User login
Extramammary Paget disease (EMPD), which was first described by Crocker1 in a patient with erythematous patches on the penis and scrotum, is morphologically identical to mammary Paget disease (MPD) of the nipple. The principal difference between EMPD and MPD is anatomic location. Extramammary Paget disease predominantly affects apocrine gland–bearing areas including the vulva, scrotum, and perianal areas. Although EMPD is not a common condition, it must be considered in the differential diagnosis for patients with chronic genital or perianal dermatitis. Primary EMPD must be distinguished from secondary epithelial involvement by an underlying invasive carcinoma that originates from sites such as the gastrointestinal or genitourinary systems (secondary EMPD).
Although multicentric primary EMPD is not uncommon among Eastern Asians, as there have been several reports in the literature from Japan,2-5 multicentric EMPD in white individuals is rare.6 We report a case of primary EMPD that was established when no underlying malignancies were detected.
Case Report
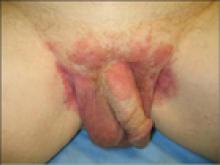
A 63-year-old white man presented to the dermatology clinic with a pruritic rash involving the groin of 8 years’ duration. Over-the-counter antifungal agents provided no improvement. Confluent erythematous and macerated plaques on the scrotum, shaft of the penis, bilateral inguinal areas, and perineum were noted on clinical examination (Figure 1). There also were well-demarcated, 4×5-cm, erythematous, velvety plaques on the right axilla and a small erythematous plaque on the left axilla. Systemic workup including colonoscopy, cystoscopy, and magnetic resonance imaging of the abdomen and pelvis were unremarkable. A positron emission tomography–computed tomography scan revealed foci of hypermetabolic activity in the bilateral inguinal nodes that were interpreted as evidence of an inflammatory process.
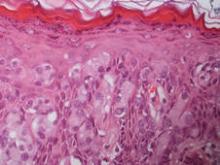
Fourteen biopsies were taken from the clinically involved regions in the genital area to delineate the extent of involvement. They all showed intraepidermal carcinoma characterized by large cells with clear cytoplasm and large hyperchromatic nuclei throughout the epidermis, which were more abundant in the basilar and lower portions of the epidermis (Figure 2). There was no evidence of underlying carcinoma in the dermis.
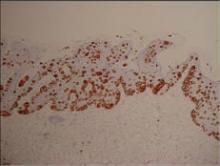
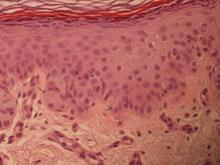
On immunohistochemical staining, the neoplastic cells were diffusely and strongly positive for cytokeratin 7 (Figure 3) and moderately positive for carcinoembryonic antigen, estrogen and progesterone receptors, and ERBB2 (formerly HER-2/neu). Additional immunostaining for cytokeratin 20 and gross cystic disease fluid protein 15 were negative. High-molecular-weight keratin staining highlighted background epithelium and spared neoplastic cells. Mucicarmine staining highlighted mucin within the cytoplasm of neoplastic cells. Ultrastructural studies showed evidence of apocrine differentiation in the neoplastic cells with deeply indented, bean-shaped nuclei and clear cytoplasm with inconspicuous organelles. Clinically unaffected skin from the periumbilical region also was biopsied. The histology demonstrated Toker cells characterized by clear cytoplasm, small vesicular nuclei, and the absence of hyperchromasia (Figure 4).
The patient was treated with 6 cycles of 5-aminolevulinic acid 20% photodynamic therapy but showed no improvement. Imiquimod cream 5% resulted in mild clinical improvement in the axillae but caused severe irritation in the groin and was discontinued after 3 months. A radiology consultation obtained 6 years prior to presentation determined that the risk associated with radiation therapy outweighed any possible benefits. Although localized disease persisted, repeated biopsies and positron emission tomography–computed tomography scans did not show any evidence of invasion or metastasis, respectively. Three years after initial presentation, groin lesions progressed to become more erythematous and vegetative. The corresponding histology demonstrated EMPD. An ancillary test (polymerase chain reaction analysis) for low- and high-risk human papillomavirus was negative.
