User login
Lesions of porokeratosis are thought to arise from disordered keratinization, though the exact pathogenesis remains uncertain. At least 5 clinical subtypes of porokeratosis have been identified: porokeratosis of Mibelli, disseminated superficial porokeratosis and disseminated superficial actinic porokeratosis (DSAP), linear porokeratosis, punctuate porokeratosis, and porokeratosis palmaris et plantaris disseminata (PPPD).1,2 Linear porokeratosis is a rare subtype with a clinical differential diagnosis that includes lichen striatus, linear lichen planus, linear verrucous epidermal nevus, segmental Darier disease, and incontinentia pigmenti.3 Definitive diagnosis of linear porokeratosis is made by histopathologic examination demonstrating a cornoid lamella, defined as a column of parakeratotic cells that lies at 45°to the surface of the epidermis and contains pyknotic basophilic nuclei.4 Patients with linear porokeratosis typically develop lesions along the lines of Blaschko in infancy or childhood.5,6 Among the different subtypes of porokeratosis, linear porokeratosis demonstrates the highest rate of malignant transformation, therefore requiring close clinical observation.7
Case Report
An 83-year-old woman presented to the outpatient clinic with a large linear plaque on the right leg that had been present since birth. Ten years prior to presentation, a portion of the lesion started to bleed; biopsy of the area was performed by an outside provider demonstrating squamous cell carcinoma (SCC), which was treated with wide local excision. One year prior to presentation, a separate portion of the plaque was biopsied by an outside provider and another diagnosis of SCC was made.
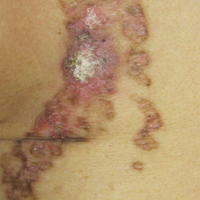
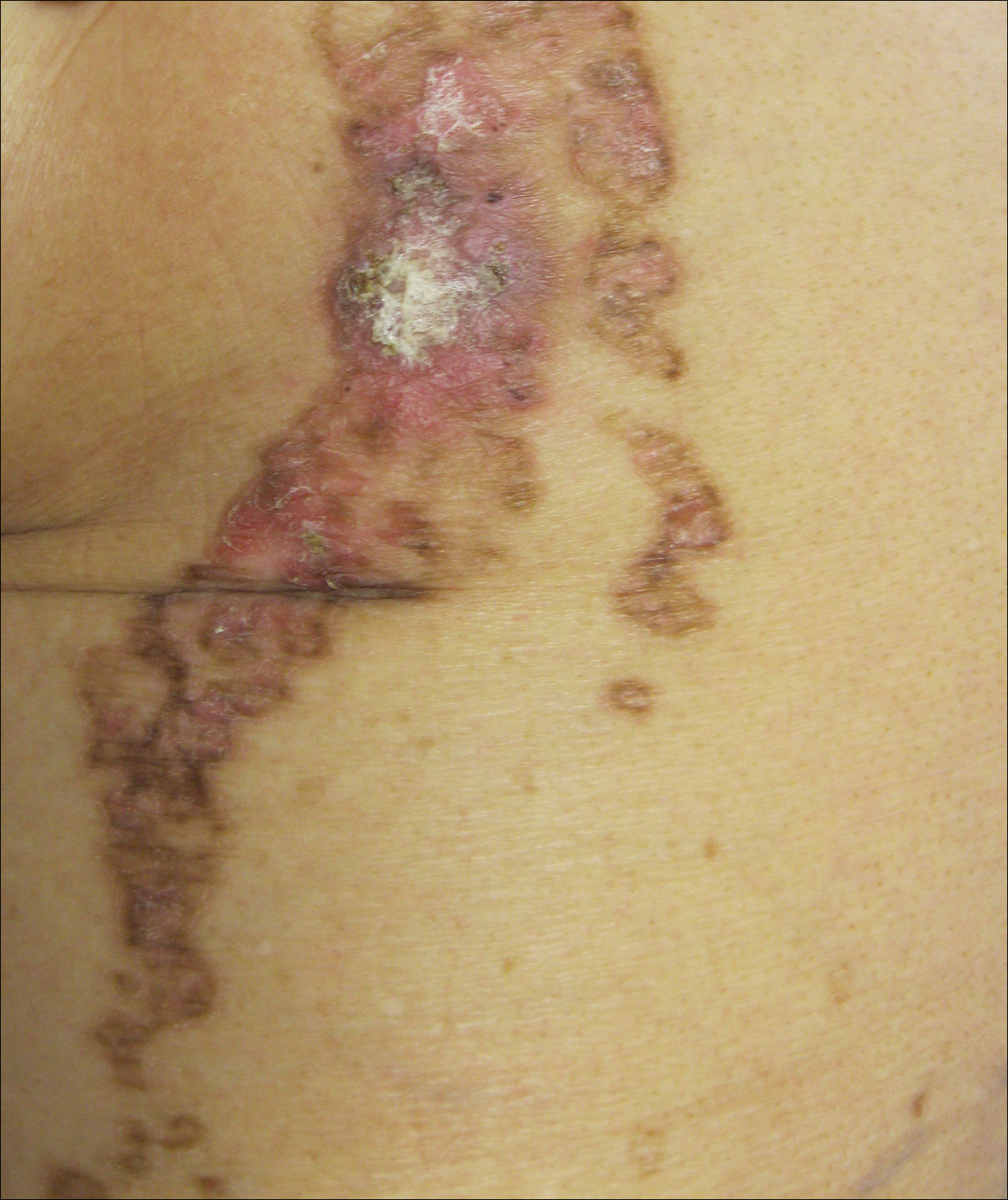
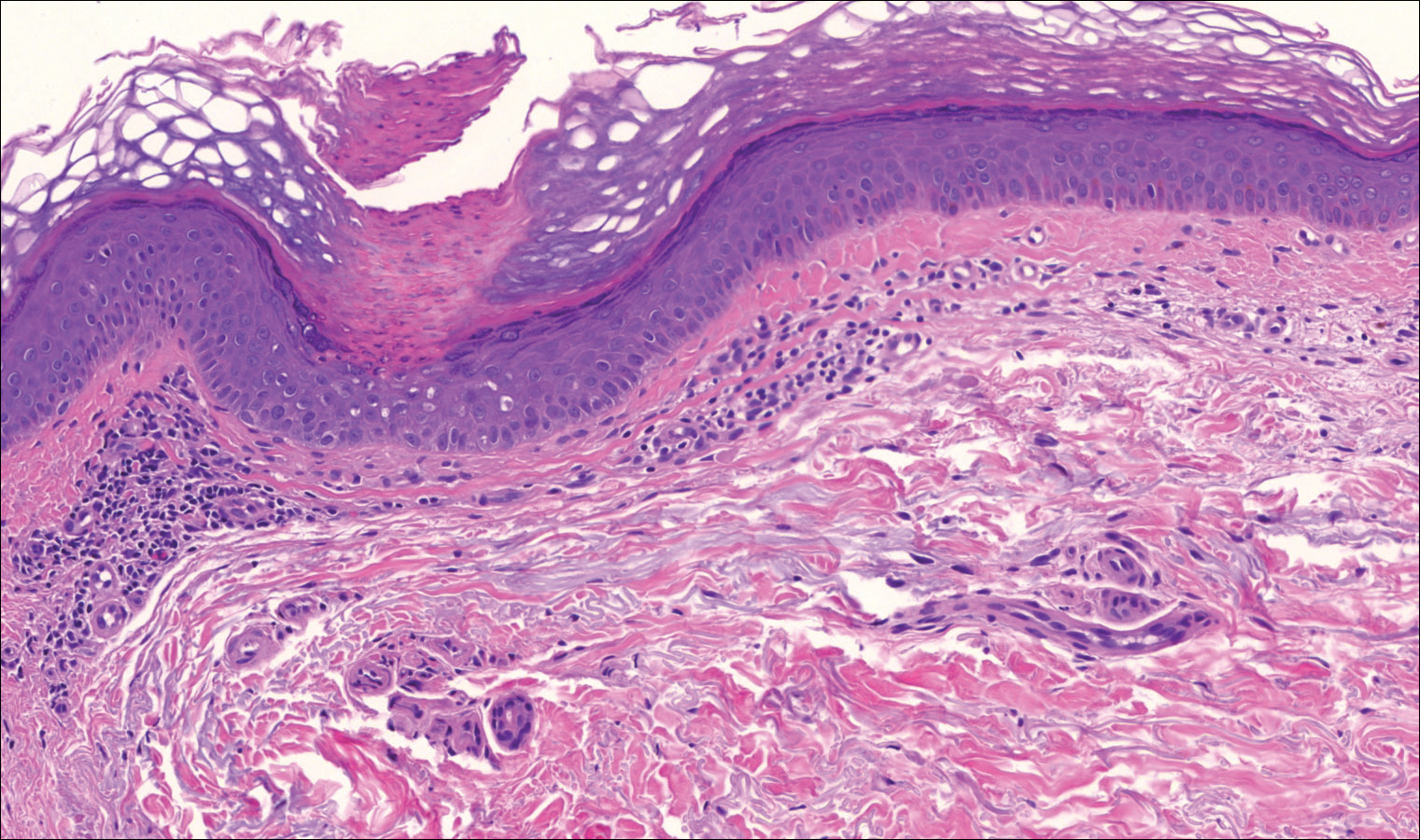
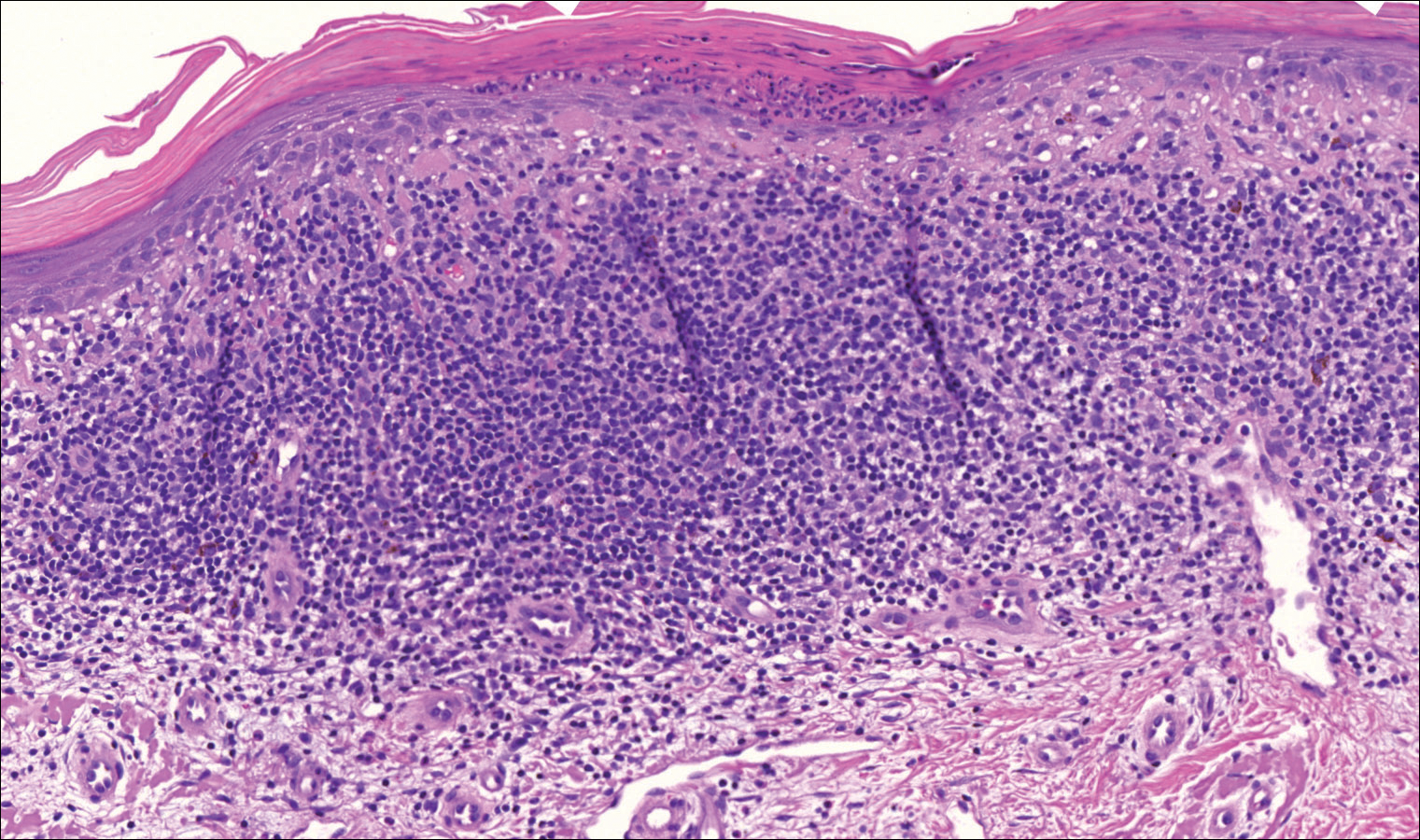
On examination performed during the initial presentation to our clinic, there was a well-demarcated tan to violaceous linear plaque present at the lower buttock and extending along the posterior leg to the skin overlying the Achilles tendon and dorsal aspect of the right foot. Within the plaque, there were areas of atrophy and areas of inflammation, induration, and hyperkeratosis (Figures 1 and 2). Two punch biopsies were performed: one from the edge of the plaque and one from a hyperkeratotic region within the plaque. Histology from the edge of the plaque demonstrated a cornoid lamella, consistent with a porokeratosis (Figure 3), whereas the histology from the hyperkeratotic region demonstrated a lichenoid infiltrate (Figure 4).

