User login
Prostate cancer is the most common malignancy in men, with an estimated 165,000 new prostate cancer diagnoses and 29,000 prostate cancer deaths occurring in the United States in 2018.1 Due to the widespread use of screening prostate-specific antigen (PSA), prostate cancer has been mainly diagnosed when the tumor is confined to the prostate. Despite definitive treatment of localized prostate cancer, some men develop systemic disease, either biochemical failure, as defined by rising PSA level, or metastatic disease.1 Several factors have been demonstrated to predict risk of relapse, including higher pretreatment PSA, higher Gleason score, and a greater anatomic extent of disease.2 In addition, the incidence of de novo metastatic prostate cancer was recently noted to be increasing. This may be due to changes in the United States Preventive Services Task Force prostate cancer screening guidelines in 2012, which recommended against screening for prostate cancer in men of any age. The updated 2018 guidelines recommend a discussion of the risks versus benefits of screening for prostate cancer for all men aged 55 to 69 years,recommend against screening for men older than 70 years, and do not have recommendations for high-risk subgroups.3
Androgen deprivation therapy (ADT) has been the cornerstone of therapy since 1941 for men with hormone-sensitive systemic disease, both in biochemically relapsed and metastatic disease.4,5 While more than 90% of patients respond to initial ADT, castration resistance is inevitable in some men.6,7 Prostate cancer will become castration-resistant typically after 18 to 24 months of ADT, with the majority of patients developing castration-resistant prostate cancer (CRPC) within 5 years of initiation of ADT.8
Pathogenesis
CRPC (previously called androgen independent prostate cancer) is defined as progression of disease despite serum total testosterone levels less than 50 ng/dL. CRPC is characterized by a rising PSA level and/or radiographic progression. One mechanism of castration resistance is genetic modification of the androgen receptor (AR), including increased expression of the wild-type AR.9 Alternatively, mutations of the steroid-binding domain may play a role in the development of castration resistance by allowing the AR to become activated by non-androgen steroid hormones or even paradoxically by antiandrogens. Studies suggest, however, that AR mutations may be seen in only 10% of prostate cancers that have developed castration resistance.10 The AR-V7 splice variant of the AR lacks an androgen binding site altogether, and may play an important role in castration resistance. In one study, the presence of this splice variant in circulating prostate cancer tumor cells predicted resistance to enzalutamide and abiraterone as well as poor outcomes.11 Intratumoral androgen synthesis also may play a role in the development of CRPC.12,13
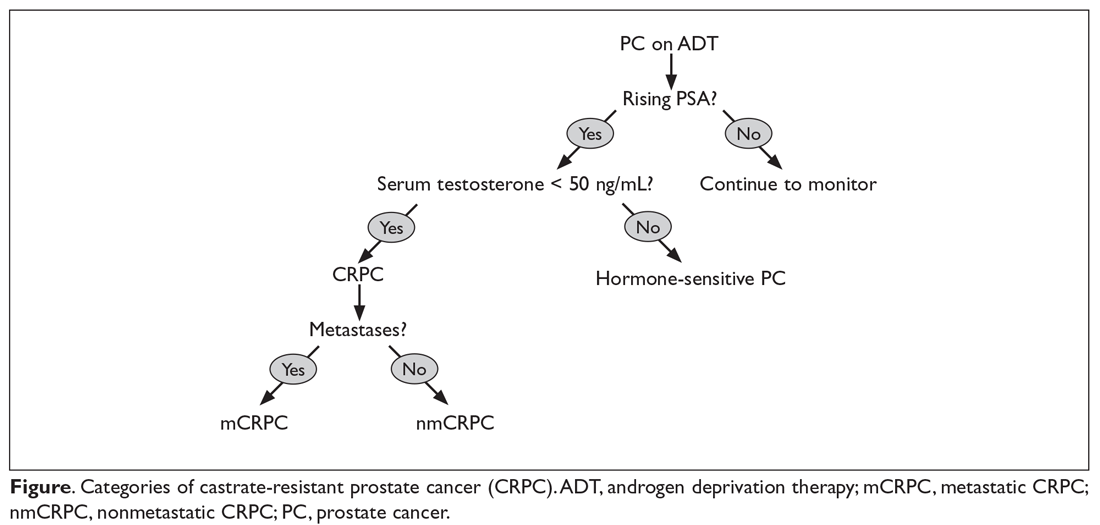
CRPC can be broadly categorized into 2 categories, metastatic (mCRPC) and nonmetastatic (nmCRPC; Figure). The exact proportion of patients entering CRPC at a nonmetastatic stage (M0) is largely unknown.14 In one study of patients at the time of diagnosis of CRPC, ≥ 84% of patients were shown to have metastases.8 In this article, we review key aspects of management of CRPC, including selection of first- and second-line therapy, and briefly discuss upcoming clinical trials.
Treatment of Nonmetastatic CRPC (M0 Disease)
Early identification of M0 CRPC is important because patients with nonmetastatic CRPC are at risk for metastasis, as demonstrated by Smith and colleagues.15 In this study that evaluated data from patients with nmCRPC in the placebo group (n = 331) of a randomized controlled trial, at 2 years 46% had developed ≥ 1 bone metastasis, 20% had died, and the median bone metastasis-free survival (MFS) was 25 months.15
Rapid PSA doubling time (PSADT) is linked to shorter time to metastasis in this group of patients. Patients with a PSADT of < 10 months had a risk for bone metastasis 12 times greater and a risk for death 4 times greater than patients with a PSADT of ≥ 10 months.16 Accordingly, observation should be reserved for those patients with a PSADT of ≥ 10 months.
Options for secondary hormonal therapy in those with a PSADT of ≤ 10 months include a first-generation antiandrogen (bicalutamide, flutamide, nilutamide), ketoconazole with hydrocortisone, and more recently second-generation antiandrogens (apalutamide or enzalutamide).
Bicalutamide competitively inhibits dihydrotestosterone and testosterone binding to the AR and is generally well-tolerated; it is given in conjunction with a GnRH agonist/castration.17 The use of other first-generation antiandrogens is limited mainly due to their toxicity profile. When compared to flutamide in a randomized, double-blinded control study, bicalutamide had significantly improved time to treatment failure.18 Due to promiscuous binding to AR, withdrawal of first-generation antiandrogen therapy has been associated with a biochemical response in a small proportion of patients, with response typically seen after 5 to 7 half-lives of the drug have elapsed.19
Although traditionally used as an antifungal agent, ketoconazole also inhibits androgen synthesis in the adrenal glands and acts as a direct cytotoxin to cancer cells.20 Ketoconazole (with hydrocortisone) has been considered as a treatment option, typically at the time of antiandrogen withdrawal. However, ketoconazole offers no survival benefit, and with the approval of abiraterone in M1 CRPC, its use has declined significantly.21 Additionally, ketoconazole poses a risk for severe hepatotoxicity and QT prolongation, and has significant interactions with numerous drugs, thereby limiting its use. Given the typically short duration of response to first-generation antiandrogens, the second-generation antiandrogens were developed and are associated with a significantly greater progression-free survival (PFS) in M0 CRPC.22,23
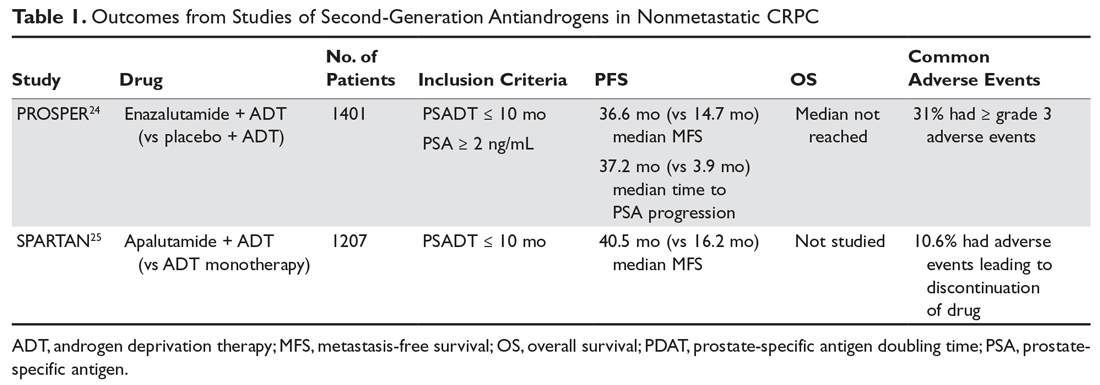
The second-generation antiandrogens enzalutamide and apalutamide not only competitively bind to the AR, inhibiting formation of the androgen/AR complex, but they also inhibit androgen/AR complex nuclear translocation and binding to nuclear DNA. In the PROSPER trial, enzalutamide significantly increased radiographic PFS and improved quality of life compared to placebo in chemotherapy-naive patients (Table 1).24 Apalutamide significantly increased MFS as well as PFS and time to PSA progression compared to placebo in the phase 3 SPARTAN trial.25 Apalutamide is generally well tolerated, with hypertension and rash being the most common severe adverse effects. Apalutamide also has less potential for central nervous system toxicities than enzalutamide. The recent approval of these agents is likely to change responses to subsequent treatments, especially in the metastatic setting.
Treatment of Metastatic CRPC (M1 Disease)
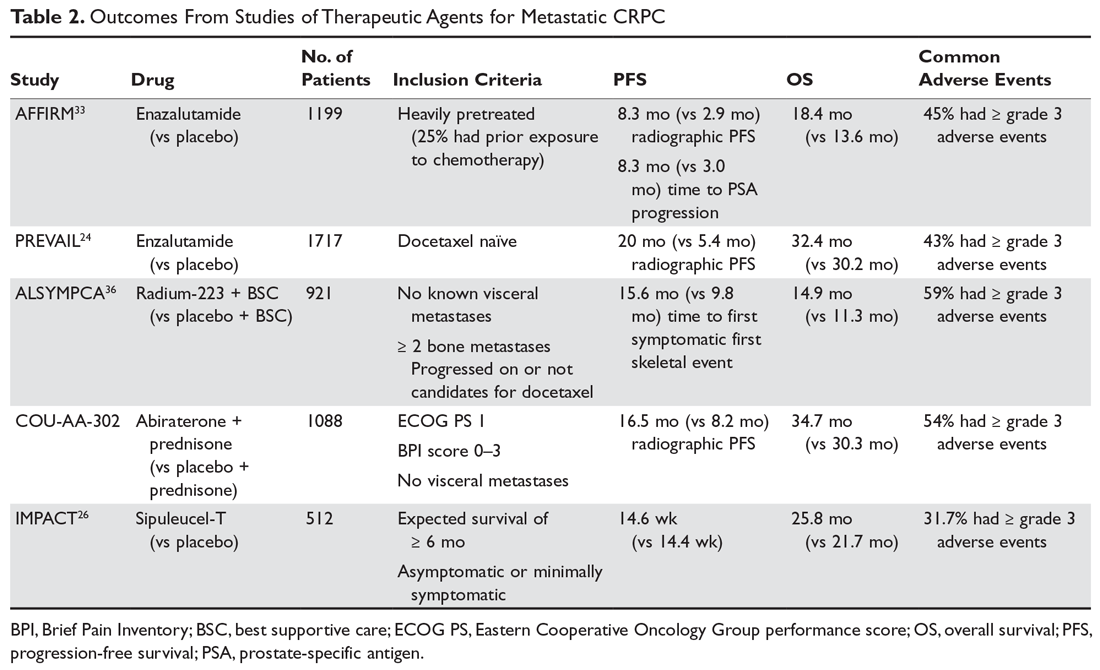
As with M0 CRPC, ADT should be continued in patients with mCRPC to maintain castration levels of testosterone while initiating additional treatments. Several drugs for the treatment of mCRPC have been approved by the US Food and Drug Administration (FDA) since 2010, including abiraterone with prednisone (or methylprednisolone), enzalutamide (but not apalutamide), radium-223, sipuleucel-T, and cabazitaxel (Table 2).
Given the availability of numerous treatment options for men with mCRPC, the sequencing of treatments should be based on careful consideration of the efficacy and adverse effect profiles of each drug as well as the anatomic and molecular characteristics of the cancer, comorbidities, and patient preference. If there is no evidence of visceral disease and the patient has an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1 with an estimated life expectancy of greater than 6 months and is minimally symptomatic, then treatment with either oral targeted agents or immunotherapy with sipuleucel-T is considered appropriate.
Sipuleucel-T is an autologous dendritic cell vaccination designed to enhance T-cell–mediated response to prostatic acid phosphatase (PAP). The treatments are prepared from leukapheresed host mononuclear cells that are then exposed to PAP fused to granulocyte-macrophage colony-stimulating factor. The activated dendritic cells are then infused back into the host once every 2 weeks for a total of 3 treatments. The main side effects of this treatment include chills, fever, and headache, but it is generally well-tolerated and has demonstrated a survival benefit.26
Both enzalutamide and abiraterone (abiraterone given with physiologic-dose steroid replacement) confer a survival benefit in chemotherapy-naive patients with M1 CRPC. Per the PREVAIL study, enzalutamide (when compared to placebo) offers a median improvement in overall survival (OS) by about 2 months and in radiographic PFS by about 14.6 months.24 Abiraterone blocks the synthesis of androgens via inhibition of CYP17 in the testes and adrenal glands. Abiraterone also confers an overall survival advantage for patients with M1 CRPC who are chemotherapy-naïve, with an estimated 25% decrease in the risk of death (hazard ratio, 0.75, P = 0.009) when compared to prednisone.27
In patients with symptomatic M1 CRPC who have visceral disease or rapidly progressive disease and who are candidates for chemotherapy, docetaxel is frequently used and is given concurrently with steroids. Docetaxel has been given for up to 10 cycles in clinical trials (assuming no progression of disease or dose-limiting toxicities were observed), and at least 6 cycles of treatment are recommended. When compared to mitoxantrone plus prednisone in the TAX 327 phase 3 trial, docetaxel plus prednisone offered a significant OS benefit of about 3 months (19.2 months versus 16.3 months).28 For patients who are not candidates for docetaxel (eg, due to preexisting peripheral neuropathy), cabazitaxel should be considered. OS is similar for mCRPC with docetaxel versus cabazitaxel when given in the first-line setting.29 Additionally, cabazitaxel dosed at 20 mg/m2 is noninferior to cabazitaxel dosed at 25 mg/m2, and the lower dose is associated with lower rates of peripheral neuropathy.30 Cabazitaxel should also be considered for mCRPC that has progressed following treatment with docetaxel, with improved OS and PFS when compared to treatment with mitoxantrone and prednisone in this setting, as shown in the TROPIC study.31 Mitoxantrone given with prednisone has been shown to improve quality of life, but it is associated with significant cardiac toxicity. Additionally, mitoxantrone does not improve disease-free survival or OS in chemotherapy-naive patients32 or in patients who have progressed on docetaxel, and therefore should not be given to patients prior to a taxane chemotherapy unless the patient absolutely cannot tolerate docetaxel or cabazitaxel.
Once a patient’s prostate cancer progresses following treatment with a taxane, the sequence in which to administer subsequent therapies should involve careful consideration of previous treatments and duration of response to each of these treatments. Both enzalutamide and abiraterone are FDA-approved for use following treatment with chemotherapy. Per the AFFIRM trial, heavily pre-treated patients (including those who have received docetaxel) have a median 5-month OS benefit with enzalutamide compared to placebo.33 Another study of M1 CRPC patients who had previously received docetaxel demonstrated an OS benefit with abiraterone (versus placebo),34 but this regimen has limited benefit in patients who have previously received both docetaxel and enzalutamide.35 A rechallenge with docetaxel therapy also can be considered if the patient’s disease responded to docetaxel in the metastatic hormone-sensitive setting.
If the patient’s metastases are limited to bone (ie, no visceral disease), then radiotherapy with radium-223 should be considered. Radium-223 is an alpha-emitting calcium-mimetic radioactive compound that tracks to bone to delay the onset of symptoms from bone metastases.36 Radium-223 also confers a median OS benefit of about 3 months.37 However, this treatment is often limited by preexisting cytopenias.
Diethylstilbestrol (1 mg daily) competes with androgens for AR binding and is also cytotoxic to androgen-sensitive and insensitive prostate cancer cells. While its efficacy is similar to bicalutamide in terms of PSA response rate and median response duration, diethylstilbestrol is associated with significantly more cardiovascular toxicity, including stroke, pulmonary embolism, and heart failure, and its use is therefore limited.38 The glucocorticoids—prednisone (5 mg orally twice daily), dexamethasone (0.5 mg daily), and hydrocortisone (40 mg daily)—inhibit pituitary synthesis of adrenocorticotropic hormone, resulting in decreased adrenal androgen synthesis. Data suggest that among the glucocorticoids, dexamethasone monotherapy may produce superior response rates compared to prednisone monotherapy.39 While the glucocorticoids do produce a PSA response, prolong time to disease progression, and can provide symptomatic relief (eg, from bone pain), they have not been shown to confer a survival benefit and therefore are not commonly used as monotherapy.
Future of CRPC Treatment
Patients with CRPC should be considered for clinical trials when available. These patients’ tumors should be assessed with next-generation sequencing for analysis of microsatellite instability (MSI) or mismatch repair (MMR) as well as the presence of other potentially targetable mutations, as this information may bring into consideration additional investigational as well as FDA-approved treatment options. As of May 2017, immunotherapy with pembrolizumab is approved for patients whose prostate cancer is deficient in MMR or has a high MSI burden based on a study of 12 solid tumor types (including prostate cancer) with deficient MMR.40 Additionally, for patients whose tumor has ≥ 1% programmed death ligand 1 (PD-L1) expression, pembrolizumab has a 17% overall response rate and confers stability of disease in 35%, with a median response duration of 13.5 months.41 Cabozantinib is a mesenchymal epithelial transition (MET) kinase and vascular endothelial growth factor receptor (VEGF-R) inhibitor. When used in heavily pre-treated patients with mCRPC, it showed a radiographic PFS benefit but no survival benefit over prednisone monotherapy.42 One study showed that for patients whose mCRPC had a homozygous deletion and/or a deleterious mutation in the homologous recombination repair genes BRCA1/2, ATM, and CHEK2 or the Fanconi anemia genes, the response rate to the poly ADP ribose polymerase (PARP) inhibitor olaparib was 88%, with a 100% response rate in those with BRCA2 mutations.43 Furthermore, mutations in these DNA repair genes predict increased sensitivity to platinum-based chemotherapy.
