User login
Case Report
A 25-year-old woman with a 5-month history of severe lichen planus (LP) on the arms, legs, and trunk presented to the emergency department with generalized blisters and erythema over the entire body, including the face and soles, of 2 days’ duration. She was evaluated for the LP 1 week prior in a referral dermatology clinic, and in addition to topical corticosteroids, she received 1 injection of 40 mg intramuscular triamcinolone acetonide. Hours following the injection she developed nausea, vomiting, and fever. The patient reported that her last menstrual period was 3 weeks prior to the current presentation.
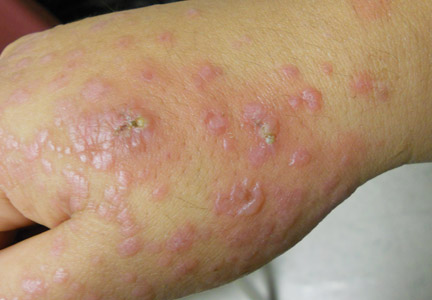
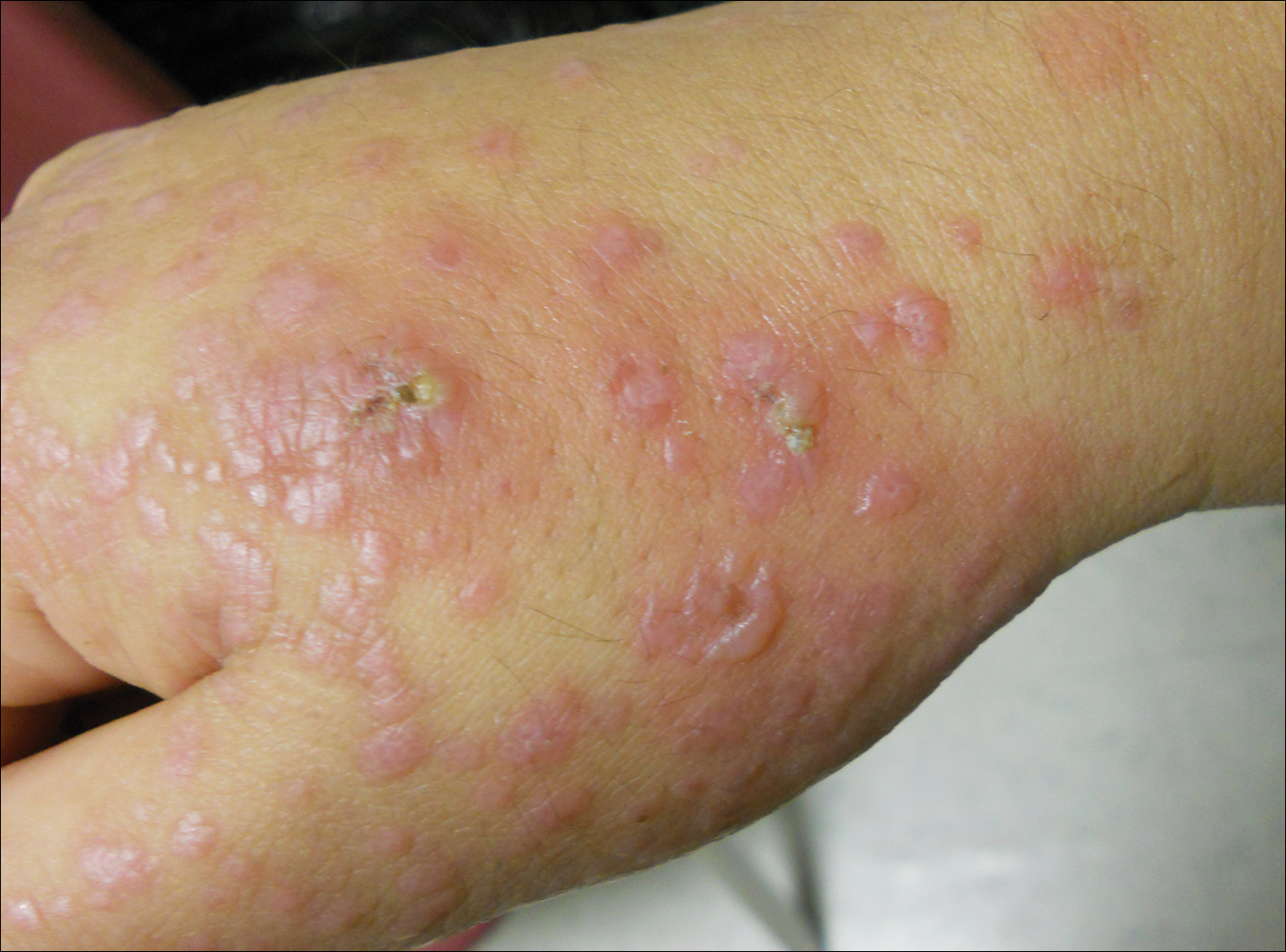
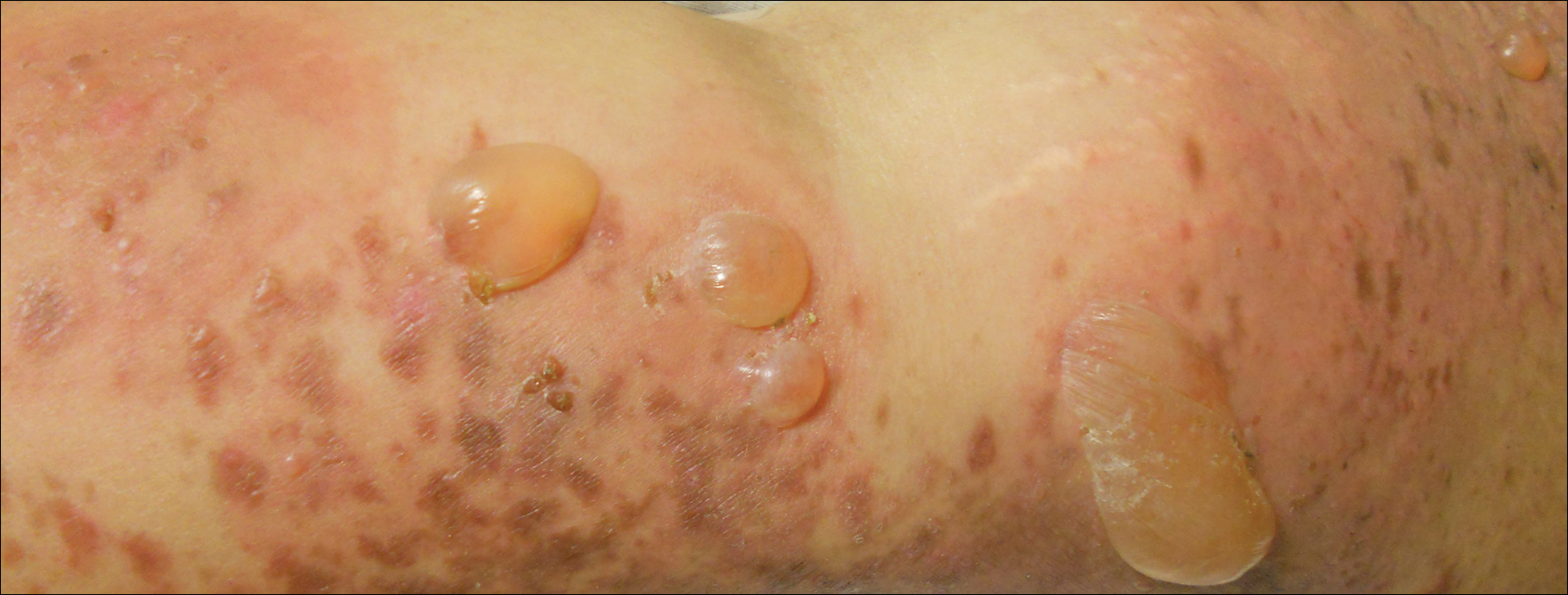
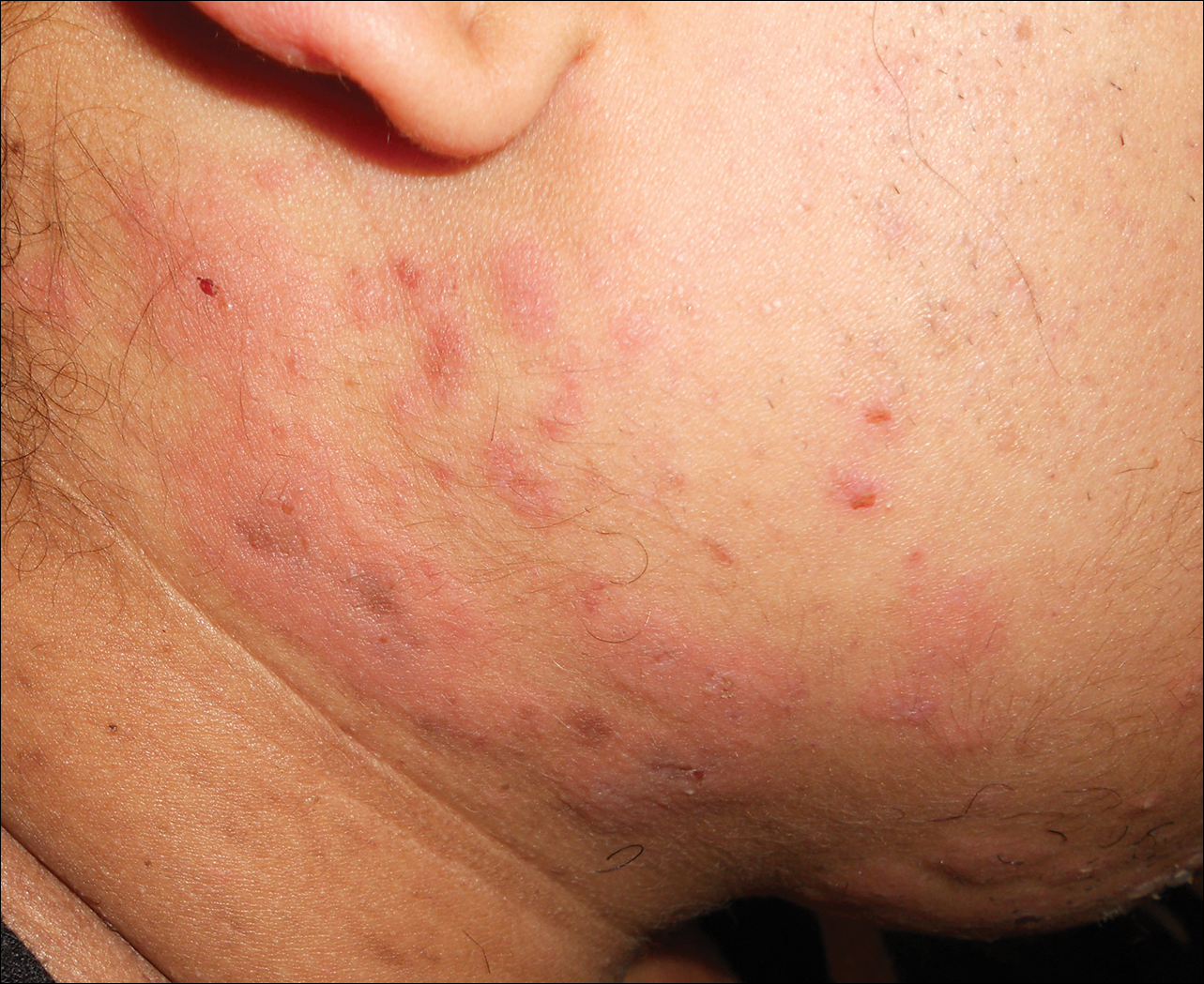
Physical examination revealed numerous lichenified, flat-topped, pink-violaceous, hyperpigmented, scaly papules and plaques (Figure 1), as well as tense, yellow, fluid-filled vesicles and bullae of various sizes on the neck, arms (Figure 2), legs, trunk, and dorsal aspect of the feet. The vesicles occurred on both normal skin and the lichenified plaques with a negative Nikolsky sign. There also were urticarial erythematous papules and plaques on the arms, trunk, neck, and face, some of which had vesicles or a violaceous dusky central hue (Figure 3). Vesicles were noted within both nostrils (nasal mucosa), and there were extremely tender erythematous patches and thick sheets of scales on the soles.
An elevated β human chorionic gonadotropin level and transvaginal ultrasonography confirmed an intrauterine pregnancy of 12 weeks’ gestation despite the patient’s report of the last menstrual period.
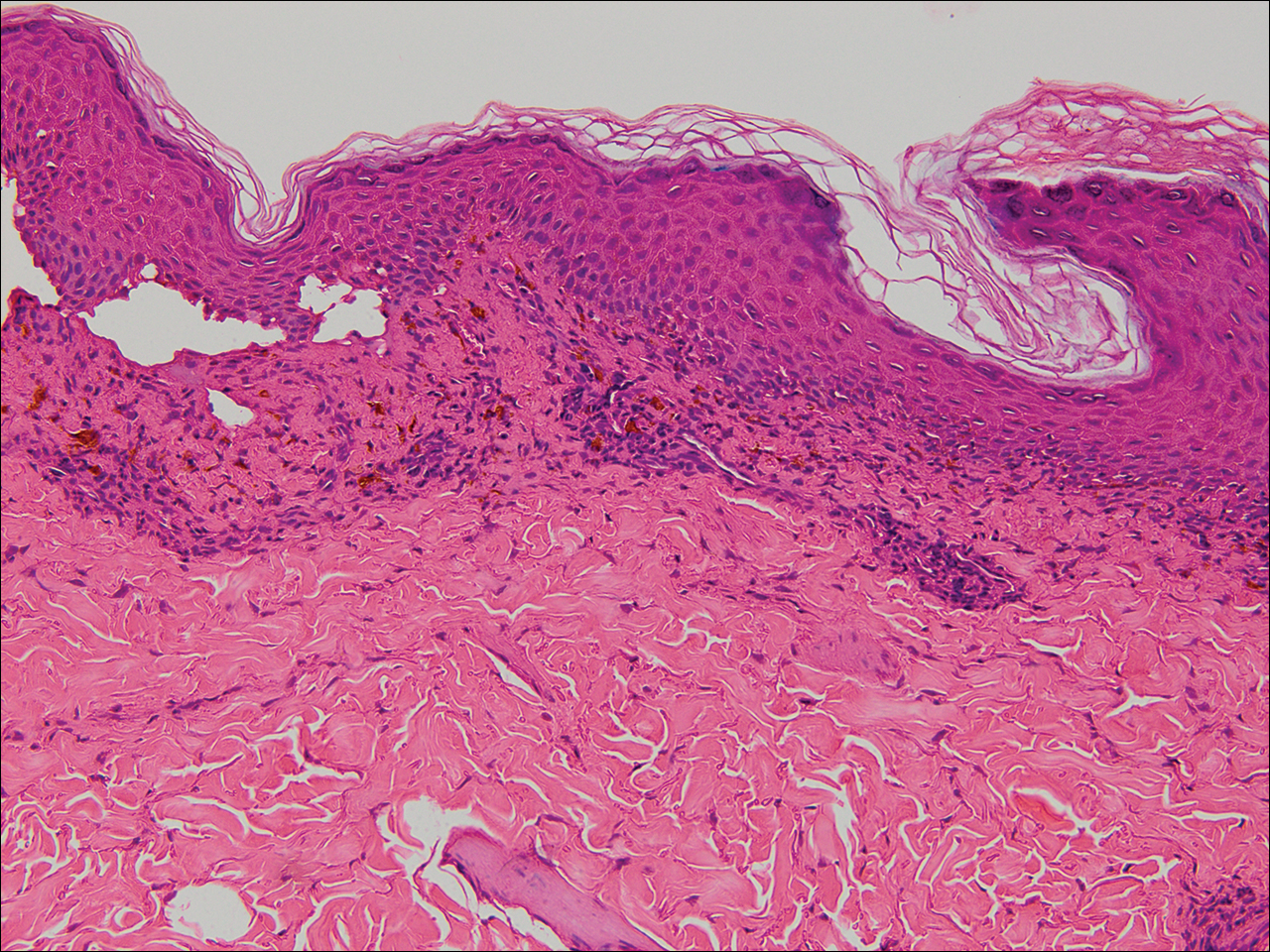
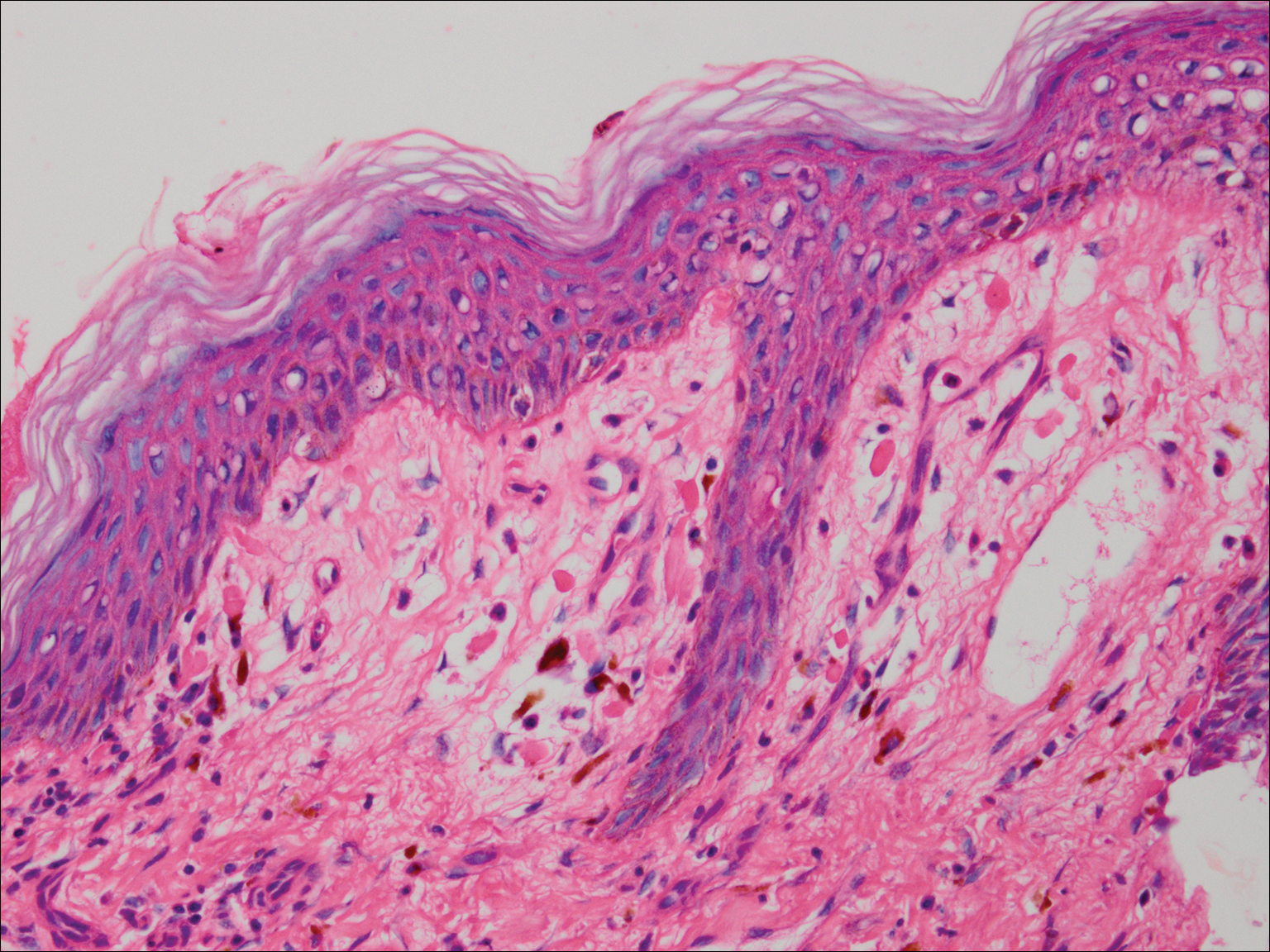
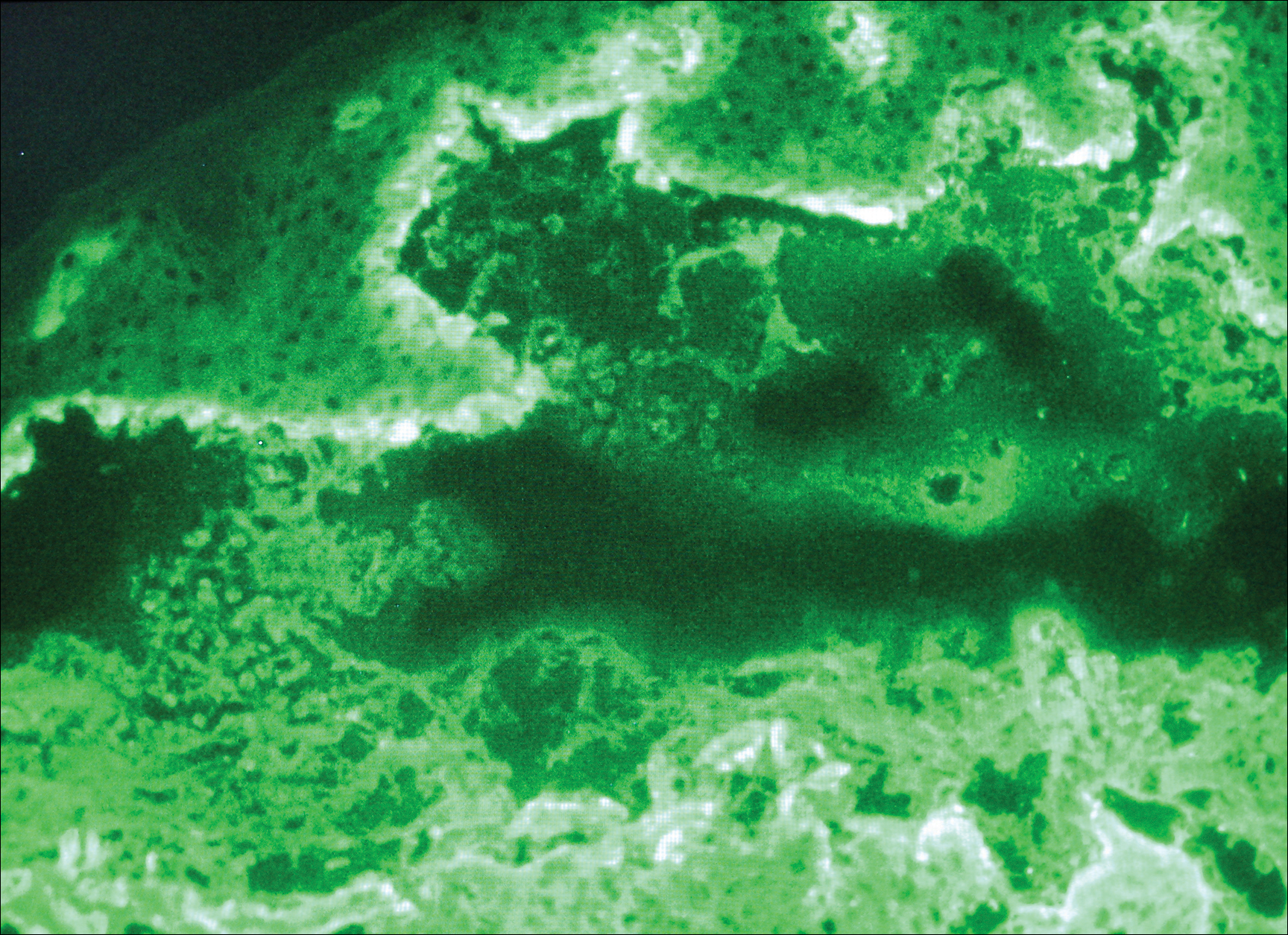
Histologic examination of a vesicle on the right arm revealed hyperkeratosis with hypergranulosis, vacuolar alteration of the basal layer with a paucicellular subepidermal vesicle, and melanophages in the superficial dermis consistent with vesicular LP (Figure 4). Histologic examination of an erythematous edematous plaque on the right upper leg revealed edema in the upper dermis with a perivascular and interstitial lymphocytic infiltrate with eosinophils. A third biopsy of a lichenoid flat-topped papule on the left arm revealed a mild bandlike infiltrate of lymphocytes and scattered eosinophils, eosinophilic colloid bodies and edema in the papillary dermis, and subepidermal vesicles and vacuolar alteration of the basal layer consistent with a vesicular lichenoid dermatitis (Figure 5). Direct immunofluorescence (DIF) of perilesional skin showed linear deposition of C3 and IgM along the basement membrane zone (BMZ) in addition to a shaggy pattern with cytoid bodies (Figure 6). There also was a faint linear deposit of IgA along the BMZ with cytoid bodies but negative for IgG. These results were interpreted as consistent with LP pemphigoides (LPP). Neither an enzyme-linked immunosorbent assay nor an immunoblot analysis was performed.
