User login
Recently, there has been a heightened interest in improving the quality and safety of the management of diabetes and hyperglycemia in the hospital.1 While observational data strongly suggests an association of hyperglycemia with morbidity and mortality in adults on general medicine and surgery units, clinical research has not yet defined the best practices for managing hyperglycemia in the hospital outside the intensive care unit (ICU). As a result, many physicians do not have a well‐formulated approach to managing hyperglycemia in the noncritically ill hospital patient, and the use of insulin therapy to attain targeted blood glucose (BG) control is often subject to practice variability, leading to suboptimal glycemic outcomes.
Practical guidelines for the management of this common clinical problem have been formulated by experts in the field, based on understanding of the physiology of glucose and insulin dynamics, the characteristics of currently available insulin preparations, and clinical experience. In 2004, in Clement et al.,2 the American Diabetes Association published a technical review promoting the use of physiologic (basal‐nutritional‐correction dose) insulin regimens in the hospital to achieve targeted glycemic outcomes. This approach has been disseminated via review articles,3 and more recently, a randomized, controlled trial demonstrated that hospitalized type 2 diabetes patients experienced better glycemic control when treated with a physiologic insulin regimen than when treated with sliding‐scale insulin alone.4 The Society of Hospital Medicine has assembled a Glycemic Control Task Force, which is charged with providing physicians and hospitals with practical tools to improve the safety and efficacy of diabetes management in the hospital. One product of this work is an educational module that serves as a tutorial on the best practice for the management of diabetes and hyperglycemia in the noncritically ill hospital patient.5 This article is based on that module, and provides a practical summary of the key concepts that will allow clinicians to confidently employ physiologic insulin regimens when caring for their hospital patients.
Case: Ms. X is a 56‐year‐old obese woman with type 2 diabetes mellitus who is admitted for treatment of an infected diabetes‐related foot ulcer. The patient will be allowed to eat dinner in a couple of hours, but the surgeons have requested that she be kept nothing by mouth (NPO) after midnight for surgical debridement in the morning. Her current weight is 100 kg, and her recent glycemic control can be summarized as having BG values that are usually in the mid‐200s (mg/dL) and a recent glycosylated hemoglobin (HbA1C) measurement of 10.9%. Her home medical regimen includes glipizide 10 mg daily, metformin 1000 mg twice daily, and 20 units of neutral protamine hagadorn (NPH) insulin at bedtime. Her blood glucose in the Emergency Department is 289 mg/dL. How should this patient's blood glucose be managed in the hospital?
PHARMACOLOGIC CONTROL OF BG IN THE HOSPITAL: INSULIN IS THE ANTIHYPERGLYCEMIC AGENT OF CHOICE
Although oral antihyperglycemic agents are frequently used in the outpatient setting, there are many potential disadvantages to using these medications in acutely ill hospital patients, as shown in Figure 1.2, 3 Oral antihyperglycemic agents, in general, are difficult to quickly titrate to effect, and have side effects that can limit their use in the hospital. Metformin can lead to lactic acidosis when it is used in clinical situations that predispose to lactate production (eg, renal failure, circulatory failure, hypoxemia). Therefore, metformin should be held in patients who have, or are at risk for, these conditions, each of which may be encountered in the hospital. Also, agents that stimulate the release of insulin, such as sulfonylureas, should be held in patients with variable nutritional intake, to prevent hypoglycemia. In contrast, insulin acts rapidly, responds in a timely fashion to dose titrations, and can be used effectively in virtually all patients and clinical situations to control BG levels. This makes insulin the treatment of choice for hyperglycemia in the hospital. Insulin can be administered via subcutaneous doses or as an intravenous infusion for cases in which rapid titration is the goal. Intravenous insulin infusions are the preferred mode of insulin delivery in the ICU setting, and may be appropriate for some noncritically ill patients in hospitals that have developed systems to safely provide them on general wards. Subcutaneous insulin is most commonly used in the noncritically ill patient population and is the focus of this article.

Although insulin is the drug of choice for managing hyperglycemia in the hospital, there are some situations when it is appropriate to continue oral antihyperglycemic medications in the hospital. These agents may be continued in hospitalized patients who are clinically stable, and who have normal nutritional intake, normal BG levels, and stable renal and cardiac function. They may also be started or resumed in the hospital if they are to be included in the discharge medication regimen once the patient is clinically stable and if it has been assured that contraindications to their use no longer exist.
Ms. X should be treated with a more robust (physiologic) insulin regimen. This statement would be true, even if she were not an inpatient with a foot infection. Her glycemic control is currently poor, as evidenced by her high HbA1C and her elevated admission BG, and is unlikely to be appreciably improved with the addition of any pharmacologic agent other than insulin. The glipizide should be held, as the patient will be NPO after midnight. Most experts would recommend holding the metformin at this time as well, since the patient will be undergoing a surgical procedure in the morning that places her at risk for predisposing factors to lactic acidosis.
INPATIENT GLYCEMIC TARGETS
At present, recommended glycemic targets for noncritically ill hospital patients are based entirely on expert opinion, as there have been no clinical studies directly comparing different glycemic targets in this patient population. However, the American College of Endocrinology, the American Association of Clinical Endocrinologists, and the American Diabetes Association do provide recommendations about glycemic targets for inpatients (Table 1).6, 7 Although controversial, these recommendations make it clear that uncontrolled hyperglycemia is no longer the accepted standard of care for hospitalized patients, and illustrate the consensus of expert opinion on the subject. Of note, many hospitals are adopting glycemic targets that are less stringent than those shown in Table 1, recognizing the challenges of controlling BG levels in hospitalized patients, and the potential risk for hypoglycemia when lower BG targets are used. Each hospital's glycemic control champions must reach consensus on a target BG range for their institution. In practice this range has been 90110 mg/dL for the lower BG limit and 140180 mg/dL for the upper BG limit. It is also important to note that the recommendations from professional organizations emphasize the need to individualize BG targets, based on the clinical circumstances of each patient.
| Organization | ICU(mg/dL) | Non‐ICU, Preprandial (mg/dL)* | Non‐ICU, Maximum(mg/dL)* |
|---|---|---|---|
| |||
| ACCE/ACE | 110 | 110 | 180 |
| ADA | 110 | 90‐130 | 180 |
Ms. X is exhibiting glycemic values far outside of the recommended upper limit of 180 mg/dL, and treatment with insulin monotherapy is the most appropriate strategy in this case.
PHYSIOLOGIC (BASAL‐BOLUS, PLUS CORRECTION DOSE) INSULIN
The management of hyperglycemia and diabetes in the inpatient setting is challenging due to the many changes that patients experience in the hospital. Hospitalized patients often experience changes in their nutritional intake and their medication regimen. In addition, hospitalized patients usually experience the stress of acute illness and are treated with medications that might impact glycemic control. Figure 2 lists some of the barriers to achieving glycemic control in hospitalized patients. The inpatient insulin program needs to be flexible enough to allow for maintenance of glycemic control in the face of tumultuous circumstances. This can best be accomplished by the use of a physiologic insulin program. This means using exogenous insulin to mimic normal physiologic insulin activity by providing the correct types and doses of insulin at the correct times.

