User login
Sleep is known to be poor among hospitalized patients for many reasons.[1] Patients may have pain, dyspnea, or other discomforts that prevent sleep. Diagnostic and therapeutic procedures, including medication administration and routine nursing care, may take place during normal sleep times. Environmental factors such as noise and light frequently remain at daytime levels during normal sleep times.[2] In response, patients frequently request pharmacological sleep aids. Unfortunately, the use of sleep medications has been linked to clinically relevant and detrimental outcomes such as delirium and falls, particularly in the elderly. For example, in the landmark study by Inouye et al., a multicomponent intervention was used to successfully reduce delirium in older (>70 years) hospitalized patients.[3] One of the successful components was nonpharmacological sleep promotion, which reduced the use of pharmacological sleep aids from 46% to 35% of patients. Most recently, Kolla and colleagues found zolpidem tartrate use to be a risk factor (odds ratio 4.37) for inpatient falls, a known risk factor for morbidity and increased healthcare costs.[4]
The scope of recent pharmacological sleep aid use in the inpatient setting is not well described. Frighetto and colleagues described the pattern of in‐hospital drug use more than 12 years ago, before the concerns above were described, with 29% of patients receiving a medication for sleep, mostly benzodiazepines.[5] In 2008, Bartick and colleagues also found a very high rate of sleep aid use (42% of patients), but additionally described in 2010 an intervention to minimize sleep disruption that successfully reduced sleep aid use by 38%.[6] Both concerns over side effects and sleep promotion efforts might have reduced the current rate of medication use. Therefore, we sought to evaluate the prescription and administration of pharmacological sleep aids in general medical and surgical inpatients at our institution. Using our electronic medical records, including preadmission and discharge medication records, we assessed new and continued usage of medications for sleep complaints following hospital admission.
METHODS
Patients and Design
Records were reviewed for all adult patient (18 years or older) admissions to 1 of 4 units (2 general medicine and 2 general surgical units) from January 1, 2013 to February 28, 2013. These units do not have specific policies to promote sleep, such as nocturnal noise and light reduction or clustering of care. Brigham and Women's Hospital (BWH) is a 793‐bed university‐affiliated teaching hospital. Approval for this retrospective chart review study was obtained from the Partners Healthcare Institutional Review Board.
BWH uses an in‐house electronic health record system, which gathers information from a wider healthcare system (Partners Healthcare). Medications, problem lists, and allergies are available from within‐system providers and prior encounters. Admitting physicians are also required to document a preadmission medication list. A computerized physician order entry (CPOE) system is used for all medication orders. Although standardized admission order sets are used, none of these sets contains a pharmacological sleep aid. There is decision support for geriatric patients (age >65 years) that may recommend reduced starting doses for some medications.[7]
Medications Monitored for Treatment of Sleep Complaints
Using our electronic medication ordering and administration system, each patient admission was reviewed for any medication that might be used for treatment of sleep complaints. The list of sleep medications was based on those commonly used for the outpatient treatment of insomnia, as well as others included based on the authors' experience as clinical inpatient pharmacists.[8, 9, 10] Admissions were reviewed for the following medications: first generation antihistamines (diphenhydramine, hydroxyzine), tricyclic antidepressants (amitriptyline, nortriptyline, desipramine), serotonin‐norepinephrine reuptake inhibitor antidepressants (mirtazapine, trazodone, nefazodone), melatonin agonists (ramelteon), nonbenzodiazepine hypnotics (zolpidem tartrate, eszopiclone), benzodiazepines (oxazepam, temazepam, lorazepam, triazolam, diazepam), typical antipsychotics (haloperidol, fluphenazine, thioridazine, chlorpromazine), and atypical antipsychotics (quetiapine fumarate, ziprasidone, olanzapine, risperidone, aripiprazole). Melatonin, which is not regulated by the US Food and Drug Administration (FDA), cannot be prescribed using our CPOE system.
Determination of Medication Administration for Treatment of Sleep Complaints
The charts of patients receiving 1 or more of these monitored medications were then reviewed by the authors to determine if the medication was indeed prescribed for insomnia/sleep. Chart documents reviewed were the patient's problem list from outpatient provider notes; admission note, including past medical history and home medications; the preadmission medication list; and the inpatient daily progress note. The medication was considered to be used for sleep complaints (as opposed to another indication) when any of the additional following inclusion criteria were met: the medication was part of the patient's home medication regimen for insomnia, the medication order indicated that the medication was for insomnia/difficulty sleeping, or the medication was administered without a specific indication between the hours of 6 pm and 6 am. The medication was not considered to be used primarily as a sleep aid if any of the following were present (exclusion criteria): utilization for an as needed reason including anxiety, agitation, itching, nausea, muscle spasm; utilization for a documented disorder including depression, anxiety, schizophrenia, bipolar disorder, alcohol withdrawal, or epilepsy; intramuscular administration of olanzapine or ziprasidone; or topical administration of diphenhydramine.
Medication Administration Characteristics
For each medication that was administered for difficulty sleeping, the following data were documented: dose in milligrams, route of administration, time of administration, administration timing directions (eg, times 1 [x1], as needed [PRN], or standing), an increase or decrease in dose during hospital stay, documentation of the medication in discharge notes or discharge medications, and documentation of development of an allergy or adverse reaction due to the medication. Changes in dose were recorded. If a patient received more than 1 study medication, each individual medication and dose was evaluated for inclusion in the study analysis. Other data collected included the total number of days of exposure to each sleep aid during admission, the date of initiation, the total number of days of exposure to more than 1 sleep aid during admission, and the location of initiation of each individual sleep aid (eg, intensive care unit, medical floor). Appropriate time of administration was defined as sleep aid administration between 9 pm and 12 am.
RESULTS
Patients
During the 59‐day study period, there were 642 patients admitted to the study units. Two hundred seventy‐six patients received 1 of the monitored medications; however, 106 patients received the medication for an indication other than insomnia/difficulty sleeping. In 2 patients, incomplete records prevented ascertainment of the motivation for using the monitored medication. Thus, 168 patients (26.2%) were determined to have received a medication for sleep complaints and were included in the study analysis (Figure 1). Table 1 lists the characteristics of the 168 patients, of whom 10 had a prior documented sleep disorder such as insomnia (6 patients), restless leg syndrome (1 patient), and obstructive sleep apnea (3 patients). The rate of sleep medication use was lower, though not drastically so, in patients 65 years of age compared to those <65 years of age.

| Patients | Value |
|---|---|
| |
| Age, y | 57.919.8a |
| Age 65 years | 70 (41.7) |
| Age 64 years | 98 (58.3) |
| Female, n (%) | 97 (57.7) |
| Ethnicity, n (%) | |
| Caucasian | 114 (67.9) |
| Black | 18 (10.7) |
| Hispanic | 13 (7.7) |
| Other | 23 (13.7) |
| Admitted to floor from: | |
| Emergency department | 95 (56.5) |
| Operating room | 30 (17.9) |
| Transferred from outside hospital | 35 (20.8) |
| Intensive care unit | 8 (4.8) |
| Admission service, n (%) | |
| Medical | 109 (64.9) |
| Surgical | 59 (35.1) |
| Hospital length of stay | 10.516.0a |
| Known sleep disorder | |
| Insomnia | 6 (3.6) |
| Restless leg syndrome | 1 (0.6) |
| Obstructive sleep apnea | 3(1.8) |
Medications Used for Treatment of Sleep Complaints
Of the 25 monitored medications, 13 were administered to patients for sleep during the study period. The most commonly administered medications (percent of patients, median dose, absolute dose range) were trazodone (30.4%, 50 mg, 12.5450 mg), lorazepam (24.4%, 0.5 mg, 0.25 mg2 mg), and zolpidem tartrate (17.9%, 10 mg, 2.5 mg10 mg) (Table 2). As only a few of these medications (diphenhydramine, ramelteon, temazepam, triazolam, zolpidem) have a formal FDA indication for insomnia, most patients (72%) were treated using an off‐label medication. Although the types of medication used did not vary substantially between young and old patients, the median doses and ranges were lower in the elderly. Admitting service did not substantially influence the medication or dose chosen for sleep complaints (data not shown).
