User login
Systemic mastocytosis is a heterogeneous disorder of stem cell origin defined by abnormal hyperplasia and accumulation of mast cells (MCs) in one or more tissues.1,2 The most commonly affected tissues are the bone marrow, gastrointestinal tract, and skin. Based on a number of major and minor criteria defined by the World Health Organization (WHO), the mastocytoses are subdivided into 7 variants that range from isolated cutaneous involvement to widespread systemic disease.1-4 The most frequently diagnosed subtype is indolent systemic mastocytosis (ISM), a chronic disorder characterized by diffuse cutaneous macules and papules as well as bone marrow involvement in the form of multifocal dense infiltrates of MCs that frequently are phenotypically positive for c-KIT and tryptase. Serum tryptase levels are nearly invariably elevated in patients with this condition.1,2
Symptoms of ISM are determined by the intermittent release of histamine and leukotrienes from hyperproliferating MCs as well as IL-6 and eosinophil chemotactic factors. As the burden of MC secretory products increases, patients experience worsening pruritus, flushing, palpitations, vomiting, and anaphylaxis in severe instances.1,2,5 The mainstay of treatment of this condition involves symptom control through the inhibition of MC mediators.1 The majority of patients respond well to antihistamines, antileukotriene agents, and oral corticosteroids during severe episodes of MC degranulation.1,2,5
Unfortunately, some patients are unable to achieve adequate symptom control through the use of mediator-targeting treatments alone. In these cases, physicians often are faced with the following treatment dilemma: Either attempt to use therapies such as interferon alfa, which is cytoreductive to MCs, or 2-chlorodeoxyadenosine to reduce the overall MC burden, or turn to newer nonimmunosuppressive second-line options. We present the case of a patient with chronic ISM with progressive cutaneous lesions and poorly controlled pruritus that was previously managed with topical corticosteroids and antihistamines who responded favorably to treatment with narrowband UVB (NB-UVB) phototherapy.
Case Report
A 57-year-old woman presented with a 10-year history of widespread red-brown macules and papules on the trunk and upper and lower extremities. The lesions were intermittently pruritic, a symptom that was exacerbated on sun and heat exposure. A skin biopsy performed by an outside dermatologist 9 years prior confirmed the presence of mastocytosis. The patient was originally treated with triamcinolone cream and oral antihistamines, which controlled her symptoms successfully for nearly a decade.
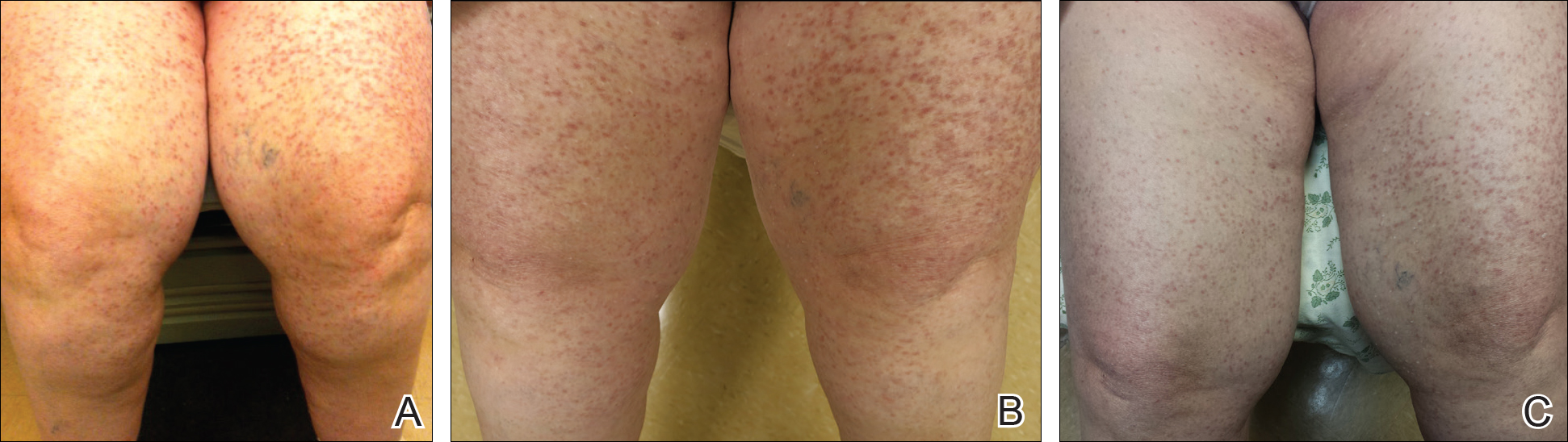
At the current presentation, the patient reported increasingly severe pruritus and lesional spread to the neck and face of 15 months’ duration. She denied any symptoms of flushing, diarrhea, syncopal episodes, or lightheadedness. Physical examination revealed a well-appearing middle-aged woman with multiple 3- to 8-mm, red-brown, blanchable macules and papules with areas coalescing into plaques that primarily involved the legs (Figure 1A); arms; back; and to a lesser extent the abdomen, neck, and face. There was no palpable lymphadenopathy.
