User login
To the Editor:
Numerous drugs have been implicated as possible causes of lichenoid drug eruptions (LDEs). We describe a case of an LDE secondary to placement of a levonorgestrel-releasing intrauterine system (IUS).
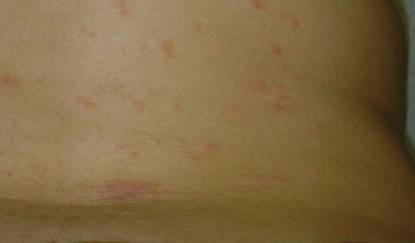
A 28-year-old woman presented with an extensive pruritic rash of 2 months’ duration. She reported that it began on the wrists; progressed inward to involve the trunk; and then became generalized over the trunk, back, wrists, and legs. A levonorgestrel-releasing IUS had been placed 6 weeks prior to the onset of the rash. She was otherwise healthy and took loratadine and pseudoephedrine on occasion for environmental allergies. On examination there were violaceous, lichenified, flat-topped, polygonal papules scattered over the arms, legs, and trunk (Figure 1). Some papules demonstrated a Köbner phenomenon. No Wickham striae or mucosal involvement was noted. Rapid plasma reagin and hepatitis panel were negative. The patient was treated empirically with fluocinonide ointment 0.05% twice daily.
|
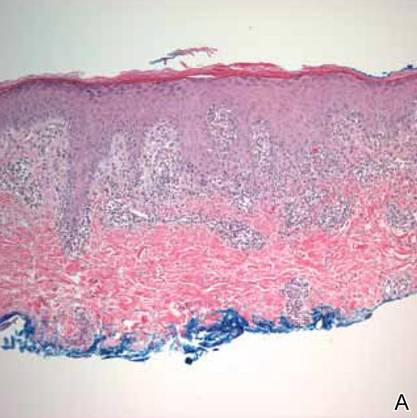
A shave biopsy was taken at the initial visit prior to steroid treatment. Histology revealed a classic lichenoid reaction pattern (Figure 2) and irregular acanthosis lying above the dense bandlike infiltrate of lymphocytes with liquefaction degeneration of the basal layer, rare Civatte bodies in the epidermis, and melanophages in the dermis.
At 5-week follow-up, the patient showed some improvement but not complete control of the lesions with topical steroids. Because the patient was on no other regular medications, we recommended a 3-month trial removal of the IUS. The patient decided to have the IUS removed and noted complete clearance of the skin lesions within 1 month. Challenge with oral or intradermal levonorgestrel was not conducted after clearance of the rash, which is a weakness in this report. Accordingly, the possibility that this patient’s condition was caused by idiopathic lichen planus, which may resolve spontaneously, cannot be ruled out. However, because the patient noted substantial improvement following removal of the device and remained symptom free 2 years after removal, we concluded that the cutaneous lesions were secondary to an LDE in response to the IUS.
It should be noted that as-needed use of pseudoephedrine and loratadine continued during this 2-year follow-up period and again the patient experienced no return of symptoms, which is particularly important because both of these agents have been associated with drug eruption patterns akin to lichenoid tissue reaction/interface dermatitis patterns. Pseudoephedrine is particularly notorious for causing nonpigmenting fixed drug eruptions such as those that heal without hyperpigmentation, while antihistamines such as loratadine have been associated with lichenoid and subacute lupus erythematosus–pattern drug reactions.1,2
Lichenoid drug reactions fall into the category of lymphocyte-rich lichenoid tissue reaction/interface dermatitis skin disorders.3 There are currently 202 different drugs reported to cause lichen planus or lichenoid eruptions as collected in Litt’s Drug Eruption & Reaction Database.4 Some of the more common causes of an LDE include angiotensin-converting enzyme inhibitors, antimalarials, calcium channel blockers, gold salts, and nonsteroidal anti-inflammatory drugs.3,4 Lichenoid eruptions typically are attributed to oral hormonal contraceptives only.5,6 An eruption in response to intrauterine levonorgestrel treatment is rare. One case report of a lichenoid eruption in response to a copper IUS was hypothesized to be due to presence of nickel salts as a manufacturing contaminant; however, the manufacturer denied the presence of the contaminant.7
The manufacturer’s information for health care professionals prescribing levonorgestrel-releasing IUS describes rashes as an adverse reaction present in less than 5% of individuals.8 Levonorgestrel-releasing IUS consists of a polyethylene frame compounded with barium sulfate, 52 mg of levonorgestrel, silicone (polydimethylsiloxane), and a monofilament brown polyethylene removal thread. The device initially releases 20 μg levonorgestrel daily, with a stable levonorgestrel plasma level of 150 to 200 pg/mL reached after the first few weeks following insertion of the device.8 Levonorgestrel is an agonist at the progesterone and androgen receptors.9 In clinical trials, levonorgestrel was implicated as the cause of increased acne, hair loss, and hirsutism as cutaneous side effects from use of levonorgestrel implants.10 However, to our knowledge, none of the other components of the levonorgestrel-releasing IUS have previously been reported to cause lichen planus or LDE.