Comment
Primary EMPD has been well documented in the literature to have a favorable prognosis if adequately treated. The few cases reported from Japan on EMPD without underlying adenocarcinoma had good outcomes.2,3 Further reports of multicentric EMPD involving 2 or 3 sites in the axillae and/or genital region did not reveal any progression to invasive carcinomas after adequate follow-up.3-9 Indeed, many of these cases, particularly those in the genital region, were aggressively treated with surgery, topical chemotherapy, immunomodulatory agents, and radiation; therefore, their natural course is not known.2 A Japanese studyconducted at 75 medical institutions (1987-1991) included 46 EMPD patients with multiple (ie, 2 or 3) sites of involvement.10 Some of the patients with a combination of genital and axillary lesions were followed without any treatment. None of the EMPD patients with axillary involvement developed invasive carcinoma after 4- to 12-year follow-up.10
Toker cells, which were first described in 1970,11 have been recognized as precursors to EMPD.12-14 Toker cells are intraepithelial cells with clear to pale-staining cytoplasm that are smaller in size than Paget cells but larger than neighboring keratinocytes. They are found in approximately 10% of normal nipples.11,14 Toker cells show vesicular chromatin, whereas Paget cells are hyperchromatic with prominent nucleoli.12 Willman et al15 examined 11 vulvectomies for the presence of Toker cells in association with mammarylike glands of the vulva. They demonstrated the presence of Toker cells in 4 (36%) of the samples.15 Additionally, Van der Putte et al16 observed Toker cells in an areolar lesion in a 47-year-old woman with MPD without underlying adenocarcinoma, suggesting that cases of MPD and EMPD confined to epithelial cells may be derived from Toker cells. Toker cells have been associated in the pathogenesis of 2 other benign entities, including clear cell papulosis17,18 and cutaneous hamartoma with pagetoid cells.19
Primary EMPD has the potential to develop in several regions of the skin that contain apocrine glands. Our patient presented with persistent genital lesions for many years without any concerns of axillary disease. Interestingly, biopsies from normal-appearing skin in the periumbilical region revealed clear cells (Figure 4). Likewise, Toker cells have been described in biopsies from clinically unaffected skin of the axillae in some cases where EMPD was identified in the genital regions.3,5 Genital lesions were the main clinical presentation and preceded axillary involvement in most instances.3,5,9 Therefore, biopsies from uninvolved apocrine sites (ie, axillary and periumbilical skin) should be considered in these patients. Based on our observation, we speculate that multicentric primary EMPD starts with Toker cell hyperplasia with a propensity to evolve into neoplasia in sites with apocrine or mammarylike glands.
Primary and secondary EMPD cannot be distinguished by histopathology and immunohistochemistry has a limited role.20,21 However, immunostaining with CDX2 (a caudal-type homeobox protein) might be helpful in differentiating primary EMPD from secondary EMPD extending from underlying anorectal adenocarcinomas.22 Primary EMPD has been treated with surgical excision.2,3,8 Despite the high recurrence rate after surgery, the prognosis for localized primary EMPD disease has been favorable.
Conclusion
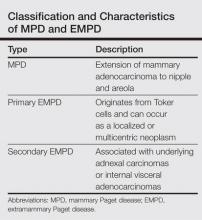
Our case suggests that Toker cell hyperplasia is a precursor to primary EMPD. In patients with established EMPD, other apocrine gland–bearing areas should be examined for multicentric disease. Lastly, the clinical course in our patient supports the hypothesis that primary multicentric EMPD has a favorable outcome. The Table lists the characteristics of MPD and EMPD and describes their development with respect to Toker cells. More studies are required to further outline the cytologic characteristics of Toker cells and to distinguish primary EMPD from secondary EMPD.
1. Crocker HR. Paget’s disease affecting the scrotum and penis. Trans Path Soc London. 1889;40:187-191.
2. Kawatsu T, Miki Y. Triple extramammary Paget’s disease. Arch Dermatol. 1971;104:316-319.
3. Makino T, Nakamura S, Nakayama H, et al. Genital Paget’s disease with clear cells in the epidermis of the axilla. J Cutan Pathol. 1998;25:568-571.
4. Inui S, Fukuhara S, Asada H, et al. Double involvement of extramammary Paget’s disease in the genitalia and axilla. J Dermatol. 2000;27:409-412.
5. Kitajima S, Yamamoto K, Tsuji T, et al. Triple extramammary Paget’s disease. Dermatol Surg. 1997;23:1035-1038.
6. Van Hamme C, Marot L, Dachelet C, et al. Paget’s extramammary disease of the axillae and perineum [in French]. Ann Dermatol Venereol. 2002;129(5, pt 1):717-719.