Supportive Care
Zoledronic acid or denosumab are FDA approved for men with CRPC and bone metastasis based on the ability of these agents to delay skeletal-related events, including pathologic fracture and spinal cord compression.46 Bisphosphonates, however, do not decrease the incidence of bone metastases.47 And while denosumab does delay the time to first bone metastasis in nmCRPC (particularly in patients with a PSADT of ≤ 6 months), it does not improve OS.48 Other supportive measures include exercise and nutrition. Moderate aerobic exercise for 150 minutes in addition to 2 or 3 strength training sessions per week is recommended by the American College of Sport Medicine to combat cancer-related fatigue.49 There are currently no dietary changes that are routinely recommended to improve the outcome of prostate cancer, but a study noted a shorter biochemical failure–free survival in men with prostate cancer who were obese and consumed a diet high in saturated fat.50
Conclusion
Prostate cancer affects more men in the United States than any other cancer. Once a patient is started on hormone therapy, in all likelihood their prostate cancer will become castration-resistant. Once prostate cancer has developed hormone resistance, there are a host of further treatment options available, including further hormone therapy, chemotherapy, immunotherapy, radiation therapy, bone-targeting agents, and clinical trials. Determining the appropriate sequence in which to use these therapies requires knowledge of the natural history of CRPC, the indications for changing therapies, the mechanism of action and adverse event profile of each treatment, and the optimal time to enroll in a clinical trial.
1. Pound CR, Partin AW, Epstein JI, Walsh PC. Prostate-specific antigen after anatomic radical retropubic prostatectomy. Patterns of recurrence and cancer control. Urol Clin North Am. 1997;24:395-406.
2. Caire AA, Sun L, Ode O, et al. Delayed prostate-specific antigen recurrence after radical prostatectomy: how to identify and what are their clinical outcomes? Urology. 2009;74:643-647.
3. US Preventive Services Task Force, Grossman DC, Curry SJ, Owens DK, et al. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1901-1913.
4. Huggins C, Hodges CV. Studies on prostatic cancer. I: The effects of castration, of estrogen, and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293-297.
5. Huggins C, Stevens RE Jr, Hodges CV. Studies on prostatic cancer. II: The effects of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941;43:209-223.
6. Pomerantz M, Kantoff P. Clinical progression to castration recurrent prostate cancer. In: Tindall DJ, James M, eds. Androgen Action in Prostate Cancer. New York: Springer; 2009:57-72.
7. Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract. 2011;65:1180-1192.
8. Sharifi N, Dahut WL, Steinberg SM, et al. A retrospective study of the time to clinical endpoints for advanced prostate cancer. BJU Int. 2005;96:985-989.
9. Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33-39.
10. Taplin ME, Rajeshkumar B, Halabi S, et al. Androgen receptor mutations in androgen-independent prostate cancer: Cancer and Leukemia Group B Study 9663. J Clin Oncol. 2003;21:2673-2678.
11. Antonarakis ES, Lu C, Luber B, et al. Clinical significance of androgen receptor splice variant-7 (AR-V7) mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first- and second-line abiraterone and enzalutamide. J Clin Oncol. 2017;35:2149-2156.
12. Kahn B, Collazo J, Kyprianou N. Androgen receptor as a driver of therapeutic resistance in advanced prostate cancer. Int J Biol Sci. 2014;10:588-595.
13. Logothetis CJ, Gallick GE, Maity SN, et al. Molecular classification of prostate cancer progression: foundation for marker-driven treatment of prostate cancer. Cancer Discov. 2013;3:849-861
14. Tombal B. Non-metastatic CRPC and asymptomatic metastatic CRPC: which treatment for which patient? Ann Oncol. 2012;23(suppl 10):251-258
15. Smith MR, Cook R, Lee KA, et al. Disease and host characteristics as predictors of time to first bone metastasis and death in men with progressive castration-resistant non-metastatic prostate cancer. Cancer. 2011;117:2077-2085.
16. Metwalli AR, Rosner IL, Cullen J, et al. Elevated alkaline phosphatase velocity strongly predicts overall survival and the risk of bone metastases in castrate-resistant prostate cancer. Urol Oncol. 2014;32:761-768
17. Akaza H, Yamaguchi A, Matsuda T, et al. Superior anti-tumor efficacy of bicalutamide 80mg in combination with luteinizing hormone-releasing hormone (LHRH) against versus LHRH agonist monotherapy as first line treatment for advanced prostate cancer: Interim results of a randomized study in Japanese patients. J Clin Oncol. 2004;34:20-28
18. Schellhammer P, Patterson AL, Sharifi R, et al. A controlled trial of bicalutamide versus flutamide, each in combination with luteinizing hormone-releasing hormone analogue therapy, in patients with advanced prostate cancer. Urology. 1995;45(5):745-752.
19. Sartor AO, Tangen CM, Hussain MH, et al. Antiandrogen withdrawal in castrate-refractory prostate cancer: a Southwest Oncology Group Trial (SWOG 9426). Cancer. 2008;112:2393-2400.
20. Eichenberger T, Trachtenberg J, Toor P, et al. Ketoconazole: a possible direct cytotoxic effect on prostate carcinoma cells. J Urol. 1989;141:190-191.
21. Small EJ, Halabi S, Dawson NA, et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583). J Clin Oncol. 2004;22:1025-1033.
22. Penson DF, Armstrong AJ, Concepcion R, et al. Enzalutamide versus bicalutamide in castration resistant prostate cancer: the STRIVE trial. J Clin Oncol. 2016;34:2098-2106.
23. Shore ND, Chowdhury S, Villers A, et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): a randomized, double-blind, phase 2 study. Lancet Oncol. 2016;199:147-154.
24. Beer TM, Armstrong AJ, Rathkopf D, et al. Enzalutamide in men with chemotherapy-naïve castration-resistant prostate cancer: extended analysis of the phase 3 PREVAIL study. Eur Urol. 2017;71:151-154.
25. Smith MR, Saad F, Chowdhury S; SPARTAN Investigators, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378:1408-1418.
26. Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411-422.
27. Ryan CJ, Smith MR, de Bono JS, et al. Randomized phase 3 trial of abiraterone acetate in men with metastatic castration-resistant prostate cancer and no prior chemotherapy. N Engl J Med. 2013;368:138-148
28. Berthold DR, Pond GR, Soban F, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol. 2008;26:242-245.
29. Oudard S, Fizazi K, Sengelov L, et al. Cabazitaxel versus docetaxel as first-line therapy for patients with metastatic castration-resistant prostate cancer: a randomized phase III trial—FIRSTANA. J Clin Oncol. 2017;35:3189-3197.
30. Eisenberger M, Hardy-Bessard A-C, Kim CS, et al. Phase III study comparing a reduced dose of cabazitaxel (20 mg/m2) and the currently approved dose (25 mg/m2) in postdocetaxel patients with metastatic castration-resistant prostate cancer—PROSELICA. J Clin Oncol. 2017;35:3198-3206.
31. de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):P1147-1154.
32. Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004;351:1502-1512.
33. Fizazi K, Scher HI, Miller K, et al. Effect of enzalutamide on time to first skeletal-related event, pain, and quality of life in men with castration-resistant prostate cancer: results from the randomised, phase 3 AFFIRM trial. Lancet Oncol. 2014;15:1147-1156.
34. de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995-2005.
35. Loriot Y, Bianchini D, Ileana E, et al. Antitumor activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide. Ann Oncol. 2013;24:1807-1812.
36. Sartor O, Coleman R, Nilsson S, et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double blind, randomized trial. Lancet Oncol. 2014;15:738-746.
37. Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013; 369:213-223.
38. Turo R, Smolski M, Esler R, et al. Diethylstilboestrol for the treatment of prostate cancer: past, present and future. Scand J Urol. 2014;48:4-14.
39. Venkitaraman R, Lorente D, Murthy V. A randomized phase 2 trial of dexamethasone versus prednisolone in castration-resistant prostate cancer. Eur Urol. 2015 67:673-679.
40. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409-413.
41. Hansen AR, Massard C, Ott PA, et al. Pembrolizumab for advanced prostate adenocarcinoma: findings of the KEYNOTE-028 study. Ann Oncol. 2018;29:1807-1813.
42. Smith M, De Bono J, Sternberg C, et al. Phase III study of cabozantinib in previously treated metastatic castration-resistant prostate cancer: COMET-1. J Clin Oncol. 2016;34:3005-3013.
43. Mateo J, Carreira S, Sandhu S, et al. DNA-Repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373:1697-1708.
44. Kentepozidis N, Soultati A, Giassas S, et al. Paclitaxel in combination with carboplatin as salvage treatment in patients with castration-resistant prostate cancer: a Hellenic oncology research group multicenter phase II study. Cancer Chemother Pharmacol. 2012;70:161-168.
