User login
Biologic agents such as those that block tumor necrosis factor (TNF) alpha have revolutionized the treatment of autoimmune diseases like rheumatoid arthritis and inflammatory bowel disease, dramatically improving disease control and quality of life. In addition, they have made true disease remission possible in some cases.
However, as with any new therapy, a variety of side effects must be considered.
WHAT ARE BIOLOGIC AGENTS?
Biologic agents are genetically engineered drugs manufactured or synthesized in vitro from molecules such as proteins, genes, and antibodies present in living organisms. By targeting specific molecular components of the inflammatory cascade, including cytokines, these drugs alter certain aspects of the body’s inflammatory response in autoimmune diseases. Because TNF inhibitors are the most widely used biologic agents in the United States, this review will focus on them.
TNF INHIBITORS
TNF inhibitors suppress the inflammatory cascade by inactivating TNF alpha, a cytokine that promotes inflammation in the intestine, synovial tissue, and other sites.1,2
Several TNF inhibitors are available. Etanercept and infliximab were the first two to receive US Food and Drug Administration (FDA) approval, and they are extensively used. Etanercept, a soluble TNF receptor given subcutaneously, was approved for treating rheumatoid arthritis in 1998. Infliximab, a chimeric monoclonal antibody (75% human and 25% mouse protein sequence) given intravenously, received FDA approval for treating Crohn disease in 1998 and for rheumatoid arthritis in 1999. Other anti-TNF agents with varying properties are also available.
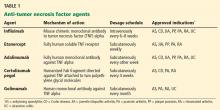
Table 1 lists anti-TNF agents approved for treating rheumatoid arthritis and inflammatory bowel disease. These are chronic, relapsing diseases that significantly and dramatically reduce quality of life, especially when they are poorly controlled.3,4 Untreated disease has been associated with malignancy, infection, pregnancy loss, and malnutrition.5–8 TNF inhibitors are aimed at patients who have moderate to severe disease or for whom previous treatments have failed, to help them achieve and maintain steroid-free remission. However, use of these agents is tempered by the risk of potentially serious side effects.
Special thought should also be given to the direct costs of the drugs (up to $30,000 per year, not counting the cost of their administration) and to the indirect costs such as time away from work to receive treatment. These are major considerations in some cases, and patients should be selected carefully for treatment with these drugs.
BEFORE STARTING THERAPY
Before starting anti-TNF therapy, several steps can reduce the risk of serious adverse events.
Take a focused history
The clinical history should include inquiries about previous bacterial, fungal, and tuberculosis infections or exposure; diabetes; and other immunocompromised states that increase the risk of acquiring potentially life-threatening infections.
Details of particular geographic areas of residence, occupational exposures, and social history should be sought. These include history of incarceration (which may put patients at risk of tuberculosis) and residence in the Ohio River valley or in the southwestern or midwestern United States (which may increase the risk of histoplasmosis, coccidioidomycosis, and other fungal infections).
Bring vaccinations up to date
Age-appropriate vaccinations should be discussed and given, ideally before starting therapy. These include influenza vaccine every year and tetanus boosters every 10 years for all and, as appropriate, varicella, human papillomavirus, and pneumococcal vaccinations. The US Centers for Disease Control and Prevention recommend an additional dose of the pneumococcal vaccine if more than 5 years have elapsed since the first one, and many clinicians opt to give it every 5 years.
In general, live-attenuated vaccines, including the intranasal influenza vaccine, are contraindicated in patients taking biologic agents.9 For patients at high risk of exposure or infection, it may be reasonable to hold the biologic agent for a period of time, vaccinate, and resume the biologic agent a month later.
Recent data suggest that the varicella zoster vaccine may be safely given to older patients with immune-mediated diseases such as rheumatoid arthritis and inflammatory bowel disease taking biologic agents.10 New guidelines from the American College of Rheumatology recommend age-appropriate vaccines for rheumatoid arthritis patients age 60 and older before biologic treatments are started. Case-by-case discussion with the subspecialist and the patient is recommended.
Screen for chronic infections
Tuberculosis screening with a purified protein derivative test or an interferon-gamma-release (Quantiferon) assay followed by chest radiography in patients with a positive test is mandatory before giving a TNF inhibitor.
Hepatitis B virus status should be determined before starting anti-TNF therapy.11
Hepatitis B vaccination has been recommended for patients with inflammatory bowel disease, but no clear recommendation exists for patients with rheumatic disease. Patients with inflammatory bowel disease tend to have low rates of response to hepatitis B vaccination12,13; possible reasons include their lack of an appropriate innate immune response to infectious agents, malnutrition, surgery, older age, and immunosuppressive drugs.14 An accelerated vaccination protocol with recombinant hepatitis B vaccine (Energix-B) in a double dose at 0, 1, and 2 months has been shown to improve response rates.15
Whenever possible, it may be better to vaccinate patients before starting immunosuppressive therapy, and to check postvaccination titers to ensure adequate response.
Perform an examination
A full physical examination with special attention to skin rashes should be performed. This may serve as a baseline to assist early detection of new rashes associated with anti-TNF therapy.
A baseline complete blood cell count and complete metabolic panel should be routinely obtained before starting therapy (and thereafter at the discretion of the physician). In conjunction with follow-up tests, they can help detect an unexpected decrease in white blood cell count or abnormal results on the liver panel.16 These baseline and follow-up tests are generally performed by the subspecialist, and the results are shared with the primary care physician.
Table 2 summarizes key information to be sought before starting a patient on a TNF inhibitor.
ADVERSE EFFECTS OF ANTI-TNF DRUGS
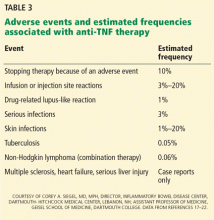
Infusion reactions, infections, cardiac arrhythmias, demyelinating disorders, skin infections, and malignancies have been reported with anti-TNF therapy. The relative frequencies of these adverse events are summarized in Table 3.17–22
NONINFECTIOUS COMPLICATIONS OF TNF INHIBITORS
Injection site reactions
When anti-TNF agents are given subcutaneously, injection site reactions are common (occurring in up to 40% of patients) and are considered minor.11 Reactions, including significant pain, typically occur within the first few months of therapy. They can last 2 to 5 days but rarely warrant stopping therapy. Treatment with ice and an antihistamine is almost always sufficient to control symptoms.
Infusion reactions with infliximab
Infliximab can cause both acute and delayed infusion reactions. Acute reactions can occur up to 24 hours after infusion but usually appear within 10 minutes of administration and are handled by the infusion suite staff. They range from the severe immunoglobulin E-mediated type I reaction, manifesting with hypotension, bronchospasm, and urticaria, to the milder anaphylactoid-type reaction, which constitutes the majority.23–25
While most primary care physicians will not encounter an acute reaction, family doctors and emergency room physicians may encounter delayed reactions, which can develop 1 to 14 days after infusion. These reactions usually resemble serum sickness and present with joint pain, fatigue, myalgia, and fever. But, unlike classic serum sickness, these reactions are generally not associated with a rash.
With nonspecific symptoms, the diagnosis may be easy to overlook. However, establishing this diagnosis is important because repeat therapy may result in a more severe reaction upon reexposure to the drug.
Once diagnosed, these reactions can be treated symptomatically with a combination of acetaminophen and diphenhydramine after discussion between the primary care physician and subspecialist.23,24
Autoimmune syndromes
Several studies have reported a small percentage of patients treated with anti-TNF agents who develop paradoxical autoimmune conditions. These range from asymptomatic immunologic alterations, including the formation of antinuclear antibodies and antibodies to double-stranded DNA, to life-threatening systemic autoimmune diseases.26,27
Autoimmune diseases associated with anti-TNF treatment include a lupus-like syndrome, vasculitides, and psoriatic skin lesions. These syndromes warrant stopping the inciting drug and, on occasion, giving corticosteroids. Most cases arise between 1 month and 1 year of starting treatment, and almost 75% resolve completely after the anti-TNF therapy is stopped.26
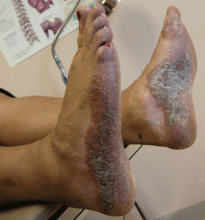
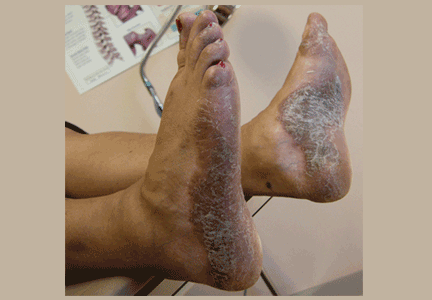
Interestingly, anti-TNF agents are approved for treating psoriasis and psoriatic arthritis, but psoriasis has paradoxically developed in patients being treated with these drugs for other autoimmune diseases. The FDA has reviewed 69 cases of new-onset psoriasis with anti-TNF therapy, including 17 pustular and 15 palmoplantar cases. The 12 most severe cases resulted in hospitalization, and symptoms resolved in most after treatment cessation.28
Fiorino et al29 counted 18 reported cases of psoriasis induced by anti-TNF therapy in patients with inflammatory bowel disease and concluded that it is rare. Harrison et al30 reported similar findings in patients with rheumatoid arthritis, with an increased incidence rate of 1.04 per 1,000 person-years. New-onset psoriasis was most common in patients treated with adalimumab. An example of the rash is seen in Figure 1.
CARDIOVASCULAR SIDE EFFECTS
Cardiovascular side effects of anti-TNF agents range from nonspecific and asymptomatic arrhythmias to worsening of heart failure.
Circulating levels of TNF are increased in patients with heart failure, and studies have evaluated the effects of TNF inhibition with infliximab on cardiac function and overall survival.31,32 The combined risk of death from any cause or hospitalization from heart failure was significantly higher in the infliximab groups, and the effects persisted for up to 5 months after stopping therapy.
Other studies22,33 have evaluated the effects of infliximab and etanercept on cardiac function and overall survival. Results showed possible exacerbation of heart failure with etanercept and increased risk of death with infliximab in patients with New York Heart Association (NYHA) class III or IV heart failure and left ventricular ejection fractions less than 35%.
Case reports have also described patients with worsening or new-onset heart failure on TNF inhibitors, including patients younger than 50 years and without identifiable cardiovascular risk factors.
Data analyses31,34,35 from large clinical registries have reported no significant increase in heart failure attributable to TNF inhibitors. However, we have concerns about the methodology of these analyses.
Currently, anti-TNF therapy is contraindicated in patients with NYHA class III or IV heart failure. Data are inconclusive for patients with class I or II heart failure. Baseline echocardiography and cardiology consultation can be considered, with close monitoring and avoidance of high doses of TNF inhibitors. If heart failure develops in a patient on anti-TNF therapy, the drug should be discontinued and the patient should be evaluated further.36
DEMYELINATING DISEASE, INCLUDING MULTIPLE SCLEROSIS
Anti-TNF agents have been associated with the onset or exacerbation of clinical symptoms and radiographic evidence of central nervous system demyelinating disorders, including multiple sclerosis.37–40 Mohan et al38 identified 19 cases of demyelinating events occurring after administration of anti-TNF agents in early 2001. In most cases, symptoms improved or resolved after therapy was stopped.
Optic neuritis,41,42 bilateral optic neuropathy,43 and aseptic meningitis44 have also been reported, but these have occurred only rarely.
How common are these effects? Postmarketing surveillance in patients with rheumatoid arthritis yields an estimated incidence of demyelinating disorders of 1 per 1,000 patient-years with adalimumab therapy.45 Complicating the assessment is an observed slight increase in risk of demyelinating conditions associated with inflammatory bowel disease.
Symptoms that should heighten the physician’s suspicion of this adverse effect include confusion, paresthesias, and ataxia. Patients on anti-TNF therapy who develop new visual symptoms should be checked for painless visual loss as a sign of early demyelinating disease.
Although data that conclusively link anti-TNF agents to multiple sclerosis are lacking, these drugs should not be initiated in patients who have a history of demyelinating disease, and treatment should be stopped promptly if the diagnosis is suspected.
MALIGNANCY
Whether anti-TNF therapy is directly linked to development of malignancies is difficult to determine. There are many confounding factors, including the risk of malignancy in underlying inflammatory disease and the concomitant use of other medications such as thiopurines, which have a known association with lymphoma.46
The incidence of lymphoma is twice as high in rheumatoid arthritis patients as in the general population.47 The risk is higher in those with more aggressive joint disease—the subset of rheumatoid arthritis patients who are more likely to be given anti-TNF agents.5
In patients with inflammatory bowel disease, the risk of cholangiocarcinoma is four times higher, and the risk of small-bowel adenocarcinoma is 16 to 18 times higher, but no increased risk of lymphoma has been identified in this population.48,49
Data from six clinical trials of infliximab, including a long-term study of its safety in Crohn disease, suggest it poses no increase in overall risk of malignancy.50–52 Similar results have been reported in patients with other rheumatic diseases.8 Information on this topic is constantly evolving, and studies range from case series to clinical trials to large patient registries.
The decision to use a TNF inhibitor should be based on the patient’s clinical picture and risk factors. Discussion of the risks and benefits of therapy with the patient should be clearly documented.
Non-Hodgkin lymphoma
Evidence about the risk of lymphoma with anti-TNF use is mixed, as up to two-thirds of patients on anti-TNF therapy have received concomitant nonbiologic immunosuppressive medications, making it difficult to determine the true risk from the biologic agents alone.53 Current evidence both supports9,53–56 and refutes7,55,57–60 the idea that anti-TNF agents increase lymphoma risk.
In patients with inflammatory bowel disease, several population-based studies have not shown a clear increase in lymphoma risk with anti-TNF use.56,59,60 Pedersen et al,61 in a meta-analysis of eight studies, confirmed these findings by showing no overall lymphoma risk in patients with inflammatory bowel disease.
However, a Canadian population-based study found a statistically significant increase in non-Hodgkin lymphoma in males with Crohn disease, with an incidence ratio of 3.63 (95% confidence interval [CI] 1.53–8.62).61 Additionally, Siegel et al62 found a significantly higher risk (6.1 cases per 10,000 patients) in patients treated with anti-TNF agents and thiopurines than in the general population (1.9 cases per 10,000 people). Although the difference was statistically significant, the overall risk is still very low.
Patients with rheumatoid arthritis seem to have a risk of lymphoma two to three times higher than in the general population. However, large population-based studies have not shown a statistically significant increase in the risk of lymphoma with anti-TNF therapy.63
Hepatosplenic T-cell lymphoma is a rare subtype of peripheral T-cell non-Hodgkin lymphoma; 25 cases have been reported in patients receiving anti-TNF therapy.64 Although the risk is extremely low (< 0.05%), physicians must carefully consider the risks and benefits of combination therapy, especially in young male patients with inflammatory bowel disease, since death is the usual outcome of this disease.65–67
Skin cancers
Wolfe and Michaud8 evaluated malignancy risk in rheumatoid arthritis patients being treated with biologic agents, including TNF inhibitors, using a large longitudinal database. These data were compared with those of the US Surveillance, Epidemiology, and End-Results (SEER) national database. No increase in the overall cancer rate was seen in rheumatoid arthritis patients (standardized incidence ratio [SIR] 1.0, 95% CI 1.0–1.1).
However, melanoma was more common in rheumatoid arthritis patients compared with SEER rates (SIR 1.7, 95% CI 1.3–2.3).8 In addition, biologic therapy was associated with a higher (but not statistically significant) risk of melanoma (odds ratio [OR] 2.3, 95% CI 0.9–5.4) and a higher risk of nonmelanoma skin cancer (OR 1.5, 95% CI 1.2–1.8), but not of other types of cancer.8
INFECTION
Patients on anti-TNF therapy are at a higher risk of infection, ranging from minor to life-threatening bacterial infections, and including the reactivation of granulomatous and fungal infections. More importantly, these agents are similar to steroids in blunting signs of infection, which may delay diagnosis and treatment.
The management of infection in patients on anti-TNF medications varies from case to case. In general, patients with a minor infection that does not require hospitalization or intravenous antibiotics can continue the biologic therapy while taking oral antibiotics. TNF inhibitors must be held in the event of a major infection.
Consultation with an infectious disease specialist is recommended, especially in complex cases.
Bacterial infections
An increased risk of minor bacterial infections such as urinary tract and respiratory infections has been well documented in several randomized control trials of anti-TNF agents, though other studies have shown no such increase in risk.33,51–59
The threshold for using antibiotics for a suspected bacterial infection is somewhat shifted in favor of treatment in patients on anti-TNF therapy. The reason is twofold: as previously noted, infections may be worse than they appear, because anti-TNF drugs can mask the signs and symptoms of a serious infection, and in patients on these drugs, an untreated bacterial infection may rapidly become life-threatening.
In general, broad-spectrum antibiotics are not warranted unless the source of infection is unclear or the patient is in danger of hemodynamic compromise.
Opportunistic infections
The association of anti-TNF agents with opportunistic infections could be viewed as an extension of their normal and intended therapeutic activity as potent immunosuppressive agents.68 Rheumatoid arthritis and inflammatory bowel disease are usually associated with conditions and situations that predispose patients to opportunistic infections, such as decreased immune response, malnutrition or malabsorption, surgeries, and concomitant immunosuppressive medications.7 Combination therapy with other immunosuppressive drugs and older age appear to markedly increase the risk of opportunistic infections, including mycobacterial and fungal infections, in patients with inflammatory bowel disease.7
Overall, opportunistic infections represent a measurable risk of anti-TNF therapy, and awareness and vigilance are important, especially in areas where opportunistic infections such as histoplasmosis and coccidiomycosis are endemic.50 Furthermore, physicians must be aware of the higher risk of opportunistic infections when multiple immunosuppressive drugs are used concurrently.