The levonorgestrel-releasing IUS has been implicated as the cause of biopsy-proven Sweet disease,11 exacerbation of preexisting seborrheic dermatitis,12 rosacea,13 and autoimmune progesterone dermatitis.14 The skin findings in these cases resolved after removal of the IUS and appropriate treatment.
Identification of the causative drug can be difficult in LDE, as timing of the eruption can vary. The latent period has been reported to range from a few months to 1 to 2 years.15 Additionally, the clinical picture is often complicated in patients with a history of different drug dosages or multiple medications. When present, the histologic features of parakeratosis and eosinophils can be clues that a lichen planus–like eruption is drug related rather than idiopathic. However, the absence of these features does not rule out a medication or environmental trigger. In this case, the time-event relationship likely indicates that the eruption was related to the levonorgestrel-releasing IUS and not triggered by other medications or not idiopathic in nature. Lichenoid drug eruptions can resolve within a few weeks or up to 2 years after drug cessation and can occasionally be complicated by partial or complete resolution and recurrence even when the drug has not been discontinued.16,17 Lichenoid drug eruptions or idiopathic lichen planus generally are treated with topical immunomodulators or corticosteroids.3
Based on the time-event relationship, morphology, distribution, and histopathologic findings, we conclude that our patient developed LDE in response to the placement of a levonorgestrel-releasing IUS. Clinicians should be aware of the possibility of LDE occurring as a rare adverse effect of these devices.
1. Shelley WB, Shelley ED. Nonpigmenting fixed drug eruption as a distinctive reaction pattern: examples caused by sensitivity to pseudoephedrine hydrochloride and tetrahydrozoline. J Am Acad Dermatol. 1987;17:403-407.
2. Crowson AN, Magro CM. Lichenoid and subacute cutaneous lupus erythematosus-like dermatitis associated with antihistamine therapy. J Cutan Pathol. 1999;26:95-99.
3. Sontheimer RD. Lichenoid tissue reaction/interface dermatitis: clinical and histological perspectives [published online ahead of print February 26, 2009]. J Invest Dermatol. 2009;129:1088-1099.
4. Litt’s Drug Eruption & Reaction Database. Boca Raton, FL: Taylor & Francis Group; 2015. http://www.drugeruptiondata.com/searchresults/index/reaction_type/id/1/char/L. Accessed June 11, 2015.
5. Coskey RJ. Eruptions due to oral contraceptives. Arch Dermatol. 1977;113:333-334.
6. Thomas P, Dalle E, Revillon B, et al. Cutaneous effects in hormonal contraception [in French]. NPN Med. 1985;5:19-24.
7. Lombardi P, Campolmi P, Sertoli A. Lichenoid dermatitis caused by nickel salts? Contact Dermatitis. 1983;9:520-521.
8. Mirena [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2014.
9. Lemus AE, Vilchis F, Damsky R, et al. Mechanism of action of levonorgestrel: in vitro metabolism and specific interactions with steroid receptors in target organs. J Steroid Biochem Mol Biol. 1992;41:881-890.
10. Brache V, Faundes A, Alvarex F, et al. Nonmenstrual adverse events during use of implantable contraceptives for women: data from clinical trials. Contraception. 2002;65:63-74.
11. Hamill M, Bowling J, Vega-Lopez F. Sweet’s syndrome and a Mirena intrauterine system. J Fam Plann Reprod Health Care. 2004;30:115-116.
12. Karri K, Mowbray D, Adams S, et al. Severe seborrhoeic dermatitis: side-effect of the Mirena intra-uterine system. Eur J Contracept Reprod Health Care. 2006;11:53-54.
13. Choudry K, Humphreys F, Menage J. Rosacea in association with the progesterone-releasing intrauterine contraceptive device. Clin Exp Dermatol. 2001;26:102.
