User login
PATIENT 1: A PERSONAL AND FAMILY HISTORY OF BREAST CANCER
A 55-year-old Ashkenazi Jewish woman presents to your clinic for her annual physical. She reports that she had been diagnosed with breast cancer 10 years ago and that it had been treated with lumpectomy. You recall that Ashkenazi Jewish ethnicity and a diagnosis of breast cancer before age 50 are red flags for a hereditary cancer syndrome, and you ask about her family history of cancer. She reports that her mother was diagnosed with breast cancer in her 60s. The patient wants to know if her daughter should start breast cancer screening.
What do you do next?
Facing increasing demands and a plethora of information to be discussed in a short time, primary care physicians may find it challenging to inform patients about the possibility of a hereditary cancer syndrome, to assess the risk, to organize genetic testing if appropriate, and to counsel patients about their management options. As our knowledge of the genetics of breast cancer continues to expand, this information will become more detailed and complex.
Nevertheless, primary care physicians can help identify patients who may have a syndrome of inherited cancer predisposition or whose family history raises concern for familial breast cancer. Patients in both groups may be candidates for genetic risk assessment, for special management options for women at high risk, or for both.
This article provides an overview of inherited conditions associated with higher breast cancer risk, and guidelines to help physicians recognize patients in their own practice for whom a genetics referral may be appropriate.
BREAST CANCER IS COMPLEX AND HETEROGENEOUS
Breast cancer is the second-leading cause of cancer deaths in women. According to the American Cancer Society, an estimated 234,340 new cases of breast cancer are expected to be diagnosed in women in the United States in 2013, and about 2,240 new cases are expected in men; 39,620 women and 410 men are expected to die of it.1
Breast cancer is a complex and heterogeneous disease, influenced by many factors, of which female sex and increasing age are the most significant. Modifiable risk factors include obesity, use of combined hormone replacement therapy, and physical inactivity. Other risk factors include dense breast tissue, having had a breast biopsy in the past, the finding of atypical hyperplasia on biopsy, a history of high-dose chest radiation, and reproductive factors that include early menarche, late menopause, nulliparity, and birth of first child after age 30.
After female sex and age, family history of the disease is the most significant risk factor for breast cancer.2 If a woman has a first-degree relative (mother, sister, daughter) with breast cancer, her risk is 1.8 times higher, and if she has a second-degree relative (aunt, grandmother) with breast cancer, her risk is 1.3 times higher.3
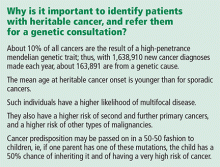
Hereditary cancer predisposition syndromes account for 5% to 10% of cases of breast cancer. These are caused by a germline mutation in a highly penetrant gene that considerably increases the risk of malignancies of the breast and other tissues. These conditions are inherited in an autosomal-dominant fashion, with age of onset tending to be significantly—several decades—younger than the median age of onset in the general population. The most common of these is hereditary breast and ovarian cancer syndrome, caused by germline mutations of the BRCA1 or BRCA2 gene.
Familial breast cancers account for 15% to 20% of cases. Here, the women who develop breast cancer have multiple family members who are also affected but without an obvious inheritance pattern, and the age of onset is similar to that in the general population.4
Sporadic forms of breast cancer account for the remaining 70% to 80% of cases. Their development can be attributed mainly to nonhereditary causes, such as the environmental and personal risk factors listed above. In general, sporadic forms of breast cancer occur at older ages, with no particular inheritance pattern and with frequency of occurrence in a family comparable to that in the general population.
IS A GENETICS CONSULTATION NEEDED?
In the case described above, the primary care physician gathered basic information about the patient’s cancer-related personal and family history. Asking a few key questions (Table 1)5,6 can help physicians understand two important things: whether a more detailed assessment of genetic risk and counseling by a genetics professional are indicated, and whether the patient would benefit from additional cancer screening and prevention.
Table 2 summarizes the National Comprehensive Cancer Network’s recommendations for cancer genetics consultation.5 These red flags for a hereditary breast cancer syndrome can help primary care providers identify patients for whom a cancer genetics referral is appropriate. Of note: the maternal and paternal family histories are equally important.
Because our patient was diagnosed with breast cancer before age 50 and is of Ashkenazi Jewish ethnicity, she meets these criteria and warrants a cancer genetics consultation.
What is a cancer-focused genetic counseling session?
The tenets of genetic counseling, described previously in this series,7 are relevant to hereditary cancer syndromes. Cancer risk assessment and genetic counseling constitute the process of identifying and counseling individuals at risk of familial or hereditary cancer.8
As in other genetic counseling scenarios, a detailed pedigree (family tree) is taken, and this information, along with the patient’s personal medical history, allows a genetics specialist to determine if the presentation is most suggestive of sporadic, familial, or hereditary cancer.
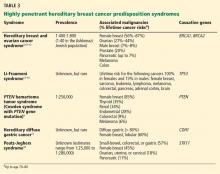
A common misconception among patients is that there is a single genetic test for hereditary breast cancer, when in fact many highly penetrant predisposition genes have been linked to heightened risk (see below). The syndromes summarized in Table 35,9–18 are part of the differential diagnosis for every patient presenting with a personal or family history of breast cancer, and the detailed information from the personal and family history, ascertained during the assessment, ensures the right syndrome is explored within a family.
Cancer-focused genetic counseling may also help a patient or family process the psychological and emotional responses that can occur when cancer risk is discussed: eg, fear of cancer and death; guilt a parent may feel for passing on a genetic predisposition; and survivor guilt experienced by family members who test negative.
Genetic counselors are trained to recognize patients who may benefit from additional counseling. Not all patients pursuing cancer-focused genetic testing need a thorough evaluation by a psychologist, unlike those with adult-onset neurodegenerative conditions such as Huntington disease. Rather, the genetic counselor discusses the psychological implications of cancer-focused genetic testing and can refer the patient to a psychologist, therapist, social worker, or others if he or she feels the patient may benefit.8
Some patients come to a genetic counseling session with concerns about whether their insurance will pay for testing, and about whether they will face discrimination because of the testing results. In most situations, genetic testing is deemed medically necessary and is covered by the patient’s insurance. When testing is necessary, genetic counselors are skilled at preauthorizing it and writing letters of medical necessity. They are also familiar with laws and regulations that protect patients, such as the Genetic Information Nondiscrimination Act, which protects patients from insurance and employment discrimination.
Because a cancer-focused genetic counseling session typically lasts 1 hour, the counselor has enough time to address these and any other concerns that might prevent a patient who is otherwise interested in genetic testing from pursuing it.
HOW CAN GENETIC TESTING HELP?
Genetic testing for hereditary cancer syndromes can have personal benefit for the patient and at-risk family members.
Note that the syndromes in Table 3 all increase the risk of more than one type of cancer. Patients with these syndromes frequently receive care from multiple subspecialists to mitigate those risks. Guidelines exist for each of these syndromes and, if followed, may prevent the morbidity and possibly death from the genotype-specific cancers that would otherwise be in the patient’s future. For patients found to have a hereditary cancer syndrome, medical management options include more-frequent cancer screening or surveillance, prophylactic surgery, and preventive medical treatment, which will be reviewed in a future article in this series.
Identifying the specific mutation in one family member allows at-risk relatives, both female and male, to then take advantage of predictive testing, with genetic counseling. If they test positive for the risk-increasing mutation, they too can take advantage of the management options for people at high risk. If they test negative, they can continue to undergo the same screening as recommended for the general population. Also, they may be relieved to know that their cancer risk is no greater than that in the general population.
The American Society of Clinical Oncology9 recommends genetic counseling and testing when all of the following are true:
- There is a personal or family history suggesting genetic cancer susceptibility
- The test can be adequately interpreted
- The results will aid in the diagnosis or influence the medical or surgical management of the patient or family at hereditary risk of cancer.
Professional society guidelines also recommend that genetic testing be done only with genetic counseling before and after.5,6,8 The National Society of Genetic Counselors provides a list of clinical genetic counselors, organized by geographical area, at www.nsgc.org.
PATIENT 1 RECEIVES GENETIC TESTING AND COUNSELING
Let’s return to the Ashkenazi Jewish patient who has a personal and family history of breast cancer, whom you referred for cancer genetics consultation and who attends this appointment. A detailed personal and family history is gathered, and a brief physical examination is done, which reveals that the patient has macrocephaly and a history of multiple uterine fibroids.
The genetic differential diagnosis for your patient includes hereditary breast and ovarian cancer syndrome (resulting from mutations in the BRCA1 and BRCA2 genes) and Cowden syndrome (from mutations in the PTEN gene) (TABLE 3). The counselor uses BRCAPRO, a statistical risk-assessment tool that estimates a patient’s risk of harboring a BRCA1 or BRCA2 mutation based on ethnicity and personal and family history of cancer, and finds her risk to be 31%. In view of this risk, genetic testing for BRCA1 and BRCA2 is offered after a detailed discussion of the genetic differential diagnosis, the implications of a positive vs a negative test result, the possibility of finding gene changes (variants) of unknown significance, and the implications of the test results for family members.