Several treatment options directed at the entire lesion were offered to the patient, but she declined these therapies and opted to address only those areas with clinical features of SCC, such as hyperkeratosis, bleeding, and rapid growth. Although biopsies performed by an outside provider were consistent with SCC, it had not been detected on biopsy performed during her initial visit to our clinic.
The patient was educated on the risk associated with her condition and instructed to follow up every 6 months to monitor for the development of SCC.
Comment
Porokeratosis is a disorder of keratinization with at least 5 clinical subtypes that share histologic similarities: porokeratosis of Mibelli, disseminated superficial porokeratosis and DSAP, linear porokeratosis, punctate porokeratosis, and PPPD.1,2 Other less common variants of porokeratosis include porokeratosis ptychotropica (a verrucous variant confined to the perianal area) and congenital unilateral linear porokeratosis.8,9
Linear porokeratosis appears in infancy or childhood with plaques that follow the lines of Blaschko.5,6 Most commonly, it presents unilaterally with annular plaques and linear hyperkeratotic papules that preferentially affect the extremities, though it also may present in a more generalized form or appear in a zosteriform pattern.10,11 Linear porokeratosis affects fewer than 20,000 individuals in the United States and accounts for fewer than 13% of all porokeratosis cases.12,13
Despite its relatively low prevalence, early identification of linear porokeratosis is important due to its high oncogenic potential, with malignant transformation to basal cell carcinoma or, more commonly, SCC reported in 19% of reported cases.1,5,7,14 The malignant transformation rate of linear porokeratosis is reported to be higher than rates seen in other porokeratosis subtypes (9.5%, 7.6%, and 3.4% for PPPD, porokeratosis of Mibelli, and DSAP, respectively).7 The risk of malignant transformation from porokeratosis increases with exposure to ionizing radiation, duration of the lesion, larger or coalescing lesions, and advanced age.7,15,16 Histologic studies have provided support for correlation between lesion size and oncogenic potential, with greater numbers of mitotic cells and more abnormal DNA ploidy seen in larger lesions.17
Histopathology
All subtypes of porokeratosis share certain histopathologic features that aid in the diagnosis of the disorder.18 Identification of the clinically observed hyperkeratotic ridged border or cornoid lamella is the primary means of definitively diagnosing porokeratosis; however, cornoid lamellae may be observed in other conditions, including verruca vulgaris and actinic keratosis.4,14
The cornoid lamella appears as a skewed column of densely packed parakeratotic cells with pyknotic basophilic nuclei extending through the stratum corneum from an epidermal invagination.4 Directly beneath the cornoid lamella, the granular layer is markedly diminished or absent, and cells of the stratum spinosum may demonstrate vacuolar changes or dyskeratosis.4,19 The superficial layer of the cornoid lamella may appear to be more centrifugally located and the cornoid lamella may be seen in several locations throughout the lesion.2,20 The degree of epidermal invagination, which is present under the cornoid lamella, varies by porokeratosis subtype; the central portion of the lesion may contain epidermis that ranges from hyperplastic to atrophic.2 Shumack et al21 noted that histologic changes under the cornoid lamella may include a lichenoid tissue reaction, papillary dermal lymphocytic infiltrate, vacuolar changes, dyskeratosis, and liquefaction degeneration of the basal layer. Because many of these histologic features also can be identified in lichen planus, a biopsy of the edge of lesions of porokeratosis is essential for making the correct diagnosis.
Heritability
Although linear porokeratosis has no identified pattern of inheritance and appears sporadic in onset, reports have described concomitant occurrence of linear porokeratosis and DSAP as well as linear porokeratosis arising in children of parents who have a diagnosis of DSAP.5,18,22,23 Based on these findings, it has been hypothesized that linear porokeratosis may represent a mosaic or segmental form of autosomal-dominant inherited subtypes of porokeratosis, such as DSAP.5 According to this hypothesis, loss of heterozygosity in patients with a DSAP mutation during early embryogenesis leads to proliferation of cells that are homozygous or hemizygous for the underlying mutation along lines of Blaschko.24 It has been suggested that the allelic loss implicated in the development of linear porokeratosis is the first step in a multistage process of carcinogenesis, which may help to explain the higher rates of malignant transformation that can be seen in linear porokeratosis.24
Management
Several treatment options exist for porokeratosis, including cryotherapy, topical 5-fluorouracil with or without adjunctive retinoid treatment, topical imiquimod, CO2 laser, shave and linear excision, curettage, dermabrasion, and oral acitretin for widespread lesions.1,25-29 One case report detailed successful treatment of adult-onset linear porokeratosis with tacrolimus ointment 0.1%.30 Treatments for porokeratosis demonstrate variable degrees of success, with the aim of eradicating the clonal population of mutant keratinocytes.2 Additionally, protection from UV radiation should be encouraged, especially in patients who have lesions that occur in areas of high actinic damage.1
Conclusion
We report of a case of linear porokeratosis with associated multiple SCCs that developed within the lesion. Definitive diagnosis of linear porokeratosis is important due to the higher rate of malignant transformation than the rate seen in other porokeratoses. In larger lesions, appropriate sampling and orientation of the pathology specimen is essential for identifying cornoid lamellae, thus allowing for appropriate follow-up and management. Several treatment options are available, though evidence for the effectiveness of any particular therapy is lacking. Research has shed light on possible genetic and molecular abnormalities in linear porokeratosis, but the exact pathogenesis of the disorder remains unclear.
