User login
Stretch marks (striae cutis distensae) are a common disfiguring skin condition characterized by linear bands of atrophic-appearing skin.1 The prevalence of stretch marks associated with pregnancy ranges from 50% to 90%.2 Although stretch marks do not pose a health risk, they often cause burning, itching, and emotional distress, and they can have a deep psychological impact on patients, particularly in young healthy women who are commonly affected by this condition.3
The cause of stretch marks currently is unknown, but they are known to develop in a variety of physiological and pathological states (eg, pregnancy, adolescent growth spurts, obesity, large weight gain, Cushing syndrome, Marfan syndrome, diabetes mellitus, long-term systemic or topical steroid use).2-5 Clinically, newly formed stretch marks present as pink or purple linear lesions without substantial depression of the skin (striae rubra). Over time, the lesions lose their pigmentation, becoming depressed, atrophic, and white (striae alba).2,3,6 The most commonly affected sites are the breasts, upper arms, abdomen, buttocks, and thighs.3,4
Regardless of the etiology, the same histologic changes can be noted in the epidermis of all stretch marks, such as atrophy and loss of rete ridges, with features that are similar to scarring.3 Additionally, reorganization and diminution of the elastic fiber network of skin can be observed.7
A variety of treatment strategies are available for stretch marks, including topical preparations (eg, tretinoin, glycolic acid) and lasers.4 With current methods, no consistently effective therapies have been established yet. In this article, we present the results of a systematic review of the literature to address the effectiveness and safety of the available treatment options for stretch marks.
Methods
A literature search for randomized controlled trials (RCTs) related to the treatment of stretch marks was conducted on March 13, 2013, using the Cochrane Central Register of Controlled Trials, PubMed (from 1966), Embase (from 1974), Chinese Biomedical Literature Database (from 1978), China National Knowledge Infrastructure (from 1994), Chinese Science Journals Database (from 1989), and Wanfang Data (from 1995). Search terms included stretch marks, stretch mark, striae atrophicae, striae distensae, striae gravidarum, striae rubra, striae alba, lineae albicantes, striae, kikkisa, and random*.
We attempted to contact the original investigators of the 25 articles assessed for eligibility by e-mail to identify the randomization and answer other methodology questions to ensure that the studies included in the analysis were RCTs. Each of the 8 RCTs selected for inclusion was assessed independently by 2 investigators (L.L. and H.M.), and data extraction also was performed independently. Any differences in opinion were resolved by discussion. The risk of biases were assessed and 5 domains—random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data—were judged for each study included in the analysis using the Cochrane Collaboration’s domain-based evaluation tool as described in the Cochrane Handbook for Systematic Reviews of Interventions.8 Publication bias was not assessed due to insufficient data.
Studies ultimately were classified into 3 categories based on the risk of bias: (1) low risk of bias or low risk of bias for all key domains; (2) unclear risk of bias or unclear risk of bias for 1 or more key domains; and (3) high risk of bias or high risk of 1 or more key domains.
Results
Search Results
Figure 1 presents the literature search results. Of 300 total search results, 8 RCTs were selected for assessment,9-16 which included a total of 240 patients (Table). The investigators of all 8 reports were contacted, but only 2 responses were received.11,14 The full text of one article could not be obtained; therefore, we could not confirm that it was a true RCT and excluded it.17
Risk of Bias
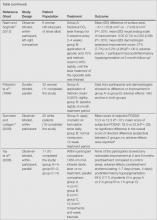
The risk of bias in methodology was evaluated for all 8 RCTs and the judgments were given for each domain (Figure 2). All the included studies claimed to be RCTs, but only 37.5% (3/8) of them used adequate randomizations, which were from a including computer-generated code,10 a table of randomized numbers,13 or the Microsoft Excel RND function (from the author by e-mail).14 The randomization methods in the other 5 studies were unclear. Allocation concealment was adequate in 1 trial13 but was unclear in the others. Three trials were double-blinded with the participants and outcome assessors blinded10,13,16; in 2 of these studies investigators also were blinded.10,13 There were 5 single-blinded trials; in 3 of these trials the outcome assessors were blinded12,14,15 and 1 was investigator-blinded.9 The other study was stated to be single-blinded but with no further detail.11 Due to the nature of the experimental design in 2 of the trials12,15 (ie, effects of laser therapy compared to topical treatment or no therapy), participants could not be blinded to treatment types; however, participants were blinded in 1 trial that compared different types of lasers.16 Investigators from all studies reported participants who did not complete the trial or were lost to follow-up, ranging from 0% to 65.6%. Two trials reported no loss of follow-up.11,12 Most trials had losses less than 20% except Pribanich et al13 who reported a loss of 65.6% of participants. One trial included a full analysis set,9 and none of the studies included an intention-to-treat analysis.
The overall risk of bias was assessed for each study and none could be categorized as low risk. Six studies had 1 or more domains assessed as high risk of bias and were classified as high risk of bias.9,11-15 The remaining 2 studies without high-risk domains had one or more domains assessed as unclear10,16 and were therefore considered to be at unclear risk of bias overall.