Laboratory results revealed a complete blood cell count and basic metabolic profile within reference range; however, the serum tryptase level was elevated at 65 ng/mL (reference range, <11.4 ng/mL). A positron emission tomography–computed tomography scan was negative, as well as a c-KIT mutation analysis. A review of the skin biopsy from 9 years prior demonstrated slight acanthosis with dermal proliferation of mononuclear cells (Figure 2A), some of which had abundant cytoplasm and oval-shaped nuclei. There were few eosinophils and marked dermal telangiectasias. Giemsa stain revealed increased numbers of MCs in the upper dermis (Figure 2B). A bone marrow biopsy performed 9 years later showed multifocal lesions composed of MCs with associated lymphoid aggregates without notable myelodyspoiesis (or myeloproliferative neoplasm). These features were all consistent with WHO criteria for ISM. Based on the most current clinical, laboratory, and histopathologic findings, the patient was diagnosed with category IB ISM.

The patient’s symptoms had remained stable for 9 years with a regimen of triamcinolone cream 0.1% twice daily, doxepin cream 5% daily as needed, and oral fexofenadine 180 mg once daily. The patient continues to use topical steroids and oral antihistamines. Due to inadequate symptom control, breakthrough pruritus, and the development of new skin lesions on the head and neck, she was started on NB-UVB treatment 2 months after presentation. The patient’s symptoms and the extent of cutaneous maculopapular lesions improved after 20 light treatments (Figure 1B), with even more dramatic results after 40 cycles of therapy (Figure 1C). Overall, the lower legs have proved most recalcitrant to this treatment modality. She is currently continuing to receive NB-UVB treatment twice weekly.
Comment
Systemic mastocytosis is a heterogeneous disorder characterized by the proliferation and accumulation of atypical MCs in tissues, principally in the bone marrow and skin, though involvement of the gastrointestinal tract, liver, spleen, and lymphatic system also have been reported.1,2,6 The WHO classification of mastocytosis divides this condition into 7 subtypes.4 Indolent systemic mastocytosis is the most common variant.2,6 The etiology of ISM is not fully understood, but there is evidence suggesting that an activating mutation of KIT proto-oncogene receptor tyrosine kinase, KIT (usually D816V), present in the MCs of nearly 80% of patients with ISM may be involved.1,3-5,7 Patients occasionally present with predominantly cutaneous findings but typically seek medical attention due to the recurrent systemic symptoms of the disease (eg, pruritus, flushing, syncope, palpitations, headache, dyspepsia, vomiting, diarrhea), which are related to the release of MC mediators.1,2
The management of ISM is complex and based primarily on symptom reduction without alteration of disease course.1,2,5,7 Patients should avoid symptom triggers such as heat, humidity, emotional and physical stress, alcohol, and certain medications (ie, aspirin, opioids, radiocontrast agents).7 Patients are initially treated with histamine H1- and H2-receptor antagonists to alleviate MC mediator release symptoms.1,2,8 Although H1 blockers are most effective in mitigating cutaneous symptoms and limiting pruritus, H2 blockers are used to control gastric hypersecretion and dyspepsia.2 Proton pump inhibitors are useful in patients with peptic ulcer disease who are unresponsive to H2-receptor antagonist therapy.2,7 Cromolyn sodium and ketotifen fumarate are MC stabilizers that help prevent degranulation, which is helpful in relieving most major ISM symptoms. Leukotriene antagonists, such as zafirlukast, montelukast sodium, or zileuton, also may be employed to target the proinflammatory and pruritogenic leukotrienes, also products of the MC protein.2,7 Imatinib mesylate and masitinib mesylate, both tyrosine kinase inhibitors, have been shown to improve symptoms and reduce MC mediator levels in ISM; however, most patients harbor the resistant KIT D816V mutation, which limits the utility of this medication.Patients with sensitive KIT mutations or those who have the wild-type KIT D816 mutation may be more appropriate candidates for imatinib or masitinib therapy, which can ameliorate symptoms of flushing, pruritus, and depression.7-10 Treatment with omalizumab, a humanized murine anti-IgE monoclonal antibody, can be effective in treating recurrent, treatment-refractory anaphylaxis in ISM patients.5,7
Symptoms unresponsive to these therapies can be effectively treated with a short course of oral corticosteroids,6,7 while MC cytoreductive therapies such as interferon alfa or 2-chlorodeoxyadenosine (cladribine/2-CdA) are reserved for refractory cases.2,7 Alternative therapies such as NB-UVB2 or psoralen plus UVA phototherapy11 also have demonstrated success in treating ISM symptoms. In the past, NB-UVB has shown efficacy in controlling pruriginous conditions ranging from chronic urticaria12,13 to atopic dermatitis14 to psoriasis.15 This evidence has spurred studies to evaluate if NB-UVB has a role in the management of uncontrolled cases of cutaneous and ISM.2,13,16,17 To date, the evidence has been promising. The majority of patients treated with this regimen report subjective reduction in pruritus in addition to clinical cutaneous disease burden.2,11 Also, laboratory analysis demonstrates decreased levels of tryptase in patients utilizing NB-UVB phototherapy.2 Thus far, the use of NB-UVB phototherapy in the treatment of pruriginous disorders such as ISM has not been associated with any severe side effects such as increased rates of anaphylaxis, though some research has suggested that this therapy may lower the threshold for patients to develop symptomatic dermographism.12 Overall, patients treated with NB-UVB phototherapy report improved quality of life related to more effective symptom control.16
Although ISM is currently considered an incurable chronic condition,6 this case illustrates that symptomatic management is possible, even in cases of long-standing, severe disease. Patients should still be encouraged to avoid triggering factors and be vigilant in preventing potential anaphylaxis. However, NB-UVB phototherapy provides a supplemental or alternative treatment choice when other therapies have failed. We hope that the success of NB-UVB demonstrated in this case provides further evidence that this light-based therapy is a valuable treatment option in mastocytosis patients with unremitting or poorly controlled symptoms.
- Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. St. Louis, MO: Mosby/Elsevier; 2012.
- Brazzelli V, Grasso V, Manna G, et al. Indolent systemic mastocytosis treated with narrow-band UVB phototherapy: study of five cases [published online May 13, 2011]. J Eur Acad Dermatol Venereol. 2012;26:465-469.
- Pardanani A, Lim KH, Lasho TL, et al. WHO subvariants of indolent mastocytosis: clinical details and prognostic evaluation in 159 consecutive adults. Blood. 2010;115:150-151.
- Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes [published online April 8, 2009]. Blood. 2009;114:937-951.
- Wolff K, Komar M, Petzelbauer P. Clinical and histopathological aspects of cutaneous mastocytosis. Leuk Res. 2001;25:519-528.
- Marone G, Spadaro G, Granata F, et al. Treatment of mastocytosis: pharmacologic basis and current concepts. Leuk Res. 2001;25:583-594.
- Pardanani A. How I treat patients with indolent and smoldering mastocytosis (rare conditions but difficult to manage)[published online February 20, 2013]. Blood. 2013;121:3085-3094.
- Hartmann K, Henz BM. Mastocytosis: recent advances in defining the disease. Br J Dermatol. 2001;144:682-695.
- Vega-Ruiz A, Cortes JE, Sever M, et al. Phase II study of imatinib mesylate as therapy for patients with systemic mastocytosis. Leuk Res. 2009;33:1481-1484.
- Lortholary O, Chandesris MO, Bulai Livideanu C, et al. Masitinib for treatment of severely symptomatic indolent systemic mastocytosis: a randomised, placebo-controlled, phase 3 study. Lancet. 2017;389:612-620.
- Godt O, Proksch E, Streit V, et al. Short-and long-term effectiveness of oral and bath PUVA therapy in urticaria pigmentosa and systemic mastocytosis. Dermatology. 1997;1:35-39.
- Berroeta L, Clark C, Ibbotson SH, et al. Narrow-band (TL-01) ultraviolet B phototherapy for chronic urticaria. Clin Exp Dermatol. 2004;29:91-99.
- Engin B, Ozdemir M, Balevi A, et al. Treatment of chronic urticaria with narrowband ultraviolet B phototherapy: a randomized controlled trial. Acta Derm Venereol. 2008;3:247-251.
- Meduri NB, Vandergriff T, Rasmussen H, et al. Phototherapy in the management of atopic dermatitis: a systemic review. Photodermatol Photoimmunol Photomed. 2007;23:106-112.
- Nguyen T, Gattu S, Pugashetti R, et al. Practice of phototherapy in the treatment of moderate-to severe psoriasis. Curr Probl Dermatol. 2009;38:59-78.
- Brazzelli V, Grassi S, Merante S, et al. Narrow-band UVB phototherapy and psoralen-ultraviolet A photochemotherapy in the treatment of cutaneous mastocytosis: a study in 20 patients. Photodermatol Photoimmunol Photomed. 2016;32:238-246.
- Prignano F, Troiano M, Lotti T. Cutaneous mastocytosis: successful treatment with narrowband ultraviolet B phototherapy. Clin Exp Dermatol. 2010;35:914-915.
