User login
Although antiretroviral therapy for human immunodeficiency virus (HIV)‐infected patients reduces viral load dramatically and improves immune function, some patients experience a clinical deterioration within the first few months of therapy because of an exuberant and dysregulated immune responsethe immune reconstitution inflammatory syndrome (IRIS). The exaggerated immune response associated with this syndrome can be stimulated by either antigens from infectious agents (typically a mycobacterium or cryptococcus) or from autoantigens, giving rise to a heterogeneous range of clinical manifestations.1 IRIS may present as an inflammatory reaction that unmasks a previously untreated infection or as a paradoxical worsening of an infection that is being treated appropriately. Although most cases of IRIS are mild and self‐limited, some patients require aggressive treatment.1
Case Report
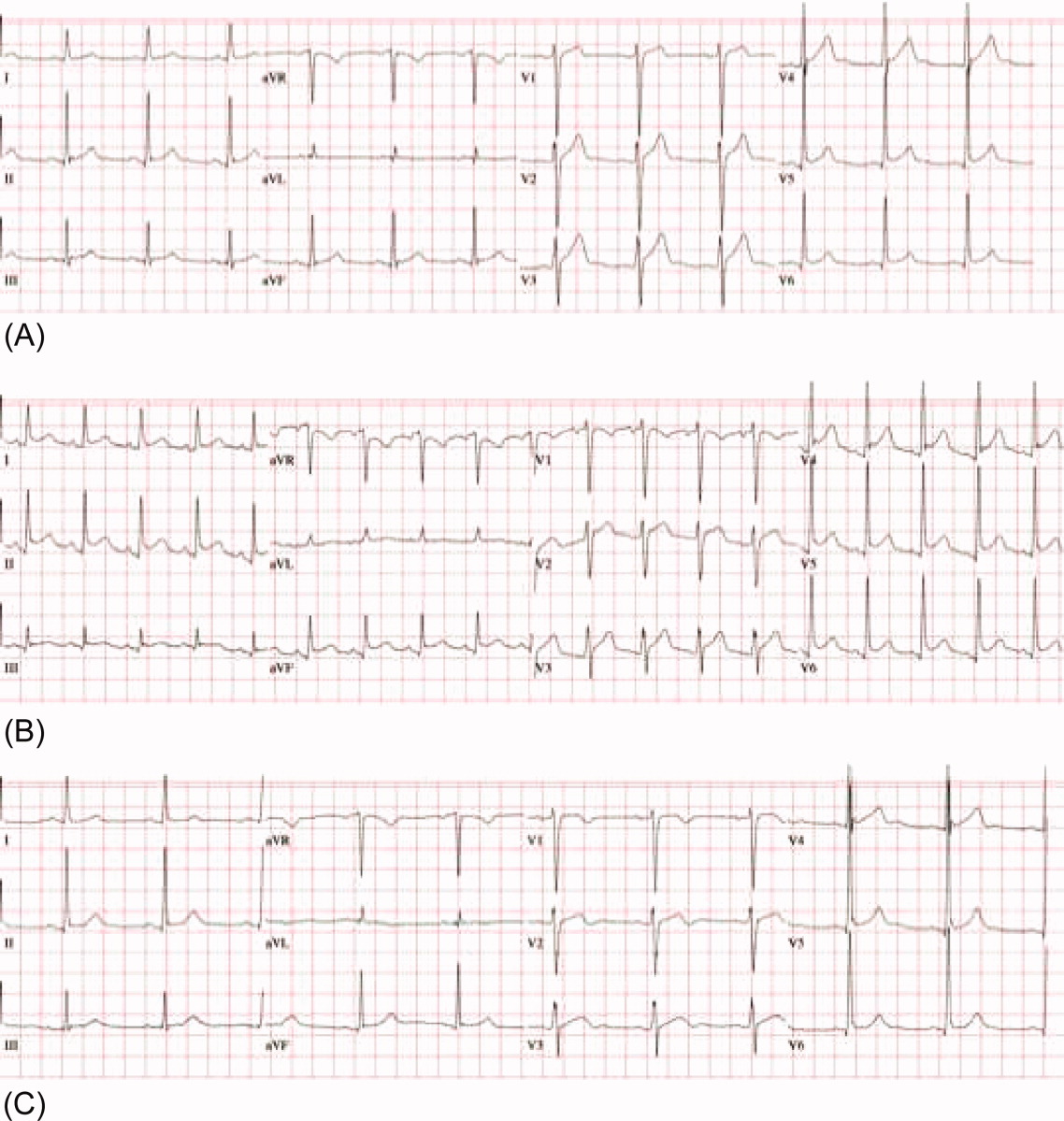
A 53‐year‐old man was evaluated for a 5‐day history of intermittent chest pain. He had been diagnosed with HIV/acquired immune deficiency syndrome (AIDS) 11 years ago but he had not been compliant with therapy. Seven years