- Curkova AK, Hegyi J, Kozub P, et al. A case of linear porokeratosis treated with photodynamic therapy with confocal microscopy surveillance. Dermatol Ther. 2014;27:144-147.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Saunders; 2012.
- Behera B, Devi B, Nayak BB, et al. Giant inflammatory linear verrucous epidermal nevus: successfully treated with full thickness excision and skin grafting. Indian J Dermatol. 2013;58:461-463.
- Wade TR, Ackerman AB. Cornoid lamellation. a histologic reaction pattern. Am J Dermatopathol. 1980;2:5-15.
- Curnow P, Foley P, Baker C. Multiple squamous cell carcinomas complicating linear porokeratosis. Australas J Dermatol. 2003;44:136-139.
- Rahbari H, Cordero AA, Mehregan AH. Linear porokeratosis. a distinctive clinical variant of porokeratosis of Mibelli. Arch Dermatol. 1974;109:526-528.
- Sasson M, Krain AD. Porokeratosis and cutaneous malignancy. a review. Dermatol Surg. 1996;22:339-342.
- Yeo J, Winhoven S, Tallon B. Porokeratosis ptychotropica: a rare and evolving variant of porokeratosis. J Cutan Pathol. 2013;40:1042-1047.
- Scola N, Skrygan M, Wieland U, et al. Altered gene expression in squamous cell carcinoma arising from congenital unilateral linear porokeratosis. Clin Exp Dermatol. 2012;37:781-785.
- Sertznig P, von Felbert V, Megahed M. Porokeratosis: present concepts. J Eur Acad Dermatol Venereol. 2012;26:404-412.
- Goldner R. Zosteriform porokeratosis of Mibelli. Arch Dermatol. 1971;104:425-426.
- Malhotra SK, Puri KJ, Goyal T, et al. Linear porokeratosis. Dermatol Online J. 2007;13:15.
- Leow YH, Soon YH, Tham SN. A report of 31 cases of porokeratosis at the National Skin Centre. Ann Acad Med Singapore. 1996;25:837-841.
- Vivas AC, Maderal AD, Kirsner RS. Giant ulcerating squamous cell carcinoma arising from linear porokeratosis: a case study. Ostomy Wound Manage. 2012;58:18-20.
- Arranz-Salas I, Sanz-Trelles A, Ojeda DB. p53 alterations in porokeratosis. J Cutan Pathol. 2003;30:455-458.
- Otsuka F, Someya T, Ishibashi Y. Porokeratosis and malignant skin tumors. J Cancer Res Clin Oncol. 1991;117:55-60.
- Otsuka F, Umebayashi Y, Watanabe S, et al. Porokeratosis large skin lesions are susceptible to skin cancer development: histological and cytological explanation for the susceptibility. J Cancer Res Clin Oncol. 1993;119:395-400.
- Lohrer R, Neumann-Acikel A, Eming R, et al. A case of linear porokeratosis superimposed on disseminated superficial actinic porokeratosis. Case Rep Dermatol. 2010;2:130-134.
- Biswas A. Cornoid lamellation revisited: apropos of porokeratosis with emphasis on unusual clinicopathological variants. Am J Dermatopathol. 2015;37:145-155.
- Reed RJ, Leone P. Porokeratosis—a mutant clonal keratosis of the epidermis. I. histogenesis. Arch Dermatol. 1970;101:340-347.
- Shumack S, Commens C, Kossard S. Disseminated superficial actinic porokeratosis. a histological review of 61 cases with particular reference to lymphocytic inflammation. Am J Dermatopathol. 1991;13:26-31.
- Murase J, Gilliam AC. Disseminated superficial actinic porokeratosis co-existing with linear and verrucous porokeratosis in an elderly woman: update on the genetics and clinical expression of porokeratosis. J Am Acad Dermatol. 2010;63:886-891.
- Commens CA, Shumack SP. Linear porokeratosis in two families with disseminated superficial actinic porokeratosis. Pediatr Dermatol. 1987;4:209-214.
- Happle R. Cancer proneness of linear porokeratosis may be explained by allelic loss. Dermatology. 1997;195:20-25.
- Rabbin PE, Baldwin HE. Treatment of porokeratosis of Mibelli with CO2 laser vaporization versus surgical excision with split-thickness skin graft. a comparison. J Dermatol Surg Oncol. 1993;19:199-202.
- Spencer JM, Katz BE. Successful treatment of porokeratosis of Mibelli with diamond fraise dermabrasion. Arch Dermatol. 1992;128:1187-1188.
- Venkatarajan S, LeLeux TM, Yang D, et al. Porokeratosis of Mibelli: successful treatment with 5 percent topical imiquimod and topical 5 percent 5-fluorouracil. Dermatol Online J. 2010;16:10.
- McDonald SG, Peterka ES. Porokeratosis (Mibelli): treatment with topical 5-fluorouracil. J Am Acad Dermatol. 1983;8:107-110.
- Shumack SP, Commens CA. Disseminated superficial actinic porokeratosis: a clinical study. J Am Acad Dermatol. 1989;20:1015-1022.
- Parks AC, Conner KJ, Armstrong CA. Long-term clearance of linear porokeratosis with tacrolimus, 0.1%, ointment. JAMA Dermatol. 2014;150:194-196.
Lesions of porokeratosis are thought to arise from disordered keratinization, though the exact pathogenesis remains uncertain. At least 5 clinical subtypes of porokeratosis have been identified: porokeratosis of Mibelli, disseminated superficial porokeratosis and disseminated superficial actinic porokeratosis (DSAP), linear porokeratosis, punctuate porokeratosis, and porokeratosis palmaris et plantaris disseminata (PPPD).1,2 Linear porokeratosis is a rare subtype with a clinical differential diagnosis that includes lichen striatus, linear lichen planus, linear verrucous epidermal nevus, segmental Darier disease, and incontinentia pigmenti.3 Definitive diagnosis of linear porokeratosis is made by histopathologic examination demonstrating a cornoid lamella, defined as a column of parakeratotic cells that lies at 45°to the surface of the epidermis and contains pyknotic basophilic nuclei.4 Patients with linear porokeratosis typically develop lesions along the lines of Blaschko in infancy or childhood.5,6 Among the different subtypes of porokeratosis, linear porokeratosis demonstrates the highest rate of malignant transformation, therefore requiring close clinical observation.7
Case Report
An 83-year-old woman presented to the outpatient clinic with a large linear plaque on the right leg that had been present since birth. Ten years prior to presentation, a portion of the lesion started to bleed; biopsy of the area was performed by an outside provider demonstrating squamous cell carcinoma (SCC), which was treated with wide local excision. One year prior to presentation, a separate portion of the plaque was biopsied by an outside provider and another diagnosis of SCC was made.
