User login
How to spot heritable breast cancer: A primary care physician’s guide
PATIENT 1: A PERSONAL AND FAMILY HISTORY OF BREAST CANCER
A 55-year-old Ashkenazi Jewish woman presents to your clinic for her annual physical. She reports that she had been diagnosed with breast cancer 10 years ago and that it had been treated with lumpectomy. You recall that Ashkenazi Jewish ethnicity and a diagnosis of breast cancer before age 50 are red flags for a hereditary cancer syndrome, and you ask about her family history of cancer. She reports that her mother was diagnosed with breast cancer in her 60s. The patient wants to know if her daughter should start breast cancer screening.
What do you do next?
Facing increasing demands and a plethora of information to be discussed in a short time, primary care physicians may find it challenging to inform patients about the possibility of a hereditary cancer syndrome, to assess the risk, to organize genetic testing if appropriate, and to counsel patients about their management options. As our knowledge of the genetics of breast cancer continues to expand, this information will become more detailed and complex.
Nevertheless, primary care physicians can help identify patients who may have a syndrome of inherited cancer predisposition or whose family history raises concern for familial breast cancer. Patients in both groups may be candidates for genetic risk assessment, for special management options for women at high risk, or for both.
This article provides an overview of inherited conditions associated with higher breast cancer risk, and guidelines to help physicians recognize patients in their own practice for whom a genetics referral may be appropriate.
BREAST CANCER IS COMPLEX AND HETEROGENEOUS
Breast cancer is the second-leading cause of cancer deaths in women. According to the American Cancer Society, an estimated 234,340 new cases of breast cancer are expected to be diagnosed in women in the United States in 2013, and about 2,240 new cases are expected in men; 39,620 women and 410 men are expected to die of it.1
Breast cancer is a complex and heterogeneous disease, influenced by many factors, of which female sex and increasing age are the most significant. Modifiable risk factors include obesity, use of combined hormone replacement therapy, and physical inactivity. Other risk factors include dense breast tissue, having had a breast biopsy in the past, the finding of atypical hyperplasia on biopsy, a history of high-dose chest radiation, and reproductive factors that include early menarche, late menopause, nulliparity, and birth of first child after age 30.
After female sex and age, family history of the disease is the most significant risk factor for breast cancer.2 If a woman has a first-degree relative (mother, sister, daughter) with breast cancer, her risk is 1.8 times higher, and if she has a second-degree relative (aunt, grandmother) with breast cancer, her risk is 1.3 times higher.3
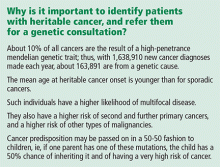
Hereditary cancer predisposition syndromes account for 5% to 10% of cases of breast cancer. These are caused by a germline mutation in a highly penetrant gene that considerably increases the risk of malignancies of the breast and other tissues. These conditions are inherited in an autosomal-dominant fashion, with age of onset tending to be significantly—several decades—younger than the median age of onset in the general population. The most common of these is hereditary breast and ovarian cancer syndrome, caused by germline mutations of the BRCA1 or BRCA2 gene.
Familial breast cancers account for 15% to 20% of cases. Here, the women who develop breast cancer have multiple family members who are also affected but without an obvious inheritance pattern, and the age of onset is similar to that in the general population.4
Sporadic forms of breast cancer account for the remaining 70% to 80% of cases. Their development can be attributed mainly to nonhereditary causes, such as the environmental and personal risk factors listed above. In general, sporadic forms of breast cancer occur at older ages, with no particular inheritance pattern and with frequency of occurrence in a family comparable to that in the general population.
IS A GENETICS CONSULTATION NEEDED?
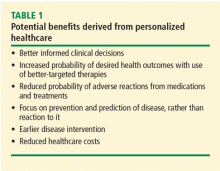
In the case described above, the primary care physician gathered basic information about the patient’s cancer-related personal and family history. Asking a few key questions (Table 1)5,6 can help physicians understand two important things: whether a more detailed assessment of genetic risk and counseling by a genetics professional are indicated, and whether the patient would benefit from additional cancer screening and prevention.
Table 2 summarizes the National Comprehensive Cancer Network’s recommendations for cancer genetics consultation.5 These red flags for a hereditary breast cancer syndrome can help primary care providers identify patients for whom a cancer genetics referral is appropriate. Of note: the maternal and paternal family histories are equally important.
Because our patient was diagnosed with breast cancer before age 50 and is of Ashkenazi Jewish ethnicity, she meets these criteria and warrants a cancer genetics consultation.
What is a cancer-focused genetic counseling session?
The tenets of genetic counseling, described previously in this series,7 are relevant to hereditary cancer syndromes. Cancer risk assessment and genetic counseling constitute the process of identifying and counseling individuals at risk of familial or hereditary cancer.8
As in other genetic counseling scenarios, a detailed pedigree (family tree) is taken, and this information, along with the patient’s personal medical history, allows a genetics specialist to determine if the presentation is most suggestive of sporadic, familial, or hereditary cancer.
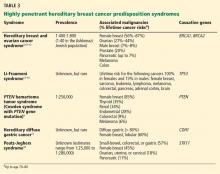
A common misconception among patients is that there is a single genetic test for hereditary breast cancer, when in fact many highly penetrant predisposition genes have been linked to heightened risk (see below). The syndromes summarized in Table 35,9–18 are part of the differential diagnosis for every patient presenting with a personal or family history of breast cancer, and the detailed information from the personal and family history, ascertained during the assessment, ensures the right syndrome is explored within a family.
Cancer-focused genetic counseling may also help a patient or family process the psychological and emotional responses that can occur when cancer risk is discussed: eg, fear of cancer and death; guilt a parent may feel for passing on a genetic predisposition; and survivor guilt experienced by family members who test negative.
Genetic counselors are trained to recognize patients who may benefit from additional counseling. Not all patients pursuing cancer-focused genetic testing need a thorough evaluation by a psychologist, unlike those with adult-onset neurodegenerative conditions such as Huntington disease. Rather, the genetic counselor discusses the psychological implications of cancer-focused genetic testing and can refer the patient to a psychologist, therapist, social worker, or others if he or she feels the patient may benefit.8
Some patients come to a genetic counseling session with concerns about whether their insurance will pay for testing, and about whether they will face discrimination because of the testing results. In most situations, genetic testing is deemed medically necessary and is covered by the patient’s insurance. When testing is necessary, genetic counselors are skilled at preauthorizing it and writing letters of medical necessity. They are also familiar with laws and regulations that protect patients, such as the Genetic Information Nondiscrimination Act, which protects patients from insurance and employment discrimination.
Because a cancer-focused genetic counseling session typically lasts 1 hour, the counselor has enough time to address these and any other concerns that might prevent a patient who is otherwise interested in genetic testing from pursuing it.
HOW CAN GENETIC TESTING HELP?
Genetic testing for hereditary cancer syndromes can have personal benefit for the patient and at-risk family members.
Note that the syndromes in Table 3 all increase the risk of more than one type of cancer. Patients with these syndromes frequently receive care from multiple subspecialists to mitigate those risks. Guidelines exist for each of these syndromes and, if followed, may prevent the morbidity and possibly death from the genotype-specific cancers that would otherwise be in the patient’s future. For patients found to have a hereditary cancer syndrome, medical management options include more-frequent cancer screening or surveillance, prophylactic surgery, and preventive medical treatment, which will be reviewed in a future article in this series.
Identifying the specific mutation in one family member allows at-risk relatives, both female and male, to then take advantage of predictive testing, with genetic counseling. If they test positive for the risk-increasing mutation, they too can take advantage of the management options for people at high risk. If they test negative, they can continue to undergo the same screening as recommended for the general population. Also, they may be relieved to know that their cancer risk is no greater than that in the general population.
The American Society of Clinical Oncology9 recommends genetic counseling and testing when all of the following are true:
- There is a personal or family history suggesting genetic cancer susceptibility
- The test can be adequately interpreted
- The results will aid in the diagnosis or influence the medical or surgical management of the patient or family at hereditary risk of cancer.
Professional society guidelines also recommend that genetic testing be done only with genetic counseling before and after.5,6,8 The National Society of Genetic Counselors provides a list of clinical genetic counselors, organized by geographical area, at www.nsgc.org.
PATIENT 1 RECEIVES GENETIC TESTING AND COUNSELING
Let’s return to the Ashkenazi Jewish patient who has a personal and family history of breast cancer, whom you referred for cancer genetics consultation and who attends this appointment. A detailed personal and family history is gathered, and a brief physical examination is done, which reveals that the patient has macrocephaly and a history of multiple uterine fibroids.
The genetic differential diagnosis for your patient includes hereditary breast and ovarian cancer syndrome (resulting from mutations in the BRCA1 and BRCA2 genes) and Cowden syndrome (from mutations in the PTEN gene) (TABLE 3). The counselor uses BRCAPRO, a statistical risk-assessment tool that estimates a patient’s risk of harboring a BRCA1 or BRCA2 mutation based on ethnicity and personal and family history of cancer, and finds her risk to be 31%. In view of this risk, genetic testing for BRCA1 and BRCA2 is offered after a detailed discussion of the genetic differential diagnosis, the implications of a positive vs a negative test result, the possibility of finding gene changes (variants) of unknown significance, and the implications of the test results for family members.
Your patient elects to pursue BRCA1 and BRCA2 genetic testing and the results are negative—no mutations in either gene are found. PTEN testing is recommended next, which your patient elects to undergo. A mutation in the PTEN gene is found, indicating that she has Cowden syndrome. This result and its implications are discussed in a posttest genetic counseling session.
Cowden syndrome is an autosomal-dominant condition that carries a heightened risk of benign and malignant neoplasms, including a lifetime risk of breast cancer of up to 85%, with the average age at diagnosis in the 40s. Mutations in the PTEN gene also predispose to other cancer types, including nonmedullary thyroid, uterine, renal, and colorectal cancers, as well as melanoma.9 Multiple benign skin lesions and gastrointestinal polyposis are common.20
During the appointment, medical management options for patients with PTEN mutations are presented (Table 4).9 Given that your patient’s breast cancer was initially treated with lumpectomy, her remaining breast tissue is at risk of a second malignancy. She has never undergone thyroid imaging, colonoscopy, or kidney imaging. She reports that lately she has had occasional abnormal uterine bleeding and pain, which she believes are caused by her uterine fibroids. Given these symptoms and in light of her PTEN mutation, hysterectomy may be presented to her as an option. The genetics team sends a detailed clinical note directly to the primary care physician so they can coordinate and “quarterback” the patient’s care.
Like many patients, your patient is very concerned about how this information may affect her daughter. She first expresses some guilt at having to tell her daughter that she may have “given” her a risk of cancer. However, during the course of the genetic counseling session, she accepts that she could not have prevented her daughter from possibly inheriting this mutation, and understands that sharing this information will enable her daughter to pursue testing to help her understand her own risks.
When a known mutation exists in the family, as is the case with your patient, predictive testing only for that mutation gives a 100% accurate result. During a separate genetic counseling appointment, the patient’s daughter opts to proceed with testing and is found to be negative for her mother’s PTEN mutation.
WHAT HAPPENS WHEN GENETIC TESTING IS NOT INDICATED?
Cancer genetic risk assessment and counseling provides benefits even when genetic testing is not indicated. In some situations genetic testing is not warranted, but referral for heightened surveillance for breast cancer is deemed necessary. Patients who have a personal or family history of cancer can still gain from a detailed assessment of their personal and family history and may come away relieved after learning that they or their family members are not at high risk of developing cancer. Such patients or families may be classified as demonstrating either familial or sporadic breast cancer diagnoses.
Familial breast cancer
Familial breast cancers, believed to account for 15% to 20% of all cases of breast cancer, share features with hereditary breast cancer syndromes.4 In affected families, the frequency of breast cancer is higher than in the general population (multiple family members may be affected), and the age of onset tends to be close to that in the general population.
Members of a family with familial breast cancer who have not yet developed the disease may be at increased risk of it. Several risk-assessment tools (the Gail, Tyrer-Cuzick, Claus, and other models)21–25 use personal and family history to estimate breast cancer risk.
Depending on the assessed risk, additional options for screening and surveillance are available. The American Cancer Society recommends magnetic resonance imaging (MRI) in addition to annual mammography for women whose lifetime risk of breast cancer is greater than 20%. They also recommend that women at moderately increased risk (ie, 15%–20% lifetime risk) talk to their doctor about the benefits and limitations of adding MRI screening to yearly mammography.1
Sporadic breast cancer
Sporadic forms of breast cancer account for 70% to 80% of cases of breast cancer. Sporadic breast cancers are thought to have mainly nonhereditary causes, with environment and personal risk factors playing a large role.
Women with apparently sporadic breast cancers are diagnosed at or beyond the average age at diagnosis in the general population and do not have a family history that suggests either a hereditary cancer syndrome or familial breast cancer. If they undergo a cancer risk assessment, they may be relieved to learn that other women in their family do not have a high probability of being affected, and that they themselves do not appear to be at increased risk of other malignancies.
PATIENT 2: NEGATIVE TEST RESULTS ARE SOMETIMES ‘UNINFORMATIVE’
A healthy 35-year-old woman is referred for a genetics consultation by her gynecologist because her mother developed breast cancer at age 40 and died of the disease. A detailed personal and family history and risk assessment are done. After pretest genetic counseling, testing for BRCA1 and BRCA2 mutations (hereditary breast and ovarian cancer syndrome) is ordered, and the patient’s test results are negative. Risk assessment determines that no other hereditary cancer syndrome is likely. Therefore, no other genetic testing is offered at this time.
Genetic testing is most informative when performed first on the family member at highest risk of having a mutation. For families with breast cancer, this is typically the person with cancer diagnosed at the earliest age.
Unfortunately, sometimes these family members cannot be tested because they are deceased or otherwise unavailable. In such situations, it is acceptable to offer testing to a close, unaffected relative, such as your patient. Pretest genetic counseling in these circumstances is key, highlighting the fact that negative (normal) results would be uninformative. In your case, we cannot know whether the patient’s mother would have tested positive for a BRCA1 or BRCA2 mutation and your patient is a “true negative,” or whether her mother would have tested negative as well.
In unaffected patients with uninformative genetic testing results, medical management is based on the patient’s personal risk factors and family history of cancer. For your patient, statistical risk modeling tools (the Gail, Claus, Couch, and Tyrer-Cuzick models) determine that her risk of developing breast cancer is 22% to 28.5%, qualifying her for MRI along with yearly mammography per the American Cancer Society guidelines previously discussed.
KNOWLEDGE CONTINUES TO EXPAND
Major advances in the understanding of breast cancer susceptibility were made in the last decade through genetic linkage mapping in families that have an overabundance of members with breast cancer.26–28 Additionally, as more information is acquired, other genes predisposing to cancer or modifying cancer risk may be identified and additional knowledge gained.
With the advent of gene-panel-based testing and exome sequencing, we will incidentally discover mutations that predispose to cancer in patients in whom we were not looking for these mutations. With improving technology and value-based health care delivery, providers must continue to embrace multidisciplinary care, and genetics will become central in guiding medical management. In the event of an incidental finding suggesting susceptibility to heritable cancer, a consult to genetic counseling is recommended.
Many studies of the genetics of breast cancer are now focusing on known hereditary breast cancer syndromes and on possibilities for risk reduction, lifestyle modification, and identification of genetic variations that may increase or decrease cancer risk for an individual patient. The Center for Personalized Genetic Healthcare at Cleveland Clinic is collaborating in one such study. Titled “Risk Factor Analysis of Hereditary Breast and Ovarian Cancer Syndrome,” it is an international study led by a leading breast cancer researcher, Dr. Steven Narod from the Women’s College Research Institute in Toronto, ON. This study is focusing on women with a BRCA1 or BRCA2 mutation and their personal cancer risk factors, lifestyle choices, and overall development of cancer. This research group and others are also focusing on identifying genetic “modifiers” of cancer risk in these high-risk women.29
For patients who do not have a hereditary cancer syndrome, research is further exploring novel genes and their relation to breast cancer risk. One such study in our laboratory has found that several genes once thought only to cause an increased risk of hereditary paraganglioma may also predispose to breast and thyroid cancer.29,31 Additional research in this area is under way to clarify these risks.
GOOD SCIENCE, BAD MEDICINE?
Other research studies have identified a number of genes currently thought to be “moderately penetrant” for breast cancer risk, meaning that they may confer a risk of breast cancer slightly greater than that in the general population, but in some instances the risk has not been proven to be high enough to alter a patient’s management.32,33
Although a few clinical laboratories currently offer testing for these kinds of genes, the clinical utility of this testing is questionable. Before offering testing on a clinical basis, we need clear, consistent data on the types of cancers associated with these genes and on the lifetime percentage risk of acquiring these cancers. Currently, it is difficult to understand whether a variant in a moderately penetrant gene is the true explanation behind a patient’s breast cancer diagnosis. If such a variant is identified and family members pursue testing for it, should those family members who test negative be considered to have the same risk of cancer as the general population? And should family members testing positive be offered prophylactic surgical options?
Without more data these questions cannot be answered, and until such data are gathered, we believe that testing for moderately penetrant genes should not be performed outside of a research study. The Center for Personalized Genetic Healthcare in Cleveland Clinic’s Genomic Medicine Institute can assist in educating and coordinating patients’ enrollment in such research studies.
PUTTING IT ALL TOGETHER
Primary care physicians are the first-line providers to individuals and families, many of whom have a personal or family history of breast cancer. Identifying patients at risk of breast cancer and hereditary cancer syndromes can be challenging in this era of shortened appointment times and patients with complex medical histories.
Reviewing an individual’s personal and cancer family history is a necessary first step in considering appropriate medical management recommendations for cancer screening and prevention, the cornerstone of personalized health care. Patients with hereditary breast cancer syndromes and those with familial breast cancer can benefit from high-risk breast cancer surveillance.
Cancer genetics risk assessment ensures that the correct genetic testing is offered to the most appropriate patients, with personalized interpretation of results and provision of future management recommendations based on the individual patient’s personal and family history. Genetic counselors empower patients to make educated and informed decisions about genetic testing, cancer screening, and prevention.
As health care continues to focus more on prevention in this new era of genomic medicine and value-based delivery of health care, genetic counselors will serve as powerful allies to physicians.34
Acknowledgments: We would like to thank Dr. Colleen Clayton and Dr. Lynn Pattimakiel of the Medicine Institute, Cleveland Clinic, for their critical review of and thoughtful feedback on this manuscript.
- American Cancer Society. Breast cancer: detailed guide( 2013). http://www.cancer.org/Cancer/BreastCancer/DetailedGuide/index. Accessed November 12, 2013.
- McTiernan A, Gilligan MA, Redmond C. Assessing individual risk for breast cancer: risky business. J Clin Epidemiol 1997; 50:547–556.
- Teerlink CC, Albright FS, Lins L, Cannon-Albright LA. A comprehensive survey of cancer risks in extended families. Genet Med 2012; 14:107–114.
