User login
CASE
A 51-year-old man presents to your office to discuss lung cancer screening. He has a history of hypertension and prediabetes. His father died of lung cancer 5 years ago, at age 77. The patient stopped smoking soon thereafter; prior to that, he smoked 1 pack of cigarettes per day for 20 years. He wants to know if he should be screened for lung cancer.
The relative lack of symptoms during the early stages of lung cancer frequently results in a delayed diagnosis. This, and the speed at which the disease progresses, underscores the need for an effective screening modality. More than half of people with lung cancer die within 1 year of diagnosis.1 Excluding skin cancer, lung cancer is the second most commonly diagnosed cancer, and more people die of lung cancer than of colon, breast, and prostate cancers combined.2 In 2022, it was estimated that there would be 236,740 new cases of lung cancer and 130,180 deaths from lung cancer.1,2 The average age at diagnosis is 70 years.2

Screening modalities: Only 1 has demonstrated mortality benefit
In 1968, Wilson and Junger3 outlined the characteristics of the ideal screening test for the World Health Organization: it should limit risk to the patient, be sensitive for detecting the disease early in its course, limit false-positive results, be acceptable to the patient, and be inexpensive to the health system.3 For decades, several screening modalities for lung cancer were trialed to fit the above guidance, but many of them fell short of the most important outcome: the impact on mortality.
Sputum cytology. The use of sputum cytology, either in combination with or without chest radiography, is not recommended. Several randomized controlled trials (RCTs) have failed to demonstrate improved lung cancer detection or mortality reduction in patients screened with this modality.4
Chest radiography (CXR). Several studies have assessed the efficacy of CXR as a screening modality. The best known was the Prostate, Lung, Colon, Ovarian (PLCO) Trial.5 This multicenter RCT enrolled more than 154,000 participants, half of whom received CXR at baseline and then annually for 3 years; the other half continued usual care (no screening). After 13 years of follow-up, there were no significant differences in lung cancer detection or mortality rates between the 2 groups.5
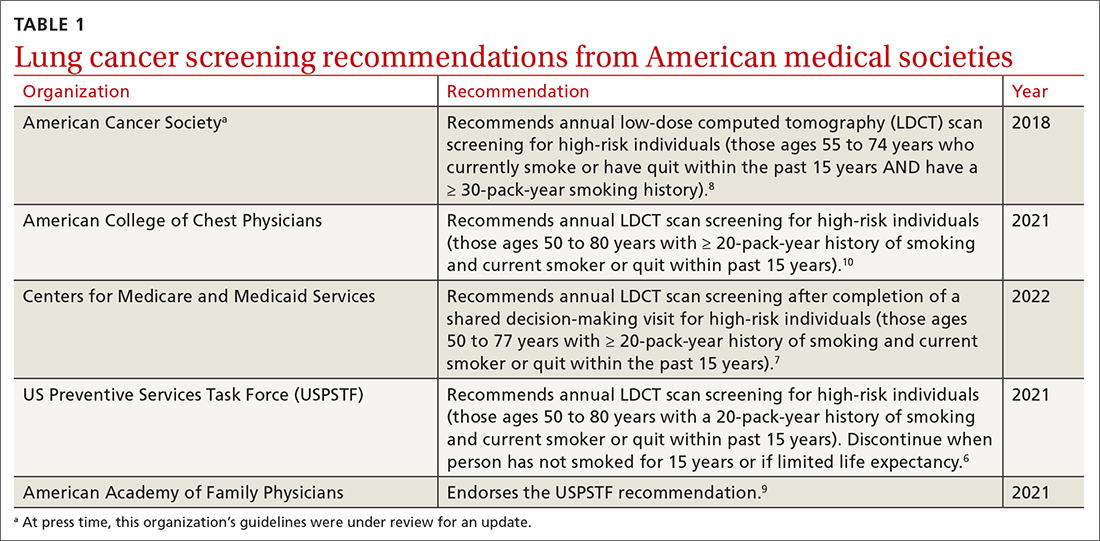
Low-dose computed tomography (LDCT). Several major medical societies recommend LDCT to screen high-risk individuals for lung cancer (TABLE 16-10). Results from 2 major RCTs have guided these recommendations.
The National Lung Screening Trial (NLST) was a multicenter RCT comparing 2 screening tests for lung cancer.11 Approximately 54,000 high-risk participants were enrolled between 2002 and 2004 and were randomized to receive annual screening with either LDCT or single-view CXR. The trial was discontinued prematurely when investigators noted a 20% reduction in lung cancer mortality in the LDCT group vs the CXR group.12 This equates to 3 fewer deaths for every 1000 people screened with LDCT vs CXR. There was also a 6% reduction in all-cause mortality noted in the LDCT vs the CXR group.12
Continue to: The NELSON trial...
The NELSON trial, conducted between 2005 and 2015, studied more than 15,000 current or former smokers ages 50 to 74 years and compared LDCT screening at various intervals to no screening.13 After 10 years, lung cancer–related mortality was reduced by 24% (or 1 less death per 1000 person-years) in men who were screened vs their unscreened counterparts.13 In contrast to the NLST, in the NELSON trial, no significant difference in all-cause mortality was observed. Subgroup analysis of the relatively small population of women included in the NELSON trial suggested a 33% reduction in 10-year mortality; however, the difference was nonsignificant between the screened and unscreened groups.13
Each of these landmark studies had characteristics that could limit the results' generalizability to the US population. In the NELSON trial, more than 80% of the study participants were male. In both trials, there was significant underrepresentation of Black, Asian, Hispanic, and other non-White people.12,13 Furthermore, participants in these studies were of higher socioeconomic status than the general US screening-eligible population.
