User login
Impact of the COVID-19 Pandemic on Care for Patients With Skin Cancer
To the Editor:
The most common malignancy in the United States is skin cancer, with melanoma accounting for the majority of skin cancer deaths.1 Despite the lack of established guidelines for routine total-body skin examinations, many patients regularly visit their dermatologist for assessment of pigmented skin lesions.2 During the COVID-19 pandemic, many patients were unable to attend in-person dermatology visits, which resulted in many high-risk individuals not receiving care or alternatively seeking virtual care for cutaneous lesions.3 There has been a lack of research in the United States exploring the utilization of teledermatology during the pandemic and its overall impact on the care of patients with a history of skin cancer. We explored the impact of the COVID-19 pandemic on care for patients with skin cancer in a large US population.

Using anonymous survey data from the 2020-2021 National Health Interview Survey,4 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with a self-reported history of skin cancer—melanoma, nonmelanoma skin cancer, or unknown skin cancer. The 3 outcome variables included having a virtual medical appointment in the past 12 months (yes/no), delaying medical care due to the COVID-19 pandemic (yes/no), and not receiving care due to the COVID-19 pandemic (yes/no). Multivariable logistic regression models evaluating the relationship between a history of skin cancer and access to care were constructed using Stata/MP 17.0 (StataCorp LLC). We controlled for patient age; education; race/ethnicity; received public assistance or welfare payments; sex; region; US citizenship status; health insurance status; comorbidities including history of hypertension, diabetes, and hypercholesterolemia; and birthplace in the United States in the logistic regression models.
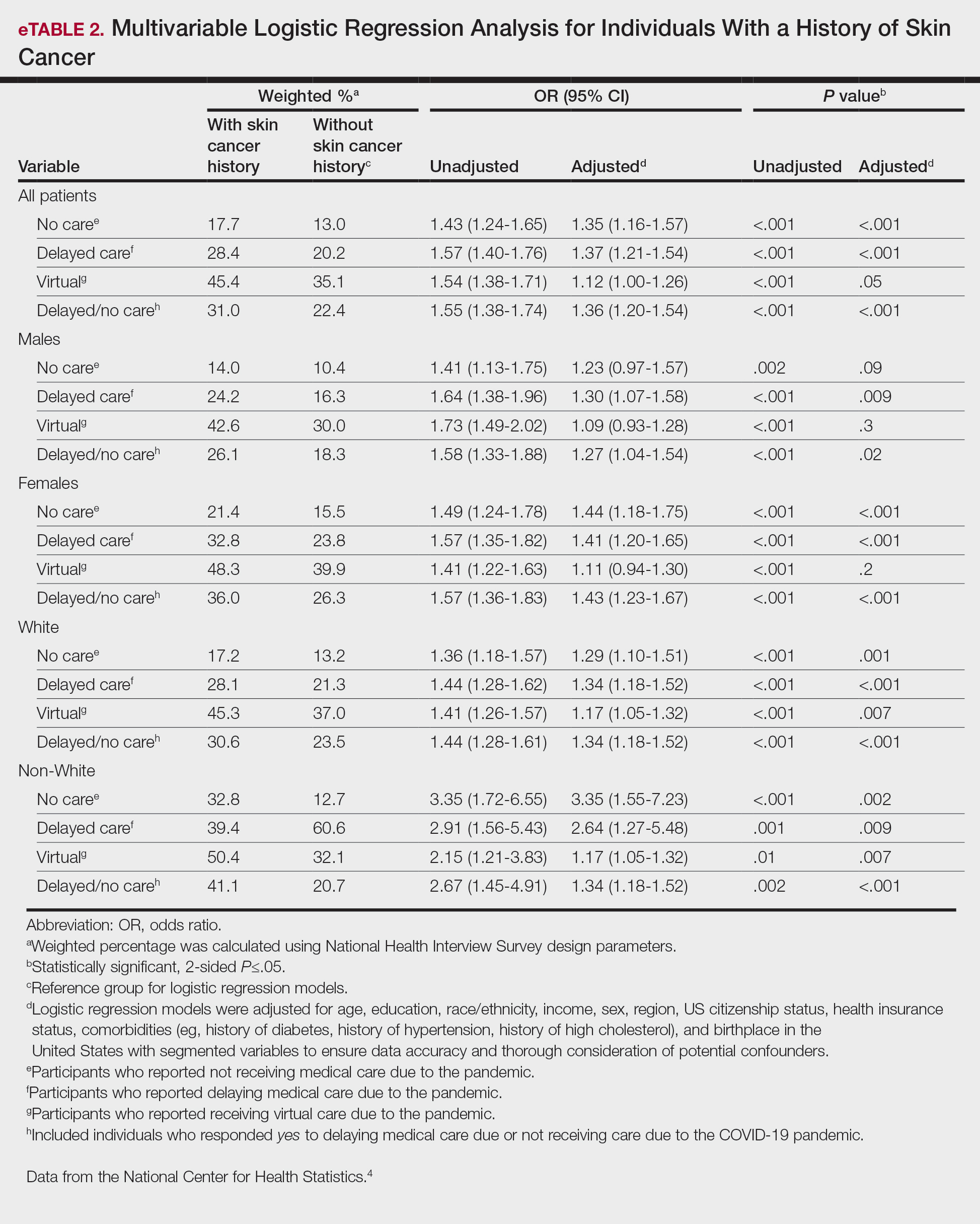
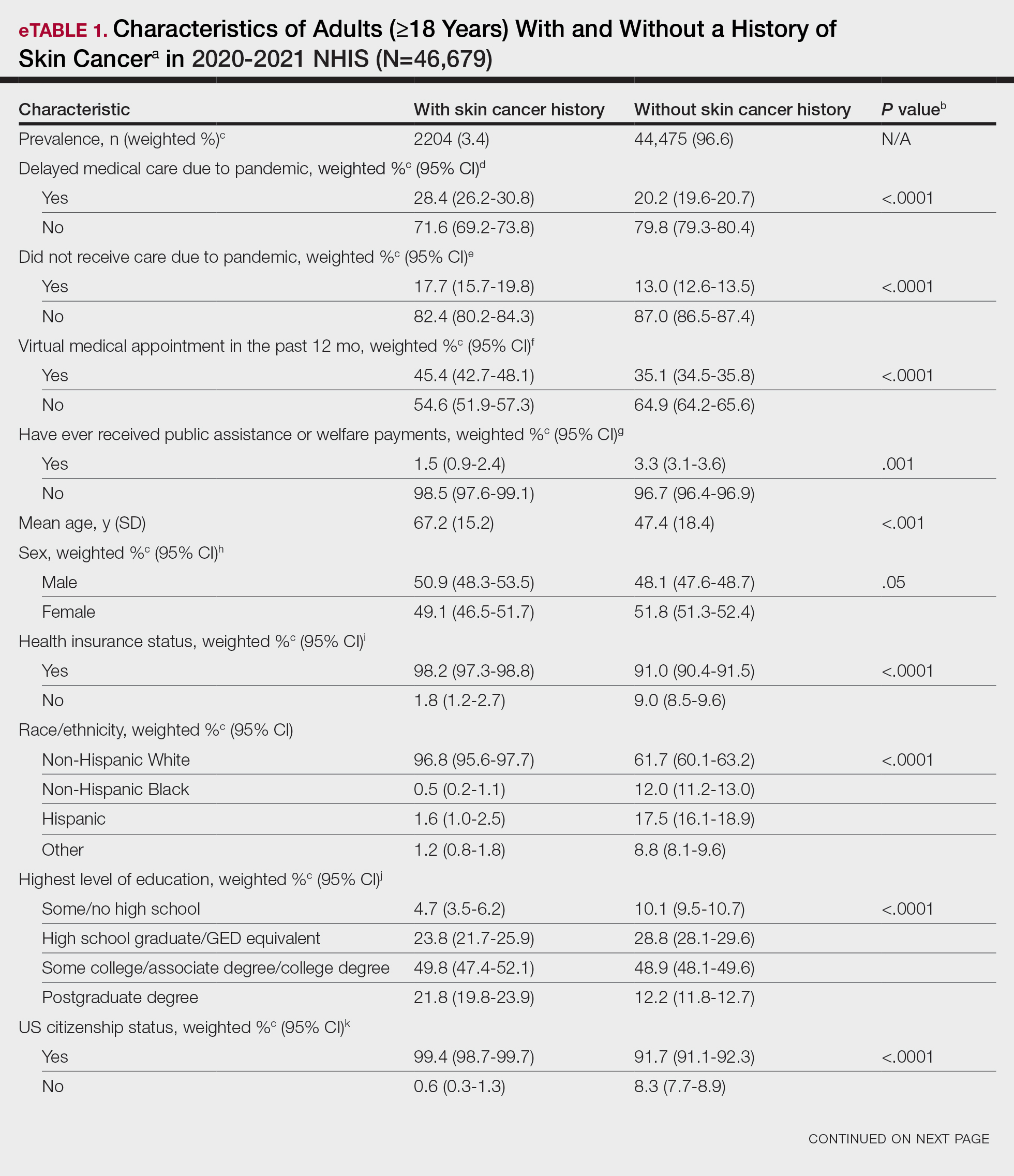
Our analysis included 46,679 patients aged 18 years or older, of whom 3.4% (weighted)(n=2204) reported a history of skin cancer (eTable 1). The weighted percentage was calculated using National Health Interview Survey design parameters (accounting for the multistage sampling design) to represent the general US population. Compared with those with no history of skin cancer, patients with a history of skin cancer were significantly more likely to delay medical care (adjusted odds ratio [AOR], 1.37; 95% CI, 1.21-1.54; P<.001) or not receive care (AOR, 1.35; 95% CI, 1.16-1.57; P<.001) due to the pandemic and were more likely to have had a virtual medical visit in the past 12 months (AOR, 1.12; 95% CI, 1.00-1.26; P=.05). Additionally, subgroup analysis revealed that females were more likely than males to forego medical care (eTable 2). β Coefficients for independent and dependent variables were further analyzed using logistic regression (eTable 3).
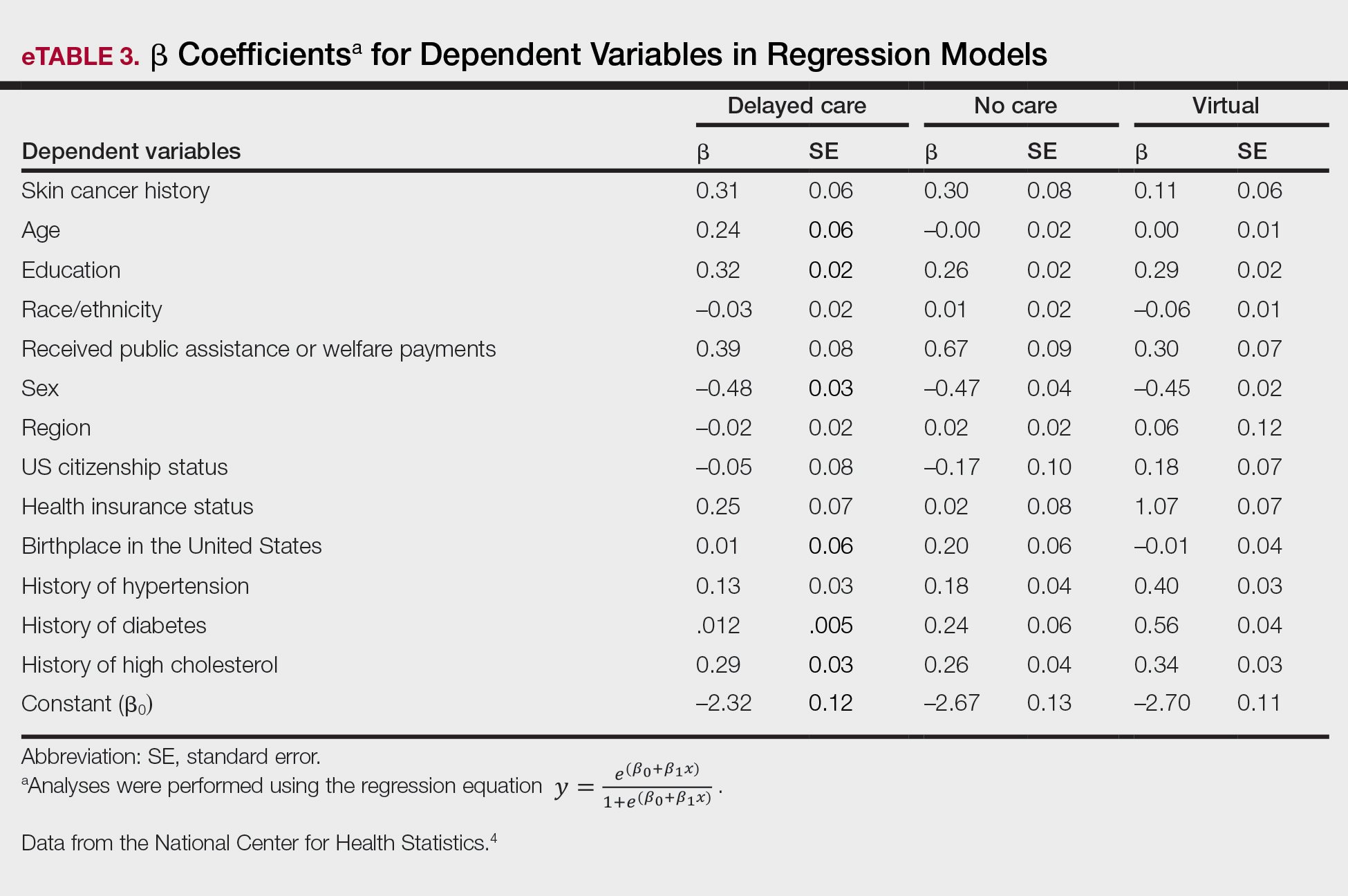
After adjusting for various potential confounders including comorbidities, our results revealed that patients with a history of skin cancer reported that they were less likely to receive in-person medical care due to the COVID-19 pandemic, as high-risk individuals with a history of skin cancer may have stopped receiving total-body skin examinations and dermatology care during the pandemic. Our findings showed that patients with a history of skin cancer were more likely than those without skin cancer to delay or forego care due to the pandemic, which may contribute to a higher incidence of advanced-stage melanomas postpandemic. Trepanowski et al5 reported an increased incidence of patients presenting with more advanced melanomas during the pandemic. Telemedicine was more commonly utilized by patients with a history of skin cancer during the pandemic.
In the future, virtual care may help limit advanced stages of skin cancer by serving as a viable alternative to in-person care.6 It has been reported that telemedicine can serve as a useful triage service reducing patient wait times.7 Teledermatology should not replace in-person care, as there is no evidence of the diagnostic accuracy of this service and many patients still will need to be seen in-person for confirmation of their diagnosis and potential biopsy. Further studies are needed to assess for missed skin cancer diagnoses due to the utilization of telemedicine.
Limitations of this study included a self-reported history of skin cancer, β coefficients that may suggest a high degree of collinearity, and lack of specific survey questions regarding dermatologic care during the COVID-19 pandemic. Further long-term studies exploring the clinical applicability and diagnostic accuracy of virtual medicine visits for cutaneous malignancies are vital, as teledermatology may play an essential role in curbing rising skin cancer rates even beyond the pandemic.
- Guy GP Jr, Thomas CC, Thompson T, et al. Vital signs: melanoma incidence and mortality trends and projections—United States, 1982-2030. MMWR Morb Mortal Wkly Rep. 2015;64:591-596.
- Whiteman DC, Olsen CM, MacGregor S, et al; QSkin Study. The effect of screening on melanoma incidence and biopsy rates. Br J Dermatol. 2022;187:515-522. doi:10.1111/bjd.21649
- Jobbágy A, Kiss N, Meznerics FA, et al. Emergency use and efficacy of an asynchronous teledermatology system as a novel tool for early diagnosis of skin cancer during the first wave of COVID-19 pandemic. Int J Environ Res Public Health. 2022;19:2699. doi:10.3390/ijerph19052699
- National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed April 19, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
- Trepanowski N, Chang MS, Zhou G, et al. Delays in melanoma presentation during the COVID-19 pandemic: a nationwide multi-institutional cohort study. J Am Acad Dermatol. 2022;87:1217-1219. doi:10.1016/j.jaad.2022.06.031
- Chiru MR, Hindocha S, Burova E, et al. Management of the two-week wait pathway for skin cancer patients, before and during the pandemic: is virtual consultation an option? J Pers Med. 2022;12:1258. doi:10.3390/jpm12081258
- Finnane A Dallest K Janda M et al. Teledermatology for the diagnosis and management of skin cancer: a systematic review. JAMA Dermatol. 2017;153:319-327. doi:10.1001/jamadermatol.2016.4361
To the Editor:
The most common malignancy in the United States is skin cancer, with melanoma accounting for the majority of skin cancer deaths.1 Despite the lack of established guidelines for routine total-body skin examinations, many patients regularly visit their dermatologist for assessment of pigmented skin lesions.2 During the COVID-19 pandemic, many patients were unable to attend in-person dermatology visits, which resulted in many high-risk individuals not receiving care or alternatively seeking virtual care for cutaneous lesions.3 There has been a lack of research in the United States exploring the utilization of teledermatology during the pandemic and its overall impact on the care of patients with a history of skin cancer. We explored the impact of the COVID-19 pandemic on care for patients with skin cancer in a large US population.


Using anonymous survey data from the 2020-2021 National Health Interview Survey,4 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with a self-reported history of skin cancer—melanoma, nonmelanoma skin cancer, or unknown skin cancer. The 3 outcome variables included having a virtual medical appointment in the past 12 months (yes/no), delaying medical care due to the COVID-19 pandemic (yes/no), and not receiving care due to the COVID-19 pandemic (yes/no). Multivariable logistic regression models evaluating the relationship between a history of skin cancer and access to care were constructed using Stata/MP 17.0 (StataCorp LLC). We controlled for patient age; education; race/ethnicity; received public assistance or welfare payments; sex; region; US citizenship status; health insurance status; comorbidities including history of hypertension, diabetes, and hypercholesterolemia; and birthplace in the United States in the logistic regression models.

Our analysis included 46,679 patients aged 18 years or older, of whom 3.4% (weighted)(n=2204) reported a history of skin cancer (eTable 1). The weighted percentage was calculated using National Health Interview Survey design parameters (accounting for the multistage sampling design) to represent the general US population. Compared with those with no history of skin cancer, patients with a history of skin cancer were significantly more likely to delay medical care (adjusted odds ratio [AOR], 1.37; 95% CI, 1.21-1.54; P<.001) or not receive care (AOR, 1.35; 95% CI, 1.16-1.57; P<.001) due to the pandemic and were more likely to have had a virtual medical visit in the past 12 months (AOR, 1.12; 95% CI, 1.00-1.26; P=.05). Additionally, subgroup analysis revealed that females were more likely than males to forego medical care (eTable 2). β Coefficients for independent and dependent variables were further analyzed using logistic regression (eTable 3).

After adjusting for various potential confounders including comorbidities, our results revealed that patients with a history of skin cancer reported that they were less likely to receive in-person medical care due to the COVID-19 pandemic, as high-risk individuals with a history of skin cancer may have stopped receiving total-body skin examinations and dermatology care during the pandemic. Our findings showed that patients with a history of skin cancer were more likely than those without skin cancer to delay or forego care due to the pandemic, which may contribute to a higher incidence of advanced-stage melanomas postpandemic. Trepanowski et al5 reported an increased incidence of patients presenting with more advanced melanomas during the pandemic. Telemedicine was more commonly utilized by patients with a history of skin cancer during the pandemic.
In the future, virtual care may help limit advanced stages of skin cancer by serving as a viable alternative to in-person care.6 It has been reported that telemedicine can serve as a useful triage service reducing patient wait times.7 Teledermatology should not replace in-person care, as there is no evidence of the diagnostic accuracy of this service and many patients still will need to be seen in-person for confirmation of their diagnosis and potential biopsy. Further studies are needed to assess for missed skin cancer diagnoses due to the utilization of telemedicine.
Limitations of this study included a self-reported history of skin cancer, β coefficients that may suggest a high degree of collinearity, and lack of specific survey questions regarding dermatologic care during the COVID-19 pandemic. Further long-term studies exploring the clinical applicability and diagnostic accuracy of virtual medicine visits for cutaneous malignancies are vital, as teledermatology may play an essential role in curbing rising skin cancer rates even beyond the pandemic.
To the Editor:
The most common malignancy in the United States is skin cancer, with melanoma accounting for the majority of skin cancer deaths.1 Despite the lack of established guidelines for routine total-body skin examinations, many patients regularly visit their dermatologist for assessment of pigmented skin lesions.2 During the COVID-19 pandemic, many patients were unable to attend in-person dermatology visits, which resulted in many high-risk individuals not receiving care or alternatively seeking virtual care for cutaneous lesions.3 There has been a lack of research in the United States exploring the utilization of teledermatology during the pandemic and its overall impact on the care of patients with a history of skin cancer. We explored the impact of the COVID-19 pandemic on care for patients with skin cancer in a large US population.


Using anonymous survey data from the 2020-2021 National Health Interview Survey,4 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with a self-reported history of skin cancer—melanoma, nonmelanoma skin cancer, or unknown skin cancer. The 3 outcome variables included having a virtual medical appointment in the past 12 months (yes/no), delaying medical care due to the COVID-19 pandemic (yes/no), and not receiving care due to the COVID-19 pandemic (yes/no). Multivariable logistic regression models evaluating the relationship between a history of skin cancer and access to care were constructed using Stata/MP 17.0 (StataCorp LLC). We controlled for patient age; education; race/ethnicity; received public assistance or welfare payments; sex; region; US citizenship status; health insurance status; comorbidities including history of hypertension, diabetes, and hypercholesterolemia; and birthplace in the United States in the logistic regression models.

Our analysis included 46,679 patients aged 18 years or older, of whom 3.4% (weighted)(n=2204) reported a history of skin cancer (eTable 1). The weighted percentage was calculated using National Health Interview Survey design parameters (accounting for the multistage sampling design) to represent the general US population. Compared with those with no history of skin cancer, patients with a history of skin cancer were significantly more likely to delay medical care (adjusted odds ratio [AOR], 1.37; 95% CI, 1.21-1.54; P<.001) or not receive care (AOR, 1.35; 95% CI, 1.16-1.57; P<.001) due to the pandemic and were more likely to have had a virtual medical visit in the past 12 months (AOR, 1.12; 95% CI, 1.00-1.26; P=.05). Additionally, subgroup analysis revealed that females were more likely than males to forego medical care (eTable 2). β Coefficients for independent and dependent variables were further analyzed using logistic regression (eTable 3).

