User login
A 36-year-old African-American man presented to our clinic for evaluation of a chronic “rash” on his trunk, arms, and legs that had been present for 3 years. Empiric therapy for tinea corporis, nummular eczema, and confluent and reticulated papillomatosis had failed to improve his lesions.
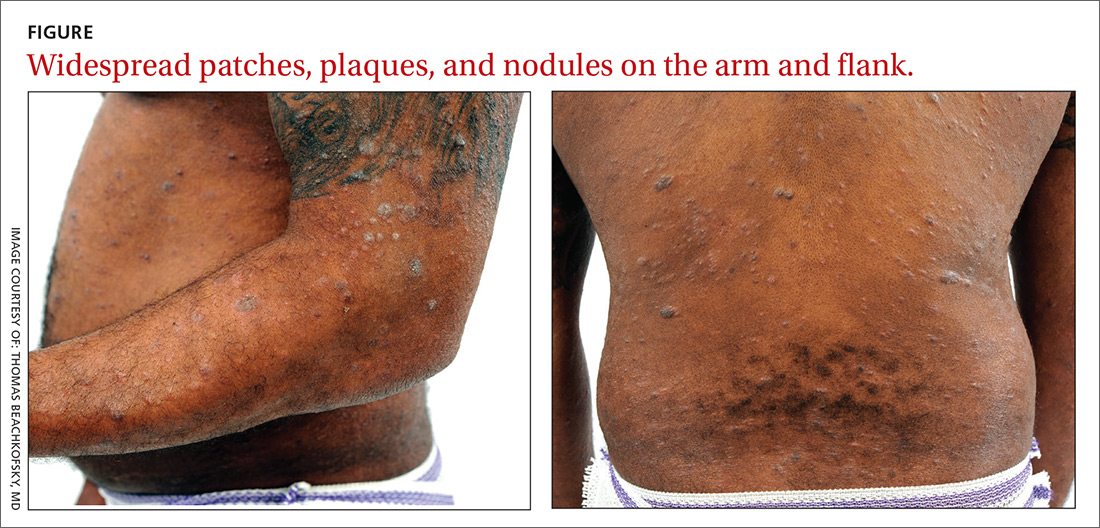
Physical examination revealed a widespread skin eruption consisting of well-demarcated, hyperpigmented, scaly patches and plaques distributed throughout the patient’s trunk and extremities. Initial biopsies of the skin lesions (performed at an outside institution prior to current presentation) were nondiagnostic.
Over the next several months, the patient developed numerous cutaneous nodules and tumors within the background of his persistent patches and plaques (FIGURE). These nodules and tumors intermittently disappeared and recurred, seemingly independent of therapies including psoralen and ultraviolet A phototherapy (PUVA), interferon, and methotrexate.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: CD30+ transformed mycosis fungoides
Repeat biopsies ultimately confirmed a diagnosis of mycosis fungoides (MF), a primary cutaneous non-Hodgkin lymphoma of T-cell origin.
Lymphomas occurring in the skin without evidence of disease in other areas of the body at the time of diagnosis are referred to as primary cutaneous lymphomas. They are broadly divided by their cell of origin into cutaneous T-cell and cutaneous B-cell lymphomas. Differentiating primary cutaneous lymphoma from systemic lymphomas with secondary skin involvement is an important distinction, as primary cutaneous lymphomas have different clinical courses and prognoses and require different treatments.1
MF is the most common type of cutaneous T-cell lymphoma (CTCL), yet it remains an uncommon diagnosis as cutaneous lymphomas comprise only 3.9% of all non-Hodgkin lymphomas.2 The CD30+ transformed variant identified in this patient is a rare subtype of MF that is associated with increased morbidity and mortality.3 Other types of CTCL include primary cutaneous anaplastic large cell lymphoma, lymphomatoid papulosis, and adult T-cell leukemia/lymphoma, all of which can resemble MF clinically and are diagnosed by histopathology.
A nonspecific presentation
Patients with MF typically present with a complaint of chronic, slowly progressive skin lesions of various sizes and morphologies including scaly patches, plaques, and tumors, often in skin distributions that are not directly exposed to the sun (often referred to as a “bathing suit” distribution).4 Pruritus typically accompanies these skin lesions, and alopecia and poikiloderma are common findings. Patients with more advanced cases may present with generalized erythroderma. This finding should raise concern for progression to Sézary syndrome, a more aggressive type of CTCL that manifests with leukemic involvement of malignant T-cells matching the T-cell clones found in the skin.2
Alopecia is a common manifestation of MF that can be clinically indistinguishable from alopecia areata.5 While isolated alopecia is unlikely to be a consequence of MF, alopecia in the setting of widespread patches and plaques should raise suspicion for a diagnosis of MF.
Continue to: Diagnostic dilemma
Diagnostic dilemma: Condition mimics benign disorders
This case illustrates the diagnostic dilemma posed by MF due to its propensity to mimic more benign skin disorders, both clinically and histologically.2 One literature review of MF cases in which the diagnosis was not suspected clinically but was eventually confirmed histopathologically found that 25 unique dermatoses can be mimicked clinically by MF.6 The more common conditions that MF can be mistaken for are discussed below. In light of the diagnostic challenge posed by this multitude of morphologic presentations, referral to Dermatology for histopathologic evaluation is a reasonable next step when empiric treatment fails to improve symptoms of these common skin disorders.
Chronic atopic dermatitis (eczema) is characterized by dry skin and severe pruritis, usually associated with lichenified plaques and a history of atopic disease. The pruritic, scaly plaques typical of MF may be misdiagnosed as eczema; however, a distribution involving the “bathing suit” area is more suggestive of MF. Patients with atopic dermatitis are more likely to display a flexural distribution.2
Tinea corporis typically presents as annular, erythematous, pruritic, scaly patches or plaques that are similar to the scaling lesions of MF. Tinea corporis can be distinguished from MF via potassium hydroxide preparation of skin scrapings from an active lesion, which should demonstrate the segmented hyphae characteristic of this infection. If lesions of this type fail to respond to topical antifungal therapy, a skin biopsy may be warranted for further evaluation.
Nummular eczema, like tinea corporis, presents with round, coin-shaped patches that are highly pruritic and can be difficult to distinguish from the pruritic patches of MF. Unlike MF, however, nummular eczema typically is observed in patients with diffusely dry skin7; the lack of this finding should prompt consideration of alternative diagnoses, including MF.
Psoriasis classically presents with symmetrically distributed, well-demarcated plaques with prominent scaling that are usually asymptomatic, but may be associated with pruritis. Psoriatic plaques may be difficult to distinguish clinically from the plaques of MF, but their distribution may offer clues; psoriasis typically follows an extensor surface distribution, whereas MF is more commonly identified in a “bathing suit” or generalized distribution.4
Continue to: Tx based on disease extent, impact on quality of life
Tx based on disease extent, impact on quality of life
Staging of MF is the primary determinant of treatment and involves evaluation of skin, lymph node, viscera, and blood involvement. Early-stage MF has a favorable prognosis and can be effectively managed with skin-directed treatments. These include topical corticosteroids, topical nitrogen mustard agents, topical retinoids, and phototherapy (PUVA).8 Total skin electron beam therapy has been proven effective in the treatment of refractory and extensive MF, and local radiation therapy also is an effective treatment for isolated cutaneous tumors. Prolonged complete remissions of early-stage MF have been achieved using skin-directed treatments.8
In contrast, advanced MF often is treatment refractory and carries a poor prognosis. Systemic treatments such as interferon therapy, oral retinoids, extracorporeal photopheresis, and chemotherapy are added in patients with advanced disease after skin-directed therapy fails to adequately control tumor burden.
Our patient initially was treated with PUVA as well as 6 million U of interferon alfa-2B 3 times weekly, which he eventually elected to discontinue after 8 months because he wanted to father a child. His disease became more widespread after discontinuing these treatments despite interim therapy with clobetasol ointment .05%. His larger nodules and tumors were intermittently treated with local radiation with good response. Methotrexate 15 mg weekly was initiated following his decline after discontinuing PUVA and interferon treatment. Treatment with methotrexate resulted in an overall decrease in his tumor burden and several months of clinical stability.
Approximately 6 months after initiating methotrexate, his condition notably worsened. He developed generalized erythroderma, systemic symptoms including fever, chills, and unintentional 9.07-kg weight loss, in addition to new findings of palmoplantar keratoderma and nail dystrophy. In light of this systemic progression and concern for developing Sézary syndrome, extracorporeal photopheresis, bexarotene (an oral retinoid) 75 mg twice daily, and interferon alfa-2B 5 million U 3 times weekly were initiated. His condition continued to decline despite these therapies, culminating in a hospital admission for sepsis.
The patient recovered, and his regimen was changed to PUVA 3 times weekly in addition to treatment with intravenous brentuximab 1.8 mg/kg, a monoclonal antibody that targets CD30 receptors. Unfortunately, his condition continued to decline on this treatment regimen, which was subsequently abandoned in favor of chemotherapy with gemcitabine 1980 mg/250 mL normal saline. The patient was improving clinically with each cycle of gemcitabine and the plan was to transition him to extracorporeal photopheresis and bexarotene for maintenance therapy; however, the patient succumbed to his disease and passed away at the age of 37.
CORRESPONDENCE
Jonathan Banta, MD, PSC 103, Box 3613, APO, AE 09603 jonathan.c.banta.mil@mail.mil
1. Whittaker S, Knobler R, Ortiz P, et al. Major achievements of the EORTC Cutaneous Lymphoma Task Force (CLTF). EJC Suppl. 2012;10:46-50.
2. Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part I. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-205.e16.
3. Kadin ME, Hughey LC, Wood GS. Large-cell transformation of mycosis fungoides-differential diagnosis with implications for clinical management: a consensus statement of the US Cutaneous Lymphoma Consortium. J Am Acad Dermatol. 2014;70:374-376.
4. Pimpinelli N, Olsen EA, Santucci M, et al; International Society for Cutaneous Lymphoma. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063.
5. Bi MY, Curry JL, Christiano AM, et al. The spectrum of hair loss in patients with mycosis fungoides and Sézary syndrome. J Am Acad Dermatol. 2011;64:53-63.
6. Zackheim HS, McCalmont TH. Mycosis fungoides: the great imitator. J Am Acad Dermatol. 2002;47:914-918.
7. Jiamton S, Tangjaturonrusamee C, Kulthanan K. Clinical features and aggravating factors in nummular eczema in Thais. Asian Pac J Allergy Immunol. 2013;31:36-42.
8. Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-223.e17.
A 36-year-old African-American man presented to our clinic for evaluation of a chronic “rash” on his trunk, arms, and legs that had been present for 3 years. Empiric therapy for tinea corporis, nummular eczema, and confluent and reticulated papillomatosis had failed to improve his lesions.
Physical examination revealed a widespread skin eruption consisting of well-demarcated, hyperpigmented, scaly patches and plaques distributed throughout the patient’s trunk and extremities. Initial biopsies of the skin lesions (performed at an outside institution prior to current presentation) were nondiagnostic.
Over the next several months, the patient developed numerous cutaneous nodules and tumors within the background of his persistent patches and plaques (FIGURE). These nodules and tumors intermittently disappeared and recurred, seemingly independent of therapies including psoralen and ultraviolet A phototherapy (PUVA), interferon, and methotrexate.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: CD30+ transformed mycosis fungoides
Repeat biopsies ultimately confirmed a diagnosis of mycosis fungoides (MF), a primary cutaneous non-Hodgkin lymphoma of T-cell origin.