7. Kao GF, Graham JH, Helwig EB. Paget’s disease of the ectopic breast with an underlying intraductal carcinoma: report of a case. J Cutan Pathol. 1986;13:59-66.
8. Murrell TW Jr, McMullan FH. Extramammary Paget’s disease. a report of two cases. Arch Dermatol. 1962;85:600-613.
9. Koseki S, Mitsuhashi Y, Yoshikawa K, et al. A case of triple extramammary Paget’s disease. J Dermatol. 1997;24:535-538.
10. Ishihara K. Statistical study of extramammary Paget’s disease in Japan [in Japanese]. Skin Cancer. 1994;9:38.
11. Toker C. Clear cells of the nipple epidermis. Cancer. 1970;25:601-610.
12. Marucci G, Betts CM, Golouh R, et al. Toker cells are probably precursors of Paget cell carcinoma: a morphological and ultrastructural description. Virchows Archiv. 2002;441:117-123.
13. Belousova IE, Kazakov DV, Michal M, et al. Vulvar toker cells: the long-awaited missing link: a proposal for an origin-based histogenetic classification of extramammary Paget disease. Am J Dermatopathol. 2006;28:84-86.
14. Val-Bernal JF. Diego C, Rodriguez-Villar D, et al. The nipple-areola complex epidermis: a prospective systemic study in adult autopsies. Am J Dermatopathology. 2010;32:787-93.
15. Willman JH, Golitz LE, Fitzpatrick JE. Vulvar clear cells of Toker: precursors of extramammary Paget’s disease. Am J Dermatopathol. 2005;27:185-188.
16. Van der Putte SC, Toonstra J, Hennipman A. Mammary Paget’s disease confined to the areola and associated with multifocal Toker cell hyperplasia. Am J Dermatopathol. 1995;17:487-493.
17. Kuo TT, Chan HL, Hsueh S. Clear cell papulosis of the skin. a new entity with histogenetic implications for cutaneous Paget’s disease. Am J Surg Pathol. 1987;11:827-834.
18. Kuo TT, Huang CL, Chan HL, et al. Clear cell papulosis: report of three cases of a newly recognized disease. J Am Acad Dermatol. 1995;33(2, pt 1):230-233.
19. Piérard-Franchimont C, Dosal FL, Estrada JA, et al. Cutaneous hamartoma with pagetoid cells. Am J Dermatopathol. 1991;13:158-161.
20. Belcher RW. Extramammary Paget’s disease. enzyme histochemical and electron microscopic study. Arch Pathol. 1972;94:59-64.
21. Ordóñez NG, Awalt H, Mackay B. Mammary and extramammary Paget’s disease. an immunocytochemical and ultrastructural study. Cancer. 1987;59:1173-1183.
22. Perrotto J, Abbott JJ, Ceilley RI, et al. The role of immunohistochemistry in discriminating primary from secondary extramammary Paget disease. Am J Dermatopathol. 2010;32:137-143.
23. Jones RE Jr, Austin C, Ackerman AB. Extramammary Paget’s disease. a critical reexamination. Am J Dermatopathol. 1979;1:101-132.
Extramammary Paget disease (EMPD), which was first described by Crocker1 in a patient with erythematous patches on the penis and scrotum, is morphologically identical to mammary Paget disease (MPD) of the nipple. The principal difference between EMPD and MPD is anatomic location. Extramammary Paget disease predominantly affects apocrine gland–bearing areas including the vulva, scrotum, and perianal areas. Although EMPD is not a common condition, it must be considered in the differential diagnosis for patients with chronic genital or perianal dermatitis. Primary EMPD must be distinguished from secondary epithelial involvement by an underlying invasive carcinoma that originates from sites such as the gastrointestinal or genitourinary systems (secondary EMPD).
Although multicentric primary EMPD is not uncommon among Eastern Asians, as there have been several reports in the literature from Japan,2-5 multicentric EMPD in white individuals is rare.6 We report a case of primary EMPD that was established when no underlying malignancies were detected.