After adjusting for various potential confounders including comorbidities, our results revealed that patients with a history of skin cancer reported that they were less likely to receive in-person medical care due to the COVID-19 pandemic, as high-risk individuals with a history of skin cancer may have stopped receiving total-body skin examinations and dermatology care during the pandemic. Our findings showed that patients with a history of skin cancer were more likely than those without skin cancer to delay or forego care due to the pandemic, which may contribute to a higher incidence of advanced-stage melanomas postpandemic. Trepanowski et al5 reported an increased incidence of patients presenting with more advanced melanomas during the pandemic. Telemedicine was more commonly utilized by patients with a history of skin cancer during the pandemic.
In the future, virtual care may help limit advanced stages of skin cancer by serving as a viable alternative to in-person care.6 It has been reported that telemedicine can serve as a useful triage service reducing patient wait times.7 Teledermatology should not replace in-person care, as there is no evidence of the diagnostic accuracy of this service and many patients still will need to be seen in-person for confirmation of their diagnosis and potential biopsy. Further studies are needed to assess for missed skin cancer diagnoses due to the utilization of telemedicine.
Limitations of this study included a self-reported history of skin cancer, β coefficients that may suggest a high degree of collinearity, and lack of specific survey questions regarding dermatologic care during the COVID-19 pandemic. Further long-term studies exploring the clinical applicability and diagnostic accuracy of virtual medicine visits for cutaneous malignancies are vital, as teledermatology may play an essential role in curbing rising skin cancer rates even beyond the pandemic.
- Guy GP Jr, Thomas CC, Thompson T, et al. Vital signs: melanoma incidence and mortality trends and projections—United States, 1982-2030. MMWR Morb Mortal Wkly Rep. 2015;64:591-596.
- Whiteman DC, Olsen CM, MacGregor S, et al; QSkin Study. The effect of screening on melanoma incidence and biopsy rates. Br J Dermatol. 2022;187:515-522. doi:10.1111/bjd.21649
- Jobbágy A, Kiss N, Meznerics FA, et al. Emergency use and efficacy of an asynchronous teledermatology system as a novel tool for early diagnosis of skin cancer during the first wave of COVID-19 pandemic. Int J Environ Res Public Health. 2022;19:2699. doi:10.3390/ijerph19052699
- National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed April 19, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
- Trepanowski N, Chang MS, Zhou G, et al. Delays in melanoma presentation during the COVID-19 pandemic: a nationwide multi-institutional cohort study. J Am Acad Dermatol. 2022;87:1217-1219. doi:10.1016/j.jaad.2022.06.031
- Chiru MR, Hindocha S, Burova E, et al. Management of the two-week wait pathway for skin cancer patients, before and during the pandemic: is virtual consultation an option? J Pers Med. 2022;12:1258. doi:10.3390/jpm12081258
- Finnane A Dallest K Janda M et al. Teledermatology for the diagnosis and management of skin cancer: a systematic review. JAMA Dermatol. 2017;153:319-327. doi:10.1001/jamadermatol.2016.4361
- Guy GP Jr, Thomas CC, Thompson T, et al. Vital signs: melanoma incidence and mortality trends and projections—United States, 1982-2030. MMWR Morb Mortal Wkly Rep. 2015;64:591-596.
- Whiteman DC, Olsen CM, MacGregor S, et al; QSkin Study. The effect of screening on melanoma incidence and biopsy rates. Br J Dermatol. 2022;187:515-522. doi:10.1111/bjd.21649
- Jobbágy A, Kiss N, Meznerics FA, et al. Emergency use and efficacy of an asynchronous teledermatology system as a novel tool for early diagnosis of skin cancer during the first wave of COVID-19 pandemic. Int J Environ Res Public Health. 2022;19:2699. doi:10.3390/ijerph19052699
- National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed April 19, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
- Trepanowski N, Chang MS, Zhou G, et al. Delays in melanoma presentation during the COVID-19 pandemic: a nationwide multi-institutional cohort study. J Am Acad Dermatol. 2022;87:1217-1219. doi:10.1016/j.jaad.2022.06.031
- Chiru MR, Hindocha S, Burova E, et al. Management of the two-week wait pathway for skin cancer patients, before and during the pandemic: is virtual consultation an option? J Pers Med. 2022;12:1258. doi:10.3390/jpm12081258
- Finnane A Dallest K Janda M et al. Teledermatology for the diagnosis and management of skin cancer: a systematic review. JAMA Dermatol. 2017;153:319-327. doi:10.1001/jamadermatol.2016.4361
PRACTICE POINTS
- The COVID-19 pandemic has altered the landscape of medicine, as many individuals are now utilizing telemedicine to receive care.
- Many individuals will continue to receive telemedicine moving forward, making it crucial to understand access to care.
Risk for COVID-19 Infection in Patients With Vitiligo
To the Editor:
Vitiligo is a depigmentation disorder that results from the loss of melanocytes in the epidermis.1 The most widely accepted pathophysiology for melanocyte destruction in vitiligo is an autoimmune process involving dysregulated cytokine production and autoreactive T-cell activation.1 Individuals with cutaneous autoinflammatory conditions currently are vital patient populations warranting research, as their susceptibility to COVID-19 infection may differ from the general population. We previously found a small increased risk for COVID-19 infection in patients with psoriasis,2 which suggests that other dermatologic conditions also may impact COVID-19 risk. The risk for COVID-19 infection in patients with vitiligo remains largely unknown. In this retrospective cohort study, we investigated the risk for COVID-19 infection in patients with vitiligo compared with those without vitiligo utilizing claims data from the COVID-19 Research Database (https://covid19researchdatabase.org/).
Claims were evaluated for patients aged 3 years and older with a vitiligo diagnosis (International Classification of Diseases, Tenth Revision [ICD-10] code L80) that was made between January 1, 2016, and January 1, 2020. Individuals without a vitiligo diagnosis during the same period were placed (4:1 ratio) in the control group and were matched with study group patients for age and sex. All comorbidity variables and vitiligo diagnoses were extracted from ICD-10 codes that were given prior to a diagnosis of COVID-19. We then constructed multivariable logistic regression models adjusting for measured confounders to evaluate if vitiligo was associated with higher risk for COVID-19 infection after January 1, 2020.
The vitiligo and nonvitiligo cohorts included 40,363 and 161,452 patients, respectively (Table 1). Logistic regression analysis with adjustment for confounding variables, including high comorbid risk factors (Table 2) revealed that patients with a diagnosis of vitiligo had significantly increased odds of COVID-19 infection compared with patients without vitiligo (adjusted odds ratio [AOR], 1.47; 95% CI, 1.37-1.57; P<.001)(Table 3). Additionally, subgroup logistic analyses for sex, age, and exclusion of patients who were HIV positive revealed that females with vitiligo had higher odds of contracting COVID-19 than males with vitiligo (Table 3).
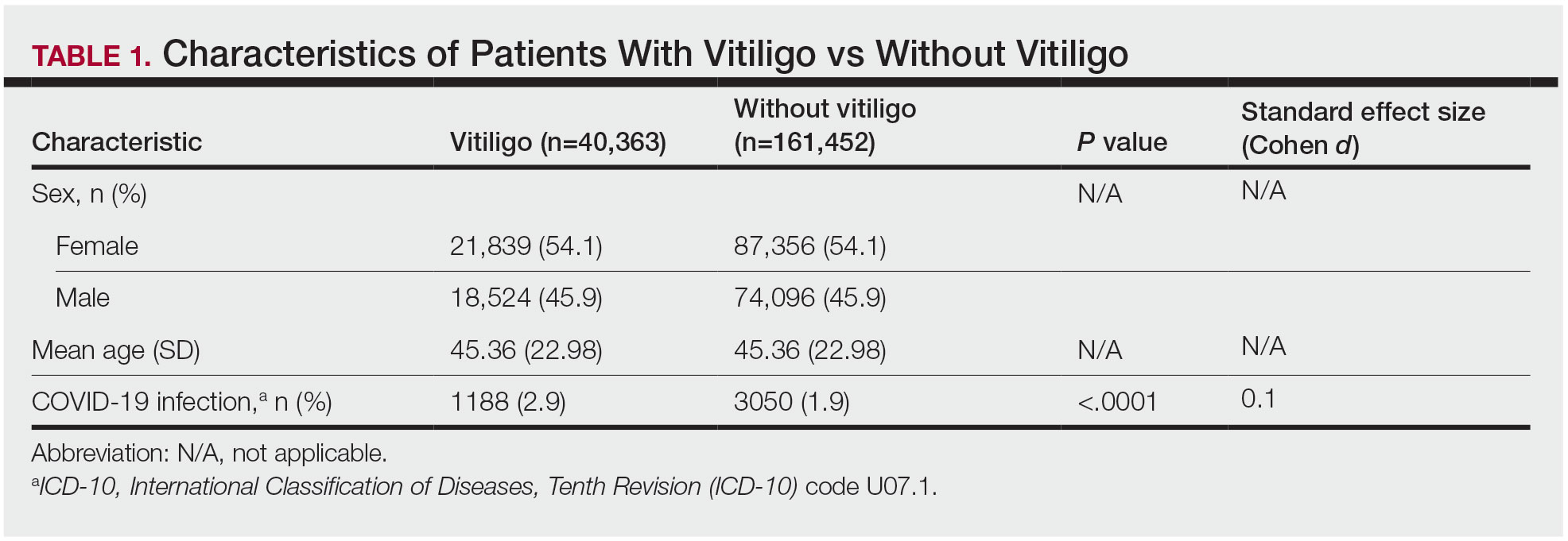
Our results showed that patients with vitiligo had a higher relative risk for contracting COVID-19 than individuals without vitiligo. It has been reported that the prevalence of COVID-19 is higher among patients with autoimmune diseases compared to the general population.3 Additionally, a handful of vitiligo patients are managed with immunosuppressive agents that may further weaken their immune response.1 Moreover, survey results from dermatologists managing vitiligo patients revealed that physicians were fairly comfortable prescribing immunosuppressants and encouraging in-office phototherapy during the COVID-19 pandemic.4 As a result, more patients may have been attending in-office visits for their phototherapy, which may have increased their risk for COVID-19. Although these factors play a role in COVID-19 infection rates, the underlying immune dysregulation in vitiligo in relation to COVID-19 remains unknown and should be further explored.
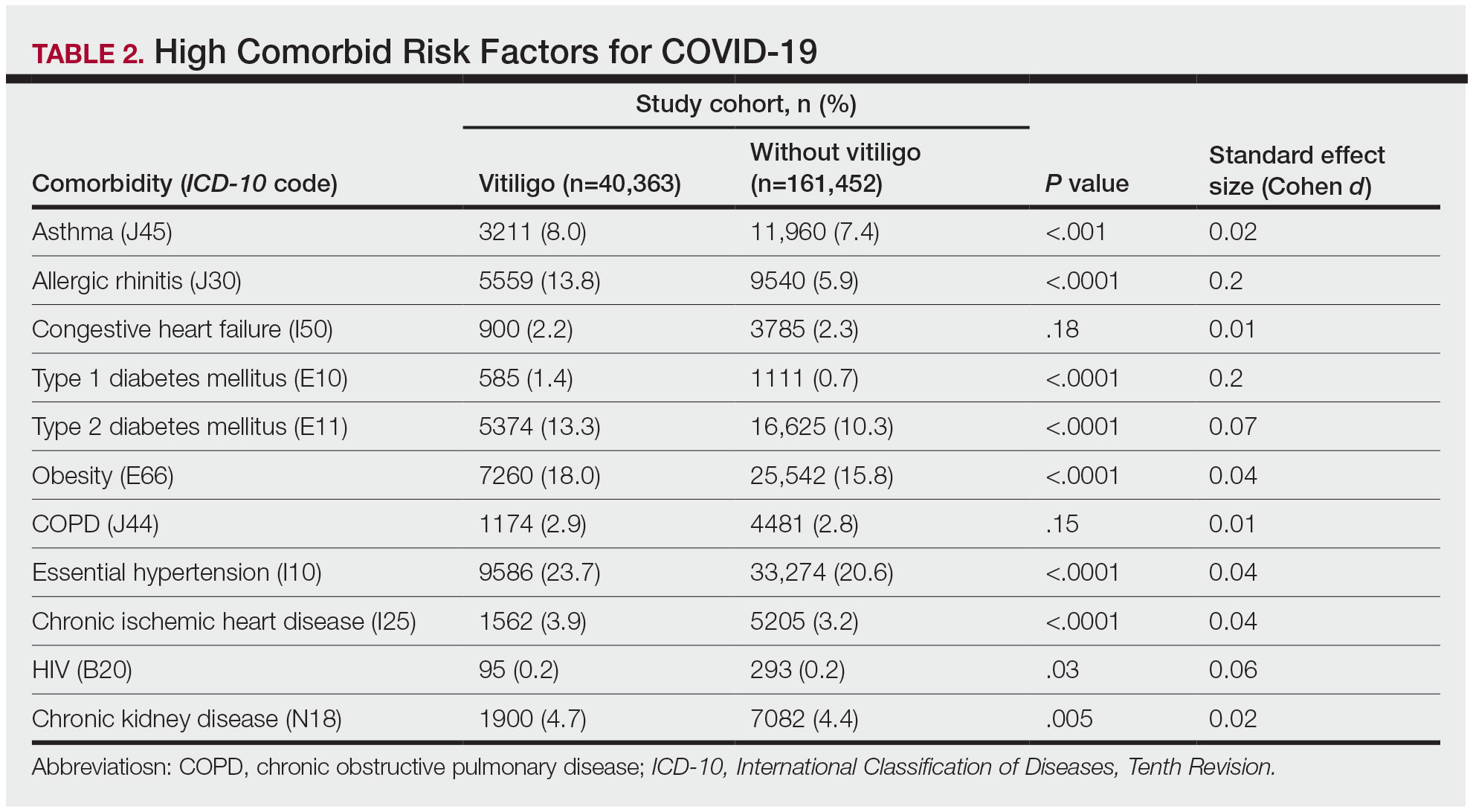
Our findings are limited by the use of ICD-10 codes, the inability to control for all potential confounding variables, the lack of data regarding the stage of vitiligo, and the absence of data for undiagnosed COVID-19 infections. In addition, patients with vitiligo may be more likely to seek care, potentially increasing their rates of COVID-19 testing. The inability to identify the stage of vitiligo during enrollment in the database may have altered our results, as individuals with active disease have increased levels of IFN-γ. Increased secretion of IFN-γ also potentially helps in the clearance of COVID-19 infection.1 Future studies should investigate this relationship via planned COVID-19 testing, identification of vitiligo stage, and controlling for other associated comorbidities.
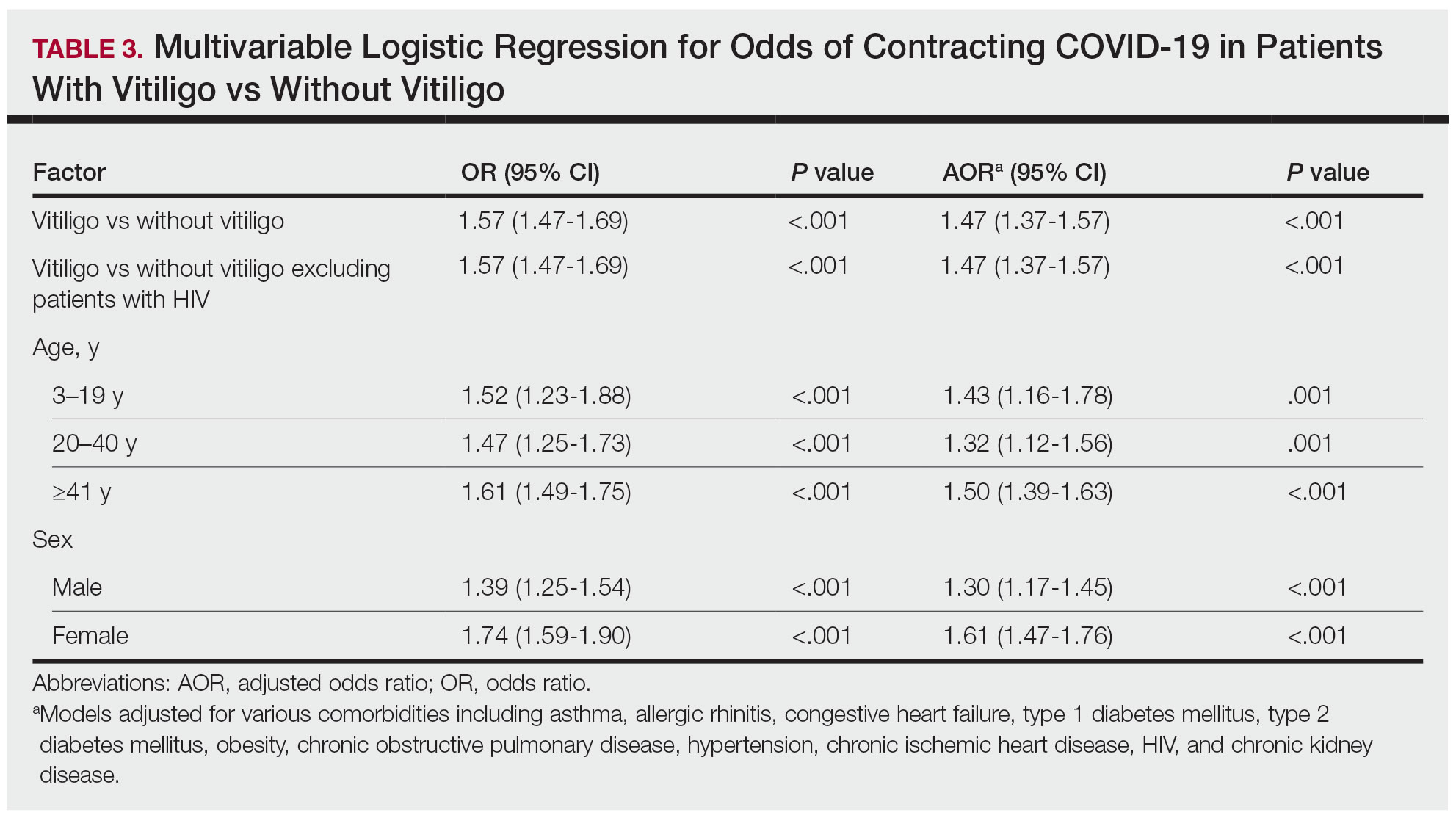
- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Wu JJ, Liu J, Thatiparthi A, et al. The risk of COVID-19 in patients with psoriasis—a retrospective cohort study [published online September 20, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.07.040
- Zhong J, Shen G, Yang H, et al. COVID-19 in patients with rheumatic disease in Hubei province, China: a multicentre retrospective observational study. Lancet Rheumatol. 2020;2:E557-E564. doi:10.1016/S2665-9913(20)30227-7
- Chatterjee M, Das A. Management of vitiligo amidst the COVID-19 pandemic: a survey and resulting consensus. Indian J Dermatol. 2021;66:479-483. doi:10.4103/ijd.ijd_859_20
To the Editor:
Vitiligo is a depigmentation disorder that results from the loss of melanocytes in the epidermis.1 The most widely accepted pathophysiology for melanocyte destruction in vitiligo is an autoimmune process involving dysregulated cytokine production and autoreactive T-cell activation.1 Individuals with cutaneous autoinflammatory conditions currently are vital patient populations warranting research, as their susceptibility to COVID-19 infection may differ from the general population. We previously found a small increased risk for COVID-19 infection in patients with psoriasis,2 which suggests that other dermatologic conditions also may impact COVID-19 risk. The risk for COVID-19 infection in patients with vitiligo remains largely unknown. In this retrospective cohort study, we investigated the risk for COVID-19 infection in patients with vitiligo compared with those without vitiligo utilizing claims data from the COVID-19 Research Database (https://covid19researchdatabase.org/).
Claims were evaluated for patients aged 3 years and older with a vitiligo diagnosis (International Classification of Diseases, Tenth Revision [ICD-10] code L80) that was made between January 1, 2016, and January 1, 2020. Individuals without a vitiligo diagnosis during the same period were placed (4:1 ratio) in the control group and were matched with study group patients for age and sex. All comorbidity variables and vitiligo diagnoses were extracted from ICD-10 codes that were given prior to a diagnosis of COVID-19. We then constructed multivariable logistic regression models adjusting for measured confounders to evaluate if vitiligo was associated with higher risk for COVID-19 infection after January 1, 2020.
The vitiligo and nonvitiligo cohorts included 40,363 and 161,452 patients, respectively (Table 1). Logistic regression analysis with adjustment for confounding variables, including high comorbid risk factors (Table 2) revealed that patients with a diagnosis of vitiligo had significantly increased odds of COVID-19 infection compared with patients without vitiligo (adjusted odds ratio [AOR], 1.47; 95% CI, 1.37-1.57; P<.001)(Table 3). Additionally, subgroup logistic analyses for sex, age, and exclusion of patients who were HIV positive revealed that females with vitiligo had higher odds of contracting COVID-19 than males with vitiligo (Table 3).

Our results showed that patients with vitiligo had a higher relative risk for contracting COVID-19 than individuals without vitiligo. It has been reported that the prevalence of COVID-19 is higher among patients with autoimmune diseases compared to the general population.3 Additionally, a handful of vitiligo patients are managed with immunosuppressive agents that may further weaken their immune response.1 Moreover, survey results from dermatologists managing vitiligo patients revealed that physicians were fairly comfortable prescribing immunosuppressants and encouraging in-office phototherapy during the COVID-19 pandemic.4 As a result, more patients may have been attending in-office visits for their phototherapy, which may have increased their risk for COVID-19. Although these factors play a role in COVID-19 infection rates, the underlying immune dysregulation in vitiligo in relation to COVID-19 remains unknown and should be further explored.

Our findings are limited by the use of ICD-10 codes, the inability to control for all potential confounding variables, the lack of data regarding the stage of vitiligo, and the absence of data for undiagnosed COVID-19 infections. In addition, patients with vitiligo may be more likely to seek care, potentially increasing their rates of COVID-19 testing. The inability to identify the stage of vitiligo during enrollment in the database may have altered our results, as individuals with active disease have increased levels of IFN-γ. Increased secretion of IFN-γ also potentially helps in the clearance of COVID-19 infection.1 Future studies should investigate this relationship via planned COVID-19 testing, identification of vitiligo stage, and controlling for other associated comorbidities.

To the Editor:
Vitiligo is a depigmentation disorder that results from the loss of melanocytes in the epidermis.1 The most widely accepted pathophysiology for melanocyte destruction in vitiligo is an autoimmune process involving dysregulated cytokine production and autoreactive T-cell activation.1 Individuals with cutaneous autoinflammatory conditions currently are vital patient populations warranting research, as their susceptibility to COVID-19 infection may differ from the general population. We previously found a small increased risk for COVID-19 infection in patients with psoriasis,2 which suggests that other dermatologic conditions also may impact COVID-19 risk. The risk for COVID-19 infection in patients with vitiligo remains largely unknown. In this retrospective cohort study, we investigated the risk for COVID-19 infection in patients with vitiligo compared with those without vitiligo utilizing claims data from the COVID-19 Research Database (https://covid19researchdatabase.org/).
Claims were evaluated for patients aged 3 years and older with a vitiligo diagnosis (International Classification of Diseases, Tenth Revision [ICD-10] code L80) that was made between January 1, 2016, and January 1, 2020. Individuals without a vitiligo diagnosis during the same period were placed (4:1 ratio) in the control group and were matched with study group patients for age and sex. All comorbidity variables and vitiligo diagnoses were extracted from ICD-10 codes that were given prior to a diagnosis of COVID-19. We then constructed multivariable logistic regression models adjusting for measured confounders to evaluate if vitiligo was associated with higher risk for COVID-19 infection after January 1, 2020.
The vitiligo and nonvitiligo cohorts included 40,363 and 161,452 patients, respectively (Table 1). Logistic regression analysis with adjustment for confounding variables, including high comorbid risk factors (Table 2) revealed that patients with a diagnosis of vitiligo had significantly increased odds of COVID-19 infection compared with patients without vitiligo (adjusted odds ratio [AOR], 1.47; 95% CI, 1.37-1.57; P<.001)(Table 3). Additionally, subgroup logistic analyses for sex, age, and exclusion of patients who were HIV positive revealed that females with vitiligo had higher odds of contracting COVID-19 than males with vitiligo (Table 3).

Our results showed that patients with vitiligo had a higher relative risk for contracting COVID-19 than individuals without vitiligo. It has been reported that the prevalence of COVID-19 is higher among patients with autoimmune diseases compared to the general population.3 Additionally, a handful of vitiligo patients are managed with immunosuppressive agents that may further weaken their immune response.1 Moreover, survey results from dermatologists managing vitiligo patients revealed that physicians were fairly comfortable prescribing immunosuppressants and encouraging in-office phototherapy during the COVID-19 pandemic.4 As a result, more patients may have been attending in-office visits for their phototherapy, which may have increased their risk for COVID-19. Although these factors play a role in COVID-19 infection rates, the underlying immune dysregulation in vitiligo in relation to COVID-19 remains unknown and should be further explored.

Our findings are limited by the use of ICD-10 codes, the inability to control for all potential confounding variables, the lack of data regarding the stage of vitiligo, and the absence of data for undiagnosed COVID-19 infections. In addition, patients with vitiligo may be more likely to seek care, potentially increasing their rates of COVID-19 testing. The inability to identify the stage of vitiligo during enrollment in the database may have altered our results, as individuals with active disease have increased levels of IFN-γ. Increased secretion of IFN-γ also potentially helps in the clearance of COVID-19 infection.1 Future studies should investigate this relationship via planned COVID-19 testing, identification of vitiligo stage, and controlling for other associated comorbidities.

- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Wu JJ, Liu J, Thatiparthi A, et al. The risk of COVID-19 in patients with psoriasis—a retrospective cohort study [published online September 20, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.07.040
- Zhong J, Shen G, Yang H, et al. COVID-19 in patients with rheumatic disease in Hubei province, China: a multicentre retrospective observational study. Lancet Rheumatol. 2020;2:E557-E564. doi:10.1016/S2665-9913(20)30227-7
- Chatterjee M, Das A. Management of vitiligo amidst the COVID-19 pandemic: a survey and resulting consensus. Indian J Dermatol. 2021;66:479-483. doi:10.4103/ijd.ijd_859_20
- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Wu JJ, Liu J, Thatiparthi A, et al. The risk of COVID-19 in patients with psoriasis—a retrospective cohort study [published online September 20, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.07.040
- Zhong J, Shen G, Yang H, et al. COVID-19 in patients with rheumatic disease in Hubei province, China: a multicentre retrospective observational study. Lancet Rheumatol. 2020;2:E557-E564. doi:10.1016/S2665-9913(20)30227-7
- Chatterjee M, Das A. Management of vitiligo amidst the COVID-19 pandemic: a survey and resulting consensus. Indian J Dermatol. 2021;66:479-483. doi:10.4103/ijd.ijd_859_20
Practice Points
- The underlying autoimmune process in vitiligo can result in various changes to the immune system.
- A diagnosis of vitiligo may alter the body’s immune response to COVID-19 infection.
Navigating Psoriasis Treatment Innovations
Psoriasis is a chronic autoimmune skin condition that affects approximately 2% to 4% of the US population and notably impacts overall quality of life.1,2 There is no cure for this long-lasting condition. Fortunately, recent developments in research have led to more targeted therapies, paving the way for a more promising transformative landscape of psoriasis management. Herein, we explore the most up-to-date advancements and developments in the realm of psoriasis care.
Emerging Systemic Therapies
Biologics are cutting-edge treatments available for moderate to severe plaque psoriasis, as IL-17A, IL-23, and tumor necrosis factor α (TNF-α) have been recognized as key targets.3
IL-17—Bimekizumab is a unique monoclonal antibody that inhibits the activity of both IL-17A and IL-17F cytokines.3 This treatment was approved by the US Food and Drug Administration (FDA) in October 2023 for patients with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy.4
Bimekizumab outperformed ustekinumab in the BE VIVID phase 3 trial, with 273 of 321 patients (85%) receiving bimekizumab vs 81 of 163 patients (50%) receiving ustekinumab experiencing at least 90% improvement in psoriasis area and severity index (PASI) score at week 16.4 In a 2020 observational study (PSO-BIO-REAL), the efficacy rate of skin clearance after 6 months of treatment with biologics was only 25% (1/4).5 Aside from moderate to severe plaque psoriasis, bimekizumab demonstrated notable improvement in patients with psoriatic arthritis who had inadequate response or intolerance to TNF-α inhibitors compared to a placebo group in the BE COMPLETE phase 3 trial.6
IL-23—Guselkumab, risankizumab, and tildrakizumab are injectable therapies approved by the FDA in 2017 for moderate to severe plaque psoriasis.3 They inhibit IL-23 signaling by targeting the p19 subunit in addition to sparing IL-12.3,7
A novel oral therapeutic peptide, JNJ-2113—the first oral IL-23 receptor antagonist peptide that blocks IL-23 signaling—has been developed, offering a new way to treat moderate to severe plaque psoriasis. Trial results from a phase 2 study (FRONTIER1) have supported JNJ-2113’s advancement into phase 3.7,8 Patients who received JNJ-2113 successfully achieved PASI75 in addition to surpassing PASI90 and PASI100 at greater proportions compared to placebo at week 16.7
The promising early results of JNJ-2113 provide patients with greater flexibility and convenience for treatment options to address the manifestations of psoriasis. Although a considerable number of patients with moderate to severe plaque psoriasis qualify for advanced therapies, a substantial proportion remain untreated. Introducing an oral route of medication administration may help overcome barriers to therapy access due to a greater preference for pills over injections.9
TNF-α Inhibitors—Adalimumab is a TNF-α inhibitor that is used to treat moderate to severe chronic plaque psoriasis in adults who are candidates for systemic phototherapy.1,10 However, one of the main barriers to initiating treatment has been cost. Biosimilars contribute to market competition, thus allowing the possibility of lower drug prices.10
There are 9 FDA-approved biosimilar products for adalimumab, with 2 having interchangeable designation. The first interchangeable biosimilar to enter the US market, adalimumab-adbm, became available in July 2023. In October 2023, adalimumab-afzb was granted interchangeable designation,11 which enables pharmacists to swiftly substitute brand products for lower-cost biosimilars, providing patients with equally safe and effective alternatives without the delay of involving the prescribing clinician.12 Pricing information indicates an initial 5% discount, which may later increase to 60%, from brand name adalimumab. Hopefully, reduced drug costs due to market competition will allow more patients to overcome barriers to therapy access.
IL-12/IL-23—Ustekinumab is a monoclonal antibody that targets IL-12 and IL-23. The FDA recently approved ustekinumab-auub as the first interchangeable ustekinumab biosimilar for the treatment of various inflammatory diseases, including moderate to severe plaque psoriasis and psoriatic arthritis.12,13 The approval of ustekinumab-auub expands therapeutic options for the treatment of diverse inflammatory diseases. As the first interchangeable biosimilar in its category, this development underscores the importance of biosimilars in providing effective and accessible treatment.12,14
Topical Innovations
In October 2023, the FDA approved an expanded indication for roflumilast cream 0.3% to treat children as young as 6 years for plaque psoriasis, even for use in intertriginous areas,15 which is a milestone given the lack of treatment options for the pediatric population because topical steroids, the most common treatment option for plaque psoriasis, can have safety concerns related to long-term use. With the advent of this steroid-free topical agent, pediatric patients have a safe and well-tolerated option for managing plaque psoriasis.16 This promising effort will now expand to trials in children as young as 2 years to test efficacy.16
Engel et al17 proposed a new algorithmic approach to the topical management of psoriasis with roflumilast cream and tapinarof cream as first-line treatments for mild disease due to their novelty in treating intertriginous areas, whereas traditional topical steroids in these areas would be inapt.17 The latest indication for roflumilast cream suggests that this proposed recommendation could be a promising and convenient enhancement to psoriasis management, potentially outperforming traditional topical corticosteroids.15,17
Final Thoughts
Innovative targeted therapies ranging from new biologic agents to broader applications of topical treatments hold the potential to transform conventional psoriasis management with greater efficacy and safety, which can help create a more effective and personalized approach with greater patient satisfaction, ultimately enhancing overall quality of life. The choice of treatment is dependent not only on the severity of the disease but also on accessibility considerations such as cost. Overall, these innovative therapies add substantial value to the treatment armamentarium for psoriasis.
- Li C, Sunhe Y, Zhou H, Dong W. Efficacy and safety evaluations of adalimumab biosimilars in the treatment of psoriasis. J Dermatolog Treat. 2023;34:2249145. doi:10.1080/09546634.2023.2249145
- Liu J, Thatiparthi A, Martin A, et al. Association between psoriasis and thyroid dysfunction among US adults in the 2009-2014 National Health and Nutrition Examination Survey [published online Mary 17, 2021]. J Am Acad Dermatol. 2022;86:897-899. doi:10.1016/j.jaad.2021.03.030
- Lee EB, Amin M, Bhutani T, et al. Emerging therapies in psoriasis: a systematic review. Cutis. 2018;101(3S):5-9.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo-controlled phase 3 trial. Lancet. 2021;397:487-498. doi:10.1016/S0140-6736(21)00125-2
- Seneschal J, Lacour JP, Bewley A, et al. A multinational, prospective, observational study to estimate complete skin clearance in patients with moderate-to-severe plaque PSOriasis treated with BIOlogics in a REAL world setting (PSO-BIO-REAL) [published online June 8, 2020]. J Eur Acad Dermatol Venereol. 2020;34:2566-2573. doi:10.1111/jdv.16568
- Merola JF, Landewé R, McInnes IB, et al. Bimekizumab in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumour necrosis factor-α inhibitors: a randomised, double-blind, placebo-controlled, phase 3 trial (BE COMPLETE)[published online December 6, 2022]. Lancet. 2023;401:38-48. doi:10.1016/S0140-6736(22)02303-0
- Janssen announces positive topline results for JNJ-2113—a novel, first and only oral IL-23 receptor antagonist peptide in development for moderate-to-severe plaque psoriasis. News release. Janssen Pharmaceutical Companies; July 4, 2023.
- Bissonnette R, Pinter A, Ferris L, et al. A Phase 2, randomized, placebo-controlled, dose-ranging study of oral JNJ-77242113 for the treatment of moderate-to-severe plaque psoriasis: FRONTIER 1. Abstract presented at: World Congress of Dermatology, July 3-8, 2023; Singapore.
- Xu Y, Sudharshan L, Hsu MA, et al. Patient preferences associated with therapies for psoriatic arthritis: a conjoint analysis. Am Health Drug Benefits. 2018;11:408-417.
- Maurelli M, Girolomoni G, Gisondi P. Cost per responder of adalimumab biosimilars versus methotrexate in patients with psoriasis: a real-life experience. J Dermatolog Treat. 2023;34:2218504. doi:10.1080/09546634.2023.2218504
- Food and Drug Administration/Center for Drug Evaluation and Research. Expiration of first interchangeable exclusivity (“FIE”) when section 351(l)(6) litigation ends prior to the submission of an application for interchangeability [memorandum]. Published October 3, 2023. Accessed January 18, 2024. https://www.fda.gov/media/173749/download
- US Food & Drug Administration. Biosimilar and interchangeable biologics: more treatment choices. Accessed January 18, 2024. https://www.fda.gov/consumers/consumer-updates/biosimilar-and-interchangeable-biologics-more-treatment-choices
- Chow V, Mytych DT, Das S, et al. Pharmacokinetic similarity of ABP 654, an ustekinumab biosimilar candidate: results from a randomized, double-blind study in healthy subjects [published online July 7, 2023]. Clin Pharmacol Drug Dev. 2023;12:863-873. doi:10.1002/cpdd.1301
- Wezlana (ustekinumab-auub) [prescribing information]. Published October 2023. Accessed January 18, 2024. www.accessdata.fda.gov/drugsatfda_docs/label/2023/761285s000,761331s000lbl.pdf
- ZORYVE (roflumilast) topical cream [prescribing information]. Westlake Village, CA: Arcutis Biotherapeutics. Revised October 2023. Accessed January 18, 2024. https://www.arcutis.com/wp-content/uploads/USPI-roflumilast-cream.pdf
- Lie E, Choi M, Wang SP, et al. Topical management of pediatric psoriasis: a review of new developments and existing therapies. Paediatr Drugs. 2024;26:9-18. doi:10.1007/s40272-023-00592-9
- Engel PV, Smith B, Javadi SS, et al. It is time to consider anew topical algorithm for psoriasis. J Am Acad Dermatol. 2023:S0190-9622(23)02906-7. doi:10.1016/j.jaad.2023.07.1048
Psoriasis is a chronic autoimmune skin condition that affects approximately 2% to 4% of the US population and notably impacts overall quality of life.1,2 There is no cure for this long-lasting condition. Fortunately, recent developments in research have led to more targeted therapies, paving the way for a more promising transformative landscape of psoriasis management. Herein, we explore the most up-to-date advancements and developments in the realm of psoriasis care.
Emerging Systemic Therapies
Biologics are cutting-edge treatments available for moderate to severe plaque psoriasis, as IL-17A, IL-23, and tumor necrosis factor α (TNF-α) have been recognized as key targets.3
IL-17—Bimekizumab is a unique monoclonal antibody that inhibits the activity of both IL-17A and IL-17F cytokines.3 This treatment was approved by the US Food and Drug Administration (FDA) in October 2023 for patients with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy.4
Bimekizumab outperformed ustekinumab in the BE VIVID phase 3 trial, with 273 of 321 patients (85%) receiving bimekizumab vs 81 of 163 patients (50%) receiving ustekinumab experiencing at least 90% improvement in psoriasis area and severity index (PASI) score at week 16.4 In a 2020 observational study (PSO-BIO-REAL), the efficacy rate of skin clearance after 6 months of treatment with biologics was only 25% (1/4).5 Aside from moderate to severe plaque psoriasis, bimekizumab demonstrated notable improvement in patients with psoriatic arthritis who had inadequate response or intolerance to TNF-α inhibitors compared to a placebo group in the BE COMPLETE phase 3 trial.6
IL-23—Guselkumab, risankizumab, and tildrakizumab are injectable therapies approved by the FDA in 2017 for moderate to severe plaque psoriasis.3 They inhibit IL-23 signaling by targeting the p19 subunit in addition to sparing IL-12.3,7
A novel oral therapeutic peptide, JNJ-2113—the first oral IL-23 receptor antagonist peptide that blocks IL-23 signaling—has been developed, offering a new way to treat moderate to severe plaque psoriasis. Trial results from a phase 2 study (FRONTIER1) have supported JNJ-2113’s advancement into phase 3.7,8 Patients who received JNJ-2113 successfully achieved PASI75 in addition to surpassing PASI90 and PASI100 at greater proportions compared to placebo at week 16.7
The promising early results of JNJ-2113 provide patients with greater flexibility and convenience for treatment options to address the manifestations of psoriasis. Although a considerable number of patients with moderate to severe plaque psoriasis qualify for advanced therapies, a substantial proportion remain untreated. Introducing an oral route of medication administration may help overcome barriers to therapy access due to a greater preference for pills over injections.9
TNF-α Inhibitors—Adalimumab is a TNF-α inhibitor that is used to treat moderate to severe chronic plaque psoriasis in adults who are candidates for systemic phototherapy.1,10 However, one of the main barriers to initiating treatment has been cost. Biosimilars contribute to market competition, thus allowing the possibility of lower drug prices.10
There are 9 FDA-approved biosimilar products for adalimumab, with 2 having interchangeable designation. The first interchangeable biosimilar to enter the US market, adalimumab-adbm, became available in July 2023. In October 2023, adalimumab-afzb was granted interchangeable designation,11 which enables pharmacists to swiftly substitute brand products for lower-cost biosimilars, providing patients with equally safe and effective alternatives without the delay of involving the prescribing clinician.12 Pricing information indicates an initial 5% discount, which may later increase to 60%, from brand name adalimumab. Hopefully, reduced drug costs due to market competition will allow more patients to overcome barriers to therapy access.
IL-12/IL-23—Ustekinumab is a monoclonal antibody that targets IL-12 and IL-23. The FDA recently approved ustekinumab-auub as the first interchangeable ustekinumab biosimilar for the treatment of various inflammatory diseases, including moderate to severe plaque psoriasis and psoriatic arthritis.12,13 The approval of ustekinumab-auub expands therapeutic options for the treatment of diverse inflammatory diseases. As the first interchangeable biosimilar in its category, this development underscores the importance of biosimilars in providing effective and accessible treatment.12,14
Topical Innovations
In October 2023, the FDA approved an expanded indication for roflumilast cream 0.3% to treat children as young as 6 years for plaque psoriasis, even for use in intertriginous areas,15 which is a milestone given the lack of treatment options for the pediatric population because topical steroids, the most common treatment option for plaque psoriasis, can have safety concerns related to long-term use. With the advent of this steroid-free topical agent, pediatric patients have a safe and well-tolerated option for managing plaque psoriasis.16 This promising effort will now expand to trials in children as young as 2 years to test efficacy.16
Engel et al17 proposed a new algorithmic approach to the topical management of psoriasis with roflumilast cream and tapinarof cream as first-line treatments for mild disease due to their novelty in treating intertriginous areas, whereas traditional topical steroids in these areas would be inapt.17 The latest indication for roflumilast cream suggests that this proposed recommendation could be a promising and convenient enhancement to psoriasis management, potentially outperforming traditional topical corticosteroids.15,17
Final Thoughts
Innovative targeted therapies ranging from new biologic agents to broader applications of topical treatments hold the potential to transform conventional psoriasis management with greater efficacy and safety, which can help create a more effective and personalized approach with greater patient satisfaction, ultimately enhancing overall quality of life. The choice of treatment is dependent not only on the severity of the disease but also on accessibility considerations such as cost. Overall, these innovative therapies add substantial value to the treatment armamentarium for psoriasis.
Psoriasis is a chronic autoimmune skin condition that affects approximately 2% to 4% of the US population and notably impacts overall quality of life.1,2 There is no cure for this long-lasting condition. Fortunately, recent developments in research have led to more targeted therapies, paving the way for a more promising transformative landscape of psoriasis management. Herein, we explore the most up-to-date advancements and developments in the realm of psoriasis care.
Emerging Systemic Therapies
Biologics are cutting-edge treatments available for moderate to severe plaque psoriasis, as IL-17A, IL-23, and tumor necrosis factor α (TNF-α) have been recognized as key targets.3
IL-17—Bimekizumab is a unique monoclonal antibody that inhibits the activity of both IL-17A and IL-17F cytokines.3 This treatment was approved by the US Food and Drug Administration (FDA) in October 2023 for patients with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy.4
Bimekizumab outperformed ustekinumab in the BE VIVID phase 3 trial, with 273 of 321 patients (85%) receiving bimekizumab vs 81 of 163 patients (50%) receiving ustekinumab experiencing at least 90% improvement in psoriasis area and severity index (PASI) score at week 16.4 In a 2020 observational study (PSO-BIO-REAL), the efficacy rate of skin clearance after 6 months of treatment with biologics was only 25% (1/4).5 Aside from moderate to severe plaque psoriasis, bimekizumab demonstrated notable improvement in patients with psoriatic arthritis who had inadequate response or intolerance to TNF-α inhibitors compared to a placebo group in the BE COMPLETE phase 3 trial.6
IL-23—Guselkumab, risankizumab, and tildrakizumab are injectable therapies approved by the FDA in 2017 for moderate to severe plaque psoriasis.3 They inhibit IL-23 signaling by targeting the p19 subunit in addition to sparing IL-12.3,7
A novel oral therapeutic peptide, JNJ-2113—the first oral IL-23 receptor antagonist peptide that blocks IL-23 signaling—has been developed, offering a new way to treat moderate to severe plaque psoriasis. Trial results from a phase 2 study (FRONTIER1) have supported JNJ-2113’s advancement into phase 3.7,8 Patients who received JNJ-2113 successfully achieved PASI75 in addition to surpassing PASI90 and PASI100 at greater proportions compared to placebo at week 16.7
The promising early results of JNJ-2113 provide patients with greater flexibility and convenience for treatment options to address the manifestations of psoriasis. Although a considerable number of patients with moderate to severe plaque psoriasis qualify for advanced therapies, a substantial proportion remain untreated. Introducing an oral route of medication administration may help overcome barriers to therapy access due to a greater preference for pills over injections.9
TNF-α Inhibitors—Adalimumab is a TNF-α inhibitor that is used to treat moderate to severe chronic plaque psoriasis in adults who are candidates for systemic phototherapy.1,10 However, one of the main barriers to initiating treatment has been cost. Biosimilars contribute to market competition, thus allowing the possibility of lower drug prices.10
There are 9 FDA-approved biosimilar products for adalimumab, with 2 having interchangeable designation. The first interchangeable biosimilar to enter the US market, adalimumab-adbm, became available in July 2023. In October 2023, adalimumab-afzb was granted interchangeable designation,11 which enables pharmacists to swiftly substitute brand products for lower-cost biosimilars, providing patients with equally safe and effective alternatives without the delay of involving the prescribing clinician.12 Pricing information indicates an initial 5% discount, which may later increase to 60%, from brand name adalimumab. Hopefully, reduced drug costs due to market competition will allow more patients to overcome barriers to therapy access.
IL-12/IL-23—Ustekinumab is a monoclonal antibody that targets IL-12 and IL-23. The FDA recently approved ustekinumab-auub as the first interchangeable ustekinumab biosimilar for the treatment of various inflammatory diseases, including moderate to severe plaque psoriasis and psoriatic arthritis.12,13 The approval of ustekinumab-auub expands therapeutic options for the treatment of diverse inflammatory diseases. As the first interchangeable biosimilar in its category, this development underscores the importance of biosimilars in providing effective and accessible treatment.12,14
Topical Innovations
In October 2023, the FDA approved an expanded indication for roflumilast cream 0.3% to treat children as young as 6 years for plaque psoriasis, even for use in intertriginous areas,15 which is a milestone given the lack of treatment options for the pediatric population because topical steroids, the most common treatment option for plaque psoriasis, can have safety concerns related to long-term use. With the advent of this steroid-free topical agent, pediatric patients have a safe and well-tolerated option for managing plaque psoriasis.16 This promising effort will now expand to trials in children as young as 2 years to test efficacy.16
Engel et al17 proposed a new algorithmic approach to the topical management of psoriasis with roflumilast cream and tapinarof cream as first-line treatments for mild disease due to their novelty in treating intertriginous areas, whereas traditional topical steroids in these areas would be inapt.17 The latest indication for roflumilast cream suggests that this proposed recommendation could be a promising and convenient enhancement to psoriasis management, potentially outperforming traditional topical corticosteroids.15,17
Final Thoughts
Innovative targeted therapies ranging from new biologic agents to broader applications of topical treatments hold the potential to transform conventional psoriasis management with greater efficacy and safety, which can help create a more effective and personalized approach with greater patient satisfaction, ultimately enhancing overall quality of life. The choice of treatment is dependent not only on the severity of the disease but also on accessibility considerations such as cost. Overall, these innovative therapies add substantial value to the treatment armamentarium for psoriasis.
- Li C, Sunhe Y, Zhou H, Dong W. Efficacy and safety evaluations of adalimumab biosimilars in the treatment of psoriasis. J Dermatolog Treat. 2023;34:2249145. doi:10.1080/09546634.2023.2249145
- Liu J, Thatiparthi A, Martin A, et al. Association between psoriasis and thyroid dysfunction among US adults in the 2009-2014 National Health and Nutrition Examination Survey [published online Mary 17, 2021]. J Am Acad Dermatol. 2022;86:897-899. doi:10.1016/j.jaad.2021.03.030
- Lee EB, Amin M, Bhutani T, et al. Emerging therapies in psoriasis: a systematic review. Cutis. 2018;101(3S):5-9.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo-controlled phase 3 trial. Lancet. 2021;397:487-498. doi:10.1016/S0140-6736(21)00125-2
- Seneschal J, Lacour JP, Bewley A, et al. A multinational, prospective, observational study to estimate complete skin clearance in patients with moderate-to-severe plaque PSOriasis treated with BIOlogics in a REAL world setting (PSO-BIO-REAL) [published online June 8, 2020]. J Eur Acad Dermatol Venereol. 2020;34:2566-2573. doi:10.1111/jdv.16568
- Merola JF, Landewé R, McInnes IB, et al. Bimekizumab in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumour necrosis factor-α inhibitors: a randomised, double-blind, placebo-controlled, phase 3 trial (BE COMPLETE)[published online December 6, 2022]. Lancet. 2023;401:38-48. doi:10.1016/S0140-6736(22)02303-0
- Janssen announces positive topline results for JNJ-2113—a novel, first and only oral IL-23 receptor antagonist peptide in development for moderate-to-severe plaque psoriasis. News release. Janssen Pharmaceutical Companies; July 4, 2023.
- Bissonnette R, Pinter A, Ferris L, et al. A Phase 2, randomized, placebo-controlled, dose-ranging study of oral JNJ-77242113 for the treatment of moderate-to-severe plaque psoriasis: FRONTIER 1. Abstract presented at: World Congress of Dermatology, July 3-8, 2023; Singapore.
- Xu Y, Sudharshan L, Hsu MA, et al. Patient preferences associated with therapies for psoriatic arthritis: a conjoint analysis. Am Health Drug Benefits. 2018;11:408-417.
- Maurelli M, Girolomoni G, Gisondi P. Cost per responder of adalimumab biosimilars versus methotrexate in patients with psoriasis: a real-life experience. J Dermatolog Treat. 2023;34:2218504. doi:10.1080/09546634.2023.2218504
- Food and Drug Administration/Center for Drug Evaluation and Research. Expiration of first interchangeable exclusivity (“FIE”) when section 351(l)(6) litigation ends prior to the submission of an application for interchangeability [memorandum]. Published October 3, 2023. Accessed January 18, 2024. https://www.fda.gov/media/173749/download
- US Food & Drug Administration. Biosimilar and interchangeable biologics: more treatment choices. Accessed January 18, 2024. https://www.fda.gov/consumers/consumer-updates/biosimilar-and-interchangeable-biologics-more-treatment-choices
- Chow V, Mytych DT, Das S, et al. Pharmacokinetic similarity of ABP 654, an ustekinumab biosimilar candidate: results from a randomized, double-blind study in healthy subjects [published online July 7, 2023]. Clin Pharmacol Drug Dev. 2023;12:863-873. doi:10.1002/cpdd.1301
- Wezlana (ustekinumab-auub) [prescribing information]. Published October 2023. Accessed January 18, 2024. www.accessdata.fda.gov/drugsatfda_docs/label/2023/761285s000,761331s000lbl.pdf
- ZORYVE (roflumilast) topical cream [prescribing information]. Westlake Village, CA: Arcutis Biotherapeutics. Revised October 2023. Accessed January 18, 2024. https://www.arcutis.com/wp-content/uploads/USPI-roflumilast-cream.pdf
- Lie E, Choi M, Wang SP, et al. Topical management of pediatric psoriasis: a review of new developments and existing therapies. Paediatr Drugs. 2024;26:9-18. doi:10.1007/s40272-023-00592-9
- Engel PV, Smith B, Javadi SS, et al. It is time to consider anew topical algorithm for psoriasis. J Am Acad Dermatol. 2023:S0190-9622(23)02906-7. doi:10.1016/j.jaad.2023.07.1048
- Li C, Sunhe Y, Zhou H, Dong W. Efficacy and safety evaluations of adalimumab biosimilars in the treatment of psoriasis. J Dermatolog Treat. 2023;34:2249145. doi:10.1080/09546634.2023.2249145
- Liu J, Thatiparthi A, Martin A, et al. Association between psoriasis and thyroid dysfunction among US adults in the 2009-2014 National Health and Nutrition Examination Survey [published online Mary 17, 2021]. J Am Acad Dermatol. 2022;86:897-899. doi:10.1016/j.jaad.2021.03.030
- Lee EB, Amin M, Bhutani T, et al. Emerging therapies in psoriasis: a systematic review. Cutis. 2018;101(3S):5-9.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo-controlled phase 3 trial. Lancet. 2021;397:487-498. doi:10.1016/S0140-6736(21)00125-2
- Seneschal J, Lacour JP, Bewley A, et al. A multinational, prospective, observational study to estimate complete skin clearance in patients with moderate-to-severe plaque PSOriasis treated with BIOlogics in a REAL world setting (PSO-BIO-REAL) [published online June 8, 2020]. J Eur Acad Dermatol Venereol. 2020;34:2566-2573. doi:10.1111/jdv.16568
- Merola JF, Landewé R, McInnes IB, et al. Bimekizumab in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumour necrosis factor-α inhibitors: a randomised, double-blind, placebo-controlled, phase 3 trial (BE COMPLETE)[published online December 6, 2022]. Lancet. 2023;401:38-48. doi:10.1016/S0140-6736(22)02303-0
- Janssen announces positive topline results for JNJ-2113—a novel, first and only oral IL-23 receptor antagonist peptide in development for moderate-to-severe plaque psoriasis. News release. Janssen Pharmaceutical Companies; July 4, 2023.
- Bissonnette R, Pinter A, Ferris L, et al. A Phase 2, randomized, placebo-controlled, dose-ranging study of oral JNJ-77242113 for the treatment of moderate-to-severe plaque psoriasis: FRONTIER 1. Abstract presented at: World Congress of Dermatology, July 3-8, 2023; Singapore.
- Xu Y, Sudharshan L, Hsu MA, et al. Patient preferences associated with therapies for psoriatic arthritis: a conjoint analysis. Am Health Drug Benefits. 2018;11:408-417.
- Maurelli M, Girolomoni G, Gisondi P. Cost per responder of adalimumab biosimilars versus methotrexate in patients with psoriasis: a real-life experience. J Dermatolog Treat. 2023;34:2218504. doi:10.1080/09546634.2023.2218504
- Food and Drug Administration/Center for Drug Evaluation and Research. Expiration of first interchangeable exclusivity (“FIE”) when section 351(l)(6) litigation ends prior to the submission of an application for interchangeability [memorandum]. Published October 3, 2023. Accessed January 18, 2024. https://www.fda.gov/media/173749/download
- US Food & Drug Administration. Biosimilar and interchangeable biologics: more treatment choices. Accessed January 18, 2024. https://www.fda.gov/consumers/consumer-updates/biosimilar-and-interchangeable-biologics-more-treatment-choices
- Chow V, Mytych DT, Das S, et al. Pharmacokinetic similarity of ABP 654, an ustekinumab biosimilar candidate: results from a randomized, double-blind study in healthy subjects [published online July 7, 2023]. Clin Pharmacol Drug Dev. 2023;12:863-873. doi:10.1002/cpdd.1301
- Wezlana (ustekinumab-auub) [prescribing information]. Published October 2023. Accessed January 18, 2024. www.accessdata.fda.gov/drugsatfda_docs/label/2023/761285s000,761331s000lbl.pdf
- ZORYVE (roflumilast) topical cream [prescribing information]. Westlake Village, CA: Arcutis Biotherapeutics. Revised October 2023. Accessed January 18, 2024. https://www.arcutis.com/wp-content/uploads/USPI-roflumilast-cream.pdf
- Lie E, Choi M, Wang SP, et al. Topical management of pediatric psoriasis: a review of new developments and existing therapies. Paediatr Drugs. 2024;26:9-18. doi:10.1007/s40272-023-00592-9
- Engel PV, Smith B, Javadi SS, et al. It is time to consider anew topical algorithm for psoriasis. J Am Acad Dermatol. 2023:S0190-9622(23)02906-7. doi:10.1016/j.jaad.2023.07.1048
Association Between Atopic Dermatitis and Chronic Obstructive Pulmonary Disease Among US Adults in the 1999-2006 NHANES Survey
To the Editor:
Atopic dermatitis (AD) is an inflammatory skin condition that affects approximately 16.5 million adults in the United States.1 Atopic dermatitis is associated with skin barrier dysfunction and the activation of type 2 inflammatory cytokines. Multiorgan involvement of AD has been demonstrated, as patients with AD are more prone to asthma, allergic rhinitis, and other systemic diseases.2 In 2020, Smirnova et al3 reported a significant association (adjusted odds ratio [AOR], 1.58; 95% CI, 1.30-1.92) between AD and chronic obstructive pulmonary disease (COPD) in a large Swedish population. Currently, there is a lack of research evaluating the association between AD and COPD in a population of US adults. Therefore, we explored the association between AD and COPD (chronic bronchitis or emphysema) in a population of US adults utilizing the 1999-2006 National Health and Nutrition Examination Survey (NHANES), as these were the latest data for AD available in NHANES.4
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 1999-2006 NHANES database. Three outcome variables—emphysema, chronic bronchitis, and COPD—and numerous confounding variables for each participant were extracted from the NHANES database. The original cohort consisted of 13,134 participants, and 43 patients were excluded from our analysis owing to the lack of response to survey questions regarding AD and COPD status. The relationship between AD and COPD was evaluated by multivariable logistic regression analyses utilizing Stata/MP 17 (StataCorp LLC). In our logistic regression models, we controlled for age, sex, race/ethnicity, education, income, tobacco usage, diabetes mellitus and asthma status, and body mass index (eTable).
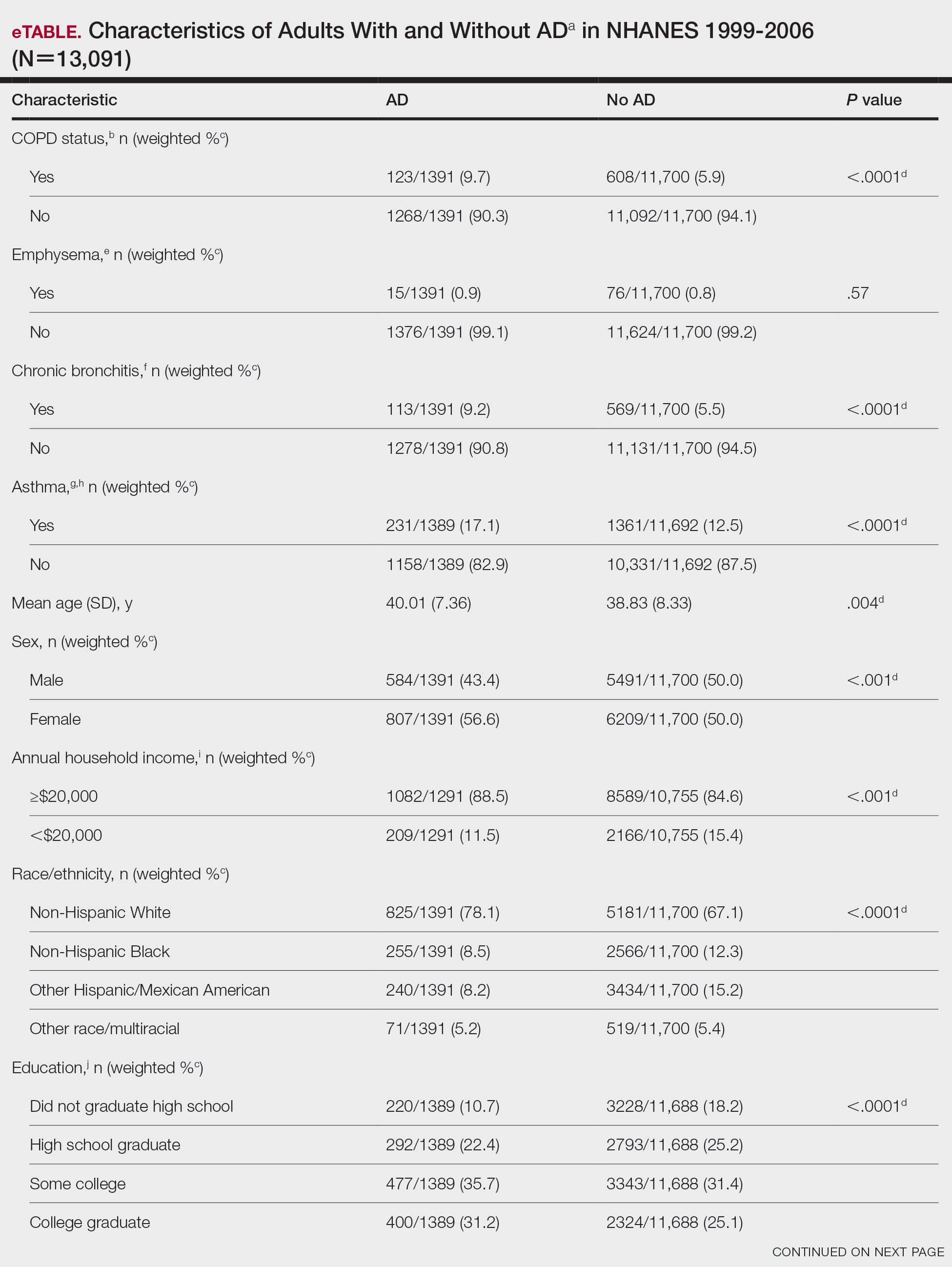
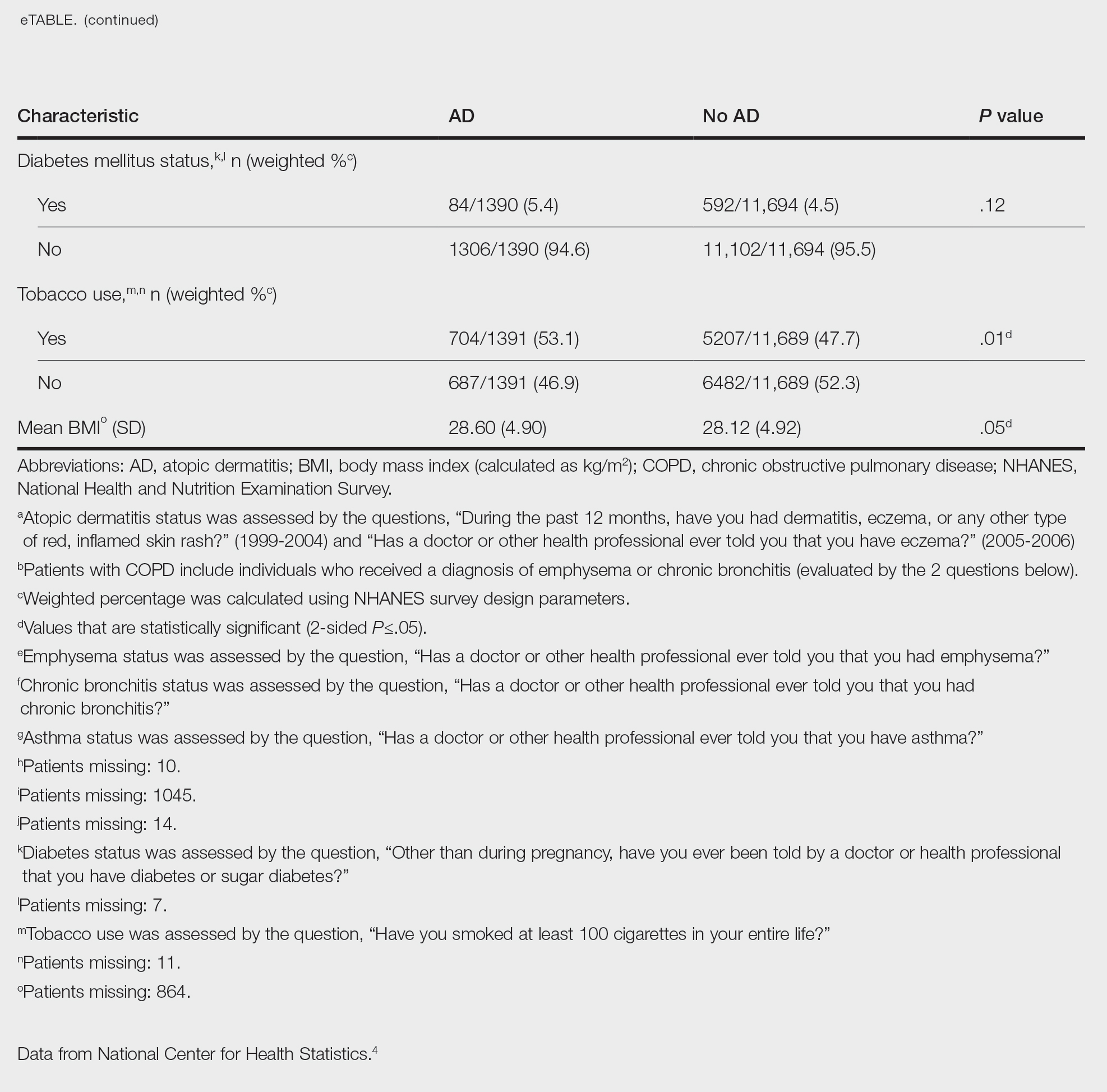
Our study consisted of 13,091 participants. Multivariable logistic regressions were utilized to examine the association between AD and COPD (Table). Approximately 12.5% (weighted) of the patients in our analysis had AD. Additionally, 9.7% (weighted) of patients with AD had received a diagnosis of COPD; conversely, 5.9% (weighted) of patients without AD had received a diagnosis of COPD. More patients with AD reported a diagnosis of chronic bronchitis (9.2%) rather than emphysema (0.9%). Our analysis revealed a significant association between AD and COPD among adults aged 20 to 59 years (AOR, 1.43; 95% CI, 1.13-1.80; P=.003) after controlling for potential confounding variables. Subsequently, we performed subgroup analyses, including exclusion of patients with an asthma diagnosis, to further explore the association between AD and COPD. After excluding participants with asthma, there was still a significant association between AD and COPD (AOR, 1.57; 95% CI, 1.14-2.16; P=.007). Moreover, the odds of receiving a COPD diagnosis were significantly higher among male patients with AD (AOR, 1.54; 95% CI, 1.06-2.25; P=.03).
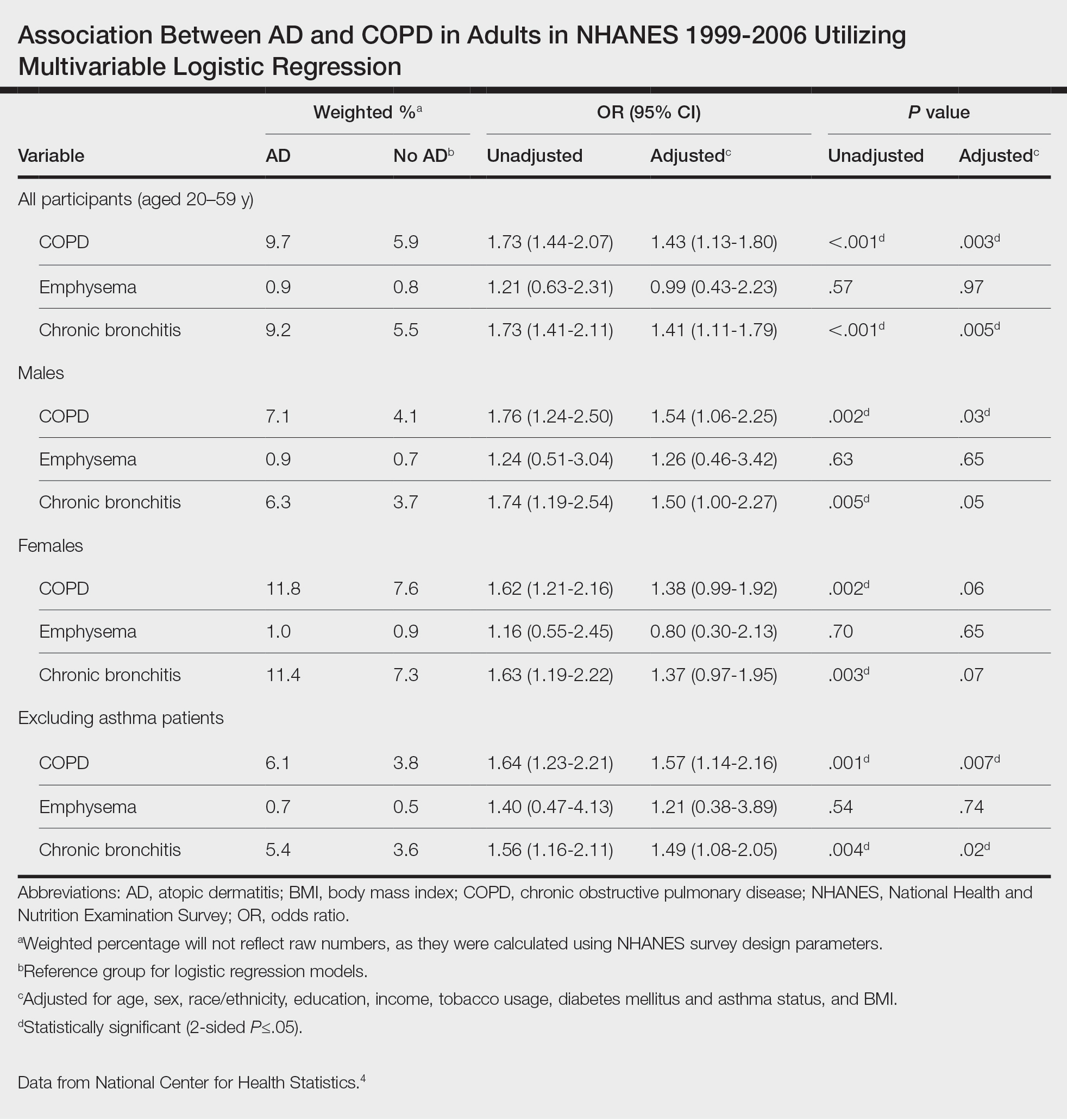
Our results support the association between AD and COPD, more specifically chronic bronchitis. This finding may be due to similar pathogenic mechanisms in both conditions, including overlapping cytokine production and immune pathways.5 Additionally, Harazin et al6 discussed the role of a novel gene, collagen 29A1 (COL29A1), in the pathogenesis of AD, COPD, and asthma. Variations in this gene may predispose patients to not only atopic diseases but also COPD.6
Limitations of our study include self-reported diagnoses and lack of patients older than 59 years. Self-reported diagnoses could have resulted in some misclassification of COPD, as some individuals may have reported a diagnosis of COPD rather than their true diagnosis of asthma. We mitigated this limitation by constructing a subpopulation model with exclusion of individuals with asthma. Further studies with spirometry-diagnosed COPD are needed to explore this relationship and the potential contributory pathophysiologic mechanisms. Understanding this association may increase awareness of potential comorbidities and assist clinicians with adequate management of patients with AD.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic Dermatitis in America Study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590. doi:10.1016/j.jid.2018.08.028
- Darlenski R, Kazandjieva J, Hristakieva E, et al. Atopic dermatitis as a systemic disease. Clin Dermatol. 2014;32:409-413. doi:10.1016/j.clindermatol.2013.11.007
- Smirnova J, Montgomery S, Lindberg M, et al. Associations of self-reported atopic dermatitis with comorbid conditions in adults: a population-based cross-sectional study. BMC Dermatol. 2020;20:23. doi:10.1186/s12895-020-00117-8
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://wwwn.cdc.gov/nchs/nhanes/
- Kawayama T, Okamoto M, Imaoka H, et al. Interleukin-18 in pulmonary inflammatory diseases. J Interferon Cytokine Res. 2012;32:443-449. doi:10.1089/jir.2012.0029
- Harazin M, Parwez Q, Petrasch-Parwez E, et al. Variation in the COL29A1 gene in German patients with atopic dermatitis, asthma and chronic obstructive pulmonary disease. J Dermatol. 2010;37:740-742. doi:10.1111/j.1346-8138.2010.00923.x
To the Editor:
Atopic dermatitis (AD) is an inflammatory skin condition that affects approximately 16.5 million adults in the United States.1 Atopic dermatitis is associated with skin barrier dysfunction and the activation of type 2 inflammatory cytokines. Multiorgan involvement of AD has been demonstrated, as patients with AD are more prone to asthma, allergic rhinitis, and other systemic diseases.2 In 2020, Smirnova et al3 reported a significant association (adjusted odds ratio [AOR], 1.58; 95% CI, 1.30-1.92) between AD and chronic obstructive pulmonary disease (COPD) in a large Swedish population. Currently, there is a lack of research evaluating the association between AD and COPD in a population of US adults. Therefore, we explored the association between AD and COPD (chronic bronchitis or emphysema) in a population of US adults utilizing the 1999-2006 National Health and Nutrition Examination Survey (NHANES), as these were the latest data for AD available in NHANES.4
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 1999-2006 NHANES database. Three outcome variables—emphysema, chronic bronchitis, and COPD—and numerous confounding variables for each participant were extracted from the NHANES database. The original cohort consisted of 13,134 participants, and 43 patients were excluded from our analysis owing to the lack of response to survey questions regarding AD and COPD status. The relationship between AD and COPD was evaluated by multivariable logistic regression analyses utilizing Stata/MP 17 (StataCorp LLC). In our logistic regression models, we controlled for age, sex, race/ethnicity, education, income, tobacco usage, diabetes mellitus and asthma status, and body mass index (eTable).