Your patient elects to pursue BRCA1 and BRCA2 genetic testing and the results are negative—no mutations in either gene are found. PTEN testing is recommended next, which your patient elects to undergo. A mutation in the PTEN gene is found, indicating that she has Cowden syndrome. This result and its implications are discussed in a posttest genetic counseling session.
Cowden syndrome is an autosomal-dominant condition that carries a heightened risk of benign and malignant neoplasms, including a lifetime risk of breast cancer of up to 85%, with the average age at diagnosis in the 40s. Mutations in the PTEN gene also predispose to other cancer types, including nonmedullary thyroid, uterine, renal, and colorectal cancers, as well as melanoma.9 Multiple benign skin lesions and gastrointestinal polyposis are common.20
During the appointment, medical management options for patients with PTEN mutations are presented (Table 4).9 Given that your patient’s breast cancer was initially treated with lumpectomy, her remaining breast tissue is at risk of a second malignancy. She has never undergone thyroid imaging, colonoscopy, or kidney imaging. She reports that lately she has had occasional abnormal uterine bleeding and pain, which she believes are caused by her uterine fibroids. Given these symptoms and in light of her PTEN mutation, hysterectomy may be presented to her as an option. The genetics team sends a detailed clinical note directly to the primary care physician so they can coordinate and “quarterback” the patient’s care.
Like many patients, your patient is very concerned about how this information may affect her daughter. She first expresses some guilt at having to tell her daughter that she may have “given” her a risk of cancer. However, during the course of the genetic counseling session, she accepts that she could not have prevented her daughter from possibly inheriting this mutation, and understands that sharing this information will enable her daughter to pursue testing to help her understand her own risks.
When a known mutation exists in the family, as is the case with your patient, predictive testing only for that mutation gives a 100% accurate result. During a separate genetic counseling appointment, the patient’s daughter opts to proceed with testing and is found to be negative for her mother’s PTEN mutation.
WHAT HAPPENS WHEN GENETIC TESTING IS NOT INDICATED?
Cancer genetic risk assessment and counseling provides benefits even when genetic testing is not indicated. In some situations genetic testing is not warranted, but referral for heightened surveillance for breast cancer is deemed necessary. Patients who have a personal or family history of cancer can still gain from a detailed assessment of their personal and family history and may come away relieved after learning that they or their family members are not at high risk of developing cancer. Such patients or families may be classified as demonstrating either familial or sporadic breast cancer diagnoses.
Familial breast cancer
Familial breast cancers, believed to account for 15% to 20% of all cases of breast cancer, share features with hereditary breast cancer syndromes.4 In affected families, the frequency of breast cancer is higher than in the general population (multiple family members may be affected), and the age of onset tends to be close to that in the general population.
Members of a family with familial breast cancer who have not yet developed the disease may be at increased risk of it. Several risk-assessment tools (the Gail, Tyrer-Cuzick, Claus, and other models)21–25 use personal and family history to estimate breast cancer risk.
Depending on the assessed risk, additional options for screening and surveillance are available. The American Cancer Society recommends magnetic resonance imaging (MRI) in addition to annual mammography for women whose lifetime risk of breast cancer is greater than 20%. They also recommend that women at moderately increased risk (ie, 15%–20% lifetime risk) talk to their doctor about the benefits and limitations of adding MRI screening to yearly mammography.1
Sporadic breast cancer
Sporadic forms of breast cancer account for 70% to 80% of cases of breast cancer. Sporadic breast cancers are thought to have mainly nonhereditary causes, with environment and personal risk factors playing a large role.
Women with apparently sporadic breast cancers are diagnosed at or beyond the average age at diagnosis in the general population and do not have a family history that suggests either a hereditary cancer syndrome or familial breast cancer. If they undergo a cancer risk assessment, they may be relieved to learn that other women in their family do not have a high probability of being affected, and that they themselves do not appear to be at increased risk of other malignancies.
PATIENT 2: NEGATIVE TEST RESULTS ARE SOMETIMES ‘UNINFORMATIVE’
A healthy 35-year-old woman is referred for a genetics consultation by her gynecologist because her mother developed breast cancer at age 40 and died of the disease. A detailed personal and family history and risk assessment are done. After pretest genetic counseling, testing for BRCA1 and BRCA2 mutations (hereditary breast and ovarian cancer syndrome) is ordered, and the patient’s test results are negative. Risk assessment determines that no other hereditary cancer syndrome is likely. Therefore, no other genetic testing is offered at this time.
Genetic testing is most informative when performed first on the family member at highest risk of having a mutation. For families with breast cancer, this is typically the person with cancer diagnosed at the earliest age.
Unfortunately, sometimes these family members cannot be tested because they are deceased or otherwise unavailable. In such situations, it is acceptable to offer testing to a close, unaffected relative, such as your patient. Pretest genetic counseling in these circumstances is key, highlighting the fact that negative (normal) results would be uninformative. In your case, we cannot know whether the patient’s mother would have tested positive for a BRCA1 or BRCA2 mutation and your patient is a “true negative,” or whether her mother would have tested negative as well.
In unaffected patients with uninformative genetic testing results, medical management is based on the patient’s personal risk factors and family history of cancer. For your patient, statistical risk modeling tools (the Gail, Claus, Couch, and Tyrer-Cuzick models) determine that her risk of developing breast cancer is 22% to 28.5%, qualifying her for MRI along with yearly mammography per the American Cancer Society guidelines previously discussed.
KNOWLEDGE CONTINUES TO EXPAND
Major advances in the understanding of breast cancer susceptibility were made in the last decade through genetic linkage mapping in families that have an overabundance of members with breast cancer.26–28 Additionally, as more information is acquired, other genes predisposing to cancer or modifying cancer risk may be identified and additional knowledge gained.
With the advent of gene-panel-based testing and exome sequencing, we will incidentally discover mutations that predispose to cancer in patients in whom we were not looking for these mutations. With improving technology and value-based health care delivery, providers must continue to embrace multidisciplinary care, and genetics will become central in guiding medical management. In the event of an incidental finding suggesting susceptibility to heritable cancer, a consult to genetic counseling is recommended.
Many studies of the genetics of breast cancer are now focusing on known hereditary breast cancer syndromes and on possibilities for risk reduction, lifestyle modification, and identification of genetic variations that may increase or decrease cancer risk for an individual patient. The Center for Personalized Genetic Healthcare at Cleveland Clinic is collaborating in one such study. Titled “Risk Factor Analysis of Hereditary Breast and Ovarian Cancer Syndrome,” it is an international study led by a leading breast cancer researcher, Dr. Steven Narod from the Women’s College Research Institute in Toronto, ON. This study is focusing on women with a BRCA1 or BRCA2 mutation and their personal cancer risk factors, lifestyle choices, and overall development of cancer. This research group and others are also focusing on identifying genetic “modifiers” of cancer risk in these high-risk women.29
For patients who do not have a hereditary cancer syndrome, research is further exploring novel genes and their relation to breast cancer risk. One such study in our laboratory has found that several genes once thought only to cause an increased risk of hereditary paraganglioma may also predispose to breast and thyroid cancer.29,31 Additional research in this area is under way to clarify these risks.
GOOD SCIENCE, BAD MEDICINE?
Other research studies have identified a number of genes currently thought to be “moderately penetrant” for breast cancer risk, meaning that they may confer a risk of breast cancer slightly greater than that in the general population, but in some instances the risk has not been proven to be high enough to alter a patient’s management.32,33
Although a few clinical laboratories currently offer testing for these kinds of genes, the clinical utility of this testing is questionable. Before offering testing on a clinical basis, we need clear, consistent data on the types of cancers associated with these genes and on the lifetime percentage risk of acquiring these cancers. Currently, it is difficult to understand whether a variant in a moderately penetrant gene is the true explanation behind a patient’s breast cancer diagnosis. If such a variant is identified and family members pursue testing for it, should those family members who test negative be considered to have the same risk of cancer as the general population? And should family members testing positive be offered prophylactic surgical options?
Without more data these questions cannot be answered, and until such data are gathered, we believe that testing for moderately penetrant genes should not be performed outside of a research study. The Center for Personalized Genetic Healthcare in Cleveland Clinic’s Genomic Medicine Institute can assist in educating and coordinating patients’ enrollment in such research studies.
PUTTING IT ALL TOGETHER
Primary care physicians are the first-line providers to individuals and families, many of whom have a personal or family history of breast cancer. Identifying patients at risk of breast cancer and hereditary cancer syndromes can be challenging in this era of shortened appointment times and patients with complex medical histories.
Reviewing an individual’s personal and cancer family history is a necessary first step in considering appropriate medical management recommendations for cancer screening and prevention, the cornerstone of personalized health care. Patients with hereditary breast cancer syndromes and those with familial breast cancer can benefit from high-risk breast cancer surveillance.
