User login
Box
1
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
Box
2
This icon represents the patient's case. Each paragraph that follows represents the discussant's thoughts.
A 67‐year‐old male presented to an outside hospital with a 1‐day history of fevers up to 39.4C, bilateral upper extremity weakness, and confusion. Forty‐eight hours prior to his presentation he had undergone uncomplicated bilateral carpal tunnel release surgery for the complaint of bilateral upper extremity paresthesias.
Bilateral carpal tunnel syndrome should prompt consideration of systemic diseases that infiltrate or impinge both canals (eg, rheumatoid arthritis, acromegaly, hypothyroidism, amyloidosis), although it is most frequently explained by a bilateral repetitive stress (eg, workplace typing). The development of upper extremity weakness suggests that an alternative condition such as cervical myelopathy, bilateral radiculopathy, or a rapidly progressive peripheral neuropathy may be responsible for his paresthesias. It would be unusual for a central nervous system process to selectively cause bilateral upper extremity weakness. Occasionally, patients emerge from surgery with limb weakness caused by peripheral nerve injury sustained from malpositioning of the extremity, but this would have been evident immediately following the operation.
Postoperative fevers are frequently unexplained, but require a search for common healthcare‐associated infections, such as pneumonia, urinary tract infection, intravenous catheter thrombophlebitis, wound infection, or Clostridium difficile colitis. However, such complications are unlikely following an ambulatory procedure. Confusion and fever together point to a central nervous system infection (meningoencephalitis or brain abscess) or a systemic infection that has impaired cognition. Malignancies can cause fever and altered mental status, but these are typically asynchronous events.
His past medical history was notable for hypertension, dyslipidemia, gout, actinic keratosis, and gastroesophageal reflux. His surgical history included bilateral knee replacements, repair of a left rotator cuff injury, and a herniorrhaphy. He was a nonsmoker who consumed 4 to 6 beers daily. His medications included clonidine, colchicine, atorvastatin, extended release metoprolol, triamterene‐hydrochlorothiazide, probenecid, and as‐needed ibuprofen and omeprazole.
Upon presentation he was cooperative and in no distress. Temperature was 38.9C, pulse 119 beats per minute, blood pressure 140/90 mm Hg, and oxygen saturation 94% on room air. He was noted to have logical thinking but impaired concentration. His upper extremity movement was restricted because of postoperative discomfort and swelling rather than true weakness. The rest of the exam was normal.
Metabolic, infectious, structural (intracranial), and toxic disorders can cause altered mental status. His heavy alcohol use puts him at risk for alcohol withdrawal and infections (such as Listeria meningitis), both of which may explain his fever and altered mental status. Signs and symptoms of meningitis are absent at this time. His knee prostheses could have harbored an infection preoperatively and therefore warrant close examination. Patients sometimes have adverse reactions to medications they have been prescribed but are not exposed to until hospitalization, although his surgical procedure was likely done on an outpatient basis. Empiric thiamine should be administered early given his confusion and alcohol habits.
Basic laboratories revealed a hemoglobin of 11.2 g/dL, white blood cell (WBC) count of 6,900/mm3 with 75% neutrophils, platelets of 206,000/mm3. Mean corpuscular volume was 97 mm3. Serum albumin was 2.4 g/dl, sodium 134 mmol/L, potassium 3.9 mmol/L, blood urea nitrogen 12 mg/dL, and creatinine 0.9 mg/dL. The aspartate aminotransferase was 93 U/L, alanine aminotransferase 73 U/L, alkaline phosphatase 254 U/L, and total bilirubin 1.0 mg/dL. Urinalysis was normal. Over the next 16 days fevers and waxing and waning mentation continued. The following studies were normal or negative: blood and urine cultures; transthoracic echocardiogram, antinuclear antibodies, hepatitis B surface antigen, hepatitis C antibody, and human immunodeficiency virus antibody; magnetic resonance imaging of the brain, electroencephalogram, and lower extremity venous ultrasound.
Hypoalbuminemia may signal chronic illness, hypoproduction from liver disease (caused by his heavy alcohol use), or losses from the kidney or gastrointestinal tract. His anemia may reflect chronic disease or point toward a specific underlying disorder. For example, fever and anemia could arise from hemolytic processes such as thrombotic thrombocytopenic purpura or clostridial infections.
An extensive workup has not revealed a cause for his prolonged fever (eg, infection, malignancy, autoimmune condition, or toxin). Likewise, an explanation for confusion is lacking. Because systemic illness and structural brain disease have not been uncovered, a lumbar puncture is indicated.
A lumbar puncture under fluoroscopic guidance revealed a cerebrospinal fluid (CSF) WBC count of 6/mm3, red blood cell count (RBC) 2255/mm3, protein 49 mg/dL, and glucose 54 mg/dL. The WBC differential was not reported. No growth was reported on bacterial cultures. Polymerase chain reactions for enterovirus and herpes simplex viruses 1 and 2 were negative. Cryptococcal antigen and Venereal Disease Research Laboratory serologies were also negative.
A CSF WBC count of 6 is out of the normal range, but could be explained by a traumatic tap given the elevated RBC; the protein and glucose are likewise at the border of normal. Collectively, these are nonspecific findings that could point to an infectious or noninfectious cause of intrathecal or paraspinous inflammation, but are not suggestive of bacterial meningitis.
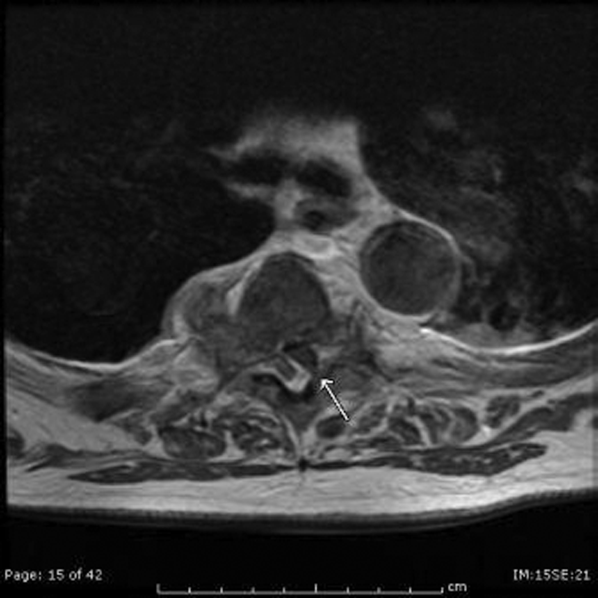
The patient developed pneumonia, for which he received ertapenem. On hospital day 17 he was intubated for hypoxia and respiratory distress and was extubated after 4 days of mechanical ventilation. Increasing weakness in all extremities prompted magnetic resonance imaging of the spine, which revealed fluid and enhancement involving the soft tissues around C3‐C4 and C5‐C6, raising concerns for discitis and osteomyelitis. Possible septic arthritis at the C3‐C4 and C4‐C5 facets was noted. Ring enhancing fluid collections from T2‐T8 compatible with an epidural abscess with cord compression at T4‐T5 and T6‐T7 were seen. Enhancement and fluid involving the facet joints between T2‐T7 was also consistent with septic arthritis (Figure 1).
