User login
Background
Psoriasis is a complex, multifactorial, systemic disease that is associated with numerous neurologic comorbidities, including stroke, multiple sclerosis, epilepsy, migraine, restless leg syndrome, Parkinson disease, and less frequently Guillain-Barré syndrome and myasthenia gravis. Anxiety and depression also are frequently seen in patients with psoriasis.1 In recent years, heightened understanding of the pathogenesis and disease mechanisms involved in psoriasis has led to the development of therapies designed to help to control the chronic inflammation associated with the disease, such as immunobiologics and small molecules.2
Although tremendous effort has gone into elucidating the immunologic underpinnings of psoriasis (certainly a worthwhile endeavor), less attention has been given to the role the nervous system plays in its pathogenesis.3,4 Nonetheless, clinical evidence suggests that the nervous system plays an important role in the pathophysiology of psoriasis and is deserving of further investigation.3
Nerves and Neuropeptides
Psychological stress is known to exacerbate psoriasis, which points to the involvement of the nervous system in psoriasis.3,5,6 In addition to provoking the sympathetic response, psychological stressors have been shown to affect the peripheral nervous system in psoriasis by modulating the skin’s network of nerves and neuropeptides.6-11 A small study divided patients with psoriasis into low-stress and high-stress groups based on their clinical examinations and answers to questionnaires. Immunohistochemical analysis showed patients in the high-stress group had elevated levels of calcitonin gene-related peptide and vasoactive intestinal polypeptide as well as reduced levels of the neuropeptide-degrading enzyme chymase compared to the low-stress group.12 Two later studies showed calcitonin gene-related peptide stimulates keratinocyte proliferation3,13 and is found at increased levels in psoriatic skin.3,14 Similarly, higher quantities of vasoactive intestinal peptide-positive nerve fibers in the epidermis and dermis are found in psoriatic plaques compared to nonlesional and normal skin.3,15
Early research suggested that substance P (SP) released from cutaneous nerve fibers causes a local neurogenic response that elicits psoriasis in predisposed individuals.16 However, there have been conflicting reports of both higher and lower levels of SP in involved and noninvolved skin in patients with psoriasis compared with healthy individuals, making the role of SP in psoriasis ambiguous.3,15,17
Nerve growth factor (NGF), a principal mediator of neurogenic inflammation, also is suspected of playing a role in the pathogenesis of psoriasis.3,6 Studies have shown NGF prevents apoptosis of keratinocytes, activates T cells, and is found in higher levels in psoriatic skin compared to controls.3,18,19
Neuropeptides also may play a contributory role in the itching and Köbner phenomenon that are seen with psoriasis.3 The Köbner phenomenon refers to the formation of psoriatic lesions in uninvolved skin of patients with psoriasis following cutaneous trauma.20 Increased levels of NGF in nonlesional skin of patients with psoriasis are believed to contribute to the development of psoriatic plaques following trauma by triggering an inflammatory response that upregulates other neuropeptides, such as SP and calcitonin gene-related peptide.3 These neuropeptides generate keratinocyte proliferation, which in turn further increase NGF expression; as such, a cycle of inflammation and formation of psoriatic lesions is engendered.3,5 A noteworthy correlation also has been shown between the severity of pruritus and density of NGF-immunoreactive keratinocytes, high-affinity NGF receptors, protein gene product 9.5–immunoreactive intraepidermal fibers, and immunoreactive vessels for E-selectin.3,21
Spontaneous Clearing of Psoriasis
Spontaneous remission of psoriasis after cerebrovascular accident was first described in a case report published in 1998.22 Other cases have reported protective effects from psoriasis and psoriatic arthritis in limbs affected by poliomyelitis.23,24 Conversely, recurrence of skin lesions in areas corresponding to nervous system injury also have been reported in cases in which patients regained neurologic function; when permanent nerve damage was sustained, psoriasis did not recur,4 which confirms that peripheral nerves play a role in the pathogenesis of psoriasis.3 It is believed that peripheral nerve damage leads to reduced secretion of neuropeptides, and central nervous system injury can propagate similar downstream effects.3,25

