User login
Impact of the COVID-19 Pandemic on Care for Patients With Skin Cancer
To the Editor:
The most common malignancy in the United States is skin cancer, with melanoma accounting for the majority of skin cancer deaths.1 Despite the lack of established guidelines for routine total-body skin examinations, many patients regularly visit their dermatologist for assessment of pigmented skin lesions.2 During the COVID-19 pandemic, many patients were unable to attend in-person dermatology visits, which resulted in many high-risk individuals not receiving care or alternatively seeking virtual care for cutaneous lesions.3 There has been a lack of research in the United States exploring the utilization of teledermatology during the pandemic and its overall impact on the care of patients with a history of skin cancer. We explored the impact of the COVID-19 pandemic on care for patients with skin cancer in a large US population.

Using anonymous survey data from the 2020-2021 National Health Interview Survey,4 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with a self-reported history of skin cancer—melanoma, nonmelanoma skin cancer, or unknown skin cancer. The 3 outcome variables included having a virtual medical appointment in the past 12 months (yes/no), delaying medical care due to the COVID-19 pandemic (yes/no), and not receiving care due to the COVID-19 pandemic (yes/no). Multivariable logistic regression models evaluating the relationship between a history of skin cancer and access to care were constructed using Stata/MP 17.0 (StataCorp LLC). We controlled for patient age; education; race/ethnicity; received public assistance or welfare payments; sex; region; US citizenship status; health insurance status; comorbidities including history of hypertension, diabetes, and hypercholesterolemia; and birthplace in the United States in the logistic regression models.
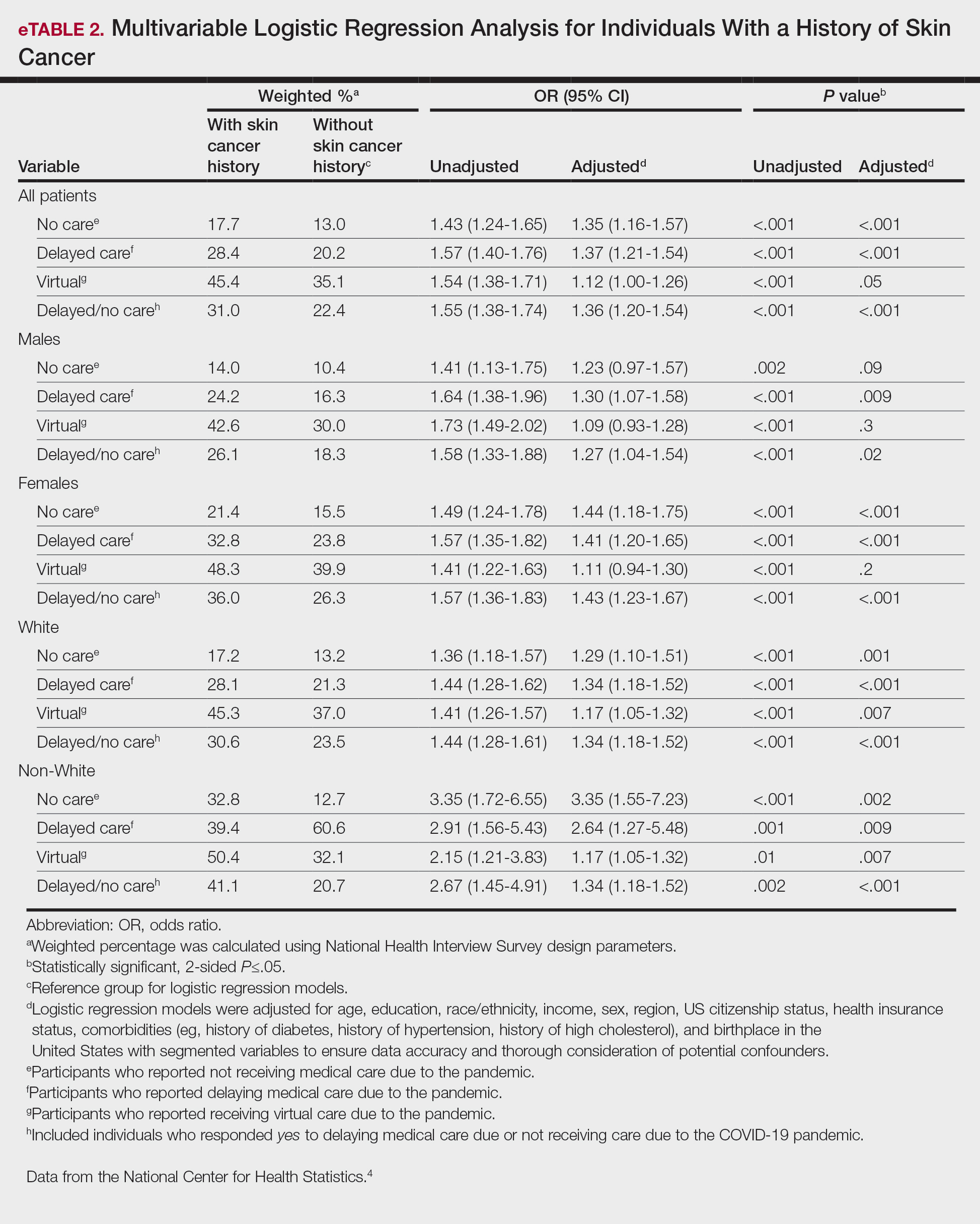
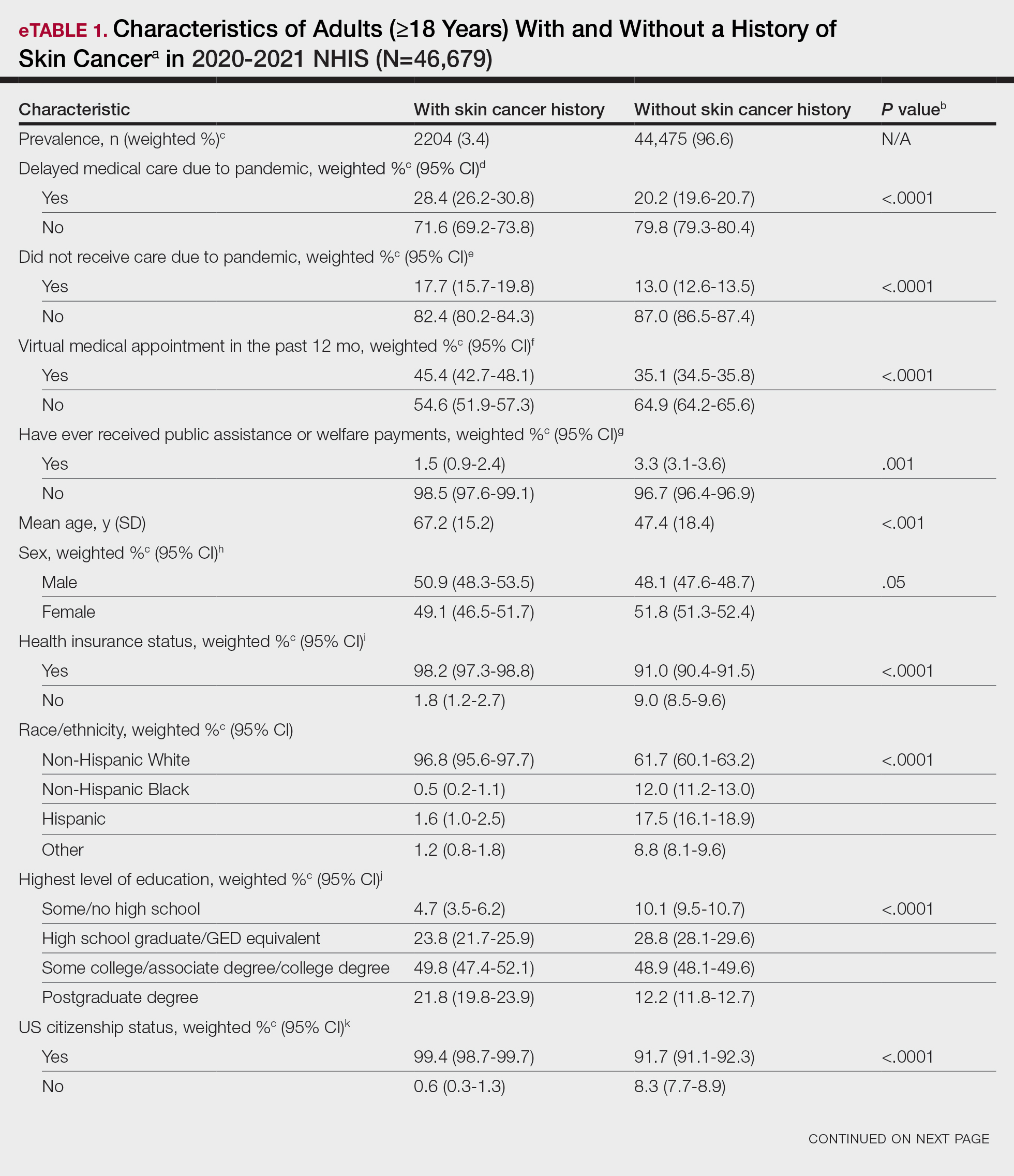
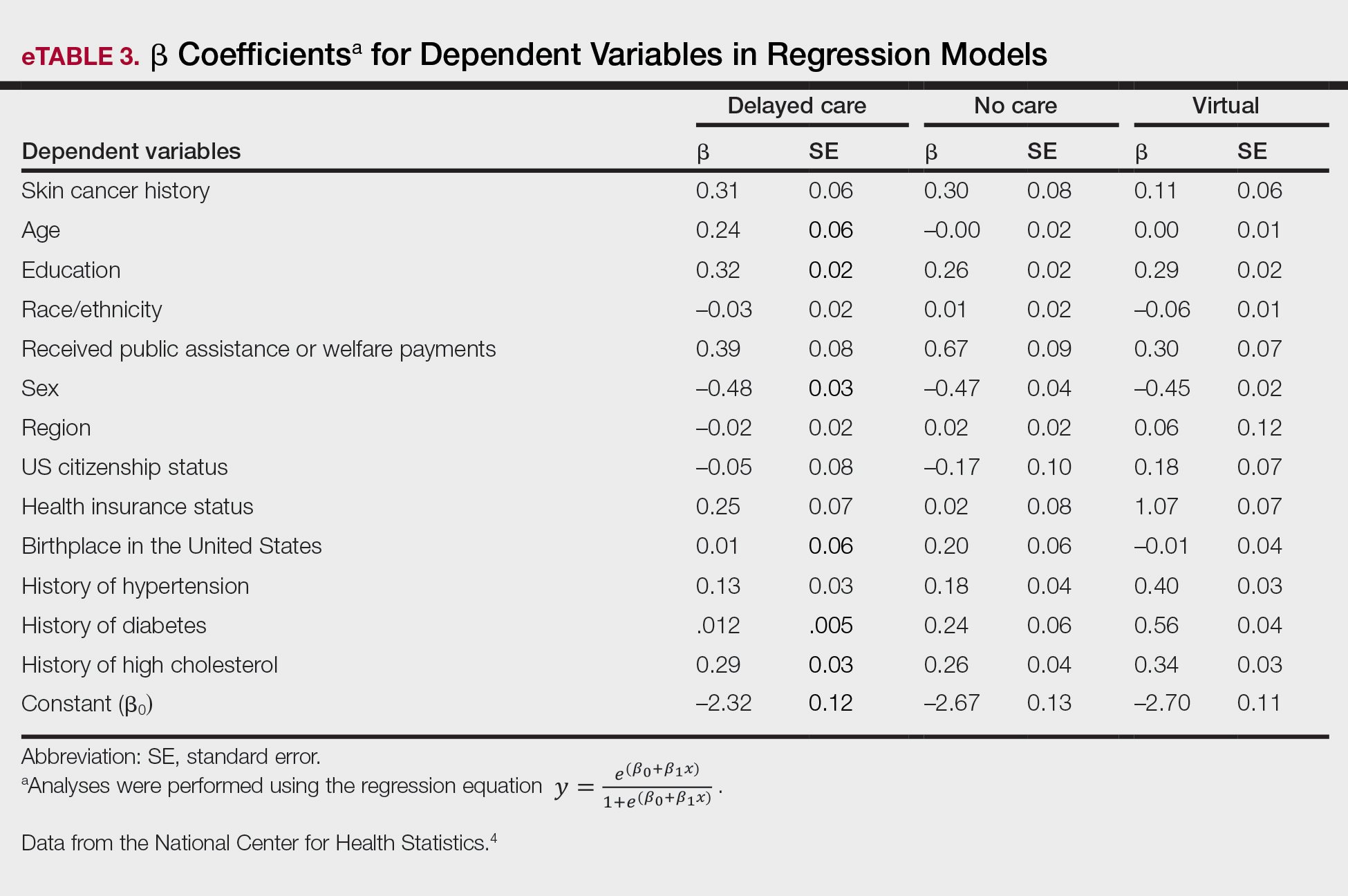
Our analysis included 46,679 patients aged 18 years or older, of whom 3.4% (weighted)(n=2204) reported a history of skin cancer (eTable 1). The weighted percentage was calculated using National Health Interview Survey design parameters (accounting for the multistage sampling design) to represent the general US population. Compared with those with no history of skin cancer, patients with a history of skin cancer were significantly more likely to delay medical care (adjusted odds ratio [AOR], 1.37; 95% CI, 1.21-1.54; P<.001) or not receive care (AOR, 1.35; 95% CI, 1.16-1.57; P<.001) due to the pandemic and were more likely to have had a virtual medical visit in the past 12 months (AOR, 1.12; 95% CI, 1.00-1.26; P=.05). Additionally, subgroup analysis revealed that females were more likely than males to forego medical care (eTable 2). β Coefficients for independent and dependent variables were further analyzed using logistic regression (eTable 3).
After adjusting for various potential confounders including comorbidities, our results revealed that patients with a history of skin cancer reported that they were less likely to receive in-person medical care due to the COVID-19 pandemic, as high-risk individuals with a history of skin cancer may have stopped receiving total-body skin examinations and dermatology care during the pandemic. Our findings showed that patients with a history of skin cancer were more likely than those without skin cancer to delay or forego care due to the pandemic, which may contribute to a higher incidence of advanced-stage melanomas postpandemic. Trepanowski et al5 reported an increased incidence of patients presenting with more advanced melanomas during the pandemic. Telemedicine was more commonly utilized by patients with a history of skin cancer during the pandemic.