A physiologic insulin regimen can be conceptualized as having 3 separate components: basal insulin, nutritional (or prandial/meal) insulin, and correction dose (or supplemental) insulin.2 A patient's total daily dose (TDD) of insulin is the sum of all of these, and represents the amount of insulin that a patient requires over the course of 1 day while receiving adequate nutrition. Basal insulin is the insulin normally released continuously by the pancreas, even when fasting. This serves to suppress glucose and ketone production. When nutrition is ingested, there is a surge in the level of glucose in the blood, and this surge is accompanied by rapid secretion of additional insulin to allow for the appropriate utilization of the glucose. The insulin that is secreted in response to nutritional intake is referred to as nutritional insulin. About one‐half of the total daily insulin secreted by a healthy normal human serves a basal function, and about one‐half is secreted in response to nutritional intake.8 Understanding this bit of physiology (the 50/50 rule) is very helpful for creating flexible regimens using exogenous insulin. Although the 50/50 rule is useful in many circumstances, there are some notable exceptions. In some cases, basal insulin might be expected to be less than one‐half of the TDD (eg, enteral feeds, as discussed below). Additional correction dose insulin is given to correct hyperglycemia that occurs despite scheduled doses of basal and nutritional insulin.
It is an important practical consideration to note that when a person with diabetes is acutely ill or stressed, as is commonly the case in the hospital setting, total daily insulin requirements increase. This is due to the action of insulin counterregulatory hormones such as catecholamines, cortisol, growth hormone, and glucagon. The hospitalized patient is therefore likely to require a TDD that is higher than that required when well. This is particularly true for the basal insulin dose. Conversely, insulin requirements will decrease as a patient recovers from acute illness, and it may be necessary to lower insulin doses as BG levels decrease during convalescence.
Exogenous basal insulin is provided as a long‐acting or intermediate‐acting, low‐peaking or nonpeaking insulin (eg, glargine or detemir), that allows for a consistent level of basal insulin (Figure 3). This insulin is provided even when the person is not receiving any nutrition. Although twice daily NPH insulin can be used to provide basal insulin, the peak (as shown in Figure 3) is likely to exceed the level of insulin that is truly required for basal needs, which can result in hypoglycemia. In theory, NPH insulin would be less physiologic than glargine or detemir, although no studies have compared these insulins in the hospital setting. When using NPH insulin as a basal insulin in a patient who is designated NPO, the dose should be reduced by one‐third to one‐half to avoid hypoglycemia that may occur when it peaks.2
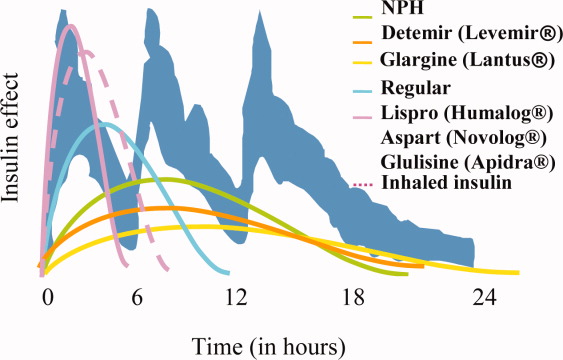
Exogenous nutritional insulin must be provided in a way that matches the nutrition that is being provided to the patient. For example, a patient who is receiving nutritional boluses (ie, meals or bolus tube feeds) can be given rapid‐acting insulin (eg, aspart, glulisine, lispro) along with each nutritional bolus to cover the glycemic peak that is caused by the meal. The rapid‐acting analog insulins can also be given at the end of a meal or bolus tube feed for cases in which it is not clear if the nutrition will be well tolerated. A reduction in the insulin dose proportionate to the amount of nutrition actually taken can then be made to decrease the risk of subsequent hypoglycemia. Regular insulin can also be given in anticipation of a meal or tube feed, but its later peak (as shown in Figure 3) requires that it be given 30 minutes before a meal is ingested, the timing of which is a challenge on most nursing units. Patients who are not receiving any nutrition should not receive nutritional insulin. And, patients receiving alternative forms of nutrition will require different nutritional insulin regimens to adequately cover their nutritional glycemic loads, as discussed below.
The separate provision of basal and nutritional insulin results in a highly‐flexible insulin program that can provide basal insulin to patients even when they are not receiving significant nutrition, and can be easily adjusted to provide appropriate nutritional insulin to match actual nutritional delivery.
Correction‐dose insulin is the small amount of insulin that is given to patients, in addition to basal and nutritional insulin, to correct hyperglycemia. Correction‐dose insulin is usually provided as rapid‐acting or regular insulin (usually the same type as the nutritional insulin), and is given in a dose that is specifically designed to reduce the patient's BG back into the target range. It is usually given at the same time as the nutritional insulin in patients who are receiving nutrition (or every 4 to 6 hours in patients who are not). Correction‐dose insulin is often written in a stepped format, to provide the appropriate amount of insulin for a given BG value. It differs from the traditional sliding‐scale in that it is not used alone (but rather as 1 component of a physiologic program), and in that it is customized to match the insulin sensitivity for each patient. Most standardized order sets for subcutaneous insulin provide several different correction‐dose scales to choose from, depending on the patient's weight or total daily insulin requirement.
If correction‐dose insulin is required consistently, or in high doses, it suggests a need to modify the basal and/or nutritional insulin. A proportion of the total number of units of correction‐dose insulin given in the preceding 24 hours can be distributed into basal and nutritional insulin doses for the next day if there is ongoing need for significant correction‐doses of insulin. A well‐designed, physiologic insulin regimen should provide targeted glycemic control, without a need for constantly adding large correctional boluses.
Some insulins do not fit neatly into either basal or nutritional insulin categories. For example, mixed insulins (eg, 70/30, rapid‐analog/NPH mixtures) combine basal and nutritional insulins to form either a double‐peaking insulin or an intermediate‐peaking insulin. The use of this type of insulin makes it impossible to manipulate the basal and nutritional components separately to enable attainment of BG targets. Therefore, the role of this type of insulin is limited in the hospital setting. Mixed insulins may, however, be started once the patient is clinically stable if they will be part of the discharge regimen.
Diabetes and hyperglycemia in the hospitalized patient require active management, and there are no autopilot insulin regimens. The use of sliding scale insulin alone to manage hyperglycemia is a common practice in hospitals.9 However, this is an historic practice that is based on the erroneous idea that BG can be managed with a reactive strategy. When sliding‐scale insulin is used as the sole modality of insulin therapy, insulin is provided only after metabolic control has been lost, and usually does not provide an appropriate dose of insulin, considering basal, nutritional, and correctional needs. The end result is poor glycemic control.9, 10 A recent randomized, controlled trial demonstrated that a physiologic insulin regimen is indeed superior to a standardized insulin sliding‐scale for managing inpatient hyperglycemia.4
Ms. X should be given an insulin regimen that includes basal, nutritional, and correctional components. The provision of separate basal and nutritional insulin will allow the clinicians to provide the patient with basal insulin even when her nutritional insulin is held, and to easily modify her nutritional insulin, depending on her nutritional intake.
PHYSIOLOGIC INSULIN: A PRACTICAL APPROACH
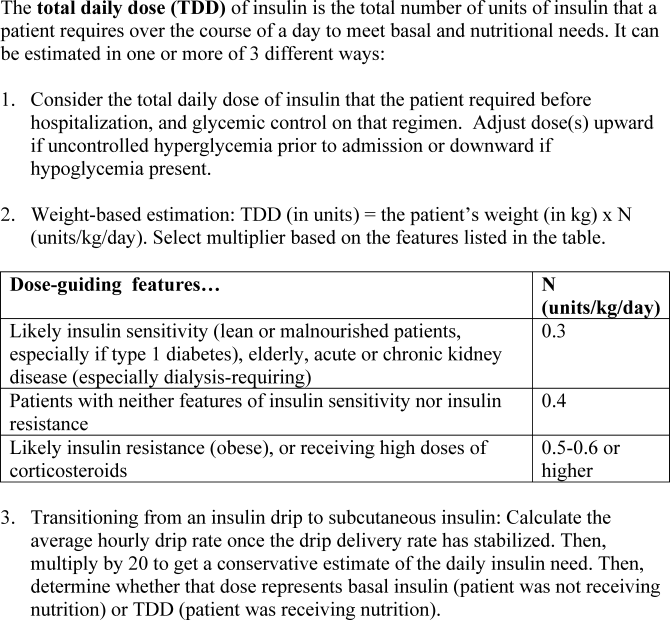
The use of physiologic insulin in the hospital can be facilitated by considering a stepwise approach (Figure 4).5 The first step is to estimate the amount of insulin that the patient will require over the course of a day if taking adequate nutrition (this is the TDD). If the patient has been treated with subcutaneous insulin before being admitted to the hospital, the clinician can use the outpatient TDD to gauge the insulin needs. To do this, the clinician simply adds up the total number of units of insulin that a patient takes at home in a day. Using this method to estimate the patient's TDD can be very helpful, even if the clinician plans to use different types of insulin while the patient is hospitalized. When using this approach, one should consider the patient's prior metabolic control on the existing regimen (ie, if the patient's glycemic control was poor on the preexisting regimen, an increase in the TDD would be necessary). In addition, clinicians should recognize that insulin requirements usually increase when patients are acutely ill, as discussed above. Managing diabetes and hyperglycemia in the hospital is very different than doing so in the outpatient arena, and hospitalists should not feel bound by the outpatient regimen.
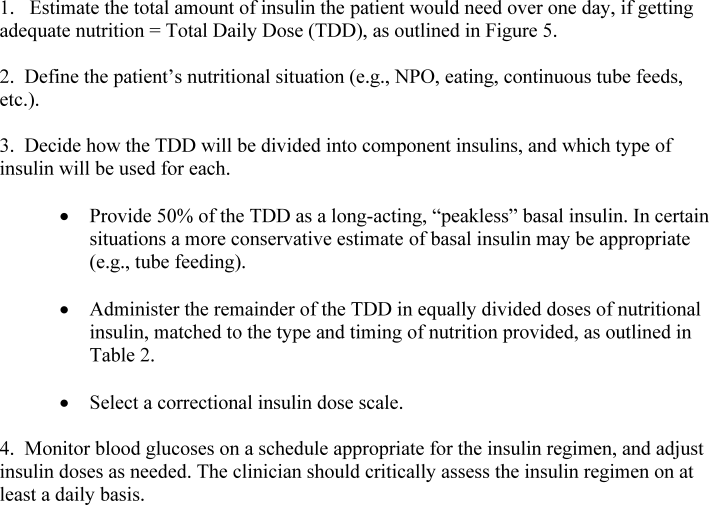
In addition, a weight‐based estimation of the TDD can be very helpful in determining a starting dose of insulin in a hospitalized patient (see Figure 5). An estimate of 0.4 units/kg of body weight provides a conservative starting point for the TDD for most patients. Occasionally, a lower starting dose of 0.3 units/kg of body weight might be safer for those patients who are likely to be very sensitive to insulin, or who are otherwise at increased at risk for hypoglycemia (see Figure 5). Patients who are overweight or obese often require considerably higher TDDs in the range of 0.5‐0.6 (or even more) units/kg of body weight. Some may require over 1 unit/kg of body weight for their TDD. Therefore, the doses provided by these calculations represent conservative estimates in most patients. Hospitalists should be able to confidently make these dose estimates and avoid dependence on nonphysiologic regimens such as sliding‐scale insulin alone.
The presence of risk factors for hypoglycemia or hyperglycemia should temper the dose calculations. Clinical conditions associated with hyperglycemia include obesity, certain medications (eg, glucocorticoids, catecholamines, tacrolimus, cyclosporine), and changes in nutritional intake (Figure 2). Clinical conditions associated with hypoglycemia are summarized in Figure 6. It is important to recognize that these calculations are intended to give the clinician a safe and rational starting point for insulin dosing. More important than the calculations is the careful monitoring of BG levels and timely modifications of the insulin regimen that follow.