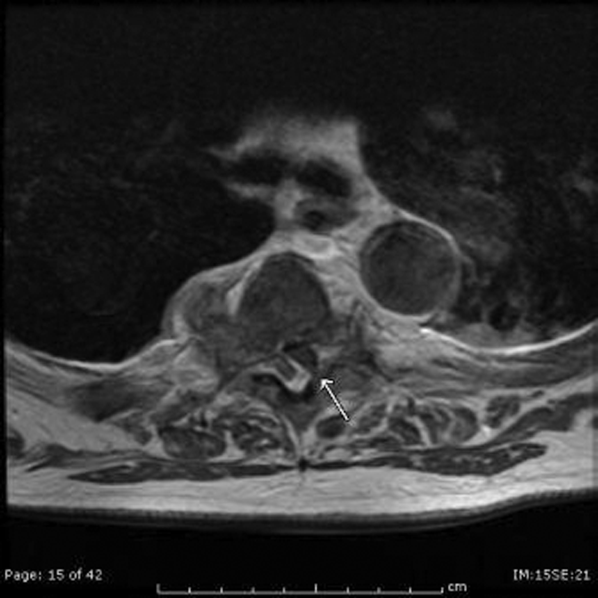
His pneumonia appears to have developed many days into his hospitalization, and therefore is unlikely to account for his initial fever and confusion. Blood cultures and echocardiogram have not suggested an endovascular infection that could account for such widespread vertebral and epidural deposition. A wide number of bacteria can cause epidural abscesses and septic arthritis, most commonly Staphylococcus aureus. Less common pathogens with a predilection for osteoarticular involvement, such as Brucella species, warrant consideration when there is appropriate epidemiologic risk.
Systemic bacterial infection remains a concern with his alcoholism rendering him partially immunosuppressed. However, a large number of adjacent spinal joints harboring a bacterial infection is unusual, and a working diagnosis of multilevel spinal infection, therefore, should prompt consideration of noninfectious processes. When a patient develops a swollen peripheral joint and fever in the postoperative setting, gout or pseudogout is a leading consideration. That same thinking should be applied to the vertebrae, where spinal gout can manifest. Surgery itself or associated changes in alcohol consumption patterns or changes in medications (at least 4 of which are relevant to goutcolchicine, hydrochlorothiazide, probenecid, and ibuprofen) could predispose him to a flare.
Aspiration of the epidural collection yielded a negative Gram stain and culture. He developed swelling in the bilateral proximal interphalangeal joints and was treated with steroids and colchicine for suspected gout flare. Vancomycin and piperacillin‐tazobactam were initiated, and on hospital day 22 the patient was transferred to another hospital for further evaluation by neurosurgery.
The negative Gram stain and culture argues against septic arthritis, but these are imperfect tests and will not detect atypical pathogens (eg, spinal tuberculosis). Reexamination of the aspirate for urate and calcium pyrophosphate crystals would be useful. Initiation of steroids in the setting of potentially undiagnosed infection requires a careful risk/benefit analysis. It may be reasonable to treat the patient with colchicine alone while withholding steroids and avoiding nonsteroidal agents in case invasive procedures are planned.
On exam his temperature was 36C, blood pressure 156/92 mm Hg, pulse 100 beats per minute, respirations 21 per minute, and oxygenation 97% on room air. He was not in acute distress and was only oriented to self. Bilateral 2+ lower extremity pitting edema up to the knees was noted. Examination of the heart and lungs was unremarkable. Gouty tophi were noted over both elbows. His joints were normal.
Cranial nerves IIXII were normal. Motor exam revealed normal muscle tone and bulk. Muscle strength was approximately 3/5 in the right upper extremity and 4+/5 in the left upper extremity. Bilateral lower extremity strength was 3/5 in hip flexion, knee flexion, and knee extension. Dorsiflexion and plantar flexion were approximately 2/5 bilaterally. Sensation was intact to light touch and pinprick, and proprioception was normal. Gait was not tested. A Foley catheter was in place.
This examination confirms ongoing encephalopathy and incomplete quadriplegia. The lower extremity weakness is nearly equal proximally and distally, which can be seen with an advanced peripheral neuropathy but is more characteristic of myelopathy. The expected concomitant sensory deficit of myelopathy is not present, although this may be difficult to detect in a confused patient. Reflex testing would help in distinguishing myelopathy (favored because of the imaging findings) from a rapid progressive peripheral motor neuropathy (eg, acute inflammatory demyelinating polyneuropathy or acute intermittent porphyria).
The pitting edema likely represents fluid overload, which can be nonspecific after prolonged immobility during hospitalization; hypoalbuminemia is oftentimes speculated to play a role when this develops. His alcohol use puts him at risk for heart failure (although there is no evidence of this on exam) and liver disease (which his liver function tests suggest). The tophi speak to the extent and chronicity of his hyperuricemia.
On arrival he reported recent onset diarrhea. Medications at transfer included metoprolol, omeprazole, prednisone, piperacillin/tazobactam, vancomycin, and colchicine; acetaminophen, bisacodyl, diphenhydramine, fentanyl, subcutaneous insulin, and labetalol were administered as needed. Laboratory studies included a hemoglobin of 9.5 g/dL, WBC count of 7,300/mm3 with 95% neutrophils, platelets 301,000/mm3, sodium 151 mmol/L, potassium 2.9 mmol/L, blood urea nitrogen 76 mg/dL, creatinine 2.0 mg/dL, aspartate aminotransferase 171 U/L, and alanine aminotransferase 127 U/L. Serum albumin was 1.7 g/dL.
At least 3 of his medicationsdiphenhydramine, fentanyl, and prednisonemay be contributing to his ongoing altered mental status, which may be further compounded by hypernatremia. Although his liver disease remains uncharacterized, hepatic encephalopathy may be contributing to his confusion as well.
Colchicine is likely responsible for his diarrhea, which would be the most readily available explanation for his hypernatremia, hypokalemia, and acute kidney injury (AKI). Acute kidney injury could result from progressive liver disease (hepatorenal syndrome), decreased arterial perfusion (suggested by third spacing or his diarrhea), acute tubular necrosis (from infection or medication), or urinary retention secondary to catheter obstruction. Acute hyperuricemia can also cause AKI (urate nephropathy).
Anemia has progressed and requires evaluation for blood loss as well as hemolysis. Hepatotoxicity from any of his medications (eg, acetaminophen) must be considered. Coagulation studies and review of the previous abdominal computed tomography would help determine the extent of his liver disease.
Neurosurgical consultation was obtained and the patient and his family elected to proceed with a thoracic laminectomy. Cheesy fluid was identified at the facet joints at T6‐T7, which was found to contain rare deposits of monosodium urate crystals. Surgical specimen cultures were sterile. His mental status and strength slowly improved to baseline following the surgery. He was discharged on postoperative day 7 to a rehabilitation facility. On the telephone follow‐up he reported that he has regained his strength completely.
The fluid analysis and clinical course confirms spinal gout. The presenting encephalopathy remains unexplained; I am unaware of gout leading to altered mental status.
