User login
Nivolumab, an immune checkpoint modulator, acts by binding to the programmed cell death 1 (PD-1) receptor on T cells, which blocks the inhibition of T cells. Nivolumab ultimately leads to stimulation of the T-cell response1 and overcomes evasive adaptations of certain cancers. Cutaneous adverse events (AEs) have been reported in approximately 20% to 40% of patients treated with the anti–PD-1 class of drugs, including nivolumab.2-4 The most common cutaneous AEs include pruritus; vitiligo; and various forms of rash, such as lichenoid dermatitis, psoriasiform eruptions, and bullous pemphigoid.1-3,5-7 We report a patient with non–small cell lung cancer being treated with nivolumab who developed a bullous lichenoid eruption consistent with the diagnosis of lichen planus pemphigoides (LPP).
Case Report
An 87-year-old woman presented with a pruritic rash on the trunk and extremities of 3 weeks’ duration. Her medical history included stage IV non–small cell lung cancer, congestive heart failure, coronary artery disease, chronic kidney disease, and hypertension. Her long-term medications were ipratropium-albuterol, alendronate, amlodipine, aspirin, carvedilol, colesevelam, probiotic granules, and bumetanide. She was previously treated with carboplatin and docetaxel, which were discontinued secondary to fatigue, diarrhea, poor appetite, loss of taste, and a nonspecific rash. Six months later (approximately 3 months prior to the onset of cutaneous symptoms), she was started on nivolumab monotherapy every 14 days for a total of 9 infusions.