The second step in developing a physiologic insulin regimen is to determine the patient's nutritional regimen. The third step, then, is to decide how the TDD will be distributed into basal and nutritional insulins, and which insulin will be used for each. As noted above, for most patients, approximately one‐half of the TDD will be provided as basal insulin, and the other one‐half given as nutritional insulin. When patients are receiving nutrition, the insulin must be given in a way that matches the timing of nutrition delivery (eg, with each meal or bolus tube feeding), and provides appropriate insulin coverage for the nutrition that is being provided.
Nutritional insulin will not be given if the patient is not receiving any nutrition, in which case basal insulin and correction‐dose insulin will usually be continued. Table 2 shows the preferred insulin regimens for a variety of different nutritional circumstances, as put forth by the Society of Hospital Medicine (SHM) Glycemic Control Task Force.5
| Nutritional Situation | Necessary Insulin Components | Preferred Regimen* |
|---|---|---|
| ||
| NPO (or clear liquids) | Basal insulin: 50% of TDD. Nutritional insulin: None. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: None. Correctional insulin: Regular insulin q 6 hours or RAA insulin q 4 hours. Other comments: Dextrose infusion (eg, D5 containing solution at 75‐150 cc/hour) recommended when nutrition is held. An IV insulin infusion is preferred for management of prolonged fasts or fasting type 1 diabetes patients. |
| Eating meals | Basal insulin: 50% of TDD. Nutritional insulin: 50% of TDD, divided equally before each meal. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin with meals. Correctional insulin: RAA insulin q AC and HS (reduced dose at HS). |
| Bolus tube feeds | Basal insulin: 40% of TDD. Nutritional insulin: 60% of the TDD, divided equally before each bolus feed. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin with each bolus. Correctional insulin: RAA insulin with each bolus. |
| Continuous tube feeds | Basal insulin: 40% (conservative) of TDD. Nutritional insulin: 60% of the TDD in divided doses. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin q 4 hours or regular insulin q 6 hours. Correctional insulin: Should match nutritional insulin choice. |
| Parenteral nutrition | Insulin is usually given parenterally, with the nutrition | Initially, a separate insulin drip allows for accurate dose‐finding. Then, 80% of amount determined as TDD using drip is added to subsequent TPN bags as regular insulin. Use correctional subcutaneous insulin doses cautiously, in addition |
For Ms. X, the first step is to determine a TDD estimate. This patient weighs 100 kg, and is obese. Therefore, a TDD of 0.5 units/kg (50 units) is appropriate. This dose may be an underestimate of her insulin needs, but it is a conservative, reasonable starting point. The patient's basal insulin could be provided by giving one‐half of the TDD as basal insulin, such as glargine, 25 units daily. In this case, the patient will be eating dinner soon, but then will be NPO after midnight for surgery. Appropriate nutritional insulin could be provided by giving one‐third of her total nutritional insulin before this meal as a rapid‐acting insulin analog (25 units nutritional insulin per day divided by 3 meals results in a dose of 8 units per meal). After she eats dinner, additional nutritional insulin will not be given until she resumes her diet postoperatively. While NPO, her basal insulin should be continued, and her blood glucose should be checked every 4‐6 hours. An appropriate correction‐dose insulin scale should be chosen to provide a supplemental insulin dose with each bedside test of the blood glucose level, if and only if, hyperglycemia is present.
Case continued: The patient is given 8 units of lispro insulin before her dinner, and is also given a dose of 25 units of glargine insulin. She is held NPO after midnight and dextrose‐containing fluid is provided intravenously overnight at a maintenance rate. In the morning her blood glucose is 161 mg/dL before surgery. Surgery goes well, and at lunch her blood glucose is 179 mg/dL, and she is given a food tray. However, the patient says that she feels mildly nauseated, and is not sure that she will be able to eat her lunch. How should her nutritional insulin be managed in this situation?
MATCHING NUTRITION AND NUTRITIONAL INSULIN: A DIFFICULT CHALLENGE
It is the provision of the correct type and amount of nutritional insulin at the right time that is most challenging in the hospital. In the hospital, a patient's nutritional intake is often interrupted. Patients might be made NPO as part of the treatment plan, or may not be able to take nutrition by mouth because of specific medical conditions. In some cases, enteral or parenteral feeding is used to replace or enhance oral feeding. Even when alternate routes of nutrition are employed, sudden interruptions in nutrition still remain common (eg, the feeding tube falls out). Ultimately, to provide the best possible care to a diabetes patient, the nutritional insulin that is delivered must match the actual nutritional delivery (Table 2). Ideally, each institution should choose a preferred, standardized approach for each nutritional situation.
Even if institutions standardize their approach to nutritional insulin delivery in general, clinicians must be able to accurately respond to the unplanned variations in nutrition that occur in the hospital. One example of this is the patient who is expected to eat meals, but who becomes suddenly unable to do so, such as in the case above. In cases like this, the best approach is to hold the patient's nutritional insulin and allow the patient to attempt to eat the provided meal. Then, a rapid‐acting insulin analog can be given just after the meal, in proportion to the amount of the meal that was eaten. If the patient really is unable to tolerate any of the meal, then no nutritional insulin is provided. If the patient does tolerate a portion of the meal (eg, 50% of the meal is consumed), then a corresponding amount of insulin is given (eg, 50% of the scheduled nutritional insulin is given). The quick onset of the rapid‐acting insulin analogs allows near‐physiologic effect, even when they are given after the meal.
Ms. X should be allowed to eat as much of the meal as she can tolerate. Afterward, her intake can be assessed, and insulin can be provided in proportion to the amount of the meal that was eaten, as described above.
Bolus Tube Feeds
Patients who are given bolus enteral feeds are typically treated like patients who are eating meals. Like meals, bolus tube feeds are nutritional boluses that should be covered with nutritional insulin boluses. Because the hyperglycemia that sometimes accompanies bolus tube feeds is partly related to the glycemic load of this type of feedings, it is reasonable to provide 60% of the TDD of insulin as nutritional insulin when using this type of nutrition.
Continuous Tube Feeds
Patients who are given continuous enteral nutrition are somewhat different than patients who are eating meals. Continuous tube feed patients receive nutrition on a continuous basis. Therefore, these patients must also receive nutritional insulin in a way that provides continuous coverage. There is no proven superior insulin regimen for the continuously tube fed patient. Because the nutrition is being provided continuously, providing all of the TDD of insulin as a long‐acting, nonpeaking insulin might be considered the most physiologic regimen. However, if such a strategy is used and the tube feeding is interrupted for some reason, the patient will be in danger of hypoglycemia for the duration of action of the basal insulin. For this reason, the SHM Glycemic Control Task Force and many endocrinologists recommend using a long‐acting basal insulin at a dose that would provide a conservative estimate of the basal component (eg, 40% or less of the TDD), and dividing the remainder of the insulin and giving it as scheduled regular (every 6 hours) or rapid‐acting insulin (every 4 hours) for the nutritional coverage.
If tube feeds are interrupted, an infusion of 10% dextrose given intravenously at the same rate as the tube feed had been running (or the equivalent) can be provided to avoid hypoglycemia until the effect of the nutritional insulin has dissipated. It is reasonable to create a standing order to notify the physician, or start an alternate source of dextrose, in the case of tube feed interruption.
Parenteral Nutrition
For patients receiving parenteral nutrition, regular insulin, mixed with the parenteral nutrition, is safe and effective. Subcutaneous correction‐dose insulin is often used, in addition to the insulin that is mixed with the nutrition. When starting parenteral nutrition, the initial use of a separate insulin infusion can help in estimating the TDD of insulin that will be required.
TYPE 1 DIABETES
Because type 2 diabetes is more prevalent than type 1 diabetes, hospitalists will manage this form of diabetes most often. However, it is crucial that hospitalists are also able to manage type 1 diabetes in the hospital. For the most part, the principles presented in this article apply to all types of diabetes patients. This section outlines some special considerations to remember when caring for type 1 diabetes patients in the hospital that will assure prevention of diabetic ketoacidosis in patients with type 1 diabetes.
Type 1 diabetes patients completely lack endogenous insulin production. Therefore, these patients require exogenous insulin to be provided at all times. Type 1 diabetes patients need to be provided continuous, exogenous basal insulin, even when fasting, to suppress gluconeogenesis and ketone production. Failure to provide basal insulin to a type 1 diabetes patient can lead to the rapid development (in hours) of ketoacidosis. When receiving nutrition, the patient with type 1 diabetes must also be provided with nutritional insulin to control postprandial BGs. Whereas many type 2 diabetes patients may produce sufficient endogenous insulin to meet basal requirements when fasting (ie, they produce enough basal insulin to maintain metabolic stability when they are not taking in nutrition), this is never the case for type 1 diabetes patients.
In addition, type 1 diabetes patients typically exhibit less insulin resistance than type 2 diabetes patients, especially if they are not obese. Therefore, type 1 diabetes patients often have TDDs of insulin that are lower than those of type 2 patients. This is reflected in the recommendations in Figure 5, with the TDD of insulin estimate for a lean type 1 diabetes patient of 0.3 units/kg/day.
CONTINUOUS SUBCUTANEOUS INSULIN INFUSIONS (INSULIN PUMPS) IN THE HOSPITAL
Continuous subcutaneous insulin infusion therapy (CSII), often referred to as insulin pump therapy, involves the use of a pump to provide a continuous flow of subcutaneous basal insulin (usually a rapid‐acting analog) through a needle that is left in place. This basal rate is adjustable, and therefore can be customized to meet variable needs over a 24‐hour period. When the patient takes in nutrition, a bolus of the same insulin is given, via the pump, at a dose that is appropriate to cover the nutritional intake. The advantages of CSII therapy are the capacity for precision and flexibility of the basal insulin delivery (compared to the use of a once‐daily or twice‐daily dose of long‐acting insulin analog), and the lack of a need to inject insulin boluses (which are delivered via the pump). This type of therapy is preferred by some diabetes patients, and although it is not highly prevalent in most areas, it is common enough that hospitalists must have a plan for managing it in their practices.
There are many barriers to the use of CSII in the hospital. Most of these barriers are related to the need for constant management of the pump. Most hospitalists and nurses do not have the expertise to manage this therapy in the hospital. Although the patient (or caregiver) might have the expertise to manage the pump, this is an acceptable option only if the patient is competent to manage the pump. Patient competence to use the pump must be formally assessed and documented, and the patient must agree to perform the many components of care related to managing the pump (eg, documenting the basal rate and boluses given, documenting BGs, providing tubing and other supplies).
Many hospitals currently choose a policy of converting insulin pump therapy to standard subcutaneous insulin treatment for most hospitalized patients. Usually the conversion of CSII to a physiologic subcutaneous insulin regimen (as detailed in this article) is fairly straightforwardthe basal insulin will be given as long‐acting, low‐peaking insulin, and nutritional and correction‐dose insulin can then be added as a rapid‐acting analog, in accord with the patient's needs.
Hospitals that admit a large number of insulin pump patients, or those that choose to use pumps routinely in the hospital, should create a formal policy for pump use.11 It has been suggested that the policy should assure that there is formal assessment of the patient's competence to manage the pump, that there is professional oversight of the pump management (usually via endocrinology and diabetes educator consultation), that contraindications for CSII use are clearly stated, and that there is a formal mechanism for engaging the patient and informing him of his roles and responsibilities (eg, a written agreement). The clinician must write insulin orders in the medical record that specify the basal and bolus insulin doses which are being used. Pump use may be limited to floors where nurses receive at least basic education in the principles of CSII pumps.
CARE TRANSITIONS IN THE HOSPITAL
Transitioning from an Intravenous Insulin Infusion to a Subcutaneous Insulin Regimen
In the hospital, it is often necessary to switch a patient from an intravenous (IV) insulin infusion to a subcutaneous (SC) insulin regimen. When doing this, the clinician must decide how much SC insulin the patient will require. As discussed earlier, the TDD may be estimated based on home insulin doses or the patient's weight. However, for a patient who is treated with an IV insulin infusion, current insulin requirements can be estimated based on the recent IV drip rate. This is the preferred method for identifying a TDD in these patients, as the insulin delivery rate at the time of drip discontinuation provides a way of determining current insulin requirements.
Regardless of the dose of SC insulin that is chosen, it is important that SC insulin be delivered well in advance of the discontinuation of the IV insulin. Because the duration of action of IV insulin is on the order of 7 minutes, the patient may become rapidly hyperglycemic or develop ketoacidosis (in type 1 diabetes) in a matter of hours if the IV insulin infusion is discontinued before the SC insulin is active. Insulin infusion should not be stopped for at least 1 hour after the SC delivery of rapid‐acting or regular insulin, and at least 2‐3 hours after the SC delivery of intermediate‐acting or long‐acting insulin.2
Discharge Transition
The hospital discharge is another challenging transition for the patient with diabetes. While hospitalized, a diabetes patient's medication regimen will likely be altered to maintain metabolic control. At the time of discharge, the patient should be provided with an appropriate medication regimen. Moreover, the patient must be educated about any new medication or other changes that will be part of the new outpatient management routine. It is also important to assure that the patient does not have knowledge deficits related to diabetes survival skills. The Joint Commission has recently put forth the expectation that such education will be provided prior to hospital discharge.12 Areas outlined for this core diabetes self‐management education include: the definition of diabetes; finger‐stick BG monitoring; glycemic targets; insulin self‐administration; hypoglycemia prevention, recognition, and treatment; hyperglycemia recognition; sick day guidelines; and when to call a clinician for help. Communication of the discharge diabetes management plan to the patient's primary care provider should also be undertaken.
Please see the article entitled Bridge Over Troubled Waters: Safe and Effective Transitions for the Inpatient with Hyperglycemia in this supplement for additional details about both the IV to SC transition and the discharge transition.
CONCLUSIONS
Understanding the basic principles of the physiologic (basal, nutritional, and correction‐dose) insulin regimen will allow clinicians to formulate safe and effective insulin regimens in virtually any clinical situation. Simple steps can allow safe estimates of initial doses and titration toward glycemic goals. Additional information and case studies can be found in a Society of Hospital Medicine Task Force Educational Module,5 available online.
Additional resources for improving glycemic control in hospital patients are available online at the Glycemic Control Resource Room (
- ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control: A call to action.Diabetes Care.2006:29:1955–1962.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- .Management of hyperglycemia in the hospital setting.N Engl J Med.2006;355:1903–1911.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (Rabbit 2 trial).Diabetes Care.2007;30:2181–2186.
- ,; for the Society of Hospital Medicine Glycemic Control Task Force. Educational module: management of diabetes and hyperglycemia in the hospital patient: focus on subcutaneous insulin use in the non‐critically ill, adult patient. Published January 2007 on the Society of Hospital Medicine Website. Available at:http://www.hospitalmedicine.org/ResourceRoomRedesign/html/11Ed_Resources/01_Teaching_Slide.cfm. Accessed August2008.
- American College of Endocrinology Task Force on Inpatient Diabetes and Metabolic Control.American College of Endocrinology Position Statement on Inpatient Diabetes and Metabolic Control.Endocr Pract.2004;10:77–82.
- American Diabetes Association.Standards of Medical Care in Diabetes, 2006.Diabetes Care.2006;29(supp 1):s4–s42.
- Larsen PR,Kronenberg HM,Melmed S,Polonsky KS, editors.Williams Textbook of Endocrinology.10th ed.Philadelphia, PA:Elsevier Science;2003.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,.Glycemic control and sliding scale use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,, et al.Use of continuous subcutaneous insulin infusion therapy in the hospital setting: Proposed guidelines and outcome measures.Diabetes Educ.2005;31:849–857.
- Inpatient Diabetes Certification. Joint Commission. Available at:http://www.jointcommission.org/CertificationPrograms/Inpatient+Diabetes. Accessed April2008.
Recently, there has been a heightened interest in improving the quality and safety of the management of diabetes and hyperglycemia in the hospital.1 While observational data strongly suggests an association of hyperglycemia with morbidity and mortality in adults on general medicine and surgery units, clinical research has not yet defined the best practices for managing hyperglycemia in the hospital outside the intensive care unit (ICU). As a result, many physicians do not have a well‐formulated approach to managing hyperglycemia in the noncritically ill hospital patient, and the use of insulin therapy to attain targeted blood glucose (BG) control is often subject to practice variability, leading to suboptimal glycemic outcomes.
Practical guidelines for the management of this common clinical problem have been formulated by experts in the field, based on understanding of the physiology of glucose and insulin dynamics, the characteristics of currently available insulin preparations, and clinical experience. In 2004, in Clement et al.,2 the American Diabetes Association published a technical review promoting the use of physiologic (basal‐nutritional‐correction dose) insulin regimens in the hospital to achieve targeted glycemic outcomes. This approach has been disseminated via review articles,3 and more recently, a randomized, controlled trial demonstrated that hospitalized type 2 diabetes patients experienced better glycemic control when treated with a physiologic insulin regimen than when treated with sliding‐scale insulin alone.4 The Society of Hospital Medicine has assembled a Glycemic Control Task Force, which is charged with providing physicians and hospitals with practical tools to improve the safety and efficacy of diabetes management in the hospital. One product of this work is an educational module that serves as a tutorial on the best practice for the management of diabetes and hyperglycemia in the noncritically ill hospital patient.5 This article is based on that module, and provides a practical summary of the key concepts that will allow clinicians to confidently employ physiologic insulin regimens when caring for their hospital patients.
Case: Ms. X is a 56‐year‐old obese woman with type 2 diabetes mellitus who is admitted for treatment of an infected diabetes‐related foot ulcer. The patient will be allowed to eat dinner in a couple of hours, but the surgeons have requested that she be kept nothing by mouth (NPO) after midnight for surgical debridement in the morning. Her current weight is 100 kg, and her recent glycemic control can be summarized as having BG values that are usually in the mid‐200s (mg/dL) and a recent glycosylated hemoglobin (HbA1C) measurement of 10.9%. Her home medical regimen includes glipizide 10 mg daily, metformin 1000 mg twice daily, and 20 units of neutral protamine hagadorn (NPH) insulin at bedtime. Her blood glucose in the Emergency Department is 289 mg/dL. How should this patient's blood glucose be managed in the hospital?
PHARMACOLOGIC CONTROL OF BG IN THE HOSPITAL: INSULIN IS THE ANTIHYPERGLYCEMIC AGENT OF CHOICE
Although oral antihyperglycemic agents are frequently used in the outpatient setting, there are many potential disadvantages to using these medications in acutely ill hospital patients, as shown in Figure 1.2, 3 Oral antihyperglycemic agents, in general, are difficult to quickly titrate to effect, and have side effects that can limit their use in the hospital. Metformin can lead to lactic acidosis when it is used in clinical situations that predispose to lactate production (eg, renal failure, circulatory failure, hypoxemia). Therefore, metformin should be held in patients who have, or are at risk for, these conditions, each of which may be encountered in the hospital. Also, agents that stimulate the release of insulin, such as sulfonylureas, should be held in patients with variable nutritional intake, to prevent hypoglycemia. In contrast, insulin acts rapidly, responds in a timely fashion to dose titrations, and can be used effectively in virtually all patients and clinical situations to control BG levels. This makes insulin the treatment of choice for hyperglycemia in the hospital. Insulin can be administered via subcutaneous doses or as an intravenous infusion for cases in which rapid titration is the goal. Intravenous insulin infusions are the preferred mode of insulin delivery in the ICU setting, and may be appropriate for some noncritically ill patients in hospitals that have developed systems to safely provide them on general wards. Subcutaneous insulin is most commonly used in the noncritically ill patient population and is the focus of this article.