Case Report
A 63-year-old white man presented to the dermatology clinic with a pruritic rash involving the groin of 8 years’ duration. Over-the-counter antifungal agents provided no improvement. Confluent erythematous and macerated plaques on the scrotum, shaft of the penis, bilateral inguinal areas, and perineum were noted on clinical examination (Figure 1). There also were well-demarcated, 4×5-cm, erythematous, velvety plaques on the right axilla and a small erythematous plaque on the left axilla. Systemic workup including colonoscopy, cystoscopy, and magnetic resonance imaging of the abdomen and pelvis were unremarkable. A positron emission tomography–computed tomography scan revealed foci of hypermetabolic activity in the bilateral inguinal nodes that were interpreted as evidence of an inflammatory process.
Fourteen biopsies were taken from the clinically involved regions in the genital area to delineate the extent of involvement. They all showed intraepidermal carcinoma characterized by large cells with clear cytoplasm and large hyperchromatic nuclei throughout the epidermis, which were more abundant in the basilar and lower portions of the epidermis (Figure 2). There was no evidence of underlying carcinoma in the dermis.
On immunohistochemical staining, the neoplastic cells were diffusely and strongly positive for cytokeratin 7 (Figure 3) and moderately positive for carcinoembryonic antigen, estrogen and progesterone receptors, and ERBB2 (formerly HER-2/neu). Additional immunostaining for cytokeratin 20 and gross cystic disease fluid protein 15 were negative. High-molecular-weight keratin staining highlighted background epithelium and spared neoplastic cells. Mucicarmine staining highlighted mucin within the cytoplasm of neoplastic cells. Ultrastructural studies showed evidence of apocrine differentiation in the neoplastic cells with deeply indented, bean-shaped nuclei and clear cytoplasm with inconspicuous organelles. Clinically unaffected skin from the periumbilical region also was biopsied. The histology demonstrated Toker cells characterized by clear cytoplasm, small vesicular nuclei, and the absence of hyperchromasia (Figure 4).
The patient was treated with 6 cycles of 5-aminolevulinic acid 20% photodynamic therapy but showed no improvement. Imiquimod cream 5% resulted in mild clinical improvement in the axillae but caused severe irritation in the groin and was discontinued after 3 months. A radiology consultation obtained 6 years prior to presentation determined that the risk associated with radiation therapy outweighed any possible benefits. Although localized disease persisted, repeated biopsies and positron emission tomography–computed tomography scans did not show any evidence of invasion or metastasis, respectively. Three years after initial presentation, groin lesions progressed to become more erythematous and vegetative. The corresponding histology demonstrated EMPD. An ancillary test (polymerase chain reaction analysis) for low- and high-risk human papillomavirus was negative.
Comment
Primary EMPD has been well documented in the literature to have a favorable prognosis if adequately treated. The few cases reported from Japan on EMPD without underlying adenocarcinoma had good outcomes.2,3 Further reports of multicentric EMPD involving 2 or 3 sites in the axillae and/or genital region did not reveal any progression to invasive carcinomas after adequate follow-up.3-9 Indeed, many of these cases, particularly those in the genital region, were aggressively treated with surgery, topical chemotherapy, immunomodulatory agents, and radiation; therefore, their natural course is not known.2 A Japanese studyconducted at 75 medical institutions (1987-1991) included 46 EMPD patients with multiple (ie, 2 or 3) sites of involvement.10 Some of the patients with a combination of genital and axillary lesions were followed without any treatment. None of the EMPD patients with axillary involvement developed invasive carcinoma after 4- to 12-year follow-up.10
Toker cells, which were first described in 1970,11 have been recognized as precursors to EMPD.12-14 Toker cells are intraepithelial cells with clear to pale-staining cytoplasm that are smaller in size than Paget cells but larger than neighboring keratinocytes. They are found in approximately 10% of normal nipples.11,14 Toker cells show vesicular chromatin, whereas Paget cells are hyperchromatic with prominent nucleoli.12 Willman et al15 examined 11 vulvectomies for the presence of Toker cells in association with mammarylike glands of the vulva. They demonstrated the presence of Toker cells in 4 (36%) of the samples.15 Additionally, Van der Putte et al16 observed Toker cells in an areolar lesion in a 47-year-old woman with MPD without underlying adenocarcinoma, suggesting that cases of MPD and EMPD confined to epithelial cells may be derived from Toker cells. Toker cells have been associated in the pathogenesis of 2 other benign entities, including clear cell papulosis17,18 and cutaneous hamartoma with pagetoid cells.19
Primary EMPD has the potential to develop in several regions of the skin that contain apocrine glands. Our patient presented with persistent genital lesions for many years without any concerns of axillary disease. Interestingly, biopsies from normal-appearing skin in the periumbilical region revealed clear cells (Figure 4). Likewise, Toker cells have been described in biopsies from clinically unaffected skin of the axillae in some cases where EMPD was identified in the genital regions.3,5 Genital lesions were the main clinical presentation and preceded axillary involvement in most instances.3,5,9 Therefore, biopsies from uninvolved apocrine sites (ie, axillary and periumbilical skin) should be considered in these patients. Based on our observation, we speculate that multicentric primary EMPD starts with Toker cell hyperplasia with a propensity to evolve into neoplasia in sites with apocrine or mammarylike glands.
