User login
Despite repeated warnings for more than 25 years by the Institute for Safe Medication Practices (ISMP) and other organizations, one of the major causes of medication errors is the ongoing use of potentially dangerous abbreviations and dose expressions. Root cause analyses information contained in the Joint Commission Sentinel Event Database shows that the underlying factors contributing to many of these medication errors are illegible or confusing handwriting by clinicians and the failure of healthcare providers to communicate clearly with one another.
Symbols and abbreviations are frequently used to save time and effort when writing prescriptions and documenting in patient charts; however, some symbols and abbreviations have the potential for misinterpretation or confusion. Examples of especially problematic abbreviations include “U” for “units” and “µg” for “micrograms.” When “U” is handwritten, it can often look like a zero. There are numerous case reports where the root cause of sentinel events related to insulin dosage has been the interpretation of a “U” as a zero. Using the abbreviation “µg” instead of “mcg” has also been the source of errors because when handwritten, the symbol “µ” can look like an “m.” The use of trailing zeros (e.g., 2.0 versus 2) or use of a leading decimal point without a leading zero (e.g. .2 instead of 0.2) are other dangerous order-writing practices. The decimal point is sometimes not seen when orders are handwritten using trailing zeros or no leading zeros. Misinterpretation of such orders could lead to a 10-fold dosing error.
A New Approach
As part of efforts to improve patient safety, the Joint Commission has long worked with hospitals to develop practical, cost-effective strategies that can be implemented at organizations regardless of unique characteristics, such as ownership, size, or location. One such Joint Commission initiative is a National Patient Safety Goal to improve communication. This goal and one of its requirements specifically addresses the role that abbreviations, acronyms, symbols, and dose designations play in medication errors.
The Joint Commission began establishing National Patient Safety Goals in 2002 as a means to target critical areas where patient safety can be improved through specific action in healthcare organizations. The resulting National Patient Safety Goals are designed to give focus to evidence-based or expert consensus-based, well-defined, practical, and cost-effective actions that have potential for significant improvement in the safety of individuals receiving care. New Goals are recommended annually by the Sentinel Event Advisory Group, a Joint Commission-appointed, multidisciplinary group of patient safety experts.
JCAHO Expectations
In order to comply with the National Patient Safety Goal related to abbreviations, an organization must conduct a thorough review of its approved abbreviation list and develop a list of unacceptable abbreviations and symbols with the involvement of physicians. In addition, organizations must do the following to meet this goal:
- The list of prohibited abbreviations, acronyms, symbols, and dose designations must be implemented for all handwritten, patient-specific communications, not just medication orders;
- These requirements apply to printed or electronic communications;
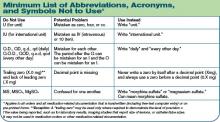
- This goal requires organizations to achieve 100% compliance with a reasonably comprehensive list of prohibited dangerous abbreviations, acronyms, symbols, and dose designations. This list need not be as extensive as some published lists, but must, at a minimum, include a set of Joint Commission-specified dangerous abbreviations, acro-nyms, symbols, and dose designations (see “Minimum List of Abbreviations, Acronyms, and Symbols Not to Use,” top right) and
- An abbreviation on the “do not use” list should not be used in any of its forms—uppercase or lowercase, with or without periods.
In addition to this minimum list, each organization should consider which abbreviations, acronyms, symbols, and dose designations it commonly uses; examine the risks associated with usage; and develop strategies to reduce usage. Hospitals also may wish to look to expert resources such as the Institute of Safe Medication Practices (ISMP)—available at www.ismp.org/Tools/abbreviationslist.pdf—and the National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP)—available at www.nccmerp.org/dangerousAbbrev.html—to develop a list of prohibited abbreviations. Finally, organizations may wish to consider the Joint Commission list (see table below) of abbreviations, symbols, and acronyms for future possible inclusion in the official “do not use” list.
Risk Reduction Strategies
To comply with this National Patient Safety Goal Requirement, hospitals may wish to consider implementing the following risk reduction strategies:
- Examine medication error data in the organization. By identifying and selecting an organization-specific set of prohibited abbreviations, it will be much easier to gain support to eliminate certain abbreviations that have been found to be problematic at that organization.
- Provide simplified alternative abbreviations. For example, some staff members may resist writing out “international units” in place of “IU.” A simpler alternative such as “Intl Units” may be a solution.
- Make the list visible. Print the list on brightly colored paper or stickers and place it in patient charts.
- Provide staff with pocket-sized cards with the “do not use” list.
- Print the list in the margin or bottom of the physician order sheets and/or progress notes.