Although insulin is the drug of choice for managing hyperglycemia in the hospital, there are some situations when it is appropriate to continue oral antihyperglycemic medications in the hospital. These agents may be continued in hospitalized patients who are clinically stable, and who have normal nutritional intake, normal BG levels, and stable renal and cardiac function. They may also be started or resumed in the hospital if they are to be included in the discharge medication regimen once the patient is clinically stable and if it has been assured that contraindications to their use no longer exist.
Ms. X should be treated with a more robust (physiologic) insulin regimen. This statement would be true, even if she were not an inpatient with a foot infection. Her glycemic control is currently poor, as evidenced by her high HbA1C and her elevated admission BG, and is unlikely to be appreciably improved with the addition of any pharmacologic agent other than insulin. The glipizide should be held, as the patient will be NPO after midnight. Most experts would recommend holding the metformin at this time as well, since the patient will be undergoing a surgical procedure in the morning that places her at risk for predisposing factors to lactic acidosis.
INPATIENT GLYCEMIC TARGETS
At present, recommended glycemic targets for noncritically ill hospital patients are based entirely on expert opinion, as there have been no clinical studies directly comparing different glycemic targets in this patient population. However, the American College of Endocrinology, the American Association of Clinical Endocrinologists, and the American Diabetes Association do provide recommendations about glycemic targets for inpatients (Table 1).6, 7 Although controversial, these recommendations make it clear that uncontrolled hyperglycemia is no longer the accepted standard of care for hospitalized patients, and illustrate the consensus of expert opinion on the subject. Of note, many hospitals are adopting glycemic targets that are less stringent than those shown in Table 1, recognizing the challenges of controlling BG levels in hospitalized patients, and the potential risk for hypoglycemia when lower BG targets are used. Each hospital's glycemic control champions must reach consensus on a target BG range for their institution. In practice this range has been 90110 mg/dL for the lower BG limit and 140180 mg/dL for the upper BG limit. It is also important to note that the recommendations from professional organizations emphasize the need to individualize BG targets, based on the clinical circumstances of each patient.
| Organization | ICU(mg/dL) | Non‐ICU, Preprandial (mg/dL)* | Non‐ICU, Maximum(mg/dL)* |
|---|---|---|---|
| |||
| ACCE/ACE | 110 | 110 | 180 |
| ADA | 110 | 90‐130 | 180 |
Ms. X is exhibiting glycemic values far outside of the recommended upper limit of 180 mg/dL, and treatment with insulin monotherapy is the most appropriate strategy in this case.
PHYSIOLOGIC (BASAL‐BOLUS, PLUS CORRECTION DOSE) INSULIN
The management of hyperglycemia and diabetes in the inpatient setting is challenging due to the many changes that patients experience in the hospital. Hospitalized patients often experience changes in their nutritional intake and their medication regimen. In addition, hospitalized patients usually experience the stress of acute illness and are treated with medications that might impact glycemic control. Figure 2 lists some of the barriers to achieving glycemic control in hospitalized patients. The inpatient insulin program needs to be flexible enough to allow for maintenance of glycemic control in the face of tumultuous circumstances. This can best be accomplished by the use of a physiologic insulin program. This means using exogenous insulin to mimic normal physiologic insulin activity by providing the correct types and doses of insulin at the correct times.

A physiologic insulin regimen can be conceptualized as having 3 separate components: basal insulin, nutritional (or prandial/meal) insulin, and correction dose (or supplemental) insulin.2 A patient's total daily dose (TDD) of insulin is the sum of all of these, and represents the amount of insulin that a patient requires over the course of 1 day while receiving adequate nutrition. Basal insulin is the insulin normally released continuously by the pancreas, even when fasting. This serves to suppress glucose and ketone production. When nutrition is ingested, there is a surge in the level of glucose in the blood, and this surge is accompanied by rapid secretion of additional insulin to allow for the appropriate utilization of the glucose. The insulin that is secreted in response to nutritional intake is referred to as nutritional insulin. About one‐half of the total daily insulin secreted by a healthy normal human serves a basal function, and about one‐half is secreted in response to nutritional intake.8 Understanding this bit of physiology (the 50/50 rule) is very helpful for creating flexible regimens using exogenous insulin. Although the 50/50 rule is useful in many circumstances, there are some notable exceptions. In some cases, basal insulin might be expected to be less than one‐half of the TDD (eg, enteral feeds, as discussed below). Additional correction dose insulin is given to correct hyperglycemia that occurs despite scheduled doses of basal and nutritional insulin.
It is an important practical consideration to note that when a person with diabetes is acutely ill or stressed, as is commonly the case in the hospital setting, total daily insulin requirements increase. This is due to the action of insulin counterregulatory hormones such as catecholamines, cortisol, growth hormone, and glucagon. The hospitalized patient is therefore likely to require a TDD that is higher than that required when well. This is particularly true for the basal insulin dose. Conversely, insulin requirements will decrease as a patient recovers from acute illness, and it may be necessary to lower insulin doses as BG levels decrease during convalescence.
Exogenous basal insulin is provided as a long‐acting or intermediate‐acting, low‐peaking or nonpeaking insulin (eg, glargine or detemir), that allows for a consistent level of basal insulin (Figure 3). This insulin is provided even when the person is not receiving any nutrition. Although twice daily NPH insulin can be used to provide basal insulin, the peak (as shown in Figure 3) is likely to exceed the level of insulin that is truly required for basal needs, which can result in hypoglycemia. In theory, NPH insulin would be less physiologic than glargine or detemir, although no studies have compared these insulins in the hospital setting. When using NPH insulin as a basal insulin in a patient who is designated NPO, the dose should be reduced by one‐third to one‐half to avoid hypoglycemia that may occur when it peaks.2

Exogenous nutritional insulin must be provided in a way that matches the nutrition that is being provided to the patient. For example, a patient who is receiving nutritional boluses (ie, meals or bolus tube feeds) can be given rapid‐acting insulin (eg, aspart, glulisine, lispro) along with each nutritional bolus to cover the glycemic peak that is caused by the meal. The rapid‐acting analog insulins can also be given at the end of a meal or bolus tube feed for cases in which it is not clear if the nutrition will be well tolerated. A reduction in the insulin dose proportionate to the amount of nutrition actually taken can then be made to decrease the risk of subsequent hypoglycemia. Regular insulin can also be given in anticipation of a meal or tube feed, but its later peak (as shown in Figure 3) requires that it be given 30 minutes before a meal is ingested, the timing of which is a challenge on most nursing units. Patients who are not receiving any nutrition should not receive nutritional insulin. And, patients receiving alternative forms of nutrition will require different nutritional insulin regimens to adequately cover their nutritional glycemic loads, as discussed below.
The separate provision of basal and nutritional insulin results in a highly‐flexible insulin program that can provide basal insulin to patients even when they are not receiving significant nutrition, and can be easily adjusted to provide appropriate nutritional insulin to match actual nutritional delivery.
Correction‐dose insulin is the small amount of insulin that is given to patients, in addition to basal and nutritional insulin, to correct hyperglycemia. Correction‐dose insulin is usually provided as rapid‐acting or regular insulin (usually the same type as the nutritional insulin), and is given in a dose that is specifically designed to reduce the patient's BG back into the target range. It is usually given at the same time as the nutritional insulin in patients who are receiving nutrition (or every 4 to 6 hours in patients who are not). Correction‐dose insulin is often written in a stepped format, to provide the appropriate amount of insulin for a given BG value. It differs from the traditional sliding‐scale in that it is not used alone (but rather as 1 component of a physiologic program), and in that it is customized to match the insulin sensitivity for each patient. Most standardized order sets for subcutaneous insulin provide several different correction‐dose scales to choose from, depending on the patient's weight or total daily insulin requirement.
If correction‐dose insulin is required consistently, or in high doses, it suggests a need to modify the basal and/or nutritional insulin. A proportion of the total number of units of correction‐dose insulin given in the preceding 24 hours can be distributed into basal and nutritional insulin doses for the next day if there is ongoing need for significant correction‐doses of insulin. A well‐designed, physiologic insulin regimen should provide targeted glycemic control, without a need for constantly adding large correctional boluses.
Some insulins do not fit neatly into either basal or nutritional insulin categories. For example, mixed insulins (eg, 70/30, rapid‐analog/NPH mixtures) combine basal and nutritional insulins to form either a double‐peaking insulin or an intermediate‐peaking insulin. The use of this type of insulin makes it impossible to manipulate the basal and nutritional components separately to enable attainment of BG targets. Therefore, the role of this type of insulin is limited in the hospital setting. Mixed insulins may, however, be started once the patient is clinically stable if they will be part of the discharge regimen.
Diabetes and hyperglycemia in the hospitalized patient require active management, and there are no autopilot insulin regimens. The use of sliding scale insulin alone to manage hyperglycemia is a common practice in hospitals.9 However, this is an historic practice that is based on the erroneous idea that BG can be managed with a reactive strategy. When sliding‐scale insulin is used as the sole modality of insulin therapy, insulin is provided only after metabolic control has been lost, and usually does not provide an appropriate dose of insulin, considering basal, nutritional, and correctional needs. The end result is poor glycemic control.9, 10 A recent randomized, controlled trial demonstrated that a physiologic insulin regimen is indeed superior to a standardized insulin sliding‐scale for managing inpatient hyperglycemia.4
Ms. X should be given an insulin regimen that includes basal, nutritional, and correctional components. The provision of separate basal and nutritional insulin will allow the clinicians to provide the patient with basal insulin even when her nutritional insulin is held, and to easily modify her nutritional insulin, depending on her nutritional intake.
PHYSIOLOGIC INSULIN: A PRACTICAL APPROACH
The use of physiologic insulin in the hospital can be facilitated by considering a stepwise approach (Figure 4).5 The first step is to estimate the amount of insulin that the patient will require over the course of a day if taking adequate nutrition (this is the TDD). If the patient has been treated with subcutaneous insulin before being admitted to the hospital, the clinician can use the outpatient TDD to gauge the insulin needs. To do this, the clinician simply adds up the total number of units of insulin that a patient takes at home in a day. Using this method to estimate the patient's TDD can be very helpful, even if the clinician plans to use different types of insulin while the patient is hospitalized. When using this approach, one should consider the patient's prior metabolic control on the existing regimen (ie, if the patient's glycemic control was poor on the preexisting regimen, an increase in the TDD would be necessary). In addition, clinicians should recognize that insulin requirements usually increase when patients are acutely ill, as discussed above. Managing diabetes and hyperglycemia in the hospital is very different than doing so in the outpatient arena, and hospitalists should not feel bound by the outpatient regimen.