45. Smith M, De Bono J, Sternberg C, et al. Phase III study of cabozantinib in previously treated metastatic castration-resistant prostate cancer: COMET-1. J Clin Oncol. 2016;34:3005-3013.
46. Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458-1468.
47. Wirth M, Tammela T, Cicalese V, et al. Prevention of bone metastases in patients with high-risk nonmetastatic prostate cancer treated with zoledronic acid: efficacy and safety results of the Zometa European Study (ZEUS). Eur Urol. 2015;67:482-491.
48. Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastases-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomized, placebo-controlled trial. Lancet. 2012;379(9810):39-46.
49. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409-1426.
50. Strom SS, Yamamura Y, Flores-Sandoval FN, et al. Prostate cancer in Mexican-Americans: identification of risk factors. Prostate. 2008;68:563-570.
Prostate cancer is the most common malignancy in men, with an estimated 165,000 new prostate cancer diagnoses and 29,000 prostate cancer deaths occurring in the United States in 2018.1 Due to the widespread use of screening prostate-specific antigen (PSA), prostate cancer has been mainly diagnosed when the tumor is confined to the prostate. Despite definitive treatment of localized prostate cancer, some men develop systemic disease, either biochemical failure, as defined by rising PSA level, or metastatic disease.1 Several factors have been demonstrated to predict risk of relapse, including higher pretreatment PSA, higher Gleason score, and a greater anatomic extent of disease.2 In addition, the incidence of de novo metastatic prostate cancer was recently noted to be increasing. This may be due to changes in the United States Preventive Services Task Force prostate cancer screening guidelines in 2012, which recommended against screening for prostate cancer in men of any age. The updated 2018 guidelines recommend a discussion of the risks versus benefits of screening for prostate cancer for all men aged 55 to 69 years,recommend against screening for men older than 70 years, and do not have recommendations for high-risk subgroups.3
Androgen deprivation therapy (ADT) has been the cornerstone of therapy since 1941 for men with hormone-sensitive systemic disease, both in biochemically relapsed and metastatic disease.4,5 While more than 90% of patients respond to initial ADT, castration resistance is inevitable in some men.6,7 Prostate cancer will become castration-resistant typically after 18 to 24 months of ADT, with the majority of patients developing castration-resistant prostate cancer (CRPC) within 5 years of initiation of ADT.8
Pathogenesis
CRPC (previously called androgen independent prostate cancer) is defined as progression of disease despite serum total testosterone levels less than 50 ng/dL. CRPC is characterized by a rising PSA level and/or radiographic progression. One mechanism of castration resistance is genetic modification of the androgen receptor (AR), including increased expression of the wild-type AR.9 Alternatively, mutations of the steroid-binding domain may play a role in the development of castration resistance by allowing the AR to become activated by non-androgen steroid hormones or even paradoxically by antiandrogens. Studies suggest, however, that AR mutations may be seen in only 10% of prostate cancers that have developed castration resistance.10 The AR-V7 splice variant of the AR lacks an androgen binding site altogether, and may play an important role in castration resistance. In one study, the presence of this splice variant in circulating prostate cancer tumor cells predicted resistance to enzalutamide and abiraterone as well as poor outcomes.11 Intratumoral androgen synthesis also may play a role in the development of CRPC.12,13
CRPC can be broadly categorized into 2 categories, metastatic (mCRPC) and nonmetastatic (nmCRPC; Figure). The exact proportion of patients entering CRPC at a nonmetastatic stage (M0) is largely unknown.14 In one study of patients at the time of diagnosis of CRPC, ≥ 84% of patients were shown to have metastases.8 In this article, we review key aspects of management of CRPC, including selection of first- and second-line therapy, and briefly discuss upcoming clinical trials.
Treatment of Nonmetastatic CRPC (M0 Disease)
Early identification of M0 CRPC is important because patients with nonmetastatic CRPC are at risk for metastasis, as demonstrated by Smith and colleagues.15 In this study that evaluated data from patients with nmCRPC in the placebo group (n = 331) of a randomized controlled trial, at 2 years 46% had developed ≥ 1 bone metastasis, 20% had died, and the median bone metastasis-free survival (MFS) was 25 months.15
Rapid PSA doubling time (PSADT) is linked to shorter time to metastasis in this group of patients. Patients with a PSADT of < 10 months had a risk for bone metastasis 12 times greater and a risk for death 4 times greater than patients with a PSADT of ≥ 10 months.16 Accordingly, observation should be reserved for those patients with a PSADT of ≥ 10 months.
Options for secondary hormonal therapy in those with a PSADT of ≤ 10 months include a first-generation antiandrogen (bicalutamide, flutamide, nilutamide), ketoconazole with hydrocortisone, and more recently second-generation antiandrogens (apalutamide or enzalutamide).
Bicalutamide competitively inhibits dihydrotestosterone and testosterone binding to the AR and is generally well-tolerated; it is given in conjunction with a GnRH agonist/castration.17 The use of other first-generation antiandrogens is limited mainly due to their toxicity profile. When compared to flutamide in a randomized, double-blinded control study, bicalutamide had significantly improved time to treatment failure.18 Due to promiscuous binding to AR, withdrawal of first-generation antiandrogen therapy has been associated with a biochemical response in a small proportion of patients, with response typically seen after 5 to 7 half-lives of the drug have elapsed.19
Although traditionally used as an antifungal agent, ketoconazole also inhibits androgen synthesis in the adrenal glands and acts as a direct cytotoxin to cancer cells.20 Ketoconazole (with hydrocortisone) has been considered as a treatment option, typically at the time of antiandrogen withdrawal. However, ketoconazole offers no survival benefit, and with the approval of abiraterone in M1 CRPC, its use has declined significantly.21 Additionally, ketoconazole poses a risk for severe hepatotoxicity and QT prolongation, and has significant interactions with numerous drugs, thereby limiting its use. Given the typically short duration of response to first-generation antiandrogens, the second-generation antiandrogens were developed and are associated with a significantly greater progression-free survival (PFS) in M0 CRPC.22,23
The second-generation antiandrogens enzalutamide and apalutamide not only competitively bind to the AR, inhibiting formation of the androgen/AR complex, but they also inhibit androgen/AR complex nuclear translocation and binding to nuclear DNA. In the PROSPER trial, enzalutamide significantly increased radiographic PFS and improved quality of life compared to placebo in chemotherapy-naive patients (Table 1).24 Apalutamide significantly increased MFS as well as PFS and time to PSA progression compared to placebo in the phase 3 SPARTAN trial.25 Apalutamide is generally well tolerated, with hypertension and rash being the most common severe adverse effects. Apalutamide also has less potential for central nervous system toxicities than enzalutamide. The recent approval of these agents is likely to change responses to subsequent treatments, especially in the metastatic setting.
Treatment of Metastatic CRPC (M1 Disease)
As with M0 CRPC, ADT should be continued in patients with mCRPC to maintain castration levels of testosterone while initiating additional treatments. Several drugs for the treatment of mCRPC have been approved by the US Food and Drug Administration (FDA) since 2010, including abiraterone with prednisone (or methylprednisolone), enzalutamide (but not apalutamide), radium-223, sipuleucel-T, and cabazitaxel (Table 2).
Given the availability of numerous treatment options for men with mCRPC, the sequencing of treatments should be based on careful consideration of the efficacy and adverse effect profiles of each drug as well as the anatomic and molecular characteristics of the cancer, comorbidities, and patient preference. If there is no evidence of visceral disease and the patient has an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1 with an estimated life expectancy of greater than 6 months and is minimally symptomatic, then treatment with either oral targeted agents or immunotherapy with sipuleucel-T is considered appropriate.
Sipuleucel-T is an autologous dendritic cell vaccination designed to enhance T-cell–mediated response to prostatic acid phosphatase (PAP). The treatments are prepared from leukapheresed host mononuclear cells that are then exposed to PAP fused to granulocyte-macrophage colony-stimulating factor. The activated dendritic cells are then infused back into the host once every 2 weeks for a total of 3 treatments. The main side effects of this treatment include chills, fever, and headache, but it is generally well-tolerated and has demonstrated a survival benefit.26
Both enzalutamide and abiraterone (abiraterone given with physiologic-dose steroid replacement) confer a survival benefit in chemotherapy-naive patients with M1 CRPC. Per the PREVAIL study, enzalutamide (when compared to placebo) offers a median improvement in overall survival (OS) by about 2 months and in radiographic PFS by about 14.6 months.24 Abiraterone blocks the synthesis of androgens via inhibition of CYP17 in the testes and adrenal glands. Abiraterone also confers an overall survival advantage for patients with M1 CRPC who are chemotherapy-naïve, with an estimated 25% decrease in the risk of death (hazard ratio, 0.75, P = 0.009) when compared to prednisone.27
In patients with symptomatic M1 CRPC who have visceral disease or rapidly progressive disease and who are candidates for chemotherapy, docetaxel is frequently used and is given concurrently with steroids. Docetaxel has been given for up to 10 cycles in clinical trials (assuming no progression of disease or dose-limiting toxicities were observed), and at least 6 cycles of treatment are recommended. When compared to mitoxantrone plus prednisone in the TAX 327 phase 3 trial, docetaxel plus prednisone offered a significant OS benefit of about 3 months (19.2 months versus 16.3 months).28 For patients who are not candidates for docetaxel (eg, due to preexisting peripheral neuropathy), cabazitaxel should be considered. OS is similar for mCRPC with docetaxel versus cabazitaxel when given in the first-line setting.29 Additionally, cabazitaxel dosed at 20 mg/m2 is noninferior to cabazitaxel dosed at 25 mg/m2, and the lower dose is associated with lower rates of peripheral neuropathy.30 Cabazitaxel should also be considered for mCRPC that has progressed following treatment with docetaxel, with improved OS and PFS when compared to treatment with mitoxantrone and prednisone in this setting, as shown in the TROPIC study.31 Mitoxantrone given with prednisone has been shown to improve quality of life, but it is associated with significant cardiac toxicity. Additionally, mitoxantrone does not improve disease-free survival or OS in chemotherapy-naive patients32 or in patients who have progressed on docetaxel, and therefore should not be given to patients prior to a taxane chemotherapy unless the patient absolutely cannot tolerate docetaxel or cabazitaxel.