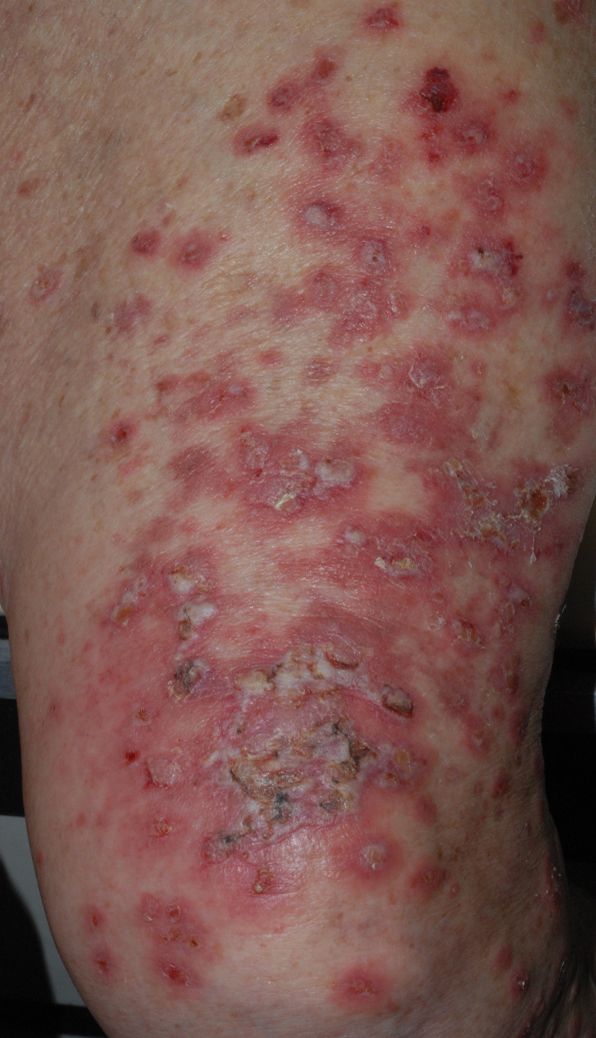
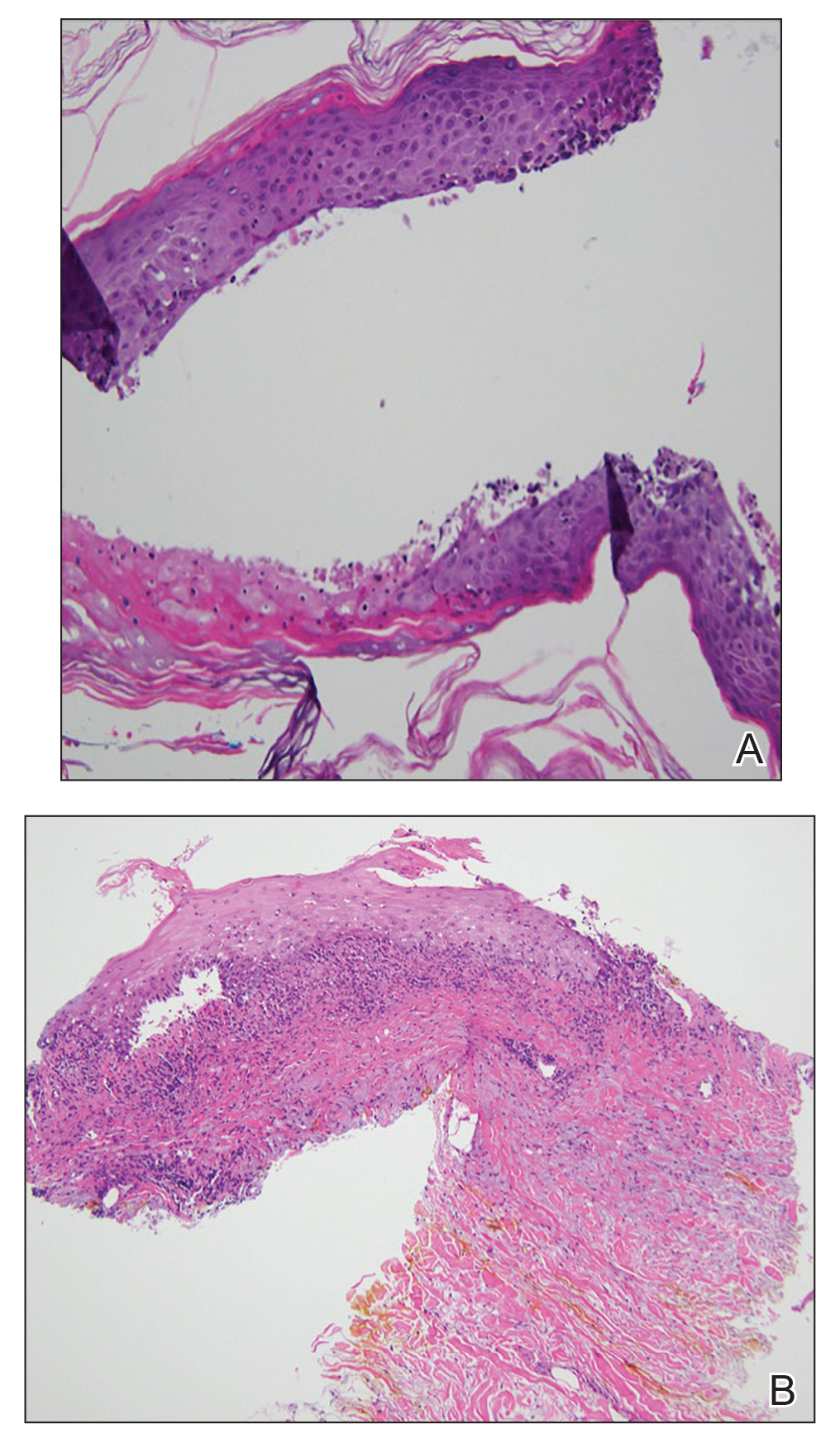
At the current presentation, physical examination revealed erythematous crusted erosions on the trunk and extremities and 1 flaccid bulla on the back. A punch biopsy revealed lichenoid dermatitis. The patient returned 2 weeks later with worsening of cutaneous manifestations, including more blisters and erosions. Figure 1 shows the clinical appearance of the eruption on the patient’s leg. At this time, additional biopsies revealed a subepidermal bullous lichenoid eruption with eosinophils (Figure 2). Direct immunofluorescence (DIF) was negative; however, indirect immunofluorescence (IIF) revealed weak linear staining for IgG antibodies along the basement membrane zone on monkey esophagus substrate. Examination of salt-split skin was noncontributory. The patient improved with a 2-week oral prednisone taper (starting at 40 mg daily). The dose was decreased incrementally over the course of 2 weeks from 40 mg to 20 mg to 0 mg. Because of the presumed grade 3 (severe) cutaneous drug eruption linked to nivolumab and further discussion with the medical oncology team, the patient decided to cease therapy. Since cessation of therapy, she has been seen twice for follow-up. At 2-month follow-up, she presented with drastic improvement of the eruption, and at 1 year she has continued to forego any further treatment for the stable and nonprogressing malignancy.
