User login
Narcolepsy was originally described in the late 1800s by the French physician Jean-Baptiste-Edouard Gélineau, who reported the case of a wine merchant suffering from somnolence. In this first description, he coined the term narcolepsie by joining the Greek words narke (numbness or stupor) and lepsis (attack).1
Since then, the disorder has been further characterized, and some insight into its biological underpinnings has been established. Importantly, treatments have improved and expanded, facilitating its management and thereby improving quality of life for those with the disorder.
This review focuses on clinically relevant features of the disorder and proposes management strategies.
CLINICAL FEATURES
Narcolepsy is characterized by instability of sleep-wake transitions.
Daytime sleepiness
Clinically, narcolepsy manifests with excessive daytime sleepiness that can be personally and socially disabling. Cataplexy, sleep paralysis, and hypnagogic or hypnopompic hallucinations can also be present,2,3 but they are not necessary for diagnosis. In fact, a minority of patients with narcolepsy have all these symptoms.4 Narcolepsy is divided into type 1 (with cataplexy) and type 2 (without cataplexy).2
Sleepiness tends to be worse with inactivity, and sleep can often be irresistible. Sleep attacks can come on suddenly and may be brief enough to manifest as a lapse in consciousness.
Short naps tend to be refreshing. Rapid eye movement (REM) latency—the interval between falling asleep and the onset of the REM sleep—is short in narcolepsy, and since the REM stage is when dreaming occurs, naps often include dreaming. Therefore, when taking a history, it is worthwhile to ask patients whether they dream during naps; a yes answer supports the diagnosis of narcolepsy.5
In children, sleepiness can manifest in reduced concentration and behavioral issues.6 Napping after age 5 or 6 is considered abnormal and may reflect pathologic sleepiness.7
Cataplexy
Cataplexy—transient muscle weakness triggered by emotion—is a specific feature of narcolepsy type 1. It often begins in the facial muscles and can manifest with slackening of the jaw or brief dropping of the head. However, episodes can be more dramatic and, if the trunk and limb muscles are affected, can result in collapsing to the ground.
Cataplexy usually has its onset at about the same time as the sleepiness associated with narcolepsy, but it can arise even years later.8 Episodes can last from a few seconds to 2 minutes. Consciousness is always preserved. A range of emotions can trigger cataplexy, but typically the emotion is a positive one such as laughter or excitement.9 Deep tendon reflexes disappear in cataplexy, so checking reflexes during a witnessed episode can be clinically valuable.2
Cataplexy can worsen with stress and insufficient sleep, occasionally with “status cataplecticus,” in which repeated, persistent episodes of cataplexy occur over several hours.8 Status cataplecticus can be spontaneous or an effect of withdrawal from anticataplectic medications.2
Cataplexy is thought to represent intrusion of REM sleep and its associated muscle atonia during wakefulness.
Sleep paralysis, hallucinations
Sleep paralysis and hallucinations are other features of narcolepsy that reflect this REM dissociation from sleep.
Sleep paralysis occurs most commonly upon awakening, but sometimes just before sleep onset. In most cases, it is manifested by inability to move the limbs or speak, lasting several seconds or, in rare cases, minutes at a time. Sleep paralysis can be associated with a sensation of fear or suffocation, especially when initially experienced.8
Hypnopompic hallucinations, occurring upon awakening, are more common than hypnagogic hallucinations, which are experienced before falling asleep. The hallucinations are often vivid and usually visual, although other types of hallucinations are possible. Unlike those that occur in psychotic disorders, the hallucinations tend to be associated with preserved insight that they are not real.10
Notably, both sleep paralysis and hallucinations are nonspecific symptoms that are common in the general population.8,11,12
Fragmented sleep
Although they are very sleepy, people with narcolepsy generally cannot stay asleep for very long. Their sleep tends to be extremely fragmented, and they often wake up several times a night.2
This sleep pattern reflects the inherent instability of sleep-wake transitions in narcolepsy. In fact, over a 24-hour period, adults with narcolepsy have a normal amount of sleep.13 In children, however, when narcolepsy first arises, the 24-hour sleep time can increase abruptly and can sometimes be associated with persistent cataplexy that can manifest as a clumsy gait.14
Weight gain, obstructive sleep apnea
Weight gain is common, particularly after symptom onset, and especially in children. As a result, obesity is a frequent comorbidity.15 Because obstructive sleep apnea can consequently develop, all patients with narcolepsy require screening for sleep-disordered breathing.
Other sleep disorders often accompany narcolepsy and are more common than in the general population.16 In a study incorporating both clinical and polysomnographic data of 100 patients with narcolepsy, insomnia was the most common comorbid sleep disorder, with a prevalence of 28%; others were REM sleep behavior disorder (24%), restless legs syndrome (24%), obstructive sleep apnea (21%), and non-REM parasomnias.17
PSYCHOSOCIAL CONSEQUENCES
Narcolepsy has significant psychosocial consequences. As a result of their symptoms, people with narcolepsy may not be able to meet academic or work-related demands.
Additionally, their risk of a motor vehicle accident is 3 to 4 times higher than in the general population, and more than one-third of patients have been in an accident due to sleepiness.18 There is some evidence to show that treatment eliminates this risk.19
Few systematic studies have examined mood disorders in narcolepsy. However, studies tend to show a higher prevalence of psychiatric disorders than in the general population, with depression and anxiety the most com-mon.20,21
DIAGNOSIS IS OFTEN DELAYED
The prevalence of narcolepsy type 1 is between 25 and 100 per 100,000 people.22 In a Mayo Clinic study,23 the incidence of narcolepsy type 1 was estimated to be 0.74 per 100,000 person-years. Epidemiologic data on narcolepsy type 2 are sparse, but patients with narcolepsy without cataplexy are thought to represent only 36% of all narcolepsy patients.23
Diagnosis is often delayed, with the average time between the onset of symptoms and the diagnosis ranging from 8 to 22 years. With increasing awareness, the efficiency of the diagnostic process is improving, and this delay is expected to lessen accordingly.24
Symptoms most commonly arise in the second decade; but the age at onset ranges significantly, between the first and fifth decades. Narcolepsy has a bimodal distribution in incidence, with the biggest peak at approximately age 15 and second smaller peak in the mid-30s. Some studies have suggested a slight male predominance.23,25
DIAGNOSIS
History is key
The history should include specific questions about the hallmark features of narcolepsy, including cataplexy, sleep paralysis, and sleep-related hallucinations. For individual assessment of subjective sleepiness, the Epworth Sleepiness Scale or Pediatric Daytime Sleepiness Scale can be administered quickly in the office setting.26,27
The Epworth score is calculated from the self-rated likelihood of falling asleep in 8 different situations, with possible scores of 0 (would never doze) to 3 (high chance of dozing) on each question, for a total possible score of 0 to 24. Normal total scores are between 0 and 10, while scores greater than 10 reflect pathologic sleepiness. Scores on the Epworth Sleepiness Scale in those with narcolepsy tend to reflect moderate to severe sleepiness, or at least 13, as opposed to patients with obstructive sleep apnea, whose scores commonly reflect milder sleepiness.28
Testing with actigraphy and polysomnography
It is imperative to rule out insufficient sleep and other sleep disorders as a cause of daytime sleepiness. This can be done with a careful clinical history, actigraphy with sleep logs, and polysomnography.
In the 2 to 4 weeks before actigraphy and subsequent testing, all medications with alerting or sedating properties (including antidepressants) should be tapered off to prevent influence on the results of the study.
Delayed sleep-phase disorder presents at a similar age as narcolepsy and can be associated with similar degrees of sleepiness. However, individuals with delayed sleep phase disorder have an inappropriately timed sleep-wake cycle so that there is a shift in their desired sleep onset and awakening times. It is common—prevalence estimates vary but average about 1% in the general population.29
Insufficient sleep syndrome is even more common, especially in teenagers and young adults, with increasing family, social, and academic demands. Sleep needs vary across the life span. A teenager needs 8 to 10 hours of sleep per night, and a young adult needs 7 to 9 hours. A study of 1,285 high school students found that 10.4% were not getting enough sleep.30
If actigraphy data suggest a circadian rhythm disorder or insufficient sleep that could explain the symptoms of sleepiness, then further testing should be halted and these specific issues should be addressed. In these cases, working with the patient toward maintaining a regular sleep-wake schedule with 7 to 8 hours of nightly sleep will often resolve symptoms.
If actigraphy demonstrates the patient is maintaining a regular sleep schedule and allowing adequate time for nightly sleep, the next step is polysomnography.
Polysomnography is performed to detect other disorders that can disrupt sleep, such as sleep-disordered breathing or periodic limb movement disorder.2,5 In addition, polysomnography can provide assurance that adequate sleep was obtained prior to the next step in testing.
Multiple sleep latency test
If sufficient sleep is obtained on polysomnograpy (at least 6 hours for an adult) and no other sleep disorder is identified, a multiple sleep latency test is performed. A urine toxicology screen is typically performed on the day of the test to ensure that drugs are not affecting the results.
The multiple sleep latency test consists of 4 to 5 nap opportunities at 2-hour intervals in a quiet dark room conducive to sleep, during which both sleep and REM latency are recorded. The sleep latency of those with narcolepsy is significantly shortened, and the diagnosis of narcolepsy requires an average sleep latency of less than 8 minutes.
Given the propensity for REM sleep in narcolepsy, another essential feature for diagnosis is the sleep-onset REM period (SOREMP). A SOREMP is defined as a REM latency of less than 15 minutes. A diagnosis of narcolepsy re-quires a SOREMP in at least 2 of the naps in a multiple sleep latency test (or 1 nap if the shortened REM latency is seen during polysomnography).31
The multiple sleep latency test has an imperfect sensitivity, though, and should be repeated when there is a high suspicion of narcolepsy.32–34 It is not completely specific either, and false-positive results occur. In fact, SOREMPs can be seen in the general population, particularly in those with a circadian rhythm disorder, insufficient sleep, or sleep-disordered breathing. Two or more SOREMPs in an multiple sleep latency test can be seen in a small proportion of the general population.35 The results of a multiple sleep latency test should be interpreted in the clinical context.
Differential diagnosis
Narcolepsy type 1 is distinguished from type 2 by the presence of cataplexy. A cerebrospinal fluid hypocretin 1 level of 110 pg/mL or less, or less than one-third of the mean value obtained in normal individuals, can substitute for the multiple sleep latency test in diagnosing narcolepsy type 1.31 Currently, hypocretin testing is generally not performed in clinical practice, although it may become a routine part of the narcolepsy evaluation in the future.
Thus, according to the International Classification of Sleep Disorders, 3rd edition,31 the diagnosis of narcolepsy type 1 requires excessive daytime sleepiness for at least 3 months that cannot be explained by another sleep disorder, medical or neurologic disorder, mental disorder, medication use, or substance use disorder, and at least 1 of the following:
- Cataplexy and mean sleep latency of 8 minutes or less with at least 2 SOREMPs on multiple sleep latency testing (1 of which can be on the preceding night’s polysomography)
- Cerebrospinal fluid hypocretin 1 levels less than 110 pg/mL or one-third the baseline normal levels and mean sleep latency ≤ 8 minutes with ≥ 2 SOREMPs on multiple sleep latency testing.
Similarly, the diagnosis of narcolepsy type 2 requires excessive daytime sleepiness for at least 3 months that cannot be explained by another sleep disorder, medical or neurological disorder, mental disorder, medication use, or substance use disorder, plus:
- Mean sleep latency of 8 minutes or less with at least 2 SOREMPs on multiple sleep latency testing.
Idiopathic hypersomnia, another disorder of central hypersomnolence, is also characterized by disabling sleepiness. It is diagnostically differentiated from narcolepsy, as there are fewer than 2 SOREMPs. As opposed to narcolepsy, in which naps tend to be refreshing, even prolonged naps in idiopathic hypersomnia are often not helpful in restoring wakefulness. In idiopathic hypersomnia, sleep is usually not fragmented, and there are few nocturnal arousals. Sleep times can often be prolonged as well, whereas in narcolepsy total sleep time through the day may not be increased but is not consolidated.
Kleine-Levin syndrome is a rarer disorder of hypersomnia. It is episodic compared with the relatively persistent sleepiness in narcolepsy and idiopathic hypersomnia. Periods of hypersomnia occur intermittently for days to weeks and are accompanied by cognitive and behavioral changes including hyperphagia and hypersexuality.4
LINKED TO HYPOCRETIN DEFICIENCY
Over the past 2 decades, the underlying pathophysiology of narcolepsy type 1 has been better characterized.