| Medication | All Patients, N=168, n (%) | Patients <65 Years Old, n=98, n (%) | Patients >65 Years Old, n=70, n (%) |
|---|---|---|---|
| |||
| Trazodoneb | 51 (30.4) | 29 (29.6) | 22 (31.4) |
| Median dose | 50 | 50 | 25 |
| Dose range | 12.5450 | 25450 | 12.5200 |
| Lorazepamc | 41 (24.4) | 24 (24.5) | 17 (24.3) |
| Median dose | 0.5 | 1 | 0.25 |
| Dose range | 0.252 | 0.252 | 0.251 |
| Zolpidem tartratec | 30 (17.9) | 20 (20.4) | 10 (14.3) |
| Median dose | 10 | 10 | 5 |
| Dose range | 2.510 | 2.510 | 2.510 |
| Quetiapine fumarate | 21 (12.5) | 9 (9.2) | 12 (17.1) |
| Median dose | 50 | 50 | 25 |
| Dose range | 12.5300 | 12.5300 | 12.5100 |
| Haloperidol | 18 (10.7) | 7 (7.1) | 11 (15.7) |
| Median dose | 1 | 5 | 1 |
| Dose range | 0.2510 | 0.510 | 0.251 |
| Diphenhydraminec | 16 (9.5) | 12 (12.2) | 4 (5.7) |
| Median dose | 25 | 25 | 12.5 |
| Dose range | 12.550 | 12.550 | 12.525 |
| Mirtazapine | 7 (4.2) | 3 (3.1) | 4 (5.7) |
| Median dose | 15 | 30 | 7.5 |
| Dose range | 7.545 | 7.530 | 7.545 |
| Olanzapine | 5 (3.6) | 3 (3.1) | 2 (2.9) |
| Median dose | 5 | 5 | 2.5 |
| Dose range | 2.512.5 | 512.5 | 2.52.5 |
| Amitriptyline | 5 (3.0) | 4 (4.1) | 1 (1.4) |
| Median dose | 25 | 25 | 25 |
| Dose range | 25100 | 25100 | |
| Diazepam | 5 (3.0) | 3 (3.1) | 2 (2.9) |
| Median dose | 5 | 5 | 10 |
| Dose range | 510 | ||
| Oxazepam | 2 (1.2) | 0 | 2 (2.9) |
| Median dose | 10 | 10 | |
| Dose range | 1010 | 1010 | |
| Temazepamc | 1 (0.6) | 0 | 1 (1.4) |
| Median dose | 15 | 15 | |
| Dose range | |||
| Hydroxyzine | 1 (0.6) | 1 (1.0) | 0 |
| Median dose | 50 | 50 | |
| Dose range | |||
Initiation, Duration, and Changes to Medications for Treatment of Sleep Complaints
None of the medication orders were part of a standardized order set. The sleep medication for the majority of patients (n=108, 64.3%) was initiated during their time on the study units (general inpatient hospital wards). Most patients (n=90, 53.6%) were ordered for a sleep aid within 48 hours of admission to the hospital. The patients who received medication for sleep had a median length of stay of 6 (interquartile range [IQR], 311) days on the study units, and received medication for a median of 2 (IQR, 15) days (Table 3). One hundred twenty patients (71.4%) were continued on a sleep aid until discharge. Essentially the same percentage of patients experienced an increase (14.9%) or decrease (14.9%) in the dose of their sleep aid during admission. Although most patients received 1 medication for sleep throughout their admission, almost one‐quarter of the patients were given 2 or more medications for sleep during their admission to the floor, sometimes including multiple medications on the same night.
| Variable | Patients, N=168 |
|---|---|
| |
| Total sleep aids each patient received during hospital length of stay, n (%) | |
| 1 sleep aid | 132 (78.6) |
| 2 sleep aids | 28 (16.7) |
| 3 sleep aids | 6 (3.6) |
| 4 sleep aids | 2 (1.2) |
| Patients who received multiple sleep aids for 1 or more days during hospital length of stay, n (%) | 20 (11.9) |
| Length of stay on study units, d | 6 [310.75]a |
| Length of sleep aid therapy on study units, d | 2 [15]a |
Of patients not known to be previously on sleep aid therapy, 40 (34.4%) of them were discharged home on a sleep aid.
Medication Administration Characteristics
Sleep medications were prescribed most frequently as standing orders (63.7%), rather than x1 (17.7%) or PRN (18.6%). Although the majority of sleep medications were administered between the hours of 9 pm and 12 am, more than 35% of doses were given outside of this range (Figure 2).
DISCUSSION
Our results confirm the continued frequent use of pharmacological sleep aids in the hospital setting, even in the elderly, despite recent concerns regarding the use of certain sleep medications. Additional, novel findings of our study are: (1) medications used for sleep complaints in the hospital are frequently those without a formal indication for sleep, (2) medications for sleep complaints are frequently administered too early or too late at night to be consistent with good sleep hygiene, and (3) many patients never previously on a medication for insomnia are discharged with a prescription for a sleep aid.
Despite recent warnings regarding side effects especially in the elderly, our rate of medication use has only slightly improved from prior reports from more than a decade ago.[3, 5] This high rate of use is likely due to a combination of patient, clinician, and environmental factors. In our sample, the sleep aid orders were not part of an order set; thus, the orders were either the result of patient request or in response to a patient report of poor sleep. Patients and clinicians may perceive medications for sleep as highly effective and safe, despite evidence to the contrary. Another factor is that both patients and clinicians may be unaware of nonpharmacological interventions that might improve sleep. Similarly, hospital environmental factors (noise, light) may be so disruptive as to preclude these interventions or opportunities for adequate sleep. Thus, the continued high use of medication for sleep is due in part to the lack of patient and clinician education and the difficulty in changing the hospital environment and culture, especially with only limited data on the value of sleep during recovery from illness.
Clinicians typically receive little training regarding sleep or its importance. In fact, most clinicians do not assess or communicate about the patient's quality of sleep.[11] Many may not know that there is little evidence of benefit of pharmacological sleep aids in the hospital. For example, a recent report found, in contrast to the authors' hypothesis, no changes in sleep architecture or duration using 10 mg of zolpidem tartrate in postoperative patients.[12] In our study, we found that some patients required an increase in the dose of their medications or were transitioned to a different sleep aid class (suggesting that sleep aids were ineffective). Alternatively, some patients' sleep aids were discontinued during hospital admission, again, likely due to perceived ineffectiveness or perhaps side effects. It is possible that the effectiveness of these medications might be influenced by timing of administration. This too was variable in our study. Proper clinician education around sleep hygiene might prevent early medication administration (which might lead to middle‐of‐the‐night awakenings) or delayed administration (which will delay the sleep phase), as was frequently seen in our cohort.
Despite emerging evidence of the importance of sleep in maintaining adequate immune, cardiovascular, and cognitive function, there are limited data regarding the benefits of sleep during acute illness.[13, 14, 15, 16] In the absence of compelling data, promoting sleep in the hospital has been difficult. The successful interventions used by Inouye and Bartick and their colleagues to minimize sedative hypnotic use included: a bedtime routine (eg, milk or herbal tea, relaxation tapes or music, back massage, toilet at bedtime), unit‐wide noise‐reduction strategies (eg, silent pill crushers, vibrating beepers, quiet hallways, and noise‐monitoring equipment that alerted staff above a certain decibel level), and schedule adjustments to allow sleep (eg, rescheduling of medications, intravenous fluids, and procedures), all of which require substantial clinician care or may not be possible in more acutely ill patients. Although such changes might be costly, sleep promotion and minimization of sleep aids continues to be part of a strategy that reduces delirium, hospital costs, and hospital length of stay.[16] From a patient perspective, many are interested in nondrug alternatives, especially those who have never used medications before, but few are told of them.[17]
Novel findings of our study include the types of medications used for sleep in the hospital. We found that a variety of medications and classes of medications were prescribed by clinicians for sleep complaints during hospitalization. This variability is due to a number of factors including the lack of rigorous data in this area, well‐established guidelines, or clinician education. We speculate that the high rates of use of nonbenzodiazepine and non‐ gamma‐aminobutyric acid (GABA)ergic agents, such as trazodone and quetiapine, reflect concerns about the use of medications such as zolpidem. Conversely, this means patients are increasingly treated with medications without formal FDA labeling for sleep. It does appear at least that the median doses of medication prescribed were lower in those over age 65 years compared to younger patients, although we cannot determine whether this reflects physician awareness or effective decision support used during computerized order entry. The geriatric decision support recommends a reduced dose for some (eg, trazodone, haloperidol) but not all (eg, quetiapine, lorazepam) of the monitored medications.