Widespread coalescent lesions with crusted and hemorrhagic bullae were present on the thigh and knee.
Comment
Immunotherapy
The interaction between the PD-1 receptor and its ligands, programmed death ligand 1 (PD-L1) and programmed death ligand 2, is an immune checkpoint.8,9 Under normal physiologic conditions, this checkpoint serves to prevent autoimmunity.10 When the PD-1 receptor is left unbound, T cells are more inclined to mount an immune response. If the receptor is ligand bound, the response of T cells is suppressed via mechanisms such as anergy or apoptosis.8 Tumor cells are known to produce PD-L1 as an adaptive resistance mechanism to evade immunity.8 Nivolumab is a human monoclonal antibody that targets the PD-1 receptor, thereby preventing the interaction with its ligand and allowing for unsuppressed activity of T cells.10 This therapy ultimately blocks the tumor’s local immune suppression mechanisms, which allows T cells to recognize cancer antigens.10
Adverse Events
Dermatologic AEs are among the most common with nivolumab treatment. In a pooled retrospective analysis of melanoma patients, Weber et al9 found that 34% of 576 patients experience cutaneous any-grade AEs associated with nivolumab treatment, most commonly pruritus. It has been well documented that anti–PD-1 therapy AEs of the skin as well as other organ systems have a delayed onset of at least 1 month.9 The average time of onset for bullous eruptions associated with anti–PD-1 therapy has been reported to be approximately 12 weeks, with a range of 7 to 16.1 weeks.11 Our patient had a bullous eruption with an onset of 12 weeks following initiation of treatment.
Although lichenoid reactions appear to be relatively common AEs of anti–PD-1 therapy,2,5,6 only a small number of cases of bullous pemphigoid eruptions have been reported.7 It has been hypothesized that blockade of the PD-1/PD-L1 pathway increases production of hemidesmosomal protein BP180 autoantibody, which is involved in the pathogenesis of LPP.7 Bullous eruptions have not been reported in the use of anticytotoxic T-lymphocyte–associated protein 4 agents, which could indicate that such eruptions are specific to the anti–PD-1 class of drugs.7
Diagnosis
Our patient represents a rare drug reaction involving both lichenoid and bullous components. Our differential diagnosis included drug-induced bullous lichen planus (BLP) and drug-induced LPP. Differentiation of these diagnoses can be difficult. In fact, in 2017 Fujii et al12 found reason to reprise the hypothesis that BLP is a transitional step toward LPP. The histologic evaluation of LPP differs depending on the type of lesion biopsied and can be indistinguishable from BLP as well as bullous pemphigoid. Therefore, clinical history and immunofluorescence should be used to make a diagnosis. Lichen planus pemphigoides typically will have linear IgG deposition along the basement membrane zone on both DIF and IIF, findings that will be negative in patients with BLP.13 Direct immunofluorescence findings in BLP include shaggy deposits of fibrin along the basement membrane zone. In this patient, DIF was negative, which may have been caused by variability among lesions in LPP, but IIF was positive. Given the clinicopathologic correlation, the diagnosis of LPP was made. Further studies, such as immunoblot and enzyme-linked immunosorbent assay, also can be used to aid diagnosis.
A similar presentation has been documented in a patient with metastatic melanoma.14 The diagnosis in this patient was LPP induced by pembrolizumab, which is another agent within the anti–PD-1 class. The Naranjo probability scale scored our patient’s eruption as a possible adverse drug reaction.15 Thus, other etiologies, such as a paraneoplastic process, cannot be completely ruled out. However, our patient has not had recurrence after 1 year, and the timing of the eruption appeared to be related to drug therapy, making alternative etiologies less likely.
