User login
Atypical vascular lesions (AVLs) are rare flesh-colored, erythematous, or violaceous macules, patches, papules, or plaques that may occur following adjuvant radiation in breast cancer patients who have undergone conservative lumpectomy.1,2 They range in size from 1 mm to 6 cm and are most often confined to the radiation field. Presentation occurs 1 to 20 years following radiation, though the lesions most often present within 5 years.1,2 Although generally considered benign, 2 of 29 cases of AVLs progressed to angiosarcoma over a 5-year follow-up period in a retrospective clinicopathologic study.1
Atypical vascular lesions show considerable histologic and clinical overlap with radiation-induced angiosarcomas (RIAs), making differentiation between the two challenging.3,4 Mentzel et al5 compared benign, atypical, and malignant postradiation vascular lesions with nonradiation-associated angiosarcomas and found that RIAs were highly variable histopathologically, ranging from well differentiated to poorly differentiated, with atypia ranging from mild to severe. Radiation-induced angiosarcomas could be distinguished from AVLs and nonradiation-associated angiosarcomas by their oncogene amplification and protein expression profiles. Most strikingly, they found amplification of the MYC oncogene by fluorescence in situ hybridization in the nucleus of almost all the RIA cells, which was not seen in AVLs or nonradiation-associated angiosarcomas. Similarly, they found positive nuclear staining for MYC protein by immunohistochemistry in the nucleus of almost all cases of RIA but not in AVL or nonradiation-associated angiosarcomas, making MYC staining a useful diagnostic marker.5 In contrast, a study by Patton et al1 concluded that AVLs demonstrate morphologic patterns and clinical outcomes that suggest they are precursors of angiosarcoma rather than just markers of risk.
Atypical vascular lesions and RIAs usually follow a total radiation dose of 40 to 50 Gy, but RIAs typically are diagnosed later (approximately 10 years following exposure).6,7 Although RIAs are rare, they are known to be aggressive and often high grade, with a median survival of less than 5 years.6,7 Survival is poor even with radical surgical treatment.8 We present a patient with at least 29 AVLs following breast-conserving surgery and radiation and suggest the need for increased awareness of the elevated risk for RIA in patients with numerous benign AVLs.
Case Report
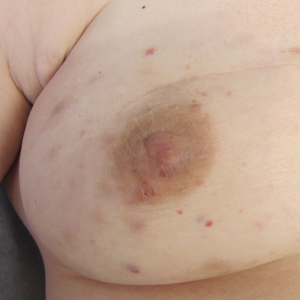
A 43-year-old woman with a history of breast cancer who underwent breast-conserving lumpectomy and adjuvant radiation presented to dermatology upon referral from surgical oncology for multiple lesions on the right breast (Figure 1). Seven years prior to presentation she was diagnosed with grade 3 poorly differentiated invasive ductal carcinoma with lobular features in the right breast that was positive for human epidermal growth factor receptor 2 but negative for estrogen or progesterone receptors. She was given neoadjuvant treatment with trastuzumab, docetaxel, and carboplatin prior to conservation lumpectomy with adjuvant radiation. She received a total dose of 50.4 Gy in 28 fractions of 1.8 Gy each over 1 month, with a final boost of 10 Gy in 5 fractions of 2 Gy, each with local skin irritation as the only concern posttreatment.
She initially presented to dermatology approximately 3 years after radiotherapy (5 years prior to current presentation) with lesions on the breast that had been present for 6 to 9 months. Physical examination showed 2 firm, painless, 4- to 5-mm papules on the right upper breast. The patient was reassured that the lesions were not suspicious for malignancy; however, 3 years later she presented to surgical oncology with 8 bluish papules or macules (all approximately 4 mm in diameter) on the right breast. These lesions were biopsied and examined by 2 institutions. Pathology of the initial punch biopsy favored a diagnosis of AVLs, though the possibility of RIA could not be ruled out without a complete excisional biopsy. Two excisional biopsies a month later were again consistent with AVLs. In all cases, the lesions were negative for MYC protein. The patient was again reassured but referred to dermatology for a second opinion.