Systemic mastocytosis is a heterogeneous disorder of stem cell origin defined by abnormal hyperplasia and accumulation of mast cells (MCs) in one or more tissues.1,2 The most commonly affected tissues are the bone marrow, gastrointestinal tract, and skin. Based on a number of major and minor criteria defined by the World Health Organization (WHO), the mastocytoses are subdivided into 7 variants that range from isolated cutaneous involvement to widespread systemic disease.1-4 The most frequently diagnosed subtype is indolent systemic mastocytosis (ISM), a chronic disorder characterized by diffuse cutaneous macules and papules as well as bone marrow involvement in the form of multifocal dense infiltrates of MCs that frequently are phenotypically positive for c-KIT and tryptase. Serum tryptase levels are nearly invariably elevated in patients with this condition.1,2
Symptoms of ISM are determined by the intermittent release of histamine and leukotrienes from hyperproliferating MCs as well as IL-6 and eosinophil chemotactic factors. As the burden of MC secretory products increases, patients experience worsening pruritus, flushing, palpitations, vomiting, and anaphylaxis in severe instances.1,2,5 The mainstay of treatment of this condition involves symptom control through the inhibition of MC mediators.1 The majority of patients respond well to antihistamines, antileukotriene agents, and oral corticosteroids during severe episodes of MC degranulation.1,2,5
Unfortunately, some patients are unable to achieve adequate symptom control through the use of mediator-targeting treatments alone. In these cases, physicians often are faced with the following treatment dilemma: Either attempt to use therapies such as interferon alfa, which is cytoreductive to MCs, or 2-chlorodeoxyadenosine to reduce the overall MC burden, or turn to newer nonimmunosuppressive second-line options. We present the case of a patient with chronic ISM with progressive cutaneous lesions and poorly controlled pruritus that was previously managed with topical corticosteroids and antihistamines who responded favorably to treatment with narrowband UVB (NB-UVB) phototherapy.
Case Report
A 57-year-old woman presented with a 10-year history of widespread red-brown macules and papules on the trunk and upper and lower extremities. The lesions were intermittently pruritic, a symptom that was exacerbated on sun and heat exposure. A skin biopsy performed by an outside dermatologist 9 years prior confirmed the presence of mastocytosis. The patient was originally treated with triamcinolone cream and oral antihistamines, which controlled her symptoms successfully for nearly a decade.
At the current presentation, the patient reported increasingly severe pruritus and lesional spread to the neck and face of 15 months’ duration. She denied any symptoms of flushing, diarrhea, syncopal episodes, or lightheadedness. Physical examination revealed a well-appearing middle-aged woman with multiple 3- to 8-mm, red-brown, blanchable macules and papules with areas coalescing into plaques that primarily involved the legs (Figure 1A); arms; back; and to a lesser extent the abdomen, neck, and face. There was no palpable lymphadenopathy.

Laboratory results revealed a complete blood cell count and basic metabolic profile within reference range; however, the serum tryptase level was elevated at 65 ng/mL (reference range, <11.4 ng/mL). A positron emission tomography–computed tomography scan was negative, as well as a c-KIT mutation analysis. A review of the skin biopsy from 9 years prior demonstrated slight acanthosis with dermal proliferation of mononuclear cells (Figure 2A), some of which had abundant cytoplasm and oval-shaped nuclei. There were few eosinophils and marked dermal telangiectasias. Giemsa stain revealed increased numbers of MCs in the upper dermis (Figure 2B). A bone marrow biopsy performed 9 years later showed multifocal lesions composed of MCs with associated lymphoid aggregates without notable myelodyspoiesis (or myeloproliferative neoplasm). These features were all consistent with WHO criteria for ISM. Based on the most current clinical, laboratory, and histopathologic findings, the patient was diagnosed with category IB ISM.

The patient’s symptoms had remained stable for 9 years with a regimen of triamcinolone cream 0.1% twice daily, doxepin cream 5% daily as needed, and oral fexofenadine 180 mg once daily. The patient continues to use topical steroids and oral antihistamines. Due to inadequate symptom control, breakthrough pruritus, and the development of new skin lesions on the head and neck, she was started on NB-UVB treatment 2 months after presentation. The patient’s symptoms and the extent of cutaneous maculopapular lesions improved after 20 light treatments (Figure 1B), with even more dramatic results after 40 cycles of therapy (Figure 1C). Overall, the lower legs have proved most recalcitrant to this treatment modality. She is currently continuing to receive NB-UVB treatment twice weekly.