On examination performed during the initial presentation to our clinic, there was a well-demarcated tan to violaceous linear plaque present at the lower buttock and extending along the posterior leg to the skin overlying the Achilles tendon and dorsal aspect of the right foot. Within the plaque, there were areas of atrophy and areas of inflammation, induration, and hyperkeratosis (Figures 1 and 2). Two punch biopsies were performed: one from the edge of the plaque and one from a hyperkeratotic region within the plaque. Histology from the edge of the plaque demonstrated a cornoid lamella, consistent with a porokeratosis (Figure 3), whereas the histology from the hyperkeratotic region demonstrated a lichenoid infiltrate (Figure 4).




Several treatment options directed at the entire lesion were offered to the patient, but she declined these therapies and opted to address only those areas with clinical features of SCC, such as hyperkeratosis, bleeding, and rapid growth. Although biopsies performed by an outside provider were consistent with SCC, it had not been detected on biopsy performed during her initial visit to our clinic.
The patient was educated on the risk associated with her condition and instructed to follow up every 6 months to monitor for the development of SCC.
Comment
Porokeratosis is a disorder of keratinization with at least 5 clinical subtypes that share histologic similarities: porokeratosis of Mibelli, disseminated superficial porokeratosis and DSAP, linear porokeratosis, punctate porokeratosis, and PPPD.1,2 Other less common variants of porokeratosis include porokeratosis ptychotropica (a verrucous variant confined to the perianal area) and congenital unilateral linear porokeratosis.8,9
Linear porokeratosis appears in infancy or childhood with plaques that follow the lines of Blaschko.5,6 Most commonly, it presents unilaterally with annular plaques and linear hyperkeratotic papules that preferentially affect the extremities, though it also may present in a more generalized form or appear in a zosteriform pattern.10,11 Linear porokeratosis affects fewer than 20,000 individuals in the United States and accounts for fewer than 13% of all porokeratosis cases.12,13
Despite its relatively low prevalence, early identification of linear porokeratosis is important due to its high oncogenic potential, with malignant transformation to basal cell carcinoma or, more commonly, SCC reported in 19% of reported cases.1,5,7,14 The malignant transformation rate of linear porokeratosis is reported to be higher than rates seen in other porokeratosis subtypes (9.5%, 7.6%, and 3.4% for PPPD, porokeratosis of Mibelli, and DSAP, respectively).7 The risk of malignant transformation from porokeratosis increases with exposure to ionizing radiation, duration of the lesion, larger or coalescing lesions, and advanced age.7,15,16 Histologic studies have provided support for correlation between lesion size and oncogenic potential, with greater numbers of mitotic cells and more abnormal DNA ploidy seen in larger lesions.17
Histopathology
All subtypes of porokeratosis share certain histopathologic features that aid in the diagnosis of the disorder.18 Identification of the clinically observed hyperkeratotic ridged border or cornoid lamella is the primary means of definitively diagnosing porokeratosis; however, cornoid lamellae may be observed in other conditions, including verruca vulgaris and actinic keratosis.4,14
The cornoid lamella appears as a skewed column of densely packed parakeratotic cells with pyknotic basophilic nuclei extending through the stratum corneum from an epidermal invagination.4 Directly beneath the cornoid lamella, the granular layer is markedly diminished or absent, and cells of the stratum spinosum may demonstrate vacuolar changes or dyskeratosis.4,19 The superficial layer of the cornoid lamella may appear to be more centrifugally located and the cornoid lamella may be seen in several locations throughout the lesion.2,20 The degree of epidermal invagination, which is present under the cornoid lamella, varies by porokeratosis subtype; the central portion of the lesion may contain epidermis that ranges from hyperplastic to atrophic.2 Shumack et al21 noted that histologic changes under the cornoid lamella may include a lichenoid tissue reaction, papillary dermal lymphocytic infiltrate, vacuolar changes, dyskeratosis, and liquefaction degeneration of the basal layer. Because many of these histologic features also can be identified in lichen planus, a biopsy of the edge of lesions of porokeratosis is essential for making the correct diagnosis.
Heritability
Although linear porokeratosis has no identified pattern of inheritance and appears sporadic in onset, reports have described concomitant occurrence of linear porokeratosis and DSAP as well as linear porokeratosis arising in children of parents who have a diagnosis of DSAP.5,18,22,23 Based on these findings, it has been hypothesized that linear porokeratosis may represent a mosaic or segmental form of autosomal-dominant inherited subtypes of porokeratosis, such as DSAP.5 According to this hypothesis, loss of heterozygosity in patients with a DSAP mutation during early embryogenesis leads to proliferation of cells that are homozygous or hemizygous for the underlying mutation along lines of Blaschko.24 It has been suggested that the allelic loss implicated in the development of linear porokeratosis is the first step in a multistage process of carcinogenesis, which may help to explain the higher rates of malignant transformation that can be seen in linear porokeratosis.24
Management
Several treatment options exist for porokeratosis, including cryotherapy, topical 5-fluorouracil with or without adjunctive retinoid treatment, topical imiquimod, CO2 laser, shave and linear excision, curettage, dermabrasion, and oral acitretin for widespread lesions.1,25-29 One case report detailed successful treatment of adult-onset linear porokeratosis with tacrolimus ointment 0.1%.30 Treatments for porokeratosis demonstrate variable degrees of success, with the aim of eradicating the clonal population of mutant keratinocytes.2 Additionally, protection from UV radiation should be encouraged, especially in patients who have lesions that occur in areas of high actinic damage.1
Conclusion
We report of a case of linear porokeratosis with associated multiple SCCs that developed within the lesion. Definitive diagnosis of linear porokeratosis is important due to the higher rate of malignant transformation than the rate seen in other porokeratoses. In larger lesions, appropriate sampling and orientation of the pathology specimen is essential for identifying cornoid lamellae, thus allowing for appropriate follow-up and management. Several treatment options are available, though evidence for the effectiveness of any particular therapy is lacking. Research has shed light on possible genetic and molecular abnormalities in linear porokeratosis, but the exact pathogenesis of the disorder remains unclear.
