User login
Our apologies, dear reader. It seems we have gotten ahead of ourselves. While we were writing about the Allergen of the Year, systemic dermatitis, and patch testing in children, we forgot to start with the basics. Let us remedy that. This is the first of a 2-part series addressing the basics of patch testing. In this article, we examine patch test systems, allergens, patch test readings, testing while on medications, and patch testing pearls and pitfalls. Let us begin!
Patch Test Systems
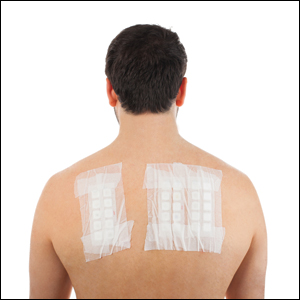
There are 2 patch test systems in North America: the Thin-layer Rapid Use Epicutaneous (T.R.U.E.) test (SmartPractice), which is approved by the US Food and Drug Administration for those 6 years and older, and the chamber method.
The T.R.U.E. test consists of 3 panels with 35 allergens and 1 negative control. The T.R.U.E. package insert1 describes surgical tape with individual polyester patches, each coated with an allergen film. Benefits of T.R.U.E. include ease of use (ie, easy storage and preparation, quick and straightforward application) and a readily identifiable set of allergens. The main drawback of T.R.U.E. is that only a limited number of allergens are tested, and as a result, it may miss the identification of some contact allergies. In an analysis of the 2015-2016 North American Contact Dermatitis Group (NACDG) patch test screening series, 25% to 40% of positive patch tests would have been missed if patch testing was performed with T.R.U.E. alone.2
Chamber method patch testing describes the process by which allergens are loaded into either metal or plastic chambers and then applied to the patient’s skin. The major benefit of the chamber method is that patches may be truly customized for the patient. The chamber method is time and labor intensive for patch preparation and application. Most comprehensive patch test clinics in North America use the chamber method, including the NACDG.
Patch test chambers largely can be divided into 2 categories: metal (aluminum) or plastic. Aluminum chambers, also known as Finn chambers, traditionally are used in patch testing. There are rare reports of hypersensitivity to aluminum chambers with associated diffuse positive patch test reactions,3,4 which may be more common in the pediatric population and likely is due to the fact that aluminum is present as an adjuvant in many childhood vaccines. As a precaution, some patch text experts recommend using plastic chambers in children younger than 16 to 18 years (M.R. and A.R.A., personal communication). Metal chambers require the additional application of diffusion discs for liquid allergens, and plastic chambers typically already contain the necessary diffusion discs. Finn chambers traditionally are applied with hypoallergenic porous surgical tape, but a waterproof tape also is available. To keep the chambers in place for the necessary 48 hours, additional tape may be applied over the patches.
Allergens
In patch test clinics, many dermatologists use a standard or screening allergen series. An appropriate standard series encompasses allergens that are most likely to be positive and relevant in the tested population. Some patch test experts recommend that allergens with a positive patch test frequency of greater than 0.5% to 1% should be included in a standard series.5 However, geographic differences in positive reactions can influence which allergens are appropriate to include. As a result, there is no universal standard series. Examples of standard or screening series include the American Contact Dermatitis Society (ACDS) allergen series,6 North American Baseline Series or North American 80 Comprehensive Series, European Baseline Series, NACDG series,2 and the Pediatric Baseline Series,7 as well as many other country- or region-specific series. There currently are 2 major commercial allergen distribution companies—Chemotechnique Diagnostics/Dormer Laboratories (series, individual allergens) and SmartPractice/allerGEAZE (series, individual allergens, T.R.U.E.).
In addition to a properly selected standard or screening series, supplemental patch testing with additional allergens can increase the diagnostic yield. Numerous supplemental series exist, including cosmetic, dental, textile, rubber, adhesive, plastics, and glue, among many others. In the NACDG 2015-2016 patch test cycle, it was found that 23% of 5597 patients reacted to an allergen that was not present on the NACDG screening series.2
In some situations, it is appropriate to patch test patient products, or nonstandard allergens. An abundance of caution, understanding of patch testing, and experience is necessary; for example, some chemicals are not recommended for testing, such as cleaning products, certain industrial chemicals, and those that may be carcinogens. We frequently consult De Groot’s Patch Testing8 for recommended allergen test concentrations and vehicles.
Patch Test Readings
The timing of the patch test reading is an important component of the test. Most North American comprehensive patch test clinics perform both first and delayed readings. After application, patches remain in place for 48 hours and then are removed, and a first reading is completed. Results are recorded as +/− (weak/doubtful), + (mild), ++ (strong), +++ (very strong), irritant, and negative.2 Many patch test specialists use side lighting to achieve the best reading and palpate to confirm the presence of induration; panel alignment devices commonly are utilized. There are some scenarios where shorter or longer application times are indicated, but this is beyond the scope of this article. A second, or delayed, reading should be completed 72 to 144 hours after initial application. We usually complete the delayed reading at 96 to 120 hours.