earlier he had been treated for 9 months with isoniazid for a positive tuberculin skin test. Three months before admission, he developed methicillin‐resistant Staphylococcus aureus skin abscesses and was found to have a CD4 count of 1/L and a HIV viral load of over 400,000 copies/mL. He finished a course of vancomycin, and was started on lopinavir, ritonavir, abacavir, lamivudine, and zidovudine. Five days before admission, he was evaluated in the emergency department for intermittent chest pain and described using cocaine. There was only J‐point elevation on the electrocardiogram (Figure 1A), serial cardiac enzymes were negative, and he was discharged home. However, despite discontinuation of cocaine use, his chest pain worsened, became pleuritic, and was associated with dyspnea, which prompted this admission. Physical examination was remarkable only for tachycardia, although the electrocardiogram now revealed diffuse ST segment (ST) elevation and PR segment (PR) depression, consistent with acute pericarditis (Figure 1B). Serial cardiac enzymes, viral studies, and bacterial, fungal, and mycobacterial blood cultures were negative. His CD4 count was 16/L, and the HIV viral load was 870 copies/mL.
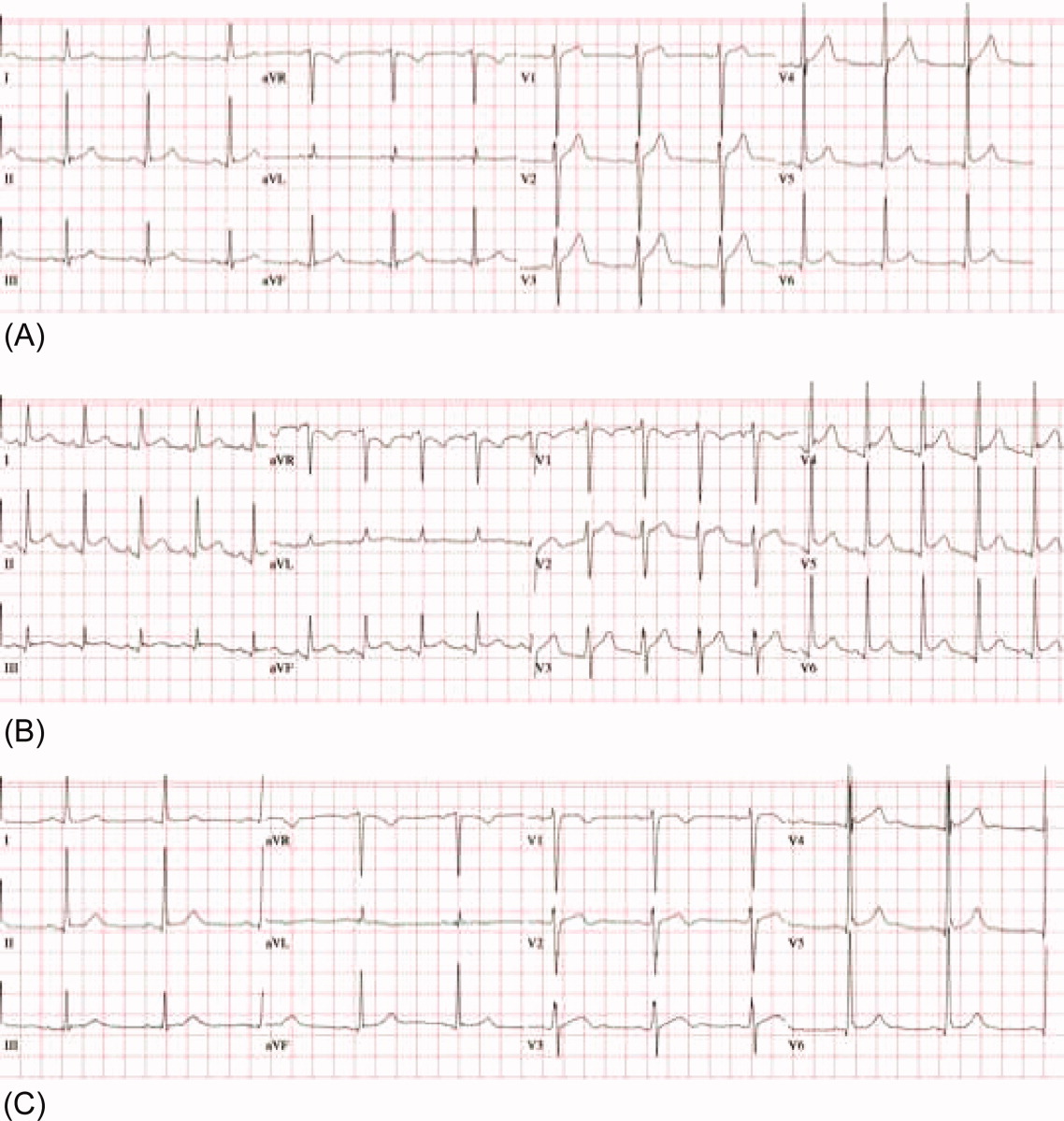
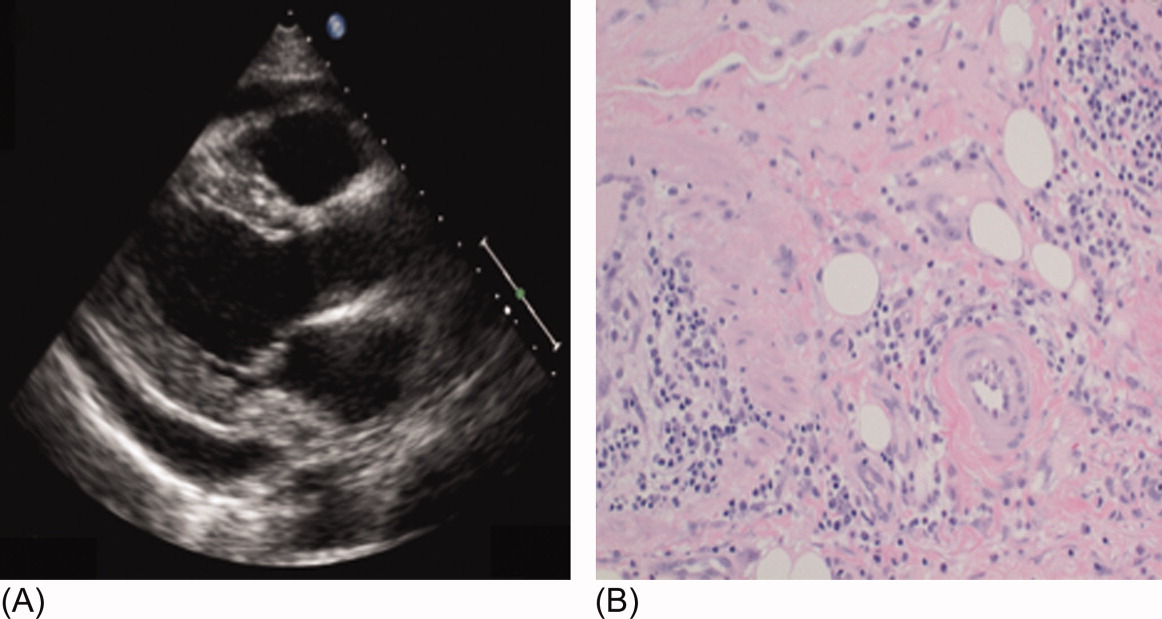
The patient was treated with high‐dose ibuprofen and colchicine, but mild chest pain and electrocardiogram changes persisted, and he developed a friction rub. A chest computed tomography (CT) scan was negative for pulmonary embolism and revealed no significant intra‐thoracic pathology, except for a moderate pericardial effusion that was confirmed by transthoracic echocardiogram (Figure 2A). There was no echocardiographic evidence of tamponade. He underwent thoracoscopic pericardial and mediastinal lymph node biopsy, along with drainage of the pericardial effusion. Pericardial biopsy showed acute on chronic inflammation consistent with pericarditis (Figure 2B) and culture was positive for Mycobacterium Avium Complex (MAC). He was treated with clarithromycin, ethambutol, and prednisone, and his antiretroviral medications were continued. At 2, 6, and 12 months follow‐up, he was asymptomatic, the electrocardiogram had normalized (Figure 1C), and the echocardiogram showed no effusion or evidence of pericardial constriction.
