User login
BARCELONA – (Assessment in Work Productivity and the Relationship with Cognitive Symptoms).
“We found that as patients rated themselves as improved in terms of cognition – ‘I can think better,’ ‘I can focus,’ ‘I’m concentrating better’ – there was a strong correlation at 12 weeks and later extended to 1 year with improved work productivity by as much as 75%. It’s pretty dramatic,” lead investigator Pratap Chokka, MD, said in an interview at the annual congress of the European College of Neuropsychopharmacology.
AtWoRK was a multicenter, open-label, naturalistic intervention study in which 219 gainfully employed Canadian adults with major depressive disorder (MDD) who had presented to primary care physicians or psychiatrists were placed on vortioxetine (Trintellex) flexibly dosed at 10-20 mg/day and scheduled for routine follow-up visits every 4 weeks for 52 weeks.
This was a patient population with severe depression, severe cognitive dysfunction, severe anxiety, and substantial functional impairment as reflected in their baseline scores on a variety of validated measures (see graphic). The study was designed to emulate real-world clinical practice.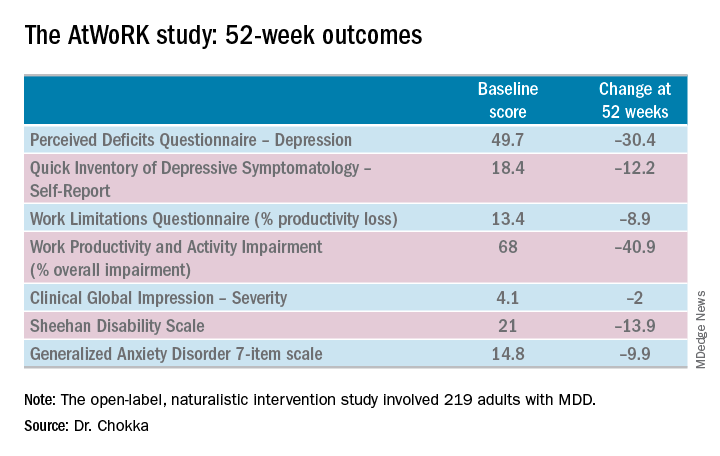
“We know that patients with depression are very impaired in terms of work productivity. Depressed patients really suffer from absenteeism and presenteeism [reduced productivity at work caused by depression]. And very few naturalistic studies have been done in working patients with depression,” according to Dr. Chokka. “The randomized trials are really important. They show us that a drug is working. But in terms of the real world that I work in, I need to have effectiveness: Does the drug work in patients with comorbid conditions, problems in their home lives, who are maybe drinking alcohol? Those are cases we’d rule out from participation in the RCTs.”
“The patients in our study walked into our clinics saying, ‘You know what, doctor, my mind isn’t working very good. I’m depressed, I can’t think, I can’t focus, I’m missing work, my boss is on my case, I’m making errors. I need help.’ These are the kinds of practicalities we wanted to address,” explained Dr. Chokka, a psychiatrist at Grey Nuns Community Hospital in Edmonton, Can.
The primary endpoint in AtWoRK was the correlation between changes in patients’ self-reported cognitive symptoms on the 20-item Perceived Deficits Questionnaire–Depression (PDQ-D-20) and changes in work productivity loss measured on the Work Limitations Questionnaire (WLQ) at week 12. Those 12-week results were recently published (CNS Spectr. 2018 May 24:1-10). At the ECNP congress, Dr. Chokka presented the expanded 52-week outcomes.
The correlation between change from baseline to week 12 in PDQ-D-20 and change in WLQ was strong (r = 0.606), and it remained strong at week 52 (r = 0.731; P less than .001).
At 52 weeks on vortioxetine, 77% of patients fulfilled criteria for MDD response, which required at least a 50% reduction in Quick Inventory of Depressive Symptomatology – Self-Report (QIDS-SR) score from baseline, and 56% for disease remission, which meant the QIDS-SR score was 5 or less. The response and remission rates were 71% and 45%, respectively, in the 107 subjects for whom the drug was the first treatment for their current MDD episode and 83% and 67% for the 112 switched to vortioxetine at study outset because the antidepressant they’d been on was ineffective.
Subjects also displayed significant improvement at 12 and 52 weeks in mood as assessed using QIDS-SR and global functioning as measured using the Sheehan Disability Scale (SDS). Of note, however, improvement in cognitive symptoms was independent of and not predictive of improvement in overall depressive symptoms on the QIDS-SR. Nor did improvement in depressive symptoms predict functional outcomes as assessed by the WLQ or SDS.