Cancer genetics risk assessment ensures that the correct genetic testing is offered to the most appropriate patients, with personalized interpretation of results and provision of future management recommendations based on the individual patient’s personal and family history. Genetic counselors empower patients to make educated and informed decisions about genetic testing, cancer screening, and prevention.
As health care continues to focus more on prevention in this new era of genomic medicine and value-based delivery of health care, genetic counselors will serve as powerful allies to physicians.34
Acknowledgments: We would like to thank Dr. Colleen Clayton and Dr. Lynn Pattimakiel of the Medicine Institute, Cleveland Clinic, for their critical review of and thoughtful feedback on this manuscript.
- American Cancer Society. Breast cancer: detailed guide( 2013). http://www.cancer.org/Cancer/BreastCancer/DetailedGuide/index. Accessed November 12, 2013.
- McTiernan A, Gilligan MA, Redmond C. Assessing individual risk for breast cancer: risky business. J Clin Epidemiol 1997; 50:547–556.
- Teerlink CC, Albright FS, Lins L, Cannon-Albright LA. A comprehensive survey of cancer risks in extended families. Genet Med 2012; 14:107–114.
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology. Breast cancer risk reduction (version 1.2013). http://www.nccn.org. Accessed November 21, 2013.
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology. Genetic/familial high risk assessment: breast and ovarian (version 4.2013). http://www.nccn.org. Accessed November 21, 2013.
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology. Breast cancer screening and diagnosis (version 2.2013). http://www.nccn.org. Accessed November 21, 2013.
- Mester JL, Schreiber AH, Moran RT. Genetic counselors: your partners in clinical practice. Cleve Clin J Med 2012; 79:560–568.
- Trepanier A, Ahrens M, McKinnon W, et al; National Society of Genetic Counselors. Genetic cancer risk assessment and counseling: recommendations of the National Society of Genetic Counselors. J Genet Couns 2004; 13:83–114.
- Tan MH, Mester JL, Ngeow J, Rybicki LA, Orloff MS, Eng C. Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res 2012; 18:400–407.
- Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet 1998; 62:676–689.
- Liede A, Karlan BY, Narod SA. Cancer risks for male carriers of germline mutations in BRCA1 or BRCA2: a review of the literature. J Clin Oncol 2004; 22:735–742.
- Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med 1997; 336:1401–1408.
- Birch JM, Hartley AL, Tricker KJ, et al. Prevalence and diversity of constitutional mutations in the p53 gene among 21 Li-Fraumeni families. Cancer Res 1994; 54:1298–1304.
- Chompret A, Brugières L, Ronsin M, et al. P53 germline mutations in childhood cancers and cancer risk for carrier individuals. Br J Cancer 2000; 82:1932–1937.
- Gonzalez KD, Noltner KA, Buzin CH, et al. Beyond Li Fraumeni syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol 2009; 27:1250–1256.
- Varley JM. Germline TP53 mutations and Li-Fraumeni syndrome. Hum Mutat 2003; 21:313–320.
- Fitzgerald RC, Hardwick R, Huntsman D, et al; International Gastric Cancer Linkage Consortium. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet 2010; 47:436–444.
- Hearle N, Schumacher V, Menko FH, et al. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res 2006; 12:3209–3215.
- American Society of Clinical Oncology. American Society of Clinical Oncology policy statement update: genetic testing for cancer susceptibility. J Clin Oncol 2003; 21:2397–2406.
- Mester J, Eng C. When overgrowth bumps into cancer: the PTEN-opathies. Am J Med Genet C Semin Med Genet 2013; 163:114–121.
- Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer. Implications for risk prediction. Cancer 1994; 73:643–651.
- Couch FJ, DeShano ML, Blackwood MA, et al. BRCA1 mutations in women attending clinics that evaluate the risk of breast cancer. N Engl J Med 1997; 336:1409–1415.
- Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med 2004; 23:1111–1130.
- Gail MH, Anderson WF, Garcia-Closas M, Sherman ME. Absolute risk models for subtypes of breast cancer. J Natl Cancer Inst 2007; 99:1657–1659.
- Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 1989; 81:1879–1886.
- Kent P, O’Donoghue JM, O’Hanlon DM, Kerin MJ, Maher DJ, Given HF. Linkage analysis and the susceptibility gene (BRCA-1) in familial breast cancer. Eur J Surg Oncol 1995; 21:240–241.
- Easton DF, Bishop DT, Ford D, Crockford GP. Genetic linkage analysis in familial breast and ovarian cancer: results from 214 families. The Breast Cancer Linkage Consortium. Am J Hum Genet 1993; 52:678–701.
- Ormiston W. Hereditary breast cancer. Eur J Cancer Care (Engl) 1996; 5:13–20.
- Couch FJ, Wang X, McGuffog L, et al. Genome-wide association study in BRCA1 mutation carriers identifies novel loci associated with breast and ovarian cancer risk. PLoS Genet 2013; 9:e1003212.
- Bennett KL, Mester J, Eng C. Germline epigenetic regulation of KILLIN in Cowden and Cowden-like syndrome. JAMA 2010; 304:2724–2731.
- Ni Y, He X, Chen J, et al. Germline SDHx variants modify breast and thyroid cancer risks in Cowden and Cowden-like syndrome via FAD/NAD-dependent destabilization of p53. Hum Mol Genet 2012; 21:300–310.
- Casadei S, Norquist BM, Walsh T, et al. Contribution of inherited mutations in the BRCA2-interacting protein PALB2 to familial breast cancer. Cancer Res 2011; 71:2222–2229.
- Walsh T, Lee MK, Casadei S, et al. Detection of inherited mutations for breast and ovarian cancer using genomic capture and massively parallel sequencing. Proc Natl Acad Sci U S A 2010; 107:12629–12633.
- Eng C. Molecular genetics to genomic medicine: at the heart of value-based delivery of healthcare. Mol Genet Genom Med 2013; 1:4–6.
PATIENT 1: A PERSONAL AND FAMILY HISTORY OF BREAST CANCER
A 55-year-old Ashkenazi Jewish woman presents to your clinic for her annual physical. She reports that she had been diagnosed with breast cancer 10 years ago and that it had been treated with lumpectomy. You recall that Ashkenazi Jewish ethnicity and a diagnosis of breast cancer before age 50 are red flags for a hereditary cancer syndrome, and you ask about her family history of cancer. She reports that her mother was diagnosed with breast cancer in her 60s. The patient wants to know if her daughter should start breast cancer screening.
What do you do next?
Facing increasing demands and a plethora of information to be discussed in a short time, primary care physicians may find it challenging to inform patients about the possibility of a hereditary cancer syndrome, to assess the risk, to organize genetic testing if appropriate, and to counsel patients about their management options. As our knowledge of the genetics of breast cancer continues to expand, this information will become more detailed and complex.
Nevertheless, primary care physicians can help identify patients who may have a syndrome of inherited cancer predisposition or whose family history raises concern for familial breast cancer. Patients in both groups may be candidates for genetic risk assessment, for special management options for women at high risk, or for both.
This article provides an overview of inherited conditions associated with higher breast cancer risk, and guidelines to help physicians recognize patients in their own practice for whom a genetics referral may be appropriate.
BREAST CANCER IS COMPLEX AND HETEROGENEOUS
Breast cancer is the second-leading cause of cancer deaths in women. According to the American Cancer Society, an estimated 234,340 new cases of breast cancer are expected to be diagnosed in women in the United States in 2013, and about 2,240 new cases are expected in men; 39,620 women and 410 men are expected to die of it.1
Breast cancer is a complex and heterogeneous disease, influenced by many factors, of which female sex and increasing age are the most significant. Modifiable risk factors include obesity, use of combined hormone replacement therapy, and physical inactivity. Other risk factors include dense breast tissue, having had a breast biopsy in the past, the finding of atypical hyperplasia on biopsy, a history of high-dose chest radiation, and reproductive factors that include early menarche, late menopause, nulliparity, and birth of first child after age 30.
After female sex and age, family history of the disease is the most significant risk factor for breast cancer.2 If a woman has a first-degree relative (mother, sister, daughter) with breast cancer, her risk is 1.8 times higher, and if she has a second-degree relative (aunt, grandmother) with breast cancer, her risk is 1.3 times higher.3
Hereditary cancer predisposition syndromes account for 5% to 10% of cases of breast cancer. These are caused by a germline mutation in a highly penetrant gene that considerably increases the risk of malignancies of the breast and other tissues. These conditions are inherited in an autosomal-dominant fashion, with age of onset tending to be significantly—several decades—younger than the median age of onset in the general population. The most common of these is hereditary breast and ovarian cancer syndrome, caused by germline mutations of the BRCA1 or BRCA2 gene.