COMMENTARY
Gout is an inflammatory condition triggered by the deposition of monosodium urate crystals in tissues in association with hyperuricemia.[1] Based on the 20072008 National Health and Nutrition Examination Survey, the prevalence of gout among US adults was 3.9% (8.3 million individuals).[2] These rates are increasing and are thought to be spurred by the aging population, increasing rates of obesity, and changing dietary habits including increases in the consumption of soft drinks and red meat.[3, 4, 5] The development of gout during hospitalization can prolong length of stay, and the implementation of a management protocol appears to help decrease treatment delays and the inappropriate discontinuation of gout prophylaxis.[6, 7] Surgery, with its associated physiologic stressors, can trigger gout, which is often polyarticular and presents with fever leading to testing and consultations for the febrile episode.[8]
Gout is an ancient disease that is familiar to most clinicians. In 1666, Daniel Sennert, a German physician, described gout as the physician's shame because of its infrequent recognition.[9] Clinical gout spans 3 stages: asymptomatic hyperuricemia, acute and intercritical gout, and chronic gouty arthritis. The typical acute presentation is monoarticular with the abrupt onset of pain, swelling, warmth, and erythema in a peripheral joint. It manifests most characteristically in the first metatarsophalangeal joint (podagra), but also frequently involves the midfoot, ankle, knee, and wrist and sometimes affects multiple joints simultaneously (polyarticular gout).[1, 10] The visualization of monosodium urate crystals either in synovial fluid or from a tophus is diagnostic of gout; however, guidelines recognize that a classic presentation of gout may be diagnosed based on clinical criteria alone.[11] Dual energy computerized tomography and ultrasonography are emerging as techniques for the visualization of monosodium urate crystals; however, they are not currently routinely recommended.[12]
There are many unusual presentations of gout, with an increase in such reports paralleling both the overall increase in the prevalence of gout and improvements in available imaging techniques.[13] Atypical presentations present diagnostic challenges and are often caused by tophaceous deposits in unusual locations. Reports of atypical gout have described entrapment neuropathies (eg, gouty deposits inducing carpal tunnel syndrome), ocular gout manifested as conjunctival deposits and uveitis, pancreatic gout presenting as a mass, and dermatologic manifestations including panniculitis.[13, 14]
Spinal gout (also known as axial gout) manifests when crystal‐induced inflammation, erosive arthritis, and tophaceous deposits occur along the spinal column. A cross‐sectional study of patients with poorly controlled gout reported the prevalence of spinal gout diagnosed by computerized tomography to be 35%. These radiographic findings were not consistently correlated with back pain.[15] Imaging features that are suggestive of spinal gout include intra‐articular and juxta‐articular erosions with sclerotic margins and density greater than the surrounding muscle. Periosteal new bone formation adjacent to bony destruction can form overhanging edges.[16] When retrospectively presented with the final diagnosis, the radiologist at our institution noted that the appearance was typical gout in an atypical location.
Spinal gout can be confused with spinal metastasis, infection, and stenosis. It can remain asymptomatic or present with back pain, radiculopathy, or cord compression. The lumbar spine is the most frequently affected site.[17, 18] Many patients with spinal gout have had chronic tophaceous gout with radiologic evidence of erosions in the peripheral joints.[15] Patients with spinal gout also have elevated urate levels and markers of inflammation.[18] Surgical decompression and stabilization is recommended when there is frank cord compression, progressive neurologic compromise, or lack of improvement with gout therapy alone.[18]
This patient's male gender, history of gout, hypertension, alcohol consumption, and thiazide diuretic use placed him at an increased risk of a gout attack.[19, 20] The possible interruption of urate‐lowering therapy for the surgical procedure and surgery itself further heightened his risk of suffering acute gouty arthritis in the perioperative period.[21] The patient's encephalopathy may have masked back pain and precluded an accurate neurologic exam. There is one case report to our knowledge describing encephalopathy that improved with colchicine and was possibly related to gout.[22] This patient's encephalopathy was deemed multifactorial and attributed to alcohol withdrawal, medications (including opioids and steroids), and infection (pneumonia).
Gout is best known for its peripheral arthritis and is rarely invoked in the consideration of spinal and myelopathic processes where more pressing competing diagnoses, such as infection and malignancy, are typically considered. In addition, when surgical specimens are submitted for examination for pathology in formaldehyde (rather than alcohol), monosodium urate crystals are dissolved and are thus difficult to identify in the specimen.
This case reminds us that gout remains a diagnostic challenge and should be considered in the differential of an inflammatory process. Recognition of the multifaceted nature of gout can allow for the earlier recognition and treatment of the less typical presentations of this ancient malady.
KEY TEACHING POINTS
- Crystalline disease is a common cause of postoperative arthritis.
- Gout (and pseudogout) should be considered in cases of focal inflammation (detected by examination or imaging) when the evidence or predisposition for infection is limited or nonexistent.
- Spinal gout presents with back pain, radiculopathy, or cord compression and may be confused with spinal metastasis, infection, and stenosis.
Acknowledgements
The authors thank Dr. Kari Waddell and Elaine Bammerlin for their assistance in the preparation of this manuscript.
Disclosure: Nothing to report.
- , . Clinical features and treatment of gout. In: Firestein GS, Budd RC, Gabriel SE, McInnes IB, O'Dell JR, eds. Kelley's Textbook of Rheumatology. Vol 2. 9th ed. Philadelphia, PA: Elsevier/Saunders; 2013:1544–1575.
- , , . Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63(10):3136–3141.
- , , , . Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol. 2004;31(8):1582–1587.
- , , , , . Purine‐rich foods, dairy and protein intake, and the risk of gout in men. New Engl J Med. 2004;350(11):1093–1103.
- , , . Fructose‐rich beverages and risk of gout in women. JAMA. 2010;304(20):2270–2278.
- , . Healthcare burden of in‐hospital gout. Intern Med J. 2012;42(11):1261–1263.
- , , , , , . Improved management of acute gout during hospitalization following introduction of a protocol. Int J Rheum Dis. 2012;15(6):512–520.
- , , . Postsurgical gout. Am Surg. 1995;61(1):56–59.
- , . Evolution of modern medicine. Arch Intern Med. 1960;105(4):640–644.
- . Clinical practice. Gout. N Engl J Med. 2011;364(5):443–452.
- . Management of gout: a 57‐year‐old man with a history of podagra, hyperuricemia, and mild renal insufficiency. JAMA. 2012;308(20):2133–2141.
- , , , et al. Diagnostic imaging of gout: comparison of high‐resolution US versus conventional X‐ray. Eur Radiol. 2008;18(3):621–630.
- , . The broad spectrum of urate crystal deposition: unusual presentations of gouty tophi. Semin Arthritis Rheum. 2012;42(2):146–154.
- , . Unusual clinical presentations of gout. Curr Opin Rheumatol. 2010;22(2):181–187.
- , , , , , . Correlates of axial gout: a cross‐sectional study. J Rheumatol. 2012;39(7):1445–1449.
- , , , , , . Axial gouty arthropathy. Am J Med Sci. 2009;338(2):140–146.
- , , . Axial (spinal) gout. Curr Rheumatol Rep. 2012;14(2):161–164.
- , , , . Spinal gout in a renal transplant patient: a case report and literature review. Surg Neurol. 2007;67(1):65–73.
- , , , et al. Alcohol consumption as a trigger of recurrent gout attacks. Am J Med. 2006;119(9):800.e11–800.e16.
- , , , , , . Recent diuretic use and the risk of recurrent gout attacks: the online case‐crossover gout study. J Rheumatol. 2006;33(7):1341–1345.
- , , , , . Clinical features and risk factors of postsurgical gout. Ann Rheum Dis. 2008;67(9):1271–1275.
- , , , . Gouty encephalopathy: myth or reality [in French]? Rev Med Interne. 1997;18(6):474–476.
Box
1
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
Box
2
This icon represents the patient's case. Each paragraph that follows represents the discussant's thoughts.
A 67‐year‐old male presented to an outside hospital with a 1‐day history of fevers up to 39.4C, bilateral upper extremity weakness, and confusion. Forty‐eight hours prior to his presentation he had undergone uncomplicated bilateral carpal tunnel release surgery for the complaint of bilateral upper extremity paresthesias.
Bilateral carpal tunnel syndrome should prompt consideration of systemic diseases that infiltrate or impinge both canals (eg, rheumatoid arthritis, acromegaly, hypothyroidism, amyloidosis), although it is most frequently explained by a bilateral repetitive stress (eg, workplace typing). The development of upper extremity weakness suggests that an alternative condition such as cervical myelopathy, bilateral radiculopathy, or a rapidly progressive peripheral neuropathy may be responsible for his paresthesias. It would be unusual for a central nervous system process to selectively cause bilateral upper extremity weakness. Occasionally, patients emerge from surgery with limb weakness caused by peripheral nerve injury sustained from malpositioning of the extremity, but this would have been evident immediately following the operation.