14. Pereira A, Coker A. Hypersensitivity to Mirena—a rare complication. J Obstet Gynaecol. 2003;23:81.
15. Halevy S, Shai A. Lichenoid drug eruptions. J Am Acad Dermatol. 1993;29(2, pt 1):249-255.
16. Seehafer JR, Rogers RS 3rd, Fleming CR, et al. Lichen planus-like lesions caused by penicillamine in primary biliary cirrhosis. Arch Dermatol. 1981;117:140-142.
17. Anderson TE. Lichen planus following quinidine therapy. Br J Dermatol. 1967;79:500.
To the Editor:
Numerous drugs have been implicated as possible causes of lichenoid drug eruptions (LDEs). We describe a case of an LDE secondary to placement of a levonorgestrel-releasing intrauterine system (IUS).
A 28-year-old woman presented with an extensive pruritic rash of 2 months’ duration. She reported that it began on the wrists; progressed inward to involve the trunk; and then became generalized over the trunk, back, wrists, and legs. A levonorgestrel-releasing IUS had been placed 6 weeks prior to the onset of the rash. She was otherwise healthy and took loratadine and pseudoephedrine on occasion for environmental allergies. On examination there were violaceous, lichenified, flat-topped, polygonal papules scattered over the arms, legs, and trunk (Figure 1). Some papules demonstrated a Köbner phenomenon. No Wickham striae or mucosal involvement was noted. Rapid plasma reagin and hepatitis panel were negative. The patient was treated empirically with fluocinonide ointment 0.05% twice daily.

|
A shave biopsy was taken at the initial visit prior to steroid treatment. Histology revealed a classic lichenoid reaction pattern (Figure 2) and irregular acanthosis lying above the dense bandlike infiltrate of lymphocytes with liquefaction degeneration of the basal layer, rare Civatte bodies in the epidermis, and melanophages in the dermis.
At 5-week follow-up, the patient showed some improvement but not complete control of the lesions with topical steroids. Because the patient was on no other regular medications, we recommended a 3-month trial removal of the IUS. The patient decided to have the IUS removed and noted complete clearance of the skin lesions within 1 month. Challenge with oral or intradermal levonorgestrel was not conducted after clearance of the rash, which is a weakness in this report. Accordingly, the possibility that this patient’s condition was caused by idiopathic lichen planus, which may resolve spontaneously, cannot be ruled out. However, because the patient noted substantial improvement following removal of the device and remained symptom free 2 years after removal, we concluded that the cutaneous lesions were secondary to an LDE in response to the IUS.
It should be noted that as-needed use of pseudoephedrine and loratadine continued during this 2-year follow-up period and again the patient experienced no return of symptoms, which is particularly important because both of these agents have been associated with drug eruption patterns akin to lichenoid tissue reaction/interface dermatitis patterns. Pseudoephedrine is particularly notorious for causing nonpigmenting fixed drug eruptions such as those that heal without hyperpigmentation, while antihistamines such as loratadine have been associated with lichenoid and subacute lupus erythematosus–pattern drug reactions.1,2
Lichenoid drug reactions fall into the category of lymphocyte-rich lichenoid tissue reaction/interface dermatitis skin disorders.3 There are currently 202 different drugs reported to cause lichen planus or lichenoid eruptions as collected in Litt’s Drug Eruption & Reaction Database.4 Some of the more common causes of an LDE include angiotensin-converting enzyme inhibitors, antimalarials, calcium channel blockers, gold salts, and nonsteroidal anti-inflammatory drugs.3,4 Lichenoid eruptions typically are attributed to oral hormonal contraceptives only.5,6 An eruption in response to intrauterine levonorgestrel treatment is rare. One case report of a lichenoid eruption in response to a copper IUS was hypothesized to be due to presence of nickel salts as a manufacturing contaminant; however, the manufacturer denied the presence of the contaminant.7
The manufacturer’s information for health care professionals prescribing levonorgestrel-releasing IUS describes rashes as an adverse reaction present in less than 5% of individuals.8 Levonorgestrel-releasing IUS consists of a polyethylene frame compounded with barium sulfate, 52 mg of levonorgestrel, silicone (polydimethylsiloxane), and a monofilament brown polyethylene removal thread. The device initially releases 20 μg levonorgestrel daily, with a stable levonorgestrel plasma level of 150 to 200 pg/mL reached after the first few weeks following insertion of the device.8 Levonorgestrel is an agonist at the progesterone and androgen receptors.9 In clinical trials, levonorgestrel was implicated as the cause of increased acne, hair loss, and hirsutism as cutaneous side effects from use of levonorgestrel implants.10 However, to our knowledge, none of the other components of the levonorgestrel-releasing IUS have previously been reported to cause lichen planus or LDE.