Effects of Treatments
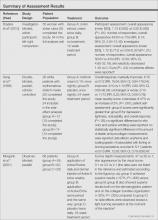
Among the 8 studies we assessed, there were different treatments, methods of comparison, product concentrations, and times of application. The methods for assessing outcomes (eg, the size and severity of stretch marks) also were varied. Therefore, it is difficult to perform a meta-analysis of the data, and all the evidence was from individual studies. A summary of the results is presented in the Table.
All of the studies we evaluated assessed clinical improvement. Three studies reported the effects of topical tretinoin on stretch marks.10,12,13 A small parallel study with unclear risk of bias indicated that white participants with erythematous stretch marks seemed to have a better response to treatment with tretinoin cream 0.1% for 24 weeks versus placebo.10 However, there was no significant difference between tretinoin cream 0.025% and placebo for patients with abdominal striae in another trial.13 The latter trial was performed with low risk of bias in methodology, but the dropout rate was high (65.6%), with only 11 of 32 participants completing the trial. It is likely that the small number of patients makes the power too low to detect significant differences between tretinoin cream 0.025% and placebo if such a difference indeed existed.13 Because the outcomes in these 2 trials were assessed in different ways, it is difficult to perform a meta-analysis on the data. More adverse effects, mainly erythema and scaling associated with itching or burning sensations, were reported with the higher concentration (0.05%) of tretinoin.10 Another study at a high risk of bias found that the combined use of tretinoin cream 0.05% and glycolic acid 10% was not as effective as fractional CO2 laser therapy in improving the appearance of striae alba.12 There also were 3 studies comparing the effects of laser therapy with another treatment or no treatment.12,15,16 Two within-participant comparison studies with unclear or high risk of bias compared CO2 fractional laser therapy with other active treatment methods in female participants with striae alba.12,16 No difference between the fractional CO2 laser and the 1550-nm nonablative fractional erbium:glass laser was reported,16 but the fractional CO2 laser may be more effective than the topical therapy.12 A small study (11 participants) at high risk of bias reported negative results for the 1450-nm mid-infrared diode laser compared to no treatment.15 Data on the adverse effects of laser therapy were available from these studies. Postinflammatory hyperpigmentation was found in all the 3 studies12,15,16 and posttreatment erythema was mentioned in 2 studies.15,16 Based on the individual studies, treatment with a cosmetic oil formulation was more effective than a moisturizer in improving clinical presentation of stretch marks in white patients.14 Women with striae rubra showed better response to treatment with onion extract cream versus no treatment.9 Limited data from 1 study showed that combined use of Active B (sodium ascorbyl phosphate, 3-aminopropyl-L-ascorbyl phosphate, carboxybetaglucan, hyaluronic acid) and Active A (hyaluronic acid, sodium salt 2 mg, sodium carboxymethyl betaglucan 0.1 mg, ascorbic acid 0.5 mg, arginine 1 mg, sodium chloride 9 mg, sterile water) might be more effective than the use of Active B or placebo.11 These 3 studies are at high risk of bias and no obvious adverse effects were reported.9,11,14
Comment
In the 8 trials included in our assessment, 5 used a within-participant design in which 2 different treatments were randomly administered to the left and right sides of the body, respectively.9,12,14-16 Because the comparison of treatments was made based on results in the same patient versus 2 different treatment groups, the results may be more accurate. In the studies we reviewed, only 3 were placebo-controlled, which may only provide limited evidence on the comparative efficacy of the treatments used in these studies.10,11,13 Most treatments were evaluated in single studies, and most studies had a small number of participants (range, 6–66 participants). A considerable number of the total participants withdrew from their respective studies or were lost during follow-up. In some cases, no reason was given,13 but in the others, it was because of an obvious side effect16 or noncompliance.14 Overall, the methodology quality was low, especially the methods of randomization and allocation concealment. Unsuccessful attempts to contact the original investigators made it difficult to make accurate assessments of the risk of bias in most of the studies included in our assessment. No study met all the risk of bias criteria, and none were classified as having a low risk of bias.
The impact of industry sponsorship on the direction and completeness of the results of the studies we reviewed is unclear. One study was funded by a grant from the manufacturer of the study product,14 and the medication used in another study was supplied by the manufacturer.13 Another study was supported in part by a company that had no part in the conduct, analysis, or reporting of the study.10 In one instance, the authors were employees of the manufacturer of the study product.9 The remaining studies made no declaration.11,12,15,16
Thus the evidence from this review was insufficient to provide clear guidelines for practice. Because the results were based on a small number of patients and were of high or unclear risk of bias, caution must be taken when comparing the efficacy of the treatments administered in these studies; however, given the negligible reported side effects, tretinoin cream 0.1%, a cosmetic oil formulation, onion extract cream, or the combined use of Active A and Active B could reasonably be considered for the treatment of stretch marks. Laser therapies such as the fractional CO2 laser or the 1550-nm fractional erbium:glass laser may be another effective choice.
