User login
Molluscum contagiosum virus (MCV) infection causes the cutaneous lesions we call molluscum. Molluscum has become common in the last 30 years. Deciding the best course of therapy requires some fundamental understanding about how MCV relates to the following factors: epidemiology, childhood immunity and vaccination, clinical features, comorbidities, and quality of life. Treatment depends on many factors, including presence or absence of atopic dermatitis (AD) and/or pruritus, other symptoms, cosmetic location, and the child’s concern about the lesions. Therapeutics include destructive and immunologic therapies, the latter geared toward increasing immune response.
Epidemiology
Molluscum contagiosum virus is the solo member of the Molluscipoxvirus genus. Infection with MCV causes benign growth or tumors in the skin (ie, molluscum). The infection is slow to clear because the virus reduces the host’s immunity.1,2 Molluscum contagiosum virus is a double-stranded DNA virus that affects keratinocytes and genetically carries the tools for its own replication (ie, DNA-dependent RNA polymerase). The virus has a few subtypes—I/Ia, II, III, and IV—with MCV-I predominating in children and healthy humans and MCV-II in patients with human immunodeficiency virus.1,2 Typing is experimental and is not standardly performed in clinical practice. Molluscum contagiosum virus produces a variety of factors that block the host’s immune response, prolonging infection and preventing erythema and inflammatory response.3
Molluscum contagiosum virus is transmitted through skin-to-skin contact and fomites, including shared towels, bathtubs, spas, bath sponges, and pool equipment.2,4,5 Transmission from household contact and bathing together has been noted in pediatric patients with MCV. Based on the data it can be posited that the lesions are softer when wet and more readily release viral particles or fomites, and fomites may be left on surfaces, especially when a child is wet.6,7 Propensity for infection occurs in patients with AD and in immunosuppressed hosts, including children with human immunodeficiency virus and iatrogenic immunosuppression caused by chemotherapy.1,2,8 Contact sports can increase the risk of transmission, and outbreaks have occurred in pools,5,9 day-care facilities,10 and sports settings.11 Cases of congenital and vertically transmitted molluscum have been documented.12,13 Sexual transmission of MCV may be seen in adolescents who are sexually active. Although child-to-child transmission can occur in the groin area from shared equipment, transmission via sexual abuse also is possible.14 Bargman15 has mentioned the isolated genital location and lack of contact with other infected children as concerning features. Latency of new lesion appearance is anywhere from 1 to 50 days from the date of inoculation; therefore, new lesions are possible and expected even after therapy has been effective in eradicating visible lesions.10 Although clearance has been reported in 6 to 12 months, one pediatric study demonstrated 70% clearance by 1.5 years, suggesting the disease often is more prolonged.16 One-third of children will experience signs of inflammation, such as pruritus and/or erythema. Rare side effects include bacterial superinfection and hypersensitivity.2
One Dutch study from 1994, the largest database survey of children to date, cited a 17% cumulative incidence of molluscum in children by reviewing the data from 103 general practices.17 In a survey and review of molluscum by Braue et al,18 annual rates in populations vary but seem to maximize at approximately 6% to 7%. Sturt et al19 reviewed the prevalence in the indigenous West Sepik section of New Guinea and noted annual incidence rates of 6% in children younger than 10 years (range, 1.8%–10.9%). Epidemics occur and can produce large numbers of cases in a short time period.18 The cumulative prevalence in early childhood may be as high as 22%, as Sturt et al19 observed in children younger than 10 years.
Rising incidence and therefore rising lifetime prevalence appear to have been an issue in the last few decades. Data from the Indian Health Service have demonstrated increases in MCV in Native American children between 2001 and 2005.20 In adults, the data support a steady increase of molluscum from 1988-2007, with a 3-fold increase from 1988-1997 to 1998-2007 in a Spanish study.21 Better population-based data are needed.
Childhood Immunity and Vaccination
Sequence homology between MC133L, a protein of MCV, with vaccinia virus suggests overlapping genes.22 Therefore, it is conceptually possible that the rise in incidence of MCV since the 1980s relates to the loss of herd immunity to variola due to lack of vaccination for smallpox, which has not been offered in the United States since 1972.23 Childhood immunity to MCV varies among studies, but it appears that children do develop antibodies to molluscum in the setting of forming an immune response. Because the rise in molluscum incidence began after the smallpox vaccine was discontinued, the factors appear related; however, the scientific data do not support the theory of a relationship. Mitchell24 has shown that a patient can develop antibodies in response to ground molluscum bodies inoculated into the skin; however, vaccination against molluscum and natural infection do not appear to produce antibodies that would cross-react and protect against other poxviruses, including vaccinia or fowl pox infections.25 Cell-mediated immunity also is required to clear MCV and may account for the inflammatory appearance of lesions as they resolve.26
Demonstrated factors that account for the rise in MCV incidence, aside from alterations in vaccination practices, include spread through sports,9 swimming,11 and AD,7 which have become more commonplace in the United States in the last few decades, supporting the theory that they may be the cause of the increase in childhood MCV infections. Another cause may be the ability of MCV to create factors that stem host immune response.1
Clinical Features
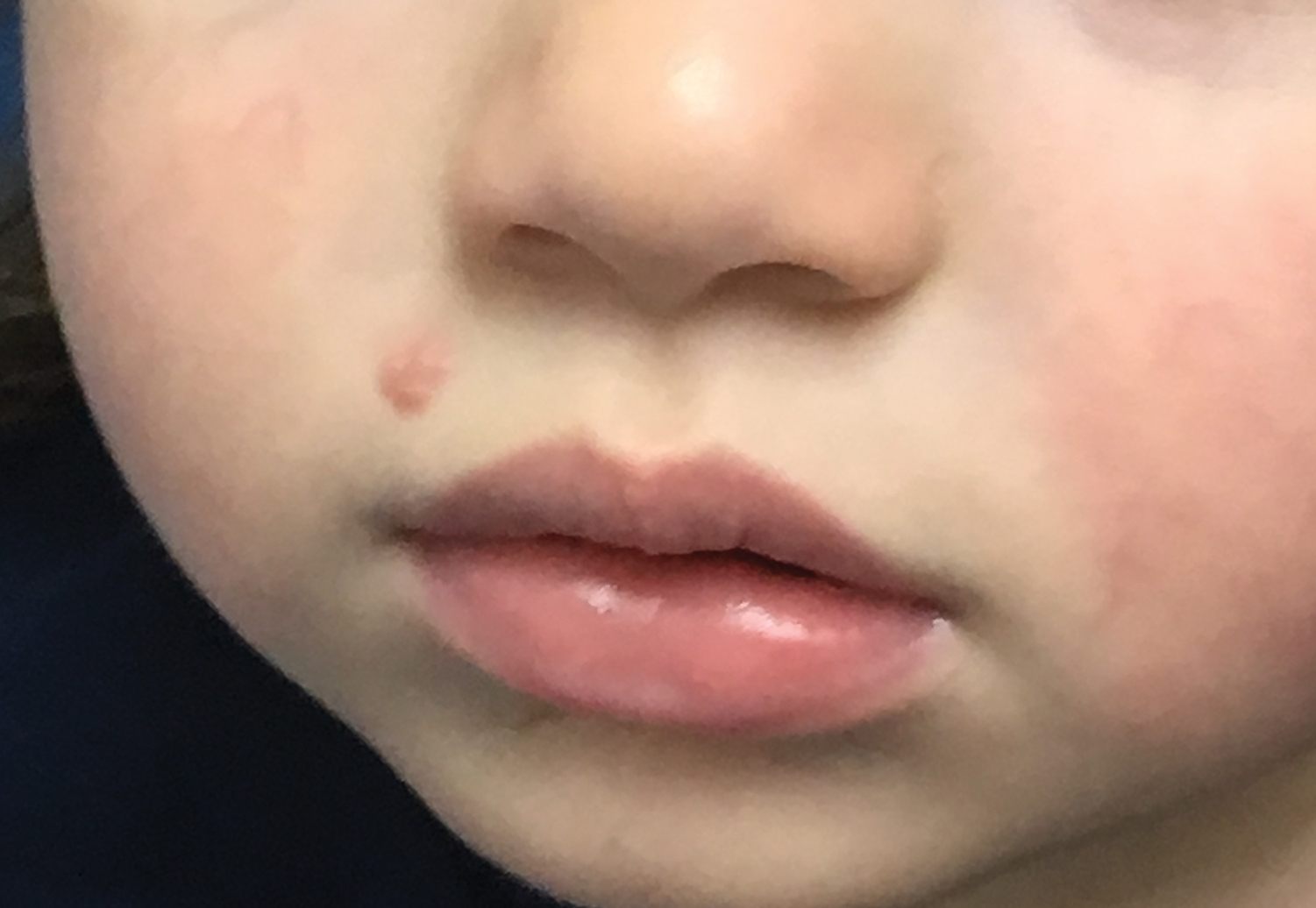
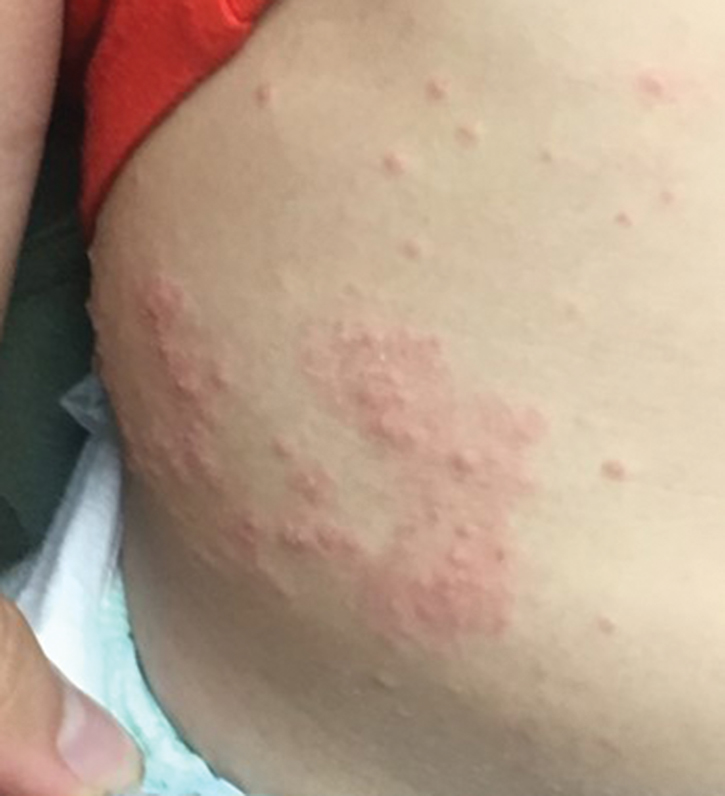