Reports of psoriasis remission in the wake of peripheral and central nervous system injury from surgical nerve resection as well as cerebrovascular accident, as documented in the case presented here, provide clinical evidence in support of the neurocutaneous pathway’s role in psoriasis.3,4 Several reports have described clinical improvement of psoriasis following sensory cutaneous nerve damage, suggesting inflammation of the cutaneous nerves may be involved in the pathogenesis of psoriasis.3,6 Clearance of psoriatic plaques at the site of injury occurred following nerve resection; after reinnervation of the affected areas, disease recurrence occurred.6,26-28 More recently, cutaneous denervation was shown to improve acanthosis and IL-23 expression in mice with psoriasiform skin.3,25 Intradermal injections of calcitonin gene-related peptide and/or a SP agonist into the denervated areas reversed this denervation-mediated improvement.3,25
Bottom Line
This case report describes spontaneous clearing of psoriasis following a cerebrovascular accident. Improvement in psoriasis in the absence of neural inputs suggest the nervous system plays a crucial role in the development of psoriatic disease.4 A better understanding of the neuropeptides involved in the neurologic-mediated clearance of psoriasis may contribute to the development of improved targeted therapies, specifically designed to target the neurologic aspects of psoriasis.3 Neuropeptides such as nerve growth factor, calcitonin gene-related peptide, and vasoactive intestinal peptide, and possibly SP may play an important role in the pathogenesis of psoriasis and may one day be ideal targets for novel therapies.
- Amanat M, Salehi M, Rezaei N. Neurological and psychiatric disorders in psoriasis. Rev Neurosci. 2018;29:805-813.
- Eberle FC, Brück J, Holstein J, et al. Recent advances in understanding psoriasis [published April 28, 2016]. F1000Res. doi:10.12688/f1000research.7927.1.
- Lee EB, Reynolds KA, Pithadia DJ, et al. Clearance of psoriasis after ischemic stroke. Cutis. 2019;103:74-76.
- Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
- Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
- Kwon CW, Fried RG, Nousari Y, et al. Psoriasis: psychosomatic, somatopsychic, or both? Clin Dermatol. 2018;36:698-703.
- Lotti T, D’Erme AM, Hercogová J. The role of neuropeptides in the control of regional immunity. Clin Dermatol. 2014;32:633-645.
- Hall JM, Cruser D, Podawiltz A, et al. Psychological stress and the cutaneous immune response: roles of the HPA axis and the sympathetic nervous system in atopic dermatitis and psoriasis [published online August 30, 2012]. Dermatol Res Pract. 2012;2012:403908.
- Raychaudhuri SK, Raychaudhuri SP. NGF and its receptor system: a new dimension in the pathogenesis of psoriasis and psoriatic arthritis. Ann N Y Acad Sci. 2009;1173:470-477.
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243-251.
- Levi-Montalcini R, Skaper SD, Dal Toso R, et al. Nerve growth factor: from neurotrophin to neurokine. Trends Neurosci. 1996;19:514-520.
- Harvima IT, Viinamäki H, Naukkarinen A, et al. Association of cutaneous mast cells and sensory nerves with psychic stress in psoriasis. Psychother Psychosom. 1993;60:168-176.
- He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
- Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
- Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
- Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
- Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
- Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
- Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
- Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29:231-236.
- Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
- Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
- Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomyelitis residual paralysis. Br J Dermatol. 2014;171:429-431.
- Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
- Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
- Farber EM, Lanigan SW, Boer J. The role of cutaneous sensory nerves in the maintenance of psoriasis. Int J Dermatol. 1990;29:418-420.
- Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
- Perlman HH. Remission of psoriasis vulgaris from the use of nerve-blocking agents. Arch Dermatol. 1972;105:128-129.
