User login
SAN DIEGO – but glycemic control was similar after either treatment.
However, researchers have yet to directly compare bariatric surgery with new dual and even triple agonists that are in development.
The review by Shohinee Sarma, MD, MPH, and Patricia Palcu, MD, from the University of Toronto, was published in Obesity. Dr. Sarma also presented the findings virtually at the Obesity journal symposium at ObesityWeek® 2022.
Eric Ravussin, PhD, outgoing editor-in-chief of Obesity, explained to in an interview that this is one of five articles the editors chose from about 20 papers submitted for consideration for the symposium, and it was selected because it is a first review and meta-analysis of this direct comparison.
It showed that in “a straight head-to-head comparison, weight loss is larger by about 20 kg (44 lb) with bariatric surgery versus a GLP-1 agonist, but the improvement in glycemia (carbohydrate metabolism) was similar,” said Dr. Ravussin, from Pennington Biomedical Research Center, Louisiana State University, Baton Rouge.
Study limitations, which the authors also acknowledge, include that this was a small review of small studies: There were only six studies and 322 patients.
Moreover, the data are from 2007 to 2017, and newer weight-loss drugs are more potent.
Most studies in the review compared bariatric surgery with liraglutide, Dr. Ravussin noted, whereas, “we have now better GLP-1 agonists like semaglutide,” as well as drugs that are combinations of a GLP-1 agonist with another agonist or agonists.
“Tirzepatide, for example, which is a combination of a GLP-1 agonist and a [glucose-dependent insulinotropic polypeptide (GIP) agonist], is showing results that are very close to weight loss with bariatric surgery,” he observed.
There are quite a few other drugs in development, too, he continued, which are going to approach the weight loss obtained with bariatric surgery.
Novo Nordisk is coming out with a combination of an amylin analog (cagrilintide) and a GLP-1 agonist (semaglutide), he noted. “There are others coming in with GLP-1 and glucagon [dual agonists], and there is even a ... combo called triple G, which is a glucagon, GLP-1, and GIP [agonist].”
We now need a head-to-head comparison between bariatric surgery versus a combination drug like tirzepatide in a large population, he said.
“This is an exciting period,” Dr. Ravussin summarized, “because, 10 years ago, nobody thought that [results with] pharmacotherapy can approach bariatric surgery. Now we have other drugs that are still in development that are going to approach really close bariatric surgery.”
In an email to this news organization, Dr. Sarma noted that “due to the potent weight loss and glycemic benefits of GLP-1 agonists, patients who wish to avoid the risks of bariatric surgery may wish to discuss the option of medical therapy with their health professionals.”
“For next steps,” she said, “we need long-term studies comparing the weight-lowering, glycemic, and cardiovascular benefits of GLP-1 agonists in comparison to bariatric surgery for better counseling in obesity treatment.”
Three RCTs, three observational studies
The researchers searched the literature for randomized controlled trials (RCTs) and observational studies up to April 21, 2021, which directly compared absolute weight loss with a GLP-1 agonist – liraglutide, dulaglutide, semaglutide, exenatide, lixisenatide, and albiglutide (which are approved by the U.S. Food and Drug Administration or Health Canada) – versus any type of bariatric surgery including Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy, gastric banding, and biliopancreatic diversion.
The studies included patients aged 18 and older with a body mass index (BMI) greater than 25 kg/m2.
Secondary outcomes included change in BMI, and for patients with type 2 diabetes, change in A1c.
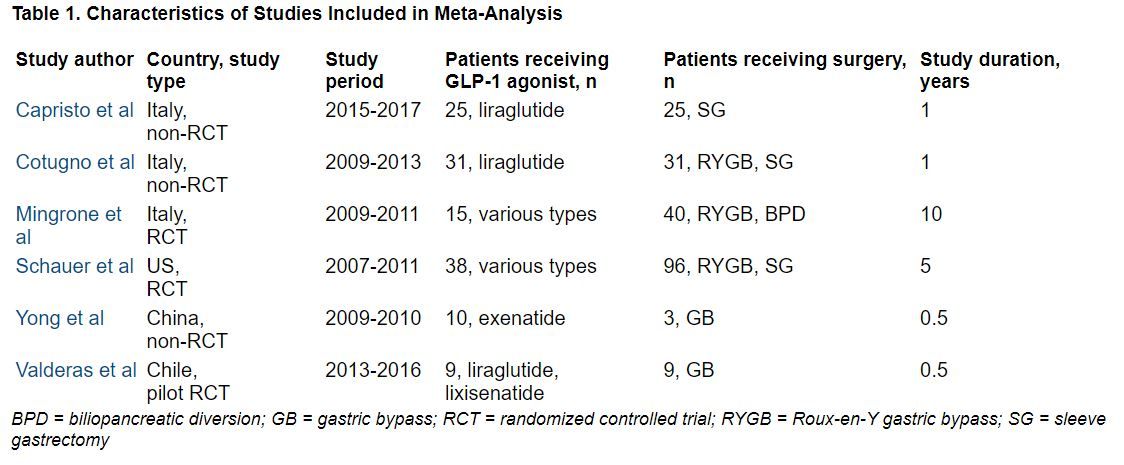
The researchers identified three RCTs and three observational studies, with diverse drugs and diverse types of bariatric surgery, which enrolled 13 to 134 patients, with follow-up from 6 months to 10 years.