In the future, virtual care may help limit advanced stages of skin cancer by serving as a viable alternative to in-person care.6 It has been reported that telemedicine can serve as a useful triage service reducing patient wait times.7 Teledermatology should not replace in-person care, as there is no evidence of the diagnostic accuracy of this service and many patients still will need to be seen in-person for confirmation of their diagnosis and potential biopsy. Further studies are needed to assess for missed skin cancer diagnoses due to the utilization of telemedicine.
Limitations of this study included a self-reported history of skin cancer, β coefficients that may suggest a high degree of collinearity, and lack of specific survey questions regarding dermatologic care during the COVID-19 pandemic. Further long-term studies exploring the clinical applicability and diagnostic accuracy of virtual medicine visits for cutaneous malignancies are vital, as teledermatology may play an essential role in curbing rising skin cancer rates even beyond the pandemic.
- Guy GP Jr, Thomas CC, Thompson T, et al. Vital signs: melanoma incidence and mortality trends and projections—United States, 1982-2030. MMWR Morb Mortal Wkly Rep. 2015;64:591-596.
- Whiteman DC, Olsen CM, MacGregor S, et al; QSkin Study. The effect of screening on melanoma incidence and biopsy rates. Br J Dermatol. 2022;187:515-522. doi:10.1111/bjd.21649
- Jobbágy A, Kiss N, Meznerics FA, et al. Emergency use and efficacy of an asynchronous teledermatology system as a novel tool for early diagnosis of skin cancer during the first wave of COVID-19 pandemic. Int J Environ Res Public Health. 2022;19:2699. doi:10.3390/ijerph19052699
- National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed April 19, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
- Trepanowski N, Chang MS, Zhou G, et al. Delays in melanoma presentation during the COVID-19 pandemic: a nationwide multi-institutional cohort study. J Am Acad Dermatol. 2022;87:1217-1219. doi:10.1016/j.jaad.2022.06.031
- Chiru MR, Hindocha S, Burova E, et al. Management of the two-week wait pathway for skin cancer patients, before and during the pandemic: is virtual consultation an option? J Pers Med. 2022;12:1258. doi:10.3390/jpm12081258
- Finnane A Dallest K Janda M et al. Teledermatology for the diagnosis and management of skin cancer: a systematic review. JAMA Dermatol. 2017;153:319-327. doi:10.1001/jamadermatol.2016.4361
To the Editor:
The most common malignancy in the United States is skin cancer, with melanoma accounting for the majority of skin cancer deaths.1 Despite the lack of established guidelines for routine total-body skin examinations, many patients regularly visit their dermatologist for assessment of pigmented skin lesions.2 During the COVID-19 pandemic, many patients were unable to attend in-person dermatology visits, which resulted in many high-risk individuals not receiving care or alternatively seeking virtual care for cutaneous lesions.3 There has been a lack of research in the United States exploring the utilization of teledermatology during the pandemic and its overall impact on the care of patients with a history of skin cancer. We explored the impact of the COVID-19 pandemic on care for patients with skin cancer in a large US population.