Management
Cessation of nivolumab therapy and a short course of oral corticosteroid therapy led to marked improvement of symptoms. Given the emergent treatment of our patient, the resolution of her symptoms cannot be solely attributed to the cessation of nivolumab or to treatment with prednisone. Oral rather than topical corticosteroids were chosen because of the severity of the eruption. Topical corticosteroids and oral antihistamines can provide relief in less severe cases of bullous reactions to anti–PD-1 therapy.7,11 This regimen also has proven to be effective in lichenoid dermatitis induced by anti–PD-1.2
Conclusion
We hope this case report will contribute to the growing body of evidence regarding recognition and management of unique reactions to cancer immunotherapies.
- Macdonald JB, Macdonald B, Golitz LE, et al. Cutaneous adverse effects of targeted therapies: part II: inhibitors of intracellular molecular signaling pathways. J Am Acad Dermatol. 2015;72:221-236; quiz 237-238.
- Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12-25.
- Abdel-Rahman O, El Halawani H, Fouad M. Risk of cutaneous toxicities in patients with solid tumors treated with immune checkpoint inhibitors: a meta-analysis. Future Oncol. 2015;11:2471-2484.
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454.
- Hwang SJ, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort [published online January 12, 2016]. J Am Acad Dermatol. 2016;74:455-461.e1.
- Sibaud V, Meyer N, Lamant L, et al. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol. 2016;28:254-263.
- Naidoo J, Schindler K, Querfeld C, et al. Autoimmune bullous skin disorders with immune checkpoint inhibitors targeting PD-1 and PD-L1. Cancer Immunol Res. 2016;4:383-389.
- Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8:328rv4.
- Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35:785-792.
- Mamalis A, Garcha M, Jagdeo J. Targeting the PD-1 pathway: a promising future for the treatment of melanoma. Arch Dermatol Res. 2014;306:511-519.
- Jour G, Glitza IC, Ellis RM, et al. Autoimmune dermatologic toxicities from immune checkpoint blockade with anti-PD-1 antibody therapy: a report on bullous skin eruptions. J Cutan Pathol. 2016;43:688-696.
- Fujii M, Takahashi I, Honma M, et al. Bullous lichen planus accompanied by elevation of serum anti-BP180 autoantibody: a possible transitional mechanism to lichen planus pemphigoides. J Dermatol. 2017;44:E124-E125.
- Arbache ST, Nogueira TG, Delgado L, et al. Immunofluorescence testing in the diagnosis of autoimmune blistering diseases: overview of 10-year experience. An Bras Dermatol. 2014;89:885-889.
- Schmidgen MI, Butsch F, Schadmand-Fischer S, et al. Pembrolizumab-induced lichen planus pemphigoides in a patient with metastatic melanoma. J Dtsch Dermatol Ges. 2017;15:742-745.
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
Nivolumab, an immune checkpoint modulator, acts by binding to the programmed cell death 1 (PD-1) receptor on T cells, which blocks the inhibition of T cells. Nivolumab ultimately leads to stimulation of the T-cell response1 and overcomes evasive adaptations of certain cancers. Cutaneous adverse events (AEs) have been reported in approximately 20% to 40% of patients treated with the anti–PD-1 class of drugs, including nivolumab.2-4 The most common cutaneous AEs include pruritus; vitiligo; and various forms of rash, such as lichenoid dermatitis, psoriasiform eruptions, and bullous pemphigoid.1-3,5-7 We report a patient with non–small cell lung cancer being treated with nivolumab who developed a bullous lichenoid eruption consistent with the diagnosis of lichen planus pemphigoides (LPP).
Case Report
An 87-year-old woman presented with a pruritic rash on the trunk and extremities of 3 weeks’ duration. Her medical history included stage IV non–small cell lung cancer, congestive heart failure, coronary artery disease, chronic kidney disease, and hypertension. Her long-term medications were ipratropium-albuterol, alendronate, amlodipine, aspirin, carvedilol, colesevelam, probiotic granules, and bumetanide. She was previously treated with carboplatin and docetaxel, which were discontinued secondary to fatigue, diarrhea, poor appetite, loss of taste, and a nonspecific rash. Six months later (approximately 3 months prior to the onset of cutaneous symptoms), she was started on nivolumab monotherapy every 14 days for a total of 9 infusions.