Discussion
This case demonstrates a unique manifestation of the IRIS associated with MAC infection, which more typically presents as peripheral, pulmonary, or intra‐abdominal lymphadenopathy.2, 3 It usually responds to MAC therapy, although intra‐abdominal disease portends a poor prognosis.3, 4 This patient has two significant risk factors for the development of IRIS: low CD4 count at the time of antiretroviral therapy and rapid viral clearance.5, 6 While his CD4 count response is lower than expected for IRIS, previous studies have shown that functional immune recovery usually precedes quantitative CD4 count recovery, and that IRIS could happen at low CD4 count.1, 7 Finally, we believe that the use of corticosteroids accounted for his rapid clinical improvement and favorable long‐term outcome, consistent with previous experience of corticosteroid use in MAC‐associated IRIS.3, 4 To our knowledge, this is the first reported case of MAC‐associated IRIS presenting as isolated acute pericarditis and pericardial effusion. In conclusion, our case illustrates that IRIS can present as an abnormal immune response to an opportunistic infection in an unusual location. Clinicians must be aware that after starting antiretroviral therapy, new symptoms, including chest pain, might represent 1 of the IRISs, and that corticosteroids might be beneficial when inflammation is severe.
Acknowledgements
The authors thank Dr. Arthur Evans for his comments.
- ,,,,.Immune reconstitution inflammatory syndrome in HIV‐infected patients receiving antiretroviral therapy.Drugs.2008;68:191–208.
- ,,,,.The imaging features of nontuberculous mycobacterial immune reconstitution syndrome.J Comput Assist Tomogr.2009;33:242–246.
- ,,, et al.Nontuberculous mycobacterial immune reconstitution syndrome in HIV‐infected patients: spectrum of disease and long‐term follow‐up.Clin Infect Dis.2005;41:1483–1497.
- ,,,,,.Mycobacterium avium complex immune reconstitution inflammatory syndrome: long term outcomes.J Transl Med.2007;5:50–56.
- ,,, et al.Incidence and risk factors for immune reconstitution inflammatory syndrome during highly active antiretroviral therapy.AIDS.2005;19:399–406.
- ,,,.Incidence and risk factors for immune reconstitution inflammatory syndrome in an ethnically diverse HIV type 1‐infected cohort.Clin Infect Dis.2006;42:418–427.
- ,,.Immune reconstitution disease associated with mycobacterial infections in HIV‐infected individuals receiving antiretrovirals.Lancet Infect Dis.2005;5:361–373.
Although antiretroviral therapy for human immunodeficiency virus (HIV)‐infected patients reduces viral load dramatically and improves immune function, some patients experience a clinical deterioration within the first few months of therapy because of an exuberant and dysregulated immune responsethe immune reconstitution inflammatory syndrome (IRIS). The exaggerated immune response associated with this syndrome can be stimulated by either antigens from infectious agents (typically a mycobacterium or cryptococcus) or from autoantigens, giving rise to a heterogeneous range of clinical manifestations.1 IRIS may present as an inflammatory reaction that unmasks a previously untreated infection or as a paradoxical worsening of an infection that is being treated appropriately. Although most cases of IRIS are mild and self‐limited, some patients require aggressive treatment.1
Case Report
A 53‐year‐old man was evaluated for a 5‐day history of intermittent chest pain. He had been diagnosed with HIV/acquired immune deficiency syndrome (AIDS) 11 years ago but he had not been compliant with therapy. Seven years earlier he had been treated for 9 months with isoniazid for a positive tuberculin skin test. Three months before admission, he developed methicillin‐resistant Staphylococcus aureus skin abscesses and was found to have a CD4 count of 1/L and a HIV viral load of over 400,000 copies/mL. He finished a course of vancomycin, and was started on lopinavir, ritonavir, abacavir, lamivudine, and zidovudine. Five days before admission, he was evaluated in the emergency department for intermittent chest pain and described using cocaine. There was only J‐point elevation on the electrocardiogram (Figure 1A), serial cardiac enzymes were negative, and he was discharged home. However, despite discontinuation of cocaine use, his chest pain worsened, became pleuritic, and was associated with dyspnea, which prompted this admission. Physical examination was remarkable only for tachycardia, although the electrocardiogram now revealed diffuse ST segment (ST) elevation and PR segment (PR) depression, consistent with acute pericarditis (Figure 1B). Serial cardiac enzymes, viral studies, and bacterial, fungal, and mycobacterial blood cultures were negative. His CD4 count was 16/L, and the HIV viral load was 870 copies/mL.