Conclusion
In future investigations of stretch mark treatments, more high-quality, placebo-controlled trials are needed. One important issue is the varied outcome assessment among different studies, which makes the evaluation and pooling of different studies difficult. Therefore, future RCTs should measure clinical features with a uniform score system such as the visual analog scale or the patient and observer scar assessment scale and try to avoid individual made-up system to assess the outcome. Furthermore, quality-of-life assessment was not included in any of the reports we evaluated; rather all 8 studies focused on changes in the appearance of stretch marks only. Given the deep psychological impact that stretch marks can have on patients, measures for quality-of-life assessment, such as the dermatology life quality index, should be incorporated into future study designs to improve the relevance of the trial and allow comparisons among studies using different interventions.
1. Viennet C, Bride J, Cohen-Letessier A, et al. Mechanical behavior of fibroblasts included in collagen lattices. J Soc Biol. 2001;195:427-430.
2. Chang AL, Agredano YZ, Kimball AB. Risk factors associated with striae gravidarum. J Am Acad Dermatol. 2004;51:881-885.
3. Salter SA, Kimball AB. Striae gravidarum. Clin Dermatol. 2006;24:97-100.
4. Elsaie ML, Baumann LS, Elsaaiee LT. Striae distensae (stretch marks) and different modalities of therapy: an update. Dermatol Surg. 2009;35:563-573.
5. Singh G, Kumar LP. Striae distensae. Indian J Dermatol Venereol Leprol. 2005;71:370-372.
6. Jiménez GP, Flores F, Berman B, et al. Treatment of striae rubra and striae alba with the 585-nm pulsed-dye laser. Dermatol Surg. 2003;29:362-365.
7. Watson RE, Parry EJ, Humphries JD, et al. Fibrillin microfibrils are reduced in skin exhibiting striae distensae. Br J Dermatol. 1998;138:931-937.
8. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Oxford, United Kingdom: The Cochrane Collaboration; 2011. http://handbook.cochrane.org/. Accessed July 8, 2014.
9. Draelos ZD, Gold MH, Kaur M, et al. Evaluation of an onion extract, Centella asiatica, and hyaluronic acid cream in the appearance of striae rubra. Skinmed. 2010;8:80-86.
10. Kang S, Kim KJ, Griffiths CE, et al. Topical tretinoin (retinoic acid) improves early stretch marks. Arch Dermatol. 1996;132:519-526.
11. Morganti P, Palombo P, Fabrizi G, et al. Biweekly in-office injectable treatment of striae distensae vs a long-term daily use of topical vitamin C. J Appl Cosmetol. 2001;19:107-112.
12. Naein FF, Soghrati M. Fractional CO2 laser as an effective modality in treatment of striae alba in skin types III and IV. J Res Med Sci. 2012;17:928-933.
13. Pribanich S, Simpson FG, Held B, et al. Low-dose tretinoin does not improve striae distensae: a double-blind, placebo-controlled study. Cutis. 1994;54:121-124.
14. Summers B, Lategan M. The effect of a topically-applied cosmetic oil formulation on striae distensae. SA Fam Pract. 2009;51:332-336.
15. Tay YK, Kwok C, Tan E. Non-ablative 1,450-nm diode laser treatment of striae distensae. Lasers Surg Med. 2006;38:196-199.
16. Yang YJ, Lee GY. Treatment of striae distensae with nonablative fractional laser versus ablative CO2 fractional laser: a randomized controlled trial. Ann Dermatol. 2011;23:481-489.
17. Joshi J, Donga SB, Pandya MA. A comparative study of Savarnakara Ghrita and Savarnakara Cream in the management of Kikkisa w.s.r. to Striae Gravidarum. Ayu. 2008;29:260-265.
Stretch marks (striae cutis distensae) are a common disfiguring skin condition characterized by linear bands of atrophic-appearing skin.1 The prevalence of stretch marks associated with pregnancy ranges from 50% to 90%.2 Although stretch marks do not pose a health risk, they often cause burning, itching, and emotional distress, and they can have a deep psychological impact on patients, particularly in young healthy women who are commonly affected by this condition.3
The cause of stretch marks currently is unknown, but they are known to develop in a variety of physiological and pathological states (eg, pregnancy, adolescent growth spurts, obesity, large weight gain, Cushing syndrome, Marfan syndrome, diabetes mellitus, long-term systemic or topical steroid use).2-5 Clinically, newly formed stretch marks present as pink or purple linear lesions without substantial depression of the skin (striae rubra). Over time, the lesions lose their pigmentation, becoming depressed, atrophic, and white (striae alba).2,3,6 The most commonly affected sites are the breasts, upper arms, abdomen, buttocks, and thighs.3,4
Regardless of the etiology, the same histologic changes can be noted in the epidermis of all stretch marks, such as atrophy and loss of rete ridges, with features that are similar to scarring.3 Additionally, reorganization and diminution of the elastic fiber network of skin can be observed.7
A variety of treatment strategies are available for stretch marks, including topical preparations (eg, tretinoin, glycolic acid) and lasers.4 With current methods, no consistently effective therapies have been established yet. In this article, we present the results of a systematic review of the literature to address the effectiveness and safety of the available treatment options for stretch marks.