At this time, LDCT is the only lung cancer screening modality that has shown benefit for both disease-related and all-cause mortality, in the populations that were studied. Based on the NLST, the number needed to screen (NNS) with LDCT to prevent 1 lung cancer–related death is 308. The NNS to prevent 1 death from any cause is 219.6
Updated evidence has led to a consensus on screening criteria
Many national societies endorse annual screening with LDCT in high-risk individuals (TABLE 16-10). Risk assessment for the purpose of lung cancer screening includes a detailed review of smoking history and age. The risk of lung cancer increases with advancing age and with cumulative quantity and duration of smoking, but decreases with increasing time since quitting. Therefore, a detailed smoking history should include total number of pack-years, current smoking status, and, if applicable, when smoking cessation occurred.
In 2021, the US Preventive Services Task Force (USPSTF) updated their 2013 lung cancer screening recommendations, expanding the screening age range and lowering the smoking history threshold for triggering initiation of screening.6 The impetus for the update was emerging evidence from systematic reviews, RCTs, and the Cancer Intervention and Surveillance Modeling Network (CISNET) that could help to determine the optimal age for screening and identify high-risk groups. For example, the NELSON trial, combined with results from CISNET modeling data, showed an empirical benefit for screening those ages 50 to 55 years.6
Continue to: As a result...
As a result, the USPSTF now recommends annual lung cancer screening with LDCT for any adult ages 50 to 80 years who has a 20-pack-year smoking history and currently smokes or has quit within the past 15 years.6 Screening should be discontinued once a person has not smoked for 15 years, develops a health problem that substantially limits life expectancy, or is not willing to have curative lung surgery.6
Expanding the screening eligibility may also address racial and gender disparities in health care. Black people and women who smoke have a higher risk for lung cancer at a lower intensity of smoking.6
Following the USPSTF update, the American College of Chest Physicians and the Centers for Medicare and Medicaid Services published updated guidance that aligns with USPSTF’s recommendations to lower the age and pack-year qualifications for initiating screening.7,10 The American Cancer Society is currently reviewing its 2018 guidelines on lung cancer screening.14TABLE 16-10 summarizes the guidance on lung cancer screening from these medical societies.
Effective screening could save lives (and money)
A smoker’s risk for lung cancer is 20 times higher than that of a nonsmoker15,16; 55% of lung cancer deaths in women and 70% in men are attributed to smoking.17 Once diagnosed with lung cancer, more than 50% of people will die within 1 year.1 This underpins the need for a lung cancer screening modality that reduces mortality. Large RCTs, including the NLST and NELSONtrials, have shown that screening high-risk individuals with LDCT can significantly reduce lung cancer–related death when compared to no screening or screening with CXR alone.11,13
There is controversy surrounding the cost benefit of implementing a nationwide lung cancer screening program. However, recent use of microsimulation models has shown LDCT to be a cost-effective strategy, with an average cost of $81,000 per quality-adjusted life-year, which is below the threshold of $100,000 to be considered cost effective.18 Expanding the upper age limit for screening leads to a greater reduction in mortality but increases treatment costs and overdiagnosis rates, and overall does not improve quality-adjusted life-years.18
Continue to: Potential harms
Potential harms: False-positives and related complications
Screening for lung cancer is not without its risks. Harms from screening typically result from false-positive test results leading to overdiagnosis, anxiety and distress, unnecessary invasive tests or procedures, and increased costs.19TABLE 26,19-23 lists specific complications from lung cancer screening with LDCT.
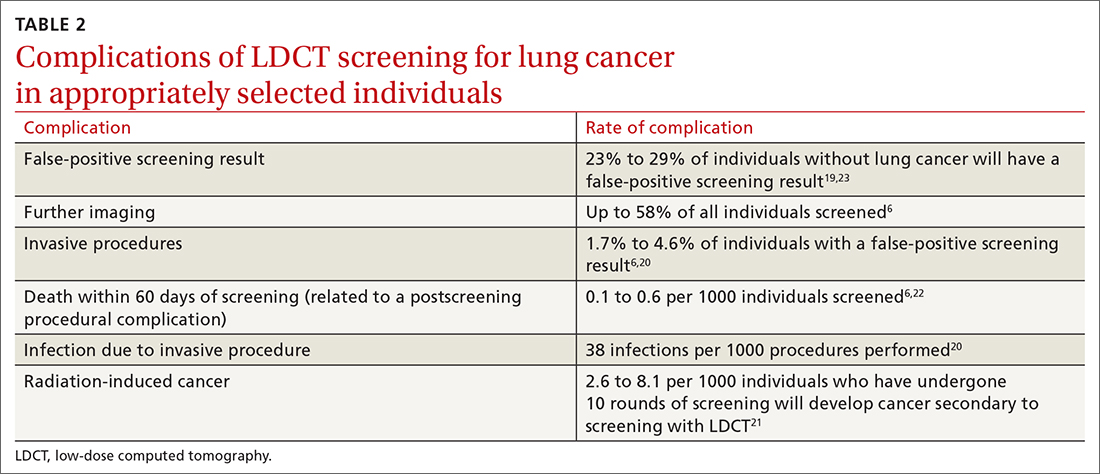
The false-positive rate is not trivial. For every 1000 patients screened, 250 people will have a positive LDCT finding but will not have lung cancer.19 Furthermore, about 1 in every 2000 individuals who screen positive, but who do not have lung cancer, die as a result of complications from the ensuing work-up.6
Annual LDCT screening increases the risk of radiation-induced cancer by approximately 0.05% over 10 years.21 The absolute risk is generally low but not insignificant. However, the mortality benefits previously outlined are significantly more robust in both absolute and relative terms vs the 10-year risk of radiation-induced cancer.
Lastly, it is important to note that the NELSON trial and NLST included a limited number of LDCT scans. Current guidelines for lung cancer screening with LDCT, including those from the USPSTF, recommend screening annually. We do not know the cumulative harm of annual LDCT over a 20- or 30-year period for those who would qualify (ie, current smokers).
If you screen, you must be able to act on the results
Effective screening programs should extend beyond the LDCT scan itself. The studies that have shown a benefit of LDCT were done at large academic centers that had the appropriate radiologic, pathologic, and surgical infrastructure to interpret and act on results and offer further diagnostic or treatment procedures.