Comment
Systemic mastocytosis is a heterogeneous disorder characterized by the proliferation and accumulation of atypical MCs in tissues, principally in the bone marrow and skin, though involvement of the gastrointestinal tract, liver, spleen, and lymphatic system also have been reported.1,2,6 The WHO classification of mastocytosis divides this condition into 7 subtypes.4 Indolent systemic mastocytosis is the most common variant.2,6 The etiology of ISM is not fully understood, but there is evidence suggesting that an activating mutation of KIT proto-oncogene receptor tyrosine kinase, KIT (usually D816V), present in the MCs of nearly 80% of patients with ISM may be involved.1,3-5,7 Patients occasionally present with predominantly cutaneous findings but typically seek medical attention due to the recurrent systemic symptoms of the disease (eg, pruritus, flushing, syncope, palpitations, headache, dyspepsia, vomiting, diarrhea), which are related to the release of MC mediators.1,2
The management of ISM is complex and based primarily on symptom reduction without alteration of disease course.1,2,5,7 Patients should avoid symptom triggers such as heat, humidity, emotional and physical stress, alcohol, and certain medications (ie, aspirin, opioids, radiocontrast agents).7 Patients are initially treated with histamine H1- and H2-receptor antagonists to alleviate MC mediator release symptoms.1,2,8 Although H1 blockers are most effective in mitigating cutaneous symptoms and limiting pruritus, H2 blockers are used to control gastric hypersecretion and dyspepsia.2 Proton pump inhibitors are useful in patients with peptic ulcer disease who are unresponsive to H2-receptor antagonist therapy.2,7 Cromolyn sodium and ketotifen fumarate are MC stabilizers that help prevent degranulation, which is helpful in relieving most major ISM symptoms. Leukotriene antagonists, such as zafirlukast, montelukast sodium, or zileuton, also may be employed to target the proinflammatory and pruritogenic leukotrienes, also products of the MC protein.2,7 Imatinib mesylate and masitinib mesylate, both tyrosine kinase inhibitors, have been shown to improve symptoms and reduce MC mediator levels in ISM; however, most patients harbor the resistant KIT D816V mutation, which limits the utility of this medication.Patients with sensitive KIT mutations or those who have the wild-type KIT D816 mutation may be more appropriate candidates for imatinib or masitinib therapy, which can ameliorate symptoms of flushing, pruritus, and depression.7-10 Treatment with omalizumab, a humanized murine anti-IgE monoclonal antibody, can be effective in treating recurrent, treatment-refractory anaphylaxis in ISM patients.5,7
Symptoms unresponsive to these therapies can be effectively treated with a short course of oral corticosteroids,6,7 while MC cytoreductive therapies such as interferon alfa or 2-chlorodeoxyadenosine (cladribine/2-CdA) are reserved for refractory cases.2,7 Alternative therapies such as NB-UVB2 or psoralen plus UVA phototherapy11 also have demonstrated success in treating ISM symptoms. In the past, NB-UVB has shown efficacy in controlling pruriginous conditions ranging from chronic urticaria12,13 to atopic dermatitis14 to psoriasis.15 This evidence has spurred studies to evaluate if NB-UVB has a role in the management of uncontrolled cases of cutaneous and ISM.2,13,16,17 To date, the evidence has been promising. The majority of patients treated with this regimen report subjective reduction in pruritus in addition to clinical cutaneous disease burden.2,11 Also, laboratory analysis demonstrates decreased levels of tryptase in patients utilizing NB-UVB phototherapy.2 Thus far, the use of NB-UVB phototherapy in the treatment of pruriginous disorders such as ISM has not been associated with any severe side effects such as increased rates of anaphylaxis, though some research has suggested that this therapy may lower the threshold for patients to develop symptomatic dermographism.12 Overall, patients treated with NB-UVB phototherapy report improved quality of life related to more effective symptom control.16
Although ISM is currently considered an incurable chronic condition,6 this case illustrates that symptomatic management is possible, even in cases of long-standing, severe disease. Patients should still be encouraged to avoid triggering factors and be vigilant in preventing potential anaphylaxis. However, NB-UVB phototherapy provides a supplemental or alternative treatment choice when other therapies have failed. We hope that the success of NB-UVB demonstrated in this case provides further evidence that this light-based therapy is a valuable treatment option in mastocytosis patients with unremitting or poorly controlled symptoms.
Systemic mastocytosis is a heterogeneous disorder of stem cell origin defined by abnormal hyperplasia and accumulation of mast cells (MCs) in one or more tissues.1,2 The most commonly affected tissues are the bone marrow, gastrointestinal tract, and skin. Based on a number of major and minor criteria defined by the World Health Organization (WHO), the mastocytoses are subdivided into 7 variants that range from isolated cutaneous involvement to widespread systemic disease.1-4 The most frequently diagnosed subtype is indolent systemic mastocytosis (ISM), a chronic disorder characterized by diffuse cutaneous macules and papules as well as bone marrow involvement in the form of multifocal dense infiltrates of MCs that frequently are phenotypically positive for c-KIT and tryptase. Serum tryptase levels are nearly invariably elevated in patients with this condition.1,2
Symptoms of ISM are determined by the intermittent release of histamine and leukotrienes from hyperproliferating MCs as well as IL-6 and eosinophil chemotactic factors. As the burden of MC secretory products increases, patients experience worsening pruritus, flushing, palpitations, vomiting, and anaphylaxis in severe instances.1,2,5 The mainstay of treatment of this condition involves symptom control through the inhibition of MC mediators.1 The majority of patients respond well to antihistamines, antileukotriene agents, and oral corticosteroids during severe episodes of MC degranulation.1,2,5
Unfortunately, some patients are unable to achieve adequate symptom control through the use of mediator-targeting treatments alone. In these cases, physicians often are faced with the following treatment dilemma: Either attempt to use therapies such as interferon alfa, which is cytoreductive to MCs, or 2-chlorodeoxyadenosine to reduce the overall MC burden, or turn to newer nonimmunosuppressive second-line options. We present the case of a patient with chronic ISM with progressive cutaneous lesions and poorly controlled pruritus that was previously managed with topical corticosteroids and antihistamines who responded favorably to treatment with narrowband UVB (NB-UVB) phototherapy.