Familial breast cancers account for 15% to 20% of cases. Here, the women who develop breast cancer have multiple family members who are also affected but without an obvious inheritance pattern, and the age of onset is similar to that in the general population.4
Sporadic forms of breast cancer account for the remaining 70% to 80% of cases. Their development can be attributed mainly to nonhereditary causes, such as the environmental and personal risk factors listed above. In general, sporadic forms of breast cancer occur at older ages, with no particular inheritance pattern and with frequency of occurrence in a family comparable to that in the general population.
IS A GENETICS CONSULTATION NEEDED?
In the case described above, the primary care physician gathered basic information about the patient’s cancer-related personal and family history. Asking a few key questions (Table 1)5,6 can help physicians understand two important things: whether a more detailed assessment of genetic risk and counseling by a genetics professional are indicated, and whether the patient would benefit from additional cancer screening and prevention.
Table 2 summarizes the National Comprehensive Cancer Network’s recommendations for cancer genetics consultation.5 These red flags for a hereditary breast cancer syndrome can help primary care providers identify patients for whom a cancer genetics referral is appropriate. Of note: the maternal and paternal family histories are equally important.
Because our patient was diagnosed with breast cancer before age 50 and is of Ashkenazi Jewish ethnicity, she meets these criteria and warrants a cancer genetics consultation.
What is a cancer-focused genetic counseling session?
The tenets of genetic counseling, described previously in this series,7 are relevant to hereditary cancer syndromes. Cancer risk assessment and genetic counseling constitute the process of identifying and counseling individuals at risk of familial or hereditary cancer.8
As in other genetic counseling scenarios, a detailed pedigree (family tree) is taken, and this information, along with the patient’s personal medical history, allows a genetics specialist to determine if the presentation is most suggestive of sporadic, familial, or hereditary cancer.
A common misconception among patients is that there is a single genetic test for hereditary breast cancer, when in fact many highly penetrant predisposition genes have been linked to heightened risk (see below). The syndromes summarized in Table 35,9–18 are part of the differential diagnosis for every patient presenting with a personal or family history of breast cancer, and the detailed information from the personal and family history, ascertained during the assessment, ensures the right syndrome is explored within a family.
Cancer-focused genetic counseling may also help a patient or family process the psychological and emotional responses that can occur when cancer risk is discussed: eg, fear of cancer and death; guilt a parent may feel for passing on a genetic predisposition; and survivor guilt experienced by family members who test negative.
Genetic counselors are trained to recognize patients who may benefit from additional counseling. Not all patients pursuing cancer-focused genetic testing need a thorough evaluation by a psychologist, unlike those with adult-onset neurodegenerative conditions such as Huntington disease. Rather, the genetic counselor discusses the psychological implications of cancer-focused genetic testing and can refer the patient to a psychologist, therapist, social worker, or others if he or she feels the patient may benefit.8
Some patients come to a genetic counseling session with concerns about whether their insurance will pay for testing, and about whether they will face discrimination because of the testing results. In most situations, genetic testing is deemed medically necessary and is covered by the patient’s insurance. When testing is necessary, genetic counselors are skilled at preauthorizing it and writing letters of medical necessity. They are also familiar with laws and regulations that protect patients, such as the Genetic Information Nondiscrimination Act, which protects patients from insurance and employment discrimination.
Because a cancer-focused genetic counseling session typically lasts 1 hour, the counselor has enough time to address these and any other concerns that might prevent a patient who is otherwise interested in genetic testing from pursuing it.
HOW CAN GENETIC TESTING HELP?
Genetic testing for hereditary cancer syndromes can have personal benefit for the patient and at-risk family members.
Note that the syndromes in Table 3 all increase the risk of more than one type of cancer. Patients with these syndromes frequently receive care from multiple subspecialists to mitigate those risks. Guidelines exist for each of these syndromes and, if followed, may prevent the morbidity and possibly death from the genotype-specific cancers that would otherwise be in the patient’s future. For patients found to have a hereditary cancer syndrome, medical management options include more-frequent cancer screening or surveillance, prophylactic surgery, and preventive medical treatment, which will be reviewed in a future article in this series.
Identifying the specific mutation in one family member allows at-risk relatives, both female and male, to then take advantage of predictive testing, with genetic counseling. If they test positive for the risk-increasing mutation, they too can take advantage of the management options for people at high risk. If they test negative, they can continue to undergo the same screening as recommended for the general population. Also, they may be relieved to know that their cancer risk is no greater than that in the general population.
The American Society of Clinical Oncology9 recommends genetic counseling and testing when all of the following are true:
- There is a personal or family history suggesting genetic cancer susceptibility
- The test can be adequately interpreted
- The results will aid in the diagnosis or influence the medical or surgical management of the patient or family at hereditary risk of cancer.
Professional society guidelines also recommend that genetic testing be done only with genetic counseling before and after.5,6,8 The National Society of Genetic Counselors provides a list of clinical genetic counselors, organized by geographical area, at www.nsgc.org.
PATIENT 1 RECEIVES GENETIC TESTING AND COUNSELING
Let’s return to the Ashkenazi Jewish patient who has a personal and family history of breast cancer, whom you referred for cancer genetics consultation and who attends this appointment. A detailed personal and family history is gathered, and a brief physical examination is done, which reveals that the patient has macrocephaly and a history of multiple uterine fibroids.
The genetic differential diagnosis for your patient includes hereditary breast and ovarian cancer syndrome (resulting from mutations in the BRCA1 and BRCA2 genes) and Cowden syndrome (from mutations in the PTEN gene) (TABLE 3). The counselor uses BRCAPRO, a statistical risk-assessment tool that estimates a patient’s risk of harboring a BRCA1 or BRCA2 mutation based on ethnicity and personal and family history of cancer, and finds her risk to be 31%. In view of this risk, genetic testing for BRCA1 and BRCA2 is offered after a detailed discussion of the genetic differential diagnosis, the implications of a positive vs a negative test result, the possibility of finding gene changes (variants) of unknown significance, and the implications of the test results for family members.
Your patient elects to pursue BRCA1 and BRCA2 genetic testing and the results are negative—no mutations in either gene are found. PTEN testing is recommended next, which your patient elects to undergo. A mutation in the PTEN gene is found, indicating that she has Cowden syndrome. This result and its implications are discussed in a posttest genetic counseling session.
Cowden syndrome is an autosomal-dominant condition that carries a heightened risk of benign and malignant neoplasms, including a lifetime risk of breast cancer of up to 85%, with the average age at diagnosis in the 40s. Mutations in the PTEN gene also predispose to other cancer types, including nonmedullary thyroid, uterine, renal, and colorectal cancers, as well as melanoma.9 Multiple benign skin lesions and gastrointestinal polyposis are common.20
During the appointment, medical management options for patients with PTEN mutations are presented (Table 4).9 Given that your patient’s breast cancer was initially treated with lumpectomy, her remaining breast tissue is at risk of a second malignancy. She has never undergone thyroid imaging, colonoscopy, or kidney imaging. She reports that lately she has had occasional abnormal uterine bleeding and pain, which she believes are caused by her uterine fibroids. Given these symptoms and in light of her PTEN mutation, hysterectomy may be presented to her as an option. The genetics team sends a detailed clinical note directly to the primary care physician so they can coordinate and “quarterback” the patient’s care.
Like many patients, your patient is very concerned about how this information may affect her daughter. She first expresses some guilt at having to tell her daughter that she may have “given” her a risk of cancer. However, during the course of the genetic counseling session, she accepts that she could not have prevented her daughter from possibly inheriting this mutation, and understands that sharing this information will enable her daughter to pursue testing to help her understand her own risks.
When a known mutation exists in the family, as is the case with your patient, predictive testing only for that mutation gives a 100% accurate result. During a separate genetic counseling appointment, the patient’s daughter opts to proceed with testing and is found to be negative for her mother’s PTEN mutation.
WHAT HAPPENS WHEN GENETIC TESTING IS NOT INDICATED?
Cancer genetic risk assessment and counseling provides benefits even when genetic testing is not indicated. In some situations genetic testing is not warranted, but referral for heightened surveillance for breast cancer is deemed necessary. Patients who have a personal or family history of cancer can still gain from a detailed assessment of their personal and family history and may come away relieved after learning that they or their family members are not at high risk of developing cancer. Such patients or families may be classified as demonstrating either familial or sporadic breast cancer diagnoses.
Familial breast cancer
Familial breast cancers, believed to account for 15% to 20% of all cases of breast cancer, share features with hereditary breast cancer syndromes.4 In affected families, the frequency of breast cancer is higher than in the general population (multiple family members may be affected), and the age of onset tends to be close to that in the general population.
Members of a family with familial breast cancer who have not yet developed the disease may be at increased risk of it. Several risk-assessment tools (the Gail, Tyrer-Cuzick, Claus, and other models)21–25 use personal and family history to estimate breast cancer risk.
