User login
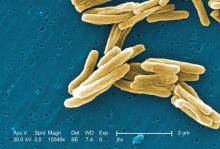
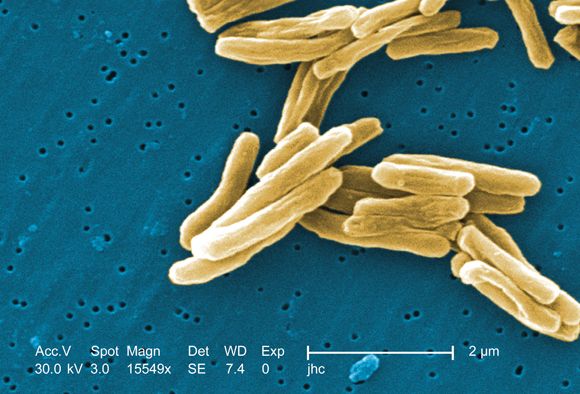
In patients with HIV, treatment with prednisone for 4 weeks after antiretroviral therapy initiation significantly reduced the risk of tuberculosis-associated immune reconstitution inflammatory syndrome (IRIS). The results from the randomized, double-blind, placebo-controlled trial were published in the New England Journal of Medicine (2018 Nov 14. doi: 10.1056/NEJMoa1800762).
A total of 240 patients were enrolled in the study, with a median age of 36 years; 60% were men, and 73% had microbiologically confirmed tuberculosis. The median CD4 count of the patients was 49 cells/mcL and the median HIV type 1 RNA viral load was 5.5 log10 copies/mL. A total of 120 patients were assigned to each group, with 18 patients lost to follow-up or withdrawn. Tuberculosis-associated IRIS was diagnosed in 39 patients (32.5%) in the prednisone group and in 56 (46.7%) in the placebo group, yielding a relative IRIS risk of 0.70 (95% confidence interval, 0.51-0.96; P = .03), according to the researchers for the PredART (Preventing TB-IRIS in High-Risk Patients: a Randomized Placebo-Controlled Trial of Prednisone) study team.
We covered this story before it was published in the journal. Find our coverage from the Conference on Retroviruses & Opportunistic Infections at the link below.
In patients with HIV, treatment with prednisone for 4 weeks after antiretroviral therapy initiation significantly reduced the risk of tuberculosis-associated immune reconstitution inflammatory syndrome (IRIS). The results from the randomized, double-blind, placebo-controlled trial were published in the New England Journal of Medicine (2018 Nov 14. doi: 10.1056/NEJMoa1800762).
A total of 240 patients were enrolled in the study, with a median age of 36 years; 60% were men, and 73% had microbiologically confirmed tuberculosis. The median CD4 count of the patients was 49 cells/mcL and the median HIV type 1 RNA viral load was 5.5 log10 copies/mL. A total of 120 patients were assigned to each group, with 18 patients lost to follow-up or withdrawn. Tuberculosis-associated IRIS was diagnosed in 39 patients (32.5%) in the prednisone group and in 56 (46.7%) in the placebo group, yielding a relative IRIS risk of 0.70 (95% confidence interval, 0.51-0.96; P = .03), according to the researchers for the PredART (Preventing TB-IRIS in High-Risk Patients: a Randomized Placebo-Controlled Trial of Prednisone) study team.
We covered this story before it was published in the journal. Find our coverage from the Conference on Retroviruses & Opportunistic Infections at the link below.
In patients with HIV, treatment with prednisone for 4 weeks after antiretroviral therapy initiation significantly reduced the risk of tuberculosis-associated immune reconstitution inflammatory syndrome (IRIS). The results from the randomized, double-blind, placebo-controlled trial were published in the New England Journal of Medicine (2018 Nov 14. doi: 10.1056/NEJMoa1800762).
A total of 240 patients were enrolled in the study, with a median age of 36 years; 60% were men, and 73% had microbiologically confirmed tuberculosis. The median CD4 count of the patients was 49 cells/mcL and the median HIV type 1 RNA viral load was 5.5 log10 copies/mL. A total of 120 patients were assigned to each group, with 18 patients lost to follow-up or withdrawn. Tuberculosis-associated IRIS was diagnosed in 39 patients (32.5%) in the prednisone group and in 56 (46.7%) in the placebo group, yielding a relative IRIS risk of 0.70 (95% confidence interval, 0.51-0.96; P = .03), according to the researchers for the PredART (Preventing TB-IRIS in High-Risk Patients: a Randomized Placebo-Controlled Trial of Prednisone) study team.
We covered this story before it was published in the journal. Find our coverage from the Conference on Retroviruses & Opportunistic Infections at the link below.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE