User login
Molluscum contagiosum virus (MCV) is a common pediatric viral infection of the skin and/or mucous membranes.1 It has been noted in increasingly younger patient populations, ranging from congenital cases resulting from perinatal/vertical transmission to transmission from cobathing and pool usage.2,3
An association between MCV infection and atopic dermatitis (AD) has been reported to be caused by a predisposition to prolonged and severe cutaneous viral infections.4 However, the exact nature of the relationship between MCV and AD is unknown.
The purpose of this study was to identify pediatric patients with AD onset or flare of AD triggered by MCV infection as well as to characterize the setting under which MCV may trigger AD onset or flares in children.
Methods
Medical records for 50 children with prior or current MCV infection who presented sequentially to an outpatient pediatric dermatology practice over a 1-month period were identified. Institutional review board approval was obtained.
Results
The age range of the 50 patients with MCV infection was 1 to 13 years, with an average age of 3.6 years at the onset of infection (reported by parents/guardians) and 4.5 years at presentation to the pediatric dermatology office (Table 1).
The role of cobathing is unknown; however, 62% (31/50) of patients previously or currently cobathed at home, suggesting it may be a risk factor for MCV infection. An association of MCV lesions in the popliteal region trended toward being more likely with cobathing, but the association was not statistically significant.
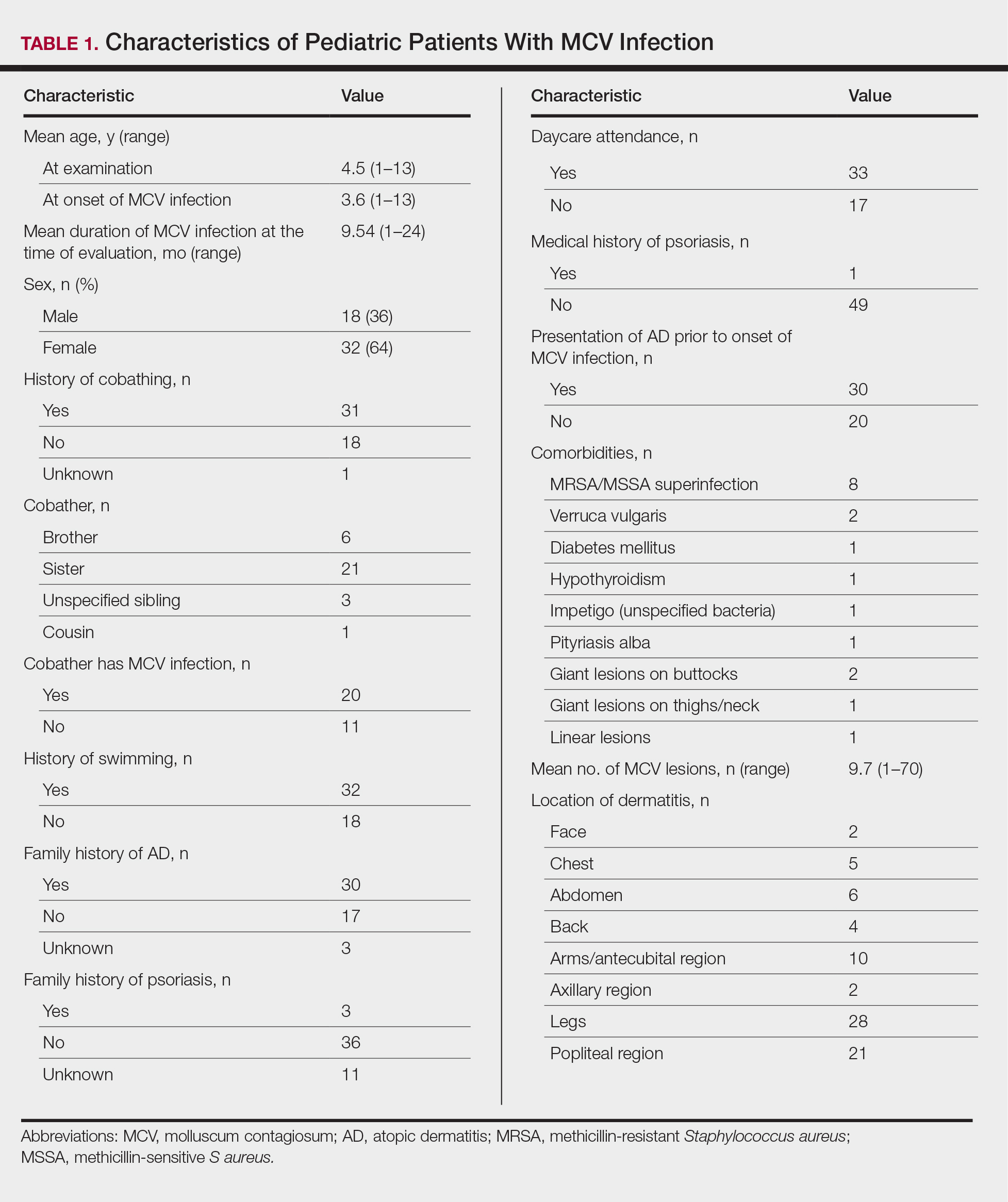
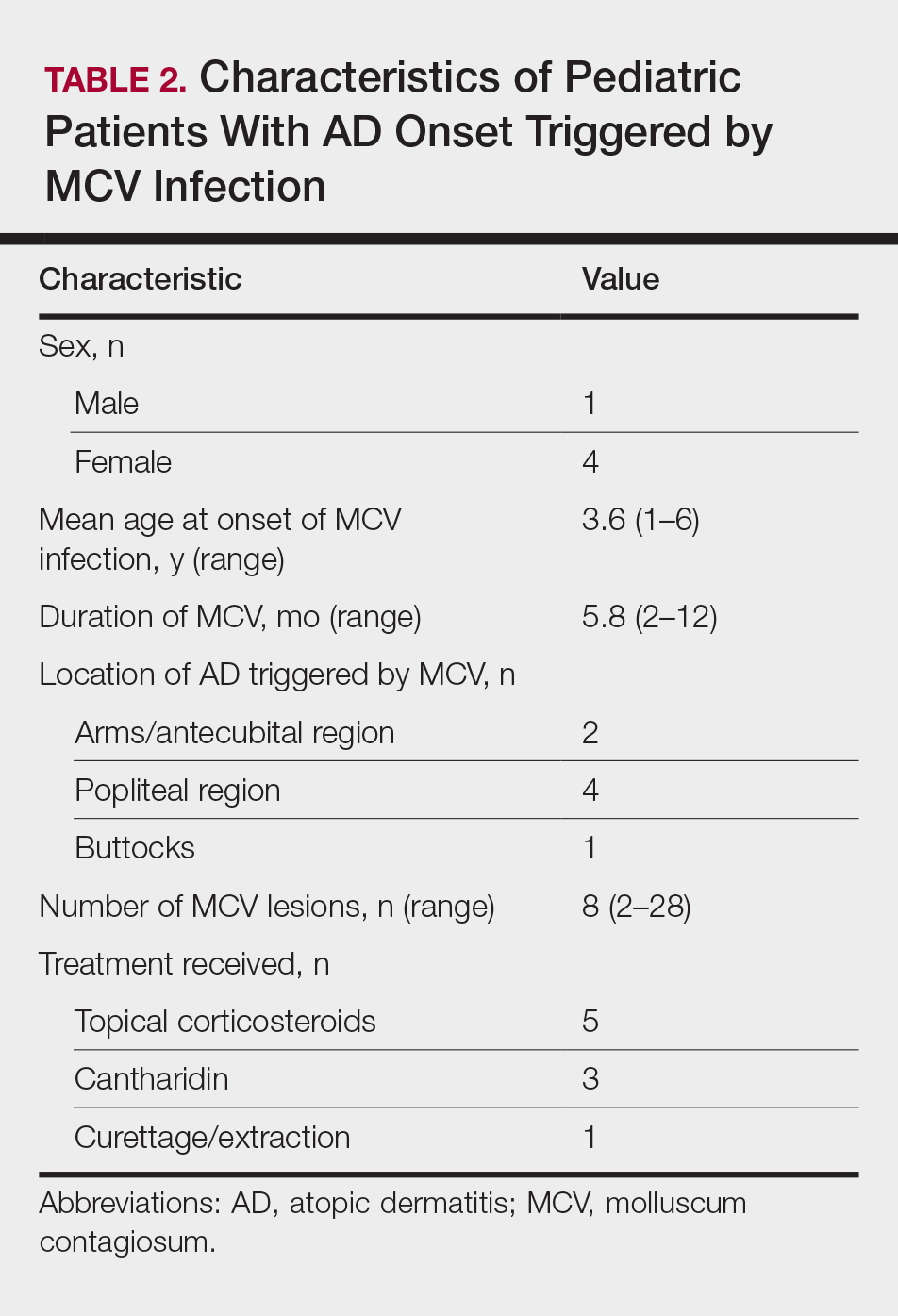
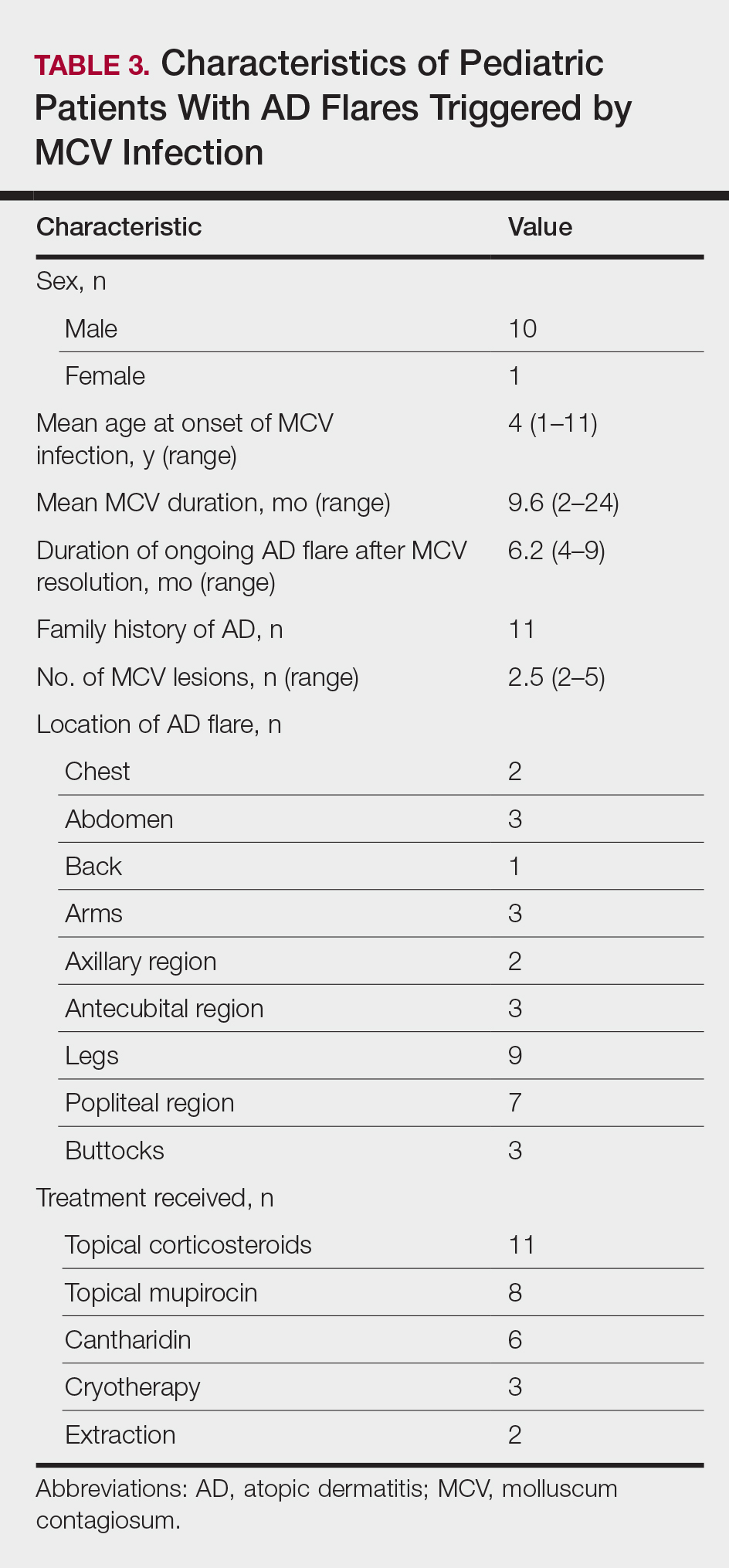
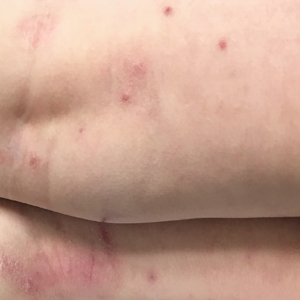
Children with AD onset triggered by MCV infection statistically were more likely to have flexural localization of MCV and AD lesions and were statistically more likely to have a family history of AD (P<.04)(Table 2). Children with AD flares triggered by MCV infection were more likely to have MCV and AD lesions of the popliteal region and legs (P<.05)(Figure) and family history of AD (P<.04)(Table 3). Location of MCV lesions on the upper and lower extremities, buttocks, and genitalia were more likely to be associated with presence of any dermatitis than facial and/or truncal lesions (P<.05). Treatment of the MCV infection did not appear to impact the course of AD when present, but prospective interventions would be needed to assess this issue.
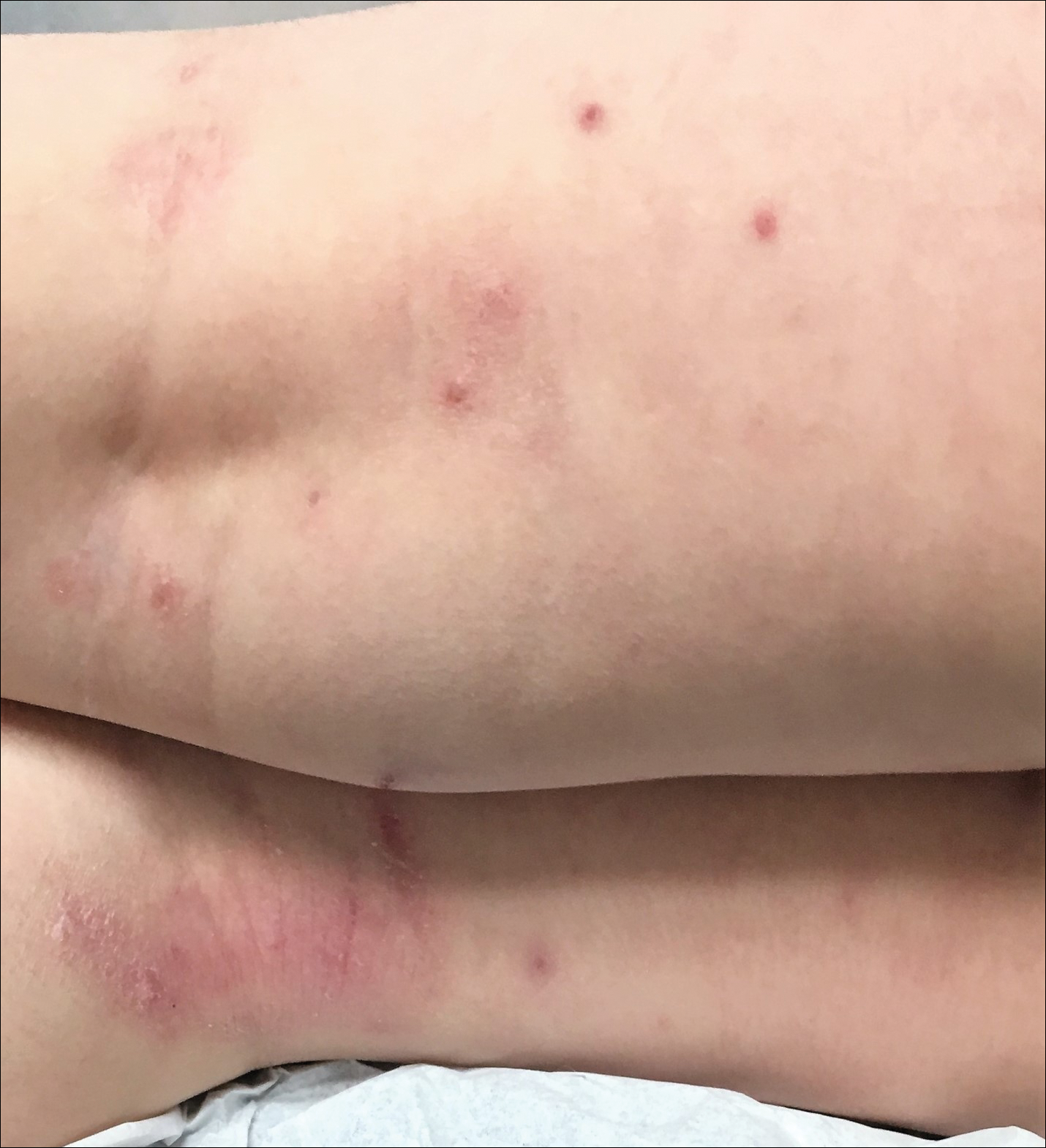
Superinfection with methicillin-resistant and methicillin-sensitive Staphylococcus aureus as well as atypical giant lesions of the intertriginous neck, inner thighs, and buttocks also were noted, but AD was uncommon in these cases. Given the limited number of cases, statistical significance could not be assessed.