Using anonymous survey data from the 2020-2021 National Health Interview Survey,4 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with a self-reported history of skin cancer—melanoma, nonmelanoma skin cancer, or unknown skin cancer. The 3 outcome variables included having a virtual medical appointment in the past 12 months (yes/no), delaying medical care due to the COVID-19 pandemic (yes/no), and not receiving care due to the COVID-19 pandemic (yes/no). Multivariable logistic regression models evaluating the relationship between a history of skin cancer and access to care were constructed using Stata/MP 17.0 (StataCorp LLC). We controlled for patient age; education; race/ethnicity; received public assistance or welfare payments; sex; region; US citizenship status; health insurance status; comorbidities including history of hypertension, diabetes, and hypercholesterolemia; and birthplace in the United States in the logistic regression models.

Our analysis included 46,679 patients aged 18 years or older, of whom 3.4% (weighted)(n=2204) reported a history of skin cancer (eTable 1). The weighted percentage was calculated using National Health Interview Survey design parameters (accounting for the multistage sampling design) to represent the general US population. Compared with those with no history of skin cancer, patients with a history of skin cancer were significantly more likely to delay medical care (adjusted odds ratio [AOR], 1.37; 95% CI, 1.21-1.54; P<.001) or not receive care (AOR, 1.35; 95% CI, 1.16-1.57; P<.001) due to the pandemic and were more likely to have had a virtual medical visit in the past 12 months (AOR, 1.12; 95% CI, 1.00-1.26; P=.05). Additionally, subgroup analysis revealed that females were more likely than males to forego medical care (eTable 2). β Coefficients for independent and dependent variables were further analyzed using logistic regression (eTable 3).

After adjusting for various potential confounders including comorbidities, our results revealed that patients with a history of skin cancer reported that they were less likely to receive in-person medical care due to the COVID-19 pandemic, as high-risk individuals with a history of skin cancer may have stopped receiving total-body skin examinations and dermatology care during the pandemic. Our findings showed that patients with a history of skin cancer were more likely than those without skin cancer to delay or forego care due to the pandemic, which may contribute to a higher incidence of advanced-stage melanomas postpandemic. Trepanowski et al5 reported an increased incidence of patients presenting with more advanced melanomas during the pandemic. Telemedicine was more commonly utilized by patients with a history of skin cancer during the pandemic.
In the future, virtual care may help limit advanced stages of skin cancer by serving as a viable alternative to in-person care.6 It has been reported that telemedicine can serve as a useful triage service reducing patient wait times.7 Teledermatology should not replace in-person care, as there is no evidence of the diagnostic accuracy of this service and many patients still will need to be seen in-person for confirmation of their diagnosis and potential biopsy. Further studies are needed to assess for missed skin cancer diagnoses due to the utilization of telemedicine.
Limitations of this study included a self-reported history of skin cancer, β coefficients that may suggest a high degree of collinearity, and lack of specific survey questions regarding dermatologic care during the COVID-19 pandemic. Further long-term studies exploring the clinical applicability and diagnostic accuracy of virtual medicine visits for cutaneous malignancies are vital, as teledermatology may play an essential role in curbing rising skin cancer rates even beyond the pandemic.
To the Editor:
The most common malignancy in the United States is skin cancer, with melanoma accounting for the majority of skin cancer deaths.1 Despite the lack of established guidelines for routine total-body skin examinations, many patients regularly visit their dermatologist for assessment of pigmented skin lesions.2 During the COVID-19 pandemic, many patients were unable to attend in-person dermatology visits, which resulted in many high-risk individuals not receiving care or alternatively seeking virtual care for cutaneous lesions.3 There has been a lack of research in the United States exploring the utilization of teledermatology during the pandemic and its overall impact on the care of patients with a history of skin cancer. We explored the impact of the COVID-19 pandemic on care for patients with skin cancer in a large US population.


Using anonymous survey data from the 2020-2021 National Health Interview Survey,4 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with a self-reported history of skin cancer—melanoma, nonmelanoma skin cancer, or unknown skin cancer. The 3 outcome variables included having a virtual medical appointment in the past 12 months (yes/no), delaying medical care due to the COVID-19 pandemic (yes/no), and not receiving care due to the COVID-19 pandemic (yes/no). Multivariable logistic regression models evaluating the relationship between a history of skin cancer and access to care were constructed using Stata/MP 17.0 (StataCorp LLC). We controlled for patient age; education; race/ethnicity; received public assistance or welfare payments; sex; region; US citizenship status; health insurance status; comorbidities including history of hypertension, diabetes, and hypercholesterolemia; and birthplace in the United States in the logistic regression models.

Our analysis included 46,679 patients aged 18 years or older, of whom 3.4% (weighted)(n=2204) reported a history of skin cancer (eTable 1). The weighted percentage was calculated using National Health Interview Survey design parameters (accounting for the multistage sampling design) to represent the general US population. Compared with those with no history of skin cancer, patients with a history of skin cancer were significantly more likely to delay medical care (adjusted odds ratio [AOR], 1.37; 95% CI, 1.21-1.54; P<.001) or not receive care (AOR, 1.35; 95% CI, 1.16-1.57; P<.001) due to the pandemic and were more likely to have had a virtual medical visit in the past 12 months (AOR, 1.12; 95% CI, 1.00-1.26; P=.05). Additionally, subgroup analysis revealed that females were more likely than males to forego medical care (eTable 2). β Coefficients for independent and dependent variables were further analyzed using logistic regression (eTable 3).