Depending on the assessed risk, additional options for screening and surveillance are available. The American Cancer Society recommends magnetic resonance imaging (MRI) in addition to annual mammography for women whose lifetime risk of breast cancer is greater than 20%. They also recommend that women at moderately increased risk (ie, 15%–20% lifetime risk) talk to their doctor about the benefits and limitations of adding MRI screening to yearly mammography.1
Sporadic breast cancer
Sporadic forms of breast cancer account for 70% to 80% of cases of breast cancer. Sporadic breast cancers are thought to have mainly nonhereditary causes, with environment and personal risk factors playing a large role.
Women with apparently sporadic breast cancers are diagnosed at or beyond the average age at diagnosis in the general population and do not have a family history that suggests either a hereditary cancer syndrome or familial breast cancer. If they undergo a cancer risk assessment, they may be relieved to learn that other women in their family do not have a high probability of being affected, and that they themselves do not appear to be at increased risk of other malignancies.
PATIENT 2: NEGATIVE TEST RESULTS ARE SOMETIMES ‘UNINFORMATIVE’
A healthy 35-year-old woman is referred for a genetics consultation by her gynecologist because her mother developed breast cancer at age 40 and died of the disease. A detailed personal and family history and risk assessment are done. After pretest genetic counseling, testing for BRCA1 and BRCA2 mutations (hereditary breast and ovarian cancer syndrome) is ordered, and the patient’s test results are negative. Risk assessment determines that no other hereditary cancer syndrome is likely. Therefore, no other genetic testing is offered at this time.
Genetic testing is most informative when performed first on the family member at highest risk of having a mutation. For families with breast cancer, this is typically the person with cancer diagnosed at the earliest age.
Unfortunately, sometimes these family members cannot be tested because they are deceased or otherwise unavailable. In such situations, it is acceptable to offer testing to a close, unaffected relative, such as your patient. Pretest genetic counseling in these circumstances is key, highlighting the fact that negative (normal) results would be uninformative. In your case, we cannot know whether the patient’s mother would have tested positive for a BRCA1 or BRCA2 mutation and your patient is a “true negative,” or whether her mother would have tested negative as well.
In unaffected patients with uninformative genetic testing results, medical management is based on the patient’s personal risk factors and family history of cancer. For your patient, statistical risk modeling tools (the Gail, Claus, Couch, and Tyrer-Cuzick models) determine that her risk of developing breast cancer is 22% to 28.5%, qualifying her for MRI along with yearly mammography per the American Cancer Society guidelines previously discussed.
KNOWLEDGE CONTINUES TO EXPAND
Major advances in the understanding of breast cancer susceptibility were made in the last decade through genetic linkage mapping in families that have an overabundance of members with breast cancer.26–28 Additionally, as more information is acquired, other genes predisposing to cancer or modifying cancer risk may be identified and additional knowledge gained.
With the advent of gene-panel-based testing and exome sequencing, we will incidentally discover mutations that predispose to cancer in patients in whom we were not looking for these mutations. With improving technology and value-based health care delivery, providers must continue to embrace multidisciplinary care, and genetics will become central in guiding medical management. In the event of an incidental finding suggesting susceptibility to heritable cancer, a consult to genetic counseling is recommended.
Many studies of the genetics of breast cancer are now focusing on known hereditary breast cancer syndromes and on possibilities for risk reduction, lifestyle modification, and identification of genetic variations that may increase or decrease cancer risk for an individual patient. The Center for Personalized Genetic Healthcare at Cleveland Clinic is collaborating in one such study. Titled “Risk Factor Analysis of Hereditary Breast and Ovarian Cancer Syndrome,” it is an international study led by a leading breast cancer researcher, Dr. Steven Narod from the Women’s College Research Institute in Toronto, ON. This study is focusing on women with a BRCA1 or BRCA2 mutation and their personal cancer risk factors, lifestyle choices, and overall development of cancer. This research group and others are also focusing on identifying genetic “modifiers” of cancer risk in these high-risk women.29
For patients who do not have a hereditary cancer syndrome, research is further exploring novel genes and their relation to breast cancer risk. One such study in our laboratory has found that several genes once thought only to cause an increased risk of hereditary paraganglioma may also predispose to breast and thyroid cancer.29,31 Additional research in this area is under way to clarify these risks.
GOOD SCIENCE, BAD MEDICINE?
Other research studies have identified a number of genes currently thought to be “moderately penetrant” for breast cancer risk, meaning that they may confer a risk of breast cancer slightly greater than that in the general population, but in some instances the risk has not been proven to be high enough to alter a patient’s management.32,33
Although a few clinical laboratories currently offer testing for these kinds of genes, the clinical utility of this testing is questionable. Before offering testing on a clinical basis, we need clear, consistent data on the types of cancers associated with these genes and on the lifetime percentage risk of acquiring these cancers. Currently, it is difficult to understand whether a variant in a moderately penetrant gene is the true explanation behind a patient’s breast cancer diagnosis. If such a variant is identified and family members pursue testing for it, should those family members who test negative be considered to have the same risk of cancer as the general population? And should family members testing positive be offered prophylactic surgical options?
Without more data these questions cannot be answered, and until such data are gathered, we believe that testing for moderately penetrant genes should not be performed outside of a research study. The Center for Personalized Genetic Healthcare in Cleveland Clinic’s Genomic Medicine Institute can assist in educating and coordinating patients’ enrollment in such research studies.
PUTTING IT ALL TOGETHER
Primary care physicians are the first-line providers to individuals and families, many of whom have a personal or family history of breast cancer. Identifying patients at risk of breast cancer and hereditary cancer syndromes can be challenging in this era of shortened appointment times and patients with complex medical histories.
Reviewing an individual’s personal and cancer family history is a necessary first step in considering appropriate medical management recommendations for cancer screening and prevention, the cornerstone of personalized health care. Patients with hereditary breast cancer syndromes and those with familial breast cancer can benefit from high-risk breast cancer surveillance.
Cancer genetics risk assessment ensures that the correct genetic testing is offered to the most appropriate patients, with personalized interpretation of results and provision of future management recommendations based on the individual patient’s personal and family history. Genetic counselors empower patients to make educated and informed decisions about genetic testing, cancer screening, and prevention.
As health care continues to focus more on prevention in this new era of genomic medicine and value-based delivery of health care, genetic counselors will serve as powerful allies to physicians.34
Acknowledgments: We would like to thank Dr. Colleen Clayton and Dr. Lynn Pattimakiel of the Medicine Institute, Cleveland Clinic, for their critical review of and thoughtful feedback on this manuscript.
PATIENT 1: A PERSONAL AND FAMILY HISTORY OF BREAST CANCER
A 55-year-old Ashkenazi Jewish woman presents to your clinic for her annual physical. She reports that she had been diagnosed with breast cancer 10 years ago and that it had been treated with lumpectomy. You recall that Ashkenazi Jewish ethnicity and a diagnosis of breast cancer before age 50 are red flags for a hereditary cancer syndrome, and you ask about her family history of cancer. She reports that her mother was diagnosed with breast cancer in her 60s. The patient wants to know if her daughter should start breast cancer screening.
What do you do next?
Facing increasing demands and a plethora of information to be discussed in a short time, primary care physicians may find it challenging to inform patients about the possibility of a hereditary cancer syndrome, to assess the risk, to organize genetic testing if appropriate, and to counsel patients about their management options. As our knowledge of the genetics of breast cancer continues to expand, this information will become more detailed and complex.
Nevertheless, primary care physicians can help identify patients who may have a syndrome of inherited cancer predisposition or whose family history raises concern for familial breast cancer. Patients in both groups may be candidates for genetic risk assessment, for special management options for women at high risk, or for both.
This article provides an overview of inherited conditions associated with higher breast cancer risk, and guidelines to help physicians recognize patients in their own practice for whom a genetics referral may be appropriate.
BREAST CANCER IS COMPLEX AND HETEROGENEOUS
Breast cancer is the second-leading cause of cancer deaths in women. According to the American Cancer Society, an estimated 234,340 new cases of breast cancer are expected to be diagnosed in women in the United States in 2013, and about 2,240 new cases are expected in men; 39,620 women and 410 men are expected to die of it.1
Breast cancer is a complex and heterogeneous disease, influenced by many factors, of which female sex and increasing age are the most significant. Modifiable risk factors include obesity, use of combined hormone replacement therapy, and physical inactivity. Other risk factors include dense breast tissue, having had a breast biopsy in the past, the finding of atypical hyperplasia on biopsy, a history of high-dose chest radiation, and reproductive factors that include early menarche, late menopause, nulliparity, and birth of first child after age 30.
After female sex and age, family history of the disease is the most significant risk factor for breast cancer.2 If a woman has a first-degree relative (mother, sister, daughter) with breast cancer, her risk is 1.8 times higher, and if she has a second-degree relative (aunt, grandmother) with breast cancer, her risk is 1.3 times higher.3
Hereditary cancer predisposition syndromes account for 5% to 10% of cases of breast cancer. These are caused by a germline mutation in a highly penetrant gene that considerably increases the risk of malignancies of the breast and other tissues. These conditions are inherited in an autosomal-dominant fashion, with age of onset tending to be significantly—several decades—younger than the median age of onset in the general population. The most common of these is hereditary breast and ovarian cancer syndrome, caused by germline mutations of the BRCA1 or BRCA2 gene.