Comment
Cutaneous infections with Malassezia have been postulated to trigger AD in infancy,1 while systemic viral infections such as varicella-zoster virus may be protective against AD when acquired in younger children.7 It appears that MCV infection in young children (eg, 3 years or younger) with specific localization to the flexural areas has the potential to trigger AD in susceptible hosts. Larger studies are needed to chart the long-term disease course of AD in these children. Due to the small size of this study, it is unclear if the rise of MCV infections since the 1980s has contributed to increased AD.8 Susceptible children appear to have a family history of AD and localization of MCV lesions on the legs, buttocks, and antecubital region. Atopic dermatitis risk appears to be highest when MCV lesions are localized to intertriginous or flexural locations.
In addition to triggering the onset of AD, MCV infection also can trigger persistent flaring of AD, especially in the popliteal region and legs. Atopic dermatitis flares can occur at any age, but they appear to cluster in preschoolers and typically are not prevented by AD or MCV treatments; however, randomized trials are needed to identify if early intervention of MCV has a preventive benefit on AD onset or flares, and longer-term observation is needed to identify true disease course modification. Reduction of the number of MCV lesions previously has been demonstrated with institution of topical corticosteroid therapy.6 Therefore, institution of atopic skin care generally is advisable in the setting of MCV infection. Future studies should address the potential use of interventions to prevent the triggering of AD onset or flares in the setting of MCV infection in children.5
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99.
- Connell CO, Oranje A, Van Gysel D, et al. Congenital molluscum contagiosum: report of four cases and review of the literature. Pediatr Dermatol. 2008;25:553-556.
- Luke JD, Silverberg NB. Vertically transmitted molluscum contagiosum infection. Pediatrics. 2010;125:E423-E425.
- Olsen JR, Piguet V, Gallacher J, et al. Molluscum contagiosum and associations with atopic eczema in children: a retrospective longitudinal study in primary care. Br J Gen Pract. 2016;66:E53-E58.
- Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32:353-357.
- Berger EM, Orlow SJ, Patel RR, et al. Experience with molluscum contagiosum and associated inflammatory reactions in a pediatric dermatology practice: the bump that rashes. Arch Dermatol. 2012;148:1257-1264.
- Silverberg JI, Norowitz KB, Kleiman E, et al. Association between varicella zoster virus infection and atopic dermatitis in early and late childhood: a case-control study. J Allergy Clin Immunol. 2010;126:300-305.
- Oriel JD. The increase in molluscum contagiosum. Br Med J (Clin Res Ed). 1987;294:74.
Molluscum contagiosum virus (MCV) is a common pediatric viral infection of the skin and/or mucous membranes.1 It has been noted in increasingly younger patient populations, ranging from congenital cases resulting from perinatal/vertical transmission to transmission from cobathing and pool usage.2,3
An association between MCV infection and atopic dermatitis (AD) has been reported to be caused by a predisposition to prolonged and severe cutaneous viral infections.4 However, the exact nature of the relationship between MCV and AD is unknown.
The purpose of this study was to identify pediatric patients with AD onset or flare of AD triggered by MCV infection as well as to characterize the setting under which MCV may trigger AD onset or flares in children.
Methods
Medical records for 50 children with prior or current MCV infection who presented sequentially to an outpatient pediatric dermatology practice over a 1-month period were identified. Institutional review board approval was obtained.
Results
The age range of the 50 patients with MCV infection was 1 to 13 years, with an average age of 3.6 years at the onset of infection (reported by parents/guardians) and 4.5 years at presentation to the pediatric dermatology office (Table 1).