Molluscum lesions have a typical appearance of pearly papules with a central dell. These lesions are lighter to flesh colored and measure 1 to 3 mm.2,4,5 The lesions cluster in the axillae and extremities and average from 10 to 20 per child.6 Lesions clear spontaneously, but new ones will continue to form until immunity is developed. Specific clinical appearances of lesions that are not pearly papules are not infrequent. Table 1 contains a short list of the manifold clinical appearances of molluscum lesions in children.1,2,7,27-35 In particular, certain clinical appearances should be considered. In small children, head and neck lesions resembling milia are not uncommon. Giant or wartlike lesions can appear on the head, neck, or gluteal region in children and are clinical mimics of condyloma or other warts (Figure 1). Giant lesions also can grow in the subcutaneous space and mimic a cyst or abscess.27 Erosive lesions mimicking eczema vaccinatum can be seen (Figure 2), but dermoscopy may demonstrate central dells in some lesions. Other viral processes mimicked include Gianotti Crosti–like lesions (Figure 3) that appear when a papular id reaction forms over the extremities or a localized version in the axilla, mimicking unilateral laterothoracic exanthema.2,36,37 Hypersensitivity reactions are commonly noted with clearance and can be papular or demonstrate swelling and erythema, termed the beginning-of-the-end sign.38
Pruritus, erythema, and swelling can occur with clearance but do not appear in all patients. Addressing pruritus is important to prevent disease spread, as patients are likely to inoculate other areas of the skin with virus when they scratch, and lesion number is reduced with dermatitis interventions.36
Comorbidities
Molluscum lesions can occur in any child; however, the impaired immunologic status and skin barrier in patients with AD is ripe for the extensive spread of lesions that is associated with higher lesion count.36 Children with molluscum infection can experience new-onset dermatitis or triggering of AD flares, especially on the extremities, such as the antecubital and popliteal regions.7 A study of children with MCV infection demonstrated that treatment of active dermatitis reduced spread. The authors mentioned autoinoculation as the mechanism; however, these data also suggest supporting barrier state as a factor in disease spread.36 Superinfection can occur prior to6 or after therapy for lesions,37 but it is unclear if this relates to the underlying atopic diathesis. Children with molluscum have been described to have warts, psoriasis, family history of atopy, diabetes mellitus, and pityriasis alba,7 while immunosuppression of any kind is associated with molluscum and high lesion count or prolonged disease in childhood.1,2
Quality of Life
Children with molluscum who have higher lesion counts appear to be at risk for severe effects on their quality of life. Approximately 10% of children with MCV infection have been documented to have severe impairments on quality of life.39 In my practice, quality of life in children with MCV appears to be affected by many factors (Table 2).7,18,39
Treatments
Proper Skin Care and Treatment of AD
Therapy for AD and/or pruritus appears to limit lesion number in children with MCV and rashes or itch.7,36 I recommend barrier repair agents, including emollients and syndet bar cleansers, to prevent small breaks in the skin that occur with xerosis and AD and that increase itch and risk of spread. Therapy for AD and molluscum dermatitis is similar and overlapping. There is always a concern about the spread of MCV when using topical calcineurin inhibitors. I, therefore, focus the dermatitis therapeutics on topical corticosteroid–based care.6,40
Prevention of Spread
Prevention of spread begins with hygiene interventions. Cobathing is common in children with MCV and should be held off when possible. It is important for the child with MCV to avoid sharing bath towels and equipment23 and having bare skin come in contact with mats in sports. I request that children with MCV wear bathing suits that cover the areas affected.
Reassurance
The most important therapy is reassurance.41 Many parents/guardians are truly unaware that the MCV infection can last for more than a year and therefore worry over normal disease course. When counseled as to the benign course of illness and given instructions on proper skin care, the parent/guardian of a child with MCV will often opt against therapy of uncomplicated cases. On the other hand, there are medical reasons for treatment, and they support the need for intervention (Table 3). Seventy percent of lesions resolve in 1.5 years; however, of the residual infections, some may last as long as 4 years.16 It is not recommended to stop children from attending school because of MCV.
Interventional Therapy
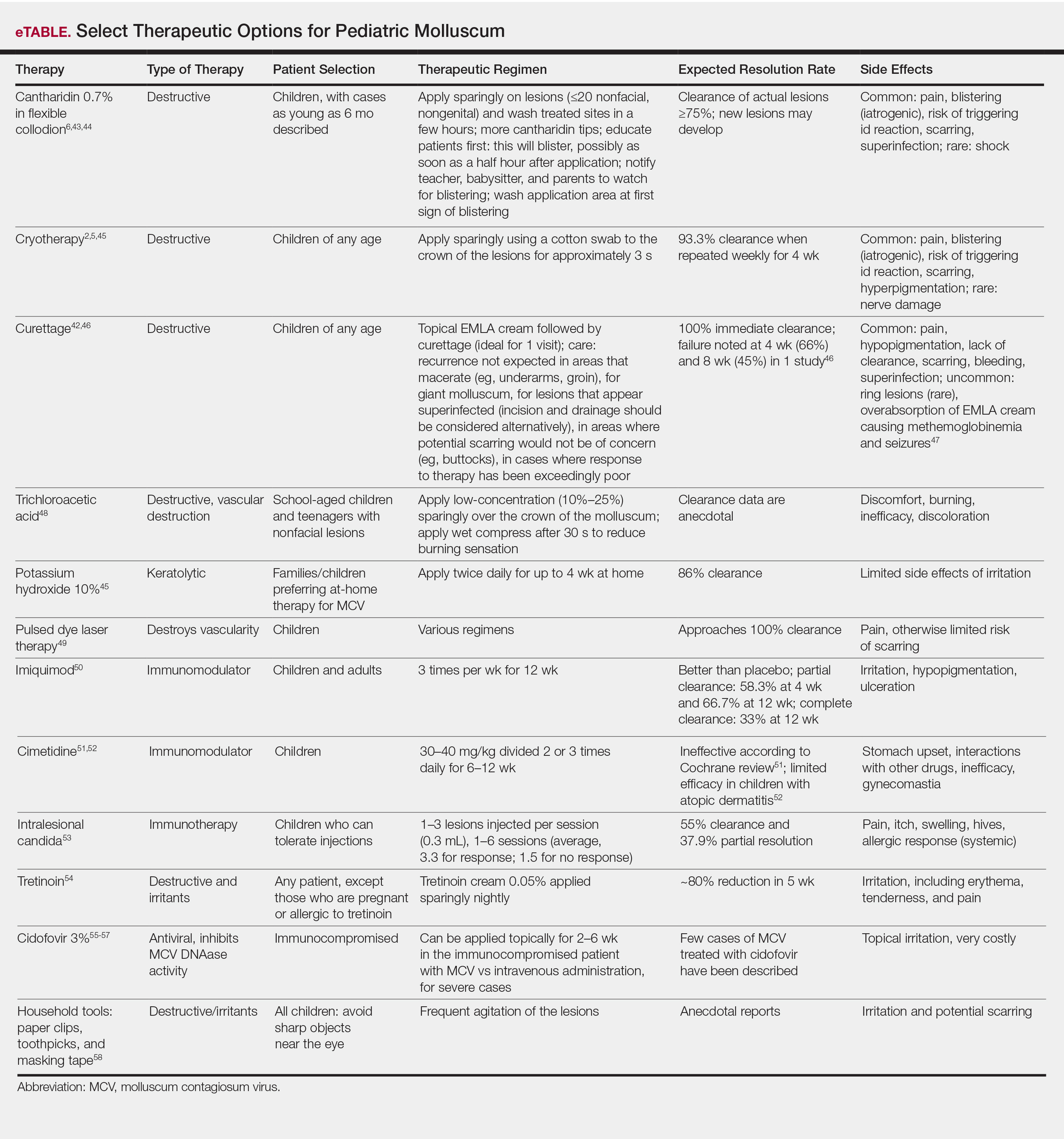
Therapeutics of MCV include destructive therapies in office (ie, cantharidin, cryotherapy, curettage, trichloroacetic acid, and glycolic acid) and at-home therapies (ie, topical retinoids, nitric oxide releasers)(eTable).2,5,6,42-58 When there are many lesions or spread is noted, immunotherapies can be used, including topical imiquimod, oral cimetidine, and intralesional Candida antigen.2,4,7 Pulsed dye laser cuts off the lesion vascular supply, while cidofovir is directly antiviral both topically and systemically, the latter reserved for severe cases in immunosuppressed adults.59 Head-to-head studies of cantharidin, curettage, topical peeling agents, and imiquimod demonstrated better satisfaction and fewer office visits with topical anesthetic and curettage on the first visit. Side effects were greatest for salicylic acid and glycolic acid; therefore, these agents are less desirable.42
Conclusion
Molluscum is a cutaneous viral infection that is common in children and has associated morbidities, including AD, pruritus, poor quality of life in some cases, and risk of contagion. Addressing the disease includes understanding its natural history and explaining it to parents/guardians. Therapeutics can be offered in cases where need is demonstrated, such as with lesions that spread and cause discomfort. Choice of therapeutics depends on the practitioner’s experience, the child’s clinical appearance, availability of therapy, and review of options with the parents/guardians. When avoidance of intervention is desired, barrier enhancement and treatment of symptomatic dermatitis are still beneficial, as are household (eg, not sharing towels) and activity (eg, adhesive bandages over active lesions) interventions to reduce transmission.