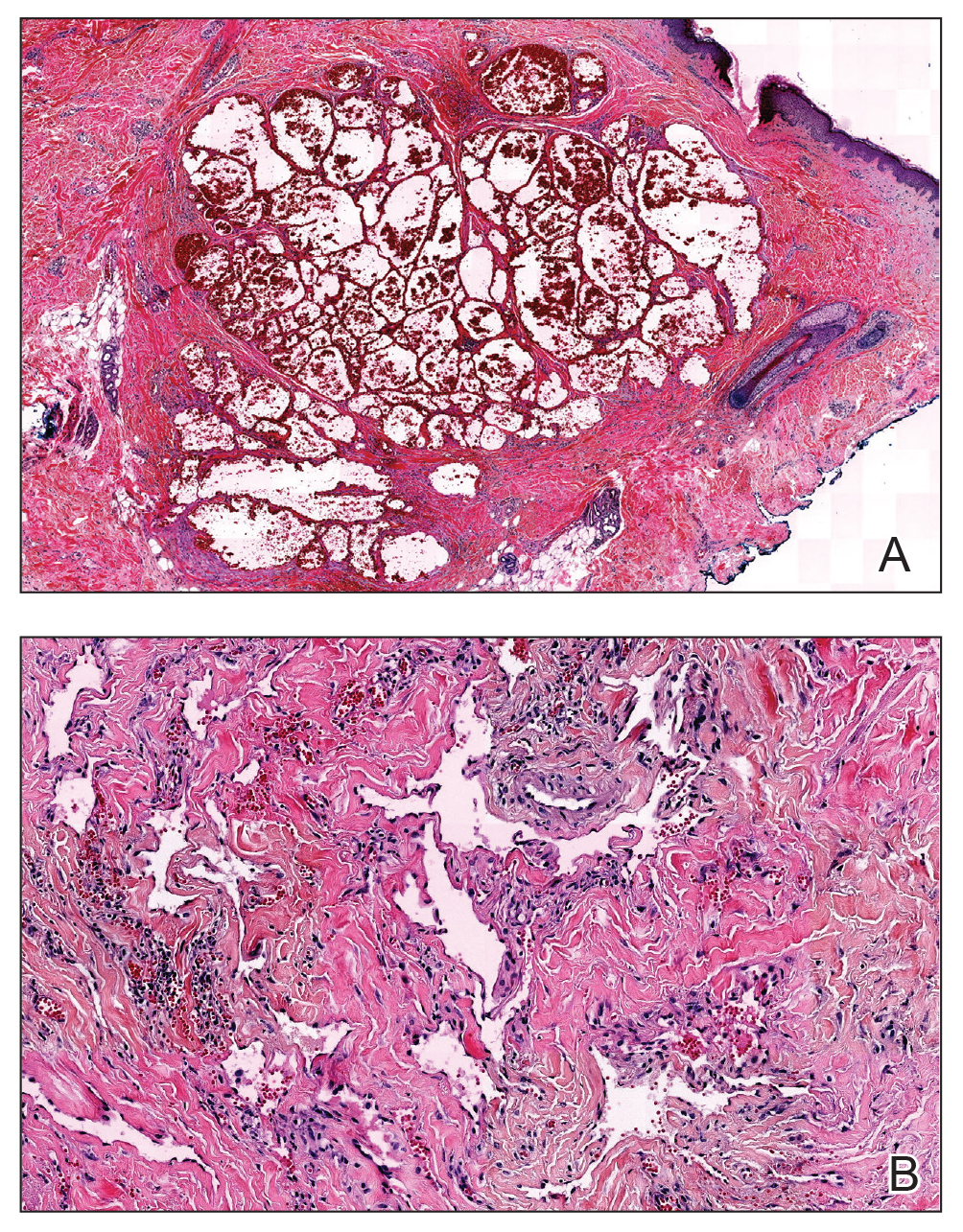
At the current presentation, physical examination showed at least 29 subcutaneous nodules on the right breast ranging in color from pink to deep blue to flesh colored with others more superficially hyperpigmented, possibly secondary to prior biopsy, and measuring 2 to 8 mm in diameter. Histopathologic examination of the biopsy specimens showed a vascular proliferation extending from the dermis into the subcutaneous tissue comprised of dilated and cavernous vascular channels lined by a single layer of endothelial cells with minimal cytologic atypia (Figure 2). There were focal areas of anastomosing slitlike vascular spaces dissecting dermal collagen. No features of malignancy, such as nuclear crowding, multilayering, or increased mitotic activity, were evident. Immunohistochemical studies for MYC protein were negative. The overall morphologic features and immunoprofile were felt to be most consistent with postradiation AVLs.
