User login
SAN FRANCISCO – An investigational immunomodulator, IMM-101, improves outcomes in patients who have advanced pancreatic cancer when combined with chemotherapy, with minimal increase in toxicity, suggest data from an open-label, phase II trial conducted in Europe.
Results reported in a poster session at the annual Gastrointestinal Cancers Symposium cosponsored by the AGA Institute, the American Society of Clinical Oncology, ASTRO, and the Society of Surgical Oncology showed that median progression-free survival was about 1 month longer for patients given IMM-101 plus gemcitabine than for patients given gemcitabine alone; the gain exceeded 2 months among the subgroup having metastatic disease. The combination was associated with slightly higher rates of certain toxicities that seemed to stem from being on gemcitabine for longer.
“I think IMM-101 brings to the field something that’s missing,” lead investigator Dr. Angus G. Dalgleish, a professor and medical oncologist at St. George’s University of London, commented in an interview. “Everybody has been doing doublets, triplets, etcetera. All they do is guarantee increased toxicity and, in some cases, misery to the patient, whereas this allows you to start treatment and stabilize [the cancer].”
“This shows that actually boosting the immune response in a nonspecific manner before we do anything else may well become the gold standard when you add in any other treatment,” he added. “And I think it’s a non–chemo specific thing and a non–tumor specific thing, because we have done all the preliminary work in melanoma. We’ve also got very encouraging data in prostate and are starting prostate studies this year. And I would like to see it go into a whole host of ones where chemo needs a nonspecific boost, like ovarian [cancer].”
Of note, the trial opened just before FOLFIRINOX (irinotecan plus 5-fluorouracil plus leucovorin plus oxaliplatin) became available to patients in Europe, and many of the more fit eligible patients opted for that therapy instead. “I think this makes the survival benefit even more remarkable,” Dr. Dalgleish stated.