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology. Breast cancer risk reduction (version 1.2013). http://www.nccn.org. Accessed November 21, 2013.
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology. Genetic/familial high risk assessment: breast and ovarian (version 4.2013). http://www.nccn.org. Accessed November 21, 2013.
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology. Breast cancer screening and diagnosis (version 2.2013). http://www.nccn.org. Accessed November 21, 2013.
- Mester JL, Schreiber AH, Moran RT. Genetic counselors: your partners in clinical practice. Cleve Clin J Med 2012; 79:560–568.
- Trepanier A, Ahrens M, McKinnon W, et al; National Society of Genetic Counselors. Genetic cancer risk assessment and counseling: recommendations of the National Society of Genetic Counselors. J Genet Couns 2004; 13:83–114.
- Tan MH, Mester JL, Ngeow J, Rybicki LA, Orloff MS, Eng C. Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res 2012; 18:400–407.
- Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet 1998; 62:676–689.
- Liede A, Karlan BY, Narod SA. Cancer risks for male carriers of germline mutations in BRCA1 or BRCA2: a review of the literature. J Clin Oncol 2004; 22:735–742.
- Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med 1997; 336:1401–1408.
- Birch JM, Hartley AL, Tricker KJ, et al. Prevalence and diversity of constitutional mutations in the p53 gene among 21 Li-Fraumeni families. Cancer Res 1994; 54:1298–1304.
- Chompret A, Brugières L, Ronsin M, et al. P53 germline mutations in childhood cancers and cancer risk for carrier individuals. Br J Cancer 2000; 82:1932–1937.
- Gonzalez KD, Noltner KA, Buzin CH, et al. Beyond Li Fraumeni syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol 2009; 27:1250–1256.
- Varley JM. Germline TP53 mutations and Li-Fraumeni syndrome. Hum Mutat 2003; 21:313–320.
- Fitzgerald RC, Hardwick R, Huntsman D, et al; International Gastric Cancer Linkage Consortium. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet 2010; 47:436–444.
- Hearle N, Schumacher V, Menko FH, et al. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res 2006; 12:3209–3215.
- American Society of Clinical Oncology. American Society of Clinical Oncology policy statement update: genetic testing for cancer susceptibility. J Clin Oncol 2003; 21:2397–2406.
- Mester J, Eng C. When overgrowth bumps into cancer: the PTEN-opathies. Am J Med Genet C Semin Med Genet 2013; 163:114–121.
- Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer. Implications for risk prediction. Cancer 1994; 73:643–651.
- Couch FJ, DeShano ML, Blackwood MA, et al. BRCA1 mutations in women attending clinics that evaluate the risk of breast cancer. N Engl J Med 1997; 336:1409–1415.
- Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med 2004; 23:1111–1130.
- Gail MH, Anderson WF, Garcia-Closas M, Sherman ME. Absolute risk models for subtypes of breast cancer. J Natl Cancer Inst 2007; 99:1657–1659.
- Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 1989; 81:1879–1886.
- Kent P, O’Donoghue JM, O’Hanlon DM, Kerin MJ, Maher DJ, Given HF. Linkage analysis and the susceptibility gene (BRCA-1) in familial breast cancer. Eur J Surg Oncol 1995; 21:240–241.
- Easton DF, Bishop DT, Ford D, Crockford GP. Genetic linkage analysis in familial breast and ovarian cancer: results from 214 families. The Breast Cancer Linkage Consortium. Am J Hum Genet 1993; 52:678–701.
- Ormiston W. Hereditary breast cancer. Eur J Cancer Care (Engl) 1996; 5:13–20.
- Couch FJ, Wang X, McGuffog L, et al. Genome-wide association study in BRCA1 mutation carriers identifies novel loci associated with breast and ovarian cancer risk. PLoS Genet 2013; 9:e1003212.
- Bennett KL, Mester J, Eng C. Germline epigenetic regulation of KILLIN in Cowden and Cowden-like syndrome. JAMA 2010; 304:2724–2731.
- Ni Y, He X, Chen J, et al. Germline SDHx variants modify breast and thyroid cancer risks in Cowden and Cowden-like syndrome via FAD/NAD-dependent destabilization of p53. Hum Mol Genet 2012; 21:300–310.
- Casadei S, Norquist BM, Walsh T, et al. Contribution of inherited mutations in the BRCA2-interacting protein PALB2 to familial breast cancer. Cancer Res 2011; 71:2222–2229.
- Walsh T, Lee MK, Casadei S, et al. Detection of inherited mutations for breast and ovarian cancer using genomic capture and massively parallel sequencing. Proc Natl Acad Sci U S A 2010; 107:12629–12633.
- Eng C. Molecular genetics to genomic medicine: at the heart of value-based delivery of healthcare. Mol Genet Genom Med 2013; 1:4–6.
PATIENT 1: A PERSONAL AND FAMILY HISTORY OF BREAST CANCER
A 55-year-old Ashkenazi Jewish woman presents to your clinic for her annual physical. She reports that she had been diagnosed with breast cancer 10 years ago and that it had been treated with lumpectomy. You recall that Ashkenazi Jewish ethnicity and a diagnosis of breast cancer before age 50 are red flags for a hereditary cancer syndrome, and you ask about her family history of cancer. She reports that her mother was diagnosed with breast cancer in her 60s. The patient wants to know if her daughter should start breast cancer screening.
What do you do next?
Facing increasing demands and a plethora of information to be discussed in a short time, primary care physicians may find it challenging to inform patients about the possibility of a hereditary cancer syndrome, to assess the risk, to organize genetic testing if appropriate, and to counsel patients about their management options. As our knowledge of the genetics of breast cancer continues to expand, this information will become more detailed and complex.
Nevertheless, primary care physicians can help identify patients who may have a syndrome of inherited cancer predisposition or whose family history raises concern for familial breast cancer. Patients in both groups may be candidates for genetic risk assessment, for special management options for women at high risk, or for both.
This article provides an overview of inherited conditions associated with higher breast cancer risk, and guidelines to help physicians recognize patients in their own practice for whom a genetics referral may be appropriate.
BREAST CANCER IS COMPLEX AND HETEROGENEOUS
Breast cancer is the second-leading cause of cancer deaths in women. According to the American Cancer Society, an estimated 234,340 new cases of breast cancer are expected to be diagnosed in women in the United States in 2013, and about 2,240 new cases are expected in men; 39,620 women and 410 men are expected to die of it.1
Breast cancer is a complex and heterogeneous disease, influenced by many factors, of which female sex and increasing age are the most significant. Modifiable risk factors include obesity, use of combined hormone replacement therapy, and physical inactivity. Other risk factors include dense breast tissue, having had a breast biopsy in the past, the finding of atypical hyperplasia on biopsy, a history of high-dose chest radiation, and reproductive factors that include early menarche, late menopause, nulliparity, and birth of first child after age 30.
After female sex and age, family history of the disease is the most significant risk factor for breast cancer.2 If a woman has a first-degree relative (mother, sister, daughter) with breast cancer, her risk is 1.8 times higher, and if she has a second-degree relative (aunt, grandmother) with breast cancer, her risk is 1.3 times higher.3
Hereditary cancer predisposition syndromes account for 5% to 10% of cases of breast cancer. These are caused by a germline mutation in a highly penetrant gene that considerably increases the risk of malignancies of the breast and other tissues. These conditions are inherited in an autosomal-dominant fashion, with age of onset tending to be significantly—several decades—younger than the median age of onset in the general population. The most common of these is hereditary breast and ovarian cancer syndrome, caused by germline mutations of the BRCA1 or BRCA2 gene.
Familial breast cancers account for 15% to 20% of cases. Here, the women who develop breast cancer have multiple family members who are also affected but without an obvious inheritance pattern, and the age of onset is similar to that in the general population.4
Sporadic forms of breast cancer account for the remaining 70% to 80% of cases. Their development can be attributed mainly to nonhereditary causes, such as the environmental and personal risk factors listed above. In general, sporadic forms of breast cancer occur at older ages, with no particular inheritance pattern and with frequency of occurrence in a family comparable to that in the general population.
IS A GENETICS CONSULTATION NEEDED?
In the case described above, the primary care physician gathered basic information about the patient’s cancer-related personal and family history. Asking a few key questions (Table 1)5,6 can help physicians understand two important things: whether a more detailed assessment of genetic risk and counseling by a genetics professional are indicated, and whether the patient would benefit from additional cancer screening and prevention.
Table 2 summarizes the National Comprehensive Cancer Network’s recommendations for cancer genetics consultation.5 These red flags for a hereditary breast cancer syndrome can help primary care providers identify patients for whom a cancer genetics referral is appropriate. Of note: the maternal and paternal family histories are equally important.
Because our patient was diagnosed with breast cancer before age 50 and is of Ashkenazi Jewish ethnicity, she meets these criteria and warrants a cancer genetics consultation.
What is a cancer-focused genetic counseling session?
The tenets of genetic counseling, described previously in this series,7 are relevant to hereditary cancer syndromes. Cancer risk assessment and genetic counseling constitute the process of identifying and counseling individuals at risk of familial or hereditary cancer.8
As in other genetic counseling scenarios, a detailed pedigree (family tree) is taken, and this information, along with the patient’s personal medical history, allows a genetics specialist to determine if the presentation is most suggestive of sporadic, familial, or hereditary cancer.
A common misconception among patients is that there is a single genetic test for hereditary breast cancer, when in fact many highly penetrant predisposition genes have been linked to heightened risk (see below). The syndromes summarized in Table 35,9–18 are part of the differential diagnosis for every patient presenting with a personal or family history of breast cancer, and the detailed information from the personal and family history, ascertained during the assessment, ensures the right syndrome is explored within a family.
Cancer-focused genetic counseling may also help a patient or family process the psychological and emotional responses that can occur when cancer risk is discussed: eg, fear of cancer and death; guilt a parent may feel for passing on a genetic predisposition; and survivor guilt experienced by family members who test negative.
Genetic counselors are trained to recognize patients who may benefit from additional counseling. Not all patients pursuing cancer-focused genetic testing need a thorough evaluation by a psychologist, unlike those with adult-onset neurodegenerative conditions such as Huntington disease. Rather, the genetic counselor discusses the psychological implications of cancer-focused genetic testing and can refer the patient to a psychologist, therapist, social worker, or others if he or she feels the patient may benefit.8
Some patients come to a genetic counseling session with concerns about whether their insurance will pay for testing, and about whether they will face discrimination because of the testing results. In most situations, genetic testing is deemed medically necessary and is covered by the patient’s insurance. When testing is necessary, genetic counselors are skilled at preauthorizing it and writing letters of medical necessity. They are also familiar with laws and regulations that protect patients, such as the Genetic Information Nondiscrimination Act, which protects patients from insurance and employment discrimination.
Because a cancer-focused genetic counseling session typically lasts 1 hour, the counselor has enough time to address these and any other concerns that might prevent a patient who is otherwise interested in genetic testing from pursuing it.
HOW CAN GENETIC TESTING HELP?
Genetic testing for hereditary cancer syndromes can have personal benefit for the patient and at-risk family members.
Note that the syndromes in Table 3 all increase the risk of more than one type of cancer. Patients with these syndromes frequently receive care from multiple subspecialists to mitigate those risks. Guidelines exist for each of these syndromes and, if followed, may prevent the morbidity and possibly death from the genotype-specific cancers that would otherwise be in the patient’s future. For patients found to have a hereditary cancer syndrome, medical management options include more-frequent cancer screening or surveillance, prophylactic surgery, and preventive medical treatment, which will be reviewed in a future article in this series.
Identifying the specific mutation in one family member allows at-risk relatives, both female and male, to then take advantage of predictive testing, with genetic counseling. If they test positive for the risk-increasing mutation, they too can take advantage of the management options for people at high risk. If they test negative, they can continue to undergo the same screening as recommended for the general population. Also, they may be relieved to know that their cancer risk is no greater than that in the general population.
The American Society of Clinical Oncology9 recommends genetic counseling and testing when all of the following are true:
- There is a personal or family history suggesting genetic cancer susceptibility
- The test can be adequately interpreted
- The results will aid in the diagnosis or influence the medical or surgical management of the patient or family at hereditary risk of cancer.
Professional society guidelines also recommend that genetic testing be done only with genetic counseling before and after.5,6,8 The National Society of Genetic Counselors provides a list of clinical genetic counselors, organized by geographical area, at www.nsgc.org.
PATIENT 1 RECEIVES GENETIC TESTING AND COUNSELING
Let’s return to the Ashkenazi Jewish patient who has a personal and family history of breast cancer, whom you referred for cancer genetics consultation and who attends this appointment. A detailed personal and family history is gathered, and a brief physical examination is done, which reveals that the patient has macrocephaly and a history of multiple uterine fibroids.
The genetic differential diagnosis for your patient includes hereditary breast and ovarian cancer syndrome (resulting from mutations in the BRCA1 and BRCA2 genes) and Cowden syndrome (from mutations in the PTEN gene) (TABLE 3). The counselor uses BRCAPRO, a statistical risk-assessment tool that estimates a patient’s risk of harboring a BRCA1 or BRCA2 mutation based on ethnicity and personal and family history of cancer, and finds her risk to be 31%. In view of this risk, genetic testing for BRCA1 and BRCA2 is offered after a detailed discussion of the genetic differential diagnosis, the implications of a positive vs a negative test result, the possibility of finding gene changes (variants) of unknown significance, and the implications of the test results for family members.
Your patient elects to pursue BRCA1 and BRCA2 genetic testing and the results are negative—no mutations in either gene are found. PTEN testing is recommended next, which your patient elects to undergo. A mutation in the PTEN gene is found, indicating that she has Cowden syndrome. This result and its implications are discussed in a posttest genetic counseling session.
Cowden syndrome is an autosomal-dominant condition that carries a heightened risk of benign and malignant neoplasms, including a lifetime risk of breast cancer of up to 85%, with the average age at diagnosis in the 40s. Mutations in the PTEN gene also predispose to other cancer types, including nonmedullary thyroid, uterine, renal, and colorectal cancers, as well as melanoma.9 Multiple benign skin lesions and gastrointestinal polyposis are common.20
During the appointment, medical management options for patients with PTEN mutations are presented (Table 4).9 Given that your patient’s breast cancer was initially treated with lumpectomy, her remaining breast tissue is at risk of a second malignancy. She has never undergone thyroid imaging, colonoscopy, or kidney imaging. She reports that lately she has had occasional abnormal uterine bleeding and pain, which she believes are caused by her uterine fibroids. Given these symptoms and in light of her PTEN mutation, hysterectomy may be presented to her as an option. The genetics team sends a detailed clinical note directly to the primary care physician so they can coordinate and “quarterback” the patient’s care.
Like many patients, your patient is very concerned about how this information may affect her daughter. She first expresses some guilt at having to tell her daughter that she may have “given” her a risk of cancer. However, during the course of the genetic counseling session, she accepts that she could not have prevented her daughter from possibly inheriting this mutation, and understands that sharing this information will enable her daughter to pursue testing to help her understand her own risks.
When a known mutation exists in the family, as is the case with your patient, predictive testing only for that mutation gives a 100% accurate result. During a separate genetic counseling appointment, the patient’s daughter opts to proceed with testing and is found to be negative for her mother’s PTEN mutation.
WHAT HAPPENS WHEN GENETIC TESTING IS NOT INDICATED?
Cancer genetic risk assessment and counseling provides benefits even when genetic testing is not indicated. In some situations genetic testing is not warranted, but referral for heightened surveillance for breast cancer is deemed necessary. Patients who have a personal or family history of cancer can still gain from a detailed assessment of their personal and family history and may come away relieved after learning that they or their family members are not at high risk of developing cancer. Such patients or families may be classified as demonstrating either familial or sporadic breast cancer diagnoses.
Familial breast cancer
Familial breast cancers, believed to account for 15% to 20% of all cases of breast cancer, share features with hereditary breast cancer syndromes.4 In affected families, the frequency of breast cancer is higher than in the general population (multiple family members may be affected), and the age of onset tends to be close to that in the general population.
Members of a family with familial breast cancer who have not yet developed the disease may be at increased risk of it. Several risk-assessment tools (the Gail, Tyrer-Cuzick, Claus, and other models)21–25 use personal and family history to estimate breast cancer risk.
Depending on the assessed risk, additional options for screening and surveillance are available. The American Cancer Society recommends magnetic resonance imaging (MRI) in addition to annual mammography for women whose lifetime risk of breast cancer is greater than 20%. They also recommend that women at moderately increased risk (ie, 15%–20% lifetime risk) talk to their doctor about the benefits and limitations of adding MRI screening to yearly mammography.1
Sporadic breast cancer
Sporadic forms of breast cancer account for 70% to 80% of cases of breast cancer. Sporadic breast cancers are thought to have mainly nonhereditary causes, with environment and personal risk factors playing a large role.
Women with apparently sporadic breast cancers are diagnosed at or beyond the average age at diagnosis in the general population and do not have a family history that suggests either a hereditary cancer syndrome or familial breast cancer. If they undergo a cancer risk assessment, they may be relieved to learn that other women in their family do not have a high probability of being affected, and that they themselves do not appear to be at increased risk of other malignancies.
PATIENT 2: NEGATIVE TEST RESULTS ARE SOMETIMES ‘UNINFORMATIVE’
A healthy 35-year-old woman is referred for a genetics consultation by her gynecologist because her mother developed breast cancer at age 40 and died of the disease. A detailed personal and family history and risk assessment are done. After pretest genetic counseling, testing for BRCA1 and BRCA2 mutations (hereditary breast and ovarian cancer syndrome) is ordered, and the patient’s test results are negative. Risk assessment determines that no other hereditary cancer syndrome is likely. Therefore, no other genetic testing is offered at this time.
Genetic testing is most informative when performed first on the family member at highest risk of having a mutation. For families with breast cancer, this is typically the person with cancer diagnosed at the earliest age.
Unfortunately, sometimes these family members cannot be tested because they are deceased or otherwise unavailable. In such situations, it is acceptable to offer testing to a close, unaffected relative, such as your patient. Pretest genetic counseling in these circumstances is key, highlighting the fact that negative (normal) results would be uninformative. In your case, we cannot know whether the patient’s mother would have tested positive for a BRCA1 or BRCA2 mutation and your patient is a “true negative,” or whether her mother would have tested negative as well.
In unaffected patients with uninformative genetic testing results, medical management is based on the patient’s personal risk factors and family history of cancer. For your patient, statistical risk modeling tools (the Gail, Claus, Couch, and Tyrer-Cuzick models) determine that her risk of developing breast cancer is 22% to 28.5%, qualifying her for MRI along with yearly mammography per the American Cancer Society guidelines previously discussed.
KNOWLEDGE CONTINUES TO EXPAND
Major advances in the understanding of breast cancer susceptibility were made in the last decade through genetic linkage mapping in families that have an overabundance of members with breast cancer.26–28 Additionally, as more information is acquired, other genes predisposing to cancer or modifying cancer risk may be identified and additional knowledge gained.