Postoperative fevers are frequently unexplained, but require a search for common healthcare‐associated infections, such as pneumonia, urinary tract infection, intravenous catheter thrombophlebitis, wound infection, or Clostridium difficile colitis. However, such complications are unlikely following an ambulatory procedure. Confusion and fever together point to a central nervous system infection (meningoencephalitis or brain abscess) or a systemic infection that has impaired cognition. Malignancies can cause fever and altered mental status, but these are typically asynchronous events.
His past medical history was notable for hypertension, dyslipidemia, gout, actinic keratosis, and gastroesophageal reflux. His surgical history included bilateral knee replacements, repair of a left rotator cuff injury, and a herniorrhaphy. He was a nonsmoker who consumed 4 to 6 beers daily. His medications included clonidine, colchicine, atorvastatin, extended release metoprolol, triamterene‐hydrochlorothiazide, probenecid, and as‐needed ibuprofen and omeprazole.
Upon presentation he was cooperative and in no distress. Temperature was 38.9C, pulse 119 beats per minute, blood pressure 140/90 mm Hg, and oxygen saturation 94% on room air. He was noted to have logical thinking but impaired concentration. His upper extremity movement was restricted because of postoperative discomfort and swelling rather than true weakness. The rest of the exam was normal.
Metabolic, infectious, structural (intracranial), and toxic disorders can cause altered mental status. His heavy alcohol use puts him at risk for alcohol withdrawal and infections (such as Listeria meningitis), both of which may explain his fever and altered mental status. Signs and symptoms of meningitis are absent at this time. His knee prostheses could have harbored an infection preoperatively and therefore warrant close examination. Patients sometimes have adverse reactions to medications they have been prescribed but are not exposed to until hospitalization, although his surgical procedure was likely done on an outpatient basis. Empiric thiamine should be administered early given his confusion and alcohol habits.
Basic laboratories revealed a hemoglobin of 11.2 g/dL, white blood cell (WBC) count of 6,900/mm3 with 75% neutrophils, platelets of 206,000/mm3. Mean corpuscular volume was 97 mm3. Serum albumin was 2.4 g/dl, sodium 134 mmol/L, potassium 3.9 mmol/L, blood urea nitrogen 12 mg/dL, and creatinine 0.9 mg/dL. The aspartate aminotransferase was 93 U/L, alanine aminotransferase 73 U/L, alkaline phosphatase 254 U/L, and total bilirubin 1.0 mg/dL. Urinalysis was normal. Over the next 16 days fevers and waxing and waning mentation continued. The following studies were normal or negative: blood and urine cultures; transthoracic echocardiogram, antinuclear antibodies, hepatitis B surface antigen, hepatitis C antibody, and human immunodeficiency virus antibody; magnetic resonance imaging of the brain, electroencephalogram, and lower extremity venous ultrasound.
Hypoalbuminemia may signal chronic illness, hypoproduction from liver disease (caused by his heavy alcohol use), or losses from the kidney or gastrointestinal tract. His anemia may reflect chronic disease or point toward a specific underlying disorder. For example, fever and anemia could arise from hemolytic processes such as thrombotic thrombocytopenic purpura or clostridial infections.
An extensive workup has not revealed a cause for his prolonged fever (eg, infection, malignancy, autoimmune condition, or toxin). Likewise, an explanation for confusion is lacking. Because systemic illness and structural brain disease have not been uncovered, a lumbar puncture is indicated.
A lumbar puncture under fluoroscopic guidance revealed a cerebrospinal fluid (CSF) WBC count of 6/mm3, red blood cell count (RBC) 2255/mm3, protein 49 mg/dL, and glucose 54 mg/dL. The WBC differential was not reported. No growth was reported on bacterial cultures. Polymerase chain reactions for enterovirus and herpes simplex viruses 1 and 2 were negative. Cryptococcal antigen and Venereal Disease Research Laboratory serologies were also negative.
A CSF WBC count of 6 is out of the normal range, but could be explained by a traumatic tap given the elevated RBC; the protein and glucose are likewise at the border of normal. Collectively, these are nonspecific findings that could point to an infectious or noninfectious cause of intrathecal or paraspinous inflammation, but are not suggestive of bacterial meningitis.
The patient developed pneumonia, for which he received ertapenem. On hospital day 17 he was intubated for hypoxia and respiratory distress and was extubated after 4 days of mechanical ventilation. Increasing weakness in all extremities prompted magnetic resonance imaging of the spine, which revealed fluid and enhancement involving the soft tissues around C3‐C4 and C5‐C6, raising concerns for discitis and osteomyelitis. Possible septic arthritis at the C3‐C4 and C4‐C5 facets was noted. Ring enhancing fluid collections from T2‐T8 compatible with an epidural abscess with cord compression at T4‐T5 and T6‐T7 were seen. Enhancement and fluid involving the facet joints between T2‐T7 was also consistent with septic arthritis (Figure 1).

His pneumonia appears to have developed many days into his hospitalization, and therefore is unlikely to account for his initial fever and confusion. Blood cultures and echocardiogram have not suggested an endovascular infection that could account for such widespread vertebral and epidural deposition. A wide number of bacteria can cause epidural abscesses and septic arthritis, most commonly Staphylococcus aureus. Less common pathogens with a predilection for osteoarticular involvement, such as Brucella species, warrant consideration when there is appropriate epidemiologic risk.
Systemic bacterial infection remains a concern with his alcoholism rendering him partially immunosuppressed. However, a large number of adjacent spinal joints harboring a bacterial infection is unusual, and a working diagnosis of multilevel spinal infection, therefore, should prompt consideration of noninfectious processes. When a patient develops a swollen peripheral joint and fever in the postoperative setting, gout or pseudogout is a leading consideration. That same thinking should be applied to the vertebrae, where spinal gout can manifest. Surgery itself or associated changes in alcohol consumption patterns or changes in medications (at least 4 of which are relevant to goutcolchicine, hydrochlorothiazide, probenecid, and ibuprofen) could predispose him to a flare.
Aspiration of the epidural collection yielded a negative Gram stain and culture. He developed swelling in the bilateral proximal interphalangeal joints and was treated with steroids and colchicine for suspected gout flare. Vancomycin and piperacillin‐tazobactam were initiated, and on hospital day 22 the patient was transferred to another hospital for further evaluation by neurosurgery.
The negative Gram stain and culture argues against septic arthritis, but these are imperfect tests and will not detect atypical pathogens (eg, spinal tuberculosis). Reexamination of the aspirate for urate and calcium pyrophosphate crystals would be useful. Initiation of steroids in the setting of potentially undiagnosed infection requires a careful risk/benefit analysis. It may be reasonable to treat the patient with colchicine alone while withholding steroids and avoiding nonsteroidal agents in case invasive procedures are planned.
On exam his temperature was 36C, blood pressure 156/92 mm Hg, pulse 100 beats per minute, respirations 21 per minute, and oxygenation 97% on room air. He was not in acute distress and was only oriented to self. Bilateral 2+ lower extremity pitting edema up to the knees was noted. Examination of the heart and lungs was unremarkable. Gouty tophi were noted over both elbows. His joints were normal.
Cranial nerves IIXII were normal. Motor exam revealed normal muscle tone and bulk. Muscle strength was approximately 3/5 in the right upper extremity and 4+/5 in the left upper extremity. Bilateral lower extremity strength was 3/5 in hip flexion, knee flexion, and knee extension. Dorsiflexion and plantar flexion were approximately 2/5 bilaterally. Sensation was intact to light touch and pinprick, and proprioception was normal. Gait was not tested. A Foley catheter was in place.