IMM-101 is a heat-killed whole-cell vaccine that is given intradermally and induces protective CD8 T-cell responses. “Although we had good data on this agent for melanoma, we noticed that it sort of boosted the immune system in all cancers [in which] we could show you get immune suppression,” he noted. Additionally, preclinical data hinted at synergy with gemcitabine.
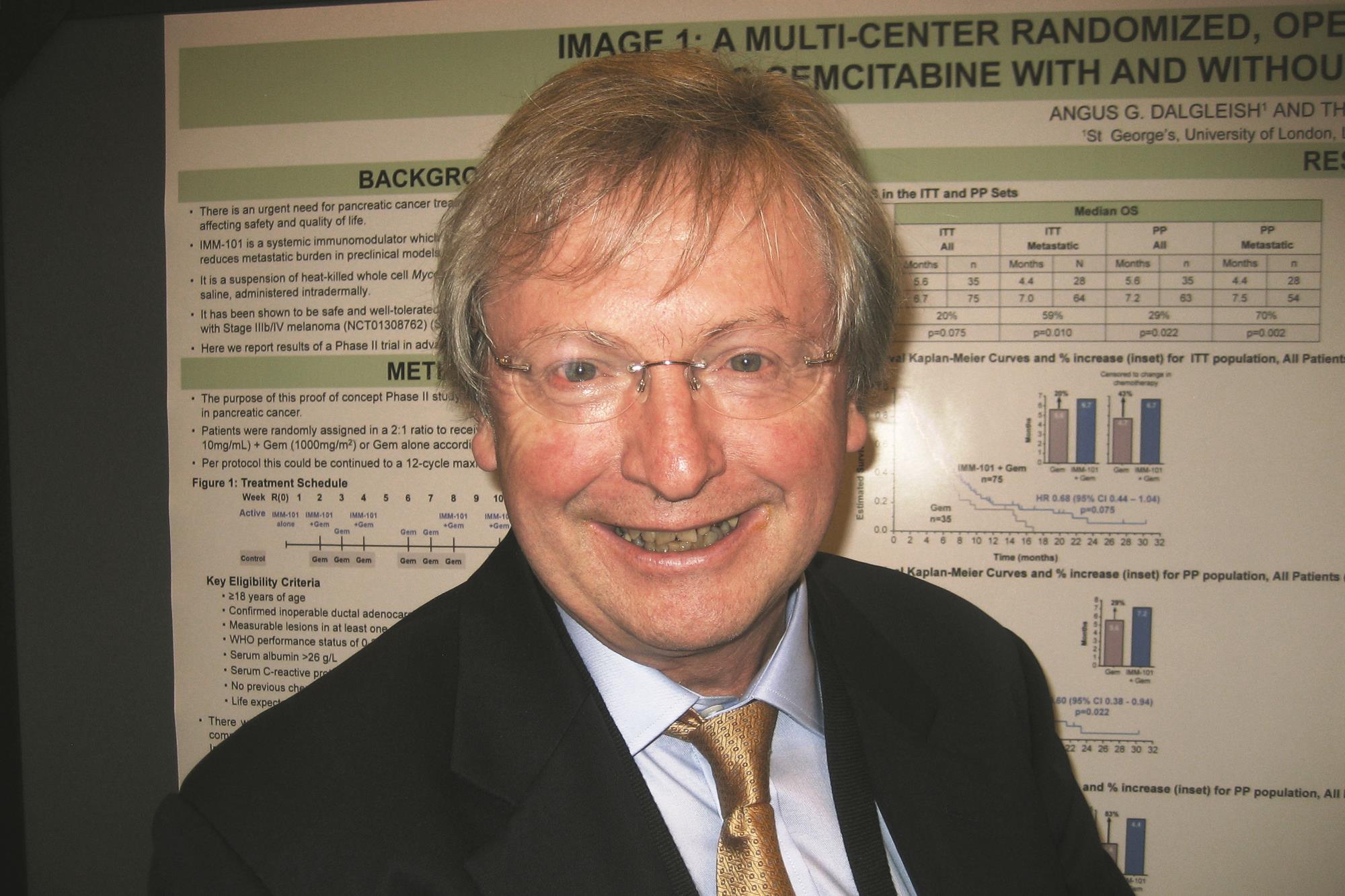
The trial, known as IMAGE 1 (Immune Modulation And Gemcitabine Evaluation 1) and sponsored by manufacturer Immodulon Therapeutics, enrolled 110 patients with stage III or IV pancreatic cancer who had not previously received any chemotherapy. The patients were randomized 2:1 to receive gemcitabine (Gemzar) either with or without IMM-101.
Intention-to-treat analyses showed that median overall survival, the primary endpoint, was 6.7 months with IMM-101 and 5.6 months without it in the entire trial population (hazard ratio, 0.68; P = .075). The difference was significant among the subset of patients having metastatic disease (7.0 vs. 4.4 months; HR, 0.54; P = .010).
“You are getting a quick effect here, 2-4 months,” Dr. Dalgleish pointed out. “I’ve done several vaccine studies where we don’t even get the split [in survival curves] for 12 months. ... So that’s absolutely staggering that we get the split so early on.”
“In the metastatic group, the survival advantage is really quite dramatic,” he added. “And unlike many other studies that we have been involved in, you are getting this tail. ... these patients are still continuing to survive.”
One patient given the combination experienced progression and was then given nab-paclitaxel (Abraxane), and had a complete response to that agent, according to Dr. Dalgleish. He speculated that it may have been due to the immune priming by IMM-101.
In per protocol analyses, the survival benefit was significant in both the entire population and the patients with metastatic disease.
Patients in the IMM-101 group also had superior progression-free survival, seen in the entire trial population (4.4 vs. 2.4 months; HR, 0.51; P = .003) and in the subgroup with metastases (4.4 vs. 2.3 months; HR, 0.40; P less than .001).
Apart from injection site reactions in some patients, IMM-101 was well tolerated, according to Dr. Dalgleish.