At the time, surgical oncology felt that the risk of radical mastectomy outweighed the risk of angiosarcoma due to the absence of frank angiosarcoma and the patient’s notable comorbidities, including diabetes mellitus, cerebrovascular disease, peripheral vascular disease, and smoking; however, after reviewing the literature and considering the difficulty of following such a large number of lesions, the dermatology team brought the patient’s case to the multidisciplinary cutaneous tumor board at the University of Massachusetts (Worcester, Massachusetts). In consensus, the tumor board recommended radical mastectomy despite the comorbidities, given her young age and the potential risk for malignant transformation of any one of the numerous AVLs to angiosarcoma.
Postmastectomy pathology showed multiple scattered foci of AVLs ranging from 1.5 to 4 mm in the dermis, similar to those seen on prior biopsies, with no frank evidence of RIA. At 3-year follow-up, the patient has had no recurrence of AVLs or findings suggestive of RIA. There were no reported complications.
Comment
Conservative breast cancer surgery and radiotherapy are becoming more prevalent for breast cancer treatment, thus the number of patients likely to present with AVLs has increased. These patients are at risk for transformation to RIAs.6 It is important for clinicians to be aware of the diagnosis of both AVLs and RIAs and their management given their more frequent presentation. In most cases, one or a few AVLs are present, and excision is the treatment of choice. In a retrospective study by Brenn and Fletcher3 examining 16 patients with AVLs and 26 patients with RIA, the majority of cases of AVL had a single lesion and the maximum number of AVLs was 4. One patient in their study had 30 AVLs (each 3–4 mm in diameter), and she was diagnosed with RIA.3 Our patient—with at least 29 identifiable AVL lesions—was felt to be at considerable risk for developing RIA, as the only other case reported with this many AVLs developed RIA.1 Given the large number of lesions, it was neither feasible to excise each one individually nor monitor all of them for malignant transformation.
Our case demonstrates the important role dermatologists may play in orchestrating care by a multispecialty team including oncology, radiation oncology, surgery, and plastic surgery. In our patient, a close examination of the literature by the dermatology team led to recognition of the potentially elevated risk for malignant transformation. The dermatology team also brought the case for review at the tumor board.
Although future studies are required to determine the relationship between AVL burden and the risk for progression to RIA, it is clear that a multidisciplinary approach and careful consideration of the current literature can prevent unnecessary morbidity and mortality for patients with this increasingly common problem.
- Patton KT, Deyrup AT, Weiss SW. Atypical vascular lesions after surgery and radiation of the breast: a clinicopathologic study of 32 cases analyzing histologic heterogeneity and association with angiosarcoma. Am J Surg Pathol. 2008;32:943-950.
- Mandrell J, Mehta S, McClure S. Atypical vascular lesion of the breast. J Am Acad Dermatol. 2010;63:337-340.