The patient was treated with high‐dose ibuprofen and colchicine, but mild chest pain and electrocardiogram changes persisted, and he developed a friction rub. A chest computed tomography (CT) scan was negative for pulmonary embolism and revealed no significant intra‐thoracic pathology, except for a moderate pericardial effusion that was confirmed by transthoracic echocardiogram (Figure 2A). There was no echocardiographic evidence of tamponade. He underwent thoracoscopic pericardial and mediastinal lymph node biopsy, along with drainage of the pericardial effusion. Pericardial biopsy showed acute on chronic inflammation consistent with pericarditis (Figure 2B) and culture was positive for Mycobacterium Avium Complex (MAC). He was treated with clarithromycin, ethambutol, and prednisone, and his antiretroviral medications were continued. At 2, 6, and 12 months follow‐up, he was asymptomatic, the electrocardiogram had normalized (Figure 1C), and the echocardiogram showed no effusion or evidence of pericardial constriction.

Discussion
This case demonstrates a unique manifestation of the IRIS associated with MAC infection, which more typically presents as peripheral, pulmonary, or intra‐abdominal lymphadenopathy.2, 3 It usually responds to MAC therapy, although intra‐abdominal disease portends a poor prognosis.3, 4 This patient has two significant risk factors for the development of IRIS: low CD4 count at the time of antiretroviral therapy and rapid viral clearance.5, 6 While his CD4 count response is lower than expected for IRIS, previous studies have shown that functional immune recovery usually precedes quantitative CD4 count recovery, and that IRIS could happen at low CD4 count.1, 7 Finally, we believe that the use of corticosteroids accounted for his rapid clinical improvement and favorable long‐term outcome, consistent with previous experience of corticosteroid use in MAC‐associated IRIS.3, 4 To our knowledge, this is the first reported case of MAC‐associated IRIS presenting as isolated acute pericarditis and pericardial effusion. In conclusion, our case illustrates that IRIS can present as an abnormal immune response to an opportunistic infection in an unusual location. Clinicians must be aware that after starting antiretroviral therapy, new symptoms, including chest pain, might represent 1 of the IRISs, and that corticosteroids might be beneficial when inflammation is severe.
Acknowledgements
The authors thank Dr. Arthur Evans for his comments.
Although antiretroviral therapy for human immunodeficiency virus (HIV)‐infected patients reduces viral load dramatically and improves immune function, some patients experience a clinical deterioration within the first few months of therapy because of an exuberant and dysregulated immune responsethe immune reconstitution inflammatory syndrome (IRIS). The exaggerated immune response associated with this syndrome can be stimulated by either antigens from infectious agents (typically a mycobacterium or cryptococcus) or from autoantigens, giving rise to a heterogeneous range of clinical manifestations.1 IRIS may present as an inflammatory reaction that unmasks a previously untreated infection or as a paradoxical worsening of an infection that is being treated appropriately. Although most cases of IRIS are mild and self‐limited, some patients require aggressive treatment.1
Case Report
A 53‐year‐old man was evaluated for a 5‐day history of intermittent chest pain. He had been diagnosed with HIV/acquired immune deficiency syndrome (AIDS) 11 years ago but he had not been compliant with therapy. Seven years earlier he had been treated for 9 months with isoniazid for a positive tuberculin skin test. Three months before admission, he developed methicillin‐resistant Staphylococcus aureus skin abscesses and was found to have a CD4 count of 1/L and a HIV viral load of over 400,000 copies/mL. He finished a course of vancomycin, and was started on lopinavir, ritonavir, abacavir, lamivudine, and zidovudine. Five days before admission, he was evaluated in the emergency department for intermittent chest pain and described using cocaine. There was only J‐point elevation on the electrocardiogram (Figure 1A), serial cardiac enzymes were negative, and he was discharged home. However, despite discontinuation of cocaine use, his chest pain worsened, became pleuritic, and was associated with dyspnea, which prompted this admission. Physical examination was remarkable only for tachycardia, although the electrocardiogram now revealed diffuse ST segment (ST) elevation and PR segment (PR) depression, consistent with acute pericarditis (Figure 1B). Serial cardiac enzymes, viral studies, and bacterial, fungal, and mycobacterial blood cultures were negative. His CD4 count was 16/L, and the HIV viral load was 870 copies/mL.