Methods
A literature search for randomized controlled trials (RCTs) related to the treatment of stretch marks was conducted on March 13, 2013, using the Cochrane Central Register of Controlled Trials, PubMed (from 1966), Embase (from 1974), Chinese Biomedical Literature Database (from 1978), China National Knowledge Infrastructure (from 1994), Chinese Science Journals Database (from 1989), and Wanfang Data (from 1995). Search terms included stretch marks, stretch mark, striae atrophicae, striae distensae, striae gravidarum, striae rubra, striae alba, lineae albicantes, striae, kikkisa, and random*.
We attempted to contact the original investigators of the 25 articles assessed for eligibility by e-mail to identify the randomization and answer other methodology questions to ensure that the studies included in the analysis were RCTs. Each of the 8 RCTs selected for inclusion was assessed independently by 2 investigators (L.L. and H.M.), and data extraction also was performed independently. Any differences in opinion were resolved by discussion. The risk of biases were assessed and 5 domains—random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data—were judged for each study included in the analysis using the Cochrane Collaboration’s domain-based evaluation tool as described in the Cochrane Handbook for Systematic Reviews of Interventions.8 Publication bias was not assessed due to insufficient data.
Studies ultimately were classified into 3 categories based on the risk of bias: (1) low risk of bias or low risk of bias for all key domains; (2) unclear risk of bias or unclear risk of bias for 1 or more key domains; and (3) high risk of bias or high risk of 1 or more key domains.
Results
Search Results
Figure 1 presents the literature search results. Of 300 total search results, 8 RCTs were selected for assessment,9-16 which included a total of 240 patients (Table). The investigators of all 8 reports were contacted, but only 2 responses were received.11,14 The full text of one article could not be obtained; therefore, we could not confirm that it was a true RCT and excluded it.17
Risk of Bias
The risk of bias in methodology was evaluated for all 8 RCTs and the judgments were given for each domain (Figure 2). All the included studies claimed to be RCTs, but only 37.5% (3/8) of them used adequate randomizations, which were from a including computer-generated code,10 a table of randomized numbers,13 or the Microsoft Excel RND function (from the author by e-mail).14 The randomization methods in the other 5 studies were unclear. Allocation concealment was adequate in 1 trial13 but was unclear in the others. Three trials were double-blinded with the participants and outcome assessors blinded10,13,16; in 2 of these studies investigators also were blinded.10,13 There were 5 single-blinded trials; in 3 of these trials the outcome assessors were blinded12,14,15 and 1 was investigator-blinded.9 The other study was stated to be single-blinded but with no further detail.11 Due to the nature of the experimental design in 2 of the trials12,15 (ie, effects of laser therapy compared to topical treatment or no therapy), participants could not be blinded to treatment types; however, participants were blinded in 1 trial that compared different types of lasers.16 Investigators from all studies reported participants who did not complete the trial or were lost to follow-up, ranging from 0% to 65.6%. Two trials reported no loss of follow-up.11,12 Most trials had losses less than 20% except Pribanich et al13 who reported a loss of 65.6% of participants. One trial included a full analysis set,9 and none of the studies included an intention-to-treat analysis.
The overall risk of bias was assessed for each study and none could be categorized as low risk. Six studies had 1 or more domains assessed as high risk of bias and were classified as high risk of bias.9,11-15 The remaining 2 studies without high-risk domains had one or more domains assessed as unclear10,16 and were therefore considered to be at unclear risk of bias overall.
Effects of Treatments
Among the 8 studies we assessed, there were different treatments, methods of comparison, product concentrations, and times of application. The methods for assessing outcomes (eg, the size and severity of stretch marks) also were varied. Therefore, it is difficult to perform a meta-analysis of the data, and all the evidence was from individual studies. A summary of the results is presented in the Table.