In addition, a weight‐based estimation of the TDD can be very helpful in determining a starting dose of insulin in a hospitalized patient (see Figure 5). An estimate of 0.4 units/kg of body weight provides a conservative starting point for the TDD for most patients. Occasionally, a lower starting dose of 0.3 units/kg of body weight might be safer for those patients who are likely to be very sensitive to insulin, or who are otherwise at increased at risk for hypoglycemia (see Figure 5). Patients who are overweight or obese often require considerably higher TDDs in the range of 0.5‐0.6 (or even more) units/kg of body weight. Some may require over 1 unit/kg of body weight for their TDD. Therefore, the doses provided by these calculations represent conservative estimates in most patients. Hospitalists should be able to confidently make these dose estimates and avoid dependence on nonphysiologic regimens such as sliding‐scale insulin alone.

The presence of risk factors for hypoglycemia or hyperglycemia should temper the dose calculations. Clinical conditions associated with hyperglycemia include obesity, certain medications (eg, glucocorticoids, catecholamines, tacrolimus, cyclosporine), and changes in nutritional intake (Figure 2). Clinical conditions associated with hypoglycemia are summarized in Figure 6. It is important to recognize that these calculations are intended to give the clinician a safe and rational starting point for insulin dosing. More important than the calculations is the careful monitoring of BG levels and timely modifications of the insulin regimen that follow.

The second step in developing a physiologic insulin regimen is to determine the patient's nutritional regimen. The third step, then, is to decide how the TDD will be distributed into basal and nutritional insulins, and which insulin will be used for each. As noted above, for most patients, approximately one‐half of the TDD will be provided as basal insulin, and the other one‐half given as nutritional insulin. When patients are receiving nutrition, the insulin must be given in a way that matches the timing of nutrition delivery (eg, with each meal or bolus tube feeding), and provides appropriate insulin coverage for the nutrition that is being provided.
Nutritional insulin will not be given if the patient is not receiving any nutrition, in which case basal insulin and correction‐dose insulin will usually be continued. Table 2 shows the preferred insulin regimens for a variety of different nutritional circumstances, as put forth by the Society of Hospital Medicine (SHM) Glycemic Control Task Force.5
| Nutritional Situation | Necessary Insulin Components | Preferred Regimen* |
|---|---|---|
| ||
| NPO (or clear liquids) | Basal insulin: 50% of TDD. Nutritional insulin: None. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: None. Correctional insulin: Regular insulin q 6 hours or RAA insulin q 4 hours. Other comments: Dextrose infusion (eg, D5 containing solution at 75‐150 cc/hour) recommended when nutrition is held. An IV insulin infusion is preferred for management of prolonged fasts or fasting type 1 diabetes patients. |
| Eating meals | Basal insulin: 50% of TDD. Nutritional insulin: 50% of TDD, divided equally before each meal. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin with meals. Correctional insulin: RAA insulin q AC and HS (reduced dose at HS). |
| Bolus tube feeds | Basal insulin: 40% of TDD. Nutritional insulin: 60% of the TDD, divided equally before each bolus feed. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin with each bolus. Correctional insulin: RAA insulin with each bolus. |
| Continuous tube feeds | Basal insulin: 40% (conservative) of TDD. Nutritional insulin: 60% of the TDD in divided doses. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin q 4 hours or regular insulin q 6 hours. Correctional insulin: Should match nutritional insulin choice. |
| Parenteral nutrition | Insulin is usually given parenterally, with the nutrition | Initially, a separate insulin drip allows for accurate dose‐finding. Then, 80% of amount determined as TDD using drip is added to subsequent TPN bags as regular insulin. Use correctional subcutaneous insulin doses cautiously, in addition |
For Ms. X, the first step is to determine a TDD estimate. This patient weighs 100 kg, and is obese. Therefore, a TDD of 0.5 units/kg (50 units) is appropriate. This dose may be an underestimate of her insulin needs, but it is a conservative, reasonable starting point. The patient's basal insulin could be provided by giving one‐half of the TDD as basal insulin, such as glargine, 25 units daily. In this case, the patient will be eating dinner soon, but then will be NPO after midnight for surgery. Appropriate nutritional insulin could be provided by giving one‐third of her total nutritional insulin before this meal as a rapid‐acting insulin analog (25 units nutritional insulin per day divided by 3 meals results in a dose of 8 units per meal). After she eats dinner, additional nutritional insulin will not be given until she resumes her diet postoperatively. While NPO, her basal insulin should be continued, and her blood glucose should be checked every 4‐6 hours. An appropriate correction‐dose insulin scale should be chosen to provide a supplemental insulin dose with each bedside test of the blood glucose level, if and only if, hyperglycemia is present.
Case continued: The patient is given 8 units of lispro insulin before her dinner, and is also given a dose of 25 units of glargine insulin. She is held NPO after midnight and dextrose‐containing fluid is provided intravenously overnight at a maintenance rate. In the morning her blood glucose is 161 mg/dL before surgery. Surgery goes well, and at lunch her blood glucose is 179 mg/dL, and she is given a food tray. However, the patient says that she feels mildly nauseated, and is not sure that she will be able to eat her lunch. How should her nutritional insulin be managed in this situation?
MATCHING NUTRITION AND NUTRITIONAL INSULIN: A DIFFICULT CHALLENGE
It is the provision of the correct type and amount of nutritional insulin at the right time that is most challenging in the hospital. In the hospital, a patient's nutritional intake is often interrupted. Patients might be made NPO as part of the treatment plan, or may not be able to take nutrition by mouth because of specific medical conditions. In some cases, enteral or parenteral feeding is used to replace or enhance oral feeding. Even when alternate routes of nutrition are employed, sudden interruptions in nutrition still remain common (eg, the feeding tube falls out). Ultimately, to provide the best possible care to a diabetes patient, the nutritional insulin that is delivered must match the actual nutritional delivery (Table 2). Ideally, each institution should choose a preferred, standardized approach for each nutritional situation.
Even if institutions standardize their approach to nutritional insulin delivery in general, clinicians must be able to accurately respond to the unplanned variations in nutrition that occur in the hospital. One example of this is the patient who is expected to eat meals, but who becomes suddenly unable to do so, such as in the case above. In cases like this, the best approach is to hold the patient's nutritional insulin and allow the patient to attempt to eat the provided meal. Then, a rapid‐acting insulin analog can be given just after the meal, in proportion to the amount of the meal that was eaten. If the patient really is unable to tolerate any of the meal, then no nutritional insulin is provided. If the patient does tolerate a portion of the meal (eg, 50% of the meal is consumed), then a corresponding amount of insulin is given (eg, 50% of the scheduled nutritional insulin is given). The quick onset of the rapid‐acting insulin analogs allows near‐physiologic effect, even when they are given after the meal.
Ms. X should be allowed to eat as much of the meal as she can tolerate. Afterward, her intake can be assessed, and insulin can be provided in proportion to the amount of the meal that was eaten, as described above.
Bolus Tube Feeds
Patients who are given bolus enteral feeds are typically treated like patients who are eating meals. Like meals, bolus tube feeds are nutritional boluses that should be covered with nutritional insulin boluses. Because the hyperglycemia that sometimes accompanies bolus tube feeds is partly related to the glycemic load of this type of feedings, it is reasonable to provide 60% of the TDD of insulin as nutritional insulin when using this type of nutrition.
Continuous Tube Feeds
Patients who are given continuous enteral nutrition are somewhat different than patients who are eating meals. Continuous tube feed patients receive nutrition on a continuous basis. Therefore, these patients must also receive nutritional insulin in a way that provides continuous coverage. There is no proven superior insulin regimen for the continuously tube fed patient. Because the nutrition is being provided continuously, providing all of the TDD of insulin as a long‐acting, nonpeaking insulin might be considered the most physiologic regimen. However, if such a strategy is used and the tube feeding is interrupted for some reason, the patient will be in danger of hypoglycemia for the duration of action of the basal insulin. For this reason, the SHM Glycemic Control Task Force and many endocrinologists recommend using a long‐acting basal insulin at a dose that would provide a conservative estimate of the basal component (eg, 40% or less of the TDD), and dividing the remainder of the insulin and giving it as scheduled regular (every 6 hours) or rapid‐acting insulin (every 4 hours) for the nutritional coverage.
If tube feeds are interrupted, an infusion of 10% dextrose given intravenously at the same rate as the tube feed had been running (or the equivalent) can be provided to avoid hypoglycemia until the effect of the nutritional insulin has dissipated. It is reasonable to create a standing order to notify the physician, or start an alternate source of dextrose, in the case of tube feed interruption.
Parenteral Nutrition
For patients receiving parenteral nutrition, regular insulin, mixed with the parenteral nutrition, is safe and effective. Subcutaneous correction‐dose insulin is often used, in addition to the insulin that is mixed with the nutrition. When starting parenteral nutrition, the initial use of a separate insulin infusion can help in estimating the TDD of insulin that will be required.
TYPE 1 DIABETES
Because type 2 diabetes is more prevalent than type 1 diabetes, hospitalists will manage this form of diabetes most often. However, it is crucial that hospitalists are also able to manage type 1 diabetes in the hospital. For the most part, the principles presented in this article apply to all types of diabetes patients. This section outlines some special considerations to remember when caring for type 1 diabetes patients in the hospital that will assure prevention of diabetic ketoacidosis in patients with type 1 diabetes.
Type 1 diabetes patients completely lack endogenous insulin production. Therefore, these patients require exogenous insulin to be provided at all times. Type 1 diabetes patients need to be provided continuous, exogenous basal insulin, even when fasting, to suppress gluconeogenesis and ketone production. Failure to provide basal insulin to a type 1 diabetes patient can lead to the rapid development (in hours) of ketoacidosis. When receiving nutrition, the patient with type 1 diabetes must also be provided with nutritional insulin to control postprandial BGs. Whereas many type 2 diabetes patients may produce sufficient endogenous insulin to meet basal requirements when fasting (ie, they produce enough basal insulin to maintain metabolic stability when they are not taking in nutrition), this is never the case for type 1 diabetes patients.
In addition, type 1 diabetes patients typically exhibit less insulin resistance than type 2 diabetes patients, especially if they are not obese. Therefore, type 1 diabetes patients often have TDDs of insulin that are lower than those of type 2 patients. This is reflected in the recommendations in Figure 5, with the TDD of insulin estimate for a lean type 1 diabetes patient of 0.3 units/kg/day.
CONTINUOUS SUBCUTANEOUS INSULIN INFUSIONS (INSULIN PUMPS) IN THE HOSPITAL
Continuous subcutaneous insulin infusion therapy (CSII), often referred to as insulin pump therapy, involves the use of a pump to provide a continuous flow of subcutaneous basal insulin (usually a rapid‐acting analog) through a needle that is left in place. This basal rate is adjustable, and therefore can be customized to meet variable needs over a 24‐hour period. When the patient takes in nutrition, a bolus of the same insulin is given, via the pump, at a dose that is appropriate to cover the nutritional intake. The advantages of CSII therapy are the capacity for precision and flexibility of the basal insulin delivery (compared to the use of a once‐daily or twice‐daily dose of long‐acting insulin analog), and the lack of a need to inject insulin boluses (which are delivered via the pump). This type of therapy is preferred by some diabetes patients, and although it is not highly prevalent in most areas, it is common enough that hospitalists must have a plan for managing it in their practices.
There are many barriers to the use of CSII in the hospital. Most of these barriers are related to the need for constant management of the pump. Most hospitalists and nurses do not have the expertise to manage this therapy in the hospital. Although the patient (or caregiver) might have the expertise to manage the pump, this is an acceptable option only if the patient is competent to manage the pump. Patient competence to use the pump must be formally assessed and documented, and the patient must agree to perform the many components of care related to managing the pump (eg, documenting the basal rate and boluses given, documenting BGs, providing tubing and other supplies).
Many hospitals currently choose a policy of converting insulin pump therapy to standard subcutaneous insulin treatment for most hospitalized patients. Usually the conversion of CSII to a physiologic subcutaneous insulin regimen (as detailed in this article) is fairly straightforwardthe basal insulin will be given as long‐acting, low‐peaking insulin, and nutritional and correction‐dose insulin can then be added as a rapid‐acting analog, in accord with the patient's needs.
Hospitals that admit a large number of insulin pump patients, or those that choose to use pumps routinely in the hospital, should create a formal policy for pump use.11 It has been suggested that the policy should assure that there is formal assessment of the patient's competence to manage the pump, that there is professional oversight of the pump management (usually via endocrinology and diabetes educator consultation), that contraindications for CSII use are clearly stated, and that there is a formal mechanism for engaging the patient and informing him of his roles and responsibilities (eg, a written agreement). The clinician must write insulin orders in the medical record that specify the basal and bolus insulin doses which are being used. Pump use may be limited to floors where nurses receive at least basic education in the principles of CSII pumps.
CARE TRANSITIONS IN THE HOSPITAL
Transitioning from an Intravenous Insulin Infusion to a Subcutaneous Insulin Regimen
In the hospital, it is often necessary to switch a patient from an intravenous (IV) insulin infusion to a subcutaneous (SC) insulin regimen. When doing this, the clinician must decide how much SC insulin the patient will require. As discussed earlier, the TDD may be estimated based on home insulin doses or the patient's weight. However, for a patient who is treated with an IV insulin infusion, current insulin requirements can be estimated based on the recent IV drip rate. This is the preferred method for identifying a TDD in these patients, as the insulin delivery rate at the time of drip discontinuation provides a way of determining current insulin requirements.
Regardless of the dose of SC insulin that is chosen, it is important that SC insulin be delivered well in advance of the discontinuation of the IV insulin. Because the duration of action of IV insulin is on the order of 7 minutes, the patient may become rapidly hyperglycemic or develop ketoacidosis (in type 1 diabetes) in a matter of hours if the IV insulin infusion is discontinued before the SC insulin is active. Insulin infusion should not be stopped for at least 1 hour after the SC delivery of rapid‐acting or regular insulin, and at least 2‐3 hours after the SC delivery of intermediate‐acting or long‐acting insulin.2
Discharge Transition
The hospital discharge is another challenging transition for the patient with diabetes. While hospitalized, a diabetes patient's medication regimen will likely be altered to maintain metabolic control. At the time of discharge, the patient should be provided with an appropriate medication regimen. Moreover, the patient must be educated about any new medication or other changes that will be part of the new outpatient management routine. It is also important to assure that the patient does not have knowledge deficits related to diabetes survival skills. The Joint Commission has recently put forth the expectation that such education will be provided prior to hospital discharge.12 Areas outlined for this core diabetes self‐management education include: the definition of diabetes; finger‐stick BG monitoring; glycemic targets; insulin self‐administration; hypoglycemia prevention, recognition, and treatment; hyperglycemia recognition; sick day guidelines; and when to call a clinician for help. Communication of the discharge diabetes management plan to the patient's primary care provider should also be undertaken.
Please see the article entitled Bridge Over Troubled Waters: Safe and Effective Transitions for the Inpatient with Hyperglycemia in this supplement for additional details about both the IV to SC transition and the discharge transition.
CONCLUSIONS
Understanding the basic principles of the physiologic (basal, nutritional, and correction‐dose) insulin regimen will allow clinicians to formulate safe and effective insulin regimens in virtually any clinical situation. Simple steps can allow safe estimates of initial doses and titration toward glycemic goals. Additional information and case studies can be found in a Society of Hospital Medicine Task Force Educational Module,5 available online.
Additional resources for improving glycemic control in hospital patients are available online at the Glycemic Control Resource Room (
Recently, there has been a heightened interest in improving the quality and safety of the management of diabetes and hyperglycemia in the hospital.1 While observational data strongly suggests an association of hyperglycemia with morbidity and mortality in adults on general medicine and surgery units, clinical research has not yet defined the best practices for managing hyperglycemia in the hospital outside the intensive care unit (ICU). As a result, many physicians do not have a well‐formulated approach to managing hyperglycemia in the noncritically ill hospital patient, and the use of insulin therapy to attain targeted blood glucose (BG) control is often subject to practice variability, leading to suboptimal glycemic outcomes.
Practical guidelines for the management of this common clinical problem have been formulated by experts in the field, based on understanding of the physiology of glucose and insulin dynamics, the characteristics of currently available insulin preparations, and clinical experience. In 2004, in Clement et al.,2 the American Diabetes Association published a technical review promoting the use of physiologic (basal‐nutritional‐correction dose) insulin regimens in the hospital to achieve targeted glycemic outcomes. This approach has been disseminated via review articles,3 and more recently, a randomized, controlled trial demonstrated that hospitalized type 2 diabetes patients experienced better glycemic control when treated with a physiologic insulin regimen than when treated with sliding‐scale insulin alone.4 The Society of Hospital Medicine has assembled a Glycemic Control Task Force, which is charged with providing physicians and hospitals with practical tools to improve the safety and efficacy of diabetes management in the hospital. One product of this work is an educational module that serves as a tutorial on the best practice for the management of diabetes and hyperglycemia in the noncritically ill hospital patient.5 This article is based on that module, and provides a practical summary of the key concepts that will allow clinicians to confidently employ physiologic insulin regimens when caring for their hospital patients.
Case: Ms. X is a 56‐year‐old obese woman with type 2 diabetes mellitus who is admitted for treatment of an infected diabetes‐related foot ulcer. The patient will be allowed to eat dinner in a couple of hours, but the surgeons have requested that she be kept nothing by mouth (NPO) after midnight for surgical debridement in the morning. Her current weight is 100 kg, and her recent glycemic control can be summarized as having BG values that are usually in the mid‐200s (mg/dL) and a recent glycosylated hemoglobin (HbA1C) measurement of 10.9%. Her home medical regimen includes glipizide 10 mg daily, metformin 1000 mg twice daily, and 20 units of neutral protamine hagadorn (NPH) insulin at bedtime. Her blood glucose in the Emergency Department is 289 mg/dL. How should this patient's blood glucose be managed in the hospital?
PHARMACOLOGIC CONTROL OF BG IN THE HOSPITAL: INSULIN IS THE ANTIHYPERGLYCEMIC AGENT OF CHOICE
Although oral antihyperglycemic agents are frequently used in the outpatient setting, there are many potential disadvantages to using these medications in acutely ill hospital patients, as shown in Figure 1.2, 3 Oral antihyperglycemic agents, in general, are difficult to quickly titrate to effect, and have side effects that can limit their use in the hospital. Metformin can lead to lactic acidosis when it is used in clinical situations that predispose to lactate production (eg, renal failure, circulatory failure, hypoxemia). Therefore, metformin should be held in patients who have, or are at risk for, these conditions, each of which may be encountered in the hospital. Also, agents that stimulate the release of insulin, such as sulfonylureas, should be held in patients with variable nutritional intake, to prevent hypoglycemia. In contrast, insulin acts rapidly, responds in a timely fashion to dose titrations, and can be used effectively in virtually all patients and clinical situations to control BG levels. This makes insulin the treatment of choice for hyperglycemia in the hospital. Insulin can be administered via subcutaneous doses or as an intravenous infusion for cases in which rapid titration is the goal. Intravenous insulin infusions are the preferred mode of insulin delivery in the ICU setting, and may be appropriate for some noncritically ill patients in hospitals that have developed systems to safely provide them on general wards. Subcutaneous insulin is most commonly used in the noncritically ill patient population and is the focus of this article.

