User login
SAN DIEGO – is poised to become a game-changer in the minimally invasive aesthetics field.
A brainchild of dermatologists and plastic surgeons at Massachusetts General Hospital, Boston, the device features a proprietary needle design that makes a series of high throughput microexcisions in epidermal and dermal tissue, with minimal downtime and without using thermal energy.
“The idea is to get more significant improvement of tissue laxity by fractionally removing the skin,” Mathew M. Avram, MD, JD, explained at the annual Masters of Aesthetics Symposium. “You can do a facelift by cutting the skin on the side and pulling it back. This is skin tightening and improvement of wrinkles with a thousand micro punches. It’s called fractional tissue extraction.”
Instead of relying on laser, heat, light, or radiofrequency, the device uses needle mechanics to extract microsized cores of full-thickness skin below the size threshold that causes scarring. “Then, you have biomechanical remodeling; you close the channels right away,” said Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital. “If you remove skin that is smaller than 500 micrometers in size, no scar is left behind.”
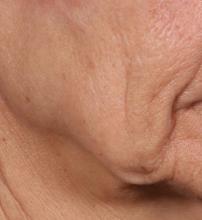
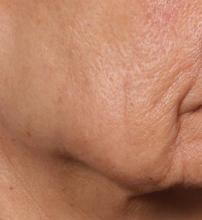
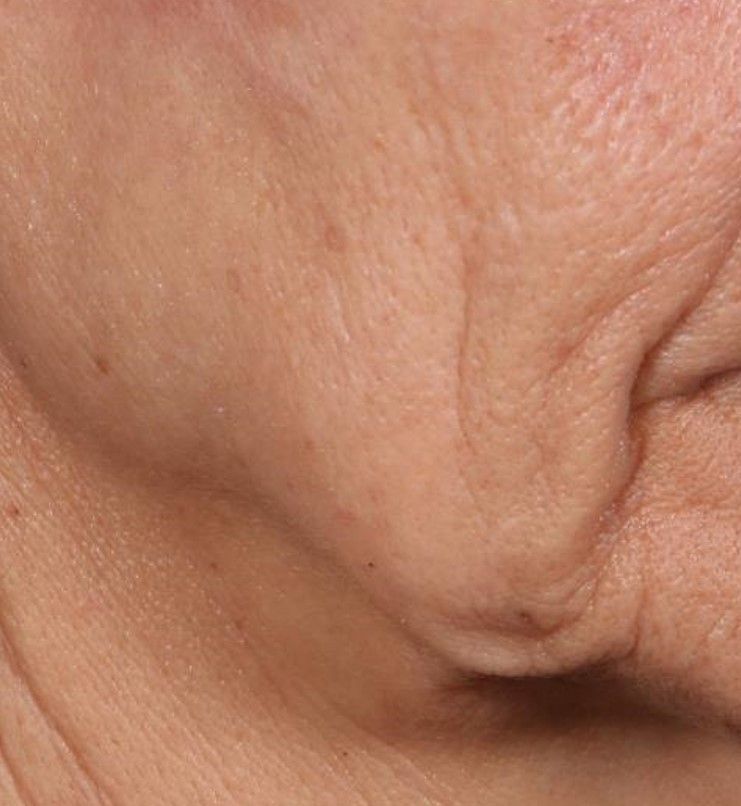
In trials of the device being carried out by Cytrellis Biosystems, more than 100 patients have been treated one to two times. During a separate presentation, one of the device investigators, Jill S. Waibel, MD, said that areas of treatment have included the upper and lower cheeks, perioral areas, and the submentum. On average, the amount of skin removed during each treatment session ranges from 5% to 8.5% and the mean down time is 3.8 days. According to combined data from two studies of 30 patients who had 60 areas treated and were followed at 90 or 180 days, subjects experienced an average 1.1 grade improvement on the Lemperle Rating Scale and showed an 80% improvement in moderate or severe wrinkles. In addition, 91% of investigators rated treatment areas as “improved” or “very much improved” on the Global Aesthetic Improvement Scale, and 88% of subjects were “satisfied” or “extremely satisfied” with the results.
“The safety profile of this device is amazing,” said Dr. Waibel, a dermatologist and owner of the Miami Dermatology and Laser Institute. “It provides an entirely new mode of treatment for skin laxity through highly approachable tissue removal with minimal to no pain or downtime. You can visually see the cores close through Optical Coherence Tomography before patients even leave the office. There have also been virtually no side effects except in one patient at another site who had minor postinflammatory hyperpigmentation.”