We found that many patients, even those who were never previously known to have insomnia, were discharged with a prescription for a sleep medication. Our study design is limited in assessing whether this prescription was needed or not, that is, whether or not the patient will have insomnia (sleep difficulty despite adequate opportunity for sleep) after hospital discharge. However, other studies have suggested that acute illness can be a precipitant for insomnia. Some of this literature has focused on patients in the intensive care unit, but it seems reasonable that patients on general medical and surgical wards (not having come through the intensive care units) might also be at risk for insomnia.[18] A study by Zisberg and colleagues in an elderly Israeli cohort found hospitalization (even without intensive care unit stay) to be both a starting point and a stopping point for chronic sleep medication use.[19] Alternatively, patients may not continue to suffer from insomnia after discharge, and thus the prescription for a sleep aid is inappropriate, as it is likely to have no benefit but may carry risk.[20] Regardless of whether hospital‐acquired insomnia persists past discharge, our findings suggest that some patients will start on chronic medication use for insomnia. Importantly, these patients may have limited understanding of the reason for their prescription, medication risks and benefits, and are unlikely to receive guidance on sleep hygiene or referral to a sleep specialist if needed. In our case, the high rate of prescriptions likely reflects the way in which inpatient medications can be added as a discharge medication automatically. This represents an area for improvement at our institution.
Limitations
This retrospective, single‐center study has several limitations. First, although our results are specific to our institution, our use of pharmacological sleep aids is similar to those previously reported in the literature. Our results are consistent in some ways with the changing trends in outpatient management of insomnia, in which trazodone and quetiapine are now frequently used. Second, we rely on the medical record for prior documentation of insomnia and/or use of medications for insomnia. However, our rate of prior diagnosis of insomnia or medication use of 8.3% is consistent with epidemiological studies.[21] Third, in this retrospective study we may have included some medications in our analysis that may have been given for indications other than sleep promotion, such as medications for anxiety or agitation. However, sleep promotion may have been an intended benefit of the medication choice. Fourth, we did not follow patients after discharge to know whether they continued with sleep medication use outside the hospital. Finally, in this retrospective chart review, we focused on utilization metrics, not on efficacy (which we can only infer) or adverse effects, such as altered mental status or falls. Moreover, we did not compare those patients who did and who did not receive any medication for sleep. However, such work will be crucial in future studies.
CONCLUSIONS
Despite increasing evidence of risks such as delirium or falls, pharmacological sleep aid use in hospitalized patients, even the elderly, remains common. A variety of medications are used, with variable administration times, which likely reflects the few rigorous studies or guidelines for the use of pharmacological sleep aids in hospitalized patients. Many patients not known to be on medications for sleep before admission leave the hospital with a sleep aid prescription. Our results suggest the need to better understand the factors that contribute to the high rate of sleep aid use in hospitalized patients. Clinician education regarding sleep, and nonpharmacological strategies to improve sleep in the hospital, are also needed.
Disclosure: Nothing to report.
- , , , . Sleep in hospitalized medical patients, part 1: factors affecting sleep. J Hosp Med. 2008;3(6):473–482.
- . Sleep and the sleep environment of older adults in acute care settings. J Gerontol Nurs. 2008;34(6):15–21.
- , , , et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669–676.
- , , , . Zolpidem is independently associated with increased risk of inpatient falls. J Hosp Med. 2013;8(1):1–6.
- , , , , , . An assessment of quality of sleep and the use of drugs with sedating properties in hospitalized adult patients. Health Qual Life Outcomes. 2004;2:17.
- , , , , . Decrease in as‐needed sedative use by limiting nighttime sleep disruptions from hospital staff. J Hosp Med. 2010;5(3):E20–E24.
- , , , , , . Guided prescription of psychotropic medications for geriatric inpatients. Arch Intern Med. 2005;165(7):802–807.
- , , , . National use of prescription medications for insomnia: NHANES 1999–2010. Sleep. 2014;37(2):343–349.
- , . Safety of low doses of quetiapine when used for insomnia. Ann Pharmacother. 2012;46(5):718–722.
- , . Ten‐year trends in the pharmacological treatment of insomnia. Sleep. 1999;22(3):371–375.
- , , , . How do clinicians assess, communicate about, and manage patient sleep in the hospital? J Nurs Adm. 2013;43(6):342–347.
- , , . Postoperative sleep disturbances after zolpidem treatment in fast‐track hip and knee replacement. J Clin Sleep Med. 2014;10(3):321–326.
- , , . Sleep deprivation after septic insult increases mortality independent of age. J Trauma. 2009;66(1):50–54.
- , , , , , . Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. Faseb J. 1996;10(5):643–653.
- , , , et al. Objective sleep duration and quality in hospitalized older adults: associations with blood pressure and mood. J Am Geriatr Soc. 2011;59(11):2185–2186.
- , , , et al. Quality improvement and cost savings with multicomponent delirium interventions: replication of the Hospital Elder Life Program in a community hospital. Psychosomatics. 2013;54(3):219–226.
- , , , , . Hospitalized patients' preference in the treatment of insomnia: pharmacological versus non‐pharmacological. Can J Clin Pharmacol. 2003;10(2):89–92.
- , , , et al. Post‐discharge insomnia symptoms are associated with quality of life impairment among survivors of acute lung injury. Sleep Med. 2012;13(8):1106–1109.
- , , , , , . Hospitalization as a turning point for sleep medication use in older adults: prospective cohort study. Drugs Aging. 2012;29(7):565–576.
- , , , et al. Inappropriate medication prescriptions in elderly adults surviving an intensive care unit hospitalization. J Am Geriatr Soc. 2013;61(7):1128–1134.
- , . Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262(11):1479–1484.
Sleep is known to be poor among hospitalized patients for many reasons.[1] Patients may have pain, dyspnea, or other discomforts that prevent sleep. Diagnostic and therapeutic procedures, including medication administration and routine nursing care, may take place during normal sleep times. Environmental factors such as noise and light frequently remain at daytime levels during normal sleep times.[2] In response, patients frequently request pharmacological sleep aids. Unfortunately, the use of sleep medications has been linked to clinically relevant and detrimental outcomes such as delirium and falls, particularly in the elderly. For example, in the landmark study by Inouye et al., a multicomponent intervention was used to successfully reduce delirium in older (>70 years) hospitalized patients.[3] One of the successful components was nonpharmacological sleep promotion, which reduced the use of pharmacological sleep aids from 46% to 35% of patients. Most recently, Kolla and colleagues found zolpidem tartrate use to be a risk factor (odds ratio 4.37) for inpatient falls, a known risk factor for morbidity and increased healthcare costs.[4]
The scope of recent pharmacological sleep aid use in the inpatient setting is not well described. Frighetto and colleagues described the pattern of in‐hospital drug use more than 12 years ago, before the concerns above were described, with 29% of patients receiving a medication for sleep, mostly benzodiazepines.[5] In 2008, Bartick and colleagues also found a very high rate of sleep aid use (42% of patients), but additionally described in 2010 an intervention to minimize sleep disruption that successfully reduced sleep aid use by 38%.[6] Both concerns over side effects and sleep promotion efforts might have reduced the current rate of medication use. Therefore, we sought to evaluate the prescription and administration of pharmacological sleep aids in general medical and surgical inpatients at our institution. Using our electronic medical records, including preadmission and discharge medication records, we assessed new and continued usage of medications for sleep complaints following hospital admission.
METHODS
Patients and Design
Records were reviewed for all adult patient (18 years or older) admissions to 1 of 4 units (2 general medicine and 2 general surgical units) from January 1, 2013 to February 28, 2013. These units do not have specific policies to promote sleep, such as nocturnal noise and light reduction or clustering of care. Brigham and Women's Hospital (BWH) is a 793‐bed university‐affiliated teaching hospital. Approval for this retrospective chart review study was obtained from the Partners Healthcare Institutional Review Board.
BWH uses an in‐house electronic health record system, which gathers information from a wider healthcare system (Partners Healthcare). Medications, problem lists, and allergies are available from within‐system providers and prior encounters. Admitting physicians are also required to document a preadmission medication list. A computerized physician order entry (CPOE) system is used for all medication orders. Although standardized admission order sets are used, none of these sets contains a pharmacological sleep aid. There is decision support for geriatric patients (age >65 years) that may recommend reduced starting doses for some medications.[7]
Medications Monitored for Treatment of Sleep Complaints
Using our electronic medication ordering and administration system, each patient admission was reviewed for any medication that might be used for treatment of sleep complaints. The list of sleep medications was based on those commonly used for the outpatient treatment of insomnia, as well as others included based on the authors' experience as clinical inpatient pharmacists.[8, 9, 10] Admissions were reviewed for the following medications: first generation antihistamines (diphenhydramine, hydroxyzine), tricyclic antidepressants (amitriptyline, nortriptyline, desipramine), serotonin‐norepinephrine reuptake inhibitor antidepressants (mirtazapine, trazodone, nefazodone), melatonin agonists (ramelteon), nonbenzodiazepine hypnotics (zolpidem tartrate, eszopiclone), benzodiazepines (oxazepam, temazepam, lorazepam, triazolam, diazepam), typical antipsychotics (haloperidol, fluphenazine, thioridazine, chlorpromazine), and atypical antipsychotics (quetiapine fumarate, ziprasidone, olanzapine, risperidone, aripiprazole). Melatonin, which is not regulated by the US Food and Drug Administration (FDA), cannot be prescribed using our CPOE system.