Background
Psoriasis is a complex, multifactorial, systemic disease that is associated with numerous neurologic comorbidities, including stroke, multiple sclerosis, epilepsy, migraine, restless leg syndrome, Parkinson disease, and less frequently Guillain-Barré syndrome and myasthenia gravis. Anxiety and depression also are frequently seen in patients with psoriasis.1 In recent years, heightened understanding of the pathogenesis and disease mechanisms involved in psoriasis has led to the development of therapies designed to help to control the chronic inflammation associated with the disease, such as immunobiologics and small molecules.2

Although tremendous effort has gone into elucidating the immunologic underpinnings of psoriasis (certainly a worthwhile endeavor), less attention has been given to the role the nervous system plays in its pathogenesis.3,4 Nonetheless, clinical evidence suggests that the nervous system plays an important role in the pathophysiology of psoriasis and is deserving of further investigation.3
Nerves and Neuropeptides
Psychological stress is known to exacerbate psoriasis, which points to the involvement of the nervous system in psoriasis.3,5,6 In addition to provoking the sympathetic response, psychological stressors have been shown to affect the peripheral nervous system in psoriasis by modulating the skin’s network of nerves and neuropeptides.6-11 A small study divided patients with psoriasis into low-stress and high-stress groups based on their clinical examinations and answers to questionnaires. Immunohistochemical analysis showed patients in the high-stress group had elevated levels of calcitonin gene-related peptide and vasoactive intestinal polypeptide as well as reduced levels of the neuropeptide-degrading enzyme chymase compared to the low-stress group.12 Two later studies showed calcitonin gene-related peptide stimulates keratinocyte proliferation3,13 and is found at increased levels in psoriatic skin.3,14 Similarly, higher quantities of vasoactive intestinal peptide-positive nerve fibers in the epidermis and dermis are found in psoriatic plaques compared to nonlesional and normal skin.3,15

Early research suggested that substance P (SP) released from cutaneous nerve fibers causes a local neurogenic response that elicits psoriasis in predisposed individuals.16 However, there have been conflicting reports of both higher and lower levels of SP in involved and noninvolved skin in patients with psoriasis compared with healthy individuals, making the role of SP in psoriasis ambiguous.3,15,17
Nerve growth factor (NGF), a principal mediator of neurogenic inflammation, also is suspected of playing a role in the pathogenesis of psoriasis.3,6 Studies have shown NGF prevents apoptosis of keratinocytes, activates T cells, and is found in higher levels in psoriatic skin compared to controls.3,18,19

Neuropeptides also may play a contributory role in the itching and Köbner phenomenon that are seen with psoriasis.3 The Köbner phenomenon refers to the formation of psoriatic lesions in uninvolved skin of patients with psoriasis following cutaneous trauma.20 Increased levels of NGF in nonlesional skin of patients with psoriasis are believed to contribute to the development of psoriatic plaques following trauma by triggering an inflammatory response that upregulates other neuropeptides, such as SP and calcitonin gene-related peptide.3 These neuropeptides generate keratinocyte proliferation, which in turn further increase NGF expression; as such, a cycle of inflammation and formation of psoriatic lesions is engendered.3,5 A noteworthy correlation also has been shown between the severity of pruritus and density of NGF-immunoreactive keratinocytes, high-affinity NGF receptors, protein gene product 9.5–immunoreactive intraepidermal fibers, and immunoreactive vessels for E-selectin.3,21
Spontaneous Clearing of Psoriasis
Spontaneous remission of psoriasis after cerebrovascular accident was first described in a case report published in 1998.22 Other cases have reported protective effects from psoriasis and psoriatic arthritis in limbs affected by poliomyelitis.23,24 Conversely, recurrence of skin lesions in areas corresponding to nervous system injury also have been reported in cases in which patients regained neurologic function; when permanent nerve damage was sustained, psoriasis did not recur,4 which confirms that peripheral nerves play a role in the pathogenesis of psoriasis.3 It is believed that peripheral nerve damage leads to reduced secretion of neuropeptides, and central nervous system injury can propagate similar downstream effects.3,25

Reports of psoriasis remission in the wake of peripheral and central nervous system injury from surgical nerve resection as well as cerebrovascular accident, as documented in the case presented here, provide clinical evidence in support of the neurocutaneous pathway’s role in psoriasis.3,4 Several reports have described clinical improvement of psoriasis following sensory cutaneous nerve damage, suggesting inflammation of the cutaneous nerves may be involved in the pathogenesis of psoriasis.3,6 Clearance of psoriatic plaques at the site of injury occurred following nerve resection; after reinnervation of the affected areas, disease recurrence occurred.6,26-28 More recently, cutaneous denervation was shown to improve acanthosis and IL-23 expression in mice with psoriasiform skin.3,25 Intradermal injections of calcitonin gene-related peptide and/or a SP agonist into the denervated areas reversed this denervation-mediated improvement.3,25
Bottom Line
This case report describes spontaneous clearing of psoriasis following a cerebrovascular accident. Improvement in psoriasis in the absence of neural inputs suggest the nervous system plays a crucial role in the development of psoriatic disease.4 A better understanding of the neuropeptides involved in the neurologic-mediated clearance of psoriasis may contribute to the development of improved targeted therapies, specifically designed to target the neurologic aspects of psoriasis.3 Neuropeptides such as nerve growth factor, calcitonin gene-related peptide, and vasoactive intestinal peptide, and possibly SP may play an important role in the pathogenesis of psoriasis and may one day be ideal targets for novel therapies.