With the advent of gene-panel-based testing and exome sequencing, we will incidentally discover mutations that predispose to cancer in patients in whom we were not looking for these mutations. With improving technology and value-based health care delivery, providers must continue to embrace multidisciplinary care, and genetics will become central in guiding medical management. In the event of an incidental finding suggesting susceptibility to heritable cancer, a consult to genetic counseling is recommended.
Many studies of the genetics of breast cancer are now focusing on known hereditary breast cancer syndromes and on possibilities for risk reduction, lifestyle modification, and identification of genetic variations that may increase or decrease cancer risk for an individual patient. The Center for Personalized Genetic Healthcare at Cleveland Clinic is collaborating in one such study. Titled “Risk Factor Analysis of Hereditary Breast and Ovarian Cancer Syndrome,” it is an international study led by a leading breast cancer researcher, Dr. Steven Narod from the Women’s College Research Institute in Toronto, ON. This study is focusing on women with a BRCA1 or BRCA2 mutation and their personal cancer risk factors, lifestyle choices, and overall development of cancer. This research group and others are also focusing on identifying genetic “modifiers” of cancer risk in these high-risk women.29
For patients who do not have a hereditary cancer syndrome, research is further exploring novel genes and their relation to breast cancer risk. One such study in our laboratory has found that several genes once thought only to cause an increased risk of hereditary paraganglioma may also predispose to breast and thyroid cancer.29,31 Additional research in this area is under way to clarify these risks.
GOOD SCIENCE, BAD MEDICINE?
Other research studies have identified a number of genes currently thought to be “moderately penetrant” for breast cancer risk, meaning that they may confer a risk of breast cancer slightly greater than that in the general population, but in some instances the risk has not been proven to be high enough to alter a patient’s management.32,33
Although a few clinical laboratories currently offer testing for these kinds of genes, the clinical utility of this testing is questionable. Before offering testing on a clinical basis, we need clear, consistent data on the types of cancers associated with these genes and on the lifetime percentage risk of acquiring these cancers. Currently, it is difficult to understand whether a variant in a moderately penetrant gene is the true explanation behind a patient’s breast cancer diagnosis. If such a variant is identified and family members pursue testing for it, should those family members who test negative be considered to have the same risk of cancer as the general population? And should family members testing positive be offered prophylactic surgical options?
Without more data these questions cannot be answered, and until such data are gathered, we believe that testing for moderately penetrant genes should not be performed outside of a research study. The Center for Personalized Genetic Healthcare in Cleveland Clinic’s Genomic Medicine Institute can assist in educating and coordinating patients’ enrollment in such research studies.
PUTTING IT ALL TOGETHER
Primary care physicians are the first-line providers to individuals and families, many of whom have a personal or family history of breast cancer. Identifying patients at risk of breast cancer and hereditary cancer syndromes can be challenging in this era of shortened appointment times and patients with complex medical histories.
Reviewing an individual’s personal and cancer family history is a necessary first step in considering appropriate medical management recommendations for cancer screening and prevention, the cornerstone of personalized health care. Patients with hereditary breast cancer syndromes and those with familial breast cancer can benefit from high-risk breast cancer surveillance.
Cancer genetics risk assessment ensures that the correct genetic testing is offered to the most appropriate patients, with personalized interpretation of results and provision of future management recommendations based on the individual patient’s personal and family history. Genetic counselors empower patients to make educated and informed decisions about genetic testing, cancer screening, and prevention.
As health care continues to focus more on prevention in this new era of genomic medicine and value-based delivery of health care, genetic counselors will serve as powerful allies to physicians.34
Acknowledgments: We would like to thank Dr. Colleen Clayton and Dr. Lynn Pattimakiel of the Medicine Institute, Cleveland Clinic, for their critical review of and thoughtful feedback on this manuscript.
PATIENT 1: A PERSONAL AND FAMILY HISTORY OF BREAST CANCER
A 55-year-old Ashkenazi Jewish woman presents to your clinic for her annual physical. She reports that she had been diagnosed with breast cancer 10 years ago and that it had been treated with lumpectomy. You recall that Ashkenazi Jewish ethnicity and a diagnosis of breast cancer before age 50 are red flags for a hereditary cancer syndrome, and you ask about her family history of cancer. She reports that her mother was diagnosed with breast cancer in her 60s. The patient wants to know if her daughter should start breast cancer screening.
What do you do next?
Facing increasing demands and a plethora of information to be discussed in a short time, primary care physicians may find it challenging to inform patients about the possibility of a hereditary cancer syndrome, to assess the risk, to organize genetic testing if appropriate, and to counsel patients about their management options. As our knowledge of the genetics of breast cancer continues to expand, this information will become more detailed and complex.
Nevertheless, primary care physicians can help identify patients who may have a syndrome of inherited cancer predisposition or whose family history raises concern for familial breast cancer. Patients in both groups may be candidates for genetic risk assessment, for special management options for women at high risk, or for both.
This article provides an overview of inherited conditions associated with higher breast cancer risk, and guidelines to help physicians recognize patients in their own practice for whom a genetics referral may be appropriate.
BREAST CANCER IS COMPLEX AND HETEROGENEOUS
Breast cancer is the second-leading cause of cancer deaths in women. According to the American Cancer Society, an estimated 234,340 new cases of breast cancer are expected to be diagnosed in women in the United States in 2013, and about 2,240 new cases are expected in men; 39,620 women and 410 men are expected to die of it.1
Breast cancer is a complex and heterogeneous disease, influenced by many factors, of which female sex and increasing age are the most significant. Modifiable risk factors include obesity, use of combined hormone replacement therapy, and physical inactivity. Other risk factors include dense breast tissue, having had a breast biopsy in the past, the finding of atypical hyperplasia on biopsy, a history of high-dose chest radiation, and reproductive factors that include early menarche, late menopause, nulliparity, and birth of first child after age 30.
After female sex and age, family history of the disease is the most significant risk factor for breast cancer.2 If a woman has a first-degree relative (mother, sister, daughter) with breast cancer, her risk is 1.8 times higher, and if she has a second-degree relative (aunt, grandmother) with breast cancer, her risk is 1.3 times higher.3
Hereditary cancer predisposition syndromes account for 5% to 10% of cases of breast cancer. These are caused by a germline mutation in a highly penetrant gene that considerably increases the risk of malignancies of the breast and other tissues. These conditions are inherited in an autosomal-dominant fashion, with age of onset tending to be significantly—several decades—younger than the median age of onset in the general population. The most common of these is hereditary breast and ovarian cancer syndrome, caused by germline mutations of the BRCA1 or BRCA2 gene.
Familial breast cancers account for 15% to 20% of cases. Here, the women who develop breast cancer have multiple family members who are also affected but without an obvious inheritance pattern, and the age of onset is similar to that in the general population.4
Sporadic forms of breast cancer account for the remaining 70% to 80% of cases. Their development can be attributed mainly to nonhereditary causes, such as the environmental and personal risk factors listed above. In general, sporadic forms of breast cancer occur at older ages, with no particular inheritance pattern and with frequency of occurrence in a family comparable to that in the general population.
IS A GENETICS CONSULTATION NEEDED?
In the case described above, the primary care physician gathered basic information about the patient’s cancer-related personal and family history. Asking a few key questions (Table 1)5,6 can help physicians understand two important things: whether a more detailed assessment of genetic risk and counseling by a genetics professional are indicated, and whether the patient would benefit from additional cancer screening and prevention.
Table 2 summarizes the National Comprehensive Cancer Network’s recommendations for cancer genetics consultation.5 These red flags for a hereditary breast cancer syndrome can help primary care providers identify patients for whom a cancer genetics referral is appropriate. Of note: the maternal and paternal family histories are equally important.
Because our patient was diagnosed with breast cancer before age 50 and is of Ashkenazi Jewish ethnicity, she meets these criteria and warrants a cancer genetics consultation.
What is a cancer-focused genetic counseling session?
The tenets of genetic counseling, described previously in this series,7 are relevant to hereditary cancer syndromes. Cancer risk assessment and genetic counseling constitute the process of identifying and counseling individuals at risk of familial or hereditary cancer.8
As in other genetic counseling scenarios, a detailed pedigree (family tree) is taken, and this information, along with the patient’s personal medical history, allows a genetics specialist to determine if the presentation is most suggestive of sporadic, familial, or hereditary cancer.
A common misconception among patients is that there is a single genetic test for hereditary breast cancer, when in fact many highly penetrant predisposition genes have been linked to heightened risk (see below). The syndromes summarized in Table 35,9–18 are part of the differential diagnosis for every patient presenting with a personal or family history of breast cancer, and the detailed information from the personal and family history, ascertained during the assessment, ensures the right syndrome is explored within a family.
Cancer-focused genetic counseling may also help a patient or family process the psychological and emotional responses that can occur when cancer risk is discussed: eg, fear of cancer and death; guilt a parent may feel for passing on a genetic predisposition; and survivor guilt experienced by family members who test negative.
Genetic counselors are trained to recognize patients who may benefit from additional counseling. Not all patients pursuing cancer-focused genetic testing need a thorough evaluation by a psychologist, unlike those with adult-onset neurodegenerative conditions such as Huntington disease. Rather, the genetic counselor discusses the psychological implications of cancer-focused genetic testing and can refer the patient to a psychologist, therapist, social worker, or others if he or she feels the patient may benefit.8
Some patients come to a genetic counseling session with concerns about whether their insurance will pay for testing, and about whether they will face discrimination because of the testing results. In most situations, genetic testing is deemed medically necessary and is covered by the patient’s insurance. When testing is necessary, genetic counselors are skilled at preauthorizing it and writing letters of medical necessity. They are also familiar with laws and regulations that protect patients, such as the Genetic Information Nondiscrimination Act, which protects patients from insurance and employment discrimination.
Because a cancer-focused genetic counseling session typically lasts 1 hour, the counselor has enough time to address these and any other concerns that might prevent a patient who is otherwise interested in genetic testing from pursuing it.
HOW CAN GENETIC TESTING HELP?
Genetic testing for hereditary cancer syndromes can have personal benefit for the patient and at-risk family members.
Note that the syndromes in Table 3 all increase the risk of more than one type of cancer. Patients with these syndromes frequently receive care from multiple subspecialists to mitigate those risks. Guidelines exist for each of these syndromes and, if followed, may prevent the morbidity and possibly death from the genotype-specific cancers that would otherwise be in the patient’s future. For patients found to have a hereditary cancer syndrome, medical management options include more-frequent cancer screening or surveillance, prophylactic surgery, and preventive medical treatment, which will be reviewed in a future article in this series.
Identifying the specific mutation in one family member allows at-risk relatives, both female and male, to then take advantage of predictive testing, with genetic counseling. If they test positive for the risk-increasing mutation, they too can take advantage of the management options for people at high risk. If they test negative, they can continue to undergo the same screening as recommended for the general population. Also, they may be relieved to know that their cancer risk is no greater than that in the general population.
The American Society of Clinical Oncology9 recommends genetic counseling and testing when all of the following are true:
- There is a personal or family history suggesting genetic cancer susceptibility
- The test can be adequately interpreted
- The results will aid in the diagnosis or influence the medical or surgical management of the patient or family at hereditary risk of cancer.
Professional society guidelines also recommend that genetic testing be done only with genetic counseling before and after.5,6,8 The National Society of Genetic Counselors provides a list of clinical genetic counselors, organized by geographical area, at www.nsgc.org.
PATIENT 1 RECEIVES GENETIC TESTING AND COUNSELING
Let’s return to the Ashkenazi Jewish patient who has a personal and family history of breast cancer, whom you referred for cancer genetics consultation and who attends this appointment. A detailed personal and family history is gathered, and a brief physical examination is done, which reveals that the patient has macrocephaly and a history of multiple uterine fibroids.
The genetic differential diagnosis for your patient includes hereditary breast and ovarian cancer syndrome (resulting from mutations in the BRCA1 and BRCA2 genes) and Cowden syndrome (from mutations in the PTEN gene) (TABLE 3). The counselor uses BRCAPRO, a statistical risk-assessment tool that estimates a patient’s risk of harboring a BRCA1 or BRCA2 mutation based on ethnicity and personal and family history of cancer, and finds her risk to be 31%. In view of this risk, genetic testing for BRCA1 and BRCA2 is offered after a detailed discussion of the genetic differential diagnosis, the implications of a positive vs a negative test result, the possibility of finding gene changes (variants) of unknown significance, and the implications of the test results for family members.
Your patient elects to pursue BRCA1 and BRCA2 genetic testing and the results are negative—no mutations in either gene are found. PTEN testing is recommended next, which your patient elects to undergo. A mutation in the PTEN gene is found, indicating that she has Cowden syndrome. This result and its implications are discussed in a posttest genetic counseling session.
Cowden syndrome is an autosomal-dominant condition that carries a heightened risk of benign and malignant neoplasms, including a lifetime risk of breast cancer of up to 85%, with the average age at diagnosis in the 40s. Mutations in the PTEN gene also predispose to other cancer types, including nonmedullary thyroid, uterine, renal, and colorectal cancers, as well as melanoma.9 Multiple benign skin lesions and gastrointestinal polyposis are common.20
During the appointment, medical management options for patients with PTEN mutations are presented (Table 4).9 Given that your patient’s breast cancer was initially treated with lumpectomy, her remaining breast tissue is at risk of a second malignancy. She has never undergone thyroid imaging, colonoscopy, or kidney imaging. She reports that lately she has had occasional abnormal uterine bleeding and pain, which she believes are caused by her uterine fibroids. Given these symptoms and in light of her PTEN mutation, hysterectomy may be presented to her as an option. The genetics team sends a detailed clinical note directly to the primary care physician so they can coordinate and “quarterback” the patient’s care.
Like many patients, your patient is very concerned about how this information may affect her daughter. She first expresses some guilt at having to tell her daughter that she may have “given” her a risk of cancer. However, during the course of the genetic counseling session, she accepts that she could not have prevented her daughter from possibly inheriting this mutation, and understands that sharing this information will enable her daughter to pursue testing to help her understand her own risks.
When a known mutation exists in the family, as is the case with your patient, predictive testing only for that mutation gives a 100% accurate result. During a separate genetic counseling appointment, the patient’s daughter opts to proceed with testing and is found to be negative for her mother’s PTEN mutation.
WHAT HAPPENS WHEN GENETIC TESTING IS NOT INDICATED?
Cancer genetic risk assessment and counseling provides benefits even when genetic testing is not indicated. In some situations genetic testing is not warranted, but referral for heightened surveillance for breast cancer is deemed necessary. Patients who have a personal or family history of cancer can still gain from a detailed assessment of their personal and family history and may come away relieved after learning that they or their family members are not at high risk of developing cancer. Such patients or families may be classified as demonstrating either familial or sporadic breast cancer diagnoses.
Familial breast cancer
Familial breast cancers, believed to account for 15% to 20% of all cases of breast cancer, share features with hereditary breast cancer syndromes.4 In affected families, the frequency of breast cancer is higher than in the general population (multiple family members may be affected), and the age of onset tends to be close to that in the general population.
Members of a family with familial breast cancer who have not yet developed the disease may be at increased risk of it. Several risk-assessment tools (the Gail, Tyrer-Cuzick, Claus, and other models)21–25 use personal and family history to estimate breast cancer risk.
Depending on the assessed risk, additional options for screening and surveillance are available. The American Cancer Society recommends magnetic resonance imaging (MRI) in addition to annual mammography for women whose lifetime risk of breast cancer is greater than 20%. They also recommend that women at moderately increased risk (ie, 15%–20% lifetime risk) talk to their doctor about the benefits and limitations of adding MRI screening to yearly mammography.1
Sporadic breast cancer
Sporadic forms of breast cancer account for 70% to 80% of cases of breast cancer. Sporadic breast cancers are thought to have mainly nonhereditary causes, with environment and personal risk factors playing a large role.
Women with apparently sporadic breast cancers are diagnosed at or beyond the average age at diagnosis in the general population and do not have a family history that suggests either a hereditary cancer syndrome or familial breast cancer. If they undergo a cancer risk assessment, they may be relieved to learn that other women in their family do not have a high probability of being affected, and that they themselves do not appear to be at increased risk of other malignancies.
PATIENT 2: NEGATIVE TEST RESULTS ARE SOMETIMES ‘UNINFORMATIVE’
A healthy 35-year-old woman is referred for a genetics consultation by her gynecologist because her mother developed breast cancer at age 40 and died of the disease. A detailed personal and family history and risk assessment are done. After pretest genetic counseling, testing for BRCA1 and BRCA2 mutations (hereditary breast and ovarian cancer syndrome) is ordered, and the patient’s test results are negative. Risk assessment determines that no other hereditary cancer syndrome is likely. Therefore, no other genetic testing is offered at this time.
Genetic testing is most informative when performed first on the family member at highest risk of having a mutation. For families with breast cancer, this is typically the person with cancer diagnosed at the earliest age.
Unfortunately, sometimes these family members cannot be tested because they are deceased or otherwise unavailable. In such situations, it is acceptable to offer testing to a close, unaffected relative, such as your patient. Pretest genetic counseling in these circumstances is key, highlighting the fact that negative (normal) results would be uninformative. In your case, we cannot know whether the patient’s mother would have tested positive for a BRCA1 or BRCA2 mutation and your patient is a “true negative,” or whether her mother would have tested negative as well.
In unaffected patients with uninformative genetic testing results, medical management is based on the patient’s personal risk factors and family history of cancer. For your patient, statistical risk modeling tools (the Gail, Claus, Couch, and Tyrer-Cuzick models) determine that her risk of developing breast cancer is 22% to 28.5%, qualifying her for MRI along with yearly mammography per the American Cancer Society guidelines previously discussed.
KNOWLEDGE CONTINUES TO EXPAND
Major advances in the understanding of breast cancer susceptibility were made in the last decade through genetic linkage mapping in families that have an overabundance of members with breast cancer.26–28 Additionally, as more information is acquired, other genes predisposing to cancer or modifying cancer risk may be identified and additional knowledge gained.
With the advent of gene-panel-based testing and exome sequencing, we will incidentally discover mutations that predispose to cancer in patients in whom we were not looking for these mutations. With improving technology and value-based health care delivery, providers must continue to embrace multidisciplinary care, and genetics will become central in guiding medical management. In the event of an incidental finding suggesting susceptibility to heritable cancer, a consult to genetic counseling is recommended.
Many studies of the genetics of breast cancer are now focusing on known hereditary breast cancer syndromes and on possibilities for risk reduction, lifestyle modification, and identification of genetic variations that may increase or decrease cancer risk for an individual patient. The Center for Personalized Genetic Healthcare at Cleveland Clinic is collaborating in one such study. Titled “Risk Factor Analysis of Hereditary Breast and Ovarian Cancer Syndrome,” it is an international study led by a leading breast cancer researcher, Dr. Steven Narod from the Women’s College Research Institute in Toronto, ON. This study is focusing on women with a BRCA1 or BRCA2 mutation and their personal cancer risk factors, lifestyle choices, and overall development of cancer. This research group and others are also focusing on identifying genetic “modifiers” of cancer risk in these high-risk women.29
For patients who do not have a hereditary cancer syndrome, research is further exploring novel genes and their relation to breast cancer risk. One such study in our laboratory has found that several genes once thought only to cause an increased risk of hereditary paraganglioma may also predispose to breast and thyroid cancer.29,31 Additional research in this area is under way to clarify these risks.