This examination confirms ongoing encephalopathy and incomplete quadriplegia. The lower extremity weakness is nearly equal proximally and distally, which can be seen with an advanced peripheral neuropathy but is more characteristic of myelopathy. The expected concomitant sensory deficit of myelopathy is not present, although this may be difficult to detect in a confused patient. Reflex testing would help in distinguishing myelopathy (favored because of the imaging findings) from a rapid progressive peripheral motor neuropathy (eg, acute inflammatory demyelinating polyneuropathy or acute intermittent porphyria).
The pitting edema likely represents fluid overload, which can be nonspecific after prolonged immobility during hospitalization; hypoalbuminemia is oftentimes speculated to play a role when this develops. His alcohol use puts him at risk for heart failure (although there is no evidence of this on exam) and liver disease (which his liver function tests suggest). The tophi speak to the extent and chronicity of his hyperuricemia.
On arrival he reported recent onset diarrhea. Medications at transfer included metoprolol, omeprazole, prednisone, piperacillin/tazobactam, vancomycin, and colchicine; acetaminophen, bisacodyl, diphenhydramine, fentanyl, subcutaneous insulin, and labetalol were administered as needed. Laboratory studies included a hemoglobin of 9.5 g/dL, WBC count of 7,300/mm3 with 95% neutrophils, platelets 301,000/mm3, sodium 151 mmol/L, potassium 2.9 mmol/L, blood urea nitrogen 76 mg/dL, creatinine 2.0 mg/dL, aspartate aminotransferase 171 U/L, and alanine aminotransferase 127 U/L. Serum albumin was 1.7 g/dL.
At least 3 of his medicationsdiphenhydramine, fentanyl, and prednisonemay be contributing to his ongoing altered mental status, which may be further compounded by hypernatremia. Although his liver disease remains uncharacterized, hepatic encephalopathy may be contributing to his confusion as well.
Colchicine is likely responsible for his diarrhea, which would be the most readily available explanation for his hypernatremia, hypokalemia, and acute kidney injury (AKI). Acute kidney injury could result from progressive liver disease (hepatorenal syndrome), decreased arterial perfusion (suggested by third spacing or his diarrhea), acute tubular necrosis (from infection or medication), or urinary retention secondary to catheter obstruction. Acute hyperuricemia can also cause AKI (urate nephropathy).
Anemia has progressed and requires evaluation for blood loss as well as hemolysis. Hepatotoxicity from any of his medications (eg, acetaminophen) must be considered. Coagulation studies and review of the previous abdominal computed tomography would help determine the extent of his liver disease.
Neurosurgical consultation was obtained and the patient and his family elected to proceed with a thoracic laminectomy. Cheesy fluid was identified at the facet joints at T6‐T7, which was found to contain rare deposits of monosodium urate crystals. Surgical specimen cultures were sterile. His mental status and strength slowly improved to baseline following the surgery. He was discharged on postoperative day 7 to a rehabilitation facility. On the telephone follow‐up he reported that he has regained his strength completely.
The fluid analysis and clinical course confirms spinal gout. The presenting encephalopathy remains unexplained; I am unaware of gout leading to altered mental status.
COMMENTARY
Gout is an inflammatory condition triggered by the deposition of monosodium urate crystals in tissues in association with hyperuricemia.[1] Based on the 20072008 National Health and Nutrition Examination Survey, the prevalence of gout among US adults was 3.9% (8.3 million individuals).[2] These rates are increasing and are thought to be spurred by the aging population, increasing rates of obesity, and changing dietary habits including increases in the consumption of soft drinks and red meat.[3, 4, 5] The development of gout during hospitalization can prolong length of stay, and the implementation of a management protocol appears to help decrease treatment delays and the inappropriate discontinuation of gout prophylaxis.[6, 7] Surgery, with its associated physiologic stressors, can trigger gout, which is often polyarticular and presents with fever leading to testing and consultations for the febrile episode.[8]
Gout is an ancient disease that is familiar to most clinicians. In 1666, Daniel Sennert, a German physician, described gout as the physician's shame because of its infrequent recognition.[9] Clinical gout spans 3 stages: asymptomatic hyperuricemia, acute and intercritical gout, and chronic gouty arthritis. The typical acute presentation is monoarticular with the abrupt onset of pain, swelling, warmth, and erythema in a peripheral joint. It manifests most characteristically in the first metatarsophalangeal joint (podagra), but also frequently involves the midfoot, ankle, knee, and wrist and sometimes affects multiple joints simultaneously (polyarticular gout).[1, 10] The visualization of monosodium urate crystals either in synovial fluid or from a tophus is diagnostic of gout; however, guidelines recognize that a classic presentation of gout may be diagnosed based on clinical criteria alone.[11] Dual energy computerized tomography and ultrasonography are emerging as techniques for the visualization of monosodium urate crystals; however, they are not currently routinely recommended.[12]
There are many unusual presentations of gout, with an increase in such reports paralleling both the overall increase in the prevalence of gout and improvements in available imaging techniques.[13] Atypical presentations present diagnostic challenges and are often caused by tophaceous deposits in unusual locations. Reports of atypical gout have described entrapment neuropathies (eg, gouty deposits inducing carpal tunnel syndrome), ocular gout manifested as conjunctival deposits and uveitis, pancreatic gout presenting as a mass, and dermatologic manifestations including panniculitis.[13, 14]
Spinal gout (also known as axial gout) manifests when crystal‐induced inflammation, erosive arthritis, and tophaceous deposits occur along the spinal column. A cross‐sectional study of patients with poorly controlled gout reported the prevalence of spinal gout diagnosed by computerized tomography to be 35%. These radiographic findings were not consistently correlated with back pain.[15] Imaging features that are suggestive of spinal gout include intra‐articular and juxta‐articular erosions with sclerotic margins and density greater than the surrounding muscle. Periosteal new bone formation adjacent to bony destruction can form overhanging edges.[16] When retrospectively presented with the final diagnosis, the radiologist at our institution noted that the appearance was typical gout in an atypical location.
Spinal gout can be confused with spinal metastasis, infection, and stenosis. It can remain asymptomatic or present with back pain, radiculopathy, or cord compression. The lumbar spine is the most frequently affected site.[17, 18] Many patients with spinal gout have had chronic tophaceous gout with radiologic evidence of erosions in the peripheral joints.[15] Patients with spinal gout also have elevated urate levels and markers of inflammation.[18] Surgical decompression and stabilization is recommended when there is frank cord compression, progressive neurologic compromise, or lack of improvement with gout therapy alone.[18]
This patient's male gender, history of gout, hypertension, alcohol consumption, and thiazide diuretic use placed him at an increased risk of a gout attack.[19, 20] The possible interruption of urate‐lowering therapy for the surgical procedure and surgery itself further heightened his risk of suffering acute gouty arthritis in the perioperative period.[21] The patient's encephalopathy may have masked back pain and precluded an accurate neurologic exam. There is one case report to our knowledge describing encephalopathy that improved with colchicine and was possibly related to gout.[22] This patient's encephalopathy was deemed multifactorial and attributed to alcohol withdrawal, medications (including opioids and steroids), and infection (pneumonia).
Gout is best known for its peripheral arthritis and is rarely invoked in the consideration of spinal and myelopathic processes where more pressing competing diagnoses, such as infection and malignancy, are typically considered. In addition, when surgical specimens are submitted for examination for pathology in formaldehyde (rather than alcohol), monosodium urate crystals are dissolved and are thus difficult to identify in the specimen.