Certain patch test reactions may peak at different times, with fragrances often reacting earlier, and metals, topical antibiotics, and textile dyes reacting later.9 In the scenario of delayed peak reactions, third readings may be indicated.
Neglecting to complete a delayed reading is a potential pitfall and can increase the risk for both false-positive and false-negative reactions.10,11 In 1996, Uter et al10 published a large study of 9946 patients who were patch tested over a 4-year period. The authors compared patch test reactions at 48 and 72 hours and found that 34.5% of all positive reactions occurred at 72 hours; an additional 15.1% were positive at 96 hours. Importantly, one reading at 48 hours missed approximately one-third of positive patch test reactions, emphasizing the importance of delayed patch test readings.10 Furthermore, another study of 9997 consecutively patch tested patients examined reactions that were either negative or doubtful between days 3 or 4 and followed to see which of those reactions were positive at days 6 or 7. Of the negative reactions, the authors found that 4.4% were positive on days 6 or 7, and of the doubtful reactions, 9.1% were positive on days 6 or 7, meaning that up to 13.5% of positive reactions can be missed when a later reading is not performed.11
Medications During Patch Testing
Topical Medications
Topical medications generally can be continued during patch testing; however, patients should not apply topical medications to the patch test application site. Ideally, there should be no topical medication applied to the patch test application site for 1 to 2 weeks prior to patch test placement.12 Use of topical medications such as corticosteroids, calcineurin inhibitors, and theoretically even phosphodiesterase 4 inhibitors can not only result in suppression of positive patch test reactions but also can make patch adherence difficult.
Phototherapy
Phototherapy can result in local cutaneous immune suppression; therefore, it is recommended that it not be applied to the patch test area either during the patch test process or for 1 to 2 weeks prior to patch test application. In addition, if heat or sweating are generated during phototherapy, they can affect the success of patch testing by poor patch adherence and/or disruption of allergen distribution.
Systemic Medications
Oral antihistamines do not affect patch testing and can be continued during the patch test process.
It is ideal to avoid systemic immunomodulatory agents during the patch test process, but they occasionally are unavoidable, either because they are necessary to manage other medical conditions or because they are needed to achieve clear enough skin to proceed with patch testing. If it is required, prednisone is not recommended to exceed 10 mg daily.12,13 If intramuscular triamcinolone acetonide has been administered, patch testing should occur at least 1 month after the most recent injection.12 Oral methotrexate can probably be continued during patch testing but should be kept at the lowest possible dose and should be held during the week of testing, if possible. Adalimumab, etanercept, infliximab, and ustekinumab can be continued, as they are unlikely to interfere with patch testing.12 There are reports of positive patch test reactions on dupilumab,14,15 and some authors have described the response as variable and potentially allergen dependent.16,17 We believe that it generally is acceptable to continue dupilumab during patch testing. Data on cyclosporine during patch testing are mixed, and caution is advised as higher doses may suppress a positive patch test. Azathioprine and mycophenolate should be avoided, if possible.12
Pearls and Pitfalls
A few tips along the way can help assure your success in patch testing.
- Proper patient counseling determines a successful test. Provide your patient with verbal and written instructions about the patch test process, patch care, and any other necessary information.
- A simple sponge bath is permissible during patch testing provided the back stays dry. One of the authors (A.R.A.) advises patients to sit in a small amount of water in a bathtub to bathe, wash only the front of the body in the shower, and wash hair in the sink.
- No sweating, swimming, heavy exercise, or heavy physical labor. If your patient is planning to run a marathon the week of patch testing, they will be sorely disappointed when you tell them no sweating or showering is allowed! Patients with an occupation that requires physical labor may require a work excuse.
- Tape does not adhere to areas of the skin with excess hair. A scissor trim or electric shave will help the patches stay occluded and in place. We use an electric razor with a disposable replaceable head. A traditional straight razor should not be used, as it can increase the risk for folliculitis, which can make patch readings quite difficult.
- Securing the patches in place with an extra layer of tape provides added security. Large sheets of transparent medical dressings work particularly well for children or if there is difficulty with tape adherence.
Avoid application of patches to areas of the skin with tattoos. In theory, tattooed skin may have a decreased immune response, and tattoo pigment can obscure results.18 However, this is sometimes unavoidable, and Fowler and McTigue18 described a case of successful patch testing on a diffusely tattooed back.
- Avoid skin lesions (eg, scars, seborrheic keratoses, dermatitis) that can affect tape application, patch adherence, or patch readings.