At the current presentation, physical examination revealed erythematous crusted erosions on the trunk and extremities and 1 flaccid bulla on the back. A punch biopsy revealed lichenoid dermatitis. The patient returned 2 weeks later with worsening of cutaneous manifestations, including more blisters and erosions. Figure 1 shows the clinical appearance of the eruption on the patient’s leg. At this time, additional biopsies revealed a subepidermal bullous lichenoid eruption with eosinophils (Figure 2). Direct immunofluorescence (DIF) was negative; however, indirect immunofluorescence (IIF) revealed weak linear staining for IgG antibodies along the basement membrane zone on monkey esophagus substrate. Examination of salt-split skin was noncontributory. The patient improved with a 2-week oral prednisone taper (starting at 40 mg daily). The dose was decreased incrementally over the course of 2 weeks from 40 mg to 20 mg to 0 mg. Because of the presumed grade 3 (severe) cutaneous drug eruption linked to nivolumab and further discussion with the medical oncology team, the patient decided to cease therapy. Since cessation of therapy, she has been seen twice for follow-up. At 2-month follow-up, she presented with drastic improvement of the eruption, and at 1 year she has continued to forego any further treatment for the stable and nonprogressing malignancy.
Widespread coalescent lesions with crusted and hemorrhagic bullae were present on the thigh and knee.
Comment
Immunotherapy
The interaction between the PD-1 receptor and its ligands, programmed death ligand 1 (PD-L1) and programmed death ligand 2, is an immune checkpoint.8,9 Under normal physiologic conditions, this checkpoint serves to prevent autoimmunity.10 When the PD-1 receptor is left unbound, T cells are more inclined to mount an immune response. If the receptor is ligand bound, the response of T cells is suppressed via mechanisms such as anergy or apoptosis.8 Tumor cells are known to produce PD-L1 as an adaptive resistance mechanism to evade immunity.8 Nivolumab is a human monoclonal antibody that targets the PD-1 receptor, thereby preventing the interaction with its ligand and allowing for unsuppressed activity of T cells.10 This therapy ultimately blocks the tumor’s local immune suppression mechanisms, which allows T cells to recognize cancer antigens.10
Adverse Events
Dermatologic AEs are among the most common with nivolumab treatment. In a pooled retrospective analysis of melanoma patients, Weber et al9 found that 34% of 576 patients experience cutaneous any-grade AEs associated with nivolumab treatment, most commonly pruritus. It has been well documented that anti–PD-1 therapy AEs of the skin as well as other organ systems have a delayed onset of at least 1 month.9 The average time of onset for bullous eruptions associated with anti–PD-1 therapy has been reported to be approximately 12 weeks, with a range of 7 to 16.1 weeks.11 Our patient had a bullous eruption with an onset of 12 weeks following initiation of treatment.
Although lichenoid reactions appear to be relatively common AEs of anti–PD-1 therapy,2,5,6 only a small number of cases of bullous pemphigoid eruptions have been reported.7 It has been hypothesized that blockade of the PD-1/PD-L1 pathway increases production of hemidesmosomal protein BP180 autoantibody, which is involved in the pathogenesis of LPP.7 Bullous eruptions have not been reported in the use of anticytotoxic T-lymphocyte–associated protein 4 agents, which could indicate that such eruptions are specific to the anti–PD-1 class of drugs.7
Diagnosis
Our patient represents a rare drug reaction involving both lichenoid and bullous components. Our differential diagnosis included drug-induced bullous lichen planus (BLP) and drug-induced LPP. Differentiation of these diagnoses can be difficult. In fact, in 2017 Fujii et al12 found reason to reprise the hypothesis that BLP is a transitional step toward LPP. The histologic evaluation of LPP differs depending on the type of lesion biopsied and can be indistinguishable from BLP as well as bullous pemphigoid. Therefore, clinical history and immunofluorescence should be used to make a diagnosis. Lichen planus pemphigoides typically will have linear IgG deposition along the basement membrane zone on both DIF and IIF, findings that will be negative in patients with BLP.13 Direct immunofluorescence findings in BLP include shaggy deposits of fibrin along the basement membrane zone. In this patient, DIF was negative, which may have been caused by variability among lesions in LPP, but IIF was positive. Given the clinicopathologic correlation, the diagnosis of LPP was made. Further studies, such as immunoblot and enzyme-linked immunosorbent assay, also can be used to aid diagnosis.