Because the patient was pregnant and had failed to respond to topical and intramuscular corticosteroids, she was started on intravenous methylprednisolone in the emergency department until new lesions stopped appearing. She was then discharged home on oral prednisone 50 mg (0.5 mg/kg/d), with close observation by her obstetrician. She also used clobetasol propionate ointment 0.05% for more severe lesions and triamcinolone acetonide cream 0.1% for less severe lesions until lesions resolved.
During treatment, the patient developed cellulitis on the leg that presented as pustules and erythema at a site of an eroded bulla, inframammary and axillary cutaneous candidiasis, and hyperglycemia at 19 weeks’ gestation. The cutaneous infections resolved with oral clindamycin 300 mg 3 times daily for 10 days. Topical mupirocin was used to treat the cellulitis and a mixture of zinc oxide, econazole cream, and desonide cream twice daily treated the candidiasis. Her obstetrician managed the hyperglycemia.
The bullous lesions and LP completely resolved after 2 months of treatment with oral prednisone 50 mg daily. The patient tolerated a corticosteroid taper (dose decreased by 5 mg every 2 weeks) until arriving at 10 mg, which was then decreased to 7.5 mg until delivery. A cesarean delivery was performed due to a large-for-gestational-age fetus, and an internist was consulted for the necessary precautions to increase the steroid dose during delivery due to the stress of the surgery and the risk for a hypothalamic crisis. There were no peripartum complications, and the baby was born without cutaneous lesions and remains healthy 1 year later. The patient remained disease free over 2 months postpartum, until new LP lesions developed without vesicles or bullae, which were then controlled with topical therapy. She was subsequently lost to follow-up.
Comment
Kaposi first described LPP in 1892 and used the term lichen ruber pemphigoides to describe a case of typical LP together with a widespread bullous eruption. Lichen planus pemphigoides is characterized by tense blisters that arise on lesions of LP as well as on skin unaffected by LP. In contrast, bullous LP blisters are confined to LP lesions only and occur from intense lichenoid inflammation and extensive liquefactive degeneration of basal keratinocytes. The vesicle formation in LPP is a result of autoantibodies to the bullous pemphigoid (BP) antigen BPAg2, which can be explained by the epitope spreading epiphenomenon whereby epidermotropic cytotoxic T cells damage the basal keratinocytes in LP by targeting unknown epidermal antigens, resulting in the exposure of BP180 and therefore instigating the autoimmune response.1 The process of epitope spreading takes months to develop; the mean duration of LP before LPP is 8 weeks in children and 12 weeks in adults,2 which is comparable to the current case.
Pathogenesis
Lichen planus pemphigoides usually is idiopathic; however, there have been cases reported in association with various medications including calcium channel blockers such as diltiazem, Chinese herbs,3 simvastatin,4 ramipril,5,6 captopril,7 psoralen plus UVA phototherapy,8 and cinnarizine.9 In addition, in a case-controlled study, the use of neuroleptics or diuretics was found to be a risk factor for LPP development.10
This case is unique because it shows an association of LPP with an intrauterine pregnancy. Despite the fact that we did not perform the required studies to determine the exact cause, there probably exists an association between LPP and the pregnancy, as the patient presented with a 5-month history of severe LP prior to vesicle formation. The patient only developed the vesicular lesions during pregnancy, which were later controlled with systemic steroids and then recurred postpartum only as LP lesions, suggesting that the patient’s pregnancy may have contributed in the pathogenesis as an inducing factor. We suspect that the LP was aggravated by the pregnancy and continued to worsen, so much as to cause epitope spreading and lead to the bullous eruption at the end of the first trimester.2
Differential Diagnosis
Initially, we suspected a diagnosis of pemphigoid gestationis (PG), previously known as herpes gestationis. The classic presentation of PG starts with an intense pruritus followed by the emergence of pruritic urticarial papules and plaques in the umbilical or periumbilical areas. The lesions may become targetlike or polycyclic and may spread to other areas of the trunk, arms, and legs, often including the palms and soles.11-15 Just as in our case, vesicles and bullous lesions appear at both the site of the urticarial plaques as well as on normal skin.16 The clinical features noted in our patient that were not typical of PG included the multiple lesions on the face and inside the nostrils. Only 20% of PG cases are associated with mucosal involvement,11,12,15 and there are no documented reports of PG occurring in a patient with LP, according to a PubMed search of articles indexed for MEDLINE using the search terms pemphigoid gestationis, herpes gestationis, and lichen planus.
Lichen planus pemphigoides can be easily differentiated from BP. Lichen planus pemphigoides occurs in younger patients, with a mean age of 35 years, unlike BP, which commonly affects elderly men.17 Lichen planus pemphigoides also is less severe and has a better response to treatment than BP. It also affects the palms and soles, which are rarely affected in BP. There are no reports in the literature of BP developing during pregnancy, according to a PubMed search using the terms bullous pemphigoid and pregnancy. However, LPP and BP share a common antibody, the BP180 antigen, and differences exist in the epitope where the antibody binds in each condition.18,19
Diagnosing LPP
In LPP, DIF typically shows linear deposits of IgG, IgM, IgA, fibrinogen, and C3 along the BMZ, of which IgG and C3 are most commonly seen.3 Our patient had linear deposition of C3, IgM, and IgA along the BMZ, which excluded bullous LP from the differential diagnosis. Bullous LP is not an autoimmune condition but rather is on the severe spectrum of LP where Max Joseph spaces become so large so as to lead to vesicle and bullae formation. In addition to the linear deposit at the BMZ, LPP typically reveals immunoglobulin (mainly IgM but also IgA), C3, and fibrinogen staining of colloid bodies in the papillary dermis on DIF; however, some cases of LPP only present with a linear deposition of C3 along the BMZ, which is why, similar to PG, these diagnoses by DIF are similar. Direct immunofluorescence of PG reveals linear IgG1 and IgG3 along the BMZ. IgG1 and IgG3 immunoglobulins are known to fix complement better than other immunoglobulins, thus linear C3 along the BMZ is the most consistently positive immunoreactant. Less common positive immunoreactivity with the same pattern has been seen with IgA, IgM, C1, and C4 (Table).14,15,18 The lack of linear IgG and the presence of IgM is more suggestive of LPP.
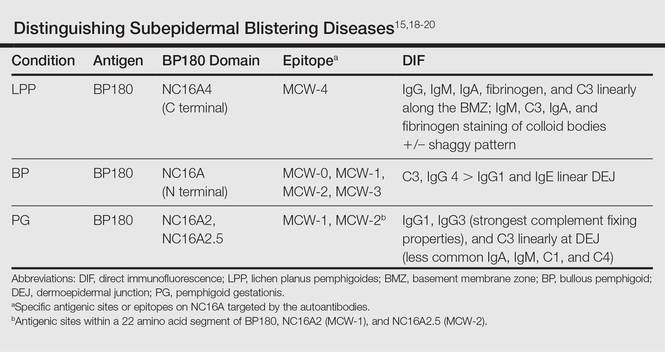
The differential diagnosis of the subepidermal autoimmune blistering diseases associated with antibodies against BP180, including BP, LPP, and PG, often is challenging.15 However, LPP can now be distinguished by immunological studies including immunoblot analysis of the immunodominant region of NC16A of the BP180 antigen and the immunoglobulin subclass that reacts to 180-, 200-,20 and 230-kDa antigens within the BMZ (Table).15,18-20 The Table summarizes the different autoantibodies, antigens, and epitopes to distinguish subepidermal autoimmune blistering diseases.