Our study consisted of 13,091 participants. Multivariable logistic regressions were utilized to examine the association between AD and COPD (Table). Approximately 12.5% (weighted) of the patients in our analysis had AD. Additionally, 9.7% (weighted) of patients with AD had received a diagnosis of COPD; conversely, 5.9% (weighted) of patients without AD had received a diagnosis of COPD. More patients with AD reported a diagnosis of chronic bronchitis (9.2%) rather than emphysema (0.9%). Our analysis revealed a significant association between AD and COPD among adults aged 20 to 59 years (AOR, 1.43; 95% CI, 1.13-1.80; P=.003) after controlling for potential confounding variables. Subsequently, we performed subgroup analyses, including exclusion of patients with an asthma diagnosis, to further explore the association between AD and COPD. After excluding participants with asthma, there was still a significant association between AD and COPD (AOR, 1.57; 95% CI, 1.14-2.16; P=.007). Moreover, the odds of receiving a COPD diagnosis were significantly higher among male patients with AD (AOR, 1.54; 95% CI, 1.06-2.25; P=.03).

Our results support the association between AD and COPD, more specifically chronic bronchitis. This finding may be due to similar pathogenic mechanisms in both conditions, including overlapping cytokine production and immune pathways.5 Additionally, Harazin et al6 discussed the role of a novel gene, collagen 29A1 (COL29A1), in the pathogenesis of AD, COPD, and asthma. Variations in this gene may predispose patients to not only atopic diseases but also COPD.6
Limitations of our study include self-reported diagnoses and lack of patients older than 59 years. Self-reported diagnoses could have resulted in some misclassification of COPD, as some individuals may have reported a diagnosis of COPD rather than their true diagnosis of asthma. We mitigated this limitation by constructing a subpopulation model with exclusion of individuals with asthma. Further studies with spirometry-diagnosed COPD are needed to explore this relationship and the potential contributory pathophysiologic mechanisms. Understanding this association may increase awareness of potential comorbidities and assist clinicians with adequate management of patients with AD.
To the Editor:
Atopic dermatitis (AD) is an inflammatory skin condition that affects approximately 16.5 million adults in the United States.1 Atopic dermatitis is associated with skin barrier dysfunction and the activation of type 2 inflammatory cytokines. Multiorgan involvement of AD has been demonstrated, as patients with AD are more prone to asthma, allergic rhinitis, and other systemic diseases.2 In 2020, Smirnova et al3 reported a significant association (adjusted odds ratio [AOR], 1.58; 95% CI, 1.30-1.92) between AD and chronic obstructive pulmonary disease (COPD) in a large Swedish population. Currently, there is a lack of research evaluating the association between AD and COPD in a population of US adults. Therefore, we explored the association between AD and COPD (chronic bronchitis or emphysema) in a population of US adults utilizing the 1999-2006 National Health and Nutrition Examination Survey (NHANES), as these were the latest data for AD available in NHANES.4
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 1999-2006 NHANES database. Three outcome variables—emphysema, chronic bronchitis, and COPD—and numerous confounding variables for each participant were extracted from the NHANES database. The original cohort consisted of 13,134 participants, and 43 patients were excluded from our analysis owing to the lack of response to survey questions regarding AD and COPD status. The relationship between AD and COPD was evaluated by multivariable logistic regression analyses utilizing Stata/MP 17 (StataCorp LLC). In our logistic regression models, we controlled for age, sex, race/ethnicity, education, income, tobacco usage, diabetes mellitus and asthma status, and body mass index (eTable).


Our study consisted of 13,091 participants. Multivariable logistic regressions were utilized to examine the association between AD and COPD (Table). Approximately 12.5% (weighted) of the patients in our analysis had AD. Additionally, 9.7% (weighted) of patients with AD had received a diagnosis of COPD; conversely, 5.9% (weighted) of patients without AD had received a diagnosis of COPD. More patients with AD reported a diagnosis of chronic bronchitis (9.2%) rather than emphysema (0.9%). Our analysis revealed a significant association between AD and COPD among adults aged 20 to 59 years (AOR, 1.43; 95% CI, 1.13-1.80; P=.003) after controlling for potential confounding variables. Subsequently, we performed subgroup analyses, including exclusion of patients with an asthma diagnosis, to further explore the association between AD and COPD. After excluding participants with asthma, there was still a significant association between AD and COPD (AOR, 1.57; 95% CI, 1.14-2.16; P=.007). Moreover, the odds of receiving a COPD diagnosis were significantly higher among male patients with AD (AOR, 1.54; 95% CI, 1.06-2.25; P=.03).

Our results support the association between AD and COPD, more specifically chronic bronchitis. This finding may be due to similar pathogenic mechanisms in both conditions, including overlapping cytokine production and immune pathways.5 Additionally, Harazin et al6 discussed the role of a novel gene, collagen 29A1 (COL29A1), in the pathogenesis of AD, COPD, and asthma. Variations in this gene may predispose patients to not only atopic diseases but also COPD.6
Limitations of our study include self-reported diagnoses and lack of patients older than 59 years. Self-reported diagnoses could have resulted in some misclassification of COPD, as some individuals may have reported a diagnosis of COPD rather than their true diagnosis of asthma. We mitigated this limitation by constructing a subpopulation model with exclusion of individuals with asthma. Further studies with spirometry-diagnosed COPD are needed to explore this relationship and the potential contributory pathophysiologic mechanisms. Understanding this association may increase awareness of potential comorbidities and assist clinicians with adequate management of patients with AD.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic Dermatitis in America Study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590. doi:10.1016/j.jid.2018.08.028
- Darlenski R, Kazandjieva J, Hristakieva E, et al. Atopic dermatitis as a systemic disease. Clin Dermatol. 2014;32:409-413. doi:10.1016/j.clindermatol.2013.11.007
- Smirnova J, Montgomery S, Lindberg M, et al. Associations of self-reported atopic dermatitis with comorbid conditions in adults: a population-based cross-sectional study. BMC Dermatol. 2020;20:23. doi:10.1186/s12895-020-00117-8
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://wwwn.cdc.gov/nchs/nhanes/
- Kawayama T, Okamoto M, Imaoka H, et al. Interleukin-18 in pulmonary inflammatory diseases. J Interferon Cytokine Res. 2012;32:443-449. doi:10.1089/jir.2012.0029
- Harazin M, Parwez Q, Petrasch-Parwez E, et al. Variation in the COL29A1 gene in German patients with atopic dermatitis, asthma and chronic obstructive pulmonary disease. J Dermatol. 2010;37:740-742. doi:10.1111/j.1346-8138.2010.00923.x
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic Dermatitis in America Study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590. doi:10.1016/j.jid.2018.08.028
- Darlenski R, Kazandjieva J, Hristakieva E, et al. Atopic dermatitis as a systemic disease. Clin Dermatol. 2014;32:409-413. doi:10.1016/j.clindermatol.2013.11.007
- Smirnova J, Montgomery S, Lindberg M, et al. Associations of self-reported atopic dermatitis with comorbid conditions in adults: a population-based cross-sectional study. BMC Dermatol. 2020;20:23. doi:10.1186/s12895-020-00117-8
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://wwwn.cdc.gov/nchs/nhanes/
- Kawayama T, Okamoto M, Imaoka H, et al. Interleukin-18 in pulmonary inflammatory diseases. J Interferon Cytokine Res. 2012;32:443-449. doi:10.1089/jir.2012.0029
- Harazin M, Parwez Q, Petrasch-Parwez E, et al. Variation in the COL29A1 gene in German patients with atopic dermatitis, asthma and chronic obstructive pulmonary disease. J Dermatol. 2010;37:740-742. doi:10.1111/j.1346-8138.2010.00923.x
Practice Points
- Various comorbidities are associated with atopic dermatitis (AD). Currently, research exploring the association between AD and chronic obstructive pulmonary disease is limited.
- Understanding the systemic diseases associated with inflammatory skin diseases can assist with adequate patient management.
Impact of the COVID-19 Pandemic on Care for Patients With Atopic Dermatitis
To the Editor:
Atopic dermatitis (AD) is a widely prevalent dermatologic condition that can severely impact a patient’s quality of life.1 Individuals with AD have been substantially affected during the COVID-19 pandemic due to the increased use of irritants, decreased access to care, and rise in psychological stress.1,2 These factors have resulted in lower quality of life and worsening dermatologic symptoms for many AD patients over the last few years.1 One major potential contributory component of these findings is decreased accessibility to in-office care during the pandemic, with a shift to telemedicine instead. Accessibility to care during the COVID-19 pandemic for AD patients compared to those without AD remains unknown. Therefore, we explored the impact of the COVID-19 pandemic on care for patients with AD in a large US population.
Using anonymous survey data from the 2021 National Health Interview Survey,3 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with AD compared to those without AD. We assigned the following 3 survey questions as outcome variables to assess access to care: delayed medical care due to COVID-19 pandemic (yes/no), did not get care due to COVID-19 pandemic (yes/no), and virtual medical appointment in the last 12 months (yes/no). In Table 1, numerous categorical survey variables, including sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region, were analyzed using χ2 testing to evaluate for differences among individuals with and without AD. Multivariable logistic regression models evaluating the relationship between AD and access to care were constructed using Stata/MP 17 (StataCorp LLC). In our analysis we controlled for age, sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region.
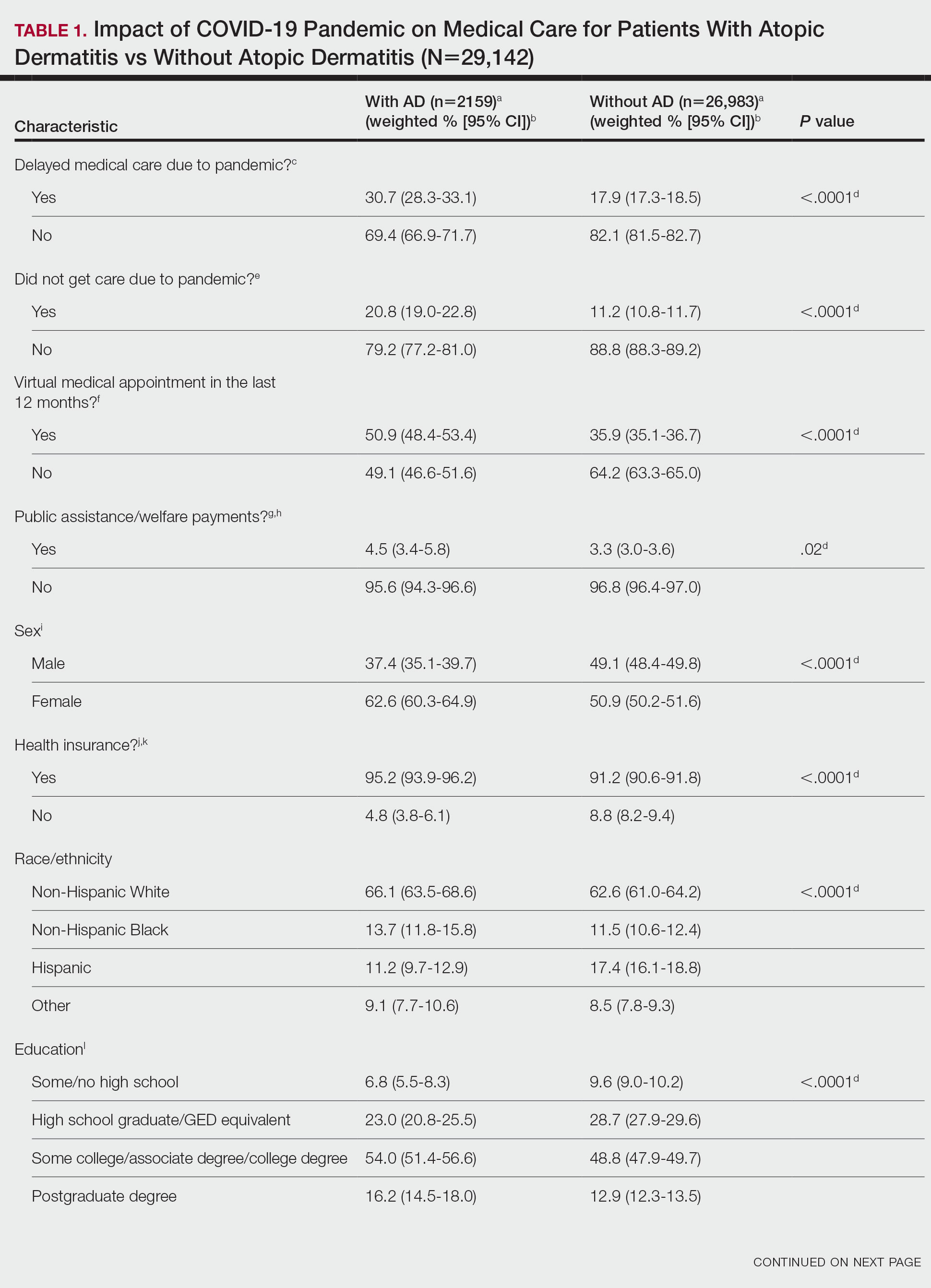
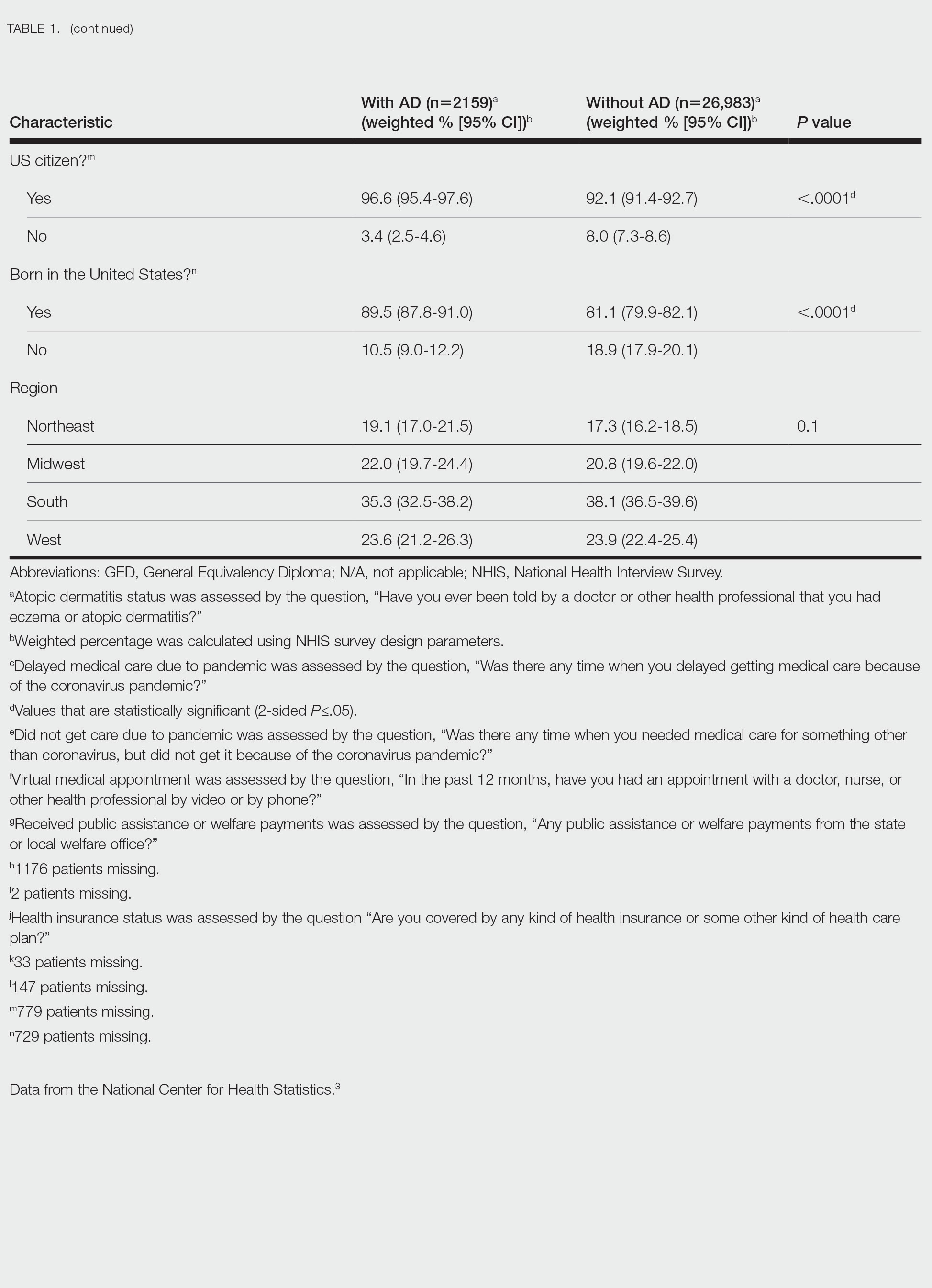
There were 29,142 adult patients (aged ≥18 years) included in our analysis. Approximately 7.4% (weighted) of individuals had AD (Table 1). After adjusting for confounding variables, patients with AD had a higher odds of delaying medical care due to the COVID-19 pandemic (adjusted odds ratio [AOR], 1.91; 95% CI, 1.69-2.16; P<.001), not receiving care due to the COVID-19 pandemic (AOR, 1.94; 95% CI, 1.71-2.22; P<.001), and having a virtual medical visit in the last 12 months (AOR, 1.72; 95% CI, 1.54-1.93; P<.001)(Table 2) compared with patients without AD.
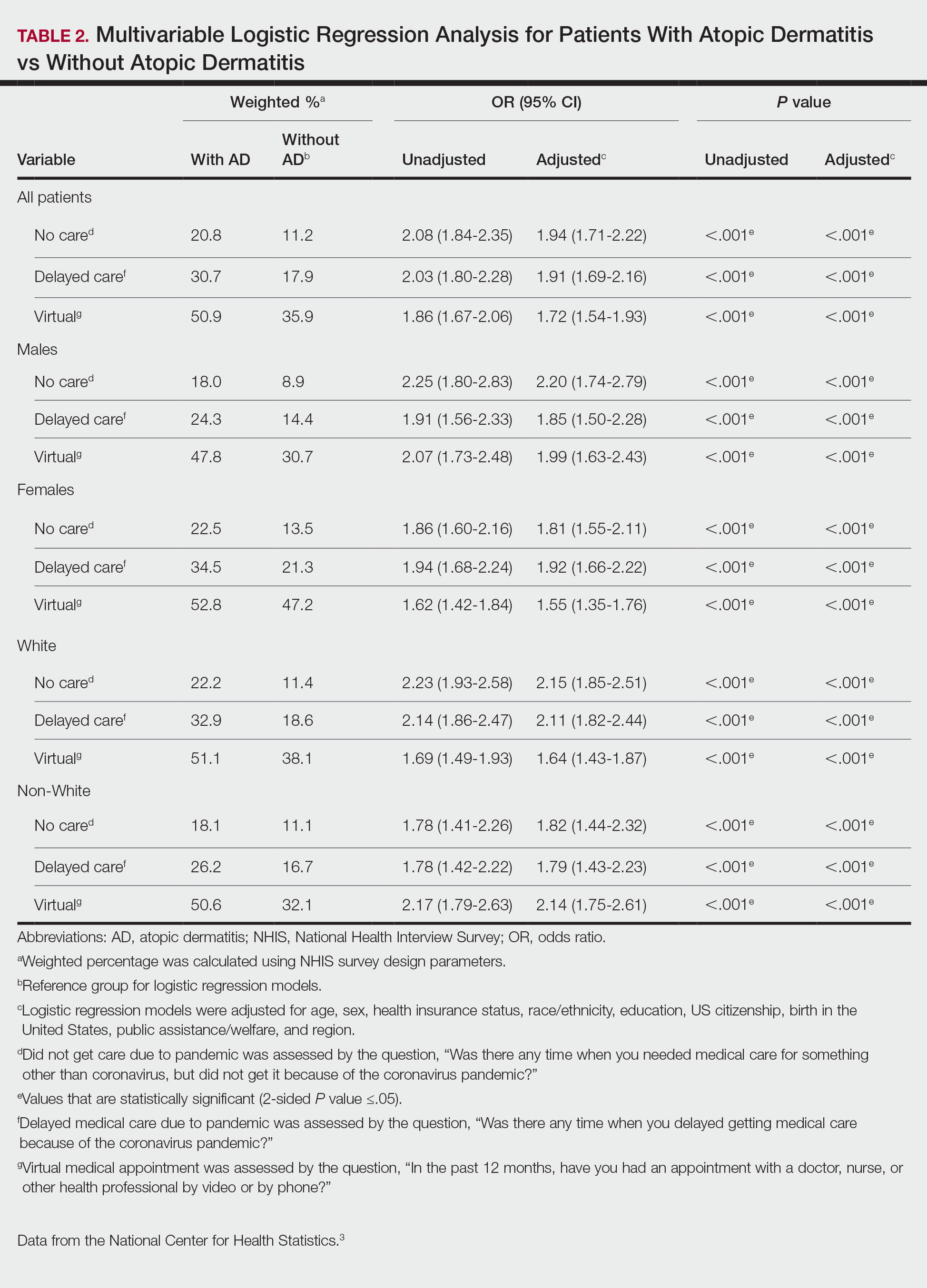
Our findings support the association between AD and decreased access to in-person care due to the COVID-19 pandemic. Moreover, telemedicine was utilized more among individuals with AD, possibly due to the accessibility of diagnostic tools for dermatologic diagnoses, such as high-quality photographs.4 According to Trinidad et al,4 telemedicine became an invaluable tool for dermatology hospitalists during the COVID-19 pandemic, as many physicians were able to comfortably diagnose patients with cutaneous diseases without an in-person visit. Utilizing telemedicine for patient care can help reduce the risk for COVID-19 transmission while also providing quality care for individuals living in rural areas.5 Chiricozzi et al6 discussed the importance of telemedicine in Italy during the pandemic, as many AD patients were able to maintain control of their disease while on systemic treatments.
Limitations of this study include self-reported measures; inability to compare patients with AD to individuals with other cutaneous diseases; and additional potential confounders, such as chronic comorbidities. Future studies should evaluate the use of telemedicine and access to care among individuals with other common skin diseases and help determine why such discrepancies exist. Understanding the difficulties in access to care and the viable alternatives in place may increase awareness and assist clinicians with adequate management of patients with AD.
1. Sieniawska J, Lesiak A, Cia˛z˙yn´ski K, et al. Impact of the COVID-19 pandemic on atopic dermatitis patients. Int J Environ Res Public Health. 2022;19:1734. doi:10.3390/ijerph19031734
2. Pourani MR, Ganji R, Dashti T, et al. Impact of COVID-19 pandemic on patients with atopic dermatitis [in Spanish]. Actas Dermosifiliogr. 2022;113:T286-T293. doi:10.1016/j.ad.2021.08.004
3. National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
4. Trinidad J, Gabel CK, Han JJ, et al. Telemedicine and dermatology hospital consultations during the COVID-19 pandemic: a multi-centre observational study on resource utilization and conversion to in-person consultations during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. 2022;36:E323-E325. doi:10.1111/jdv.17898
5. Marasca C, Annunziata MC, Camela E, et al. Teledermatology and inflammatory skin conditions during COVID-19 era: new perspectives and applications. J Clin Med. 2022;11:1511. doi:10.3390/jcm11061511
6. Chiricozzi A, Talamonti M, De Simone C, et al. Management of patients with atopic dermatitis undergoing systemic therapy during COVID-19 pandemic in Italy: data from the DA-COVID-19 registry. Allergy. 2021;76:1813-1824. doi:10.1111/all.14767
To the Editor:
Atopic dermatitis (AD) is a widely prevalent dermatologic condition that can severely impact a patient’s quality of life.1 Individuals with AD have been substantially affected during the COVID-19 pandemic due to the increased use of irritants, decreased access to care, and rise in psychological stress.1,2 These factors have resulted in lower quality of life and worsening dermatologic symptoms for many AD patients over the last few years.1 One major potential contributory component of these findings is decreased accessibility to in-office care during the pandemic, with a shift to telemedicine instead. Accessibility to care during the COVID-19 pandemic for AD patients compared to those without AD remains unknown. Therefore, we explored the impact of the COVID-19 pandemic on care for patients with AD in a large US population.
Using anonymous survey data from the 2021 National Health Interview Survey,3 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with AD compared to those without AD. We assigned the following 3 survey questions as outcome variables to assess access to care: delayed medical care due to COVID-19 pandemic (yes/no), did not get care due to COVID-19 pandemic (yes/no), and virtual medical appointment in the last 12 months (yes/no). In Table 1, numerous categorical survey variables, including sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region, were analyzed using χ2 testing to evaluate for differences among individuals with and without AD. Multivariable logistic regression models evaluating the relationship between AD and access to care were constructed using Stata/MP 17 (StataCorp LLC). In our analysis we controlled for age, sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region.


There were 29,142 adult patients (aged ≥18 years) included in our analysis. Approximately 7.4% (weighted) of individuals had AD (Table 1). After adjusting for confounding variables, patients with AD had a higher odds of delaying medical care due to the COVID-19 pandemic (adjusted odds ratio [AOR], 1.91; 95% CI, 1.69-2.16; P<.001), not receiving care due to the COVID-19 pandemic (AOR, 1.94; 95% CI, 1.71-2.22; P<.001), and having a virtual medical visit in the last 12 months (AOR, 1.72; 95% CI, 1.54-1.93; P<.001)(Table 2) compared with patients without AD.

Our findings support the association between AD and decreased access to in-person care due to the COVID-19 pandemic. Moreover, telemedicine was utilized more among individuals with AD, possibly due to the accessibility of diagnostic tools for dermatologic diagnoses, such as high-quality photographs.4 According to Trinidad et al,4 telemedicine became an invaluable tool for dermatology hospitalists during the COVID-19 pandemic, as many physicians were able to comfortably diagnose patients with cutaneous diseases without an in-person visit. Utilizing telemedicine for patient care can help reduce the risk for COVID-19 transmission while also providing quality care for individuals living in rural areas.5 Chiricozzi et al6 discussed the importance of telemedicine in Italy during the pandemic, as many AD patients were able to maintain control of their disease while on systemic treatments.
Limitations of this study include self-reported measures; inability to compare patients with AD to individuals with other cutaneous diseases; and additional potential confounders, such as chronic comorbidities. Future studies should evaluate the use of telemedicine and access to care among individuals with other common skin diseases and help determine why such discrepancies exist. Understanding the difficulties in access to care and the viable alternatives in place may increase awareness and assist clinicians with adequate management of patients with AD.
To the Editor:
Atopic dermatitis (AD) is a widely prevalent dermatologic condition that can severely impact a patient’s quality of life.1 Individuals with AD have been substantially affected during the COVID-19 pandemic due to the increased use of irritants, decreased access to care, and rise in psychological stress.1,2 These factors have resulted in lower quality of life and worsening dermatologic symptoms for many AD patients over the last few years.1 One major potential contributory component of these findings is decreased accessibility to in-office care during the pandemic, with a shift to telemedicine instead. Accessibility to care during the COVID-19 pandemic for AD patients compared to those without AD remains unknown. Therefore, we explored the impact of the COVID-19 pandemic on care for patients with AD in a large US population.
Using anonymous survey data from the 2021 National Health Interview Survey,3 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with AD compared to those without AD. We assigned the following 3 survey questions as outcome variables to assess access to care: delayed medical care due to COVID-19 pandemic (yes/no), did not get care due to COVID-19 pandemic (yes/no), and virtual medical appointment in the last 12 months (yes/no). In Table 1, numerous categorical survey variables, including sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region, were analyzed using χ2 testing to evaluate for differences among individuals with and without AD. Multivariable logistic regression models evaluating the relationship between AD and access to care were constructed using Stata/MP 17 (StataCorp LLC). In our analysis we controlled for age, sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region.


There were 29,142 adult patients (aged ≥18 years) included in our analysis. Approximately 7.4% (weighted) of individuals had AD (Table 1). After adjusting for confounding variables, patients with AD had a higher odds of delaying medical care due to the COVID-19 pandemic (adjusted odds ratio [AOR], 1.91; 95% CI, 1.69-2.16; P<.001), not receiving care due to the COVID-19 pandemic (AOR, 1.94; 95% CI, 1.71-2.22; P<.001), and having a virtual medical visit in the last 12 months (AOR, 1.72; 95% CI, 1.54-1.93; P<.001)(Table 2) compared with patients without AD.