Continue to: Prior to screening...
Prior to screening for lung cancer with LDCT, documentation of shared decision-making between the patient and the clinician is necessary.7 This discussion should include the potential benefits and harms of screening, potential results and likelihood of follow-up diagnostic testing, the false-positive rate of LDCT lung cancer screening, and cumulative radiation exposure. In addition, screening should be considered only if the patient is willing to be screened annually, is willing to pursue follow-up scans and procedures (including lung biopsy) if deemed necessary, and does not have comorbid conditions that significantly limit life expectancy.
Smoking cessation: The most important change to make
Smoking cessation is the single most important risk-modifying behavior to reduce one’s chance of developing lung cancer. At age 40, smokers have a 2-fold increase in all-cause mortality compared to age-matched nonsmokers. This rises to a 3-fold increase by the age of 70.16
Smoking cessation reduces the risk of lung cancer by 20% after 5 years, 30% to 50% after 10 years, and up to 70% after 15 years.24 In its guidelines, the American Thoracic Society recommends varenicline (Chantix) for all smokers to assist with smoking cessation.25
CASE
This 51-year-old patient with at least a 20-pack-year history of smoking should be commended for giving up smoking. Based on the USPSTF recommendations, he should be screened annually with LDCT for the next 10 years.
Screening to save more lives
The results of 2 large multicenter RCTs have led to the recent recommendation for lung cancer screening of high-risk adults with the use of LDCT. Screening with LDCT has been shown to reduce disease-related mortality and likely be cost effective in the long term.
Screening with LDCT should be part of a multidisciplinary system that has the infrastructure not only to perform the screening, but also to diagnose and appropriately follow up and treat patients whose results are concerning. The risk of false-positive results leading to increased anxiety, overdiagnosis, and unnecessary procedures points to the importance of proper patient selection, counseling, and shared decision-making. Smoking cessation remains the most important disease-modifying behavior one can make to reduce their risk for lung cancer.
CORRESPONDENCE
Carlton J. Covey, MD, 101 Bodin Circle, David Grant Medical Center, Travis Air Force Base, Fairfield, CA, 94545; carlcovey24@gmail.com
1. National Cancer Institute. Cancer Stat Facts: lung and bronchus cancer. Accessed October 12, 2022. https://seer.cancer.gov/statfacts/html/lungb.html
2. American Cancer Society. Key statistics for lung cancer. Accessed October 12, 2022. https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html
3. Wilson JMG, Junger G. Principles and Practice of Screening for Disease. World Health Organization; 1968:21-25, 100. https://apps.who.int/iris/handle/10665/37650
4. Humphrey LL, Teutsch S, Johnson M. Lung cancer screening with sputum cytologic examination, chest radiography, and computed tomography: an update for the United States preventive services task force. Ann Intern Med. 2004;140:740-753. doi: 10.7326/0003-4819-140-9-200405040-00015
5. Oken MM, Hocking WG, Kvale PA, et al. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA. 2011;306:1865-1873. doi: 10.1001/jama.2011.1591
6. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:962-970. doi: 10.1001/jama.2021.1117
7. Centers for Medicare & Medicaid Services. Screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439R). Accessed October 14, 2022. www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=304
8. Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2018: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2018;68:297-316. doi: 10.3322/caac.21446
9. American Academy of Family Physicians. AAFP updates recommendation on lung cancer screening. Published April 6, 2021. Accessed October 12, 2022. www.aafp.org/news/health-of-the-public/20210406lungcancer.html
10. Mazzone PJ, Silvestri GA, Souter LH, et al. Screening for lung cancer: CHEST Guideline and Expert Panel Report. CHEST. 2021;160:E427-E494. doi: 10.1016/j.chest.2021.06.063
11. The National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi: 10.1056/NEJMoa1102873
12. The National Lung Screening Trial Research Team. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368:1980-1991. doi: 10.1056/NEJMoa1209120
13. de Koning HJ, van der Aalst CM, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503-513. doi: 10.1056/NEJMoa1911793
14. American Cancer Society. Lung cancer screening guidelines. Accessed October 14, 2022. www.cancer.org/health-care-professionals/american-cancer-society-prevention-early-detection-guidelines/lung-cancer-screening-guidelines.html
15. Pirie K, Peto R, Reeves GK, et al. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet. 2013;381:133-141. doi: 10.1016/S0140-6736(12)61720-6
16. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE
17. O’Keefe LM, Gemma T, Huxley R, et al. Smoking as a risk factor for lung cancer in women and men: a systematic review and meta-analysis. BMJ Open. 2018;8:e021611. doi: 10.1136/bmjopen-2018-021611
18. Criss SD, Pianpian C, Bastani M, et al. Cost-effectiveness analysis of lung cancer screening in the United States: a comparative modeling study. Ann Intern Med. 2019;171:796-805. doi: 10.7326/M19-0322
19. Lazris A, Roth RA. Lung cancer screening: pros and cons. Am Fam Physician. 2019;99:740-742.
20. Ali MU, Miller J, Peirson L, et al. Screening for lung cancer: a systematic review and meta-analysis. Prev Med. 2016;89:301-314. doi: 10.1016/j.ypmed.2016.04.015
21. Rampinelli C, De Marco P, Origgi D, et al. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: secondary analysis of trial data and risk-benefit analysis. BMJ. 2017;356:j347. doi: 10.1136/bmj.j347
22. Manser RL, Lethaby A, Irving LB, et al. Screening for lung cancer. Cochrane Database Syst Rev. 2013;CD001991. doi: 10.1002/14651858.CD001991.pub3
23. Mazzone PJ, Silvestri GA, Patel S, et al. Screening for lung cancer: CHEST guideline and expert panel report. CHEST. 2018;153:954-985. doi: 10.1016/j.chest.2018.01.016
24. US Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking. and Health. Smoking Cessation: A Report of the Surgeon General. US Department of Health and Human Services; 2020. www.ncbi.nlm.nih.gov/books/NBK555591/
25. Leone FT, Zhang Y, Evers-Casey S, et al, on behalf of the American Thoracic Society Assembly on Clinical Problems. Initiating pharmacologic treatment in tobacco-dependent adults: an official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202:e5-e31. doi: 10.1164/rccm.202005-1982ST
CASE
A 51-year-old man presents to your office to discuss lung cancer screening. He has a history of hypertension and prediabetes. His father died of lung cancer 5 years ago, at age 77. The patient stopped smoking soon thereafter; prior to that, he smoked 1 pack of cigarettes per day for 20 years. He wants to know if he should be screened for lung cancer.