- Shisler JL. Immune evasion strategies of molluscum contagiosum virus. Adv Virus Res. 2015;92:201-252.
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99.
- Moss B, Shisler JL, Xiang Y, et al. Immune-defense molecules of molluscum contagiosum virus, a human poxvirus. Trends Microbiol. 2000;8:473-477.
- Silverberg NB. Warts and molluscum in children. Adv Dermatol. 2004;20:23-73.
- Choong KY, Roberts LJ. Molluscum contagiosum, swimming and bathing: a clinical analysis. Australas J Dermatol. 1999;40:89-92.
- Silverberg NB, Sidbury R, Mancini AJ. Childhood molluscum contagiosum: experience with cantharidin therapy in 300 patients. J Am Acad Dermatol. 2000;43:503-507.
- Silverberg NB. Molluscum contagiosum virus infection can trigger atopic dermatitis disease onset or flare. Cutis. 2018;102:191-194.
- Ajithkumar VT, Sasidharanpillai S, Muhammed K, et al. Disseminated molluscum contagiosum following chemotherapy: a therapeutic challenge. Indian J Dermatol Venereol Leprol. 2017;83:516.
- Oren B, Wende SO. An outbreak of molluscum contagiosum in a kibbutz. Infection. 1991;19:159-161.
- Molluscum contagiosum. Healthy Children website. https://www.healthychildren.org/English/health-issues/conditions/skin/Pages/Molluscum-Contagiosum.aspx. Updated November 21, 2015. Accessed October 16, 2019.
- Peterson AR, Nash E, Anderson BJ. Infectious disease in contact sports. Sports Health. 2019;11:47-58.
- Connell CO, Oranje A, Van Gysel D, et al. Congenital molluscum contagiosum: report of four cases and review of the literature. Pediatr Dermatol. 2008;25:553-556.
- Luke JD, Silverberg NB. Vertically transmitted molluscum contagiosum infection. Pediatrics. 2010;125:E423-E425.
- Mendiratta V, Agarwal S, Chander R. Reappraisal of sexually transmitted infections in children: a hospital-based study from an urban area. Indian J Sex Transm Dis AIDS. 2014;35:25-28.
- Bargman H. Genital molluscum contagiosum in children: evidence of sexual abuse? CMAJ. 1986;135:432-433.
- Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32:353-357.
- Koning S, Bruijnzeels MA, van Suijlekom-Smit LW, et al. Molluscum contagiosum in Dutch general practice. Br J Gen Pract. 1994;44:417-419.
- Braue A, Ross G, Varigos G, et al. Epidemiology and impact of childhood molluscum contagiosum: a case series and critical review of the literature. Pediatr Dermatol. 2005;22:287-294.
- Sturt RJ, Muller HK, Francis GD. Molluscum contagiosum in villages of the West Sepik District of New Guinea. Med J Aust. 1971;2:751-754.
- Reynolds MG, Homan RC, Yorita Christensen KL, et al. The incidence of molluscum contagiosum among American Indians and Alaska Natives. PLoS One. 2009;4:e5255.
- Villa L, Varela JA, Otero L, et al. Molluscum contagiosum: a 20-year study in a sexually transmitted infections unit. Sex Transm Dis. 2010;37:423-424.
- Watanabe T, Morikawa S, Suzuki K, et al. Two major antigenic polypeptides of molluscum contagiosum virus. J Infect Dis. 1998;177:284-292.
- Vaccine basics. Centers for Disease Control and Prevention website. https://www.cdc.gov/smallpox/vaccine-basics/index.html. Updated July 12, 2017. Accessed October 16, 2019.
- Mitchell JC. Observations on the virus of molluscum contagiosum. Br J Exp Pathol. 1953;34:44-49.
- Konya J, Thompson CH. Molluscum contagiosum virus: antibody responses in patients with clinical lesions and its sero-epidemiology in a representative Australian population. J Infect Dis. 1999;179:701-704.
- Steffen C, Markman JA. Spontaneous disappearance of molluscum contagiosum. Arch Dermatol. 1980;116:923-924.
- Uzuncakmak TK, Kuru BC, Zemheri EI, et al. Isolated giant molluscum contagiosum mimicking epidermoid cyst. Dermatol Pract Concept. 2016;6:71-73.
- Persechino S, Abruzzese C, Caperchi C, et al. Condyloma acuminata and mollusca contagiosa: a giant manifestation in a patient with lupus. Skinmed. 2014;12:310-311.
- Kim SK, Do JE, Kang HY, et al. Giant molluscum contagiosum of immunocompetent children occurring on the anogenital area. Eur J Dermatol. 2007;17:537-538.
- Alam MS, Shrirao N. Giant molluscum contagiosum presenting as lid neoplasm in an immunocompetent child. Dermatol Online J. 2016;22. pii:13030/qt56v567gn.
- Krishnamurthy J, Nagappa DK. The cytology of molluscum contagiosum mimicking skin adnexal tumor. J Cytol. 2010;27:74-75.
- Baek YS, Oh CH, Song HJ, et al. Asymmetrical periflexural exanthem of childhood with concurrence of molluscum contagiosum infection. Clin Exp Dermatol. 2011;36:676-677.
- Lee HJ, Kwon JA, Kim JW. Erythema multiforme-like molluscum dermatitis. Acta Derm Venereol. 2002;82:217-218.
- Lee YB, Choi HJ, Park HJ, et al. Two cases of erythema multiforme associated with molluscum contagiosum. Int J Dermatol. 2009;48:659-660.
- Vasily DB, Bhatia SG. Erythema annulare centrifugum and molluscum contagiosum.
- Berger EM, Orlow SJ, Patel RR, et al. Experience with molluscum contagiosum and associated inflammatory reactions in a pediatric dermatology practice: the bump that rashes. Arch Dermatol. 2012;148:1257-1264.
- Groner A, Laing-Grayman D, Silverberg NB. Outpatient pediatric community-acquired methicillin-resistant Staphylococcus aureus: a polymorphous clinical disease. Cutis. 2008;81:115-122.
- Butala N, Siegfried E, Weissler A. Molluscum BOTE sign: a predictor of imminent resolution. Pediatrics. 2013;131:E1650-E1653.
- Olsen JR, Gallagher J, Finlay AY, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190-195.
- Goksugur N, Ozbostanci B, Goksugur SB. Molluscum contagiosum infection associated with pimecrolimus use in pityriasis alba. Pediatr Dermatol. 2007;24:E63-E65.
- Lee R, Schwartz RA. Pediatric molluscum contagiosum: reflections on the last challenging poxvirus infection, part 1. Cutis. 2010;86:230-236.
- Hanna D, Hatami A, Powell J, et al. A prospective randomized trial comparing the efficacy and adverse effects of four recognized treatments of molluscum contagiosum in children. Pediatr Dermatol. 2006;23:574-579.
