Article

Lung cancer screening: New evidence, updated guidance
- Author:
- Carlton J. Covey, MD
- Stephen D. Cagle Jr, MD
Emerging evidence supports lower thresholds for age and smoking history when screening for lung cancer. Here’s how the USPSTF and others have...
Article
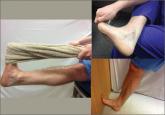
Plantar Fasciitis: How Best to Treat?
- Author:
- Carlton J. Covey, MD
- Mark D. Mulder, MD
In addition to stretching exercises and orthotics, consider steroid injections as part of your first-line treatment options. For recalcitrant pain...
Article
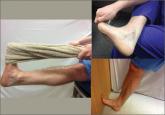
Plantar fasciitis: How best to treat?
- Author:
- Carlton J. Covey, MD
- Mark D. Mulder, MD
In addition to stretching exercises and orthotics, consider steroid injections as part of your first-line treatment options. For recalcitrant pain...