Narcolepsy type 1 has been linked to a deficiency in hypocretin in the central nervous system.36 Hypocretin (also known as orexin) is a hormone produced in the hypothalamus that acts on multiple brain regions and maintains alertness. For unclear reasons, hypothalamic neurons producing hypocretin are selectively reduced in narcolepsy type 1. Hypocretin also stabilizes wakefulness and inhibits REM sleep; therefore, hypocretin deficiency can lead to inappropriate intrusions of REM sleep onto wakefulness, leading to the hallmark features of narcolepsy—cataplexy, sleep-related hallucinations, and sleep paralysis.37 According to one theory, cataplexy is triggered by emotional stimuli because of a pathway between the medial prefrontal cortex and the amygdala to the pons.38
Cerebrospinal fluid levels of hypocretin in patients with narcolepsy type 2 tend to be normal, and the biologic underpinnings of narcolepsy type 2 remain mysterious. However, in the subgroup of those with narcolepsy type 2 in which hypocretin is low, many individuals go on to develop cataplexy, thereby evolving to narcolepsy type 1.36
POSSIBLE AUTOIMMUNE BASIS
Narcolepsy is typically a sporadic disorder, although familial cases have been described. The risk of a parent with narcolepsy having a child who is affected is approximately 1%.5
Narcolepsy type 1 is strongly associated with HLA-DQB1*0602, with up to 95% of those affected having at least one allele.39 Having 2 copies of the allele further increases the risk of developing narcolepsy.40 However, this allele is far from specific for narcolepsy with cataplexy, as it occurs in 12% to 38% of the general population.41 Therefore, HLA typing currently has limited clinical utility. The exact cause is as yet unknown, but substantial literature proposes an autoimmune basis of the disorder, given the strong association with the HLA subtype.42–44
After the 2009 H1N1 influenza pandemic, there was a significant increase in the incidence of narcolepsy with cataplexy, which again sparked interest in an autoimmune etiology underlying the disorder. Pandemrix, an H1N1 vaccine produced as a result of the 2009 pandemic, appeared to also be associated with an increase in the incidence of narcolepsy. An association with other upper respiratory infections has also been noted, further supporting a possible autoimmune basis.
A few studies have looked for serum autoantibodies involved in the pathogenesis of narcolepsy. Thus far, only one has been identified, an antibody to Tribbles homolog 2, found in 20% to 40% of those with new onset of nar-colepsy.42–44
TREATMENTS FOR DAYTIME SLEEPINESS
As with many chronic disorders, the treatment of narcolepsy consists of symptomatic rather than curative management, which can be done through both pharmacologic and nonpharmacologic means.
Nondrug measures
Scheduled naps lasting 15 to 20 minutes can help improve alertness.45 A consistent sleep schedule with good sleep hygiene, ensuring sufficient nightly sleep, is also important. In one study, the combination of scheduled naps and regular nocturnal sleep times reduced the level of daytime sleepiness and unintentional daytime sleep. Daytime naps were most helpful for those with the highest degree of daytime sleepiness.45
Strategic use of caffeine can be helpful and can reduce dependence on pharmacologic treatment.
Screening should be performed routinely for other sleep disorders, such as sleep-disordered breathing, which should be treated if identified.5,18 When being treated for other medical conditions, individuals with narcolepsy should avoid medications that can cause sedation, such as opiates or barbiturates; alcohol should be minimized or avoided.
Networking with other individuals with narcolepsy through support groups such as Narcolepsy Network can be valuable for learning coping skills and connecting with community resources. Psychological counseling for the patient, and sometimes the family, can also be useful. School-age children may need special accommodations such as schedule adjustments to allow for scheduled naps or frequent breaks to maintain alertness.
People with narcolepsy tend to function better in careers that do not require long periods of sitting, as sleepiness tends to be worse, but instead offer flexibility and require higher levels of activity that tend to combat sleepiness. They should not work as commercial drivers.18
Medications
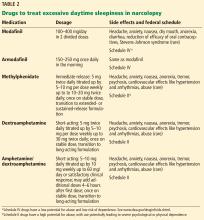
While behavioral interventions in narcolepsy are vital, they are rarely sufficient, and drugs that promote daytime wakefulness are used as an adjunct (Table 2).46
Realistic expectations should be established when starting, as some degree of residual sleepiness usually remains even with optimal medical therapy. Medications should be strategically scheduled to maximize alertness during necessary times such as at work or school or during driving. Patients should specifically be counseled to avoid driving if sleepy.18,47
Modafinil is often used as a first-line therapy, given its favorable side-effect profile and low potential for abuse. Its pharmacologic action has been debated but it probably acts as a selective dopamine reuptake inhibitor. It is typically taken twice daily (upon waking and early afternoon) and is usually well tolerated.
Potential side effects include headache, nausea, dry mouth, anorexia, diarrhea, and, rarely, Stevens-Johnson syndrome. Cardiovascular side effects are minimal, making it a favorable choice in older patients.18,48
A trial in 283 patients showed significantly lower levels of sleepiness in patients taking modafinil 200 mg or 400 mg than in a control group. Other trials have supported these findings and showed improved driving performance on modafinil.18
Notably, modafinil can increase the metabolism of oral contraceptives, thereby reducing their efficacy. Women of childbearing age should be warned about this interaction and should be transitioned to nonhormonal forms of contraception.2,47
Armodafinil, a purified R-isomer of modafinil, has a longer half-life and requires only once-daily dosing.5
If modafinil or armodafinil fails to optimally manage daytime sleepiness, a traditional stimulant such as methylphenidate or an amphetamine is often used.
Methylphenidate and amphetamines primarily inhibit the reuptake and increase the release of the monoamines, mainly dopamine, and to a lesser degree serotonin and norepinephrine.
These drugs have more significant adverse effects that can involve the cardiovascular system, causing hypertension and arrhythmias. Anorexia, weight loss, and, particularly with high doses, psychosis can occur.49
These drugs should be avoided in patients with a history of significant cardiovascular disease. Before starting stimulant therapy, a thorough cardiovascular examination should be done, often including electrocardiography to ensure there is no baseline arrhythmia.
Patients on these medications should be followed closely to ensure that blood pressure, pulse, and weight are not negatively affected.18,50 Addiction and tolerance can develop with these drugs, and follow-up should include assessment for dependence. Some states may require prescription drug monitoring to ensure the drugs are not being abused or diverted.
Short- and long-acting formulations of both methylphenidate and amphetamines are available, and a long-acting form is often used in conjunction with a short-acting form as needed.18
Addiction and drug-seeking behavior can develop but are unusual in those taking stimulants to treat narcolepsy.49
Follow-up
Residual daytime sleepiness can be measured subjectively through the Epworth Sleepiness Scale on follow-up. If necessary, a maintenance-of-wakefulness test can provide an objective assessment of treatment efficacy.18
As narcolepsy is a chronic disorder, treatment should evolve with time. Most medications that treat narcolepsy are categorized by the US Food and Drug Administration as pregnancy category C, as we do not have adequate studies in human pregnancies to evaluate their effects. When a patient with narcolepsy becomes pregnant, she should be counseled about the risks and benefits of remaining on therapy. Treatment should balance the risks of sleepiness with the potential risks of remaining on medications.50 In the elderly, as cardiovascular comorbidities tend to increase, the risks and benefits of therapy should be routinely reevaluated.
For cataplexy
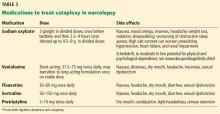
Sodium oxybate,51–53 the most potent anticataplectic drug, is the sodium salt of gamma hydroxybutyrate, a metabolite of gamma-aminobutyric acid. Sodium oxybate can be prescribed in the United States, Canada, and Europe. The American Academy of Sleep Medicine recommends sodium oxybate for cataplexy, daytime sleepiness, and disrupted sleep based on 3 level-1 studies and 2 level-4 studies.46
Sodium oxybate increases slow-wave sleep, improves sleep continuity, and often helps to mitigate daytime sleepiness. Due to its short half-life, its administration is unusual: the first dose is taken before bedtime and the second dose 2.5 to 4 hours later. Some patients set an alarm clock to take the second dose, while others awaken spontaneously to take the second dose. Most patients find that with adherence to dosing and safety instructions, sodium oxybate can serve as a highly effective form of treatment of both excessive sleepiness and cataplexy and may reduce the need for stimulant-based therapies.
The most common adverse effects are nausea, mood swings, and enuresis. Occasionally, psychosis can result and limit use of the drug. Obstructive sleep apnea can also develop or worsen.52 Because of its high salt content, sodium oxybate should be used with caution in those with heart failure, hypertension, or renal impairment. Its relative, gamma hydroxybutyrate, causes rapid sedation and has been notorious for illegal use as a date rape drug.
In the United States, sodium oxybate is distributed only through a central pharmacy to mitigate potential abuse. Due to this system, the rates of diversion are extremely low, estimated in a postmarketing analysis to be 1 instance per 5,200 patients treated. In the same study, abuse and dependence were both rare as well, about 1 case for every 2,600 and 6,500 patients treated.6,18,52,53
Antidepressants promote the action of norepinephrine and, to a lesser degree, serotonin, thereby suppressing REM sleep.
Venlafaxine, a serotonin-norepinephrine reuptake inhibitor, is often used as a first-line treatment for cataplexy. Selective serotonin reuptake inhibitors such as fluoxetine are also used with success. Tricyclic antidepressants such as protriptyline or clomipramine are extremely effective for cataplexy, but are rarely used due to their adverse effects.2,47
FUTURE WORK
While our understanding of narcolepsy has advanced, there are still gaps in our knowledge of the disorder—namely, the specific trigger for the loss of hypocretin neurons in type 1 narcolepsy and the underlying pathophysiology of type 2.
A number of emerging therapies target the hypocretin system, including peptide replacement, neuronal transplant, and immunotherapy preventing hypocretin neuronal cell death.50,54,55 Additional drugs designed to improve alertness that do not involve the hypocretin system are also being developed, including a histamine inverse agonist.50,56 Sodium oxybate and modafinil, although currently approved for use in adults, are still off-label in pediatric practice. Studies of the safety and efficacy of these medications in children are needed.7,57
- Gélineau J. De la narcolepsie. Gazette des Hôpitaux Civils et Militaires 1880; part a, 53:626–628, part b, 54:635–637.
- Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet 2007; 369(9560):499–511. doi:10.1016/S0140-6736(07)60237-2
- Scammell TE. Clinical features and diagnosis of narcolepsy in adults. In: Eichler AF, ed. UpToDate. Waltham, MA: UpToDate; 2018. www.uptodate.com. Accessed October 31, 2018.
- Morrish E, King MA, Smith IE, Shneerson JM. Factors associated with a delay in the diagnosis of narcolepsy. Sleep Med 2004; 5(1):37–41. pmid:14725825
- Scammell TE. Narcolepsy. N Engl J Med 2015; 373(27):2654–2662. doi:10.1056/NEJMra1500587
- Babiker MO, Prasad M. Narcolepsy in children: a diagnostic and management approach. Pediatr Neurol 2015; 52(6):557–565. doi:10.1016/j.pediatrneurol.2015.02.020
- Kotagal S. Narcolepsy in children. In: UpToDate, Eichler AF, ed. UpToDate, Waltham, MA. www.uptodate.com. Accessed October 31, 2018.
- Scammell TE. The neurobiology, diagnosis, and treatment of narcolepsy. Ann Neurol 2003; 53(2):154–166. doi:10.1002/ana.10444
- Overeem S, van Nues SJ, van der Zande WL, Donjacour CE, van Mierlo P, Lammers GJ. The clinical features of cataplexy: a questionnaire study in narcolepsy patients with and without hypocretin-1 deficiency. Sleep Med 2011; 12(1):12–18. doi:10.1016/j.sleep.2010.05.010
- Plazzi G, Fabbri C, Pizza F, Serretti A. Schizophrenia-like symptoms in narcolepsy type 1: shared and distinctive clinical characteristics. Neuropsychobiology 2015; 71(4):218–224. doi:10.1159/000432400
- Ohayon MM. Prevalence of hallucinations and their pathological associations in the general population. Psychiatry Res 2000; 97(2-3):153–164. pmid:11166087
- Sharpless BA, Barber JP. Lifetime prevalence rates of sleep paralysis: a systematic review. Sleep Med Rev 2011;5(5):311–315. doi:10.1016/j.smrv.2011.01.007
- Broughton R, Dunham W, Newman J, Lutley K, Duschesne P, Rivers M. Ambulatory 24 hour sleep-wake monitoring in narcolepsy-cataplexy compared to matched controls. Electroencephalogr Clin Neurophysiol 1988; 70(6):473–481. pmid:2461281
- Pizza F, Franceschini C, Peltola H, et al. Clinical and polysomnographic course of childhood narcolepsy with cataplexy. Brain 2013; 136(pt 12):3787–3795. doi:10.1093/brain/awt277
- Kotagal S, Krahn LE, Slocumb N. A putative link between childhood narcolepsy and obesity. Sleep Med 2004; 5(2):147–150. doi:10.1016/j.sleep.2003.10.006
- Pizza F, Tartarotti S, Poryazova R, Baumann CR, Bassetti CL. Sleep-disordered breathing and periodic limb movements in narcolepsy with cataplexy: a systematic analysis of 35 consecutive patients. Eur Neurol 2013; 70(1-2):22–26. doi:10.1159/000348719
- Frauscher B, Ehrmann L, Mitterling T, et al. Delayed diagnosis, range of severity, and multiple sleep comorbidities: a clinical and polysomnographic analysis of 100 patients of the Innsbruck narcolepsy cohort. J Clin Sleep Med 2013; 9(8):805–812. doi:10.5664/jcsm.2926
- Scammell TE. Treatment of narcolepsy in adults. In: Eichler AF, ed. UpToDate, Waltham, MA. www.uptodate.com. Accessed October 31, 2018.