- Coloe Dosal J, Stewart PW, Lin JA, et al. Cantharidin for the treatment of molluscum contagiosum: a prospective, double-blinded, placebo-controlled trial. Pediatr Dermatol. 2014;31:440-449.
- Vakharia PP, Chopra R, Silverberg NB, et al. Efficacy and safety of topical cantharidin treatment for molluscum contagiosum and warts: a systematic review. Am J Clin Dermatol. 2018;19:791-803.
- Handjani F, Behazin E, Sadati MS. Comparison of 10% potassium hydroxide solution versus cryotherapy in the treatment of molluscum contagiosum: an open randomized clinical trial. J Dermatolog Treat. 2014;25:249-250.
- Simonart T, De Maertelaer V. Curettage treatment for molluscum contagiosum: a follow-up survey study. Br J Dermatol. 2008;159:1144-1147.
- Cho YS, Chung BY, Park CW, et al. Seizures and methemoglobinemia after topical application of eutectic mixture of lidocaine and prilocaine on a 3.5-year-old child with molluscum contagiosum and atopic dermatitis. Pediatr Dermatol. 2016;33:E284-E285.
- Bard S, Shiman MI, Bellman B, et al. Treatment of facial molluscum contagiosum with trichloroacetic acid. Pediatr Dermatol. 2009;26:425-426.
- Griffith RD, Yazdani Abyaneh MA, Falto-Aizpurua L, et al. Pulsed dye laser therapy for molluscum contagiosum: a systematic review. J Drugs Dermatol. 2014;13:1349-1352.
- Theos AU, Cummins R, Silverberg NB, et al. Effectiveness of imiquimod cream 5% for treating childhood molluscum contagiosum in a double-blind, randomized pilot trial. Cutis. 2004;74:134-138, 141-142.
- van der Wouden JC, Menke J, Gajadin S, et al. Interventions for cutaneous molluscum contagiosum. Cochrane Database Syst Rev. 2006:CD004767.
- Cunningham BB, Paller AS, Garzon M. Inefficacy of oral cimetidine for nonatopic children with molluscum contagiosum. Pediatr Dermatol. 1998;15:71-72.
- Enns LL, Evans MS. Intralesional immunotherapy with Candida antigen for the treatment of molluscum contagiosum in children. Pediatr Dermatol. 2011;28:254-258.
- Rajouria EA, Amatya A, Karn D. Comparative study of 5% potassium hydroxide solution versus 0.05% tretinoin cream for molluscum contagiosum in children. Kathmandu Univ Med J (KUMJ). 2011;9:291-294.
- Briand S, Milpied B, Navas D, et al. 1% topical cidofovir used as last alternative to treat viral infections. J Eur Acad Dermatol Venereol. 2008;22:249-250.
- Zabawski EJ Jr, Cockerell CJ. Topical cidofovir for molluscum contagiosum in children. Pediatr Dermatol. 1999;16:414-415.
- Watanabe T. Cidofovir diphosphate inhibits molluscum contagiosum virus DNA polymerase activity. J Invest Dermatol. 2008;128:1327-1329.
- Lindau MS, Munar MY. Use of duct tape occlusion in the treatment of recurrent molluscum contagiosum. Pediatr Dermatol. 2004;21:609.
- Silverberg N. Pediatric molluscum contagiosum: optimal treatment strategies. Paediatr Drugs. 2003;5:505-512.
Molluscum contagiosum virus (MCV) infection causes the cutaneous lesions we call molluscum. Molluscum has become common in the last 30 years. Deciding the best course of therapy requires some fundamental understanding about how MCV relates to the following factors: epidemiology, childhood immunity and vaccination, clinical features, comorbidities, and quality of life. Treatment depends on many factors, including presence or absence of atopic dermatitis (AD) and/or pruritus, other symptoms, cosmetic location, and the child’s concern about the lesions. Therapeutics include destructive and immunologic therapies, the latter geared toward increasing immune response.
Epidemiology
Molluscum contagiosum virus is the solo member of the Molluscipoxvirus genus. Infection with MCV causes benign growth or tumors in the skin (ie, molluscum). The infection is slow to clear because the virus reduces the host’s immunity.1,2 Molluscum contagiosum virus is a double-stranded DNA virus that affects keratinocytes and genetically carries the tools for its own replication (ie, DNA-dependent RNA polymerase). The virus has a few subtypes—I/Ia, II, III, and IV—with MCV-I predominating in children and healthy humans and MCV-II in patients with human immunodeficiency virus.1,2 Typing is experimental and is not standardly performed in clinical practice. Molluscum contagiosum virus produces a variety of factors that block the host’s immune response, prolonging infection and preventing erythema and inflammatory response.3
Molluscum contagiosum virus is transmitted through skin-to-skin contact and fomites, including shared towels, bathtubs, spas, bath sponges, and pool equipment.2,4,5 Transmission from household contact and bathing together has been noted in pediatric patients with MCV. Based on the data it can be posited that the lesions are softer when wet and more readily release viral particles or fomites, and fomites may be left on surfaces, especially when a child is wet.6,7 Propensity for infection occurs in patients with AD and in immunosuppressed hosts, including children with human immunodeficiency virus and iatrogenic immunosuppression caused by chemotherapy.1,2,8 Contact sports can increase the risk of transmission, and outbreaks have occurred in pools,5,9 day-care facilities,10 and sports settings.11 Cases of congenital and vertically transmitted molluscum have been documented.12,13 Sexual transmission of MCV may be seen in adolescents who are sexually active. Although child-to-child transmission can occur in the groin area from shared equipment, transmission via sexual abuse also is possible.14 Bargman15 has mentioned the isolated genital location and lack of contact with other infected children as concerning features. Latency of new lesion appearance is anywhere from 1 to 50 days from the date of inoculation; therefore, new lesions are possible and expected even after therapy has been effective in eradicating visible lesions.10 Although clearance has been reported in 6 to 12 months, one pediatric study demonstrated 70% clearance by 1.5 years, suggesting the disease often is more prolonged.16 One-third of children will experience signs of inflammation, such as pruritus and/or erythema. Rare side effects include bacterial superinfection and hypersensitivity.2
One Dutch study from 1994, the largest database survey of children to date, cited a 17% cumulative incidence of molluscum in children by reviewing the data from 103 general practices.17 In a survey and review of molluscum by Braue et al,18 annual rates in populations vary but seem to maximize at approximately 6% to 7%. Sturt et al19 reviewed the prevalence in the indigenous West Sepik section of New Guinea and noted annual incidence rates of 6% in children younger than 10 years (range, 1.8%–10.9%). Epidemics occur and can produce large numbers of cases in a short time period.18 The cumulative prevalence in early childhood may be as high as 22%, as Sturt et al19 observed in children younger than 10 years.
Rising incidence and therefore rising lifetime prevalence appear to have been an issue in the last few decades. Data from the Indian Health Service have demonstrated increases in MCV in Native American children between 2001 and 2005.20 In adults, the data support a steady increase of molluscum from 1988-2007, with a 3-fold increase from 1988-1997 to 1998-2007 in a Spanish study.21 Better population-based data are needed.