- Brenn T, Fletcher CD. Radiation-associated cutaneous atypical vascular lesions and angiosarcoma: clinicopathologic analysis of 42 cases. Am J Surg Pathol. 2005;29:983-996.
- Losch A, Chilek KD, Zirwas MJ. Post-radiation atypical vascular proliferation mimicking angiosarcoma eight months following breast-conserving therapy for breast carcinoma. J Clin Aesthet Dermatol. 2011;4:47-48.
- Mentzel T, Schildhaus HU, Palmedo G, et al. Postradiation cutaneous angiosarcoma after treatment of breast carcinoma is characterized by MYC amplification in contrast to atypical vascular lesions after radiotherapy and control cases: clinicopathological, immunohistochemical and molecular analysis of 66 cases. Mod Pathol. 2012;25:75-85.
- Tahir M, Hendry P, Baird L, et al. Radiation induced angiosarcoma a sequela of radiotherapy for breast cancer following conservative surgery. Int Semin Surg Oncol. 2006;3:26.
- Hillenbrand T, Menge F, Hohenberger P, et al. Primary and secondary angiosarcomas: a comparative single-center analysis. Clin Sarcoma Res. 2015;5:14.
- Seinen JM, Styring E, Verstappen V, et al. Radiation-associated angiosarcoma after breast cancer: high recurrence rate and poor survival despite surgical treatment with R0 resection. Ann Surg Oncol. 2012;19:2700-2706.
Atypical vascular lesions (AVLs) are rare flesh-colored, erythematous, or violaceous macules, patches, papules, or plaques that may occur following adjuvant radiation in breast cancer patients who have undergone conservative lumpectomy.1,2 They range in size from 1 mm to 6 cm and are most often confined to the radiation field. Presentation occurs 1 to 20 years following radiation, though the lesions most often present within 5 years.1,2 Although generally considered benign, 2 of 29 cases of AVLs progressed to angiosarcoma over a 5-year follow-up period in a retrospective clinicopathologic study.1
Atypical vascular lesions show considerable histologic and clinical overlap with radiation-induced angiosarcomas (RIAs), making differentiation between the two challenging.3,4 Mentzel et al5 compared benign, atypical, and malignant postradiation vascular lesions with nonradiation-associated angiosarcomas and found that RIAs were highly variable histopathologically, ranging from well differentiated to poorly differentiated, with atypia ranging from mild to severe. Radiation-induced angiosarcomas could be distinguished from AVLs and nonradiation-associated angiosarcomas by their oncogene amplification and protein expression profiles. Most strikingly, they found amplification of the MYC oncogene by fluorescence in situ hybridization in the nucleus of almost all the RIA cells, which was not seen in AVLs or nonradiation-associated angiosarcomas. Similarly, they found positive nuclear staining for MYC protein by immunohistochemistry in the nucleus of almost all cases of RIA but not in AVL or nonradiation-associated angiosarcomas, making MYC staining a useful diagnostic marker.5 In contrast, a study by Patton et al1 concluded that AVLs demonstrate morphologic patterns and clinical outcomes that suggest they are precursors of angiosarcoma rather than just markers of risk.
Atypical vascular lesions and RIAs usually follow a total radiation dose of 40 to 50 Gy, but RIAs typically are diagnosed later (approximately 10 years following exposure).6,7 Although RIAs are rare, they are known to be aggressive and often high grade, with a median survival of less than 5 years.6,7 Survival is poor even with radical surgical treatment.8 We present a patient with at least 29 AVLs following breast-conserving surgery and radiation and suggest the need for increased awareness of the elevated risk for RIA in patients with numerous benign AVLs.