Lesions of porokeratosis are thought to arise from disordered keratinization, though the exact pathogenesis remains uncertain. At least 5 clinical subtypes of porokeratosis have been identified: porokeratosis of Mibelli, disseminated superficial porokeratosis and disseminated superficial actinic porokeratosis (DSAP), linear porokeratosis, punctuate porokeratosis, and porokeratosis palmaris et plantaris disseminata (PPPD).1,2 Linear porokeratosis is a rare subtype with a clinical differential diagnosis that includes lichen striatus, linear lichen planus, linear verrucous epidermal nevus, segmental Darier disease, and incontinentia pigmenti.3 Definitive diagnosis of linear porokeratosis is made by histopathologic examination demonstrating a cornoid lamella, defined as a column of parakeratotic cells that lies at 45°to the surface of the epidermis and contains pyknotic basophilic nuclei.4 Patients with linear porokeratosis typically develop lesions along the lines of Blaschko in infancy or childhood.5,6 Among the different subtypes of porokeratosis, linear porokeratosis demonstrates the highest rate of malignant transformation, therefore requiring close clinical observation.7
Case Report
An 83-year-old woman presented to the outpatient clinic with a large linear plaque on the right leg that had been present since birth. Ten years prior to presentation, a portion of the lesion started to bleed; biopsy of the area was performed by an outside provider demonstrating squamous cell carcinoma (SCC), which was treated with wide local excision. One year prior to presentation, a separate portion of the plaque was biopsied by an outside provider and another diagnosis of SCC was made.
On examination performed during the initial presentation to our clinic, there was a well-demarcated tan to violaceous linear plaque present at the lower buttock and extending along the posterior leg to the skin overlying the Achilles tendon and dorsal aspect of the right foot. Within the plaque, there were areas of atrophy and areas of inflammation, induration, and hyperkeratosis (Figures 1 and 2). Two punch biopsies were performed: one from the edge of the plaque and one from a hyperkeratotic region within the plaque. Histology from the edge of the plaque demonstrated a cornoid lamella, consistent with a porokeratosis (Figure 3), whereas the histology from the hyperkeratotic region demonstrated a lichenoid infiltrate (Figure 4).




Several treatment options directed at the entire lesion were offered to the patient, but she declined these therapies and opted to address only those areas with clinical features of SCC, such as hyperkeratosis, bleeding, and rapid growth. Although biopsies performed by an outside provider were consistent with SCC, it had not been detected on biopsy performed during her initial visit to our clinic.
The patient was educated on the risk associated with her condition and instructed to follow up every 6 months to monitor for the development of SCC.
Comment
Porokeratosis is a disorder of keratinization with at least 5 clinical subtypes that share histologic similarities: porokeratosis of Mibelli, disseminated superficial porokeratosis and DSAP, linear porokeratosis, punctate porokeratosis, and PPPD.1,2 Other less common variants of porokeratosis include porokeratosis ptychotropica (a verrucous variant confined to the perianal area) and congenital unilateral linear porokeratosis.8,9
Linear porokeratosis appears in infancy or childhood with plaques that follow the lines of Blaschko.5,6 Most commonly, it presents unilaterally with annular plaques and linear hyperkeratotic papules that preferentially affect the extremities, though it also may present in a more generalized form or appear in a zosteriform pattern.10,11 Linear porokeratosis affects fewer than 20,000 individuals in the United States and accounts for fewer than 13% of all porokeratosis cases.12,13
Despite its relatively low prevalence, early identification of linear porokeratosis is important due to its high oncogenic potential, with malignant transformation to basal cell carcinoma or, more commonly, SCC reported in 19% of reported cases.1,5,7,14 The malignant transformation rate of linear porokeratosis is reported to be higher than rates seen in other porokeratosis subtypes (9.5%, 7.6%, and 3.4% for PPPD, porokeratosis of Mibelli, and DSAP, respectively).7 The risk of malignant transformation from porokeratosis increases with exposure to ionizing radiation, duration of the lesion, larger or coalescing lesions, and advanced age.7,15,16 Histologic studies have provided support for correlation between lesion size and oncogenic potential, with greater numbers of mitotic cells and more abnormal DNA ploidy seen in larger lesions.17
Histopathology
All subtypes of porokeratosis share certain histopathologic features that aid in the diagnosis of the disorder.18 Identification of the clinically observed hyperkeratotic ridged border or cornoid lamella is the primary means of definitively diagnosing porokeratosis; however, cornoid lamellae may be observed in other conditions, including verruca vulgaris and actinic keratosis.4,14
The cornoid lamella appears as a skewed column of densely packed parakeratotic cells with pyknotic basophilic nuclei extending through the stratum corneum from an epidermal invagination.4 Directly beneath the cornoid lamella, the granular layer is markedly diminished or absent, and cells of the stratum spinosum may demonstrate vacuolar changes or dyskeratosis.4,19 The superficial layer of the cornoid lamella may appear to be more centrifugally located and the cornoid lamella may be seen in several locations throughout the lesion.2,20 The degree of epidermal invagination, which is present under the cornoid lamella, varies by porokeratosis subtype; the central portion of the lesion may contain epidermis that ranges from hyperplastic to atrophic.2 Shumack et al21 noted that histologic changes under the cornoid lamella may include a lichenoid tissue reaction, papillary dermal lymphocytic infiltrate, vacuolar changes, dyskeratosis, and liquefaction degeneration of the basal layer. Because many of these histologic features also can be identified in lichen planus, a biopsy of the edge of lesions of porokeratosis is essential for making the correct diagnosis.