Final Interpretation
The first step to excellent patch testing is understanding the patch test process. Patch test systems include T.R.U.E. and the chamber method. There are several allergen screening series, and the best series for each patient is determined based on geographic region, exposures, and allergen prevalence. The timing and practice of the patch test reading is vital, and physicians should be cognizant of medications and phototherapy use during the patch test process. An understanding of common pearls and pitfalls makes the difference between a good and great patch tester.
Now that you are an expert in performing the test, watch out for part 2 of this series on patch test interpretation, relevance, education, and counseling. Happy testing!
- T.R.U.E TEST [package insert]. Phoenix, AZ: SmartPractice; 1994.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Ward JM, Walsh RK, Bellet JS, et al. Allergic contact dermatitis to aluminum-based chambers during routine patch testing. Paper presented at: American Contact Dermatitis Society Annual Meeting; March 19, 2020; Denver, CO.
- Deleuran MG, Ahlström MG, Zachariae C, et al. Patch test reactivity to aluminum chambers. Contact Dermatitis. 2019;81:318-319.
- Bruze M, Condé-Salazar L, Goossens A, et al. European Society of Contact Dermatitis. thoughts on sensitizers in a standard patch test series. Contact Dermatitis. 1999;41:241-250.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2017 update. Dermatitis. 2017;28:141-143.
- Yu J, Atwater AR, Brod B, et al. Pediatric baseline patch test series: Pediatric Contact Dermatitis Workgroup. Dermatitis. 2018;29:206-212.
- De Groot AC. Patch Testing: Test Concentrations and Vehicles for 4900 Chemicals. 4th ed. Wapserveen, The Netherlands: Acdegroot Publishing; 2018.
- Chaudhry HM, Drage LA, El-Azhary RA, et al. Delayed patch-test reading after 5 days: an update from the Mayo Clinic Contact Dermatitis Group. Dermatitis. 2017;28:253-260.
- Uter WJ, Geier J, Schnuch A. Good clinical practice in patch testing: readings beyond day 2 are necessary: a confirmatory analysis. Members of the Information Network of Departments of Dermatology. Am J Contact Dermat. 1996;7:231-237.
- Madsen JT, Andersen KE. Outcome of a second patch test reading of T.R.U.E. Tests® on D6/7. Contact Dermatitis. 2013;68:94-97.
- Lampel H, Atwater AR. Patch testing tools of the trade: use of immunosuppressants and antihistamines during patch testing. J Dermatol Nurses’ Assoc. 2016;8:209-211.
- Fowler JF, Maibach HI, Zirwas M, et al. Effects of immunomodulatory agents on patch testing: expert opinion 2012. Dermatitis. 2012;23:301-303.
- Puza CJ, Atwater AR. Positive patch test reaction in a patient taking dupilumab. Dermatitis. 2018;29:89.
- Hoot JW, Douglas JD, Falo LD. Patch testing in a patient on dupilumab. Dermatitis. 2018;29:164.
- Stout M, Silverberg JI. Variable impact of dupilumab on patch testing results and allergic contact dermatitis in adults with atopic dermatitis. J Am Acad Dermatol. 2019;81:157-162.
- Raffi J, Botto N. Patch testing and allergen-specific inhibition in a patient taking dupilumab. JAMA Dermatol. 2019;155:120-121.
- Fowler JF, McTigue MK. Patch testing over tattoos. Am J Contact Dermat. 2002;13:19-20.
Our apologies, dear reader. It seems we have gotten ahead of ourselves. While we were writing about the Allergen of the Year, systemic dermatitis, and patch testing in children, we forgot to start with the basics. Let us remedy that. This is the first of a 2-part series addressing the basics of patch testing. In this article, we examine patch test systems, allergens, patch test readings, testing while on medications, and patch testing pearls and pitfalls. Let us begin!
Patch Test Systems
There are 2 patch test systems in North America: the Thin-layer Rapid Use Epicutaneous (T.R.U.E.) test (SmartPractice), which is approved by the US Food and Drug Administration for those 6 years and older, and the chamber method.
The T.R.U.E. test consists of 3 panels with 35 allergens and 1 negative control. The T.R.U.E. package insert1 describes surgical tape with individual polyester patches, each coated with an allergen film. Benefits of T.R.U.E. include ease of use (ie, easy storage and preparation, quick and straightforward application) and a readily identifiable set of allergens. The main drawback of T.R.U.E. is that only a limited number of allergens are tested, and as a result, it may miss the identification of some contact allergies. In an analysis of the 2015-2016 North American Contact Dermatitis Group (NACDG) patch test screening series, 25% to 40% of positive patch tests would have been missed if patch testing was performed with T.R.U.E. alone.2
Chamber method patch testing describes the process by which allergens are loaded into either metal or plastic chambers and then applied to the patient’s skin. The major benefit of the chamber method is that patches may be truly customized for the patient. The chamber method is time and labor intensive for patch preparation and application. Most comprehensive patch test clinics in North America use the chamber method, including the NACDG.