Granulomatous infections such as tuberculosis
Anti-TNF agents increase the risk of de novo granulomatous infections and of reactivating such infections. Granuloma formation and intracellular destruction of mycobacteria depend on TNF. TNF is important in maintaining the anatomic integrity of granulomas where these organisms have been sequestered, and blocking TNF leads to breakdown of granulomas and release of virulent organisms.69,70
TNF inhibitors increase the risk of reactivation of latent tuberculosis infection. The risk is greater with infliximab and adalimumab than with etanercept,71,72 and it has been described with certolizumab.74 Study results are varied thus far but show a risk of tuberculosis reactivation five to 30 times higher than in the general population, with tremendous variability in risk depending on background rates of previous exposure.
The absence of typical tuberculosis symptoms further complicates care in these cases. Fever, weight loss, and night sweats tend to be TNF-mediated and are therefore masked by anti-TNF agents, leading to atypical presentations. In addition, active tuberculosis infection associated with TNF inhibitors is more likely to involve extrapulmonary sites such as the skin and musculoskeletal system and to be disseminated at presentation.
A paradoxical worsening of tuberculosis symptoms may also be seen in patients with latent tuberculosis reactivation, especially after discontinuing anti-TNF therapy. This is thought to result from an immune reconstitution inflammatory syndrome.
The pretreatment evaluation should include a history of risk factors, a physical examination, and either a tuberculin skin test or an interferon-gamma-release assay. Interferon-gamma-release assays are particularly helpful in patients who have received bacille Calmette-Guérin vaccination. In patients who test positive or have been exposed, tuberculosis treatment should begin 4 weeks before starting anti-TNF therapy, though the optimal timing of antituberculosis agents is still controversial.74–77
If tuberculosis develops in a patient on anti-TNF therapy, he or she should receive antituberculosis drugs. Anti-TNF therapy should be stopped and should be resumed after 2 months only if no other treatment option is available.75
Invasive opportunistic fungal infections
Invasive opportunistic fungal infections have been reported with anti-TNF therapy, including histoplasmosis and coccidioidomycosis.78–80 Most patients who had histoplasmosis were treated with other immunosuppressive therapies and resided in or were raised near the Ohio or Mississippi River valleys, where this disease is endemic. Similarly, most cases of coccidioidomycosis were in endemic areas of Arizona, California, and Nevada, and patients on concomitant immunosuppressive therapy.78
Currently, there is no evidence to recommend obtaining Histoplasma capsulatum or Coccidioides immitis serologies before initiating anti-TNF therapy in patients in endemic areas.81 However, patients must be instructed to seek medical attention quickly for pulmonary or febrile illnesses.
Viral hepatitis infections
The data on hepatitis B and hepatitis C in patients on biologic therapies are mostly limited to case reports.
Hepatitis B. A small prospective study from Spain followed the liver biochemistry tests and hepatitis B status of 80 patients with Crohn disease treated with infliximab. Of three patients who were chronic hepatitis B carriers before starting infliximab, two experienced reactivation of hepatitis B after discontinuing infliximab, and one ultimately died. The third patient was treated with lamivudine concurrently with infliximab without clinical or biochemical changes during or after therapy.82
Similar findings were observed in two patients with rheumatoid arthritis on treatment with infliximab. One of the patients required liver transplantation, and both were treated with lamivudine, resulting in normalization of liver function test results.83,84
Recent reviews indicate that despite these findings, hepatitis B reactivation after anti-TNF withdrawal may not be common.85 There are limited data on hepatitis B reactivation and associated liver dysfunction in patients with inflammatory bowel disease treated with immunosuppressants. In a retrospective multicenter trial by Loras et al86 in patients with inflammatory bowel disease who had viral hepatitis, 36% of patients positive for hepatitis B surface antigen developed liver dysfunction, and six patients developed liver failure. In that study, treatment with more than two immunosuppressants was an independent predictor of hepatitis B reactivation.
Prolonged immunosuppression (longer than 3 months) has also been identified as an independent predictor of liver dysfunction (OR 3.06; 95% 95% CI 1.02–9.16).87
The European Association for the Study of the Liver and the American Association for the Study of Liver Diseases recommend starting lamivudine before chemotherapy or immunomodulator or immunosuppressive therapy in hepatitis B virus carriers and continuing preventive treatment for at least 6 months after stopping immunomodulating drugs. Lamivudine at a dose of 100 mg/day may reduce the risk of reactivation of hepatitis B.88–90 Tenofovir and entecavir may be useful alternatives in patients with hepatitis B who have never received nucleoside analogues. Hepatitis B reactivation did not occur in any of the 16 patients who received preventive entecavir treatment while receiving immunosuppressive treatments.89,91
In patients receiving immunosuppressive therapy, hepatitis B reactivation is associated with significant morbidity and mortality. Although risk factors for reactivation of hepatitis B virus infection have been identified, we recommend preventive treatment for all carriers positive for hepatitis B surface antigen. This should be done regardless of the number, type, and dosage of immunosuppressants and regardless of hepatitis B virus DNA levels.90
The frequency of hepatitis B and hepatitis C infection in patients with Crohn disease has been reported to be as high as 24%. The high incidence is thought to be secondary to multiple blood transfusions and surgeries.92 The use of biologic agents, including anti-TNF agents, in chronic hepatitis B virus- or hepatitis C virus-infected patients can lead to enhanced viral replication and hepatitis exacerbation.
Although active viral replication can occur during treatment with biologic agents, reactivation or exacerbation can also occur after the anti-TNF agent is stopped.82 This finding has prompted the recommendation that all candidates for biologic therapy be tested for hepatitis B immunization status, followed by immunization in nonimmune patients before starting anti-TNF therapy.93,94
Hepatitis C. There are no guidelines that adequately address the use of anti-TNF agents in patients with chronic hepatitis C infection.
Several small retrospective studies in rheumatoid arthritis patients with hepatitis C have shown that TNF inhibitors can be safely used without worsening liver function tests or changing the viral load.95–98 This is reassuring and provides the subspecialist with another treatment option, as other therapies such as disease-modifying antirheumatic drugs and steroids are known to aggravate viral hepatitis and increase the risk of viremia.96
Although small retrospective studies and one large randomized double-blind placebo-controlled trial have shown TNF inhibitors to be relatively safe in rheumatoid arthritis patients with hepatitis C, their use in these patients should be considered only with caution if they have evidence of hepatic synthetic dysfunction (eg, hypoalbuminemia, thrombocytopenia, increased international normalized ratio). The American College of Rheumatology recommends avoiding TNF inhibitors in Child-Pugh classes B and C.99
PREGNANCY
Physicians caring for patients with rheumatoid arthritis and inflammatory bowel disease must be aware of how these diseases affect fecundity and fertility and how the medications can affect conception and pregnancy. Many patients have difficulty conceiving while their autoimmune disease is active, and better disease control may improve fecundity and result in unanticipated pregnancy. Patients should be advised of the need for contraception if pregnancy is ill-advised or undesired.
Many patients seek advice about teratogenicity before conceiving, or seek guidance about rheumatoid arthritis and inflammatory bowel disease treatment while pregnant.
Several studies have reported a higher risk of adverse pregnancy outcomes in patients with rheumatoid arthritis and inflammatory bowel disease than in the general population.99–105 In inflammatory bowel disease, the odds of a premature delivery or having a low-birth-weight child are twice as high as in the normal population.100,103 Higher rates of cesarean delivery and stillbirth have also been reported. The main predisposing factor appears to be the disease activity at the time of conception, as active disease seems to be linked to adverse pregnancy outcomes.
Treatment with anti-TNF agents may rapidly achieve and maintain remission, raising the question of the safety of continued anti-TNF use during pregnancy. The FDA classifies anti-TNF agents as category B drugs, as animal studies have not demonstrated fetal risk, and no well-controlled prospective study has yet been conducted with pregnant women.
In an observational study, Schnitzler et al105 assessed the outcomes of 42 pregnancies in 35 patients with inflammatory bowel disease receiving either infliximab or adalimumab during pregnancy, compared with 56 pregnancies in 45 healthy patients without inflammatory bowel disease. There was no statistical difference in abortion rates between patients receiving anti-TNF agents and healthy women without inflammatory bowel disease (21% vs 14%, P = .4234). There was also no significant difference observed in birth weight, birth length, or cranial circumference of the children between the two groups. However, pregnancies with direct exposure to anti-TNF agents resulted in a higher frequency of premature delivery (25% vs 6%, P = .023).
Similar results were noted from the Crohn’s Therapy, Resource, Evaluation, and Assessment Tool, or TREAT, registry, as well as from a large systematic review by Vinet et al106 and case reports of rheumatoid arthritis and inflammatory bowel disease in women exposed to anti-TNF agents during pregnancy.
In addition, results from a recent systematic review of 38 studies of anti-TNF use and fetal risk, with a total of 437 women (189 on infliximab, 230 on adalimumab, 18 on certolizumab pegol), showed similar results.107 In pregnancies exposed to anti-TNF agents, the rates of congenital abnormalities (3.4%), fetal deaths (8.5%), and preterm births (2.7%) were similar to those in the general population.
For patients in disease remission on TNF inhibitors, it is reasonable to continue these agents during pregnancy after careful discussion with the patient. Fetal safety and infant immunization response after delivery are the primary concerns in these cases.
Both infliximab and adalimumab cross the placenta and remain detectable in the baby’s circulation 4 months (for adalimumab) to 6 months (for infliximab) after delivery. It is currently recommended that infliximab be stopped at 32 weeks of gestation for the remainder of the pregnancy and that adalimumab be stopped at 34 to 36 weeks, given a planned 40-week gestation.
Certolizumab does not cross the placenta in significant amounts and should be continued throughout pregnancy; drug levels in infants were shown to be less than 2 μg/mL, even when dosed the week of delivery.108–112
TAKE-HOME POINTS
- All health care providers of patients with rheumatoid arthritis and inflammatory bowel disease should be familiar with anti-TNF agents used in treating these diseases.
- The benefits of controlling the disease far outweigh the risks of therapy when used appropriately.
- Care of these patients should be multidisciplinary, with clear communication between primary care physician and specialist.
- Patient education and monitoring combined with prompt communication between primary care physician and specialist are key.
- Wijbrandts CA, Dijkgraaf MG, Kraan MC, et al. The clinical response to infliximab in rheumatoid arthritis is in part dependent on pretreatment tumour necrosis factor alpha expression in the synovium. Ann Rheum Dis 2008; 67:1139–1144.
- Sfikakis PP. The first decade of biologic TNF antagonists in clinical practice: lessons learned, unresolved issues and future directions. Curr Dir Autoimmun 2010; 11:180–210.
- Casellas F, Alcalá MJ, Prieto L, Miró JR, Malagelada JR. Assessment of the influence of disease activity on the quality of life of patients with inflammatory bowel disease using a short questionnaire. Am J Gastroenterol 2004; 99:457–461.
- Casellas F, López-Vivancos J, Badia X, Vilaseca J, Malagelada JR. Influence of inflammatory bowel disease on different dimensions of quality of life. Eur J Gastroenterol Hepatol 2001; 13:567–572.
- Baecklund E, Iliadou A, Askling J, et al. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum 2006; 54:692–701.
- Franklin J, Lunt M, Bunn D, Symmons D, Silman A. Incidence of lymphoma in a large primary care derived cohort of cases of inflammatory polyarthritis. Ann Rheum Dis 2006; 65:617–622.
- Toruner M, Loftus EV, Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology 2008; 134:929–936.
- Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum 2007; 56:2886–2895.
- Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1997; 46:1–24.
- Zhang J, Xie F, Delzell E, et al. Association between vaccination for herpes zoster and risk of herpes zoster infection among older patients with selected immune-mediated diseases. JAMA 2012; 308:43–49.
- Moreland LW, Baumgartner SW, Schiff MH, et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med 1997; 337:141–147.
- Altunöz ME, Senates E, Yesil A, Calhan T, Ovünç AO. Patients with inflammatory bowel disease have a lower response rate to HBV vaccination compared to controls. Dig Dis Sci 2012; 57:1039–1044.
- Gisbert JP, Villagrasa JR, Rodríguez-Nogueiras A, Chaparro M. Efficacy of hepatitis B vaccination and revaccination and factors impacting on response in patients with inflammatory bowel disease. Am J Gastroenterol 2012; 107:1460–1466.
- Carrera E, Manzano R, Garrido E. Efficacy of the vaccination in inflammatory bowel disease. World J Gastroenterol 2013; 19:1349–1353.
- Gisbert JP, Menchén L, García-Sánchez V, Marín I, Villagrasa JR, Chaparro M. Comparison of the effectiveness of two protocols for vaccination (standard and double dosage) against hepatitis B virus in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2012; 35:1379–1385.
- Ferkolj I. How to improve the safety of biologic therapy in Crohn’s disease. J Physiol Pharmacol 2009; 60(suppl 7):67–70.
- Siegel CA. The risks of biologic therapy for inflammatory bowel disease. In:Bernstein ED, editor. The Inflammatory Bowel Disease Yearbook, volume 6. London, UK: Remedica, 2010:89–108.
- Remicade (infliximab) Package Insert. Horsham, PA: Janssen Biotech, Inc; 2013. http://www.remicade.com/shared/product/remicade/prescribing-information.pdf. Accessed January 2, 2014.
- Vermeire S, Noman M, Van Assche G, et al. Autoimmunity associated with anti-tumor necrosis factor alpha treatment in Crohn’s disease: a prospective cohort study. Gastroenterology 2003; 125:32–39.
- Cush JJ. Biological drug use: US perspectives on indications and monitoring. Ann Rheum Dis 2005; 64(suppl 4):iv18–iv23.
- TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. The Lenercept Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group. Neurology 1999; 53:457–465.
- Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT; Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation 2003; 107:3133–3140.
- Cheifetz A, Mayer L. Monoclonal antibodies, immunogenicity, and associated infusion reactions. Mt Sinai J Med 2005; 72:250–256.
- Cheifetz A, Smedley M, Martin S, et al. The incidence and management of infusion reactions to infliximab: a large center experience. Am J Gastroenterol 2003; 98:1315–1324.
- Vultaggio A, Matucci A, Nencini F, et al. Anti-infliximab IgE and non-IgE antibodies and induction of infusion-related severe anaphylactic reactions. Allergy 2010; 65:657–661.
- Ramos-Casals M, Brito-Zerón P, Muñoz S, et al. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine (Baltimore) 2007; 86:242–251.
- Stallmach A, Hagel S, Bruns T. Adverse effects of biologics used for treating IBD. Best Pract Res Clin Gastroenterol 2010; 24:167–182.
- Haagsma CJ, Blom HJ, van Riel PL, et al. Influence of sulphasalazine, methotrexate, and the combination of both on plasma homocysteine concentrations in patients with rheumatoid arthritis. Ann Rheum Dis 1999; 58:79–84.
- Fiorino G, Allez M, Malesci A, Danese S. Review article: anti TNF-alpha induced psoriasis in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2009; 29:921–927.
- Harrison MJ, Dixon WG, Watson KD, et al; British Society for Rheumatology Biologics Register Control Centre Consortium; BSRBR. Rates of new-onset psoriasis in patients with rheumatoid arthritis receiving anti-tumour necrosis factor alpha therapy: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis 2009; 68:209–215.
- Marchesoni A, Zaccara E, Gorla R, et al. TNF-alpha antagonist survival rate in a cohort of rheumatoid arthritis patients observed under conditions of standard clinical practice. Ann N Y Acad Sci 2009; 1173:837–846.
- Tomas L, Lazurova I, Pundova L, et al. Acute and long-term effect of infliximab on humoral and echocardiographic parameters in patients with chronic inflammatory diseases. Clin Rheumatol 2013 32:61–66.
- Senel S, Cobankara V, Taskoylu O, et al. The safety and efficacy of etanercept on cardiac functions and lipid profile in patients with active rheumatoid arthritis. J Investig Med 2012; 60:62–65.
- Al-Aly Z, Pan H, Zeringue A, et al. Tumor necrosis factor-a blockade, cardiovascular outcomes, and survival in rheumatoid arthritis. Transl Res 2011; 157:10–18.
- Listing J, Strangfeld A, Kekow J, et al. Does tumor necrosis factor alpha inhibition promote or prevent heart failure in patients with rheumatoid arthritis? Arthritis Rheum 2008; 58:667–677.
- Wolfe F, Michaud K. Heart failure in rheumatoid arthritis: rates, predictors, and the effect of anti-tumor necrosis factor therapy. Am J Med 2004; 116:305–311.
- Enayati PJ, Papadakis KA. Association of anti-tumor necrosis factor therapy with the development of multiple sclerosis. J Clin Gastroenterol 2005; 39:303–306.
- Mohan N, Edwards ET, Cupps TR, et al. Demyelination occurring during anti-tumor necrosis factor alpha therapy for inflammatory arthritides. Arthritis Rheum 2001; 44:2862–2869.
- Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med 2004; 350:876–885.
- Thomas CW, Weinshenker BG, Sandborn WJ. Demyelination during anti-tumor necrosis factor alpha therapy with infliximab for Crohn’s disease. Inflamm Bowel Dis 2004; 10:28–31.
- Foroozan R, Buono LM, Sergott RC, Savino PJ. Retrobulbar optic neuritis associated with infliximab. Arch Ophthalmol 2002; 120:985–987.
- Strong BY, Erny BC, Herzenberg H, Razzeca KJ. Retrobulbar optic neuritis associated with infliximab in a patient with Crohn disease. Ann Intern Med 2004; 140:W34.
- ten Tusscher MP, Jacobs PJ, Busch MJ, de Graaf L, Diemont WL. Bilateral anterior toxic optic neuropathy and the use of infliximab. BMJ 2003; 326:579.
- Hegde N, Gayomali C, Rich MW. Infliximab-induced headache and infliximab-induced meningitis: two ends of the same spectrum? South Med J 2005; 98:564–566.
- Schiff MH, Burmester GR, Kent JD, et al. Safety analyses of adalimumab (HUMIRA) in global clinical trials and US postmarketing surveillance of patients with rheumatoid arthritis. Ann Rheum Dis 2006; 65:889–894.
- Beaugerie L, Brousse N, Bouvier AM, et al; CESAME Study Group. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet 2009; 374:1617–1625.