Once a patient’s prostate cancer progresses following treatment with a taxane, the sequence in which to administer subsequent therapies should involve careful consideration of previous treatments and duration of response to each of these treatments. Both enzalutamide and abiraterone are FDA-approved for use following treatment with chemotherapy. Per the AFFIRM trial, heavily pre-treated patients (including those who have received docetaxel) have a median 5-month OS benefit with enzalutamide compared to placebo.33 Another study of M1 CRPC patients who had previously received docetaxel demonstrated an OS benefit with abiraterone (versus placebo),34 but this regimen has limited benefit in patients who have previously received both docetaxel and enzalutamide.35 A rechallenge with docetaxel therapy also can be considered if the patient’s disease responded to docetaxel in the metastatic hormone-sensitive setting.
If the patient’s metastases are limited to bone (ie, no visceral disease), then radiotherapy with radium-223 should be considered. Radium-223 is an alpha-emitting calcium-mimetic radioactive compound that tracks to bone to delay the onset of symptoms from bone metastases.36 Radium-223 also confers a median OS benefit of about 3 months.37 However, this treatment is often limited by preexisting cytopenias.
Diethylstilbestrol (1 mg daily) competes with androgens for AR binding and is also cytotoxic to androgen-sensitive and insensitive prostate cancer cells. While its efficacy is similar to bicalutamide in terms of PSA response rate and median response duration, diethylstilbestrol is associated with significantly more cardiovascular toxicity, including stroke, pulmonary embolism, and heart failure, and its use is therefore limited.38 The glucocorticoids—prednisone (5 mg orally twice daily), dexamethasone (0.5 mg daily), and hydrocortisone (40 mg daily)—inhibit pituitary synthesis of adrenocorticotropic hormone, resulting in decreased adrenal androgen synthesis. Data suggest that among the glucocorticoids, dexamethasone monotherapy may produce superior response rates compared to prednisone monotherapy.39 While the glucocorticoids do produce a PSA response, prolong time to disease progression, and can provide symptomatic relief (eg, from bone pain), they have not been shown to confer a survival benefit and therefore are not commonly used as monotherapy.
Future of CRPC Treatment
Patients with CRPC should be considered for clinical trials when available. These patients’ tumors should be assessed with next-generation sequencing for analysis of microsatellite instability (MSI) or mismatch repair (MMR) as well as the presence of other potentially targetable mutations, as this information may bring into consideration additional investigational as well as FDA-approved treatment options. As of May 2017, immunotherapy with pembrolizumab is approved for patients whose prostate cancer is deficient in MMR or has a high MSI burden based on a study of 12 solid tumor types (including prostate cancer) with deficient MMR.40 Additionally, for patients whose tumor has ≥ 1% programmed death ligand 1 (PD-L1) expression, pembrolizumab has a 17% overall response rate and confers stability of disease in 35%, with a median response duration of 13.5 months.41 Cabozantinib is a mesenchymal epithelial transition (MET) kinase and vascular endothelial growth factor receptor (VEGF-R) inhibitor. When used in heavily pre-treated patients with mCRPC, it showed a radiographic PFS benefit but no survival benefit over prednisone monotherapy.42 One study showed that for patients whose mCRPC had a homozygous deletion and/or a deleterious mutation in the homologous recombination repair genes BRCA1/2, ATM, and CHEK2 or the Fanconi anemia genes, the response rate to the poly ADP ribose polymerase (PARP) inhibitor olaparib was 88%, with a 100% response rate in those with BRCA2 mutations.43 Furthermore, mutations in these DNA repair genes predict increased sensitivity to platinum-based chemotherapy.
Supportive Care
Zoledronic acid or denosumab are FDA approved for men with CRPC and bone metastasis based on the ability of these agents to delay skeletal-related events, including pathologic fracture and spinal cord compression.46 Bisphosphonates, however, do not decrease the incidence of bone metastases.47 And while denosumab does delay the time to first bone metastasis in nmCRPC (particularly in patients with a PSADT of ≤ 6 months), it does not improve OS.48 Other supportive measures include exercise and nutrition. Moderate aerobic exercise for 150 minutes in addition to 2 or 3 strength training sessions per week is recommended by the American College of Sport Medicine to combat cancer-related fatigue.49 There are currently no dietary changes that are routinely recommended to improve the outcome of prostate cancer, but a study noted a shorter biochemical failure–free survival in men with prostate cancer who were obese and consumed a diet high in saturated fat.50
Conclusion
Prostate cancer affects more men in the United States than any other cancer. Once a patient is started on hormone therapy, in all likelihood their prostate cancer will become castration-resistant. Once prostate cancer has developed hormone resistance, there are a host of further treatment options available, including further hormone therapy, chemotherapy, immunotherapy, radiation therapy, bone-targeting agents, and clinical trials. Determining the appropriate sequence in which to use these therapies requires knowledge of the natural history of CRPC, the indications for changing therapies, the mechanism of action and adverse event profile of each treatment, and the optimal time to enroll in a clinical trial.
Prostate cancer is the most common malignancy in men, with an estimated 165,000 new prostate cancer diagnoses and 29,000 prostate cancer deaths occurring in the United States in 2018.1 Due to the widespread use of screening prostate-specific antigen (PSA), prostate cancer has been mainly diagnosed when the tumor is confined to the prostate. Despite definitive treatment of localized prostate cancer, some men develop systemic disease, either biochemical failure, as defined by rising PSA level, or metastatic disease.1 Several factors have been demonstrated to predict risk of relapse, including higher pretreatment PSA, higher Gleason score, and a greater anatomic extent of disease.2 In addition, the incidence of de novo metastatic prostate cancer was recently noted to be increasing. This may be due to changes in the United States Preventive Services Task Force prostate cancer screening guidelines in 2012, which recommended against screening for prostate cancer in men of any age. The updated 2018 guidelines recommend a discussion of the risks versus benefits of screening for prostate cancer for all men aged 55 to 69 years,recommend against screening for men older than 70 years, and do not have recommendations for high-risk subgroups.3
Androgen deprivation therapy (ADT) has been the cornerstone of therapy since 1941 for men with hormone-sensitive systemic disease, both in biochemically relapsed and metastatic disease.4,5 While more than 90% of patients respond to initial ADT, castration resistance is inevitable in some men.6,7 Prostate cancer will become castration-resistant typically after 18 to 24 months of ADT, with the majority of patients developing castration-resistant prostate cancer (CRPC) within 5 years of initiation of ADT.8
Pathogenesis
CRPC (previously called androgen independent prostate cancer) is defined as progression of disease despite serum total testosterone levels less than 50 ng/dL. CRPC is characterized by a rising PSA level and/or radiographic progression. One mechanism of castration resistance is genetic modification of the androgen receptor (AR), including increased expression of the wild-type AR.9 Alternatively, mutations of the steroid-binding domain may play a role in the development of castration resistance by allowing the AR to become activated by non-androgen steroid hormones or even paradoxically by antiandrogens. Studies suggest, however, that AR mutations may be seen in only 10% of prostate cancers that have developed castration resistance.10 The AR-V7 splice variant of the AR lacks an androgen binding site altogether, and may play an important role in castration resistance. In one study, the presence of this splice variant in circulating prostate cancer tumor cells predicted resistance to enzalutamide and abiraterone as well as poor outcomes.11 Intratumoral androgen synthesis also may play a role in the development of CRPC.12,13
CRPC can be broadly categorized into 2 categories, metastatic (mCRPC) and nonmetastatic (nmCRPC; Figure). The exact proportion of patients entering CRPC at a nonmetastatic stage (M0) is largely unknown.14 In one study of patients at the time of diagnosis of CRPC, ≥ 84% of patients were shown to have metastases.8 In this article, we review key aspects of management of CRPC, including selection of first- and second-line therapy, and briefly discuss upcoming clinical trials.
