User login
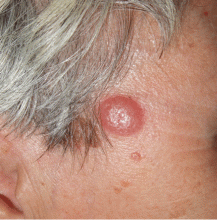
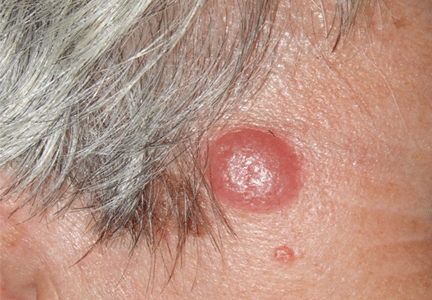
Q: Which is the most likely diagnosis?
- Basal cell carcinoma
- Squamous cell carcinoma
- Lymphocytoma cutis
- Amelanotic melanoma
- Pyogenic granuloma
A: The correct answer is lymphocytoma cutis. The differential diagnosis of a pink papule on the face of a middle-aged person includes nonmelanoma skin cancer, lymphoma, lymphocytoma cutis, metastatic disease, certain infections, Jessner lymphocytic infiltrate, connective tissue disease, and some adnexal tumors. Histologic study is a useful diagnostic aid in this context.
Basal cell carcinoma is the most common cutaneous malignant neoplasm, and although these tumors rarely metastasize, they are capable of gross tissue destruction, particularly those lesions arising on the face. Clinically, this tumor presents as a shiny, pearly nodule with telangiectasias on the surface, as in our patient, but skin biopsy shows large basaloid lobules of varying shape and size forming a relatively circumscribed mass with a “palisade” around the rim of the lobule.
Squamous cell carcinoma manifests as shallow ulcers, often with a keratinous crust and elevated, indurate borders, but also as plaques or nodules. The clinical diagnosis should be confirmed with skin biopsy, which reveals atypical keratinocytes extending from the epidermis to the dermis with dyskeratosis, intercellular bridges, variable central keratinization, and horn pearl formation, depending on the differentiation of the tumor.
Amelanotic melanoma is nonpigmented and appears as a pink nodule mimicking basal cell carcinoma or squamous cell carcinoma. Histologic study is necessary for the diagnosis, and shows an atypical proliferation of melanocytic cells in the epidermis and dermis.
Pyogenic granuloma is a very common benign vascular lesion considered to be a hyperplastic process or a vascular neoplasm. The lesion typically presents as a red or bluish papule or polyp that bleeds easily, and a reddish homogeneous area surrounded by a white “collarette” is found in most cases. Histologic features of an early lesion resemble granulation tissue and include lobules of capillaries and venules that often radiate from larger, more central vessels.
LYMPHOCYTOMA CUTIS: KEY FEATURES
Lymphocytoma cutis (pseudolymphoma) is a benign reactive polyclonal and inflammatory disorder that most frequently includes B lymphocytes, with a smaller population of T lymphocytes. It infiltrates the skin and resembles rudimentary germinal follicles, as in the present case. The lesion usually presents as an asymptomatic red-brown or violet papule or nodule, 3 mm to 5 cm in diameter, most often on the face, chest, or upper extremities.1 The lesion may be solitary, as in our patient, but lesions may also be grouped or numerous and widespread. It is three times more common in women than in men. It may resolve spontaneously, but it may also recur.
In Europe, lymphocytoma cutis occurs most often in B burgdorferi infection after a tick bite. Lymphocytoma cutis occurs in 1.3% of cases of B burgdorferi infection,2 although other infectious, physical, or chemical agents may produce the same reaction pattern. Tattooing (particularly red areas), acupuncture, vaccination, arthropod reactions, hyposensitization antigen reaction, and ingestion of drug have been implicated in this form of lymphoid hyperplasia.3,4
DIAGNOSTIC CHALLENGES
Lymphocytoma cutis can be challenging to diagnose, and although it can be suspected clinically, incisional biopsy is usually necessary in order to differentiate it from cutaneous B lymphoma.5
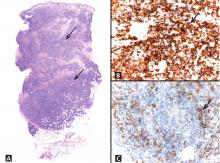
The infiltrate is predominantly nodular (> 90%) and located in the upper and mid dermis (“top heavy”) in lymphocytoma cutis, whereas it can be nodular or diffuse in cutaneous B lymphoma, with sharply demarcated borders that are convex rather than concave. Lymphoid follicles with germinal centers are sometimes present, and the interfollicular cellular population is polymorphic in lymphocytoma cutis (lymphocytes, plasma cells, histiocytes, eosinophils). In lymphocytoma cutis, cells express the phenotype of mature B lymphocytes (CD20, CD79a) and show regular and sharply demarcated networks of CD21+ follicular dendritic cells, whereas in cutaneous B lymphoma these networks are irregular. Light chains are usually polyclonal, although monoclonal populations of B cell in cases of cutaneous lymphocytoma cutis have been described. Extracutaneous involvement is possible in cutaneous B lymphoma but is usually absent in lymphocytoma cutis.