- Ekström K, Hjalgrim H, Brandt L, et al. Risk of malignant lymphomas in patients with rheumatoid arthritis and in their first-degree relatives. Arthritis Rheum 2003; 48:963–970.
- Lakatos PL, Lakatos L. Risk for colorectal cancer in ulcerative colitis: changes, causes and management strategies. World J Gastroenterol 2008; 14:3937–3947.
- Persson PG, Karlén P, Bernell O, et al. Crohn’s disease and cancer: a population-based cohort study. Gastroenterology 1994; 107:1675–1679.
- de Silva S, Devlin S, Panaccione R. Optimizing the safety of biologic therapy for IBD. Nat Rev Gastroenterol Hepatol 2010; 7:93–101.
- Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infections and mortality in association with therapies for Crohn’s disease: TREAT registry. Clin Gastroenterol Hepatol 2006; 4:621–630.
- Peyrin-Biroulet L, Deltenre P, de Suray N, Branche J, Sandborn WJ, Colombel JF. Efficacy and safety of tumor necrosis factor antagonists in Crohn’s disease: meta-analysis of placebo-controlled trials. Clin Gastroenterol Hepatol 2008; 6:644–653.
- Hanauer SB, Feagan BG, Lichtenstein GR, et al; ACCENT I Study Group. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 2002; 359:1541–1549.
- Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology 2007; 132:52–65.
- Sandborn WJ, Feagan BG, Stoinov S, et al; PRECISE 1 Study Investigators. Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med 2007; 357:228–238.
- Sandborn WJ, Hanauer SB, Rutgeerts P, et al. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut 2007; 56:1232–1239.
- Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology 2006; 130:323–333.
- Rutgeerts P, D’Haens G, Targan S, et al. Efficacy and safety of retreatment with anti-tumor necrosis factor antibody (infliximab) to maintain remission in Crohn’s disease. Gastroenterology 1999; 117:761–769.
- Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005; 353:2462–2476.
- Pedersen N, Duricova D, Elkjaer M, Gamborg M, Munkholm P, Jess T. Risk of extra-intestinal cancer in inflammatory bowel disease: meta-analysis of population-based cohort studies. Am J Gastroenterol 2010; 105:1480–1487.
- Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer 2001; 91:854–862.
- Siegel CA, Marden SM, Persing SN, Larson RJ, Sands BE. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn’s disease: a meta-analysis. Clin Gastroenterol Hepatol 2009; 7:874–881.
- Wolfe F, Michaud K. Lympyhoma in rheumatoid arthritis. The effect of methotrexate and anti-tumor necrosis factor therapy in 18,572 patients. Arthritis Rheum 2004; 50:1740–1751.
- Parakkal D, Sifuentes H, Semer R, Ehrenpreis ED. Hepatosplenic T-cell lymphoma in patients receiving TNF-a inhibitor therapy: expanding the groups at risk. Eur J Gastroenterol Hepatol 2011; 23:1150–1156.
- Rosh JR, Gross T, Mamula P, Griffiths A, Hyams J. Hepatosplenic T-cell lymphoma in adolescents and young adults with Crohn’s disease: a cautionary tale? Inflamm Bowel Dis 2007; 13:1024–1030.
- Shale M, Kanfer E, Panaccione R, Ghosh S. Hepatosplenic T cell lymphoma in inflammatory bowel disease. Gut 2008; 57:1639–1641.
- Thai A, Prindiville T. Hepatosplenic T-cell lymphoma and inflammatory bowel disease. J Crohns Colitis 2010; 4:511–522.
- Viget N, Vernier-Massouille G, Salmon-Ceron D, Yazdanpanah Y, Colombel JF. Opportunistic infections in patients with inflammatory bowel disease: prevention and diagnosis. Gut 2008; 57:549–558.
- Bekker LG, Freeman S, Murray PJ, Ryffel B, Kaplan G. TNF-alpha controls intracellular mycobacterial growth by both inducible nitric oxide synthase-dependent and inducible nitric oxide synthase-independent pathways. J Immunol 2001; 166:6728–6734.
- Roach DR, Bean AG, Demangel C, France MP, Briscoe H, Britton WJ. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J Immunol 2002; 168:4620–4627.
- Gómez-Reino JJ, Carmona L, Valverde VR, Mola EM, Montero MDBIOBADASER Group. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum 2003; 48:2122–2127.
- Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med 2001; 345:1098–1104.
- Smolen J, Landewé RB, Mease P, et al. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomised controlled trial. Ann Rheum Dis 2009; 68:797–804.
- Demkow U, Broniarek-Samson B, Filewska M, et al. Prevalence of latent tuberculosis infection in health care workers in Poland assessed by interferon-gamma whole blood and tuberculin skin tests. J Physiol Pharmacol 2008; 59(suppl 6):209–217.
- Pache I, Rogler G, Felley C. TNF-alpha blockers in inflammatory bowel diseases: practical consensus recommendations and a user’s guide. Swiss Med Wkly 2009; 139:278–287.
- Rahier JF, Ben-Horin S, Chowers Y, et al; European Crohn’s and Colitis Organisation (ECCO). European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis 2009; 3:47–91.
- Rahier JF, Yazdanpanah Y, Colombel JF, Travis S. The European (ECCO) consensus on infection in IBD: what does it change for the clinician? Gut 2009; 58:1313–1315.
- Bergstrom L, Yocum DE, Ampel NM, et al. Increased risk of coccidioidomycosis in patients treated with tumor necrosis factor alpha antagonists. Arthritis Rheum 2004; 50:1959–1966.
- Lee JH, Slifman NR, Gershon SK, et al. Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor alpha antagonists infliximab and etanercept. Arthritis Rheum 2002; 46:2565–2570.
- Wood KL, Hage CA, Knox KS, et al. Histoplasmosis after treatment with anti-tumor necrosis factor-alpha therapy. Am J Respir Crit Care Med 2003; 167:1279–1282.
- Reddy JG, Loftus EV. Safety of infliximab and other biologic agents in the inflammatory bowel diseases. Gastroenterol Clin North Am 2006; 35:837–855.
- Esteve M, Saro C, González-Huix F, Suarez F, Forné M, Viver JM. Chronic hepatitis B reactivation following infliximab therapy in Crohn’s disease patients: need for primary prophylaxis. Gut 2004; 53:1363–1365.
- Michel M, Duvoux C, Hezode C, Cherqui D. Fulminant hepatitis after infliximab in a patient with hepatitis B virus treated for an adult onset Still’s disease. J Rheumatol 2003; 30:1624–1625.
- Ostuni P, Botsios C, Punzi L, Sfriso P, Todesco S. Hepatitis B reactivation in a chronic hepatitis B surface antigen carrier with rheumatoid arthritis treated with infliximab and low dose methotrexate. Ann Rheum Dis 2003; 62:686–687.
- Pérez-Alvarez R, Díaz-Lagares C, García-Hernández F, et al; BIOGEAS Study Group. Hepatitis B virus (HBV) reactivation in patients receiving tumor necrosis factor (TNF)-targeted therapy: analysis of 257 cases. Medicine (Baltimore) 2011; 90:359–371.
- Loras C, Gisbert JP, Mínguez M, et al; REPENTINA study; GETECCU (Grupo Español de Enfermedades de Crohn y Colitis Ulcerosa) Group. Liver dysfunction related to hepatitis B and C in patients with inflammatory bowel disease treated with immunosuppressive therapy. Gut 2010; 59:1340–1346.
- Park SH, Yang SK, Lim YS, et al. Clinical courses of chronic hepatitis B virus infection and inflammatory bowel disease in patients with both diseases. Inflamm Bowel Dis 2012; 18:2004–2010.
- Loomba R, Rowley A, Wesley R, et al. Systematic review: the effect of preventive lamivudine on hepatitis B reactivation during chemotherapy. Ann Intern Med 2008; 148:519–528.
- Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology 2009; 50:661–662.
- European Association For The Study Of The Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol 2009; 50:227–242.
- Watanabe M, Shibuya A, Takada J, et al. Entecavir is an optional agent to prevent hepatitis B virus (HBV) reactivation: a review of 16 patients. Eur J Intern Med 2010; 21:333–337.
- Biancone L, Pavia M, Del Vecchio Blanco G, et al; Italian Group for the Study of the Colon and Rectum (GISC). Hepatitis B and C virus infection in Crohn’s disease. Inflamm Bowel Dis 2001; 7:287–294.
- Melmed GY. Vaccination strategies for patients with inflammatory bowel disease on immunomodulators and biologics. Inflamm Bowel Dis 2009; 15:1410–1416.
- Melmed GY, Ippoliti AF, Papadakis KA, et al. Patients with inflammatory bowel disease are at risk for vaccine-preventable illnesses. Am J Gastroenterol 2006; 101:1834–1840.
- Ferri C, Ferraccioli G, Ferrari D, et al. Safety of anti-tumor necrosis factor-alpha therapy in patients with rheumatoid arthritis and chronic hepatitis C virus infection. J Rheumatol 2008; 35:1944–1949.
- Mok MY, Ng WL, Yuen MF, Wong RW, Lau CS. Safety of disease modifying anti-rheumatic agents in rheumatoid arthritis patients with chronic viral hepatitis. Clin Exp Rheumatol 2000; 18:363–368.
- Vassilopoulos D, Calabrese LH. Risks of immunosuppressive therapies including biologic agents in patients with rheumatic diseases and coexisting chronic viral infections. Curr Opin Rheumatol 2007; 19:619–625.
- Vassilopoulos D, Apostolopoulou A, Hadziyannis E, et al. Long-term safety of anti-TNF treatment in patients with rheumatic diseases and chronic or resolved hepatitis B virus infection. Ann Rheum Dis 2010; 69:1352–1355.
- Saag KG, Teng GG, Patkar NM, et al; American College of Rheumatology. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum 2008; 59:762–784.
- Cornish J, Tan E, Teare J, et al. A meta-analysis on the influence of inflammatory bowel disease on pregnancy. Gut 2007; 56:830–837.
- Dominitz JA, Young JC, Boyko EJ. Outcomes of infants born to mothers with inflammatory bowel disease: a population-based cohort study. Am J Gastroenterol 2002; 97:641–648.
- Kornfeld D, Cnattingius S, Ekbom A. Pregnancy outcomes in women with inflammatory bowel disease—a population-based cohort study. Am J Obstet Gynecol 1997; 177:942–946.
- Mahadevan U, Sandborn WJ, Li DK, Hakimian S, Kane S, Corley DA. Pregnancy outcomes in women with inflammatory bowel disease: a large community-based study from Northern California. Gastroenterology 2007; 133:1106–1112.
- Nguyen GC, Boudreau H, Harris ML, Maxwell CV. Outcomes of obstetric hospitalizations among women with inflammatory bowel disease in the United States. Clin Gastroenterol Hepatol 2009; 7:329–334.
- Schnitzler F, Fidder H, Ferrante M, et al. Outcome of pregnancy in women with inflammatory bowel disease treated with antitumor necrosis factor therapy. Inflamm Bowel Dis 2011; 17:1846–1854.
- Vinet E, Pineau C, Gordon C, Clarke AE, Bernatsky S. Biologic therapy and pregnancy outcomes in women with rheumatic diseases. Arthritis Rheum 2009; 61:587–592.
- Guidi L, Pugliese D, Armuzzi A. Update on the management of inflammatory bowel disease: specific role of adalimumab. Clin Exp Gastroenterol 2011; 4:163–172.
- Mahadevan U. Continuing immunomodulators and biologic medications in pregnant IBD patients – pro. IInflamm Bowel Dis 2007; 13:1439–1440.
- Mahadevan U. Gastrointestinal medications in pregnancy. Best Pract Res Clin Gastroenterol 2007; 21:849–877.
- Mahadevan U, Cucchiara S, Hyams JS, et al. The London Position Statement of the World Congress of Gastroenterology on Biological Therapy for IBD with the European Crohn’s and Colitis Organisation: pregnancy and pediatrics. Am J Gastroenterol 2011; 106:214–223.
- Mahadevan U, Kane S. Use of infliximab in pregnancy. Am J Gastroenterol 2010; 105:219–220.
- Vasiliauskas EA, Church JA, Silverman N, Barry M, Targan SR, Dubinsky MC. Case report: evidence for transplacental transfer of maternally administered infliximab to the newborn. Clin Gastroenterol Hepatol 2006; 4:1255–1258.
Biologic agents such as those that block tumor necrosis factor (TNF) alpha have revolutionized the treatment of autoimmune diseases like rheumatoid arthritis and inflammatory bowel disease, dramatically improving disease control and quality of life. In addition, they have made true disease remission possible in some cases.
However, as with any new therapy, a variety of side effects must be considered.
WHAT ARE BIOLOGIC AGENTS?
Biologic agents are genetically engineered drugs manufactured or synthesized in vitro from molecules such as proteins, genes, and antibodies present in living organisms. By targeting specific molecular components of the inflammatory cascade, including cytokines, these drugs alter certain aspects of the body’s inflammatory response in autoimmune diseases. Because TNF inhibitors are the most widely used biologic agents in the United States, this review will focus on them.
TNF INHIBITORS
TNF inhibitors suppress the inflammatory cascade by inactivating TNF alpha, a cytokine that promotes inflammation in the intestine, synovial tissue, and other sites.1,2
Several TNF inhibitors are available. Etanercept and infliximab were the first two to receive US Food and Drug Administration (FDA) approval, and they are extensively used. Etanercept, a soluble TNF receptor given subcutaneously, was approved for treating rheumatoid arthritis in 1998. Infliximab, a chimeric monoclonal antibody (75% human and 25% mouse protein sequence) given intravenously, received FDA approval for treating Crohn disease in 1998 and for rheumatoid arthritis in 1999. Other anti-TNF agents with varying properties are also available.
Table 1 lists anti-TNF agents approved for treating rheumatoid arthritis and inflammatory bowel disease. These are chronic, relapsing diseases that significantly and dramatically reduce quality of life, especially when they are poorly controlled.3,4 Untreated disease has been associated with malignancy, infection, pregnancy loss, and malnutrition.5–8 TNF inhibitors are aimed at patients who have moderate to severe disease or for whom previous treatments have failed, to help them achieve and maintain steroid-free remission. However, use of these agents is tempered by the risk of potentially serious side effects.
Special thought should also be given to the direct costs of the drugs (up to $30,000 per year, not counting the cost of their administration) and to the indirect costs such as time away from work to receive treatment. These are major considerations in some cases, and patients should be selected carefully for treatment with these drugs.
BEFORE STARTING THERAPY
Before starting anti-TNF therapy, several steps can reduce the risk of serious adverse events.
Take a focused history
The clinical history should include inquiries about previous bacterial, fungal, and tuberculosis infections or exposure; diabetes; and other immunocompromised states that increase the risk of acquiring potentially life-threatening infections.
Details of particular geographic areas of residence, occupational exposures, and social history should be sought. These include history of incarceration (which may put patients at risk of tuberculosis) and residence in the Ohio River valley or in the southwestern or midwestern United States (which may increase the risk of histoplasmosis, coccidioidomycosis, and other fungal infections).
Bring vaccinations up to date
Age-appropriate vaccinations should be discussed and given, ideally before starting therapy. These include influenza vaccine every year and tetanus boosters every 10 years for all and, as appropriate, varicella, human papillomavirus, and pneumococcal vaccinations. The US Centers for Disease Control and Prevention recommend an additional dose of the pneumococcal vaccine if more than 5 years have elapsed since the first one, and many clinicians opt to give it every 5 years.
In general, live-attenuated vaccines, including the intranasal influenza vaccine, are contraindicated in patients taking biologic agents.9 For patients at high risk of exposure or infection, it may be reasonable to hold the biologic agent for a period of time, vaccinate, and resume the biologic agent a month later.
Recent data suggest that the varicella zoster vaccine may be safely given to older patients with immune-mediated diseases such as rheumatoid arthritis and inflammatory bowel disease taking biologic agents.10 New guidelines from the American College of Rheumatology recommend age-appropriate vaccines for rheumatoid arthritis patients age 60 and older before biologic treatments are started. Case-by-case discussion with the subspecialist and the patient is recommended.
Screen for chronic infections
Tuberculosis screening with a purified protein derivative test or an interferon-gamma-release (Quantiferon) assay followed by chest radiography in patients with a positive test is mandatory before giving a TNF inhibitor.
Hepatitis B virus status should be determined before starting anti-TNF therapy.11
Hepatitis B vaccination has been recommended for patients with inflammatory bowel disease, but no clear recommendation exists for patients with rheumatic disease. Patients with inflammatory bowel disease tend to have low rates of response to hepatitis B vaccination12,13; possible reasons include their lack of an appropriate innate immune response to infectious agents, malnutrition, surgery, older age, and immunosuppressive drugs.14 An accelerated vaccination protocol with recombinant hepatitis B vaccine (Energix-B) in a double dose at 0, 1, and 2 months has been shown to improve response rates.15
Whenever possible, it may be better to vaccinate patients before starting immunosuppressive therapy, and to check postvaccination titers to ensure adequate response.
Perform an examination
A full physical examination with special attention to skin rashes should be performed. This may serve as a baseline to assist early detection of new rashes associated with anti-TNF therapy.
A baseline complete blood cell count and complete metabolic panel should be routinely obtained before starting therapy (and thereafter at the discretion of the physician). In conjunction with follow-up tests, they can help detect an unexpected decrease in white blood cell count or abnormal results on the liver panel.16 These baseline and follow-up tests are generally performed by the subspecialist, and the results are shared with the primary care physician.
Table 2 summarizes key information to be sought before starting a patient on a TNF inhibitor.