The device allows for local and scarless treatment of wrinkles in the areas in which they form, she continued, so results are natural and true to the underlying anatomy. “While its target testing has been in more severely lax patients, I think it has a great future for younger patients who want to stave off a future face lift,” Dr. Waibel said. “Initial treatments required preoperative lidocaine injections. However, recent trials using more tolerable analgesic methods have shown that this may not even be necessary. This is very exciting new technology and I have high hopes for the future of this device.”
Histological analysis from baseline to 60-90 days post treatment showed homogenization of elastosis, which signals reorganization of the papillary dermis, Dr. Avram said. It also showed a decrease in the grenz zone, rete ridge flattening, a slight increase in the collagen-to-elastin ratio, and no scarring. Pending clearance, he said, the device could be commercially available in 2020.
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, Soliton, and Zalea. He also reported having ownership and/or shareholder interest in Cytrellis, Invasix, and Zalea and intellectual property rights with Cytrellis.
Dr. Waibel disclosed that she has conducted clinical research for AbbVie, Aquavit, Cytrellis, Lumenis, Lutronic, Michelson Diagnostics, RegenX, Sciton, Sebacia, and Syneron/Candela. She is also a consultant for RegenX, Strata, and Syneron/Candela and is a member of the advisory board for Dominion Technologies, Sciton, and Sebacia.
SAN DIEGO – is poised to become a game-changer in the minimally invasive aesthetics field.
A brainchild of dermatologists and plastic surgeons at Massachusetts General Hospital, Boston, the device features a proprietary needle design that makes a series of high throughput microexcisions in epidermal and dermal tissue, with minimal downtime and without using thermal energy.
“The idea is to get more significant improvement of tissue laxity by fractionally removing the skin,” Mathew M. Avram, MD, JD, explained at the annual Masters of Aesthetics Symposium. “You can do a facelift by cutting the skin on the side and pulling it back. This is skin tightening and improvement of wrinkles with a thousand micro punches. It’s called fractional tissue extraction.”
Instead of relying on laser, heat, light, or radiofrequency, the device uses needle mechanics to extract microsized cores of full-thickness skin below the size threshold that causes scarring. “Then, you have biomechanical remodeling; you close the channels right away,” said Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital. “If you remove skin that is smaller than 500 micrometers in size, no scar is left behind.”
In trials of the device being carried out by Cytrellis Biosystems, more than 100 patients have been treated one to two times. During a separate presentation, one of the device investigators, Jill S. Waibel, MD, said that areas of treatment have included the upper and lower cheeks, perioral areas, and the submentum. On average, the amount of skin removed during each treatment session ranges from 5% to 8.5% and the mean down time is 3.8 days. According to combined data from two studies of 30 patients who had 60 areas treated and were followed at 90 or 180 days, subjects experienced an average 1.1 grade improvement on the Lemperle Rating Scale and showed an 80% improvement in moderate or severe wrinkles. In addition, 91% of investigators rated treatment areas as “improved” or “very much improved” on the Global Aesthetic Improvement Scale, and 88% of subjects were “satisfied” or “extremely satisfied” with the results.
“The safety profile of this device is amazing,” said Dr. Waibel, a dermatologist and owner of the Miami Dermatology and Laser Institute. “It provides an entirely new mode of treatment for skin laxity through highly approachable tissue removal with minimal to no pain or downtime. You can visually see the cores close through Optical Coherence Tomography before patients even leave the office. There have also been virtually no side effects except in one patient at another site who had minor postinflammatory hyperpigmentation.”
The device allows for local and scarless treatment of wrinkles in the areas in which they form, she continued, so results are natural and true to the underlying anatomy. “While its target testing has been in more severely lax patients, I think it has a great future for younger patients who want to stave off a future face lift,” Dr. Waibel said. “Initial treatments required preoperative lidocaine injections. However, recent trials using more tolerable analgesic methods have shown that this may not even be necessary. This is very exciting new technology and I have high hopes for the future of this device.”