Treatment of Nonmetastatic CRPC (M0 Disease)
Early identification of M0 CRPC is important because patients with nonmetastatic CRPC are at risk for metastasis, as demonstrated by Smith and colleagues.15 In this study that evaluated data from patients with nmCRPC in the placebo group (n = 331) of a randomized controlled trial, at 2 years 46% had developed ≥ 1 bone metastasis, 20% had died, and the median bone metastasis-free survival (MFS) was 25 months.15
Rapid PSA doubling time (PSADT) is linked to shorter time to metastasis in this group of patients. Patients with a PSADT of < 10 months had a risk for bone metastasis 12 times greater and a risk for death 4 times greater than patients with a PSADT of ≥ 10 months.16 Accordingly, observation should be reserved for those patients with a PSADT of ≥ 10 months.
Options for secondary hormonal therapy in those with a PSADT of ≤ 10 months include a first-generation antiandrogen (bicalutamide, flutamide, nilutamide), ketoconazole with hydrocortisone, and more recently second-generation antiandrogens (apalutamide or enzalutamide).
Bicalutamide competitively inhibits dihydrotestosterone and testosterone binding to the AR and is generally well-tolerated; it is given in conjunction with a GnRH agonist/castration.17 The use of other first-generation antiandrogens is limited mainly due to their toxicity profile. When compared to flutamide in a randomized, double-blinded control study, bicalutamide had significantly improved time to treatment failure.18 Due to promiscuous binding to AR, withdrawal of first-generation antiandrogen therapy has been associated with a biochemical response in a small proportion of patients, with response typically seen after 5 to 7 half-lives of the drug have elapsed.19
Although traditionally used as an antifungal agent, ketoconazole also inhibits androgen synthesis in the adrenal glands and acts as a direct cytotoxin to cancer cells.20 Ketoconazole (with hydrocortisone) has been considered as a treatment option, typically at the time of antiandrogen withdrawal. However, ketoconazole offers no survival benefit, and with the approval of abiraterone in M1 CRPC, its use has declined significantly.21 Additionally, ketoconazole poses a risk for severe hepatotoxicity and QT prolongation, and has significant interactions with numerous drugs, thereby limiting its use. Given the typically short duration of response to first-generation antiandrogens, the second-generation antiandrogens were developed and are associated with a significantly greater progression-free survival (PFS) in M0 CRPC.22,23
The second-generation antiandrogens enzalutamide and apalutamide not only competitively bind to the AR, inhibiting formation of the androgen/AR complex, but they also inhibit androgen/AR complex nuclear translocation and binding to nuclear DNA. In the PROSPER trial, enzalutamide significantly increased radiographic PFS and improved quality of life compared to placebo in chemotherapy-naive patients (Table 1).24 Apalutamide significantly increased MFS as well as PFS and time to PSA progression compared to placebo in the phase 3 SPARTAN trial.25 Apalutamide is generally well tolerated, with hypertension and rash being the most common severe adverse effects. Apalutamide also has less potential for central nervous system toxicities than enzalutamide. The recent approval of these agents is likely to change responses to subsequent treatments, especially in the metastatic setting.
Treatment of Metastatic CRPC (M1 Disease)
As with M0 CRPC, ADT should be continued in patients with mCRPC to maintain castration levels of testosterone while initiating additional treatments. Several drugs for the treatment of mCRPC have been approved by the US Food and Drug Administration (FDA) since 2010, including abiraterone with prednisone (or methylprednisolone), enzalutamide (but not apalutamide), radium-223, sipuleucel-T, and cabazitaxel (Table 2).
Given the availability of numerous treatment options for men with mCRPC, the sequencing of treatments should be based on careful consideration of the efficacy and adverse effect profiles of each drug as well as the anatomic and molecular characteristics of the cancer, comorbidities, and patient preference. If there is no evidence of visceral disease and the patient has an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1 with an estimated life expectancy of greater than 6 months and is minimally symptomatic, then treatment with either oral targeted agents or immunotherapy with sipuleucel-T is considered appropriate.
Sipuleucel-T is an autologous dendritic cell vaccination designed to enhance T-cell–mediated response to prostatic acid phosphatase (PAP). The treatments are prepared from leukapheresed host mononuclear cells that are then exposed to PAP fused to granulocyte-macrophage colony-stimulating factor. The activated dendritic cells are then infused back into the host once every 2 weeks for a total of 3 treatments. The main side effects of this treatment include chills, fever, and headache, but it is generally well-tolerated and has demonstrated a survival benefit.26
Both enzalutamide and abiraterone (abiraterone given with physiologic-dose steroid replacement) confer a survival benefit in chemotherapy-naive patients with M1 CRPC. Per the PREVAIL study, enzalutamide (when compared to placebo) offers a median improvement in overall survival (OS) by about 2 months and in radiographic PFS by about 14.6 months.24 Abiraterone blocks the synthesis of androgens via inhibition of CYP17 in the testes and adrenal glands. Abiraterone also confers an overall survival advantage for patients with M1 CRPC who are chemotherapy-naïve, with an estimated 25% decrease in the risk of death (hazard ratio, 0.75, P = 0.009) when compared to prednisone.27
In patients with symptomatic M1 CRPC who have visceral disease or rapidly progressive disease and who are candidates for chemotherapy, docetaxel is frequently used and is given concurrently with steroids. Docetaxel has been given for up to 10 cycles in clinical trials (assuming no progression of disease or dose-limiting toxicities were observed), and at least 6 cycles of treatment are recommended. When compared to mitoxantrone plus prednisone in the TAX 327 phase 3 trial, docetaxel plus prednisone offered a significant OS benefit of about 3 months (19.2 months versus 16.3 months).28 For patients who are not candidates for docetaxel (eg, due to preexisting peripheral neuropathy), cabazitaxel should be considered. OS is similar for mCRPC with docetaxel versus cabazitaxel when given in the first-line setting.29 Additionally, cabazitaxel dosed at 20 mg/m2 is noninferior to cabazitaxel dosed at 25 mg/m2, and the lower dose is associated with lower rates of peripheral neuropathy.30 Cabazitaxel should also be considered for mCRPC that has progressed following treatment with docetaxel, with improved OS and PFS when compared to treatment with mitoxantrone and prednisone in this setting, as shown in the TROPIC study.31 Mitoxantrone given with prednisone has been shown to improve quality of life, but it is associated with significant cardiac toxicity. Additionally, mitoxantrone does not improve disease-free survival or OS in chemotherapy-naive patients32 or in patients who have progressed on docetaxel, and therefore should not be given to patients prior to a taxane chemotherapy unless the patient absolutely cannot tolerate docetaxel or cabazitaxel.
Once a patient’s prostate cancer progresses following treatment with a taxane, the sequence in which to administer subsequent therapies should involve careful consideration of previous treatments and duration of response to each of these treatments. Both enzalutamide and abiraterone are FDA-approved for use following treatment with chemotherapy. Per the AFFIRM trial, heavily pre-treated patients (including those who have received docetaxel) have a median 5-month OS benefit with enzalutamide compared to placebo.33 Another study of M1 CRPC patients who had previously received docetaxel demonstrated an OS benefit with abiraterone (versus placebo),34 but this regimen has limited benefit in patients who have previously received both docetaxel and enzalutamide.35 A rechallenge with docetaxel therapy also can be considered if the patient’s disease responded to docetaxel in the metastatic hormone-sensitive setting.
If the patient’s metastases are limited to bone (ie, no visceral disease), then radiotherapy with radium-223 should be considered. Radium-223 is an alpha-emitting calcium-mimetic radioactive compound that tracks to bone to delay the onset of symptoms from bone metastases.36 Radium-223 also confers a median OS benefit of about 3 months.37 However, this treatment is often limited by preexisting cytopenias.
Diethylstilbestrol (1 mg daily) competes with androgens for AR binding and is also cytotoxic to androgen-sensitive and insensitive prostate cancer cells. While its efficacy is similar to bicalutamide in terms of PSA response rate and median response duration, diethylstilbestrol is associated with significantly more cardiovascular toxicity, including stroke, pulmonary embolism, and heart failure, and its use is therefore limited.38 The glucocorticoids—prednisone (5 mg orally twice daily), dexamethasone (0.5 mg daily), and hydrocortisone (40 mg daily)—inhibit pituitary synthesis of adrenocorticotropic hormone, resulting in decreased adrenal androgen synthesis. Data suggest that among the glucocorticoids, dexamethasone monotherapy may produce superior response rates compared to prednisone monotherapy.39 While the glucocorticoids do produce a PSA response, prolong time to disease progression, and can provide symptomatic relief (eg, from bone pain), they have not been shown to confer a survival benefit and therefore are not commonly used as monotherapy.
Future of CRPC Treatment
Patients with CRPC should be considered for clinical trials when available. These patients’ tumors should be assessed with next-generation sequencing for analysis of microsatellite instability (MSI) or mismatch repair (MMR) as well as the presence of other potentially targetable mutations, as this information may bring into consideration additional investigational as well as FDA-approved treatment options. As of May 2017, immunotherapy with pembrolizumab is approved for patients whose prostate cancer is deficient in MMR or has a high MSI burden based on a study of 12 solid tumor types (including prostate cancer) with deficient MMR.40 Additionally, for patients whose tumor has ≥ 1% programmed death ligand 1 (PD-L1) expression, pembrolizumab has a 17% overall response rate and confers stability of disease in 35%, with a median response duration of 13.5 months.41 Cabozantinib is a mesenchymal epithelial transition (MET) kinase and vascular endothelial growth factor receptor (VEGF-R) inhibitor. When used in heavily pre-treated patients with mCRPC, it showed a radiographic PFS benefit but no survival benefit over prednisone monotherapy.42 One study showed that for patients whose mCRPC had a homozygous deletion and/or a deleterious mutation in the homologous recombination repair genes BRCA1/2, ATM, and CHEK2 or the Fanconi anemia genes, the response rate to the poly ADP ribose polymerase (PARP) inhibitor olaparib was 88%, with a 100% response rate in those with BRCA2 mutations.43 Furthermore, mutations in these DNA repair genes predict increased sensitivity to platinum-based chemotherapy.