Our findings support the association between AD and decreased access to in-person care due to the COVID-19 pandemic. Moreover, telemedicine was utilized more among individuals with AD, possibly due to the accessibility of diagnostic tools for dermatologic diagnoses, such as high-quality photographs.4 According to Trinidad et al,4 telemedicine became an invaluable tool for dermatology hospitalists during the COVID-19 pandemic, as many physicians were able to comfortably diagnose patients with cutaneous diseases without an in-person visit. Utilizing telemedicine for patient care can help reduce the risk for COVID-19 transmission while also providing quality care for individuals living in rural areas.5 Chiricozzi et al6 discussed the importance of telemedicine in Italy during the pandemic, as many AD patients were able to maintain control of their disease while on systemic treatments.
Limitations of this study include self-reported measures; inability to compare patients with AD to individuals with other cutaneous diseases; and additional potential confounders, such as chronic comorbidities. Future studies should evaluate the use of telemedicine and access to care among individuals with other common skin diseases and help determine why such discrepancies exist. Understanding the difficulties in access to care and the viable alternatives in place may increase awareness and assist clinicians with adequate management of patients with AD.
1. Sieniawska J, Lesiak A, Cia˛z˙yn´ski K, et al. Impact of the COVID-19 pandemic on atopic dermatitis patients. Int J Environ Res Public Health. 2022;19:1734. doi:10.3390/ijerph19031734
2. Pourani MR, Ganji R, Dashti T, et al. Impact of COVID-19 pandemic on patients with atopic dermatitis [in Spanish]. Actas Dermosifiliogr. 2022;113:T286-T293. doi:10.1016/j.ad.2021.08.004
3. National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
4. Trinidad J, Gabel CK, Han JJ, et al. Telemedicine and dermatology hospital consultations during the COVID-19 pandemic: a multi-centre observational study on resource utilization and conversion to in-person consultations during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. 2022;36:E323-E325. doi:10.1111/jdv.17898
5. Marasca C, Annunziata MC, Camela E, et al. Teledermatology and inflammatory skin conditions during COVID-19 era: new perspectives and applications. J Clin Med. 2022;11:1511. doi:10.3390/jcm11061511
6. Chiricozzi A, Talamonti M, De Simone C, et al. Management of patients with atopic dermatitis undergoing systemic therapy during COVID-19 pandemic in Italy: data from the DA-COVID-19 registry. Allergy. 2021;76:1813-1824. doi:10.1111/all.14767
1. Sieniawska J, Lesiak A, Cia˛z˙yn´ski K, et al. Impact of the COVID-19 pandemic on atopic dermatitis patients. Int J Environ Res Public Health. 2022;19:1734. doi:10.3390/ijerph19031734
2. Pourani MR, Ganji R, Dashti T, et al. Impact of COVID-19 pandemic on patients with atopic dermatitis [in Spanish]. Actas Dermosifiliogr. 2022;113:T286-T293. doi:10.1016/j.ad.2021.08.004
3. National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
4. Trinidad J, Gabel CK, Han JJ, et al. Telemedicine and dermatology hospital consultations during the COVID-19 pandemic: a multi-centre observational study on resource utilization and conversion to in-person consultations during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. 2022;36:E323-E325. doi:10.1111/jdv.17898
5. Marasca C, Annunziata MC, Camela E, et al. Teledermatology and inflammatory skin conditions during COVID-19 era: new perspectives and applications. J Clin Med. 2022;11:1511. doi:10.3390/jcm11061511
6. Chiricozzi A, Talamonti M, De Simone C, et al. Management of patients with atopic dermatitis undergoing systemic therapy during COVID-19 pandemic in Italy: data from the DA-COVID-19 registry. Allergy. 2021;76:1813-1824. doi:10.1111/all.14767
Practice Points
- The landscape of dermatology has seen major changes due to the COVID-19 pandemic, as many patients now utilize telemedicine to receive care.
- Understanding accessibility to in-person care for patients with atopic dermatitis during the COVID-19 pandemic can assist with the development of methods to enhance management.
Financial Insecurity Among US Adults With Psoriasis
To the Editor:
Approximately 3% of the US population, or 6.9 million adults, is affected by psoriasis.1 Psoriasis has a substantial impact on quality of life and is associated with increased health care expenses and medication costs. In 2013, it was reported that the estimated US annual cost—direct, indirect, intangible, and comorbidity costs—of psoriasis for adults was $112 billion.2 We investigated the prevalence and sociodemographic characteristics of adult psoriasis patients (aged ≥20 years) with financial insecurity utilizing the 2009–2014 National Health and Nutrition Examination Survey (NHANES) data.3
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database to evaluate financial insecurity. Financial insecurity was evaluated by 2 outcome variables. The primary outcome variable was assessed by the question “Are you covered by health insurance or some other kind of health care plan (including health insurance obtained through employment or purchased directly as well as government programs like Medicare and Medicaid that provide medical care or help pay medical bills)?”3 Our secondary outcome variable was evaluated by a reported annual household income of less than $20,000. P values in Table 1 were calculated using Pearson χ2 tests. In Table 2, multivariate logistic regressions were performed using Stata/MP 17 (StataCorp LLC) to analyze associations between outcome variables and sociodemographic characteristics. Additionally, we controlled for age, race/ethnicity, sex, education, marital status, US citizenship status, and tobacco use. Subsequently, relationships with P<.05 were considered statistically significant.
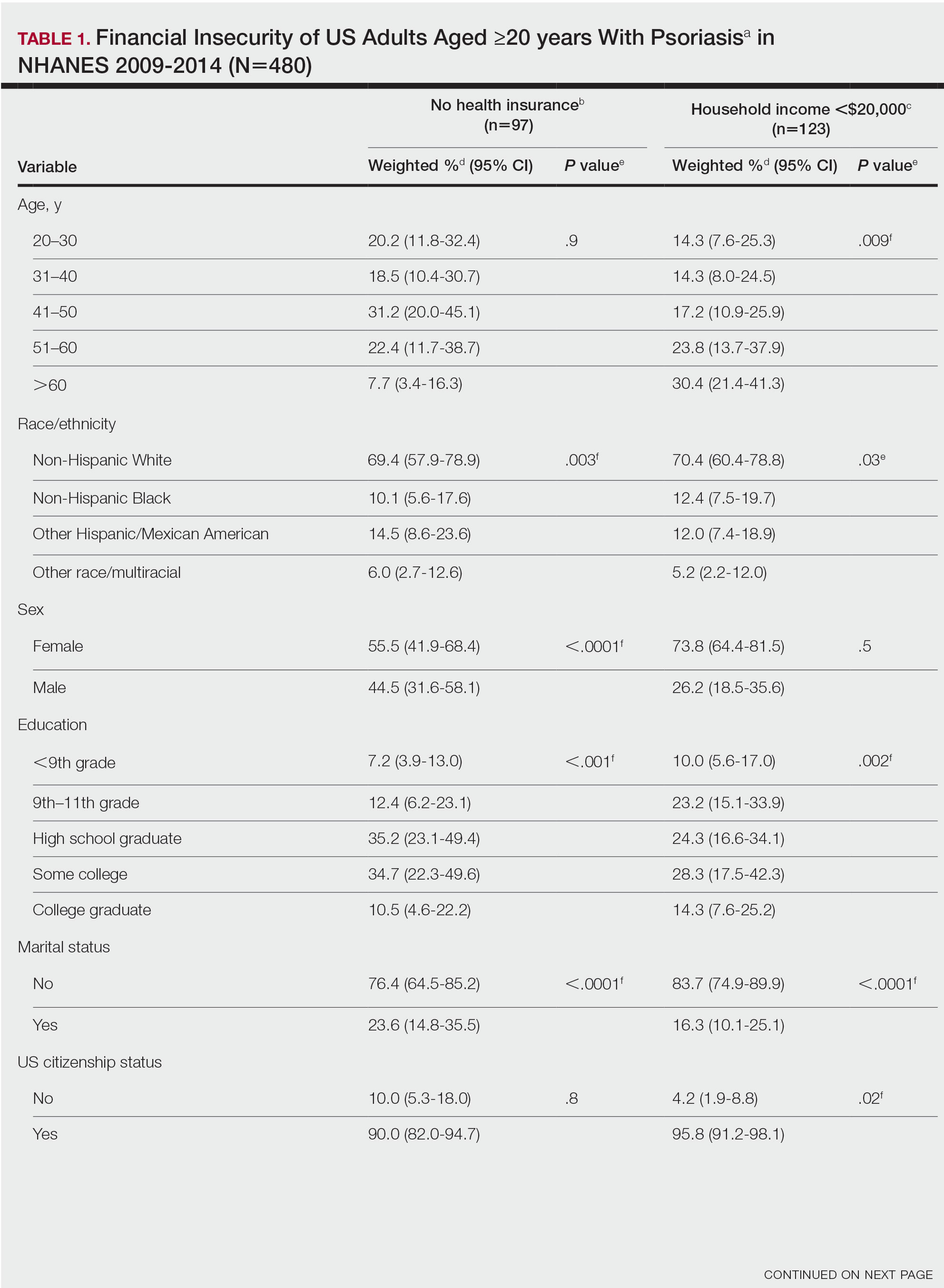

Our analysis comprised 480 individuals with psoriasis; 40 individuals were excluded from our analysis because they did not report annual household income and health insurance status (Table 1). Among the 480 individuals with psoriasis, approximately 16% (weighted) reported a lack of health insurance, and approximately 17% (weighted) reported an annual household income of less than $20,000. Among those who reported an annual household income of less than $20,000, approximately 38% (weighted) of them reported that they did not have health insurance.
Multivariate logistic regression analyses revealed that elderly individuals (aged >60 years), college graduates, married individuals, and US citizens had decreased odds of lacking health insurance (Table 2). Additionally, those with a history of tobacco use (adjusted odds ratio [AOR] 2.02; 95% CI, 1.00-4.05) were associated with lacking health insurance. Non-Hispanic Black individuals (AOR 2.26; 95% CI, 1.09-4.71) and US citizens (AOR 5.01; 95% CI, 1.28-19.63) had a significant association with an annual household income of less than $20,000 (P<.05). Lastly, males, those with education beyond ninth grade, and married individuals had a significantly decreased odds of having an annual household income of less than $20,000 (P<.05)(Table 2).
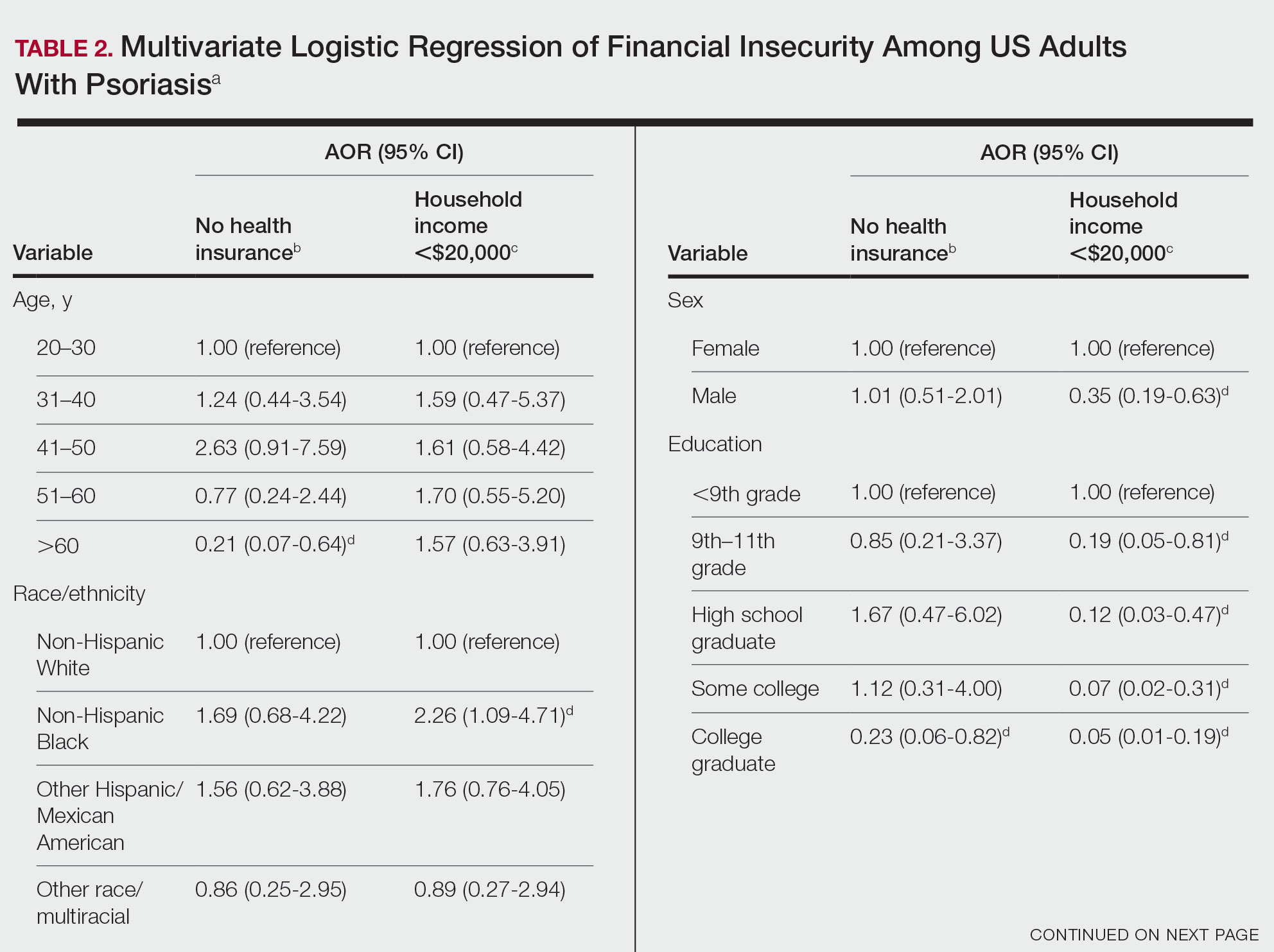
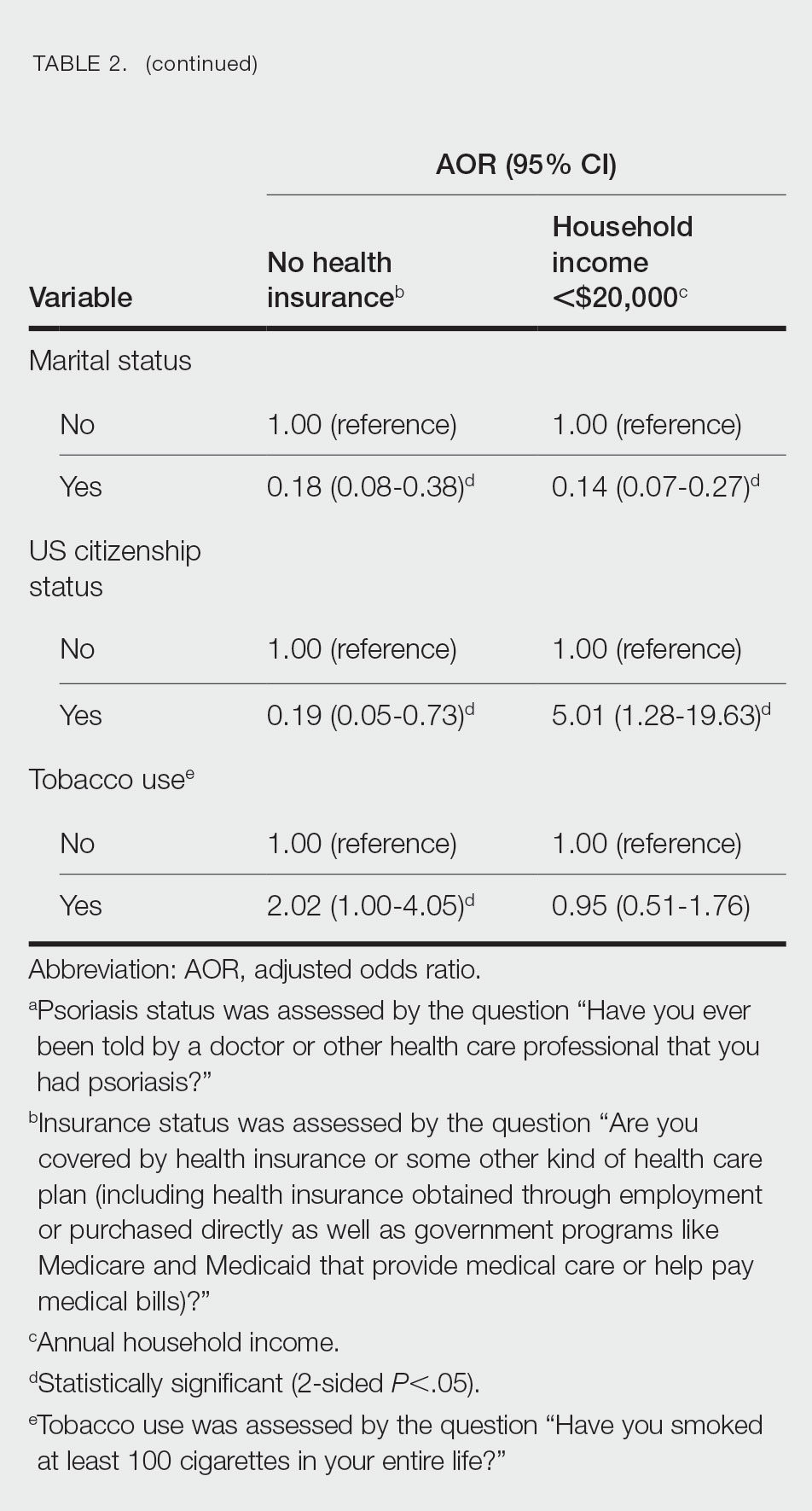
Our findings indicate that certain sociodemographic groups of psoriasis patients have an increased risk for being financially insecure. It is important to evaluate the cost of treatment, number of necessary visits to the office, and cost of transportation, as these factors can serve as a major economic burden to patients being managed for psoriasis.4 Additionally, the cost of biologics has been increasing over time.5 Taking all of this into account when caring for psoriasis patients is crucial, as understanding the financial status of patients can assist with determining appropriate individualized treatment regimens.
- Liu J, Thatiparthi A, Martin A, et al. Prevalence of psoriasis among adults in the US 2009-2010 and 2013-2014 National Health and Nutrition Examination Surveys. J Am Acad Dermatol. 2021;84:767-769. doi:10.1016/j.jaad.2020.10.035
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658. doi:10.1001/jamadermatol.2014.3593
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Maya-Rico AM, Londoño-García Á, Palacios-Barahona AU, et al. Out-of-pocket costs for patients with psoriasis in an outpatient dermatology referral service. An Bras Dermatol. 2021;96:295-300. doi:10.1016/j.abd.2020.09.004
- Cheng J, Feldman SR. The cost of biologics for psoriasis is increasing. Drugs Context. 2014;3:212266. doi:10.7573/dic.212266
To the Editor:
Approximately 3% of the US population, or 6.9 million adults, is affected by psoriasis.1 Psoriasis has a substantial impact on quality of life and is associated with increased health care expenses and medication costs. In 2013, it was reported that the estimated US annual cost—direct, indirect, intangible, and comorbidity costs—of psoriasis for adults was $112 billion.2 We investigated the prevalence and sociodemographic characteristics of adult psoriasis patients (aged ≥20 years) with financial insecurity utilizing the 2009–2014 National Health and Nutrition Examination Survey (NHANES) data.3
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database to evaluate financial insecurity. Financial insecurity was evaluated by 2 outcome variables. The primary outcome variable was assessed by the question “Are you covered by health insurance or some other kind of health care plan (including health insurance obtained through employment or purchased directly as well as government programs like Medicare and Medicaid that provide medical care or help pay medical bills)?”3 Our secondary outcome variable was evaluated by a reported annual household income of less than $20,000. P values in Table 1 were calculated using Pearson χ2 tests. In Table 2, multivariate logistic regressions were performed using Stata/MP 17 (StataCorp LLC) to analyze associations between outcome variables and sociodemographic characteristics. Additionally, we controlled for age, race/ethnicity, sex, education, marital status, US citizenship status, and tobacco use. Subsequently, relationships with P<.05 were considered statistically significant.


Our analysis comprised 480 individuals with psoriasis; 40 individuals were excluded from our analysis because they did not report annual household income and health insurance status (Table 1). Among the 480 individuals with psoriasis, approximately 16% (weighted) reported a lack of health insurance, and approximately 17% (weighted) reported an annual household income of less than $20,000. Among those who reported an annual household income of less than $20,000, approximately 38% (weighted) of them reported that they did not have health insurance.
Multivariate logistic regression analyses revealed that elderly individuals (aged >60 years), college graduates, married individuals, and US citizens had decreased odds of lacking health insurance (Table 2). Additionally, those with a history of tobacco use (adjusted odds ratio [AOR] 2.02; 95% CI, 1.00-4.05) were associated with lacking health insurance. Non-Hispanic Black individuals (AOR 2.26; 95% CI, 1.09-4.71) and US citizens (AOR 5.01; 95% CI, 1.28-19.63) had a significant association with an annual household income of less than $20,000 (P<.05). Lastly, males, those with education beyond ninth grade, and married individuals had a significantly decreased odds of having an annual household income of less than $20,000 (P<.05)(Table 2).


Our findings indicate that certain sociodemographic groups of psoriasis patients have an increased risk for being financially insecure. It is important to evaluate the cost of treatment, number of necessary visits to the office, and cost of transportation, as these factors can serve as a major economic burden to patients being managed for psoriasis.4 Additionally, the cost of biologics has been increasing over time.5 Taking all of this into account when caring for psoriasis patients is crucial, as understanding the financial status of patients can assist with determining appropriate individualized treatment regimens.
To the Editor:
Approximately 3% of the US population, or 6.9 million adults, is affected by psoriasis.1 Psoriasis has a substantial impact on quality of life and is associated with increased health care expenses and medication costs. In 2013, it was reported that the estimated US annual cost—direct, indirect, intangible, and comorbidity costs—of psoriasis for adults was $112 billion.2 We investigated the prevalence and sociodemographic characteristics of adult psoriasis patients (aged ≥20 years) with financial insecurity utilizing the 2009–2014 National Health and Nutrition Examination Survey (NHANES) data.3
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database to evaluate financial insecurity. Financial insecurity was evaluated by 2 outcome variables. The primary outcome variable was assessed by the question “Are you covered by health insurance or some other kind of health care plan (including health insurance obtained through employment or purchased directly as well as government programs like Medicare and Medicaid that provide medical care or help pay medical bills)?”3 Our secondary outcome variable was evaluated by a reported annual household income of less than $20,000. P values in Table 1 were calculated using Pearson χ2 tests. In Table 2, multivariate logistic regressions were performed using Stata/MP 17 (StataCorp LLC) to analyze associations between outcome variables and sociodemographic characteristics. Additionally, we controlled for age, race/ethnicity, sex, education, marital status, US citizenship status, and tobacco use. Subsequently, relationships with P<.05 were considered statistically significant.


Our analysis comprised 480 individuals with psoriasis; 40 individuals were excluded from our analysis because they did not report annual household income and health insurance status (Table 1). Among the 480 individuals with psoriasis, approximately 16% (weighted) reported a lack of health insurance, and approximately 17% (weighted) reported an annual household income of less than $20,000. Among those who reported an annual household income of less than $20,000, approximately 38% (weighted) of them reported that they did not have health insurance.
Multivariate logistic regression analyses revealed that elderly individuals (aged >60 years), college graduates, married individuals, and US citizens had decreased odds of lacking health insurance (Table 2). Additionally, those with a history of tobacco use (adjusted odds ratio [AOR] 2.02; 95% CI, 1.00-4.05) were associated with lacking health insurance. Non-Hispanic Black individuals (AOR 2.26; 95% CI, 1.09-4.71) and US citizens (AOR 5.01; 95% CI, 1.28-19.63) had a significant association with an annual household income of less than $20,000 (P<.05). Lastly, males, those with education beyond ninth grade, and married individuals had a significantly decreased odds of having an annual household income of less than $20,000 (P<.05)(Table 2).


Our findings indicate that certain sociodemographic groups of psoriasis patients have an increased risk for being financially insecure. It is important to evaluate the cost of treatment, number of necessary visits to the office, and cost of transportation, as these factors can serve as a major economic burden to patients being managed for psoriasis.4 Additionally, the cost of biologics has been increasing over time.5 Taking all of this into account when caring for psoriasis patients is crucial, as understanding the financial status of patients can assist with determining appropriate individualized treatment regimens.
- Liu J, Thatiparthi A, Martin A, et al. Prevalence of psoriasis among adults in the US 2009-2010 and 2013-2014 National Health and Nutrition Examination Surveys. J Am Acad Dermatol. 2021;84:767-769. doi:10.1016/j.jaad.2020.10.035
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658. doi:10.1001/jamadermatol.2014.3593
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Maya-Rico AM, Londoño-García Á, Palacios-Barahona AU, et al. Out-of-pocket costs for patients with psoriasis in an outpatient dermatology referral service. An Bras Dermatol. 2021;96:295-300. doi:10.1016/j.abd.2020.09.004
- Cheng J, Feldman SR. The cost of biologics for psoriasis is increasing. Drugs Context. 2014;3:212266. doi:10.7573/dic.212266
- Liu J, Thatiparthi A, Martin A, et al. Prevalence of psoriasis among adults in the US 2009-2010 and 2013-2014 National Health and Nutrition Examination Surveys. J Am Acad Dermatol. 2021;84:767-769. doi:10.1016/j.jaad.2020.10.035
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658. doi:10.1001/jamadermatol.2014.3593
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Maya-Rico AM, Londoño-García Á, Palacios-Barahona AU, et al. Out-of-pocket costs for patients with psoriasis in an outpatient dermatology referral service. An Bras Dermatol. 2021;96:295-300. doi:10.1016/j.abd.2020.09.004
- Cheng J, Feldman SR. The cost of biologics for psoriasis is increasing. Drugs Context. 2014;3:212266. doi:10.7573/dic.212266
Practice Points
- The economic burden on patients with psoriasis has been rising over time, as the disease impacts many aspects of patients’ lives.
- Various sociodemographic groups among patients with psoriasis are financially insecure. Knowing which groups are at higher risk for poor outcomes due to financial insecurity can assist with appropriate treatment regimens.
Association Between Psoriasis and Obesity Among US Adults in the 2009-2014 National Health and Nutrition Examination Survey
To the Editor:
Psoriasis is an immune-mediated dermatologic condition that is associated with various comorbidities, including obesity.1 The underlying pathophysiology of psoriasis has been extensively studied, and recent research has discussed the role of obesity in IL-17 secretion.2 The relationship between being overweight/obese and having psoriasis has been documented in the literature.1,2 However, this association in a recent population is lacking. We sought to investigate the association between psoriasis and obesity utilizing a representative US population of adults—the 2009-2014 National Health and Nutrition Examination Survey (NHANES) data,3 which contains the most recent psoriasis data.
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database. Three 2-year cycles of NHANES data were combined to create our 2009 to 2014 dataset. In the Table, numerous variables including age, sex, household income, race/ethnicity, education, diabetes status, tobacco use, body mass index (BMI), waist circumference, and being called overweight by a health care provider were analyzed using χ2 or t test analyses to evaluate for differences among those with and without psoriasis. Diabetes status was assessed by the question “Other than during pregnancy, have you ever been told by a doctor or health professional that you have diabetes or sugar diabetes?” Tobacco use was assessed by the question “Have you smoked at least 100 cigarettes in your entire life?” Psoriasis status was assessed by a self-reported response to the question “Have you ever been told by a doctor or other health care professional that you had psoriasis?” Three different outcome variables were used to determine if patients were overweight or obese: BMI, waist circumference, and response to the question “Has a doctor or other health professional ever told you that you were overweight?” Obesity was defined as having a BMI of 30 or higher or waist circumference of 102 cm or more in males and 88 cm or more in females.4 Being overweight was defined as having a BMI of 25 to 29.99 or response of Yes to “Has a doctor or other health professional ever told you that you were overweight?”
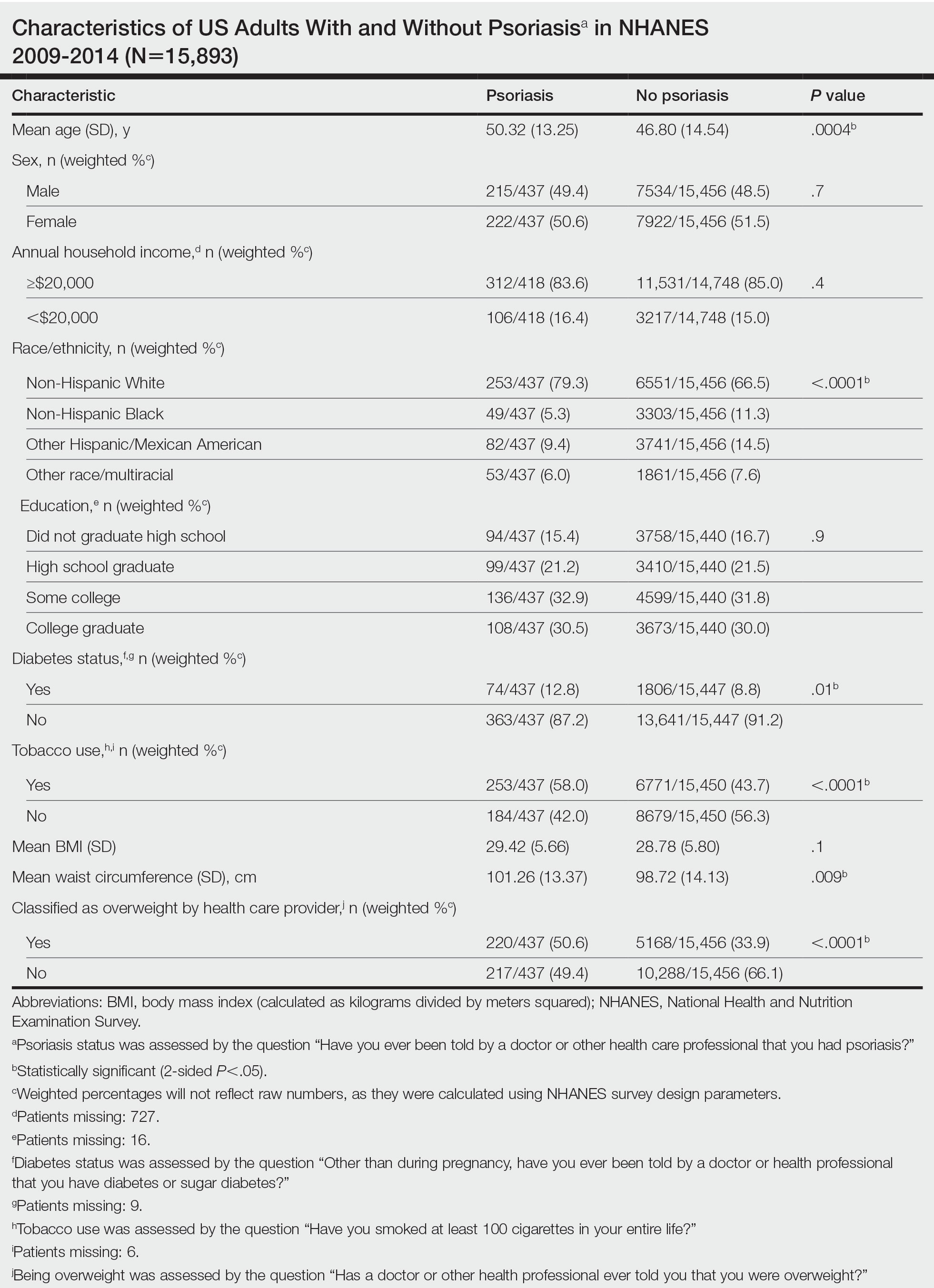
Initially, there were 17,547 participants 20 years and older from 2009 to 2014, but 1654 participants were excluded because of missing data for obesity or psoriasis; therefore, 15,893 patients were included in our analysis. Multivariable logistic regressions were utilized to examine the association between psoriasis and being overweight/obese (eTable). Additionally, the models were adjusted based on age, sex, household income, race/ethnicity, diabetes status, and tobacco use. All data processing and analysis were performed in Stata/MP 17 (StataCorp LLC). P<.05 was considered statistically significant.
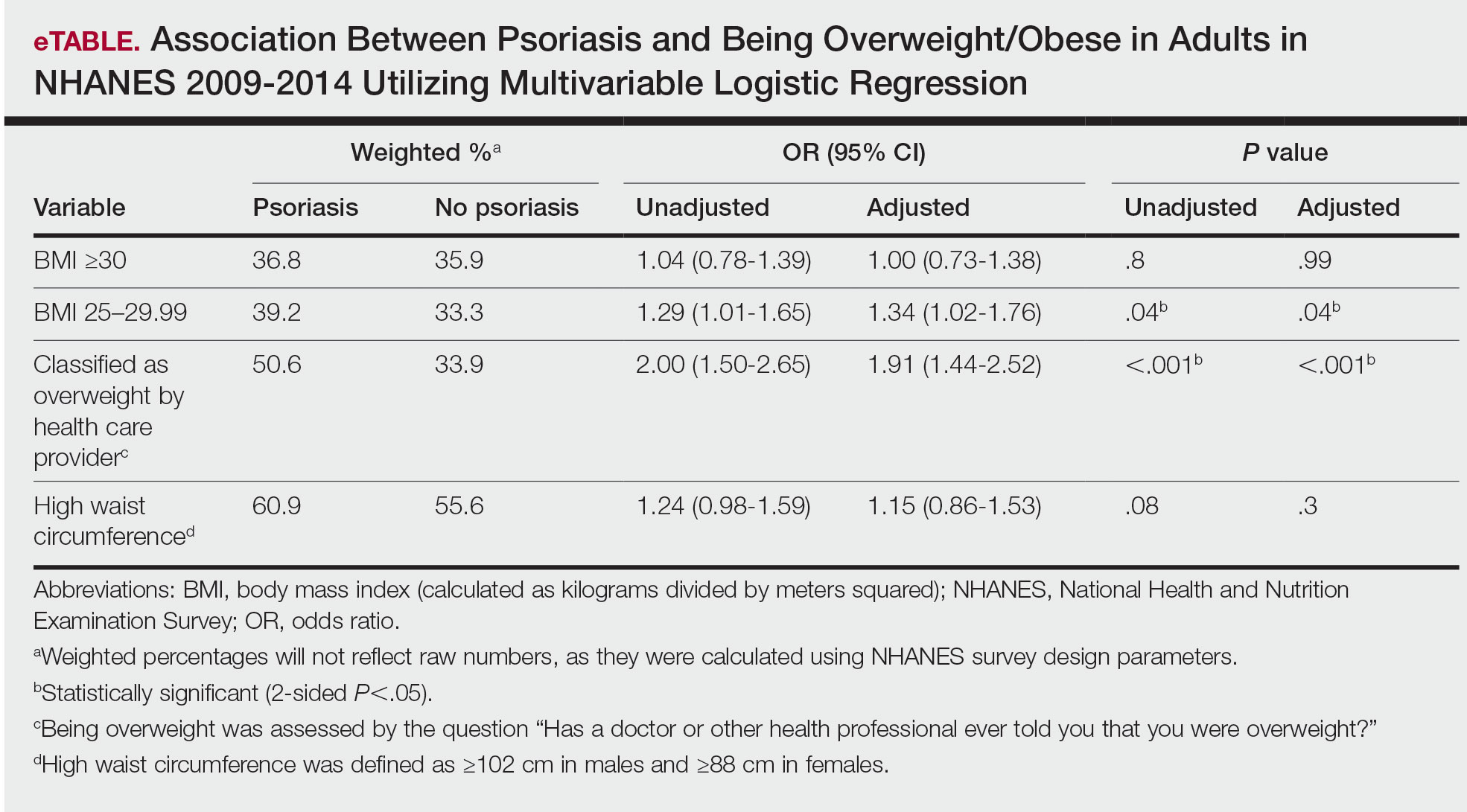
The Table shows characteristics of US adults with and without psoriasis in NHANES 2009-2014. We found that the variables of interest evaluating body weight that were significantly different on analysis between patients with and without psoriasis included waist circumference—patients with psoriasis had a significantly higher waist circumference (P=.009)—and being told by a health care provider that they are overweight (P<.0001), which supports the findings by Love et al,5 who reported abdominal obesity was the most common feature of metabolic syndrome exhibited among patients with psoriasis.
Multivariable logistic regression analysis (eTable) revealed that there was a significant association between psoriasis and BMI of 25 to 29.99 (adjusted odds ratio [AOR], 1.34; 95% CI, 1.02-1.76; P=.04) and being told by a health care provider that they are overweight (AOR, 1.91; 95% CI, 1.44-2.52; P<.001). After adjusting for confounding variables, there was no significant association between psoriasis and a BMI of 30 or higher (AOR, 1.00; 95% CI, 0.73-1.38; P=.99) or a waist circumference of 102 cm or more in males and 88 cm or more in females (AOR, 1.15; 95% CI, 0.86-1.53; P=.3).
Our findings suggest that a few variables indicative of being overweight or obese are associated with psoriasis. This relationship most likely is due to increased adipokine, including resistin, levels in overweight individuals, resulting in a proinflammatory state.6 It has been suggested that BMI alone is not a definitive marker for measuring fat storage levels in individuals. People can have a normal or slightly elevated BMI but possess excessive adiposity, resulting in chronic inflammation.6 Therefore, our findings of a significant association between psoriasis and being told by a health care provider that they are overweight might be a stronger measurement for possessing excessive fat, as this is likely due to clinical judgment rather than BMI measurement.
Moreover, it should be noted that the potential reason for the lack of association between BMI of 30 or higher and psoriasis in our analysis may be a result of BMI serving as a poor measurement for adiposity. Additionally, Armstrong and colleagues7 discussed that the association between BMI and psoriasis was stronger for patients with moderate to severe psoriasis. Our study consisted of NHANES data for self-reported psoriasis diagnoses without a psoriasis severity index, making it difficult to extrapolate which individuals had mild or moderate to severe psoriasis, which may have contributed to our finding of no association between BMI of 30 or higher and psoriasis.
The self-reported nature of the survey questions and lack of questions regarding psoriasis severity serve as limitations to the study. Both obesity and psoriasis can have various systemic consequences, such as cardiovascular disease, due to the development of an inflammatory state.8 Future studies may explore other body measurements that indicate being overweight or obese and the potential synergistic relationship of obesity and psoriasis severity, optimizing the development of effective treatment plans.
- Jensen P, Skov L. Psoriasis and obesity. Dermatology. 2016;232:633-639.
- Xu C, Ji J, Su T, et al. The association of psoriasis and obesity: focusing on IL-17A-related immunological mechanisms. Int J Dermatol Venereol. 2021;4:116-121.
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Ross R, Neeland IJ, Yamashita S, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol. 2020;16:177-189.
- Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003-2006. Arch Dermatol. 2011;147:419-424.
- Paroutoglou K, Papadavid E, Christodoulatos GS, et al. Deciphering the association between psoriasis and obesity: current evidence and treatment considerations. Curr Obes Rep. 2020;9:165-178.
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012;2:E54.
- Hamminga EA, van der Lely AJ, Neumann HAM, et al. Chronic inflammation in psoriasis and obesity: implications for therapy. Med Hypotheses. 2006;67:768-773.
To the Editor:
Psoriasis is an immune-mediated dermatologic condition that is associated with various comorbidities, including obesity.1 The underlying pathophysiology of psoriasis has been extensively studied, and recent research has discussed the role of obesity in IL-17 secretion.2 The relationship between being overweight/obese and having psoriasis has been documented in the literature.1,2 However, this association in a recent population is lacking. We sought to investigate the association between psoriasis and obesity utilizing a representative US population of adults—the 2009-2014 National Health and Nutrition Examination Survey (NHANES) data,3 which contains the most recent psoriasis data.
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database. Three 2-year cycles of NHANES data were combined to create our 2009 to 2014 dataset. In the Table, numerous variables including age, sex, household income, race/ethnicity, education, diabetes status, tobacco use, body mass index (BMI), waist circumference, and being called overweight by a health care provider were analyzed using χ2 or t test analyses to evaluate for differences among those with and without psoriasis. Diabetes status was assessed by the question “Other than during pregnancy, have you ever been told by a doctor or health professional that you have diabetes or sugar diabetes?” Tobacco use was assessed by the question “Have you smoked at least 100 cigarettes in your entire life?” Psoriasis status was assessed by a self-reported response to the question “Have you ever been told by a doctor or other health care professional that you had psoriasis?” Three different outcome variables were used to determine if patients were overweight or obese: BMI, waist circumference, and response to the question “Has a doctor or other health professional ever told you that you were overweight?” Obesity was defined as having a BMI of 30 or higher or waist circumference of 102 cm or more in males and 88 cm or more in females.4 Being overweight was defined as having a BMI of 25 to 29.99 or response of Yes to “Has a doctor or other health professional ever told you that you were overweight?”