GOOD SCIENCE, BAD MEDICINE?
Other research studies have identified a number of genes currently thought to be “moderately penetrant” for breast cancer risk, meaning that they may confer a risk of breast cancer slightly greater than that in the general population, but in some instances the risk has not been proven to be high enough to alter a patient’s management.32,33
Although a few clinical laboratories currently offer testing for these kinds of genes, the clinical utility of this testing is questionable. Before offering testing on a clinical basis, we need clear, consistent data on the types of cancers associated with these genes and on the lifetime percentage risk of acquiring these cancers. Currently, it is difficult to understand whether a variant in a moderately penetrant gene is the true explanation behind a patient’s breast cancer diagnosis. If such a variant is identified and family members pursue testing for it, should those family members who test negative be considered to have the same risk of cancer as the general population? And should family members testing positive be offered prophylactic surgical options?
Without more data these questions cannot be answered, and until such data are gathered, we believe that testing for moderately penetrant genes should not be performed outside of a research study. The Center for Personalized Genetic Healthcare in Cleveland Clinic’s Genomic Medicine Institute can assist in educating and coordinating patients’ enrollment in such research studies.
PUTTING IT ALL TOGETHER
Primary care physicians are the first-line providers to individuals and families, many of whom have a personal or family history of breast cancer. Identifying patients at risk of breast cancer and hereditary cancer syndromes can be challenging in this era of shortened appointment times and patients with complex medical histories.
Reviewing an individual’s personal and cancer family history is a necessary first step in considering appropriate medical management recommendations for cancer screening and prevention, the cornerstone of personalized health care. Patients with hereditary breast cancer syndromes and those with familial breast cancer can benefit from high-risk breast cancer surveillance.
Cancer genetics risk assessment ensures that the correct genetic testing is offered to the most appropriate patients, with personalized interpretation of results and provision of future management recommendations based on the individual patient’s personal and family history. Genetic counselors empower patients to make educated and informed decisions about genetic testing, cancer screening, and prevention.
As health care continues to focus more on prevention in this new era of genomic medicine and value-based delivery of health care, genetic counselors will serve as powerful allies to physicians.34
Acknowledgments: We would like to thank Dr. Colleen Clayton and Dr. Lynn Pattimakiel of the Medicine Institute, Cleveland Clinic, for their critical review of and thoughtful feedback on this manuscript.
- American Cancer Society. Breast cancer: detailed guide( 2013). http://www.cancer.org/Cancer/BreastCancer/DetailedGuide/index. Accessed November 12, 2013.
- McTiernan A, Gilligan MA, Redmond C. Assessing individual risk for breast cancer: risky business. J Clin Epidemiol 1997; 50:547–556.
- Teerlink CC, Albright FS, Lins L, Cannon-Albright LA. A comprehensive survey of cancer risks in extended families. Genet Med 2012; 14:107–114.
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology. Breast cancer risk reduction (version 1.2013). http://www.nccn.org. Accessed November 21, 2013.
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology. Genetic/familial high risk assessment: breast and ovarian (version 4.2013). http://www.nccn.org. Accessed November 21, 2013.
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology. Breast cancer screening and diagnosis (version 2.2013). http://www.nccn.org. Accessed November 21, 2013.
- Mester JL, Schreiber AH, Moran RT. Genetic counselors: your partners in clinical practice. Cleve Clin J Med 2012; 79:560–568.
- Trepanier A, Ahrens M, McKinnon W, et al; National Society of Genetic Counselors. Genetic cancer risk assessment and counseling: recommendations of the National Society of Genetic Counselors. J Genet Couns 2004; 13:83–114.
- Tan MH, Mester JL, Ngeow J, Rybicki LA, Orloff MS, Eng C. Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res 2012; 18:400–407.
- Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet 1998; 62:676–689.
- Liede A, Karlan BY, Narod SA. Cancer risks for male carriers of germline mutations in BRCA1 or BRCA2: a review of the literature. J Clin Oncol 2004; 22:735–742.
- Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med 1997; 336:1401–1408.
- Birch JM, Hartley AL, Tricker KJ, et al. Prevalence and diversity of constitutional mutations in the p53 gene among 21 Li-Fraumeni families. Cancer Res 1994; 54:1298–1304.
- Chompret A, Brugières L, Ronsin M, et al. P53 germline mutations in childhood cancers and cancer risk for carrier individuals. Br J Cancer 2000; 82:1932–1937.
- Gonzalez KD, Noltner KA, Buzin CH, et al. Beyond Li Fraumeni syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol 2009; 27:1250–1256.
- Varley JM. Germline TP53 mutations and Li-Fraumeni syndrome. Hum Mutat 2003; 21:313–320.
- Fitzgerald RC, Hardwick R, Huntsman D, et al; International Gastric Cancer Linkage Consortium. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet 2010; 47:436–444.
- Hearle N, Schumacher V, Menko FH, et al. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res 2006; 12:3209–3215.
- American Society of Clinical Oncology. American Society of Clinical Oncology policy statement update: genetic testing for cancer susceptibility. J Clin Oncol 2003; 21:2397–2406.
- Mester J, Eng C. When overgrowth bumps into cancer: the PTEN-opathies. Am J Med Genet C Semin Med Genet 2013; 163:114–121.
- Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer. Implications for risk prediction. Cancer 1994; 73:643–651.
- Couch FJ, DeShano ML, Blackwood MA, et al. BRCA1 mutations in women attending clinics that evaluate the risk of breast cancer. N Engl J Med 1997; 336:1409–1415.
- Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med 2004; 23:1111–1130.
- Gail MH, Anderson WF, Garcia-Closas M, Sherman ME. Absolute risk models for subtypes of breast cancer. J Natl Cancer Inst 2007; 99:1657–1659.
- Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 1989; 81:1879–1886.
- Kent P, O’Donoghue JM, O’Hanlon DM, Kerin MJ, Maher DJ, Given HF. Linkage analysis and the susceptibility gene (BRCA-1) in familial breast cancer. Eur J Surg Oncol 1995; 21:240–241.
- Easton DF, Bishop DT, Ford D, Crockford GP. Genetic linkage analysis in familial breast and ovarian cancer: results from 214 families. The Breast Cancer Linkage Consortium. Am J Hum Genet 1993; 52:678–701.
- Ormiston W. Hereditary breast cancer. Eur J Cancer Care (Engl) 1996; 5:13–20.
- Couch FJ, Wang X, McGuffog L, et al. Genome-wide association study in BRCA1 mutation carriers identifies novel loci associated with breast and ovarian cancer risk. PLoS Genet 2013; 9:e1003212.
- Bennett KL, Mester J, Eng C. Germline epigenetic regulation of KILLIN in Cowden and Cowden-like syndrome. JAMA 2010; 304:2724–2731.
- Ni Y, He X, Chen J, et al. Germline SDHx variants modify breast and thyroid cancer risks in Cowden and Cowden-like syndrome via FAD/NAD-dependent destabilization of p53. Hum Mol Genet 2012; 21:300–310.
- Casadei S, Norquist BM, Walsh T, et al. Contribution of inherited mutations in the BRCA2-interacting protein PALB2 to familial breast cancer. Cancer Res 2011; 71:2222–2229.
- Walsh T, Lee MK, Casadei S, et al. Detection of inherited mutations for breast and ovarian cancer using genomic capture and massively parallel sequencing. Proc Natl Acad Sci U S A 2010; 107:12629–12633.
- Eng C. Molecular genetics to genomic medicine: at the heart of value-based delivery of healthcare. Mol Genet Genom Med 2013; 1:4–6.
- American Cancer Society. Breast cancer: detailed guide( 2013). http://www.cancer.org/Cancer/BreastCancer/DetailedGuide/index. Accessed November 12, 2013.
- McTiernan A, Gilligan MA, Redmond C. Assessing individual risk for breast cancer: risky business. J Clin Epidemiol 1997; 50:547–556.
- Teerlink CC, Albright FS, Lins L, Cannon-Albright LA. A comprehensive survey of cancer risks in extended families. Genet Med 2012; 14:107–114.
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology. Breast cancer risk reduction (version 1.2013). http://www.nccn.org. Accessed November 21, 2013.
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology. Genetic/familial high risk assessment: breast and ovarian (version 4.2013). http://www.nccn.org. Accessed November 21, 2013.
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology. Breast cancer screening and diagnosis (version 2.2013). http://www.nccn.org. Accessed November 21, 2013.
- Mester JL, Schreiber AH, Moran RT. Genetic counselors: your partners in clinical practice. Cleve Clin J Med 2012; 79:560–568.
- Trepanier A, Ahrens M, McKinnon W, et al; National Society of Genetic Counselors. Genetic cancer risk assessment and counseling: recommendations of the National Society of Genetic Counselors. J Genet Couns 2004; 13:83–114.
- Tan MH, Mester JL, Ngeow J, Rybicki LA, Orloff MS, Eng C. Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res 2012; 18:400–407.
- Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet 1998; 62:676–689.
- Liede A, Karlan BY, Narod SA. Cancer risks for male carriers of germline mutations in BRCA1 or BRCA2: a review of the literature. J Clin Oncol 2004; 22:735–742.
- Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med 1997; 336:1401–1408.
- Birch JM, Hartley AL, Tricker KJ, et al. Prevalence and diversity of constitutional mutations in the p53 gene among 21 Li-Fraumeni families. Cancer Res 1994; 54:1298–1304.
- Chompret A, Brugières L, Ronsin M, et al. P53 germline mutations in childhood cancers and cancer risk for carrier individuals. Br J Cancer 2000; 82:1932–1937.
- Gonzalez KD, Noltner KA, Buzin CH, et al. Beyond Li Fraumeni syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol 2009; 27:1250–1256.
- Varley JM. Germline TP53 mutations and Li-Fraumeni syndrome. Hum Mutat 2003; 21:313–320.
- Fitzgerald RC, Hardwick R, Huntsman D, et al; International Gastric Cancer Linkage Consortium. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet 2010; 47:436–444.
- Hearle N, Schumacher V, Menko FH, et al. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res 2006; 12:3209–3215.
- American Society of Clinical Oncology. American Society of Clinical Oncology policy statement update: genetic testing for cancer susceptibility. J Clin Oncol 2003; 21:2397–2406.
- Mester J, Eng C. When overgrowth bumps into cancer: the PTEN-opathies. Am J Med Genet C Semin Med Genet 2013; 163:114–121.
- Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer. Implications for risk prediction. Cancer 1994; 73:643–651.
- Couch FJ, DeShano ML, Blackwood MA, et al. BRCA1 mutations in women attending clinics that evaluate the risk of breast cancer. N Engl J Med 1997; 336:1409–1415.
- Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med 2004; 23:1111–1130.
- Gail MH, Anderson WF, Garcia-Closas M, Sherman ME. Absolute risk models for subtypes of breast cancer. J Natl Cancer Inst 2007; 99:1657–1659.
- Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 1989; 81:1879–1886.
- Kent P, O’Donoghue JM, O’Hanlon DM, Kerin MJ, Maher DJ, Given HF. Linkage analysis and the susceptibility gene (BRCA-1) in familial breast cancer. Eur J Surg Oncol 1995; 21:240–241.
- Easton DF, Bishop DT, Ford D, Crockford GP. Genetic linkage analysis in familial breast and ovarian cancer: results from 214 families. The Breast Cancer Linkage Consortium. Am J Hum Genet 1993; 52:678–701.
- Ormiston W. Hereditary breast cancer. Eur J Cancer Care (Engl) 1996; 5:13–20.
- Couch FJ, Wang X, McGuffog L, et al. Genome-wide association study in BRCA1 mutation carriers identifies novel loci associated with breast and ovarian cancer risk. PLoS Genet 2013; 9:e1003212.
- Bennett KL, Mester J, Eng C. Germline epigenetic regulation of KILLIN in Cowden and Cowden-like syndrome. JAMA 2010; 304:2724–2731.
- Ni Y, He X, Chen J, et al. Germline SDHx variants modify breast and thyroid cancer risks in Cowden and Cowden-like syndrome via FAD/NAD-dependent destabilization of p53. Hum Mol Genet 2012; 21:300–310.
- Casadei S, Norquist BM, Walsh T, et al. Contribution of inherited mutations in the BRCA2-interacting protein PALB2 to familial breast cancer. Cancer Res 2011; 71:2222–2229.
- Walsh T, Lee MK, Casadei S, et al. Detection of inherited mutations for breast and ovarian cancer using genomic capture and massively parallel sequencing. Proc Natl Acad Sci U S A 2010; 107:12629–12633.
- Eng C. Molecular genetics to genomic medicine: at the heart of value-based delivery of healthcare. Mol Genet Genom Med 2013; 1:4–6.
KEY POINTS
- Primary care physicians play a critical role in identifying patients at risk of inherited health problems.
- Hereditary cancers are important to detect because the age of onset is early, multiple primary cancers can develop, and cancer predisposition may be inherited.
- Hereditary syndromes account for only a minority of cases of breast cancer, but women who bear the responsible mutations have an extremely high risk.
- Patients with hereditary breast cancer syndromes and those with familial breast cancer can benefit from heightened surveillance for breast cancer.
- Cancer genetics risk assessment ensures that the correct genetic testing is offered to the most appropriate patients, with personalized interpretation of results and provision of future management recommendations based on the individual patient’s personal and family history.
Personalizing patient care
The concept and promise of personalized health care have been anticipated for decades. Yet in its breadth and in the way we would like it practiced, it is in its infancy.
Personalized health care aims to individualize care by integrating a person’s unique clinical, molecular (ie, genetic, genomic), and environmental information. Applied not only when the patient is sick but also when he or she is well, it builds on and enhances our current standards of care.
As Sir William Osler recognized more than a century ago, “Variability is the law of life, and as no two faces are the same, so no two bodies are alike, and no two individuals react alike and behave alike under the abnormal conditions which we know as disease.”1
PREDICTING THE RISK OF DISEASE
For years, we have attempted to predict and stratify the risk of disease, and the Human Genome Project has given us a new set of tools to help understand the complexity of disease and its variability.
We know and have known for decades—and in some cultures, for centuries—that family history is the most clinically validated tool for predicting the risk of disease.
Nevertheless, evidence suggests that we physicians are not collecting adequate family histories, and because of this, we are missing opportunities to intervene and prevent diseases predicted by the family history. The current standard of care is to use family medical histories to hone genetic differential diagnoses, and based on the differential diagnoses, to target specific genes to test in the setting of genetic counseling. Current genetic testing is used for molecular diagnoses and predictive testing so that gene-specific clinical management can be subsequently tailored.
PREDICTING RESPONSE TO TREATMENT
The use of personalized health care to predict response to treatment is a novel and constantly evolving practice.
ABO blood typing is a form of genetics-based personalization of safe transfusion that dates back to World War II.
A prominent, recent success story is in cancer treatment. For example, the American Society of Clinical Oncology now recommends that tumors from patients with node-negative, estrogen-receptor-positive breast cancer be evaluated with the Oncotype DX assay.2 This test measures the expression of 21 genes, and the score obtained identifies patients most likely to benefit from adjuvant chemotherapy. A similar 12-gene expression signature has been developed for colon cancer, and others have been developed for hematologic cancers.2,3 As with other new but apparently valid tests, the risk scores derived are sensitive at the extremes but ambiguous in the mid-ranges. We anticipate many more developments in this field.
In the field of pharmacogenomics, there is evidence to suggest that prior knowledge of CYP2C9 and VKORC1 genotypes enhances outcomes for patients starting treatment with warfarin. The US Food and Drug Administration revised the label on warfarin in February 2010, suggesting that genotypes be taken into consideration when the drug is prescribed.4
However, clinicians have been slow to adopt genotype testing when prescribing warfarin. Some cite the paucity of large, randomized, controlled trials demonstrating clinical utility of genotype-informed prescribing. Others cite concern that warfarin will soon become obsolete with the arrival of newer anticoagulants (such as factor X inhibitors) that do not carry warfarin’s adverse effects, and these genotypes will therefore become moot. Perhaps, as we move forward and new drugs are developed, companion genotype tests could be developed at the same time to be used with them.5
IMPROVING CARE, SAVING MONEY, AND EMPOWERING PATIENTS
The goal of personalized health care, by customizing treatments (medication types and dosages) and preventive strategies, is to optimize medical care and improve outcomes for each patient. It could improve the quality of care by targeting interventions and reducing adverse events, topics that are important to all of us in the current environment of health care reform.
A personalized approach might also, in the long run, decrease the cost of health care by driving appropriate utilization of resources.
Lastly, the true value of personalized health care may be in its potential to improve patient satisfaction and to empower our patients to work with us towards better health.
WE LAUNCH A NEW SERIES
To keep physicians up-to-date on progress in personalized health care, the Cleveland Clinic Journal of Medicine will present a series of articles on the topic. The series, to run once a quarter, begins in this issue, on page 331, with an article on the importance of the family history as a piece of genetic information that can help to predict the risk of disease and inform preventive care plans. Future topics will include the role of genetics and genomics in personalized care of patients with breast and colorectal cancers; the genetic counselor as a part of the health care team; pharmacogenomics; and ethical, legal, and societal considerations.
Our goal in this series is to provide practical information to help our readers incorporate personalized approaches into daily practice. In addition, as patients become more interested in and informed about personalized health care, we hope this information will help clinicians to effectively coach them about its potential benefits and risks. We also hope this information will enable our readers to ask the right questions so that patient and health care provider can work together to help the patient grow old gracefully.
As the series unfolds, we ask you to send us feedback and to suggest other topics in personalized health care you would like us to cover in this series.
- Osler W. Aequanimitas, With Other Addresses to Medical Students, Nurses and Practitioners of Medicine. 2nd edition. Philadelphia, PA: P. Blakiston’s Sone & Co, 1906:348.
- McDermott U, Downing JR, Stratton MR. Genomics and the continuum of cancer care. N Engl J Med 2011; 364:340–350.
- Eng C. Microenvironmental protection in diffuse large-B-cell lymphoma. N Engl J Med 2008; 359:2379–2381.
- Wang L, McLeod HL, Weinshilboum RM. Genomics and drug response. N Engl J Med 2011; 364:1144–1153.
- Hamburg MA, Collins FS. The path to personalized medicine. N Engl J Med 2010; 363:301–304.
The concept and promise of personalized health care have been anticipated for decades. Yet in its breadth and in the way we would like it practiced, it is in its infancy.
Personalized health care aims to individualize care by integrating a person’s unique clinical, molecular (ie, genetic, genomic), and environmental information. Applied not only when the patient is sick but also when he or she is well, it builds on and enhances our current standards of care.
