User login
Debra P is a 62-year-old African American woman who calls your office to report that she has an upcoming routine colonoscopy planned in 2 weeks. She has been taking warfarin for the past 2 years for ischemic stroke prevention secondary to atrial fibrillation (AF), and her gastroenterologist recommended that she contact her family physician (FP) to discuss periprocedural anticoagulation plans. Ms. P is currently taking warfarin 5 mg on Mondays, Wednesdays, and Fridays, and 2.5 mg all other days of the week. Her international normalized ratio (INR) was 2.3 when it was last checked 2 weeks ago, and it has been stable and within goal range for the past 6 months. Her medical history includes AF, well-controlled hypertension, and type 2 diabetes mellitus, as well as gout and stage 3 chronic kidney disease. Ms. P denies any history of stroke or transient ischemic attack (TIA). She is requesting instructions on how to manage her warfarin before and after her upcoming colonoscopy.
Jerry Q is a 68-year-old Caucasian man with longstanding osteoarthritis who is scheduled to undergo a total left knee arthroplasty in one week. His orthopedic surgeon recommended that he contact his FP for instructions regarding managing apixaban perioperatively. Jerry has been taking apixaban 5 mg bid for the past 9 months due to a history of recurrent deep vein thrombosis (DVT) and pulmonary embolism (PE) (both unprovoked). Mr. Q had been taking warfarin following his first DVT 4 years ago, but, after reporting that INR monitoring was a burden, he was started on apixaban. The patient has normal renal function and is relatively healthy otherwise. How should apixaban be managed before and after his upcoming surgery?
Each year, approximately 15% to 20% of patients taking an oral anticoagulant undergo a procedure that carries a heightened risk for bleeding.1,2 Stopping oral anticoagulation is often necessary before—and sometimes briefly after—many of these procedures in order to minimize the risk of bleeding.3 This means that countless decisions must be made by health care providers each year regarding if, when, and how to pause and resume oral anticoagulation. These decisions are not always straightforward, especially when you consider the risks for thrombosis and bleeding that are unique to the procedure and to the individual patient.
With these variables in mind, the health care provider must make decisions regarding anticoagulation during the periprocedural period based on the following 5 questions:
- Will this patient need to have his/her oral anticoagulant stopped prior to the procedure?
- If the patient’s oral anticoagulation needs to be held, when should it be stopped and for how long?
- Will periprocedural bridging with a parenteral anticoagulant be necessary prior to the procedure?
- When should the patient resume his or her oral anticoagulant after the procedure, and at what dosage?
- Will bridging with a parenteral anticoagulant be necessary after the procedure?
Before addressing these 5 questions, though, physicians must assess patients’ thrombotic and bleeding risks.4-6
Anticoagulant regimens and the risks of discontinuing them
The 2 most common indications for long-term oral anticoagulation are venous thromboembolism (VTE), which occurs in approximately one million Americans every year,7,8 and stroke prevention in the setting of AF (AF occurs in 3-6 million US adults per year).6
Warfarin is also often used in patients with mechanical heart valves for long-term stroke prevention; however, direct oral anticoagulants (DOACs) are not recommended for patients with mechanical heart valves because trials have not yet demonstrated their safety or efficacy in this population.4,5,9
Who’s at highest risk for an acute thromboembolic event?