In Dr. Chokka’s view, these findings have clear implications for clinical practice: “In the past we thought that, if we can get the mood better, things will all get better. We now we know that treating depression is about more than just getting the mood better.”
Vortioxetine is an antidepressant with multiple agonist and antagonist effects on various 5-HT serotonin receptors.
The AtWoRK study was supported by Lundbeck Canada. Dr. Chokka reported receiving research grants from and serving on advisory boards and as a speaker for that company and others.
SOURCE: Chokka P. ECNP, P.022.
BARCELONA – (Assessment in Work Productivity and the Relationship with Cognitive Symptoms).
“We found that as patients rated themselves as improved in terms of cognition – ‘I can think better,’ ‘I can focus,’ ‘I’m concentrating better’ – there was a strong correlation at 12 weeks and later extended to 1 year with improved work productivity by as much as 75%. It’s pretty dramatic,” lead investigator Pratap Chokka, MD, said in an interview at the annual congress of the European College of Neuropsychopharmacology.
AtWoRK was a multicenter, open-label, naturalistic intervention study in which 219 gainfully employed Canadian adults with major depressive disorder (MDD) who had presented to primary care physicians or psychiatrists were placed on vortioxetine (Trintellex) flexibly dosed at 10-20 mg/day and scheduled for routine follow-up visits every 4 weeks for 52 weeks.
This was a patient population with severe depression, severe cognitive dysfunction, severe anxiety, and substantial functional impairment as reflected in their baseline scores on a variety of validated measures (see graphic). The study was designed to emulate real-world clinical practice.
“We know that patients with depression are very impaired in terms of work productivity. Depressed patients really suffer from absenteeism and presenteeism [reduced productivity at work caused by depression]. And very few naturalistic studies have been done in working patients with depression,” according to Dr. Chokka. “The randomized trials are really important. They show us that a drug is working. But in terms of the real world that I work in, I need to have effectiveness: Does the drug work in patients with comorbid conditions, problems in their home lives, who are maybe drinking alcohol? Those are cases we’d rule out from participation in the RCTs.”
“The patients in our study walked into our clinics saying, ‘You know what, doctor, my mind isn’t working very good. I’m depressed, I can’t think, I can’t focus, I’m missing work, my boss is on my case, I’m making errors. I need help.’ These are the kinds of practicalities we wanted to address,” explained Dr. Chokka, a psychiatrist at Grey Nuns Community Hospital in Edmonton, Can.
The primary endpoint in AtWoRK was the correlation between changes in patients’ self-reported cognitive symptoms on the 20-item Perceived Deficits Questionnaire–Depression (PDQ-D-20) and changes in work productivity loss measured on the Work Limitations Questionnaire (WLQ) at week 12. Those 12-week results were recently published (CNS Spectr. 2018 May 24:1-10). At the ECNP congress, Dr. Chokka presented the expanded 52-week outcomes.
The correlation between change from baseline to week 12 in PDQ-D-20 and change in WLQ was strong (r = 0.606), and it remained strong at week 52 (r = 0.731; P less than .001).
At 52 weeks on vortioxetine, 77% of patients fulfilled criteria for MDD response, which required at least a 50% reduction in Quick Inventory of Depressive Symptomatology – Self-Report (QIDS-SR) score from baseline, and 56% for disease remission, which meant the QIDS-SR score was 5 or less. The response and remission rates were 71% and 45%, respectively, in the 107 subjects for whom the drug was the first treatment for their current MDD episode and 83% and 67% for the 112 switched to vortioxetine at study outset because the antidepressant they’d been on was ineffective.
Subjects also displayed significant improvement at 12 and 52 weeks in mood as assessed using QIDS-SR and global functioning as measured using the Sheehan Disability Scale (SDS). Of note, however, improvement in cognitive symptoms was independent of and not predictive of improvement in overall depressive symptoms on the QIDS-SR. Nor did improvement in depressive symptoms predict functional outcomes as assessed by the WLQ or SDS.
In Dr. Chokka’s view, these findings have clear implications for clinical practice: “In the past we thought that, if we can get the mood better, things will all get better. We now we know that treating depression is about more than just getting the mood better.”
Vortioxetine is an antidepressant with multiple agonist and antagonist effects on various 5-HT serotonin receptors.
The AtWoRK study was supported by Lundbeck Canada. Dr. Chokka reported receiving research grants from and serving on advisory boards and as a speaker for that company and others.
SOURCE: Chokka P. ECNP, P.022.
BARCELONA – (Assessment in Work Productivity and the Relationship with Cognitive Symptoms).