Case Report
A 43-year-old woman with a history of breast cancer who underwent breast-conserving lumpectomy and adjuvant radiation presented to dermatology upon referral from surgical oncology for multiple lesions on the right breast (Figure 1). Seven years prior to presentation she was diagnosed with grade 3 poorly differentiated invasive ductal carcinoma with lobular features in the right breast that was positive for human epidermal growth factor receptor 2 but negative for estrogen or progesterone receptors. She was given neoadjuvant treatment with trastuzumab, docetaxel, and carboplatin prior to conservation lumpectomy with adjuvant radiation. She received a total dose of 50.4 Gy in 28 fractions of 1.8 Gy each over 1 month, with a final boost of 10 Gy in 5 fractions of 2 Gy, each with local skin irritation as the only concern posttreatment.
She initially presented to dermatology approximately 3 years after radiotherapy (5 years prior to current presentation) with lesions on the breast that had been present for 6 to 9 months. Physical examination showed 2 firm, painless, 4- to 5-mm papules on the right upper breast. The patient was reassured that the lesions were not suspicious for malignancy; however, 3 years later she presented to surgical oncology with 8 bluish papules or macules (all approximately 4 mm in diameter) on the right breast. These lesions were biopsied and examined by 2 institutions. Pathology of the initial punch biopsy favored a diagnosis of AVLs, though the possibility of RIA could not be ruled out without a complete excisional biopsy. Two excisional biopsies a month later were again consistent with AVLs. In all cases, the lesions were negative for MYC protein. The patient was again reassured but referred to dermatology for a second opinion.
At the current presentation, physical examination showed at least 29 subcutaneous nodules on the right breast ranging in color from pink to deep blue to flesh colored with others more superficially hyperpigmented, possibly secondary to prior biopsy, and measuring 2 to 8 mm in diameter. Histopathologic examination of the biopsy specimens showed a vascular proliferation extending from the dermis into the subcutaneous tissue comprised of dilated and cavernous vascular channels lined by a single layer of endothelial cells with minimal cytologic atypia (Figure 2). There were focal areas of anastomosing slitlike vascular spaces dissecting dermal collagen. No features of malignancy, such as nuclear crowding, multilayering, or increased mitotic activity, were evident. Immunohistochemical studies for MYC protein were negative. The overall morphologic features and immunoprofile were felt to be most consistent with postradiation AVLs.

At the time, surgical oncology felt that the risk of radical mastectomy outweighed the risk of angiosarcoma due to the absence of frank angiosarcoma and the patient’s notable comorbidities, including diabetes mellitus, cerebrovascular disease, peripheral vascular disease, and smoking; however, after reviewing the literature and considering the difficulty of following such a large number of lesions, the dermatology team brought the patient’s case to the multidisciplinary cutaneous tumor board at the University of Massachusetts (Worcester, Massachusetts). In consensus, the tumor board recommended radical mastectomy despite the comorbidities, given her young age and the potential risk for malignant transformation of any one of the numerous AVLs to angiosarcoma.
Postmastectomy pathology showed multiple scattered foci of AVLs ranging from 1.5 to 4 mm in the dermis, similar to those seen on prior biopsies, with no frank evidence of RIA. At 3-year follow-up, the patient has had no recurrence of AVLs or findings suggestive of RIA. There were no reported complications.
Comment
Conservative breast cancer surgery and radiotherapy are becoming more prevalent for breast cancer treatment, thus the number of patients likely to present with AVLs has increased. These patients are at risk for transformation to RIAs.6 It is important for clinicians to be aware of the diagnosis of both AVLs and RIAs and their management given their more frequent presentation. In most cases, one or a few AVLs are present, and excision is the treatment of choice. In a retrospective study by Brenn and Fletcher3 examining 16 patients with AVLs and 26 patients with RIA, the majority of cases of AVL had a single lesion and the maximum number of AVLs was 4. One patient in their study had 30 AVLs (each 3–4 mm in diameter), and she was diagnosed with RIA.3 Our patient—with at least 29 identifiable AVL lesions—was felt to be at considerable risk for developing RIA, as the only other case reported with this many AVLs developed RIA.1 Given the large number of lesions, it was neither feasible to excise each one individually nor monitor all of them for malignant transformation.