Patch test chambers largely can be divided into 2 categories: metal (aluminum) or plastic. Aluminum chambers, also known as Finn chambers, traditionally are used in patch testing. There are rare reports of hypersensitivity to aluminum chambers with associated diffuse positive patch test reactions,3,4 which may be more common in the pediatric population and likely is due to the fact that aluminum is present as an adjuvant in many childhood vaccines. As a precaution, some patch text experts recommend using plastic chambers in children younger than 16 to 18 years (M.R. and A.R.A., personal communication). Metal chambers require the additional application of diffusion discs for liquid allergens, and plastic chambers typically already contain the necessary diffusion discs. Finn chambers traditionally are applied with hypoallergenic porous surgical tape, but a waterproof tape also is available. To keep the chambers in place for the necessary 48 hours, additional tape may be applied over the patches.
Allergens
In patch test clinics, many dermatologists use a standard or screening allergen series. An appropriate standard series encompasses allergens that are most likely to be positive and relevant in the tested population. Some patch test experts recommend that allergens with a positive patch test frequency of greater than 0.5% to 1% should be included in a standard series.5 However, geographic differences in positive reactions can influence which allergens are appropriate to include. As a result, there is no universal standard series. Examples of standard or screening series include the American Contact Dermatitis Society (ACDS) allergen series,6 North American Baseline Series or North American 80 Comprehensive Series, European Baseline Series, NACDG series,2 and the Pediatric Baseline Series,7 as well as many other country- or region-specific series. There currently are 2 major commercial allergen distribution companies—Chemotechnique Diagnostics/Dormer Laboratories (series, individual allergens) and SmartPractice/allerGEAZE (series, individual allergens, T.R.U.E.).
In addition to a properly selected standard or screening series, supplemental patch testing with additional allergens can increase the diagnostic yield. Numerous supplemental series exist, including cosmetic, dental, textile, rubber, adhesive, plastics, and glue, among many others. In the NACDG 2015-2016 patch test cycle, it was found that 23% of 5597 patients reacted to an allergen that was not present on the NACDG screening series.2
In some situations, it is appropriate to patch test patient products, or nonstandard allergens. An abundance of caution, understanding of patch testing, and experience is necessary; for example, some chemicals are not recommended for testing, such as cleaning products, certain industrial chemicals, and those that may be carcinogens. We frequently consult De Groot’s Patch Testing8 for recommended allergen test concentrations and vehicles.
Patch Test Readings
The timing of the patch test reading is an important component of the test. Most North American comprehensive patch test clinics perform both first and delayed readings. After application, patches remain in place for 48 hours and then are removed, and a first reading is completed. Results are recorded as +/− (weak/doubtful), + (mild), ++ (strong), +++ (very strong), irritant, and negative.2 Many patch test specialists use side lighting to achieve the best reading and palpate to confirm the presence of induration; panel alignment devices commonly are utilized. There are some scenarios where shorter or longer application times are indicated, but this is beyond the scope of this article. A second, or delayed, reading should be completed 72 to 144 hours after initial application. We usually complete the delayed reading at 96 to 120 hours.
Certain patch test reactions may peak at different times, with fragrances often reacting earlier, and metals, topical antibiotics, and textile dyes reacting later.9 In the scenario of delayed peak reactions, third readings may be indicated.
Neglecting to complete a delayed reading is a potential pitfall and can increase the risk for both false-positive and false-negative reactions.10,11 In 1996, Uter et al10 published a large study of 9946 patients who were patch tested over a 4-year period. The authors compared patch test reactions at 48 and 72 hours and found that 34.5% of all positive reactions occurred at 72 hours; an additional 15.1% were positive at 96 hours. Importantly, one reading at 48 hours missed approximately one-third of positive patch test reactions, emphasizing the importance of delayed patch test readings.10 Furthermore, another study of 9997 consecutively patch tested patients examined reactions that were either negative or doubtful between days 3 or 4 and followed to see which of those reactions were positive at days 6 or 7. Of the negative reactions, the authors found that 4.4% were positive on days 6 or 7, and of the doubtful reactions, 9.1% were positive on days 6 or 7, meaning that up to 13.5% of positive reactions can be missed when a later reading is not performed.11
Medications During Patch Testing
Topical Medications
Topical medications generally can be continued during patch testing; however, patients should not apply topical medications to the patch test application site. Ideally, there should be no topical medication applied to the patch test application site for 1 to 2 weeks prior to patch test placement.12 Use of topical medications such as corticosteroids, calcineurin inhibitors, and theoretically even phosphodiesterase 4 inhibitors can not only result in suppression of positive patch test reactions but also can make patch adherence difficult.