Although insulin is the drug of choice for managing hyperglycemia in the hospital, there are some situations when it is appropriate to continue oral antihyperglycemic medications in the hospital. These agents may be continued in hospitalized patients who are clinically stable, and who have normal nutritional intake, normal BG levels, and stable renal and cardiac function. They may also be started or resumed in the hospital if they are to be included in the discharge medication regimen once the patient is clinically stable and if it has been assured that contraindications to their use no longer exist.
Ms. X should be treated with a more robust (physiologic) insulin regimen. This statement would be true, even if she were not an inpatient with a foot infection. Her glycemic control is currently poor, as evidenced by her high HbA1C and her elevated admission BG, and is unlikely to be appreciably improved with the addition of any pharmacologic agent other than insulin. The glipizide should be held, as the patient will be NPO after midnight. Most experts would recommend holding the metformin at this time as well, since the patient will be undergoing a surgical procedure in the morning that places her at risk for predisposing factors to lactic acidosis.
INPATIENT GLYCEMIC TARGETS
At present, recommended glycemic targets for noncritically ill hospital patients are based entirely on expert opinion, as there have been no clinical studies directly comparing different glycemic targets in this patient population. However, the American College of Endocrinology, the American Association of Clinical Endocrinologists, and the American Diabetes Association do provide recommendations about glycemic targets for inpatients (Table 1).6, 7 Although controversial, these recommendations make it clear that uncontrolled hyperglycemia is no longer the accepted standard of care for hospitalized patients, and illustrate the consensus of expert opinion on the subject. Of note, many hospitals are adopting glycemic targets that are less stringent than those shown in Table 1, recognizing the challenges of controlling BG levels in hospitalized patients, and the potential risk for hypoglycemia when lower BG targets are used. Each hospital's glycemic control champions must reach consensus on a target BG range for their institution. In practice this range has been 90110 mg/dL for the lower BG limit and 140180 mg/dL for the upper BG limit. It is also important to note that the recommendations from professional organizations emphasize the need to individualize BG targets, based on the clinical circumstances of each patient.
| Organization | ICU(mg/dL) | Non‐ICU, Preprandial (mg/dL)* | Non‐ICU, Maximum(mg/dL)* |
|---|---|---|---|
| |||
| ACCE/ACE | 110 | 110 | 180 |
| ADA | 110 | 90‐130 | 180 |
Ms. X is exhibiting glycemic values far outside of the recommended upper limit of 180 mg/dL, and treatment with insulin monotherapy is the most appropriate strategy in this case.
PHYSIOLOGIC (BASAL‐BOLUS, PLUS CORRECTION DOSE) INSULIN
The management of hyperglycemia and diabetes in the inpatient setting is challenging due to the many changes that patients experience in the hospital. Hospitalized patients often experience changes in their nutritional intake and their medication regimen. In addition, hospitalized patients usually experience the stress of acute illness and are treated with medications that might impact glycemic control. Figure 2 lists some of the barriers to achieving glycemic control in hospitalized patients. The inpatient insulin program needs to be flexible enough to allow for maintenance of glycemic control in the face of tumultuous circumstances. This can best be accomplished by the use of a physiologic insulin program. This means using exogenous insulin to mimic normal physiologic insulin activity by providing the correct types and doses of insulin at the correct times.