The relative lack of symptoms during the early stages of lung cancer frequently results in a delayed diagnosis. This, and the speed at which the disease progresses, underscores the need for an effective screening modality. More than half of people with lung cancer die within 1 year of diagnosis.1 Excluding skin cancer, lung cancer is the second most commonly diagnosed cancer, and more people die of lung cancer than of colon, breast, and prostate cancers combined.2 In 2022, it was estimated that there would be 236,740 new cases of lung cancer and 130,180 deaths from lung cancer.1,2 The average age at diagnosis is 70 years.2

Screening modalities: Only 1 has demonstrated mortality benefit
In 1968, Wilson and Junger3 outlined the characteristics of the ideal screening test for the World Health Organization: it should limit risk to the patient, be sensitive for detecting the disease early in its course, limit false-positive results, be acceptable to the patient, and be inexpensive to the health system.3 For decades, several screening modalities for lung cancer were trialed to fit the above guidance, but many of them fell short of the most important outcome: the impact on mortality.
Sputum cytology. The use of sputum cytology, either in combination with or without chest radiography, is not recommended. Several randomized controlled trials (RCTs) have failed to demonstrate improved lung cancer detection or mortality reduction in patients screened with this modality.4
Chest radiography (CXR). Several studies have assessed the efficacy of CXR as a screening modality. The best known was the Prostate, Lung, Colon, Ovarian (PLCO) Trial.5 This multicenter RCT enrolled more than 154,000 participants, half of whom received CXR at baseline and then annually for 3 years; the other half continued usual care (no screening). After 13 years of follow-up, there were no significant differences in lung cancer detection or mortality rates between the 2 groups.5
Low-dose computed tomography (LDCT). Several major medical societies recommend LDCT to screen high-risk individuals for lung cancer (TABLE 16-10). Results from 2 major RCTs have guided these recommendations.

The National Lung Screening Trial (NLST) was a multicenter RCT comparing 2 screening tests for lung cancer.11 Approximately 54,000 high-risk participants were enrolled between 2002 and 2004 and were randomized to receive annual screening with either LDCT or single-view CXR. The trial was discontinued prematurely when investigators noted a 20% reduction in lung cancer mortality in the LDCT group vs the CXR group.12 This equates to 3 fewer deaths for every 1000 people screened with LDCT vs CXR. There was also a 6% reduction in all-cause mortality noted in the LDCT vs the CXR group.12
Continue to: The NELSON trial...
The NELSON trial, conducted between 2005 and 2015, studied more than 15,000 current or former smokers ages 50 to 74 years and compared LDCT screening at various intervals to no screening.13 After 10 years, lung cancer–related mortality was reduced by 24% (or 1 less death per 1000 person-years) in men who were screened vs their unscreened counterparts.13 In contrast to the NLST, in the NELSON trial, no significant difference in all-cause mortality was observed. Subgroup analysis of the relatively small population of women included in the NELSON trial suggested a 33% reduction in 10-year mortality; however, the difference was nonsignificant between the screened and unscreened groups.13
Each of these landmark studies had characteristics that could limit the results' generalizability to the US population. In the NELSON trial, more than 80% of the study participants were male. In both trials, there was significant underrepresentation of Black, Asian, Hispanic, and other non-White people.12,13 Furthermore, participants in these studies were of higher socioeconomic status than the general US screening-eligible population.
At this time, LDCT is the only lung cancer screening modality that has shown benefit for both disease-related and all-cause mortality, in the populations that were studied. Based on the NLST, the number needed to screen (NNS) with LDCT to prevent 1 lung cancer–related death is 308. The NNS to prevent 1 death from any cause is 219.6
Updated evidence has led to a consensus on screening criteria
Many national societies endorse annual screening with LDCT in high-risk individuals (TABLE 16-10). Risk assessment for the purpose of lung cancer screening includes a detailed review of smoking history and age. The risk of lung cancer increases with advancing age and with cumulative quantity and duration of smoking, but decreases with increasing time since quitting. Therefore, a detailed smoking history should include total number of pack-years, current smoking status, and, if applicable, when smoking cessation occurred.
In 2021, the US Preventive Services Task Force (USPSTF) updated their 2013 lung cancer screening recommendations, expanding the screening age range and lowering the smoking history threshold for triggering initiation of screening.6 The impetus for the update was emerging evidence from systematic reviews, RCTs, and the Cancer Intervention and Surveillance Modeling Network (CISNET) that could help to determine the optimal age for screening and identify high-risk groups. For example, the NELSON trial, combined with results from CISNET modeling data, showed an empirical benefit for screening those ages 50 to 55 years.6
Continue to: As a result...
As a result, the USPSTF now recommends annual lung cancer screening with LDCT for any adult ages 50 to 80 years who has a 20-pack-year smoking history and currently smokes or has quit within the past 15 years.6 Screening should be discontinued once a person has not smoked for 15 years, develops a health problem that substantially limits life expectancy, or is not willing to have curative lung surgery.6
Expanding the screening eligibility may also address racial and gender disparities in health care. Black people and women who smoke have a higher risk for lung cancer at a lower intensity of smoking.6
Following the USPSTF update, the American College of Chest Physicians and the Centers for Medicare and Medicaid Services published updated guidance that aligns with USPSTF’s recommendations to lower the age and pack-year qualifications for initiating screening.7,10 The American Cancer Society is currently reviewing its 2018 guidelines on lung cancer screening.14TABLE 16-10 summarizes the guidance on lung cancer screening from these medical societies.