Initially, there were 17,547 participants 20 years and older from 2009 to 2014, but 1654 participants were excluded because of missing data for obesity or psoriasis; therefore, 15,893 patients were included in our analysis. Multivariable logistic regressions were utilized to examine the association between psoriasis and being overweight/obese (eTable). Additionally, the models were adjusted based on age, sex, household income, race/ethnicity, diabetes status, and tobacco use. All data processing and analysis were performed in Stata/MP 17 (StataCorp LLC). P<.05 was considered statistically significant.

The Table shows characteristics of US adults with and without psoriasis in NHANES 2009-2014. We found that the variables of interest evaluating body weight that were significantly different on analysis between patients with and without psoriasis included waist circumference—patients with psoriasis had a significantly higher waist circumference (P=.009)—and being told by a health care provider that they are overweight (P<.0001), which supports the findings by Love et al,5 who reported abdominal obesity was the most common feature of metabolic syndrome exhibited among patients with psoriasis.
Multivariable logistic regression analysis (eTable) revealed that there was a significant association between psoriasis and BMI of 25 to 29.99 (adjusted odds ratio [AOR], 1.34; 95% CI, 1.02-1.76; P=.04) and being told by a health care provider that they are overweight (AOR, 1.91; 95% CI, 1.44-2.52; P<.001). After adjusting for confounding variables, there was no significant association between psoriasis and a BMI of 30 or higher (AOR, 1.00; 95% CI, 0.73-1.38; P=.99) or a waist circumference of 102 cm or more in males and 88 cm or more in females (AOR, 1.15; 95% CI, 0.86-1.53; P=.3).
Our findings suggest that a few variables indicative of being overweight or obese are associated with psoriasis. This relationship most likely is due to increased adipokine, including resistin, levels in overweight individuals, resulting in a proinflammatory state.6 It has been suggested that BMI alone is not a definitive marker for measuring fat storage levels in individuals. People can have a normal or slightly elevated BMI but possess excessive adiposity, resulting in chronic inflammation.6 Therefore, our findings of a significant association between psoriasis and being told by a health care provider that they are overweight might be a stronger measurement for possessing excessive fat, as this is likely due to clinical judgment rather than BMI measurement.
Moreover, it should be noted that the potential reason for the lack of association between BMI of 30 or higher and psoriasis in our analysis may be a result of BMI serving as a poor measurement for adiposity. Additionally, Armstrong and colleagues7 discussed that the association between BMI and psoriasis was stronger for patients with moderate to severe psoriasis. Our study consisted of NHANES data for self-reported psoriasis diagnoses without a psoriasis severity index, making it difficult to extrapolate which individuals had mild or moderate to severe psoriasis, which may have contributed to our finding of no association between BMI of 30 or higher and psoriasis.
The self-reported nature of the survey questions and lack of questions regarding psoriasis severity serve as limitations to the study. Both obesity and psoriasis can have various systemic consequences, such as cardiovascular disease, due to the development of an inflammatory state.8 Future studies may explore other body measurements that indicate being overweight or obese and the potential synergistic relationship of obesity and psoriasis severity, optimizing the development of effective treatment plans.
To the Editor:
Psoriasis is an immune-mediated dermatologic condition that is associated with various comorbidities, including obesity.1 The underlying pathophysiology of psoriasis has been extensively studied, and recent research has discussed the role of obesity in IL-17 secretion.2 The relationship between being overweight/obese and having psoriasis has been documented in the literature.1,2 However, this association in a recent population is lacking. We sought to investigate the association between psoriasis and obesity utilizing a representative US population of adults—the 2009-2014 National Health and Nutrition Examination Survey (NHANES) data,3 which contains the most recent psoriasis data.
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database. Three 2-year cycles of NHANES data were combined to create our 2009 to 2014 dataset. In the Table, numerous variables including age, sex, household income, race/ethnicity, education, diabetes status, tobacco use, body mass index (BMI), waist circumference, and being called overweight by a health care provider were analyzed using χ2 or t test analyses to evaluate for differences among those with and without psoriasis. Diabetes status was assessed by the question “Other than during pregnancy, have you ever been told by a doctor or health professional that you have diabetes or sugar diabetes?” Tobacco use was assessed by the question “Have you smoked at least 100 cigarettes in your entire life?” Psoriasis status was assessed by a self-reported response to the question “Have you ever been told by a doctor or other health care professional that you had psoriasis?” Three different outcome variables were used to determine if patients were overweight or obese: BMI, waist circumference, and response to the question “Has a doctor or other health professional ever told you that you were overweight?” Obesity was defined as having a BMI of 30 or higher or waist circumference of 102 cm or more in males and 88 cm or more in females.4 Being overweight was defined as having a BMI of 25 to 29.99 or response of Yes to “Has a doctor or other health professional ever told you that you were overweight?”

Initially, there were 17,547 participants 20 years and older from 2009 to 2014, but 1654 participants were excluded because of missing data for obesity or psoriasis; therefore, 15,893 patients were included in our analysis. Multivariable logistic regressions were utilized to examine the association between psoriasis and being overweight/obese (eTable). Additionally, the models were adjusted based on age, sex, household income, race/ethnicity, diabetes status, and tobacco use. All data processing and analysis were performed in Stata/MP 17 (StataCorp LLC). P<.05 was considered statistically significant.

The Table shows characteristics of US adults with and without psoriasis in NHANES 2009-2014. We found that the variables of interest evaluating body weight that were significantly different on analysis between patients with and without psoriasis included waist circumference—patients with psoriasis had a significantly higher waist circumference (P=.009)—and being told by a health care provider that they are overweight (P<.0001), which supports the findings by Love et al,5 who reported abdominal obesity was the most common feature of metabolic syndrome exhibited among patients with psoriasis.
Multivariable logistic regression analysis (eTable) revealed that there was a significant association between psoriasis and BMI of 25 to 29.99 (adjusted odds ratio [AOR], 1.34; 95% CI, 1.02-1.76; P=.04) and being told by a health care provider that they are overweight (AOR, 1.91; 95% CI, 1.44-2.52; P<.001). After adjusting for confounding variables, there was no significant association between psoriasis and a BMI of 30 or higher (AOR, 1.00; 95% CI, 0.73-1.38; P=.99) or a waist circumference of 102 cm or more in males and 88 cm or more in females (AOR, 1.15; 95% CI, 0.86-1.53; P=.3).
Our findings suggest that a few variables indicative of being overweight or obese are associated with psoriasis. This relationship most likely is due to increased adipokine, including resistin, levels in overweight individuals, resulting in a proinflammatory state.6 It has been suggested that BMI alone is not a definitive marker for measuring fat storage levels in individuals. People can have a normal or slightly elevated BMI but possess excessive adiposity, resulting in chronic inflammation.6 Therefore, our findings of a significant association between psoriasis and being told by a health care provider that they are overweight might be a stronger measurement for possessing excessive fat, as this is likely due to clinical judgment rather than BMI measurement.
Moreover, it should be noted that the potential reason for the lack of association between BMI of 30 or higher and psoriasis in our analysis may be a result of BMI serving as a poor measurement for adiposity. Additionally, Armstrong and colleagues7 discussed that the association between BMI and psoriasis was stronger for patients with moderate to severe psoriasis. Our study consisted of NHANES data for self-reported psoriasis diagnoses without a psoriasis severity index, making it difficult to extrapolate which individuals had mild or moderate to severe psoriasis, which may have contributed to our finding of no association between BMI of 30 or higher and psoriasis.
The self-reported nature of the survey questions and lack of questions regarding psoriasis severity serve as limitations to the study. Both obesity and psoriasis can have various systemic consequences, such as cardiovascular disease, due to the development of an inflammatory state.8 Future studies may explore other body measurements that indicate being overweight or obese and the potential synergistic relationship of obesity and psoriasis severity, optimizing the development of effective treatment plans.
- Jensen P, Skov L. Psoriasis and obesity. Dermatology. 2016;232:633-639.
- Xu C, Ji J, Su T, et al. The association of psoriasis and obesity: focusing on IL-17A-related immunological mechanisms. Int J Dermatol Venereol. 2021;4:116-121.
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Ross R, Neeland IJ, Yamashita S, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol. 2020;16:177-189.
- Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003-2006. Arch Dermatol. 2011;147:419-424.
- Paroutoglou K, Papadavid E, Christodoulatos GS, et al. Deciphering the association between psoriasis and obesity: current evidence and treatment considerations. Curr Obes Rep. 2020;9:165-178.
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012;2:E54.
- Hamminga EA, van der Lely AJ, Neumann HAM, et al. Chronic inflammation in psoriasis and obesity: implications for therapy. Med Hypotheses. 2006;67:768-773.
- Jensen P, Skov L. Psoriasis and obesity. Dermatology. 2016;232:633-639.
- Xu C, Ji J, Su T, et al. The association of psoriasis and obesity: focusing on IL-17A-related immunological mechanisms. Int J Dermatol Venereol. 2021;4:116-121.
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Ross R, Neeland IJ, Yamashita S, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol. 2020;16:177-189.
- Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003-2006. Arch Dermatol. 2011;147:419-424.
- Paroutoglou K, Papadavid E, Christodoulatos GS, et al. Deciphering the association between psoriasis and obesity: current evidence and treatment considerations. Curr Obes Rep. 2020;9:165-178.
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012;2:E54.
- Hamminga EA, van der Lely AJ, Neumann HAM, et al. Chronic inflammation in psoriasis and obesity: implications for therapy. Med Hypotheses. 2006;67:768-773.
Practice Points
- There are many comorbidities that are associated with psoriasis, making it crucial to evaluate for these diseases in patients with psoriasis.
- Obesity may be a contributing factor to psoriasis development due to the role of IL-17 secretion.
Adverse Effects of the COVID-19 Vaccine in Patients With Psoriasis
To the Editor:
Because the SARS-CoV-2 virus is constantly changing, routine vaccination to prevent COVID-19 infection is recommended. The messenger RNA (mRNA) vaccines from Pfizer-BioNTech and Moderna as well as the Ad26.COV2.S (Johnson & Johnson) and NVX-CoV2373 (Novavax) vaccines are the most commonly used COVID-19 vaccines in the United States. Adverse effects following vaccination against SARS-CoV-2 are well documented; recent studies report a small incidence of adverse effects in the general population, with most being minor (eg, headache, fever, muscle pain).1,2 Interestingly, reports of exacerbation of psoriasis and new-onset psoriasis following COVID-19 vaccination suggest a potential association.3,4 However, the literature investigating the vaccine adverse effect profile in this demographic is scarce. We examined the incidence of adverse effects from SARS-CoV-2 vaccines in patients with psoriasis.
This retrospective cohort study used the COVID-19 Research Database (https://covid19researchdatabase.org/) to examine the adverse effects following the first and second doses of the mRNA vaccines in patients with and without psoriasis. The sample size for the Ad26.COV2.S vaccine was too small to analyze.
Claims were evaluated from August to October 2021 for 2 diagnoses of psoriasis prior to January 1, 2020, using the International Classification of Diseases, Tenth Revision (ICD-10) code L40.9 to increase the positive predictive value and ensure that the diagnosis preceded the COVID-19 pandemic. Patients younger than 18 years and those who did not receive 2 doses of a SARS-CoV-2 vaccine were excluded. Controls who did not have a diagnosis of psoriasis were matched for age, sex, and hypertension at a 4:1 ratio. Hypertension represented the most common comorbidity that could feasibly be controlled for in this study population. Other comorbidities recorded included obesity, type 2 diabetes mellitus, congestive heart failure, asthma, chronic obstructive pulmonary disease, chronic ischemic heart disease, rhinitis, and chronic kidney disease.
Common adverse effects as long as 30 days after vaccination were identified using ICD-10 codes. Adverse effects of interest were anaphylactic reaction, initial encounter of adverse effect of viral vaccines, fever, allergic urticaria, weakness, altered mental status, malaise, allergic reaction, chest pain, symptoms involving circulatory or respiratory systems, localized rash, axillary lymphadenopathy, infection, and myocarditis.5 Poisson regression was performed using Stata 17 analytical software.
We identified 4273 patients with psoriasis and 17,092 controls who received mRNA COVID-19 vaccines (Table). Adjusted odds ratios (aORs) for doses 1 and 2 were calculated for each vaccine (eTable). Adverse effects with sufficient data to generate an aOR included weakness, altered mental status, malaise, chest pain, and symptoms involving the circulatory or respiratory system. The aORs for allergic urticaria and initial encounter of adverse effect of viral vaccines were only calculated for the Moderna mRNA vaccine due to low sample size.

This study demonstrated that patients with psoriasis do not appear to have a significantly increased risk of adverse effects from mRNA SARS-CoV-2 vaccines. Although the ORs in this study were not significant, most recorded adverse effects demonstrated an aOR less than 1, suggesting that there might be a lower risk of certain adverse effects in psoriasis patients. This could be explained by the immunomodulatory effects of certain systemic psoriasis treatments that might influence the adverse effect presentation.

The study is limited by the lack of treatment data, small sample size, and the fact that it did not assess flares or worsening of psoriasis with the vaccines. Underreporting of adverse effects by patients and underdiagnosis of adverse effects secondary to SARS-CoV-2 vaccines due to its novel nature, incompletely understood consequences, and limited ICD-10 codes associated with adverse effects all contributed to the small sample size.
Our findings suggest that the risk for immediate adverse effects from the mRNA SARS-CoV-2 vaccines is not increased among psoriasis patients. However, the impact of immunomodulatory agents on vaccine efficacy and expected adverse effects should be investigated. As more individuals receive the COVID-19 vaccine, the adverse effect profile in patients with psoriasis is an important area of investigation.
- Singh A, Khillan R, Mishra Y, et al. The safety profile of COVID-19 vaccinations in the United States. Am J Infect Control. 2022;50:15-19. doi: 10.1016/j.ajic.2021.10.015
- Beatty AL, Peyser ND, Butcher XE, et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw Open. 2021;4:e2140364. doi:10.1001/jamanetworkopen.2021.40364
- Bellinato F, Maurelli M, Gisondi P, et al. Cutaneous adverse reactions associated with SARS-CoV-2 vaccines. J Clin Med. 2021;10:5344. doi:10.3390/jcm10225344
- Elamin S, Hinds F, Tolland J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin Exp Dermatol. 2022;47:153-155. doi:10.1111/ced.14895
- Remer EE. Coding COVID-19 vaccination. ICD10monitor. Published March 2, 2021. Updated October 18, 2022. Accessed January 17, 2023. https://icd10monitor.medlearn.com/coding-covid-19-vaccination/
To the Editor:
Because the SARS-CoV-2 virus is constantly changing, routine vaccination to prevent COVID-19 infection is recommended. The messenger RNA (mRNA) vaccines from Pfizer-BioNTech and Moderna as well as the Ad26.COV2.S (Johnson & Johnson) and NVX-CoV2373 (Novavax) vaccines are the most commonly used COVID-19 vaccines in the United States. Adverse effects following vaccination against SARS-CoV-2 are well documented; recent studies report a small incidence of adverse effects in the general population, with most being minor (eg, headache, fever, muscle pain).1,2 Interestingly, reports of exacerbation of psoriasis and new-onset psoriasis following COVID-19 vaccination suggest a potential association.3,4 However, the literature investigating the vaccine adverse effect profile in this demographic is scarce. We examined the incidence of adverse effects from SARS-CoV-2 vaccines in patients with psoriasis.
This retrospective cohort study used the COVID-19 Research Database (https://covid19researchdatabase.org/) to examine the adverse effects following the first and second doses of the mRNA vaccines in patients with and without psoriasis. The sample size for the Ad26.COV2.S vaccine was too small to analyze.
Claims were evaluated from August to October 2021 for 2 diagnoses of psoriasis prior to January 1, 2020, using the International Classification of Diseases, Tenth Revision (ICD-10) code L40.9 to increase the positive predictive value and ensure that the diagnosis preceded the COVID-19 pandemic. Patients younger than 18 years and those who did not receive 2 doses of a SARS-CoV-2 vaccine were excluded. Controls who did not have a diagnosis of psoriasis were matched for age, sex, and hypertension at a 4:1 ratio. Hypertension represented the most common comorbidity that could feasibly be controlled for in this study population. Other comorbidities recorded included obesity, type 2 diabetes mellitus, congestive heart failure, asthma, chronic obstructive pulmonary disease, chronic ischemic heart disease, rhinitis, and chronic kidney disease.
Common adverse effects as long as 30 days after vaccination were identified using ICD-10 codes. Adverse effects of interest were anaphylactic reaction, initial encounter of adverse effect of viral vaccines, fever, allergic urticaria, weakness, altered mental status, malaise, allergic reaction, chest pain, symptoms involving circulatory or respiratory systems, localized rash, axillary lymphadenopathy, infection, and myocarditis.5 Poisson regression was performed using Stata 17 analytical software.
We identified 4273 patients with psoriasis and 17,092 controls who received mRNA COVID-19 vaccines (Table). Adjusted odds ratios (aORs) for doses 1 and 2 were calculated for each vaccine (eTable). Adverse effects with sufficient data to generate an aOR included weakness, altered mental status, malaise, chest pain, and symptoms involving the circulatory or respiratory system. The aORs for allergic urticaria and initial encounter of adverse effect of viral vaccines were only calculated for the Moderna mRNA vaccine due to low sample size.

This study demonstrated that patients with psoriasis do not appear to have a significantly increased risk of adverse effects from mRNA SARS-CoV-2 vaccines. Although the ORs in this study were not significant, most recorded adverse effects demonstrated an aOR less than 1, suggesting that there might be a lower risk of certain adverse effects in psoriasis patients. This could be explained by the immunomodulatory effects of certain systemic psoriasis treatments that might influence the adverse effect presentation.

The study is limited by the lack of treatment data, small sample size, and the fact that it did not assess flares or worsening of psoriasis with the vaccines. Underreporting of adverse effects by patients and underdiagnosis of adverse effects secondary to SARS-CoV-2 vaccines due to its novel nature, incompletely understood consequences, and limited ICD-10 codes associated with adverse effects all contributed to the small sample size.
Our findings suggest that the risk for immediate adverse effects from the mRNA SARS-CoV-2 vaccines is not increased among psoriasis patients. However, the impact of immunomodulatory agents on vaccine efficacy and expected adverse effects should be investigated. As more individuals receive the COVID-19 vaccine, the adverse effect profile in patients with psoriasis is an important area of investigation.
To the Editor:
Because the SARS-CoV-2 virus is constantly changing, routine vaccination to prevent COVID-19 infection is recommended. The messenger RNA (mRNA) vaccines from Pfizer-BioNTech and Moderna as well as the Ad26.COV2.S (Johnson & Johnson) and NVX-CoV2373 (Novavax) vaccines are the most commonly used COVID-19 vaccines in the United States. Adverse effects following vaccination against SARS-CoV-2 are well documented; recent studies report a small incidence of adverse effects in the general population, with most being minor (eg, headache, fever, muscle pain).1,2 Interestingly, reports of exacerbation of psoriasis and new-onset psoriasis following COVID-19 vaccination suggest a potential association.3,4 However, the literature investigating the vaccine adverse effect profile in this demographic is scarce. We examined the incidence of adverse effects from SARS-CoV-2 vaccines in patients with psoriasis.
This retrospective cohort study used the COVID-19 Research Database (https://covid19researchdatabase.org/) to examine the adverse effects following the first and second doses of the mRNA vaccines in patients with and without psoriasis. The sample size for the Ad26.COV2.S vaccine was too small to analyze.
Claims were evaluated from August to October 2021 for 2 diagnoses of psoriasis prior to January 1, 2020, using the International Classification of Diseases, Tenth Revision (ICD-10) code L40.9 to increase the positive predictive value and ensure that the diagnosis preceded the COVID-19 pandemic. Patients younger than 18 years and those who did not receive 2 doses of a SARS-CoV-2 vaccine were excluded. Controls who did not have a diagnosis of psoriasis were matched for age, sex, and hypertension at a 4:1 ratio. Hypertension represented the most common comorbidity that could feasibly be controlled for in this study population. Other comorbidities recorded included obesity, type 2 diabetes mellitus, congestive heart failure, asthma, chronic obstructive pulmonary disease, chronic ischemic heart disease, rhinitis, and chronic kidney disease.
Common adverse effects as long as 30 days after vaccination were identified using ICD-10 codes. Adverse effects of interest were anaphylactic reaction, initial encounter of adverse effect of viral vaccines, fever, allergic urticaria, weakness, altered mental status, malaise, allergic reaction, chest pain, symptoms involving circulatory or respiratory systems, localized rash, axillary lymphadenopathy, infection, and myocarditis.5 Poisson regression was performed using Stata 17 analytical software.
We identified 4273 patients with psoriasis and 17,092 controls who received mRNA COVID-19 vaccines (Table). Adjusted odds ratios (aORs) for doses 1 and 2 were calculated for each vaccine (eTable). Adverse effects with sufficient data to generate an aOR included weakness, altered mental status, malaise, chest pain, and symptoms involving the circulatory or respiratory system. The aORs for allergic urticaria and initial encounter of adverse effect of viral vaccines were only calculated for the Moderna mRNA vaccine due to low sample size.

This study demonstrated that patients with psoriasis do not appear to have a significantly increased risk of adverse effects from mRNA SARS-CoV-2 vaccines. Although the ORs in this study were not significant, most recorded adverse effects demonstrated an aOR less than 1, suggesting that there might be a lower risk of certain adverse effects in psoriasis patients. This could be explained by the immunomodulatory effects of certain systemic psoriasis treatments that might influence the adverse effect presentation.