After adjusting for various potential confounders including comorbidities, our results revealed that patients with a history of skin cancer reported that they were less likely to receive in-person medical care due to the COVID-19 pandemic, as high-risk individuals with a history of skin cancer may have stopped receiving total-body skin examinations and dermatology care during the pandemic. Our findings showed that patients with a history of skin cancer were more likely than those without skin cancer to delay or forego care due to the pandemic, which may contribute to a higher incidence of advanced-stage melanomas postpandemic. Trepanowski et al5 reported an increased incidence of patients presenting with more advanced melanomas during the pandemic. Telemedicine was more commonly utilized by patients with a history of skin cancer during the pandemic.
In the future, virtual care may help limit advanced stages of skin cancer by serving as a viable alternative to in-person care.6 It has been reported that telemedicine can serve as a useful triage service reducing patient wait times.7 Teledermatology should not replace in-person care, as there is no evidence of the diagnostic accuracy of this service and many patients still will need to be seen in-person for confirmation of their diagnosis and potential biopsy. Further studies are needed to assess for missed skin cancer diagnoses due to the utilization of telemedicine.
Limitations of this study included a self-reported history of skin cancer, β coefficients that may suggest a high degree of collinearity, and lack of specific survey questions regarding dermatologic care during the COVID-19 pandemic. Further long-term studies exploring the clinical applicability and diagnostic accuracy of virtual medicine visits for cutaneous malignancies are vital, as teledermatology may play an essential role in curbing rising skin cancer rates even beyond the pandemic.
- Guy GP Jr, Thomas CC, Thompson T, et al. Vital signs: melanoma incidence and mortality trends and projections—United States, 1982-2030. MMWR Morb Mortal Wkly Rep. 2015;64:591-596.
- Whiteman DC, Olsen CM, MacGregor S, et al; QSkin Study. The effect of screening on melanoma incidence and biopsy rates. Br J Dermatol. 2022;187:515-522. doi:10.1111/bjd.21649
- Jobbágy A, Kiss N, Meznerics FA, et al. Emergency use and efficacy of an asynchronous teledermatology system as a novel tool for early diagnosis of skin cancer during the first wave of COVID-19 pandemic. Int J Environ Res Public Health. 2022;19:2699. doi:10.3390/ijerph19052699
- National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed April 19, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
- Trepanowski N, Chang MS, Zhou G, et al. Delays in melanoma presentation during the COVID-19 pandemic: a nationwide multi-institutional cohort study. J Am Acad Dermatol. 2022;87:1217-1219. doi:10.1016/j.jaad.2022.06.031
- Chiru MR, Hindocha S, Burova E, et al. Management of the two-week wait pathway for skin cancer patients, before and during the pandemic: is virtual consultation an option? J Pers Med. 2022;12:1258. doi:10.3390/jpm12081258
- Finnane A Dallest K Janda M et al. Teledermatology for the diagnosis and management of skin cancer: a systematic review. JAMA Dermatol. 2017;153:319-327. doi:10.1001/jamadermatol.2016.4361
- Guy GP Jr, Thomas CC, Thompson T, et al. Vital signs: melanoma incidence and mortality trends and projections—United States, 1982-2030. MMWR Morb Mortal Wkly Rep. 2015;64:591-596.
- Whiteman DC, Olsen CM, MacGregor S, et al; QSkin Study. The effect of screening on melanoma incidence and biopsy rates. Br J Dermatol. 2022;187:515-522. doi:10.1111/bjd.21649
- Jobbágy A, Kiss N, Meznerics FA, et al. Emergency use and efficacy of an asynchronous teledermatology system as a novel tool for early diagnosis of skin cancer during the first wave of COVID-19 pandemic. Int J Environ Res Public Health. 2022;19:2699. doi:10.3390/ijerph19052699
- National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed April 19, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
- Trepanowski N, Chang MS, Zhou G, et al. Delays in melanoma presentation during the COVID-19 pandemic: a nationwide multi-institutional cohort study. J Am Acad Dermatol. 2022;87:1217-1219. doi:10.1016/j.jaad.2022.06.031
- Chiru MR, Hindocha S, Burova E, et al. Management of the two-week wait pathway for skin cancer patients, before and during the pandemic: is virtual consultation an option? J Pers Med. 2022;12:1258. doi:10.3390/jpm12081258
- Finnane A Dallest K Janda M et al. Teledermatology for the diagnosis and management of skin cancer: a systematic review. JAMA Dermatol. 2017;153:319-327. doi:10.1001/jamadermatol.2016.4361
PRACTICE POINTS
- The COVID-19 pandemic has altered the landscape of medicine, as many individuals are now utilizing telemedicine to receive care.
- Many individuals will continue to receive telemedicine moving forward, making it crucial to understand access to care.
Financial Insecurity Among US Adults With Psoriasis
To the Editor:
Approximately 3% of the US population, or 6.9 million adults, is affected by psoriasis.1 Psoriasis has a substantial impact on quality of life and is associated with increased health care expenses and medication costs. In 2013, it was reported that the estimated US annual cost—direct, indirect, intangible, and comorbidity costs—of psoriasis for adults was $112 billion.2 We investigated the prevalence and sociodemographic characteristics of adult psoriasis patients (aged ≥20 years) with financial insecurity utilizing the 2009–2014 National Health and Nutrition Examination Survey (NHANES) data.3
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database to evaluate financial insecurity. Financial insecurity was evaluated by 2 outcome variables. The primary outcome variable was assessed by the question “Are you covered by health insurance or some other kind of health care plan (including health insurance obtained through employment or purchased directly as well as government programs like Medicare and Medicaid that provide medical care or help pay medical bills)?”3 Our secondary outcome variable was evaluated by a reported annual household income of less than $20,000. P values in Table 1 were calculated using Pearson χ2 tests. In Table 2, multivariate logistic regressions were performed using Stata/MP 17 (StataCorp LLC) to analyze associations between outcome variables and sociodemographic characteristics. Additionally, we controlled for age, race/ethnicity, sex, education, marital status, US citizenship status, and tobacco use. Subsequently, relationships with P<.05 were considered statistically significant.
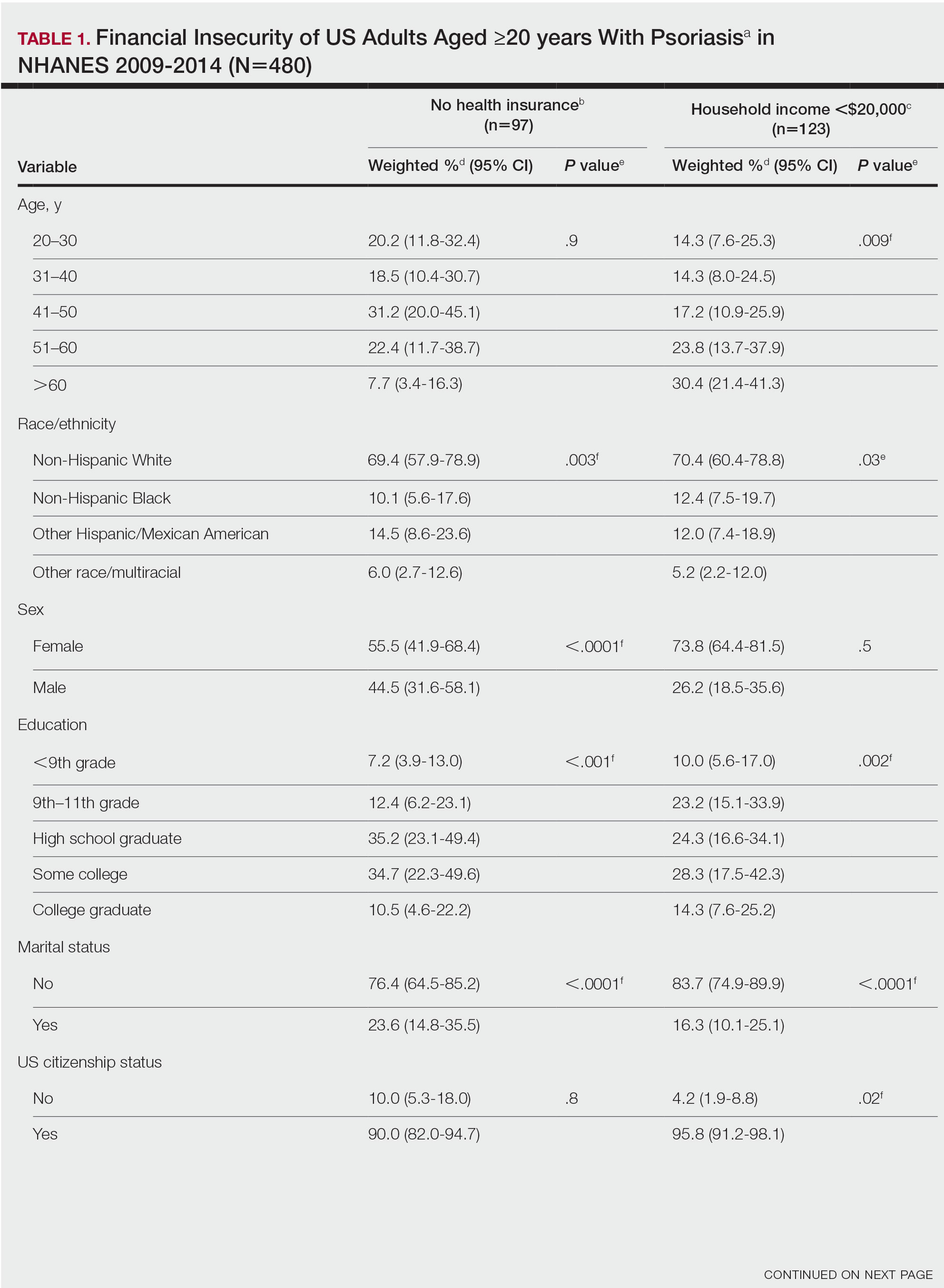