Determination of Medication Administration for Treatment of Sleep Complaints
The charts of patients receiving 1 or more of these monitored medications were then reviewed by the authors to determine if the medication was indeed prescribed for insomnia/sleep. Chart documents reviewed were the patient's problem list from outpatient provider notes; admission note, including past medical history and home medications; the preadmission medication list; and the inpatient daily progress note. The medication was considered to be used for sleep complaints (as opposed to another indication) when any of the additional following inclusion criteria were met: the medication was part of the patient's home medication regimen for insomnia, the medication order indicated that the medication was for insomnia/difficulty sleeping, or the medication was administered without a specific indication between the hours of 6 pm and 6 am. The medication was not considered to be used primarily as a sleep aid if any of the following were present (exclusion criteria): utilization for an as needed reason including anxiety, agitation, itching, nausea, muscle spasm; utilization for a documented disorder including depression, anxiety, schizophrenia, bipolar disorder, alcohol withdrawal, or epilepsy; intramuscular administration of olanzapine or ziprasidone; or topical administration of diphenhydramine.
Medication Administration Characteristics
For each medication that was administered for difficulty sleeping, the following data were documented: dose in milligrams, route of administration, time of administration, administration timing directions (eg, times 1 [x1], as needed [PRN], or standing), an increase or decrease in dose during hospital stay, documentation of the medication in discharge notes or discharge medications, and documentation of development of an allergy or adverse reaction due to the medication. Changes in dose were recorded. If a patient received more than 1 study medication, each individual medication and dose was evaluated for inclusion in the study analysis. Other data collected included the total number of days of exposure to each sleep aid during admission, the date of initiation, the total number of days of exposure to more than 1 sleep aid during admission, and the location of initiation of each individual sleep aid (eg, intensive care unit, medical floor). Appropriate time of administration was defined as sleep aid administration between 9 pm and 12 am.
RESULTS
Patients
During the 59‐day study period, there were 642 patients admitted to the study units. Two hundred seventy‐six patients received 1 of the monitored medications; however, 106 patients received the medication for an indication other than insomnia/difficulty sleeping. In 2 patients, incomplete records prevented ascertainment of the motivation for using the monitored medication. Thus, 168 patients (26.2%) were determined to have received a medication for sleep complaints and were included in the study analysis (Figure 1). Table 1 lists the characteristics of the 168 patients, of whom 10 had a prior documented sleep disorder such as insomnia (6 patients), restless leg syndrome (1 patient), and obstructive sleep apnea (3 patients). The rate of sleep medication use was lower, though not drastically so, in patients 65 years of age compared to those <65 years of age.

| Patients | Value |
|---|---|
| |
| Age, y | 57.919.8a |
| Age 65 years | 70 (41.7) |
| Age 64 years | 98 (58.3) |
| Female, n (%) | 97 (57.7) |
| Ethnicity, n (%) | |
| Caucasian | 114 (67.9) |
| Black | 18 (10.7) |
| Hispanic | 13 (7.7) |
| Other | 23 (13.7) |
| Admitted to floor from: | |
| Emergency department | 95 (56.5) |
| Operating room | 30 (17.9) |
| Transferred from outside hospital | 35 (20.8) |
| Intensive care unit | 8 (4.8) |
| Admission service, n (%) | |
| Medical | 109 (64.9) |
| Surgical | 59 (35.1) |
| Hospital length of stay | 10.516.0a |
| Known sleep disorder | |
| Insomnia | 6 (3.6) |
| Restless leg syndrome | 1 (0.6) |
| Obstructive sleep apnea | 3(1.8) |
Medications Used for Treatment of Sleep Complaints
Of the 25 monitored medications, 13 were administered to patients for sleep during the study period. The most commonly administered medications (percent of patients, median dose, absolute dose range) were trazodone (30.4%, 50 mg, 12.5450 mg), lorazepam (24.4%, 0.5 mg, 0.25 mg2 mg), and zolpidem tartrate (17.9%, 10 mg, 2.5 mg10 mg) (Table 2). As only a few of these medications (diphenhydramine, ramelteon, temazepam, triazolam, zolpidem) have a formal FDA indication for insomnia, most patients (72%) were treated using an off‐label medication. Although the types of medication used did not vary substantially between young and old patients, the median doses and ranges were lower in the elderly. Admitting service did not substantially influence the medication or dose chosen for sleep complaints (data not shown).
| Medication | All Patients, N=168, n (%) | Patients <65 Years Old, n=98, n (%) | Patients >65 Years Old, n=70, n (%) |
|---|---|---|---|
| |||
| Trazodoneb | 51 (30.4) | 29 (29.6) | 22 (31.4) |
| Median dose | 50 | 50 | 25 |
| Dose range | 12.5450 | 25450 | 12.5200 |
| Lorazepamc | 41 (24.4) | 24 (24.5) | 17 (24.3) |
| Median dose | 0.5 | 1 | 0.25 |
| Dose range | 0.252 | 0.252 | 0.251 |
| Zolpidem tartratec | 30 (17.9) | 20 (20.4) | 10 (14.3) |
| Median dose | 10 | 10 | 5 |
| Dose range | 2.510 | 2.510 | 2.510 |
| Quetiapine fumarate | 21 (12.5) | 9 (9.2) | 12 (17.1) |
| Median dose | 50 | 50 | 25 |
| Dose range | 12.5300 | 12.5300 | 12.5100 |
| Haloperidol | 18 (10.7) | 7 (7.1) | 11 (15.7) |
| Median dose | 1 | 5 | 1 |
| Dose range | 0.2510 | 0.510 | 0.251 |
| Diphenhydraminec | 16 (9.5) | 12 (12.2) | 4 (5.7) |
| Median dose | 25 | 25 | 12.5 |
| Dose range | 12.550 | 12.550 | 12.525 |
| Mirtazapine | 7 (4.2) | 3 (3.1) | 4 (5.7) |
| Median dose | 15 | 30 | 7.5 |
| Dose range | 7.545 | 7.530 | 7.545 |
| Olanzapine | 5 (3.6) | 3 (3.1) | 2 (2.9) |
| Median dose | 5 | 5 | 2.5 |
| Dose range | 2.512.5 | 512.5 | 2.52.5 |
| Amitriptyline | 5 (3.0) | 4 (4.1) | 1 (1.4) |
| Median dose | 25 | 25 | 25 |
| Dose range | 25100 | 25100 | |
| Diazepam | 5 (3.0) | 3 (3.1) | 2 (2.9) |
| Median dose | 5 | 5 | 10 |
| Dose range | 510 | ||
| Oxazepam | 2 (1.2) | 0 | 2 (2.9) |
| Median dose | 10 | 10 | |
| Dose range | 1010 | 1010 | |
| Temazepamc | 1 (0.6) | 0 | 1 (1.4) |
| Median dose | 15 | 15 | |
| Dose range | |||
| Hydroxyzine | 1 (0.6) | 1 (1.0) | 0 |
| Median dose | 50 | 50 | |
| Dose range | |||
Initiation, Duration, and Changes to Medications for Treatment of Sleep Complaints
None of the medication orders were part of a standardized order set. The sleep medication for the majority of patients (n=108, 64.3%) was initiated during their time on the study units (general inpatient hospital wards). Most patients (n=90, 53.6%) were ordered for a sleep aid within 48 hours of admission to the hospital. The patients who received medication for sleep had a median length of stay of 6 (interquartile range [IQR], 311) days on the study units, and received medication for a median of 2 (IQR, 15) days (Table 3). One hundred twenty patients (71.4%) were continued on a sleep aid until discharge. Essentially the same percentage of patients experienced an increase (14.9%) or decrease (14.9%) in the dose of their sleep aid during admission. Although most patients received 1 medication for sleep throughout their admission, almost one‐quarter of the patients were given 2 or more medications for sleep during their admission to the floor, sometimes including multiple medications on the same night.
| Variable | Patients, N=168 |
|---|---|
| |
| Total sleep aids each patient received during hospital length of stay, n (%) | |
| 1 sleep aid | 132 (78.6) |
| 2 sleep aids | 28 (16.7) |
| 3 sleep aids | 6 (3.6) |
| 4 sleep aids | 2 (1.2) |
| Patients who received multiple sleep aids for 1 or more days during hospital length of stay, n (%) | 20 (11.9) |
| Length of stay on study units, d | 6 [310.75]a |
| Length of sleep aid therapy on study units, d | 2 [15]a |
Of patients not known to be previously on sleep aid therapy, 40 (34.4%) of them were discharged home on a sleep aid.