Heritability
Although linear porokeratosis has no identified pattern of inheritance and appears sporadic in onset, reports have described concomitant occurrence of linear porokeratosis and DSAP as well as linear porokeratosis arising in children of parents who have a diagnosis of DSAP.5,18,22,23 Based on these findings, it has been hypothesized that linear porokeratosis may represent a mosaic or segmental form of autosomal-dominant inherited subtypes of porokeratosis, such as DSAP.5 According to this hypothesis, loss of heterozygosity in patients with a DSAP mutation during early embryogenesis leads to proliferation of cells that are homozygous or hemizygous for the underlying mutation along lines of Blaschko.24 It has been suggested that the allelic loss implicated in the development of linear porokeratosis is the first step in a multistage process of carcinogenesis, which may help to explain the higher rates of malignant transformation that can be seen in linear porokeratosis.24
Management
Several treatment options exist for porokeratosis, including cryotherapy, topical 5-fluorouracil with or without adjunctive retinoid treatment, topical imiquimod, CO2 laser, shave and linear excision, curettage, dermabrasion, and oral acitretin for widespread lesions.1,25-29 One case report detailed successful treatment of adult-onset linear porokeratosis with tacrolimus ointment 0.1%.30 Treatments for porokeratosis demonstrate variable degrees of success, with the aim of eradicating the clonal population of mutant keratinocytes.2 Additionally, protection from UV radiation should be encouraged, especially in patients who have lesions that occur in areas of high actinic damage.1
Conclusion
We report of a case of linear porokeratosis with associated multiple SCCs that developed within the lesion. Definitive diagnosis of linear porokeratosis is important due to the higher rate of malignant transformation than the rate seen in other porokeratoses. In larger lesions, appropriate sampling and orientation of the pathology specimen is essential for identifying cornoid lamellae, thus allowing for appropriate follow-up and management. Several treatment options are available, though evidence for the effectiveness of any particular therapy is lacking. Research has shed light on possible genetic and molecular abnormalities in linear porokeratosis, but the exact pathogenesis of the disorder remains unclear.
- Curkova AK, Hegyi J, Kozub P, et al. A case of linear porokeratosis treated with photodynamic therapy with confocal microscopy surveillance. Dermatol Ther. 2014;27:144-147.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Saunders; 2012.
- Behera B, Devi B, Nayak BB, et al. Giant inflammatory linear verrucous epidermal nevus: successfully treated with full thickness excision and skin grafting. Indian J Dermatol. 2013;58:461-463.
- Wade TR, Ackerman AB. Cornoid lamellation. a histologic reaction pattern. Am J Dermatopathol. 1980;2:5-15.
- Curnow P, Foley P, Baker C. Multiple squamous cell carcinomas complicating linear porokeratosis. Australas J Dermatol. 2003;44:136-139.
- Rahbari H, Cordero AA, Mehregan AH. Linear porokeratosis. a distinctive clinical variant of porokeratosis of Mibelli. Arch Dermatol. 1974;109:526-528.
- Sasson M, Krain AD. Porokeratosis and cutaneous malignancy. a review. Dermatol Surg. 1996;22:339-342.
- Yeo J, Winhoven S, Tallon B. Porokeratosis ptychotropica: a rare and evolving variant of porokeratosis. J Cutan Pathol. 2013;40:1042-1047.
- Scola N, Skrygan M, Wieland U, et al. Altered gene expression in squamous cell carcinoma arising from congenital unilateral linear porokeratosis. Clin Exp Dermatol. 2012;37:781-785.
- Sertznig P, von Felbert V, Megahed M. Porokeratosis: present concepts. J Eur Acad Dermatol Venereol. 2012;26:404-412.
- Goldner R. Zosteriform porokeratosis of Mibelli. Arch Dermatol. 1971;104:425-426.
- Malhotra SK, Puri KJ, Goyal T, et al. Linear porokeratosis. Dermatol Online J. 2007;13:15.
- Leow YH, Soon YH, Tham SN. A report of 31 cases of porokeratosis at the National Skin Centre. Ann Acad Med Singapore. 1996;25:837-841.
- Vivas AC, Maderal AD, Kirsner RS. Giant ulcerating squamous cell carcinoma arising from linear porokeratosis: a case study. Ostomy Wound Manage. 2012;58:18-20.
- Arranz-Salas I, Sanz-Trelles A, Ojeda DB. p53 alterations in porokeratosis. J Cutan Pathol. 2003;30:455-458.
- Otsuka F, Someya T, Ishibashi Y. Porokeratosis and malignant skin tumors. J Cancer Res Clin Oncol. 1991;117:55-60.
- Otsuka F, Umebayashi Y, Watanabe S, et al. Porokeratosis large skin lesions are susceptible to skin cancer development: histological and cytological explanation for the susceptibility. J Cancer Res Clin Oncol. 1993;119:395-400.
- Lohrer R, Neumann-Acikel A, Eming R, et al. A case of linear porokeratosis superimposed on disseminated superficial actinic porokeratosis. Case Rep Dermatol. 2010;2:130-134.
- Biswas A. Cornoid lamellation revisited: apropos of porokeratosis with emphasis on unusual clinicopathological variants. Am J Dermatopathol. 2015;37:145-155.
- Reed RJ, Leone P. Porokeratosis—a mutant clonal keratosis of the epidermis. I. histogenesis. Arch Dermatol. 1970;101:340-347.
- Shumack S, Commens C, Kossard S. Disseminated superficial actinic porokeratosis. a histological review of 61 cases with particular reference to lymphocytic inflammation. Am J Dermatopathol. 1991;13:26-31.
- Murase J, Gilliam AC. Disseminated superficial actinic porokeratosis co-existing with linear and verrucous porokeratosis in an elderly woman: update on the genetics and clinical expression of porokeratosis. J Am Acad Dermatol. 2010;63:886-891.
- Commens CA, Shumack SP. Linear porokeratosis in two families with disseminated superficial actinic porokeratosis. Pediatr Dermatol. 1987;4:209-214.
- Happle R. Cancer proneness of linear porokeratosis may be explained by allelic loss. Dermatology. 1997;195:20-25.
- Rabbin PE, Baldwin HE. Treatment of porokeratosis of Mibelli with CO2 laser vaporization versus surgical excision with split-thickness skin graft. a comparison. J Dermatol Surg Oncol. 1993;19:199-202.
- Spencer JM, Katz BE. Successful treatment of porokeratosis of Mibelli with diamond fraise dermabrasion. Arch Dermatol. 1992;128:1187-1188.