Effective screening could save lives (and money)
A smoker’s risk for lung cancer is 20 times higher than that of a nonsmoker15,16; 55% of lung cancer deaths in women and 70% in men are attributed to smoking.17 Once diagnosed with lung cancer, more than 50% of people will die within 1 year.1 This underpins the need for a lung cancer screening modality that reduces mortality. Large RCTs, including the NLST and NELSONtrials, have shown that screening high-risk individuals with LDCT can significantly reduce lung cancer–related death when compared to no screening or screening with CXR alone.11,13
There is controversy surrounding the cost benefit of implementing a nationwide lung cancer screening program. However, recent use of microsimulation models has shown LDCT to be a cost-effective strategy, with an average cost of $81,000 per quality-adjusted life-year, which is below the threshold of $100,000 to be considered cost effective.18 Expanding the upper age limit for screening leads to a greater reduction in mortality but increases treatment costs and overdiagnosis rates, and overall does not improve quality-adjusted life-years.18
Continue to: Potential harms
Potential harms: False-positives and related complications
Screening for lung cancer is not without its risks. Harms from screening typically result from false-positive test results leading to overdiagnosis, anxiety and distress, unnecessary invasive tests or procedures, and increased costs.19TABLE 26,19-23 lists specific complications from lung cancer screening with LDCT.

The false-positive rate is not trivial. For every 1000 patients screened, 250 people will have a positive LDCT finding but will not have lung cancer.19 Furthermore, about 1 in every 2000 individuals who screen positive, but who do not have lung cancer, die as a result of complications from the ensuing work-up.6
Annual LDCT screening increases the risk of radiation-induced cancer by approximately 0.05% over 10 years.21 The absolute risk is generally low but not insignificant. However, the mortality benefits previously outlined are significantly more robust in both absolute and relative terms vs the 10-year risk of radiation-induced cancer.
Lastly, it is important to note that the NELSON trial and NLST included a limited number of LDCT scans. Current guidelines for lung cancer screening with LDCT, including those from the USPSTF, recommend screening annually. We do not know the cumulative harm of annual LDCT over a 20- or 30-year period for those who would qualify (ie, current smokers).
If you screen, you must be able to act on the results
Effective screening programs should extend beyond the LDCT scan itself. The studies that have shown a benefit of LDCT were done at large academic centers that had the appropriate radiologic, pathologic, and surgical infrastructure to interpret and act on results and offer further diagnostic or treatment procedures.
Continue to: Prior to screening...
Prior to screening for lung cancer with LDCT, documentation of shared decision-making between the patient and the clinician is necessary.7 This discussion should include the potential benefits and harms of screening, potential results and likelihood of follow-up diagnostic testing, the false-positive rate of LDCT lung cancer screening, and cumulative radiation exposure. In addition, screening should be considered only if the patient is willing to be screened annually, is willing to pursue follow-up scans and procedures (including lung biopsy) if deemed necessary, and does not have comorbid conditions that significantly limit life expectancy.
Smoking cessation: The most important change to make
Smoking cessation is the single most important risk-modifying behavior to reduce one’s chance of developing lung cancer. At age 40, smokers have a 2-fold increase in all-cause mortality compared to age-matched nonsmokers. This rises to a 3-fold increase by the age of 70.16
Smoking cessation reduces the risk of lung cancer by 20% after 5 years, 30% to 50% after 10 years, and up to 70% after 15 years.24 In its guidelines, the American Thoracic Society recommends varenicline (Chantix) for all smokers to assist with smoking cessation.25
CASE
This 51-year-old patient with at least a 20-pack-year history of smoking should be commended for giving up smoking. Based on the USPSTF recommendations, he should be screened annually with LDCT for the next 10 years.
Screening to save more lives
The results of 2 large multicenter RCTs have led to the recent recommendation for lung cancer screening of high-risk adults with the use of LDCT. Screening with LDCT has been shown to reduce disease-related mortality and likely be cost effective in the long term.
Screening with LDCT should be part of a multidisciplinary system that has the infrastructure not only to perform the screening, but also to diagnose and appropriately follow up and treat patients whose results are concerning. The risk of false-positive results leading to increased anxiety, overdiagnosis, and unnecessary procedures points to the importance of proper patient selection, counseling, and shared decision-making. Smoking cessation remains the most important disease-modifying behavior one can make to reduce their risk for lung cancer.
CORRESPONDENCE
Carlton J. Covey, MD, 101 Bodin Circle, David Grant Medical Center, Travis Air Force Base, Fairfield, CA, 94545; carlcovey24@gmail.com
CASE
A 51-year-old man presents to your office to discuss lung cancer screening. He has a history of hypertension and prediabetes. His father died of lung cancer 5 years ago, at age 77. The patient stopped smoking soon thereafter; prior to that, he smoked 1 pack of cigarettes per day for 20 years. He wants to know if he should be screened for lung cancer.
The relative lack of symptoms during the early stages of lung cancer frequently results in a delayed diagnosis. This, and the speed at which the disease progresses, underscores the need for an effective screening modality. More than half of people with lung cancer die within 1 year of diagnosis.1 Excluding skin cancer, lung cancer is the second most commonly diagnosed cancer, and more people die of lung cancer than of colon, breast, and prostate cancers combined.2 In 2022, it was estimated that there would be 236,740 new cases of lung cancer and 130,180 deaths from lung cancer.1,2 The average age at diagnosis is 70 years.2

Screening modalities: Only 1 has demonstrated mortality benefit
In 1968, Wilson and Junger3 outlined the characteristics of the ideal screening test for the World Health Organization: it should limit risk to the patient, be sensitive for detecting the disease early in its course, limit false-positive results, be acceptable to the patient, and be inexpensive to the health system.3 For decades, several screening modalities for lung cancer were trialed to fit the above guidance, but many of them fell short of the most important outcome: the impact on mortality.