Medication Administration Characteristics
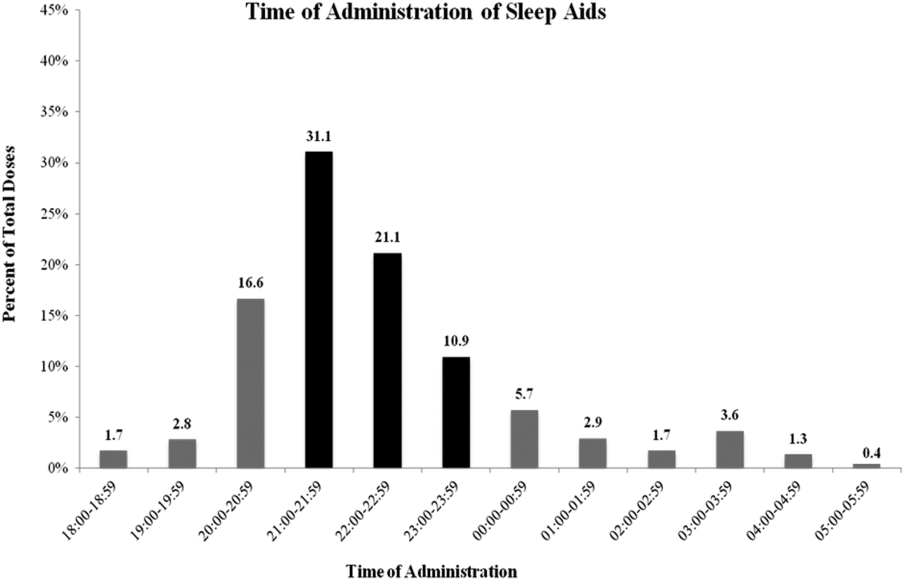
Sleep medications were prescribed most frequently as standing orders (63.7%), rather than x1 (17.7%) or PRN (18.6%). Although the majority of sleep medications were administered between the hours of 9 pm and 12 am, more than 35% of doses were given outside of this range (Figure 2).

DISCUSSION
Our results confirm the continued frequent use of pharmacological sleep aids in the hospital setting, even in the elderly, despite recent concerns regarding the use of certain sleep medications. Additional, novel findings of our study are: (1) medications used for sleep complaints in the hospital are frequently those without a formal indication for sleep, (2) medications for sleep complaints are frequently administered too early or too late at night to be consistent with good sleep hygiene, and (3) many patients never previously on a medication for insomnia are discharged with a prescription for a sleep aid.
Despite recent warnings regarding side effects especially in the elderly, our rate of medication use has only slightly improved from prior reports from more than a decade ago.[3, 5] This high rate of use is likely due to a combination of patient, clinician, and environmental factors. In our sample, the sleep aid orders were not part of an order set; thus, the orders were either the result of patient request or in response to a patient report of poor sleep. Patients and clinicians may perceive medications for sleep as highly effective and safe, despite evidence to the contrary. Another factor is that both patients and clinicians may be unaware of nonpharmacological interventions that might improve sleep. Similarly, hospital environmental factors (noise, light) may be so disruptive as to preclude these interventions or opportunities for adequate sleep. Thus, the continued high use of medication for sleep is due in part to the lack of patient and clinician education and the difficulty in changing the hospital environment and culture, especially with only limited data on the value of sleep during recovery from illness.
Clinicians typically receive little training regarding sleep or its importance. In fact, most clinicians do not assess or communicate about the patient's quality of sleep.[11] Many may not know that there is little evidence of benefit of pharmacological sleep aids in the hospital. For example, a recent report found, in contrast to the authors' hypothesis, no changes in sleep architecture or duration using 10 mg of zolpidem tartrate in postoperative patients.[12] In our study, we found that some patients required an increase in the dose of their medications or were transitioned to a different sleep aid class (suggesting that sleep aids were ineffective). Alternatively, some patients' sleep aids were discontinued during hospital admission, again, likely due to perceived ineffectiveness or perhaps side effects. It is possible that the effectiveness of these medications might be influenced by timing of administration. This too was variable in our study. Proper clinician education around sleep hygiene might prevent early medication administration (which might lead to middle‐of‐the‐night awakenings) or delayed administration (which will delay the sleep phase), as was frequently seen in our cohort.
Despite emerging evidence of the importance of sleep in maintaining adequate immune, cardiovascular, and cognitive function, there are limited data regarding the benefits of sleep during acute illness.[13, 14, 15, 16] In the absence of compelling data, promoting sleep in the hospital has been difficult. The successful interventions used by Inouye and Bartick and their colleagues to minimize sedative hypnotic use included: a bedtime routine (eg, milk or herbal tea, relaxation tapes or music, back massage, toilet at bedtime), unit‐wide noise‐reduction strategies (eg, silent pill crushers, vibrating beepers, quiet hallways, and noise‐monitoring equipment that alerted staff above a certain decibel level), and schedule adjustments to allow sleep (eg, rescheduling of medications, intravenous fluids, and procedures), all of which require substantial clinician care or may not be possible in more acutely ill patients. Although such changes might be costly, sleep promotion and minimization of sleep aids continues to be part of a strategy that reduces delirium, hospital costs, and hospital length of stay.[16] From a patient perspective, many are interested in nondrug alternatives, especially those who have never used medications before, but few are told of them.[17]
Novel findings of our study include the types of medications used for sleep in the hospital. We found that a variety of medications and classes of medications were prescribed by clinicians for sleep complaints during hospitalization. This variability is due to a number of factors including the lack of rigorous data in this area, well‐established guidelines, or clinician education. We speculate that the high rates of use of nonbenzodiazepine and non‐ gamma‐aminobutyric acid (GABA)ergic agents, such as trazodone and quetiapine, reflect concerns about the use of medications such as zolpidem. Conversely, this means patients are increasingly treated with medications without formal FDA labeling for sleep. It does appear at least that the median doses of medication prescribed were lower in those over age 65 years compared to younger patients, although we cannot determine whether this reflects physician awareness or effective decision support used during computerized order entry. The geriatric decision support recommends a reduced dose for some (eg, trazodone, haloperidol) but not all (eg, quetiapine, lorazepam) of the monitored medications.
We found that many patients, even those who were never previously known to have insomnia, were discharged with a prescription for a sleep medication. Our study design is limited in assessing whether this prescription was needed or not, that is, whether or not the patient will have insomnia (sleep difficulty despite adequate opportunity for sleep) after hospital discharge. However, other studies have suggested that acute illness can be a precipitant for insomnia. Some of this literature has focused on patients in the intensive care unit, but it seems reasonable that patients on general medical and surgical wards (not having come through the intensive care units) might also be at risk for insomnia.[18] A study by Zisberg and colleagues in an elderly Israeli cohort found hospitalization (even without intensive care unit stay) to be both a starting point and a stopping point for chronic sleep medication use.[19] Alternatively, patients may not continue to suffer from insomnia after discharge, and thus the prescription for a sleep aid is inappropriate, as it is likely to have no benefit but may carry risk.[20] Regardless of whether hospital‐acquired insomnia persists past discharge, our findings suggest that some patients will start on chronic medication use for insomnia. Importantly, these patients may have limited understanding of the reason for their prescription, medication risks and benefits, and are unlikely to receive guidance on sleep hygiene or referral to a sleep specialist if needed. In our case, the high rate of prescriptions likely reflects the way in which inpatient medications can be added as a discharge medication automatically. This represents an area for improvement at our institution.
Limitations
This retrospective, single‐center study has several limitations. First, although our results are specific to our institution, our use of pharmacological sleep aids is similar to those previously reported in the literature. Our results are consistent in some ways with the changing trends in outpatient management of insomnia, in which trazodone and quetiapine are now frequently used. Second, we rely on the medical record for prior documentation of insomnia and/or use of medications for insomnia. However, our rate of prior diagnosis of insomnia or medication use of 8.3% is consistent with epidemiological studies.[21] Third, in this retrospective study we may have included some medications in our analysis that may have been given for indications other than sleep promotion, such as medications for anxiety or agitation. However, sleep promotion may have been an intended benefit of the medication choice. Fourth, we did not follow patients after discharge to know whether they continued with sleep medication use outside the hospital. Finally, in this retrospective chart review, we focused on utilization metrics, not on efficacy (which we can only infer) or adverse effects, such as altered mental status or falls. Moreover, we did not compare those patients who did and who did not receive any medication for sleep. However, such work will be crucial in future studies.