The study is limited by the lack of treatment data, small sample size, and the fact that it did not assess flares or worsening of psoriasis with the vaccines. Underreporting of adverse effects by patients and underdiagnosis of adverse effects secondary to SARS-CoV-2 vaccines due to its novel nature, incompletely understood consequences, and limited ICD-10 codes associated with adverse effects all contributed to the small sample size.
Our findings suggest that the risk for immediate adverse effects from the mRNA SARS-CoV-2 vaccines is not increased among psoriasis patients. However, the impact of immunomodulatory agents on vaccine efficacy and expected adverse effects should be investigated. As more individuals receive the COVID-19 vaccine, the adverse effect profile in patients with psoriasis is an important area of investigation.
- Singh A, Khillan R, Mishra Y, et al. The safety profile of COVID-19 vaccinations in the United States. Am J Infect Control. 2022;50:15-19. doi: 10.1016/j.ajic.2021.10.015
- Beatty AL, Peyser ND, Butcher XE, et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw Open. 2021;4:e2140364. doi:10.1001/jamanetworkopen.2021.40364
- Bellinato F, Maurelli M, Gisondi P, et al. Cutaneous adverse reactions associated with SARS-CoV-2 vaccines. J Clin Med. 2021;10:5344. doi:10.3390/jcm10225344
- Elamin S, Hinds F, Tolland J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin Exp Dermatol. 2022;47:153-155. doi:10.1111/ced.14895
- Remer EE. Coding COVID-19 vaccination. ICD10monitor. Published March 2, 2021. Updated October 18, 2022. Accessed January 17, 2023. https://icd10monitor.medlearn.com/coding-covid-19-vaccination/
- Singh A, Khillan R, Mishra Y, et al. The safety profile of COVID-19 vaccinations in the United States. Am J Infect Control. 2022;50:15-19. doi: 10.1016/j.ajic.2021.10.015
- Beatty AL, Peyser ND, Butcher XE, et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw Open. 2021;4:e2140364. doi:10.1001/jamanetworkopen.2021.40364
- Bellinato F, Maurelli M, Gisondi P, et al. Cutaneous adverse reactions associated with SARS-CoV-2 vaccines. J Clin Med. 2021;10:5344. doi:10.3390/jcm10225344
- Elamin S, Hinds F, Tolland J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin Exp Dermatol. 2022;47:153-155. doi:10.1111/ced.14895
- Remer EE. Coding COVID-19 vaccination. ICD10monitor. Published March 2, 2021. Updated October 18, 2022. Accessed January 17, 2023. https://icd10monitor.medlearn.com/coding-covid-19-vaccination/
PRACTICE POINTS
- Patients who have psoriasis do not appear to have an increased incidence of adverse effects from messenger RNA COVID-19 vaccines.
- Clinicians can safely recommend COVID-19 vaccines to patients who have psoriasis.
New Treatments for Psoriasis: An Update on a Therapeutic Frontier
The landscape of psoriasis treatments has undergone rapid change within the last decade, and the dizzying speed of drug development has not slowed, with 4 notable entries into the psoriasis treatment armamentarium within the last year: tapinarof, roflumilast, deucravacitinib, and spesolimab. Several others are in late-stage development, and these therapies represent new mechanisms, pathways, and delivery systems that will meaningfully broaden the spectrum of treatment choices for our patients. However, it can be quite difficult to keep track of all of the medication options. This review aims to present the mechanisms and data on both newly available therapeutics for psoriasis and products in the pipeline that may have a major impact on our treatment paradigm for psoriasis in the near future.
Topical Treatments
Tapinarof—Tapinarof is a topical aryl hydrocarbon receptor (AhR)–modulating agent derived from a secondary metabolite produced by a bacterial symbiont of entomopathogenic nematodes.1 Tapinarof binds and activates AhR, inducing a signaling cascade that suppresses the expression of helper T cells TH17 and TH22, upregulates skin barrier protein expression, and reduces epidermal oxidative stress.2 This is a familiar mechanism, as AhR agonism is one of the pathways modulated by coal tar. Tapinarof’s overall effects on immune function, skin barrier integrity, and antioxidant activity show great promise for the treatment of plaque psoriasis.
Two phase 3 trials (N=1025) evaluated the efficacy and safety of once-daily tapinarof cream 1% for plaque psoriasis.3 A physician global assessment (PGA) score of 0/1 occurred in 35.4% to 40.2% of patients in the tapinarof group and in 6.0% of patients in the vehicle group. At week 12, 36.1% to 47.6% of patients treated with daily applications of tapinarof cream achieved a 75% reduction in their baseline psoriasis area and severity index (PASI 75) score compared with 6.9% to 10.2% in the vehicle group.3 In a long-term extension study, a substantial remittive effect of at least 4 months off tapinarof therapy was observed in patients who achieved complete clearance (PGA=0).4 Use of tapinarof cream was associated with folliculitis in up to 23.5% of patients.3,4
Roflumilast—
Topical roflumilast is a selective, highly potent PDE-4 inhibitor with greater affinity for PDE-4 compared to crisaborole and apremilast.8 Two phase 3 trials (N=881) evaluated the efficacy and safety profile of roflumilast cream for plaque psoriasis, with a particular interest in its use for intertriginous areas.9 At week 8, 37.5% to 42.4% of roflumilast-treated patients achieved investigator global assessment (IGA) success compared with 6.1% to 6.9% of vehicle-treated patients. Intertriginous IGA success was observed in 68.1% to 71.2% of patients treated with roflumilast cream compared with 13.8% to 18.5% of vehicle-treated patients. At 8-week follow-up, 39.0% to 41.6% of roflumilast-treated patients achieved PASI 75 vs 5.3% to 7.6% of patients in the vehicle group. Few stinging, burning, or application-site reactions were reported with roflumilast, along with rare instances of gastrointestinal AEs (<4%).9
Oral Therapy
Deucravacitinib—Tyrosine kinase 2 (TYK2) mediates the intracellular signaling of the TH17 and TH1 inflammatory cytokines IL-12/IL-23 and type I interferons, respectively, the former of which are critical in the development of psoriasis via the Janus kinase (JAK) signal transducer and activator of transcription pathway.10 Deucravacitinib is an oral selective TYK2 allosteric inhibitor that binds to the regulatory domain of the enzyme rather than the active catalytic domain, where other TYK2 and JAK1, JAK2, and JAK3 inhibitors bind.11 This unique inhibitory mechanism accounts for the high functional selectivity of deucravacitinib for TYK2 vs the closely related JAK1, JAK2, and JAK3 kinases, thus avoiding the pitfall of prior JAK inhibitors that were associated with major AEs, including an increased risk for serious infections, malignancies, and thrombosis.12 The selective suppression of the inflammatory TYK2 pathway has the potential to shift future therapeutic targets to a narrower range of receptors that may contribute to favorable benefit-risk profiles.
Two phase 3 trials (N=1686) compared the efficacy and safety of deucravacitinib vs placebo and apremilast in adults with moderate to severe plaque psoriasis.13,14 At week 16, 53.0% to 58.4% of deucravacitinib-treated patients achieved PASI 75 compared with 35.1% to 39.8% of apremilast-treated patients. At 16-week follow-up, static PGA response was observed in 49.5% to 53.6% of patients in the deucravacitinib group and 32.1% to 33.9% of the apremilast group. The most frequent AEs associated with deucravacitinib therapy were nasopharyngitis and upper respiratory tract infection, whereas headache, diarrhea, and nausea were more common with apremilast. Treatment with deucravacitinib caused no meaningful changes in laboratory parameters, which are known to change with JAK1, JAK2, and JAK3 inhibitors.13,14 A long-term extension study demonstrated that deucravacitinib had persistent efficacy and consistent safety for up to 2 years.15
Other TYK2 Inhibitors in the Pipeline
Novel oral allosteric TYK2 inhibitors—VTX958 and NDI-034858—and the competitive TYK2 inhibitor PF-06826647 are being developed. Theoretically, these new allosteric inhibitors possess unique structural properties to provide greater TYK2 suppression while bypassing JAK1, JAK2, and JAK3 pathways that may contribute to improved efficacy and safety profiles compared with other TYK2 inhibitors such as deucravacitinib. The results of a phase 1b trial (ClinicalTrials.gov Identifier NCT04999839) showed a dose-dependent reduction of disease severity associated with NDI-034858 treatment for patients with moderate to severe plaque psoriasis, albeit in only 26 patients. At week 4, PASI 50 was achieved in 13%, 57%, and 40% of patients in the 5-, 10-, and 30-mg groups, respectively, compared with 0% in the placebo group.16 In a phase 2 trial of 179 patients, 46.5% and 33.0% of patients treated with 400 and 200 mg of PF-06826647, respectively, achieved PASI 90 at week 16. Conversely, dose-dependent laboratory abnormalities were observed with PF-06826647, including anemia, neutropenia, and increases in creatine phosphokinase.17 At high concentrations, PF-06826647 may disrupt JAK signaling pathways involved in hematopoiesis and renal functions owing to its mode of action as a competitive inhibitor. Overall, these agents are much farther from market, and long-term studies with larger diverse patient cohorts are required to adequately assess the efficacy and safety data of these novel oral TYK2 inhibitors for patients with psoriasis.
EDP1815—EDP1815 is an oral preparation of a single strain of Prevotella histicola being developed for the treatment of inflammatory diseases, including psoriasis. EDP1815 interacts with host intestinal immune cells through the small intestinal axis (SINTAX) to suppress systemic inflammation across the TH1, TH2, and TH17 pathways. Therapy triggers broad immunomodulatory effects without causing systemic absorption, colonic colonization, or modification of the gut microbiome.18 In a phase 2 study (NCT04603027), the primary end point analysis, mean percentage change in PASI between treatment and placebo, demonstrated that at week 16, EDP1815 was superior to placebo with 80% to 90% probability across each cohort. At week 16, 25% to 32% of patients across the 3 cohorts treated with EDP1815 achieved PASI 50 compared with 12% of patients receiving placebo. Gastrointestinal AEs were comparable between treatment and placebo groups. These results suggest that SINTAX-targeted therapies may provide efficacious and safe immunomodulatory effects for patients with mild to moderate psoriasis, who often have limited treatment options. Although improvements may be mild, SINTAX-targeted therapies can be seen as a particularly attractive adjunctive treatment for patients with severe psoriasis taking other medications or as part of a treatment approach for a patient with milder psoriasis.
Biologics
Bimekizumab—Bimekizumab is a monoclonal IgG1 antibody that selectively inhibits IL-17A and IL-17F. Although IL-17A is a more potent cytokine, IL-17F may be more highly expressed in psoriatic lesional skin and independently contribute to the activation of proinflammatory signaling pathways implicated in the pathophysiology of psoriasis.19 Evidence suggests that dual inhibition of IL-17A and IL-17F may provide more complete suppression of inflammation and improved clinical responses than IL-17A inhibition alone.20
Prior bimekizumab phase 3 clinical studies have shown both rapid and durable clinical improvements in skin clearance compared with placebo.21 Three phase 3 trials—BE VIVID (N=567),22 BE SURE (N=478),23 and BE RADIANT (N=743)24—assessed the efficacy and safety of bimekizumab vs the IL-12/IL-23 inhibitor ustekinumab, the tumor necrosis factor inhibitor adalimumab, and the selective IL-17A inhibitor secukinumab, respectively. At week 4, significantly more patients treated with bimekizumab (71%–77%) achieved PASI 75 than patients treated with ustekinumab (15%; P<.0001), adalimumab (31.4%; P<.001), or secukinumab (47.3%; P<.001).22-24 After 16 weeks of treatment, PASI 90 was achieved by 85% to 86.2%, 50%, and 47.2% of patients treated with bimekizumab, ustekinumab, and adalimumab, respectively.22,23 At week 16, PASI 100 was observed in 59% to 61.7%, 21%, 23.9%, and 48.9% of patients treated with bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively. An IGA response (score of 0/1) at week 16 was achieved by 84% to 85.5%, 53%, 57.2%, and 78.6% of patients receiving bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively.22-24
The most common AEs in bimekizumab-treated patients were nasopharyngitis, oral candidiasis, and upper respiratory tract infection.22-24 The dual inhibition of IL-17A and IL-17F suppresses host defenses against Candida at the oral mucosa, increasing the incidence of bimekizumab-associated oral candidiasis.25 Despite the increased risk of Candida infections, these data suggest that inhibition of both IL-17A and IL-17F with bimekizumab may provide faster and greater clinical benefit for patients with moderate to severe plaque psoriasis than inhibition of IL-17A alone and other biologic therapies, as the PASI 100 clearance rates across the multiple comparator trials and the placebo-controlled pivotal trial are consistently the highest among any biologic for the treatment of psoriasis.
Spesolimab—The IL-36 pathway and IL-36 receptor genes have been linked to the pathogenesis of generalized pustular psoriasis.26 In a phase 2 trial, 19 of 35 patients (54%) receiving an intravenous dose of spesolimab, an IL-36 receptor inhibitor, had a generalized pustular psoriasis PGA pustulation subscore of 0 (no visible pustules) at the end of week 1 vs 6% of patients in the placebo group.27 A generalized pustular psoriasis PGA total score of 0 or 1 was observed in 43% (15/35) of spesolimab-treated patients compared with 11% (2/18) of patients in the placebo group. The most common AEs in patients treated with spesolimab were minor infections.27 Two open-label phase 3 trials—NCT05200247 and NCT05239039—are underway to determine the long-term efficacy and safety of spesolimab in patients with generalized pustular psoriasis.
Conclusion
Although we have seen a renaissance in psoriasis therapies with the advent of biologics in the last 20 years, recent evidence shows that more innovation is underway. Just in the last year, 2 new mechanisms for treating psoriasis topically without steroids have come to fruition, and there have not been truly novel mechanisms for treating psoriasis topically since approvals for tazarotene and calcipotriene in the 1990s. An entirely new class—TYK2 inhibitors—was developed and landed in psoriasis first, greatly improving the efficacy measures attained with oral medications in general. Finally, an orphan diagnosis got its due with an ambitiously designed study looking at a previously unheard-of 1-week end point, but it comes for one of the few true dermatologic emergencies we encounter, generalized pustular psoriasis. We are fortunate to have so many meaningful new treatments available to us, and it is invigorating to see that even more efficacious biologics and treatments are coming, along with novel concepts such as a treatment affecting the microbiome. Now, we just need to make sure that our patients have the access they deserve to the wide array of available treatments.
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J Am Acad Dermatol. 2021;84:1059-1067.
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137:2110-2119.
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229.
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806.
- Card GL, England BP, Suzuki Y, et al. Structural basis for the activity of drugs that inhibit phosphodiesterases. Structure. 2004;12:2233-2247.
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis (Auckl). 2021;11:21-29.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Dong C, Virtucio C, Zemska O, et al. Treatment of skin inflammation with benzoxaborole phosphodiesterase inhibitors: selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J Pharmacol Exp Ther. 2016;358:413-422.
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084.
- Nogueira M, Puig L, Torres T. JAK inhibitors for treatment of psoriasis: focus on selective tyk2 inhibitors. Drugs. 2020;80:341-352.
- Wrobleski ST, Moslin R, Lin S, et al. Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J Med Chem. 2019;62:8973-8995.
- Chimalakonda A, Burke J, Cheng L, et al. Selectivity profile of the tyrosine kinase 2 inhibitor deucravacitinib compared with janus kinase 1/2/3 inhibitors. Dermatol Ther (Heidelb). 2021;11:1763-1776.
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program for Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51.
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39.
- Warren RB, Sofen H, Imafuku S, et al. POS1046 deucravacitinib long-term efficacy and safety in plaque psoriasis: 2-year results from the phase 3 POETYK PSO program [abstract]. Ann Rheum Dis. 2022;81(suppl 1):841.
- McElwee JJ, Garcet S, Li X, et al. Analysis of histologic, molecular and clinical improvement in moderate-to-severe psoriasis: results from a Phase 1b trial of the novel allosteric TYK2 inhibitor NDI-034858. Poster presented at: American Academy of Dermatology Annual Meeting; March 25, 2022; Boston, MA.
- Tehlirian C, Singh RSP, Pradhan V, et al. Oral tyrosine kinase 2 inhibitor PF-06826647 demonstrates efficacy and an acceptable safety profile in participants with moderate-to-severe plaque psoriasis in a phase 2b, randomized, double-blind, placebo-controlled study. J Am Acad Dermatol. 2022;87:333-342.
- Hilliard-Barth K, Cormack T, Ramani K, et al. Immune mechanisms of the systemic effects of EDP1815: an orally delivered, gut-restricted microbial drug candidate for the treatment of inflammatory diseases. Poster presented at: Society for Mucosal Immunology Virtual Congress; July 20-22, 2021, Cambridge, MA.
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532.
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894.
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475-486.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397:487-498.
- Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 2021;385:130-141.
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152.
- Blauvelt A, Lebwohl MG, Bissonnette R. IL-23/IL-17A dysfunction phenotypes inform possible clinical effects from anti-IL-17A therapies. J Invest Dermatol. 2015;135:1946-1953.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440.
The landscape of psoriasis treatments has undergone rapid change within the last decade, and the dizzying speed of drug development has not slowed, with 4 notable entries into the psoriasis treatment armamentarium within the last year: tapinarof, roflumilast, deucravacitinib, and spesolimab. Several others are in late-stage development, and these therapies represent new mechanisms, pathways, and delivery systems that will meaningfully broaden the spectrum of treatment choices for our patients. However, it can be quite difficult to keep track of all of the medication options. This review aims to present the mechanisms and data on both newly available therapeutics for psoriasis and products in the pipeline that may have a major impact on our treatment paradigm for psoriasis in the near future.
Topical Treatments
Tapinarof—Tapinarof is a topical aryl hydrocarbon receptor (AhR)–modulating agent derived from a secondary metabolite produced by a bacterial symbiont of entomopathogenic nematodes.1 Tapinarof binds and activates AhR, inducing a signaling cascade that suppresses the expression of helper T cells TH17 and TH22, upregulates skin barrier protein expression, and reduces epidermal oxidative stress.2 This is a familiar mechanism, as AhR agonism is one of the pathways modulated by coal tar. Tapinarof’s overall effects on immune function, skin barrier integrity, and antioxidant activity show great promise for the treatment of plaque psoriasis.
Two phase 3 trials (N=1025) evaluated the efficacy and safety of once-daily tapinarof cream 1% for plaque psoriasis.3 A physician global assessment (PGA) score of 0/1 occurred in 35.4% to 40.2% of patients in the tapinarof group and in 6.0% of patients in the vehicle group. At week 12, 36.1% to 47.6% of patients treated with daily applications of tapinarof cream achieved a 75% reduction in their baseline psoriasis area and severity index (PASI 75) score compared with 6.9% to 10.2% in the vehicle group.3 In a long-term extension study, a substantial remittive effect of at least 4 months off tapinarof therapy was observed in patients who achieved complete clearance (PGA=0).4 Use of tapinarof cream was associated with folliculitis in up to 23.5% of patients.3,4
Roflumilast—
Topical roflumilast is a selective, highly potent PDE-4 inhibitor with greater affinity for PDE-4 compared to crisaborole and apremilast.8 Two phase 3 trials (N=881) evaluated the efficacy and safety profile of roflumilast cream for plaque psoriasis, with a particular interest in its use for intertriginous areas.9 At week 8, 37.5% to 42.4% of roflumilast-treated patients achieved investigator global assessment (IGA) success compared with 6.1% to 6.9% of vehicle-treated patients. Intertriginous IGA success was observed in 68.1% to 71.2% of patients treated with roflumilast cream compared with 13.8% to 18.5% of vehicle-treated patients. At 8-week follow-up, 39.0% to 41.6% of roflumilast-treated patients achieved PASI 75 vs 5.3% to 7.6% of patients in the vehicle group. Few stinging, burning, or application-site reactions were reported with roflumilast, along with rare instances of gastrointestinal AEs (<4%).9
Oral Therapy
Deucravacitinib—Tyrosine kinase 2 (TYK2) mediates the intracellular signaling of the TH17 and TH1 inflammatory cytokines IL-12/IL-23 and type I interferons, respectively, the former of which are critical in the development of psoriasis via the Janus kinase (JAK) signal transducer and activator of transcription pathway.10 Deucravacitinib is an oral selective TYK2 allosteric inhibitor that binds to the regulatory domain of the enzyme rather than the active catalytic domain, where other TYK2 and JAK1, JAK2, and JAK3 inhibitors bind.11 This unique inhibitory mechanism accounts for the high functional selectivity of deucravacitinib for TYK2 vs the closely related JAK1, JAK2, and JAK3 kinases, thus avoiding the pitfall of prior JAK inhibitors that were associated with major AEs, including an increased risk for serious infections, malignancies, and thrombosis.12 The selective suppression of the inflammatory TYK2 pathway has the potential to shift future therapeutic targets to a narrower range of receptors that may contribute to favorable benefit-risk profiles.
Two phase 3 trials (N=1686) compared the efficacy and safety of deucravacitinib vs placebo and apremilast in adults with moderate to severe plaque psoriasis.13,14 At week 16, 53.0% to 58.4% of deucravacitinib-treated patients achieved PASI 75 compared with 35.1% to 39.8% of apremilast-treated patients. At 16-week follow-up, static PGA response was observed in 49.5% to 53.6% of patients in the deucravacitinib group and 32.1% to 33.9% of the apremilast group. The most frequent AEs associated with deucravacitinib therapy were nasopharyngitis and upper respiratory tract infection, whereas headache, diarrhea, and nausea were more common with apremilast. Treatment with deucravacitinib caused no meaningful changes in laboratory parameters, which are known to change with JAK1, JAK2, and JAK3 inhibitors.13,14 A long-term extension study demonstrated that deucravacitinib had persistent efficacy and consistent safety for up to 2 years.15
Other TYK2 Inhibitors in the Pipeline
Novel oral allosteric TYK2 inhibitors—VTX958 and NDI-034858—and the competitive TYK2 inhibitor PF-06826647 are being developed. Theoretically, these new allosteric inhibitors possess unique structural properties to provide greater TYK2 suppression while bypassing JAK1, JAK2, and JAK3 pathways that may contribute to improved efficacy and safety profiles compared with other TYK2 inhibitors such as deucravacitinib. The results of a phase 1b trial (ClinicalTrials.gov Identifier NCT04999839) showed a dose-dependent reduction of disease severity associated with NDI-034858 treatment for patients with moderate to severe plaque psoriasis, albeit in only 26 patients. At week 4, PASI 50 was achieved in 13%, 57%, and 40% of patients in the 5-, 10-, and 30-mg groups, respectively, compared with 0% in the placebo group.16 In a phase 2 trial of 179 patients, 46.5% and 33.0% of patients treated with 400 and 200 mg of PF-06826647, respectively, achieved PASI 90 at week 16. Conversely, dose-dependent laboratory abnormalities were observed with PF-06826647, including anemia, neutropenia, and increases in creatine phosphokinase.17 At high concentrations, PF-06826647 may disrupt JAK signaling pathways involved in hematopoiesis and renal functions owing to its mode of action as a competitive inhibitor. Overall, these agents are much farther from market, and long-term studies with larger diverse patient cohorts are required to adequately assess the efficacy and safety data of these novel oral TYK2 inhibitors for patients with psoriasis.
EDP1815—EDP1815 is an oral preparation of a single strain of Prevotella histicola being developed for the treatment of inflammatory diseases, including psoriasis. EDP1815 interacts with host intestinal immune cells through the small intestinal axis (SINTAX) to suppress systemic inflammation across the TH1, TH2, and TH17 pathways. Therapy triggers broad immunomodulatory effects without causing systemic absorption, colonic colonization, or modification of the gut microbiome.18 In a phase 2 study (NCT04603027), the primary end point analysis, mean percentage change in PASI between treatment and placebo, demonstrated that at week 16, EDP1815 was superior to placebo with 80% to 90% probability across each cohort. At week 16, 25% to 32% of patients across the 3 cohorts treated with EDP1815 achieved PASI 50 compared with 12% of patients receiving placebo. Gastrointestinal AEs were comparable between treatment and placebo groups. These results suggest that SINTAX-targeted therapies may provide efficacious and safe immunomodulatory effects for patients with mild to moderate psoriasis, who often have limited treatment options. Although improvements may be mild, SINTAX-targeted therapies can be seen as a particularly attractive adjunctive treatment for patients with severe psoriasis taking other medications or as part of a treatment approach for a patient with milder psoriasis.
Biologics
Bimekizumab—Bimekizumab is a monoclonal IgG1 antibody that selectively inhibits IL-17A and IL-17F. Although IL-17A is a more potent cytokine, IL-17F may be more highly expressed in psoriatic lesional skin and independently contribute to the activation of proinflammatory signaling pathways implicated in the pathophysiology of psoriasis.19 Evidence suggests that dual inhibition of IL-17A and IL-17F may provide more complete suppression of inflammation and improved clinical responses than IL-17A inhibition alone.20
Prior bimekizumab phase 3 clinical studies have shown both rapid and durable clinical improvements in skin clearance compared with placebo.21 Three phase 3 trials—BE VIVID (N=567),22 BE SURE (N=478),23 and BE RADIANT (N=743)24—assessed the efficacy and safety of bimekizumab vs the IL-12/IL-23 inhibitor ustekinumab, the tumor necrosis factor inhibitor adalimumab, and the selective IL-17A inhibitor secukinumab, respectively. At week 4, significantly more patients treated with bimekizumab (71%–77%) achieved PASI 75 than patients treated with ustekinumab (15%; P<.0001), adalimumab (31.4%; P<.001), or secukinumab (47.3%; P<.001).22-24 After 16 weeks of treatment, PASI 90 was achieved by 85% to 86.2%, 50%, and 47.2% of patients treated with bimekizumab, ustekinumab, and adalimumab, respectively.22,23 At week 16, PASI 100 was observed in 59% to 61.7%, 21%, 23.9%, and 48.9% of patients treated with bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively. An IGA response (score of 0/1) at week 16 was achieved by 84% to 85.5%, 53%, 57.2%, and 78.6% of patients receiving bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively.22-24
The most common AEs in bimekizumab-treated patients were nasopharyngitis, oral candidiasis, and upper respiratory tract infection.22-24 The dual inhibition of IL-17A and IL-17F suppresses host defenses against Candida at the oral mucosa, increasing the incidence of bimekizumab-associated oral candidiasis.25 Despite the increased risk of Candida infections, these data suggest that inhibition of both IL-17A and IL-17F with bimekizumab may provide faster and greater clinical benefit for patients with moderate to severe plaque psoriasis than inhibition of IL-17A alone and other biologic therapies, as the PASI 100 clearance rates across the multiple comparator trials and the placebo-controlled pivotal trial are consistently the highest among any biologic for the treatment of psoriasis.
Spesolimab—The IL-36 pathway and IL-36 receptor genes have been linked to the pathogenesis of generalized pustular psoriasis.26 In a phase 2 trial, 19 of 35 patients (54%) receiving an intravenous dose of spesolimab, an IL-36 receptor inhibitor, had a generalized pustular psoriasis PGA pustulation subscore of 0 (no visible pustules) at the end of week 1 vs 6% of patients in the placebo group.27 A generalized pustular psoriasis PGA total score of 0 or 1 was observed in 43% (15/35) of spesolimab-treated patients compared with 11% (2/18) of patients in the placebo group. The most common AEs in patients treated with spesolimab were minor infections.27 Two open-label phase 3 trials—NCT05200247 and NCT05239039—are underway to determine the long-term efficacy and safety of spesolimab in patients with generalized pustular psoriasis.
Conclusion
Although we have seen a renaissance in psoriasis therapies with the advent of biologics in the last 20 years, recent evidence shows that more innovation is underway. Just in the last year, 2 new mechanisms for treating psoriasis topically without steroids have come to fruition, and there have not been truly novel mechanisms for treating psoriasis topically since approvals for tazarotene and calcipotriene in the 1990s. An entirely new class—TYK2 inhibitors—was developed and landed in psoriasis first, greatly improving the efficacy measures attained with oral medications in general. Finally, an orphan diagnosis got its due with an ambitiously designed study looking at a previously unheard-of 1-week end point, but it comes for one of the few true dermatologic emergencies we encounter, generalized pustular psoriasis. We are fortunate to have so many meaningful new treatments available to us, and it is invigorating to see that even more efficacious biologics and treatments are coming, along with novel concepts such as a treatment affecting the microbiome. Now, we just need to make sure that our patients have the access they deserve to the wide array of available treatments.
The landscape of psoriasis treatments has undergone rapid change within the last decade, and the dizzying speed of drug development has not slowed, with 4 notable entries into the psoriasis treatment armamentarium within the last year: tapinarof, roflumilast, deucravacitinib, and spesolimab. Several others are in late-stage development, and these therapies represent new mechanisms, pathways, and delivery systems that will meaningfully broaden the spectrum of treatment choices for our patients. However, it can be quite difficult to keep track of all of the medication options. This review aims to present the mechanisms and data on both newly available therapeutics for psoriasis and products in the pipeline that may have a major impact on our treatment paradigm for psoriasis in the near future.
Topical Treatments
Tapinarof—Tapinarof is a topical aryl hydrocarbon receptor (AhR)–modulating agent derived from a secondary metabolite produced by a bacterial symbiont of entomopathogenic nematodes.1 Tapinarof binds and activates AhR, inducing a signaling cascade that suppresses the expression of helper T cells TH17 and TH22, upregulates skin barrier protein expression, and reduces epidermal oxidative stress.2 This is a familiar mechanism, as AhR agonism is one of the pathways modulated by coal tar. Tapinarof’s overall effects on immune function, skin barrier integrity, and antioxidant activity show great promise for the treatment of plaque psoriasis.
Two phase 3 trials (N=1025) evaluated the efficacy and safety of once-daily tapinarof cream 1% for plaque psoriasis.3 A physician global assessment (PGA) score of 0/1 occurred in 35.4% to 40.2% of patients in the tapinarof group and in 6.0% of patients in the vehicle group. At week 12, 36.1% to 47.6% of patients treated with daily applications of tapinarof cream achieved a 75% reduction in their baseline psoriasis area and severity index (PASI 75) score compared with 6.9% to 10.2% in the vehicle group.3 In a long-term extension study, a substantial remittive effect of at least 4 months off tapinarof therapy was observed in patients who achieved complete clearance (PGA=0).4 Use of tapinarof cream was associated with folliculitis in up to 23.5% of patients.3,4
Roflumilast—
Topical roflumilast is a selective, highly potent PDE-4 inhibitor with greater affinity for PDE-4 compared to crisaborole and apremilast.8 Two phase 3 trials (N=881) evaluated the efficacy and safety profile of roflumilast cream for plaque psoriasis, with a particular interest in its use for intertriginous areas.9 At week 8, 37.5% to 42.4% of roflumilast-treated patients achieved investigator global assessment (IGA) success compared with 6.1% to 6.9% of vehicle-treated patients. Intertriginous IGA success was observed in 68.1% to 71.2% of patients treated with roflumilast cream compared with 13.8% to 18.5% of vehicle-treated patients. At 8-week follow-up, 39.0% to 41.6% of roflumilast-treated patients achieved PASI 75 vs 5.3% to 7.6% of patients in the vehicle group. Few stinging, burning, or application-site reactions were reported with roflumilast, along with rare instances of gastrointestinal AEs (<4%).9
Oral Therapy
Deucravacitinib—Tyrosine kinase 2 (TYK2) mediates the intracellular signaling of the TH17 and TH1 inflammatory cytokines IL-12/IL-23 and type I interferons, respectively, the former of which are critical in the development of psoriasis via the Janus kinase (JAK) signal transducer and activator of transcription pathway.10 Deucravacitinib is an oral selective TYK2 allosteric inhibitor that binds to the regulatory domain of the enzyme rather than the active catalytic domain, where other TYK2 and JAK1, JAK2, and JAK3 inhibitors bind.11 This unique inhibitory mechanism accounts for the high functional selectivity of deucravacitinib for TYK2 vs the closely related JAK1, JAK2, and JAK3 kinases, thus avoiding the pitfall of prior JAK inhibitors that were associated with major AEs, including an increased risk for serious infections, malignancies, and thrombosis.12 The selective suppression of the inflammatory TYK2 pathway has the potential to shift future therapeutic targets to a narrower range of receptors that may contribute to favorable benefit-risk profiles.
Two phase 3 trials (N=1686) compared the efficacy and safety of deucravacitinib vs placebo and apremilast in adults with moderate to severe plaque psoriasis.13,14 At week 16, 53.0% to 58.4% of deucravacitinib-treated patients achieved PASI 75 compared with 35.1% to 39.8% of apremilast-treated patients. At 16-week follow-up, static PGA response was observed in 49.5% to 53.6% of patients in the deucravacitinib group and 32.1% to 33.9% of the apremilast group. The most frequent AEs associated with deucravacitinib therapy were nasopharyngitis and upper respiratory tract infection, whereas headache, diarrhea, and nausea were more common with apremilast. Treatment with deucravacitinib caused no meaningful changes in laboratory parameters, which are known to change with JAK1, JAK2, and JAK3 inhibitors.13,14 A long-term extension study demonstrated that deucravacitinib had persistent efficacy and consistent safety for up to 2 years.15
Other TYK2 Inhibitors in the Pipeline
Novel oral allosteric TYK2 inhibitors—VTX958 and NDI-034858—and the competitive TYK2 inhibitor PF-06826647 are being developed. Theoretically, these new allosteric inhibitors possess unique structural properties to provide greater TYK2 suppression while bypassing JAK1, JAK2, and JAK3 pathways that may contribute to improved efficacy and safety profiles compared with other TYK2 inhibitors such as deucravacitinib. The results of a phase 1b trial (ClinicalTrials.gov Identifier NCT04999839) showed a dose-dependent reduction of disease severity associated with NDI-034858 treatment for patients with moderate to severe plaque psoriasis, albeit in only 26 patients. At week 4, PASI 50 was achieved in 13%, 57%, and 40% of patients in the 5-, 10-, and 30-mg groups, respectively, compared with 0% in the placebo group.16 In a phase 2 trial of 179 patients, 46.5% and 33.0% of patients treated with 400 and 200 mg of PF-06826647, respectively, achieved PASI 90 at week 16. Conversely, dose-dependent laboratory abnormalities were observed with PF-06826647, including anemia, neutropenia, and increases in creatine phosphokinase.17 At high concentrations, PF-06826647 may disrupt JAK signaling pathways involved in hematopoiesis and renal functions owing to its mode of action as a competitive inhibitor. Overall, these agents are much farther from market, and long-term studies with larger diverse patient cohorts are required to adequately assess the efficacy and safety data of these novel oral TYK2 inhibitors for patients with psoriasis.
EDP1815—EDP1815 is an oral preparation of a single strain of Prevotella histicola being developed for the treatment of inflammatory diseases, including psoriasis. EDP1815 interacts with host intestinal immune cells through the small intestinal axis (SINTAX) to suppress systemic inflammation across the TH1, TH2, and TH17 pathways. Therapy triggers broad immunomodulatory effects without causing systemic absorption, colonic colonization, or modification of the gut microbiome.18 In a phase 2 study (NCT04603027), the primary end point analysis, mean percentage change in PASI between treatment and placebo, demonstrated that at week 16, EDP1815 was superior to placebo with 80% to 90% probability across each cohort. At week 16, 25% to 32% of patients across the 3 cohorts treated with EDP1815 achieved PASI 50 compared with 12% of patients receiving placebo. Gastrointestinal AEs were comparable between treatment and placebo groups. These results suggest that SINTAX-targeted therapies may provide efficacious and safe immunomodulatory effects for patients with mild to moderate psoriasis, who often have limited treatment options. Although improvements may be mild, SINTAX-targeted therapies can be seen as a particularly attractive adjunctive treatment for patients with severe psoriasis taking other medications or as part of a treatment approach for a patient with milder psoriasis.
Biologics
Bimekizumab—Bimekizumab is a monoclonal IgG1 antibody that selectively inhibits IL-17A and IL-17F. Although IL-17A is a more potent cytokine, IL-17F may be more highly expressed in psoriatic lesional skin and independently contribute to the activation of proinflammatory signaling pathways implicated in the pathophysiology of psoriasis.19 Evidence suggests that dual inhibition of IL-17A and IL-17F may provide more complete suppression of inflammation and improved clinical responses than IL-17A inhibition alone.20
Prior bimekizumab phase 3 clinical studies have shown both rapid and durable clinical improvements in skin clearance compared with placebo.21 Three phase 3 trials—BE VIVID (N=567),22 BE SURE (N=478),23 and BE RADIANT (N=743)24—assessed the efficacy and safety of bimekizumab vs the IL-12/IL-23 inhibitor ustekinumab, the tumor necrosis factor inhibitor adalimumab, and the selective IL-17A inhibitor secukinumab, respectively. At week 4, significantly more patients treated with bimekizumab (71%–77%) achieved PASI 75 than patients treated with ustekinumab (15%; P<.0001), adalimumab (31.4%; P<.001), or secukinumab (47.3%; P<.001).22-24 After 16 weeks of treatment, PASI 90 was achieved by 85% to 86.2%, 50%, and 47.2% of patients treated with bimekizumab, ustekinumab, and adalimumab, respectively.22,23 At week 16, PASI 100 was observed in 59% to 61.7%, 21%, 23.9%, and 48.9% of patients treated with bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively. An IGA response (score of 0/1) at week 16 was achieved by 84% to 85.5%, 53%, 57.2%, and 78.6% of patients receiving bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively.22-24
The most common AEs in bimekizumab-treated patients were nasopharyngitis, oral candidiasis, and upper respiratory tract infection.22-24 The dual inhibition of IL-17A and IL-17F suppresses host defenses against Candida at the oral mucosa, increasing the incidence of bimekizumab-associated oral candidiasis.25 Despite the increased risk of Candida infections, these data suggest that inhibition of both IL-17A and IL-17F with bimekizumab may provide faster and greater clinical benefit for patients with moderate to severe plaque psoriasis than inhibition of IL-17A alone and other biologic therapies, as the PASI 100 clearance rates across the multiple comparator trials and the placebo-controlled pivotal trial are consistently the highest among any biologic for the treatment of psoriasis.
Spesolimab—The IL-36 pathway and IL-36 receptor genes have been linked to the pathogenesis of generalized pustular psoriasis.26 In a phase 2 trial, 19 of 35 patients (54%) receiving an intravenous dose of spesolimab, an IL-36 receptor inhibitor, had a generalized pustular psoriasis PGA pustulation subscore of 0 (no visible pustules) at the end of week 1 vs 6% of patients in the placebo group.27 A generalized pustular psoriasis PGA total score of 0 or 1 was observed in 43% (15/35) of spesolimab-treated patients compared with 11% (2/18) of patients in the placebo group. The most common AEs in patients treated with spesolimab were minor infections.27 Two open-label phase 3 trials—NCT05200247 and NCT05239039—are underway to determine the long-term efficacy and safety of spesolimab in patients with generalized pustular psoriasis.
Conclusion
Although we have seen a renaissance in psoriasis therapies with the advent of biologics in the last 20 years, recent evidence shows that more innovation is underway. Just in the last year, 2 new mechanisms for treating psoriasis topically without steroids have come to fruition, and there have not been truly novel mechanisms for treating psoriasis topically since approvals for tazarotene and calcipotriene in the 1990s. An entirely new class—TYK2 inhibitors—was developed and landed in psoriasis first, greatly improving the efficacy measures attained with oral medications in general. Finally, an orphan diagnosis got its due with an ambitiously designed study looking at a previously unheard-of 1-week end point, but it comes for one of the few true dermatologic emergencies we encounter, generalized pustular psoriasis. We are fortunate to have so many meaningful new treatments available to us, and it is invigorating to see that even more efficacious biologics and treatments are coming, along with novel concepts such as a treatment affecting the microbiome. Now, we just need to make sure that our patients have the access they deserve to the wide array of available treatments.
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J Am Acad Dermatol. 2021;84:1059-1067.
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137:2110-2119.
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229.
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806.
- Card GL, England BP, Suzuki Y, et al. Structural basis for the activity of drugs that inhibit phosphodiesterases. Structure. 2004;12:2233-2247.
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis (Auckl). 2021;11:21-29.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Dong C, Virtucio C, Zemska O, et al. Treatment of skin inflammation with benzoxaborole phosphodiesterase inhibitors: selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J Pharmacol Exp Ther. 2016;358:413-422.
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084.
- Nogueira M, Puig L, Torres T. JAK inhibitors for treatment of psoriasis: focus on selective tyk2 inhibitors. Drugs. 2020;80:341-352.
- Wrobleski ST, Moslin R, Lin S, et al. Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J Med Chem. 2019;62:8973-8995.
- Chimalakonda A, Burke J, Cheng L, et al. Selectivity profile of the tyrosine kinase 2 inhibitor deucravacitinib compared with janus kinase 1/2/3 inhibitors. Dermatol Ther (Heidelb). 2021;11:1763-1776.
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program for Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51.
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39.
- Warren RB, Sofen H, Imafuku S, et al. POS1046 deucravacitinib long-term efficacy and safety in plaque psoriasis: 2-year results from the phase 3 POETYK PSO program [abstract]. Ann Rheum Dis. 2022;81(suppl 1):841.
- McElwee JJ, Garcet S, Li X, et al. Analysis of histologic, molecular and clinical improvement in moderate-to-severe psoriasis: results from a Phase 1b trial of the novel allosteric TYK2 inhibitor NDI-034858. Poster presented at: American Academy of Dermatology Annual Meeting; March 25, 2022; Boston, MA.
- Tehlirian C, Singh RSP, Pradhan V, et al. Oral tyrosine kinase 2 inhibitor PF-06826647 demonstrates efficacy and an acceptable safety profile in participants with moderate-to-severe plaque psoriasis in a phase 2b, randomized, double-blind, placebo-controlled study. J Am Acad Dermatol. 2022;87:333-342.
- Hilliard-Barth K, Cormack T, Ramani K, et al. Immune mechanisms of the systemic effects of EDP1815: an orally delivered, gut-restricted microbial drug candidate for the treatment of inflammatory diseases. Poster presented at: Society for Mucosal Immunology Virtual Congress; July 20-22, 2021, Cambridge, MA.
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532.
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894.
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475-486.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397:487-498.
- Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 2021;385:130-141.
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152.
- Blauvelt A, Lebwohl MG, Bissonnette R. IL-23/IL-17A dysfunction phenotypes inform possible clinical effects from anti-IL-17A therapies. J Invest Dermatol. 2015;135:1946-1953.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440.
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J Am Acad Dermatol. 2021;84:1059-1067.
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137:2110-2119.
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229.
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806.
- Card GL, England BP, Suzuki Y, et al. Structural basis for the activity of drugs that inhibit phosphodiesterases. Structure. 2004;12:2233-2247.
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis (Auckl). 2021;11:21-29.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Dong C, Virtucio C, Zemska O, et al. Treatment of skin inflammation with benzoxaborole phosphodiesterase inhibitors: selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J Pharmacol Exp Ther. 2016;358:413-422.
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084.
- Nogueira M, Puig L, Torres T. JAK inhibitors for treatment of psoriasis: focus on selective tyk2 inhibitors. Drugs. 2020;80:341-352.
- Wrobleski ST, Moslin R, Lin S, et al. Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J Med Chem. 2019;62:8973-8995.
- Chimalakonda A, Burke J, Cheng L, et al. Selectivity profile of the tyrosine kinase 2 inhibitor deucravacitinib compared with janus kinase 1/2/3 inhibitors. Dermatol Ther (Heidelb). 2021;11:1763-1776.
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program for Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51.
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39.
- Warren RB, Sofen H, Imafuku S, et al. POS1046 deucravacitinib long-term efficacy and safety in plaque psoriasis: 2-year results from the phase 3 POETYK PSO program [abstract]. Ann Rheum Dis. 2022;81(suppl 1):841.
- McElwee JJ, Garcet S, Li X, et al. Analysis of histologic, molecular and clinical improvement in moderate-to-severe psoriasis: results from a Phase 1b trial of the novel allosteric TYK2 inhibitor NDI-034858. Poster presented at: American Academy of Dermatology Annual Meeting; March 25, 2022; Boston, MA.
- Tehlirian C, Singh RSP, Pradhan V, et al. Oral tyrosine kinase 2 inhibitor PF-06826647 demonstrates efficacy and an acceptable safety profile in participants with moderate-to-severe plaque psoriasis in a phase 2b, randomized, double-blind, placebo-controlled study. J Am Acad Dermatol. 2022;87:333-342.
- Hilliard-Barth K, Cormack T, Ramani K, et al. Immune mechanisms of the systemic effects of EDP1815: an orally delivered, gut-restricted microbial drug candidate for the treatment of inflammatory diseases. Poster presented at: Society for Mucosal Immunology Virtual Congress; July 20-22, 2021, Cambridge, MA.
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532.
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894.
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475-486.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397:487-498.
- Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 2021;385:130-141.
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152.
- Blauvelt A, Lebwohl MG, Bissonnette R. IL-23/IL-17A dysfunction phenotypes inform possible clinical effects from anti-IL-17A therapies. J Invest Dermatol. 2015;135:1946-1953.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440.
PRACTICE POINTS
- Roflumilast, a phosphodiesterase 4 inhibitor, and tapinarof, an aryl hydrocarbon receptor–modulating agent, are 2 novel nonsteroidal topical treatments safe for regular long-term use on all affected areas of the skin in adult patients with plaque psoriasis.
- Deucravacitinib is an oral selective tyrosine kinase 2 allosteric inhibitor that has demonstrated a favorable safety profile and greater levels of efficacy than other available oral medications for plaque psoriasis.
- The dual inhibition of IL-17A and IL-17F with bimekizumab provides faster responses and greater clinical benefits for patients with moderate to severe plaque psoriasis than inhibition of IL-17A alone, achieving higher levels of efficacy than has been reported with any other biologic therapy.
- Spesolimab, an IL-36 receptor inhibitor, is an effective, US Food and Drug Administration–approved treatment for patients with generalized pustular psoriasis.
Insights From the 2020-2021 Dermatology Residency Match
To the Editor:
Data from the program director survey of the National Resident Matching Program offer key insights into the 2021 dermatology application process.1,2 Examination of data from the 2020 (N=12) and 2021 (N=17) program director survey regarding interviewing applicants revealed that specialty-specific letters of recommendation (LORs), personal prior knowledge of an applicant, and personal statement increased in importance by 17%, 7.4%, and 17%, respectively, whereas away rotations within the department decreased in importance by 44.9% (Table).1,2 Interestingly, for ranking applicants, programs decreased their emphasis on specialty-specific LORs by 25.8% and away rotations within the department by 22.7% and increased emphasis on personal statements by 14.7% and personal prior knowledge of an applicant by 0.8% from 2020 to 2021 (Table).1,2 These findings align with the prior recommendation to limit away rotations; data are contradictory—when comparing factors for interviewing as compared to ranking applicants—for specialty-specific LORs.
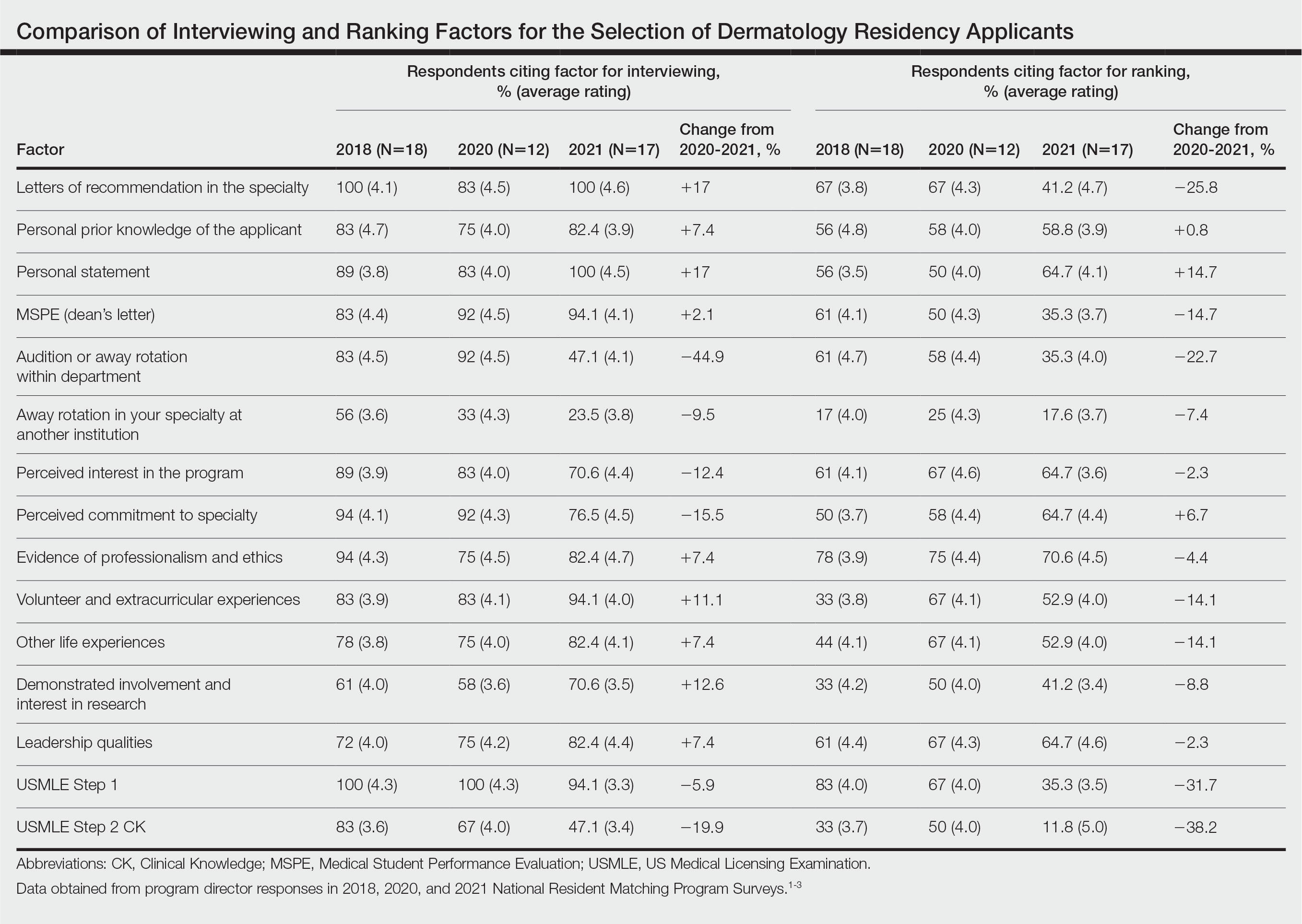
We further compared data from the otolaryngology cycle, which implemented preference signaling by which an applicant can signal their interest in a particular residency program in the 2021 Match, to data from dermatology with no preference signaling. A 90% probability of matching is estimated to require approximately 8 or 9 interviews for dermatology or 12 interviews for otolaryngology for MD senior students in 2020.4 In prior dermatology application cycles, the most highly qualified candidates constituted 7% to 21% of all applicants but were estimated to receive half of all interviews, causing a maldistribution of interviews.5,6
For the 2021 otolaryngology match, the Society of University Otolaryngologists implemented a novel preference signaling system that allowed candidates to show interest in programs by sending 5 preferences, or tokens.7 Recent data reports from the otolaryngology cycle demonstrated at least a 2-fold increase in the rate of receiving an interview invitation for signaled programs compared to the closest nonsignaled program if applicants were provided an additional token.7 Regarding overall applicant competitiveness (ie, dividing participants into quartiles based on their competitiveness), the highest increase in the overall rate of interview invitations (3.5 [total invitations/total applications]) was demonstrated for fourth-quartile (ie, “lowest quartile”) applicants compared with the increase in the overall rate of interview invitations seen in other quartiles (first quartile, an increase of 2.3; second quartile, an increase of 2.6; and third quartile, an increase of 2.4).7 We look forward to seeing the impact of preference signaling on the results of the 2022 dermatology cycle.
Despite changes in the interviewing process to accommodate COVID-19 pandemic safety recommendations, the overall dermatology postgraduate year (PGY) 2 fill rate remained unchanged from 2018 (98.6%) to 2021 (98.7%). Zero PGY-1 positions and 5 PGY-2 positions were unfilled in the 2021 Main Residency Match compared to 1 unfilled PGY-1 position and 4 unfilled PGY-2 positions in 2018.8 The coordinated interview invitation release, holistic review of applications, increased number of rankings, and virtual interviews might have helped offset potential obstacles imparted by inability to complete away rotations, inability to obtain LORs, and conducting interviews virtually.5
A limitation of our analysis is the low response rate of program directors to National Resident Matching Program surveys.
These strategies—holistic application review and coordinated interview release—may be considered in future cycles given their convenience and negligible impact on the dermatology match rate. For example, virtual interviews relieve the financial and time burdens of in-person interviews—approximately $10,000 for each US senior applicant—thus potentially allowing for a more equitable matching process.3 Inversely, in-person interviews allow participants to effectively network and form more meaningful connections while obtaining a better understanding of facilities and surrounding locales. As such, the medical community should continue to come to a consensus on the optimal format to host interviews.
- Results of the 2021 NRMP Program Director Survey. National Resident Matching Program. August 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/11/2021-PD-Survey-Report-for-WWW.pdf
- Results of the 2020 NRMP Program Director Survey. National Resident Matching Program. August 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2022/01/2020-PD-Survey.pdf
- Rojek NW, Shinkai K, Fett N. Dermatology faculty and residents’ perspectives on the dermatology residency application process: a nationwide survey. J Am Acad Dermatol. 2018;79:157-159. doi:10.1016/j.jaad.2018.01.00
- Charting Outcomes in the Match: Senior Students of U.S. MD Medical Schools. National Resident Matching Program. July 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/Charting-Outcomes-in-the-Match-2020_MD-Senior_final.pdf
- Thatiparthi A, Martin A, Liu J, et al. Preliminary outcomes of 2020-2021 dermatology residency application cycle and adverse effects of COVID-19. J Am Acad Dermatol. 2021;84:e263-e264. doi:10.1016/j.jaad.2021.03.034
- Hammoud MM, Standiford T, Carmody JB. Potential implications of COVID-19 for the 2020-2021 residency application cycle. JAMA. 2020;324:29-30. doi:10.1001/jama.2020.8911
- Interview offer rate with/without ENTSignaling. Society of University Otolaryngologists. Updated July 19, 2022. Accessed December 12, 2022. https://opdo-hns.org/mpage/signaling-updates
- Results and Data: 2021 Main Residency Match. National Resident Matching Program. May 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/MRM-Results_and-Data_2021.pdf
To the Editor:
Data from the program director survey of the National Resident Matching Program offer key insights into the 2021 dermatology application process.1,2 Examination of data from the 2020 (N=12) and 2021 (N=17) program director survey regarding interviewing applicants revealed that specialty-specific letters of recommendation (LORs), personal prior knowledge of an applicant, and personal statement increased in importance by 17%, 7.4%, and 17%, respectively, whereas away rotations within the department decreased in importance by 44.9% (Table).1,2 Interestingly, for ranking applicants, programs decreased their emphasis on specialty-specific LORs by 25.8% and away rotations within the department by 22.7% and increased emphasis on personal statements by 14.7% and personal prior knowledge of an applicant by 0.8% from 2020 to 2021 (Table).1,2 These findings align with the prior recommendation to limit away rotations; data are contradictory—when comparing factors for interviewing as compared to ranking applicants—for specialty-specific LORs.