Familial breast cancers account for 15% to 20% of cases. Here, the women who develop breast cancer have multiple family members who are also affected but without an obvious inheritance pattern, and the age of onset is similar to that in the general population.4
Sporadic forms of breast cancer account for the remaining 70% to 80% of cases. Their development can be attributed mainly to nonhereditary causes, such as the environmental and personal risk factors listed above. In general, sporadic forms of breast cancer occur at older ages, with no particular inheritance pattern and with frequency of occurrence in a family comparable to that in the general population.
IS A GENETICS CONSULTATION NEEDED?
In the case described above, the primary care physician gathered basic information about the patient’s cancer-related personal and family history. Asking a few key questions (Table 1)5,6 can help physicians understand two important things: whether a more detailed assessment of genetic risk and counseling by a genetics professional are indicated, and whether the patient would benefit from additional cancer screening and prevention.
Table 2 summarizes the National Comprehensive Cancer Network’s recommendations for cancer genetics consultation.5 These red flags for a hereditary breast cancer syndrome can help primary care providers identify patients for whom a cancer genetics referral is appropriate. Of note: the maternal and paternal family histories are equally important.
Because our patient was diagnosed with breast cancer before age 50 and is of Ashkenazi Jewish ethnicity, she meets these criteria and warrants a cancer genetics consultation.
What is a cancer-focused genetic counseling session?
The tenets of genetic counseling, described previously in this series,7 are relevant to hereditary cancer syndromes. Cancer risk assessment and genetic counseling constitute the process of identifying and counseling individuals at risk of familial or hereditary cancer.8
As in other genetic counseling scenarios, a detailed pedigree (family tree) is taken, and this information, along with the patient’s personal medical history, allows a genetics specialist to determine if the presentation is most suggestive of sporadic, familial, or hereditary cancer.
A common misconception among patients is that there is a single genetic test for hereditary breast cancer, when in fact many highly penetrant predisposition genes have been linked to heightened risk (see below). The syndromes summarized in Table 35,9–18 are part of the differential diagnosis for every patient presenting with a personal or family history of breast cancer, and the detailed information from the personal and family history, ascertained during the assessment, ensures the right syndrome is explored within a family.
Cancer-focused genetic counseling may also help a patient or family process the psychological and emotional responses that can occur when cancer risk is discussed: eg, fear of cancer and death; guilt a parent may feel for passing on a genetic predisposition; and survivor guilt experienced by family members who test negative.
Genetic counselors are trained to recognize patients who may benefit from additional counseling. Not all patients pursuing cancer-focused genetic testing need a thorough evaluation by a psychologist, unlike those with adult-onset neurodegenerative conditions such as Huntington disease. Rather, the genetic counselor discusses the psychological implications of cancer-focused genetic testing and can refer the patient to a psychologist, therapist, social worker, or others if he or she feels the patient may benefit.8
Some patients come to a genetic counseling session with concerns about whether their insurance will pay for testing, and about whether they will face discrimination because of the testing results. In most situations, genetic testing is deemed medically necessary and is covered by the patient’s insurance. When testing is necessary, genetic counselors are skilled at preauthorizing it and writing letters of medical necessity. They are also familiar with laws and regulations that protect patients, such as the Genetic Information Nondiscrimination Act, which protects patients from insurance and employment discrimination.
Because a cancer-focused genetic counseling session typically lasts 1 hour, the counselor has enough time to address these and any other concerns that might prevent a patient who is otherwise interested in genetic testing from pursuing it.
HOW CAN GENETIC TESTING HELP?
Genetic testing for hereditary cancer syndromes can have personal benefit for the patient and at-risk family members.
Note that the syndromes in Table 3 all increase the risk of more than one type of cancer. Patients with these syndromes frequently receive care from multiple subspecialists to mitigate those risks. Guidelines exist for each of these syndromes and, if followed, may prevent the morbidity and possibly death from the genotype-specific cancers that would otherwise be in the patient’s future. For patients found to have a hereditary cancer syndrome, medical management options include more-frequent cancer screening or surveillance, prophylactic surgery, and preventive medical treatment, which will be reviewed in a future article in this series.
Identifying the specific mutation in one family member allows at-risk relatives, both female and male, to then take advantage of predictive testing, with genetic counseling. If they test positive for the risk-increasing mutation, they too can take advantage of the management options for people at high risk. If they test negative, they can continue to undergo the same screening as recommended for the general population. Also, they may be relieved to know that their cancer risk is no greater than that in the general population.
The American Society of Clinical Oncology9 recommends genetic counseling and testing when all of the following are true:
- There is a personal or family history suggesting genetic cancer susceptibility
- The test can be adequately interpreted
- The results will aid in the diagnosis or influence the medical or surgical management of the patient or family at hereditary risk of cancer.
Professional society guidelines also recommend that genetic testing be done only with genetic counseling before and after.5,6,8 The National Society of Genetic Counselors provides a list of clinical genetic counselors, organized by geographical area, at www.nsgc.org.
PATIENT 1 RECEIVES GENETIC TESTING AND COUNSELING
Let’s return to the Ashkenazi Jewish patient who has a personal and family history of breast cancer, whom you referred for cancer genetics consultation and who attends this appointment. A detailed personal and family history is gathered, and a brief physical examination is done, which reveals that the patient has macrocephaly and a history of multiple uterine fibroids.
The genetic differential diagnosis for your patient includes hereditary breast and ovarian cancer syndrome (resulting from mutations in the BRCA1 and BRCA2 genes) and Cowden syndrome (from mutations in the PTEN gene) (TABLE 3). The counselor uses BRCAPRO, a statistical risk-assessment tool that estimates a patient’s risk of harboring a BRCA1 or BRCA2 mutation based on ethnicity and personal and family history of cancer, and finds her risk to be 31%. In view of this risk, genetic testing for BRCA1 and BRCA2 is offered after a detailed discussion of the genetic differential diagnosis, the implications of a positive vs a negative test result, the possibility of finding gene changes (variants) of unknown significance, and the implications of the test results for family members.
Your patient elects to pursue BRCA1 and BRCA2 genetic testing and the results are negative—no mutations in either gene are found. PTEN testing is recommended next, which your patient elects to undergo. A mutation in the PTEN gene is found, indicating that she has Cowden syndrome. This result and its implications are discussed in a posttest genetic counseling session.
Cowden syndrome is an autosomal-dominant condition that carries a heightened risk of benign and malignant neoplasms, including a lifetime risk of breast cancer of up to 85%, with the average age at diagnosis in the 40s. Mutations in the PTEN gene also predispose to other cancer types, including nonmedullary thyroid, uterine, renal, and colorectal cancers, as well as melanoma.9 Multiple benign skin lesions and gastrointestinal polyposis are common.20
During the appointment, medical management options for patients with PTEN mutations are presented (Table 4).9 Given that your patient’s breast cancer was initially treated with lumpectomy, her remaining breast tissue is at risk of a second malignancy. She has never undergone thyroid imaging, colonoscopy, or kidney imaging. She reports that lately she has had occasional abnormal uterine bleeding and pain, which she believes are caused by her uterine fibroids. Given these symptoms and in light of her PTEN mutation, hysterectomy may be presented to her as an option. The genetics team sends a detailed clinical note directly to the primary care physician so they can coordinate and “quarterback” the patient’s care.
Like many patients, your patient is very concerned about how this information may affect her daughter. She first expresses some guilt at having to tell her daughter that she may have “given” her a risk of cancer. However, during the course of the genetic counseling session, she accepts that she could not have prevented her daughter from possibly inheriting this mutation, and understands that sharing this information will enable her daughter to pursue testing to help her understand her own risks.
When a known mutation exists in the family, as is the case with your patient, predictive testing only for that mutation gives a 100% accurate result. During a separate genetic counseling appointment, the patient’s daughter opts to proceed with testing and is found to be negative for her mother’s PTEN mutation.
WHAT HAPPENS WHEN GENETIC TESTING IS NOT INDICATED?
Cancer genetic risk assessment and counseling provides benefits even when genetic testing is not indicated. In some situations genetic testing is not warranted, but referral for heightened surveillance for breast cancer is deemed necessary. Patients who have a personal or family history of cancer can still gain from a detailed assessment of their personal and family history and may come away relieved after learning that they or their family members are not at high risk of developing cancer. Such patients or families may be classified as demonstrating either familial or sporadic breast cancer diagnoses.
Familial breast cancer
Familial breast cancers, believed to account for 15% to 20% of all cases of breast cancer, share features with hereditary breast cancer syndromes.4 In affected families, the frequency of breast cancer is higher than in the general population (multiple family members may be affected), and the age of onset tends to be close to that in the general population.