Our case demonstrates the important role dermatologists may play in orchestrating care by a multispecialty team including oncology, radiation oncology, surgery, and plastic surgery. In our patient, a close examination of the literature by the dermatology team led to recognition of the potentially elevated risk for malignant transformation. The dermatology team also brought the case for review at the tumor board.
Although future studies are required to determine the relationship between AVL burden and the risk for progression to RIA, it is clear that a multidisciplinary approach and careful consideration of the current literature can prevent unnecessary morbidity and mortality for patients with this increasingly common problem.
Atypical vascular lesions (AVLs) are rare flesh-colored, erythematous, or violaceous macules, patches, papules, or plaques that may occur following adjuvant radiation in breast cancer patients who have undergone conservative lumpectomy.1,2 They range in size from 1 mm to 6 cm and are most often confined to the radiation field. Presentation occurs 1 to 20 years following radiation, though the lesions most often present within 5 years.1,2 Although generally considered benign, 2 of 29 cases of AVLs progressed to angiosarcoma over a 5-year follow-up period in a retrospective clinicopathologic study.1
Atypical vascular lesions show considerable histologic and clinical overlap with radiation-induced angiosarcomas (RIAs), making differentiation between the two challenging.3,4 Mentzel et al5 compared benign, atypical, and malignant postradiation vascular lesions with nonradiation-associated angiosarcomas and found that RIAs were highly variable histopathologically, ranging from well differentiated to poorly differentiated, with atypia ranging from mild to severe. Radiation-induced angiosarcomas could be distinguished from AVLs and nonradiation-associated angiosarcomas by their oncogene amplification and protein expression profiles. Most strikingly, they found amplification of the MYC oncogene by fluorescence in situ hybridization in the nucleus of almost all the RIA cells, which was not seen in AVLs or nonradiation-associated angiosarcomas. Similarly, they found positive nuclear staining for MYC protein by immunohistochemistry in the nucleus of almost all cases of RIA but not in AVL or nonradiation-associated angiosarcomas, making MYC staining a useful diagnostic marker.5 In contrast, a study by Patton et al1 concluded that AVLs demonstrate morphologic patterns and clinical outcomes that suggest they are precursors of angiosarcoma rather than just markers of risk.
Atypical vascular lesions and RIAs usually follow a total radiation dose of 40 to 50 Gy, but RIAs typically are diagnosed later (approximately 10 years following exposure).6,7 Although RIAs are rare, they are known to be aggressive and often high grade, with a median survival of less than 5 years.6,7 Survival is poor even with radical surgical treatment.8 We present a patient with at least 29 AVLs following breast-conserving surgery and radiation and suggest the need for increased awareness of the elevated risk for RIA in patients with numerous benign AVLs.
Case Report
A 43-year-old woman with a history of breast cancer who underwent breast-conserving lumpectomy and adjuvant radiation presented to dermatology upon referral from surgical oncology for multiple lesions on the right breast (Figure 1). Seven years prior to presentation she was diagnosed with grade 3 poorly differentiated invasive ductal carcinoma with lobular features in the right breast that was positive for human epidermal growth factor receptor 2 but negative for estrogen or progesterone receptors. She was given neoadjuvant treatment with trastuzumab, docetaxel, and carboplatin prior to conservation lumpectomy with adjuvant radiation. She received a total dose of 50.4 Gy in 28 fractions of 1.8 Gy each over 1 month, with a final boost of 10 Gy in 5 fractions of 2 Gy, each with local skin irritation as the only concern posttreatment.
She initially presented to dermatology approximately 3 years after radiotherapy (5 years prior to current presentation) with lesions on the breast that had been present for 6 to 9 months. Physical examination showed 2 firm, painless, 4- to 5-mm papules on the right upper breast. The patient was reassured that the lesions were not suspicious for malignancy; however, 3 years later she presented to surgical oncology with 8 bluish papules or macules (all approximately 4 mm in diameter) on the right breast. These lesions were biopsied and examined by 2 institutions. Pathology of the initial punch biopsy favored a diagnosis of AVLs, though the possibility of RIA could not be ruled out without a complete excisional biopsy. Two excisional biopsies a month later were again consistent with AVLs. In all cases, the lesions were negative for MYC protein. The patient was again reassured but referred to dermatology for a second opinion.