CONCLUSIONS
Despite increasing evidence of risks such as delirium or falls, pharmacological sleep aid use in hospitalized patients, even the elderly, remains common. A variety of medications are used, with variable administration times, which likely reflects the few rigorous studies or guidelines for the use of pharmacological sleep aids in hospitalized patients. Many patients not known to be on medications for sleep before admission leave the hospital with a sleep aid prescription. Our results suggest the need to better understand the factors that contribute to the high rate of sleep aid use in hospitalized patients. Clinician education regarding sleep, and nonpharmacological strategies to improve sleep in the hospital, are also needed.
Disclosure: Nothing to report.
Sleep is known to be poor among hospitalized patients for many reasons.[1] Patients may have pain, dyspnea, or other discomforts that prevent sleep. Diagnostic and therapeutic procedures, including medication administration and routine nursing care, may take place during normal sleep times. Environmental factors such as noise and light frequently remain at daytime levels during normal sleep times.[2] In response, patients frequently request pharmacological sleep aids. Unfortunately, the use of sleep medications has been linked to clinically relevant and detrimental outcomes such as delirium and falls, particularly in the elderly. For example, in the landmark study by Inouye et al., a multicomponent intervention was used to successfully reduce delirium in older (>70 years) hospitalized patients.[3] One of the successful components was nonpharmacological sleep promotion, which reduced the use of pharmacological sleep aids from 46% to 35% of patients. Most recently, Kolla and colleagues found zolpidem tartrate use to be a risk factor (odds ratio 4.37) for inpatient falls, a known risk factor for morbidity and increased healthcare costs.[4]
The scope of recent pharmacological sleep aid use in the inpatient setting is not well described. Frighetto and colleagues described the pattern of in‐hospital drug use more than 12 years ago, before the concerns above were described, with 29% of patients receiving a medication for sleep, mostly benzodiazepines.[5] In 2008, Bartick and colleagues also found a very high rate of sleep aid use (42% of patients), but additionally described in 2010 an intervention to minimize sleep disruption that successfully reduced sleep aid use by 38%.[6] Both concerns over side effects and sleep promotion efforts might have reduced the current rate of medication use. Therefore, we sought to evaluate the prescription and administration of pharmacological sleep aids in general medical and surgical inpatients at our institution. Using our electronic medical records, including preadmission and discharge medication records, we assessed new and continued usage of medications for sleep complaints following hospital admission.
METHODS
Patients and Design
Records were reviewed for all adult patient (18 years or older) admissions to 1 of 4 units (2 general medicine and 2 general surgical units) from January 1, 2013 to February 28, 2013. These units do not have specific policies to promote sleep, such as nocturnal noise and light reduction or clustering of care. Brigham and Women's Hospital (BWH) is a 793‐bed university‐affiliated teaching hospital. Approval for this retrospective chart review study was obtained from the Partners Healthcare Institutional Review Board.
BWH uses an in‐house electronic health record system, which gathers information from a wider healthcare system (Partners Healthcare). Medications, problem lists, and allergies are available from within‐system providers and prior encounters. Admitting physicians are also required to document a preadmission medication list. A computerized physician order entry (CPOE) system is used for all medication orders. Although standardized admission order sets are used, none of these sets contains a pharmacological sleep aid. There is decision support for geriatric patients (age >65 years) that may recommend reduced starting doses for some medications.[7]
Medications Monitored for Treatment of Sleep Complaints
Using our electronic medication ordering and administration system, each patient admission was reviewed for any medication that might be used for treatment of sleep complaints. The list of sleep medications was based on those commonly used for the outpatient treatment of insomnia, as well as others included based on the authors' experience as clinical inpatient pharmacists.[8, 9, 10] Admissions were reviewed for the following medications: first generation antihistamines (diphenhydramine, hydroxyzine), tricyclic antidepressants (amitriptyline, nortriptyline, desipramine), serotonin‐norepinephrine reuptake inhibitor antidepressants (mirtazapine, trazodone, nefazodone), melatonin agonists (ramelteon), nonbenzodiazepine hypnotics (zolpidem tartrate, eszopiclone), benzodiazepines (oxazepam, temazepam, lorazepam, triazolam, diazepam), typical antipsychotics (haloperidol, fluphenazine, thioridazine, chlorpromazine), and atypical antipsychotics (quetiapine fumarate, ziprasidone, olanzapine, risperidone, aripiprazole). Melatonin, which is not regulated by the US Food and Drug Administration (FDA), cannot be prescribed using our CPOE system.
Determination of Medication Administration for Treatment of Sleep Complaints
The charts of patients receiving 1 or more of these monitored medications were then reviewed by the authors to determine if the medication was indeed prescribed for insomnia/sleep. Chart documents reviewed were the patient's problem list from outpatient provider notes; admission note, including past medical history and home medications; the preadmission medication list; and the inpatient daily progress note. The medication was considered to be used for sleep complaints (as opposed to another indication) when any of the additional following inclusion criteria were met: the medication was part of the patient's home medication regimen for insomnia, the medication order indicated that the medication was for insomnia/difficulty sleeping, or the medication was administered without a specific indication between the hours of 6 pm and 6 am. The medication was not considered to be used primarily as a sleep aid if any of the following were present (exclusion criteria): utilization for an as needed reason including anxiety, agitation, itching, nausea, muscle spasm; utilization for a documented disorder including depression, anxiety, schizophrenia, bipolar disorder, alcohol withdrawal, or epilepsy; intramuscular administration of olanzapine or ziprasidone; or topical administration of diphenhydramine.
Medication Administration Characteristics
For each medication that was administered for difficulty sleeping, the following data were documented: dose in milligrams, route of administration, time of administration, administration timing directions (eg, times 1 [x1], as needed [PRN], or standing), an increase or decrease in dose during hospital stay, documentation of the medication in discharge notes or discharge medications, and documentation of development of an allergy or adverse reaction due to the medication. Changes in dose were recorded. If a patient received more than 1 study medication, each individual medication and dose was evaluated for inclusion in the study analysis. Other data collected included the total number of days of exposure to each sleep aid during admission, the date of initiation, the total number of days of exposure to more than 1 sleep aid during admission, and the location of initiation of each individual sleep aid (eg, intensive care unit, medical floor). Appropriate time of administration was defined as sleep aid administration between 9 pm and 12 am.
RESULTS
Patients
During the 59‐day study period, there were 642 patients admitted to the study units. Two hundred seventy‐six patients received 1 of the monitored medications; however, 106 patients received the medication for an indication other than insomnia/difficulty sleeping. In 2 patients, incomplete records prevented ascertainment of the motivation for using the monitored medication. Thus, 168 patients (26.2%) were determined to have received a medication for sleep complaints and were included in the study analysis (Figure 1). Table 1 lists the characteristics of the 168 patients, of whom 10 had a prior documented sleep disorder such as insomnia (6 patients), restless leg syndrome (1 patient), and obstructive sleep apnea (3 patients). The rate of sleep medication use was lower, though not drastically so, in patients 65 years of age compared to those <65 years of age.

| Patients | Value |
|---|---|
| |
| Age, y | 57.919.8a |
| Age 65 years | 70 (41.7) |
| Age 64 years | 98 (58.3) |
| Female, n (%) | 97 (57.7) |
| Ethnicity, n (%) | |
| Caucasian | 114 (67.9) |
| Black | 18 (10.7) |
| Hispanic | 13 (7.7) |
| Other | 23 (13.7) |
| Admitted to floor from: | |
| Emergency department | 95 (56.5) |
| Operating room | 30 (17.9) |
| Transferred from outside hospital | 35 (20.8) |
| Intensive care unit | 8 (4.8) |
| Admission service, n (%) | |
| Medical | 109 (64.9) |
| Surgical | 59 (35.1) |
| Hospital length of stay | 10.516.0a |
| Known sleep disorder | |
| Insomnia | 6 (3.6) |
| Restless leg syndrome | 1 (0.6) |
| Obstructive sleep apnea | 3(1.8) |
Medications Used for Treatment of Sleep Complaints
Of the 25 monitored medications, 13 were administered to patients for sleep during the study period. The most commonly administered medications (percent of patients, median dose, absolute dose range) were trazodone (30.4%, 50 mg, 12.5450 mg), lorazepam (24.4%, 0.5 mg, 0.25 mg2 mg), and zolpidem tartrate (17.9%, 10 mg, 2.5 mg10 mg) (Table 2). As only a few of these medications (diphenhydramine, ramelteon, temazepam, triazolam, zolpidem) have a formal FDA indication for insomnia, most patients (72%) were treated using an off‐label medication. Although the types of medication used did not vary substantially between young and old patients, the median doses and ranges were lower in the elderly. Admitting service did not substantially influence the medication or dose chosen for sleep complaints (data not shown).