Despite not performing these studies in our patient, we concluded that the clinical, histological, and DIF findings of this case are more consistent with LPP than with the other subepidermal blistering diseases. However, we cannot exclude the possibility of the patient having a new entity with a unique antibody from epitope spreading.
Conclusion
We present a case of lichenoid papules and plaques consistent with LP, with the development of vesicles and bullae after the first trimester of pregnancy. The clinical, pathologic, and DIF findings were highly suggestive of LPP. Although the exact pathogenic mechanism is not fully known, we suspect that pregnancy may have contributed to the origin of the disease. Further evaluation of pregnant patients with lichenoid lesions who develop blisters are needed for the elucidation of the mechanism, which may be secondary to epitope spreading that led to new autoantibody formation.
- Stingl G, Holubar K. Coexistence of lichen planus and bullous pemphigoid. an immunopathological study. Br J Dermatol. 1975;93:313-320.
- Paige DG, Bhogal BS, Black MM, et al. Lichen planus pemphigoides in a child—immunopathological findings. Clin Exp Dermatol. 1993;18:552-554.
- Xu HH, Xiao T, He CD, et al. Lichen planus pemphigoides associated with Chinese herbs. Clin Exp Dermatol. 2009;34:329-332.
- Stoebner PE, Michot C, Ligeron C, et al. Simvastatin induced lichen planus pemphigoides. Ann Dermatol Venereol. 2003;130:187-190.
- Zhu YI, Fitzpatrick JE, Kornfeld BW. Lichen planus pemphigoides associated with Ramipril. Int J Dermatol. 2006;45:1453-1455.
- Ogg GS, Bhogal BS, Hashimoto T, et al. Ramipril-associated lichen planus pemphigoides. Br J Dermatol. 1997;136:412-414.
- Flageul B, Foldes C, Wallach D, et al. Captopril-induced lichen planus pemphigoides with pemphigus-like features. a case report. Dermatologica. 1986;173:248-255.
- Kuramoto N, Kishimoto S, Shibagaki R, et al. PUVA-induced lichen planus pemphigoides. Br J Dermatol. 2000;142:509-512.
- Miyagawa S, Ohi H, Muramatsu T, et al. Lichen planus pemphigoides-like lesions induced by Cinnarizine. Br J Dermatol. 1985;112:607-613.
- Bastuji-Garin S, Joly P, Picard-Dahan C, et al. Drugs associated with bullous pemphigoid. a case-control study. Arch Dermatol. 1996;132:272-276.
- Ambros-Rudolph CM. Dermatoses of pregnancy-clues to diagnosis, fetal risk and therapy. Ann Dermatol. 2011;23:265-275.
- DiZenzo G, Calabresi V, Grosso F, et al. The intracellular and extracellular domains of BP180 antigen comprise novel epitopes targeted by pemphigoid gestationis autoantibodies. J Invest Dermatol. 2006;127:864-873.
- Jenkis RE, Hern S, Black MM. Clinical features and management of 87 patients with pemphigus gestationis. Clin Exp Dermatol. 1999;24:255-259.
- Kasperkiewicz M, Zillikens D, Schmidt E. Pemphigoid diseases: pathogenesis, diagnosis, and treatment. Autoimmunity. 2012;45:55-70.
- Cobo MF, Santi CG, Maruta CW, et al. Pemphigoid gestationis: clinical and laboratory evaluation. Clinics. 2009;64:1042-1047.
- Hsu S, Ghohestani RF, Uitto J. Lichen planus pemphigoides with IgG autoantibodies to the 180 kd bullous pemphigoid antigen (type XVII collagen). J Am Acad Dermatol. 2000;42:136-141.
- Harjai B, Mendiratta V, Kakkar S, et al. Childhood lichen planus pemphigoides—a rare entity. J Eur Acad Dermatol Venereol. 2006;20:117-118.
- Zillikens D, Caux F, Mascaro JM, et al. Autoantibodies in lichen planus pemphigoides react with a novel epitope within the C-terminal NC16A domain of BP180. J Invest Dermatol. 1999;113:117-121.
- Zillikens D. BP180 as the common autoantigen in blistering diseases with different clinical phenotypes. Keio J Med. 2002;51:21-28.
- Davis AL, Bhogal BS, Whitehead P, et al. Lichen planus pemphigoides: its relationship to bullous pemphigoid. Br J Dermatol. 1991;125:263-271.
Case Report
A 25-year-old woman with a 5-month history of severe lichen planus (LP) on the arms, legs, and trunk presented to the emergency department with generalized blisters and erythema over the entire body, including the face and soles, of 2 days’ duration. She was evaluated for the LP 1 week prior in a referral dermatology clinic, and in addition to topical corticosteroids, she received 1 injection of 40 mg intramuscular triamcinolone acetonide. Hours following the injection she developed nausea, vomiting, and fever. The patient reported that her last menstrual period was 3 weeks prior to the current presentation.
Physical examination revealed numerous lichenified, flat-topped, pink-violaceous, hyperpigmented, scaly papules and plaques (Figure 1), as well as tense, yellow, fluid-filled vesicles and bullae of various sizes on the neck, arms (Figure 2), legs, trunk, and dorsal aspect of the feet. The vesicles occurred on both normal skin and the lichenified plaques with a negative Nikolsky sign. There also were urticarial erythematous papules and plaques on the arms, trunk, neck, and face, some of which had vesicles or a violaceous dusky central hue (Figure 3). Vesicles were noted within both nostrils (nasal mucosa), and there were extremely tender erythematous patches and thick sheets of scales on the soles.



An elevated β human chorionic gonadotropin level and transvaginal ultrasonography confirmed an intrauterine pregnancy of 12 weeks’ gestation despite the patient’s report of the last menstrual period.
Histologic examination of a vesicle on the right arm revealed hyperkeratosis with hypergranulosis, vacuolar alteration of the basal layer with a paucicellular subepidermal vesicle, and melanophages in the superficial dermis consistent with vesicular LP (Figure 4). Histologic examination of an erythematous edematous plaque on the right upper leg revealed edema in the upper dermis with a perivascular and interstitial lymphocytic infiltrate with eosinophils. A third biopsy of a lichenoid flat-topped papule on the left arm revealed a mild bandlike infiltrate of lymphocytes and scattered eosinophils, eosinophilic colloid bodies and edema in the papillary dermis, and subepidermal vesicles and vacuolar alteration of the basal layer consistent with a vesicular lichenoid dermatitis (Figure 5). Direct immunofluorescence (DIF) of perilesional skin showed linear deposition of C3 and IgM along the basement membrane zone (BMZ) in addition to a shaggy pattern with cytoid bodies (Figure 6). There also was a faint linear deposit of IgA along the BMZ with cytoid bodies but negative for IgG. These results were interpreted as consistent with LP pemphigoides (LPP). Neither an enzyme-linked immunosorbent assay nor an immunoblot analysis was performed.



Because the patient was pregnant and had failed to respond to topical and intramuscular corticosteroids, she was started on intravenous methylprednisolone in the emergency department until new lesions stopped appearing. She was then discharged home on oral prednisone 50 mg (0.5 mg/kg/d), with close observation by her obstetrician. She also used clobetasol propionate ointment 0.05% for more severe lesions and triamcinolone acetonide cream 0.1% for less severe lesions until lesions resolved.
During treatment, the patient developed cellulitis on the leg that presented as pustules and erythema at a site of an eroded bulla, inframammary and axillary cutaneous candidiasis, and hyperglycemia at 19 weeks’ gestation. The cutaneous infections resolved with oral clindamycin 300 mg 3 times daily for 10 days. Topical mupirocin was used to treat the cellulitis and a mixture of zinc oxide, econazole cream, and desonide cream twice daily treated the candidiasis. Her obstetrician managed the hyperglycemia.