Primary and secondary EMPD cannot be distinguished by histopathology and immunohistochemistry has a limited role.20,21 However, immunostaining with CDX2 (a caudal-type homeobox protein) might be helpful in differentiating primary EMPD from secondary EMPD extending from underlying anorectal adenocarcinomas.22 Primary EMPD has been treated with surgical excision.2,3,8 Despite the high recurrence rate after surgery, the prognosis for localized primary EMPD disease has been favorable.
Conclusion
Our case suggests that Toker cell hyperplasia is a precursor to primary EMPD. In patients with established EMPD, other apocrine gland–bearing areas should be examined for multicentric disease. Lastly, the clinical course in our patient supports the hypothesis that primary multicentric EMPD has a favorable outcome. The Table lists the characteristics of MPD and EMPD and describes their development with respect to Toker cells. More studies are required to further outline the cytologic characteristics of Toker cells and to distinguish primary EMPD from secondary EMPD.
Extramammary Paget disease (EMPD), which was first described by Crocker1 in a patient with erythematous patches on the penis and scrotum, is morphologically identical to mammary Paget disease (MPD) of the nipple. The principal difference between EMPD and MPD is anatomic location. Extramammary Paget disease predominantly affects apocrine gland–bearing areas including the vulva, scrotum, and perianal areas. Although EMPD is not a common condition, it must be considered in the differential diagnosis for patients with chronic genital or perianal dermatitis. Primary EMPD must be distinguished from secondary epithelial involvement by an underlying invasive carcinoma that originates from sites such as the gastrointestinal or genitourinary systems (secondary EMPD).
Although multicentric primary EMPD is not uncommon among Eastern Asians, as there have been several reports in the literature from Japan,2-5 multicentric EMPD in white individuals is rare.6 We report a case of primary EMPD that was established when no underlying malignancies were detected.
Case Report
A 63-year-old white man presented to the dermatology clinic with a pruritic rash involving the groin of 8 years’ duration. Over-the-counter antifungal agents provided no improvement. Confluent erythematous and macerated plaques on the scrotum, shaft of the penis, bilateral inguinal areas, and perineum were noted on clinical examination (Figure 1). There also were well-demarcated, 4×5-cm, erythematous, velvety plaques on the right axilla and a small erythematous plaque on the left axilla. Systemic workup including colonoscopy, cystoscopy, and magnetic resonance imaging of the abdomen and pelvis were unremarkable. A positron emission tomography–computed tomography scan revealed foci of hypermetabolic activity in the bilateral inguinal nodes that were interpreted as evidence of an inflammatory process.
Fourteen biopsies were taken from the clinically involved regions in the genital area to delineate the extent of involvement. They all showed intraepidermal carcinoma characterized by large cells with clear cytoplasm and large hyperchromatic nuclei throughout the epidermis, which were more abundant in the basilar and lower portions of the epidermis (Figure 2). There was no evidence of underlying carcinoma in the dermis.
On immunohistochemical staining, the neoplastic cells were diffusely and strongly positive for cytokeratin 7 (Figure 3) and moderately positive for carcinoembryonic antigen, estrogen and progesterone receptors, and ERBB2 (formerly HER-2/neu). Additional immunostaining for cytokeratin 20 and gross cystic disease fluid protein 15 were negative. High-molecular-weight keratin staining highlighted background epithelium and spared neoplastic cells. Mucicarmine staining highlighted mucin within the cytoplasm of neoplastic cells. Ultrastructural studies showed evidence of apocrine differentiation in the neoplastic cells with deeply indented, bean-shaped nuclei and clear cytoplasm with inconspicuous organelles. Clinically unaffected skin from the periumbilical region also was biopsied. The histology demonstrated Toker cells characterized by clear cytoplasm, small vesicular nuclei, and the absence of hyperchromasia (Figure 4).