The role of cobathing is unknown; however, 62% (31/50) of patients previously or currently cobathed at home, suggesting it may be a risk factor for MCV infection. An association of MCV lesions in the popliteal region trended toward being more likely with cobathing, but the association was not statistically significant.
Children with AD onset triggered by MCV infection statistically were more likely to have flexural localization of MCV and AD lesions and were statistically more likely to have a family history of AD (P<.04)(Table 2). Children with AD flares triggered by MCV infection were more likely to have MCV and AD lesions of the popliteal region and legs (P<.05)(Figure) and family history of AD (P<.04)(Table 3). Location of MCV lesions on the upper and lower extremities, buttocks, and genitalia were more likely to be associated with presence of any dermatitis than facial and/or truncal lesions (P<.05). Treatment of the MCV infection did not appear to impact the course of AD when present, but prospective interventions would be needed to assess this issue.

Superinfection with methicillin-resistant and methicillin-sensitive Staphylococcus aureus as well as atypical giant lesions of the intertriginous neck, inner thighs, and buttocks also were noted, but AD was uncommon in these cases. Given the limited number of cases, statistical significance could not be assessed.
Comment
Cutaneous infections with Malassezia have been postulated to trigger AD in infancy,1 while systemic viral infections such as varicella-zoster virus may be protective against AD when acquired in younger children.7 It appears that MCV infection in young children (eg, 3 years or younger) with specific localization to the flexural areas has the potential to trigger AD in susceptible hosts. Larger studies are needed to chart the long-term disease course of AD in these children. Due to the small size of this study, it is unclear if the rise of MCV infections since the 1980s has contributed to increased AD.8 Susceptible children appear to have a family history of AD and localization of MCV lesions on the legs, buttocks, and antecubital region. Atopic dermatitis risk appears to be highest when MCV lesions are localized to intertriginous or flexural locations.
In addition to triggering the onset of AD, MCV infection also can trigger persistent flaring of AD, especially in the popliteal region and legs. Atopic dermatitis flares can occur at any age, but they appear to cluster in preschoolers and typically are not prevented by AD or MCV treatments; however, randomized trials are needed to identify if early intervention of MCV has a preventive benefit on AD onset or flares, and longer-term observation is needed to identify true disease course modification. Reduction of the number of MCV lesions previously has been demonstrated with institution of topical corticosteroid therapy.6 Therefore, institution of atopic skin care generally is advisable in the setting of MCV infection. Future studies should address the potential use of interventions to prevent the triggering of AD onset or flares in the setting of MCV infection in children.5
Molluscum contagiosum virus (MCV) is a common pediatric viral infection of the skin and/or mucous membranes.1 It has been noted in increasingly younger patient populations, ranging from congenital cases resulting from perinatal/vertical transmission to transmission from cobathing and pool usage.2,3
An association between MCV infection and atopic dermatitis (AD) has been reported to be caused by a predisposition to prolonged and severe cutaneous viral infections.4 However, the exact nature of the relationship between MCV and AD is unknown.
The purpose of this study was to identify pediatric patients with AD onset or flare of AD triggered by MCV infection as well as to characterize the setting under which MCV may trigger AD onset or flares in children.
Methods
Medical records for 50 children with prior or current MCV infection who presented sequentially to an outpatient pediatric dermatology practice over a 1-month period were identified. Institutional review board approval was obtained.
Results
The age range of the 50 patients with MCV infection was 1 to 13 years, with an average age of 3.6 years at the onset of infection (reported by parents/guardians) and 4.5 years at presentation to the pediatric dermatology office (Table 1).
The role of cobathing is unknown; however, 62% (31/50) of patients previously or currently cobathed at home, suggesting it may be a risk factor for MCV infection. An association of MCV lesions in the popliteal region trended toward being more likely with cobathing, but the association was not statistically significant.
Children with AD onset triggered by MCV infection statistically were more likely to have flexural localization of MCV and AD lesions and were statistically more likely to have a family history of AD (P<.04)(Table 2). Children with AD flares triggered by MCV infection were more likely to have MCV and AD lesions of the popliteal region and legs (P<.05)(Figure) and family history of AD (P<.04)(Table 3). Location of MCV lesions on the upper and lower extremities, buttocks, and genitalia were more likely to be associated with presence of any dermatitis than facial and/or truncal lesions (P<.05). Treatment of the MCV infection did not appear to impact the course of AD when present, but prospective interventions would be needed to assess this issue.