Lymphocytoma cutis typically involutes over a period of months, even with no treatment, as it did in our patient. Otherwise, there are different therapeutic options, including intralesional and topical corticosteroids, surgery, and cryosurgery.6 Photodynamic therapy with delta-aminolevulinic acid is an effective and safe modality for the treatment of lymphocytoma cutis and may be cosmetically beneficial.7
- Ploysangam T, Breneman DL, Mutasim DF. Cutaneous pseudolymphomas. J Am Acad Dermatol 1998; 38:877–895.
- Albrecht S, Hofstadter S, Artsob H, Chaban O, From L. Lymphadenosis benigna cutis resulting from Borrelia infection (Borrelia lymphocytoma). J Am Acad Dermatol 1991; 24:621–625.
- Peretz E, Grunwald MH, Cagnano E, Halevy S. Follicular B-cell pseudolymphoma. Australas J Dermatol 2000; 41:48–49.
- Hermes B, Haas N, Grabbe J, Czarnetzki BM. Foreign-body granuloma and IgE-pseudolymphoma after multiple bee stings. Br J Dermatol 1994; 130:780–784.
- Kerl H, Fink-Puches R, Cerroni L. Diagnostic criteria of primary cutaneous B-cell lymphomas and pseudolymphomas. Keio J Med 2001; 50:269–273.
- Kuflik AS, Schwartz RA. Lymphocytoma cutis: a series of five patients successfully treated with cryosurgery. J Am Acad Dermatol 1992; 26:449–452.
- Takeda H, Kaneko T, Harada K, Matsuzaki Y, Nakano H, Hanada K. Successful treatment of lymphadenosis benigna cutis with topical photodynamic therapy with delta-aminolevulinic acid. Dermatology 2005; 211:264–266.
Q: Which is the most likely diagnosis?
- Basal cell carcinoma
- Squamous cell carcinoma
- Lymphocytoma cutis
- Amelanotic melanoma
- Pyogenic granuloma
A: The correct answer is lymphocytoma cutis. The differential diagnosis of a pink papule on the face of a middle-aged person includes nonmelanoma skin cancer, lymphoma, lymphocytoma cutis, metastatic disease, certain infections, Jessner lymphocytic infiltrate, connective tissue disease, and some adnexal tumors. Histologic study is a useful diagnostic aid in this context.
Basal cell carcinoma is the most common cutaneous malignant neoplasm, and although these tumors rarely metastasize, they are capable of gross tissue destruction, particularly those lesions arising on the face. Clinically, this tumor presents as a shiny, pearly nodule with telangiectasias on the surface, as in our patient, but skin biopsy shows large basaloid lobules of varying shape and size forming a relatively circumscribed mass with a “palisade” around the rim of the lobule.
Squamous cell carcinoma manifests as shallow ulcers, often with a keratinous crust and elevated, indurate borders, but also as plaques or nodules. The clinical diagnosis should be confirmed with skin biopsy, which reveals atypical keratinocytes extending from the epidermis to the dermis with dyskeratosis, intercellular bridges, variable central keratinization, and horn pearl formation, depending on the differentiation of the tumor.
Amelanotic melanoma is nonpigmented and appears as a pink nodule mimicking basal cell carcinoma or squamous cell carcinoma. Histologic study is necessary for the diagnosis, and shows an atypical proliferation of melanocytic cells in the epidermis and dermis.
Pyogenic granuloma is a very common benign vascular lesion considered to be a hyperplastic process or a vascular neoplasm. The lesion typically presents as a red or bluish papule or polyp that bleeds easily, and a reddish homogeneous area surrounded by a white “collarette” is found in most cases. Histologic features of an early lesion resemble granulation tissue and include lobules of capillaries and venules that often radiate from larger, more central vessels.
LYMPHOCYTOMA CUTIS: KEY FEATURES
Lymphocytoma cutis (pseudolymphoma) is a benign reactive polyclonal and inflammatory disorder that most frequently includes B lymphocytes, with a smaller population of T lymphocytes. It infiltrates the skin and resembles rudimentary germinal follicles, as in the present case. The lesion usually presents as an asymptomatic red-brown or violet papule or nodule, 3 mm to 5 cm in diameter, most often on the face, chest, or upper extremities.1 The lesion may be solitary, as in our patient, but lesions may also be grouped or numerous and widespread. It is three times more common in women than in men. It may resolve spontaneously, but it may also recur.