Histological analysis from baseline to 60-90 days post treatment showed homogenization of elastosis, which signals reorganization of the papillary dermis, Dr. Avram said. It also showed a decrease in the grenz zone, rete ridge flattening, a slight increase in the collagen-to-elastin ratio, and no scarring. Pending clearance, he said, the device could be commercially available in 2020.
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, Soliton, and Zalea. He also reported having ownership and/or shareholder interest in Cytrellis, Invasix, and Zalea and intellectual property rights with Cytrellis.
Dr. Waibel disclosed that she has conducted clinical research for AbbVie, Aquavit, Cytrellis, Lumenis, Lutronic, Michelson Diagnostics, RegenX, Sciton, Sebacia, and Syneron/Candela. She is also a consultant for RegenX, Strata, and Syneron/Candela and is a member of the advisory board for Dominion Technologies, Sciton, and Sebacia.
SAN DIEGO – is poised to become a game-changer in the minimally invasive aesthetics field.
A brainchild of dermatologists and plastic surgeons at Massachusetts General Hospital, Boston, the device features a proprietary needle design that makes a series of high throughput microexcisions in epidermal and dermal tissue, with minimal downtime and without using thermal energy.
“The idea is to get more significant improvement of tissue laxity by fractionally removing the skin,” Mathew M. Avram, MD, JD, explained at the annual Masters of Aesthetics Symposium. “You can do a facelift by cutting the skin on the side and pulling it back. This is skin tightening and improvement of wrinkles with a thousand micro punches. It’s called fractional tissue extraction.”
Instead of relying on laser, heat, light, or radiofrequency, the device uses needle mechanics to extract microsized cores of full-thickness skin below the size threshold that causes scarring. “Then, you have biomechanical remodeling; you close the channels right away,” said Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital. “If you remove skin that is smaller than 500 micrometers in size, no scar is left behind.”
In trials of the device being carried out by Cytrellis Biosystems, more than 100 patients have been treated one to two times. During a separate presentation, one of the device investigators, Jill S. Waibel, MD, said that areas of treatment have included the upper and lower cheeks, perioral areas, and the submentum. On average, the amount of skin removed during each treatment session ranges from 5% to 8.5% and the mean down time is 3.8 days. According to combined data from two studies of 30 patients who had 60 areas treated and were followed at 90 or 180 days, subjects experienced an average 1.1 grade improvement on the Lemperle Rating Scale and showed an 80% improvement in moderate or severe wrinkles. In addition, 91% of investigators rated treatment areas as “improved” or “very much improved” on the Global Aesthetic Improvement Scale, and 88% of subjects were “satisfied” or “extremely satisfied” with the results.
“The safety profile of this device is amazing,” said Dr. Waibel, a dermatologist and owner of the Miami Dermatology and Laser Institute. “It provides an entirely new mode of treatment for skin laxity through highly approachable tissue removal with minimal to no pain or downtime. You can visually see the cores close through Optical Coherence Tomography before patients even leave the office. There have also been virtually no side effects except in one patient at another site who had minor postinflammatory hyperpigmentation.”
The device allows for local and scarless treatment of wrinkles in the areas in which they form, she continued, so results are natural and true to the underlying anatomy. “While its target testing has been in more severely lax patients, I think it has a great future for younger patients who want to stave off a future face lift,” Dr. Waibel said. “Initial treatments required preoperative lidocaine injections. However, recent trials using more tolerable analgesic methods have shown that this may not even be necessary. This is very exciting new technology and I have high hopes for the future of this device.”
Histological analysis from baseline to 60-90 days post treatment showed homogenization of elastosis, which signals reorganization of the papillary dermis, Dr. Avram said. It also showed a decrease in the grenz zone, rete ridge flattening, a slight increase in the collagen-to-elastin ratio, and no scarring. Pending clearance, he said, the device could be commercially available in 2020.
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, Soliton, and Zalea. He also reported having ownership and/or shareholder interest in Cytrellis, Invasix, and Zalea and intellectual property rights with Cytrellis.
Dr. Waibel disclosed that she has conducted clinical research for AbbVie, Aquavit, Cytrellis, Lumenis, Lutronic, Michelson Diagnostics, RegenX, Sciton, Sebacia, and Syneron/Candela. She is also a consultant for RegenX, Strata, and Syneron/Candela and is a member of the advisory board for Dominion Technologies, Sciton, and Sebacia.
EXPERT ANALYSIS FROM MOA 2019