Background
Psoriasis is a complex, multifactorial, systemic disease that is associated with numerous neurologic comorbidities, including stroke, multiple sclerosis, epilepsy, migraine, restless leg syndrome, Parkinson disease, and less frequently Guillain-Barré syndrome and myasthenia gravis. Anxiety and depression also are frequently seen in patients with psoriasis.1 In recent years, heightened understanding of the pathogenesis and disease mechanisms involved in psoriasis has led to the development of therapies designed to help to control the chronic inflammation associated with the disease, such as immunobiologics and small molecules.2

Although tremendous effort has gone into elucidating the immunologic underpinnings of psoriasis (certainly a worthwhile endeavor), less attention has been given to the role the nervous system plays in its pathogenesis.3,4 Nonetheless, clinical evidence suggests that the nervous system plays an important role in the pathophysiology of psoriasis and is deserving of further investigation.3
Nerves and Neuropeptides
Psychological stress is known to exacerbate psoriasis, which points to the involvement of the nervous system in psoriasis.3,5,6 In addition to provoking the sympathetic response, psychological stressors have been shown to affect the peripheral nervous system in psoriasis by modulating the skin’s network of nerves and neuropeptides.6-11 A small study divided patients with psoriasis into low-stress and high-stress groups based on their clinical examinations and answers to questionnaires. Immunohistochemical analysis showed patients in the high-stress group had elevated levels of calcitonin gene-related peptide and vasoactive intestinal polypeptide as well as reduced levels of the neuropeptide-degrading enzyme chymase compared to the low-stress group.12 Two later studies showed calcitonin gene-related peptide stimulates keratinocyte proliferation3,13 and is found at increased levels in psoriatic skin.3,14 Similarly, higher quantities of vasoactive intestinal peptide-positive nerve fibers in the epidermis and dermis are found in psoriatic plaques compared to nonlesional and normal skin.3,15

Early research suggested that substance P (SP) released from cutaneous nerve fibers causes a local neurogenic response that elicits psoriasis in predisposed individuals.16 However, there have been conflicting reports of both higher and lower levels of SP in involved and noninvolved skin in patients with psoriasis compared with healthy individuals, making the role of SP in psoriasis ambiguous.3,15,17
Nerve growth factor (NGF), a principal mediator of neurogenic inflammation, also is suspected of playing a role in the pathogenesis of psoriasis.3,6 Studies have shown NGF prevents apoptosis of keratinocytes, activates T cells, and is found in higher levels in psoriatic skin compared to controls.3,18,19

Neuropeptides also may play a contributory role in the itching and Köbner phenomenon that are seen with psoriasis.3 The Köbner phenomenon refers to the formation of psoriatic lesions in uninvolved skin of patients with psoriasis following cutaneous trauma.20 Increased levels of NGF in nonlesional skin of patients with psoriasis are believed to contribute to the development of psoriatic plaques following trauma by triggering an inflammatory response that upregulates other neuropeptides, such as SP and calcitonin gene-related peptide.3 These neuropeptides generate keratinocyte proliferation, which in turn further increase NGF expression; as such, a cycle of inflammation and formation of psoriatic lesions is engendered.3,5 A noteworthy correlation also has been shown between the severity of pruritus and density of NGF-immunoreactive keratinocytes, high-affinity NGF receptors, protein gene product 9.5–immunoreactive intraepidermal fibers, and immunoreactive vessels for E-selectin.3,21