Sputum cytology. The use of sputum cytology, either in combination with or without chest radiography, is not recommended. Several randomized controlled trials (RCTs) have failed to demonstrate improved lung cancer detection or mortality reduction in patients screened with this modality.4
Chest radiography (CXR). Several studies have assessed the efficacy of CXR as a screening modality. The best known was the Prostate, Lung, Colon, Ovarian (PLCO) Trial.5 This multicenter RCT enrolled more than 154,000 participants, half of whom received CXR at baseline and then annually for 3 years; the other half continued usual care (no screening). After 13 years of follow-up, there were no significant differences in lung cancer detection or mortality rates between the 2 groups.5
Low-dose computed tomography (LDCT). Several major medical societies recommend LDCT to screen high-risk individuals for lung cancer (TABLE 16-10). Results from 2 major RCTs have guided these recommendations.

The National Lung Screening Trial (NLST) was a multicenter RCT comparing 2 screening tests for lung cancer.11 Approximately 54,000 high-risk participants were enrolled between 2002 and 2004 and were randomized to receive annual screening with either LDCT or single-view CXR. The trial was discontinued prematurely when investigators noted a 20% reduction in lung cancer mortality in the LDCT group vs the CXR group.12 This equates to 3 fewer deaths for every 1000 people screened with LDCT vs CXR. There was also a 6% reduction in all-cause mortality noted in the LDCT vs the CXR group.12
Continue to: The NELSON trial...
The NELSON trial, conducted between 2005 and 2015, studied more than 15,000 current or former smokers ages 50 to 74 years and compared LDCT screening at various intervals to no screening.13 After 10 years, lung cancer–related mortality was reduced by 24% (or 1 less death per 1000 person-years) in men who were screened vs their unscreened counterparts.13 In contrast to the NLST, in the NELSON trial, no significant difference in all-cause mortality was observed. Subgroup analysis of the relatively small population of women included in the NELSON trial suggested a 33% reduction in 10-year mortality; however, the difference was nonsignificant between the screened and unscreened groups.13
Each of these landmark studies had characteristics that could limit the results' generalizability to the US population. In the NELSON trial, more than 80% of the study participants were male. In both trials, there was significant underrepresentation of Black, Asian, Hispanic, and other non-White people.12,13 Furthermore, participants in these studies were of higher socioeconomic status than the general US screening-eligible population.
At this time, LDCT is the only lung cancer screening modality that has shown benefit for both disease-related and all-cause mortality, in the populations that were studied. Based on the NLST, the number needed to screen (NNS) with LDCT to prevent 1 lung cancer–related death is 308. The NNS to prevent 1 death from any cause is 219.6
Updated evidence has led to a consensus on screening criteria
Many national societies endorse annual screening with LDCT in high-risk individuals (TABLE 16-10). Risk assessment for the purpose of lung cancer screening includes a detailed review of smoking history and age. The risk of lung cancer increases with advancing age and with cumulative quantity and duration of smoking, but decreases with increasing time since quitting. Therefore, a detailed smoking history should include total number of pack-years, current smoking status, and, if applicable, when smoking cessation occurred.
In 2021, the US Preventive Services Task Force (USPSTF) updated their 2013 lung cancer screening recommendations, expanding the screening age range and lowering the smoking history threshold for triggering initiation of screening.6 The impetus for the update was emerging evidence from systematic reviews, RCTs, and the Cancer Intervention and Surveillance Modeling Network (CISNET) that could help to determine the optimal age for screening and identify high-risk groups. For example, the NELSON trial, combined with results from CISNET modeling data, showed an empirical benefit for screening those ages 50 to 55 years.6
Continue to: As a result...
As a result, the USPSTF now recommends annual lung cancer screening with LDCT for any adult ages 50 to 80 years who has a 20-pack-year smoking history and currently smokes or has quit within the past 15 years.6 Screening should be discontinued once a person has not smoked for 15 years, develops a health problem that substantially limits life expectancy, or is not willing to have curative lung surgery.6
Expanding the screening eligibility may also address racial and gender disparities in health care. Black people and women who smoke have a higher risk for lung cancer at a lower intensity of smoking.6
Following the USPSTF update, the American College of Chest Physicians and the Centers for Medicare and Medicaid Services published updated guidance that aligns with USPSTF’s recommendations to lower the age and pack-year qualifications for initiating screening.7,10 The American Cancer Society is currently reviewing its 2018 guidelines on lung cancer screening.14TABLE 16-10 summarizes the guidance on lung cancer screening from these medical societies.
Effective screening could save lives (and money)
A smoker’s risk for lung cancer is 20 times higher than that of a nonsmoker15,16; 55% of lung cancer deaths in women and 70% in men are attributed to smoking.17 Once diagnosed with lung cancer, more than 50% of people will die within 1 year.1 This underpins the need for a lung cancer screening modality that reduces mortality. Large RCTs, including the NLST and NELSONtrials, have shown that screening high-risk individuals with LDCT can significantly reduce lung cancer–related death when compared to no screening or screening with CXR alone.11,13
There is controversy surrounding the cost benefit of implementing a nationwide lung cancer screening program. However, recent use of microsimulation models has shown LDCT to be a cost-effective strategy, with an average cost of $81,000 per quality-adjusted life-year, which is below the threshold of $100,000 to be considered cost effective.18 Expanding the upper age limit for screening leads to a greater reduction in mortality but increases treatment costs and overdiagnosis rates, and overall does not improve quality-adjusted life-years.18
Continue to: Potential harms
Potential harms: False-positives and related complications
Screening for lung cancer is not without its risks. Harms from screening typically result from false-positive test results leading to overdiagnosis, anxiety and distress, unnecessary invasive tests or procedures, and increased costs.19TABLE 26,19-23 lists specific complications from lung cancer screening with LDCT.