Childhood Immunity and Vaccination
Sequence homology between MC133L, a protein of MCV, with vaccinia virus suggests overlapping genes.22 Therefore, it is conceptually possible that the rise in incidence of MCV since the 1980s relates to the loss of herd immunity to variola due to lack of vaccination for smallpox, which has not been offered in the United States since 1972.23 Childhood immunity to MCV varies among studies, but it appears that children do develop antibodies to molluscum in the setting of forming an immune response. Because the rise in molluscum incidence began after the smallpox vaccine was discontinued, the factors appear related; however, the scientific data do not support the theory of a relationship. Mitchell24 has shown that a patient can develop antibodies in response to ground molluscum bodies inoculated into the skin; however, vaccination against molluscum and natural infection do not appear to produce antibodies that would cross-react and protect against other poxviruses, including vaccinia or fowl pox infections.25 Cell-mediated immunity also is required to clear MCV and may account for the inflammatory appearance of lesions as they resolve.26
Demonstrated factors that account for the rise in MCV incidence, aside from alterations in vaccination practices, include spread through sports,9 swimming,11 and AD,7 which have become more commonplace in the United States in the last few decades, supporting the theory that they may be the cause of the increase in childhood MCV infections. Another cause may be the ability of MCV to create factors that stem host immune response.1
Clinical Features
Molluscum lesions have a typical appearance of pearly papules with a central dell. These lesions are lighter to flesh colored and measure 1 to 3 mm.2,4,5 The lesions cluster in the axillae and extremities and average from 10 to 20 per child.6 Lesions clear spontaneously, but new ones will continue to form until immunity is developed. Specific clinical appearances of lesions that are not pearly papules are not infrequent. Table 1 contains a short list of the manifold clinical appearances of molluscum lesions in children.1,2,7,27-35 In particular, certain clinical appearances should be considered. In small children, head and neck lesions resembling milia are not uncommon. Giant or wartlike lesions can appear on the head, neck, or gluteal region in children and are clinical mimics of condyloma or other warts (Figure 1). Giant lesions also can grow in the subcutaneous space and mimic a cyst or abscess.27 Erosive lesions mimicking eczema vaccinatum can be seen (Figure 2), but dermoscopy may demonstrate central dells in some lesions. Other viral processes mimicked include Gianotti Crosti–like lesions (Figure 3) that appear when a papular id reaction forms over the extremities or a localized version in the axilla, mimicking unilateral laterothoracic exanthema.2,36,37 Hypersensitivity reactions are commonly noted with clearance and can be papular or demonstrate swelling and erythema, termed the beginning-of-the-end sign.38
Pruritus, erythema, and swelling can occur with clearance but do not appear in all patients. Addressing pruritus is important to prevent disease spread, as patients are likely to inoculate other areas of the skin with virus when they scratch, and lesion number is reduced with dermatitis interventions.36
Comorbidities
Molluscum lesions can occur in any child; however, the impaired immunologic status and skin barrier in patients with AD is ripe for the extensive spread of lesions that is associated with higher lesion count.36 Children with molluscum infection can experience new-onset dermatitis or triggering of AD flares, especially on the extremities, such as the antecubital and popliteal regions.7 A study of children with MCV infection demonstrated that treatment of active dermatitis reduced spread. The authors mentioned autoinoculation as the mechanism; however, these data also suggest supporting barrier state as a factor in disease spread.36 Superinfection can occur prior to6 or after therapy for lesions,37 but it is unclear if this relates to the underlying atopic diathesis. Children with molluscum have been described to have warts, psoriasis, family history of atopy, diabetes mellitus, and pityriasis alba,7 while immunosuppression of any kind is associated with molluscum and high lesion count or prolonged disease in childhood.1,2
Quality of Life
Children with molluscum who have higher lesion counts appear to be at risk for severe effects on their quality of life. Approximately 10% of children with MCV infection have been documented to have severe impairments on quality of life.39 In my practice, quality of life in children with MCV appears to be affected by many factors (Table 2).7,18,39
Treatments
Proper Skin Care and Treatment of AD
Therapy for AD and/or pruritus appears to limit lesion number in children with MCV and rashes or itch.7,36 I recommend barrier repair agents, including emollients and syndet bar cleansers, to prevent small breaks in the skin that occur with xerosis and AD and that increase itch and risk of spread. Therapy for AD and molluscum dermatitis is similar and overlapping. There is always a concern about the spread of MCV when using topical calcineurin inhibitors. I, therefore, focus the dermatitis therapeutics on topical corticosteroid–based care.6,40
Prevention of Spread
Prevention of spread begins with hygiene interventions. Cobathing is common in children with MCV and should be held off when possible. It is important for the child with MCV to avoid sharing bath towels and equipment23 and having bare skin come in contact with mats in sports. I request that children with MCV wear bathing suits that cover the areas affected.
Reassurance
The most important therapy is reassurance.41 Many parents/guardians are truly unaware that the MCV infection can last for more than a year and therefore worry over normal disease course. When counseled as to the benign course of illness and given instructions on proper skin care, the parent/guardian of a child with MCV will often opt against therapy of uncomplicated cases. On the other hand, there are medical reasons for treatment, and they support the need for intervention (Table 3). Seventy percent of lesions resolve in 1.5 years; however, of the residual infections, some may last as long as 4 years.16 It is not recommended to stop children from attending school because of MCV.
Interventional Therapy
Therapeutics of MCV include destructive therapies in office (ie, cantharidin, cryotherapy, curettage, trichloroacetic acid, and glycolic acid) and at-home therapies (ie, topical retinoids, nitric oxide releasers)(eTable).2,5,6,42-58 When there are many lesions or spread is noted, immunotherapies can be used, including topical imiquimod, oral cimetidine, and intralesional Candida antigen.2,4,7 Pulsed dye laser cuts off the lesion vascular supply, while cidofovir is directly antiviral both topically and systemically, the latter reserved for severe cases in immunosuppressed adults.59 Head-to-head studies of cantharidin, curettage, topical peeling agents, and imiquimod demonstrated better satisfaction and fewer office visits with topical anesthetic and curettage on the first visit. Side effects were greatest for salicylic acid and glycolic acid; therefore, these agents are less desirable.42
Conclusion
Molluscum is a cutaneous viral infection that is common in children and has associated morbidities, including AD, pruritus, poor quality of life in some cases, and risk of contagion. Addressing the disease includes understanding its natural history and explaining it to parents/guardians. Therapeutics can be offered in cases where need is demonstrated, such as with lesions that spread and cause discomfort. Choice of therapeutics depends on the practitioner’s experience, the child’s clinical appearance, availability of therapy, and review of options with the parents/guardians. When avoidance of intervention is desired, barrier enhancement and treatment of symptomatic dermatitis are still beneficial, as are household (eg, not sharing towels) and activity (eg, adhesive bandages over active lesions) interventions to reduce transmission.
Molluscum contagiosum virus (MCV) infection causes the cutaneous lesions we call molluscum. Molluscum has become common in the last 30 years. Deciding the best course of therapy requires some fundamental understanding about how MCV relates to the following factors: epidemiology, childhood immunity and vaccination, clinical features, comorbidities, and quality of life. Treatment depends on many factors, including presence or absence of atopic dermatitis (AD) and/or pruritus, other symptoms, cosmetic location, and the child’s concern about the lesions. Therapeutics include destructive and immunologic therapies, the latter geared toward increasing immune response.
Epidemiology
Molluscum contagiosum virus is the solo member of the Molluscipoxvirus genus. Infection with MCV causes benign growth or tumors in the skin (ie, molluscum). The infection is slow to clear because the virus reduces the host’s immunity.1,2 Molluscum contagiosum virus is a double-stranded DNA virus that affects keratinocytes and genetically carries the tools for its own replication (ie, DNA-dependent RNA polymerase). The virus has a few subtypes—I/Ia, II, III, and IV—with MCV-I predominating in children and healthy humans and MCV-II in patients with human immunodeficiency virus.1,2 Typing is experimental and is not standardly performed in clinical practice. Molluscum contagiosum virus produces a variety of factors that block the host’s immune response, prolonging infection and preventing erythema and inflammatory response.3
Molluscum contagiosum virus is transmitted through skin-to-skin contact and fomites, including shared towels, bathtubs, spas, bath sponges, and pool equipment.2,4,5 Transmission from household contact and bathing together has been noted in pediatric patients with MCV. Based on the data it can be posited that the lesions are softer when wet and more readily release viral particles or fomites, and fomites may be left on surfaces, especially when a child is wet.6,7 Propensity for infection occurs in patients with AD and in immunosuppressed hosts, including children with human immunodeficiency virus and iatrogenic immunosuppression caused by chemotherapy.1,2,8 Contact sports can increase the risk of transmission, and outbreaks have occurred in pools,5,9 day-care facilities,10 and sports settings.11 Cases of congenital and vertically transmitted molluscum have been documented.12,13 Sexual transmission of MCV may be seen in adolescents who are sexually active. Although child-to-child transmission can occur in the groin area from shared equipment, transmission via sexual abuse also is possible.14 Bargman15 has mentioned the isolated genital location and lack of contact with other infected children as concerning features. Latency of new lesion appearance is anywhere from 1 to 50 days from the date of inoculation; therefore, new lesions are possible and expected even after therapy has been effective in eradicating visible lesions.10 Although clearance has been reported in 6 to 12 months, one pediatric study demonstrated 70% clearance by 1.5 years, suggesting the disease often is more prolonged.16 One-third of children will experience signs of inflammation, such as pruritus and/or erythema. Rare side effects include bacterial superinfection and hypersensitivity.2
One Dutch study from 1994, the largest database survey of children to date, cited a 17% cumulative incidence of molluscum in children by reviewing the data from 103 general practices.17 In a survey and review of molluscum by Braue et al,18 annual rates in populations vary but seem to maximize at approximately 6% to 7%. Sturt et al19 reviewed the prevalence in the indigenous West Sepik section of New Guinea and noted annual incidence rates of 6% in children younger than 10 years (range, 1.8%–10.9%). Epidemics occur and can produce large numbers of cases in a short time period.18 The cumulative prevalence in early childhood may be as high as 22%, as Sturt et al19 observed in children younger than 10 years.
Rising incidence and therefore rising lifetime prevalence appear to have been an issue in the last few decades. Data from the Indian Health Service have demonstrated increases in MCV in Native American children between 2001 and 2005.20 In adults, the data support a steady increase of molluscum from 1988-2007, with a 3-fold increase from 1988-1997 to 1998-2007 in a Spanish study.21 Better population-based data are needed.