During follow-up, the overall mean weight loss was 22.7 kg greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 25.1 kg greater in the two non-RCTs with these data (Capristo et al. and Cotugno et al.).
The overall mean decrease in BMI was 8.2 kg/m2 greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 10.6 kg/m2 greater in the three non-RCTs with these data.
The overall mean decrease in A1c was 1.28% lower in the three RCTs with these data, and it was 0.9% lower in the one non-RCT with these data.
“In adults with obesity, bariatric surgery still confers the highest reductions in weight and BMI but confers similar effects in glycemic control when compared with GLP-1 agonists,” the researchers summarize.
Dr. Sarma received funding from the Clinical Investigator Program. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – but glycemic control was similar after either treatment.
However, researchers have yet to directly compare bariatric surgery with new dual and even triple agonists that are in development.
The review by Shohinee Sarma, MD, MPH, and Patricia Palcu, MD, from the University of Toronto, was published in Obesity. Dr. Sarma also presented the findings virtually at the Obesity journal symposium at ObesityWeek® 2022.
Eric Ravussin, PhD, outgoing editor-in-chief of Obesity, explained to in an interview that this is one of five articles the editors chose from about 20 papers submitted for consideration for the symposium, and it was selected because it is a first review and meta-analysis of this direct comparison.
It showed that in “a straight head-to-head comparison, weight loss is larger by about 20 kg (44 lb) with bariatric surgery versus a GLP-1 agonist, but the improvement in glycemia (carbohydrate metabolism) was similar,” said Dr. Ravussin, from Pennington Biomedical Research Center, Louisiana State University, Baton Rouge.
Study limitations, which the authors also acknowledge, include that this was a small review of small studies: There were only six studies and 322 patients.
Moreover, the data are from 2007 to 2017, and newer weight-loss drugs are more potent.
Most studies in the review compared bariatric surgery with liraglutide, Dr. Ravussin noted, whereas, “we have now better GLP-1 agonists like semaglutide,” as well as drugs that are combinations of a GLP-1 agonist with another agonist or agonists.
“Tirzepatide, for example, which is a combination of a GLP-1 agonist and a [glucose-dependent insulinotropic polypeptide (GIP) agonist], is showing results that are very close to weight loss with bariatric surgery,” he observed.
There are quite a few other drugs in development, too, he continued, which are going to approach the weight loss obtained with bariatric surgery.
Novo Nordisk is coming out with a combination of an amylin analog (cagrilintide) and a GLP-1 agonist (semaglutide), he noted. “There are others coming in with GLP-1 and glucagon [dual agonists], and there is even a ... combo called triple G, which is a glucagon, GLP-1, and GIP [agonist].”
We now need a head-to-head comparison between bariatric surgery versus a combination drug like tirzepatide in a large population, he said.
“This is an exciting period,” Dr. Ravussin summarized, “because, 10 years ago, nobody thought that [results with] pharmacotherapy can approach bariatric surgery. Now we have other drugs that are still in development that are going to approach really close bariatric surgery.”
In an email to this news organization, Dr. Sarma noted that “due to the potent weight loss and glycemic benefits of GLP-1 agonists, patients who wish to avoid the risks of bariatric surgery may wish to discuss the option of medical therapy with their health professionals.”
“For next steps,” she said, “we need long-term studies comparing the weight-lowering, glycemic, and cardiovascular benefits of GLP-1 agonists in comparison to bariatric surgery for better counseling in obesity treatment.”
Three RCTs, three observational studies
The researchers searched the literature for randomized controlled trials (RCTs) and observational studies up to April 21, 2021, which directly compared absolute weight loss with a GLP-1 agonist – liraglutide, dulaglutide, semaglutide, exenatide, lixisenatide, and albiglutide (which are approved by the U.S. Food and Drug Administration or Health Canada) – versus any type of bariatric surgery including Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy, gastric banding, and biliopancreatic diversion.
The studies included patients aged 18 and older with a body mass index (BMI) greater than 25 kg/m2.
Secondary outcomes included change in BMI, and for patients with type 2 diabetes, change in A1c.
The researchers identified three RCTs and three observational studies, with diverse drugs and diverse types of bariatric surgery, which enrolled 13 to 134 patients, with follow-up from 6 months to 10 years.
During follow-up, the overall mean weight loss was 22.7 kg greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 25.1 kg greater in the two non-RCTs with these data (Capristo et al. and Cotugno et al.).