Members of a family with familial breast cancer who have not yet developed the disease may be at increased risk of it. Several risk-assessment tools (the Gail, Tyrer-Cuzick, Claus, and other models)21–25 use personal and family history to estimate breast cancer risk.
Depending on the assessed risk, additional options for screening and surveillance are available. The American Cancer Society recommends magnetic resonance imaging (MRI) in addition to annual mammography for women whose lifetime risk of breast cancer is greater than 20%. They also recommend that women at moderately increased risk (ie, 15%–20% lifetime risk) talk to their doctor about the benefits and limitations of adding MRI screening to yearly mammography.1
Sporadic breast cancer
Sporadic forms of breast cancer account for 70% to 80% of cases of breast cancer. Sporadic breast cancers are thought to have mainly nonhereditary causes, with environment and personal risk factors playing a large role.
Women with apparently sporadic breast cancers are diagnosed at or beyond the average age at diagnosis in the general population and do not have a family history that suggests either a hereditary cancer syndrome or familial breast cancer. If they undergo a cancer risk assessment, they may be relieved to learn that other women in their family do not have a high probability of being affected, and that they themselves do not appear to be at increased risk of other malignancies.
PATIENT 2: NEGATIVE TEST RESULTS ARE SOMETIMES ‘UNINFORMATIVE’
A healthy 35-year-old woman is referred for a genetics consultation by her gynecologist because her mother developed breast cancer at age 40 and died of the disease. A detailed personal and family history and risk assessment are done. After pretest genetic counseling, testing for BRCA1 and BRCA2 mutations (hereditary breast and ovarian cancer syndrome) is ordered, and the patient’s test results are negative. Risk assessment determines that no other hereditary cancer syndrome is likely. Therefore, no other genetic testing is offered at this time.
Genetic testing is most informative when performed first on the family member at highest risk of having a mutation. For families with breast cancer, this is typically the person with cancer diagnosed at the earliest age.
Unfortunately, sometimes these family members cannot be tested because they are deceased or otherwise unavailable. In such situations, it is acceptable to offer testing to a close, unaffected relative, such as your patient. Pretest genetic counseling in these circumstances is key, highlighting the fact that negative (normal) results would be uninformative. In your case, we cannot know whether the patient’s mother would have tested positive for a BRCA1 or BRCA2 mutation and your patient is a “true negative,” or whether her mother would have tested negative as well.
In unaffected patients with uninformative genetic testing results, medical management is based on the patient’s personal risk factors and family history of cancer. For your patient, statistical risk modeling tools (the Gail, Claus, Couch, and Tyrer-Cuzick models) determine that her risk of developing breast cancer is 22% to 28.5%, qualifying her for MRI along with yearly mammography per the American Cancer Society guidelines previously discussed.
KNOWLEDGE CONTINUES TO EXPAND
Major advances in the understanding of breast cancer susceptibility were made in the last decade through genetic linkage mapping in families that have an overabundance of members with breast cancer.26–28 Additionally, as more information is acquired, other genes predisposing to cancer or modifying cancer risk may be identified and additional knowledge gained.
With the advent of gene-panel-based testing and exome sequencing, we will incidentally discover mutations that predispose to cancer in patients in whom we were not looking for these mutations. With improving technology and value-based health care delivery, providers must continue to embrace multidisciplinary care, and genetics will become central in guiding medical management. In the event of an incidental finding suggesting susceptibility to heritable cancer, a consult to genetic counseling is recommended.
Many studies of the genetics of breast cancer are now focusing on known hereditary breast cancer syndromes and on possibilities for risk reduction, lifestyle modification, and identification of genetic variations that may increase or decrease cancer risk for an individual patient. The Center for Personalized Genetic Healthcare at Cleveland Clinic is collaborating in one such study. Titled “Risk Factor Analysis of Hereditary Breast and Ovarian Cancer Syndrome,” it is an international study led by a leading breast cancer researcher, Dr. Steven Narod from the Women’s College Research Institute in Toronto, ON. This study is focusing on women with a BRCA1 or BRCA2 mutation and their personal cancer risk factors, lifestyle choices, and overall development of cancer. This research group and others are also focusing on identifying genetic “modifiers” of cancer risk in these high-risk women.29
For patients who do not have a hereditary cancer syndrome, research is further exploring novel genes and their relation to breast cancer risk. One such study in our laboratory has found that several genes once thought only to cause an increased risk of hereditary paraganglioma may also predispose to breast and thyroid cancer.29,31 Additional research in this area is under way to clarify these risks.
GOOD SCIENCE, BAD MEDICINE?
Other research studies have identified a number of genes currently thought to be “moderately penetrant” for breast cancer risk, meaning that they may confer a risk of breast cancer slightly greater than that in the general population, but in some instances the risk has not been proven to be high enough to alter a patient’s management.32,33
Although a few clinical laboratories currently offer testing for these kinds of genes, the clinical utility of this testing is questionable. Before offering testing on a clinical basis, we need clear, consistent data on the types of cancers associated with these genes and on the lifetime percentage risk of acquiring these cancers. Currently, it is difficult to understand whether a variant in a moderately penetrant gene is the true explanation behind a patient’s breast cancer diagnosis. If such a variant is identified and family members pursue testing for it, should those family members who test negative be considered to have the same risk of cancer as the general population? And should family members testing positive be offered prophylactic surgical options?
Without more data these questions cannot be answered, and until such data are gathered, we believe that testing for moderately penetrant genes should not be performed outside of a research study. The Center for Personalized Genetic Healthcare in Cleveland Clinic’s Genomic Medicine Institute can assist in educating and coordinating patients’ enrollment in such research studies.
PUTTING IT ALL TOGETHER
Primary care physicians are the first-line providers to individuals and families, many of whom have a personal or family history of breast cancer. Identifying patients at risk of breast cancer and hereditary cancer syndromes can be challenging in this era of shortened appointment times and patients with complex medical histories.
Reviewing an individual’s personal and cancer family history is a necessary first step in considering appropriate medical management recommendations for cancer screening and prevention, the cornerstone of personalized health care. Patients with hereditary breast cancer syndromes and those with familial breast cancer can benefit from high-risk breast cancer surveillance.
Cancer genetics risk assessment ensures that the correct genetic testing is offered to the most appropriate patients, with personalized interpretation of results and provision of future management recommendations based on the individual patient’s personal and family history. Genetic counselors empower patients to make educated and informed decisions about genetic testing, cancer screening, and prevention.
As health care continues to focus more on prevention in this new era of genomic medicine and value-based delivery of health care, genetic counselors will serve as powerful allies to physicians.34
Acknowledgments: We would like to thank Dr. Colleen Clayton and Dr. Lynn Pattimakiel of the Medicine Institute, Cleveland Clinic, for their critical review of and thoughtful feedback on this manuscript.
- American Cancer Society. Breast cancer: detailed guide( 2013). http://www.cancer.org/Cancer/BreastCancer/DetailedGuide/index. Accessed November 12, 2013.
- McTiernan A, Gilligan MA, Redmond C. Assessing individual risk for breast cancer: risky business. J Clin Epidemiol 1997; 50:547–556.
- Teerlink CC, Albright FS, Lins L, Cannon-Albright LA. A comprehensive survey of cancer risks in extended families. Genet Med 2012; 14:107–114.
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology. Breast cancer risk reduction (version 1.2013). http://www.nccn.org. Accessed November 21, 2013.
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology. Genetic/familial high risk assessment: breast and ovarian (version 4.2013). http://www.nccn.org. Accessed November 21, 2013.
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology. Breast cancer screening and diagnosis (version 2.2013). http://www.nccn.org. Accessed November 21, 2013.
- Mester JL, Schreiber AH, Moran RT. Genetic counselors: your partners in clinical practice. Cleve Clin J Med 2012; 79:560–568.
- Trepanier A, Ahrens M, McKinnon W, et al; National Society of Genetic Counselors. Genetic cancer risk assessment and counseling: recommendations of the National Society of Genetic Counselors. J Genet Couns 2004; 13:83–114.
- Tan MH, Mester JL, Ngeow J, Rybicki LA, Orloff MS, Eng C. Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res 2012; 18:400–407.
- Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet 1998; 62:676–689.
- Liede A, Karlan BY, Narod SA. Cancer risks for male carriers of germline mutations in BRCA1 or BRCA2: a review of the literature. J Clin Oncol 2004; 22:735–742.
- Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med 1997; 336:1401–1408.
- Birch JM, Hartley AL, Tricker KJ, et al. Prevalence and diversity of constitutional mutations in the p53 gene among 21 Li-Fraumeni families. Cancer Res 1994; 54:1298–1304.
- Chompret A, Brugières L, Ronsin M, et al. P53 germline mutations in childhood cancers and cancer risk for carrier individuals. Br J Cancer 2000; 82:1932–1937.
- Gonzalez KD, Noltner KA, Buzin CH, et al. Beyond Li Fraumeni syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol 2009; 27:1250–1256.