ADVERSE EFFECTS OF ANTI-TNF DRUGS
Infusion reactions, infections, cardiac arrhythmias, demyelinating disorders, skin infections, and malignancies have been reported with anti-TNF therapy. The relative frequencies of these adverse events are summarized in Table 3.17–22
NONINFECTIOUS COMPLICATIONS OF TNF INHIBITORS
Injection site reactions
When anti-TNF agents are given subcutaneously, injection site reactions are common (occurring in up to 40% of patients) and are considered minor.11 Reactions, including significant pain, typically occur within the first few months of therapy. They can last 2 to 5 days but rarely warrant stopping therapy. Treatment with ice and an antihistamine is almost always sufficient to control symptoms.
Infusion reactions with infliximab
Infliximab can cause both acute and delayed infusion reactions. Acute reactions can occur up to 24 hours after infusion but usually appear within 10 minutes of administration and are handled by the infusion suite staff. They range from the severe immunoglobulin E-mediated type I reaction, manifesting with hypotension, bronchospasm, and urticaria, to the milder anaphylactoid-type reaction, which constitutes the majority.23–25
While most primary care physicians will not encounter an acute reaction, family doctors and emergency room physicians may encounter delayed reactions, which can develop 1 to 14 days after infusion. These reactions usually resemble serum sickness and present with joint pain, fatigue, myalgia, and fever. But, unlike classic serum sickness, these reactions are generally not associated with a rash.
With nonspecific symptoms, the diagnosis may be easy to overlook. However, establishing this diagnosis is important because repeat therapy may result in a more severe reaction upon reexposure to the drug.
Once diagnosed, these reactions can be treated symptomatically with a combination of acetaminophen and diphenhydramine after discussion between the primary care physician and subspecialist.23,24
Autoimmune syndromes
Several studies have reported a small percentage of patients treated with anti-TNF agents who develop paradoxical autoimmune conditions. These range from asymptomatic immunologic alterations, including the formation of antinuclear antibodies and antibodies to double-stranded DNA, to life-threatening systemic autoimmune diseases.26,27
Autoimmune diseases associated with anti-TNF treatment include a lupus-like syndrome, vasculitides, and psoriatic skin lesions. These syndromes warrant stopping the inciting drug and, on occasion, giving corticosteroids. Most cases arise between 1 month and 1 year of starting treatment, and almost 75% resolve completely after the anti-TNF therapy is stopped.26
Interestingly, anti-TNF agents are approved for treating psoriasis and psoriatic arthritis, but psoriasis has paradoxically developed in patients being treated with these drugs for other autoimmune diseases. The FDA has reviewed 69 cases of new-onset psoriasis with anti-TNF therapy, including 17 pustular and 15 palmoplantar cases. The 12 most severe cases resulted in hospitalization, and symptoms resolved in most after treatment cessation.28
Fiorino et al29 counted 18 reported cases of psoriasis induced by anti-TNF therapy in patients with inflammatory bowel disease and concluded that it is rare. Harrison et al30 reported similar findings in patients with rheumatoid arthritis, with an increased incidence rate of 1.04 per 1,000 person-years. New-onset psoriasis was most common in patients treated with adalimumab. An example of the rash is seen in Figure 1.
CARDIOVASCULAR SIDE EFFECTS
Cardiovascular side effects of anti-TNF agents range from nonspecific and asymptomatic arrhythmias to worsening of heart failure.
Circulating levels of TNF are increased in patients with heart failure, and studies have evaluated the effects of TNF inhibition with infliximab on cardiac function and overall survival.31,32 The combined risk of death from any cause or hospitalization from heart failure was significantly higher in the infliximab groups, and the effects persisted for up to 5 months after stopping therapy.
Other studies22,33 have evaluated the effects of infliximab and etanercept on cardiac function and overall survival. Results showed possible exacerbation of heart failure with etanercept and increased risk of death with infliximab in patients with New York Heart Association (NYHA) class III or IV heart failure and left ventricular ejection fractions less than 35%.
Case reports have also described patients with worsening or new-onset heart failure on TNF inhibitors, including patients younger than 50 years and without identifiable cardiovascular risk factors.
Data analyses31,34,35 from large clinical registries have reported no significant increase in heart failure attributable to TNF inhibitors. However, we have concerns about the methodology of these analyses.
Currently, anti-TNF therapy is contraindicated in patients with NYHA class III or IV heart failure. Data are inconclusive for patients with class I or II heart failure. Baseline echocardiography and cardiology consultation can be considered, with close monitoring and avoidance of high doses of TNF inhibitors. If heart failure develops in a patient on anti-TNF therapy, the drug should be discontinued and the patient should be evaluated further.36
DEMYELINATING DISEASE, INCLUDING MULTIPLE SCLEROSIS
Anti-TNF agents have been associated with the onset or exacerbation of clinical symptoms and radiographic evidence of central nervous system demyelinating disorders, including multiple sclerosis.37–40 Mohan et al38 identified 19 cases of demyelinating events occurring after administration of anti-TNF agents in early 2001. In most cases, symptoms improved or resolved after therapy was stopped.
Optic neuritis,41,42 bilateral optic neuropathy,43 and aseptic meningitis44 have also been reported, but these have occurred only rarely.
How common are these effects? Postmarketing surveillance in patients with rheumatoid arthritis yields an estimated incidence of demyelinating disorders of 1 per 1,000 patient-years with adalimumab therapy.45 Complicating the assessment is an observed slight increase in risk of demyelinating conditions associated with inflammatory bowel disease.
Symptoms that should heighten the physician’s suspicion of this adverse effect include confusion, paresthesias, and ataxia. Patients on anti-TNF therapy who develop new visual symptoms should be checked for painless visual loss as a sign of early demyelinating disease.
Although data that conclusively link anti-TNF agents to multiple sclerosis are lacking, these drugs should not be initiated in patients who have a history of demyelinating disease, and treatment should be stopped promptly if the diagnosis is suspected.
MALIGNANCY
Whether anti-TNF therapy is directly linked to development of malignancies is difficult to determine. There are many confounding factors, including the risk of malignancy in underlying inflammatory disease and the concomitant use of other medications such as thiopurines, which have a known association with lymphoma.46
The incidence of lymphoma is twice as high in rheumatoid arthritis patients as in the general population.47 The risk is higher in those with more aggressive joint disease—the subset of rheumatoid arthritis patients who are more likely to be given anti-TNF agents.5
In patients with inflammatory bowel disease, the risk of cholangiocarcinoma is four times higher, and the risk of small-bowel adenocarcinoma is 16 to 18 times higher, but no increased risk of lymphoma has been identified in this population.48,49
Data from six clinical trials of infliximab, including a long-term study of its safety in Crohn disease, suggest it poses no increase in overall risk of malignancy.50–52 Similar results have been reported in patients with other rheumatic diseases.8 Information on this topic is constantly evolving, and studies range from case series to clinical trials to large patient registries.
The decision to use a TNF inhibitor should be based on the patient’s clinical picture and risk factors. Discussion of the risks and benefits of therapy with the patient should be clearly documented.
Non-Hodgkin lymphoma
Evidence about the risk of lymphoma with anti-TNF use is mixed, as up to two-thirds of patients on anti-TNF therapy have received concomitant nonbiologic immunosuppressive medications, making it difficult to determine the true risk from the biologic agents alone.53 Current evidence both supports9,53–56 and refutes7,55,57–60 the idea that anti-TNF agents increase lymphoma risk.
In patients with inflammatory bowel disease, several population-based studies have not shown a clear increase in lymphoma risk with anti-TNF use.56,59,60 Pedersen et al,61 in a meta-analysis of eight studies, confirmed these findings by showing no overall lymphoma risk in patients with inflammatory bowel disease.
However, a Canadian population-based study found a statistically significant increase in non-Hodgkin lymphoma in males with Crohn disease, with an incidence ratio of 3.63 (95% confidence interval [CI] 1.53–8.62).61 Additionally, Siegel et al62 found a significantly higher risk (6.1 cases per 10,000 patients) in patients treated with anti-TNF agents and thiopurines than in the general population (1.9 cases per 10,000 people). Although the difference was statistically significant, the overall risk is still very low.
Patients with rheumatoid arthritis seem to have a risk of lymphoma two to three times higher than in the general population. However, large population-based studies have not shown a statistically significant increase in the risk of lymphoma with anti-TNF therapy.63
Hepatosplenic T-cell lymphoma is a rare subtype of peripheral T-cell non-Hodgkin lymphoma; 25 cases have been reported in patients receiving anti-TNF therapy.64 Although the risk is extremely low (< 0.05%), physicians must carefully consider the risks and benefits of combination therapy, especially in young male patients with inflammatory bowel disease, since death is the usual outcome of this disease.65–67
Skin cancers
Wolfe and Michaud8 evaluated malignancy risk in rheumatoid arthritis patients being treated with biologic agents, including TNF inhibitors, using a large longitudinal database. These data were compared with those of the US Surveillance, Epidemiology, and End-Results (SEER) national database. No increase in the overall cancer rate was seen in rheumatoid arthritis patients (standardized incidence ratio [SIR] 1.0, 95% CI 1.0–1.1).
However, melanoma was more common in rheumatoid arthritis patients compared with SEER rates (SIR 1.7, 95% CI 1.3–2.3).8 In addition, biologic therapy was associated with a higher (but not statistically significant) risk of melanoma (odds ratio [OR] 2.3, 95% CI 0.9–5.4) and a higher risk of nonmelanoma skin cancer (OR 1.5, 95% CI 1.2–1.8), but not of other types of cancer.8
INFECTION
Patients on anti-TNF therapy are at a higher risk of infection, ranging from minor to life-threatening bacterial infections, and including the reactivation of granulomatous and fungal infections. More importantly, these agents are similar to steroids in blunting signs of infection, which may delay diagnosis and treatment.
The management of infection in patients on anti-TNF medications varies from case to case. In general, patients with a minor infection that does not require hospitalization or intravenous antibiotics can continue the biologic therapy while taking oral antibiotics. TNF inhibitors must be held in the event of a major infection.
Consultation with an infectious disease specialist is recommended, especially in complex cases.
Bacterial infections
An increased risk of minor bacterial infections such as urinary tract and respiratory infections has been well documented in several randomized control trials of anti-TNF agents, though other studies have shown no such increase in risk.33,51–59
The threshold for using antibiotics for a suspected bacterial infection is somewhat shifted in favor of treatment in patients on anti-TNF therapy. The reason is twofold: as previously noted, infections may be worse than they appear, because anti-TNF drugs can mask the signs and symptoms of a serious infection, and in patients on these drugs, an untreated bacterial infection may rapidly become life-threatening.
In general, broad-spectrum antibiotics are not warranted unless the source of infection is unclear or the patient is in danger of hemodynamic compromise.
Opportunistic infections
The association of anti-TNF agents with opportunistic infections could be viewed as an extension of their normal and intended therapeutic activity as potent immunosuppressive agents.68 Rheumatoid arthritis and inflammatory bowel disease are usually associated with conditions and situations that predispose patients to opportunistic infections, such as decreased immune response, malnutrition or malabsorption, surgeries, and concomitant immunosuppressive medications.7 Combination therapy with other immunosuppressive drugs and older age appear to markedly increase the risk of opportunistic infections, including mycobacterial and fungal infections, in patients with inflammatory bowel disease.7
Overall, opportunistic infections represent a measurable risk of anti-TNF therapy, and awareness and vigilance are important, especially in areas where opportunistic infections such as histoplasmosis and coccidiomycosis are endemic.50 Furthermore, physicians must be aware of the higher risk of opportunistic infections when multiple immunosuppressive drugs are used concurrently.
Granulomatous infections such as tuberculosis
Anti-TNF agents increase the risk of de novo granulomatous infections and of reactivating such infections. Granuloma formation and intracellular destruction of mycobacteria depend on TNF. TNF is important in maintaining the anatomic integrity of granulomas where these organisms have been sequestered, and blocking TNF leads to breakdown of granulomas and release of virulent organisms.69,70
TNF inhibitors increase the risk of reactivation of latent tuberculosis infection. The risk is greater with infliximab and adalimumab than with etanercept,71,72 and it has been described with certolizumab.74 Study results are varied thus far but show a risk of tuberculosis reactivation five to 30 times higher than in the general population, with tremendous variability in risk depending on background rates of previous exposure.
The absence of typical tuberculosis symptoms further complicates care in these cases. Fever, weight loss, and night sweats tend to be TNF-mediated and are therefore masked by anti-TNF agents, leading to atypical presentations. In addition, active tuberculosis infection associated with TNF inhibitors is more likely to involve extrapulmonary sites such as the skin and musculoskeletal system and to be disseminated at presentation.
A paradoxical worsening of tuberculosis symptoms may also be seen in patients with latent tuberculosis reactivation, especially after discontinuing anti-TNF therapy. This is thought to result from an immune reconstitution inflammatory syndrome.
The pretreatment evaluation should include a history of risk factors, a physical examination, and either a tuberculin skin test or an interferon-gamma-release assay. Interferon-gamma-release assays are particularly helpful in patients who have received bacille Calmette-Guérin vaccination. In patients who test positive or have been exposed, tuberculosis treatment should begin 4 weeks before starting anti-TNF therapy, though the optimal timing of antituberculosis agents is still controversial.74–77
If tuberculosis develops in a patient on anti-TNF therapy, he or she should receive antituberculosis drugs. Anti-TNF therapy should be stopped and should be resumed after 2 months only if no other treatment option is available.75
Invasive opportunistic fungal infections
Invasive opportunistic fungal infections have been reported with anti-TNF therapy, including histoplasmosis and coccidioidomycosis.78–80 Most patients who had histoplasmosis were treated with other immunosuppressive therapies and resided in or were raised near the Ohio or Mississippi River valleys, where this disease is endemic. Similarly, most cases of coccidioidomycosis were in endemic areas of Arizona, California, and Nevada, and patients on concomitant immunosuppressive therapy.78
Currently, there is no evidence to recommend obtaining Histoplasma capsulatum or Coccidioides immitis serologies before initiating anti-TNF therapy in patients in endemic areas.81 However, patients must be instructed to seek medical attention quickly for pulmonary or febrile illnesses.
Viral hepatitis infections
The data on hepatitis B and hepatitis C in patients on biologic therapies are mostly limited to case reports.
Hepatitis B. A small prospective study from Spain followed the liver biochemistry tests and hepatitis B status of 80 patients with Crohn disease treated with infliximab. Of three patients who were chronic hepatitis B carriers before starting infliximab, two experienced reactivation of hepatitis B after discontinuing infliximab, and one ultimately died. The third patient was treated with lamivudine concurrently with infliximab without clinical or biochemical changes during or after therapy.82
Similar findings were observed in two patients with rheumatoid arthritis on treatment with infliximab. One of the patients required liver transplantation, and both were treated with lamivudine, resulting in normalization of liver function test results.83,84
Recent reviews indicate that despite these findings, hepatitis B reactivation after anti-TNF withdrawal may not be common.85 There are limited data on hepatitis B reactivation and associated liver dysfunction in patients with inflammatory bowel disease treated with immunosuppressants. In a retrospective multicenter trial by Loras et al86 in patients with inflammatory bowel disease who had viral hepatitis, 36% of patients positive for hepatitis B surface antigen developed liver dysfunction, and six patients developed liver failure. In that study, treatment with more than two immunosuppressants was an independent predictor of hepatitis B reactivation.
Prolonged immunosuppression (longer than 3 months) has also been identified as an independent predictor of liver dysfunction (OR 3.06; 95% 95% CI 1.02–9.16).87
The European Association for the Study of the Liver and the American Association for the Study of Liver Diseases recommend starting lamivudine before chemotherapy or immunomodulator or immunosuppressive therapy in hepatitis B virus carriers and continuing preventive treatment for at least 6 months after stopping immunomodulating drugs. Lamivudine at a dose of 100 mg/day may reduce the risk of reactivation of hepatitis B.88–90 Tenofovir and entecavir may be useful alternatives in patients with hepatitis B who have never received nucleoside analogues. Hepatitis B reactivation did not occur in any of the 16 patients who received preventive entecavir treatment while receiving immunosuppressive treatments.89,91
In patients receiving immunosuppressive therapy, hepatitis B reactivation is associated with significant morbidity and mortality. Although risk factors for reactivation of hepatitis B virus infection have been identified, we recommend preventive treatment for all carriers positive for hepatitis B surface antigen. This should be done regardless of the number, type, and dosage of immunosuppressants and regardless of hepatitis B virus DNA levels.90
The frequency of hepatitis B and hepatitis C infection in patients with Crohn disease has been reported to be as high as 24%. The high incidence is thought to be secondary to multiple blood transfusions and surgeries.92 The use of biologic agents, including anti-TNF agents, in chronic hepatitis B virus- or hepatitis C virus-infected patients can lead to enhanced viral replication and hepatitis exacerbation.
Although active viral replication can occur during treatment with biologic agents, reactivation or exacerbation can also occur after the anti-TNF agent is stopped.82 This finding has prompted the recommendation that all candidates for biologic therapy be tested for hepatitis B immunization status, followed by immunization in nonimmune patients before starting anti-TNF therapy.93,94
Hepatitis C. There are no guidelines that adequately address the use of anti-TNF agents in patients with chronic hepatitis C infection.
Several small retrospective studies in rheumatoid arthritis patients with hepatitis C have shown that TNF inhibitors can be safely used without worsening liver function tests or changing the viral load.95–98 This is reassuring and provides the subspecialist with another treatment option, as other therapies such as disease-modifying antirheumatic drugs and steroids are known to aggravate viral hepatitis and increase the risk of viremia.96
Although small retrospective studies and one large randomized double-blind placebo-controlled trial have shown TNF inhibitors to be relatively safe in rheumatoid arthritis patients with hepatitis C, their use in these patients should be considered only with caution if they have evidence of hepatic synthetic dysfunction (eg, hypoalbuminemia, thrombocytopenia, increased international normalized ratio). The American College of Rheumatology recommends avoiding TNF inhibitors in Child-Pugh classes B and C.99
PREGNANCY
Physicians caring for patients with rheumatoid arthritis and inflammatory bowel disease must be aware of how these diseases affect fecundity and fertility and how the medications can affect conception and pregnancy. Many patients have difficulty conceiving while their autoimmune disease is active, and better disease control may improve fecundity and result in unanticipated pregnancy. Patients should be advised of the need for contraception if pregnancy is ill-advised or undesired.