Lymphomas occurring in the skin without evidence of disease in other areas of the body at the time of diagnosis are referred to as primary cutaneous lymphomas. They are broadly divided by their cell of origin into cutaneous T-cell and cutaneous B-cell lymphomas. Differentiating primary cutaneous lymphoma from systemic lymphomas with secondary skin involvement is an important distinction, as primary cutaneous lymphomas have different clinical courses and prognoses and require different treatments.1
MF is the most common type of cutaneous T-cell lymphoma (CTCL), yet it remains an uncommon diagnosis as cutaneous lymphomas comprise only 3.9% of all non-Hodgkin lymphomas.2 The CD30+ transformed variant identified in this patient is a rare subtype of MF that is associated with increased morbidity and mortality.3 Other types of CTCL include primary cutaneous anaplastic large cell lymphoma, lymphomatoid papulosis, and adult T-cell leukemia/lymphoma, all of which can resemble MF clinically and are diagnosed by histopathology.
A nonspecific presentation
Patients with MF typically present with a complaint of chronic, slowly progressive skin lesions of various sizes and morphologies including scaly patches, plaques, and tumors, often in skin distributions that are not directly exposed to the sun (often referred to as a “bathing suit” distribution).4 Pruritus typically accompanies these skin lesions, and alopecia and poikiloderma are common findings. Patients with more advanced cases may present with generalized erythroderma. This finding should raise concern for progression to Sézary syndrome, a more aggressive type of CTCL that manifests with leukemic involvement of malignant T-cells matching the T-cell clones found in the skin.2
Alopecia is a common manifestation of MF that can be clinically indistinguishable from alopecia areata.5 While isolated alopecia is unlikely to be a consequence of MF, alopecia in the setting of widespread patches and plaques should raise suspicion for a diagnosis of MF.
Continue to: Diagnostic dilemma
Diagnostic dilemma: Condition mimics benign disorders
This case illustrates the diagnostic dilemma posed by MF due to its propensity to mimic more benign skin disorders, both clinically and histologically.2 One literature review of MF cases in which the diagnosis was not suspected clinically but was eventually confirmed histopathologically found that 25 unique dermatoses can be mimicked clinically by MF.6 The more common conditions that MF can be mistaken for are discussed below. In light of the diagnostic challenge posed by this multitude of morphologic presentations, referral to Dermatology for histopathologic evaluation is a reasonable next step when empiric treatment fails to improve symptoms of these common skin disorders.
Chronic atopic dermatitis (eczema) is characterized by dry skin and severe pruritis, usually associated with lichenified plaques and a history of atopic disease. The pruritic, scaly plaques typical of MF may be misdiagnosed as eczema; however, a distribution involving the “bathing suit” area is more suggestive of MF. Patients with atopic dermatitis are more likely to display a flexural distribution.2
Tinea corporis typically presents as annular, erythematous, pruritic, scaly patches or plaques that are similar to the scaling lesions of MF. Tinea corporis can be distinguished from MF via potassium hydroxide preparation of skin scrapings from an active lesion, which should demonstrate the segmented hyphae characteristic of this infection. If lesions of this type fail to respond to topical antifungal therapy, a skin biopsy may be warranted for further evaluation.
Nummular eczema, like tinea corporis, presents with round, coin-shaped patches that are highly pruritic and can be difficult to distinguish from the pruritic patches of MF. Unlike MF, however, nummular eczema typically is observed in patients with diffusely dry skin7; the lack of this finding should prompt consideration of alternative diagnoses, including MF.
Psoriasis classically presents with symmetrically distributed, well-demarcated plaques with prominent scaling that are usually asymptomatic, but may be associated with pruritis. Psoriatic plaques may be difficult to distinguish clinically from the plaques of MF, but their distribution may offer clues; psoriasis typically follows an extensor surface distribution, whereas MF is more commonly identified in a “bathing suit” or generalized distribution.4
Continue to: Tx based on disease extent, impact on quality of life
Tx based on disease extent, impact on quality of life
Staging of MF is the primary determinant of treatment and involves evaluation of skin, lymph node, viscera, and blood involvement. Early-stage MF has a favorable prognosis and can be effectively managed with skin-directed treatments. These include topical corticosteroids, topical nitrogen mustard agents, topical retinoids, and phototherapy (PUVA).8 Total skin electron beam therapy has been proven effective in the treatment of refractory and extensive MF, and local radiation therapy also is an effective treatment for isolated cutaneous tumors. Prolonged complete remissions of early-stage MF have been achieved using skin-directed treatments.8
In contrast, advanced MF often is treatment refractory and carries a poor prognosis. Systemic treatments such as interferon therapy, oral retinoids, extracorporeal photopheresis, and chemotherapy are added in patients with advanced disease after skin-directed therapy fails to adequately control tumor burden.