Case Report
A 57-year-old woman presented with a 10-year history of widespread red-brown macules and papules on the trunk and upper and lower extremities. The lesions were intermittently pruritic, a symptom that was exacerbated on sun and heat exposure. A skin biopsy performed by an outside dermatologist 9 years prior confirmed the presence of mastocytosis. The patient was originally treated with triamcinolone cream and oral antihistamines, which controlled her symptoms successfully for nearly a decade.
At the current presentation, the patient reported increasingly severe pruritus and lesional spread to the neck and face of 15 months’ duration. She denied any symptoms of flushing, diarrhea, syncopal episodes, or lightheadedness. Physical examination revealed a well-appearing middle-aged woman with multiple 3- to 8-mm, red-brown, blanchable macules and papules with areas coalescing into plaques that primarily involved the legs (Figure 1A); arms; back; and to a lesser extent the abdomen, neck, and face. There was no palpable lymphadenopathy.

Laboratory results revealed a complete blood cell count and basic metabolic profile within reference range; however, the serum tryptase level was elevated at 65 ng/mL (reference range, <11.4 ng/mL). A positron emission tomography–computed tomography scan was negative, as well as a c-KIT mutation analysis. A review of the skin biopsy from 9 years prior demonstrated slight acanthosis with dermal proliferation of mononuclear cells (Figure 2A), some of which had abundant cytoplasm and oval-shaped nuclei. There were few eosinophils and marked dermal telangiectasias. Giemsa stain revealed increased numbers of MCs in the upper dermis (Figure 2B). A bone marrow biopsy performed 9 years later showed multifocal lesions composed of MCs with associated lymphoid aggregates without notable myelodyspoiesis (or myeloproliferative neoplasm). These features were all consistent with WHO criteria for ISM. Based on the most current clinical, laboratory, and histopathologic findings, the patient was diagnosed with category IB ISM.

The patient’s symptoms had remained stable for 9 years with a regimen of triamcinolone cream 0.1% twice daily, doxepin cream 5% daily as needed, and oral fexofenadine 180 mg once daily. The patient continues to use topical steroids and oral antihistamines. Due to inadequate symptom control, breakthrough pruritus, and the development of new skin lesions on the head and neck, she was started on NB-UVB treatment 2 months after presentation. The patient’s symptoms and the extent of cutaneous maculopapular lesions improved after 20 light treatments (Figure 1B), with even more dramatic results after 40 cycles of therapy (Figure 1C). Overall, the lower legs have proved most recalcitrant to this treatment modality. She is currently continuing to receive NB-UVB treatment twice weekly.
Comment
Systemic mastocytosis is a heterogeneous disorder characterized by the proliferation and accumulation of atypical MCs in tissues, principally in the bone marrow and skin, though involvement of the gastrointestinal tract, liver, spleen, and lymphatic system also have been reported.1,2,6 The WHO classification of mastocytosis divides this condition into 7 subtypes.4 Indolent systemic mastocytosis is the most common variant.2,6 The etiology of ISM is not fully understood, but there is evidence suggesting that an activating mutation of KIT proto-oncogene receptor tyrosine kinase, KIT (usually D816V), present in the MCs of nearly 80% of patients with ISM may be involved.1,3-5,7 Patients occasionally present with predominantly cutaneous findings but typically seek medical attention due to the recurrent systemic symptoms of the disease (eg, pruritus, flushing, syncope, palpitations, headache, dyspepsia, vomiting, diarrhea), which are related to the release of MC mediators.1,2
The management of ISM is complex and based primarily on symptom reduction without alteration of disease course.1,2,5,7 Patients should avoid symptom triggers such as heat, humidity, emotional and physical stress, alcohol, and certain medications (ie, aspirin, opioids, radiocontrast agents).7 Patients are initially treated with histamine H1- and H2-receptor antagonists to alleviate MC mediator release symptoms.1,2,8 Although H1 blockers are most effective in mitigating cutaneous symptoms and limiting pruritus, H2 blockers are used to control gastric hypersecretion and dyspepsia.2 Proton pump inhibitors are useful in patients with peptic ulcer disease who are unresponsive to H2-receptor antagonist therapy.2,7 Cromolyn sodium and ketotifen fumarate are MC stabilizers that help prevent degranulation, which is helpful in relieving most major ISM symptoms. Leukotriene antagonists, such