As Sir William Osler recognized more than a century ago, “Variability is the law of life, and as no two faces are the same, so no two bodies are alike, and no two individuals react alike and behave alike under the abnormal conditions which we know as disease.”1
PREDICTING THE RISK OF DISEASE
For years, we have attempted to predict and stratify the risk of disease, and the Human Genome Project has given us a new set of tools to help understand the complexity of disease and its variability.
We know and have known for decades—and in some cultures, for centuries—that family history is the most clinically validated tool for predicting the risk of disease.
Nevertheless, evidence suggests that we physicians are not collecting adequate family histories, and because of this, we are missing opportunities to intervene and prevent diseases predicted by the family history. The current standard of care is to use family medical histories to hone genetic differential diagnoses, and based on the differential diagnoses, to target specific genes to test in the setting of genetic counseling. Current genetic testing is used for molecular diagnoses and predictive testing so that gene-specific clinical management can be subsequently tailored.
PREDICTING RESPONSE TO TREATMENT
The use of personalized health care to predict response to treatment is a novel and constantly evolving practice.
ABO blood typing is a form of genetics-based personalization of safe transfusion that dates back to World War II.
A prominent, recent success story is in cancer treatment. For example, the American Society of Clinical Oncology now recommends that tumors from patients with node-negative, estrogen-receptor-positive breast cancer be evaluated with the Oncotype DX assay.2 This test measures the expression of 21 genes, and the score obtained identifies patients most likely to benefit from adjuvant chemotherapy. A similar 12-gene expression signature has been developed for colon cancer, and others have been developed for hematologic cancers.2,3 As with other new but apparently valid tests, the risk scores derived are sensitive at the extremes but ambiguous in the mid-ranges. We anticipate many more developments in this field.
In the field of pharmacogenomics, there is evidence to suggest that prior knowledge of CYP2C9 and VKORC1 genotypes enhances outcomes for patients starting treatment with warfarin. The US Food and Drug Administration revised the label on warfarin in February 2010, suggesting that genotypes be taken into consideration when the drug is prescribed.4
However, clinicians have been slow to adopt genotype testing when prescribing warfarin. Some cite the paucity of large, randomized, controlled trials demonstrating clinical utility of genotype-informed prescribing. Others cite concern that warfarin will soon become obsolete with the arrival of newer anticoagulants (such as factor X inhibitors) that do not carry warfarin’s adverse effects, and these genotypes will therefore become moot. Perhaps, as we move forward and new drugs are developed, companion genotype tests could be developed at the same time to be used with them.5
IMPROVING CARE, SAVING MONEY, AND EMPOWERING PATIENTS
The goal of personalized health care, by customizing treatments (medication types and dosages) and preventive strategies, is to optimize medical care and improve outcomes for each patient. It could improve the quality of care by targeting interventions and reducing adverse events, topics that are important to all of us in the current environment of health care reform.
A personalized approach might also, in the long run, decrease the cost of health care by driving appropriate utilization of resources.
Lastly, the true value of personalized health care may be in its potential to improve patient satisfaction and to empower our patients to work with us towards better health.
WE LAUNCH A NEW SERIES
To keep physicians up-to-date on progress in personalized health care, the Cleveland Clinic Journal of Medicine will present a series of articles on the topic. The series, to run once a quarter, begins in this issue, on page 331, with an article on the importance of the family history as a piece of genetic information that can help to predict the risk of disease and inform preventive care plans. Future topics will include the role of genetics and genomics in personalized care of patients with breast and colorectal cancers; the genetic counselor as a part of the health care team; pharmacogenomics; and ethical, legal, and societal considerations.
Our goal in this series is to provide practical information to help our readers incorporate personalized approaches into daily practice. In addition, as patients become more interested in and informed about personalized health care, we hope this information will help clinicians to effectively coach them about its potential benefits and risks. We also hope this information will enable our readers to ask the right questions so that patient and health care provider can work together to help the patient grow old gracefully.
As the series unfolds, we ask you to send us feedback and to suggest other topics in personalized health care you would like us to cover in this series.
The concept and promise of personalized health care have been anticipated for decades. Yet in its breadth and in the way we would like it practiced, it is in its infancy.
Personalized health care aims to individualize care by integrating a person’s unique clinical, molecular (ie, genetic, genomic), and environmental information. Applied not only when the patient is sick but also when he or she is well, it builds on and enhances our current standards of care.
As Sir William Osler recognized more than a century ago, “Variability is the law of life, and as no two faces are the same, so no two bodies are alike, and no two individuals react alike and behave alike under the abnormal conditions which we know as disease.”1
PREDICTING THE RISK OF DISEASE
For years, we have attempted to predict and stratify the risk of disease, and the Human Genome Project has given us a new set of tools to help understand the complexity of disease and its variability.
We know and have known for decades—and in some cultures, for centuries—that family history is the most clinically validated tool for predicting the risk of disease.
Nevertheless, evidence suggests that we physicians are not collecting adequate family histories, and because of this, we are missing opportunities to intervene and prevent diseases predicted by the family history. The current standard of care is to use family medical histories to hone genetic differential diagnoses, and based on the differential diagnoses, to target specific genes to test in the setting of genetic counseling. Current genetic testing is used for molecular diagnoses and predictive testing so that gene-specific clinical management can be subsequently tailored.
PREDICTING RESPONSE TO TREATMENT
The use of personalized health care to predict response to treatment is a novel and constantly evolving practice.
ABO blood typing is a form of genetics-based personalization of safe transfusion that dates back to World War II.
A prominent, recent success story is in cancer treatment. For example, the American Society of Clinical Oncology now recommends that tumors from patients with node-negative, estrogen-receptor-positive breast cancer be evaluated with the Oncotype DX assay.2 This test measures the expression of 21 genes, and the score obtained identifies patients most likely to benefit from adjuvant chemotherapy. A similar 12-gene expression signature has been developed for colon cancer, and others have been developed for hematologic cancers.2,3 As with other new but apparently valid tests, the risk scores derived are sensitive at the extremes but ambiguous in the mid-ranges. We anticipate many more developments in this field.
In the field of pharmacogenomics, there is evidence to suggest that prior knowledge of CYP2C9 and VKORC1 genotypes enhances outcomes for patients starting treatment with warfarin. The US Food and Drug Administration revised the label on warfarin in February 2010, suggesting that genotypes be taken into consideration when the drug is prescribed.4
However, clinicians have been slow to adopt genotype testing when prescribing warfarin. Some cite the paucity of large, randomized, controlled trials demonstrating clinical utility of genotype-informed prescribing. Others cite concern that warfarin will soon become obsolete with the arrival of newer anticoagulants (such as factor X inhibitors) that do not carry warfarin’s adverse effects, and these genotypes will therefore become moot. Perhaps, as we move forward and new drugs are developed, companion genotype tests could be developed at the same time to be used with them.5
IMPROVING CARE, SAVING MONEY, AND EMPOWERING PATIENTS
The goal of personalized health care, by customizing treatments (medication types and dosages) and preventive strategies, is to optimize medical care and improve outcomes for each patient. It could improve the quality of care by targeting interventions and reducing adverse events, topics that are important to all of us in the current environment of health care reform.
A personalized approach might also, in the long run, decrease the cost of health care by driving appropriate utilization of resources.
Lastly, the true value of personalized health care may be in its potential to improve patient satisfaction and to empower our patients to work with us towards better health.
WE LAUNCH A NEW SERIES
To keep physicians up-to-date on progress in personalized health care, the Cleveland Clinic Journal of Medicine will present a series of articles on the topic. The series, to run once a quarter, begins in this issue, on page 331, with an article on the importance of the family history as a piece of genetic information that can help to predict the risk of disease and inform preventive care plans. Future topics will include the role of genetics and genomics in personalized care of patients with breast and colorectal cancers; the genetic counselor as a part of the health care team; pharmacogenomics; and ethical, legal, and societal considerations.
Our goal in this series is to provide practical information to help our readers incorporate personalized approaches into daily practice. In addition, as patients become more interested in and informed about personalized health care, we hope this information will help clinicians to effectively coach them about its potential benefits and risks. We also hope this information will enable our readers to ask the right questions so that patient and health care provider can work together to help the patient grow old gracefully.
As the series unfolds, we ask you to send us feedback and to suggest other topics in personalized health care you would like us to cover in this series.
- Osler W. Aequanimitas, With Other Addresses to Medical Students, Nurses and Practitioners of Medicine. 2nd edition. Philadelphia, PA: P. Blakiston’s Sone & Co, 1906:348.
- McDermott U, Downing JR, Stratton MR. Genomics and the continuum of cancer care. N Engl J Med 2011; 364:340–350.
- Eng C. Microenvironmental protection in diffuse large-B-cell lymphoma. N Engl J Med 2008; 359:2379–2381.
- Wang L, McLeod HL, Weinshilboum RM. Genomics and drug response. N Engl J Med 2011; 364:1144–1153.
- Hamburg MA, Collins FS. The path to personalized medicine. N Engl J Med 2010; 363:301–304.
- Osler W. Aequanimitas, With Other Addresses to Medical Students, Nurses and Practitioners of Medicine. 2nd edition. Philadelphia, PA: P. Blakiston’s Sone & Co, 1906:348.
- McDermott U, Downing JR, Stratton MR. Genomics and the continuum of cancer care. N Engl J Med 2011; 364:340–350.
- Eng C. Microenvironmental protection in diffuse large-B-cell lymphoma. N Engl J Med 2008; 359:2379–2381.
- Wang L, McLeod HL, Weinshilboum RM. Genomics and drug response. N Engl J Med 2011; 364:1144–1153.
- Hamburg MA, Collins FS. The path to personalized medicine. N Engl J Med 2010; 363:301–304.
Building an innovative model for personalized healthcare
Personalized healthcare is the tailoring of medical management and patient care to the individual characteristics of each patient. This is achieved by incorporating the genetic and genomic makeup of an individual and his or her family medical history, environment, health-related behaviors, culture, and values into a complete health picture that can be used to customize care. Another level of personalization, often called personalized medicine, involves the selection of drug therapy through the use of tests to determine the genes and gene interactions that can reliably predict an individual’s response to a given therapy. This white paper focuses largely on the use of personalized healthcare as a risk prediction tool.
CURRENT STATUS OF PERSONALIZED HEALTHCARE
The case for personalized healthcare: Seeking value
To fully appreciate the need to advance the adoption of personalized healthcare into the delivery of medicine, one must consider the operation of our current healthcare system and its inefficiencies in terms of delivery and cost, its imprecision in the selection of therapies, and its inability to optimize outcomes. The framework of the US healthcare system as it is now constructed is expensive, disease-directed (instead of health- and wellness-directed), fragmented, and complex. While gross domestic product (GDP) in the United States has increased by approximately 3% per year,2 the compounded growth rate of healthcare expenditures is 6.1% per year. Healthcare in the aggregate now represents 17.6% of GDP and 27% of spending by the federal government and consumes 28% of the average household’s discretionary spending, surpassed only by housing.3
Personalized healthcare can potentially address the need for value consistent with the healthcare system’s prominent share of the US economy. The growth in healthcare spending is certain to be a target of the newly created Joint Select Committee on Deficit Reduction (created by the Budget Control Act of 2011), which is tasked with deficit reduction of at least $1.5 trillion over a 10-year period.
The need to address healthcare costs has been recognized in the Patient Protection and Affordable Care Act, a central feature of which is the creation of integrated health systems that pay for value based on quality, cost containment, and consumer experience. The legislation was enacted to transform healthcare in a variety of ways to make it more sustainable. The Patient Protection and Affordable Care Act seeks to end fragmentation by expanding the use of information technology to reorganize the delivery system and to prevent errors, shifting from volume-based incentives to incentives based on performance and outcomes, and rewarding effective healthcare delivery measures and good patient outcomes.
A shift from reactive to proactive
The premise behind personalized healthcare is the potential for more efficient healthcare, with the assumption that efficiency translates to lower cost and improved patient care.
Although healthcare reform is most often referred to in the context of improving access to care through insurance coverage mandates, true healthcare reform shifts current healthcare models from the practice of reactive medicine to the practice of proactive medicine, in which the tools of personalized healthcare (ie, genetics, genomics, and other molecular diagnostics) enable not only better quality of care but also less expensive care.
Several personalized tools have long been accepted into mainstream medicine. Two examples are the family history, which is the least expensive and most available genetic evaluation tool, and ABO blood typing for safe transfusions (as ABO blood types are alleles of a gene). In fact, much of what is now considered mainstream medical management was at one time considered new. To allow further evolution of medical practice, our challenge is to open our minds to the possibility that personalized proactive medicine can improve healthcare.
The new vision: More precise management
The trial-and-error approach to treating disease is inefficient and costly. Many drugs are effective for only about 50% of patients, often leading to switching or intensification of therapy that requires multiple patient visits.
Personalized medicine considers pharmacokinetic and other characteristics in selection of drug dosages. Genomic testing has the potential to provide clearer insight into the more successful use of currently available medicines. Treatment decisions (ie, drug and drug dosage choice) made on the basis of pharmacogenomic testing should increase adherence through greater effectiveness and fewer adverse drug reactions.
A massive amount of waste is related to pharmaceutical nonadherence and noncompliance. The New England Healthcare Institute has estimated that medication nonadherence costs the healthcare system $290 billion annually.4 Methodologies targeted at individual patients to improve adherence to drug regimens could save the healthcare system a tremendous amount of money.
Cancer management as a model for personalized healthcare. Personalization of therapy is especially suited to cancer management, given that the response to nonspecific cancer chemotherapy is suboptimal in most patients yet exposes them to adverse effects.5 Large-scale sequencing of human cancer genomes is rapidly changing the understanding of cancer biology and is identifying new targets in difficult-to-treat diseases and causes of drug resistance. Applying this information can achieve cost savings by avoiding the use of treatments that are ineffective in particular patients.
Overexpression of genetic mutations renders some cancers less susceptible to certain treatments, but has opened the door to individualized molecularly guided treatment strategies. For example, among patients with non–small cell lung cancer, mutations in the epidermal growth factor receptor (EGFR) tyrosine kinase domain predict response to EGFR tyrosine kinase inhibitors, and anaplastic lymphoma kinase (ALK) inhibitors induce response in patients harboring a mutation in EML4-ALK genes. The recognition that human epidermal growth factor receptor (HER)-2 overexpression as a result of ERBB2 gene amplification occurs in as many as 20% of human breast cancers paved the way for the development of HER-2–targeted therapies. Patients with advanced colorectal cancer whose tumors express the KRAS gene mutation do not benefit from an EGFR inhibitor, whereas those with wild-type KRAS have improved survival with EGFR inhibitor treatment.6
BARRIERS TO THE APPLICATION OF PERSONALIZED HEALTHCARE
The availability and potential of personalized healthcare services and technology is not universally recognized or appreciated by consumers and clinicians. This lack of awareness contributes to a shortage of public support and limited demand for such services. Other barriers include misperceptions regarding the impact of personalized healthcare on disease management, limited incentives to use the available technology, and a knowledge gap among healthcare providers.
Lack of awareness and support
As applications of personalized healthcare advance to the point of clinical relevance, it is important to consider strategies for effective implementation into healthcare practice. Personalized healthcare, when more fully implemented, promises to accelerate the progress that healthcare reform hopes to achieve.
A major challenge to widespread adoption of personalized healthcare is limited recognition by the public and some healthcare providers that personalized healthcare can help to achieve better value. For personalized medicine to be embraced, the concept of “helix to health,” or translation of knowledge to the clinical setting, must resonate with the general public. Despite lack of public and provider awareness, the Personalized Medicine Coalition (PMC) has documented the existence of 56 personalized treatment and diagnostic products. Further, more than 200 product labels now recommend genetic testing prior to use to identify likely responders or inform of the influence of genetic variation on safety and effectiveness.
Consumers’ confidence in the efficacy and safety of medicines they take might contribute to the absence of public support for personalized healthcare. Similarly, despite the availability of genomic tests and tools, many physicians who might be advocates for personalized healthcare do not see the relevance of genomic medicine to their practices in terms of direct benefit to patient care.7
Apart from clinicians and consumers, support is also weak among health insurers and employers, even though the return on investment for personalized healthcare may be profound. Payers await the economic outcomes data that are crucial for their commitment to personalized healthcare. In addition, some have concerns about the ethical implications of personalized healthcare (see “Managing Genomic Information Responsibly”).
Perception of impact on treatment and prevention
A frequent criticism of genomics in medicine is that a genetic diagnosis does not help with patient management. In fact, surveillance and management of patients and family members often changes in response to a genetic diagnosis; knowing which gene is involved personalizes medical management. An example is the management of hereditary nonpolyposis colorectal cancer (HNPCC), or Lynch syndrome, which is the most common form of hereditary colon cancer. For a person with HNPCC, the lifetime risk of developing colorectal cancer is approximately 80%. Lynch syndrome is caused by germline mutations in one of three major mismatch repair (MMR) genes (MLH1, MSH2, and MSH6), and it predisposes to other cancers—uterine, stomach, and ovarian—as well. In women with Lynch syndrome, the lifetime risk for uterine cancer is 40%, compared with 4% in the general population.
At least 90% of patients with Lynch syndrome can be detected through MMR testing via microsatellite instability (MSI) or immunohistochemistry (IHC).8 MSI is a cellular phenotype that indicates a deficiency in at least one DNA MMR protein.
Although 5-fluorouracil–based chemo therapy is the standard of care for treatment of colorectal cancer, it confers no survival advantage in patients with MMR-IHC null (lack of expression of the gene) or MSI-high sporadic colorectal cancer.9,10 Knowing the status of MMR proteins, therefore, would alter the decision regarding neoadjuvant and adjuvant chemotherapy.
Perception of value
Implementation of pharmacogenomics into clinical practice has lagged. One major reason is the lack of an obvious business model for a product that may only be required once in an individual patient’s lifetime.11
A second barrier to integration lies in the limited demand for pharmacogenomics from physicians. This may be related partly to limited expertise in genetics among many physicians and to significant pushback from payers against today’s costs. Without reimbursement, little incentive exists for pharmacogenomics diagnostics. The incentive for physicians is further depressed, perhaps appropriately, when randomized controlled studies fail to demonstrate improved clinical outcomes with the use of pharmacogenomicbased treatment strategies. Two such examples are genotype-guided warfarin dosing, which failed in a randomized controlled trial to improve the proportion of international normalized ratios in the therapeutic range,12 and dosing of clopidogrel based on platelet reactivity, which did not improve outcomes after percutaneous coronary intervention compared with standard dosing in a randomized double-blind clinical trial.13
A significant delay in obtaining the results of pharmacogenomics testing, which also postpones the prescribing encounter, is another major drawback.
A knowledge gap persists

At present, delivery of personalized healthcare is not part of the usual training of physicians and other healthcare providers who are the gatekeepers of medicine. Few medical schools incorporate human and medical genetics, genomics, and pharmacogenomics into their curricula. Genetics is inadequately emphasized in residency curricula outside of pediatrics, family medicine, and obstetrics/gynecology.