| Medication | All Patients, N=168, n (%) | Patients <65 Years Old, n=98, n (%) | Patients >65 Years Old, n=70, n (%) |
|---|---|---|---|
| |||
| Trazodoneb | 51 (30.4) | 29 (29.6) | 22 (31.4) |
| Median dose | 50 | 50 | 25 |
| Dose range | 12.5450 | 25450 | 12.5200 |
| Lorazepamc | 41 (24.4) | 24 (24.5) | 17 (24.3) |
| Median dose | 0.5 | 1 | 0.25 |
| Dose range | 0.252 | 0.252 | 0.251 |
| Zolpidem tartratec | 30 (17.9) | 20 (20.4) | 10 (14.3) |
| Median dose | 10 | 10 | 5 |
| Dose range | 2.510 | 2.510 | 2.510 |
| Quetiapine fumarate | 21 (12.5) | 9 (9.2) | 12 (17.1) |
| Median dose | 50 | 50 | 25 |
| Dose range | 12.5300 | 12.5300 | 12.5100 |
| Haloperidol | 18 (10.7) | 7 (7.1) | 11 (15.7) |
| Median dose | 1 | 5 | 1 |
| Dose range | 0.2510 | 0.510 | 0.251 |
| Diphenhydraminec | 16 (9.5) | 12 (12.2) | 4 (5.7) |
| Median dose | 25 | 25 | 12.5 |
| Dose range | 12.550 | 12.550 | 12.525 |
| Mirtazapine | 7 (4.2) | 3 (3.1) | 4 (5.7) |
| Median dose | 15 | 30 | 7.5 |
| Dose range | 7.545 | 7.530 | 7.545 |
| Olanzapine | 5 (3.6) | 3 (3.1) | 2 (2.9) |
| Median dose | 5 | 5 | 2.5 |
| Dose range | 2.512.5 | 512.5 | 2.52.5 |
| Amitriptyline | 5 (3.0) | 4 (4.1) | 1 (1.4) |
| Median dose | 25 | 25 | 25 |
| Dose range | 25100 | 25100 | |
| Diazepam | 5 (3.0) | 3 (3.1) | 2 (2.9) |
| Median dose | 5 | 5 | 10 |
| Dose range | 510 | ||
| Oxazepam | 2 (1.2) | 0 | 2 (2.9) |
| Median dose | 10 | 10 | |
| Dose range | 1010 | 1010 | |
| Temazepamc | 1 (0.6) | 0 | 1 (1.4) |
| Median dose | 15 | 15 | |
| Dose range | |||
| Hydroxyzine | 1 (0.6) | 1 (1.0) | 0 |
| Median dose | 50 | 50 | |
| Dose range | |||
Initiation, Duration, and Changes to Medications for Treatment of Sleep Complaints
None of the medication orders were part of a standardized order set. The sleep medication for the majority of patients (n=108, 64.3%) was initiated during their time on the study units (general inpatient hospital wards). Most patients (n=90, 53.6%) were ordered for a sleep aid within 48 hours of admission to the hospital. The patients who received medication for sleep had a median length of stay of 6 (interquartile range [IQR], 311) days on the study units, and received medication for a median of 2 (IQR, 15) days (Table 3). One hundred twenty patients (71.4%) were continued on a sleep aid until discharge. Essentially the same percentage of patients experienced an increase (14.9%) or decrease (14.9%) in the dose of their sleep aid during admission. Although most patients received 1 medication for sleep throughout their admission, almost one‐quarter of the patients were given 2 or more medications for sleep during their admission to the floor, sometimes including multiple medications on the same night.
| Variable | Patients, N=168 |
|---|---|
| |
| Total sleep aids each patient received during hospital length of stay, n (%) | |
| 1 sleep aid | 132 (78.6) |
| 2 sleep aids | 28 (16.7) |
| 3 sleep aids | 6 (3.6) |
| 4 sleep aids | 2 (1.2) |
| Patients who received multiple sleep aids for 1 or more days during hospital length of stay, n (%) | 20 (11.9) |
| Length of stay on study units, d | 6 [310.75]a |
| Length of sleep aid therapy on study units, d | 2 [15]a |
Of patients not known to be previously on sleep aid therapy, 40 (34.4%) of them were discharged home on a sleep aid.
Medication Administration Characteristics
Sleep medications were prescribed most frequently as standing orders (63.7%), rather than x1 (17.7%) or PRN (18.6%). Although the majority of sleep medications were administered between the hours of 9 pm and 12 am, more than 35% of doses were given outside of this range (Figure 2).

DISCUSSION
Our results confirm the continued frequent use of pharmacological sleep aids in the hospital setting, even in the elderly, despite recent concerns regarding the use of certain sleep medications. Additional, novel findings of our study are: (1) medications used for sleep complaints in the hospital are frequently those without a formal indication for sleep, (2) medications for sleep complaints are frequently administered too early or too late at night to be consistent with good sleep hygiene, and (3) many patients never previously on a medication for insomnia are discharged with a prescription for a sleep aid.
Despite recent warnings regarding side effects especially in the elderly, our rate of medication use has only slightly improved from prior reports from more than a decade ago.[3, 5] This high rate of use is likely due to a combination of patient, clinician, and environmental factors. In our sample, the sleep aid orders were not part of an order set; thus, the orders were either the result of patient request or in response to a patient report of poor sleep. Patients and clinicians may perceive medications for sleep as highly effective and safe, despite evidence to the contrary. Another factor is that both patients and clinicians may be unaware of nonpharmacological interventions that might improve sleep. Similarly, hospital environmental factors (noise, light) may be so disruptive as to preclude these interventions or opportunities for adequate sleep. Thus, the continued high use of medication for sleep is due in part to the lack of patient and clinician education and the difficulty in changing the hospital environment and culture, especially with only limited data on the value of sleep during recovery from illness.
Clinicians typically receive little training regarding sleep or its importance. In fact, most clinicians do not assess or communicate about the patient's quality of sleep.[11] Many may not know that there is little evidence of benefit of pharmacological sleep aids in the hospital. For example, a recent report found, in contrast to the authors' hypothesis, no changes in sleep architecture or duration using 10 mg of zolpidem tartrate in postoperative patients.[12] In our study, we found that some patients required an increase in the dose of their medications or were transitioned to a different sleep aid class (suggesting that sleep aids were ineffective). Alternatively, some patients' sleep aids were discontinued during hospital admission, again, likely due to perceived ineffectiveness or perhaps side effects. It is possible that the effectiveness of these medications might be influenced by timing of administration. This too was variable in our study. Proper clinician education around sleep hygiene might prevent early medication administration (which might lead to middle‐of‐the‐night awakenings) or delayed administration (which will delay the sleep phase), as was frequently seen in our cohort.
Despite emerging evidence of the importance of sleep in maintaining adequate immune, cardiovascular, and cognitive function, there are limited data regarding the benefits of sleep during acute illness.[13, 14, 15, 16] In the absence of compelling data, promoting sleep in the hospital has been difficult. The successful interventions used by Inouye and Bartick and their colleagues to minimize sedative hypnotic use included: a bedtime routine (eg, milk or herbal tea, relaxation tapes or music, back massage, toilet at bedtime), unit‐wide noise‐reduction strategies (eg, silent pill crushers, vibrating beepers, quiet hallways, and noise‐monitoring equipment that alerted staff above a certain decibel level), and schedule adjustments to allow sleep (eg, rescheduling of medications, intravenous fluids, and procedures), all of which require substantial clinician care or may not be possible in more acutely ill patients. Although such changes might be costly, sleep promotion and minimization of sleep aids continues to be part of a strategy that reduces delirium, hospital costs, and hospital length of stay.[16] From a patient perspective, many are interested in nondrug alternatives, especially those who have never used medications before, but few are told of them.[17]
Novel findings of our study include the types of medications used for sleep in the hospital. We found that a variety of medications and classes of medications were prescribed by clinicians for sleep complaints during hospitalization. This variability is due to a number of factors including the lack of rigorous data in this area, well‐established guidelines, or clinician education. We speculate that the high rates of use of nonbenzodiazepine and non‐ gamma‐aminobutyric acid (GABA)ergic agents, such as trazodone and quetiapine, reflect concerns about the use of medications such as zolpidem. Conversely, this means patients are increasingly treated with medications without formal FDA labeling for sleep. It does appear at least that the median doses of medication prescribed were lower in those over age 65 years compared to younger patients, although we cannot determine whether this reflects physician awareness or effective decision support used during computerized order entry. The geriatric decision support recommends a reduced dose for some (eg, trazodone, haloperidol) but not all (eg, quetiapine, lorazepam) of the monitored medications.