The false-positive rate is not trivial. For every 1000 patients screened, 250 people will have a positive LDCT finding but will not have lung cancer.19 Furthermore, about 1 in every 2000 individuals who screen positive, but who do not have lung cancer, die as a result of complications from the ensuing work-up.6
Annual LDCT screening increases the risk of radiation-induced cancer by approximately 0.05% over 10 years.21 The absolute risk is generally low but not insignificant. However, the mortality benefits previously outlined are significantly more robust in both absolute and relative terms vs the 10-year risk of radiation-induced cancer.
Lastly, it is important to note that the NELSON trial and NLST included a limited number of LDCT scans. Current guidelines for lung cancer screening with LDCT, including those from the USPSTF, recommend screening annually. We do not know the cumulative harm of annual LDCT over a 20- or 30-year period for those who would qualify (ie, current smokers).
If you screen, you must be able to act on the results
Effective screening programs should extend beyond the LDCT scan itself. The studies that have shown a benefit of LDCT were done at large academic centers that had the appropriate radiologic, pathologic, and surgical infrastructure to interpret and act on results and offer further diagnostic or treatment procedures.
Continue to: Prior to screening...
Prior to screening for lung cancer with LDCT, documentation of shared decision-making between the patient and the clinician is necessary.7 This discussion should include the potential benefits and harms of screening, potential results and likelihood of follow-up diagnostic testing, the false-positive rate of LDCT lung cancer screening, and cumulative radiation exposure. In addition, screening should be considered only if the patient is willing to be screened annually, is willing to pursue follow-up scans and procedures (including lung biopsy) if deemed necessary, and does not have comorbid conditions that significantly limit life expectancy.
Smoking cessation: The most important change to make
Smoking cessation is the single most important risk-modifying behavior to reduce one’s chance of developing lung cancer. At age 40, smokers have a 2-fold increase in all-cause mortality compared to age-matched nonsmokers. This rises to a 3-fold increase by the age of 70.16
Smoking cessation reduces the risk of lung cancer by 20% after 5 years, 30% to 50% after 10 years, and up to 70% after 15 years.24 In its guidelines, the American Thoracic Society recommends varenicline (Chantix) for all smokers to assist with smoking cessation.25
CASE
This 51-year-old patient with at least a 20-pack-year history of smoking should be commended for giving up smoking. Based on the USPSTF recommendations, he should be screened annually with LDCT for the next 10 years.
Screening to save more lives
The results of 2 large multicenter RCTs have led to the recent recommendation for lung cancer screening of high-risk adults with the use of LDCT. Screening with LDCT has been shown to reduce disease-related mortality and likely be cost effective in the long term.
Screening with LDCT should be part of a multidisciplinary system that has the infrastructure not only to perform the screening, but also to diagnose and appropriately follow up and treat patients whose results are concerning. The risk of false-positive results leading to increased anxiety, overdiagnosis, and unnecessary procedures points to the importance of proper patient selection, counseling, and shared decision-making. Smoking cessation remains the most important disease-modifying behavior one can make to reduce their risk for lung cancer.
CORRESPONDENCE
Carlton J. Covey, MD, 101 Bodin Circle, David Grant Medical Center, Travis Air Force Base, Fairfield, CA, 94545; carlcovey24@gmail.com
1. National Cancer Institute. Cancer Stat Facts: lung and bronchus cancer. Accessed October 12, 2022. https://seer.cancer.gov/statfacts/html/lungb.html
2. American Cancer Society. Key statistics for lung cancer. Accessed October 12, 2022. https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html
3. Wilson JMG, Junger G. Principles and Practice of Screening for Disease. World Health Organization; 1968:21-25, 100. https://apps.who.int/iris/handle/10665/37650
4. Humphrey LL, Teutsch S, Johnson M. Lung cancer screening with sputum cytologic examination, chest radiography, and computed tomography: an update for the United States preventive services task force. Ann Intern Med. 2004;140:740-753. doi: 10.7326/0003-4819-140-9-200405040-00015
5. Oken MM, Hocking WG, Kvale PA, et al. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA. 2011;306:1865-1873. doi: 10.1001/jama.2011.1591
6. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:962-970. doi: 10.1001/jama.2021.1117
7. Centers for Medicare & Medicaid Services. Screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439R). Accessed October 14, 2022. www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=304
8. Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2018: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2018;68:297-316. doi: 10.3322/caac.21446
9. American Academy of Family Physicians. AAFP updates recommendation on lung cancer screening. Published April 6, 2021. Accessed October 12, 2022. www.aafp.org/news/health-of-the-public/20210406lungcancer.html
10. Mazzone PJ, Silvestri GA, Souter LH, et al. Screening for lung cancer: CHEST Guideline and Expert Panel Report. CHEST. 2021;160:E427-E494. doi: 10.1016/j.chest.2021.06.063
11. The National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi: 10.1056/NEJMoa1102873
12. The National Lung Screening Trial Research Team. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368:1980-1991. doi: 10.1056/NEJMoa1209120
13. de Koning HJ, van der Aalst CM, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503-513. doi: 10.1056/NEJMoa1911793
14. American Cancer Society. Lung cancer screening guidelines. Accessed October 14, 2022. www.cancer.org/health-care-professionals/american-cancer-society-prevention-early-detection-guidelines/lung-cancer-screening-guidelines.html
15. Pirie K, Peto R, Reeves GK, et al. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet. 2013;381:133-141. doi: 10.1016/S0140-6736(12)61720-6
16. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE
17. O’Keefe LM, Gemma T, Huxley R, et al. Smoking as a risk factor for lung cancer in women and men: a systematic review and meta-analysis. BMJ Open. 2018;8:e021611. doi: 10.1136/bmjopen-2018-021611
18. Criss SD, Pianpian C, Bastani M, et al. Cost-effectiveness analysis of lung cancer screening in the United States: a comparative modeling study. Ann Intern Med. 2019;171:796-805. doi: 10.7326/M19-0322