- Pizza F, Jaussent I, Lopez R, et al. Car crashes and central disorders of hypersomnolence: a French study. PLoS One 2015; 10(6):e0129386. doi:10.1371/journal.pone.0129386
- Fortuyn HD, Lappenschaar MA, Furer JW, et al. Anxiety and mood disorders in narcolepsy: a case-control study. Gen Hosp Psychiatry 2010; 32(1):49–56. doi:10.1016/j.genhosppsych.2009.08.007
- Ruoff CM, Reaven NL, Funk SE, et al. High rates of psychiatric comorbidity in narcolepsy: findings from the Burden of Narcolepsy Disease (BOND) study of 9,312 patients in the United States. J Clin Psychiatry 2017; 78(2):171–176. doi:10.4088/JCP.15m10262
- Longstreth WT Jr, Koepsell TD, Ton TG, Hendrickson AF, van Belle G. The epidemiology of narcolepsy. Sleep. 2007; 30(1):13–26. pmid:17310860
- Silber MH, Krahn LE, Olson EJ, Pankratz VS. The epidemiology of narcolepsy in Olmsted County, Minnesota: a population-based study. Sleep 2002; 25(2):197–202. pmid:11902429
- Thorpy MJ, Krieger AC. Delayed diagnosis of narcolepsy: characterization and impact. Sleep Med 2014; 15(5):502–507. doi:10.1016/j.sleep.2014.01.015
- Dauvilliers Y, Montplaisir J, Molinari N, et al. Age at onset of narcolepsy in two large populations of patients in France and Quebec. Neurology 2001; 57(11):2029–2033. pmid:11739821
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991; 14(6):540–545. pmid:1798888
- Drake C, Nickel C, Burduvali E, Roth T, Jefferson C, Badia P. The pediatric daytime sleepiness scale (PDSS): sleep habits and school outcomes in middle-school children. Sleep 2003; 26(4):455–458. pmid:12841372
- van der Heide A, van Schie MK, Lammers GJ, et al. Comparing treatment effect measurements in narcolepsy: the sustained attention to response task, Epworth sleepiness scale and maintenance of wakefulness test. Sleep 2015; 38(7):1051–1058. doi:10.5665/sleep.4810
- Nesbitt AD. Delayed sleep-wake phase disorder. J Thorac Dis 2018; 10(suppl 1):S103–S111. doi:10.21037/jtd.2018.01.11
- Pallesen S, Saxvig IW, Molde H, Sørensen E, Wilhelmsen-Langeland A, Bjorvatn B. Brief report: behaviorally induced insufficient sleep syndrome in older adolescents: prevalence and correlates. J Adolesc 2011; 34(2):391–395. doi:10.1016/j.adolescence.2010.02.005
- American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Disorders; 2014.
- Trotti LM, Staab BA, Rye DB. Test-retest reliability of the multiple sleep latency test in narcolepsy without cataplexy and idiopathic hypersomnia. J Clin Sleep Med 2013; 9(8):789–795. doi:10.5664/jcsm.2922
- Andlauer O, Moore H, Jouhier L, et al. Nocturnal rapid eye movement sleep latency for identifying patients with narcolepsy/hypocretin deficiency. JAMA Neurol 2013; 70(7):891–902. doi:10.1001/jamaneurol.2013.1589
- Cairns A, Bogan R. Prevalence and clinical correlates of a short onset REM period (SOREMP) during routine PSG. Sleep 2015; 38(10):1575–1581. doi:10.5665/sleep.5050
- Mignot E, Lin L, Finn L, et al. Correlates of sleep-onset REM periods during the multiple sleep latency test in community adults. Brain 2006; 129(6):1609–1623. doi:10.1093/brain/awl079
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet 2000; 355(9197):39–40. doi:10.1016/S0140-6736(99)05582-8
- Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med 2000; 6(9):991–997. doi:10.1038/79690
- Oishi Y, Williams RH, Agostinelli L, et al. Role of the medial prefrontal cortex in cataplexy. J Neurosci 2013; 33(23):9743–9751. doi:10.1523/JNEUROSCI.0499-13.2013
- Mignot E, Hayduk R, Black J, Grumet FC, Guilleminault C. HLA DQB1*0602 is associated with cataplexy in 509 narcoleptic patients.. Sleep 1997; 20(11):1012–1020. pmid:9456467
- Pelin Z, Guilleminault C, Risch N, Grumet FC, Mignot E. HLA-DQB1*0602 homozygosity increases relative risk for narcolepsy but not disease severity in two ethnic groups. US Modafinil in Narcolepsy Multicenter Study Group. Tissue Antigens 1998; 51(1):96–100. pmid:9459509
- Akintomide GS, Rickards H. Narcolepsy: a review. Neuropsychiatr Dis Treat 2011; 7(1):507–518. doi:10.2147/NDT.S23624
- Mahlios J, De la Herrán-Arita AK, Mignot E. The autoimmune basis of narcolepsy. Curr Opin Neurobiol 2013; 23(5):767–773. doi:10.1016/j.conb.2013.04.013
- Degn M, Kornum BR. Type 1 narcolepsy: a CD8(+) T cell-mediated disease? Ann N Y Acad Sci 2015;1 351:80–88. doi:10.1111/nyas.12793
- Liblau RS, Vassalli A, Seifinejad A, Tafti M. Hypocretin (orexin) biology and the pathophysiology of narcolepsy with cataplexy. Lancet Neurol 2015; 14(3):318–328. doi:10.1016/S1474-4422(14)70218-2
- Rogers AE, Aldrich MS, Lin X. A comparison of three different sleep schedules for reducing daytime sleepiness in narcolepsy. Sleep 2001; 24(4):385–391. pmid:11403522
- Morgenthaler TI, Kapur VK, Brown TM, et al; Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep 2007; 30(12):1705–1711. pmid:18246980
- Mignot EJ. A practical guide to the therapy of narcolepsy and hypersomnia syndromes. Neurotherapeutics 2012; 9(4):739–752. doi:10.1007/s13311-012-0150-9
- Roth T, Schwartz JR, Hirshkowitz M, Erman MK, Dayno JM, Arora S. Evaluation of the safety of modafinil for treatment of excessive sleepiness. J Clin Sleep Med 2007; 3(6):595–602. pmid:17993041
- Auger RR, Goodman SH, Silber MH, Krahn LE, Pankratz VS, Slocumb NL. Risks of high-dose stimulants in the treatment of disorders of excessive somnolence: a case-control study. Sleep 2005; 28(6):667–672. pmid:16477952
- Abad VC, Guilleminault C. New developments in the management of narcolepsy. Nat Sci Sleep 2017; 9:39–57. doi:10.2147/NSS.S103467
- Drakatos P, Lykouras D, D’Ancona G, et al. Safety and efficacy of long-term use of sodium oxybate for narcolepsy with cataplexy in routine clinical practice. Sleep Med 2017; 35:80–84. doi:10.1016/j.sleep.2017.03.028
- Mansukhani MP, Kotagal S. Sodium oxybate in the treatment of childhood narcolepsy–cataplexy: a retrospective study. Sleep Med 2012; 13(6):606–610. doi:10.1016/j.sleep.2011.10.032
- Wang YG, Swick TJ, Carter LP, Thorpy MJ, Benowitz NL. Safety overview of postmarketing and clinical experience of sodium oxybate (Xyrem): abuse, misuse, dependence, and diversion. J Clin Sleep Med 2009; 5(4):365–371. pmid:19968016
- Weinhold SL, Seeck-Hirschner M, Nowak A, Hallschmid M, Göder R, Baier PC. The effect of intranasal orexin-A (hypocretin-1) on sleep, wakefulness and attention in narcolepsy with cataplexy. Behav Brain Res 2014; 262:8–13. doi:10.1016/j.bbr.2013.12.045
- Arias-Carrión O, Murillo-Rodriguez E. Effects of hypocretin/orexin cell transplantation on narcoleptic-like sleep behavior in rats. PLoS One 2014; 9(4):e95342. doi:10.1371/journal.pone.0095342
- Leu-Semenescu S, Nittur N, Golmard JL, Arnulf I. Effects of pitolisant, a histamine H3 inverse agonist, in drug-resistant idiopathic and symptomatic hypersomnia: a chart review. Sleep Med 2014; 15(6):681–687. doi:10.1016/j.sleep.2014.01.021
- Lecendreux M, Bruni O, Franco P, et al. Clinical experience suggests that modafinil is an effective and safe treatment for paediatric narcolepsy. J Sleep Res 2012; 21(4):481–483. doi:10.1111/j.1365-2869.2011.00991.x
Narcolepsy was originally described in the late 1800s by the French physician Jean-Baptiste-Edouard Gélineau, who reported the case of a wine merchant suffering from somnolence. In this first description, he coined the term narcolepsie by joining the Greek words narke (numbness or stupor) and lepsis (attack).1
Since then, the disorder has been further characterized, and some insight into its biological underpinnings has been established. Importantly, treatments have improved and expanded, facilitating its management and thereby improving quality of life for those with the disorder.
This review focuses on clinically relevant features of the disorder and proposes management strategies.
CLINICAL FEATURES
Narcolepsy is characterized by instability of sleep-wake transitions.
Daytime sleepiness
Clinically, narcolepsy manifests with excessive daytime sleepiness that can be personally and socially disabling. Cataplexy, sleep paralysis, and hypnagogic or hypnopompic hallucinations can also be present,2,3 but they are not necessary for diagnosis. In fact, a minority of patients with narcolepsy have all these symptoms.4 Narcolepsy is divided into type 1 (with cataplexy) and type 2 (without cataplexy).2
Sleepiness tends to be worse with inactivity, and sleep can often be irresistible. Sleep attacks can come on suddenly and may be brief enough to manifest as a lapse in consciousness.
Short naps tend to be refreshing. Rapid eye movement (REM) latency—the interval between falling asleep and the onset of the REM sleep—is short in narcolepsy, and since the REM stage is when dreaming occurs, naps often include dreaming. Therefore, when taking a history, it is worthwhile to ask patients whether they dream during naps; a yes answer supports the diagnosis of narcolepsy.5
In children, sleepiness can manifest in reduced concentration and behavioral issues.6 Napping after age 5 or 6 is considered abnormal and may reflect pathologic sleepiness.7
Cataplexy
Cataplexy—transient muscle weakness triggered by emotion—is a specific feature of narcolepsy type 1. It often begins in the facial muscles and can manifest with slackening of the jaw or brief dropping of the head. However, episodes can be more dramatic and, if the trunk and limb muscles are affected, can result in collapsing to the ground.
Cataplexy usually has its onset at about the same time as the sleepiness associated with narcolepsy, but it can arise even years later.8 Episodes can last from a few seconds to 2 minutes. Consciousness is always preserved. A range of emotions can trigger cataplexy, but typically the emotion is a positive one such as laughter or excitement.9 Deep tendon reflexes disappear in cataplexy, so checking reflexes during a witnessed episode can be clinically valuable.2
Cataplexy can worsen with stress and insufficient sleep, occasionally with “status cataplecticus,” in which repeated, persistent episodes of cataplexy occur over several hours.8 Status cataplecticus can be spontaneous or an effect of withdrawal from anticataplectic medications.2
Cataplexy is thought to represent intrusion of REM sleep and its associated muscle atonia during wakefulness.
Sleep paralysis, hallucinations
Sleep paralysis and hallucinations are other features of narcolepsy that reflect this REM dissociation from sleep.
Sleep paralysis occurs most commonly upon awakening, but sometimes just before sleep onset. In most cases, it is manifested by inability to move the limbs or speak, lasting several seconds or, in rare cases, minutes at a time. Sleep paralysis can be associated with a sensation of fear or suffocation, especially when initially experienced.8
Hypnopompic hallucinations, occurring upon awakening, are more common than hypnagogic hallucinations, which are experienced before falling asleep. The hallucinations are often vivid and usually visual, although other types of hallucinations are possible. Unlike those that occur in psychotic disorders, the hallucinations tend to be associated with preserved insight that they are not real.10
Notably, both sleep paralysis and hallucinations are nonspecific symptoms that are common in the general population.8,11,12
Fragmented sleep
Although they are very sleepy, people with narcolepsy generally cannot stay asleep for very long. Their sleep tends to be extremely fragmented, and they often wake up several times a night.2
This sleep pattern reflects the inherent instability of sleep-wake transitions in narcolepsy. In fact, over a 24-hour period, adults with narcolepsy have a normal amount of sleep.13 In children, however, when narcolepsy first arises, the 24-hour sleep time can increase abruptly and can sometimes be associated with persistent cataplexy that can manifest as a clumsy gait.14
Weight gain, obstructive sleep apnea
Weight gain is common, particularly after symptom onset, and especially in children. As a result, obesity is a frequent comorbidity.15 Because obstructive sleep apnea can consequently develop, all patients with narcolepsy require screening for sleep-disordered breathing.