Many patients seek advice about teratogenicity before conceiving, or seek guidance about rheumatoid arthritis and inflammatory bowel disease treatment while pregnant.
Several studies have reported a higher risk of adverse pregnancy outcomes in patients with rheumatoid arthritis and inflammatory bowel disease than in the general population.99–105 In inflammatory bowel disease, the odds of a premature delivery or having a low-birth-weight child are twice as high as in the normal population.100,103 Higher rates of cesarean delivery and stillbirth have also been reported. The main predisposing factor appears to be the disease activity at the time of conception, as active disease seems to be linked to adverse pregnancy outcomes.
Treatment with anti-TNF agents may rapidly achieve and maintain remission, raising the question of the safety of continued anti-TNF use during pregnancy. The FDA classifies anti-TNF agents as category B drugs, as animal studies have not demonstrated fetal risk, and no well-controlled prospective study has yet been conducted with pregnant women.
In an observational study, Schnitzler et al105 assessed the outcomes of 42 pregnancies in 35 patients with inflammatory bowel disease receiving either infliximab or adalimumab during pregnancy, compared with 56 pregnancies in 45 healthy patients without inflammatory bowel disease. There was no statistical difference in abortion rates between patients receiving anti-TNF agents and healthy women without inflammatory bowel disease (21% vs 14%, P = .4234). There was also no significant difference observed in birth weight, birth length, or cranial circumference of the children between the two groups. However, pregnancies with direct exposure to anti-TNF agents resulted in a higher frequency of premature delivery (25% vs 6%, P = .023).
Similar results were noted from the Crohn’s Therapy, Resource, Evaluation, and Assessment Tool, or TREAT, registry, as well as from a large systematic review by Vinet et al106 and case reports of rheumatoid arthritis and inflammatory bowel disease in women exposed to anti-TNF agents during pregnancy.
In addition, results from a recent systematic review of 38 studies of anti-TNF use and fetal risk, with a total of 437 women (189 on infliximab, 230 on adalimumab, 18 on certolizumab pegol), showed similar results.107 In pregnancies exposed to anti-TNF agents, the rates of congenital abnormalities (3.4%), fetal deaths (8.5%), and preterm births (2.7%) were similar to those in the general population.
For patients in disease remission on TNF inhibitors, it is reasonable to continue these agents during pregnancy after careful discussion with the patient. Fetal safety and infant immunization response after delivery are the primary concerns in these cases.
Both infliximab and adalimumab cross the placenta and remain detectable in the baby’s circulation 4 months (for adalimumab) to 6 months (for infliximab) after delivery. It is currently recommended that infliximab be stopped at 32 weeks of gestation for the remainder of the pregnancy and that adalimumab be stopped at 34 to 36 weeks, given a planned 40-week gestation.
Certolizumab does not cross the placenta in significant amounts and should be continued throughout pregnancy; drug levels in infants were shown to be less than 2 μg/mL, even when dosed the week of delivery.108–112
TAKE-HOME POINTS
- All health care providers of patients with rheumatoid arthritis and inflammatory bowel disease should be familiar with anti-TNF agents used in treating these diseases.
- The benefits of controlling the disease far outweigh the risks of therapy when used appropriately.
- Care of these patients should be multidisciplinary, with clear communication between primary care physician and specialist.
- Patient education and monitoring combined with prompt communication between primary care physician and specialist are key.
Biologic agents such as those that block tumor necrosis factor (TNF) alpha have revolutionized the treatment of autoimmune diseases like rheumatoid arthritis and inflammatory bowel disease, dramatically improving disease control and quality of life. In addition, they have made true disease remission possible in some cases.
However, as with any new therapy, a variety of side effects must be considered.
WHAT ARE BIOLOGIC AGENTS?
Biologic agents are genetically engineered drugs manufactured or synthesized in vitro from molecules such as proteins, genes, and antibodies present in living organisms. By targeting specific molecular components of the inflammatory cascade, including cytokines, these drugs alter certain aspects of the body’s inflammatory response in autoimmune diseases. Because TNF inhibitors are the most widely used biologic agents in the United States, this review will focus on them.
TNF INHIBITORS
TNF inhibitors suppress the inflammatory cascade by inactivating TNF alpha, a cytokine that promotes inflammation in the intestine, synovial tissue, and other sites.1,2
Several TNF inhibitors are available. Etanercept and infliximab were the first two to receive US Food and Drug Administration (FDA) approval, and they are extensively used. Etanercept, a soluble TNF receptor given subcutaneously, was approved for treating rheumatoid arthritis in 1998. Infliximab, a chimeric monoclonal antibody (75% human and 25% mouse protein sequence) given intravenously, received FDA approval for treating Crohn disease in 1998 and for rheumatoid arthritis in 1999. Other anti-TNF agents with varying properties are also available.
Table 1 lists anti-TNF agents approved for treating rheumatoid arthritis and inflammatory bowel disease. These are chronic, relapsing diseases that significantly and dramatically reduce quality of life, especially when they are poorly controlled.3,4 Untreated disease has been associated with malignancy, infection, pregnancy loss, and malnutrition.5–8 TNF inhibitors are aimed at patients who have moderate to severe disease or for whom previous treatments have failed, to help them achieve and maintain steroid-free remission. However, use of these agents is tempered by the risk of potentially serious side effects.
Special thought should also be given to the direct costs of the drugs (up to $30,000 per year, not counting the cost of their administration) and to the indirect costs such as time away from work to receive treatment. These are major considerations in some cases, and patients should be selected carefully for treatment with these drugs.
BEFORE STARTING THERAPY
Before starting anti-TNF therapy, several steps can reduce the risk of serious adverse events.
Take a focused history
The clinical history should include inquiries about previous bacterial, fungal, and tuberculosis infections or exposure; diabetes; and other immunocompromised states that increase the risk of acquiring potentially life-threatening infections.
Details of particular geographic areas of residence, occupational exposures, and social history should be sought. These include history of incarceration (which may put patients at risk of tuberculosis) and residence in the Ohio River valley or in the southwestern or midwestern United States (which may increase the risk of histoplasmosis, coccidioidomycosis, and other fungal infections).
Bring vaccinations up to date
Age-appropriate vaccinations should be discussed and given, ideally before starting therapy. These include influenza vaccine every year and tetanus boosters every 10 years for all and, as appropriate, varicella, human papillomavirus, and pneumococcal vaccinations. The US Centers for Disease Control and Prevention recommend an additional dose of the pneumococcal vaccine if more than 5 years have elapsed since the first one, and many clinicians opt to give it every 5 years.
In general, live-attenuated vaccines, including the intranasal influenza vaccine, are contraindicated in patients taking biologic agents.9 For patients at high risk of exposure or infection, it may be reasonable to hold the biologic agent for a period of time, vaccinate, and resume the biologic agent a month later.
Recent data suggest that the varicella zoster vaccine may be safely given to older patients with immune-mediated diseases such as rheumatoid arthritis and inflammatory bowel disease taking biologic agents.10 New guidelines from the American College of Rheumatology recommend age-appropriate vaccines for rheumatoid arthritis patients age 60 and older before biologic treatments are started. Case-by-case discussion with the subspecialist and the patient is recommended.
Screen for chronic infections
Tuberculosis screening with a purified protein derivative test or an interferon-gamma-release (Quantiferon) assay followed by chest radiography in patients with a positive test is mandatory before giving a TNF inhibitor.
Hepatitis B virus status should be determined before starting anti-TNF therapy.11
Hepatitis B vaccination has been recommended for patients with inflammatory bowel disease, but no clear recommendation exists for patients with rheumatic disease. Patients with inflammatory bowel disease tend to have low rates of response to hepatitis B vaccination12,13; possible reasons include their lack of an appropriate innate immune response to infectious agents, malnutrition, surgery, older age, and immunosuppressive drugs.14 An accelerated vaccination protocol with recombinant hepatitis B vaccine (Energix-B) in a double dose at 0, 1, and 2 months has been shown to improve response rates.15
Whenever possible, it may be better to vaccinate patients before starting immunosuppressive therapy, and to check postvaccination titers to ensure adequate response.
Perform an examination
A full physical examination with special attention to skin rashes should be performed. This may serve as a baseline to assist early detection of new rashes associated with anti-TNF therapy.
A baseline complete blood cell count and complete metabolic panel should be routinely obtained before starting therapy (and thereafter at the discretion of the physician). In conjunction with follow-up tests, they can help detect an unexpected decrease in white blood cell count or abnormal results on the liver panel.16 These baseline and follow-up tests are generally performed by the subspecialist, and the results are shared with the primary care physician.
Table 2 summarizes key information to be sought before starting a patient on a TNF inhibitor.
ADVERSE EFFECTS OF ANTI-TNF DRUGS
Infusion reactions, infections, cardiac arrhythmias, demyelinating disorders, skin infections, and malignancies have been reported with anti-TNF therapy. The relative frequencies of these adverse events are summarized in Table 3.17–22
NONINFECTIOUS COMPLICATIONS OF TNF INHIBITORS
Injection site reactions
When anti-TNF agents are given subcutaneously, injection site reactions are common (occurring in up to 40% of patients) and are considered minor.11 Reactions, including significant pain, typically occur within the first few months of therapy. They can last 2 to 5 days but rarely warrant stopping therapy. Treatment with ice and an antihistamine is almost always sufficient to control symptoms.
Infusion reactions with infliximab
Infliximab can cause both acute and delayed infusion reactions. Acute reactions can occur up to 24 hours after infusion but usually appear within 10 minutes of administration and are handled by the infusion suite staff. They range from the severe immunoglobulin E-mediated type I reaction, manifesting with hypotension, bronchospasm, and urticaria, to the milder anaphylactoid-type reaction, which constitutes the majority.23–25
While most primary care physicians will not encounter an acute reaction, family doctors and emergency room physicians may encounter delayed reactions, which can develop 1 to 14 days after infusion. These reactions usually resemble serum sickness and present with joint pain, fatigue, myalgia, and fever. But, unlike classic serum sickness, these reactions are generally not associated with a rash.
With nonspecific symptoms, the diagnosis may be easy to overlook. However, establishing this diagnosis is important because repeat therapy may result in a more severe reaction upon reexposure to the drug.
Once diagnosed, these reactions can be treated symptomatically with a combination of acetaminophen and diphenhydramine after discussion between the primary care physician and subspecialist.23,24
Autoimmune syndromes
Several studies have reported a small percentage of patients treated with anti-TNF agents who develop paradoxical autoimmune conditions. These range from asymptomatic immunologic alterations, including the formation of antinuclear antibodies and antibodies to double-stranded DNA, to life-threatening systemic autoimmune diseases.26,27
Autoimmune diseases associated with anti-TNF treatment include a lupus-like syndrome, vasculitides, and psoriatic skin lesions. These syndromes warrant stopping the inciting drug and, on occasion, giving corticosteroids. Most cases arise between 1 month and 1 year of starting treatment, and almost 75% resolve completely after the anti-TNF therapy is stopped.26
Interestingly, anti-TNF agents are approved for treating psoriasis and psoriatic arthritis, but psoriasis has paradoxically developed in patients being treated with these drugs for other autoimmune diseases. The FDA has reviewed 69 cases of new-onset psoriasis with anti-TNF therapy, including 17 pustular and 15 palmoplantar cases. The 12 most severe cases resulted in hospitalization, and symptoms resolved in most after treatment cessation.28
Fiorino et al29 counted 18 reported cases of psoriasis induced by anti-TNF therapy in patients with inflammatory bowel disease and concluded that it is rare. Harrison et al30 reported similar findings in patients with rheumatoid arthritis, with an increased incidence rate of 1.04 per 1,000 person-years. New-onset psoriasis was most common in patients treated with adalimumab. An example of the rash is seen in Figure 1.
CARDIOVASCULAR SIDE EFFECTS
Cardiovascular side effects of anti-TNF agents range from nonspecific and asymptomatic arrhythmias to worsening of heart failure.
Circulating levels of TNF are increased in patients with heart failure, and studies have evaluated the effects of TNF inhibition with infliximab on cardiac function and overall survival.31,32 The combined risk of death from any cause or hospitalization from heart failure was significantly higher in the infliximab groups, and the effects persisted for up to 5 months after stopping therapy.
Other studies22,33 have evaluated the effects of infliximab and etanercept on cardiac function and overall survival. Results showed possible exacerbation of heart failure with etanercept and increased risk of death with infliximab in patients with New York Heart Association (NYHA) class III or IV heart failure and left ventricular ejection fractions less than 35%.
Case reports have also described patients with worsening or new-onset heart failure on TNF inhibitors, including patients younger than 50 years and without identifiable cardiovascular risk factors.
Data analyses31,34,35 from large clinical registries have reported no significant increase in heart failure attributable to TNF inhibitors. However, we have concerns about the methodology of these analyses.
Currently, anti-TNF therapy is contraindicated in patients with NYHA class III or IV heart failure. Data are inconclusive for patients with class I or II heart failure. Baseline echocardiography and cardiology consultation can be considered, with close monitoring and avoidance of high doses of TNF inhibitors. If heart failure develops in a patient on anti-TNF therapy, the drug should be discontinued and the patient should be evaluated further.36
DEMYELINATING DISEASE, INCLUDING MULTIPLE SCLEROSIS
Anti-TNF agents have been associated with the onset or exacerbation of clinical symptoms and radiographic evidence of central nervous system demyelinating disorders, including multiple sclerosis.37–40 Mohan et al38 identified 19 cases of demyelinating events occurring after administration of anti-TNF agents in early 2001. In most cases, symptoms improved or resolved after therapy was stopped.
Optic neuritis,41,42 bilateral optic neuropathy,43 and aseptic meningitis44 have also been reported, but these have occurred only rarely.
How common are these effects? Postmarketing surveillance in patients with rheumatoid arthritis yields an estimated incidence of demyelinating disorders of 1 per 1,000 patient-years with adalimumab therapy.45 Complicating the assessment is an observed slight increase in risk of demyelinating conditions associated with inflammatory bowel disease.
Symptoms that should heighten the physician’s suspicion of this adverse effect include confusion, paresthesias, and ataxia. Patients on anti-TNF therapy who develop new visual symptoms should be checked for painless visual loss as a sign of early demyelinating disease.
Although data that conclusively link anti-TNF agents to multiple sclerosis are lacking, these drugs should not be initiated in patients who have a history of demyelinating disease, and treatment should be stopped promptly if the diagnosis is suspected.
MALIGNANCY
Whether anti-TNF therapy is directly linked to development of malignancies is difficult to determine. There are many confounding factors, including the risk of malignancy in underlying inflammatory disease and the concomitant use of other medications such as thiopurines, which have a known association with lymphoma.46
The incidence of lymphoma is twice as high in rheumatoid arthritis patients as in the general population.47 The risk is higher in those with more aggressive joint disease—the subset of rheumatoid arthritis patients who are more likely to be given anti-TNF agents.5
In patients with inflammatory bowel disease, the risk of cholangiocarcinoma is four times higher, and the risk of small-bowel adenocarcinoma is 16 to 18 times higher, but no increased risk of lymphoma has been identified in this population.48,49
Data from six clinical trials of infliximab, including a long-term study of its safety in Crohn disease, suggest it poses no increase in overall risk of malignancy.50–52 Similar results have been reported in patients with other rheumatic diseases.8 Information on this topic is constantly evolving, and studies range from case series to clinical trials to large patient registries.
The decision to use a TNF inhibitor should be based on the patient’s clinical picture and risk factors. Discussion of the risks and benefits of therapy with the patient should be clearly documented.
Non-Hodgkin lymphoma
Evidence about the risk of lymphoma with anti-TNF use is mixed, as up to two-thirds of patients on anti-TNF therapy have received concomitant nonbiologic immunosuppressive medications, making it difficult to determine the true risk from the biologic agents alone.53 Current evidence both supports9,53–56 and refutes7,55,57–60 the idea that anti-TNF agents increase lymphoma risk.
In patients with inflammatory bowel disease, several population-based studies have not shown a clear increase in lymphoma risk with anti-TNF use.56,59,60 Pedersen et al,61 in a meta-analysis of eight studies, confirmed these findings by showing no overall lymphoma risk in patients with inflammatory bowel disease.
However, a Canadian population-based study found a statistically significant increase in non-Hodgkin lymphoma in males with Crohn disease, with an incidence ratio of 3.63 (95% confidence interval [CI] 1.53–8.62).61 Additionally, Siegel et al62 found a significantly higher risk (6.1 cases per 10,000 patients) in patients treated with anti-TNF agents and thiopurines than in the general population (1.9 cases per 10,000 people). Although the difference was statistically significant, the overall risk is still very low.
Patients with rheumatoid arthritis seem to have a risk of lymphoma two to three times higher than in the general population. However, large population-based studies have not shown a statistically significant increase in the risk of lymphoma with anti-TNF therapy.63
Hepatosplenic T-cell lymphoma is a rare subtype of peripheral T-cell non-Hodgkin lymphoma; 25 cases have been reported in patients receiving anti-TNF therapy.64 Although the risk is extremely low (< 0.05%), physicians must carefully consider the risks and benefits of combination therapy, especially in young male patients with inflammatory bowel disease, since death is the usual outcome of this disease.65–67
Skin cancers
Wolfe and Michaud8 evaluated malignancy risk in rheumatoid arthritis patients being treated with biologic agents, including TNF inhibitors, using a large longitudinal database. These data were compared with those of the US Surveillance, Epidemiology, and End-Results (SEER) national database. No increase in the overall cancer rate was seen in rheumatoid arthritis patients (standardized incidence ratio [SIR] 1.0, 95% CI 1.0–1.1).