Supportive Care
Zoledronic acid or denosumab are FDA approved for men with CRPC and bone metastasis based on the ability of these agents to delay skeletal-related events, including pathologic fracture and spinal cord compression.46 Bisphosphonates, however, do not decrease the incidence of bone metastases.47 And while denosumab does delay the time to first bone metastasis in nmCRPC (particularly in patients with a PSADT of ≤ 6 months), it does not improve OS.48 Other supportive measures include exercise and nutrition. Moderate aerobic exercise for 150 minutes in addition to 2 or 3 strength training sessions per week is recommended by the American College of Sport Medicine to combat cancer-related fatigue.49 There are currently no dietary changes that are routinely recommended to improve the outcome of prostate cancer, but a study noted a shorter biochemical failure–free survival in men with prostate cancer who were obese and consumed a diet high in saturated fat.50
Conclusion
Prostate cancer affects more men in the United States than any other cancer. Once a patient is started on hormone therapy, in all likelihood their prostate cancer will become castration-resistant. Once prostate cancer has developed hormone resistance, there are a host of further treatment options available, including further hormone therapy, chemotherapy, immunotherapy, radiation therapy, bone-targeting agents, and clinical trials. Determining the appropriate sequence in which to use these therapies requires knowledge of the natural history of CRPC, the indications for changing therapies, the mechanism of action and adverse event profile of each treatment, and the optimal time to enroll in a clinical trial.
1. Pound CR, Partin AW, Epstein JI, Walsh PC. Prostate-specific antigen after anatomic radical retropubic prostatectomy. Patterns of recurrence and cancer control. Urol Clin North Am. 1997;24:395-406.
2. Caire AA, Sun L, Ode O, et al. Delayed prostate-specific antigen recurrence after radical prostatectomy: how to identify and what are their clinical outcomes? Urology. 2009;74:643-647.
3. US Preventive Services Task Force, Grossman DC, Curry SJ, Owens DK, et al. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1901-1913.
4. Huggins C, Hodges CV. Studies on prostatic cancer. I: The effects of castration, of estrogen, and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293-297.
5. Huggins C, Stevens RE Jr, Hodges CV. Studies on prostatic cancer. II: The effects of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941;43:209-223.
6. Pomerantz M, Kantoff P. Clinical progression to castration recurrent prostate cancer. In: Tindall DJ, James M, eds. Androgen Action in Prostate Cancer. New York: Springer; 2009:57-72.
7. Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract. 2011;65:1180-1192.
8. Sharifi N, Dahut WL, Steinberg SM, et al. A retrospective study of the time to clinical endpoints for advanced prostate cancer. BJU Int. 2005;96:985-989.
9. Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33-39.
10. Taplin ME, Rajeshkumar B, Halabi S, et al. Androgen receptor mutations in androgen-independent prostate cancer: Cancer and Leukemia Group B Study 9663. J Clin Oncol. 2003;21:2673-2678.
11. Antonarakis ES, Lu C, Luber B, et al. Clinical significance of androgen receptor splice variant-7 (AR-V7) mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first- and second-line abiraterone and enzalutamide. J Clin Oncol. 2017;35:2149-2156.
12. Kahn B, Collazo J, Kyprianou N. Androgen receptor as a driver of therapeutic resistance in advanced prostate cancer. Int J Biol Sci. 2014;10:588-595.
13. Logothetis CJ, Gallick GE, Maity SN, et al. Molecular classification of prostate cancer progression: foundation for marker-driven treatment of prostate cancer. Cancer Discov. 2013;3:849-861
14. Tombal B. Non-metastatic CRPC and asymptomatic metastatic CRPC: which treatment for which patient? Ann Oncol. 2012;23(suppl 10):251-258
15. Smith MR, Cook R, Lee KA, et al. Disease and host characteristics as predictors of time to first bone metastasis and death in men with progressive castration-resistant non-metastatic prostate cancer. Cancer. 2011;117:2077-2085.
16. Metwalli AR, Rosner IL, Cullen J, et al. Elevated alkaline phosphatase velocity strongly predicts overall survival and the risk of bone metastases in castrate-resistant prostate cancer. Urol Oncol. 2014;32:761-768
17. Akaza H, Yamaguchi A, Matsuda T, et al. Superior anti-tumor efficacy of bicalutamide 80mg in combination with luteinizing hormone-releasing hormone (LHRH) against versus LHRH agonist monotherapy as first line treatment for advanced prostate cancer: Interim results of a randomized study in Japanese patients. J Clin Oncol. 2004;34:20-28
18. Schellhammer P, Patterson AL, Sharifi R, et al. A controlled trial of bicalutamide versus flutamide, each in combination with luteinizing hormone-releasing hormone analogue therapy, in patients with advanced prostate cancer. Urology. 1995;45(5):745-752.
19. Sartor AO, Tangen CM, Hussain MH, et al. Antiandrogen withdrawal in castrate-refractory prostate cancer: a Southwest Oncology Group Trial (SWOG 9426). Cancer. 2008;112:2393-2400.
20. Eichenberger T, Trachtenberg J, Toor P, et al. Ketoconazole: a possible direct cytotoxic effect on prostate carcinoma cells. J Urol. 1989;141:190-191.
21. Small EJ, Halabi S, Dawson NA, et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583). J Clin Oncol. 2004;22:1025-1033.
22. Penson DF, Armstrong AJ, Concepcion R, et al. Enzalutamide versus bicalutamide in castration resistant prostate cancer: the STRIVE trial. J Clin Oncol. 2016;34:2098-2106.
23. Shore ND, Chowdhury S, Villers A, et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): a randomized, double-blind, phase 2 study. Lancet Oncol. 2016;199:147-154.
24. Beer TM, Armstrong AJ, Rathkopf D, et al. Enzalutamide in men with chemotherapy-naïve castration-resistant prostate cancer: extended analysis of the phase 3 PREVAIL study. Eur Urol. 2017;71:151-154.
25. Smith MR, Saad F, Chowdhury S; SPARTAN Investigators, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378:1408-1418.
26. Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411-422.
27. Ryan CJ, Smith MR, de Bono JS, et al. Randomized phase 3 trial of abiraterone acetate in men with metastatic castration-resistant prostate cancer and no prior chemotherapy. N Engl J Med. 2013;368:138-148
28. Berthold DR, Pond GR, Soban F, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol. 2008;26:242-245.
29. Oudard S, Fizazi K, Sengelov L, et al. Cabazitaxel versus docetaxel as first-line therapy for patients with metastatic castration-resistant prostate cancer: a randomized phase III trial—FIRSTANA. J Clin Oncol. 2017;35:3189-3197.
30. Eisenberger M, Hardy-Bessard A-C, Kim CS, et al. Phase III study comparing a reduced dose of cabazitaxel (20 mg/m2) and the currently approved dose (25 mg/m2) in postdocetaxel patients with metastatic castration-resistant prostate cancer—PROSELICA. J Clin Oncol. 2017;35:3198-3206.
31. de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):P1147-1154.
32. Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004;351:1502-1512.
33. Fizazi K, Scher HI, Miller K, et al. Effect of enzalutamide on time to first skeletal-related event, pain, and quality of life in men with castration-resistant prostate cancer: results from the randomised, phase 3 AFFIRM trial. Lancet Oncol. 2014;15:1147-1156.
34. de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995-2005.
35. Loriot Y, Bianchini D, Ileana E, et al. Antitumor activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide. Ann Oncol. 2013;24:1807-1812.
36. Sartor O, Coleman R, Nilsson S, et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double blind, randomized trial. Lancet Oncol. 2014;15:738-746.
37. Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013; 369:213-223.
38. Turo R, Smolski M, Esler R, et al. Diethylstilboestrol for the treatment of prostate cancer: past, present and future. Scand J Urol. 2014;48:4-14.
39. Venkitaraman R, Lorente D, Murthy V. A randomized phase 2 trial of dexamethasone versus prednisolone in castration-resistant prostate cancer. Eur Urol. 2015 67:673-679.
40. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409-413.
41. Hansen AR, Massard C, Ott PA, et al. Pembrolizumab for advanced prostate adenocarcinoma: findings of the KEYNOTE-028 study. Ann Oncol. 2018;29:1807-1813.
42. Smith M, De Bono J, Sternberg C, et al. Phase III study of cabozantinib in previously treated metastatic castration-resistant prostate cancer: COMET-1. J Clin Oncol. 2016;34:3005-3013.