This case reminds us that gout remains a diagnostic challenge and should be considered in the differential of an inflammatory process. Recognition of the multifaceted nature of gout can allow for the earlier recognition and treatment of the less typical presentations of this ancient malady.
KEY TEACHING POINTS
- Crystalline disease is a common cause of postoperative arthritis.
- Gout (and pseudogout) should be considered in cases of focal inflammation (detected by examination or imaging) when the evidence or predisposition for infection is limited or nonexistent.
- Spinal gout presents with back pain, radiculopathy, or cord compression and may be confused with spinal metastasis, infection, and stenosis.
Acknowledgements
The authors thank Dr. Kari Waddell and Elaine Bammerlin for their assistance in the preparation of this manuscript.
Disclosure: Nothing to report.
Box
1
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
Box
2
This icon represents the patient's case. Each paragraph that follows represents the discussant's thoughts.
A 67‐year‐old male presented to an outside hospital with a 1‐day history of fevers up to 39.4C, bilateral upper extremity weakness, and confusion. Forty‐eight hours prior to his presentation he had undergone uncomplicated bilateral carpal tunnel release surgery for the complaint of bilateral upper extremity paresthesias.
Bilateral carpal tunnel syndrome should prompt consideration of systemic diseases that infiltrate or impinge both canals (eg, rheumatoid arthritis, acromegaly, hypothyroidism, amyloidosis), although it is most frequently explained by a bilateral repetitive stress (eg, workplace typing). The development of upper extremity weakness suggests that an alternative condition such as cervical myelopathy, bilateral radiculopathy, or a rapidly progressive peripheral neuropathy may be responsible for his paresthesias. It would be unusual for a central nervous system process to selectively cause bilateral upper extremity weakness. Occasionally, patients emerge from surgery with limb weakness caused by peripheral nerve injury sustained from malpositioning of the extremity, but this would have been evident immediately following the operation.
Postoperative fevers are frequently unexplained, but require a search for common healthcare‐associated infections, such as pneumonia, urinary tract infection, intravenous catheter thrombophlebitis, wound infection, or Clostridium difficile colitis. However, such complications are unlikely following an ambulatory procedure. Confusion and fever together point to a central nervous system infection (meningoencephalitis or brain abscess) or a systemic infection that has impaired cognition. Malignancies can cause fever and altered mental status, but these are typically asynchronous events.
His past medical history was notable for hypertension, dyslipidemia, gout, actinic keratosis, and gastroesophageal reflux. His surgical history included bilateral knee replacements, repair of a left rotator cuff injury, and a herniorrhaphy. He was a nonsmoker who consumed 4 to 6 beers daily. His medications included clonidine, colchicine, atorvastatin, extended release metoprolol, triamterene‐hydrochlorothiazide, probenecid, and as‐needed ibuprofen and omeprazole.
Upon presentation he was cooperative and in no distress. Temperature was 38.9C, pulse 119 beats per minute, blood pressure 140/90 mm Hg, and oxygen saturation 94% on room air. He was noted to have logical thinking but impaired concentration. His upper extremity movement was restricted because of postoperative discomfort and swelling rather than true weakness. The rest of the exam was normal.
Metabolic, infectious, structural (intracranial), and toxic disorders can cause altered mental status. His heavy alcohol use puts him at risk for alcohol withdrawal and infections (such as Listeria meningitis), both of which may explain his fever and altered mental status. Signs and symptoms of meningitis are absent at this time. His knee prostheses could have harbored an infection preoperatively and therefore warrant close examination. Patients sometimes have adverse reactions to medications they have been prescribed but are not exposed to until hospitalization, although his surgical procedure was likely done on an outpatient basis. Empiric thiamine should be administered early given his confusion and alcohol habits.
Basic laboratories revealed a hemoglobin of 11.2 g/dL, white blood cell (WBC) count of 6,900/mm3 with 75% neutrophils, platelets of 206,000/mm3. Mean corpuscular volume was 97 mm3. Serum albumin was 2.4 g/dl, sodium 134 mmol/L, potassium 3.9 mmol/L, blood urea nitrogen 12 mg/dL, and creatinine 0.9 mg/dL. The aspartate aminotransferase was 93 U/L, alanine aminotransferase 73 U/L, alkaline phosphatase 254 U/L, and total bilirubin 1.0 mg/dL. Urinalysis was normal. Over the next 16 days fevers and waxing and waning mentation continued. The following studies were normal or negative: blood and urine cultures; transthoracic echocardiogram, antinuclear antibodies, hepatitis B surface antigen, hepatitis C antibody, and human immunodeficiency virus antibody; magnetic resonance imaging of the brain, electroencephalogram, and lower extremity venous ultrasound.
Hypoalbuminemia may signal chronic illness, hypoproduction from liver disease (caused by his heavy alcohol use), or losses from the kidney or gastrointestinal tract. His anemia may reflect chronic disease or point toward a specific underlying disorder. For example, fever and anemia could arise from hemolytic processes such as thrombotic thrombocytopenic purpura or clostridial infections.
An extensive workup has not revealed a cause for his prolonged fever (eg, infection, malignancy, autoimmune condition, or toxin). Likewise, an explanation for confusion is lacking. Because systemic illness and structural brain disease have not been uncovered, a lumbar puncture is indicated.
A lumbar puncture under fluoroscopic guidance revealed a cerebrospinal fluid (CSF) WBC count of 6/mm3, red blood cell count (RBC) 2255/mm3, protein 49 mg/dL, and glucose 54 mg/dL. The WBC differential was not reported. No growth was reported on bacterial cultures. Polymerase chain reactions for enterovirus and herpes simplex viruses 1 and 2 were negative. Cryptococcal antigen and Venereal Disease Research Laboratory serologies were also negative.
A CSF WBC count of 6 is out of the normal range, but could be explained by a traumatic tap given the elevated RBC; the protein and glucose are likewise at the border of normal. Collectively, these are nonspecific findings that could point to an infectious or noninfectious cause of intrathecal or paraspinous inflammation, but are not suggestive of bacterial meningitis.
The patient developed pneumonia, for which he received ertapenem. On hospital day 17 he was intubated for hypoxia and respiratory distress and was extubated after 4 days of mechanical ventilation. Increasing weakness in all extremities prompted magnetic resonance imaging of the spine, which revealed fluid and enhancement involving the soft tissues around C3‐C4 and C5‐C6, raising concerns for discitis and osteomyelitis. Possible septic arthritis at the C3‐C4 and C4‐C5 facets was noted. Ring enhancing fluid collections from T2‐T8 compatible with an epidural abscess with cord compression at T4‐T5 and T6‐T7 were seen. Enhancement and fluid involving the facet joints between T2‐T7 was also consistent with septic arthritis (Figure 1).

His pneumonia appears to have developed many days into his hospitalization, and therefore is unlikely to account for his initial fever and confusion. Blood cultures and echocardiogram have not suggested an endovascular infection that could account for such widespread vertebral and epidural deposition. A wide number of bacteria can cause epidural abscesses and septic arthritis, most commonly Staphylococcus aureus. Less common pathogens with a predilection for osteoarticular involvement, such as Brucella species, warrant consideration when there is appropriate epidemiologic risk.
Systemic bacterial infection remains a concern with his alcoholism rendering him partially immunosuppressed. However, a large number of adjacent spinal joints harboring a bacterial infection is unusual, and a working diagnosis of multilevel spinal infection, therefore, should prompt consideration of noninfectious processes. When a patient develops a swollen peripheral joint and fever in the postoperative setting, gout or pseudogout is a leading consideration. That same thinking should be applied to the vertebrae, where spinal gout can manifest. Surgery itself or associated changes in alcohol consumption patterns or changes in medications (at least 4 of which are relevant to goutcolchicine, hydrochlorothiazide, probenecid, and ibuprofen) could predispose him to a flare.