- Attach laminated copies of the list to the back of the physician order divider in the patient chart.
- Send monthly reminders to staff.
- Delete prohibited abbreviations from preprinted order sheets and other forms.
- Work with software vendors to ensure changes are made to be consistent with the list.
- Take a digital picture or scan the document containing the prohibited abbreviation and send it via e-mail directly to the offending prescriber to call attention to the issue.
- Direct the pharmacy not to accept any of the prohibited abbreviations. Orders with dangerous abbreviations or illegible handwriting must be corrected before being dispensed.
- Conduct a mock survey to test staff knowledge.
- At every staff meeting give patient safety updates, including information about the prohibited abbreviations.
- Ask all staff to sign a statement that he or she has received the list and agrees not to use the abbreviations.
- Promote a “do-not-use abbreviation of the month” policy.
- Develop and implement a policy to ensure that staff refer to the list and take steps to ensure compliance. Consider including a policy that states if an unacceptable abbreviation is used, the prescriber verifies the prescription order before it is filled.
- Monitor staff compliance with the list and offer additional education and training, as appropriate.
Conclusion
During the past decade, healthcare providers have been searching for more effective ways to reduce the risk of systems breakdowns that result in serious harm to patients. The Joint Commission is committed to working with organizations through the accreditation process on ways to anticipate and prevent errors. National Patient Safety Goals, such as the one associated with prohibited abbreviations, are one such method to promote specific improvements in patient safety. By using the principals of sound system design, organizations and providers further strengthen foundations that support safe, high-quality care. TH
Dr. Jacott is special advisor for professional relations for the Joint Commission on Accreditation of Healthcare Organizations.
Despite repeated warnings for more than 25 years by the Institute for Safe Medication Practices (ISMP) and other organizations, one of the major causes of medication errors is the ongoing use of potentially dangerous abbreviations and dose expressions. Root cause analyses information contained in the Joint Commission Sentinel Event Database shows that the underlying factors contributing to many of these medication errors are illegible or confusing handwriting by clinicians and the failure of healthcare providers to communicate clearly with one another.
Symbols and abbreviations are frequently used to save time and effort when writing prescriptions and documenting in patient charts; however, some symbols and abbreviations have the potential for misinterpretation or confusion. Examples of especially problematic abbreviations include “U” for “units” and “µg” for “micrograms.” When “U” is handwritten, it can often look like a zero. There are numerous case reports where the root cause of sentinel events related to insulin dosage has been the interpretation of a “U” as a zero. Using the abbreviation “µg” instead of “mcg” has also been the source of errors because when handwritten, the symbol “µ” can look like an “m.” The use of trailing zeros (e.g., 2.0 versus 2) or use of a leading decimal point without a leading zero (e.g. .2 instead of 0.2) are other dangerous order-writing practices. The decimal point is sometimes not seen when orders are handwritten using trailing zeros or no leading zeros. Misinterpretation of such orders could lead to a 10-fold dosing error.
A New Approach
As part of efforts to improve patient safety, the Joint Commission has long worked with hospitals to develop practical, cost-effective strategies that can be implemented at organizations regardless of unique characteristics, such as ownership, size, or location. One such Joint Commission initiative is a National Patient Safety Goal to improve communication. This goal and one of its requirements specifically addresses the role that abbreviations, acronyms, symbols, and dose designations play in medication errors.
The Joint Commission began establishing National Patient Safety Goals in 2002 as a means to target critical areas where patient safety can be improved through specific action in healthcare organizations. The resulting National Patient Safety Goals are designed to give focus to evidence-based or expert consensus-based, well-defined, practical, and cost-effective actions that have potential for significant improvement in the safety of individuals receiving care. New Goals are recommended annually by the Sentinel Event Advisory Group, a Joint Commission-appointed, multidisciplinary group of patient safety experts.
JCAHO Expectations
In order to comply with the National Patient Safety Goal related to abbreviations, an organization must conduct a thorough review of its approved abbreviation list and develop a list of unacceptable abbreviations and symbols with the involvement of physicians. In addition, organizations must do the following to meet this goal:
- The list of prohibited abbreviations, acronyms, symbols, and dose designations must be implemented for all handwritten, patient-specific communications, not just medication orders;
- These requirements apply to printed or electronic communications;
- This goal requires organizations to achieve 100% compliance with a reasonably comprehensive list of prohibited dangerous abbreviations, acronyms, symbols, and dose designations. This list need not be as extensive as some published lists, but must, at a minimum, include a set of Joint Commission-specified dangerous abbreviations, acro-nyms, symbols, and dose designations (see “Minimum List of Abbreviations, Acronyms, and Symbols Not to Use,” top right) and
- An abbreviation on the “do not use” list should not be used in any of its forms—uppercase or lowercase, with or without periods.