Our analysis comprised 480 individuals with psoriasis; 40 individuals were excluded from our analysis because they did not report annual household income and health insurance status (Table 1). Among the 480 individuals with psoriasis, approximately 16% (weighted) reported a lack of health insurance, and approximately 17% (weighted) reported an annual household income of less than $20,000. Among those who reported an annual household income of less than $20,000, approximately 38% (weighted) of them reported that they did not have health insurance.
Multivariate logistic regression analyses revealed that elderly individuals (aged >60 years), college graduates, married individuals, and US citizens had decreased odds of lacking health insurance (Table 2). Additionally, those with a history of tobacco use (adjusted odds ratio [AOR] 2.02; 95% CI, 1.00-4.05) were associated with lacking health insurance. Non-Hispanic Black individuals (AOR 2.26; 95% CI, 1.09-4.71) and US citizens (AOR 5.01; 95% CI, 1.28-19.63) had a significant association with an annual household income of less than $20,000 (P<.05). Lastly, males, those with education beyond ninth grade, and married individuals had a significantly decreased odds of having an annual household income of less than $20,000 (P<.05)(Table 2).
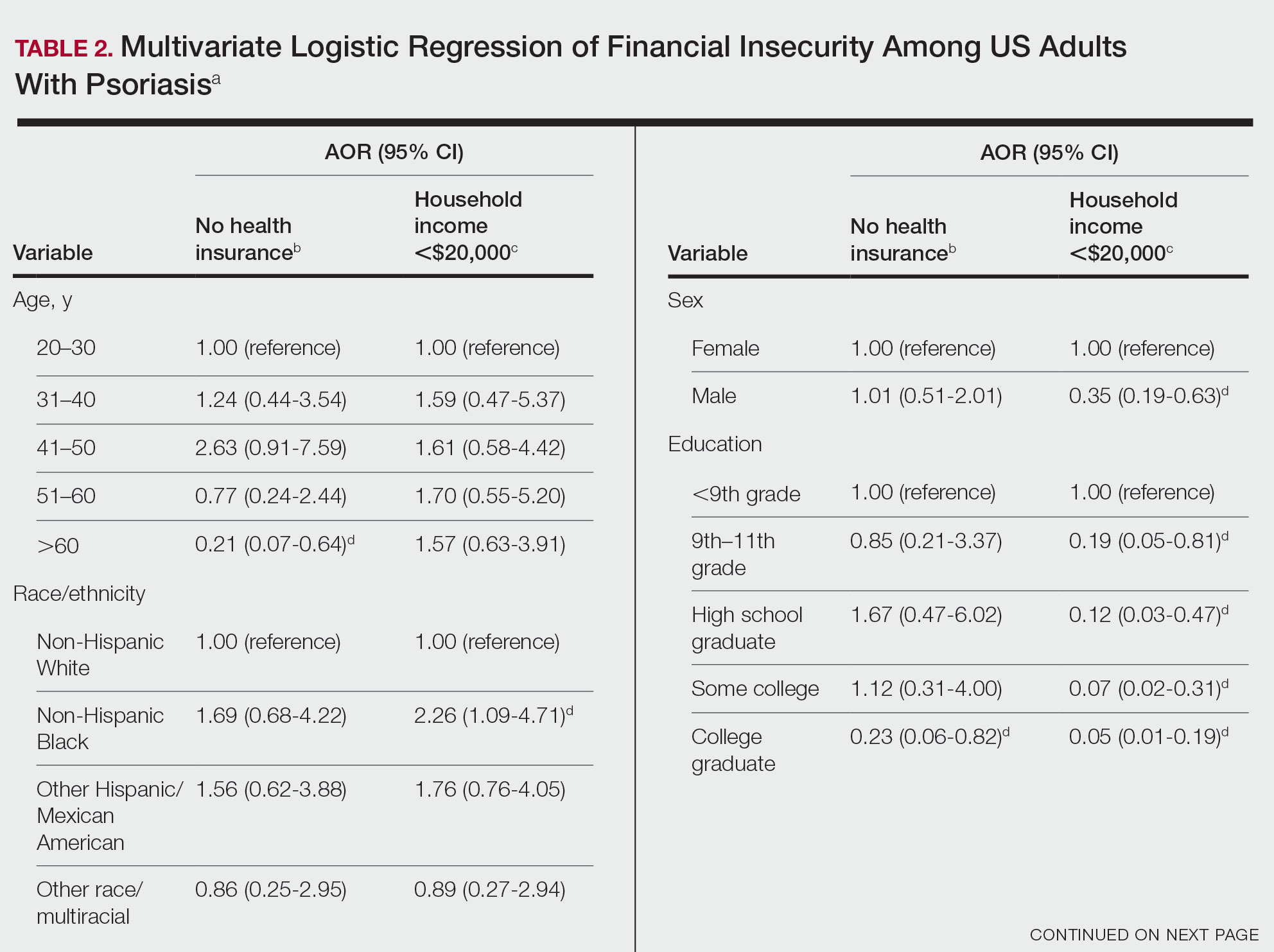
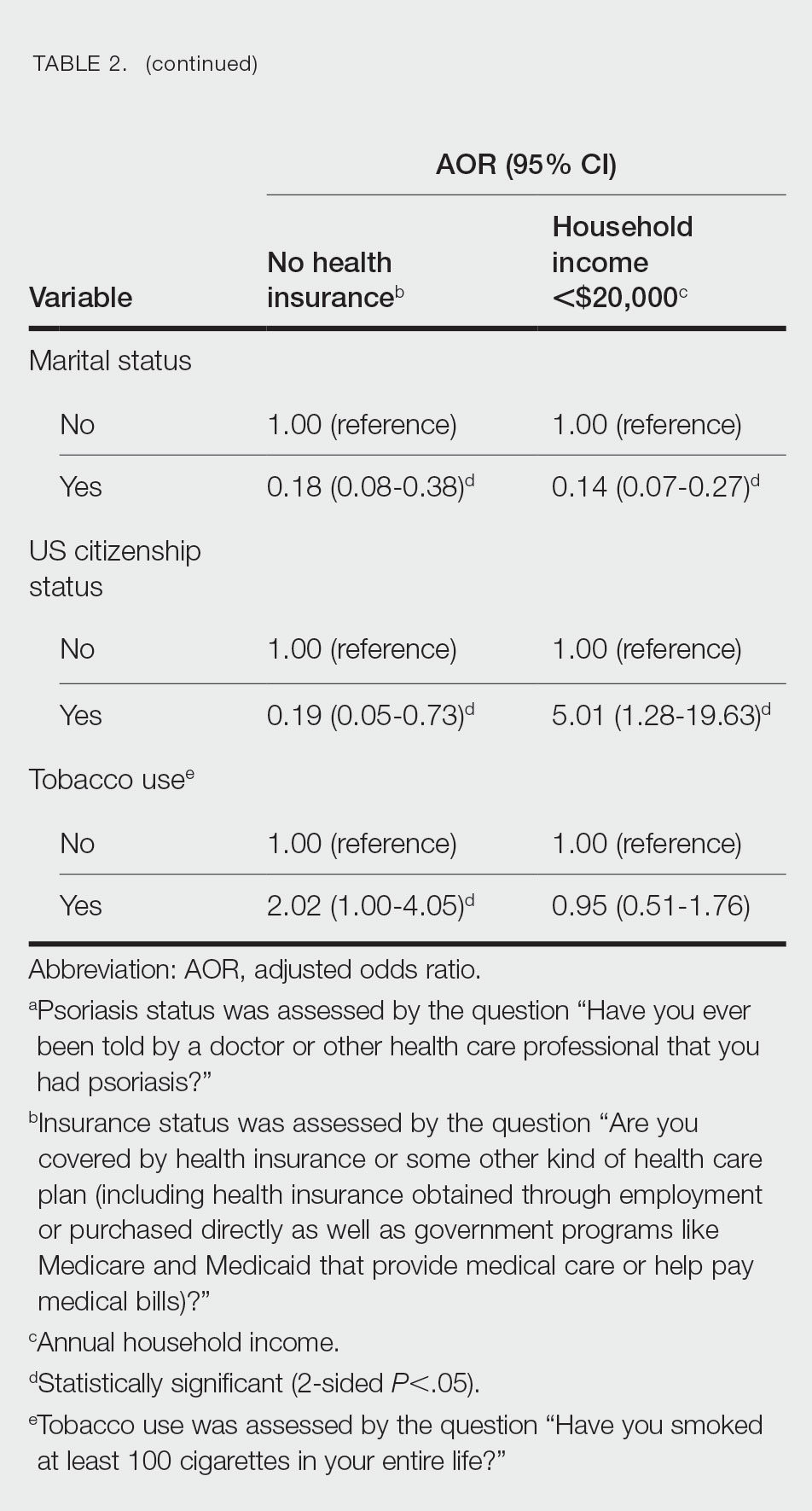
Our findings indicate that certain sociodemographic groups of psoriasis patients have an increased risk for being financially insecure. It is important to evaluate the cost of treatment, number of necessary visits to the office, and cost of transportation, as these factors can serve as a major economic burden to patients being managed for psoriasis.4 Additionally, the cost of biologics has been increasing over time.5 Taking all of this into account when caring for psoriasis patients is crucial, as understanding the financial status of patients can assist with determining appropriate individualized treatment regimens.
- Liu J, Thatiparthi A, Martin A, et al. Prevalence of psoriasis among adults in the US 2009-2010 and 2013-2014 National Health and Nutrition Examination Surveys. J Am Acad Dermatol. 2021;84:767-769. doi:10.1016/j.jaad.2020.10.035
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658. doi:10.1001/jamadermatol.2014.3593
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Maya-Rico AM, Londoño-García Á, Palacios-Barahona AU, et al. Out-of-pocket costs for patients with psoriasis in an outpatient dermatology referral service. An Bras Dermatol. 2021;96:295-300. doi:10.1016/j.abd.2020.09.004
- Cheng J, Feldman SR. The cost of biologics for psoriasis is increasing. Drugs Context. 2014;3:212266. doi:10.7573/dic.212266
To the Editor:
Approximately 3% of the US population, or 6.9 million adults, is affected by psoriasis.1 Psoriasis has a substantial impact on quality of life and is associated with increased health care expenses and medication costs. In 2013, it was reported that the estimated US annual cost—direct, indirect, intangible, and comorbidity costs—of psoriasis for adults was $112 billion.2 We investigated the prevalence and sociodemographic characteristics of adult psoriasis patients (aged ≥20 years) with financial insecurity utilizing the 2009–2014 National Health and Nutrition Examination Survey (NHANES) data.3
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database to evaluate financial insecurity. Financial insecurity was evaluated by 2 outcome variables. The primary outcome variable was assessed by the question “Are you covered by health insurance or some other kind of health care plan (including health insurance obtained through employment or purchased directly as well as government programs like Medicare and Medicaid that provide medical care or help pay medical bills)?”3 Our secondary outcome variable was evaluated by a reported annual household income of less than $20,000. P values in Table 1 were calculated using Pearson χ2 tests. In Table 2, multivariate logistic regressions were performed using Stata/MP 17 (StataCorp LLC) to analyze associations between outcome variables and sociodemographic characteristics. Additionally, we controlled for age, race/ethnicity, sex, education, marital status, US citizenship status, and tobacco use. Subsequently, relationships with P<.05 were considered statistically significant.