Our patient initially was treated with PUVA as well as 6 million U of interferon alfa-2B 3 times weekly, which he eventually elected to discontinue after 8 months because he wanted to father a child. His disease became more widespread after discontinuing these treatments despite interim therapy with clobetasol ointment .05%. His larger nodules and tumors were intermittently treated with local radiation with good response. Methotrexate 15 mg weekly was initiated following his decline after discontinuing PUVA and interferon treatment. Treatment with methotrexate resulted in an overall decrease in his tumor burden and several months of clinical stability.
Approximately 6 months after initiating methotrexate, his condition notably worsened. He developed generalized erythroderma, systemic symptoms including fever, chills, and unintentional 9.07-kg weight loss, in addition to new findings of palmoplantar keratoderma and nail dystrophy. In light of this systemic progression and concern for developing Sézary syndrome, extracorporeal photopheresis, bexarotene (an oral retinoid) 75 mg twice daily, and interferon alfa-2B 5 million U 3 times weekly were initiated. His condition continued to decline despite these therapies, culminating in a hospital admission for sepsis.
The patient recovered, and his regimen was changed to PUVA 3 times weekly in addition to treatment with intravenous brentuximab 1.8 mg/kg, a monoclonal antibody that targets CD30 receptors. Unfortunately, his condition continued to decline on this treatment regimen, which was subsequently abandoned in favor of chemotherapy with gemcitabine 1980 mg/250 mL normal saline. The patient was improving clinically with each cycle of gemcitabine and the plan was to transition him to extracorporeal photopheresis and bexarotene for maintenance therapy; however, the patient succumbed to his disease and passed away at the age of 37.
CORRESPONDENCE
Jonathan Banta, MD, PSC 103, Box 3613, APO, AE 09603 jonathan.c.banta.mil@mail.mil
A 36-year-old African-American man presented to our clinic for evaluation of a chronic “rash” on his trunk, arms, and legs that had been present for 3 years. Empiric therapy for tinea corporis, nummular eczema, and confluent and reticulated papillomatosis had failed to improve his lesions.
Physical examination revealed a widespread skin eruption consisting of well-demarcated, hyperpigmented, scaly patches and plaques distributed throughout the patient’s trunk and extremities. Initial biopsies of the skin lesions (performed at an outside institution prior to current presentation) were nondiagnostic.
Over the next several months, the patient developed numerous cutaneous nodules and tumors within the background of his persistent patches and plaques (FIGURE). These nodules and tumors intermittently disappeared and recurred, seemingly independent of therapies including psoralen and ultraviolet A phototherapy (PUVA), interferon, and methotrexate.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: CD30+ transformed mycosis fungoides
Repeat biopsies ultimately confirmed a diagnosis of mycosis fungoides (MF), a primary cutaneous non-Hodgkin lymphoma of T-cell origin.
Lymphomas occurring in the skin without evidence of disease in other areas of the body at the time of diagnosis are referred to as primary cutaneous lymphomas. They are broadly divided by their cell of origin into cutaneous T-cell and cutaneous B-cell lymphomas. Differentiating primary cutaneous lymphoma from systemic lymphomas with secondary skin involvement is an important distinction, as primary cutaneous lymphomas have different clinical courses and prognoses and require different treatments.1
MF is the most common type of cutaneous T-cell lymphoma (CTCL), yet it remains an uncommon diagnosis as cutaneous lymphomas comprise only 3.9% of all non-Hodgkin lymphomas.2 The CD30+ transformed variant identified in this patient is a rare subtype of MF that is associated with increased morbidity and mortality.3 Other types of CTCL include primary cutaneous anaplastic large cell lymphoma, lymphomatoid papulosis, and adult T-cell leukemia/lymphoma, all of which can resemble MF clinically and are diagnosed by histopathology.