Spontaneous Clearing of Psoriasis
Spontaneous remission of psoriasis after cerebrovascular accident was first described in a case report published in 1998.22 Other cases have reported protective effects from psoriasis and psoriatic arthritis in limbs affected by poliomyelitis.23,24 Conversely, recurrence of skin lesions in areas corresponding to nervous system injury also have been reported in cases in which patients regained neurologic function; when permanent nerve damage was sustained, psoriasis did not recur,4 which confirms that peripheral nerves play a role in the pathogenesis of psoriasis.3 It is believed that peripheral nerve damage leads to reduced secretion of neuropeptides, and central nervous system injury can propagate similar downstream effects.3,25

Reports of psoriasis remission in the wake of peripheral and central nervous system injury from surgical nerve resection as well as cerebrovascular accident, as documented in the case presented here, provide clinical evidence in support of the neurocutaneous pathway’s role in psoriasis.3,4 Several reports have described clinical improvement of psoriasis following sensory cutaneous nerve damage, suggesting inflammation of the cutaneous nerves may be involved in the pathogenesis of psoriasis.3,6 Clearance of psoriatic plaques at the site of injury occurred following nerve resection; after reinnervation of the affected areas, disease recurrence occurred.6,26-28 More recently, cutaneous denervation was shown to improve acanthosis and IL-23 expression in mice with psoriasiform skin.3,25 Intradermal injections of calcitonin gene-related peptide and/or a SP agonist into the denervated areas reversed this denervation-mediated improvement.3,25
Bottom Line
This case report describes spontaneous clearing of psoriasis following a cerebrovascular accident. Improvement in psoriasis in the absence of neural inputs suggest the nervous system plays a crucial role in the development of psoriatic disease.4 A better understanding of the neuropeptides involved in the neurologic-mediated clearance of psoriasis may contribute to the development of improved targeted therapies, specifically designed to target the neurologic aspects of psoriasis.3 Neuropeptides such as nerve growth factor, calcitonin gene-related peptide, and vasoactive intestinal peptide, and possibly SP may play an important role in the pathogenesis of psoriasis and may one day be ideal targets for novel therapies.
- Amanat M, Salehi M, Rezaei N. Neurological and psychiatric disorders in psoriasis. Rev Neurosci. 2018;29:805-813.
- Eberle FC, Brück J, Holstein J, et al. Recent advances in understanding psoriasis [published April 28, 2016]. F1000Res. doi:10.12688/f1000research.7927.1.
- Lee EB, Reynolds KA, Pithadia DJ, et al. Clearance of psoriasis after ischemic stroke. Cutis. 2019;103:74-76.
- Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
- Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
- Kwon CW, Fried RG, Nousari Y, et al. Psoriasis: psychosomatic, somatopsychic, or both? Clin Dermatol. 2018;36:698-703.
- Lotti T, D’Erme AM, Hercogová J. The role of neuropeptides in the control of regional immunity. Clin Dermatol. 2014;32:633-645.
- Hall JM, Cruser D, Podawiltz A, et al. Psychological stress and the cutaneous immune response: roles of the HPA axis and the sympathetic nervous system in atopic dermatitis and psoriasis [published online August 30, 2012]. Dermatol Res Pract. 2012;2012:403908.
- Raychaudhuri SK, Raychaudhuri SP. NGF and its receptor system: a new dimension in the pathogenesis of psoriasis and psoriatic arthritis. Ann N Y Acad Sci. 2009;1173:470-477.
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243-251.