Childhood Immunity and Vaccination
Sequence homology between MC133L, a protein of MCV, with vaccinia virus suggests overlapping genes.22 Therefore, it is conceptually possible that the rise in incidence of MCV since the 1980s relates to the loss of herd immunity to variola due to lack of vaccination for smallpox, which has not been offered in the United States since 1972.23 Childhood immunity to MCV varies among studies, but it appears that children do develop antibodies to molluscum in the setting of forming an immune response. Because the rise in molluscum incidence began after the smallpox vaccine was discontinued, the factors appear related; however, the scientific data do not support the theory of a relationship. Mitchell24 has shown that a patient can develop antibodies in response to ground molluscum bodies inoculated into the skin; however, vaccination against molluscum and natural infection do not appear to produce antibodies that would cross-react and protect against other poxviruses, including vaccinia or fowl pox infections.25 Cell-mediated immunity also is required to clear MCV and may account for the inflammatory appearance of lesions as they resolve.26
Demonstrated factors that account for the rise in MCV incidence, aside from alterations in vaccination practices, include spread through sports,9 swimming,11 and AD,7 which have become more commonplace in the United States in the last few decades, supporting the theory that they may be the cause of the increase in childhood MCV infections. Another cause may be the ability of MCV to create factors that stem host immune response.1
Clinical Features
Molluscum lesions have a typical appearance of pearly papules with a central dell. These lesions are lighter to flesh colored and measure 1 to 3 mm.2,4,5 The lesions cluster in the axillae and extremities and average from 10 to 20 per child.6 Lesions clear spontaneously, but new ones will continue to form until immunity is developed. Specific clinical appearances of lesions that are not pearly papules are not infrequent. Table 1 contains a short list of the manifold clinical appearances of molluscum lesions in children.1,2,7,27-35 In particular, certain clinical appearances should be considered. In small children, head and neck lesions resembling milia are not uncommon. Giant or wartlike lesions can appear on the head, neck, or gluteal region in children and are clinical mimics of condyloma or other warts (Figure 1). Giant lesions also can grow in the subcutaneous space and mimic a cyst or abscess.27 Erosive lesions mimicking eczema vaccinatum can be seen (Figure 2), but dermoscopy may demonstrate central dells in some lesions. Other viral processes mimicked include Gianotti Crosti–like lesions (Figure 3) that appear when a papular id reaction forms over the extremities or a localized version in the axilla, mimicking unilateral laterothoracic exanthema.2,36,37 Hypersensitivity reactions are commonly noted with clearance and can be papular or demonstrate swelling and erythema, termed the beginning-of-the-end sign.38
Pruritus, erythema, and swelling can occur with clearance but do not appear in all patients. Addressing pruritus is important to prevent disease spread, as patients are likely to inoculate other areas of the skin with virus when they scratch, and lesion number is reduced with dermatitis interventions.36
Comorbidities
Molluscum lesions can occur in any child; however, the impaired immunologic status and skin barrier in patients with AD is ripe for the extensive spread of lesions that is associated with higher lesion count.36 Children with molluscum infection can experience new-onset dermatitis or triggering of AD flares, especially on the extremities, such as the antecubital and popliteal regions.7 A study of children with MCV infection demonstrated that treatment of active dermatitis reduced spread. The authors mentioned autoinoculation as the mechanism; however, these data also suggest supporting barrier state as a factor in disease spread.36 Superinfection can occur prior to6 or after therapy for lesions,37 but it is unclear if this relates to the underlying atopic diathesis. Children with molluscum have been described to have warts, psoriasis, family history of atopy, diabetes mellitus, and pityriasis alba,7 while immunosuppression of any kind is associated with molluscum and high lesion count or prolonged disease in childhood.1,2
Quality of Life
Children with molluscum who have higher lesion counts appear to be at risk for severe effects on their quality of life. Approximately 10% of children with MCV infection have been documented to have severe impairments on quality of life.39 In my practice, quality of life in children with MCV appears to be affected by many factors (Table 2).7,18,39
Treatments
Proper Skin Care and Treatment of AD
Therapy for AD and/or pruritus appears to limit lesion number in children with MCV and rashes or itch.7,36 I recommend barrier repair agents, including emollients and syndet bar cleansers, to prevent small breaks in the skin that occur with xerosis and AD and that increase itch and risk of spread. Therapy for AD and molluscum dermatitis is similar and overlapping. There is always a concern about the spread of MCV when using topical calcineurin inhibitors. I, therefore, focus the dermatitis therapeutics on topical corticosteroid–based care.6,40
Prevention of Spread
Prevention of spread begins with hygiene interventions. Cobathing is common in children with MCV and should be held off when possible. It is important for the child with MCV to avoid sharing bath towels and equipment23 and having bare skin come in contact with mats in sports. I request that children with MCV wear bathing suits that cover the areas affected.
Reassurance
The most important therapy is reassurance.41 Many parents/guardians are truly unaware that the MCV infection can last for more than a year and therefore worry over normal disease course. When counseled as to the benign course of illness and given instructions on proper skin care, the parent/guardian of a child with MCV will often opt against therapy of uncomplicated cases. On the other hand, there are medical reasons for treatment, and they support the need for intervention (Table 3). Seventy percent of lesions resolve in 1.5 years; however, of the residual infections, some may last as long as 4 years.16 It is not recommended to stop children from attending school because of MCV.
Interventional Therapy
Therapeutics of MCV include destructive therapies in office (ie, cantharidin, cryotherapy, curettage, trichloroacetic acid, and glycolic acid) and at-home therapies (ie, topical retinoids, nitric oxide releasers)(eTable).2,5,6,42-58 When there are many lesions or spread is noted, immunotherapies can be used, including topical imiquimod, oral cimetidine, and intralesional Candida antigen.2,4,7 Pulsed dye laser cuts off the lesion vascular supply, while cidofovir is directly antiviral both topically and systemically, the latter reserved for severe cases in immunosuppressed adults.59 Head-to-head studies of cantharidin, curettage, topical peeling agents, and imiquimod demonstrated better satisfaction and fewer office visits with topical anesthetic and curettage on the first visit. Side effects were greatest for salicylic acid and glycolic acid; therefore, these agents are less desirable.42
Conclusion
Molluscum is a cutaneous viral infection that is common in children and has associated morbidities, including AD, pruritus, poor quality of life in some cases, and risk of contagion. Addressing the disease includes understanding its natural history and explaining it to parents/guardians. Therapeutics can be offered in cases where need is demonstrated, such as with lesions that spread and cause discomfort. Choice of therapeutics depends on the practitioner’s experience, the child’s clinical appearance, availability of therapy, and review of options with the parents/guardians. When avoidance of intervention is desired, barrier enhancement and treatment of symptomatic dermatitis are still beneficial, as are household (eg, not sharing towels) and activity (eg, adhesive bandages over active lesions) interventions to reduce transmission.
- Shisler JL. Immune evasion strategies of molluscum contagiosum virus. Adv Virus Res. 2015;92:201-252.
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99.
- Moss B, Shisler JL, Xiang Y, et al. Immune-defense molecules of molluscum contagiosum virus, a human poxvirus. Trends Microbiol. 2000;8:473-477.
- Silverberg NB. Warts and molluscum in children. Adv Dermatol. 2004;20:23-73.
- Choong KY, Roberts LJ. Molluscum contagiosum, swimming and bathing: a clinical analysis. Australas J Dermatol. 1999;40:89-92.
- Silverberg NB, Sidbury R, Mancini AJ. Childhood molluscum contagiosum: experience with cantharidin therapy in 300 patients. J Am Acad Dermatol. 2000;43:503-507.
- Silverberg NB. Molluscum contagiosum virus infection can trigger atopic dermatitis disease onset or flare. Cutis. 2018;102:191-194.
- Ajithkumar VT, Sasidharanpillai S, Muhammed K, et al. Disseminated molluscum contagiosum following chemotherapy: a therapeutic challenge. Indian J Dermatol Venereol Leprol. 2017;83:516.
- Oren B, Wende SO. An outbreak of molluscum contagiosum in a kibbutz. Infection. 1991;19:159-161.
- Molluscum contagiosum. Healthy Children website. https://www.healthychildren.org/English/health-issues/conditions/skin/Pages/Molluscum-Contagiosum.aspx. Updated November 21, 2015. Accessed October 16, 2019.
- Peterson AR, Nash E, Anderson BJ. Infectious disease in contact sports. Sports Health. 2019;11:47-58.
- Connell CO, Oranje A, Van Gysel D, et al. Congenital molluscum contagiosum: report of four cases and review of the literature. Pediatr Dermatol. 2008;25:553-556.
- Luke JD, Silverberg NB. Vertically transmitted molluscum contagiosum infection. Pediatrics. 2010;125:E423-E425.
- Mendiratta V, Agarwal S, Chander R. Reappraisal of sexually transmitted infections in children: a hospital-based study from an urban area. Indian J Sex Transm Dis AIDS. 2014;35:25-28.
- Bargman H. Genital molluscum contagiosum in children: evidence of sexual abuse? CMAJ. 1986;135:432-433.
- Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32:353-357.
- Koning S, Bruijnzeels MA, van Suijlekom-Smit LW, et al. Molluscum contagiosum in Dutch general practice. Br J Gen Pract. 1994;44:417-419.
- Braue A, Ross G, Varigos G, et al. Epidemiology and impact of childhood molluscum contagiosum: a case series and critical review of the literature. Pediatr Dermatol. 2005;22:287-294.