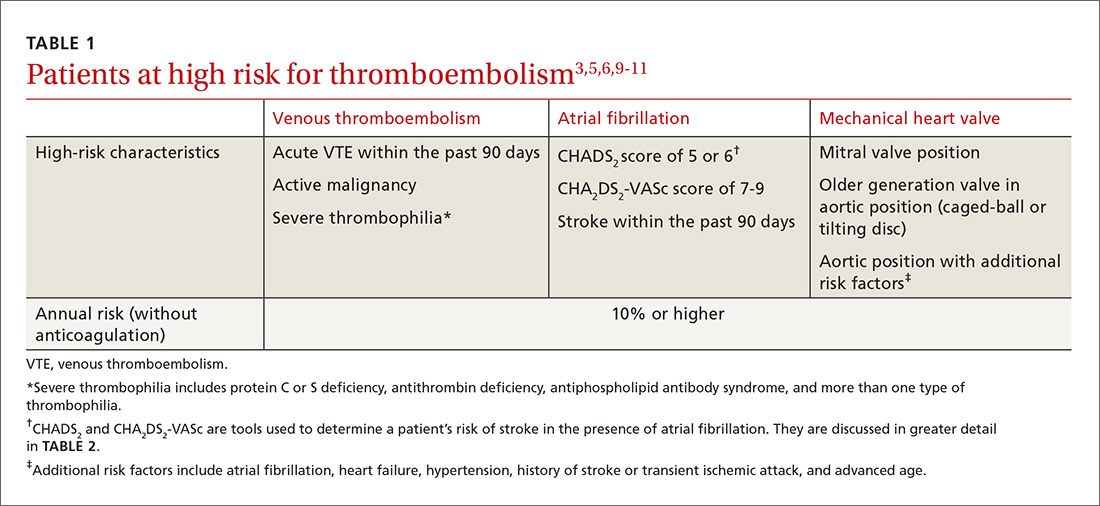
When planning for interruptions in oral anticoagulation, it is important to identify patients at highest risk for an acute thromboembolic event. Patients with 10% or higher annual risk for VTE or ischemic stroke are generally placed into this high-risk category (TABLE 13,5,6,9-11).3 Keep in mind that the absolute risk for thromboembolism during a brief period of oral coagulation interruption is relatively low, even in those patients considered to be at high risk. Using a mathematical approach (although simplistic), a patient with a 10% annual risk for a thromboembolic event would have <0.3% chance for developing such an event in the acute phase, even if their anticoagulation was withheld for up to 10 days ([10%/365 days] × 10 days).
Patients with mechanical heart valves. Nearly all patients with a mechanical heart valve are at moderate to high risk for ischemic stroke.3
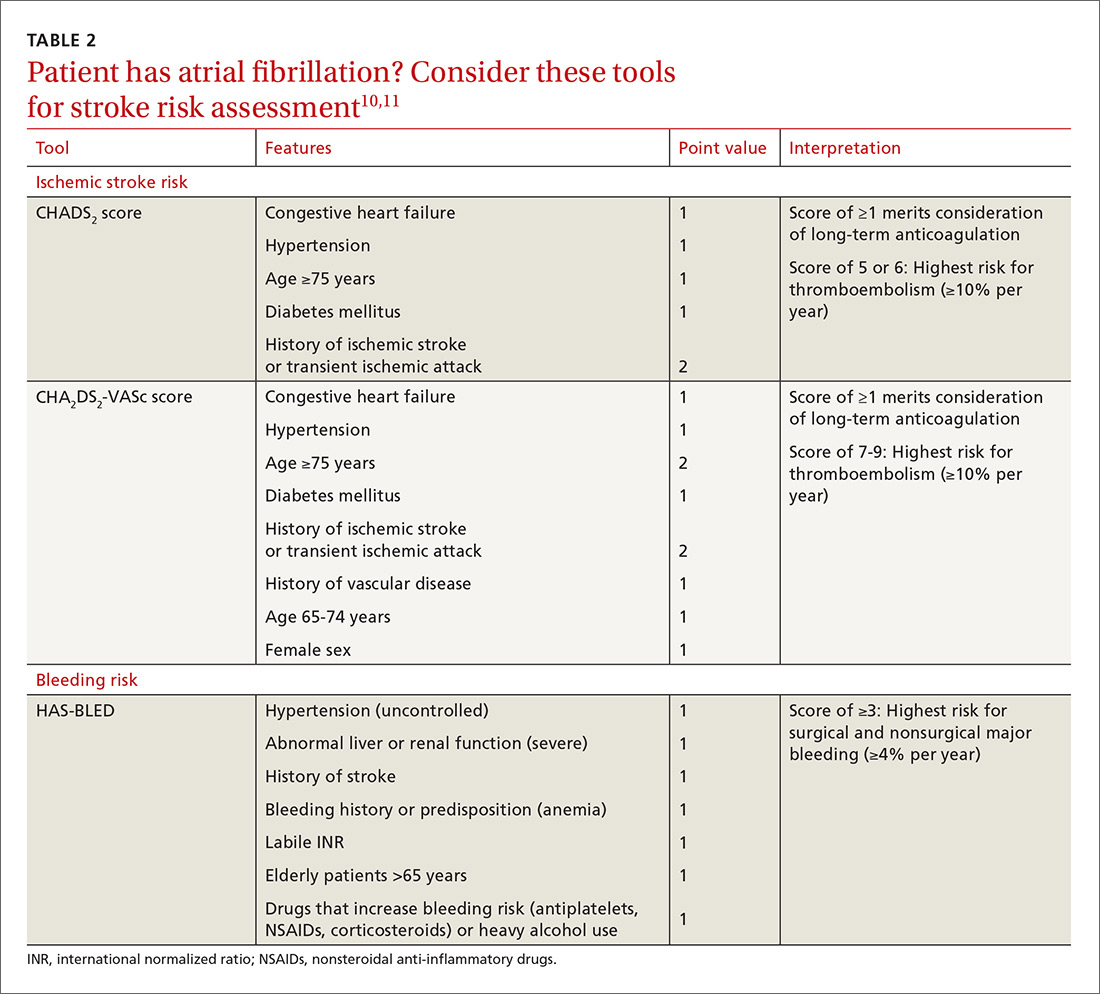
For patients with AF, the CHADS2 and CHA2DS2-VASc scoring tools can be used to estimate annual thrombosis risk based on the presence of risk factors (TABLE 210,11).6,9-11 It should be noted, however, that these scoring tools have not been validated specifically for periprocedural risk estimations. Nonetheless, the latest 2017 American College of Cardiology (ACC) guidelines recommend the use of the CHA2DS2-VASc scoring tool for making decisions regarding perioperative bridging in patients with AF.11