However, melanoma was more common in rheumatoid arthritis patients compared with SEER rates (SIR 1.7, 95% CI 1.3–2.3).8 In addition, biologic therapy was associated with a higher (but not statistically significant) risk of melanoma (odds ratio [OR] 2.3, 95% CI 0.9–5.4) and a higher risk of nonmelanoma skin cancer (OR 1.5, 95% CI 1.2–1.8), but not of other types of cancer.8
INFECTION
Patients on anti-TNF therapy are at a higher risk of infection, ranging from minor to life-threatening bacterial infections, and including the reactivation of granulomatous and fungal infections. More importantly, these agents are similar to steroids in blunting signs of infection, which may delay diagnosis and treatment.
The management of infection in patients on anti-TNF medications varies from case to case. In general, patients with a minor infection that does not require hospitalization or intravenous antibiotics can continue the biologic therapy while taking oral antibiotics. TNF inhibitors must be held in the event of a major infection.
Consultation with an infectious disease specialist is recommended, especially in complex cases.
Bacterial infections
An increased risk of minor bacterial infections such as urinary tract and respiratory infections has been well documented in several randomized control trials of anti-TNF agents, though other studies have shown no such increase in risk.33,51–59
The threshold for using antibiotics for a suspected bacterial infection is somewhat shifted in favor of treatment in patients on anti-TNF therapy. The reason is twofold: as previously noted, infections may be worse than they appear, because anti-TNF drugs can mask the signs and symptoms of a serious infection, and in patients on these drugs, an untreated bacterial infection may rapidly become life-threatening.
In general, broad-spectrum antibiotics are not warranted unless the source of infection is unclear or the patient is in danger of hemodynamic compromise.
Opportunistic infections
The association of anti-TNF agents with opportunistic infections could be viewed as an extension of their normal and intended therapeutic activity as potent immunosuppressive agents.68 Rheumatoid arthritis and inflammatory bowel disease are usually associated with conditions and situations that predispose patients to opportunistic infections, such as decreased immune response, malnutrition or malabsorption, surgeries, and concomitant immunosuppressive medications.7 Combination therapy with other immunosuppressive drugs and older age appear to markedly increase the risk of opportunistic infections, including mycobacterial and fungal infections, in patients with inflammatory bowel disease.7
Overall, opportunistic infections represent a measurable risk of anti-TNF therapy, and awareness and vigilance are important, especially in areas where opportunistic infections such as histoplasmosis and coccidiomycosis are endemic.50 Furthermore, physicians must be aware of the higher risk of opportunistic infections when multiple immunosuppressive drugs are used concurrently.
Granulomatous infections such as tuberculosis
Anti-TNF agents increase the risk of de novo granulomatous infections and of reactivating such infections. Granuloma formation and intracellular destruction of mycobacteria depend on TNF. TNF is important in maintaining the anatomic integrity of granulomas where these organisms have been sequestered, and blocking TNF leads to breakdown of granulomas and release of virulent organisms.69,70
TNF inhibitors increase the risk of reactivation of latent tuberculosis infection. The risk is greater with infliximab and adalimumab than with etanercept,71,72 and it has been described with certolizumab.74 Study results are varied thus far but show a risk of tuberculosis reactivation five to 30 times higher than in the general population, with tremendous variability in risk depending on background rates of previous exposure.
The absence of typical tuberculosis symptoms further complicates care in these cases. Fever, weight loss, and night sweats tend to be TNF-mediated and are therefore masked by anti-TNF agents, leading to atypical presentations. In addition, active tuberculosis infection associated with TNF inhibitors is more likely to involve extrapulmonary sites such as the skin and musculoskeletal system and to be disseminated at presentation.
A paradoxical worsening of tuberculosis symptoms may also be seen in patients with latent tuberculosis reactivation, especially after discontinuing anti-TNF therapy. This is thought to result from an immune reconstitution inflammatory syndrome.
The pretreatment evaluation should include a history of risk factors, a physical examination, and either a tuberculin skin test or an interferon-gamma-release assay. Interferon-gamma-release assays are particularly helpful in patients who have received bacille Calmette-Guérin vaccination. In patients who test positive or have been exposed, tuberculosis treatment should begin 4 weeks before starting anti-TNF therapy, though the optimal timing of antituberculosis agents is still controversial.74–77
If tuberculosis develops in a patient on anti-TNF therapy, he or she should receive antituberculosis drugs. Anti-TNF therapy should be stopped and should be resumed after 2 months only if no other treatment option is available.75
Invasive opportunistic fungal infections
Invasive opportunistic fungal infections have been reported with anti-TNF therapy, including histoplasmosis and coccidioidomycosis.78–80 Most patients who had histoplasmosis were treated with other immunosuppressive therapies and resided in or were raised near the Ohio or Mississippi River valleys, where this disease is endemic. Similarly, most cases of coccidioidomycosis were in endemic areas of Arizona, California, and Nevada, and patients on concomitant immunosuppressive therapy.78
Currently, there is no evidence to recommend obtaining Histoplasma capsulatum or Coccidioides immitis serologies before initiating anti-TNF therapy in patients in endemic areas.81 However, patients must be instructed to seek medical attention quickly for pulmonary or febrile illnesses.
Viral hepatitis infections
The data on hepatitis B and hepatitis C in patients on biologic therapies are mostly limited to case reports.
Hepatitis B. A small prospective study from Spain followed the liver biochemistry tests and hepatitis B status of 80 patients with Crohn disease treated with infliximab. Of three patients who were chronic hepatitis B carriers before starting infliximab, two experienced reactivation of hepatitis B after discontinuing infliximab, and one ultimately died. The third patient was treated with lamivudine concurrently with infliximab without clinical or biochemical changes during or after therapy.82
Similar findings were observed in two patients with rheumatoid arthritis on treatment with infliximab. One of the patients required liver transplantation, and both were treated with lamivudine, resulting in normalization of liver function test results.83,84
Recent reviews indicate that despite these findings, hepatitis B reactivation after anti-TNF withdrawal may not be common.85 There are limited data on hepatitis B reactivation and associated liver dysfunction in patients with inflammatory bowel disease treated with immunosuppressants. In a retrospective multicenter trial by Loras et al86 in patients with inflammatory bowel disease who had viral hepatitis, 36% of patients positive for hepatitis B surface antigen developed liver dysfunction, and six patients developed liver failure. In that study, treatment with more than two immunosuppressants was an independent predictor of hepatitis B reactivation.
Prolonged immunosuppression (longer than 3 months) has also been identified as an independent predictor of liver dysfunction (OR 3.06; 95% 95% CI 1.02–9.16).87
The European Association for the Study of the Liver and the American Association for the Study of Liver Diseases recommend starting lamivudine before chemotherapy or immunomodulator or immunosuppressive therapy in hepatitis B virus carriers and continuing preventive treatment for at least 6 months after stopping immunomodulating drugs. Lamivudine at a dose of 100 mg/day may reduce the risk of reactivation of hepatitis B.88–90 Tenofovir and entecavir may be useful alternatives in patients with hepatitis B who have never received nucleoside analogues. Hepatitis B reactivation did not occur in any of the 16 patients who received preventive entecavir treatment while receiving immunosuppressive treatments.89,91
In patients receiving immunosuppressive therapy, hepatitis B reactivation is associated with significant morbidity and mortality. Although risk factors for reactivation of hepatitis B virus infection have been identified, we recommend preventive treatment for all carriers positive for hepatitis B surface antigen. This should be done regardless of the number, type, and dosage of immunosuppressants and regardless of hepatitis B virus DNA levels.90
The frequency of hepatitis B and hepatitis C infection in patients with Crohn disease has been reported to be as high as 24%. The high incidence is thought to be secondary to multiple blood transfusions and surgeries.92 The use of biologic agents, including anti-TNF agents, in chronic hepatitis B virus- or hepatitis C virus-infected patients can lead to enhanced viral replication and hepatitis exacerbation.
Although active viral replication can occur during treatment with biologic agents, reactivation or exacerbation can also occur after the anti-TNF agent is stopped.82 This finding has prompted the recommendation that all candidates for biologic therapy be tested for hepatitis B immunization status, followed by immunization in nonimmune patients before starting anti-TNF therapy.93,94
Hepatitis C. There are no guidelines that adequately address the use of anti-TNF agents in patients with chronic hepatitis C infection.
Several small retrospective studies in rheumatoid arthritis patients with hepatitis C have shown that TNF inhibitors can be safely used without worsening liver function tests or changing the viral load.95–98 This is reassuring and provides the subspecialist with another treatment option, as other therapies such as disease-modifying antirheumatic drugs and steroids are known to aggravate viral hepatitis and increase the risk of viremia.96
Although small retrospective studies and one large randomized double-blind placebo-controlled trial have shown TNF inhibitors to be relatively safe in rheumatoid arthritis patients with hepatitis C, their use in these patients should be considered only with caution if they have evidence of hepatic synthetic dysfunction (eg, hypoalbuminemia, thrombocytopenia, increased international normalized ratio). The American College of Rheumatology recommends avoiding TNF inhibitors in Child-Pugh classes B and C.99
PREGNANCY
Physicians caring for patients with rheumatoid arthritis and inflammatory bowel disease must be aware of how these diseases affect fecundity and fertility and how the medications can affect conception and pregnancy. Many patients have difficulty conceiving while their autoimmune disease is active, and better disease control may improve fecundity and result in unanticipated pregnancy. Patients should be advised of the need for contraception if pregnancy is ill-advised or undesired.
Many patients seek advice about teratogenicity before conceiving, or seek guidance about rheumatoid arthritis and inflammatory bowel disease treatment while pregnant.
Several studies have reported a higher risk of adverse pregnancy outcomes in patients with rheumatoid arthritis and inflammatory bowel disease than in the general population.99–105 In inflammatory bowel disease, the odds of a premature delivery or having a low-birth-weight child are twice as high as in the normal population.100,103 Higher rates of cesarean delivery and stillbirth have also been reported. The main predisposing factor appears to be the disease activity at the time of conception, as active disease seems to be linked to adverse pregnancy outcomes.
Treatment with anti-TNF agents may rapidly achieve and maintain remission, raising the question of the safety of continued anti-TNF use during pregnancy. The FDA classifies anti-TNF agents as category B drugs, as animal studies have not demonstrated fetal risk, and no well-controlled prospective study has yet been conducted with pregnant women.
In an observational study, Schnitzler et al105 assessed the outcomes of 42 pregnancies in 35 patients with inflammatory bowel disease receiving either infliximab or adalimumab during pregnancy, compared with 56 pregnancies in 45 healthy patients without inflammatory bowel disease. There was no statistical difference in abortion rates between patients receiving anti-TNF agents and healthy women without inflammatory bowel disease (21% vs 14%, P = .4234). There was also no significant difference observed in birth weight, birth length, or cranial circumference of the children between the two groups. However, pregnancies with direct exposure to anti-TNF agents resulted in a higher frequency of premature delivery (25% vs 6%, P = .023).
Similar results were noted from the Crohn’s Therapy, Resource, Evaluation, and Assessment Tool, or TREAT, registry, as well as from a large systematic review by Vinet et al106 and case reports of rheumatoid arthritis and inflammatory bowel disease in women exposed to anti-TNF agents during pregnancy.
In addition, results from a recent systematic review of 38 studies of anti-TNF use and fetal risk, with a total of 437 women (189 on infliximab, 230 on adalimumab, 18 on certolizumab pegol), showed similar results.107 In pregnancies exposed to anti-TNF agents, the rates of congenital abnormalities (3.4%), fetal deaths (8.5%), and preterm births (2.7%) were similar to those in the general population.
For patients in disease remission on TNF inhibitors, it is reasonable to continue these agents during pregnancy after careful discussion with the patient. Fetal safety and infant immunization response after delivery are the primary concerns in these cases.
Both infliximab and adalimumab cross the placenta and remain detectable in the baby’s circulation 4 months (for adalimumab) to 6 months (for infliximab) after delivery. It is currently recommended that infliximab be stopped at 32 weeks of gestation for the remainder of the pregnancy and that adalimumab be stopped at 34 to 36 weeks, given a planned 40-week gestation.
Certolizumab does not cross the placenta in significant amounts and should be continued throughout pregnancy; drug levels in infants were shown to be less than 2 μg/mL, even when dosed the week of delivery.108–112
TAKE-HOME POINTS
- All health care providers of patients with rheumatoid arthritis and inflammatory bowel disease should be familiar with anti-TNF agents used in treating these diseases.
- The benefits of controlling the disease far outweigh the risks of therapy when used appropriately.
- Care of these patients should be multidisciplinary, with clear communication between primary care physician and specialist.
- Patient education and monitoring combined with prompt communication between primary care physician and specialist are key.
- Wijbrandts CA, Dijkgraaf MG, Kraan MC, et al. The clinical response to infliximab in rheumatoid arthritis is in part dependent on pretreatment tumour necrosis factor alpha expression in the synovium. Ann Rheum Dis 2008; 67:1139–1144.
- Sfikakis PP. The first decade of biologic TNF antagonists in clinical practice: lessons learned, unresolved issues and future directions. Curr Dir Autoimmun 2010; 11:180–210.
- Casellas F, Alcalá MJ, Prieto L, Miró JR, Malagelada JR. Assessment of the influence of disease activity on the quality of life of patients with inflammatory bowel disease using a short questionnaire. Am J Gastroenterol 2004; 99:457–461.
- Casellas F, López-Vivancos J, Badia X, Vilaseca J, Malagelada JR. Influence of inflammatory bowel disease on different dimensions of quality of life. Eur J Gastroenterol Hepatol 2001; 13:567–572.
- Baecklund E, Iliadou A, Askling J, et al. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum 2006; 54:692–701.
- Franklin J, Lunt M, Bunn D, Symmons D, Silman A. Incidence of lymphoma in a large primary care derived cohort of cases of inflammatory polyarthritis. Ann Rheum Dis 2006; 65:617–622.
- Toruner M, Loftus EV, Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology 2008; 134:929–936.
- Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum 2007; 56:2886–2895.
- Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1997; 46:1–24.
- Zhang J, Xie F, Delzell E, et al. Association between vaccination for herpes zoster and risk of herpes zoster infection among older patients with selected immune-mediated diseases. JAMA 2012; 308:43–49.
- Moreland LW, Baumgartner SW, Schiff MH, et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med 1997; 337:141–147.
- Altunöz ME, Senates E, Yesil A, Calhan T, Ovünç AO. Patients with inflammatory bowel disease have a lower response rate to HBV vaccination compared to controls. Dig Dis Sci 2012; 57:1039–1044.
- Gisbert JP, Villagrasa JR, Rodríguez-Nogueiras A, Chaparro M. Efficacy of hepatitis B vaccination and revaccination and factors impacting on response in patients with inflammatory bowel disease. Am J Gastroenterol 2012; 107:1460–1466.
- Carrera E, Manzano R, Garrido E. Efficacy of the vaccination in inflammatory bowel disease. World J Gastroenterol 2013; 19:1349–1353.
- Gisbert JP, Menchén L, García-Sánchez V, Marín I, Villagrasa JR, Chaparro M. Comparison of the effectiveness of two protocols for vaccination (standard and double dosage) against hepatitis B virus in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2012; 35:1379–1385.
- Ferkolj I. How to improve the safety of biologic therapy in Crohn’s disease. J Physiol Pharmacol 2009; 60(suppl 7):67–70.
- Siegel CA. The risks of biologic therapy for inflammatory bowel disease. In:Bernstein ED, editor. The Inflammatory Bowel Disease Yearbook, volume 6. London, UK: Remedica, 2010:89–108.
- Remicade (infliximab) Package Insert. Horsham, PA: Janssen Biotech, Inc; 2013. http://www.remicade.com/shared/product/remicade/prescribing-information.pdf. Accessed January 2, 2014.
- Vermeire S, Noman M, Van Assche G, et al. Autoimmunity associated with anti-tumor necrosis factor alpha treatment in Crohn’s disease: a prospective cohort study. Gastroenterology 2003; 125:32–39.
- Cush JJ. Biological drug use: US perspectives on indications and monitoring. Ann Rheum Dis 2005; 64(suppl 4):iv18–iv23.
- TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. The Lenercept Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group. Neurology 1999; 53:457–465.
- Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT; Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation 2003; 107:3133–3140.
- Cheifetz A, Mayer L. Monoclonal antibodies, immunogenicity, and associated infusion reactions. Mt Sinai J Med 2005; 72:250–256.
- Cheifetz A, Smedley M, Martin S, et al. The incidence and management of infusion reactions to infliximab: a large center experience. Am J Gastroenterol 2003; 98:1315–1324.
- Vultaggio A, Matucci A, Nencini F, et al. Anti-infliximab IgE and non-IgE antibodies and induction of infusion-related severe anaphylactic reactions. Allergy 2010; 65:657–661.
- Ramos-Casals M, Brito-Zerón P, Muñoz S, et al. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine (Baltimore) 2007; 86:242–251.
- Stallmach A, Hagel S, Bruns T. Adverse effects of biologics used for treating IBD. Best Pract Res Clin Gastroenterol 2010; 24:167–182.
- Haagsma CJ, Blom HJ, van Riel PL, et al. Influence of sulphasalazine, methotrexate, and the combination of both on plasma homocysteine concentrations in patients with rheumatoid arthritis. Ann Rheum Dis 1999; 58:79–84.
- Fiorino G, Allez M, Malesci A, Danese S. Review article: anti TNF-alpha induced psoriasis in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2009; 29:921–927.
- Harrison MJ, Dixon WG, Watson KD, et al; British Society for Rheumatology Biologics Register Control Centre Consortium; BSRBR. Rates of new-onset psoriasis in patients with rheumatoid arthritis receiving anti-tumour necrosis factor alpha therapy: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis 2009; 68:209–215.
- Marchesoni A, Zaccara E, Gorla R, et al. TNF-alpha antagonist survival rate in a cohort of rheumatoid arthritis patients observed under conditions of standard clinical practice. Ann N Y Acad Sci 2009; 1173:837–846.
- Tomas L, Lazurova I, Pundova L, et al. Acute and long-term effect of infliximab on humoral and echocardiographic parameters in patients with chronic inflammatory diseases. Clin Rheumatol 2013 32:61–66.