A nonspecific presentation
Patients with MF typically present with a complaint of chronic, slowly progressive skin lesions of various sizes and morphologies including scaly patches, plaques, and tumors, often in skin distributions that are not directly exposed to the sun (often referred to as a “bathing suit” distribution).4 Pruritus typically accompanies these skin lesions, and alopecia and poikiloderma are common findings. Patients with more advanced cases may present with generalized erythroderma. This finding should raise concern for progression to Sézary syndrome, a more aggressive type of CTCL that manifests with leukemic involvement of malignant T-cells matching the T-cell clones found in the skin.2
Alopecia is a common manifestation of MF that can be clinically indistinguishable from alopecia areata.5 While isolated alopecia is unlikely to be a consequence of MF, alopecia in the setting of widespread patches and plaques should raise suspicion for a diagnosis of MF.
Continue to: Diagnostic dilemma
Diagnostic dilemma: Condition mimics benign disorders
This case illustrates the diagnostic dilemma posed by MF due to its propensity to mimic more benign skin disorders, both clinically and histologically.2 One literature review of MF cases in which the diagnosis was not suspected clinically but was eventually confirmed histopathologically found that 25 unique dermatoses can be mimicked clinically by MF.6 The more common conditions that MF can be mistaken for are discussed below. In light of the diagnostic challenge posed by this multitude of morphologic presentations, referral to Dermatology for histopathologic evaluation is a reasonable next step when empiric treatment fails to improve symptoms of these common skin disorders.
Chronic atopic dermatitis (eczema) is characterized by dry skin and severe pruritis, usually associated with lichenified plaques and a history of atopic disease. The pruritic, scaly plaques typical of MF may be misdiagnosed as eczema; however, a distribution involving the “bathing suit” area is more suggestive of MF. Patients with atopic dermatitis are more likely to display a flexural distribution.2
Tinea corporis typically presents as annular, erythematous, pruritic, scaly patches or plaques that are similar to the scaling lesions of MF. Tinea corporis can be distinguished from MF via potassium hydroxide preparation of skin scrapings from an active lesion, which should demonstrate the segmented hyphae characteristic of this infection. If lesions of this type fail to respond to topical antifungal therapy, a skin biopsy may be warranted for further evaluation.
Nummular eczema, like tinea corporis, presents with round, coin-shaped patches that are highly pruritic and can be difficult to distinguish from the pruritic patches of MF. Unlike MF, however, nummular eczema typically is observed in patients with diffusely dry skin7; the lack of this finding should prompt consideration of alternative diagnoses, including MF.
Psoriasis classically presents with symmetrically distributed, well-demarcated plaques with prominent scaling that are usually asymptomatic, but may be associated with pruritis. Psoriatic plaques may be difficult to distinguish clinically from the plaques of MF, but their distribution may offer clues; psoriasis typically follows an extensor surface distribution, whereas MF is more commonly identified in a “bathing suit” or generalized distribution.4
Continue to: Tx based on disease extent, impact on quality of life
Tx based on disease extent, impact on quality of life
Staging of MF is the primary determinant of treatment and involves evaluation of skin, lymph node, viscera, and blood involvement. Early-stage MF has a favorable prognosis and can be effectively managed with skin-directed treatments. These include topical corticosteroids, topical nitrogen mustard agents, topical retinoids, and phototherapy (PUVA).8 Total skin electron beam therapy has been proven effective in the treatment of refractory and extensive MF, and local radiation therapy also is an effective treatment for isolated cutaneous tumors. Prolonged complete remissions of early-stage MF have been achieved using skin-directed treatments.8
In contrast, advanced MF often is treatment refractory and carries a poor prognosis. Systemic treatments such as interferon therapy, oral retinoids, extracorporeal photopheresis, and chemotherapy are added in patients with advanced disease after skin-directed therapy fails to adequately control tumor burden.