The patient was treated with high‐dose ibuprofen and colchicine, but mild chest pain and electrocardiogram changes persisted, and he developed a friction rub. A chest computed tomography (CT) scan was negative for pulmonary embolism and revealed no significant intra‐thoracic pathology, except for a moderate pericardial effusion that was confirmed by transthoracic echocardiogram (Figure 2A). There was no echocardiographic evidence of tamponade. He underwent thoracoscopic pericardial and mediastinal lymph node biopsy, along with drainage of the pericardial effusion. Pericardial biopsy showed acute on chronic inflammation consistent with pericarditis (Figure 2B) and culture was positive for Mycobacterium Avium Complex (MAC). He was treated with clarithromycin, ethambutol, and prednisone, and his antiretroviral medications were continued. At 2, 6, and 12 months follow‐up, he was asymptomatic, the electrocardiogram had normalized (Figure 1C), and the echocardiogram showed no effusion or evidence of pericardial constriction.

Discussion
This case demonstrates a unique manifestation of the IRIS associated with MAC infection, which more typically presents as peripheral, pulmonary, or intra‐abdominal lymphadenopathy.2, 3 It usually responds to MAC therapy, although intra‐abdominal disease portends a poor prognosis.3, 4 This patient has two significant risk factors for the development of IRIS: low CD4 count at the time of antiretroviral therapy and rapid viral clearance.5, 6 While his CD4 count response is lower than expected for IRIS, previous studies have shown that functional immune recovery usually precedes quantitative CD4 count recovery, and that IRIS could happen at low CD4 count.1, 7 Finally, we believe that the use of corticosteroids accounted for his rapid clinical improvement and favorable long‐term outcome, consistent with previous experience of corticosteroid use in MAC‐associated IRIS.3, 4 To our knowledge, this is the first reported case of MAC‐associated IRIS presenting as isolated acute pericarditis and pericardial effusion. In conclusion, our case illustrates that IRIS can present as an abnormal immune response to an opportunistic infection in an unusual location. Clinicians must be aware that after starting antiretroviral therapy, new symptoms, including chest pain, might represent 1 of the IRISs, and that corticosteroids might be beneficial when inflammation is severe.
Acknowledgements
The authors thank Dr. Arthur Evans for his comments.
- ,,,,.Immune reconstitution inflammatory syndrome in HIV‐infected patients receiving antiretroviral therapy.Drugs.2008;68:191–208.
- ,,,,.The imaging features of nontuberculous mycobacterial immune reconstitution syndrome.J Comput Assist Tomogr.2009;33:242–246.
- ,,, et al.Nontuberculous mycobacterial immune reconstitution syndrome in HIV‐infected patients: spectrum of disease and long‐term follow‐up.Clin Infect Dis.2005;41:1483–1497.
- ,,,,,.Mycobacterium avium complex immune reconstitution inflammatory syndrome: long term outcomes.J Transl Med.2007;5:50–56.
- ,,, et al.Incidence and risk factors for immune reconstitution inflammatory syndrome during highly active antiretroviral therapy.AIDS.2005;19:399–406.
- ,,,.Incidence and risk factors for immune reconstitution inflammatory syndrome in an ethnically diverse HIV type 1‐infected cohort.Clin Infect Dis.2006;42:418–427.
- ,,.Immune reconstitution disease associated with mycobacterial infections in HIV‐infected individuals receiving antiretrovirals.Lancet Infect Dis.2005;5:361–373.
- ,,,,.Immune reconstitution inflammatory syndrome in HIV‐infected patients receiving antiretroviral therapy.Drugs.2008;68:191–208.
- ,,,,.The imaging features of nontuberculous mycobacterial immune reconstitution syndrome.J Comput Assist Tomogr.2009;33:242–246.
- ,,, et al.Nontuberculous mycobacterial immune reconstitution syndrome in HIV‐infected patients: spectrum of disease and long‐term follow‐up.Clin Infect Dis.2005;41:1483–1497.
- ,,,,,.Mycobacterium avium complex immune reconstitution inflammatory syndrome: long term outcomes.J Transl Med.2007;5:50–56.
- ,,, et al.Incidence and risk factors for immune reconstitution inflammatory syndrome during highly active antiretroviral therapy.AIDS.2005;19:399–406.
- ,,,.Incidence and risk factors for immune reconstitution inflammatory syndrome in an ethnically diverse HIV type 1‐infected cohort.Clin Infect Dis.2006;42:418–427.
- ,,.Immune reconstitution disease associated with mycobacterial infections in HIV‐infected individuals receiving antiretrovirals.Lancet Infect Dis.2005;5:361–373.