In Europe, lymphocytoma cutis occurs most often in B burgdorferi infection after a tick bite. Lymphocytoma cutis occurs in 1.3% of cases of B burgdorferi infection,2 although other infectious, physical, or chemical agents may produce the same reaction pattern. Tattooing (particularly red areas), acupuncture, vaccination, arthropod reactions, hyposensitization antigen reaction, and ingestion of drug have been implicated in this form of lymphoid hyperplasia.3,4
DIAGNOSTIC CHALLENGES
Lymphocytoma cutis can be challenging to diagnose, and although it can be suspected clinically, incisional biopsy is usually necessary in order to differentiate it from cutaneous B lymphoma.5
The infiltrate is predominantly nodular (> 90%) and located in the upper and mid dermis (“top heavy”) in lymphocytoma cutis, whereas it can be nodular or diffuse in cutaneous B lymphoma, with sharply demarcated borders that are convex rather than concave. Lymphoid follicles with germinal centers are sometimes present, and the interfollicular cellular population is polymorphic in lymphocytoma cutis (lymphocytes, plasma cells, histiocytes, eosinophils). In lymphocytoma cutis, cells express the phenotype of mature B lymphocytes (CD20, CD79a) and show regular and sharply demarcated networks of CD21+ follicular dendritic cells, whereas in cutaneous B lymphoma these networks are irregular. Light chains are usually polyclonal, although monoclonal populations of B cell in cases of cutaneous lymphocytoma cutis have been described. Extracutaneous involvement is possible in cutaneous B lymphoma but is usually absent in lymphocytoma cutis.
Lymphocytoma cutis typically involutes over a period of months, even with no treatment, as it did in our patient. Otherwise, there are different therapeutic options, including intralesional and topical corticosteroids, surgery, and cryosurgery.6 Photodynamic therapy with delta-aminolevulinic acid is an effective and safe modality for the treatment of lymphocytoma cutis and may be cosmetically beneficial.7
Q: Which is the most likely diagnosis?
- Basal cell carcinoma
- Squamous cell carcinoma
- Lymphocytoma cutis
- Amelanotic melanoma
- Pyogenic granuloma
A: The correct answer is lymphocytoma cutis. The differential diagnosis of a pink papule on the face of a middle-aged person includes nonmelanoma skin cancer, lymphoma, lymphocytoma cutis, metastatic disease, certain infections, Jessner lymphocytic infiltrate, connective tissue disease, and some adnexal tumors. Histologic study is a useful diagnostic aid in this context.
Basal cell carcinoma is the most common cutaneous malignant neoplasm, and although these tumors rarely metastasize, they are capable of gross tissue destruction, particularly those lesions arising on the face. Clinically, this tumor presents as a shiny, pearly nodule with telangiectasias on the surface, as in our patient, but skin biopsy shows large basaloid lobules of varying shape and size forming a relatively circumscribed mass with a “palisade” around the rim of the lobule.
Squamous cell carcinoma manifests as shallow ulcers, often with a keratinous crust and elevated, indurate borders, but also as plaques or nodules. The clinical diagnosis should be confirmed with skin biopsy, which reveals atypical keratinocytes extending from the epidermis to the dermis with dyskeratosis, intercellular bridges, variable central keratinization, and horn pearl formation, depending on the differentiation of the tumor.
Amelanotic melanoma is nonpigmented and appears as a pink nodule mimicking basal cell carcinoma or squamous cell carcinoma. Histologic study is necessary for the diagnosis, and shows an atypical proliferation of melanocytic cells in the epidermis and dermis.
Pyogenic granuloma is a very common benign vascular lesion considered to be a hyperplastic process or a vascular neoplasm. The lesion typically presents as a red or bluish papule or polyp that bleeds easily, and a reddish homogeneous area surrounded by a white “collarette” is found in most cases. Histologic features of an early lesion resemble granulation tissue and include lobules of capillaries and venules that often radiate from larger, more central vessels.
LYMPHOCYTOMA CUTIS: KEY FEATURES
Lymphocytoma cutis (pseudolymphoma) is a benign reactive polyclonal and inflammatory disorder that most frequently includes B lymphocytes, with a smaller population of T lymphocytes. It infiltrates the skin and resembles rudimentary germinal follicles, as in the present case. The lesion usually presents as an asymptomatic red-brown or violet papule or nodule, 3 mm to 5 cm in diameter, most often on the face, chest, or upper extremities.1 The lesion may be solitary, as in our patient, but lesions may also be grouped or numerous and widespread. It is three times more common in women than in men. It may resolve spontaneously, but it may also recur.