“When you add gemcitabine plus ‘x’ – Iressa [gefitinib], capecitabine [Xeloda], Abraxane, etcetera – you get a benefit but at great cost,” he commented. In this trial, toxicity “is slightly worse in the combination arm, but the side effects are all those associated with the chemotherapy and the disease – nothing to do with the immune modulator, which we have given to hundreds of patients before. And that would appear to reflect the fact that those patients have been on gemcitabine for longer than the ones on gemcitabine alone.”
Quality of life data are still being analyzed, according to Dr. Dalgleish. “But ... from my personal experience, the patients do feel better on the vaccine as [it has] a kind of overall boosting effect.”
The investigators plan to take IMM-101 forward in clinical trials, he said. “But I think in the States, they might want to do that with Abraxane first line.” In the United Kingdom, the National Institute for Health and Care Excellence (NICE) recently declined Abraxane, meaning cost won’t be reimbursed if it is used, Dr. Dalgleish said. “So we may well be able to do the same study in Europe in order to get approval, although personally, I’d be very keen for conditional approval on this [trial] in the U.K. because it has a clear benefit without any toxicity. And there is a system where you can get a phase 4 approval and have postapproval monitoring.”
Dr. Dalgleish disclosed that he has a consulting or advisory role with and receives research funding from Immodulon Therapeutics.
SAN FRANCISCO – An investigational immunomodulator, IMM-101, improves outcomes in patients who have advanced pancreatic cancer when combined with chemotherapy, with minimal increase in toxicity, suggest data from an open-label, phase II trial conducted in Europe.
Results reported in a poster session at the annual Gastrointestinal Cancers Symposium cosponsored by the AGA Institute, the American Society of Clinical Oncology, ASTRO, and the Society of Surgical Oncology showed that median progression-free survival was about 1 month longer for patients given IMM-101 plus gemcitabine than for patients given gemcitabine alone; the gain exceeded 2 months among the subgroup having metastatic disease. The combination was associated with slightly higher rates of certain toxicities that seemed to stem from being on gemcitabine for longer.
“I think IMM-101 brings to the field something that’s missing,” lead investigator Dr. Angus G. Dalgleish, a professor and medical oncologist at St. George’s University of London, commented in an interview. “Everybody has been doing doublets, triplets, etcetera. All they do is guarantee increased toxicity and, in some cases, misery to the patient, whereas this allows you to start treatment and stabilize [the cancer].”
“This shows that actually boosting the immune response in a nonspecific manner before we do anything else may well become the gold standard when you add in any other treatment,” he added. “And I think it’s a non–chemo specific thing and a non–tumor specific thing, because we have done all the preliminary work in melanoma. We’ve also got very encouraging data in prostate and are starting prostate studies this year. And I would like to see it go into a whole host of ones where chemo needs a nonspecific boost, like ovarian [cancer].”
Of note, the trial opened just before FOLFIRINOX (irinotecan plus 5-fluorouracil plus leucovorin plus oxaliplatin) became available to patients in Europe, and many of the more fit eligible patients opted for that therapy instead. “I think this makes the survival benefit even more remarkable,” Dr. Dalgleish stated.
IMM-101 is a heat-killed whole-cell vaccine that is given intradermally and induces protective CD8 T-cell responses. “Although we had good data on this agent for melanoma, we noticed that it sort of boosted the immune system in all cancers [in which] we could show you get immune suppression,” he noted. Additionally, preclinical data hinted at synergy with gemcitabine.
The trial, known as IMAGE 1 (Immune Modulation And Gemcitabine Evaluation 1) and sponsored by manufacturer Immodulon Therapeutics, enrolled 110 patients with stage III or IV pancreatic cancer who had not previously received any chemotherapy. The patients were randomized 2:1 to receive gemcitabine (Gemzar) either with or without IMM-101.
Intention-to-treat analyses showed that median overall survival, the primary endpoint, was 6.7 months with IMM-101 and 5.6 months without it in the entire trial population (hazard ratio, 0.68; P = .075). The difference was significant among the subset of patients having metastatic disease (7.0 vs. 4.4 months; HR, 0.54; P = .010).