- Venkatarajan S, LeLeux TM, Yang D, et al. Porokeratosis of Mibelli: successful treatment with 5 percent topical imiquimod and topical 5 percent 5-fluorouracil. Dermatol Online J. 2010;16:10.
- McDonald SG, Peterka ES. Porokeratosis (Mibelli): treatment with topical 5-fluorouracil. J Am Acad Dermatol. 1983;8:107-110.
- Shumack SP, Commens CA. Disseminated superficial actinic porokeratosis: a clinical study. J Am Acad Dermatol. 1989;20:1015-1022.
- Parks AC, Conner KJ, Armstrong CA. Long-term clearance of linear porokeratosis with tacrolimus, 0.1%, ointment. JAMA Dermatol. 2014;150:194-196.
- Curkova AK, Hegyi J, Kozub P, et al. A case of linear porokeratosis treated with photodynamic therapy with confocal microscopy surveillance. Dermatol Ther. 2014;27:144-147.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Saunders; 2012.
- Behera B, Devi B, Nayak BB, et al. Giant inflammatory linear verrucous epidermal nevus: successfully treated with full thickness excision and skin grafting. Indian J Dermatol. 2013;58:461-463.
- Wade TR, Ackerman AB. Cornoid lamellation. a histologic reaction pattern. Am J Dermatopathol. 1980;2:5-15.
- Curnow P, Foley P, Baker C. Multiple squamous cell carcinomas complicating linear porokeratosis. Australas J Dermatol. 2003;44:136-139.
- Rahbari H, Cordero AA, Mehregan AH. Linear porokeratosis. a distinctive clinical variant of porokeratosis of Mibelli. Arch Dermatol. 1974;109:526-528.
- Sasson M, Krain AD. Porokeratosis and cutaneous malignancy. a review. Dermatol Surg. 1996;22:339-342.
- Yeo J, Winhoven S, Tallon B. Porokeratosis ptychotropica: a rare and evolving variant of porokeratosis. J Cutan Pathol. 2013;40:1042-1047.
- Scola N, Skrygan M, Wieland U, et al. Altered gene expression in squamous cell carcinoma arising from congenital unilateral linear porokeratosis. Clin Exp Dermatol. 2012;37:781-785.
- Sertznig P, von Felbert V, Megahed M. Porokeratosis: present concepts. J Eur Acad Dermatol Venereol. 2012;26:404-412.
- Goldner R. Zosteriform porokeratosis of Mibelli. Arch Dermatol. 1971;104:425-426.
- Malhotra SK, Puri KJ, Goyal T, et al. Linear porokeratosis. Dermatol Online J. 2007;13:15.
- Leow YH, Soon YH, Tham SN. A report of 31 cases of porokeratosis at the National Skin Centre. Ann Acad Med Singapore. 1996;25:837-841.
- Vivas AC, Maderal AD, Kirsner RS. Giant ulcerating squamous cell carcinoma arising from linear porokeratosis: a case study. Ostomy Wound Manage. 2012;58:18-20.
- Arranz-Salas I, Sanz-Trelles A, Ojeda DB. p53 alterations in porokeratosis. J Cutan Pathol. 2003;30:455-458.
- Otsuka F, Someya T, Ishibashi Y. Porokeratosis and malignant skin tumors. J Cancer Res Clin Oncol. 1991;117:55-60.
- Otsuka F, Umebayashi Y, Watanabe S, et al. Porokeratosis large skin lesions are susceptible to skin cancer development: histological and cytological explanation for the susceptibility. J Cancer Res Clin Oncol. 1993;119:395-400.
- Lohrer R, Neumann-Acikel A, Eming R, et al. A case of linear porokeratosis superimposed on disseminated superficial actinic porokeratosis. Case Rep Dermatol. 2010;2:130-134.
- Biswas A. Cornoid lamellation revisited: apropos of porokeratosis with emphasis on unusual clinicopathological variants. Am J Dermatopathol. 2015;37:145-155.
- Reed RJ, Leone P. Porokeratosis—a mutant clonal keratosis of the epidermis. I. histogenesis. Arch Dermatol. 1970;101:340-347.
- Shumack S, Commens C, Kossard S. Disseminated superficial actinic porokeratosis. a histological review of 61 cases with particular reference to lymphocytic inflammation. Am J Dermatopathol. 1991;13:26-31.
- Murase J, Gilliam AC. Disseminated superficial actinic porokeratosis co-existing with linear and verrucous porokeratosis in an elderly woman: update on the genetics and clinical expression of porokeratosis. J Am Acad Dermatol. 2010;63:886-891.
- Commens CA, Shumack SP. Linear porokeratosis in two families with disseminated superficial actinic porokeratosis. Pediatr Dermatol. 1987;4:209-214.
- Happle R. Cancer proneness of linear porokeratosis may be explained by allelic loss. Dermatology. 1997;195:20-25.
- Rabbin PE, Baldwin HE. Treatment of porokeratosis of Mibelli with CO2 laser vaporization versus surgical excision with split-thickness skin graft. a comparison. J Dermatol Surg Oncol. 1993;19:199-202.
- Spencer JM, Katz BE. Successful treatment of porokeratosis of Mibelli with diamond fraise dermabrasion. Arch Dermatol. 1992;128:1187-1188.
- Venkatarajan S, LeLeux TM, Yang D, et al. Porokeratosis of Mibelli: successful treatment with 5 percent topical imiquimod and topical 5 percent 5-fluorouracil. Dermatol Online J. 2010;16:10.
- McDonald SG, Peterka ES. Porokeratosis (Mibelli): treatment with topical 5-fluorouracil. J Am Acad Dermatol. 1983;8:107-110.
- Shumack SP, Commens CA. Disseminated superficial actinic porokeratosis: a clinical study. J Am Acad Dermatol. 1989;20:1015-1022.
- Parks AC, Conner KJ, Armstrong CA. Long-term clearance of linear porokeratosis with tacrolimus, 0.1%, ointment. JAMA Dermatol. 2014;150:194-196.
Practice Points
- Porokeratosis represents a heterogeneous group of skin disorders.
- Porokeratosis can be inherited in an autosomal-dominant pattern, though many patients lack a family history.
- The presence of a cornoid lamella is the characteristic finding of porokeratosis on histology.
- The rate of malignant transformation to squamous cell carcinoma is highest in linear porokeratosis, lowest in disseminated superficial actinic porokeratosis, and unreported in the punctate type.