The levonorgestrel-releasing IUS has been implicated as the cause of biopsy-proven Sweet disease,11 exacerbation of preexisting seborrheic dermatitis,12 rosacea,13 and autoimmune progesterone dermatitis.14 The skin findings in these cases resolved after removal of the IUS and appropriate treatment.
Identification of the causative drug can be difficult in LDE, as timing of the eruption can vary. The latent period has been reported to range from a few months to 1 to 2 years.15 Additionally, the clinical picture is often complicated in patients with a history of different drug dosages or multiple medications. When present, the histologic features of parakeratosis and eosinophils can be clues that a lichen planus–like eruption is drug related rather than idiopathic. However, the absence of these features does not rule out a medication or environmental trigger. In this case, the time-event relationship likely indicates that the eruption was related to the levonorgestrel-releasing IUS and not triggered by other medications or not idiopathic in nature. Lichenoid drug eruptions can resolve within a few weeks or up to 2 years after drug cessation and can occasionally be complicated by partial or complete resolution and recurrence even when the drug has not been discontinued.16,17 Lichenoid drug eruptions or idiopathic lichen planus generally are treated with topical immunomodulators or corticosteroids.3
Based on the time-event relationship, morphology, distribution, and histopathologic findings, we conclude that our patient developed LDE in response to the placement of a levonorgestrel-releasing IUS. Clinicians should be aware of the possibility of LDE occurring as a rare adverse effect of these devices.
To the Editor:
Numerous drugs have been implicated as possible causes of lichenoid drug eruptions (LDEs). We describe a case of an LDE secondary to placement of a levonorgestrel-releasing intrauterine system (IUS).
A 28-year-old woman presented with an extensive pruritic rash of 2 months’ duration. She reported that it began on the wrists; progressed inward to involve the trunk; and then became generalized over the trunk, back, wrists, and legs. A levonorgestrel-releasing IUS had been placed 6 weeks prior to the onset of the rash. She was otherwise healthy and took loratadine and pseudoephedrine on occasion for environmental allergies. On examination there were violaceous, lichenified, flat-topped, polygonal papules scattered over the arms, legs, and trunk (Figure 1). Some papules demonstrated a Köbner phenomenon. No Wickham striae or mucosal involvement was noted. Rapid plasma reagin and hepatitis panel were negative. The patient was treated empirically with fluocinonide ointment 0.05% twice daily.

|
A shave biopsy was taken at the initial visit prior to steroid treatment. Histology revealed a classic lichenoid reaction pattern (Figure 2) and irregular acanthosis lying above the dense bandlike infiltrate of lymphocytes with liquefaction degeneration of the basal layer, rare Civatte bodies in the epidermis, and melanophages in the dermis.
At 5-week follow-up, the patient showed some improvement but not complete control of the lesions with topical steroids. Because the patient was on no other regular medications, we recommended a 3-month trial removal of the IUS. The patient decided to have the IUS removed and noted complete clearance of the skin lesions within 1 month. Challenge with oral or intradermal levonorgestrel was not conducted after clearance of the rash, which is a weakness in this report. Accordingly, the possibility that this patient’s condition was caused by idiopathic lichen planus, which may resolve spontaneously, cannot be ruled out. However, because the patient noted substantial improvement following removal of the device and remained symptom free 2 years after removal, we concluded that the cutaneous lesions were secondary to an LDE in response to the IUS.