“You are getting a quick effect here, 2-4 months,” Dr. Dalgleish pointed out. “I’ve done several vaccine studies where we don’t even get the split [in survival curves] for 12 months. ... So that’s absolutely staggering that we get the split so early on.”
“In the metastatic group, the survival advantage is really quite dramatic,” he added. “And unlike many other studies that we have been involved in, you are getting this tail. ... these patients are still continuing to survive.”
One patient given the combination experienced progression and was then given nab-paclitaxel (Abraxane), and had a complete response to that agent, according to Dr. Dalgleish. He speculated that it may have been due to the immune priming by IMM-101.
In per protocol analyses, the survival benefit was significant in both the entire population and the patients with metastatic disease.
Patients in the IMM-101 group also had superior progression-free survival, seen in the entire trial population (4.4 vs. 2.4 months; HR, 0.51; P = .003) and in the subgroup with metastases (4.4 vs. 2.3 months; HR, 0.40; P less than .001).
Apart from injection site reactions in some patients, IMM-101 was well tolerated, according to Dr. Dalgleish.
“When you add gemcitabine plus ‘x’ – Iressa [gefitinib], capecitabine [Xeloda], Abraxane, etcetera – you get a benefit but at great cost,” he commented. In this trial, toxicity “is slightly worse in the combination arm, but the side effects are all those associated with the chemotherapy and the disease – nothing to do with the immune modulator, which we have given to hundreds of patients before. And that would appear to reflect the fact that those patients have been on gemcitabine for longer than the ones on gemcitabine alone.”
Quality of life data are still being analyzed, according to Dr. Dalgleish. “But ... from my personal experience, the patients do feel better on the vaccine as [it has] a kind of overall boosting effect.”
The investigators plan to take IMM-101 forward in clinical trials, he said. “But I think in the States, they might want to do that with Abraxane first line.” In the United Kingdom, the National Institute for Health and Care Excellence (NICE) recently declined Abraxane, meaning cost won’t be reimbursed if it is used, Dr. Dalgleish said. “So we may well be able to do the same study in Europe in order to get approval, although personally, I’d be very keen for conditional approval on this [trial] in the U.K. because it has a clear benefit without any toxicity. And there is a system where you can get a phase 4 approval and have postapproval monitoring.”
Dr. Dalgleish disclosed that he has a consulting or advisory role with and receives research funding from Immodulon Therapeutics.
SAN FRANCISCO – An investigational immunomodulator, IMM-101, improves outcomes in patients who have advanced pancreatic cancer when combined with chemotherapy, with minimal increase in toxicity, suggest data from an open-label, phase II trial conducted in Europe.
Results reported in a poster session at the annual Gastrointestinal Cancers Symposium cosponsored by the AGA Institute, the American Society of Clinical Oncology, ASTRO, and the Society of Surgical Oncology showed that median progression-free survival was about 1 month longer for patients given IMM-101 plus gemcitabine than for patients given gemcitabine alone; the gain exceeded 2 months among the subgroup having metastatic disease. The combination was associated with slightly higher rates of certain toxicities that seemed to stem from being on gemcitabine for longer.
“I think IMM-101 brings to the field something that’s missing,” lead investigator Dr. Angus G. Dalgleish, a professor and medical oncologist at St. George’s University of London, commented in an interview. “Everybody has been doing doublets, triplets, etcetera. All they do is guarantee increased toxicity and, in some cases, misery to the patient, whereas this allows you to start treatment and stabilize [the cancer].”
“This shows that actually boosting the immune response in a nonspecific manner before we do anything else may well become the gold standard when you add in any other treatment,” he added. “And I think it’s a non–chemo specific thing and a non–tumor specific thing, because we have done all the preliminary work in melanoma. We’ve also got very encouraging data in prostate and are starting prostate studies this year. And I would like to see it go into a whole host of ones where chemo needs a nonspecific boost, like ovarian [cancer].”
Of note, the trial opened just before FOLFIRINOX (irinotecan plus 5-fluorouracil plus leucovorin plus oxaliplatin) became available to patients in Europe, and many of the more fit eligible patients opted for that therapy instead. “I think this makes the survival benefit even more remarkable,” Dr. Dalgleish stated.