Other sleep disorders often accompany narcolepsy and are more common than in the general population.16 In a study incorporating both clinical and polysomnographic data of 100 patients with narcolepsy, insomnia was the most common comorbid sleep disorder, with a prevalence of 28%; others were REM sleep behavior disorder (24%), restless legs syndrome (24%), obstructive sleep apnea (21%), and non-REM parasomnias.17
PSYCHOSOCIAL CONSEQUENCES
Narcolepsy has significant psychosocial consequences. As a result of their symptoms, people with narcolepsy may not be able to meet academic or work-related demands.
Additionally, their risk of a motor vehicle accident is 3 to 4 times higher than in the general population, and more than one-third of patients have been in an accident due to sleepiness.18 There is some evidence to show that treatment eliminates this risk.19
Few systematic studies have examined mood disorders in narcolepsy. However, studies tend to show a higher prevalence of psychiatric disorders than in the general population, with depression and anxiety the most com-mon.20,21
DIAGNOSIS IS OFTEN DELAYED
The prevalence of narcolepsy type 1 is between 25 and 100 per 100,000 people.22 In a Mayo Clinic study,23 the incidence of narcolepsy type 1 was estimated to be 0.74 per 100,000 person-years. Epidemiologic data on narcolepsy type 2 are sparse, but patients with narcolepsy without cataplexy are thought to represent only 36% of all narcolepsy patients.23
Diagnosis is often delayed, with the average time between the onset of symptoms and the diagnosis ranging from 8 to 22 years. With increasing awareness, the efficiency of the diagnostic process is improving, and this delay is expected to lessen accordingly.24
Symptoms most commonly arise in the second decade; but the age at onset ranges significantly, between the first and fifth decades. Narcolepsy has a bimodal distribution in incidence, with the biggest peak at approximately age 15 and second smaller peak in the mid-30s. Some studies have suggested a slight male predominance.23,25
DIAGNOSIS
History is key
The history should include specific questions about the hallmark features of narcolepsy, including cataplexy, sleep paralysis, and sleep-related hallucinations. For individual assessment of subjective sleepiness, the Epworth Sleepiness Scale or Pediatric Daytime Sleepiness Scale can be administered quickly in the office setting.26,27
The Epworth score is calculated from the self-rated likelihood of falling asleep in 8 different situations, with possible scores of 0 (would never doze) to 3 (high chance of dozing) on each question, for a total possible score of 0 to 24. Normal total scores are between 0 and 10, while scores greater than 10 reflect pathologic sleepiness. Scores on the Epworth Sleepiness Scale in those with narcolepsy tend to reflect moderate to severe sleepiness, or at least 13, as opposed to patients with obstructive sleep apnea, whose scores commonly reflect milder sleepiness.28
Testing with actigraphy and polysomnography
It is imperative to rule out insufficient sleep and other sleep disorders as a cause of daytime sleepiness. This can be done with a careful clinical history, actigraphy with sleep logs, and polysomnography.
In the 2 to 4 weeks before actigraphy and subsequent testing, all medications with alerting or sedating properties (including antidepressants) should be tapered off to prevent influence on the results of the study.
Delayed sleep-phase disorder presents at a similar age as narcolepsy and can be associated with similar degrees of sleepiness. However, individuals with delayed sleep phase disorder have an inappropriately timed sleep-wake cycle so that there is a shift in their desired sleep onset and awakening times. It is common—prevalence estimates vary but average about 1% in the general population.29
Insufficient sleep syndrome is even more common, especially in teenagers and young adults, with increasing family, social, and academic demands. Sleep needs vary across the life span. A teenager needs 8 to 10 hours of sleep per night, and a young adult needs 7 to 9 hours. A study of 1,285 high school students found that 10.4% were not getting enough sleep.30
If actigraphy data suggest a circadian rhythm disorder or insufficient sleep that could explain the symptoms of sleepiness, then further testing should be halted and these specific issues should be addressed. In these cases, working with the patient toward maintaining a regular sleep-wake schedule with 7 to 8 hours of nightly sleep will often resolve symptoms.
If actigraphy demonstrates the patient is maintaining a regular sleep schedule and allowing adequate time for nightly sleep, the next step is polysomnography.
Polysomnography is performed to detect other disorders that can disrupt sleep, such as sleep-disordered breathing or periodic limb movement disorder.2,5 In addition, polysomnography can provide assurance that adequate sleep was obtained prior to the next step in testing.
Multiple sleep latency test
If sufficient sleep is obtained on polysomnograpy (at least 6 hours for an adult) and no other sleep disorder is identified, a multiple sleep latency test is performed. A urine toxicology screen is typically performed on the day of the test to ensure that drugs are not affecting the results.
The multiple sleep latency test consists of 4 to 5 nap opportunities at 2-hour intervals in a quiet dark room conducive to sleep, during which both sleep and REM latency are recorded. The sleep latency of those with narcolepsy is significantly shortened, and the diagnosis of narcolepsy requires an average sleep latency of less than 8 minutes.
Given the propensity for REM sleep in narcolepsy, another essential feature for diagnosis is the sleep-onset REM period (SOREMP). A SOREMP is defined as a REM latency of less than 15 minutes. A diagnosis of narcolepsy re-quires a SOREMP in at least 2 of the naps in a multiple sleep latency test (or 1 nap if the shortened REM latency is seen during polysomnography).31
The multiple sleep latency test has an imperfect sensitivity, though, and should be repeated when there is a high suspicion of narcolepsy.32–34 It is not completely specific either, and false-positive results occur. In fact, SOREMPs can be seen in the general population, particularly in those with a circadian rhythm disorder, insufficient sleep, or sleep-disordered breathing. Two or more SOREMPs in an multiple sleep latency test can be seen in a small proportion of the general population.35 The results of a multiple sleep latency test should be interpreted in the clinical context.
Differential diagnosis
Narcolepsy type 1 is distinguished from type 2 by the presence of cataplexy. A cerebrospinal fluid hypocretin 1 level of 110 pg/mL or less, or less than one-third of the mean value obtained in normal individuals, can substitute for the multiple sleep latency test in diagnosing narcolepsy type 1.31 Currently, hypocretin testing is generally not performed in clinical practice, although it may become a routine part of the narcolepsy evaluation in the future.
Thus, according to the International Classification of Sleep Disorders, 3rd edition,31 the diagnosis of narcolepsy type 1 requires excessive daytime sleepiness for at least 3 months that cannot be explained by another sleep disorder, medical or neurologic disorder, mental disorder, medication use, or substance use disorder, and at least 1 of the following:
- Cataplexy and mean sleep latency of 8 minutes or less with at least 2 SOREMPs on multiple sleep latency testing (1 of which can be on the preceding night’s polysomography)
- Cerebrospinal fluid hypocretin 1 levels less than 110 pg/mL or one-third the baseline normal levels and mean sleep latency ≤ 8 minutes with ≥ 2 SOREMPs on multiple sleep latency testing.
Similarly, the diagnosis of narcolepsy type 2 requires excessive daytime sleepiness for at least 3 months that cannot be explained by another sleep disorder, medical or neurological disorder, mental disorder, medication use, or substance use disorder, plus:
- Mean sleep latency of 8 minutes or less with at least 2 SOREMPs on multiple sleep latency testing.
Idiopathic hypersomnia, another disorder of central hypersomnolence, is also characterized by disabling sleepiness. It is diagnostically differentiated from narcolepsy, as there are fewer than 2 SOREMPs. As opposed to narcolepsy, in which naps tend to be refreshing, even prolonged naps in idiopathic hypersomnia are often not helpful in restoring wakefulness. In idiopathic hypersomnia, sleep is usually not fragmented, and there are few nocturnal arousals. Sleep times can often be prolonged as well, whereas in narcolepsy total sleep time through the day may not be increased but is not consolidated.
Kleine-Levin syndrome is a rarer disorder of hypersomnia. It is episodic compared with the relatively persistent sleepiness in narcolepsy and idiopathic hypersomnia. Periods of hypersomnia occur intermittently for days to weeks and are accompanied by cognitive and behavioral changes including hyperphagia and hypersexuality.4
LINKED TO HYPOCRETIN DEFICIENCY
Over the past 2 decades, the underlying pathophysiology of narcolepsy type 1 has been better characterized.
Narcolepsy type 1 has been linked to a deficiency in hypocretin in the central nervous system.36 Hypocretin (also known as orexin) is a hormone produced in the hypothalamus that acts on multiple brain regions and maintains alertness. For unclear reasons, hypothalamic neurons producing hypocretin are selectively reduced in narcolepsy type 1. Hypocretin also stabilizes wakefulness and inhibits REM sleep; therefore, hypocretin deficiency can lead to inappropriate intrusions of REM sleep onto wakefulness, leading to the hallmark features of narcolepsy—cataplexy, sleep-related hallucinations, and sleep paralysis.37 According to one theory, cataplexy is triggered by emotional stimuli because of a pathway between the medial prefrontal cortex and the amygdala to the pons.38
Cerebrospinal fluid levels of hypocretin in patients with narcolepsy type 2 tend to be normal, and the biologic underpinnings of narcolepsy type 2 remain mysterious. However, in the subgroup of those with narcolepsy type 2 in which hypocretin is low, many individuals go on to develop cataplexy, thereby evolving to narcolepsy type 1.36
POSSIBLE AUTOIMMUNE BASIS
Narcolepsy is typically a sporadic disorder, although familial cases have been described. The risk of a parent with narcolepsy having a child who is affected is approximately 1%.5
Narcolepsy type 1 is strongly associated with HLA-DQB1*0602, with up to 95% of those affected having at least one allele.39 Having 2 copies of the allele further increases the risk of developing narcolepsy.40 However, this allele is far from specific for narcolepsy with cataplexy, as it occurs in 12% to 38% of the general population.41 Therefore, HLA typing currently has limited clinical utility. The exact cause is as yet unknown, but substantial literature proposes an autoimmune basis of the disorder, given the strong association with the HLA subtype.42–44
After the 2009 H1N1 influenza pandemic, there was a significant increase in the incidence of narcolepsy with cataplexy, which again sparked interest in an autoimmune etiology underlying the disorder. Pandemrix, an H1N1 vaccine produced as a result of the 2009 pandemic, appeared to also be associated with an increase in the incidence of narcolepsy. An association with other upper respiratory infections has also been noted, further supporting a possible autoimmune basis.
A few studies have looked for serum autoantibodies involved in the pathogenesis of narcolepsy. Thus far, only one has been identified, an antibody to Tribbles homolog 2, found in 20% to 40% of those with new onset of nar-colepsy.42–44
TREATMENTS FOR DAYTIME SLEEPINESS
As with many chronic disorders, the treatment of narcolepsy consists of symptomatic rather than curative management, which can be done through both pharmacologic and nonpharmacologic means.
Nondrug measures
Scheduled naps lasting 15 to 20 minutes can help improve alertness.45 A consistent sleep schedule with good sleep hygiene, ensuring sufficient nightly sleep, is also important. In one study, the combination of scheduled naps and regular nocturnal sleep times reduced the level of daytime sleepiness and unintentional daytime sleep. Daytime naps were most helpful for those with the highest degree of daytime sleepiness.45
Strategic use of caffeine can be helpful and can reduce dependence on pharmacologic treatment.
Screening should be performed routinely for other sleep disorders, such as sleep-disordered breathing, which should be treated if identified.5,18 When being treated for other medical conditions, individuals with narcolepsy should avoid medications that can cause sedation, such as opiates or barbiturates; alcohol should be minimized or avoided.
Networking with other individuals with narcolepsy through support groups such as Narcolepsy Network can be valuable for learning coping skills and connecting with community resources. Psychological counseling for the patient, and sometimes the family, can also be useful. School-age children may need special accommodations such as schedule adjustments to allow for scheduled naps or frequent breaks to maintain alertness.
People with narcolepsy tend to function better in careers that do not require long periods of sitting, as sleepiness tends to be worse, but instead offer flexibility and require higher levels of activity that tend to combat sleepiness. They should not work as commercial drivers.18
Medications
While behavioral interventions in narcolepsy are vital, they are rarely sufficient, and drugs that promote daytime wakefulness are used as an adjunct (Table 2).46
Realistic expectations should be established when starting, as some degree of residual sleepiness usually remains even with optimal medical therapy. Medications should be strategically scheduled to maximize alertness during necessary times such as at work or school or during driving. Patients should specifically be counseled to avoid driving if sleepy.18,47
Modafinil is often used as a first-line therapy, given its favorable side-effect profile and low potential for abuse. Its pharmacologic action has been debated but it probably acts as a selective dopamine reuptake inhibitor. It is typically taken twice daily (upon waking and early afternoon) and is usually well tolerated.
Potential side effects include headache, nausea, dry mouth, anorexia, diarrhea, and, rarely, Stevens-Johnson syndrome. Cardiovascular side effects are minimal, making it a favorable choice in older patients.18,48
A trial in 283 patients showed significantly lower levels of sleepiness in patients taking modafinil 200 mg or 400 mg than in a control group. Other trials have supported these findings and showed improved driving performance on modafinil.18
Notably, modafinil can increase the metabolism of oral contraceptives, thereby reducing their efficacy. Women of childbearing age should be warned about this interaction and should be transitioned to nonhormonal forms of contraception.2,47
Armodafinil, a purified R-isomer of modafinil, has a longer half-life and requires only once-daily dosing.5
If modafinil or armodafinil fails to optimally manage daytime sleepiness, a traditional stimulant such as methylphenidate or an amphetamine is often used.