The bullous lesions and LP completely resolved after 2 months of treatment with oral prednisone 50 mg daily. The patient tolerated a corticosteroid taper (dose decreased by 5 mg every 2 weeks) until arriving at 10 mg, which was then decreased to 7.5 mg until delivery. A cesarean delivery was performed due to a large-for-gestational-age fetus, and an internist was consulted for the necessary precautions to increase the steroid dose during delivery due to the stress of the surgery and the risk for a hypothalamic crisis. There were no peripartum complications, and the baby was born without cutaneous lesions and remains healthy 1 year later. The patient remained disease free over 2 months postpartum, until new LP lesions developed without vesicles or bullae, which were then controlled with topical therapy. She was subsequently lost to follow-up.
Comment
Kaposi first described LPP in 1892 and used the term lichen ruber pemphigoides to describe a case of typical LP together with a widespread bullous eruption. Lichen planus pemphigoides is characterized by tense blisters that arise on lesions of LP as well as on skin unaffected by LP. In contrast, bullous LP blisters are confined to LP lesions only and occur from intense lichenoid inflammation and extensive liquefactive degeneration of basal keratinocytes. The vesicle formation in LPP is a result of autoantibodies to the bullous pemphigoid (BP) antigen BPAg2, which can be explained by the epitope spreading epiphenomenon whereby epidermotropic cytotoxic T cells damage the basal keratinocytes in LP by targeting unknown epidermal antigens, resulting in the exposure of BP180 and therefore instigating the autoimmune response.1 The process of epitope spreading takes months to develop; the mean duration of LP before LPP is 8 weeks in children and 12 weeks in adults,2 which is comparable to the current case.
Pathogenesis
Lichen planus pemphigoides usually is idiopathic; however, there have been cases reported in association with various medications including calcium channel blockers such as diltiazem, Chinese herbs,3 simvastatin,4 ramipril,5,6 captopril,7 psoralen plus UVA phototherapy,8 and cinnarizine.9 In addition, in a case-controlled study, the use of neuroleptics or diuretics was found to be a risk factor for LPP development.10
This case is unique because it shows an association of LPP with an intrauterine pregnancy. Despite the fact that we did not perform the required studies to determine the exact cause, there probably exists an association between LPP and the pregnancy, as the patient presented with a 5-month history of severe LP prior to vesicle formation. The patient only developed the vesicular lesions during pregnancy, which were later controlled with systemic steroids and then recurred postpartum only as LP lesions, suggesting that the patient’s pregnancy may have contributed in the pathogenesis as an inducing factor. We suspect that the LP was aggravated by the pregnancy and continued to worsen, so much as to cause epitope spreading and lead to the bullous eruption at the end of the first trimester.2
Differential Diagnosis
Initially, we suspected a diagnosis of pemphigoid gestationis (PG), previously known as herpes gestationis. The classic presentation of PG starts with an intense pruritus followed by the emergence of pruritic urticarial papules and plaques in the umbilical or periumbilical areas. The lesions may become targetlike or polycyclic and may spread to other areas of the trunk, arms, and legs, often including the palms and soles.11-15 Just as in our case, vesicles and bullous lesions appear at both the site of the urticarial plaques as well as on normal skin.16 The clinical features noted in our patient that were not typical of PG included the multiple lesions on the face and inside the nostrils. Only 20% of PG cases are associated with mucosal involvement,11,12,15 and there are no documented reports of PG occurring in a patient with LP, according to a PubMed search of articles indexed for MEDLINE using the search terms pemphigoid gestationis, herpes gestationis, and lichen planus.
Lichen planus pemphigoides can be easily differentiated from BP. Lichen planus pemphigoides occurs in younger patients, with a mean age of 35 years, unlike BP, which commonly affects elderly men.17 Lichen planus pemphigoides also is less severe and has a better response to treatment than BP. It also affects the palms and soles, which are rarely affected in BP. There are no reports in the literature of BP developing during pregnancy, according to a PubMed search using the terms bullous pemphigoid and pregnancy. However, LPP and BP share a common antibody, the BP180 antigen, and differences exist in the epitope where the antibody binds in each condition.18,19
Diagnosing LPP
In LPP, DIF typically shows linear deposits of IgG, IgM, IgA, fibrinogen, and C3 along the BMZ, of which IgG and C3 are most commonly seen.3 Our patient had linear deposition of C3, IgM, and IgA along the BMZ, which excluded bullous LP from the differential diagnosis. Bullous LP is not an autoimmune condition but rather is on the severe spectrum of LP where Max Joseph spaces become so large so as to lead to vesicle and bullae formation. In addition to the linear deposit at the BMZ, LPP typically reveals immunoglobulin (mainly IgM but also IgA), C3, and fibrinogen staining of colloid bodies in the papillary dermis on DIF; however, some cases of LPP only present with a linear deposition of C3 along the BMZ, which is why, similar to PG, these diagnoses by DIF are similar. Direct immunofluorescence of PG reveals linear IgG1 and IgG3 along the BMZ. IgG1 and IgG3 immunoglobulins are known to fix complement better than other immunoglobulins, thus linear C3 along the BMZ is the most consistently positive immunoreactant. Less common positive immunoreactivity with the same pattern has been seen with IgA, IgM, C1, and C4 (Table).14,15,18 The lack of linear IgG and the presence of IgM is more suggestive of LPP.

The differential diagnosis of the subepidermal autoimmune blistering diseases associated with antibodies against BP180, including BP, LPP, and PG, often is challenging.15 However, LPP can now be distinguished by immunological studies including immunoblot analysis of the immunodominant region of NC16A of the BP180 antigen and the immunoglobulin subclass that reacts to 180-, 200-,20 and 230-kDa antigens within the BMZ (Table).15,18-20 The Table summarizes the different autoantibodies, antigens, and epitopes to distinguish subepidermal autoimmune blistering diseases.
Despite not performing these studies in our patient, we concluded that the clinical, histological, and DIF findings of this case are more consistent with LPP than with the other subepidermal blistering diseases. However, we cannot exclude the possibility of the patient having a new entity with a unique antibody from epitope spreading.
Conclusion
We present a case of lichenoid papules and plaques consistent with LP, with the development of vesicles and bullae after the first trimester of pregnancy. The clinical, pathologic, and DIF findings were highly suggestive of LPP. Although the exact pathogenic mechanism is not fully known, we suspect that pregnancy may have contributed to the origin of the disease. Further evaluation of pregnant patients with lichenoid lesions who develop blisters are needed for the elucidation of the mechanism, which may be secondary to epitope spreading that led to new autoantibody formation.
Case Report
A 25-year-old woman with a 5-month history of severe lichen planus (LP) on the arms, legs, and trunk presented to the emergency department with generalized blisters and erythema over the entire body, including the face and soles, of 2 days’ duration. She was evaluated for the LP 1 week prior in a referral dermatology clinic, and in addition to topical corticosteroids, she received 1 injection of 40 mg intramuscular triamcinolone acetonide. Hours following the injection she developed nausea, vomiting, and fever. The patient reported that her last menstrual period was 3 weeks prior to the current presentation.
Physical examination revealed numerous lichenified, flat-topped, pink-violaceous, hyperpigmented, scaly papules and plaques (Figure 1), as well as tense, yellow, fluid-filled vesicles and bullae of various sizes on the neck, arms (Figure 2), legs, trunk, and dorsal aspect of the feet. The vesicles occurred on both normal skin and the lichenified plaques with a negative Nikolsky sign. There also were urticarial erythematous papules and plaques on the arms, trunk, neck, and face, some of which had vesicles or a violaceous dusky central hue (Figure 3). Vesicles were noted within both nostrils (nasal mucosa), and there were extremely tender erythematous patches and thick sheets of scales on the soles.



An elevated β human chorionic gonadotropin level and transvaginal ultrasonography confirmed an intrauterine pregnancy of 12 weeks’ gestation despite the patient’s report of the last menstrual period.