All of the studies we evaluated assessed clinical improvement. Three studies reported the effects of topical tretinoin on stretch marks.10,12,13 A small parallel study with unclear risk of bias indicated that white participants with erythematous stretch marks seemed to have a better response to treatment with tretinoin cream 0.1% for 24 weeks versus placebo.10 However, there was no significant difference between tretinoin cream 0.025% and placebo for patients with abdominal striae in another trial.13 The latter trial was performed with low risk of bias in methodology, but the dropout rate was high (65.6%), with only 11 of 32 participants completing the trial. It is likely that the small number of patients makes the power too low to detect significant differences between tretinoin cream 0.025% and placebo if such a difference indeed existed.13 Because the outcomes in these 2 trials were assessed in different ways, it is difficult to perform a meta-analysis on the data. More adverse effects, mainly erythema and scaling associated with itching or burning sensations, were reported with the higher concentration (0.05%) of tretinoin.10 Another study at a high risk of bias found that the combined use of tretinoin cream 0.05% and glycolic acid 10% was not as effective as fractional CO2 laser therapy in improving the appearance of striae alba.12 There also were 3 studies comparing the effects of laser therapy with another treatment or no treatment.12,15,16 Two within-participant comparison studies with unclear or high risk of bias compared CO2 fractional laser therapy with other active treatment methods in female participants with striae alba.12,16 No difference between the fractional CO2 laser and the 1550-nm nonablative fractional erbium:glass laser was reported,16 but the fractional CO2 laser may be more effective than the topical therapy.12 A small study (11 participants) at high risk of bias reported negative results for the 1450-nm mid-infrared diode laser compared to no treatment.15 Data on the adverse effects of laser therapy were available from these studies. Postinflammatory hyperpigmentation was found in all the 3 studies12,15,16 and posttreatment erythema was mentioned in 2 studies.15,16 Based on the individual studies, treatment with a cosmetic oil formulation was more effective than a moisturizer in improving clinical presentation of stretch marks in white patients.14 Women with striae rubra showed better response to treatment with onion extract cream versus no treatment.9 Limited data from 1 study showed that combined use of Active B (sodium ascorbyl phosphate, 3-aminopropyl-L-ascorbyl phosphate, carboxybetaglucan, hyaluronic acid) and Active A (hyaluronic acid, sodium salt 2 mg, sodium carboxymethyl betaglucan 0.1 mg, ascorbic acid 0.5 mg, arginine 1 mg, sodium chloride 9 mg, sterile water) might be more effective than the use of Active B or placebo.11 These 3 studies are at high risk of bias and no obvious adverse effects were reported.9,11,14
Comment
In the 8 trials included in our assessment, 5 used a within-participant design in which 2 different treatments were randomly administered to the left and right sides of the body, respectively.9,12,14-16 Because the comparison of treatments was made based on results in the same patient versus 2 different treatment groups, the results may be more accurate. In the studies we reviewed, only 3 were placebo-controlled, which may only provide limited evidence on the comparative efficacy of the treatments used in these studies.10,11,13 Most treatments were evaluated in single studies, and most studies had a small number of participants (range, 6–66 participants). A considerable number of the total participants withdrew from their respective studies or were lost during follow-up. In some cases, no reason was given,13 but in the others, it was because of an obvious side effect16 or noncompliance.14 Overall, the methodology quality was low, especially the methods of randomization and allocation concealment. Unsuccessful attempts to contact the original investigators made it difficult to make accurate assessments of the risk of bias in most of the studies included in our assessment. No study met all the risk of bias criteria, and none were classified as having a low risk of bias.
The impact of industry sponsorship on the direction and completeness of the results of the studies we reviewed is unclear. One study was funded by a grant from the manufacturer of the study product,14 and the medication used in another study was supplied by the manufacturer.13 Another study was supported in part by a company that had no part in the conduct, analysis, or reporting of the study.10 In one instance, the authors were employees of the manufacturer of the study product.9 The remaining studies made no declaration.11,12,15,16
Thus the evidence from this review was insufficient to provide clear guidelines for practice. Because the results were based on a small number of patients and were of high or unclear risk of bias, caution must be taken when comparing the efficacy of the treatments administered in these studies; however, given the negligible reported side effects, tretinoin cream 0.1%, a cosmetic oil formulation, onion extract cream, or the combined use of Active A and Active B could reasonably be considered for the treatment of stretch marks. Laser therapies such as the fractional CO2 laser or the 1550-nm fractional erbium:glass laser may be another effective choice.
Conclusion
In future investigations of stretch mark treatments, more high-quality, placebo-controlled trials are needed. One important issue is the varied outcome assessment among different studies, which makes the evaluation and pooling of different studies difficult. Therefore, future RCTs should measure clinical features with a uniform score system such as the visual analog scale or the patient and observer scar assessment scale and try to avoid individual made-up system to assess the outcome. Furthermore, quality-of-life assessment was not included in any of the reports we evaluated; rather all 8 studies focused on changes in the appearance of stretch marks only. Given the deep psychological impact that stretch marks can have on patients, measures for quality-of-life assessment, such as the dermatology life quality index, should be incorporated into future study designs to improve the relevance of the trial and allow comparisons among studies using different interventions.
Stretch marks (striae cutis distensae) are a common disfiguring skin condition characterized by linear bands of atrophic-appearing skin.1 The prevalence of stretch marks associated with pregnancy ranges from 50% to 90%.2 Although stretch marks do not pose a health risk, they often cause burning, itching, and emotional distress, and they can have a deep psychological impact on patients, particularly in young healthy women who are commonly affected by this condition.3
The cause of stretch marks currently is unknown, but they are known to develop in a variety of physiological and pathological states (eg, pregnancy, adolescent growth spurts, obesity, large weight gain, Cushing syndrome, Marfan syndrome, diabetes mellitus, long-term systemic or topical steroid use).2-5 Clinically, newly formed stretch marks present as pink or purple linear lesions without substantial depression of the skin (striae rubra). Over time, the lesions lose their pigmentation, becoming depressed, atrophic, and white (striae alba).2,3,6 The most commonly affected sites are the breasts, upper arms, abdomen, buttocks, and thighs.3,4
Regardless of the etiology, the same histologic changes can be noted in the epidermis of all stretch marks, such as atrophy and loss of rete ridges, with features that are similar to scarring.3 Additionally, reorganization and diminution of the elastic fiber network of skin can be observed.7
A variety of treatment strategies are available for stretch marks, including topical preparations (eg, tretinoin, glycolic acid) and lasers.4 With current methods, no consistently effective therapies have been established yet. In this article, we present the results of a systematic review of the literature to address the effectiveness and safety of the available treatment options for stretch marks.