as zafirlukast, montelukast sodium, or zileuton, also may be employed to target the proinflammatory and pruritogenic leukotrienes, also products of the MC protein.2,7 Imatinib mesylate and masitinib mesylate, both tyrosine kinase inhibitors, have been shown to improve symptoms and reduce MC mediator levels in ISM; however, most patients harbor the resistant KIT D816V mutation, which limits the utility of this medication.Patients with sensitive KIT mutations or those who have the wild-type KIT D816 mutation may be more appropriate candidates for imatinib or masitinib therapy, which can ameliorate symptoms of flushing, pruritus, and depression.7-10 Treatment with omalizumab, a humanized murine anti-IgE monoclonal antibody, can be effective in treating recurrent, treatment-refractory anaphylaxis in ISM patients.5,7
Symptoms unresponsive to these therapies can be effectively treated with a short course of oral corticosteroids,6,7 while MC cytoreductive therapies such as interferon alfa or 2-chlorodeoxyadenosine (cladribine/2-CdA) are reserved for refractory cases.2,7 Alternative therapies such as NB-UVB2 or psoralen plus UVA phototherapy11 also have demonstrated success in treating ISM symptoms. In the past, NB-UVB has shown efficacy in controlling pruriginous conditions ranging from chronic urticaria12,13 to atopic dermatitis14 to psoriasis.15 This evidence has spurred studies to evaluate if NB-UVB has a role in the management of uncontrolled cases of cutaneous and ISM.2,13,16,17 To date, the evidence has been promising. The majority of patients treated with this regimen report subjective reduction in pruritus in addition to clinical cutaneous disease burden.2,11 Also, laboratory analysis demonstrates decreased levels of tryptase in patients utilizing NB-UVB phototherapy.2 Thus far, the use of NB-UVB phototherapy in the treatment of pruriginous disorders such as ISM has not been associated with any severe side effects such as increased rates of anaphylaxis, though some research has suggested that this therapy may lower the threshold for patients to develop symptomatic dermographism.12 Overall, patients treated with NB-UVB phototherapy report improved quality of life related to more effective symptom control.16
Although ISM is currently considered an incurable chronic condition,6 this case illustrates that symptomatic management is possible, even in cases of long-standing, severe disease. Patients should still be encouraged to avoid triggering factors and be vigilant in preventing potential anaphylaxis. However, NB-UVB phototherapy provides a supplemental or alternative treatment choice when other therapies have failed. We hope that the success of NB-UVB demonstrated in this case provides further evidence that this light-based therapy is a valuable treatment option in mastocytosis patients with unremitting or poorly controlled symptoms.
- Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. St. Louis, MO: Mosby/Elsevier; 2012.
- Brazzelli V, Grasso V, Manna G, et al. Indolent systemic mastocytosis treated with narrow-band UVB phototherapy: study of five cases [published online May 13, 2011]. J Eur Acad Dermatol Venereol. 2012;26:465-469.
- Pardanani A, Lim KH, Lasho TL, et al. WHO subvariants of indolent mastocytosis: clinical details and prognostic evaluation in 159 consecutive adults. Blood. 2010;115:150-151.
- Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes [published online April 8, 2009]. Blood. 2009;114:937-951.
- Wolff K, Komar M, Petzelbauer P. Clinical and histopathological aspects of cutaneous mastocytosis. Leuk Res. 2001;25:519-528.
- Marone G, Spadaro G, Granata F, et al. Treatment of mastocytosis: pharmacologic basis and current concepts. Leuk Res. 2001;25:583-594.
- Pardanani A. How I treat patients with indolent and smoldering mastocytosis (rare conditions but difficult to manage)[published online February 20, 2013]. Blood. 2013;121:3085-3094.
- Hartmann K, Henz BM. Mastocytosis: recent advances in defining the disease. Br J Dermatol. 2001;144:682-695.
- Vega-Ruiz A, Cortes JE, Sever M, et al. Phase II study of imatinib mesylate as therapy for patients with systemic mastocytosis. Leuk Res. 2009;33:1481-1484.
- Lortholary O, Chandesris MO, Bulai Livideanu C, et al. Masitinib for treatment of severely symptomatic indolent systemic mastocytosis: a randomised, placebo-controlled, phase 3 study. Lancet. 2017;389:612-620.
- Godt O, Proksch E, Streit V, et al. Short-and long-term effectiveness of oral and bath PUVA therapy in urticaria pigmentosa and systemic mastocytosis. Dermatology. 1997;1:35-39.
- Berroeta L, Clark C, Ibbotson SH, et al. Narrow-band (TL-01) ultraviolet B phototherapy for chronic urticaria. Clin Exp Dermatol. 2004;29:91-99.