Histologic examination of a vesicle on the right arm revealed hyperkeratosis with hypergranulosis, vacuolar alteration of the basal layer with a paucicellular subepidermal vesicle, and melanophages in the superficial dermis consistent with vesicular LP (Figure 4). Histologic examination of an erythematous edematous plaque on the right upper leg revealed edema in the upper dermis with a perivascular and interstitial lymphocytic infiltrate with eosinophils. A third biopsy of a lichenoid flat-topped papule on the left arm revealed a mild bandlike infiltrate of lymphocytes and scattered eosinophils, eosinophilic colloid bodies and edema in the papillary dermis, and subepidermal vesicles and vacuolar alteration of the basal layer consistent with a vesicular lichenoid dermatitis (Figure 5). Direct immunofluorescence (DIF) of perilesional skin showed linear deposition of C3 and IgM along the basement membrane zone (BMZ) in addition to a shaggy pattern with cytoid bodies (Figure 6). There also was a faint linear deposit of IgA along the BMZ with cytoid bodies but negative for IgG. These results were interpreted as consistent with LP pemphigoides (LPP). Neither an enzyme-linked immunosorbent assay nor an immunoblot analysis was performed.



Because the patient was pregnant and had failed to respond to topical and intramuscular corticosteroids, she was started on intravenous methylprednisolone in the emergency department until new lesions stopped appearing. She was then discharged home on oral prednisone 50 mg (0.5 mg/kg/d), with close observation by her obstetrician. She also used clobetasol propionate ointment 0.05% for more severe lesions and triamcinolone acetonide cream 0.1% for less severe lesions until lesions resolved.
During treatment, the patient developed cellulitis on the leg that presented as pustules and erythema at a site of an eroded bulla, inframammary and axillary cutaneous candidiasis, and hyperglycemia at 19 weeks’ gestation. The cutaneous infections resolved with oral clindamycin 300 mg 3 times daily for 10 days. Topical mupirocin was used to treat the cellulitis and a mixture of zinc oxide, econazole cream, and desonide cream twice daily treated the candidiasis. Her obstetrician managed the hyperglycemia.
The bullous lesions and LP completely resolved after 2 months of treatment with oral prednisone 50 mg daily. The patient tolerated a corticosteroid taper (dose decreased by 5 mg every 2 weeks) until arriving at 10 mg, which was then decreased to 7.5 mg until delivery. A cesarean delivery was performed due to a large-for-gestational-age fetus, and an internist was consulted for the necessary precautions to increase the steroid dose during delivery due to the stress of the surgery and the risk for a hypothalamic crisis. There were no peripartum complications, and the baby was born without cutaneous lesions and remains healthy 1 year later. The patient remained disease free over 2 months postpartum, until new LP lesions developed without vesicles or bullae, which were then controlled with topical therapy. She was subsequently lost to follow-up.
Comment
Kaposi first described LPP in 1892 and used the term lichen ruber pemphigoides to describe a case of typical LP together with a widespread bullous eruption. Lichen planus pemphigoides is characterized by tense blisters that arise on lesions of LP as well as on skin unaffected by LP. In contrast, bullous LP blisters are confined to LP lesions only and occur from intense lichenoid inflammation and extensive liquefactive degeneration of basal keratinocytes. The vesicle formation in LPP is a result of autoantibodies to the bullous pemphigoid (BP) antigen BPAg2, which can be explained by the epitope spreading epiphenomenon whereby epidermotropic cytotoxic T cells damage the basal keratinocytes in LP by targeting unknown epidermal antigens, resulting in the exposure of BP180 and therefore instigating the autoimmune response.1 The process of epitope spreading takes months to develop; the mean duration of LP before LPP is 8 weeks in children and 12 weeks in adults,2 which is comparable to the current case.
Pathogenesis
Lichen planus pemphigoides usually is idiopathic; however, there have been cases reported in association with various medications including calcium channel blockers such as diltiazem, Chinese herbs,3 simvastatin,4 ramipril,5,6 captopril,7 psoralen plus UVA phototherapy,8 and cinnarizine.9 In addition, in a case-controlled study, the use of neuroleptics or diuretics was found to be a risk factor for LPP development.10
This case is unique because it shows an association of LPP with an intrauterine pregnancy. Despite the fact that we did not perform the required studies to determine the exact cause, there probably exists an association between LPP and the pregnancy, as the patient presented with a 5-month history of severe LP prior to vesicle formation. The patient only developed the vesicular lesions during pregnancy, which were later controlled with systemic steroids and then recurred postpartum only as LP lesions, suggesting that the patient’s pregnancy may have contributed in the pathogenesis as an inducing factor. We suspect that the LP was aggravated by the pregnancy and continued to worsen, so much as to cause epitope spreading and lead to the bullous eruption at the end of the first trimester.2
Differential Diagnosis
Initially, we suspected a diagnosis of pemphigoid gestationis (PG), previously known as herpes gestationis. The classic presentation of PG starts with an intense pruritus followed by the emergence of pruritic urticarial papules and plaques in the umbilical or periumbilical areas. The lesions may become targetlike or polycyclic and may spread to other areas of the trunk, arms, and legs, often including the palms and soles.11-15 Just as in our case, vesicles and bullous lesions appear at both the site of the urticarial plaques as well as on normal skin.16 The clinical features noted in our patient that were not typical of PG included the multiple lesions on the face and inside the nostrils. Only 20% of PG cases are associated with mucosal involvement,11,12,15 and there are no documented reports of PG occurring in a patient with LP, according to a PubMed search of articles indexed for MEDLINE using the search terms pemphigoid gestationis, herpes gestationis, and lichen planus.
Lichen planus pemphigoides can be easily differentiated from BP. Lichen planus pemphigoides occurs in younger patients, with a mean age of 35 years, unlike BP, which commonly affects elderly men.17 Lichen planus pemphigoides also is less severe and has a better response to treatment than BP. It also affects the palms and soles, which are rarely affected in BP. There are no reports in the literature of BP developing during pregnancy, according to a PubMed search using the terms bullous pemphigoid and pregnancy. However, LPP and BP share a common antibody, the BP180 antigen, and differences exist in the epitope where the antibody binds in each condition.18,19
Diagnosing LPP
In LPP, DIF typically shows linear deposits of IgG, IgM, IgA, fibrinogen, and C3 along the BMZ, of which IgG and C3 are most commonly seen.3 Our patient had linear deposition of C3, IgM, and IgA along the BMZ, which excluded bullous LP from the differential diagnosis. Bullous LP is not an autoimmune condition but rather is on the severe spectrum of LP where Max Joseph spaces become so large so as to lead to vesicle and bullae formation. In addition to the linear deposit at the BMZ, LPP typically reveals immunoglobulin (mainly IgM but also IgA), C3, and fibrinogen staining of colloid bodies in the papillary dermis on DIF; however, some cases of LPP only present with a linear deposition of C3 along the BMZ, which is why, similar to PG, these diagnoses by DIF are similar. Direct immunofluorescence of PG reveals linear IgG1 and IgG3 along the BMZ. IgG1 and IgG3 immunoglobulins are known to fix complement better than other immunoglobulins, thus linear C3 along the BMZ is the most consistently positive immunoreactant. Less common positive immunoreactivity with the same pattern has been seen with IgA, IgM, C1, and C4 (Table).14,15,18 The lack of linear IgG and the presence of IgM is more suggestive of LPP.

The differential diagnosis of the subepidermal autoimmune blistering diseases associated with antibodies against BP180, including BP, LPP, and PG, often is challenging.15 However, LPP can now be distinguished by immunological studies including immunoblot analysis of the immunodominant region of NC16A of the BP180 antigen and the immunoglobulin subclass that reacts to 180-, 200-,20 and 230-kDa antigens within the BMZ (Table).15,18-20 The Table summarizes the different autoantibodies, antigens, and epitopes to distinguish subepidermal autoimmune blistering diseases.
Despite not performing these studies in our patient, we concluded that the clinical, histological, and DIF findings of this case are more consistent with LPP than with the other subepidermal blistering diseases. However, we cannot exclude the possibility of the patient having a new entity with a unique antibody from epitope spreading.