A similar presentation has been documented in a patient with metastatic melanoma.14 The diagnosis in this patient was LPP induced by pembrolizumab, which is another agent within the anti–PD-1 class. The Naranjo probability scale scored our patient’s eruption as a possible adverse drug reaction.15 Thus, other etiologies, such as a paraneoplastic process, cannot be completely ruled out. However, our patient has not had recurrence after 1 year, and the timing of the eruption appeared to be related to drug therapy, making alternative etiologies less likely.
Management
Cessation of nivolumab therapy and a short course of oral corticosteroid therapy led to marked improvement of symptoms. Given the emergent treatment of our patient, the resolution of her symptoms cannot be solely attributed to the cessation of nivolumab or to treatment with prednisone. Oral rather than topical corticosteroids were chosen because of the severity of the eruption. Topical corticosteroids and oral antihistamines can provide relief in less severe cases of bullous reactions to anti–PD-1 therapy.7,11 This regimen also has proven to be effective in lichenoid dermatitis induced by anti–PD-1.2
Conclusion
We hope this case report will contribute to the growing body of evidence regarding recognition and management of unique reactions to cancer immunotherapies.
Nivolumab, an immune checkpoint modulator, acts by binding to the programmed cell death 1 (PD-1) receptor on T cells, which blocks the inhibition of T cells. Nivolumab ultimately leads to stimulation of the T-cell response1 and overcomes evasive adaptations of certain cancers. Cutaneous adverse events (AEs) have been reported in approximately 20% to 40% of patients treated with the anti–PD-1 class of drugs, including nivolumab.2-4 The most common cutaneous AEs include pruritus; vitiligo; and various forms of rash, such as lichenoid dermatitis, psoriasiform eruptions, and bullous pemphigoid.1-3,5-7 We report a patient with non–small cell lung cancer being treated with nivolumab who developed a bullous lichenoid eruption consistent with the diagnosis of lichen planus pemphigoides (LPP).
Case Report
An 87-year-old woman presented with a pruritic rash on the trunk and extremities of 3 weeks’ duration. Her medical history included stage IV non–small cell lung cancer, congestive heart failure, coronary artery disease, chronic kidney disease, and hypertension. Her long-term medications were ipratropium-albuterol, alendronate, amlodipine, aspirin, carvedilol, colesevelam, probiotic granules, and bumetanide. She was previously treated with carboplatin and docetaxel, which were discontinued secondary to fatigue, diarrhea, poor appetite, loss of taste, and a nonspecific rash. Six months later (approximately 3 months prior to the onset of cutaneous symptoms), she was started on nivolumab monotherapy every 14 days for a total of 9 infusions.
At the current presentation, physical examination revealed erythematous crusted erosions on the trunk and extremities and 1 flaccid bulla on the back. A punch biopsy revealed lichenoid dermatitis. The patient returned 2 weeks later with worsening of cutaneous manifestations, including more blisters and erosions. Figure 1 shows the clinical appearance of the eruption on the patient’s leg. At this time, additional biopsies revealed a subepidermal bullous lichenoid eruption with eosinophils (Figure 2). Direct immunofluorescence (DIF) was negative; however, indirect immunofluorescence (IIF) revealed weak linear staining for IgG antibodies along the basement membrane zone on monkey esophagus substrate. Examination of salt-split skin was noncontributory. The patient improved with a 2-week oral prednisone taper (starting at 40 mg daily). The dose was decreased incrementally over the course of 2 weeks from 40 mg to 20 mg to 0 mg. Because of the presumed grade 3 (severe) cutaneous drug eruption linked to nivolumab and further discussion with the medical oncology team, the patient decided to cease therapy. Since cessation of therapy, she has been seen twice for follow-up. At 2-month follow-up, she presented with drastic improvement of the eruption, and at 1 year she has continued to forego any further treatment for the stable and nonprogressing malignancy.
Widespread coalescent lesions with crusted and hemorrhagic bullae were present on the thigh and knee.