At the current presentation, physical examination showed at least 29 subcutaneous nodules on the right breast ranging in color from pink to deep blue to flesh colored with others more superficially hyperpigmented, possibly secondary to prior biopsy, and measuring 2 to 8 mm in diameter. Histopathologic examination of the biopsy specimens showed a vascular proliferation extending from the dermis into the subcutaneous tissue comprised of dilated and cavernous vascular channels lined by a single layer of endothelial cells with minimal cytologic atypia (Figure 2). There were focal areas of anastomosing slitlike vascular spaces dissecting dermal collagen. No features of malignancy, such as nuclear crowding, multilayering, or increased mitotic activity, were evident. Immunohistochemical studies for MYC protein were negative. The overall morphologic features and immunoprofile were felt to be most consistent with postradiation AVLs.

At the time, surgical oncology felt that the risk of radical mastectomy outweighed the risk of angiosarcoma due to the absence of frank angiosarcoma and the patient’s notable comorbidities, including diabetes mellitus, cerebrovascular disease, peripheral vascular disease, and smoking; however, after reviewing the literature and considering the difficulty of following such a large number of lesions, the dermatology team brought the patient’s case to the multidisciplinary cutaneous tumor board at the University of Massachusetts (Worcester, Massachusetts). In consensus, the tumor board recommended radical mastectomy despite the comorbidities, given her young age and the potential risk for malignant transformation of any one of the numerous AVLs to angiosarcoma.
Postmastectomy pathology showed multiple scattered foci of AVLs ranging from 1.5 to 4 mm in the dermis, similar to those seen on prior biopsies, with no frank evidence of RIA. At 3-year follow-up, the patient has had no recurrence of AVLs or findings suggestive of RIA. There were no reported complications.
Comment
Conservative breast cancer surgery and radiotherapy are becoming more prevalent for breast cancer treatment, thus the number of patients likely to present with AVLs has increased. These patients are at risk for transformation to RIAs.6 It is important for clinicians to be aware of the diagnosis of both AVLs and RIAs and their management given their more frequent presentation. In most cases, one or a few AVLs are present, and excision is the treatment of choice. In a retrospective study by Brenn and Fletcher3 examining 16 patients with AVLs and 26 patients with RIA, the majority of cases of AVL had a single lesion and the maximum number of AVLs was 4. One patient in their study had 30 AVLs (each 3–4 mm in diameter), and she was diagnosed with RIA.3 Our patient—with at least 29 identifiable AVL lesions—was felt to be at considerable risk for developing RIA, as the only other case reported with this many AVLs developed RIA.1 Given the large number of lesions, it was neither feasible to excise each one individually nor monitor all of them for malignant transformation.
Our case demonstrates the important role dermatologists may play in orchestrating care by a multispecialty team including oncology, radiation oncology, surgery, and plastic surgery. In our patient, a close examination of the literature by the dermatology team led to recognition of the potentially elevated risk for malignant transformation. The dermatology team also brought the case for review at the tumor board.
Although future studies are required to determine the relationship between AVL burden and the risk for progression to RIA, it is clear that a multidisciplinary approach and careful consideration of the current literature can prevent unnecessary morbidity and mortality for patients with this increasingly common problem.
- Patton KT, Deyrup AT, Weiss SW. Atypical vascular lesions after surgery and radiation of the breast: a clinicopathologic study of 32 cases analyzing histologic heterogeneity and association with angiosarcoma. Am J Surg Pathol. 2008;32:943-950.