Methods
A literature search for randomized controlled trials (RCTs) related to the treatment of stretch marks was conducted on March 13, 2013, using the Cochrane Central Register of Controlled Trials, PubMed (from 1966), Embase (from 1974), Chinese Biomedical Literature Database (from 1978), China National Knowledge Infrastructure (from 1994), Chinese Science Journals Database (from 1989), and Wanfang Data (from 1995). Search terms included stretch marks, stretch mark, striae atrophicae, striae distensae, striae gravidarum, striae rubra, striae alba, lineae albicantes, striae, kikkisa, and random*.
We attempted to contact the original investigators of the 25 articles assessed for eligibility by e-mail to identify the randomization and answer other methodology questions to ensure that the studies included in the analysis were RCTs. Each of the 8 RCTs selected for inclusion was assessed independently by 2 investigators (L.L. and H.M.), and data extraction also was performed independently. Any differences in opinion were resolved by discussion. The risk of biases were assessed and 5 domains—random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data—were judged for each study included in the analysis using the Cochrane Collaboration’s domain-based evaluation tool as described in the Cochrane Handbook for Systematic Reviews of Interventions.8 Publication bias was not assessed due to insufficient data.
Studies ultimately were classified into 3 categories based on the risk of bias: (1) low risk of bias or low risk of bias for all key domains; (2) unclear risk of bias or unclear risk of bias for 1 or more key domains; and (3) high risk of bias or high risk of 1 or more key domains.
Results
Search Results
Figure 1 presents the literature search results. Of 300 total search results, 8 RCTs were selected for assessment,9-16 which included a total of 240 patients (Table). The investigators of all 8 reports were contacted, but only 2 responses were received.11,14 The full text of one article could not be obtained; therefore, we could not confirm that it was a true RCT and excluded it.17
Risk of Bias
The risk of bias in methodology was evaluated for all 8 RCTs and the judgments were given for each domain (Figure 2). All the included studies claimed to be RCTs, but only 37.5% (3/8) of them used adequate randomizations, which were from a including computer-generated code,10 a table of randomized numbers,13 or the Microsoft Excel RND function (from the author by e-mail).14 The randomization methods in the other 5 studies were unclear. Allocation concealment was adequate in 1 trial13 but was unclear in the others. Three trials were double-blinded with the participants and outcome assessors blinded10,13,16; in 2 of these studies investigators also were blinded.10,13 There were 5 single-blinded trials; in 3 of these trials the outcome assessors were blinded12,14,15 and 1 was investigator-blinded.9 The other study was stated to be single-blinded but with no further detail.11 Due to the nature of the experimental design in 2 of the trials12,15 (ie, effects of laser therapy compared to topical treatment or no therapy), participants could not be blinded to treatment types; however, participants were blinded in 1 trial that compared different types of lasers.16 Investigators from all studies reported participants who did not complete the trial or were lost to follow-up, ranging from 0% to 65.6%. Two trials reported no loss of follow-up.11,12 Most trials had losses less than 20% except Pribanich et al13 who reported a loss of 65.6% of participants. One trial included a full analysis set,9 and none of the studies included an intention-to-treat analysis.
The overall risk of bias was assessed for each study and none could be categorized as low risk. Six studies had 1 or more domains assessed as high risk of bias and were classified as high risk of bias.9,11-15 The remaining 2 studies without high-risk domains had one or more domains assessed as unclear10,16 and were therefore considered to be at unclear risk of bias overall.
Effects of Treatments
Among the 8 studies we assessed, there were different treatments, methods of comparison, product concentrations, and times of application. The methods for assessing outcomes (eg, the size and severity of stretch marks) also were varied. Therefore, it is difficult to perform a meta-analysis of the data, and all the evidence was from individual studies. A summary of the results is presented in the Table.