“We found that as patients rated themselves as improved in terms of cognition – ‘I can think better,’ ‘I can focus,’ ‘I’m concentrating better’ – there was a strong correlation at 12 weeks and later extended to 1 year with improved work productivity by as much as 75%. It’s pretty dramatic,” lead investigator Pratap Chokka, MD, said in an interview at the annual congress of the European College of Neuropsychopharmacology.
AtWoRK was a multicenter, open-label, naturalistic intervention study in which 219 gainfully employed Canadian adults with major depressive disorder (MDD) who had presented to primary care physicians or psychiatrists were placed on vortioxetine (Trintellex) flexibly dosed at 10-20 mg/day and scheduled for routine follow-up visits every 4 weeks for 52 weeks.
This was a patient population with severe depression, severe cognitive dysfunction, severe anxiety, and substantial functional impairment as reflected in their baseline scores on a variety of validated measures (see graphic). The study was designed to emulate real-world clinical practice.
“We know that patients with depression are very impaired in terms of work productivity. Depressed patients really suffer from absenteeism and presenteeism [reduced productivity at work caused by depression]. And very few naturalistic studies have been done in working patients with depression,” according to Dr. Chokka. “The randomized trials are really important. They show us that a drug is working. But in terms of the real world that I work in, I need to have effectiveness: Does the drug work in patients with comorbid conditions, problems in their home lives, who are maybe drinking alcohol? Those are cases we’d rule out from participation in the RCTs.”
“The patients in our study walked into our clinics saying, ‘You know what, doctor, my mind isn’t working very good. I’m depressed, I can’t think, I can’t focus, I’m missing work, my boss is on my case, I’m making errors. I need help.’ These are the kinds of practicalities we wanted to address,” explained Dr. Chokka, a psychiatrist at Grey Nuns Community Hospital in Edmonton, Can.
The primary endpoint in AtWoRK was the correlation between changes in patients’ self-reported cognitive symptoms on the 20-item Perceived Deficits Questionnaire–Depression (PDQ-D-20) and changes in work productivity loss measured on the Work Limitations Questionnaire (WLQ) at week 12. Those 12-week results were recently published (CNS Spectr. 2018 May 24:1-10). At the ECNP congress, Dr. Chokka presented the expanded 52-week outcomes.
The correlation between change from baseline to week 12 in PDQ-D-20 and change in WLQ was strong (r = 0.606), and it remained strong at week 52 (r = 0.731; P less than .001).
At 52 weeks on vortioxetine, 77% of patients fulfilled criteria for MDD response, which required at least a 50% reduction in Quick Inventory of Depressive Symptomatology – Self-Report (QIDS-SR) score from baseline, and 56% for disease remission, which meant the QIDS-SR score was 5 or less. The response and remission rates were 71% and 45%, respectively, in the 107 subjects for whom the drug was the first treatment for their current MDD episode and 83% and 67% for the 112 switched to vortioxetine at study outset because the antidepressant they’d been on was ineffective.
Subjects also displayed significant improvement at 12 and 52 weeks in mood as assessed using QIDS-SR and global functioning as measured using the Sheehan Disability Scale (SDS). Of note, however, improvement in cognitive symptoms was independent of and not predictive of improvement in overall depressive symptoms on the QIDS-SR. Nor did improvement in depressive symptoms predict functional outcomes as assessed by the WLQ or SDS.
In Dr. Chokka’s view, these findings have clear implications for clinical practice: “In the past we thought that, if we can get the mood better, things will all get better. We now we know that treating depression is about more than just getting the mood better.”
Vortioxetine is an antidepressant with multiple agonist and antagonist effects on various 5-HT serotonin receptors.
The AtWoRK study was supported by Lundbeck Canada. Dr. Chokka reported receiving research grants from and serving on advisory boards and as a speaker for that company and others.
SOURCE: Chokka P. ECNP, P.022.
REPORTING FROM THE ECNP CONGRESS
Key clinical point: Treat cognitive symptoms of depression to improve impaired work productivity.
Major finding: Impaired work productivity in depressed patients improved greatly in response to reduction in cognitive symptoms, but not with enhanced mood.
Study details: A 52-week, multicenter, open-label study in which 219 employed adults with major depression were placed on vortioxetine and serially assessed for changes in cognitive dysfunction, mood, and work productivity.
Disclosures: The AtWoRK study was supported by Lundbeck Canada. The presenter reported receiving research grants from and serving on advisory boards and as a speaker for that company and others.
Source: Chokka P. ECNP, P.022.