Our analysis comprised 480 individuals with psoriasis; 40 individuals were excluded from our analysis because they did not report annual household income and health insurance status (Table 1). Among the 480 individuals with psoriasis, approximately 16% (weighted) reported a lack of health insurance, and approximately 17% (weighted) reported an annual household income of less than $20,000. Among those who reported an annual household income of less than $20,000, approximately 38% (weighted) of them reported that they did not have health insurance.
Multivariate logistic regression analyses revealed that elderly individuals (aged >60 years), college graduates, married individuals, and US citizens had decreased odds of lacking health insurance (Table 2). Additionally, those with a history of tobacco use (adjusted odds ratio [AOR] 2.02; 95% CI, 1.00-4.05) were associated with lacking health insurance. Non-Hispanic Black individuals (AOR 2.26; 95% CI, 1.09-4.71) and US citizens (AOR 5.01; 95% CI, 1.28-19.63) had a significant association with an annual household income of less than $20,000 (P<.05). Lastly, males, those with education beyond ninth grade, and married individuals had a significantly decreased odds of having an annual household income of less than $20,000 (P<.05)(Table 2).


Our findings indicate that certain sociodemographic groups of psoriasis patients have an increased risk for being financially insecure. It is important to evaluate the cost of treatment, number of necessary visits to the office, and cost of transportation, as these factors can serve as a major economic burden to patients being managed for psoriasis.4 Additionally, the cost of biologics has been increasing over time.5 Taking all of this into account when caring for psoriasis patients is crucial, as understanding the financial status of patients can assist with determining appropriate individualized treatment regimens.
To the Editor:
Approximately 3% of the US population, or 6.9 million adults, is affected by psoriasis.1 Psoriasis has a substantial impact on quality of life and is associated with increased health care expenses and medication costs. In 2013, it was reported that the estimated US annual cost—direct, indirect, intangible, and comorbidity costs—of psoriasis for adults was $112 billion.2 We investigated the prevalence and sociodemographic characteristics of adult psoriasis patients (aged ≥20 years) with financial insecurity utilizing the 2009–2014 National Health and Nutrition Examination Survey (NHANES) data.3
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database to evaluate financial insecurity. Financial insecurity was evaluated by 2 outcome variables. The primary outcome variable was assessed by the question “Are you covered by health insurance or some other kind of health care plan (including health insurance obtained through employment or purchased directly as well as government programs like Medicare and Medicaid that provide medical care or help pay medical bills)?”3 Our secondary outcome variable was evaluated by a reported annual household income of less than $20,000. P values in Table 1 were calculated using Pearson χ2 tests. In Table 2, multivariate logistic regressions were performed using Stata/MP 17 (StataCorp LLC) to analyze associations between outcome variables and sociodemographic characteristics. Additionally, we controlled for age, race/ethnicity, sex, education, marital status, US citizenship status, and tobacco use. Subsequently, relationships with P<.05 were considered statistically significant.


Our analysis comprised 480 individuals with psoriasis; 40 individuals were excluded from our analysis because they did not report annual household income and health insurance status (Table 1). Among the 480 individuals with psoriasis, approximately 16% (weighted) reported a lack of health insurance, and approximately 17% (weighted) reported an annual household income of less than $20,000. Among those who reported an annual household income of less than $20,000, approximately 38% (weighted) of them reported that they did not have health insurance.
Multivariate logistic regression analyses revealed that elderly individuals (aged >60 years), college graduates, married individuals, and US citizens had decreased odds of lacking health insurance (Table 2). Additionally, those with a history of tobacco use (adjusted odds ratio [AOR] 2.02; 95% CI, 1.00-4.05) were associated with lacking health insurance. Non-Hispanic Black individuals (AOR 2.26; 95% CI, 1.09-4.71) and US citizens (AOR 5.01; 95% CI, 1.28-19.63) had a significant association with an annual household income of less than $20,000 (P<.05). Lastly, males, those with education beyond ninth grade, and married individuals had a significantly decreased odds of having an annual household income of less than $20,000 (P<.05)(Table 2).