- Varley JM. Germline TP53 mutations and Li-Fraumeni syndrome. Hum Mutat 2003; 21:313–320.
- Fitzgerald RC, Hardwick R, Huntsman D, et al; International Gastric Cancer Linkage Consortium. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet 2010; 47:436–444.
- Hearle N, Schumacher V, Menko FH, et al. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res 2006; 12:3209–3215.
- American Society of Clinical Oncology. American Society of Clinical Oncology policy statement update: genetic testing for cancer susceptibility. J Clin Oncol 2003; 21:2397–2406.
- Mester J, Eng C. When overgrowth bumps into cancer: the PTEN-opathies. Am J Med Genet C Semin Med Genet 2013; 163:114–121.
- Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer. Implications for risk prediction. Cancer 1994; 73:643–651.
- Couch FJ, DeShano ML, Blackwood MA, et al. BRCA1 mutations in women attending clinics that evaluate the risk of breast cancer. N Engl J Med 1997; 336:1409–1415.
- Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med 2004; 23:1111–1130.
- Gail MH, Anderson WF, Garcia-Closas M, Sherman ME. Absolute risk models for subtypes of breast cancer. J Natl Cancer Inst 2007; 99:1657–1659.
- Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 1989; 81:1879–1886.
- Kent P, O’Donoghue JM, O’Hanlon DM, Kerin MJ, Maher DJ, Given HF. Linkage analysis and the susceptibility gene (BRCA-1) in familial breast cancer. Eur J Surg Oncol 1995; 21:240–241.
- Easton DF, Bishop DT, Ford D, Crockford GP. Genetic linkage analysis in familial breast and ovarian cancer: results from 214 families. The Breast Cancer Linkage Consortium. Am J Hum Genet 1993; 52:678–701.
- Ormiston W. Hereditary breast cancer. Eur J Cancer Care (Engl) 1996; 5:13–20.
- Couch FJ, Wang X, McGuffog L, et al. Genome-wide association study in BRCA1 mutation carriers identifies novel loci associated with breast and ovarian cancer risk. PLoS Genet 2013; 9:e1003212.
- Bennett KL, Mester J, Eng C. Germline epigenetic regulation of KILLIN in Cowden and Cowden-like syndrome. JAMA 2010; 304:2724–2731.
- Ni Y, He X, Chen J, et al. Germline SDHx variants modify breast and thyroid cancer risks in Cowden and Cowden-like syndrome via FAD/NAD-dependent destabilization of p53. Hum Mol Genet 2012; 21:300–310.
- Casadei S, Norquist BM, Walsh T, et al. Contribution of inherited mutations in the BRCA2-interacting protein PALB2 to familial breast cancer. Cancer Res 2011; 71:2222–2229.
- Walsh T, Lee MK, Casadei S, et al. Detection of inherited mutations for breast and ovarian cancer using genomic capture and massively parallel sequencing. Proc Natl Acad Sci U S A 2010; 107:12629–12633.
- Eng C. Molecular genetics to genomic medicine: at the heart of value-based delivery of healthcare. Mol Genet Genom Med 2013; 1:4–6.
- American Cancer Society. Breast cancer: detailed guide( 2013). http://www.cancer.org/Cancer/BreastCancer/DetailedGuide/index. Accessed November 12, 2013.
- McTiernan A, Gilligan MA, Redmond C. Assessing individual risk for breast cancer: risky business. J Clin Epidemiol 1997; 50:547–556.
- Teerlink CC, Albright FS, Lins L, Cannon-Albright LA. A comprehensive survey of cancer risks in extended families. Genet Med 2012; 14:107–114.
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology. Breast cancer risk reduction (version 1.2013). http://www.nccn.org. Accessed November 21, 2013.
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology. Genetic/familial high risk assessment: breast and ovarian (version 4.2013). http://www.nccn.org. Accessed November 21, 2013.
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology. Breast cancer screening and diagnosis (version 2.2013). http://www.nccn.org. Accessed November 21, 2013.
- Mester JL, Schreiber AH, Moran RT. Genetic counselors: your partners in clinical practice. Cleve Clin J Med 2012; 79:560–568.
- Trepanier A, Ahrens M, McKinnon W, et al; National Society of Genetic Counselors. Genetic cancer risk assessment and counseling: recommendations of the National Society of Genetic Counselors. J Genet Couns 2004; 13:83–114.
- Tan MH, Mester JL, Ngeow J, Rybicki LA, Orloff MS, Eng C. Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res 2012; 18:400–407.
- Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet 1998; 62:676–689.
- Liede A, Karlan BY, Narod SA. Cancer risks for male carriers of germline mutations in BRCA1 or BRCA2: a review of the literature. J Clin Oncol 2004; 22:735–742.
- Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med 1997; 336:1401–1408.
- Birch JM, Hartley AL, Tricker KJ, et al. Prevalence and diversity of constitutional mutations in the p53 gene among 21 Li-Fraumeni families. Cancer Res 1994; 54:1298–1304.
- Chompret A, Brugières L, Ronsin M, et al. P53 germline mutations in childhood cancers and cancer risk for carrier individuals. Br J Cancer 2000; 82:1932–1937.
- Gonzalez KD, Noltner KA, Buzin CH, et al. Beyond Li Fraumeni syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol 2009; 27:1250–1256.
- Varley JM. Germline TP53 mutations and Li-Fraumeni syndrome. Hum Mutat 2003; 21:313–320.
- Fitzgerald RC, Hardwick R, Huntsman D, et al; International Gastric Cancer Linkage Consortium. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet 2010; 47:436–444.
- Hearle N, Schumacher V, Menko FH, et al. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res 2006; 12:3209–3215.
- American Society of Clinical Oncology. American Society of Clinical Oncology policy statement update: genetic testing for cancer susceptibility. J Clin Oncol 2003; 21:2397–2406.
- Mester J, Eng C. When overgrowth bumps into cancer: the PTEN-opathies. Am J Med Genet C Semin Med Genet 2013; 163:114–121.
- Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer. Implications for risk prediction. Cancer 1994; 73:643–651.
- Couch FJ, DeShano ML, Blackwood MA, et al. BRCA1 mutations in women attending clinics that evaluate the risk of breast cancer. N Engl J Med 1997; 336:1409–1415.
- Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med 2004; 23:1111–1130.
- Gail MH, Anderson WF, Garcia-Closas M, Sherman ME. Absolute risk models for subtypes of breast cancer. J Natl Cancer Inst 2007; 99:1657–1659.
- Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 1989; 81:1879–1886.
- Kent P, O’Donoghue JM, O’Hanlon DM, Kerin MJ, Maher DJ, Given HF. Linkage analysis and the susceptibility gene (BRCA-1) in familial breast cancer. Eur J Surg Oncol 1995; 21:240–241.
- Easton DF, Bishop DT, Ford D, Crockford GP. Genetic linkage analysis in familial breast and ovarian cancer: results from 214 families. The Breast Cancer Linkage Consortium. Am J Hum Genet 1993; 52:678–701.
- Ormiston W. Hereditary breast cancer. Eur J Cancer Care (Engl) 1996; 5:13–20.
- Couch FJ, Wang X, McGuffog L, et al. Genome-wide association study in BRCA1 mutation carriers identifies novel loci associated with breast and ovarian cancer risk. PLoS Genet 2013; 9:e1003212.
- Bennett KL, Mester J, Eng C. Germline epigenetic regulation of KILLIN in Cowden and Cowden-like syndrome. JAMA 2010; 304:2724–2731.
- Ni Y, He X, Chen J, et al. Germline SDHx variants modify breast and thyroid cancer risks in Cowden and Cowden-like syndrome via FAD/NAD-dependent destabilization of p53. Hum Mol Genet 2012; 21:300–310.
- Casadei S, Norquist BM, Walsh T, et al. Contribution of inherited mutations in the BRCA2-interacting protein PALB2 to familial breast cancer. Cancer Res 2011; 71:2222–2229.
- Walsh T, Lee MK, Casadei S, et al. Detection of inherited mutations for breast and ovarian cancer using genomic capture and massively parallel sequencing. Proc Natl Acad Sci U S A 2010; 107:12629–12633.
- Eng C. Molecular genetics to genomic medicine: at the heart of value-based delivery of healthcare. Mol Genet Genom Med 2013; 1:4–6.
KEY POINTS
- Primary care physicians play a critical role in identifying patients at risk of inherited health problems.
- Hereditary cancers are important to detect because the age of onset is early, multiple primary cancers can develop, and cancer predisposition may be inherited.
- Hereditary syndromes account for only a minority of cases of breast cancer, but women who bear the responsible mutations have an extremely high risk.
- Patients with hereditary breast cancer syndromes and those with familial breast cancer can benefit from heightened surveillance for breast cancer.
- Cancer genetics risk assessment ensures that the correct genetic testing is offered to the most appropriate patients, with personalized interpretation of results and provision of future management recommendations based on the individual patient’s personal and family history.