The overall mean decrease in BMI was 8.2 kg/m2 greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 10.6 kg/m2 greater in the three non-RCTs with these data.
The overall mean decrease in A1c was 1.28% lower in the three RCTs with these data, and it was 0.9% lower in the one non-RCT with these data.
“In adults with obesity, bariatric surgery still confers the highest reductions in weight and BMI but confers similar effects in glycemic control when compared with GLP-1 agonists,” the researchers summarize.
Dr. Sarma received funding from the Clinical Investigator Program. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – but glycemic control was similar after either treatment.
However, researchers have yet to directly compare bariatric surgery with new dual and even triple agonists that are in development.
The review by Shohinee Sarma, MD, MPH, and Patricia Palcu, MD, from the University of Toronto, was published in Obesity. Dr. Sarma also presented the findings virtually at the Obesity journal symposium at ObesityWeek® 2022.
Eric Ravussin, PhD, outgoing editor-in-chief of Obesity, explained to in an interview that this is one of five articles the editors chose from about 20 papers submitted for consideration for the symposium, and it was selected because it is a first review and meta-analysis of this direct comparison.
It showed that in “a straight head-to-head comparison, weight loss is larger by about 20 kg (44 lb) with bariatric surgery versus a GLP-1 agonist, but the improvement in glycemia (carbohydrate metabolism) was similar,” said Dr. Ravussin, from Pennington Biomedical Research Center, Louisiana State University, Baton Rouge.
Study limitations, which the authors also acknowledge, include that this was a small review of small studies: There were only six studies and 322 patients.
Moreover, the data are from 2007 to 2017, and newer weight-loss drugs are more potent.
Most studies in the review compared bariatric surgery with liraglutide, Dr. Ravussin noted, whereas, “we have now better GLP-1 agonists like semaglutide,” as well as drugs that are combinations of a GLP-1 agonist with another agonist or agonists.
“Tirzepatide, for example, which is a combination of a GLP-1 agonist and a [glucose-dependent insulinotropic polypeptide (GIP) agonist], is showing results that are very close to weight loss with bariatric surgery,” he observed.
There are quite a few other drugs in development, too, he continued, which are going to approach the weight loss obtained with bariatric surgery.
Novo Nordisk is coming out with a combination of an amylin analog (cagrilintide) and a GLP-1 agonist (semaglutide), he noted. “There are others coming in with GLP-1 and glucagon [dual agonists], and there is even a ... combo called triple G, which is a glucagon, GLP-1, and GIP [agonist].”
We now need a head-to-head comparison between bariatric surgery versus a combination drug like tirzepatide in a large population, he said.
“This is an exciting period,” Dr. Ravussin summarized, “because, 10 years ago, nobody thought that [results with] pharmacotherapy can approach bariatric surgery. Now we have other drugs that are still in development that are going to approach really close bariatric surgery.”
In an email to this news organization, Dr. Sarma noted that “due to the potent weight loss and glycemic benefits of GLP-1 agonists, patients who wish to avoid the risks of bariatric surgery may wish to discuss the option of medical therapy with their health professionals.”
“For next steps,” she said, “we need long-term studies comparing the weight-lowering, glycemic, and cardiovascular benefits of GLP-1 agonists in comparison to bariatric surgery for better counseling in obesity treatment.”
Three RCTs, three observational studies
The researchers searched the literature for randomized controlled trials (RCTs) and observational studies up to April 21, 2021, which directly compared absolute weight loss with a GLP-1 agonist – liraglutide, dulaglutide, semaglutide, exenatide, lixisenatide, and albiglutide (which are approved by the U.S. Food and Drug Administration or Health Canada) – versus any type of bariatric surgery including Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy, gastric banding, and biliopancreatic diversion.
The studies included patients aged 18 and older with a body mass index (BMI) greater than 25 kg/m2.
Secondary outcomes included change in BMI, and for patients with type 2 diabetes, change in A1c.
The researchers identified three RCTs and three observational studies, with diverse drugs and diverse types of bariatric surgery, which enrolled 13 to 134 patients, with follow-up from 6 months to 10 years.
During follow-up, the overall mean weight loss was 22.7 kg greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 25.1 kg greater in the two non-RCTs with these data (Capristo et al. and Cotugno et al.).
The overall mean decrease in BMI was 8.2 kg/m2 greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 10.6 kg/m2 greater in the three non-RCTs with these data.
The overall mean decrease in A1c was 1.28% lower in the three RCTs with these data, and it was 0.9% lower in the one non-RCT with these data.
“In adults with obesity, bariatric surgery still confers the highest reductions in weight and BMI but confers similar effects in glycemic control when compared with GLP-1 agonists,” the researchers summarize.
Dr. Sarma received funding from the Clinical Investigator Program. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT OBESITYWEEK®