We further compared data from the otolaryngology cycle, which implemented preference signaling by which an applicant can signal their interest in a particular residency program in the 2021 Match, to data from dermatology with no preference signaling. A 90% probability of matching is estimated to require approximately 8 or 9 interviews for dermatology or 12 interviews for otolaryngology for MD senior students in 2020.4 In prior dermatology application cycles, the most highly qualified candidates constituted 7% to 21% of all applicants but were estimated to receive half of all interviews, causing a maldistribution of interviews.5,6
For the 2021 otolaryngology match, the Society of University Otolaryngologists implemented a novel preference signaling system that allowed candidates to show interest in programs by sending 5 preferences, or tokens.7 Recent data reports from the otolaryngology cycle demonstrated at least a 2-fold increase in the rate of receiving an interview invitation for signaled programs compared to the closest nonsignaled program if applicants were provided an additional token.7 Regarding overall applicant competitiveness (ie, dividing participants into quartiles based on their competitiveness), the highest increase in the overall rate of interview invitations (3.5 [total invitations/total applications]) was demonstrated for fourth-quartile (ie, “lowest quartile”) applicants compared with the increase in the overall rate of interview invitations seen in other quartiles (first quartile, an increase of 2.3; second quartile, an increase of 2.6; and third quartile, an increase of 2.4).7 We look forward to seeing the impact of preference signaling on the results of the 2022 dermatology cycle.
Despite changes in the interviewing process to accommodate COVID-19 pandemic safety recommendations, the overall dermatology postgraduate year (PGY) 2 fill rate remained unchanged from 2018 (98.6%) to 2021 (98.7%). Zero PGY-1 positions and 5 PGY-2 positions were unfilled in the 2021 Main Residency Match compared to 1 unfilled PGY-1 position and 4 unfilled PGY-2 positions in 2018.8 The coordinated interview invitation release, holistic review of applications, increased number of rankings, and virtual interviews might have helped offset potential obstacles imparted by inability to complete away rotations, inability to obtain LORs, and conducting interviews virtually.5
A limitation of our analysis is the low response rate of program directors to National Resident Matching Program surveys.
These strategies—holistic application review and coordinated interview release—may be considered in future cycles given their convenience and negligible impact on the dermatology match rate. For example, virtual interviews relieve the financial and time burdens of in-person interviews—approximately $10,000 for each US senior applicant—thus potentially allowing for a more equitable matching process.3 Inversely, in-person interviews allow participants to effectively network and form more meaningful connections while obtaining a better understanding of facilities and surrounding locales. As such, the medical community should continue to come to a consensus on the optimal format to host interviews.
To the Editor:
Data from the program director survey of the National Resident Matching Program offer key insights into the 2021 dermatology application process.1,2 Examination of data from the 2020 (N=12) and 2021 (N=17) program director survey regarding interviewing applicants revealed that specialty-specific letters of recommendation (LORs), personal prior knowledge of an applicant, and personal statement increased in importance by 17%, 7.4%, and 17%, respectively, whereas away rotations within the department decreased in importance by 44.9% (Table).1,2 Interestingly, for ranking applicants, programs decreased their emphasis on specialty-specific LORs by 25.8% and away rotations within the department by 22.7% and increased emphasis on personal statements by 14.7% and personal prior knowledge of an applicant by 0.8% from 2020 to 2021 (Table).1,2 These findings align with the prior recommendation to limit away rotations; data are contradictory—when comparing factors for interviewing as compared to ranking applicants—for specialty-specific LORs.

We further compared data from the otolaryngology cycle, which implemented preference signaling by which an applicant can signal their interest in a particular residency program in the 2021 Match, to data from dermatology with no preference signaling. A 90% probability of matching is estimated to require approximately 8 or 9 interviews for dermatology or 12 interviews for otolaryngology for MD senior students in 2020.4 In prior dermatology application cycles, the most highly qualified candidates constituted 7% to 21% of all applicants but were estimated to receive half of all interviews, causing a maldistribution of interviews.5,6
For the 2021 otolaryngology match, the Society of University Otolaryngologists implemented a novel preference signaling system that allowed candidates to show interest in programs by sending 5 preferences, or tokens.7 Recent data reports from the otolaryngology cycle demonstrated at least a 2-fold increase in the rate of receiving an interview invitation for signaled programs compared to the closest nonsignaled program if applicants were provided an additional token.7 Regarding overall applicant competitiveness (ie, dividing participants into quartiles based on their competitiveness), the highest increase in the overall rate of interview invitations (3.5 [total invitations/total applications]) was demonstrated for fourth-quartile (ie, “lowest quartile”) applicants compared with the increase in the overall rate of interview invitations seen in other quartiles (first quartile, an increase of 2.3; second quartile, an increase of 2.6; and third quartile, an increase of 2.4).7 We look forward to seeing the impact of preference signaling on the results of the 2022 dermatology cycle.
Despite changes in the interviewing process to accommodate COVID-19 pandemic safety recommendations, the overall dermatology postgraduate year (PGY) 2 fill rate remained unchanged from 2018 (98.6%) to 2021 (98.7%). Zero PGY-1 positions and 5 PGY-2 positions were unfilled in the 2021 Main Residency Match compared to 1 unfilled PGY-1 position and 4 unfilled PGY-2 positions in 2018.8 The coordinated interview invitation release, holistic review of applications, increased number of rankings, and virtual interviews might have helped offset potential obstacles imparted by inability to complete away rotations, inability to obtain LORs, and conducting interviews virtually.5
A limitation of our analysis is the low response rate of program directors to National Resident Matching Program surveys.
These strategies—holistic application review and coordinated interview release—may be considered in future cycles given their convenience and negligible impact on the dermatology match rate. For example, virtual interviews relieve the financial and time burdens of in-person interviews—approximately $10,000 for each US senior applicant—thus potentially allowing for a more equitable matching process.3 Inversely, in-person interviews allow participants to effectively network and form more meaningful connections while obtaining a better understanding of facilities and surrounding locales. As such, the medical community should continue to come to a consensus on the optimal format to host interviews.
- Results of the 2021 NRMP Program Director Survey. National Resident Matching Program. August 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/11/2021-PD-Survey-Report-for-WWW.pdf
- Results of the 2020 NRMP Program Director Survey. National Resident Matching Program. August 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2022/01/2020-PD-Survey.pdf
- Rojek NW, Shinkai K, Fett N. Dermatology faculty and residents’ perspectives on the dermatology residency application process: a nationwide survey. J Am Acad Dermatol. 2018;79:157-159. doi:10.1016/j.jaad.2018.01.00
- Charting Outcomes in the Match: Senior Students of U.S. MD Medical Schools. National Resident Matching Program. July 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/Charting-Outcomes-in-the-Match-2020_MD-Senior_final.pdf
- Thatiparthi A, Martin A, Liu J, et al. Preliminary outcomes of 2020-2021 dermatology residency application cycle and adverse effects of COVID-19. J Am Acad Dermatol. 2021;84:e263-e264. doi:10.1016/j.jaad.2021.03.034
- Hammoud MM, Standiford T, Carmody JB. Potential implications of COVID-19 for the 2020-2021 residency application cycle. JAMA. 2020;324:29-30. doi:10.1001/jama.2020.8911
- Interview offer rate with/without ENTSignaling. Society of University Otolaryngologists. Updated July 19, 2022. Accessed December 12, 2022. https://opdo-hns.org/mpage/signaling-updates
- Results and Data: 2021 Main Residency Match. National Resident Matching Program. May 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/MRM-Results_and-Data_2021.pdf
- Results of the 2021 NRMP Program Director Survey. National Resident Matching Program. August 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/11/2021-PD-Survey-Report-for-WWW.pdf
- Results of the 2020 NRMP Program Director Survey. National Resident Matching Program. August 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2022/01/2020-PD-Survey.pdf
- Rojek NW, Shinkai K, Fett N. Dermatology faculty and residents’ perspectives on the dermatology residency application process: a nationwide survey. J Am Acad Dermatol. 2018;79:157-159. doi:10.1016/j.jaad.2018.01.00
- Charting Outcomes in the Match: Senior Students of U.S. MD Medical Schools. National Resident Matching Program. July 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/Charting-Outcomes-in-the-Match-2020_MD-Senior_final.pdf
- Thatiparthi A, Martin A, Liu J, et al. Preliminary outcomes of 2020-2021 dermatology residency application cycle and adverse effects of COVID-19. J Am Acad Dermatol. 2021;84:e263-e264. doi:10.1016/j.jaad.2021.03.034
- Hammoud MM, Standiford T, Carmody JB. Potential implications of COVID-19 for the 2020-2021 residency application cycle. JAMA. 2020;324:29-30. doi:10.1001/jama.2020.8911
- Interview offer rate with/without ENTSignaling. Society of University Otolaryngologists. Updated July 19, 2022. Accessed December 12, 2022. https://opdo-hns.org/mpage/signaling-updates
- Results and Data: 2021 Main Residency Match. National Resident Matching Program. May 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/MRM-Results_and-Data_2021.pdf
PRACTICE POINTS
- Although there have been numerous changes to the dermatology interview process due to the COVID-19 pandemic, the overall fill rate for postgraduate year 2 positions remained unchanged from 2018 (prepandemic) to 2021 (postpandemic).
- Strategies to accommodate new safety recommendations for interviews may reduce the financial burden (approximately $10,000 for each senior applicant) and time constraints on applicants. These strategies should be considered for implementation in future cycles.