In Europe, lymphocytoma cutis occurs most often in B burgdorferi infection after a tick bite. Lymphocytoma cutis occurs in 1.3% of cases of B burgdorferi infection,2 although other infectious, physical, or chemical agents may produce the same reaction pattern. Tattooing (particularly red areas), acupuncture, vaccination, arthropod reactions, hyposensitization antigen reaction, and ingestion of drug have been implicated in this form of lymphoid hyperplasia.3,4
DIAGNOSTIC CHALLENGES
Lymphocytoma cutis can be challenging to diagnose, and although it can be suspected clinically, incisional biopsy is usually necessary in order to differentiate it from cutaneous B lymphoma.5
The infiltrate is predominantly nodular (> 90%) and located in the upper and mid dermis (“top heavy”) in lymphocytoma cutis, whereas it can be nodular or diffuse in cutaneous B lymphoma, with sharply demarcated borders that are convex rather than concave. Lymphoid follicles with germinal centers are sometimes present, and the interfollicular cellular population is polymorphic in lymphocytoma cutis (lymphocytes, plasma cells, histiocytes, eosinophils). In lymphocytoma cutis, cells express the phenotype of mature B lymphocytes (CD20, CD79a) and show regular and sharply demarcated networks of CD21+ follicular dendritic cells, whereas in cutaneous B lymphoma these networks are irregular. Light chains are usually polyclonal, although monoclonal populations of B cell in cases of cutaneous lymphocytoma cutis have been described. Extracutaneous involvement is possible in cutaneous B lymphoma but is usually absent in lymphocytoma cutis.
Lymphocytoma cutis typically involutes over a period of months, even with no treatment, as it did in our patient. Otherwise, there are different therapeutic options, including intralesional and topical corticosteroids, surgery, and cryosurgery.6 Photodynamic therapy with delta-aminolevulinic acid is an effective and safe modality for the treatment of lymphocytoma cutis and may be cosmetically beneficial.7
- Ploysangam T, Breneman DL, Mutasim DF. Cutaneous pseudolymphomas. J Am Acad Dermatol 1998; 38:877–895.
- Albrecht S, Hofstadter S, Artsob H, Chaban O, From L. Lymphadenosis benigna cutis resulting from Borrelia infection (Borrelia lymphocytoma). J Am Acad Dermatol 1991; 24:621–625.
- Peretz E, Grunwald MH, Cagnano E, Halevy S. Follicular B-cell pseudolymphoma. Australas J Dermatol 2000; 41:48–49.
- Hermes B, Haas N, Grabbe J, Czarnetzki BM. Foreign-body granuloma and IgE-pseudolymphoma after multiple bee stings. Br J Dermatol 1994; 130:780–784.
- Kerl H, Fink-Puches R, Cerroni L. Diagnostic criteria of primary cutaneous B-cell lymphomas and pseudolymphomas. Keio J Med 2001; 50:269–273.
- Kuflik AS, Schwartz RA. Lymphocytoma cutis: a series of five patients successfully treated with cryosurgery. J Am Acad Dermatol 1992; 26:449–452.
- Takeda H, Kaneko T, Harada K, Matsuzaki Y, Nakano H, Hanada K. Successful treatment of lymphadenosis benigna cutis with topical photodynamic therapy with delta-aminolevulinic acid. Dermatology 2005; 211:264–266.
- Ploysangam T, Breneman DL, Mutasim DF. Cutaneous pseudolymphomas. J Am Acad Dermatol 1998; 38:877–895.
- Albrecht S, Hofstadter S, Artsob H, Chaban O, From L. Lymphadenosis benigna cutis resulting from Borrelia infection (Borrelia lymphocytoma). J Am Acad Dermatol 1991; 24:621–625.
- Peretz E, Grunwald MH, Cagnano E, Halevy S. Follicular B-cell pseudolymphoma. Australas J Dermatol 2000; 41:48–49.
- Hermes B, Haas N, Grabbe J, Czarnetzki BM. Foreign-body granuloma and IgE-pseudolymphoma after multiple bee stings. Br J Dermatol 1994; 130:780–784.
- Kerl H, Fink-Puches R, Cerroni L. Diagnostic criteria of primary cutaneous B-cell lymphomas and pseudolymphomas. Keio J Med 2001; 50:269–273.
- Kuflik AS, Schwartz RA. Lymphocytoma cutis: a series of five patients successfully treated with cryosurgery. J Am Acad Dermatol 1992; 26:449–452.
- Takeda H, Kaneko T, Harada K, Matsuzaki Y, Nakano H, Hanada K. Successful treatment of lymphadenosis benigna cutis with topical photodynamic therapy with delta-aminolevulinic acid. Dermatology 2005; 211:264–266.