Superinfection with methicillin-resistant and methicillin-sensitive Staphylococcus aureus as well as atypical giant lesions of the intertriginous neck, inner thighs, and buttocks also were noted, but AD was uncommon in these cases. Given the limited number of cases, statistical significance could not be assessed.
Comment
Cutaneous infections with Malassezia have been postulated to trigger AD in infancy,1 while systemic viral infections such as varicella-zoster virus may be protective against AD when acquired in younger children.7 It appears that MCV infection in young children (eg, 3 years or younger) with specific localization to the flexural areas has the potential to trigger AD in susceptible hosts. Larger studies are needed to chart the long-term disease course of AD in these children. Due to the small size of this study, it is unclear if the rise of MCV infections since the 1980s has contributed to increased AD.8 Susceptible children appear to have a family history of AD and localization of MCV lesions on the legs, buttocks, and antecubital region. Atopic dermatitis risk appears to be highest when MCV lesions are localized to intertriginous or flexural locations.
In addition to triggering the onset of AD, MCV infection also can trigger persistent flaring of AD, especially in the popliteal region and legs. Atopic dermatitis flares can occur at any age, but they appear to cluster in preschoolers and typically are not prevented by AD or MCV treatments; however, randomized trials are needed to identify if early intervention of MCV has a preventive benefit on AD onset or flares, and longer-term observation is needed to identify true disease course modification. Reduction of the number of MCV lesions previously has been demonstrated with institution of topical corticosteroid therapy.6 Therefore, institution of atopic skin care generally is advisable in the setting of MCV infection. Future studies should address the potential use of interventions to prevent the triggering of AD onset or flares in the setting of MCV infection in children.5
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99.
- Connell CO, Oranje A, Van Gysel D, et al. Congenital molluscum contagiosum: report of four cases and review of the literature. Pediatr Dermatol. 2008;25:553-556.
- Luke JD, Silverberg NB. Vertically transmitted molluscum contagiosum infection. Pediatrics. 2010;125:E423-E425.
- Olsen JR, Piguet V, Gallacher J, et al. Molluscum contagiosum and associations with atopic eczema in children: a retrospective longitudinal study in primary care. Br J Gen Pract. 2016;66:E53-E58.
- Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32:353-357.
- Berger EM, Orlow SJ, Patel RR, et al. Experience with molluscum contagiosum and associated inflammatory reactions in a pediatric dermatology practice: the bump that rashes. Arch Dermatol. 2012;148:1257-1264.
- Silverberg JI, Norowitz KB, Kleiman E, et al. Association between varicella zoster virus infection and atopic dermatitis in early and late childhood: a case-control study. J Allergy Clin Immunol. 2010;126:300-305.
- Oriel JD. The increase in molluscum contagiosum. Br Med J (Clin Res Ed). 1987;294:74.
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99.
- Connell CO, Oranje A, Van Gysel D, et al. Congenital molluscum contagiosum: report of four cases and review of the literature. Pediatr Dermatol. 2008;25:553-556.
- Luke JD, Silverberg NB. Vertically transmitted molluscum contagiosum infection. Pediatrics. 2010;125:E423-E425.
- Olsen JR, Piguet V, Gallacher J, et al. Molluscum contagiosum and associations with atopic eczema in children: a retrospective longitudinal study in primary care. Br J Gen Pract. 2016;66:E53-E58.
- Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32:353-357.
- Berger EM, Orlow SJ, Patel RR, et al. Experience with molluscum contagiosum and associated inflammatory reactions in a pediatric dermatology practice: the bump that rashes. Arch Dermatol. 2012;148:1257-1264.
- Silverberg JI, Norowitz KB, Kleiman E, et al. Association between varicella zoster virus infection and atopic dermatitis in early and late childhood: a case-control study. J Allergy Clin Immunol. 2010;126:300-305.
- Oriel JD. The increase in molluscum contagiosum. Br Med J (Clin Res Ed). 1987;294:74.
Practice Points
- Molluscum contagiosum virus (MCV) infection appears to aggravate atopic dermatitis (AD) symptoms in a subset of pediatric patients.
- In susceptible children, the first onset of AD symptoms can occur during the course of MCV infection.