19. Lazris A, Roth RA. Lung cancer screening: pros and cons. Am Fam Physician. 2019;99:740-742.
20. Ali MU, Miller J, Peirson L, et al. Screening for lung cancer: a systematic review and meta-analysis. Prev Med. 2016;89:301-314. doi: 10.1016/j.ypmed.2016.04.015
21. Rampinelli C, De Marco P, Origgi D, et al. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: secondary analysis of trial data and risk-benefit analysis. BMJ. 2017;356:j347. doi: 10.1136/bmj.j347
22. Manser RL, Lethaby A, Irving LB, et al. Screening for lung cancer. Cochrane Database Syst Rev. 2013;CD001991. doi: 10.1002/14651858.CD001991.pub3
23. Mazzone PJ, Silvestri GA, Patel S, et al. Screening for lung cancer: CHEST guideline and expert panel report. CHEST. 2018;153:954-985. doi: 10.1016/j.chest.2018.01.016
24. US Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking. and Health. Smoking Cessation: A Report of the Surgeon General. US Department of Health and Human Services; 2020. www.ncbi.nlm.nih.gov/books/NBK555591/
25. Leone FT, Zhang Y, Evers-Casey S, et al, on behalf of the American Thoracic Society Assembly on Clinical Problems. Initiating pharmacologic treatment in tobacco-dependent adults: an official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202:e5-e31. doi: 10.1164/rccm.202005-1982ST
1. National Cancer Institute. Cancer Stat Facts: lung and bronchus cancer. Accessed October 12, 2022. https://seer.cancer.gov/statfacts/html/lungb.html
2. American Cancer Society. Key statistics for lung cancer. Accessed October 12, 2022. https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html
3. Wilson JMG, Junger G. Principles and Practice of Screening for Disease. World Health Organization; 1968:21-25, 100. https://apps.who.int/iris/handle/10665/37650
4. Humphrey LL, Teutsch S, Johnson M. Lung cancer screening with sputum cytologic examination, chest radiography, and computed tomography: an update for the United States preventive services task force. Ann Intern Med. 2004;140:740-753. doi: 10.7326/0003-4819-140-9-200405040-00015
5. Oken MM, Hocking WG, Kvale PA, et al. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA. 2011;306:1865-1873. doi: 10.1001/jama.2011.1591
6. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:962-970. doi: 10.1001/jama.2021.1117
7. Centers for Medicare & Medicaid Services. Screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439R). Accessed October 14, 2022. www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=304
8. Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2018: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2018;68:297-316. doi: 10.3322/caac.21446
9. American Academy of Family Physicians. AAFP updates recommendation on lung cancer screening. Published April 6, 2021. Accessed October 12, 2022. www.aafp.org/news/health-of-the-public/20210406lungcancer.html
10. Mazzone PJ, Silvestri GA, Souter LH, et al. Screening for lung cancer: CHEST Guideline and Expert Panel Report. CHEST. 2021;160:E427-E494. doi: 10.1016/j.chest.2021.06.063
11. The National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi: 10.1056/NEJMoa1102873
12. The National Lung Screening Trial Research Team. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368:1980-1991. doi: 10.1056/NEJMoa1209120
13. de Koning HJ, van der Aalst CM, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503-513. doi: 10.1056/NEJMoa1911793
14. American Cancer Society. Lung cancer screening guidelines. Accessed October 14, 2022. www.cancer.org/health-care-professionals/american-cancer-society-prevention-early-detection-guidelines/lung-cancer-screening-guidelines.html
15. Pirie K, Peto R, Reeves GK, et al. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet. 2013;381:133-141. doi: 10.1016/S0140-6736(12)61720-6
16. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE
17. O’Keefe LM, Gemma T, Huxley R, et al. Smoking as a risk factor for lung cancer in women and men: a systematic review and meta-analysis. BMJ Open. 2018;8:e021611. doi: 10.1136/bmjopen-2018-021611
18. Criss SD, Pianpian C, Bastani M, et al. Cost-effectiveness analysis of lung cancer screening in the United States: a comparative modeling study. Ann Intern Med. 2019;171:796-805. doi: 10.7326/M19-0322
19. Lazris A, Roth RA. Lung cancer screening: pros and cons. Am Fam Physician. 2019;99:740-742.
20. Ali MU, Miller J, Peirson L, et al. Screening for lung cancer: a systematic review and meta-analysis. Prev Med. 2016;89:301-314. doi: 10.1016/j.ypmed.2016.04.015
21. Rampinelli C, De Marco P, Origgi D, et al. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: secondary analysis of trial data and risk-benefit analysis. BMJ. 2017;356:j347. doi: 10.1136/bmj.j347
22. Manser RL, Lethaby A, Irving LB, et al. Screening for lung cancer. Cochrane Database Syst Rev. 2013;CD001991. doi: 10.1002/14651858.CD001991.pub3
23. Mazzone PJ, Silvestri GA, Patel S, et al. Screening for lung cancer: CHEST guideline and expert panel report. CHEST. 2018;153:954-985. doi: 10.1016/j.chest.2018.01.016
24. US Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking. and Health. Smoking Cessation: A Report of the Surgeon General. US Department of Health and Human Services; 2020. www.ncbi.nlm.nih.gov/books/NBK555591/
25. Leone FT, Zhang Y, Evers-Casey S, et al, on behalf of the American Thoracic Society Assembly on Clinical Problems. Initiating pharmacologic treatment in tobacco-dependent adults: an official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202:e5-e31. doi: 10.1164/rccm.202005-1982ST
PRACTICE RECOMMENDATIONS
› Recommend annual lung cancer screening for all highrisk adults ages 50 to 80 years using low-dose computed tomography. A
› Do not pursue lung cancer screening in patients who quit smoking ≥ 15 years ago, have a health problem that limits their life expectancy, or are unwilling to undergo lung surgery. A
› Recommend varenicline as first-line pharmacotherapy for smokers who would like to quit. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