It should be noted that as-needed use of pseudoephedrine and loratadine continued during this 2-year follow-up period and again the patient experienced no return of symptoms, which is particularly important because both of these agents have been associated with drug eruption patterns akin to lichenoid tissue reaction/interface dermatitis patterns. Pseudoephedrine is particularly notorious for causing nonpigmenting fixed drug eruptions such as those that heal without hyperpigmentation, while antihistamines such as loratadine have been associated with lichenoid and subacute lupus erythematosus–pattern drug reactions.1,2
Lichenoid drug reactions fall into the category of lymphocyte-rich lichenoid tissue reaction/interface dermatitis skin disorders.3 There are currently 202 different drugs reported to cause lichen planus or lichenoid eruptions as collected in Litt’s Drug Eruption & Reaction Database.4 Some of the more common causes of an LDE include angiotensin-converting enzyme inhibitors, antimalarials, calcium channel blockers, gold salts, and nonsteroidal anti-inflammatory drugs.3,4 Lichenoid eruptions typically are attributed to oral hormonal contraceptives only.5,6 An eruption in response to intrauterine levonorgestrel treatment is rare. One case report of a lichenoid eruption in response to a copper IUS was hypothesized to be due to presence of nickel salts as a manufacturing contaminant; however, the manufacturer denied the presence of the contaminant.7
The manufacturer’s information for health care professionals prescribing levonorgestrel-releasing IUS describes rashes as an adverse reaction present in less than 5% of individuals.8 Levonorgestrel-releasing IUS consists of a polyethylene frame compounded with barium sulfate, 52 mg of levonorgestrel, silicone (polydimethylsiloxane), and a monofilament brown polyethylene removal thread. The device initially releases 20 μg levonorgestrel daily, with a stable levonorgestrel plasma level of 150 to 200 pg/mL reached after the first few weeks following insertion of the device.8 Levonorgestrel is an agonist at the progesterone and androgen receptors.9 In clinical trials, levonorgestrel was implicated as the cause of increased acne, hair loss, and hirsutism as cutaneous side effects from use of levonorgestrel implants.10 However, to our knowledge, none of the other components of the levonorgestrel-releasing IUS have previously been reported to cause lichen planus or LDE.
The levonorgestrel-releasing IUS has been implicated as the cause of biopsy-proven Sweet disease,11 exacerbation of preexisting seborrheic dermatitis,12 rosacea,13 and autoimmune progesterone dermatitis.14 The skin findings in these cases resolved after removal of the IUS and appropriate treatment.
Identification of the causative drug can be difficult in LDE, as timing of the eruption can vary. The latent period has been reported to range from a few months to 1 to 2 years.15 Additionally, the clinical picture is often complicated in patients with a history of different drug dosages or multiple medications. When present, the histologic features of parakeratosis and eosinophils can be clues that a lichen planus–like eruption is drug related rather than idiopathic. However, the absence of these features does not rule out a medication or environmental trigger. In this case, the time-event relationship likely indicates that the eruption was related to the levonorgestrel-releasing IUS and not triggered by other medications or not idiopathic in nature. Lichenoid drug eruptions can resolve within a few weeks or up to 2 years after drug cessation and can occasionally be complicated by partial or complete resolution and recurrence even when the drug has not been discontinued.16,17 Lichenoid drug eruptions or idiopathic lichen planus generally are treated with topical immunomodulators or corticosteroids.3
Based on the time-event relationship, morphology, distribution, and histopathologic findings, we conclude that our patient developed LDE in response to the placement of a levonorgestrel-releasing IUS. Clinicians should be aware of the possibility of LDE occurring as a rare adverse effect of these devices.
1. Shelley WB, Shelley ED. Nonpigmenting fixed drug eruption as a distinctive reaction pattern: examples caused by sensitivity to pseudoephedrine hydrochloride and tetrahydrozoline. J Am Acad Dermatol. 1987;17:403-407.
2. Crowson AN, Magro CM. Lichenoid and subacute cutaneous lupus erythematosus-like dermatitis associated with antihistamine therapy. J Cutan Pathol. 1999;26:95-99.
3. Sontheimer RD. Lichenoid tissue reaction/interface dermatitis: clinical and histological perspectives [published online ahead of print February 26, 2009]. J Invest Dermatol. 2009;129:1088-1099.
4. Litt’s Drug Eruption & Reaction Database. Boca Raton, FL: Taylor & Francis Group; 2015. http://www.drugeruptiondata.com/searchresults/index/reaction_type/id/1/char/L. Accessed June 11, 2015.
5. Coskey RJ. Eruptions due to oral contraceptives. Arch Dermatol. 1977;113:333-334.
6. Thomas P, Dalle E, Revillon B, et al. Cutaneous effects in hormonal contraception [in French]. NPN Med. 1985;5:19-24.
7. Lombardi P, Campolmi P, Sertoli A. Lichenoid dermatitis caused by nickel salts? Contact Dermatitis. 1983;9:520-521.
8. Mirena [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2014.
9. Lemus AE, Vilchis F, Damsky R, et al. Mechanism of action of levonorgestrel: in vitro metabolism and specific interactions with steroid receptors in target organs. J Steroid Biochem Mol Biol. 1992;41:881-890.