- Engin B, Ozdemir M, Balevi A, et al. Treatment of chronic urticaria with narrowband ultraviolet B phototherapy: a randomized controlled trial. Acta Derm Venereol. 2008;3:247-251.
- Meduri NB, Vandergriff T, Rasmussen H, et al. Phototherapy in the management of atopic dermatitis: a systemic review. Photodermatol Photoimmunol Photomed. 2007;23:106-112.
- Nguyen T, Gattu S, Pugashetti R, et al. Practice of phototherapy in the treatment of moderate-to severe psoriasis. Curr Probl Dermatol. 2009;38:59-78.
- Brazzelli V, Grassi S, Merante S, et al. Narrow-band UVB phototherapy and psoralen-ultraviolet A photochemotherapy in the treatment of cutaneous mastocytosis: a study in 20 patients. Photodermatol Photoimmunol Photomed. 2016;32:238-246.
- Prignano F, Troiano M, Lotti T. Cutaneous mastocytosis: successful treatment with narrowband ultraviolet B phototherapy. Clin Exp Dermatol. 2010;35:914-915.
- Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. St. Louis, MO: Mosby/Elsevier; 2012.
- Brazzelli V, Grasso V, Manna G, et al. Indolent systemic mastocytosis treated with narrow-band UVB phototherapy: study of five cases [published online May 13, 2011]. J Eur Acad Dermatol Venereol. 2012;26:465-469.
- Pardanani A, Lim KH, Lasho TL, et al. WHO subvariants of indolent mastocytosis: clinical details and prognostic evaluation in 159 consecutive adults. Blood. 2010;115:150-151.
- Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes [published online April 8, 2009]. Blood. 2009;114:937-951.
- Wolff K, Komar M, Petzelbauer P. Clinical and histopathological aspects of cutaneous mastocytosis. Leuk Res. 2001;25:519-528.
- Marone G, Spadaro G, Granata F, et al. Treatment of mastocytosis: pharmacologic basis and current concepts. Leuk Res. 2001;25:583-594.
- Pardanani A. How I treat patients with indolent and smoldering mastocytosis (rare conditions but difficult to manage)[published online February 20, 2013]. Blood. 2013;121:3085-3094.
- Hartmann K, Henz BM. Mastocytosis: recent advances in defining the disease. Br J Dermatol. 2001;144:682-695.
- Vega-Ruiz A, Cortes JE, Sever M, et al. Phase II study of imatinib mesylate as therapy for patients with systemic mastocytosis. Leuk Res. 2009;33:1481-1484.
- Lortholary O, Chandesris MO, Bulai Livideanu C, et al. Masitinib for treatment of severely symptomatic indolent systemic mastocytosis: a randomised, placebo-controlled, phase 3 study. Lancet. 2017;389:612-620.
- Godt O, Proksch E, Streit V, et al. Short-and long-term effectiveness of oral and bath PUVA therapy in urticaria pigmentosa and systemic mastocytosis. Dermatology. 1997;1:35-39.
- Berroeta L, Clark C, Ibbotson SH, et al. Narrow-band (TL-01) ultraviolet B phototherapy for chronic urticaria. Clin Exp Dermatol. 2004;29:91-99.
- Engin B, Ozdemir M, Balevi A, et al. Treatment of chronic urticaria with narrowband ultraviolet B phototherapy: a randomized controlled trial. Acta Derm Venereol. 2008;3:247-251.
- Meduri NB, Vandergriff T, Rasmussen H, et al. Phototherapy in the management of atopic dermatitis: a systemic review. Photodermatol Photoimmunol Photomed. 2007;23:106-112.
- Nguyen T, Gattu S, Pugashetti R, et al. Practice of phototherapy in the treatment of moderate-to severe psoriasis. Curr Probl Dermatol. 2009;38:59-78.
- Brazzelli V, Grassi S, Merante S, et al. Narrow-band UVB phototherapy and psoralen-ultraviolet A photochemotherapy in the treatment of cutaneous mastocytosis: a study in 20 patients. Photodermatol Photoimmunol Photomed. 2016;32:238-246.
- Prignano F, Troiano M, Lotti T. Cutaneous mastocytosis: successful treatment with narrowband ultraviolet B phototherapy. Clin Exp Dermatol. 2010;35:914-915.
Practice Points
- Patients with cutaneous lesions and symptoms consistent with mastocytosis should be worked up for potential systemic involvement.
- Symptoms of indolent systemic mastocytosis (ISM) include pruritus, flushing, palpitations, vomiting, and anaphylaxis in severe instances.
- Most patients respond well to antihistamines, antileukotriene agents, and oral corticosteroids during severe episodes of mast cell degranulation.
- Narrowband UVB is a safe, effective, and well-tolerated treatment option for symptom control in refractory ISM cases.