- Sturt RJ, Muller HK, Francis GD. Molluscum contagiosum in villages of the West Sepik District of New Guinea. Med J Aust. 1971;2:751-754.
- Reynolds MG, Homan RC, Yorita Christensen KL, et al. The incidence of molluscum contagiosum among American Indians and Alaska Natives. PLoS One. 2009;4:e5255.
- Villa L, Varela JA, Otero L, et al. Molluscum contagiosum: a 20-year study in a sexually transmitted infections unit. Sex Transm Dis. 2010;37:423-424.
- Watanabe T, Morikawa S, Suzuki K, et al. Two major antigenic polypeptides of molluscum contagiosum virus. J Infect Dis. 1998;177:284-292.
- Vaccine basics. Centers for Disease Control and Prevention website. https://www.cdc.gov/smallpox/vaccine-basics/index.html. Updated July 12, 2017. Accessed October 16, 2019.
- Mitchell JC. Observations on the virus of molluscum contagiosum. Br J Exp Pathol. 1953;34:44-49.
- Konya J, Thompson CH. Molluscum contagiosum virus: antibody responses in patients with clinical lesions and its sero-epidemiology in a representative Australian population. J Infect Dis. 1999;179:701-704.
- Steffen C, Markman JA. Spontaneous disappearance of molluscum contagiosum. Arch Dermatol. 1980;116:923-924.
- Uzuncakmak TK, Kuru BC, Zemheri EI, et al. Isolated giant molluscum contagiosum mimicking epidermoid cyst. Dermatol Pract Concept. 2016;6:71-73.
- Persechino S, Abruzzese C, Caperchi C, et al. Condyloma acuminata and mollusca contagiosa: a giant manifestation in a patient with lupus. Skinmed. 2014;12:310-311.
- Kim SK, Do JE, Kang HY, et al. Giant molluscum contagiosum of immunocompetent children occurring on the anogenital area. Eur J Dermatol. 2007;17:537-538.
- Alam MS, Shrirao N. Giant molluscum contagiosum presenting as lid neoplasm in an immunocompetent child. Dermatol Online J. 2016;22. pii:13030/qt56v567gn.
- Krishnamurthy J, Nagappa DK. The cytology of molluscum contagiosum mimicking skin adnexal tumor. J Cytol. 2010;27:74-75.
- Baek YS, Oh CH, Song HJ, et al. Asymmetrical periflexural exanthem of childhood with concurrence of molluscum contagiosum infection. Clin Exp Dermatol. 2011;36:676-677.
- Lee HJ, Kwon JA, Kim JW. Erythema multiforme-like molluscum dermatitis. Acta Derm Venereol. 2002;82:217-218.
- Lee YB, Choi HJ, Park HJ, et al. Two cases of erythema multiforme associated with molluscum contagiosum. Int J Dermatol. 2009;48:659-660.
- Vasily DB, Bhatia SG. Erythema annulare centrifugum and molluscum contagiosum.
- Berger EM, Orlow SJ, Patel RR, et al. Experience with molluscum contagiosum and associated inflammatory reactions in a pediatric dermatology practice: the bump that rashes. Arch Dermatol. 2012;148:1257-1264.
- Groner A, Laing-Grayman D, Silverberg NB. Outpatient pediatric community-acquired methicillin-resistant Staphylococcus aureus: a polymorphous clinical disease. Cutis. 2008;81:115-122.
- Butala N, Siegfried E, Weissler A. Molluscum BOTE sign: a predictor of imminent resolution. Pediatrics. 2013;131:E1650-E1653.
- Olsen JR, Gallagher J, Finlay AY, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190-195.
- Goksugur N, Ozbostanci B, Goksugur SB. Molluscum contagiosum infection associated with pimecrolimus use in pityriasis alba. Pediatr Dermatol. 2007;24:E63-E65.
- Lee R, Schwartz RA. Pediatric molluscum contagiosum: reflections on the last challenging poxvirus infection, part 1. Cutis. 2010;86:230-236.
- Hanna D, Hatami A, Powell J, et al. A prospective randomized trial comparing the efficacy and adverse effects of four recognized treatments of molluscum contagiosum in children. Pediatr Dermatol. 2006;23:574-579.
- Coloe Dosal J, Stewart PW, Lin JA, et al. Cantharidin for the treatment of molluscum contagiosum: a prospective, double-blinded, placebo-controlled trial. Pediatr Dermatol. 2014;31:440-449.
- Vakharia PP, Chopra R, Silverberg NB, et al. Efficacy and safety of topical cantharidin treatment for molluscum contagiosum and warts: a systematic review. Am J Clin Dermatol. 2018;19:791-803.
- Handjani F, Behazin E, Sadati MS. Comparison of 10% potassium hydroxide solution versus cryotherapy in the treatment of molluscum contagiosum: an open randomized clinical trial. J Dermatolog Treat. 2014;25:249-250.
- Simonart T, De Maertelaer V. Curettage treatment for molluscum contagiosum: a follow-up survey study. Br J Dermatol. 2008;159:1144-1147.
- Cho YS, Chung BY, Park CW, et al. Seizures and methemoglobinemia after topical application of eutectic mixture of lidocaine and prilocaine on a 3.5-year-old child with molluscum contagiosum and atopic dermatitis. Pediatr Dermatol. 2016;33:E284-E285.
- Bard S, Shiman MI, Bellman B, et al. Treatment of facial molluscum contagiosum with trichloroacetic acid. Pediatr Dermatol. 2009;26:425-426.
- Griffith RD, Yazdani Abyaneh MA, Falto-Aizpurua L, et al. Pulsed dye laser therapy for molluscum contagiosum: a systematic review. J Drugs Dermatol. 2014;13:1349-1352.
- Theos AU, Cummins R, Silverberg NB, et al. Effectiveness of imiquimod cream 5% for treating childhood molluscum contagiosum in a double-blind, randomized pilot trial. Cutis. 2004;74:134-138, 141-142.
- van der Wouden JC, Menke J, Gajadin S, et al. Interventions for cutaneous molluscum contagiosum. Cochrane Database Syst Rev. 2006:CD004767.
- Cunningham BB, Paller AS, Garzon M. Inefficacy of oral cimetidine for nonatopic children with molluscum contagiosum. Pediatr Dermatol. 1998;15:71-72.
- Enns LL, Evans MS. Intralesional immunotherapy with Candida antigen for the treatment of molluscum contagiosum in children. Pediatr Dermatol. 2011;28:254-258.
- Rajouria EA, Amatya A, Karn D. Comparative study of 5% potassium hydroxide solution versus 0.05% tretinoin cream for molluscum contagiosum in children. Kathmandu Univ Med J (KUMJ). 2011;9:291-294.
- Briand S, Milpied B, Navas D, et al. 1% topical cidofovir used as last alternative to treat viral infections. J Eur Acad Dermatol Venereol. 2008;22:249-250.
- Zabawski EJ Jr, Cockerell CJ. Topical cidofovir for molluscum contagiosum in children. Pediatr Dermatol. 1999;16:414-415.
- Watanabe T. Cidofovir diphosphate inhibits molluscum contagiosum virus DNA polymerase activity. J Invest Dermatol. 2008;128:1327-1329.
- Lindau MS, Munar MY. Use of duct tape occlusion in the treatment of recurrent molluscum contagiosum. Pediatr Dermatol. 2004;21:609.
- Silverberg N. Pediatric molluscum contagiosum: optimal treatment strategies. Paediatr Drugs. 2003;5:505-512.
- Shisler JL. Immune evasion strategies of molluscum contagiosum virus. Adv Virus Res. 2015;92:201-252.
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99.
- Moss B, Shisler JL, Xiang Y, et al. Immune-defense molecules of molluscum contagiosum virus, a human poxvirus. Trends Microbiol. 2000;8:473-477.
- Silverberg NB. Warts and molluscum in children. Adv Dermatol. 2004;20:23-73.
- Choong KY, Roberts LJ. Molluscum contagiosum, swimming and bathing: a clinical analysis. Australas J Dermatol. 1999;40:89-92.
- Silverberg NB, Sidbury R, Mancini AJ. Childhood molluscum contagiosum: experience with cantharidin therapy in 300 patients. J Am Acad Dermatol. 2000;43:503-507.
- Silverberg NB. Molluscum contagiosum virus infection can trigger atopic dermatitis disease onset or flare. Cutis. 2018;102:191-194.
- Ajithkumar VT, Sasidharanpillai S, Muhammed K, et al. Disseminated molluscum contagiosum following chemotherapy: a therapeutic challenge. Indian J Dermatol Venereol Leprol. 2017;83:516.
- Oren B, Wende SO. An outbreak of molluscum contagiosum in a kibbutz. Infection. 1991;19:159-161.
- Molluscum contagiosum. Healthy Children website. https://www.healthychildren.org/English/health-issues/conditions/skin/Pages/Molluscum-Contagiosum.aspx. Updated November 21, 2015. Accessed October 16, 2019.