Aspiration of the epidural collection yielded a negative Gram stain and culture. He developed swelling in the bilateral proximal interphalangeal joints and was treated with steroids and colchicine for suspected gout flare. Vancomycin and piperacillin‐tazobactam were initiated, and on hospital day 22 the patient was transferred to another hospital for further evaluation by neurosurgery.
The negative Gram stain and culture argues against septic arthritis, but these are imperfect tests and will not detect atypical pathogens (eg, spinal tuberculosis). Reexamination of the aspirate for urate and calcium pyrophosphate crystals would be useful. Initiation of steroids in the setting of potentially undiagnosed infection requires a careful risk/benefit analysis. It may be reasonable to treat the patient with colchicine alone while withholding steroids and avoiding nonsteroidal agents in case invasive procedures are planned.
On exam his temperature was 36C, blood pressure 156/92 mm Hg, pulse 100 beats per minute, respirations 21 per minute, and oxygenation 97% on room air. He was not in acute distress and was only oriented to self. Bilateral 2+ lower extremity pitting edema up to the knees was noted. Examination of the heart and lungs was unremarkable. Gouty tophi were noted over both elbows. His joints were normal.
Cranial nerves IIXII were normal. Motor exam revealed normal muscle tone and bulk. Muscle strength was approximately 3/5 in the right upper extremity and 4+/5 in the left upper extremity. Bilateral lower extremity strength was 3/5 in hip flexion, knee flexion, and knee extension. Dorsiflexion and plantar flexion were approximately 2/5 bilaterally. Sensation was intact to light touch and pinprick, and proprioception was normal. Gait was not tested. A Foley catheter was in place.
This examination confirms ongoing encephalopathy and incomplete quadriplegia. The lower extremity weakness is nearly equal proximally and distally, which can be seen with an advanced peripheral neuropathy but is more characteristic of myelopathy. The expected concomitant sensory deficit of myelopathy is not present, although this may be difficult to detect in a confused patient. Reflex testing would help in distinguishing myelopathy (favored because of the imaging findings) from a rapid progressive peripheral motor neuropathy (eg, acute inflammatory demyelinating polyneuropathy or acute intermittent porphyria).
The pitting edema likely represents fluid overload, which can be nonspecific after prolonged immobility during hospitalization; hypoalbuminemia is oftentimes speculated to play a role when this develops. His alcohol use puts him at risk for heart failure (although there is no evidence of this on exam) and liver disease (which his liver function tests suggest). The tophi speak to the extent and chronicity of his hyperuricemia.
On arrival he reported recent onset diarrhea. Medications at transfer included metoprolol, omeprazole, prednisone, piperacillin/tazobactam, vancomycin, and colchicine; acetaminophen, bisacodyl, diphenhydramine, fentanyl, subcutaneous insulin, and labetalol were administered as needed. Laboratory studies included a hemoglobin of 9.5 g/dL, WBC count of 7,300/mm3 with 95% neutrophils, platelets 301,000/mm3, sodium 151 mmol/L, potassium 2.9 mmol/L, blood urea nitrogen 76 mg/dL, creatinine 2.0 mg/dL, aspartate aminotransferase 171 U/L, and alanine aminotransferase 127 U/L. Serum albumin was 1.7 g/dL.
At least 3 of his medicationsdiphenhydramine, fentanyl, and prednisonemay be contributing to his ongoing altered mental status, which may be further compounded by hypernatremia. Although his liver disease remains uncharacterized, hepatic encephalopathy may be contributing to his confusion as well.
Colchicine is likely responsible for his diarrhea, which would be the most readily available explanation for his hypernatremia, hypokalemia, and acute kidney injury (AKI). Acute kidney injury could result from progressive liver disease (hepatorenal syndrome), decreased arterial perfusion (suggested by third spacing or his diarrhea), acute tubular necrosis (from infection or medication), or urinary retention secondary to catheter obstruction. Acute hyperuricemia can also cause AKI (urate nephropathy).
Anemia has progressed and requires evaluation for blood loss as well as hemolysis. Hepatotoxicity from any of his medications (eg, acetaminophen) must be considered. Coagulation studies and review of the previous abdominal computed tomography would help determine the extent of his liver disease.
Neurosurgical consultation was obtained and the patient and his family elected to proceed with a thoracic laminectomy. Cheesy fluid was identified at the facet joints at T6‐T7, which was found to contain rare deposits of monosodium urate crystals. Surgical specimen cultures were sterile. His mental status and strength slowly improved to baseline following the surgery. He was discharged on postoperative day 7 to a rehabilitation facility. On the telephone follow‐up he reported that he has regained his strength completely.
The fluid analysis and clinical course confirms spinal gout. The presenting encephalopathy remains unexplained; I am unaware of gout leading to altered mental status.
COMMENTARY
Gout is an inflammatory condition triggered by the deposition of monosodium urate crystals in tissues in association with hyperuricemia.[1] Based on the 20072008 National Health and Nutrition Examination Survey, the prevalence of gout among US adults was 3.9% (8.3 million individuals).[2] These rates are increasing and are thought to be spurred by the aging population, increasing rates of obesity, and changing dietary habits including increases in the consumption of soft drinks and red meat.[3, 4, 5] The development of gout during hospitalization can prolong length of stay, and the implementation of a management protocol appears to help decrease treatment delays and the inappropriate discontinuation of gout prophylaxis.[6, 7] Surgery, with its associated physiologic stressors, can trigger gout, which is often polyarticular and presents with fever leading to testing and consultations for the febrile episode.[8]
Gout is an ancient disease that is familiar to most clinicians. In 1666, Daniel Sennert, a German physician, described gout as the physician's shame because of its infrequent recognition.[9] Clinical gout spans 3 stages: asymptomatic hyperuricemia, acute and intercritical gout, and chronic gouty arthritis. The typical acute presentation is monoarticular with the abrupt onset of pain, swelling, warmth, and erythema in a peripheral joint. It manifests most characteristically in the first metatarsophalangeal joint (podagra), but also frequently involves the midfoot, ankle, knee, and wrist and sometimes affects multiple joints simultaneously (polyarticular gout).[1, 10] The visualization of monosodium urate crystals either in synovial fluid or from a tophus is diagnostic of gout; however, guidelines recognize that a classic presentation of gout may be diagnosed based on clinical criteria alone.[11] Dual energy computerized tomography and ultrasonography are emerging as techniques for the visualization of monosodium urate crystals; however, they are not currently routinely recommended.[12]
There are many unusual presentations of gout, with an increase in such reports paralleling both the overall increase in the prevalence of gout and improvements in available imaging techniques.[13] Atypical presentations present diagnostic challenges and are often caused by tophaceous deposits in unusual locations. Reports of atypical gout have described entrapment neuropathies (eg, gouty deposits inducing carpal tunnel syndrome), ocular gout manifested as conjunctival deposits and uveitis, pancreatic gout presenting as a mass, and dermatologic manifestations including panniculitis.[13, 14]
Spinal gout (also known as axial gout) manifests when crystal‐induced inflammation, erosive arthritis, and tophaceous deposits occur along the spinal column. A cross‐sectional study of patients with poorly controlled gout reported the prevalence of spinal gout diagnosed by computerized tomography to be 35%. These radiographic findings were not consistently correlated with back pain.[15] Imaging features that are suggestive of spinal gout include intra‐articular and juxta‐articular erosions with sclerotic margins and density greater than the surrounding muscle. Periosteal new bone formation adjacent to bony destruction can form overhanging edges.[16] When retrospectively presented with the final diagnosis, the radiologist at our institution noted that the appearance was typical gout in an atypical location.