Phototherapy
Phototherapy can result in local cutaneous immune suppression; therefore, it is recommended that it not be applied to the patch test area either during the patch test process or for 1 to 2 weeks prior to patch test application. In addition, if heat or sweating are generated during phototherapy, they can affect the success of patch testing by poor patch adherence and/or disruption of allergen distribution.
Systemic Medications
Oral antihistamines do not affect patch testing and can be continued during the patch test process.
It is ideal to avoid systemic immunomodulatory agents during the patch test process, but they occasionally are unavoidable, either because they are necessary to manage other medical conditions or because they are needed to achieve clear enough skin to proceed with patch testing. If it is required, prednisone is not recommended to exceed 10 mg daily.12,13 If intramuscular triamcinolone acetonide has been administered, patch testing should occur at least 1 month after the most recent injection.12 Oral methotrexate can probably be continued during patch testing but should be kept at the lowest possible dose and should be held during the week of testing, if possible. Adalimumab, etanercept, infliximab, and ustekinumab can be continued, as they are unlikely to interfere with patch testing.12 There are reports of positive patch test reactions on dupilumab,14,15 and some authors have described the response as variable and potentially allergen dependent.16,17 We believe that it generally is acceptable to continue dupilumab during patch testing. Data on cyclosporine during patch testing are mixed, and caution is advised as higher doses may suppress a positive patch test. Azathioprine and mycophenolate should be avoided, if possible.12
Pearls and Pitfalls
A few tips along the way can help assure your success in patch testing.
- Proper patient counseling determines a successful test. Provide your patient with verbal and written instructions about the patch test process, patch care, and any other necessary information.
- A simple sponge bath is permissible during patch testing provided the back stays dry. One of the authors (A.R.A.) advises patients to sit in a small amount of water in a bathtub to bathe, wash only the front of the body in the shower, and wash hair in the sink.
- No sweating, swimming, heavy exercise, or heavy physical labor. If your patient is planning to run a marathon the week of patch testing, they will be sorely disappointed when you tell them no sweating or showering is allowed! Patients with an occupation that requires physical labor may require a work excuse.
- Tape does not adhere to areas of the skin with excess hair. A scissor trim or electric shave will help the patches stay occluded and in place. We use an electric razor with a disposable replaceable head. A traditional straight razor should not be used, as it can increase the risk for folliculitis, which can make patch readings quite difficult.
- Securing the patches in place with an extra layer of tape provides added security. Large sheets of transparent medical dressings work particularly well for children or if there is difficulty with tape adherence.
Avoid application of patches to areas of the skin with tattoos. In theory, tattooed skin may have a decreased immune response, and tattoo pigment can obscure results.18 However, this is sometimes unavoidable, and Fowler and McTigue18 described a case of successful patch testing on a diffusely tattooed back.
- Avoid skin lesions (eg, scars, seborrheic keratoses, dermatitis) that can affect tape application, patch adherence, or patch readings.
Final Interpretation
The first step to excellent patch testing is understanding the patch test process. Patch test systems include T.R.U.E. and the chamber method. There are several allergen screening series, and the best series for each patient is determined based on geographic region, exposures, and allergen prevalence. The timing and practice of the patch test reading is vital, and physicians should be cognizant of medications and phototherapy use during the patch test process. An understanding of common pearls and pitfalls makes the difference between a good and great patch tester.
Now that you are an expert in performing the test, watch out for part 2 of this series on patch test interpretation, relevance, education, and counseling. Happy testing!
Our apologies, dear reader. It seems we have gotten ahead of ourselves. While we were writing about the Allergen of the Year, systemic dermatitis, and patch testing in children, we forgot to start with the basics. Let us remedy that. This is the first of a 2-part series addressing the basics of patch testing. In this article, we examine patch test systems, allergens, patch test readings, testing while on medications, and patch testing pearls and pitfalls. Let us begin!
Patch Test Systems
There are 2 patch test systems in North America: the Thin-layer Rapid Use Epicutaneous (T.R.U.E.) test (SmartPractice), which is approved by the US Food and Drug Administration for those 6 years and older, and the chamber method.
The T.R.U.E. test consists of 3 panels with 35 allergens and 1 negative control. The T.R.U.E. package insert1 describes surgical tape with individual polyester patches, each coated with an allergen film. Benefits of T.R.U.E. include ease of use (ie, easy storage and preparation, quick and straightforward application) and a readily identifiable set of allergens. The main drawback of T.R.U.E. is that only a limited number of allergens are tested, and as a result, it may miss the identification of some contact allergies. In an analysis of the 2015-2016 North American Contact Dermatitis Group (NACDG) patch test screening series, 25% to 40% of positive patch tests would have been missed if patch testing was performed with T.R.U.E. alone.2
Chamber method patch testing describes the process by which allergens are loaded into either metal or plastic chambers and then applied to the patient’s skin. The major benefit of the chamber method is that patches may be truly customized for the patient. The chamber method is time and labor intensive for patch preparation and application. Most comprehensive patch test clinics in North America use the chamber method, including the NACDG.