The patient was treated with 6 cycles of 5-aminolevulinic acid 20% photodynamic therapy but showed no improvement. Imiquimod cream 5% resulted in mild clinical improvement in the axillae but caused severe irritation in the groin and was discontinued after 3 months. A radiology consultation obtained 6 years prior to presentation determined that the risk associated with radiation therapy outweighed any possible benefits. Although localized disease persisted, repeated biopsies and positron emission tomography–computed tomography scans did not show any evidence of invasion or metastasis, respectively. Three years after initial presentation, groin lesions progressed to become more erythematous and vegetative. The corresponding histology demonstrated EMPD. An ancillary test (polymerase chain reaction analysis) for low- and high-risk human papillomavirus was negative.
Comment
Primary EMPD has been well documented in the literature to have a favorable prognosis if adequately treated. The few cases reported from Japan on EMPD without underlying adenocarcinoma had good outcomes.2,3 Further reports of multicentric EMPD involving 2 or 3 sites in the axillae and/or genital region did not reveal any progression to invasive carcinomas after adequate follow-up.3-9 Indeed, many of these cases, particularly those in the genital region, were aggressively treated with surgery, topical chemotherapy, immunomodulatory agents, and radiation; therefore, their natural course is not known.2 A Japanese studyconducted at 75 medical institutions (1987-1991) included 46 EMPD patients with multiple (ie, 2 or 3) sites of involvement.10 Some of the patients with a combination of genital and axillary lesions were followed without any treatment. None of the EMPD patients with axillary involvement developed invasive carcinoma after 4- to 12-year follow-up.10
Toker cells, which were first described in 1970,11 have been recognized as precursors to EMPD.12-14 Toker cells are intraepithelial cells with clear to pale-staining cytoplasm that are smaller in size than Paget cells but larger than neighboring keratinocytes. They are found in approximately 10% of normal nipples.11,14 Toker cells show vesicular chromatin, whereas Paget cells are hyperchromatic with prominent nucleoli.12 Willman et al15 examined 11 vulvectomies for the presence of Toker cells in association with mammarylike glands of the vulva. They demonstrated the presence of Toker cells in 4 (36%) of the samples.15 Additionally, Van der Putte et al16 observed Toker cells in an areolar lesion in a 47-year-old woman with MPD without underlying adenocarcinoma, suggesting that cases of MPD and EMPD confined to epithelial cells may be derived from Toker cells. Toker cells have been associated in the pathogenesis of 2 other benign entities, including clear cell papulosis17,18 and cutaneous hamartoma with pagetoid cells.19
Primary EMPD has the potential to develop in several regions of the skin that contain apocrine glands. Our patient presented with persistent genital lesions for many years without any concerns of axillary disease. Interestingly, biopsies from normal-appearing skin in the periumbilical region revealed clear cells (Figure 4). Likewise, Toker cells have been described in biopsies from clinically unaffected skin of the axillae in some cases where EMPD was identified in the genital regions.3,5 Genital lesions were the main clinical presentation and preceded axillary involvement in most instances.3,5,9 Therefore, biopsies from uninvolved apocrine sites (ie, axillary and periumbilical skin) should be considered in these patients. Based on our observation, we speculate that multicentric primary EMPD starts with Toker cell hyperplasia with a propensity to evolve into neoplasia in sites with apocrine or mammarylike glands.
Primary and secondary EMPD cannot be distinguished by histopathology and immunohistochemistry has a limited role.20,21 However, immunostaining with CDX2 (a caudal-type homeobox protein) might be helpful in differentiating primary EMPD from secondary EMPD extending from underlying anorectal adenocarcinomas.22 Primary EMPD has been treated with surgical excision.2,3,8 Despite the high recurrence rate after surgery, the prognosis for localized primary EMPD disease has been favorable.
Conclusion
Our case suggests that Toker cell hyperplasia is a precursor to primary EMPD. In patients with established EMPD, other apocrine gland–bearing areas should be examined for multicentric disease. Lastly, the clinical course in our patient supports the hypothesis that primary multicentric EMPD has a favorable outcome. The Table lists the characteristics of MPD and EMPD and describes their development with respect to Toker cells. More studies are required to further outline the cytologic characteristics of Toker cells and to distinguish primary EMPD from secondary EMPD.