Spinal gout can be confused with spinal metastasis, infection, and stenosis. It can remain asymptomatic or present with back pain, radiculopathy, or cord compression. The lumbar spine is the most frequently affected site.[17, 18] Many patients with spinal gout have had chronic tophaceous gout with radiologic evidence of erosions in the peripheral joints.[15] Patients with spinal gout also have elevated urate levels and markers of inflammation.[18] Surgical decompression and stabilization is recommended when there is frank cord compression, progressive neurologic compromise, or lack of improvement with gout therapy alone.[18]
This patient's male gender, history of gout, hypertension, alcohol consumption, and thiazide diuretic use placed him at an increased risk of a gout attack.[19, 20] The possible interruption of urate‐lowering therapy for the surgical procedure and surgery itself further heightened his risk of suffering acute gouty arthritis in the perioperative period.[21] The patient's encephalopathy may have masked back pain and precluded an accurate neurologic exam. There is one case report to our knowledge describing encephalopathy that improved with colchicine and was possibly related to gout.[22] This patient's encephalopathy was deemed multifactorial and attributed to alcohol withdrawal, medications (including opioids and steroids), and infection (pneumonia).
Gout is best known for its peripheral arthritis and is rarely invoked in the consideration of spinal and myelopathic processes where more pressing competing diagnoses, such as infection and malignancy, are typically considered. In addition, when surgical specimens are submitted for examination for pathology in formaldehyde (rather than alcohol), monosodium urate crystals are dissolved and are thus difficult to identify in the specimen.
This case reminds us that gout remains a diagnostic challenge and should be considered in the differential of an inflammatory process. Recognition of the multifaceted nature of gout can allow for the earlier recognition and treatment of the less typical presentations of this ancient malady.
KEY TEACHING POINTS
- Crystalline disease is a common cause of postoperative arthritis.
- Gout (and pseudogout) should be considered in cases of focal inflammation (detected by examination or imaging) when the evidence or predisposition for infection is limited or nonexistent.
- Spinal gout presents with back pain, radiculopathy, or cord compression and may be confused with spinal metastasis, infection, and stenosis.
Acknowledgements
The authors thank Dr. Kari Waddell and Elaine Bammerlin for their assistance in the preparation of this manuscript.
Disclosure: Nothing to report.
- , . Clinical features and treatment of gout. In: Firestein GS, Budd RC, Gabriel SE, McInnes IB, O'Dell JR, eds. Kelley's Textbook of Rheumatology. Vol 2. 9th ed. Philadelphia, PA: Elsevier/Saunders; 2013:1544–1575.
- , , . Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63(10):3136–3141.
- , , , . Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol. 2004;31(8):1582–1587.
- , , , , . Purine‐rich foods, dairy and protein intake, and the risk of gout in men. New Engl J Med. 2004;350(11):1093–1103.
- , , . Fructose‐rich beverages and risk of gout in women. JAMA. 2010;304(20):2270–2278.
- , . Healthcare burden of in‐hospital gout. Intern Med J. 2012;42(11):1261–1263.
- , , , , , . Improved management of acute gout during hospitalization following introduction of a protocol. Int J Rheum Dis. 2012;15(6):512–520.
- , , . Postsurgical gout. Am Surg. 1995;61(1):56–59.
- , . Evolution of modern medicine. Arch Intern Med. 1960;105(4):640–644.
- . Clinical practice. Gout. N Engl J Med. 2011;364(5):443–452.
- . Management of gout: a 57‐year‐old man with a history of podagra, hyperuricemia, and mild renal insufficiency. JAMA. 2012;308(20):2133–2141.
- , , , et al. Diagnostic imaging of gout: comparison of high‐resolution US versus conventional X‐ray. Eur Radiol. 2008;18(3):621–630.
- , . The broad spectrum of urate crystal deposition: unusual presentations of gouty tophi. Semin Arthritis Rheum. 2012;42(2):146–154.
- , . Unusual clinical presentations of gout. Curr Opin Rheumatol. 2010;22(2):181–187.
- , , , , , . Correlates of axial gout: a cross‐sectional study. J Rheumatol. 2012;39(7):1445–1449.
- , , , , , . Axial gouty arthropathy. Am J Med Sci. 2009;338(2):140–146.
- , , . Axial (spinal) gout. Curr Rheumatol Rep. 2012;14(2):161–164.
- , , , . Spinal gout in a renal transplant patient: a case report and literature review. Surg Neurol. 2007;67(1):65–73.
- , , , et al. Alcohol consumption as a trigger of recurrent gout attacks. Am J Med. 2006;119(9):800.e11–800.e16.
- , , , , , . Recent diuretic use and the risk of recurrent gout attacks: the online case‐crossover gout study. J Rheumatol. 2006;33(7):1341–1345.
- , , , , . Clinical features and risk factors of postsurgical gout. Ann Rheum Dis. 2008;67(9):1271–1275.
- , , , . Gouty encephalopathy: myth or reality [in French]? Rev Med Interne. 1997;18(6):474–476.
- , . Clinical features and treatment of gout. In: Firestein GS, Budd RC, Gabriel SE, McInnes IB, O'Dell JR, eds. Kelley's Textbook of Rheumatology. Vol 2. 9th ed. Philadelphia, PA: Elsevier/Saunders; 2013:1544–1575.
- , , . Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63(10):3136–3141.
- , , , . Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol. 2004;31(8):1582–1587.
- , , , , . Purine‐rich foods, dairy and protein intake, and the risk of gout in men. New Engl J Med. 2004;350(11):1093–1103.
- , , . Fructose‐rich beverages and risk of gout in women. JAMA. 2010;304(20):2270–2278.
- , . Healthcare burden of in‐hospital gout. Intern Med J. 2012;42(11):1261–1263.
- , , , , , . Improved management of acute gout during hospitalization following introduction of a protocol. Int J Rheum Dis. 2012;15(6):512–520.
- , , . Postsurgical gout. Am Surg. 1995;61(1):56–59.
- , . Evolution of modern medicine. Arch Intern Med. 1960;105(4):640–644.
- . Clinical practice. Gout. N Engl J Med. 2011;364(5):443–452.
- . Management of gout: a 57‐year‐old man with a history of podagra, hyperuricemia, and mild renal insufficiency. JAMA. 2012;308(20):2133–2141.
- , , , et al. Diagnostic imaging of gout: comparison of high‐resolution US versus conventional X‐ray. Eur Radiol. 2008;18(3):621–630.
- , . The broad spectrum of urate crystal deposition: unusual presentations of gouty tophi. Semin Arthritis Rheum. 2012;42(2):146–154.
- , . Unusual clinical presentations of gout. Curr Opin Rheumatol. 2010;22(2):181–187.
- , , , , , . Correlates of axial gout: a cross‐sectional study. J Rheumatol. 2012;39(7):1445–1449.
- , , , , , . Axial gouty arthropathy. Am J Med Sci. 2009;338(2):140–146.
- , , . Axial (spinal) gout. Curr Rheumatol Rep. 2012;14(2):161–164.
- , , , . Spinal gout in a renal transplant patient: a case report and literature review. Surg Neurol. 2007;67(1):65–73.
- , , , et al. Alcohol consumption as a trigger of recurrent gout attacks. Am J Med. 2006;119(9):800.e11–800.e16.
- , , , , , . Recent diuretic use and the risk of recurrent gout attacks: the online case‐crossover gout study. J Rheumatol. 2006;33(7):1341–1345.
- , , , , . Clinical features and risk factors of postsurgical gout. Ann Rheum Dis. 2008;67(9):1271–1275.
- , , , . Gouty encephalopathy: myth or reality [in French]? Rev Med Interne. 1997;18(6):474–476.