- Senel S, Cobankara V, Taskoylu O, et al. The safety and efficacy of etanercept on cardiac functions and lipid profile in patients with active rheumatoid arthritis. J Investig Med 2012; 60:62–65.
- Al-Aly Z, Pan H, Zeringue A, et al. Tumor necrosis factor-a blockade, cardiovascular outcomes, and survival in rheumatoid arthritis. Transl Res 2011; 157:10–18.
- Listing J, Strangfeld A, Kekow J, et al. Does tumor necrosis factor alpha inhibition promote or prevent heart failure in patients with rheumatoid arthritis? Arthritis Rheum 2008; 58:667–677.
- Wolfe F, Michaud K. Heart failure in rheumatoid arthritis: rates, predictors, and the effect of anti-tumor necrosis factor therapy. Am J Med 2004; 116:305–311.
- Enayati PJ, Papadakis KA. Association of anti-tumor necrosis factor therapy with the development of multiple sclerosis. J Clin Gastroenterol 2005; 39:303–306.
- Mohan N, Edwards ET, Cupps TR, et al. Demyelination occurring during anti-tumor necrosis factor alpha therapy for inflammatory arthritides. Arthritis Rheum 2001; 44:2862–2869.
- Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med 2004; 350:876–885.
- Thomas CW, Weinshenker BG, Sandborn WJ. Demyelination during anti-tumor necrosis factor alpha therapy with infliximab for Crohn’s disease. Inflamm Bowel Dis 2004; 10:28–31.
- Foroozan R, Buono LM, Sergott RC, Savino PJ. Retrobulbar optic neuritis associated with infliximab. Arch Ophthalmol 2002; 120:985–987.
- Strong BY, Erny BC, Herzenberg H, Razzeca KJ. Retrobulbar optic neuritis associated with infliximab in a patient with Crohn disease. Ann Intern Med 2004; 140:W34.
- ten Tusscher MP, Jacobs PJ, Busch MJ, de Graaf L, Diemont WL. Bilateral anterior toxic optic neuropathy and the use of infliximab. BMJ 2003; 326:579.
- Hegde N, Gayomali C, Rich MW. Infliximab-induced headache and infliximab-induced meningitis: two ends of the same spectrum? South Med J 2005; 98:564–566.
- Schiff MH, Burmester GR, Kent JD, et al. Safety analyses of adalimumab (HUMIRA) in global clinical trials and US postmarketing surveillance of patients with rheumatoid arthritis. Ann Rheum Dis 2006; 65:889–894.
- Beaugerie L, Brousse N, Bouvier AM, et al; CESAME Study Group. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet 2009; 374:1617–1625.
- Ekström K, Hjalgrim H, Brandt L, et al. Risk of malignant lymphomas in patients with rheumatoid arthritis and in their first-degree relatives. Arthritis Rheum 2003; 48:963–970.
- Lakatos PL, Lakatos L. Risk for colorectal cancer in ulcerative colitis: changes, causes and management strategies. World J Gastroenterol 2008; 14:3937–3947.
- Persson PG, Karlén P, Bernell O, et al. Crohn’s disease and cancer: a population-based cohort study. Gastroenterology 1994; 107:1675–1679.
- de Silva S, Devlin S, Panaccione R. Optimizing the safety of biologic therapy for IBD. Nat Rev Gastroenterol Hepatol 2010; 7:93–101.
- Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infections and mortality in association with therapies for Crohn’s disease: TREAT registry. Clin Gastroenterol Hepatol 2006; 4:621–630.
- Peyrin-Biroulet L, Deltenre P, de Suray N, Branche J, Sandborn WJ, Colombel JF. Efficacy and safety of tumor necrosis factor antagonists in Crohn’s disease: meta-analysis of placebo-controlled trials. Clin Gastroenterol Hepatol 2008; 6:644–653.
- Hanauer SB, Feagan BG, Lichtenstein GR, et al; ACCENT I Study Group. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 2002; 359:1541–1549.
- Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology 2007; 132:52–65.
- Sandborn WJ, Feagan BG, Stoinov S, et al; PRECISE 1 Study Investigators. Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med 2007; 357:228–238.
- Sandborn WJ, Hanauer SB, Rutgeerts P, et al. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut 2007; 56:1232–1239.
- Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology 2006; 130:323–333.
- Rutgeerts P, D’Haens G, Targan S, et al. Efficacy and safety of retreatment with anti-tumor necrosis factor antibody (infliximab) to maintain remission in Crohn’s disease. Gastroenterology 1999; 117:761–769.
- Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005; 353:2462–2476.
- Pedersen N, Duricova D, Elkjaer M, Gamborg M, Munkholm P, Jess T. Risk of extra-intestinal cancer in inflammatory bowel disease: meta-analysis of population-based cohort studies. Am J Gastroenterol 2010; 105:1480–1487.
- Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer 2001; 91:854–862.
- Siegel CA, Marden SM, Persing SN, Larson RJ, Sands BE. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn’s disease: a meta-analysis. Clin Gastroenterol Hepatol 2009; 7:874–881.
- Wolfe F, Michaud K. Lympyhoma in rheumatoid arthritis. The effect of methotrexate and anti-tumor necrosis factor therapy in 18,572 patients. Arthritis Rheum 2004; 50:1740–1751.
- Parakkal D, Sifuentes H, Semer R, Ehrenpreis ED. Hepatosplenic T-cell lymphoma in patients receiving TNF-a inhibitor therapy: expanding the groups at risk. Eur J Gastroenterol Hepatol 2011; 23:1150–1156.
- Rosh JR, Gross T, Mamula P, Griffiths A, Hyams J. Hepatosplenic T-cell lymphoma in adolescents and young adults with Crohn’s disease: a cautionary tale? Inflamm Bowel Dis 2007; 13:1024–1030.
- Shale M, Kanfer E, Panaccione R, Ghosh S. Hepatosplenic T cell lymphoma in inflammatory bowel disease. Gut 2008; 57:1639–1641.
- Thai A, Prindiville T. Hepatosplenic T-cell lymphoma and inflammatory bowel disease. J Crohns Colitis 2010; 4:511–522.
- Viget N, Vernier-Massouille G, Salmon-Ceron D, Yazdanpanah Y, Colombel JF. Opportunistic infections in patients with inflammatory bowel disease: prevention and diagnosis. Gut 2008; 57:549–558.
- Bekker LG, Freeman S, Murray PJ, Ryffel B, Kaplan G. TNF-alpha controls intracellular mycobacterial growth by both inducible nitric oxide synthase-dependent and inducible nitric oxide synthase-independent pathways. J Immunol 2001; 166:6728–6734.
- Roach DR, Bean AG, Demangel C, France MP, Briscoe H, Britton WJ. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J Immunol 2002; 168:4620–4627.
- Gómez-Reino JJ, Carmona L, Valverde VR, Mola EM, Montero MDBIOBADASER Group. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum 2003; 48:2122–2127.
- Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med 2001; 345:1098–1104.
- Smolen J, Landewé RB, Mease P, et al. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomised controlled trial. Ann Rheum Dis 2009; 68:797–804.
- Demkow U, Broniarek-Samson B, Filewska M, et al. Prevalence of latent tuberculosis infection in health care workers in Poland assessed by interferon-gamma whole blood and tuberculin skin tests. J Physiol Pharmacol 2008; 59(suppl 6):209–217.
- Pache I, Rogler G, Felley C. TNF-alpha blockers in inflammatory bowel diseases: practical consensus recommendations and a user’s guide. Swiss Med Wkly 2009; 139:278–287.
- Rahier JF, Ben-Horin S, Chowers Y, et al; European Crohn’s and Colitis Organisation (ECCO). European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis 2009; 3:47–91.
- Rahier JF, Yazdanpanah Y, Colombel JF, Travis S. The European (ECCO) consensus on infection in IBD: what does it change for the clinician? Gut 2009; 58:1313–1315.
- Bergstrom L, Yocum DE, Ampel NM, et al. Increased risk of coccidioidomycosis in patients treated with tumor necrosis factor alpha antagonists. Arthritis Rheum 2004; 50:1959–1966.
- Lee JH, Slifman NR, Gershon SK, et al. Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor alpha antagonists infliximab and etanercept. Arthritis Rheum 2002; 46:2565–2570.
- Wood KL, Hage CA, Knox KS, et al. Histoplasmosis after treatment with anti-tumor necrosis factor-alpha therapy. Am J Respir Crit Care Med 2003; 167:1279–1282.
- Reddy JG, Loftus EV. Safety of infliximab and other biologic agents in the inflammatory bowel diseases. Gastroenterol Clin North Am 2006; 35:837–855.
- Esteve M, Saro C, González-Huix F, Suarez F, Forné M, Viver JM. Chronic hepatitis B reactivation following infliximab therapy in Crohn’s disease patients: need for primary prophylaxis. Gut 2004; 53:1363–1365.
- Michel M, Duvoux C, Hezode C, Cherqui D. Fulminant hepatitis after infliximab in a patient with hepatitis B virus treated for an adult onset Still’s disease. J Rheumatol 2003; 30:1624–1625.
- Ostuni P, Botsios C, Punzi L, Sfriso P, Todesco S. Hepatitis B reactivation in a chronic hepatitis B surface antigen carrier with rheumatoid arthritis treated with infliximab and low dose methotrexate. Ann Rheum Dis 2003; 62:686–687.
- Pérez-Alvarez R, Díaz-Lagares C, García-Hernández F, et al; BIOGEAS Study Group. Hepatitis B virus (HBV) reactivation in patients receiving tumor necrosis factor (TNF)-targeted therapy: analysis of 257 cases. Medicine (Baltimore) 2011; 90:359–371.
- Loras C, Gisbert JP, Mínguez M, et al; REPENTINA study; GETECCU (Grupo Español de Enfermedades de Crohn y Colitis Ulcerosa) Group. Liver dysfunction related to hepatitis B and C in patients with inflammatory bowel disease treated with immunosuppressive therapy. Gut 2010; 59:1340–1346.
- Park SH, Yang SK, Lim YS, et al. Clinical courses of chronic hepatitis B virus infection and inflammatory bowel disease in patients with both diseases. Inflamm Bowel Dis 2012; 18:2004–2010.
- Loomba R, Rowley A, Wesley R, et al. Systematic review: the effect of preventive lamivudine on hepatitis B reactivation during chemotherapy. Ann Intern Med 2008; 148:519–528.
- Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology 2009; 50:661–662.
- European Association For The Study Of The Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol 2009; 50:227–242.
- Watanabe M, Shibuya A, Takada J, et al. Entecavir is an optional agent to prevent hepatitis B virus (HBV) reactivation: a review of 16 patients. Eur J Intern Med 2010; 21:333–337.
- Biancone L, Pavia M, Del Vecchio Blanco G, et al; Italian Group for the Study of the Colon and Rectum (GISC). Hepatitis B and C virus infection in Crohn’s disease. Inflamm Bowel Dis 2001; 7:287–294.
- Melmed GY. Vaccination strategies for patients with inflammatory bowel disease on immunomodulators and biologics. Inflamm Bowel Dis 2009; 15:1410–1416.
- Melmed GY, Ippoliti AF, Papadakis KA, et al. Patients with inflammatory bowel disease are at risk for vaccine-preventable illnesses. Am J Gastroenterol 2006; 101:1834–1840.
- Ferri C, Ferraccioli G, Ferrari D, et al. Safety of anti-tumor necrosis factor-alpha therapy in patients with rheumatoid arthritis and chronic hepatitis C virus infection. J Rheumatol 2008; 35:1944–1949.
- Mok MY, Ng WL, Yuen MF, Wong RW, Lau CS. Safety of disease modifying anti-rheumatic agents in rheumatoid arthritis patients with chronic viral hepatitis. Clin Exp Rheumatol 2000; 18:363–368.
- Vassilopoulos D, Calabrese LH. Risks of immunosuppressive therapies including biologic agents in patients with rheumatic diseases and coexisting chronic viral infections. Curr Opin Rheumatol 2007; 19:619–625.
- Vassilopoulos D, Apostolopoulou A, Hadziyannis E, et al. Long-term safety of anti-TNF treatment in patients with rheumatic diseases and chronic or resolved hepatitis B virus infection. Ann Rheum Dis 2010; 69:1352–1355.
- Saag KG, Teng GG, Patkar NM, et al; American College of Rheumatology. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum 2008; 59:762–784.
- Cornish J, Tan E, Teare J, et al. A meta-analysis on the influence of inflammatory bowel disease on pregnancy. Gut 2007; 56:830–837.
- Dominitz JA, Young JC, Boyko EJ. Outcomes of infants born to mothers with inflammatory bowel disease: a population-based cohort study. Am J Gastroenterol 2002; 97:641–648.
- Kornfeld D, Cnattingius S, Ekbom A. Pregnancy outcomes in women with inflammatory bowel disease—a population-based cohort study. Am J Obstet Gynecol 1997; 177:942–946.
- Mahadevan U, Sandborn WJ, Li DK, Hakimian S, Kane S, Corley DA. Pregnancy outcomes in women with inflammatory bowel disease: a large community-based study from Northern California. Gastroenterology 2007; 133:1106–1112.
- Nguyen GC, Boudreau H, Harris ML, Maxwell CV. Outcomes of obstetric hospitalizations among women with inflammatory bowel disease in the United States. Clin Gastroenterol Hepatol 2009; 7:329–334.
- Schnitzler F, Fidder H, Ferrante M, et al. Outcome of pregnancy in women with inflammatory bowel disease treated with antitumor necrosis factor therapy. Inflamm Bowel Dis 2011; 17:1846–1854.
- Vinet E, Pineau C, Gordon C, Clarke AE, Bernatsky S. Biologic therapy and pregnancy outcomes in women with rheumatic diseases. Arthritis Rheum 2009; 61:587–592.
- Guidi L, Pugliese D, Armuzzi A. Update on the management of inflammatory bowel disease: specific role of adalimumab. Clin Exp Gastroenterol 2011; 4:163–172.
- Mahadevan U. Continuing immunomodulators and biologic medications in pregnant IBD patients – pro. IInflamm Bowel Dis 2007; 13:1439–1440.
- Mahadevan U. Gastrointestinal medications in pregnancy. Best Pract Res Clin Gastroenterol 2007; 21:849–877.
- Mahadevan U, Cucchiara S, Hyams JS, et al. The London Position Statement of the World Congress of Gastroenterology on Biological Therapy for IBD with the European Crohn’s and Colitis Organisation: pregnancy and pediatrics. Am J Gastroenterol 2011; 106:214–223.
- Mahadevan U, Kane S. Use of infliximab in pregnancy. Am J Gastroenterol 2010; 105:219–220.
- Vasiliauskas EA, Church JA, Silverman N, Barry M, Targan SR, Dubinsky MC. Case report: evidence for transplacental transfer of maternally administered infliximab to the newborn. Clin Gastroenterol Hepatol 2006; 4:1255–1258.
- Wijbrandts CA, Dijkgraaf MG, Kraan MC, et al. The clinical response to infliximab in rheumatoid arthritis is in part dependent on pretreatment tumour necrosis factor alpha expression in the synovium. Ann Rheum Dis 2008; 67:1139–1144.
- Sfikakis PP. The first decade of biologic TNF antagonists in clinical practice: lessons learned, unresolved issues and future directions. Curr Dir Autoimmun 2010; 11:180–210.
- Casellas F, Alcalá MJ, Prieto L, Miró JR, Malagelada JR. Assessment of the influence of disease activity on the quality of life of patients with inflammatory bowel disease using a short questionnaire. Am J Gastroenterol 2004; 99:457–461.
- Casellas F, López-Vivancos J, Badia X, Vilaseca J, Malagelada JR. Influence of inflammatory bowel disease on different dimensions of quality of life. Eur J Gastroenterol Hepatol 2001; 13:567–572.
- Baecklund E, Iliadou A, Askling J, et al. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum 2006; 54:692–701.
- Franklin J, Lunt M, Bunn D, Symmons D, Silman A. Incidence of lymphoma in a large primary care derived cohort of cases of inflammatory polyarthritis. Ann Rheum Dis 2006; 65:617–622.
- Toruner M, Loftus EV, Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology 2008; 134:929–936.
- Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum 2007; 56:2886–2895.
- Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1997; 46:1–24.
- Zhang J, Xie F, Delzell E, et al. Association between vaccination for herpes zoster and risk of herpes zoster infection among older patients with selected immune-mediated diseases. JAMA 2012; 308:43–49.
- Moreland LW, Baumgartner SW, Schiff MH, et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med 1997; 337:141–147.
- Altunöz ME, Senates E, Yesil A, Calhan T, Ovünç AO. Patients with inflammatory bowel disease have a lower response rate to HBV vaccination compared to controls. Dig Dis Sci 2012; 57:1039–1044.
- Gisbert JP, Villagrasa JR, Rodríguez-Nogueiras A, Chaparro M. Efficacy of hepatitis B vaccination and revaccination and factors impacting on response in patients with inflammatory bowel disease. Am J Gastroenterol 2012; 107:1460–1466.
- Carrera E, Manzano R, Garrido E. Efficacy of the vaccination in inflammatory bowel disease. World J Gastroenterol 2013; 19:1349–1353.
- Gisbert JP, Menchén L, García-Sánchez V, Marín I, Villagrasa JR, Chaparro M. Comparison of the effectiveness of two protocols for vaccination (standard and double dosage) against hepatitis B virus in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2012; 35:1379–1385.
- Ferkolj I. How to improve the safety of biologic therapy in Crohn’s disease. J Physiol Pharmacol 2009; 60(suppl 7):67–70.
- Siegel CA. The risks of biologic therapy for inflammatory bowel disease. In:Bernstein ED, editor. The Inflammatory Bowel Disease Yearbook, volume 6. London, UK: Remedica, 2010:89–108.
- Remicade (infliximab) Package Insert. Horsham, PA: Janssen Biotech, Inc; 2013. http://www.remicade.com/shared/product/remicade/prescribing-information.pdf. Accessed January 2, 2014.