1. Crocker HR. Paget’s disease affecting the scrotum and penis. Trans Path Soc London. 1889;40:187-191.
2. Kawatsu T, Miki Y. Triple extramammary Paget’s disease. Arch Dermatol. 1971;104:316-319.
3. Makino T, Nakamura S, Nakayama H, et al. Genital Paget’s disease with clear cells in the epidermis of the axilla. J Cutan Pathol. 1998;25:568-571.
4. Inui S, Fukuhara S, Asada H, et al. Double involvement of extramammary Paget’s disease in the genitalia and axilla. J Dermatol. 2000;27:409-412.
5. Kitajima S, Yamamoto K, Tsuji T, et al. Triple extramammary Paget’s disease. Dermatol Surg. 1997;23:1035-1038.
6. Van Hamme C, Marot L, Dachelet C, et al. Paget’s extramammary disease of the axillae and perineum [in French]. Ann Dermatol Venereol. 2002;129(5, pt 1):717-719.
7. Kao GF, Graham JH, Helwig EB. Paget’s disease of the ectopic breast with an underlying intraductal carcinoma: report of a case. J Cutan Pathol. 1986;13:59-66.
8. Murrell TW Jr, McMullan FH. Extramammary Paget’s disease. a report of two cases. Arch Dermatol. 1962;85:600-613.
9. Koseki S, Mitsuhashi Y, Yoshikawa K, et al. A case of triple extramammary Paget’s disease. J Dermatol. 1997;24:535-538.
10. Ishihara K. Statistical study of extramammary Paget’s disease in Japan [in Japanese]. Skin Cancer. 1994;9:38.
11. Toker C. Clear cells of the nipple epidermis. Cancer. 1970;25:601-610.
12. Marucci G, Betts CM, Golouh R, et al. Toker cells are probably precursors of Paget cell carcinoma: a morphological and ultrastructural description. Virchows Archiv. 2002;441:117-123.
13. Belousova IE, Kazakov DV, Michal M, et al. Vulvar toker cells: the long-awaited missing link: a proposal for an origin-based histogenetic classification of extramammary Paget disease. Am J Dermatopathol. 2006;28:84-86.
14. Val-Bernal JF. Diego C, Rodriguez-Villar D, et al. The nipple-areola complex epidermis: a prospective systemic study in adult autopsies. Am J Dermatopathology. 2010;32:787-93.
15. Willman JH, Golitz LE, Fitzpatrick JE. Vulvar clear cells of Toker: precursors of extramammary Paget’s disease. Am J Dermatopathol. 2005;27:185-188.
16. Van der Putte SC, Toonstra J, Hennipman A. Mammary Paget’s disease confined to the areola and associated with multifocal Toker cell hyperplasia. Am J Dermatopathol. 1995;17:487-493.
17. Kuo TT, Chan HL, Hsueh S. Clear cell papulosis of the skin. a new entity with histogenetic implications for cutaneous Paget’s disease. Am J Surg Pathol. 1987;11:827-834.
18. Kuo TT, Huang CL, Chan HL, et al. Clear cell papulosis: report of three cases of a newly recognized disease. J Am Acad Dermatol. 1995;33(2, pt 1):230-233.
19. Piérard-Franchimont C, Dosal FL, Estrada JA, et al. Cutaneous hamartoma with pagetoid cells. Am J Dermatopathol. 1991;13:158-161.
20. Belcher RW. Extramammary Paget’s disease. enzyme histochemical and electron microscopic study. Arch Pathol. 1972;94:59-64.
21. Ordóñez NG, Awalt H, Mackay B. Mammary and extramammary Paget’s disease. an immunocytochemical and ultrastructural study. Cancer. 1987;59:1173-1183.
22. Perrotto J, Abbott JJ, Ceilley RI, et al. The role of immunohistochemistry in discriminating primary from secondary extramammary Paget disease. Am J Dermatopathol. 2010;32:137-143.
23. Jones RE Jr, Austin C, Ackerman AB. Extramammary Paget’s disease. a critical reexamination. Am J Dermatopathol. 1979;1:101-132.