Our findings indicate that certain sociodemographic groups of psoriasis patients have an increased risk for being financially insecure. It is important to evaluate the cost of treatment, number of necessary visits to the office, and cost of transportation, as these factors can serve as a major economic burden to patients being managed for psoriasis.4 Additionally, the cost of biologics has been increasing over time.5 Taking all of this into account when caring for psoriasis patients is crucial, as understanding the financial status of patients can assist with determining appropriate individualized treatment regimens.
- Liu J, Thatiparthi A, Martin A, et al. Prevalence of psoriasis among adults in the US 2009-2010 and 2013-2014 National Health and Nutrition Examination Surveys. J Am Acad Dermatol. 2021;84:767-769. doi:10.1016/j.jaad.2020.10.035
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658. doi:10.1001/jamadermatol.2014.3593
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Maya-Rico AM, Londoño-García Á, Palacios-Barahona AU, et al. Out-of-pocket costs for patients with psoriasis in an outpatient dermatology referral service. An Bras Dermatol. 2021;96:295-300. doi:10.1016/j.abd.2020.09.004
- Cheng J, Feldman SR. The cost of biologics for psoriasis is increasing. Drugs Context. 2014;3:212266. doi:10.7573/dic.212266
- Liu J, Thatiparthi A, Martin A, et al. Prevalence of psoriasis among adults in the US 2009-2010 and 2013-2014 National Health and Nutrition Examination Surveys. J Am Acad Dermatol. 2021;84:767-769. doi:10.1016/j.jaad.2020.10.035
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658. doi:10.1001/jamadermatol.2014.3593
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Maya-Rico AM, Londoño-García Á, Palacios-Barahona AU, et al. Out-of-pocket costs for patients with psoriasis in an outpatient dermatology referral service. An Bras Dermatol. 2021;96:295-300. doi:10.1016/j.abd.2020.09.004
- Cheng J, Feldman SR. The cost of biologics for psoriasis is increasing. Drugs Context. 2014;3:212266. doi:10.7573/dic.212266
Practice Points
- The economic burden on patients with psoriasis has been rising over time, as the disease impacts many aspects of patients’ lives.
- Various sociodemographic groups among patients with psoriasis are financially insecure. Knowing which groups are at higher risk for poor outcomes due to financial insecurity can assist with appropriate treatment regimens.
Adverse Effects of the COVID-19 Vaccine in Patients With Psoriasis
To the Editor:
Because the SARS-CoV-2 virus is constantly changing, routine vaccination to prevent COVID-19 infection is recommended. The messenger RNA (mRNA) vaccines from Pfizer-BioNTech and Moderna as well as the Ad26.COV2.S (Johnson & Johnson) and NVX-CoV2373 (Novavax) vaccines are the most commonly used COVID-19 vaccines in the United States. Adverse effects following vaccination against SARS-CoV-2 are well documented; recent studies report a small incidence of adverse effects in the general population, with most being minor (eg, headache, fever, muscle pain).1,2 Interestingly, reports of exacerbation of psoriasis and new-onset psoriasis following COVID-19 vaccination suggest a potential association.3,4 However, the literature investigating the vaccine adverse effect profile in this demographic is scarce. We examined the incidence of adverse effects from SARS-CoV-2 vaccines in patients with psoriasis.
This retrospective cohort study used the COVID-19 Research Database (https://covid19researchdatabase.org/) to examine the adverse effects following the first and second doses of the mRNA vaccines in patients with and without psoriasis. The sample size for the Ad26.COV2.S vaccine was too small to analyze.
Claims were evaluated from August to October 2021 for 2 diagnoses of psoriasis prior to January 1, 2020, using the International Classification of Diseases, Tenth Revision (ICD-10) code L40.9 to increase the positive predictive value and ensure that the diagnosis preceded the COVID-19 pandemic. Patients younger than 18 years and those who did not receive 2 doses of a SARS-CoV-2 vaccine were excluded. Controls who did not have a diagnosis of psoriasis were matched for age, sex, and hypertension at a 4:1 ratio. Hypertension represented the most common comorbidity that could feasibly be controlled for in this study population. Other comorbidities recorded included obesity, type 2 diabetes mellitus, congestive heart failure, asthma, chronic obstructive pulmonary disease, chronic ischemic heart disease, rhinitis, and chronic kidney disease.
Common adverse effects as long as 30 days after vaccination were identified using ICD-10 codes. Adverse effects of interest were anaphylactic reaction, initial encounter of adverse effect of viral vaccines, fever, allergic urticaria, weakness, altered mental status, malaise, allergic reaction, chest pain, symptoms involving circulatory or respiratory systems, localized rash, axillary lymphadenopathy, infection, and myocarditis.5 Poisson regression was performed using Stata 17 analytical software.
We identified 4273 patients with psoriasis and 17,092 controls who received mRNA COVID-19 vaccines (Table). Adjusted odds ratios (aORs) for doses 1 and 2 were calculated for each vaccine (eTable). Adverse effects with sufficient data to generate an aOR included weakness, altered mental status, malaise, chest pain, and symptoms involving the circulatory or respiratory system. The aORs for allergic urticaria and initial encounter of adverse effect of viral vaccines were only calculated for the Moderna mRNA vaccine due to low sample size.

This study demonstrated that patients with psoriasis do not appear to have a significantly increased risk of adverse effects from mRNA SARS-CoV-2 vaccines. Although the ORs in this study were not significant, most recorded adverse effects demonstrated an aOR less than 1, suggesting that there might be a lower risk of certain adverse effects in psoriasis patients. This could be explained by the immunomodulatory effects of certain systemic psoriasis treatments that might influence the adverse effect presentation.

The study is limited by the lack of treatment data, small sample size, and the fact that it did not assess flares or worsening of psoriasis with the vaccines. Underreporting of adverse effects by patients and underdiagnosis of adverse effects secondary to SARS-CoV-2 vaccines due to its novel nature, incompletely understood consequences, and limited ICD-10 codes associated with adverse effects all contributed to the small sample size.
Our findings suggest that the risk for immediate adverse effects from the mRNA SARS-CoV-2 vaccines is not increased among psoriasis patients. However, the impact of immunomodulatory agents on vaccine efficacy and expected adverse effects should be investigated. As more individuals receive the COVID-19 vaccine, the adverse effect profile in patients with psoriasis is an important area of investigation.
- Singh A, Khillan R, Mishra Y, et al. The safety profile of COVID-19 vaccinations in the United States. Am J Infect Control. 2022;50:15-19. doi: 10.1016/j.ajic.2021.10.015
- Beatty AL, Peyser ND, Butcher XE, et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw Open. 2021;4:e2140364. doi:10.1001/jamanetworkopen.2021.40364
- Bellinato F, Maurelli M, Gisondi P, et al. Cutaneous adverse reactions associated with SARS-CoV-2 vaccines. J Clin Med. 2021;10:5344. doi:10.3390/jcm10225344
- Elamin S, Hinds F, Tolland J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin Exp Dermatol. 2022;47:153-155. doi:10.1111/ced.14895
- Remer EE. Coding COVID-19 vaccination. ICD10monitor. Published March 2, 2021. Updated October 18, 2022. Accessed January 17, 2023. https://icd10monitor.medlearn.com/coding-covid-19-vaccination/
To the Editor:
Because the SARS-CoV-2 virus is constantly changing, routine vaccination to prevent COVID-19 infection is recommended. The messenger RNA (mRNA) vaccines from Pfizer-BioNTech and Moderna as well as the Ad26.COV2.S (Johnson & Johnson) and NVX-CoV2373 (Novavax) vaccines are the most commonly used COVID-19 vaccines in the United States. Adverse effects following vaccination against SARS-CoV-2 are well documented; recent studies report a small incidence of adverse effects in the general population, with most being minor (eg, headache, fever, muscle pain).1,2 Interestingly, reports of exacerbation of psoriasis and new-onset psoriasis following COVID-19 vaccination suggest a potential association.3,4 However, the literature investigating the vaccine adverse effect profile in this demographic is scarce. We examined the incidence of adverse effects from SARS-CoV-2 vaccines in patients with psoriasis.
This retrospective cohort study used the COVID-19 Research Database (https://covid19researchdatabase.org/) to examine the adverse effects following the first and second doses of the mRNA vaccines in patients with and without psoriasis. The sample size for the Ad26.COV2.S vaccine was too small to analyze.
Claims were evaluated from August to October 2021 for 2 diagnoses of psoriasis prior to January 1, 2020, using the International Classification of Diseases, Tenth Revision (ICD-10) code L40.9 to increase the positive predictive value and ensure that the diagnosis preceded the COVID-19 pandemic. Patients younger than 18 years and those who did not receive 2 doses of a SARS-CoV-2 vaccine were excluded. Controls who did not have a diagnosis of psoriasis were matched for age, sex, and hypertension at a 4:1 ratio. Hypertension represented the most common comorbidity that could feasibly be controlled for in this study population. Other comorbidities recorded included obesity, type 2 diabetes mellitus, congestive heart failure, asthma, chronic obstructive pulmonary disease, chronic ischemic heart disease, rhinitis, and chronic kidney disease.
Common adverse effects as long as 30 days after vaccination were identified using ICD-10 codes. Adverse effects of interest were anaphylactic reaction, initial encounter of adverse effect of viral vaccines, fever, allergic urticaria, weakness, altered mental status, malaise, allergic reaction, chest pain, symptoms involving circulatory or respiratory systems, localized rash, axillary lymphadenopathy, infection, and myocarditis.5 Poisson regression was performed using Stata 17 analytical software.
We identified 4273 patients with psoriasis and 17,092 controls who received mRNA COVID-19 vaccines (Table). Adjusted odds ratios (aORs) for doses 1 and 2 were calculated for each vaccine (eTable). Adverse effects with sufficient data to generate an aOR included weakness, altered mental status, malaise, chest pain, and symptoms involving the circulatory or respiratory system. The aORs for allergic urticaria and initial encounter of adverse effect of viral vaccines were only calculated for the Moderna mRNA vaccine due to low sample size.