Patch test chambers largely can be divided into 2 categories: metal (aluminum) or plastic. Aluminum chambers, also known as Finn chambers, traditionally are used in patch testing. There are rare reports of hypersensitivity to aluminum chambers with associated diffuse positive patch test reactions,3,4 which may be more common in the pediatric population and likely is due to the fact that aluminum is present as an adjuvant in many childhood vaccines. As a precaution, some patch text experts recommend using plastic chambers in children younger than 16 to 18 years (M.R. and A.R.A., personal communication). Metal chambers require the additional application of diffusion discs for liquid allergens, and plastic chambers typically already contain the necessary diffusion discs. Finn chambers traditionally are applied with hypoallergenic porous surgical tape, but a waterproof tape also is available. To keep the chambers in place for the necessary 48 hours, additional tape may be applied over the patches.
Allergens
In patch test clinics, many dermatologists use a standard or screening allergen series. An appropriate standard series encompasses allergens that are most likely to be positive and relevant in the tested population. Some patch test experts recommend that allergens with a positive patch test frequency of greater than 0.5% to 1% should be included in a standard series.5 However, geographic differences in positive reactions can influence which allergens are appropriate to include. As a result, there is no universal standard series. Examples of standard or screening series include the American Contact Dermatitis Society (ACDS) allergen series,6 North American Baseline Series or North American 80 Comprehensive Series, European Baseline Series, NACDG series,2 and the Pediatric Baseline Series,7 as well as many other country- or region-specific series. There currently are 2 major commercial allergen distribution companies—Chemotechnique Diagnostics/Dormer Laboratories (series, individual allergens) and SmartPractice/allerGEAZE (series, individual allergens, T.R.U.E.).
In addition to a properly selected standard or screening series, supplemental patch testing with additional allergens can increase the diagnostic yield. Numerous supplemental series exist, including cosmetic, dental, textile, rubber, adhesive, plastics, and glue, among many others. In the NACDG 2015-2016 patch test cycle, it was found that 23% of 5597 patients reacted to an allergen that was not present on the NACDG screening series.2
In some situations, it is appropriate to patch test patient products, or nonstandard allergens. An abundance of caution, understanding of patch testing, and experience is necessary; for example, some chemicals are not recommended for testing, such as cleaning products, certain industrial chemicals, and those that may be carcinogens. We frequently consult De Groot’s Patch Testing8 for recommended allergen test concentrations and vehicles.
Patch Test Readings
The timing of the patch test reading is an important component of the test. Most North American comprehensive patch test clinics perform both first and delayed readings. After application, patches remain in place for 48 hours and then are removed, and a first reading is completed. Results are recorded as +/− (weak/doubtful), + (mild), ++ (strong), +++ (very strong), irritant, and negative.2 Many patch test specialists use side lighting to achieve the best reading and palpate to confirm the presence of induration; panel alignment devices commonly are utilized. There are some scenarios where shorter or longer application times are indicated, but this is beyond the scope of this article. A second, or delayed, reading should be completed 72 to 144 hours after initial application. We usually complete the delayed reading at 96 to 120 hours.
Certain patch test reactions may peak at different times, with fragrances often reacting earlier, and metals, topical antibiotics, and textile dyes reacting later.9 In the scenario of delayed peak reactions, third readings may be indicated.
Neglecting to complete a delayed reading is a potential pitfall and can increase the risk for both false-positive and false-negative reactions.10,11 In 1996, Uter et al10 published a large study of 9946 patients who were patch tested over a 4-year period. The authors compared patch test reactions at 48 and 72 hours and found that 34.5% of all positive reactions occurred at 72 hours; an additional 15.1% were positive at 96 hours. Importantly, one reading at 48 hours missed approximately one-third of positive patch test reactions, emphasizing the importance of delayed patch test readings.10 Furthermore, another study of 9997 consecutively patch tested patients examined reactions that were either negative or doubtful between days 3 or 4 and followed to see which of those reactions were positive at days 6 or 7. Of the negative reactions, the authors found that 4.4% were positive on days 6 or 7, and of the doubtful reactions, 9.1% were positive on days 6 or 7, meaning that up to 13.5% of positive reactions can be missed when a later reading is not performed.11
Medications During Patch Testing
Topical Medications
Topical medications generally can be continued during patch testing; however, patients should not apply topical medications to the patch test application site. Ideally, there should be no topical medication applied to the patch test application site for 1 to 2 weeks prior to patch test placement.12 Use of topical medications such as corticosteroids, calcineurin inhibitors, and theoretically even phosphodiesterase 4 inhibitors can not only result in suppression of positive patch test reactions but also can make patch adherence difficult.