A physiologic insulin regimen can be conceptualized as having 3 separate components: basal insulin, nutritional (or prandial/meal) insulin, and correction dose (or supplemental) insulin.2 A patient's total daily dose (TDD) of insulin is the sum of all of these, and represents the amount of insulin that a patient requires over the course of 1 day while receiving adequate nutrition. Basal insulin is the insulin normally released continuously by the pancreas, even when fasting. This serves to suppress glucose and ketone production. When nutrition is ingested, there is a surge in the level of glucose in the blood, and this surge is accompanied by rapid secretion of additional insulin to allow for the appropriate utilization of the glucose. The insulin that is secreted in response to nutritional intake is referred to as nutritional insulin. About one‐half of the total daily insulin secreted by a healthy normal human serves a basal function, and about one‐half is secreted in response to nutritional intake.8 Understanding this bit of physiology (the 50/50 rule) is very helpful for creating flexible regimens using exogenous insulin. Although the 50/50 rule is useful in many circumstances, there are some notable exceptions. In some cases, basal insulin might be expected to be less than one‐half of the TDD (eg, enteral feeds, as discussed below). Additional correction dose insulin is given to correct hyperglycemia that occurs despite scheduled doses of basal and nutritional insulin.
It is an important practical consideration to note that when a person with diabetes is acutely ill or stressed, as is commonly the case in the hospital setting, total daily insulin requirements increase. This is due to the action of insulin counterregulatory hormones such as catecholamines, cortisol, growth hormone, and glucagon. The hospitalized patient is therefore likely to require a TDD that is higher than that required when well. This is particularly true for the basal insulin dose. Conversely, insulin requirements will decrease as a patient recovers from acute illness, and it may be necessary to lower insulin doses as BG levels decrease during convalescence.
Exogenous basal insulin is provided as a long‐acting or intermediate‐acting, low‐peaking or nonpeaking insulin (eg, glargine or detemir), that allows for a consistent level of basal insulin (Figure 3). This insulin is provided even when the person is not receiving any nutrition. Although twice daily NPH insulin can be used to provide basal insulin, the peak (as shown in Figure 3) is likely to exceed the level of insulin that is truly required for basal needs, which can result in hypoglycemia. In theory, NPH insulin would be less physiologic than glargine or detemir, although no studies have compared these insulins in the hospital setting. When using NPH insulin as a basal insulin in a patient who is designated NPO, the dose should be reduced by one‐third to one‐half to avoid hypoglycemia that may occur when it peaks.2

Exogenous nutritional insulin must be provided in a way that matches the nutrition that is being provided to the patient. For example, a patient who is receiving nutritional boluses (ie, meals or bolus tube feeds) can be given rapid‐acting insulin (eg, aspart, glulisine, lispro) along with each nutritional bolus to cover the glycemic peak that is caused by the meal. The rapid‐acting analog insulins can also be given at the end of a meal or bolus tube feed for cases in which it is not clear if the nutrition will be well tolerated. A reduction in the insulin dose proportionate to the amount of nutrition actually taken can then be made to decrease the risk of subsequent hypoglycemia. Regular insulin can also be given in anticipation of a meal or tube feed, but its later peak (as shown in Figure 3) requires that it be given 30 minutes before a meal is ingested, the timing of which is a challenge on most nursing units. Patients who are not receiving any nutrition should not receive nutritional insulin. And, patients receiving alternative forms of nutrition will require different nutritional insulin regimens to adequately cover their nutritional glycemic loads, as discussed below.
The separate provision of basal and nutritional insulin results in a highly‐flexible insulin program that can provide basal insulin to patients even when they are not receiving significant nutrition, and can be easily adjusted to provide appropriate nutritional insulin to match actual nutritional delivery.
Correction‐dose insulin is the small amount of insulin that is given to patients, in addition to basal and nutritional insulin, to correct hyperglycemia. Correction‐dose insulin is usually provided as rapid‐acting or regular insulin (usually the same type as the nutritional insulin), and is given in a dose that is specifically designed to reduce the patient's BG back into the target range. It is usually given at the same time as the nutritional insulin in patients who are receiving nutrition (or every 4 to 6 hours in patients who are not). Correction‐dose insulin is often written in a stepped format, to provide the appropriate amount of insulin for a given BG value. It differs from the traditional sliding‐scale in that it is not used alone (but rather as 1 component of a physiologic program), and in that it is customized to match the insulin sensitivity for each patient. Most standardized order sets for subcutaneous insulin provide several different correction‐dose scales to choose from, depending on the patient's weight or total daily insulin requirement.
If correction‐dose insulin is required consistently, or in high doses, it suggests a need to modify the basal and/or nutritional insulin. A proportion of the total number of units of correction‐dose insulin given in the preceding 24 hours can be distributed into basal and nutritional insulin doses for the next day if there is ongoing need for significant correction‐doses of insulin. A well‐designed, physiologic insulin regimen should provide targeted glycemic control, without a need for constantly adding large correctional boluses.
Some insulins do not fit neatly into either basal or nutritional insulin categories. For example, mixed insulins (eg, 70/30, rapid‐analog/NPH mixtures) combine basal and nutritional insulins to form either a double‐peaking insulin or an intermediate‐peaking insulin. The use of this type of insulin makes it impossible to manipulate the basal and nutritional components separately to enable attainment of BG targets. Therefore, the role of this type of insulin is limited in the hospital setting. Mixed insulins may, however, be started once the patient is clinically stable if they will be part of the discharge regimen.
Diabetes and hyperglycemia in the hospitalized patient require active management, and there are no autopilot insulin regimens. The use of sliding scale insulin alone to manage hyperglycemia is a common practice in hospitals.9 However, this is an historic practice that is based on the erroneous idea that BG can be managed with a reactive strategy. When sliding‐scale insulin is used as the sole modality of insulin therapy, insulin is provided only after metabolic control has been lost, and usually does not provide an appropriate dose of insulin, considering basal, nutritional, and correctional needs. The end result is poor glycemic control.9, 10 A recent randomized, controlled trial demonstrated that a physiologic insulin regimen is indeed superior to a standardized insulin sliding‐scale for managing inpatient hyperglycemia.4
Ms. X should be given an insulin regimen that includes basal, nutritional, and correctional components. The provision of separate basal and nutritional insulin will allow the clinicians to provide the patient with basal insulin even when her nutritional insulin is held, and to easily modify her nutritional insulin, depending on her nutritional intake.
PHYSIOLOGIC INSULIN: A PRACTICAL APPROACH
The use of physiologic insulin in the hospital can be facilitated by considering a stepwise approach (Figure 4).5 The first step is to estimate the amount of insulin that the patient will require over the course of a day if taking adequate nutrition (this is the TDD). If the patient has been treated with subcutaneous insulin before being admitted to the hospital, the clinician can use the outpatient TDD to gauge the insulin needs. To do this, the clinician simply adds up the total number of units of insulin that a patient takes at home in a day. Using this method to estimate the patient's TDD can be very helpful, even if the clinician plans to use different types of insulin while the patient is hospitalized. When using this approach, one should consider the patient's prior metabolic control on the existing regimen (ie, if the patient's glycemic control was poor on the preexisting regimen, an increase in the TDD would be necessary). In addition, clinicians should recognize that insulin requirements usually increase when patients are acutely ill, as discussed above. Managing diabetes and hyperglycemia in the hospital is very different than doing so in the outpatient arena, and hospitalists should not feel bound by the outpatient regimen.

In addition, a weight‐based estimation of the TDD can be very helpful in determining a starting dose of insulin in a hospitalized patient (see Figure 5). An estimate of 0.4 units/kg of body weight provides a conservative starting point for the TDD for most patients. Occasionally, a lower starting dose of 0.3 units/kg of body weight might be safer for those patients who are likely to be very sensitive to insulin, or who are otherwise at increased at risk for hypoglycemia (see Figure 5). Patients who are overweight or obese often require considerably higher TDDs in the range of 0.5‐0.6 (or even more) units/kg of body weight. Some may require over 1 unit/kg of body weight for their TDD. Therefore, the doses provided by these calculations represent conservative estimates in most patients. Hospitalists should be able to confidently make these dose estimates and avoid dependence on nonphysiologic regimens such as sliding‐scale insulin alone.

The presence of risk factors for hypoglycemia or hyperglycemia should temper the dose calculations. Clinical conditions associated with hyperglycemia include obesity, certain medications (eg, glucocorticoids, catecholamines, tacrolimus, cyclosporine), and changes in nutritional intake (Figure 2). Clinical conditions associated with hypoglycemia are summarized in Figure 6. It is important to recognize that these calculations are intended to give the clinician a safe and rational starting point for insulin dosing. More important than the calculations is the careful monitoring of BG levels and timely modifications of the insulin regimen that follow.