The resulting knowledge gap is a fundamental factor in the lack of interest in using genomics in clinical medicine. Educating consumers and physicians at all levels, including specialty societies as well as insurers, will be key to expanding utilization of personalized healthcare. Educating payers and providing them with more data on economic outcomes associated with personalized healthcare will be necessary for adoption into clinical practice; implementation will lag as long as reimbursement decisions do not support personalized approaches to medicine.
As DNA sequencing technology has become less expensive and more powerful, companies have begun to market personal genomic testing. As a result, patients who use these services will increasingly want to discuss the results with their physicians. A significant number of clinicians are unfamiliar with personal genomic testing and emerging genetic testing options. In one survey of physicians who attended educational sessions that discussed recent developments in clinical genetics, only 37% indicated that they were familiar with recent genetic research that affected their patients.14
Targeted education will enhance physicians’ understanding of probabilities and risk estimates from the use of genomic testing; it will also improve recognition of potential causes of patient anxiety, gene variants of unknown significance, and follow-up tests and procedures that can add to expense. Nonphysician healthcare providers (ie, nurses and physician assistants) of direct care also will benefit from education.
INTEGRATING PERSONALIZED HEALTHCARE INTO CLINICAL PRACTICE
Practice standardization and an overhaul of the health information technology (HIT) infrastructure are needed if we are to reap the potential benefits of personalized healthcare. Creative approaches to practitioner education, which are being used in some institutions, must become more widespread. Similarly, the models for successful integration of personalized healthcare that have been achieved in some settings also can be implemented in other institutions.
Data collection and integration must be prioritized
Personalized healthcare can be both predictive and preventive, but moving past the disruptive phase of personalized healthcare will require a radical transformation of the healthcare “ecosystem” and HIT infrastructure.
Although data collection in the current system is extensive, data sharing and data management are inadequate. The pace at which HIT links clinical and genetic information must be accelerated. HIT will expedite innovation and implementation of personalized healthcare, allowing greater integration of data to permit improved data analysis capability. The ultimate goal is to create an interoperable system that connects these data across hospitals and clinicians to help clinicians interpret genomic and other risk information to better inform patient care.
Fully integrated health systems support better coordination of care and optimize the treatment of individual patients: linking research findings, treatment guidelines, treatment outcomes based on genetic profiles, and the individual patient’s own genetic profile will help to personalize treatments. Genomic information added to an individual’s electronic medical record along with improved data-sharing will facilitate clinicians’ ability to retrieve outcomes data based on patient characteristics.
Care models must be standardized, evidence-based practices must be executed, and care must be coordinated yet decentralized. In this way, clinicians can use the electronic medical record as an interoperable patient record to determine a personalized pathway to patient management. Standardization reduces variability in practice and permits seamless execution of care. Automation is imperative to achieving standardization, irrespective of the care supervisor. Investments must therefore be made to stimulate electronic medical record decision support.
In addition, larger data sets will be needed to identify the types of patients likely to respond to a treatment. Ideal data sets would be large enough to have adequate statistical power, be publicly available, standardize the collection of data with respect to response to therapy and toxicity, and contain data on concomitant collections of biologic samples.
Reimbursement must keep pace with medical advances
Payer willingness to reimburse for genomic tests and treatments will determine the pace of integration of personalized healthcare into clinical practice. Evidence that enhanced value can be derived from personalized approaches to medicine must be generated before personalized healthcare gains widespread acceptance by payers.
In addition, care-coordinated models must be developed to promote a value-based agenda that facilitates physician accountability and encourages clinical integration.
Innovative approaches are needed to educate providers
Development of point-of-care tools. Because information overload and lack of time are obstacles to clinicians’ efforts to incorporate genomic information into clinical practice, emphasis must be placed on genomic applications that have demonstrated utility. Engaging busy clinicians with point-of-care tools will maximize the relevance of the genomic information they receive and encourage effective use of their time. Decision-making should be supported through automatic risk assessment and management recommendations.
Educational tools. The National Coalition for Health Professional Education in Genetics (NCHPEG) was borne out of the recognition that the pace of genomic discovery far exceeds the pace at which healthcare providers can be educated. Its vision is to improve healthcare through informed use of genomic resources. NCHPEG is a member-based organization whose stakeholders include professional societies, hospitals, advocacy groups, and industry; it attempts to identify the specific educational needs for particular target audiences and then address these needs. It achieves its goals through the use of point-of-care tools and educational programs for continuing medical education credit.
One NCHPEG tool is the Pregnancy and Health Profile, which is a risk assessment and screening tool that attempts to improve the identification of women and babies at risk of developing genetic disease. It collects personal and family history information, performs a risk assessment for the clinician, and provides clinical decision support and education.
Another example of an educational tool is the “Genes to Society” curriculum initiated by The Johns Hopkins University School of Medicine in August 2009. The curriculum is being used as “the foundation for the scientific and clinical career development of future physicians.”15
Using personal genomic testing for education. The number of direct-to-consumer genomic tests is growing, and their market penetration will only increase as the cost of supplying a personal genome continues to decline. Whole genome scanning is being offered with the promise of identifying genetic predisposition to multiple diseases.
Participation in personal genomic testing may be a useful educational tool. Medical students, residents, and practicing physicians who participate in testing may be better equipped to advise patients about the processes involved and the potential utility and limitations of direct-to-consumer genotyping.14
Some companies that offer direct-to-consumer genomic testing provide telephone support from genetic counselors to help clients and their healthcare providers manage genetic information. Counselor services include identifying hereditary risks and reviewing diagnostic, preventive, and early-detection options.
Implementing pharmacogenomics into practice: Decision support systems are needed
A genomic decision support system that guides medication prescribing is needed to implement pharmacogenomic diagnostics. For such a system to achieve the goal of selecting the best medication for each individual, it must do the following:
- Test all polymorphisms relevant to the prescribing of any medication
- Be completed with no out-of-pocket cost to the patient
- Be performed before the patient requires the medication
- Provide results that will be interpreted as part of an individualized pharmacogenomics consult.11
The 1200 Patients Project, a pilot research study under way at the Center for Personalized Therapeutics at the University of Chicago, is attempting to demonstrate the feasibility of incorporating pharmacogenomic testing into routine clinical practice for medication treatment decisions. DNA samples from patients who are taking at least one prescription medication are being tested for differences in genes that may suggest greater effectiveness or an increased risk of side effects from certain medications.
Solutions in practice
Cleveland Clinic’s genetics-based management of Lynch syndrome, the integration of genetics services during patient appointments at Cleveland Clinic, and a coordinated approach at The Ohio State University Medical Center are examples of practical applications of personalized healthcare.
Colorectal cancer management. One example of a personalized approach to medicine that improves health outcome while achieving cost savings is the genetics-based approach to HNPCC (Lynch syndrome) at Cleveland Clinic.
Early identification of Lynch syndrome by screening all colorectal cancer patients has been shown to save $250,000 per life-year gained in the United States.16 All colorectal cancers resected at the Cleveland Clinic main campus are routinely screened for MSI and IHC, and the process is embedded into the routine pathology workflow. With the patients’ foreknowledge, a gastrointestinal cancer genetics counselor scans the list of MSI and IHC results each week. Patients who are MSI-high or IHC-null are invited to receive genetic counseling and consider germline single-gene testing guided by the IHC results. With this active approach, patient uptake is 80%; in comparison, with a passive approach (MSI/IHC results are placed in the pathology report), the uptake is 14%17 (B. Leach and C. Eng, unpublished data, 2011).The successful application of the active approach requires the close cooperation of multiple disciplines, including members of the Cleveland Clinic Genomic Medicine, Pathology & Laboratory Medicine, and Digestive Disease Institutes.18
Integrating genetics-based care at Cleveland Clinic. Time delays for genetics services and limited collaboration with managing physicians who are not genetics specialists reduces genetics-based access and availability. Broad access to genetics clinical services is a means of clinical integration of genetics-enabled care. Providing patients and healthcare providers with easy access and short wait times is vital for clinical integration of genetics-enabled personalized healthcare.
As part of a patient-centered focus on medicine, clinical genetics services have been integrated throughout Cleveland Clinic. The system has two genetics clinics at its main campus and has embedded multiple genetics satellites within its nongenetics clinics, easing access. Genetics counselors are stationed in the same areas of practice as referring providers. Although patient encounters have increased at the medical genetics clinic in the Genomic Medicine Institute, genetics consultations no longer require an extra trip to the clinic since they are integrated into existing appointments. With this approach, large numbers of patients can be seen with no wait times.
Coordinated care at The Ohio State University Medical Center. The Center for Personalized Health Care at The Ohio State University Medical Center (OSUMC) embraces a systems-based care-coordinated model that improves care by executing standardized processes and automating routine tasks. The Institute for Systems Biology, which was established to develop genomics, wellness, and chronic disease biomarkers, collaborates with OSUMC on pilot projects in chronic disease, including cancer.
The OSUMC has a closed system in which it is the payer, employer, and provider of healthcare. This closed system serves as an ideal testing ground for reform. Goals include intervention in disease before symptoms appear and maintenance of wellness. The data from these demonstration projects should facilitate adoption of personalized healthcare by improving physician acceptance of personalized approaches and satisfying payers that personalized healthcare is cost-effective.

- Personalized medicine. Coriell Institute for Medical Research Web site. http://www.coriell.org/personalized-medicine. Updated 2011. Accessed December 27, 2011.
- The 2012 Statistical Abstract. U.S. Census Bureau Web site. http://www.census.gov/compendia/statab/cats/income_expenditures_poverty_wealth/gross_domestic_product_gdp.html. Updated September 27, 2011. Accessed December 22, 2011.
- National health expenditure fact sheet. Center for Medicare & Medicaid Services (CMS) Web site. https://www.cms.gov/NationalHealthExpendData/25_NHE_Fact_Sheet.asp. Updated November 4, 2011. Accessed December 22, 2011.
- New England Healthcare Institute (NEHI). Thinking outside the pillbox: A system-wide approach to improving patient medication adherence for chronic disease. NEHI Web site. http://www.nehi.net/publications/44/thinking_outside_the_pillbox_a_systemwide_approach_to_improving_patient_medication_adherence_for_chronic_disease. Published August 12, 2009. Accessed December 22, 2011.
- Spear BB, Heath-Chiozzi M, Huff J. Clinical application of pharmacogenetics. Trends Mol Med 2001; 7:201–204.
- Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008; 359:1757–1765.
- Feero WG, Green ED. Genomics education for healthcare professionals in the 21st century. JAMA 2011; 306:989–990.
- Meyers M, Wagner MW, Hwang HS, Kinsella TJ, Boothman DA. Role of the hMLH1 DNA mismatch repair protein in flouropyrimidine-mediated cell death and cell cycle responses. Cancer Res 2001; 61:5193–5201.
- Carethers JM, Chauhan DP, Fink D, et al. Mismatch repair proficiency and in vitro response to 5-fluorouracil. Gastroenterology 1999; 117:123–131.
- Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 2003; 349:247–257.
- Ratain MJ. Personalized medicine: building the GPS to take us there. Clin Pharmacol Ther 2007; 81:321–322.
- Anderson JL, Horne BD, Stevens SM, et al; Couma-Gen Investigators. Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation [published online ahead of print November 7, 2007]. Circulation 2007; 116:2563–2570. doi: 10.1161/CIRCULATIONAHA.107.737312
- Price MJ, Angiolillo DJ, Teirstein PS, et al .Platelet reactivity and cardiovascular outcomes after percutaneous coronary intervention: a time-dependent analysis of the Gauging Responsiveness with a VerifyNow P2Y12 assay: Impact on Thrombosis and Safety (GRAVITAS) trial [published online ahead of print August 29, 2011]. Circulation 2011; 124:1132–1137. doi: 10.1161/CIRCULATIONAHA.111.029165
- Sharp RR, Goldlust ME, Eng C. Addressing gaps in physician education using personal genomic testing. Genet Med 2011; 13:750–751.
- Wiener CM, Thomas PA, Goodspeed E, Valle D, Nichols DG. “Genes to society”—the logic and process of the new curriculum for the Johns Hopkins University School of Medicine. Acad Med 2010; 85:498–506.
- Ladabaum U, Wang G, Terdiman J, et al. Strategies to identify the Lynch syndrome among patients with colorectal cancer: a costeffectiveness analysis. Ann Intern Med 2011; 155:69–79.
- Leach B, Eng C, Kalady M, et al. Sharing the responsibility: multidisciplinary model improves colorectal cancer microsatellite testing. Paper presented at: InSight 2009 Annual Conference: September 2009; Orlando, FL.
- Manolio TA, Chisolm R, Ozenberger B, et al. Implementing genomic medicine in the clinic: the future is here. Genet Med Forthcoming.
Personalized healthcare is the tailoring of medical management and patient care to the individual characteristics of each patient. This is achieved by incorporating the genetic and genomic makeup of an individual and his or her family medical history, environment, health-related behaviors, culture, and values into a complete health picture that can be used to customize care. Another level of personalization, often called personalized medicine, involves the selection of drug therapy through the use of tests to determine the genes and gene interactions that can reliably predict an individual’s response to a given therapy. This white paper focuses largely on the use of personalized healthcare as a risk prediction tool.
CURRENT STATUS OF PERSONALIZED HEALTHCARE
The case for personalized healthcare: Seeking value
To fully appreciate the need to advance the adoption of personalized healthcare into the delivery of medicine, one must consider the operation of our current healthcare system and its inefficiencies in terms of delivery and cost, its imprecision in the selection of therapies, and its inability to optimize outcomes. The framework of the US healthcare system as it is now constructed is expensive, disease-directed (instead of health- and wellness-directed), fragmented, and complex. While gross domestic product (GDP) in the United States has increased by approximately 3% per year,2 the compounded growth rate of healthcare expenditures is 6.1% per year. Healthcare in the aggregate now represents 17.6% of GDP and 27% of spending by the federal government and consumes 28% of the average household’s discretionary spending, surpassed only by housing.3
Personalized healthcare can potentially address the need for value consistent with the healthcare system’s prominent share of the US economy. The growth in healthcare spending is certain to be a target of the newly created Joint Select Committee on Deficit Reduction (created by the Budget Control Act of 2011), which is tasked with deficit reduction of at least $1.5 trillion over a 10-year period.
The need to address healthcare costs has been recognized in the Patient Protection and Affordable Care Act, a central feature of which is the creation of integrated health systems that pay for value based on quality, cost containment, and consumer experience. The legislation was enacted to transform healthcare in a variety of ways to make it more sustainable. The Patient Protection and Affordable Care Act seeks to end fragmentation by expanding the use of information technology to reorganize the delivery system and to prevent errors, shifting from volume-based incentives to incentives based on performance and outcomes, and rewarding effective healthcare delivery measures and good patient outcomes.
A shift from reactive to proactive
The premise behind personalized healthcare is the potential for more efficient healthcare, with the assumption that efficiency translates to lower cost and improved patient care.
Although healthcare reform is most often referred to in the context of improving access to care through insurance coverage mandates, true healthcare reform shifts current healthcare models from the practice of reactive medicine to the practice of proactive medicine, in which the tools of personalized healthcare (ie, genetics, genomics, and other molecular diagnostics) enable not only better quality of care but also less expensive care.
Several personalized tools have long been accepted into mainstream medicine. Two examples are the family history, which is the least expensive and most available genetic evaluation tool, and ABO blood typing for safe transfusions (as ABO blood types are alleles of a gene). In fact, much of what is now considered mainstream medical management was at one time considered new. To allow further evolution of medical practice, our challenge is to open our minds to the possibility that personalized proactive medicine can improve healthcare.
The new vision: More precise management
The trial-and-error approach to treating disease is inefficient and costly. Many drugs are effective for only about 50% of patients, often leading to switching or intensification of therapy that requires multiple patient visits.
Personalized medicine considers pharmacokinetic and other characteristics in selection of drug dosages. Genomic testing has the potential to provide clearer insight into the more successful use of currently available medicines. Treatment decisions (ie, drug and drug dosage choice) made on the basis of pharmacogenomic testing should increase adherence through greater effectiveness and fewer adverse drug reactions.
A massive amount of waste is related to pharmaceutical nonadherence and noncompliance. The New England Healthcare Institute has estimated that medication nonadherence costs the healthcare system $290 billion annually.4 Methodologies targeted at individual patients to improve adherence to drug regimens could save the healthcare system a tremendous amount of money.
Cancer management as a model for personalized healthcare. Personalization of therapy is especially suited to cancer management, given that the response to nonspecific cancer chemotherapy is suboptimal in most patients yet exposes them to adverse effects.5 Large-scale sequencing of human cancer genomes is rapidly changing the understanding of cancer biology and is identifying new targets in difficult-to-treat diseases and causes of drug resistance. Applying this information can achieve cost savings by avoiding the use of treatments that are ineffective in particular patients.
Overexpression of genetic mutations renders some cancers less susceptible to certain treatments, but has opened the door to individualized molecularly guided treatment strategies. For example, among patients with non–small cell lung cancer, mutations in the epidermal growth factor receptor (EGFR) tyrosine kinase domain predict response to EGFR tyrosine kinase inhibitors, and anaplastic lymphoma kinase (ALK) inhibitors induce response in patients harboring a mutation in EML4-ALK genes. The recognition that human epidermal growth factor receptor (HER)-2 overexpression as a result of ERBB2 gene amplification occurs in as many as 20% of human breast cancers paved the way for the development of HER-2–targeted therapies. Patients with advanced colorectal cancer whose tumors express the KRAS gene mutation do not benefit from an EGFR inhibitor, whereas those with wild-type KRAS have improved survival with EGFR inhibitor treatment.6
BARRIERS TO THE APPLICATION OF PERSONALIZED HEALTHCARE
The availability and potential of personalized healthcare services and technology is not universally recognized or appreciated by consumers and clinicians. This lack of awareness contributes to a shortage of public support and limited demand for such services. Other barriers include misperceptions regarding the impact of personalized healthcare on disease management, limited incentives to use the available technology, and a knowledge gap among healthcare providers.
Lack of awareness and support
As applications of personalized healthcare advance to the point of clinical relevance, it is important to consider strategies for effective implementation into healthcare practice. Personalized healthcare, when more fully implemented, promises to accelerate the progress that healthcare reform hopes to achieve.
A major challenge to widespread adoption of personalized healthcare is limited recognition by the public and some healthcare providers that personalized healthcare can help to achieve better value. For personalized medicine to be embraced, the concept of “helix to health,” or translation of knowledge to the clinical setting, must resonate with the general public. Despite lack of public and provider awareness, the Personalized Medicine Coalition (PMC) has documented the existence of 56 personalized treatment and diagnostic products. Further, more than 200 product labels now recommend genetic testing prior to use to identify likely responders or inform of the influence of genetic variation on safety and effectiveness.