Phototherapy
Phototherapy can result in local cutaneous immune suppression; therefore, it is recommended that it not be applied to the patch test area either during the patch test process or for 1 to 2 weeks prior to patch test application. In addition, if heat or sweating are generated during phototherapy, they can affect the success of patch testing by poor patch adherence and/or disruption of allergen distribution.
Systemic Medications
Oral antihistamines do not affect patch testing and can be continued during the patch test process.
It is ideal to avoid systemic immunomodulatory agents during the patch test process, but they occasionally are unavoidable, either because they are necessary to manage other medical conditions or because they are needed to achieve clear enough skin to proceed with patch testing. If it is required, prednisone is not recommended to exceed 10 mg daily.12,13 If intramuscular triamcinolone acetonide has been administered, patch testing should occur at least 1 month after the most recent injection.12 Oral methotrexate can probably be continued during patch testing but should be kept at the lowest possible dose and should be held during the week of testing, if possible. Adalimumab, etanercept, infliximab, and ustekinumab can be continued, as they are unlikely to interfere with patch testing.12 There are reports of positive patch test reactions on dupilumab,14,15 and some authors have described the response as variable and potentially allergen dependent.16,17 We believe that it generally is acceptable to continue dupilumab during patch testing. Data on cyclosporine during patch testing are mixed, and caution is advised as higher doses may suppress a positive patch test. Azathioprine and mycophenolate should be avoided, if possible.12
Pearls and Pitfalls
A few tips along the way can help assure your success in patch testing.
- Proper patient counseling determines a successful test. Provide your patient with verbal and written instructions about the patch test process, patch care, and any other necessary information.
- A simple sponge bath is permissible during patch testing provided the back stays dry. One of the authors (A.R.A.) advises patients to sit in a small amount of water in a bathtub to bathe, wash only the front of the body in the shower, and wash hair in the sink.
- No sweating, swimming, heavy exercise, or heavy physical labor. If your patient is planning to run a marathon the week of patch testing, they will be sorely disappointed when you tell them no sweating or showering is allowed! Patients with an occupation that requires physical labor may require a work excuse.
- Tape does not adhere to areas of the skin with excess hair. A scissor trim or electric shave will help the patches stay occluded and in place. We use an electric razor with a disposable replaceable head. A traditional straight razor should not be used, as it can increase the risk for folliculitis, which can make patch readings quite difficult.
- Securing the patches in place with an extra layer of tape provides added security. Large sheets of transparent medical dressings work particularly well for children or if there is difficulty with tape adherence.
Avoid application of patches to areas of the skin with tattoos. In theory, tattooed skin may have a decreased immune response, and tattoo pigment can obscure results.18 However, this is sometimes unavoidable, and Fowler and McTigue18 described a case of successful patch testing on a diffusely tattooed back.
- Avoid skin lesions (eg, scars, seborrheic keratoses, dermatitis) that can affect tape application, patch adherence, or patch readings.
Final Interpretation
The first step to excellent patch testing is understanding the patch test process. Patch test systems include T.R.U.E. and the chamber method. There are several allergen screening series, and the best series for each patient is determined based on geographic region, exposures, and allergen prevalence. The timing and practice of the patch test reading is vital, and physicians should be cognizant of medications and phototherapy use during the patch test process. An understanding of common pearls and pitfalls makes the difference between a good and great patch tester.
Now that you are an expert in performing the test, watch out for part 2 of this series on patch test interpretation, relevance, education, and counseling. Happy testing!
- T.R.U.E TEST [package insert]. Phoenix, AZ: SmartPractice; 1994.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Ward JM, Walsh RK, Bellet JS, et al. Allergic contact dermatitis to aluminum-based chambers during routine patch testing. Paper presented at: American Contact Dermatitis Society Annual Meeting; March 19, 2020; Denver, CO.
- Deleuran MG, Ahlström MG, Zachariae C, et al. Patch test reactivity to aluminum chambers. Contact Dermatitis. 2019;81:318-319.
- Bruze M, Condé-Salazar L, Goossens A, et al. European Society of Contact Dermatitis. thoughts on sensitizers in a standard patch test series. Contact Dermatitis. 1999;41:241-250.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2017 update. Dermatitis. 2017;28:141-143.
- Yu J, Atwater AR, Brod B, et al. Pediatric baseline patch test series: Pediatric Contact Dermatitis Workgroup. Dermatitis. 2018;29:206-212.
- De Groot AC. Patch Testing: Test Concentrations and Vehicles for 4900 Chemicals. 4th ed. Wapserveen, The Netherlands: Acdegroot Publishing; 2018.