In addition to this minimum list, each organization should consider which abbreviations, acronyms, symbols, and dose designations it commonly uses; examine the risks associated with usage; and develop strategies to reduce usage. Hospitals also may wish to look to expert resources such as the Institute of Safe Medication Practices (ISMP)—available at www.ismp.org/Tools/abbreviationslist.pdf—and the National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP)—available at www.nccmerp.org/dangerousAbbrev.html—to develop a list of prohibited abbreviations. Finally, organizations may wish to consider the Joint Commission list (see table below) of abbreviations, symbols, and acronyms for future possible inclusion in the official “do not use” list.
Risk Reduction Strategies
To comply with this National Patient Safety Goal Requirement, hospitals may wish to consider implementing the following risk reduction strategies:
- Examine medication error data in the organization. By identifying and selecting an organization-specific set of prohibited abbreviations, it will be much easier to gain support to eliminate certain abbreviations that have been found to be problematic at that organization.
- Provide simplified alternative abbreviations. For example, some staff members may resist writing out “international units” in place of “IU.” A simpler alternative such as “Intl Units” may be a solution.
- Make the list visible. Print the list on brightly colored paper or stickers and place it in patient charts.
- Provide staff with pocket-sized cards with the “do not use” list.
- Print the list in the margin or bottom of the physician order sheets and/or progress notes.
- Attach laminated copies of the list to the back of the physician order divider in the patient chart.
- Send monthly reminders to staff.
- Delete prohibited abbreviations from preprinted order sheets and other forms.
- Work with software vendors to ensure changes are made to be consistent with the list.
- Take a digital picture or scan the document containing the prohibited abbreviation and send it via e-mail directly to the offending prescriber to call attention to the issue.
- Direct the pharmacy not to accept any of the prohibited abbreviations. Orders with dangerous abbreviations or illegible handwriting must be corrected before being dispensed.
- Conduct a mock survey to test staff knowledge.
- At every staff meeting give patient safety updates, including information about the prohibited abbreviations.
- Ask all staff to sign a statement that he or she has received the list and agrees not to use the abbreviations.
- Promote a “do-not-use abbreviation of the month” policy.
- Develop and implement a policy to ensure that staff refer to the list and take steps to ensure compliance. Consider including a policy that states if an unacceptable abbreviation is used, the prescriber verifies the prescription order before it is filled.
- Monitor staff compliance with the list and offer additional education and training, as appropriate.
Conclusion
During the past decade, healthcare providers have been searching for more effective ways to reduce the risk of systems breakdowns that result in serious harm to patients. The Joint Commission is committed to working with organizations through the accreditation process on ways to anticipate and prevent errors. National Patient Safety Goals, such as the one associated with prohibited abbreviations, are one such method to promote specific improvements in patient safety. By using the principals of sound system design, organizations and providers further strengthen foundations that support safe, high-quality care. TH
Dr. Jacott is special advisor for professional relations for the Joint Commission on Accreditation of Healthcare Organizations.
Despite repeated warnings for more than 25 years by the Institute for Safe Medication Practices (ISMP) and other organizations, one of the major causes of medication errors is the ongoing use of potentially dangerous abbreviations and dose expressions. Root cause analyses information contained in the Joint Commission Sentinel Event Database shows that the underlying factors contributing to many of these medication errors are illegible or confusing handwriting by clinicians and the failure of healthcare providers to communicate clearly with one another.
Symbols and abbreviations are frequently used to save time and effort when writing prescriptions and documenting in patient charts; however, some symbols and abbreviations have the potential for misinterpretation or confusion. Examples of especially problematic abbreviations include “U” for “units” and “µg” for “micrograms.” When “U” is handwritten, it can often look like a zero. There are numerous case reports where the root cause of sentinel events related to insulin dosage has been the interpretation of a “U” as a zero. Using the abbreviation “µg” instead of “mcg” has also been the source of errors because when handwritten, the symbol “µ” can look like an “m.” The use of trailing zeros (e.g., 2.0 versus 2) or use of a leading decimal point without a leading zero (e.g. .2 instead of 0.2) are other dangerous order-writing practices. The decimal point is sometimes not seen when orders are handwritten using trailing zeros or no leading zeros. Misinterpretation of such orders could lead to a 10-fold dosing error.