Consumers’ confidence in the efficacy and safety of medicines they take might contribute to the absence of public support for personalized healthcare. Similarly, despite the availability of genomic tests and tools, many physicians who might be advocates for personalized healthcare do not see the relevance of genomic medicine to their practices in terms of direct benefit to patient care.7
Apart from clinicians and consumers, support is also weak among health insurers and employers, even though the return on investment for personalized healthcare may be profound. Payers await the economic outcomes data that are crucial for their commitment to personalized healthcare. In addition, some have concerns about the ethical implications of personalized healthcare (see “Managing Genomic Information Responsibly”).
Perception of impact on treatment and prevention
A frequent criticism of genomics in medicine is that a genetic diagnosis does not help with patient management. In fact, surveillance and management of patients and family members often changes in response to a genetic diagnosis; knowing which gene is involved personalizes medical management. An example is the management of hereditary nonpolyposis colorectal cancer (HNPCC), or Lynch syndrome, which is the most common form of hereditary colon cancer. For a person with HNPCC, the lifetime risk of developing colorectal cancer is approximately 80%. Lynch syndrome is caused by germline mutations in one of three major mismatch repair (MMR) genes (MLH1, MSH2, and MSH6), and it predisposes to other cancers—uterine, stomach, and ovarian—as well. In women with Lynch syndrome, the lifetime risk for uterine cancer is 40%, compared with 4% in the general population.
At least 90% of patients with Lynch syndrome can be detected through MMR testing via microsatellite instability (MSI) or immunohistochemistry (IHC).8 MSI is a cellular phenotype that indicates a deficiency in at least one DNA MMR protein.
Although 5-fluorouracil–based chemo therapy is the standard of care for treatment of colorectal cancer, it confers no survival advantage in patients with MMR-IHC null (lack of expression of the gene) or MSI-high sporadic colorectal cancer.9,10 Knowing the status of MMR proteins, therefore, would alter the decision regarding neoadjuvant and adjuvant chemotherapy.
Perception of value
Implementation of pharmacogenomics into clinical practice has lagged. One major reason is the lack of an obvious business model for a product that may only be required once in an individual patient’s lifetime.11
A second barrier to integration lies in the limited demand for pharmacogenomics from physicians. This may be related partly to limited expertise in genetics among many physicians and to significant pushback from payers against today’s costs. Without reimbursement, little incentive exists for pharmacogenomics diagnostics. The incentive for physicians is further depressed, perhaps appropriately, when randomized controlled studies fail to demonstrate improved clinical outcomes with the use of pharmacogenomicbased treatment strategies. Two such examples are genotype-guided warfarin dosing, which failed in a randomized controlled trial to improve the proportion of international normalized ratios in the therapeutic range,12 and dosing of clopidogrel based on platelet reactivity, which did not improve outcomes after percutaneous coronary intervention compared with standard dosing in a randomized double-blind clinical trial.13
A significant delay in obtaining the results of pharmacogenomics testing, which also postpones the prescribing encounter, is another major drawback.

A knowledge gap persists

At present, delivery of personalized healthcare is not part of the usual training of physicians and other healthcare providers who are the gatekeepers of medicine. Few medical schools incorporate human and medical genetics, genomics, and pharmacogenomics into their curricula. Genetics is inadequately emphasized in residency curricula outside of pediatrics, family medicine, and obstetrics/gynecology.
The resulting knowledge gap is a fundamental factor in the lack of interest in using genomics in clinical medicine. Educating consumers and physicians at all levels, including specialty societies as well as insurers, will be key to expanding utilization of personalized healthcare. Educating payers and providing them with more data on economic outcomes associated with personalized healthcare will be necessary for adoption into clinical practice; implementation will lag as long as reimbursement decisions do not support personalized approaches to medicine.
As DNA sequencing technology has become less expensive and more powerful, companies have begun to market personal genomic testing. As a result, patients who use these services will increasingly want to discuss the results with their physicians. A significant number of clinicians are unfamiliar with personal genomic testing and emerging genetic testing options. In one survey of physicians who attended educational sessions that discussed recent developments in clinical genetics, only 37% indicated that they were familiar with recent genetic research that affected their patients.14
Targeted education will enhance physicians’ understanding of probabilities and risk estimates from the use of genomic testing; it will also improve recognition of potential causes of patient anxiety, gene variants of unknown significance, and follow-up tests and procedures that can add to expense. Nonphysician healthcare providers (ie, nurses and physician assistants) of direct care also will benefit from education.
INTEGRATING PERSONALIZED HEALTHCARE INTO CLINICAL PRACTICE
Practice standardization and an overhaul of the health information technology (HIT) infrastructure are needed if we are to reap the potential benefits of personalized healthcare. Creative approaches to practitioner education, which are being used in some institutions, must become more widespread. Similarly, the models for successful integration of personalized healthcare that have been achieved in some settings also can be implemented in other institutions.
Data collection and integration must be prioritized
Personalized healthcare can be both predictive and preventive, but moving past the disruptive phase of personalized healthcare will require a radical transformation of the healthcare “ecosystem” and HIT infrastructure.
Although data collection in the current system is extensive, data sharing and data management are inadequate. The pace at which HIT links clinical and genetic information must be accelerated. HIT will expedite innovation and implementation of personalized healthcare, allowing greater integration of data to permit improved data analysis capability. The ultimate goal is to create an interoperable system that connects these data across hospitals and clinicians to help clinicians interpret genomic and other risk information to better inform patient care.
Fully integrated health systems support better coordination of care and optimize the treatment of individual patients: linking research findings, treatment guidelines, treatment outcomes based on genetic profiles, and the individual patient’s own genetic profile will help to personalize treatments. Genomic information added to an individual’s electronic medical record along with improved data-sharing will facilitate clinicians’ ability to retrieve outcomes data based on patient characteristics.
Care models must be standardized, evidence-based practices must be executed, and care must be coordinated yet decentralized. In this way, clinicians can use the electronic medical record as an interoperable patient record to determine a personalized pathway to patient management. Standardization reduces variability in practice and permits seamless execution of care. Automation is imperative to achieving standardization, irrespective of the care supervisor. Investments must therefore be made to stimulate electronic medical record decision support.
In addition, larger data sets will be needed to identify the types of patients likely to respond to a treatment. Ideal data sets would be large enough to have adequate statistical power, be publicly available, standardize the collection of data with respect to response to therapy and toxicity, and contain data on concomitant collections of biologic samples.
Reimbursement must keep pace with medical advances
Payer willingness to reimburse for genomic tests and treatments will determine the pace of integration of personalized healthcare into clinical practice. Evidence that enhanced value can be derived from personalized approaches to medicine must be generated before personalized healthcare gains widespread acceptance by payers.
In addition, care-coordinated models must be developed to promote a value-based agenda that facilitates physician accountability and encourages clinical integration.
Innovative approaches are needed to educate providers
Development of point-of-care tools. Because information overload and lack of time are obstacles to clinicians’ efforts to incorporate genomic information into clinical practice, emphasis must be placed on genomic applications that have demonstrated utility. Engaging busy clinicians with point-of-care tools will maximize the relevance of the genomic information they receive and encourage effective use of their time. Decision-making should be supported through automatic risk assessment and management recommendations.
Educational tools. The National Coalition for Health Professional Education in Genetics (NCHPEG) was borne out of the recognition that the pace of genomic discovery far exceeds the pace at which healthcare providers can be educated. Its vision is to improve healthcare through informed use of genomic resources. NCHPEG is a member-based organization whose stakeholders include professional societies, hospitals, advocacy groups, and industry; it attempts to identify the specific educational needs for particular target audiences and then address these needs. It achieves its goals through the use of point-of-care tools and educational programs for continuing medical education credit.
One NCHPEG tool is the Pregnancy and Health Profile, which is a risk assessment and screening tool that attempts to improve the identification of women and babies at risk of developing genetic disease. It collects personal and family history information, performs a risk assessment for the clinician, and provides clinical decision support and education.
Another example of an educational tool is the “Genes to Society” curriculum initiated by The Johns Hopkins University School of Medicine in August 2009. The curriculum is being used as “the foundation for the scientific and clinical career development of future physicians.”15
Using personal genomic testing for education. The number of direct-to-consumer genomic tests is growing, and their market penetration will only increase as the cost of supplying a personal genome continues to decline. Whole genome scanning is being offered with the promise of identifying genetic predisposition to multiple diseases.
Participation in personal genomic testing may be a useful educational tool. Medical students, residents, and practicing physicians who participate in testing may be better equipped to advise patients about the processes involved and the potential utility and limitations of direct-to-consumer genotyping.14
Some companies that offer direct-to-consumer genomic testing provide telephone support from genetic counselors to help clients and their healthcare providers manage genetic information. Counselor services include identifying hereditary risks and reviewing diagnostic, preventive, and early-detection options.
Implementing pharmacogenomics into practice: Decision support systems are needed
A genomic decision support system that guides medication prescribing is needed to implement pharmacogenomic diagnostics. For such a system to achieve the goal of selecting the best medication for each individual, it must do the following:
- Test all polymorphisms relevant to the prescribing of any medication
- Be completed with no out-of-pocket cost to the patient
- Be performed before the patient requires the medication
- Provide results that will be interpreted as part of an individualized pharmacogenomics consult.11
The 1200 Patients Project, a pilot research study under way at the Center for Personalized Therapeutics at the University of Chicago, is attempting to demonstrate the feasibility of incorporating pharmacogenomic testing into routine clinical practice for medication treatment decisions. DNA samples from patients who are taking at least one prescription medication are being tested for differences in genes that may suggest greater effectiveness or an increased risk of side effects from certain medications.
Solutions in practice
Cleveland Clinic’s genetics-based management of Lynch syndrome, the integration of genetics services during patient appointments at Cleveland Clinic, and a coordinated approach at The Ohio State University Medical Center are examples of practical applications of personalized healthcare.
Colorectal cancer management. One example of a personalized approach to medicine that improves health outcome while achieving cost savings is the genetics-based approach to HNPCC (Lynch syndrome) at Cleveland Clinic.
Early identification of Lynch syndrome by screening all colorectal cancer patients has been shown to save $250,000 per life-year gained in the United States.16 All colorectal cancers resected at the Cleveland Clinic main campus are routinely screened for MSI and IHC, and the process is embedded into the routine pathology workflow. With the patients’ foreknowledge, a gastrointestinal cancer genetics counselor scans the list of MSI and IHC results each week. Patients who are MSI-high or IHC-null are invited to receive genetic counseling and consider germline single-gene testing guided by the IHC results. With this active approach, patient uptake is 80%; in comparison, with a passive approach (MSI/IHC results are placed in the pathology report), the uptake is 14%17 (B. Leach and C. Eng, unpublished data, 2011).The successful application of the active approach requires the close cooperation of multiple disciplines, including members of the Cleveland Clinic Genomic Medicine, Pathology & Laboratory Medicine, and Digestive Disease Institutes.18
Integrating genetics-based care at Cleveland Clinic. Time delays for genetics services and limited collaboration with managing physicians who are not genetics specialists reduces genetics-based access and availability. Broad access to genetics clinical services is a means of clinical integration of genetics-enabled care. Providing patients and healthcare providers with easy access and short wait times is vital for clinical integration of genetics-enabled personalized healthcare.
As part of a patient-centered focus on medicine, clinical genetics services have been integrated throughout Cleveland Clinic. The system has two genetics clinics at its main campus and has embedded multiple genetics satellites within its nongenetics clinics, easing access. Genetics counselors are stationed in the same areas of practice as referring providers. Although patient encounters have increased at the medical genetics clinic in the Genomic Medicine Institute, genetics consultations no longer require an extra trip to the clinic since they are integrated into existing appointments. With this approach, large numbers of patients can be seen with no wait times.
Coordinated care at The Ohio State University Medical Center. The Center for Personalized Health Care at The Ohio State University Medical Center (OSUMC) embraces a systems-based care-coordinated model that improves care by executing standardized processes and automating routine tasks. The Institute for Systems Biology, which was established to develop genomics, wellness, and chronic disease biomarkers, collaborates with OSUMC on pilot projects in chronic disease, including cancer.
The OSUMC has a closed system in which it is the payer, employer, and provider of healthcare. This closed system serves as an ideal testing ground for reform. Goals include intervention in disease before symptoms appear and maintenance of wellness. The data from these demonstration projects should facilitate adoption of personalized healthcare by improving physician acceptance of personalized approaches and satisfying payers that personalized healthcare is cost-effective.

Personalized healthcare is the tailoring of medical management and patient care to the individual characteristics of each patient. This is achieved by incorporating the genetic and genomic makeup of an individual and his or her family medical history, environment, health-related behaviors, culture, and values into a complete health picture that can be used to customize care. Another level of personalization, often called personalized medicine, involves the selection of drug therapy through the use of tests to determine the genes and gene interactions that can reliably predict an individual’s response to a given therapy. This white paper focuses largely on the use of personalized healthcare as a risk prediction tool.
CURRENT STATUS OF PERSONALIZED HEALTHCARE
The case for personalized healthcare: Seeking value
To fully appreciate the need to advance the adoption of personalized healthcare into the delivery of medicine, one must consider the operation of our current healthcare system and its inefficiencies in terms of delivery and cost, its imprecision in the selection of therapies, and its inability to optimize outcomes. The framework of the US healthcare system as it is now constructed is expensive, disease-directed (instead of health- and wellness-directed), fragmented, and complex. While gross domestic product (GDP) in the United States has increased by approximately 3% per year,2 the compounded growth rate of healthcare expenditures is 6.1% per year. Healthcare in the aggregate now represents 17.6% of GDP and 27% of spending by the federal government and consumes 28% of the average household’s discretionary spending, surpassed only by housing.3
Personalized healthcare can potentially address the need for value consistent with the healthcare system’s prominent share of the US economy. The growth in healthcare spending is certain to be a target of the newly created Joint Select Committee on Deficit Reduction (created by the Budget Control Act of 2011), which is tasked with deficit reduction of at least $1.5 trillion over a 10-year period.
The need to address healthcare costs has been recognized in the Patient Protection and Affordable Care Act, a central feature of which is the creation of integrated health systems that pay for value based on quality, cost containment, and consumer experience. The legislation was enacted to transform healthcare in a variety of ways to make it more sustainable. The Patient Protection and Affordable Care Act seeks to end fragmentation by expanding the use of information technology to reorganize the delivery system and to prevent errors, shifting from volume-based incentives to incentives based on performance and outcomes, and rewarding effective healthcare delivery measures and good patient outcomes.
A shift from reactive to proactive
The premise behind personalized healthcare is the potential for more efficient healthcare, with the assumption that efficiency translates to lower cost and improved patient care.
Although healthcare reform is most often referred to in the context of improving access to care through insurance coverage mandates, true healthcare reform shifts current healthcare models from the practice of reactive medicine to the practice of proactive medicine, in which the tools of personalized healthcare (ie, genetics, genomics, and other molecular diagnostics) enable not only better quality of care but also less expensive care.
Several personalized tools have long been accepted into mainstream medicine. Two examples are the family history, which is the least expensive and most available genetic evaluation tool, and ABO blood typing for safe transfusions (as ABO blood types are alleles of a gene). In fact, much of what is now considered mainstream medical management was at one time considered new. To allow further evolution of medical practice, our challenge is to open our minds to the possibility that personalized proactive medicine can improve healthcare.
The new vision: More precise management
The trial-and-error approach to treating disease is inefficient and costly. Many drugs are effective for only about 50% of patients, often leading to switching or intensification of therapy that requires multiple patient visits.
Personalized medicine considers pharmacokinetic and other characteristics in selection of drug dosages. Genomic testing has the potential to provide clearer insight into the more successful use of currently available medicines. Treatment decisions (ie, drug and drug dosage choice) made on the basis of pharmacogenomic testing should increase adherence through greater effectiveness and fewer adverse drug reactions.
A massive amount of waste is related to pharmaceutical nonadherence and noncompliance. The New England Healthcare Institute has estimated that medication nonadherence costs the healthcare system $290 billion annually.4 Methodologies targeted at individual patients to improve adherence to drug regimens could save the healthcare system a tremendous amount of money.
Cancer management as a model for personalized healthcare. Personalization of therapy is especially suited to cancer management, given that the response to nonspecific cancer chemotherapy is suboptimal in most patients yet exposes them to adverse effects.5 Large-scale sequencing of human cancer genomes is rapidly changing the understanding of cancer biology and is identifying new targets in difficult-to-treat diseases and causes of drug resistance. Applying this information can achieve cost savings by avoiding the use of treatments that are ineffective in particular patients.
Overexpression of genetic mutations renders some cancers less susceptible to certain treatments, but has opened the door to individualized molecularly guided treatment strategies. For example, among patients with non–small cell lung cancer, mutations in the epidermal growth factor receptor (EGFR) tyrosine kinase domain predict response to EGFR tyrosine kinase inhibitors, and anaplastic lymphoma kinase (ALK) inhibitors induce response in patients harboring a mutation in EML4-ALK genes. The recognition that human epidermal growth factor receptor (HER)-2 overexpression as a result of ERBB2 gene amplification occurs in as many as 20% of human breast cancers paved the way for the development of HER-2–targeted therapies. Patients with advanced colorectal cancer whose tumors express the KRAS gene mutation do not benefit from an EGFR inhibitor, whereas those with wild-type KRAS have improved survival with EGFR inhibitor treatment.6
BARRIERS TO THE APPLICATION OF PERSONALIZED HEALTHCARE
The availability and potential of personalized healthcare services and technology is not universally recognized or appreciated by consumers and clinicians. This lack of awareness contributes to a shortage of public support and limited demand for such services. Other barriers include misperceptions regarding the impact of personalized healthcare on disease management, limited incentives to use the available technology, and a knowledge gap among healthcare providers.
Lack of awareness and support
As applications of personalized healthcare advance to the point of clinical relevance, it is important to consider strategies for effective implementation into healthcare practice. Personalized healthcare, when more fully implemented, promises to accelerate the progress that healthcare reform hopes to achieve.
A major challenge to widespread adoption of personalized healthcare is limited recognition by the public and some healthcare providers that personalized healthcare can help to achieve better value. For personalized medicine to be embraced, the concept of “helix to health,” or translation of knowledge to the clinical setting, must resonate with the general public. Despite lack of public and provider awareness, the Personalized Medicine Coalition (PMC) has documented the existence of 56 personalized treatment and diagnostic products. Further, more than 200 product labels now recommend genetic testing prior to use to identify likely responders or inform of the influence of genetic variation on safety and effectiveness.
Consumers’ confidence in the efficacy and safety of medicines they take might contribute to the absence of public support for personalized healthcare. Similarly, despite the availability of genomic tests and tools, many physicians who might be advocates for personalized healthcare do not see the relevance of genomic medicine to their practices in terms of direct benefit to patient care.7
Apart from clinicians and consumers, support is also weak among health insurers and employers, even though the return on investment for personalized healthcare may be profound. Payers await the economic outcomes data that are crucial for their commitment to personalized healthcare. In addition, some have concerns about the ethical implications of personalized healthcare (see “Managing Genomic Information Responsibly”).