- Mandrell J, Mehta S, McClure S. Atypical vascular lesion of the breast. J Am Acad Dermatol. 2010;63:337-340.
- Brenn T, Fletcher CD. Radiation-associated cutaneous atypical vascular lesions and angiosarcoma: clinicopathologic analysis of 42 cases. Am J Surg Pathol. 2005;29:983-996.
- Losch A, Chilek KD, Zirwas MJ. Post-radiation atypical vascular proliferation mimicking angiosarcoma eight months following breast-conserving therapy for breast carcinoma. J Clin Aesthet Dermatol. 2011;4:47-48.
- Mentzel T, Schildhaus HU, Palmedo G, et al. Postradiation cutaneous angiosarcoma after treatment of breast carcinoma is characterized by MYC amplification in contrast to atypical vascular lesions after radiotherapy and control cases: clinicopathological, immunohistochemical and molecular analysis of 66 cases. Mod Pathol. 2012;25:75-85.
- Tahir M, Hendry P, Baird L, et al. Radiation induced angiosarcoma a sequela of radiotherapy for breast cancer following conservative surgery. Int Semin Surg Oncol. 2006;3:26.
- Hillenbrand T, Menge F, Hohenberger P, et al. Primary and secondary angiosarcomas: a comparative single-center analysis. Clin Sarcoma Res. 2015;5:14.
- Seinen JM, Styring E, Verstappen V, et al. Radiation-associated angiosarcoma after breast cancer: high recurrence rate and poor survival despite surgical treatment with R0 resection. Ann Surg Oncol. 2012;19:2700-2706.
- Patton KT, Deyrup AT, Weiss SW. Atypical vascular lesions after surgery and radiation of the breast: a clinicopathologic study of 32 cases analyzing histologic heterogeneity and association with angiosarcoma. Am J Surg Pathol. 2008;32:943-950.
- Mandrell J, Mehta S, McClure S. Atypical vascular lesion of the breast. J Am Acad Dermatol. 2010;63:337-340.
- Brenn T, Fletcher CD. Radiation-associated cutaneous atypical vascular lesions and angiosarcoma: clinicopathologic analysis of 42 cases. Am J Surg Pathol. 2005;29:983-996.
- Losch A, Chilek KD, Zirwas MJ. Post-radiation atypical vascular proliferation mimicking angiosarcoma eight months following breast-conserving therapy for breast carcinoma. J Clin Aesthet Dermatol. 2011;4:47-48.
- Mentzel T, Schildhaus HU, Palmedo G, et al. Postradiation cutaneous angiosarcoma after treatment of breast carcinoma is characterized by MYC amplification in contrast to atypical vascular lesions after radiotherapy and control cases: clinicopathological, immunohistochemical and molecular analysis of 66 cases. Mod Pathol. 2012;25:75-85.
- Tahir M, Hendry P, Baird L, et al. Radiation induced angiosarcoma a sequela of radiotherapy for breast cancer following conservative surgery. Int Semin Surg Oncol. 2006;3:26.
- Hillenbrand T, Menge F, Hohenberger P, et al. Primary and secondary angiosarcomas: a comparative single-center analysis. Clin Sarcoma Res. 2015;5:14.
- Seinen JM, Styring E, Verstappen V, et al. Radiation-associated angiosarcoma after breast cancer: high recurrence rate and poor survival despite surgical treatment with R0 resection. Ann Surg Oncol. 2012;19:2700-2706.
Practice Points
- Atypical vascular lesions (AVLs) of the breast have been reported in breast cancer patients following radiation treatment.
- Conservative breast cancer surgery and radiotherapy are becoming more prevalent for breast cancer treatment, thus the number of patients likely to present with AVLs has increased.
- Differentiation between AVLs and radiation-induced angiosarcomas (RIAs) can be challenging due to considerable histologic and clinical overlap; therefore, it is important for clinicians to be aware of the diagnosis and management of both AVLs and RIAs.