Methylphenidate and amphetamines primarily inhibit the reuptake and increase the release of the monoamines, mainly dopamine, and to a lesser degree serotonin and norepinephrine.
These drugs have more significant adverse effects that can involve the cardiovascular system, causing hypertension and arrhythmias. Anorexia, weight loss, and, particularly with high doses, psychosis can occur.49
These drugs should be avoided in patients with a history of significant cardiovascular disease. Before starting stimulant therapy, a thorough cardiovascular examination should be done, often including electrocardiography to ensure there is no baseline arrhythmia.
Patients on these medications should be followed closely to ensure that blood pressure, pulse, and weight are not negatively affected.18,50 Addiction and tolerance can develop with these drugs, and follow-up should include assessment for dependence. Some states may require prescription drug monitoring to ensure the drugs are not being abused or diverted.
Short- and long-acting formulations of both methylphenidate and amphetamines are available, and a long-acting form is often used in conjunction with a short-acting form as needed.18
Addiction and drug-seeking behavior can develop but are unusual in those taking stimulants to treat narcolepsy.49
Follow-up
Residual daytime sleepiness can be measured subjectively through the Epworth Sleepiness Scale on follow-up. If necessary, a maintenance-of-wakefulness test can provide an objective assessment of treatment efficacy.18
As narcolepsy is a chronic disorder, treatment should evolve with time. Most medications that treat narcolepsy are categorized by the US Food and Drug Administration as pregnancy category C, as we do not have adequate studies in human pregnancies to evaluate their effects. When a patient with narcolepsy becomes pregnant, she should be counseled about the risks and benefits of remaining on therapy. Treatment should balance the risks of sleepiness with the potential risks of remaining on medications.50 In the elderly, as cardiovascular comorbidities tend to increase, the risks and benefits of therapy should be routinely reevaluated.
For cataplexy
Sodium oxybate,51–53 the most potent anticataplectic drug, is the sodium salt of gamma hydroxybutyrate, a metabolite of gamma-aminobutyric acid. Sodium oxybate can be prescribed in the United States, Canada, and Europe. The American Academy of Sleep Medicine recommends sodium oxybate for cataplexy, daytime sleepiness, and disrupted sleep based on 3 level-1 studies and 2 level-4 studies.46
Sodium oxybate increases slow-wave sleep, improves sleep continuity, and often helps to mitigate daytime sleepiness. Due to its short half-life, its administration is unusual: the first dose is taken before bedtime and the second dose 2.5 to 4 hours later. Some patients set an alarm clock to take the second dose, while others awaken spontaneously to take the second dose. Most patients find that with adherence to dosing and safety instructions, sodium oxybate can serve as a highly effective form of treatment of both excessive sleepiness and cataplexy and may reduce the need for stimulant-based therapies.
The most common adverse effects are nausea, mood swings, and enuresis. Occasionally, psychosis can result and limit use of the drug. Obstructive sleep apnea can also develop or worsen.52 Because of its high salt content, sodium oxybate should be used with caution in those with heart failure, hypertension, or renal impairment. Its relative, gamma hydroxybutyrate, causes rapid sedation and has been notorious for illegal use as a date rape drug.
In the United States, sodium oxybate is distributed only through a central pharmacy to mitigate potential abuse. Due to this system, the rates of diversion are extremely low, estimated in a postmarketing analysis to be 1 instance per 5,200 patients treated. In the same study, abuse and dependence were both rare as well, about 1 case for every 2,600 and 6,500 patients treated.6,18,52,53
Antidepressants promote the action of norepinephrine and, to a lesser degree, serotonin, thereby suppressing REM sleep.
Venlafaxine, a serotonin-norepinephrine reuptake inhibitor, is often used as a first-line treatment for cataplexy. Selective serotonin reuptake inhibitors such as fluoxetine are also used with success. Tricyclic antidepressants such as protriptyline or clomipramine are extremely effective for cataplexy, but are rarely used due to their adverse effects.2,47
FUTURE WORK
While our understanding of narcolepsy has advanced, there are still gaps in our knowledge of the disorder—namely, the specific trigger for the loss of hypocretin neurons in type 1 narcolepsy and the underlying pathophysiology of type 2.
A number of emerging therapies target the hypocretin system, including peptide replacement, neuronal transplant, and immunotherapy preventing hypocretin neuronal cell death.50,54,55 Additional drugs designed to improve alertness that do not involve the hypocretin system are also being developed, including a histamine inverse agonist.50,56 Sodium oxybate and modafinil, although currently approved for use in adults, are still off-label in pediatric practice. Studies of the safety and efficacy of these medications in children are needed.7,57
Narcolepsy was originally described in the late 1800s by the French physician Jean-Baptiste-Edouard Gélineau, who reported the case of a wine merchant suffering from somnolence. In this first description, he coined the term narcolepsie by joining the Greek words narke (numbness or stupor) and lepsis (attack).1
Since then, the disorder has been further characterized, and some insight into its biological underpinnings has been established. Importantly, treatments have improved and expanded, facilitating its management and thereby improving quality of life for those with the disorder.
This review focuses on clinically relevant features of the disorder and proposes management strategies.
CLINICAL FEATURES
Narcolepsy is characterized by instability of sleep-wake transitions.
Daytime sleepiness
Clinically, narcolepsy manifests with excessive daytime sleepiness that can be personally and socially disabling. Cataplexy, sleep paralysis, and hypnagogic or hypnopompic hallucinations can also be present,2,3 but they are not necessary for diagnosis. In fact, a minority of patients with narcolepsy have all these symptoms.4 Narcolepsy is divided into type 1 (with cataplexy) and type 2 (without cataplexy).2
Sleepiness tends to be worse with inactivity, and sleep can often be irresistible. Sleep attacks can come on suddenly and may be brief enough to manifest as a lapse in consciousness.
Short naps tend to be refreshing. Rapid eye movement (REM) latency—the interval between falling asleep and the onset of the REM sleep—is short in narcolepsy, and since the REM stage is when dreaming occurs, naps often include dreaming. Therefore, when taking a history, it is worthwhile to ask patients whether they dream during naps; a yes answer supports the diagnosis of narcolepsy.5
In children, sleepiness can manifest in reduced concentration and behavioral issues.6 Napping after age 5 or 6 is considered abnormal and may reflect pathologic sleepiness.7
Cataplexy
Cataplexy—transient muscle weakness triggered by emotion—is a specific feature of narcolepsy type 1. It often begins in the facial muscles and can manifest with slackening of the jaw or brief dropping of the head. However, episodes can be more dramatic and, if the trunk and limb muscles are affected, can result in collapsing to the ground.
Cataplexy usually has its onset at about the same time as the sleepiness associated with narcolepsy, but it can arise even years later.8 Episodes can last from a few seconds to 2 minutes. Consciousness is always preserved. A range of emotions can trigger cataplexy, but typically the emotion is a positive one such as laughter or excitement.9 Deep tendon reflexes disappear in cataplexy, so checking reflexes during a witnessed episode can be clinically valuable.2
Cataplexy can worsen with stress and insufficient sleep, occasionally with “status cataplecticus,” in which repeated, persistent episodes of cataplexy occur over several hours.8 Status cataplecticus can be spontaneous or an effect of withdrawal from anticataplectic medications.2
Cataplexy is thought to represent intrusion of REM sleep and its associated muscle atonia during wakefulness.
Sleep paralysis, hallucinations
Sleep paralysis and hallucinations are other features of narcolepsy that reflect this REM dissociation from sleep.
Sleep paralysis occurs most commonly upon awakening, but sometimes just before sleep onset. In most cases, it is manifested by inability to move the limbs or speak, lasting several seconds or, in rare cases, minutes at a time. Sleep paralysis can be associated with a sensation of fear or suffocation, especially when initially experienced.8
Hypnopompic hallucinations, occurring upon awakening, are more common than hypnagogic hallucinations, which are experienced before falling asleep. The hallucinations are often vivid and usually visual, although other types of hallucinations are possible. Unlike those that occur in psychotic disorders, the hallucinations tend to be associated with preserved insight that they are not real.10
Notably, both sleep paralysis and hallucinations are nonspecific symptoms that are common in the general population.8,11,12
Fragmented sleep
Although they are very sleepy, people with narcolepsy generally cannot stay asleep for very long. Their sleep tends to be extremely fragmented, and they often wake up several times a night.2
This sleep pattern reflects the inherent instability of sleep-wake transitions in narcolepsy. In fact, over a 24-hour period, adults with narcolepsy have a normal amount of sleep.13 In children, however, when narcolepsy first arises, the 24-hour sleep time can increase abruptly and can sometimes be associated with persistent cataplexy that can manifest as a clumsy gait.14
Weight gain, obstructive sleep apnea
Weight gain is common, particularly after symptom onset, and especially in children. As a result, obesity is a frequent comorbidity.15 Because obstructive sleep apnea can consequently develop, all patients with narcolepsy require screening for sleep-disordered breathing.
Other sleep disorders often accompany narcolepsy and are more common than in the general population.16 In a study incorporating both clinical and polysomnographic data of 100 patients with narcolepsy, insomnia was the most common comorbid sleep disorder, with a prevalence of 28%; others were REM sleep behavior disorder (24%), restless legs syndrome (24%), obstructive sleep apnea (21%), and non-REM parasomnias.17
PSYCHOSOCIAL CONSEQUENCES
Narcolepsy has significant psychosocial consequences. As a result of their symptoms, people with narcolepsy may not be able to meet academic or work-related demands.
Additionally, their risk of a motor vehicle accident is 3 to 4 times higher than in the general population, and more than one-third of patients have been in an accident due to sleepiness.18 There is some evidence to show that treatment eliminates this risk.19
Few systematic studies have examined mood disorders in narcolepsy. However, studies tend to show a higher prevalence of psychiatric disorders than in the general population, with depression and anxiety the most com-mon.20,21
DIAGNOSIS IS OFTEN DELAYED
The prevalence of narcolepsy type 1 is between 25 and 100 per 100,000 people.22 In a Mayo Clinic study,23 the incidence of narcolepsy type 1 was estimated to be 0.74 per 100,000 person-years. Epidemiologic data on narcolepsy type 2 are sparse, but patients with narcolepsy without cataplexy are thought to represent only 36% of all narcolepsy patients.23
Diagnosis is often delayed, with the average time between the onset of symptoms and the diagnosis ranging from 8 to 22 years. With increasing awareness, the efficiency of the diagnostic process is improving, and this delay is expected to lessen accordingly.24
Symptoms most commonly arise in the second decade; but the age at onset ranges significantly, between the first and fifth decades. Narcolepsy has a bimodal distribution in incidence, with the biggest peak at approximately age 15 and second smaller peak in the mid-30s. Some studies have suggested a slight male predominance.23,25
DIAGNOSIS
History is key
The history should include specific questions about the hallmark features of narcolepsy, including cataplexy, sleep paralysis, and sleep-related hallucinations. For individual assessment of subjective sleepiness, the Epworth Sleepiness Scale or Pediatric Daytime Sleepiness Scale can be administered quickly in the office setting.26,27
The Epworth score is calculated from the self-rated likelihood of falling asleep in 8 different situations, with possible scores of 0 (would never doze) to 3 (high chance of dozing) on each question, for a total possible score of 0 to 24. Normal total scores are between 0 and 10, while scores greater than 10 reflect pathologic sleepiness. Scores on the Epworth Sleepiness Scale in those with narcolepsy tend to reflect moderate to severe sleepiness, or at least 13, as opposed to patients with obstructive sleep apnea, whose scores commonly reflect milder sleepiness.28
Testing with actigraphy and polysomnography
It is imperative to rule out insufficient sleep and other sleep disorders as a cause of daytime sleepiness. This can be done with a careful clinical history, actigraphy with sleep logs, and polysomnography.
In the 2 to 4 weeks before actigraphy and subsequent testing, all medications with alerting or sedating properties (including antidepressants) should be tapered off to prevent influence on the results of the study.
Delayed sleep-phase disorder presents at a similar age as narcolepsy and can be associated with similar degrees of sleepiness. However, individuals with delayed sleep phase disorder have an inappropriately timed sleep-wake cycle so that there is a shift in their desired sleep onset and awakening times. It is common—prevalence estimates vary but average about 1% in the general population.29
Insufficient sleep syndrome is even more common, especially in teenagers and young adults, with increasing family, social, and academic demands. Sleep needs vary across the life span. A teenager needs 8 to 10 hours of sleep per night, and a young adult needs 7 to 9 hours. A study of 1,285 high school students found that 10.4% were not getting enough sleep.30
If actigraphy data suggest a circadian rhythm disorder or insufficient sleep that could explain the symptoms of sleepiness, then further testing should be halted and these specific issues should be addressed. In these cases, working with the patient toward maintaining a regular sleep-wake schedule with 7 to 8 hours of nightly sleep will often resolve symptoms.