We found that many patients, even those who were never previously known to have insomnia, were discharged with a prescription for a sleep medication. Our study design is limited in assessing whether this prescription was needed or not, that is, whether or not the patient will have insomnia (sleep difficulty despite adequate opportunity for sleep) after hospital discharge. However, other studies have suggested that acute illness can be a precipitant for insomnia. Some of this literature has focused on patients in the intensive care unit, but it seems reasonable that patients on general medical and surgical wards (not having come through the intensive care units) might also be at risk for insomnia.[18] A study by Zisberg and colleagues in an elderly Israeli cohort found hospitalization (even without intensive care unit stay) to be both a starting point and a stopping point for chronic sleep medication use.[19] Alternatively, patients may not continue to suffer from insomnia after discharge, and thus the prescription for a sleep aid is inappropriate, as it is likely to have no benefit but may carry risk.[20] Regardless of whether hospital‐acquired insomnia persists past discharge, our findings suggest that some patients will start on chronic medication use for insomnia. Importantly, these patients may have limited understanding of the reason for their prescription, medication risks and benefits, and are unlikely to receive guidance on sleep hygiene or referral to a sleep specialist if needed. In our case, the high rate of prescriptions likely reflects the way in which inpatient medications can be added as a discharge medication automatically. This represents an area for improvement at our institution.
Limitations
This retrospective, single‐center study has several limitations. First, although our results are specific to our institution, our use of pharmacological sleep aids is similar to those previously reported in the literature. Our results are consistent in some ways with the changing trends in outpatient management of insomnia, in which trazodone and quetiapine are now frequently used. Second, we rely on the medical record for prior documentation of insomnia and/or use of medications for insomnia. However, our rate of prior diagnosis of insomnia or medication use of 8.3% is consistent with epidemiological studies.[21] Third, in this retrospective study we may have included some medications in our analysis that may have been given for indications other than sleep promotion, such as medications for anxiety or agitation. However, sleep promotion may have been an intended benefit of the medication choice. Fourth, we did not follow patients after discharge to know whether they continued with sleep medication use outside the hospital. Finally, in this retrospective chart review, we focused on utilization metrics, not on efficacy (which we can only infer) or adverse effects, such as altered mental status or falls. Moreover, we did not compare those patients who did and who did not receive any medication for sleep. However, such work will be crucial in future studies.
CONCLUSIONS
Despite increasing evidence of risks such as delirium or falls, pharmacological sleep aid use in hospitalized patients, even the elderly, remains common. A variety of medications are used, with variable administration times, which likely reflects the few rigorous studies or guidelines for the use of pharmacological sleep aids in hospitalized patients. Many patients not known to be on medications for sleep before admission leave the hospital with a sleep aid prescription. Our results suggest the need to better understand the factors that contribute to the high rate of sleep aid use in hospitalized patients. Clinician education regarding sleep, and nonpharmacological strategies to improve sleep in the hospital, are also needed.
Disclosure: Nothing to report.
- , , , . Sleep in hospitalized medical patients, part 1: factors affecting sleep. J Hosp Med. 2008;3(6):473–482.
- . Sleep and the sleep environment of older adults in acute care settings. J Gerontol Nurs. 2008;34(6):15–21.
- , , , et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669–676.
- , , , . Zolpidem is independently associated with increased risk of inpatient falls. J Hosp Med. 2013;8(1):1–6.
- , , , , , . An assessment of quality of sleep and the use of drugs with sedating properties in hospitalized adult patients. Health Qual Life Outcomes. 2004;2:17.
- , , , , . Decrease in as‐needed sedative use by limiting nighttime sleep disruptions from hospital staff. J Hosp Med. 2010;5(3):E20–E24.
- , , , , , . Guided prescription of psychotropic medications for geriatric inpatients. Arch Intern Med. 2005;165(7):802–807.
- , , , . National use of prescription medications for insomnia: NHANES 1999–2010. Sleep. 2014;37(2):343–349.
- , . Safety of low doses of quetiapine when used for insomnia. Ann Pharmacother. 2012;46(5):718–722.
- , . Ten‐year trends in the pharmacological treatment of insomnia. Sleep. 1999;22(3):371–375.
- , , , . How do clinicians assess, communicate about, and manage patient sleep in the hospital? J Nurs Adm. 2013;43(6):342–347.
- , , . Postoperative sleep disturbances after zolpidem treatment in fast‐track hip and knee replacement. J Clin Sleep Med. 2014;10(3):321–326.
- , , . Sleep deprivation after septic insult increases mortality independent of age. J Trauma. 2009;66(1):50–54.
- , , , , , . Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. Faseb J. 1996;10(5):643–653.
- , , , et al. Objective sleep duration and quality in hospitalized older adults: associations with blood pressure and mood. J Am Geriatr Soc. 2011;59(11):2185–2186.
- , , , et al. Quality improvement and cost savings with multicomponent delirium interventions: replication of the Hospital Elder Life Program in a community hospital. Psychosomatics. 2013;54(3):219–226.
- , , , , . Hospitalized patients' preference in the treatment of insomnia: pharmacological versus non‐pharmacological. Can J Clin Pharmacol. 2003;10(2):89–92.
- , , , et al. Post‐discharge insomnia symptoms are associated with quality of life impairment among survivors of acute lung injury. Sleep Med. 2012;13(8):1106–1109.
- , , , , , . Hospitalization as a turning point for sleep medication use in older adults: prospective cohort study. Drugs Aging. 2012;29(7):565–576.
- , , , et al. Inappropriate medication prescriptions in elderly adults surviving an intensive care unit hospitalization. J Am Geriatr Soc. 2013;61(7):1128–1134.
- , . Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262(11):1479–1484.
- , , , . Sleep in hospitalized medical patients, part 1: factors affecting sleep. J Hosp Med. 2008;3(6):473–482.
- . Sleep and the sleep environment of older adults in acute care settings. J Gerontol Nurs. 2008;34(6):15–21.
- , , , et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669–676.
- , , , . Zolpidem is independently associated with increased risk of inpatient falls. J Hosp Med. 2013;8(1):1–6.
- , , , , , . An assessment of quality of sleep and the use of drugs with sedating properties in hospitalized adult patients. Health Qual Life Outcomes. 2004;2:17.
- , , , , . Decrease in as‐needed sedative use by limiting nighttime sleep disruptions from hospital staff. J Hosp Med. 2010;5(3):E20–E24.
- , , , , , . Guided prescription of psychotropic medications for geriatric inpatients. Arch Intern Med. 2005;165(7):802–807.
- , , , . National use of prescription medications for insomnia: NHANES 1999–2010. Sleep. 2014;37(2):343–349.
- , . Safety of low doses of quetiapine when used for insomnia. Ann Pharmacother. 2012;46(5):718–722.
- , . Ten‐year trends in the pharmacological treatment of insomnia. Sleep. 1999;22(3):371–375.
- , , , . How do clinicians assess, communicate about, and manage patient sleep in the hospital? J Nurs Adm. 2013;43(6):342–347.
- , , . Postoperative sleep disturbances after zolpidem treatment in fast‐track hip and knee replacement. J Clin Sleep Med. 2014;10(3):321–326.
- , , . Sleep deprivation after septic insult increases mortality independent of age. J Trauma. 2009;66(1):50–54.
- , , , , , . Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. Faseb J. 1996;10(5):643–653.
- , , , et al. Objective sleep duration and quality in hospitalized older adults: associations with blood pressure and mood. J Am Geriatr Soc. 2011;59(11):2185–2186.
- , , , et al. Quality improvement and cost savings with multicomponent delirium interventions: replication of the Hospital Elder Life Program in a community hospital. Psychosomatics. 2013;54(3):219–226.
- , , , , . Hospitalized patients' preference in the treatment of insomnia: pharmacological versus non‐pharmacological. Can J Clin Pharmacol. 2003;10(2):89–92.
- , , , et al. Post‐discharge insomnia symptoms are associated with quality of life impairment among survivors of acute lung injury. Sleep Med. 2012;13(8):1106–1109.
- , , , , , . Hospitalization as a turning point for sleep medication use in older adults: prospective cohort study. Drugs Aging. 2012;29(7):565–576.
- , , , et al. Inappropriate medication prescriptions in elderly adults surviving an intensive care unit hospitalization. J Am Geriatr Soc. 2013;61(7):1128–1134.
- , . Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262(11):1479–1484.
© 2014 Society of Hospital Medicine