A New Approach
As part of efforts to improve patient safety, the Joint Commission has long worked with hospitals to develop practical, cost-effective strategies that can be implemented at organizations regardless of unique characteristics, such as ownership, size, or location. One such Joint Commission initiative is a National Patient Safety Goal to improve communication. This goal and one of its requirements specifically addresses the role that abbreviations, acronyms, symbols, and dose designations play in medication errors.
The Joint Commission began establishing National Patient Safety Goals in 2002 as a means to target critical areas where patient safety can be improved through specific action in healthcare organizations. The resulting National Patient Safety Goals are designed to give focus to evidence-based or expert consensus-based, well-defined, practical, and cost-effective actions that have potential for significant improvement in the safety of individuals receiving care. New Goals are recommended annually by the Sentinel Event Advisory Group, a Joint Commission-appointed, multidisciplinary group of patient safety experts.
JCAHO Expectations
In order to comply with the National Patient Safety Goal related to abbreviations, an organization must conduct a thorough review of its approved abbreviation list and develop a list of unacceptable abbreviations and symbols with the involvement of physicians. In addition, organizations must do the following to meet this goal:
- The list of prohibited abbreviations, acronyms, symbols, and dose designations must be implemented for all handwritten, patient-specific communications, not just medication orders;
- These requirements apply to printed or electronic communications;
- This goal requires organizations to achieve 100% compliance with a reasonably comprehensive list of prohibited dangerous abbreviations, acronyms, symbols, and dose designations. This list need not be as extensive as some published lists, but must, at a minimum, include a set of Joint Commission-specified dangerous abbreviations, acro-nyms, symbols, and dose designations (see “Minimum List of Abbreviations, Acronyms, and Symbols Not to Use,” top right) and
- An abbreviation on the “do not use” list should not be used in any of its forms—uppercase or lowercase, with or without periods.
In addition to this minimum list, each organization should consider which abbreviations, acronyms, symbols, and dose designations it commonly uses; examine the risks associated with usage; and develop strategies to reduce usage. Hospitals also may wish to look to expert resources such as the Institute of Safe Medication Practices (ISMP)—available at www.ismp.org/Tools/abbreviationslist.pdf—and the National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP)—available at www.nccmerp.org/dangerousAbbrev.html—to develop a list of prohibited abbreviations. Finally, organizations may wish to consider the Joint Commission list (see table below) of abbreviations, symbols, and acronyms for future possible inclusion in the official “do not use” list.
Risk Reduction Strategies
To comply with this National Patient Safety Goal Requirement, hospitals may wish to consider implementing the following risk reduction strategies:
- Examine medication error data in the organization. By identifying and selecting an organization-specific set of prohibited abbreviations, it will be much easier to gain support to eliminate certain abbreviations that have been found to be problematic at that organization.
- Provide simplified alternative abbreviations. For example, some staff members may resist writing out “international units” in place of “IU.” A simpler alternative such as “Intl Units” may be a solution.
- Make the list visible. Print the list on brightly colored paper or stickers and place it in patient charts.
- Provide staff with pocket-sized cards with the “do not use” list.
- Print the list in the margin or bottom of the physician order sheets and/or progress notes.
- Attach laminated copies of the list to the back of the physician order divider in the patient chart.
- Send monthly reminders to staff.
- Delete prohibited abbreviations from preprinted order sheets and other forms.
- Work with software vendors to ensure changes are made to be consistent with the list.
- Take a digital picture or scan the document containing the prohibited abbreviation and send it via e-mail directly to the offending prescriber to call attention to the issue.
- Direct the pharmacy not to accept any of the prohibited abbreviations. Orders with dangerous abbreviations or illegible handwriting must be corrected before being dispensed.
- Conduct a mock survey to test staff knowledge.
- At every staff meeting give patient safety updates, including information about the prohibited abbreviations.
- Ask all staff to sign a statement that he or she has received the list and agrees not to use the abbreviations.
- Promote a “do-not-use abbreviation of the month” policy.
- Develop and implement a policy to ensure that staff refer to the list and take steps to ensure compliance. Consider including a policy that states if an unacceptable abbreviation is used, the prescriber verifies the prescription order before it is filled.
- Monitor staff compliance with the list and offer additional education and training, as appropriate.
Conclusion
During the past decade, healthcare providers have been searching for more effective ways to reduce the risk of systems breakdowns that result in serious harm to patients. The Joint Commission is committed to working with organizations through the accreditation process on ways to anticipate and prevent errors. National Patient Safety Goals, such as the one associated with prohibited abbreviations, are one such method to promote specific improvements in patient safety. By using the principals of sound system design, organizations and providers further strengthen foundations that support safe, high-quality care. TH
Dr. Jacott is special advisor for professional relations for the Joint Commission on Accreditation of Healthcare Organizations.