All of the studies we evaluated assessed clinical improvement. Three studies reported the effects of topical tretinoin on stretch marks.10,12,13 A small parallel study with unclear risk of bias indicated that white participants with erythematous stretch marks seemed to have a better response to treatment with tretinoin cream 0.1% for 24 weeks versus placebo.10 However, there was no significant difference between tretinoin cream 0.025% and placebo for patients with abdominal striae in another trial.13 The latter trial was performed with low risk of bias in methodology, but the dropout rate was high (65.6%), with only 11 of 32 participants completing the trial. It is likely that the small number of patients makes the power too low to detect significant differences between tretinoin cream 0.025% and placebo if such a difference indeed existed.13 Because the outcomes in these 2 trials were assessed in different ways, it is difficult to perform a meta-analysis on the data. More adverse effects, mainly erythema and scaling associated with itching or burning sensations, were reported with the higher concentration (0.05%) of tretinoin.10 Another study at a high risk of bias found that the combined use of tretinoin cream 0.05% and glycolic acid 10% was not as effective as fractional CO2 laser therapy in improving the appearance of striae alba.12 There also were 3 studies comparing the effects of laser therapy with another treatment or no treatment.12,15,16 Two within-participant comparison studies with unclear or high risk of bias compared CO2 fractional laser therapy with other active treatment methods in female participants with striae alba.12,16 No difference between the fractional CO2 laser and the 1550-nm nonablative fractional erbium:glass laser was reported,16 but the fractional CO2 laser may be more effective than the topical therapy.12 A small study (11 participants) at high risk of bias reported negative results for the 1450-nm mid-infrared diode laser compared to no treatment.15 Data on the adverse effects of laser therapy were available from these studies. Postinflammatory hyperpigmentation was found in all the 3 studies12,15,16 and posttreatment erythema was mentioned in 2 studies.15,16 Based on the individual studies, treatment with a cosmetic oil formulation was more effective than a moisturizer in improving clinical presentation of stretch marks in white patients.14 Women with striae rubra showed better response to treatment with onion extract cream versus no treatment.9 Limited data from 1 study showed that combined use of Active B (sodium ascorbyl phosphate, 3-aminopropyl-L-ascorbyl phosphate, carboxybetaglucan, hyaluronic acid) and Active A (hyaluronic acid, sodium salt 2 mg, sodium carboxymethyl betaglucan 0.1 mg, ascorbic acid 0.5 mg, arginine 1 mg, sodium chloride 9 mg, sterile water) might be more effective than the use of Active B or placebo.11 These 3 studies are at high risk of bias and no obvious adverse effects were reported.9,11,14
Comment
In the 8 trials included in our assessment, 5 used a within-participant design in which 2 different treatments were randomly administered to the left and right sides of the body, respectively.9,12,14-16 Because the comparison of treatments was made based on results in the same patient versus 2 different treatment groups, the results may be more accurate. In the studies we reviewed, only 3 were placebo-controlled, which may only provide limited evidence on the comparative efficacy of the treatments used in these studies.10,11,13 Most treatments were evaluated in single studies, and most studies had a small number of participants (range, 6–66 participants). A considerable number of the total participants withdrew from their respective studies or were lost during follow-up. In some cases, no reason was given,13 but in the others, it was because of an obvious side effect16 or noncompliance.14 Overall, the methodology quality was low, especially the methods of randomization and allocation concealment. Unsuccessful attempts to contact the original investigators made it difficult to make accurate assessments of the risk of bias in most of the studies included in our assessment. No study met all the risk of bias criteria, and none were classified as having a low risk of bias.
The impact of industry sponsorship on the direction and completeness of the results of the studies we reviewed is unclear. One study was funded by a grant from the manufacturer of the study product,14 and the medication used in another study was supplied by the manufacturer.13 Another study was supported in part by a company that had no part in the conduct, analysis, or reporting of the study.10 In one instance, the authors were employees of the manufacturer of the study product.9 The remaining studies made no declaration.11,12,15,16
Thus the evidence from this review was insufficient to provide clear guidelines for practice. Because the results were based on a small number of patients and were of high or unclear risk of bias, caution must be taken when comparing the efficacy of the treatments administered in these studies; however, given the negligible reported side effects, tretinoin cream 0.1%, a cosmetic oil formulation, onion extract cream, or the combined use of Active A and Active B could reasonably be considered for the treatment of stretch marks. Laser therapies such as the fractional CO2 laser or the 1550-nm fractional erbium:glass laser may be another effective choice.
Conclusion
In future investigations of stretch mark treatments, more high-quality, placebo-controlled trials are needed. One important issue is the varied outcome assessment among different studies, which makes the evaluation and pooling of different studies difficult. Therefore, future RCTs should measure clinical features with a uniform score system such as the visual analog scale or the patient and observer scar assessment scale and try to avoid individual made-up system to assess the outcome. Furthermore, quality-of-life assessment was not included in any of the reports we evaluated; rather all 8 studies focused on changes in the appearance of stretch marks only. Given the deep psychological impact that stretch marks can have on patients, measures for quality-of-life assessment, such as the dermatology life quality index, should be incorporated into future study designs to improve the relevance of the trial and allow comparisons among studies using different interventions.
1. Viennet C, Bride J, Cohen-Letessier A, et al. Mechanical behavior of fibroblasts included in collagen lattices. J Soc Biol. 2001;195:427-430.
2. Chang AL, Agredano YZ, Kimball AB. Risk factors associated with striae gravidarum. J Am Acad Dermatol. 2004;51:881-885.
3. Salter SA, Kimball AB. Striae gravidarum. Clin Dermatol. 2006;24:97-100.
4. Elsaie ML, Baumann LS, Elsaaiee LT. Striae distensae (stretch marks) and different modalities of therapy: an update. Dermatol Surg. 2009;35:563-573.
5. Singh G, Kumar LP. Striae distensae. Indian J Dermatol Venereol Leprol. 2005;71:370-372.
6. Jiménez GP, Flores F, Berman B, et al. Treatment of striae rubra and striae alba with the 585-nm pulsed-dye laser. Dermatol Surg. 2003;29:362-365.
7. Watson RE, Parry EJ, Humphries JD, et al. Fibrillin microfibrils are reduced in skin exhibiting striae distensae. Br J Dermatol. 1998;138:931-937.
8. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Oxford, United Kingdom: The Cochrane Collaboration; 2011. http://handbook.cochrane.org/. Accessed July 8, 2014.
9. Draelos ZD, Gold MH, Kaur M, et al. Evaluation of an onion extract, Centella asiatica, and hyaluronic acid cream in the appearance of striae rubra. Skinmed. 2010;8:80-86.
10. Kang S, Kim KJ, Griffiths CE, et al. Topical tretinoin (retinoic acid) improves early stretch marks. Arch Dermatol. 1996;132:519-526.
11. Morganti P, Palombo P, Fabrizi G, et al. Biweekly in-office injectable treatment of striae distensae vs a long-term daily use of topical vitamin C. J Appl Cosmetol. 2001;19:107-112.
12. Naein FF, Soghrati M. Fractional CO2 laser as an effective modality in treatment of striae alba in skin types III and IV. J Res Med Sci. 2012;17:928-933.
13. Pribanich S, Simpson FG, Held B, et al. Low-dose tretinoin does not improve striae distensae: a double-blind, placebo-controlled study. Cutis. 1994;54:121-124.
14. Summers B, Lategan M. The effect of a topically-applied cosmetic oil formulation on striae distensae. SA Fam Pract. 2009;51:332-336.
15. Tay YK, Kwok C, Tan E. Non-ablative 1,450-nm diode laser treatment of striae distensae. Lasers Surg Med. 2006;38:196-199.
16. Yang YJ, Lee GY. Treatment of striae distensae with nonablative fractional laser versus ablative CO2 fractional laser: a randomized controlled trial. Ann Dermatol. 2011;23:481-489.
17. Joshi J, Donga SB, Pandya MA. A comparative study of Savarnakara Ghrita and Savarnakara Cream in the management of Kikkisa w.s.r. to Striae Gravidarum. Ayu. 2008;29:260-265.
1. Viennet C, Bride J, Cohen-Letessier A, et al. Mechanical behavior of fibroblasts included in collagen lattices. J Soc Biol. 2001;195:427-430.
2. Chang AL, Agredano YZ, Kimball AB. Risk factors associated with striae gravidarum. J Am Acad Dermatol. 2004;51:881-885.
3. Salter SA, Kimball AB. Striae gravidarum. Clin Dermatol. 2006;24:97-100.
4. Elsaie ML, Baumann LS, Elsaaiee LT. Striae distensae (stretch marks) and different modalities of therapy: an update. Dermatol Surg. 2009;35:563-573.
5. Singh G, Kumar LP. Striae distensae. Indian J Dermatol Venereol Leprol. 2005;71:370-372.
6. Jiménez GP, Flores F, Berman B, et al. Treatment of striae rubra and striae alba with the 585-nm pulsed-dye laser. Dermatol Surg. 2003;29:362-365.
7. Watson RE, Parry EJ, Humphries JD, et al. Fibrillin microfibrils are reduced in skin exhibiting striae distensae. Br J Dermatol. 1998;138:931-937.
8. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Oxford, United Kingdom: The Cochrane Collaboration; 2011. http://handbook.cochrane.org/. Accessed July 8, 2014.
9. Draelos ZD, Gold MH, Kaur M, et al. Evaluation of an onion extract, Centella asiatica, and hyaluronic acid cream in the appearance of striae rubra. Skinmed. 2010;8:80-86.
10. Kang S, Kim KJ, Griffiths CE, et al. Topical tretinoin (retinoic acid) improves early stretch marks. Arch Dermatol. 1996;132:519-526.
11. Morganti P, Palombo P, Fabrizi G, et al. Biweekly in-office injectable treatment of striae distensae vs a long-term daily use of topical vitamin C. J Appl Cosmetol. 2001;19:107-112.
12. Naein FF, Soghrati M. Fractional CO2 laser as an effective modality in treatment of striae alba in skin types III and IV. J Res Med Sci. 2012;17:928-933.
13. Pribanich S, Simpson FG, Held B, et al. Low-dose tretinoin does not improve striae distensae: a double-blind, placebo-controlled study. Cutis. 1994;54:121-124.
14. Summers B, Lategan M. The effect of a topically-applied cosmetic oil formulation on striae distensae. SA Fam Pract. 2009;51:332-336.
15. Tay YK, Kwok C, Tan E. Non-ablative 1,450-nm diode laser treatment of striae distensae. Lasers Surg Med. 2006;38:196-199.
16. Yang YJ, Lee GY. Treatment of striae distensae with nonablative fractional laser versus ablative CO2 fractional laser: a randomized controlled trial. Ann Dermatol. 2011;23:481-489.
17. Joshi J, Donga SB, Pandya MA. A comparative study of Savarnakara Ghrita and Savarnakara Cream in the management of Kikkisa w.s.r. to Striae Gravidarum. Ayu. 2008;29:260-265.
Practice Points
- Given the negligible reported side effects, tretinoin cream 0.1%, a cosmetic oil formulation, onion extract cream, or the combined use of Active A and Active B could be considered for the treatment of stretch marks, though the evidence is insufficient.
- High-quality, randomized, placebo-controlled trials are needed in the future.