IMM-101 is a heat-killed whole-cell vaccine that is given intradermally and induces protective CD8 T-cell responses. “Although we had good data on this agent for melanoma, we noticed that it sort of boosted the immune system in all cancers [in which] we could show you get immune suppression,” he noted. Additionally, preclinical data hinted at synergy with gemcitabine.
The trial, known as IMAGE 1 (Immune Modulation And Gemcitabine Evaluation 1) and sponsored by manufacturer Immodulon Therapeutics, enrolled 110 patients with stage III or IV pancreatic cancer who had not previously received any chemotherapy. The patients were randomized 2:1 to receive gemcitabine (Gemzar) either with or without IMM-101.
Intention-to-treat analyses showed that median overall survival, the primary endpoint, was 6.7 months with IMM-101 and 5.6 months without it in the entire trial population (hazard ratio, 0.68; P = .075). The difference was significant among the subset of patients having metastatic disease (7.0 vs. 4.4 months; HR, 0.54; P = .010).
“You are getting a quick effect here, 2-4 months,” Dr. Dalgleish pointed out. “I’ve done several vaccine studies where we don’t even get the split [in survival curves] for 12 months. ... So that’s absolutely staggering that we get the split so early on.”
“In the metastatic group, the survival advantage is really quite dramatic,” he added. “And unlike many other studies that we have been involved in, you are getting this tail. ... these patients are still continuing to survive.”
One patient given the combination experienced progression and was then given nab-paclitaxel (Abraxane), and had a complete response to that agent, according to Dr. Dalgleish. He speculated that it may have been due to the immune priming by IMM-101.
In per protocol analyses, the survival benefit was significant in both the entire population and the patients with metastatic disease.
Patients in the IMM-101 group also had superior progression-free survival, seen in the entire trial population (4.4 vs. 2.4 months; HR, 0.51; P = .003) and in the subgroup with metastases (4.4 vs. 2.3 months; HR, 0.40; P less than .001).
Apart from injection site reactions in some patients, IMM-101 was well tolerated, according to Dr. Dalgleish.
“When you add gemcitabine plus ‘x’ – Iressa [gefitinib], capecitabine [Xeloda], Abraxane, etcetera – you get a benefit but at great cost,” he commented. In this trial, toxicity “is slightly worse in the combination arm, but the side effects are all those associated with the chemotherapy and the disease – nothing to do with the immune modulator, which we have given to hundreds of patients before. And that would appear to reflect the fact that those patients have been on gemcitabine for longer than the ones on gemcitabine alone.”
Quality of life data are still being analyzed, according to Dr. Dalgleish. “But ... from my personal experience, the patients do feel better on the vaccine as [it has] a kind of overall boosting effect.”
The investigators plan to take IMM-101 forward in clinical trials, he said. “But I think in the States, they might want to do that with Abraxane first line.” In the United Kingdom, the National Institute for Health and Care Excellence (NICE) recently declined Abraxane, meaning cost won’t be reimbursed if it is used, Dr. Dalgleish said. “So we may well be able to do the same study in Europe in order to get approval, although personally, I’d be very keen for conditional approval on this [trial] in the U.K. because it has a clear benefit without any toxicity. And there is a system where you can get a phase 4 approval and have postapproval monitoring.”
Dr. Dalgleish disclosed that he has a consulting or advisory role with and receives research funding from Immodulon Therapeutics.
AT THE GASTROINTESTINAL CANCERS SYMPOSIUM
Key clinical point: IMM-101 appears safe and efficacious when added to gemcitabine as first-line therapy.
Major finding: Median overall survival was 6.7 months with IMM-101 and 5.6 months without it.
Data source: A randomized, open-label, phase II trial among 110 patients with advanced pancreatic cancer.
Disclosures: Dr. Dalgleish disclosed that he has a consulting or advisory role with and receives research funding from Immodulon Therapeutics. The trial was sponsored by Immodulon Therapeutics.