If actigraphy demonstrates the patient is maintaining a regular sleep schedule and allowing adequate time for nightly sleep, the next step is polysomnography.
Polysomnography is performed to detect other disorders that can disrupt sleep, such as sleep-disordered breathing or periodic limb movement disorder.2,5 In addition, polysomnography can provide assurance that adequate sleep was obtained prior to the next step in testing.
Multiple sleep latency test
If sufficient sleep is obtained on polysomnograpy (at least 6 hours for an adult) and no other sleep disorder is identified, a multiple sleep latency test is performed. A urine toxicology screen is typically performed on the day of the test to ensure that drugs are not affecting the results.
The multiple sleep latency test consists of 4 to 5 nap opportunities at 2-hour intervals in a quiet dark room conducive to sleep, during which both sleep and REM latency are recorded. The sleep latency of those with narcolepsy is significantly shortened, and the diagnosis of narcolepsy requires an average sleep latency of less than 8 minutes.
Given the propensity for REM sleep in narcolepsy, another essential feature for diagnosis is the sleep-onset REM period (SOREMP). A SOREMP is defined as a REM latency of less than 15 minutes. A diagnosis of narcolepsy re-quires a SOREMP in at least 2 of the naps in a multiple sleep latency test (or 1 nap if the shortened REM latency is seen during polysomnography).31
The multiple sleep latency test has an imperfect sensitivity, though, and should be repeated when there is a high suspicion of narcolepsy.32–34 It is not completely specific either, and false-positive results occur. In fact, SOREMPs can be seen in the general population, particularly in those with a circadian rhythm disorder, insufficient sleep, or sleep-disordered breathing. Two or more SOREMPs in an multiple sleep latency test can be seen in a small proportion of the general population.35 The results of a multiple sleep latency test should be interpreted in the clinical context.
Differential diagnosis
Narcolepsy type 1 is distinguished from type 2 by the presence of cataplexy. A cerebrospinal fluid hypocretin 1 level of 110 pg/mL or less, or less than one-third of the mean value obtained in normal individuals, can substitute for the multiple sleep latency test in diagnosing narcolepsy type 1.31 Currently, hypocretin testing is generally not performed in clinical practice, although it may become a routine part of the narcolepsy evaluation in the future.
Thus, according to the International Classification of Sleep Disorders, 3rd edition,31 the diagnosis of narcolepsy type 1 requires excessive daytime sleepiness for at least 3 months that cannot be explained by another sleep disorder, medical or neurologic disorder, mental disorder, medication use, or substance use disorder, and at least 1 of the following:
- Cataplexy and mean sleep latency of 8 minutes or less with at least 2 SOREMPs on multiple sleep latency testing (1 of which can be on the preceding night’s polysomography)
- Cerebrospinal fluid hypocretin 1 levels less than 110 pg/mL or one-third the baseline normal levels and mean sleep latency ≤ 8 minutes with ≥ 2 SOREMPs on multiple sleep latency testing.
Similarly, the diagnosis of narcolepsy type 2 requires excessive daytime sleepiness for at least 3 months that cannot be explained by another sleep disorder, medical or neurological disorder, mental disorder, medication use, or substance use disorder, plus:
- Mean sleep latency of 8 minutes or less with at least 2 SOREMPs on multiple sleep latency testing.
Idiopathic hypersomnia, another disorder of central hypersomnolence, is also characterized by disabling sleepiness. It is diagnostically differentiated from narcolepsy, as there are fewer than 2 SOREMPs. As opposed to narcolepsy, in which naps tend to be refreshing, even prolonged naps in idiopathic hypersomnia are often not helpful in restoring wakefulness. In idiopathic hypersomnia, sleep is usually not fragmented, and there are few nocturnal arousals. Sleep times can often be prolonged as well, whereas in narcolepsy total sleep time through the day may not be increased but is not consolidated.
Kleine-Levin syndrome is a rarer disorder of hypersomnia. It is episodic compared with the relatively persistent sleepiness in narcolepsy and idiopathic hypersomnia. Periods of hypersomnia occur intermittently for days to weeks and are accompanied by cognitive and behavioral changes including hyperphagia and hypersexuality.4
LINKED TO HYPOCRETIN DEFICIENCY
Over the past 2 decades, the underlying pathophysiology of narcolepsy type 1 has been better characterized.
Narcolepsy type 1 has been linked to a deficiency in hypocretin in the central nervous system.36 Hypocretin (also known as orexin) is a hormone produced in the hypothalamus that acts on multiple brain regions and maintains alertness. For unclear reasons, hypothalamic neurons producing hypocretin are selectively reduced in narcolepsy type 1. Hypocretin also stabilizes wakefulness and inhibits REM sleep; therefore, hypocretin deficiency can lead to inappropriate intrusions of REM sleep onto wakefulness, leading to the hallmark features of narcolepsy—cataplexy, sleep-related hallucinations, and sleep paralysis.37 According to one theory, cataplexy is triggered by emotional stimuli because of a pathway between the medial prefrontal cortex and the amygdala to the pons.38
Cerebrospinal fluid levels of hypocretin in patients with narcolepsy type 2 tend to be normal, and the biologic underpinnings of narcolepsy type 2 remain mysterious. However, in the subgroup of those with narcolepsy type 2 in which hypocretin is low, many individuals go on to develop cataplexy, thereby evolving to narcolepsy type 1.36
POSSIBLE AUTOIMMUNE BASIS
Narcolepsy is typically a sporadic disorder, although familial cases have been described. The risk of a parent with narcolepsy having a child who is affected is approximately 1%.5
Narcolepsy type 1 is strongly associated with HLA-DQB1*0602, with up to 95% of those affected having at least one allele.39 Having 2 copies of the allele further increases the risk of developing narcolepsy.40 However, this allele is far from specific for narcolepsy with cataplexy, as it occurs in 12% to 38% of the general population.41 Therefore, HLA typing currently has limited clinical utility. The exact cause is as yet unknown, but substantial literature proposes an autoimmune basis of the disorder, given the strong association with the HLA subtype.42–44
After the 2009 H1N1 influenza pandemic, there was a significant increase in the incidence of narcolepsy with cataplexy, which again sparked interest in an autoimmune etiology underlying the disorder. Pandemrix, an H1N1 vaccine produced as a result of the 2009 pandemic, appeared to also be associated with an increase in the incidence of narcolepsy. An association with other upper respiratory infections has also been noted, further supporting a possible autoimmune basis.
A few studies have looked for serum autoantibodies involved in the pathogenesis of narcolepsy. Thus far, only one has been identified, an antibody to Tribbles homolog 2, found in 20% to 40% of those with new onset of nar-colepsy.42–44
TREATMENTS FOR DAYTIME SLEEPINESS
As with many chronic disorders, the treatment of narcolepsy consists of symptomatic rather than curative management, which can be done through both pharmacologic and nonpharmacologic means.
Nondrug measures
Scheduled naps lasting 15 to 20 minutes can help improve alertness.45 A consistent sleep schedule with good sleep hygiene, ensuring sufficient nightly sleep, is also important. In one study, the combination of scheduled naps and regular nocturnal sleep times reduced the level of daytime sleepiness and unintentional daytime sleep. Daytime naps were most helpful for those with the highest degree of daytime sleepiness.45
Strategic use of caffeine can be helpful and can reduce dependence on pharmacologic treatment.
Screening should be performed routinely for other sleep disorders, such as sleep-disordered breathing, which should be treated if identified.5,18 When being treated for other medical conditions, individuals with narcolepsy should avoid medications that can cause sedation, such as opiates or barbiturates; alcohol should be minimized or avoided.
Networking with other individuals with narcolepsy through support groups such as Narcolepsy Network can be valuable for learning coping skills and connecting with community resources. Psychological counseling for the patient, and sometimes the family, can also be useful. School-age children may need special accommodations such as schedule adjustments to allow for scheduled naps or frequent breaks to maintain alertness.
People with narcolepsy tend to function better in careers that do not require long periods of sitting, as sleepiness tends to be worse, but instead offer flexibility and require higher levels of activity that tend to combat sleepiness. They should not work as commercial drivers.18
Medications
While behavioral interventions in narcolepsy are vital, they are rarely sufficient, and drugs that promote daytime wakefulness are used as an adjunct (Table 2).46
Realistic expectations should be established when starting, as some degree of residual sleepiness usually remains even with optimal medical therapy. Medications should be strategically scheduled to maximize alertness during necessary times such as at work or school or during driving. Patients should specifically be counseled to avoid driving if sleepy.18,47
Modafinil is often used as a first-line therapy, given its favorable side-effect profile and low potential for abuse. Its pharmacologic action has been debated but it probably acts as a selective dopamine reuptake inhibitor. It is typically taken twice daily (upon waking and early afternoon) and is usually well tolerated.
Potential side effects include headache, nausea, dry mouth, anorexia, diarrhea, and, rarely, Stevens-Johnson syndrome. Cardiovascular side effects are minimal, making it a favorable choice in older patients.18,48
A trial in 283 patients showed significantly lower levels of sleepiness in patients taking modafinil 200 mg or 400 mg than in a control group. Other trials have supported these findings and showed improved driving performance on modafinil.18
Notably, modafinil can increase the metabolism of oral contraceptives, thereby reducing their efficacy. Women of childbearing age should be warned about this interaction and should be transitioned to nonhormonal forms of contraception.2,47
Armodafinil, a purified R-isomer of modafinil, has a longer half-life and requires only once-daily dosing.5
If modafinil or armodafinil fails to optimally manage daytime sleepiness, a traditional stimulant such as methylphenidate or an amphetamine is often used.
Methylphenidate and amphetamines primarily inhibit the reuptake and increase the release of the monoamines, mainly dopamine, and to a lesser degree serotonin and norepinephrine.
These drugs have more significant adverse effects that can involve the cardiovascular system, causing hypertension and arrhythmias. Anorexia, weight loss, and, particularly with high doses, psychosis can occur.49
These drugs should be avoided in patients with a history of significant cardiovascular disease. Before starting stimulant therapy, a thorough cardiovascular examination should be done, often including electrocardiography to ensure there is no baseline arrhythmia.
Patients on these medications should be followed closely to ensure that blood pressure, pulse, and weight are not negatively affected.18,50 Addiction and tolerance can develop with these drugs, and follow-up should include assessment for dependence. Some states may require prescription drug monitoring to ensure the drugs are not being abused or diverted.
Short- and long-acting formulations of both methylphenidate and amphetamines are available, and a long-acting form is often used in conjunction with a short-acting form as needed.18
Addiction and drug-seeking behavior can develop but are unusual in those taking stimulants to treat narcolepsy.49
Follow-up
Residual daytime sleepiness can be measured subjectively through the Epworth Sleepiness Scale on follow-up. If necessary, a maintenance-of-wakefulness test can provide an objective assessment of treatment efficacy.18
As narcolepsy is a chronic disorder, treatment should evolve with time. Most medications that treat narcolepsy are categorized by the US Food and Drug Administration as pregnancy category C, as we do not have adequate studies in human pregnancies to evaluate their effects. When a patient with narcolepsy becomes pregnant, she should be counseled about the risks and benefits of remaining on therapy. Treatment should balance the risks of sleepiness with the potential risks of remaining on medications.50 In the elderly, as cardiovascular comorbidities tend to increase, the risks and benefits of therapy should be routinely reevaluated.
For cataplexy
Sodium oxybate,51–53 the most potent anticataplectic drug, is the sodium salt of gamma hydroxybutyrate, a metabolite of gamma-aminobutyric acid. Sodium oxybate can be prescribed in the United States, Canada, and Europe. The American Academy of Sleep Medicine recommends sodium oxybate for cataplexy, daytime sleepiness, and disrupted sleep based on 3 level-1 studies and 2 level-4 studies.46
Sodium oxybate increases slow-wave sleep, improves sleep continuity, and often helps to mitigate daytime sleepiness. Due to its short half-life, its administration is unusual: the first dose is taken before bedtime and the second dose 2.5 to 4 hours later. Some patients set an alarm clock to take the second dose, while others awaken spontaneously to take the second dose. Most patients find that with adherence to dosing and safety instructions, sodium oxybate can serve as a highly effective form of treatment of both excessive sleepiness and cataplexy and may reduce the need for stimulant-based therapies.
The most common adverse effects are nausea, mood swings, and enuresis. Occasionally, psychosis can result and limit use of the drug. Obstructive sleep apnea can also develop or worsen.52 Because of its high salt content, sodium oxybate should be used with caution in those with heart failure, hypertension, or renal impairment. Its relative, gamma hydroxybutyrate, causes rapid sedation and has been notorious for illegal use as a date rape drug.
In the United States, sodium oxybate is distributed only through a central pharmacy to mitigate potential abuse. Due to this system, the rates of diversion are extremely low, estimated in a postmarketing analysis to be 1 instance per 5,200 patients treated. In the same study, abuse and dependence were both rare as well, about 1 case for every 2,600 and 6,500 patients treated.6,18,52,53
Antidepressants promote the action of norepinephrine and, to a lesser degree, serotonin, thereby suppressing REM sleep.