- Chaudhry HM, Drage LA, El-Azhary RA, et al. Delayed patch-test reading after 5 days: an update from the Mayo Clinic Contact Dermatitis Group. Dermatitis. 2017;28:253-260.
- Uter WJ, Geier J, Schnuch A. Good clinical practice in patch testing: readings beyond day 2 are necessary: a confirmatory analysis. Members of the Information Network of Departments of Dermatology. Am J Contact Dermat. 1996;7:231-237.
- Madsen JT, Andersen KE. Outcome of a second patch test reading of T.R.U.E. Tests® on D6/7. Contact Dermatitis. 2013;68:94-97.
- Lampel H, Atwater AR. Patch testing tools of the trade: use of immunosuppressants and antihistamines during patch testing. J Dermatol Nurses’ Assoc. 2016;8:209-211.
- Fowler JF, Maibach HI, Zirwas M, et al. Effects of immunomodulatory agents on patch testing: expert opinion 2012. Dermatitis. 2012;23:301-303.
- Puza CJ, Atwater AR. Positive patch test reaction in a patient taking dupilumab. Dermatitis. 2018;29:89.
- Hoot JW, Douglas JD, Falo LD. Patch testing in a patient on dupilumab. Dermatitis. 2018;29:164.
- Stout M, Silverberg JI. Variable impact of dupilumab on patch testing results and allergic contact dermatitis in adults with atopic dermatitis. J Am Acad Dermatol. 2019;81:157-162.
- Raffi J, Botto N. Patch testing and allergen-specific inhibition in a patient taking dupilumab. JAMA Dermatol. 2019;155:120-121.
- Fowler JF, McTigue MK. Patch testing over tattoos. Am J Contact Dermat. 2002;13:19-20.
- T.R.U.E TEST [package insert]. Phoenix, AZ: SmartPractice; 1994.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Ward JM, Walsh RK, Bellet JS, et al. Allergic contact dermatitis to aluminum-based chambers during routine patch testing. Paper presented at: American Contact Dermatitis Society Annual Meeting; March 19, 2020; Denver, CO.
- Deleuran MG, Ahlström MG, Zachariae C, et al. Patch test reactivity to aluminum chambers. Contact Dermatitis. 2019;81:318-319.
- Bruze M, Condé-Salazar L, Goossens A, et al. European Society of Contact Dermatitis. thoughts on sensitizers in a standard patch test series. Contact Dermatitis. 1999;41:241-250.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2017 update. Dermatitis. 2017;28:141-143.
- Yu J, Atwater AR, Brod B, et al. Pediatric baseline patch test series: Pediatric Contact Dermatitis Workgroup. Dermatitis. 2018;29:206-212.
- De Groot AC. Patch Testing: Test Concentrations and Vehicles for 4900 Chemicals. 4th ed. Wapserveen, The Netherlands: Acdegroot Publishing; 2018.
- Chaudhry HM, Drage LA, El-Azhary RA, et al. Delayed patch-test reading after 5 days: an update from the Mayo Clinic Contact Dermatitis Group. Dermatitis. 2017;28:253-260.
- Uter WJ, Geier J, Schnuch A. Good clinical practice in patch testing: readings beyond day 2 are necessary: a confirmatory analysis. Members of the Information Network of Departments of Dermatology. Am J Contact Dermat. 1996;7:231-237.
- Madsen JT, Andersen KE. Outcome of a second patch test reading of T.R.U.E. Tests® on D6/7. Contact Dermatitis. 2013;68:94-97.
- Lampel H, Atwater AR. Patch testing tools of the trade: use of immunosuppressants and antihistamines during patch testing. J Dermatol Nurses’ Assoc. 2016;8:209-211.
- Fowler JF, Maibach HI, Zirwas M, et al. Effects of immunomodulatory agents on patch testing: expert opinion 2012. Dermatitis. 2012;23:301-303.
- Puza CJ, Atwater AR. Positive patch test reaction in a patient taking dupilumab. Dermatitis. 2018;29:89.
- Hoot JW, Douglas JD, Falo LD. Patch testing in a patient on dupilumab. Dermatitis. 2018;29:164.
- Stout M, Silverberg JI. Variable impact of dupilumab on patch testing results and allergic contact dermatitis in adults with atopic dermatitis. J Am Acad Dermatol. 2019;81:157-162.
- Raffi J, Botto N. Patch testing and allergen-specific inhibition in a patient taking dupilumab. JAMA Dermatol. 2019;155:120-121.
- Fowler JF, McTigue MK. Patch testing over tattoos. Am J Contact Dermat. 2002;13:19-20.
Practice Points
- There are 2 basic patch testing systems: the T.R.U.E. test and the chamber method.
- Patches should be applied to the upper back and should be removed after 48 hours. A delayed reading is necessary and should be performed 72 to 144 hours after placement.
- Certain oral and topical medications and phototherapy may interfere with patch test results.