Comment
Immunotherapy
The interaction between the PD-1 receptor and its ligands, programmed death ligand 1 (PD-L1) and programmed death ligand 2, is an immune checkpoint.8,9 Under normal physiologic conditions, this checkpoint serves to prevent autoimmunity.10 When the PD-1 receptor is left unbound, T cells are more inclined to mount an immune response. If the receptor is ligand bound, the response of T cells is suppressed via mechanisms such as anergy or apoptosis.8 Tumor cells are known to produce PD-L1 as an adaptive resistance mechanism to evade immunity.8 Nivolumab is a human monoclonal antibody that targets the PD-1 receptor, thereby preventing the interaction with its ligand and allowing for unsuppressed activity of T cells.10 This therapy ultimately blocks the tumor’s local immune suppression mechanisms, which allows T cells to recognize cancer antigens.10
Adverse Events
Dermatologic AEs are among the most common with nivolumab treatment. In a pooled retrospective analysis of melanoma patients, Weber et al9 found that 34% of 576 patients experience cutaneous any-grade AEs associated with nivolumab treatment, most commonly pruritus. It has been well documented that anti–PD-1 therapy AEs of the skin as well as other organ systems have a delayed onset of at least 1 month.9 The average time of onset for bullous eruptions associated with anti–PD-1 therapy has been reported to be approximately 12 weeks, with a range of 7 to 16.1 weeks.11 Our patient had a bullous eruption with an onset of 12 weeks following initiation of treatment.
Although lichenoid reactions appear to be relatively common AEs of anti–PD-1 therapy,2,5,6 only a small number of cases of bullous pemphigoid eruptions have been reported.7 It has been hypothesized that blockade of the PD-1/PD-L1 pathway increases production of hemidesmosomal protein BP180 autoantibody, which is involved in the pathogenesis of LPP.7 Bullous eruptions have not been reported in the use of anticytotoxic T-lymphocyte–associated protein 4 agents, which could indicate that such eruptions are specific to the anti–PD-1 class of drugs.7
Diagnosis
Our patient represents a rare drug reaction involving both lichenoid and bullous components. Our differential diagnosis included drug-induced bullous lichen planus (BLP) and drug-induced LPP. Differentiation of these diagnoses can be difficult. In fact, in 2017 Fujii et al12 found reason to reprise the hypothesis that BLP is a transitional step toward LPP. The histologic evaluation of LPP differs depending on the type of lesion biopsied and can be indistinguishable from BLP as well as bullous pemphigoid. Therefore, clinical history and immunofluorescence should be used to make a diagnosis. Lichen planus pemphigoides typically will have linear IgG deposition along the basement membrane zone on both DIF and IIF, findings that will be negative in patients with BLP.13 Direct immunofluorescence findings in BLP include shaggy deposits of fibrin along the basement membrane zone. In this patient, DIF was negative, which may have been caused by variability among lesions in LPP, but IIF was positive. Given the clinicopathologic correlation, the diagnosis of LPP was made. Further studies, such as immunoblot and enzyme-linked immunosorbent assay, also can be used to aid diagnosis.
A similar presentation has been documented in a patient with metastatic melanoma.14 The diagnosis in this patient was LPP induced by pembrolizumab, which is another agent within the anti–PD-1 class. The Naranjo probability scale scored our patient’s eruption as a possible adverse drug reaction.15 Thus, other etiologies, such as a paraneoplastic process, cannot be completely ruled out. However, our patient has not had recurrence after 1 year, and the timing of the eruption appeared to be related to drug therapy, making alternative etiologies less likely.
Management
Cessation of nivolumab therapy and a short course of oral corticosteroid therapy led to marked improvement of symptoms. Given the emergent treatment of our patient, the resolution of her symptoms cannot be solely attributed to the cessation of nivolumab or to treatment with prednisone. Oral rather than topical corticosteroids were chosen because of the severity of the eruption. Topical corticosteroids and oral antihistamines can provide relief in less severe cases of bullous reactions to anti–PD-1 therapy.7,11 This regimen also has proven to be effective in lichenoid dermatitis induced by anti–PD-1.2
Conclusion
We hope this case report will contribute to the growing body of evidence regarding recognition and management of unique reactions to cancer immunotherapies.
- Macdonald JB, Macdonald B, Golitz LE, et al. Cutaneous adverse effects of targeted therapies: part II: inhibitors of intracellular molecular signaling pathways. J Am Acad Dermatol. 2015;72:221-236; quiz 237-238.
- Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12-25.
- Abdel-Rahman O, El Halawani H, Fouad M. Risk of cutaneous toxicities in patients with solid tumors treated with immune checkpoint inhibitors: a meta-analysis. Future Oncol. 2015;11:2471-2484.