Venlafaxine, a serotonin-norepinephrine reuptake inhibitor, is often used as a first-line treatment for cataplexy. Selective serotonin reuptake inhibitors such as fluoxetine are also used with success. Tricyclic antidepressants such as protriptyline or clomipramine are extremely effective for cataplexy, but are rarely used due to their adverse effects.2,47
FUTURE WORK
While our understanding of narcolepsy has advanced, there are still gaps in our knowledge of the disorder—namely, the specific trigger for the loss of hypocretin neurons in type 1 narcolepsy and the underlying pathophysiology of type 2.
A number of emerging therapies target the hypocretin system, including peptide replacement, neuronal transplant, and immunotherapy preventing hypocretin neuronal cell death.50,54,55 Additional drugs designed to improve alertness that do not involve the hypocretin system are also being developed, including a histamine inverse agonist.50,56 Sodium oxybate and modafinil, although currently approved for use in adults, are still off-label in pediatric practice. Studies of the safety and efficacy of these medications in children are needed.7,57
- Gélineau J. De la narcolepsie. Gazette des Hôpitaux Civils et Militaires 1880; part a, 53:626–628, part b, 54:635–637.
- Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet 2007; 369(9560):499–511. doi:10.1016/S0140-6736(07)60237-2
- Scammell TE. Clinical features and diagnosis of narcolepsy in adults. In: Eichler AF, ed. UpToDate. Waltham, MA: UpToDate; 2018. www.uptodate.com. Accessed October 31, 2018.
- Morrish E, King MA, Smith IE, Shneerson JM. Factors associated with a delay in the diagnosis of narcolepsy. Sleep Med 2004; 5(1):37–41. pmid:14725825
- Scammell TE. Narcolepsy. N Engl J Med 2015; 373(27):2654–2662. doi:10.1056/NEJMra1500587
- Babiker MO, Prasad M. Narcolepsy in children: a diagnostic and management approach. Pediatr Neurol 2015; 52(6):557–565. doi:10.1016/j.pediatrneurol.2015.02.020
- Kotagal S. Narcolepsy in children. In: UpToDate, Eichler AF, ed. UpToDate, Waltham, MA. www.uptodate.com. Accessed October 31, 2018.
- Scammell TE. The neurobiology, diagnosis, and treatment of narcolepsy. Ann Neurol 2003; 53(2):154–166. doi:10.1002/ana.10444
- Overeem S, van Nues SJ, van der Zande WL, Donjacour CE, van Mierlo P, Lammers GJ. The clinical features of cataplexy: a questionnaire study in narcolepsy patients with and without hypocretin-1 deficiency. Sleep Med 2011; 12(1):12–18. doi:10.1016/j.sleep.2010.05.010
- Plazzi G, Fabbri C, Pizza F, Serretti A. Schizophrenia-like symptoms in narcolepsy type 1: shared and distinctive clinical characteristics. Neuropsychobiology 2015; 71(4):218–224. doi:10.1159/000432400
- Ohayon MM. Prevalence of hallucinations and their pathological associations in the general population. Psychiatry Res 2000; 97(2-3):153–164. pmid:11166087
- Sharpless BA, Barber JP. Lifetime prevalence rates of sleep paralysis: a systematic review. Sleep Med Rev 2011;5(5):311–315. doi:10.1016/j.smrv.2011.01.007
- Broughton R, Dunham W, Newman J, Lutley K, Duschesne P, Rivers M. Ambulatory 24 hour sleep-wake monitoring in narcolepsy-cataplexy compared to matched controls. Electroencephalogr Clin Neurophysiol 1988; 70(6):473–481. pmid:2461281
- Pizza F, Franceschini C, Peltola H, et al. Clinical and polysomnographic course of childhood narcolepsy with cataplexy. Brain 2013; 136(pt 12):3787–3795. doi:10.1093/brain/awt277
- Kotagal S, Krahn LE, Slocumb N. A putative link between childhood narcolepsy and obesity. Sleep Med 2004; 5(2):147–150. doi:10.1016/j.sleep.2003.10.006
- Pizza F, Tartarotti S, Poryazova R, Baumann CR, Bassetti CL. Sleep-disordered breathing and periodic limb movements in narcolepsy with cataplexy: a systematic analysis of 35 consecutive patients. Eur Neurol 2013; 70(1-2):22–26. doi:10.1159/000348719
- Frauscher B, Ehrmann L, Mitterling T, et al. Delayed diagnosis, range of severity, and multiple sleep comorbidities: a clinical and polysomnographic analysis of 100 patients of the Innsbruck narcolepsy cohort. J Clin Sleep Med 2013; 9(8):805–812. doi:10.5664/jcsm.2926
- Scammell TE. Treatment of narcolepsy in adults. In: Eichler AF, ed. UpToDate, Waltham, MA. www.uptodate.com. Accessed October 31, 2018.
- Pizza F, Jaussent I, Lopez R, et al. Car crashes and central disorders of hypersomnolence: a French study. PLoS One 2015; 10(6):e0129386. doi:10.1371/journal.pone.0129386
- Fortuyn HD, Lappenschaar MA, Furer JW, et al. Anxiety and mood disorders in narcolepsy: a case-control study. Gen Hosp Psychiatry 2010; 32(1):49–56. doi:10.1016/j.genhosppsych.2009.08.007
- Ruoff CM, Reaven NL, Funk SE, et al. High rates of psychiatric comorbidity in narcolepsy: findings from the Burden of Narcolepsy Disease (BOND) study of 9,312 patients in the United States. J Clin Psychiatry 2017; 78(2):171–176. doi:10.4088/JCP.15m10262
- Longstreth WT Jr, Koepsell TD, Ton TG, Hendrickson AF, van Belle G. The epidemiology of narcolepsy. Sleep. 2007; 30(1):13–26. pmid:17310860
- Silber MH, Krahn LE, Olson EJ, Pankratz VS. The epidemiology of narcolepsy in Olmsted County, Minnesota: a population-based study. Sleep 2002; 25(2):197–202. pmid:11902429
- Thorpy MJ, Krieger AC. Delayed diagnosis of narcolepsy: characterization and impact. Sleep Med 2014; 15(5):502–507. doi:10.1016/j.sleep.2014.01.015
- Dauvilliers Y, Montplaisir J, Molinari N, et al. Age at onset of narcolepsy in two large populations of patients in France and Quebec. Neurology 2001; 57(11):2029–2033. pmid:11739821
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991; 14(6):540–545. pmid:1798888
- Drake C, Nickel C, Burduvali E, Roth T, Jefferson C, Badia P. The pediatric daytime sleepiness scale (PDSS): sleep habits and school outcomes in middle-school children. Sleep 2003; 26(4):455–458. pmid:12841372
- van der Heide A, van Schie MK, Lammers GJ, et al. Comparing treatment effect measurements in narcolepsy: the sustained attention to response task, Epworth sleepiness scale and maintenance of wakefulness test. Sleep 2015; 38(7):1051–1058. doi:10.5665/sleep.4810
- Nesbitt AD. Delayed sleep-wake phase disorder. J Thorac Dis 2018; 10(suppl 1):S103–S111. doi:10.21037/jtd.2018.01.11
- Pallesen S, Saxvig IW, Molde H, Sørensen E, Wilhelmsen-Langeland A, Bjorvatn B. Brief report: behaviorally induced insufficient sleep syndrome in older adolescents: prevalence and correlates. J Adolesc 2011; 34(2):391–395. doi:10.1016/j.adolescence.2010.02.005
- American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Disorders; 2014.
- Trotti LM, Staab BA, Rye DB. Test-retest reliability of the multiple sleep latency test in narcolepsy without cataplexy and idiopathic hypersomnia. J Clin Sleep Med 2013; 9(8):789–795. doi:10.5664/jcsm.2922
- Andlauer O, Moore H, Jouhier L, et al. Nocturnal rapid eye movement sleep latency for identifying patients with narcolepsy/hypocretin deficiency. JAMA Neurol 2013; 70(7):891–902. doi:10.1001/jamaneurol.2013.1589
- Cairns A, Bogan R. Prevalence and clinical correlates of a short onset REM period (SOREMP) during routine PSG. Sleep 2015; 38(10):1575–1581. doi:10.5665/sleep.5050
- Mignot E, Lin L, Finn L, et al. Correlates of sleep-onset REM periods during the multiple sleep latency test in community adults. Brain 2006; 129(6):1609–1623. doi:10.1093/brain/awl079
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet 2000; 355(9197):39–40. doi:10.1016/S0140-6736(99)05582-8
- Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med 2000; 6(9):991–997. doi:10.1038/79690
- Oishi Y, Williams RH, Agostinelli L, et al. Role of the medial prefrontal cortex in cataplexy. J Neurosci 2013; 33(23):9743–9751. doi:10.1523/JNEUROSCI.0499-13.2013
- Mignot E, Hayduk R, Black J, Grumet FC, Guilleminault C. HLA DQB1*0602 is associated with cataplexy in 509 narcoleptic patients.. Sleep 1997; 20(11):1012–1020. pmid:9456467
- Pelin Z, Guilleminault C, Risch N, Grumet FC, Mignot E. HLA-DQB1*0602 homozygosity increases relative risk for narcolepsy but not disease severity in two ethnic groups. US Modafinil in Narcolepsy Multicenter Study Group. Tissue Antigens 1998; 51(1):96–100. pmid:9459509
- Akintomide GS, Rickards H. Narcolepsy: a review. Neuropsychiatr Dis Treat 2011; 7(1):507–518. doi:10.2147/NDT.S23624
- Mahlios J, De la Herrán-Arita AK, Mignot E. The autoimmune basis of narcolepsy. Curr Opin Neurobiol 2013; 23(5):767–773. doi:10.1016/j.conb.2013.04.013
- Degn M, Kornum BR. Type 1 narcolepsy: a CD8(+) T cell-mediated disease? Ann N Y Acad Sci 2015;1 351:80–88. doi:10.1111/nyas.12793
- Liblau RS, Vassalli A, Seifinejad A, Tafti M. Hypocretin (orexin) biology and the pathophysiology of narcolepsy with cataplexy. Lancet Neurol 2015; 14(3):318–328. doi:10.1016/S1474-4422(14)70218-2
- Rogers AE, Aldrich MS, Lin X. A comparison of three different sleep schedules for reducing daytime sleepiness in narcolepsy. Sleep 2001; 24(4):385–391. pmid:11403522
- Morgenthaler TI, Kapur VK, Brown TM, et al; Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep 2007; 30(12):1705–1711. pmid:18246980
- Mignot EJ. A practical guide to the therapy of narcolepsy and hypersomnia syndromes. Neurotherapeutics 2012; 9(4):739–752. doi:10.1007/s13311-012-0150-9
- Roth T, Schwartz JR, Hirshkowitz M, Erman MK, Dayno JM, Arora S. Evaluation of the safety of modafinil for treatment of excessive sleepiness. J Clin Sleep Med 2007; 3(6):595–602. pmid:17993041
- Auger RR, Goodman SH, Silber MH, Krahn LE, Pankratz VS, Slocumb NL. Risks of high-dose stimulants in the treatment of disorders of excessive somnolence: a case-control study. Sleep 2005; 28(6):667–672. pmid:16477952
- Abad VC, Guilleminault C. New developments in the management of narcolepsy. Nat Sci Sleep 2017; 9:39–57. doi:10.2147/NSS.S103467
- Drakatos P, Lykouras D, D’Ancona G, et al. Safety and efficacy of long-term use of sodium oxybate for narcolepsy with cataplexy in routine clinical practice. Sleep Med 2017; 35:80–84. doi:10.1016/j.sleep.2017.03.028
- Mansukhani MP, Kotagal S. Sodium oxybate in the treatment of childhood narcolepsy–cataplexy: a retrospective study. Sleep Med 2012; 13(6):606–610. doi:10.1016/j.sleep.2011.10.032
- Wang YG, Swick TJ, Carter LP, Thorpy MJ, Benowitz NL. Safety overview of postmarketing and clinical experience of sodium oxybate (Xyrem): abuse, misuse, dependence, and diversion. J Clin Sleep Med 2009; 5(4):365–371. pmid:19968016
- Weinhold SL, Seeck-Hirschner M, Nowak A, Hallschmid M, Göder R, Baier PC. The effect of intranasal orexin-A (hypocretin-1) on sleep, wakefulness and attention in narcolepsy with cataplexy. Behav Brain Res 2014; 262:8–13. doi:10.1016/j.bbr.2013.12.045
- Arias-Carrión O, Murillo-Rodriguez E. Effects of hypocretin/orexin cell transplantation on narcoleptic-like sleep behavior in rats. PLoS One 2014; 9(4):e95342. doi:10.1371/journal.pone.0095342
- Leu-Semenescu S, Nittur N, Golmard JL, Arnulf I. Effects of pitolisant, a histamine H3 inverse agonist, in drug-resistant idiopathic and symptomatic hypersomnia: a chart review. Sleep Med 2014; 15(6):681–687. doi:10.1016/j.sleep.2014.01.021
- Lecendreux M, Bruni O, Franco P, et al. Clinical experience suggests that modafinil is an effective and safe treatment for paediatric narcolepsy. J Sleep Res 2012; 21(4):481–483. doi:10.1111/j.1365-2869.2011.00991.x
- Gélineau J. De la narcolepsie. Gazette des Hôpitaux Civils et Militaires 1880; part a, 53:626–628, part b, 54:635–637.
- Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet 2007; 369(9560):499–511. doi:10.1016/S0140-6736(07)60237-2
- Scammell TE. Clinical features and diagnosis of narcolepsy in adults. In: Eichler AF, ed. UpToDate. Waltham, MA: UpToDate; 2018. www.uptodate.com. Accessed October 31, 2018.
- Morrish E, King MA, Smith IE, Shneerson JM. Factors associated with a delay in the diagnosis of narcolepsy. Sleep Med 2004; 5(1):37–41. pmid:14725825
- Scammell TE. Narcolepsy. N Engl J Med 2015; 373(27):2654–2662. doi:10.1056/NEJMra1500587
- Babiker MO, Prasad M. Narcolepsy in children: a diagnostic and management approach. Pediatr Neurol 2015; 52(6):557–565. doi:10.1016/j.pediatrneurol.2015.02.020
- Kotagal S. Narcolepsy in children. In: UpToDate, Eichler AF, ed. UpToDate, Waltham, MA. www.uptodate.com. Accessed October 31, 2018.
- Scammell TE. The neurobiology, diagnosis, and treatment of narcolepsy. Ann Neurol 2003; 53(2):154–166. doi:10.1002/ana.10444
- Overeem S, van Nues SJ, van der Zande WL, Donjacour CE, van Mierlo P, Lammers GJ. The clinical features of cataplexy: a questionnaire study in narcolepsy patients with and without hypocretin-1 deficiency. Sleep Med 2011; 12(1):12–18. doi:10.1016/j.sleep.2010.05.010
- Plazzi G, Fabbri C, Pizza F, Serretti A. Schizophrenia-like symptoms in narcolepsy type 1: shared and distinctive clinical characteristics. Neuropsychobiology 2015; 71(4):218–224. doi:10.1159/000432400
- Ohayon MM. Prevalence of hallucinations and their pathological associations in the general population. Psychiatry Res 2000; 97(2-3):153–164. pmid:11166087
- Sharpless BA, Barber JP. Lifetime prevalence rates of sleep paralysis: a systematic review. Sleep Med Rev 2011;5(5):311–315. doi:10.1016/j.smrv.2011.01.007
- Broughton R, Dunham W, Newman J, Lutley K, Duschesne P, Rivers M. Ambulatory 24 hour sleep-wake monitoring in narcolepsy-cataplexy compared to matched controls. Electroencephalogr Clin Neurophysiol 1988; 70(6):473–481. pmid:2461281
- Pizza F, Franceschini C, Peltola H, et al. Clinical and polysomnographic course of childhood narcolepsy with cataplexy. Brain 2013; 136(pt 12):3787–3795. doi:10.1093/brain/awt277
- Kotagal S, Krahn LE, Slocumb N. A putative link between childhood narcolepsy and obesity. Sleep Med 2004; 5(2):147–150. doi:10.1016/j.sleep.2003.10.006
- Pizza F, Tartarotti S, Poryazova R, Baumann CR, Bassetti CL. Sleep-disordered breathing and periodic limb movements in narcolepsy with cataplexy: a systematic analysis of 35 consecutive patients. Eur Neurol 2013; 70(1-2):22–26. doi:10.1159/000348719
- Frauscher B, Ehrmann L, Mitterling T, et al. Delayed diagnosis, range of severity, and multiple sleep comorbidities: a clinical and polysomnographic analysis of 100 patients of the Innsbruck narcolepsy cohort. J Clin Sleep Med 2013; 9(8):805–812. doi:10.5664/jcsm.2926
- Scammell TE. Treatment of narcolepsy in adults. In: Eichler AF, ed. UpToDate, Waltham, MA. www.uptodate.com. Accessed October 31, 2018.
- Pizza F, Jaussent I, Lopez R, et al. Car crashes and central disorders of hypersomnolence: a French study. PLoS One 2015; 10(6):e0129386. doi:10.1371/journal.pone.0129386
- Fortuyn HD, Lappenschaar MA, Furer JW, et al. Anxiety and mood disorders in narcolepsy: a case-control study. Gen Hosp Psychiatry 2010; 32(1):49–56. doi:10.1016/j.genhosppsych.2009.08.007
- Ruoff CM, Reaven NL, Funk SE, et al. High rates of psychiatric comorbidity in narcolepsy: findings from the Burden of Narcolepsy Disease (BOND) study of 9,312 patients in the United States. J Clin Psychiatry 2017; 78(2):171–176. doi:10.4088/JCP.15m10262
- Longstreth WT Jr, Koepsell TD, Ton TG, Hendrickson AF, van Belle G. The epidemiology of narcolepsy. Sleep. 2007; 30(1):13–26. pmid:17310860
- Silber MH, Krahn LE, Olson EJ, Pankratz VS. The epidemiology of narcolepsy in Olmsted County, Minnesota: a population-based study. Sleep 2002; 25(2):197–202. pmid:11902429
- Thorpy MJ, Krieger AC. Delayed diagnosis of narcolepsy: characterization and impact. Sleep Med 2014; 15(5):502–507. doi:10.1016/j.sleep.2014.01.015
- Dauvilliers Y, Montplaisir J, Molinari N, et al. Age at onset of narcolepsy in two large populations of patients in France and Quebec. Neurology 2001; 57(11):2029–2033. pmid:11739821
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991; 14(6):540–545. pmid:1798888
- Drake C, Nickel C, Burduvali E, Roth T, Jefferson C, Badia P. The pediatric daytime sleepiness scale (PDSS): sleep habits and school outcomes in middle-school children. Sleep 2003; 26(4):455–458. pmid:12841372
- van der Heide A, van Schie MK, Lammers GJ, et al. Comparing treatment effect measurements in narcolepsy: the sustained attention to response task, Epworth sleepiness scale and maintenance of wakefulness test. Sleep 2015; 38(7):1051–1058. doi:10.5665/sleep.4810
- Nesbitt AD. Delayed sleep-wake phase disorder. J Thorac Dis 2018; 10(suppl 1):S103–S111. doi:10.21037/jtd.2018.01.11
- Pallesen S, Saxvig IW, Molde H, Sørensen E, Wilhelmsen-Langeland A, Bjorvatn B. Brief report: behaviorally induced insufficient sleep syndrome in older adolescents: prevalence and correlates. J Adolesc 2011; 34(2):391–395. doi:10.1016/j.adolescence.2010.02.005
- American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Disorders; 2014.
- Trotti LM, Staab BA, Rye DB. Test-retest reliability of the multiple sleep latency test in narcolepsy without cataplexy and idiopathic hypersomnia. J Clin Sleep Med 2013; 9(8):789–795. doi:10.5664/jcsm.2922
- Andlauer O, Moore H, Jouhier L, et al. Nocturnal rapid eye movement sleep latency for identifying patients with narcolepsy/hypocretin deficiency. JAMA Neurol 2013; 70(7):891–902. doi:10.1001/jamaneurol.2013.1589
- Cairns A, Bogan R. Prevalence and clinical correlates of a short onset REM period (SOREMP) during routine PSG. Sleep 2015; 38(10):1575–1581. doi:10.5665/sleep.5050
- Mignot E, Lin L, Finn L, et al. Correlates of sleep-onset REM periods during the multiple sleep latency test in community adults. Brain 2006; 129(6):1609–1623. doi:10.1093/brain/awl079
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet 2000; 355(9197):39–40. doi:10.1016/S0140-6736(99)05582-8
- Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med 2000; 6(9):991–997. doi:10.1038/79690
- Oishi Y, Williams RH, Agostinelli L, et al. Role of the medial prefrontal cortex in cataplexy. J Neurosci 2013; 33(23):9743–9751. doi:10.1523/JNEUROSCI.0499-13.2013
- Mignot E, Hayduk R, Black J, Grumet FC, Guilleminault C. HLA DQB1*0602 is associated with cataplexy in 509 narcoleptic patients.. Sleep 1997; 20(11):1012–1020. pmid:9456467
- Pelin Z, Guilleminault C, Risch N, Grumet FC, Mignot E. HLA-DQB1*0602 homozygosity increases relative risk for narcolepsy but not disease severity in two ethnic groups. US Modafinil in Narcolepsy Multicenter Study Group. Tissue Antigens 1998; 51(1):96–100. pmid:9459509
- Akintomide GS, Rickards H. Narcolepsy: a review. Neuropsychiatr Dis Treat 2011; 7(1):507–518. doi:10.2147/NDT.S23624
- Mahlios J, De la Herrán-Arita AK, Mignot E. The autoimmune basis of narcolepsy. Curr Opin Neurobiol 2013; 23(5):767–773. doi:10.1016/j.conb.2013.04.013
- Degn M, Kornum BR. Type 1 narcolepsy: a CD8(+) T cell-mediated disease? Ann N Y Acad Sci 2015;1 351:80–88. doi:10.1111/nyas.12793
- Liblau RS, Vassalli A, Seifinejad A, Tafti M. Hypocretin (orexin) biology and the pathophysiology of narcolepsy with cataplexy. Lancet Neurol 2015; 14(3):318–328. doi:10.1016/S1474-4422(14)70218-2
- Rogers AE, Aldrich MS, Lin X. A comparison of three different sleep schedules for reducing daytime sleepiness in narcolepsy. Sleep 2001; 24(4):385–391. pmid:11403522
- Morgenthaler TI, Kapur VK, Brown TM, et al; Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep 2007; 30(12):1705–1711. pmid:18246980
- Mignot EJ. A practical guide to the therapy of narcolepsy and hypersomnia syndromes. Neurotherapeutics 2012; 9(4):739–752. doi:10.1007/s13311-012-0150-9
- Roth T, Schwartz JR, Hirshkowitz M, Erman MK, Dayno JM, Arora S. Evaluation of the safety of modafinil for treatment of excessive sleepiness. J Clin Sleep Med 2007; 3(6):595–602. pmid:17993041
- Auger RR, Goodman SH, Silber MH, Krahn LE, Pankratz VS, Slocumb NL. Risks of high-dose stimulants in the treatment of disorders of excessive somnolence: a case-control study. Sleep 2005; 28(6):667–672. pmid:16477952
- Abad VC, Guilleminault C. New developments in the management of narcolepsy. Nat Sci Sleep 2017; 9:39–57. doi:10.2147/NSS.S103467
- Drakatos P, Lykouras D, D’Ancona G, et al. Safety and efficacy of long-term use of sodium oxybate for narcolepsy with cataplexy in routine clinical practice. Sleep Med 2017; 35:80–84. doi:10.1016/j.sleep.2017.03.028
- Mansukhani MP, Kotagal S. Sodium oxybate in the treatment of childhood narcolepsy–cataplexy: a retrospective study. Sleep Med 2012; 13(6):606–610. doi:10.1016/j.sleep.2011.10.032
- Wang YG, Swick TJ, Carter LP, Thorpy MJ, Benowitz NL. Safety overview of postmarketing and clinical experience of sodium oxybate (Xyrem): abuse, misuse, dependence, and diversion. J Clin Sleep Med 2009; 5(4):365–371. pmid:19968016
- Weinhold SL, Seeck-Hirschner M, Nowak A, Hallschmid M, Göder R, Baier PC. The effect of intranasal orexin-A (hypocretin-1) on sleep, wakefulness and attention in narcolepsy with cataplexy. Behav Brain Res 2014; 262:8–13. doi:10.1016/j.bbr.2013.12.045
- Arias-Carrión O, Murillo-Rodriguez E. Effects of hypocretin/orexin cell transplantation on narcoleptic-like sleep behavior in rats. PLoS One 2014; 9(4):e95342. doi:10.1371/journal.pone.0095342
- Leu-Semenescu S, Nittur N, Golmard JL, Arnulf I. Effects of pitolisant, a histamine H3 inverse agonist, in drug-resistant idiopathic and symptomatic hypersomnia: a chart review. Sleep Med 2014; 15(6):681–687. doi:10.1016/j.sleep.2014.01.021
- Lecendreux M, Bruni O, Franco P, et al. Clinical experience suggests that modafinil is an effective and safe treatment for paediatric narcolepsy. J Sleep Res 2012; 21(4):481–483. doi:10.1111/j.1365-2869.2011.00991.x
KEY POINTS
- Features of narcolepsy include daytime sleepiness, sleep attacks, cataplexy (in narcolepsy type 1), sleep paralysis, and sleep-related hallucinations.
- People with narcolepsy feel sleepy and can fall asleep quickly, but they do not stay asleep for long. They go into rapid eye movement sleep soon after falling asleep. Total sleep time is normal, but sleep is fragmented.
- Scheduled naps lasting 15 to 20 minutes can improve alertness. A consistent sleep schedule with good sleep hygiene is also important.
- Modafinil, methylphenidate, and amphetamines are used to manage daytime sleepiness, and sodium oxybate and antidepressants are used for cataplexy.