- Levi-Montalcini R, Skaper SD, Dal Toso R, et al. Nerve growth factor: from neurotrophin to neurokine. Trends Neurosci. 1996;19:514-520.
- Harvima IT, Viinamäki H, Naukkarinen A, et al. Association of cutaneous mast cells and sensory nerves with psychic stress in psoriasis. Psychother Psychosom. 1993;60:168-176.
- He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
- Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
- Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
- Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
- Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
- Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
- Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
- Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29:231-236.
- Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
- Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
- Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomyelitis residual paralysis. Br J Dermatol. 2014;171:429-431.
- Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
- Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
- Farber EM, Lanigan SW, Boer J. The role of cutaneous sensory nerves in the maintenance of psoriasis. Int J Dermatol. 1990;29:418-420.
- Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
- Perlman HH. Remission of psoriasis vulgaris from the use of nerve-blocking agents. Arch Dermatol. 1972;105:128-129.
- Amanat M, Salehi M, Rezaei N. Neurological and psychiatric disorders in psoriasis. Rev Neurosci. 2018;29:805-813.
- Eberle FC, Brück J, Holstein J, et al. Recent advances in understanding psoriasis [published April 28, 2016]. F1000Res. doi:10.12688/f1000research.7927.1.
- Lee EB, Reynolds KA, Pithadia DJ, et al. Clearance of psoriasis after ischemic stroke. Cutis. 2019;103:74-76.
- Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
- Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
- Kwon CW, Fried RG, Nousari Y, et al. Psoriasis: psychosomatic, somatopsychic, or both? Clin Dermatol. 2018;36:698-703.
- Lotti T, D’Erme AM, Hercogová J. The role of neuropeptides in the control of regional immunity. Clin Dermatol. 2014;32:633-645.
- Hall JM, Cruser D, Podawiltz A, et al. Psychological stress and the cutaneous immune response: roles of the HPA axis and the sympathetic nervous system in atopic dermatitis and psoriasis [published online August 30, 2012]. Dermatol Res Pract. 2012;2012:403908.
- Raychaudhuri SK, Raychaudhuri SP. NGF and its receptor system: a new dimension in the pathogenesis of psoriasis and psoriatic arthritis. Ann N Y Acad Sci. 2009;1173:470-477.
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243-251.
- Levi-Montalcini R, Skaper SD, Dal Toso R, et al. Nerve growth factor: from neurotrophin to neurokine. Trends Neurosci. 1996;19:514-520.
- Harvima IT, Viinamäki H, Naukkarinen A, et al. Association of cutaneous mast cells and sensory nerves with psychic stress in psoriasis. Psychother Psychosom. 1993;60:168-176.
- He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
- Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
- Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
- Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
- Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
- Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
- Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
- Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29:231-236.
- Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
- Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
- Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomyelitis residual paralysis. Br J Dermatol. 2014;171:429-431.
- Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
- Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
- Farber EM, Lanigan SW, Boer J. The role of cutaneous sensory nerves in the maintenance of psoriasis. Int J Dermatol. 1990;29:418-420.
- Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
- Perlman HH. Remission of psoriasis vulgaris from the use of nerve-blocking agents. Arch Dermatol. 1972;105:128-129.