Perception of impact on treatment and prevention
A frequent criticism of genomics in medicine is that a genetic diagnosis does not help with patient management. In fact, surveillance and management of patients and family members often changes in response to a genetic diagnosis; knowing which gene is involved personalizes medical management. An example is the management of hereditary nonpolyposis colorectal cancer (HNPCC), or Lynch syndrome, which is the most common form of hereditary colon cancer. For a person with HNPCC, the lifetime risk of developing colorectal cancer is approximately 80%. Lynch syndrome is caused by germline mutations in one of three major mismatch repair (MMR) genes (MLH1, MSH2, and MSH6), and it predisposes to other cancers—uterine, stomach, and ovarian—as well. In women with Lynch syndrome, the lifetime risk for uterine cancer is 40%, compared with 4% in the general population.
At least 90% of patients with Lynch syndrome can be detected through MMR testing via microsatellite instability (MSI) or immunohistochemistry (IHC).8 MSI is a cellular phenotype that indicates a deficiency in at least one DNA MMR protein.
Although 5-fluorouracil–based chemo therapy is the standard of care for treatment of colorectal cancer, it confers no survival advantage in patients with MMR-IHC null (lack of expression of the gene) or MSI-high sporadic colorectal cancer.9,10 Knowing the status of MMR proteins, therefore, would alter the decision regarding neoadjuvant and adjuvant chemotherapy.
Perception of value
Implementation of pharmacogenomics into clinical practice has lagged. One major reason is the lack of an obvious business model for a product that may only be required once in an individual patient’s lifetime.11
A second barrier to integration lies in the limited demand for pharmacogenomics from physicians. This may be related partly to limited expertise in genetics among many physicians and to significant pushback from payers against today’s costs. Without reimbursement, little incentive exists for pharmacogenomics diagnostics. The incentive for physicians is further depressed, perhaps appropriately, when randomized controlled studies fail to demonstrate improved clinical outcomes with the use of pharmacogenomicbased treatment strategies. Two such examples are genotype-guided warfarin dosing, which failed in a randomized controlled trial to improve the proportion of international normalized ratios in the therapeutic range,12 and dosing of clopidogrel based on platelet reactivity, which did not improve outcomes after percutaneous coronary intervention compared with standard dosing in a randomized double-blind clinical trial.13
A significant delay in obtaining the results of pharmacogenomics testing, which also postpones the prescribing encounter, is another major drawback.

A knowledge gap persists

At present, delivery of personalized healthcare is not part of the usual training of physicians and other healthcare providers who are the gatekeepers of medicine. Few medical schools incorporate human and medical genetics, genomics, and pharmacogenomics into their curricula. Genetics is inadequately emphasized in residency curricula outside of pediatrics, family medicine, and obstetrics/gynecology.
The resulting knowledge gap is a fundamental factor in the lack of interest in using genomics in clinical medicine. Educating consumers and physicians at all levels, including specialty societies as well as insurers, will be key to expanding utilization of personalized healthcare. Educating payers and providing them with more data on economic outcomes associated with personalized healthcare will be necessary for adoption into clinical practice; implementation will lag as long as reimbursement decisions do not support personalized approaches to medicine.
As DNA sequencing technology has become less expensive and more powerful, companies have begun to market personal genomic testing. As a result, patients who use these services will increasingly want to discuss the results with their physicians. A significant number of clinicians are unfamiliar with personal genomic testing and emerging genetic testing options. In one survey of physicians who attended educational sessions that discussed recent developments in clinical genetics, only 37% indicated that they were familiar with recent genetic research that affected their patients.14
Targeted education will enhance physicians’ understanding of probabilities and risk estimates from the use of genomic testing; it will also improve recognition of potential causes of patient anxiety, gene variants of unknown significance, and follow-up tests and procedures that can add to expense. Nonphysician healthcare providers (ie, nurses and physician assistants) of direct care also will benefit from education.
INTEGRATING PERSONALIZED HEALTHCARE INTO CLINICAL PRACTICE
Practice standardization and an overhaul of the health information technology (HIT) infrastructure are needed if we are to reap the potential benefits of personalized healthcare. Creative approaches to practitioner education, which are being used in some institutions, must become more widespread. Similarly, the models for successful integration of personalized healthcare that have been achieved in some settings also can be implemented in other institutions.
Data collection and integration must be prioritized
Personalized healthcare can be both predictive and preventive, but moving past the disruptive phase of personalized healthcare will require a radical transformation of the healthcare “ecosystem” and HIT infrastructure.
Although data collection in the current system is extensive, data sharing and data management are inadequate. The pace at which HIT links clinical and genetic information must be accelerated. HIT will expedite innovation and implementation of personalized healthcare, allowing greater integration of data to permit improved data analysis capability. The ultimate goal is to create an interoperable system that connects these data across hospitals and clinicians to help clinicians interpret genomic and other risk information to better inform patient care.
Fully integrated health systems support better coordination of care and optimize the treatment of individual patients: linking research findings, treatment guidelines, treatment outcomes based on genetic profiles, and the individual patient’s own genetic profile will help to personalize treatments. Genomic information added to an individual’s electronic medical record along with improved data-sharing will facilitate clinicians’ ability to retrieve outcomes data based on patient characteristics.
Care models must be standardized, evidence-based practices must be executed, and care must be coordinated yet decentralized. In this way, clinicians can use the electronic medical record as an interoperable patient record to determine a personalized pathway to patient management. Standardization reduces variability in practice and permits seamless execution of care. Automation is imperative to achieving standardization, irrespective of the care supervisor. Investments must therefore be made to stimulate electronic medical record decision support.
In addition, larger data sets will be needed to identify the types of patients likely to respond to a treatment. Ideal data sets would be large enough to have adequate statistical power, be publicly available, standardize the collection of data with respect to response to therapy and toxicity, and contain data on concomitant collections of biologic samples.
Reimbursement must keep pace with medical advances
Payer willingness to reimburse for genomic tests and treatments will determine the pace of integration of personalized healthcare into clinical practice. Evidence that enhanced value can be derived from personalized approaches to medicine must be generated before personalized healthcare gains widespread acceptance by payers.
In addition, care-coordinated models must be developed to promote a value-based agenda that facilitates physician accountability and encourages clinical integration.
Innovative approaches are needed to educate providers
Development of point-of-care tools. Because information overload and lack of time are obstacles to clinicians’ efforts to incorporate genomic information into clinical practice, emphasis must be placed on genomic applications that have demonstrated utility. Engaging busy clinicians with point-of-care tools will maximize the relevance of the genomic information they receive and encourage effective use of their time. Decision-making should be supported through automatic risk assessment and management recommendations.
Educational tools. The National Coalition for Health Professional Education in Genetics (NCHPEG) was borne out of the recognition that the pace of genomic discovery far exceeds the pace at which healthcare providers can be educated. Its vision is to improve healthcare through informed use of genomic resources. NCHPEG is a member-based organization whose stakeholders include professional societies, hospitals, advocacy groups, and industry; it attempts to identify the specific educational needs for particular target audiences and then address these needs. It achieves its goals through the use of point-of-care tools and educational programs for continuing medical education credit.
One NCHPEG tool is the Pregnancy and Health Profile, which is a risk assessment and screening tool that attempts to improve the identification of women and babies at risk of developing genetic disease. It collects personal and family history information, performs a risk assessment for the clinician, and provides clinical decision support and education.
Another example of an educational tool is the “Genes to Society” curriculum initiated by The Johns Hopkins University School of Medicine in August 2009. The curriculum is being used as “the foundation for the scientific and clinical career development of future physicians.”15
Using personal genomic testing for education. The number of direct-to-consumer genomic tests is growing, and their market penetration will only increase as the cost of supplying a personal genome continues to decline. Whole genome scanning is being offered with the promise of identifying genetic predisposition to multiple diseases.
Participation in personal genomic testing may be a useful educational tool. Medical students, residents, and practicing physicians who participate in testing may be better equipped to advise patients about the processes involved and the potential utility and limitations of direct-to-consumer genotyping.14
Some companies that offer direct-to-consumer genomic testing provide telephone support from genetic counselors to help clients and their healthcare providers manage genetic information. Counselor services include identifying hereditary risks and reviewing diagnostic, preventive, and early-detection options.
Implementing pharmacogenomics into practice: Decision support systems are needed
A genomic decision support system that guides medication prescribing is needed to implement pharmacogenomic diagnostics. For such a system to achieve the goal of selecting the best medication for each individual, it must do the following:
- Test all polymorphisms relevant to the prescribing of any medication
- Be completed with no out-of-pocket cost to the patient
- Be performed before the patient requires the medication
- Provide results that will be interpreted as part of an individualized pharmacogenomics consult.11
The 1200 Patients Project, a pilot research study under way at the Center for Personalized Therapeutics at the University of Chicago, is attempting to demonstrate the feasibility of incorporating pharmacogenomic testing into routine clinical practice for medication treatment decisions. DNA samples from patients who are taking at least one prescription medication are being tested for differences in genes that may suggest greater effectiveness or an increased risk of side effects from certain medications.
Solutions in practice
Cleveland Clinic’s genetics-based management of Lynch syndrome, the integration of genetics services during patient appointments at Cleveland Clinic, and a coordinated approach at The Ohio State University Medical Center are examples of practical applications of personalized healthcare.
Colorectal cancer management. One example of a personalized approach to medicine that improves health outcome while achieving cost savings is the genetics-based approach to HNPCC (Lynch syndrome) at Cleveland Clinic.
Early identification of Lynch syndrome by screening all colorectal cancer patients has been shown to save $250,000 per life-year gained in the United States.16 All colorectal cancers resected at the Cleveland Clinic main campus are routinely screened for MSI and IHC, and the process is embedded into the routine pathology workflow. With the patients’ foreknowledge, a gastrointestinal cancer genetics counselor scans the list of MSI and IHC results each week. Patients who are MSI-high or IHC-null are invited to receive genetic counseling and consider germline single-gene testing guided by the IHC results. With this active approach, patient uptake is 80%; in comparison, with a passive approach (MSI/IHC results are placed in the pathology report), the uptake is 14%17 (B. Leach and C. Eng, unpublished data, 2011).The successful application of the active approach requires the close cooperation of multiple disciplines, including members of the Cleveland Clinic Genomic Medicine, Pathology & Laboratory Medicine, and Digestive Disease Institutes.18
Integrating genetics-based care at Cleveland Clinic. Time delays for genetics services and limited collaboration with managing physicians who are not genetics specialists reduces genetics-based access and availability. Broad access to genetics clinical services is a means of clinical integration of genetics-enabled care. Providing patients and healthcare providers with easy access and short wait times is vital for clinical integration of genetics-enabled personalized healthcare.
As part of a patient-centered focus on medicine, clinical genetics services have been integrated throughout Cleveland Clinic. The system has two genetics clinics at its main campus and has embedded multiple genetics satellites within its nongenetics clinics, easing access. Genetics counselors are stationed in the same areas of practice as referring providers. Although patient encounters have increased at the medical genetics clinic in the Genomic Medicine Institute, genetics consultations no longer require an extra trip to the clinic since they are integrated into existing appointments. With this approach, large numbers of patients can be seen with no wait times.
Coordinated care at The Ohio State University Medical Center. The Center for Personalized Health Care at The Ohio State University Medical Center (OSUMC) embraces a systems-based care-coordinated model that improves care by executing standardized processes and automating routine tasks. The Institute for Systems Biology, which was established to develop genomics, wellness, and chronic disease biomarkers, collaborates with OSUMC on pilot projects in chronic disease, including cancer.
The OSUMC has a closed system in which it is the payer, employer, and provider of healthcare. This closed system serves as an ideal testing ground for reform. Goals include intervention in disease before symptoms appear and maintenance of wellness. The data from these demonstration projects should facilitate adoption of personalized healthcare by improving physician acceptance of personalized approaches and satisfying payers that personalized healthcare is cost-effective.

- Personalized medicine. Coriell Institute for Medical Research Web site. http://www.coriell.org/personalized-medicine. Updated 2011. Accessed December 27, 2011.
- The 2012 Statistical Abstract. U.S. Census Bureau Web site. http://www.census.gov/compendia/statab/cats/income_expenditures_poverty_wealth/gross_domestic_product_gdp.html. Updated September 27, 2011. Accessed December 22, 2011.
- National health expenditure fact sheet. Center for Medicare & Medicaid Services (CMS) Web site. https://www.cms.gov/NationalHealthExpendData/25_NHE_Fact_Sheet.asp. Updated November 4, 2011. Accessed December 22, 2011.
- New England Healthcare Institute (NEHI). Thinking outside the pillbox: A system-wide approach to improving patient medication adherence for chronic disease. NEHI Web site. http://www.nehi.net/publications/44/thinking_outside_the_pillbox_a_systemwide_approach_to_improving_patient_medication_adherence_for_chronic_disease. Published August 12, 2009. Accessed December 22, 2011.
- Spear BB, Heath-Chiozzi M, Huff J. Clinical application of pharmacogenetics. Trends Mol Med 2001; 7:201–204.
- Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008; 359:1757–1765.
- Feero WG, Green ED. Genomics education for healthcare professionals in the 21st century. JAMA 2011; 306:989–990.
- Meyers M, Wagner MW, Hwang HS, Kinsella TJ, Boothman DA. Role of the hMLH1 DNA mismatch repair protein in flouropyrimidine-mediated cell death and cell cycle responses. Cancer Res 2001; 61:5193–5201.
- Carethers JM, Chauhan DP, Fink D, et al. Mismatch repair proficiency and in vitro response to 5-fluorouracil. Gastroenterology 1999; 117:123–131.
- Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 2003; 349:247–257.
- Ratain MJ. Personalized medicine: building the GPS to take us there. Clin Pharmacol Ther 2007; 81:321–322.
- Anderson JL, Horne BD, Stevens SM, et al; Couma-Gen Investigators. Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation [published online ahead of print November 7, 2007]. Circulation 2007; 116:2563–2570. doi: 10.1161/CIRCULATIONAHA.107.737312
- Price MJ, Angiolillo DJ, Teirstein PS, et al .Platelet reactivity and cardiovascular outcomes after percutaneous coronary intervention: a time-dependent analysis of the Gauging Responsiveness with a VerifyNow P2Y12 assay: Impact on Thrombosis and Safety (GRAVITAS) trial [published online ahead of print August 29, 2011]. Circulation 2011; 124:1132–1137. doi: 10.1161/CIRCULATIONAHA.111.029165
- Sharp RR, Goldlust ME, Eng C. Addressing gaps in physician education using personal genomic testing. Genet Med 2011; 13:750–751.
- Wiener CM, Thomas PA, Goodspeed E, Valle D, Nichols DG. “Genes to society”—the logic and process of the new curriculum for the Johns Hopkins University School of Medicine. Acad Med 2010; 85:498–506.
- Ladabaum U, Wang G, Terdiman J, et al. Strategies to identify the Lynch syndrome among patients with colorectal cancer: a costeffectiveness analysis. Ann Intern Med 2011; 155:69–79.
- Leach B, Eng C, Kalady M, et al. Sharing the responsibility: multidisciplinary model improves colorectal cancer microsatellite testing. Paper presented at: InSight 2009 Annual Conference: September 2009; Orlando, FL.
- Manolio TA, Chisolm R, Ozenberger B, et al. Implementing genomic medicine in the clinic: the future is here. Genet Med Forthcoming.
- Personalized medicine. Coriell Institute for Medical Research Web site. http://www.coriell.org/personalized-medicine. Updated 2011. Accessed December 27, 2011.
- The 2012 Statistical Abstract. U.S. Census Bureau Web site. http://www.census.gov/compendia/statab/cats/income_expenditures_poverty_wealth/gross_domestic_product_gdp.html. Updated September 27, 2011. Accessed December 22, 2011.
- National health expenditure fact sheet. Center for Medicare & Medicaid Services (CMS) Web site. https://www.cms.gov/NationalHealthExpendData/25_NHE_Fact_Sheet.asp. Updated November 4, 2011. Accessed December 22, 2011.
- New England Healthcare Institute (NEHI). Thinking outside the pillbox: A system-wide approach to improving patient medication adherence for chronic disease. NEHI Web site. http://www.nehi.net/publications/44/thinking_outside_the_pillbox_a_systemwide_approach_to_improving_patient_medication_adherence_for_chronic_disease. Published August 12, 2009. Accessed December 22, 2011.
- Spear BB, Heath-Chiozzi M, Huff J. Clinical application of pharmacogenetics. Trends Mol Med 2001; 7:201–204.
- Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008; 359:1757–1765.
- Feero WG, Green ED. Genomics education for healthcare professionals in the 21st century. JAMA 2011; 306:989–990.
- Meyers M, Wagner MW, Hwang HS, Kinsella TJ, Boothman DA. Role of the hMLH1 DNA mismatch repair protein in flouropyrimidine-mediated cell death and cell cycle responses. Cancer Res 2001; 61:5193–5201.
- Carethers JM, Chauhan DP, Fink D, et al. Mismatch repair proficiency and in vitro response to 5-fluorouracil. Gastroenterology 1999; 117:123–131.
- Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 2003; 349:247–257.
- Ratain MJ. Personalized medicine: building the GPS to take us there. Clin Pharmacol Ther 2007; 81:321–322.
- Anderson JL, Horne BD, Stevens SM, et al; Couma-Gen Investigators. Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation [published online ahead of print November 7, 2007]. Circulation 2007; 116:2563–2570. doi: 10.1161/CIRCULATIONAHA.107.737312
- Price MJ, Angiolillo DJ, Teirstein PS, et al .Platelet reactivity and cardiovascular outcomes after percutaneous coronary intervention: a time-dependent analysis of the Gauging Responsiveness with a VerifyNow P2Y12 assay: Impact on Thrombosis and Safety (GRAVITAS) trial [published online ahead of print August 29, 2011]. Circulation 2011; 124:1132–1137. doi: 10.1161/CIRCULATIONAHA.111.029165
- Sharp RR, Goldlust ME, Eng C. Addressing gaps in physician education using personal genomic testing. Genet Med 2011; 13:750–751.
- Wiener CM, Thomas PA, Goodspeed E, Valle D, Nichols DG. “Genes to society”—the logic and process of the new curriculum for the Johns Hopkins University School of Medicine. Acad Med 2010; 85:498–506.
- Ladabaum U, Wang G, Terdiman J, et al. Strategies to identify the Lynch syndrome among patients with colorectal cancer: a costeffectiveness analysis. Ann Intern Med 2011; 155:69–79.
- Leach B, Eng C, Kalady M, et al. Sharing the responsibility: multidisciplinary model improves colorectal cancer microsatellite testing. Paper presented at: InSight 2009 Annual Conference: September 2009; Orlando, FL.
- Manolio TA, Chisolm R, Ozenberger B, et al. Implementing genomic medicine in the clinic: the future is here. Genet Med Forthcoming.