10. Brache V, Faundes A, Alvarex F, et al. Nonmenstrual adverse events during use of implantable contraceptives for women: data from clinical trials. Contraception. 2002;65:63-74.
11. Hamill M, Bowling J, Vega-Lopez F. Sweet’s syndrome and a Mirena intrauterine system. J Fam Plann Reprod Health Care. 2004;30:115-116.
12. Karri K, Mowbray D, Adams S, et al. Severe seborrhoeic dermatitis: side-effect of the Mirena intra-uterine system. Eur J Contracept Reprod Health Care. 2006;11:53-54.
13. Choudry K, Humphreys F, Menage J. Rosacea in association with the progesterone-releasing intrauterine contraceptive device. Clin Exp Dermatol. 2001;26:102.
14. Pereira A, Coker A. Hypersensitivity to Mirena—a rare complication. J Obstet Gynaecol. 2003;23:81.
15. Halevy S, Shai A. Lichenoid drug eruptions. J Am Acad Dermatol. 1993;29(2, pt 1):249-255.
16. Seehafer JR, Rogers RS 3rd, Fleming CR, et al. Lichen planus-like lesions caused by penicillamine in primary biliary cirrhosis. Arch Dermatol. 1981;117:140-142.
17. Anderson TE. Lichen planus following quinidine therapy. Br J Dermatol. 1967;79:500.
1. Shelley WB, Shelley ED. Nonpigmenting fixed drug eruption as a distinctive reaction pattern: examples caused by sensitivity to pseudoephedrine hydrochloride and tetrahydrozoline. J Am Acad Dermatol. 1987;17:403-407.
2. Crowson AN, Magro CM. Lichenoid and subacute cutaneous lupus erythematosus-like dermatitis associated with antihistamine therapy. J Cutan Pathol. 1999;26:95-99.
3. Sontheimer RD. Lichenoid tissue reaction/interface dermatitis: clinical and histological perspectives [published online ahead of print February 26, 2009]. J Invest Dermatol. 2009;129:1088-1099.
4. Litt’s Drug Eruption & Reaction Database. Boca Raton, FL: Taylor & Francis Group; 2015. http://www.drugeruptiondata.com/searchresults/index/reaction_type/id/1/char/L. Accessed June 11, 2015.
5. Coskey RJ. Eruptions due to oral contraceptives. Arch Dermatol. 1977;113:333-334.
6. Thomas P, Dalle E, Revillon B, et al. Cutaneous effects in hormonal contraception [in French]. NPN Med. 1985;5:19-24.
7. Lombardi P, Campolmi P, Sertoli A. Lichenoid dermatitis caused by nickel salts? Contact Dermatitis. 1983;9:520-521.
8. Mirena [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2014.
9. Lemus AE, Vilchis F, Damsky R, et al. Mechanism of action of levonorgestrel: in vitro metabolism and specific interactions with steroid receptors in target organs. J Steroid Biochem Mol Biol. 1992;41:881-890.
10. Brache V, Faundes A, Alvarex F, et al. Nonmenstrual adverse events during use of implantable contraceptives for women: data from clinical trials. Contraception. 2002;65:63-74.
11. Hamill M, Bowling J, Vega-Lopez F. Sweet’s syndrome and a Mirena intrauterine system. J Fam Plann Reprod Health Care. 2004;30:115-116.
12. Karri K, Mowbray D, Adams S, et al. Severe seborrhoeic dermatitis: side-effect of the Mirena intra-uterine system. Eur J Contracept Reprod Health Care. 2006;11:53-54.
13. Choudry K, Humphreys F, Menage J. Rosacea in association with the progesterone-releasing intrauterine contraceptive device. Clin Exp Dermatol. 2001;26:102.
14. Pereira A, Coker A. Hypersensitivity to Mirena—a rare complication. J Obstet Gynaecol. 2003;23:81.
15. Halevy S, Shai A. Lichenoid drug eruptions. J Am Acad Dermatol. 1993;29(2, pt 1):249-255.
16. Seehafer JR, Rogers RS 3rd, Fleming CR, et al. Lichen planus-like lesions caused by penicillamine in primary biliary cirrhosis. Arch Dermatol. 1981;117:140-142.
17. Anderson TE. Lichen planus following quinidine therapy. Br J Dermatol. 1967;79:500.