacolon, shock, or organ system failure should obtain prompt surgical consultation. Antibiotic treatment should consist of a combination of higher doses of oral vancomycin and intravenous metronidazole (See Table 2).

In addition to occasional treatment failures, a vexing characteristic of CDAD is its frequent recurrence rate, which may range from 15% to 30% or higher.11 The approach to recurrences is twofold: treatment of the C. difficile itself, and attempts to restore the colonic microbiome. The antibiotic treatment of the first recurrence of CDAD consists of either a 10-day course of fidaxomicin or a tapered, pulsed dose of vancomycin, which may be more effective than a repeat 10-day course of oral vancomycin.12 Although the treatment is unchanged for subsequent recurrences, the guidelines suggest consideration of rifaximin after a course of vancomycin (See Table 2).
Probiotics have been investigated as a means of restoring the colonic microbiome. Use of probiotics for both primary and secondary prevention of CDAD has resulted in conflicting data, with pooled analyses showing some benefit, while randomized controlled trials demonstrate less benefit.13 In addition, reports of bloodstream infections with Lactobacillus in frail patients and Saccharomyces in immunocompromised patients and those with central venous catheters raise doubts regarding their safety in certain patient populations.13 The IDSA/SHEA guidelines make no recommendations about the use of probiotics for the prevention of CDAD at this time.
Fecal microbiota transplant (FMT), however, does appear to be effective, especially in comparison to antibiotics alone in patients with multiple recurrences of CDAD.13 The IDSA/SHEA guidelines recommend consideration for FMT after the second recurrence of CDAD. The Fecal Microbiota Transplantation Workgroup has also proposed a set of guidelines for consideration of FMT when available (See Table 3).

Application of data
The recent IDSA/SHEA guidelines have revised the treatment paradigm for CDAD. Most notably, metronidazole is no longer recommended for treatment of either initial or subsequent episodes of mild to severe CDAD, except when the cost of treatment may preclude the use of more effective therapies.
Initial episodes of mild to severe infection should be treated with either oral vancomycin or fidaxomicin. Recurrent episodes of CDAD should be treated with an agent different from that used for the initial episode, or with a pulsed, tapered regimen of oral vancomycin. FMT, where available, should be considered with multiple recurrences, or with moderate to severe infection not responding to standard therapy.
Fulminant CDAD, characterized by hypotension, shock, severe ileus, or megacolon, is a life-threatening medical emergency with a high mortality rate. Treatment should include high-dose oral vancomycin and intravenous metronidazole, with consideration of rectal vancomycin in patients with ileus. Immediate surgical consultation should be obtained to weigh the benefits of colectomy.
Back to our case
Our patient was treated with a 10-day course of vancomycin because this was uncomplicated CDAD and was her initial episode. Were she to develop a recurrence, she could be treated with a pulsed, tapered vancomycin regimen or fidaxomicin.
Bottom line
Vancomycin and fidaxomicin are recommended for the initial episode as well as recurrent CDAD. FMT should be considered for patients with multiple episodes of CDAD or treatment failures.
Dr. Roberts, Dr. Hillman, and Dr. Manian are hospitalists at Massachusetts General Hospital in Boston.
References
1. Louie TJ et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011 Feb 3;364:422-31. doi: 10.1056/NEJMoa0910812.
2. Burnham CA et al. Diagnosis of Clostridium difficile infection: an ongoing conundrum for clinicians and for clinical laboratories. Clin Microbiol Rev. 2013 Jul;26:604-30. doi: 10.1128/CMR.00016-13.
3. McDonald LC et al. Clinical Practice Guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018 Mar 19;66:987-94. doi: 10.1093/cid/ciy149.
4. Hensgens MP et al. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J Antimicrob Chemother. 2012 Mar;67:742-8. doi: 10.1093/jac/dkr508. Epub 2011 Dec 6.
5. Chitnis AS et al. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med. 2013 Jul 22;173:1359-67. doi: 10.1001/jamainternmed.2013.7056.
6. Solomon DA et al. ID learning unit: Understanding and interpreting testing for Clostridium difficile. Open Forum Infectious Diseases. 2014 Mar;1(1);ofu007. doi: 10.1093/ofid/ofu007.
7. Johnson S et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014 Aug 1;59(3):345-54. doi: 10.1093/cid/ciu313. Epub 2014 May 5.
8. https://m.goodrx.com/fidaxomicin, accessed June 24, 2018.
9. Van Hise NW et al. Efficacy of oral vancomycin in preventing recurrent Clostridium difficile infection in patients treated with systemic antimicrobial agents. Clin Infect Dis. 2016 Sep 1;63:651-3. doi: 10.1093/cid/ciw401. Epub 2016 Jun 17.
10. Sailhamer EA et al. Fulminant Clostridium difficile colitis: Patterns of care and predictors of mortality. Arch Surg. 2009;144:433-9. doi: 10.1001/archsurg.2009.51.
11. Zar FA et al. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302-7. doi: 10.1086/519265. Epub 2007 Jun 19.
12. Bakken JS et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011;9:1044-9. doi: 10.1016/j.cgh.2011.08.014. Epub 2011 Aug 24.
13. Crow JR et al. Probiotics and fecal microbiota transplant for primary and secondary prevention of Clostridium difficile infection. Pharmacotherapy. 2015 Nov;35:1016-25. doi: 10.1002/phar.1644. Epub 2015 Nov 2.
Additional reading
1. McDonald LC et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018 Mar 19;66:987-94. doi: 10.1093/cid/ciy149.
2. Burnham CA et al. Diagnosis of Clostridium difficile infection: an ongoing conundrum for clinicians and for clinical laboratories. Clin Microbiol Rev. 2013 Jul;26:604-30. doi: 10.1128/CMR.00016-13.
3. Crow JR, Davis SL, Chaykosky DM, Smith TT, Smith JM. Probiotics and fecal microbiota transplant for primary and secondary prevention of Clostridium difficile infection. Pharmacotherapy. 2015 Nov; 35:1016-25. doi: 10.1002/phar.1644. Epub 2015 Nov 2. Review.
Key points
1. Metronidazole is inferior to oral vancomycin and fidaxomicin for clinical cure of CDAD. The IDSA/SHEA guidelines now recommend a 10-day course of oral vancomycin or fidaxomicin for nonfulminant cases of CDAD.
2. For fulminant CDAD, the IDSA/SHEA guidelines suggest an increased dose of vancomycin and the addition of IV metronidazole. In such cases, surgical consultation should also be obtained.
3. After the second recurrence of Clostridium difficile infection, hospitalists should consider referral for FMT where available.
Quiz
The recent IDSA/SHEA guidelines no longer recommend metronidazole in the treatment of CDAD, except for which of the following scenarios (best answer)?
A. Treatment of a first episode of nonfulminant CDAD.
B. Treatment of recurrent CDAD following an initial course of oral vancomycin.
C. Treatment of fulminant infection with IV metronidazole in addition to oral or rectal vancomycin.
D. For prophylaxis following fecal microbiota transplant.
Answer: C. In fulminant infection, concurrent ileus may interfere with appropriate delivery of oral vancomycin to the colon. Adding intravenous metronidazole can allow this antibiotic to reach the bowel. Adding intravenous metronidazole to oral vancomycin is also recommended by IDSA/SHEA guidelines in cases of fulminant CDAD. Evidence from high-quality randomized controlled trials has shown that vancomycin is superior to oral metronidazole for treatment of initial and recurrent episodes of CDAD. There is no evidence to support the use of metronidazole for recurrent CDAD following an initial course of oral vancomycin or for prophylaxis following FMT.