43. Mateo J, Carreira S, Sandhu S, et al. DNA-Repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373:1697-1708.
44. Kentepozidis N, Soultati A, Giassas S, et al. Paclitaxel in combination with carboplatin as salvage treatment in patients with castration-resistant prostate cancer: a Hellenic oncology research group multicenter phase II study. Cancer Chemother Pharmacol. 2012;70:161-168.
45. Smith M, De Bono J, Sternberg C, et al. Phase III study of cabozantinib in previously treated metastatic castration-resistant prostate cancer: COMET-1. J Clin Oncol. 2016;34:3005-3013.
46. Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458-1468.
47. Wirth M, Tammela T, Cicalese V, et al. Prevention of bone metastases in patients with high-risk nonmetastatic prostate cancer treated with zoledronic acid: efficacy and safety results of the Zometa European Study (ZEUS). Eur Urol. 2015;67:482-491.
48. Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastases-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomized, placebo-controlled trial. Lancet. 2012;379(9810):39-46.
49. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409-1426.
50. Strom SS, Yamamura Y, Flores-Sandoval FN, et al. Prostate cancer in Mexican-Americans: identification of risk factors. Prostate. 2008;68:563-570.
1. Pound CR, Partin AW, Epstein JI, Walsh PC. Prostate-specific antigen after anatomic radical retropubic prostatectomy. Patterns of recurrence and cancer control. Urol Clin North Am. 1997;24:395-406.
2. Caire AA, Sun L, Ode O, et al. Delayed prostate-specific antigen recurrence after radical prostatectomy: how to identify and what are their clinical outcomes? Urology. 2009;74:643-647.
3. US Preventive Services Task Force, Grossman DC, Curry SJ, Owens DK, et al. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1901-1913.
4. Huggins C, Hodges CV. Studies on prostatic cancer. I: The effects of castration, of estrogen, and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293-297.
5. Huggins C, Stevens RE Jr, Hodges CV. Studies on prostatic cancer. II: The effects of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941;43:209-223.
6. Pomerantz M, Kantoff P. Clinical progression to castration recurrent prostate cancer. In: Tindall DJ, James M, eds. Androgen Action in Prostate Cancer. New York: Springer; 2009:57-72.
7. Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract. 2011;65:1180-1192.
8. Sharifi N, Dahut WL, Steinberg SM, et al. A retrospective study of the time to clinical endpoints for advanced prostate cancer. BJU Int. 2005;96:985-989.
9. Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33-39.
10. Taplin ME, Rajeshkumar B, Halabi S, et al. Androgen receptor mutations in androgen-independent prostate cancer: Cancer and Leukemia Group B Study 9663. J Clin Oncol. 2003;21:2673-2678.
11. Antonarakis ES, Lu C, Luber B, et al. Clinical significance of androgen receptor splice variant-7 (AR-V7) mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first- and second-line abiraterone and enzalutamide. J Clin Oncol. 2017;35:2149-2156.
12. Kahn B, Collazo J, Kyprianou N. Androgen receptor as a driver of therapeutic resistance in advanced prostate cancer. Int J Biol Sci. 2014;10:588-595.
13. Logothetis CJ, Gallick GE, Maity SN, et al. Molecular classification of prostate cancer progression: foundation for marker-driven treatment of prostate cancer. Cancer Discov. 2013;3:849-861
14. Tombal B. Non-metastatic CRPC and asymptomatic metastatic CRPC: which treatment for which patient? Ann Oncol. 2012;23(suppl 10):251-258
15. Smith MR, Cook R, Lee KA, et al. Disease and host characteristics as predictors of time to first bone metastasis and death in men with progressive castration-resistant non-metastatic prostate cancer. Cancer. 2011;117:2077-2085.
16. Metwalli AR, Rosner IL, Cullen J, et al. Elevated alkaline phosphatase velocity strongly predicts overall survival and the risk of bone metastases in castrate-resistant prostate cancer. Urol Oncol. 2014;32:761-768
17. Akaza H, Yamaguchi A, Matsuda T, et al. Superior anti-tumor efficacy of bicalutamide 80mg in combination with luteinizing hormone-releasing hormone (LHRH) against versus LHRH agonist monotherapy as first line treatment for advanced prostate cancer: Interim results of a randomized study in Japanese patients. J Clin Oncol. 2004;34:20-28
18. Schellhammer P, Patterson AL, Sharifi R, et al. A controlled trial of bicalutamide versus flutamide, each in combination with luteinizing hormone-releasing hormone analogue therapy, in patients with advanced prostate cancer. Urology. 1995;45(5):745-752.
19. Sartor AO, Tangen CM, Hussain MH, et al. Antiandrogen withdrawal in castrate-refractory prostate cancer: a Southwest Oncology Group Trial (SWOG 9426). Cancer. 2008;112:2393-2400.
20. Eichenberger T, Trachtenberg J, Toor P, et al. Ketoconazole: a possible direct cytotoxic effect on prostate carcinoma cells. J Urol. 1989;141:190-191.
21. Small EJ, Halabi S, Dawson NA, et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583). J Clin Oncol. 2004;22:1025-1033.
22. Penson DF, Armstrong AJ, Concepcion R, et al. Enzalutamide versus bicalutamide in castration resistant prostate cancer: the STRIVE trial. J Clin Oncol. 2016;34:2098-2106.
23. Shore ND, Chowdhury S, Villers A, et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): a randomized, double-blind, phase 2 study. Lancet Oncol. 2016;199:147-154.
24. Beer TM, Armstrong AJ, Rathkopf D, et al. Enzalutamide in men with chemotherapy-naïve castration-resistant prostate cancer: extended analysis of the phase 3 PREVAIL study. Eur Urol. 2017;71:151-154.
25. Smith MR, Saad F, Chowdhury S; SPARTAN Investigators, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378:1408-1418.
26. Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411-422.
27. Ryan CJ, Smith MR, de Bono JS, et al. Randomized phase 3 trial of abiraterone acetate in men with metastatic castration-resistant prostate cancer and no prior chemotherapy. N Engl J Med. 2013;368:138-148
28. Berthold DR, Pond GR, Soban F, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol. 2008;26:242-245.
29. Oudard S, Fizazi K, Sengelov L, et al. Cabazitaxel versus docetaxel as first-line therapy for patients with metastatic castration-resistant prostate cancer: a randomized phase III trial—FIRSTANA. J Clin Oncol. 2017;35:3189-3197.
30. Eisenberger M, Hardy-Bessard A-C, Kim CS, et al. Phase III study comparing a reduced dose of cabazitaxel (20 mg/m2) and the currently approved dose (25 mg/m2) in postdocetaxel patients with metastatic castration-resistant prostate cancer—PROSELICA. J Clin Oncol. 2017;35:3198-3206.
31. de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):P1147-1154.
32. Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004;351:1502-1512.
33. Fizazi K, Scher HI, Miller K, et al. Effect of enzalutamide on time to first skeletal-related event, pain, and quality of life in men with castration-resistant prostate cancer: results from the randomised, phase 3 AFFIRM trial. Lancet Oncol. 2014;15:1147-1156.
34. de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995-2005.
35. Loriot Y, Bianchini D, Ileana E, et al. Antitumor activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide. Ann Oncol. 2013;24:1807-1812.
36. Sartor O, Coleman R, Nilsson S, et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double blind, randomized trial. Lancet Oncol. 2014;15:738-746.
37. Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013; 369:213-223.
38. Turo R, Smolski M, Esler R, et al. Diethylstilboestrol for the treatment of prostate cancer: past, present and future. Scand J Urol. 2014;48:4-14.
39. Venkitaraman R, Lorente D, Murthy V. A randomized phase 2 trial of dexamethasone versus prednisolone in castration-resistant prostate cancer. Eur Urol. 2015 67:673-679.
40. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409-413.
41. Hansen AR, Massard C, Ott PA, et al. Pembrolizumab for advanced prostate adenocarcinoma: findings of the KEYNOTE-028 study. Ann Oncol. 2018;29:1807-1813.
42. Smith M, De Bono J, Sternberg C, et al. Phase III study of cabozantinib in previously treated metastatic castration-resistant prostate cancer: COMET-1. J Clin Oncol. 2016;34:3005-3013.
43. Mateo J, Carreira S, Sandhu S, et al. DNA-Repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373:1697-1708.
44. Kentepozidis N, Soultati A, Giassas S, et al. Paclitaxel in combination with carboplatin as salvage treatment in patients with castration-resistant prostate cancer: a Hellenic oncology research group multicenter phase II study. Cancer Chemother Pharmacol. 2012;70:161-168.
45. Smith M, De Bono J, Sternberg C, et al. Phase III study of cabozantinib in previously treated metastatic castration-resistant prostate cancer: COMET-1. J Clin Oncol. 2016;34:3005-3013.
46. Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458-1468.
47. Wirth M, Tammela T, Cicalese V, et al. Prevention of bone metastases in patients with high-risk nonmetastatic prostate cancer treated with zoledronic acid: efficacy and safety results of the Zometa European Study (ZEUS). Eur Urol. 2015;67:482-491.
48. Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastases-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomized, placebo-controlled trial. Lancet. 2012;379(9810):39-46.
49. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409-1426.
50. Strom SS, Yamamura Y, Flores-Sandoval FN, et al. Prostate cancer in Mexican-Americans: identification of risk factors. Prostate. 2008;68:563-570.