- Peterson AR, Nash E, Anderson BJ. Infectious disease in contact sports. Sports Health. 2019;11:47-58.
- Connell CO, Oranje A, Van Gysel D, et al. Congenital molluscum contagiosum: report of four cases and review of the literature. Pediatr Dermatol. 2008;25:553-556.
- Luke JD, Silverberg NB. Vertically transmitted molluscum contagiosum infection. Pediatrics. 2010;125:E423-E425.
- Mendiratta V, Agarwal S, Chander R. Reappraisal of sexually transmitted infections in children: a hospital-based study from an urban area. Indian J Sex Transm Dis AIDS. 2014;35:25-28.
- Bargman H. Genital molluscum contagiosum in children: evidence of sexual abuse? CMAJ. 1986;135:432-433.
- Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32:353-357.
- Koning S, Bruijnzeels MA, van Suijlekom-Smit LW, et al. Molluscum contagiosum in Dutch general practice. Br J Gen Pract. 1994;44:417-419.
- Braue A, Ross G, Varigos G, et al. Epidemiology and impact of childhood molluscum contagiosum: a case series and critical review of the literature. Pediatr Dermatol. 2005;22:287-294.
- Sturt RJ, Muller HK, Francis GD. Molluscum contagiosum in villages of the West Sepik District of New Guinea. Med J Aust. 1971;2:751-754.
- Reynolds MG, Homan RC, Yorita Christensen KL, et al. The incidence of molluscum contagiosum among American Indians and Alaska Natives. PLoS One. 2009;4:e5255.
- Villa L, Varela JA, Otero L, et al. Molluscum contagiosum: a 20-year study in a sexually transmitted infections unit. Sex Transm Dis. 2010;37:423-424.
- Watanabe T, Morikawa S, Suzuki K, et al. Two major antigenic polypeptides of molluscum contagiosum virus. J Infect Dis. 1998;177:284-292.
- Vaccine basics. Centers for Disease Control and Prevention website. https://www.cdc.gov/smallpox/vaccine-basics/index.html. Updated July 12, 2017. Accessed October 16, 2019.
- Mitchell JC. Observations on the virus of molluscum contagiosum. Br J Exp Pathol. 1953;34:44-49.
- Konya J, Thompson CH. Molluscum contagiosum virus: antibody responses in patients with clinical lesions and its sero-epidemiology in a representative Australian population. J Infect Dis. 1999;179:701-704.
- Steffen C, Markman JA. Spontaneous disappearance of molluscum contagiosum. Arch Dermatol. 1980;116:923-924.
- Uzuncakmak TK, Kuru BC, Zemheri EI, et al. Isolated giant molluscum contagiosum mimicking epidermoid cyst. Dermatol Pract Concept. 2016;6:71-73.
- Persechino S, Abruzzese C, Caperchi C, et al. Condyloma acuminata and mollusca contagiosa: a giant manifestation in a patient with lupus. Skinmed. 2014;12:310-311.
- Kim SK, Do JE, Kang HY, et al. Giant molluscum contagiosum of immunocompetent children occurring on the anogenital area. Eur J Dermatol. 2007;17:537-538.
- Alam MS, Shrirao N. Giant molluscum contagiosum presenting as lid neoplasm in an immunocompetent child. Dermatol Online J. 2016;22. pii:13030/qt56v567gn.
- Krishnamurthy J, Nagappa DK. The cytology of molluscum contagiosum mimicking skin adnexal tumor. J Cytol. 2010;27:74-75.
- Baek YS, Oh CH, Song HJ, et al. Asymmetrical periflexural exanthem of childhood with concurrence of molluscum contagiosum infection. Clin Exp Dermatol. 2011;36:676-677.
- Lee HJ, Kwon JA, Kim JW. Erythema multiforme-like molluscum dermatitis. Acta Derm Venereol. 2002;82:217-218.
- Lee YB, Choi HJ, Park HJ, et al. Two cases of erythema multiforme associated with molluscum contagiosum. Int J Dermatol. 2009;48:659-660.
- Vasily DB, Bhatia SG. Erythema annulare centrifugum and molluscum contagiosum.
- Berger EM, Orlow SJ, Patel RR, et al. Experience with molluscum contagiosum and associated inflammatory reactions in a pediatric dermatology practice: the bump that rashes. Arch Dermatol. 2012;148:1257-1264.
- Groner A, Laing-Grayman D, Silverberg NB. Outpatient pediatric community-acquired methicillin-resistant Staphylococcus aureus: a polymorphous clinical disease. Cutis. 2008;81:115-122.
- Butala N, Siegfried E, Weissler A. Molluscum BOTE sign: a predictor of imminent resolution. Pediatrics. 2013;131:E1650-E1653.
- Olsen JR, Gallagher J, Finlay AY, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190-195.
- Goksugur N, Ozbostanci B, Goksugur SB. Molluscum contagiosum infection associated with pimecrolimus use in pityriasis alba. Pediatr Dermatol. 2007;24:E63-E65.
- Lee R, Schwartz RA. Pediatric molluscum contagiosum: reflections on the last challenging poxvirus infection, part 1. Cutis. 2010;86:230-236.
- Hanna D, Hatami A, Powell J, et al. A prospective randomized trial comparing the efficacy and adverse effects of four recognized treatments of molluscum contagiosum in children. Pediatr Dermatol. 2006;23:574-579.
- Coloe Dosal J, Stewart PW, Lin JA, et al. Cantharidin for the treatment of molluscum contagiosum: a prospective, double-blinded, placebo-controlled trial. Pediatr Dermatol. 2014;31:440-449.
- Vakharia PP, Chopra R, Silverberg NB, et al. Efficacy and safety of topical cantharidin treatment for molluscum contagiosum and warts: a systematic review. Am J Clin Dermatol. 2018;19:791-803.
- Handjani F, Behazin E, Sadati MS. Comparison of 10% potassium hydroxide solution versus cryotherapy in the treatment of molluscum contagiosum: an open randomized clinical trial. J Dermatolog Treat. 2014;25:249-250.
- Simonart T, De Maertelaer V. Curettage treatment for molluscum contagiosum: a follow-up survey study. Br J Dermatol. 2008;159:1144-1147.
- Cho YS, Chung BY, Park CW, et al. Seizures and methemoglobinemia after topical application of eutectic mixture of lidocaine and prilocaine on a 3.5-year-old child with molluscum contagiosum and atopic dermatitis. Pediatr Dermatol. 2016;33:E284-E285.
- Bard S, Shiman MI, Bellman B, et al. Treatment of facial molluscum contagiosum with trichloroacetic acid. Pediatr Dermatol. 2009;26:425-426.
- Griffith RD, Yazdani Abyaneh MA, Falto-Aizpurua L, et al. Pulsed dye laser therapy for molluscum contagiosum: a systematic review. J Drugs Dermatol. 2014;13:1349-1352.
- Theos AU, Cummins R, Silverberg NB, et al. Effectiveness of imiquimod cream 5% for treating childhood molluscum contagiosum in a double-blind, randomized pilot trial. Cutis. 2004;74:134-138, 141-142.
- van der Wouden JC, Menke J, Gajadin S, et al. Interventions for cutaneous molluscum contagiosum. Cochrane Database Syst Rev. 2006:CD004767.
- Cunningham BB, Paller AS, Garzon M. Inefficacy of oral cimetidine for nonatopic children with molluscum contagiosum. Pediatr Dermatol. 1998;15:71-72.
- Enns LL, Evans MS. Intralesional immunotherapy with Candida antigen for the treatment of molluscum contagiosum in children. Pediatr Dermatol. 2011;28:254-258.
- Rajouria EA, Amatya A, Karn D. Comparative study of 5% potassium hydroxide solution versus 0.05% tretinoin cream for molluscum contagiosum in children. Kathmandu Univ Med J (KUMJ). 2011;9:291-294.
- Briand S, Milpied B, Navas D, et al. 1% topical cidofovir used as last alternative to treat viral infections. J Eur Acad Dermatol Venereol. 2008;22:249-250.
- Zabawski EJ Jr, Cockerell CJ. Topical cidofovir for molluscum contagiosum in children. Pediatr Dermatol. 1999;16:414-415.
- Watanabe T. Cidofovir diphosphate inhibits molluscum contagiosum virus DNA polymerase activity. J Invest Dermatol. 2008;128:1327-1329.
- Lindau MS, Munar MY. Use of duct tape occlusion in the treatment of recurrent molluscum contagiosum. Pediatr Dermatol. 2004;21:609.
- Silverberg N. Pediatric molluscum contagiosum: optimal treatment strategies. Paediatr Drugs. 2003;5:505-512.
Practice Points
- Molluscum appears as pearly papules with a central dell (ie, umbilicated).
- Caused by a poxvirus, the disease is very contagious and transferred via skin-to-skin contact or fomites.
- One-third of children with molluscum will develop symptoms of local erythema, swelling, or pruritus.
- Diagnosis usually is clinical.
- Children are primarily managed through observation; however, cantharidin, cryotherapy, or curettage can be used for symptomatic or cosmetically concerning lesions.