1. Crocker HR. Paget’s disease affecting the scrotum and penis. Trans Path Soc London. 1889;40:187-191.
2. Kawatsu T, Miki Y. Triple extramammary Paget’s disease. Arch Dermatol. 1971;104:316-319.
3. Makino T, Nakamura S, Nakayama H, et al. Genital Paget’s disease with clear cells in the epidermis of the axilla. J Cutan Pathol. 1998;25:568-571.
4. Inui S, Fukuhara S, Asada H, et al. Double involvement of extramammary Paget’s disease in the genitalia and axilla. J Dermatol. 2000;27:409-412.
5. Kitajima S, Yamamoto K, Tsuji T, et al. Triple extramammary Paget’s disease. Dermatol Surg. 1997;23:1035-1038.
6. Van Hamme C, Marot L, Dachelet C, et al. Paget’s extramammary disease of the axillae and perineum [in French]. Ann Dermatol Venereol. 2002;129(5, pt 1):717-719.
7. Kao GF, Graham JH, Helwig EB. Paget’s disease of the ectopic breast with an underlying intraductal carcinoma: report of a case. J Cutan Pathol. 1986;13:59-66.
8. Murrell TW Jr, McMullan FH. Extramammary Paget’s disease. a report of two cases. Arch Dermatol. 1962;85:600-613.
9. Koseki S, Mitsuhashi Y, Yoshikawa K, et al. A case of triple extramammary Paget’s disease. J Dermatol. 1997;24:535-538.
10. Ishihara K. Statistical study of extramammary Paget’s disease in Japan [in Japanese]. Skin Cancer. 1994;9:38.
11. Toker C. Clear cells of the nipple epidermis. Cancer. 1970;25:601-610.
12. Marucci G, Betts CM, Golouh R, et al. Toker cells are probably precursors of Paget cell carcinoma: a morphological and ultrastructural description. Virchows Archiv. 2002;441:117-123.
13. Belousova IE, Kazakov DV, Michal M, et al. Vulvar toker cells: the long-awaited missing link: a proposal for an origin-based histogenetic classification of extramammary Paget disease. Am J Dermatopathol. 2006;28:84-86.
14. Val-Bernal JF. Diego C, Rodriguez-Villar D, et al. The nipple-areola complex epidermis: a prospective systemic study in adult autopsies. Am J Dermatopathology. 2010;32:787-93.
15. Willman JH, Golitz LE, Fitzpatrick JE. Vulvar clear cells of Toker: precursors of extramammary Paget’s disease. Am J Dermatopathol. 2005;27:185-188.
16. Van der Putte SC, Toonstra J, Hennipman A. Mammary Paget’s disease confined to the areola and associated with multifocal Toker cell hyperplasia. Am J Dermatopathol. 1995;17:487-493.
17. Kuo TT, Chan HL, Hsueh S. Clear cell papulosis of the skin. a new entity with histogenetic implications for cutaneous Paget’s disease. Am J Surg Pathol. 1987;11:827-834.
18. Kuo TT, Huang CL, Chan HL, et al. Clear cell papulosis: report of three cases of a newly recognized disease. J Am Acad Dermatol. 1995;33(2, pt 1):230-233.
19. Piérard-Franchimont C, Dosal FL, Estrada JA, et al. Cutaneous hamartoma with pagetoid cells. Am J Dermatopathol. 1991;13:158-161.
20. Belcher RW. Extramammary Paget’s disease. enzyme histochemical and electron microscopic study. Arch Pathol. 1972;94:59-64.
21. Ordóñez NG, Awalt H, Mackay B. Mammary and extramammary Paget’s disease. an immunocytochemical and ultrastructural study. Cancer. 1987;59:1173-1183.
22. Perrotto J, Abbott JJ, Ceilley RI, et al. The role of immunohistochemistry in discriminating primary from secondary extramammary Paget disease. Am J Dermatopathol. 2010;32:137-143.
23. Jones RE Jr, Austin C, Ackerman AB. Extramammary Paget’s disease. a critical reexamination. Am J Dermatopathol. 1979;1:101-132.
Practice Points
- Toker cells are epithelial clear cells found in apocrine gland–bearing areas of the skin.
- Toker cell hyperplasia may be a precursor to primary extramammary Paget disease (EMPD).
- In patients with established EMPD, it is important to examine other parts of the skin with apocrine glands for multicentric disease.
- Primary multicentric EMPD has a favorable outcome.