- Vermeire S, Noman M, Van Assche G, et al. Autoimmunity associated with anti-tumor necrosis factor alpha treatment in Crohn’s disease: a prospective cohort study. Gastroenterology 2003; 125:32–39.
- Cush JJ. Biological drug use: US perspectives on indications and monitoring. Ann Rheum Dis 2005; 64(suppl 4):iv18–iv23.
- TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. The Lenercept Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group. Neurology 1999; 53:457–465.
- Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT; Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation 2003; 107:3133–3140.
- Cheifetz A, Mayer L. Monoclonal antibodies, immunogenicity, and associated infusion reactions. Mt Sinai J Med 2005; 72:250–256.
- Cheifetz A, Smedley M, Martin S, et al. The incidence and management of infusion reactions to infliximab: a large center experience. Am J Gastroenterol 2003; 98:1315–1324.
- Vultaggio A, Matucci A, Nencini F, et al. Anti-infliximab IgE and non-IgE antibodies and induction of infusion-related severe anaphylactic reactions. Allergy 2010; 65:657–661.
- Ramos-Casals M, Brito-Zerón P, Muñoz S, et al. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine (Baltimore) 2007; 86:242–251.
- Stallmach A, Hagel S, Bruns T. Adverse effects of biologics used for treating IBD. Best Pract Res Clin Gastroenterol 2010; 24:167–182.
- Haagsma CJ, Blom HJ, van Riel PL, et al. Influence of sulphasalazine, methotrexate, and the combination of both on plasma homocysteine concentrations in patients with rheumatoid arthritis. Ann Rheum Dis 1999; 58:79–84.
- Fiorino G, Allez M, Malesci A, Danese S. Review article: anti TNF-alpha induced psoriasis in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2009; 29:921–927.
- Harrison MJ, Dixon WG, Watson KD, et al; British Society for Rheumatology Biologics Register Control Centre Consortium; BSRBR. Rates of new-onset psoriasis in patients with rheumatoid arthritis receiving anti-tumour necrosis factor alpha therapy: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis 2009; 68:209–215.
- Marchesoni A, Zaccara E, Gorla R, et al. TNF-alpha antagonist survival rate in a cohort of rheumatoid arthritis patients observed under conditions of standard clinical practice. Ann N Y Acad Sci 2009; 1173:837–846.
- Tomas L, Lazurova I, Pundova L, et al. Acute and long-term effect of infliximab on humoral and echocardiographic parameters in patients with chronic inflammatory diseases. Clin Rheumatol 2013 32:61–66.
- Senel S, Cobankara V, Taskoylu O, et al. The safety and efficacy of etanercept on cardiac functions and lipid profile in patients with active rheumatoid arthritis. J Investig Med 2012; 60:62–65.
- Al-Aly Z, Pan H, Zeringue A, et al. Tumor necrosis factor-a blockade, cardiovascular outcomes, and survival in rheumatoid arthritis. Transl Res 2011; 157:10–18.
- Listing J, Strangfeld A, Kekow J, et al. Does tumor necrosis factor alpha inhibition promote or prevent heart failure in patients with rheumatoid arthritis? Arthritis Rheum 2008; 58:667–677.
- Wolfe F, Michaud K. Heart failure in rheumatoid arthritis: rates, predictors, and the effect of anti-tumor necrosis factor therapy. Am J Med 2004; 116:305–311.
- Enayati PJ, Papadakis KA. Association of anti-tumor necrosis factor therapy with the development of multiple sclerosis. J Clin Gastroenterol 2005; 39:303–306.
- Mohan N, Edwards ET, Cupps TR, et al. Demyelination occurring during anti-tumor necrosis factor alpha therapy for inflammatory arthritides. Arthritis Rheum 2001; 44:2862–2869.
- Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med 2004; 350:876–885.
- Thomas CW, Weinshenker BG, Sandborn WJ. Demyelination during anti-tumor necrosis factor alpha therapy with infliximab for Crohn’s disease. Inflamm Bowel Dis 2004; 10:28–31.
- Foroozan R, Buono LM, Sergott RC, Savino PJ. Retrobulbar optic neuritis associated with infliximab. Arch Ophthalmol 2002; 120:985–987.
- Strong BY, Erny BC, Herzenberg H, Razzeca KJ. Retrobulbar optic neuritis associated with infliximab in a patient with Crohn disease. Ann Intern Med 2004; 140:W34.
- ten Tusscher MP, Jacobs PJ, Busch MJ, de Graaf L, Diemont WL. Bilateral anterior toxic optic neuropathy and the use of infliximab. BMJ 2003; 326:579.
- Hegde N, Gayomali C, Rich MW. Infliximab-induced headache and infliximab-induced meningitis: two ends of the same spectrum? South Med J 2005; 98:564–566.
- Schiff MH, Burmester GR, Kent JD, et al. Safety analyses of adalimumab (HUMIRA) in global clinical trials and US postmarketing surveillance of patients with rheumatoid arthritis. Ann Rheum Dis 2006; 65:889–894.
- Beaugerie L, Brousse N, Bouvier AM, et al; CESAME Study Group. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet 2009; 374:1617–1625.
- Ekström K, Hjalgrim H, Brandt L, et al. Risk of malignant lymphomas in patients with rheumatoid arthritis and in their first-degree relatives. Arthritis Rheum 2003; 48:963–970.
- Lakatos PL, Lakatos L. Risk for colorectal cancer in ulcerative colitis: changes, causes and management strategies. World J Gastroenterol 2008; 14:3937–3947.
- Persson PG, Karlén P, Bernell O, et al. Crohn’s disease and cancer: a population-based cohort study. Gastroenterology 1994; 107:1675–1679.
- de Silva S, Devlin S, Panaccione R. Optimizing the safety of biologic therapy for IBD. Nat Rev Gastroenterol Hepatol 2010; 7:93–101.
- Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infections and mortality in association with therapies for Crohn’s disease: TREAT registry. Clin Gastroenterol Hepatol 2006; 4:621–630.
- Peyrin-Biroulet L, Deltenre P, de Suray N, Branche J, Sandborn WJ, Colombel JF. Efficacy and safety of tumor necrosis factor antagonists in Crohn’s disease: meta-analysis of placebo-controlled trials. Clin Gastroenterol Hepatol 2008; 6:644–653.
- Hanauer SB, Feagan BG, Lichtenstein GR, et al; ACCENT I Study Group. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 2002; 359:1541–1549.
- Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology 2007; 132:52–65.
- Sandborn WJ, Feagan BG, Stoinov S, et al; PRECISE 1 Study Investigators. Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med 2007; 357:228–238.
- Sandborn WJ, Hanauer SB, Rutgeerts P, et al. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut 2007; 56:1232–1239.
- Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology 2006; 130:323–333.
- Rutgeerts P, D’Haens G, Targan S, et al. Efficacy and safety of retreatment with anti-tumor necrosis factor antibody (infliximab) to maintain remission in Crohn’s disease. Gastroenterology 1999; 117:761–769.
- Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005; 353:2462–2476.
- Pedersen N, Duricova D, Elkjaer M, Gamborg M, Munkholm P, Jess T. Risk of extra-intestinal cancer in inflammatory bowel disease: meta-analysis of population-based cohort studies. Am J Gastroenterol 2010; 105:1480–1487.
- Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer 2001; 91:854–862.
- Siegel CA, Marden SM, Persing SN, Larson RJ, Sands BE. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn’s disease: a meta-analysis. Clin Gastroenterol Hepatol 2009; 7:874–881.
- Wolfe F, Michaud K. Lympyhoma in rheumatoid arthritis. The effect of methotrexate and anti-tumor necrosis factor therapy in 18,572 patients. Arthritis Rheum 2004; 50:1740–1751.
- Parakkal D, Sifuentes H, Semer R, Ehrenpreis ED. Hepatosplenic T-cell lymphoma in patients receiving TNF-a inhibitor therapy: expanding the groups at risk. Eur J Gastroenterol Hepatol 2011; 23:1150–1156.
- Rosh JR, Gross T, Mamula P, Griffiths A, Hyams J. Hepatosplenic T-cell lymphoma in adolescents and young adults with Crohn’s disease: a cautionary tale? Inflamm Bowel Dis 2007; 13:1024–1030.
- Shale M, Kanfer E, Panaccione R, Ghosh S. Hepatosplenic T cell lymphoma in inflammatory bowel disease. Gut 2008; 57:1639–1641.
- Thai A, Prindiville T. Hepatosplenic T-cell lymphoma and inflammatory bowel disease. J Crohns Colitis 2010; 4:511–522.
- Viget N, Vernier-Massouille G, Salmon-Ceron D, Yazdanpanah Y, Colombel JF. Opportunistic infections in patients with inflammatory bowel disease: prevention and diagnosis. Gut 2008; 57:549–558.
- Bekker LG, Freeman S, Murray PJ, Ryffel B, Kaplan G. TNF-alpha controls intracellular mycobacterial growth by both inducible nitric oxide synthase-dependent and inducible nitric oxide synthase-independent pathways. J Immunol 2001; 166:6728–6734.
- Roach DR, Bean AG, Demangel C, France MP, Briscoe H, Britton WJ. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J Immunol 2002; 168:4620–4627.
- Gómez-Reino JJ, Carmona L, Valverde VR, Mola EM, Montero MDBIOBADASER Group. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum 2003; 48:2122–2127.
- Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med 2001; 345:1098–1104.
- Smolen J, Landewé RB, Mease P, et al. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomised controlled trial. Ann Rheum Dis 2009; 68:797–804.
- Demkow U, Broniarek-Samson B, Filewska M, et al. Prevalence of latent tuberculosis infection in health care workers in Poland assessed by interferon-gamma whole blood and tuberculin skin tests. J Physiol Pharmacol 2008; 59(suppl 6):209–217.
- Pache I, Rogler G, Felley C. TNF-alpha blockers in inflammatory bowel diseases: practical consensus recommendations and a user’s guide. Swiss Med Wkly 2009; 139:278–287.
- Rahier JF, Ben-Horin S, Chowers Y, et al; European Crohn’s and Colitis Organisation (ECCO). European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis 2009; 3:47–91.
- Rahier JF, Yazdanpanah Y, Colombel JF, Travis S. The European (ECCO) consensus on infection in IBD: what does it change for the clinician? Gut 2009; 58:1313–1315.
- Bergstrom L, Yocum DE, Ampel NM, et al. Increased risk of coccidioidomycosis in patients treated with tumor necrosis factor alpha antagonists. Arthritis Rheum 2004; 50:1959–1966.
- Lee JH, Slifman NR, Gershon SK, et al. Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor alpha antagonists infliximab and etanercept. Arthritis Rheum 2002; 46:2565–2570.
- Wood KL, Hage CA, Knox KS, et al. Histoplasmosis after treatment with anti-tumor necrosis factor-alpha therapy. Am J Respir Crit Care Med 2003; 167:1279–1282.
- Reddy JG, Loftus EV. Safety of infliximab and other biologic agents in the inflammatory bowel diseases. Gastroenterol Clin North Am 2006; 35:837–855.
- Esteve M, Saro C, González-Huix F, Suarez F, Forné M, Viver JM. Chronic hepatitis B reactivation following infliximab therapy in Crohn’s disease patients: need for primary prophylaxis. Gut 2004; 53:1363–1365.
- Michel M, Duvoux C, Hezode C, Cherqui D. Fulminant hepatitis after infliximab in a patient with hepatitis B virus treated for an adult onset Still’s disease. J Rheumatol 2003; 30:1624–1625.
- Ostuni P, Botsios C, Punzi L, Sfriso P, Todesco S. Hepatitis B reactivation in a chronic hepatitis B surface antigen carrier with rheumatoid arthritis treated with infliximab and low dose methotrexate. Ann Rheum Dis 2003; 62:686–687.
- Pérez-Alvarez R, Díaz-Lagares C, García-Hernández F, et al; BIOGEAS Study Group. Hepatitis B virus (HBV) reactivation in patients receiving tumor necrosis factor (TNF)-targeted therapy: analysis of 257 cases. Medicine (Baltimore) 2011; 90:359–371.
- Loras C, Gisbert JP, Mínguez M, et al; REPENTINA study; GETECCU (Grupo Español de Enfermedades de Crohn y Colitis Ulcerosa) Group. Liver dysfunction related to hepatitis B and C in patients with inflammatory bowel disease treated with immunosuppressive therapy. Gut 2010; 59:1340–1346.
- Park SH, Yang SK, Lim YS, et al. Clinical courses of chronic hepatitis B virus infection and inflammatory bowel disease in patients with both diseases. Inflamm Bowel Dis 2012; 18:2004–2010.
- Loomba R, Rowley A, Wesley R, et al. Systematic review: the effect of preventive lamivudine on hepatitis B reactivation during chemotherapy. Ann Intern Med 2008; 148:519–528.
- Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology 2009; 50:661–662.
- European Association For The Study Of The Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol 2009; 50:227–242.
- Watanabe M, Shibuya A, Takada J, et al. Entecavir is an optional agent to prevent hepatitis B virus (HBV) reactivation: a review of 16 patients. Eur J Intern Med 2010; 21:333–337.
- Biancone L, Pavia M, Del Vecchio Blanco G, et al; Italian Group for the Study of the Colon and Rectum (GISC). Hepatitis B and C virus infection in Crohn’s disease. Inflamm Bowel Dis 2001; 7:287–294.
- Melmed GY. Vaccination strategies for patients with inflammatory bowel disease on immunomodulators and biologics. Inflamm Bowel Dis 2009; 15:1410–1416.
- Melmed GY, Ippoliti AF, Papadakis KA, et al. Patients with inflammatory bowel disease are at risk for vaccine-preventable illnesses. Am J Gastroenterol 2006; 101:1834–1840.
- Ferri C, Ferraccioli G, Ferrari D, et al. Safety of anti-tumor necrosis factor-alpha therapy in patients with rheumatoid arthritis and chronic hepatitis C virus infection. J Rheumatol 2008; 35:1944–1949.
- Mok MY, Ng WL, Yuen MF, Wong RW, Lau CS. Safety of disease modifying anti-rheumatic agents in rheumatoid arthritis patients with chronic viral hepatitis. Clin Exp Rheumatol 2000; 18:363–368.
- Vassilopoulos D, Calabrese LH. Risks of immunosuppressive therapies including biologic agents in patients with rheumatic diseases and coexisting chronic viral infections. Curr Opin Rheumatol 2007; 19:619–625.
- Vassilopoulos D, Apostolopoulou A, Hadziyannis E, et al. Long-term safety of anti-TNF treatment in patients with rheumatic diseases and chronic or resolved hepatitis B virus infection. Ann Rheum Dis 2010; 69:1352–1355.
- Saag KG, Teng GG, Patkar NM, et al; American College of Rheumatology. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum 2008; 59:762–784.
- Cornish J, Tan E, Teare J, et al. A meta-analysis on the influence of inflammatory bowel disease on pregnancy. Gut 2007; 56:830–837.
- Dominitz JA, Young JC, Boyko EJ. Outcomes of infants born to mothers with inflammatory bowel disease: a population-based cohort study. Am J Gastroenterol 2002; 97:641–648.
- Kornfeld D, Cnattingius S, Ekbom A. Pregnancy outcomes in women with inflammatory bowel disease—a population-based cohort study. Am J Obstet Gynecol 1997; 177:942–946.
- Mahadevan U, Sandborn WJ, Li DK, Hakimian S, Kane S, Corley DA. Pregnancy outcomes in women with inflammatory bowel disease: a large community-based study from Northern California. Gastroenterology 2007; 133:1106–1112.
- Nguyen GC, Boudreau H, Harris ML, Maxwell CV. Outcomes of obstetric hospitalizations among women with inflammatory bowel disease in the United States. Clin Gastroenterol Hepatol 2009; 7:329–334.
- Schnitzler F, Fidder H, Ferrante M, et al. Outcome of pregnancy in women with inflammatory bowel disease treated with antitumor necrosis factor therapy. Inflamm Bowel Dis 2011; 17:1846–1854.
- Vinet E, Pineau C, Gordon C, Clarke AE, Bernatsky S. Biologic therapy and pregnancy outcomes in women with rheumatic diseases. Arthritis Rheum 2009; 61:587–592.
- Guidi L, Pugliese D, Armuzzi A. Update on the management of inflammatory bowel disease: specific role of adalimumab. Clin Exp Gastroenterol 2011; 4:163–172.
- Mahadevan U. Continuing immunomodulators and biologic medications in pregnant IBD patients – pro. IInflamm Bowel Dis 2007; 13:1439–1440.
- Mahadevan U. Gastrointestinal medications in pregnancy. Best Pract Res Clin Gastroenterol 2007; 21:849–877.
- Mahadevan U, Cucchiara S, Hyams JS, et al. The London Position Statement of the World Congress of Gastroenterology on Biological Therapy for IBD with the European Crohn’s and Colitis Organisation: pregnancy and pediatrics. Am J Gastroenterol 2011; 106:214–223.
- Mahadevan U, Kane S. Use of infliximab in pregnancy. Am J Gastroenterol 2010; 105:219–220.
- Vasiliauskas EA, Church JA, Silverman N, Barry M, Targan SR, Dubinsky MC. Case report: evidence for transplacental transfer of maternally administered infliximab to the newborn. Clin Gastroenterol Hepatol 2006; 4:1255–1258.
KEY POINTS
- Over the past 10 years, TNF inhibitors have substantially altered the management of autoimmune diseases such as rheumatoid arthritis and inflammatory bowel disease.
- Safety concerns include risks of infection, reactivation of latent infection (eg, fungal infection, granulomatous infection), malignancy, and autoimmune and neurologic effects.
- Before treating, take a complete history, including exposure to latent infections and geographic considerations, and bring patients’ immunizations up to date.
- Regular clinical and laboratory monitoring during treatment helps optimize therapy and minimize the risk of adverse effects.
- Physicians must be aware of atypical presentations of infection and understand how their treatment may differ in patients on biologic therapy.