The second step in developing a physiologic insulin regimen is to determine the patient's nutritional regimen. The third step, then, is to decide how the TDD will be distributed into basal and nutritional insulins, and which insulin will be used for each. As noted above, for most patients, approximately one‐half of the TDD will be provided as basal insulin, and the other one‐half given as nutritional insulin. When patients are receiving nutrition, the insulin must be given in a way that matches the timing of nutrition delivery (eg, with each meal or bolus tube feeding), and provides appropriate insulin coverage for the nutrition that is being provided.
Nutritional insulin will not be given if the patient is not receiving any nutrition, in which case basal insulin and correction‐dose insulin will usually be continued. Table 2 shows the preferred insulin regimens for a variety of different nutritional circumstances, as put forth by the Society of Hospital Medicine (SHM) Glycemic Control Task Force.5
| Nutritional Situation | Necessary Insulin Components | Preferred Regimen* |
|---|---|---|
| ||
| NPO (or clear liquids) | Basal insulin: 50% of TDD. Nutritional insulin: None. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: None. Correctional insulin: Regular insulin q 6 hours or RAA insulin q 4 hours. Other comments: Dextrose infusion (eg, D5 containing solution at 75‐150 cc/hour) recommended when nutrition is held. An IV insulin infusion is preferred for management of prolonged fasts or fasting type 1 diabetes patients. |
| Eating meals | Basal insulin: 50% of TDD. Nutritional insulin: 50% of TDD, divided equally before each meal. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin with meals. Correctional insulin: RAA insulin q AC and HS (reduced dose at HS). |
| Bolus tube feeds | Basal insulin: 40% of TDD. Nutritional insulin: 60% of the TDD, divided equally before each bolus feed. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin with each bolus. Correctional insulin: RAA insulin with each bolus. |
| Continuous tube feeds | Basal insulin: 40% (conservative) of TDD. Nutritional insulin: 60% of the TDD in divided doses. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin q 4 hours or regular insulin q 6 hours. Correctional insulin: Should match nutritional insulin choice. |
| Parenteral nutrition | Insulin is usually given parenterally, with the nutrition | Initially, a separate insulin drip allows for accurate dose‐finding. Then, 80% of amount determined as TDD using drip is added to subsequent TPN bags as regular insulin. Use correctional subcutaneous insulin doses cautiously, in addition |
For Ms. X, the first step is to determine a TDD estimate. This patient weighs 100 kg, and is obese. Therefore, a TDD of 0.5 units/kg (50 units) is appropriate. This dose may be an underestimate of her insulin needs, but it is a conservative, reasonable starting point. The patient's basal insulin could be provided by giving one‐half of the TDD as basal insulin, such as glargine, 25 units daily. In this case, the patient will be eating dinner soon, but then will be NPO after midnight for surgery. Appropriate nutritional insulin could be provided by giving one‐third of her total nutritional insulin before this meal as a rapid‐acting insulin analog (25 units nutritional insulin per day divided by 3 meals results in a dose of 8 units per meal). After she eats dinner, additional nutritional insulin will not be given until she resumes her diet postoperatively. While NPO, her basal insulin should be continued, and her blood glucose should be checked every 4‐6 hours. An appropriate correction‐dose insulin scale should be chosen to provide a supplemental insulin dose with each bedside test of the blood glucose level, if and only if, hyperglycemia is present.
Case continued: The patient is given 8 units of lispro insulin before her dinner, and is also given a dose of 25 units of glargine insulin. She is held NPO after midnight and dextrose‐containing fluid is provided intravenously overnight at a maintenance rate. In the morning her blood glucose is 161 mg/dL before surgery. Surgery goes well, and at lunch her blood glucose is 179 mg/dL, and she is given a food tray. However, the patient says that she feels mildly nauseated, and is not sure that she will be able to eat her lunch. How should her nutritional insulin be managed in this situation?
MATCHING NUTRITION AND NUTRITIONAL INSULIN: A DIFFICULT CHALLENGE
It is the provision of the correct type and amount of nutritional insulin at the right time that is most challenging in the hospital. In the hospital, a patient's nutritional intake is often interrupted. Patients might be made NPO as part of the treatment plan, or may not be able to take nutrition by mouth because of specific medical conditions. In some cases, enteral or parenteral feeding is used to replace or enhance oral feeding. Even when alternate routes of nutrition are employed, sudden interruptions in nutrition still remain common (eg, the feeding tube falls out). Ultimately, to provide the best possible care to a diabetes patient, the nutritional insulin that is delivered must match the actual nutritional delivery (Table 2). Ideally, each institution should choose a preferred, standardized approach for each nutritional situation.
Even if institutions standardize their approach to nutritional insulin delivery in general, clinicians must be able to accurately respond to the unplanned variations in nutrition that occur in the hospital. One example of this is the patient who is expected to eat meals, but who becomes suddenly unable to do so, such as in the case above. In cases like this, the best approach is to hold the patient's nutritional insulin and allow the patient to attempt to eat the provided meal. Then, a rapid‐acting insulin analog can be given just after the meal, in proportion to the amount of the meal that was eaten. If the patient really is unable to tolerate any of the meal, then no nutritional insulin is provided. If the patient does tolerate a portion of the meal (eg, 50% of the meal is consumed), then a corresponding amount of insulin is given (eg, 50% of the scheduled nutritional insulin is given). The quick onset of the rapid‐acting insulin analogs allows near‐physiologic effect, even when they are given after the meal.
Ms. X should be allowed to eat as much of the meal as she can tolerate. Afterward, her intake can be assessed, and insulin can be provided in proportion to the amount of the meal that was eaten, as described above.
Bolus Tube Feeds
Patients who are given bolus enteral feeds are typically treated like patients who are eating meals. Like meals, bolus tube feeds are nutritional boluses that should be covered with nutritional insulin boluses. Because the hyperglycemia that sometimes accompanies bolus tube feeds is partly related to the glycemic load of this type of feedings, it is reasonable to provide 60% of the TDD of insulin as nutritional insulin when using this type of nutrition.
Continuous Tube Feeds
Patients who are given continuous enteral nutrition are somewhat different than patients who are eating meals. Continuous tube feed patients receive nutrition on a continuous basis. Therefore, these patients must also receive nutritional insulin in a way that provides continuous coverage. There is no proven superior insulin regimen for the continuously tube fed patient. Because the nutrition is being provided continuously, providing all of the TDD of insulin as a long‐acting, nonpeaking insulin might be considered the most physiologic regimen. However, if such a strategy is used and the tube feeding is interrupted for some reason, the patient will be in danger of hypoglycemia for the duration of action of the basal insulin. For this reason, the SHM Glycemic Control Task Force and many endocrinologists recommend using a long‐acting basal insulin at a dose that would provide a conservative estimate of the basal component (eg, 40% or less of the TDD), and dividing the remainder of the insulin and giving it as scheduled regular (every 6 hours) or rapid‐acting insulin (every 4 hours) for the nutritional coverage.
If tube feeds are interrupted, an infusion of 10% dextrose given intravenously at the same rate as the tube feed had been running (or the equivalent) can be provided to avoid hypoglycemia until the effect of the nutritional insulin has dissipated. It is reasonable to create a standing order to notify the physician, or start an alternate source of dextrose, in the case of tube feed interruption.
Parenteral Nutrition
For patients receiving parenteral nutrition, regular insulin, mixed with the parenteral nutrition, is safe and effective. Subcutaneous correction‐dose insulin is often used, in addition to the insulin that is mixed with the nutrition. When starting parenteral nutrition, the initial use of a separate insulin infusion can help in estimating the TDD of insulin that will be required.
TYPE 1 DIABETES
Because type 2 diabetes is more prevalent than type 1 diabetes, hospitalists will manage this form of diabetes most often. However, it is crucial that hospitalists are also able to manage type 1 diabetes in the hospital. For the most part, the principles presented in this article apply to all types of diabetes patients. This section outlines some special considerations to remember when caring for type 1 diabetes patients in the hospital that will assure prevention of diabetic ketoacidosis in patients with type 1 diabetes.
Type 1 diabetes patients completely lack endogenous insulin production. Therefore, these patients require exogenous insulin to be provided at all times. Type 1 diabetes patients need to be provided continuous, exogenous basal insulin, even when fasting, to suppress gluconeogenesis and ketone production. Failure to provide basal insulin to a type 1 diabetes patient can lead to the rapid development (in hours) of ketoacidosis. When receiving nutrition, the patient with type 1 diabetes must also be provided with nutritional insulin to control postprandial BGs. Whereas many type 2 diabetes patients may produce sufficient endogenous insulin to meet basal requirements when fasting (ie, they produce enough basal insulin to maintain metabolic stability when they are not taking in nutrition), this is never the case for type 1 diabetes patients.
In addition, type 1 diabetes patients typically exhibit less insulin resistance than type 2 diabetes patients, especially if they are not obese. Therefore, type 1 diabetes patients often have TDDs of insulin that are lower than those of type 2 patients. This is reflected in the recommendations in Figure 5, with the TDD of insulin estimate for a lean type 1 diabetes patient of 0.3 units/kg/day.
CONTINUOUS SUBCUTANEOUS INSULIN INFUSIONS (INSULIN PUMPS) IN THE HOSPITAL
Continuous subcutaneous insulin infusion therapy (CSII), often referred to as insulin pump therapy, involves the use of a pump to provide a continuous flow of subcutaneous basal insulin (usually a rapid‐acting analog) through a needle that is left in place. This basal rate is adjustable, and therefore can be customized to meet variable needs over a 24‐hour period. When the patient takes in nutrition, a bolus of the same insulin is given, via the pump, at a dose that is appropriate to cover the nutritional intake. The advantages of CSII therapy are the capacity for precision and flexibility of the basal insulin delivery (compared to the use of a once‐daily or twice‐daily dose of long‐acting insulin analog), and the lack of a need to inject insulin boluses (which are delivered via the pump). This type of therapy is preferred by some diabetes patients, and although it is not highly prevalent in most areas, it is common enough that hospitalists must have a plan for managing it in their practices.
There are many barriers to the use of CSII in the hospital. Most of these barriers are related to the need for constant management of the pump. Most hospitalists and nurses do not have the expertise to manage this therapy in the hospital. Although the patient (or caregiver) might have the expertise to manage the pump, this is an acceptable option only if the patient is competent to manage the pump. Patient competence to use the pump must be formally assessed and documented, and the patient must agree to perform the many components of care related to managing the pump (eg, documenting the basal rate and boluses given, documenting BGs, providing tubing and other supplies).
Many hospitals currently choose a policy of converting insulin pump therapy to standard subcutaneous insulin treatment for most hospitalized patients. Usually the conversion of CSII to a physiologic subcutaneous insulin regimen (as detailed in this article) is fairly straightforwardthe basal insulin will be given as long‐acting, low‐peaking insulin, and nutritional and correction‐dose insulin can then be added as a rapid‐acting analog, in accord with the patient's needs.
Hospitals that admit a large number of insulin pump patients, or those that choose to use pumps routinely in the hospital, should create a formal policy for pump use.11 It has been suggested that the policy should assure that there is formal assessment of the patient's competence to manage the pump, that there is professional oversight of the pump management (usually via endocrinology and diabetes educator consultation), that contraindications for CSII use are clearly stated, and that there is a formal mechanism for engaging the patient and informing him of his roles and responsibilities (eg, a written agreement). The clinician must write insulin orders in the medical record that specify the basal and bolus insulin doses which are being used. Pump use may be limited to floors where nurses receive at least basic education in the principles of CSII pumps.
CARE TRANSITIONS IN THE HOSPITAL
Transitioning from an Intravenous Insulin Infusion to a Subcutaneous Insulin Regimen
In the hospital, it is often necessary to switch a patient from an intravenous (IV) insulin infusion to a subcutaneous (SC) insulin regimen. When doing this, the clinician must decide how much SC insulin the patient will require. As discussed earlier, the TDD may be estimated based on home insulin doses or the patient's weight. However, for a patient who is treated with an IV insulin infusion, current insulin requirements can be estimated based on the recent IV drip rate. This is the preferred method for identifying a TDD in these patients, as the insulin delivery rate at the time of drip discontinuation provides a way of determining current insulin requirements.
Regardless of the dose of SC insulin that is chosen, it is important that SC insulin be delivered well in advance of the discontinuation of the IV insulin. Because the duration of action of IV insulin is on the order of 7 minutes, the patient may become rapidly hyperglycemic or develop ketoacidosis (in type 1 diabetes) in a matter of hours if the IV insulin infusion is discontinued before the SC insulin is active. Insulin infusion should not be stopped for at least 1 hour after the SC delivery of rapid‐acting or regular insulin, and at least 2‐3 hours after the SC delivery of intermediate‐acting or long‐acting insulin.2
Discharge Transition
The hospital discharge is another challenging transition for the patient with diabetes. While hospitalized, a diabetes patient's medication regimen will likely be altered to maintain metabolic control. At the time of discharge, the patient should be provided with an appropriate medication regimen. Moreover, the patient must be educated about any new medication or other changes that will be part of the new outpatient management routine. It is also important to assure that the patient does not have knowledge deficits related to diabetes survival skills. The Joint Commission has recently put forth the expectation that such education will be provided prior to hospital discharge.12 Areas outlined for this core diabetes self‐management education include: the definition of diabetes; finger‐stick BG monitoring; glycemic targets; insulin self‐administration; hypoglycemia prevention, recognition, and treatment; hyperglycemia recognition; sick day guidelines; and when to call a clinician for help. Communication of the discharge diabetes management plan to the patient's primary care provider should also be undertaken.
Please see the article entitled Bridge Over Troubled Waters: Safe and Effective Transitions for the Inpatient with Hyperglycemia in this supplement for additional details about both the IV to SC transition and the discharge transition.
CONCLUSIONS
Understanding the basic principles of the physiologic (basal, nutritional, and correction‐dose) insulin regimen will allow clinicians to formulate safe and effective insulin regimens in virtually any clinical situation. Simple steps can allow safe estimates of initial doses and titration toward glycemic goals. Additional information and case studies can be found in a Society of Hospital Medicine Task Force Educational Module,5 available online.
Additional resources for improving glycemic control in hospital patients are available online at the Glycemic Control Resource Room (
- ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control: A call to action.Diabetes Care.2006:29:1955–1962.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- .Management of hyperglycemia in the hospital setting.N Engl J Med.2006;355:1903–1911.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (Rabbit 2 trial).Diabetes Care.2007;30:2181–2186.
- ,; for the Society of Hospital Medicine Glycemic Control Task Force. Educational module: management of diabetes and hyperglycemia in the hospital patient: focus on subcutaneous insulin use in the non‐critically ill, adult patient. Published January 2007 on the Society of Hospital Medicine Website. Available at:http://www.hospitalmedicine.org/ResourceRoomRedesign/html/11Ed_Resources/01_Teaching_Slide.cfm. Accessed August2008.
- American College of Endocrinology Task Force on Inpatient Diabetes and Metabolic Control.American College of Endocrinology Position Statement on Inpatient Diabetes and Metabolic Control.Endocr Pract.2004;10:77–82.
- American Diabetes Association.Standards of Medical Care in Diabetes, 2006.Diabetes Care.2006;29(supp 1):s4–s42.
- Larsen PR,Kronenberg HM,Melmed S,Polonsky KS, editors.Williams Textbook of Endocrinology.10th ed.Philadelphia, PA:Elsevier Science;2003.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,.Glycemic control and sliding scale use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,, et al.Use of continuous subcutaneous insulin infusion therapy in the hospital setting: Proposed guidelines and outcome measures.Diabetes Educ.2005;31:849–857.
- Inpatient Diabetes Certification. Joint Commission. Available at:http://www.jointcommission.org/CertificationPrograms/Inpatient+Diabetes. Accessed April2008.
- ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control: A call to action.Diabetes Care.2006:29:1955–1962.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- .Management of hyperglycemia in the hospital setting.N Engl J Med.2006;355:1903–1911.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (Rabbit 2 trial).Diabetes Care.2007;30:2181–2186.
- ,; for the Society of Hospital Medicine Glycemic Control Task Force. Educational module: management of diabetes and hyperglycemia in the hospital patient: focus on subcutaneous insulin use in the non‐critically ill, adult patient. Published January 2007 on the Society of Hospital Medicine Website. Available at:http://www.hospitalmedicine.org/ResourceRoomRedesign/html/11Ed_Resources/01_Teaching_Slide.cfm. Accessed August2008.
- American College of Endocrinology Task Force on Inpatient Diabetes and Metabolic Control.American College of Endocrinology Position Statement on Inpatient Diabetes and Metabolic Control.Endocr Pract.2004;10:77–82.
- American Diabetes Association.Standards of Medical Care in Diabetes, 2006.Diabetes Care.2006;29(supp 1):s4–s42.
- Larsen PR,Kronenberg HM,Melmed S,Polonsky KS, editors.Williams Textbook of Endocrinology.10th ed.Philadelphia, PA:Elsevier Science;2003.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,.Glycemic control and sliding scale use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,, et al.Use of continuous subcutaneous insulin infusion therapy in the hospital setting: Proposed guidelines and outcome measures.Diabetes Educ.2005;31:849–857.
- Inpatient Diabetes Certification. Joint Commission. Available at:http://www.jointcommission.org/CertificationPrograms/Inpatient+Diabetes. Accessed April2008.