The Case
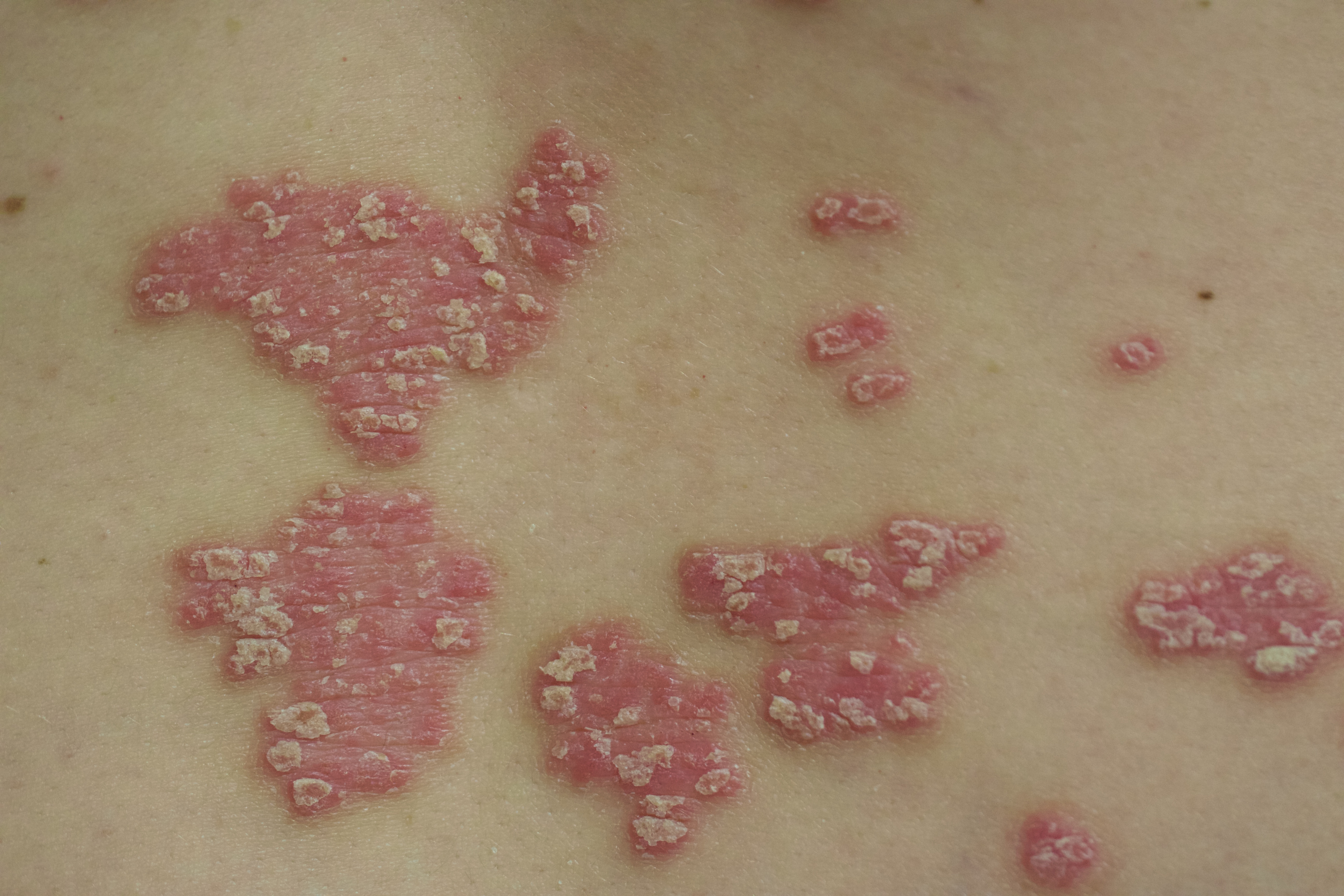
A 52-year-old man with psoriasis presented to the dermatology clinic for follow-up. The patient had been using topical clobetasol and apremilast with limited success; however, he had not yet tried biologic therapy. Physical examination revealed erythematous, scaly, indurated papules and plaques on the chest, abdomen, back, arms, and legs, consistent with psoriasis. Affected body surface area was approximately 10%.
Treatment
Ustekinumab was prescribed, but the patient did not pick it up from the pharmacy.
Approximately 1 month later, the patient presented to the emergency department with left-sided weakness and numbness. He was subsequently hospitalized for treatment of stroke. During hospitalization, the patient was started on lisinopril, aspirin, and atorvastatin. He also was given subcu-taneous enoxaparin with plans to initiate warfarin as an outpatient. No therapies for the treatment of psoriasis were given during his admission. Three days after being admitted, he was discharged to a skilled nursing facility.
Patient Outcome
Three months following discharge, the patient returned to the dermatology clinic for follow-up. After his stroke, he reported that his psoriasis had cleared and had not returned. Physical examination revealed his skin was clear of psoriatic lesions.