Conclusion
We present a case of lichenoid papules and plaques consistent with LP, with the development of vesicles and bullae after the first trimester of pregnancy. The clinical, pathologic, and DIF findings were highly suggestive of LPP. Although the exact pathogenic mechanism is not fully known, we suspect that pregnancy may have contributed to the origin of the disease. Further evaluation of pregnant patients with lichenoid lesions who develop blisters are needed for the elucidation of the mechanism, which may be secondary to epitope spreading that led to new autoantibody formation.
- Stingl G, Holubar K. Coexistence of lichen planus and bullous pemphigoid. an immunopathological study. Br J Dermatol. 1975;93:313-320.
- Paige DG, Bhogal BS, Black MM, et al. Lichen planus pemphigoides in a child—immunopathological findings. Clin Exp Dermatol. 1993;18:552-554.
- Xu HH, Xiao T, He CD, et al. Lichen planus pemphigoides associated with Chinese herbs. Clin Exp Dermatol. 2009;34:329-332.
- Stoebner PE, Michot C, Ligeron C, et al. Simvastatin induced lichen planus pemphigoides. Ann Dermatol Venereol. 2003;130:187-190.
- Zhu YI, Fitzpatrick JE, Kornfeld BW. Lichen planus pemphigoides associated with Ramipril. Int J Dermatol. 2006;45:1453-1455.
- Ogg GS, Bhogal BS, Hashimoto T, et al. Ramipril-associated lichen planus pemphigoides. Br J Dermatol. 1997;136:412-414.
- Flageul B, Foldes C, Wallach D, et al. Captopril-induced lichen planus pemphigoides with pemphigus-like features. a case report. Dermatologica. 1986;173:248-255.
- Kuramoto N, Kishimoto S, Shibagaki R, et al. PUVA-induced lichen planus pemphigoides. Br J Dermatol. 2000;142:509-512.
- Miyagawa S, Ohi H, Muramatsu T, et al. Lichen planus pemphigoides-like lesions induced by Cinnarizine. Br J Dermatol. 1985;112:607-613.
- Bastuji-Garin S, Joly P, Picard-Dahan C, et al. Drugs associated with bullous pemphigoid. a case-control study. Arch Dermatol. 1996;132:272-276.
- Ambros-Rudolph CM. Dermatoses of pregnancy-clues to diagnosis, fetal risk and therapy. Ann Dermatol. 2011;23:265-275.
- DiZenzo G, Calabresi V, Grosso F, et al. The intracellular and extracellular domains of BP180 antigen comprise novel epitopes targeted by pemphigoid gestationis autoantibodies. J Invest Dermatol. 2006;127:864-873.
- Jenkis RE, Hern S, Black MM. Clinical features and management of 87 patients with pemphigus gestationis. Clin Exp Dermatol. 1999;24:255-259.
- Kasperkiewicz M, Zillikens D, Schmidt E. Pemphigoid diseases: pathogenesis, diagnosis, and treatment. Autoimmunity. 2012;45:55-70.
- Cobo MF, Santi CG, Maruta CW, et al. Pemphigoid gestationis: clinical and laboratory evaluation. Clinics. 2009;64:1042-1047.
- Hsu S, Ghohestani RF, Uitto J. Lichen planus pemphigoides with IgG autoantibodies to the 180 kd bullous pemphigoid antigen (type XVII collagen). J Am Acad Dermatol. 2000;42:136-141.
- Harjai B, Mendiratta V, Kakkar S, et al. Childhood lichen planus pemphigoides—a rare entity. J Eur Acad Dermatol Venereol. 2006;20:117-118.
- Zillikens D, Caux F, Mascaro JM, et al. Autoantibodies in lichen planus pemphigoides react with a novel epitope within the C-terminal NC16A domain of BP180. J Invest Dermatol. 1999;113:117-121.
- Zillikens D. BP180 as the common autoantigen in blistering diseases with different clinical phenotypes. Keio J Med. 2002;51:21-28.
- Davis AL, Bhogal BS, Whitehead P, et al. Lichen planus pemphigoides: its relationship to bullous pemphigoid. Br J Dermatol. 1991;125:263-271.
- Stingl G, Holubar K. Coexistence of lichen planus and bullous pemphigoid. an immunopathological study. Br J Dermatol. 1975;93:313-320.
- Paige DG, Bhogal BS, Black MM, et al. Lichen planus pemphigoides in a child—immunopathological findings. Clin Exp Dermatol. 1993;18:552-554.
- Xu HH, Xiao T, He CD, et al. Lichen planus pemphigoides associated with Chinese herbs. Clin Exp Dermatol. 2009;34:329-332.
- Stoebner PE, Michot C, Ligeron C, et al. Simvastatin induced lichen planus pemphigoides. Ann Dermatol Venereol. 2003;130:187-190.
- Zhu YI, Fitzpatrick JE, Kornfeld BW. Lichen planus pemphigoides associated with Ramipril. Int J Dermatol. 2006;45:1453-1455.
- Ogg GS, Bhogal BS, Hashimoto T, et al. Ramipril-associated lichen planus pemphigoides. Br J Dermatol. 1997;136:412-414.
- Flageul B, Foldes C, Wallach D, et al. Captopril-induced lichen planus pemphigoides with pemphigus-like features. a case report. Dermatologica. 1986;173:248-255.
- Kuramoto N, Kishimoto S, Shibagaki R, et al. PUVA-induced lichen planus pemphigoides. Br J Dermatol. 2000;142:509-512.
- Miyagawa S, Ohi H, Muramatsu T, et al. Lichen planus pemphigoides-like lesions induced by Cinnarizine. Br J Dermatol. 1985;112:607-613.
- Bastuji-Garin S, Joly P, Picard-Dahan C, et al. Drugs associated with bullous pemphigoid. a case-control study. Arch Dermatol. 1996;132:272-276.
- Ambros-Rudolph CM. Dermatoses of pregnancy-clues to diagnosis, fetal risk and therapy. Ann Dermatol. 2011;23:265-275.
- DiZenzo G, Calabresi V, Grosso F, et al. The intracellular and extracellular domains of BP180 antigen comprise novel epitopes targeted by pemphigoid gestationis autoantibodies. J Invest Dermatol. 2006;127:864-873.
- Jenkis RE, Hern S, Black MM. Clinical features and management of 87 patients with pemphigus gestationis. Clin Exp Dermatol. 1999;24:255-259.
- Kasperkiewicz M, Zillikens D, Schmidt E. Pemphigoid diseases: pathogenesis, diagnosis, and treatment. Autoimmunity. 2012;45:55-70.
- Cobo MF, Santi CG, Maruta CW, et al. Pemphigoid gestationis: clinical and laboratory evaluation. Clinics. 2009;64:1042-1047.
- Hsu S, Ghohestani RF, Uitto J. Lichen planus pemphigoides with IgG autoantibodies to the 180 kd bullous pemphigoid antigen (type XVII collagen). J Am Acad Dermatol. 2000;42:136-141.
- Harjai B, Mendiratta V, Kakkar S, et al. Childhood lichen planus pemphigoides—a rare entity. J Eur Acad Dermatol Venereol. 2006;20:117-118.
- Zillikens D, Caux F, Mascaro JM, et al. Autoantibodies in lichen planus pemphigoides react with a novel epitope within the C-terminal NC16A domain of BP180. J Invest Dermatol. 1999;113:117-121.
- Zillikens D. BP180 as the common autoantigen in blistering diseases with different clinical phenotypes. Keio J Med. 2002;51:21-28.
- Davis AL, Bhogal BS, Whitehead P, et al. Lichen planus pemphigoides: its relationship to bullous pemphigoid. Br J Dermatol. 1991;125:263-271.
Practice Points
- Lichen planus pemphigoides (LPP) is characterized by tense blisters that arise not only on lichen planus lesions such as bullous lichen planus but also on skin unaffected by lichen planus.
- In LPP, the autoantibodies specifically target the MCW-4 epitope of the NC16A4 domain of the bullous pemphigoid antigen BPAg2, distinguishing it from other autoimmune blistering diseases against the NC16A domain.