This study demonstrated that patients with psoriasis do not appear to have a significantly increased risk of adverse effects from mRNA SARS-CoV-2 vaccines. Although the ORs in this study were not significant, most recorded adverse effects demonstrated an aOR less than 1, suggesting that there might be a lower risk of certain adverse effects in psoriasis patients. This could be explained by the immunomodulatory effects of certain systemic psoriasis treatments that might influence the adverse effect presentation.

The study is limited by the lack of treatment data, small sample size, and the fact that it did not assess flares or worsening of psoriasis with the vaccines. Underreporting of adverse effects by patients and underdiagnosis of adverse effects secondary to SARS-CoV-2 vaccines due to its novel nature, incompletely understood consequences, and limited ICD-10 codes associated with adverse effects all contributed to the small sample size.
Our findings suggest that the risk for immediate adverse effects from the mRNA SARS-CoV-2 vaccines is not increased among psoriasis patients. However, the impact of immunomodulatory agents on vaccine efficacy and expected adverse effects should be investigated. As more individuals receive the COVID-19 vaccine, the adverse effect profile in patients with psoriasis is an important area of investigation.
To the Editor:
Because the SARS-CoV-2 virus is constantly changing, routine vaccination to prevent COVID-19 infection is recommended. The messenger RNA (mRNA) vaccines from Pfizer-BioNTech and Moderna as well as the Ad26.COV2.S (Johnson & Johnson) and NVX-CoV2373 (Novavax) vaccines are the most commonly used COVID-19 vaccines in the United States. Adverse effects following vaccination against SARS-CoV-2 are well documented; recent studies report a small incidence of adverse effects in the general population, with most being minor (eg, headache, fever, muscle pain).1,2 Interestingly, reports of exacerbation of psoriasis and new-onset psoriasis following COVID-19 vaccination suggest a potential association.3,4 However, the literature investigating the vaccine adverse effect profile in this demographic is scarce. We examined the incidence of adverse effects from SARS-CoV-2 vaccines in patients with psoriasis.
This retrospective cohort study used the COVID-19 Research Database (https://covid19researchdatabase.org/) to examine the adverse effects following the first and second doses of the mRNA vaccines in patients with and without psoriasis. The sample size for the Ad26.COV2.S vaccine was too small to analyze.
Claims were evaluated from August to October 2021 for 2 diagnoses of psoriasis prior to January 1, 2020, using the International Classification of Diseases, Tenth Revision (ICD-10) code L40.9 to increase the positive predictive value and ensure that the diagnosis preceded the COVID-19 pandemic. Patients younger than 18 years and those who did not receive 2 doses of a SARS-CoV-2 vaccine were excluded. Controls who did not have a diagnosis of psoriasis were matched for age, sex, and hypertension at a 4:1 ratio. Hypertension represented the most common comorbidity that could feasibly be controlled for in this study population. Other comorbidities recorded included obesity, type 2 diabetes mellitus, congestive heart failure, asthma, chronic obstructive pulmonary disease, chronic ischemic heart disease, rhinitis, and chronic kidney disease.
Common adverse effects as long as 30 days after vaccination were identified using ICD-10 codes. Adverse effects of interest were anaphylactic reaction, initial encounter of adverse effect of viral vaccines, fever, allergic urticaria, weakness, altered mental status, malaise, allergic reaction, chest pain, symptoms involving circulatory or respiratory systems, localized rash, axillary lymphadenopathy, infection, and myocarditis.5 Poisson regression was performed using Stata 17 analytical software.
We identified 4273 patients with psoriasis and 17,092 controls who received mRNA COVID-19 vaccines (Table). Adjusted odds ratios (aORs) for doses 1 and 2 were calculated for each vaccine (eTable). Adverse effects with sufficient data to generate an aOR included weakness, altered mental status, malaise, chest pain, and symptoms involving the circulatory or respiratory system. The aORs for allergic urticaria and initial encounter of adverse effect of viral vaccines were only calculated for the Moderna mRNA vaccine due to low sample size.

This study demonstrated that patients with psoriasis do not appear to have a significantly increased risk of adverse effects from mRNA SARS-CoV-2 vaccines. Although the ORs in this study were not significant, most recorded adverse effects demonstrated an aOR less than 1, suggesting that there might be a lower risk of certain adverse effects in psoriasis patients. This could be explained by the immunomodulatory effects of certain systemic psoriasis treatments that might influence the adverse effect presentation.

The study is limited by the lack of treatment data, small sample size, and the fact that it did not assess flares or worsening of psoriasis with the vaccines. Underreporting of adverse effects by patients and underdiagnosis of adverse effects secondary to SARS-CoV-2 vaccines due to its novel nature, incompletely understood consequences, and limited ICD-10 codes associated with adverse effects all contributed to the small sample size.
Our findings suggest that the risk for immediate adverse effects from the mRNA SARS-CoV-2 vaccines is not increased among psoriasis patients. However, the impact of immunomodulatory agents on vaccine efficacy and expected adverse effects should be investigated. As more individuals receive the COVID-19 vaccine, the adverse effect profile in patients with psoriasis is an important area of investigation.
- Singh A, Khillan R, Mishra Y, et al. The safety profile of COVID-19 vaccinations in the United States. Am J Infect Control. 2022;50:15-19. doi: 10.1016/j.ajic.2021.10.015
- Beatty AL, Peyser ND, Butcher XE, et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw Open. 2021;4:e2140364. doi:10.1001/jamanetworkopen.2021.40364
- Bellinato F, Maurelli M, Gisondi P, et al. Cutaneous adverse reactions associated with SARS-CoV-2 vaccines. J Clin Med. 2021;10:5344. doi:10.3390/jcm10225344
- Elamin S, Hinds F, Tolland J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin Exp Dermatol. 2022;47:153-155. doi:10.1111/ced.14895
- Remer EE. Coding COVID-19 vaccination. ICD10monitor. Published March 2, 2021. Updated October 18, 2022. Accessed January 17, 2023. https://icd10monitor.medlearn.com/coding-covid-19-vaccination/
- Singh A, Khillan R, Mishra Y, et al. The safety profile of COVID-19 vaccinations in the United States. Am J Infect Control. 2022;50:15-19. doi: 10.1016/j.ajic.2021.10.015
- Beatty AL, Peyser ND, Butcher XE, et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw Open. 2021;4:e2140364. doi:10.1001/jamanetworkopen.2021.40364
- Bellinato F, Maurelli M, Gisondi P, et al. Cutaneous adverse reactions associated with SARS-CoV-2 vaccines. J Clin Med. 2021;10:5344. doi:10.3390/jcm10225344
- Elamin S, Hinds F, Tolland J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin Exp Dermatol. 2022;47:153-155. doi:10.1111/ced.14895
- Remer EE. Coding COVID-19 vaccination. ICD10monitor. Published March 2, 2021. Updated October 18, 2022. Accessed January 17, 2023. https://icd10monitor.medlearn.com/coding-covid-19-vaccination/
PRACTICE POINTS
- Patients who have psoriasis do not appear to have an increased incidence of adverse effects from messenger RNA COVID-19 vaccines.
- Clinicians can safely recommend COVID-19 vaccines to patients who have psoriasis.