Our patient initially was treated with PUVA as well as 6 million U of interferon alfa-2B 3 times weekly, which he eventually elected to discontinue after 8 months because he wanted to father a child. His disease became more widespread after discontinuing these treatments despite interim therapy with clobetasol ointment .05%. His larger nodules and tumors were intermittently treated with local radiation with good response. Methotrexate 15 mg weekly was initiated following his decline after discontinuing PUVA and interferon treatment. Treatment with methotrexate resulted in an overall decrease in his tumor burden and several months of clinical stability.
Approximately 6 months after initiating methotrexate, his condition notably worsened. He developed generalized erythroderma, systemic symptoms including fever, chills, and unintentional 9.07-kg weight loss, in addition to new findings of palmoplantar keratoderma and nail dystrophy. In light of this systemic progression and concern for developing Sézary syndrome, extracorporeal photopheresis, bexarotene (an oral retinoid) 75 mg twice daily, and interferon alfa-2B 5 million U 3 times weekly were initiated. His condition continued to decline despite these therapies, culminating in a hospital admission for sepsis.
The patient recovered, and his regimen was changed to PUVA 3 times weekly in addition to treatment with intravenous brentuximab 1.8 mg/kg, a monoclonal antibody that targets CD30 receptors. Unfortunately, his condition continued to decline on this treatment regimen, which was subsequently abandoned in favor of chemotherapy with gemcitabine 1980 mg/250 mL normal saline. The patient was improving clinically with each cycle of gemcitabine and the plan was to transition him to extracorporeal photopheresis and bexarotene for maintenance therapy; however, the patient succumbed to his disease and passed away at the age of 37.
CORRESPONDENCE
Jonathan Banta, MD, PSC 103, Box 3613, APO, AE 09603 jonathan.c.banta.mil@mail.mil
1. Whittaker S, Knobler R, Ortiz P, et al. Major achievements of the EORTC Cutaneous Lymphoma Task Force (CLTF). EJC Suppl. 2012;10:46-50.
2. Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part I. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-205.e16.
3. Kadin ME, Hughey LC, Wood GS. Large-cell transformation of mycosis fungoides-differential diagnosis with implications for clinical management: a consensus statement of the US Cutaneous Lymphoma Consortium. J Am Acad Dermatol. 2014;70:374-376.
4. Pimpinelli N, Olsen EA, Santucci M, et al; International Society for Cutaneous Lymphoma. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063.
5. Bi MY, Curry JL, Christiano AM, et al. The spectrum of hair loss in patients with mycosis fungoides and Sézary syndrome. J Am Acad Dermatol. 2011;64:53-63.
6. Zackheim HS, McCalmont TH. Mycosis fungoides: the great imitator. J Am Acad Dermatol. 2002;47:914-918.
7. Jiamton S, Tangjaturonrusamee C, Kulthanan K. Clinical features and aggravating factors in nummular eczema in Thais. Asian Pac J Allergy Immunol. 2013;31:36-42.
8. Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-223.e17.
1. Whittaker S, Knobler R, Ortiz P, et al. Major achievements of the EORTC Cutaneous Lymphoma Task Force (CLTF). EJC Suppl. 2012;10:46-50.
2. Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part I. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-205.e16.
3. Kadin ME, Hughey LC, Wood GS. Large-cell transformation of mycosis fungoides-differential diagnosis with implications for clinical management: a consensus statement of the US Cutaneous Lymphoma Consortium. J Am Acad Dermatol. 2014;70:374-376.
4. Pimpinelli N, Olsen EA, Santucci M, et al; International Society for Cutaneous Lymphoma. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063.
5. Bi MY, Curry JL, Christiano AM, et al. The spectrum of hair loss in patients with mycosis fungoides and Sézary syndrome. J Am Acad Dermatol. 2011;64:53-63.
6. Zackheim HS, McCalmont TH. Mycosis fungoides: the great imitator. J Am Acad Dermatol. 2002;47:914-918.
7. Jiamton S, Tangjaturonrusamee C, Kulthanan K. Clinical features and aggravating factors in nummular eczema in Thais. Asian Pac J Allergy Immunol. 2013;31:36-42.
8. Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-223.e17.