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454.
- Hwang SJ, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort [published online January 12, 2016]. J Am Acad Dermatol. 2016;74:455-461.e1.
- Sibaud V, Meyer N, Lamant L, et al. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol. 2016;28:254-263.
- Naidoo J, Schindler K, Querfeld C, et al. Autoimmune bullous skin disorders with immune checkpoint inhibitors targeting PD-1 and PD-L1. Cancer Immunol Res. 2016;4:383-389.
- Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8:328rv4.
- Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35:785-792.
- Mamalis A, Garcha M, Jagdeo J. Targeting the PD-1 pathway: a promising future for the treatment of melanoma. Arch Dermatol Res. 2014;306:511-519.
- Jour G, Glitza IC, Ellis RM, et al. Autoimmune dermatologic toxicities from immune checkpoint blockade with anti-PD-1 antibody therapy: a report on bullous skin eruptions. J Cutan Pathol. 2016;43:688-696.
- Fujii M, Takahashi I, Honma M, et al. Bullous lichen planus accompanied by elevation of serum anti-BP180 autoantibody: a possible transitional mechanism to lichen planus pemphigoides. J Dermatol. 2017;44:E124-E125.
- Arbache ST, Nogueira TG, Delgado L, et al. Immunofluorescence testing in the diagnosis of autoimmune blistering diseases: overview of 10-year experience. An Bras Dermatol. 2014;89:885-889.
- Schmidgen MI, Butsch F, Schadmand-Fischer S, et al. Pembrolizumab-induced lichen planus pemphigoides in a patient with metastatic melanoma. J Dtsch Dermatol Ges. 2017;15:742-745.
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
- Macdonald JB, Macdonald B, Golitz LE, et al. Cutaneous adverse effects of targeted therapies: part II: inhibitors of intracellular molecular signaling pathways. J Am Acad Dermatol. 2015;72:221-236; quiz 237-238.
- Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12-25.
- Abdel-Rahman O, El Halawani H, Fouad M. Risk of cutaneous toxicities in patients with solid tumors treated with immune checkpoint inhibitors: a meta-analysis. Future Oncol. 2015;11:2471-2484.
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454.
- Hwang SJ, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort [published online January 12, 2016]. J Am Acad Dermatol. 2016;74:455-461.e1.
- Sibaud V, Meyer N, Lamant L, et al. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol. 2016;28:254-263.
- Naidoo J, Schindler K, Querfeld C, et al. Autoimmune bullous skin disorders with immune checkpoint inhibitors targeting PD-1 and PD-L1. Cancer Immunol Res. 2016;4:383-389.
- Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8:328rv4.
- Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35:785-792.
- Mamalis A, Garcha M, Jagdeo J. Targeting the PD-1 pathway: a promising future for the treatment of melanoma. Arch Dermatol Res. 2014;306:511-519.
- Jour G, Glitza IC, Ellis RM, et al. Autoimmune dermatologic toxicities from immune checkpoint blockade with anti-PD-1 antibody therapy: a report on bullous skin eruptions. J Cutan Pathol. 2016;43:688-696.
- Fujii M, Takahashi I, Honma M, et al. Bullous lichen planus accompanied by elevation of serum anti-BP180 autoantibody: a possible transitional mechanism to lichen planus pemphigoides. J Dermatol. 2017;44:E124-E125.
- Arbache ST, Nogueira TG, Delgado L, et al. Immunofluorescence testing in the diagnosis of autoimmune blistering diseases: overview of 10-year experience. An Bras Dermatol. 2014;89:885-889.
- Schmidgen MI, Butsch F, Schadmand-Fischer S, et al. Pembrolizumab-induced lichen planus pemphigoides in a patient with metastatic melanoma. J Dtsch Dermatol Ges. 2017;15:742-745.
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
Practice Points
- Dermatologists should be aware that lichen planus pemphigoides is within the spectrum of toxicity for patients treated with nivolumab.
- Bullous eruptions related to anti–programmed cell death 1 agents tend to appear 4 months after initiation of therapy.
- A severe cutaneous toxicity of a checkpoint inhibitor should be managed using oral corticosteroids with consideration of withdrawing the offending agent.