Patients with previous VTE. Multiple aspects of a patient’s past medical history need to be taken into account when estimating annual and acute risk for VTE. Patients at the highest risk for VTE recurrence (annual VTE risk ≥10%) include those with recent VTE (past 90 days), active malignancy, and/or severe thrombophilias (TABLE 13,5,6,9-11).3,5,6 Patients without any of these features can still be at moderate risk for recurrent VTE, as a single VTE without a clear provoking factor can confer a 5% to 10% annualized risk for recurrence.12,13 Previous proximal DVT and PE are associated with a higher risk for recurrence than a distal DVT, and males have a higher recurrence risk than females.5,12 There are scoring tools, such as DASH (D-dimer, Age, Sex, Hormones) and the “Men Continue and HERDOO2,” that can help estimate annualized risk for VTE recurrence; however, they are not validated (nor particularly useful) when making decisions in the perioperative period.14,15
Additional risk factors. Consider additional risk factors for thromboembolism, including estrogen/hormone replacement therapy, pregnancy, leg or hip fractures, immobility, trauma, spinal cord injury, central venous lines, congestive heart failure, thrombophilia, increased age, obesity, and varicose veins.5,16
In addition, some surgeries have a higher inherent risk for thrombosis. Major orthopedic surgery (knee and hip arthroplasty, hip fracture surgery) and surgeries for major trauma or spinal cord injuries are associated with an exceedingly high rate of VTE.17 Similarly, coronary artery bypass surgery, heart valve replacement, and carotid endarterectomies carry the highest risk for acute ischemic stroke.3
Who’s at highest risk for bleeding?
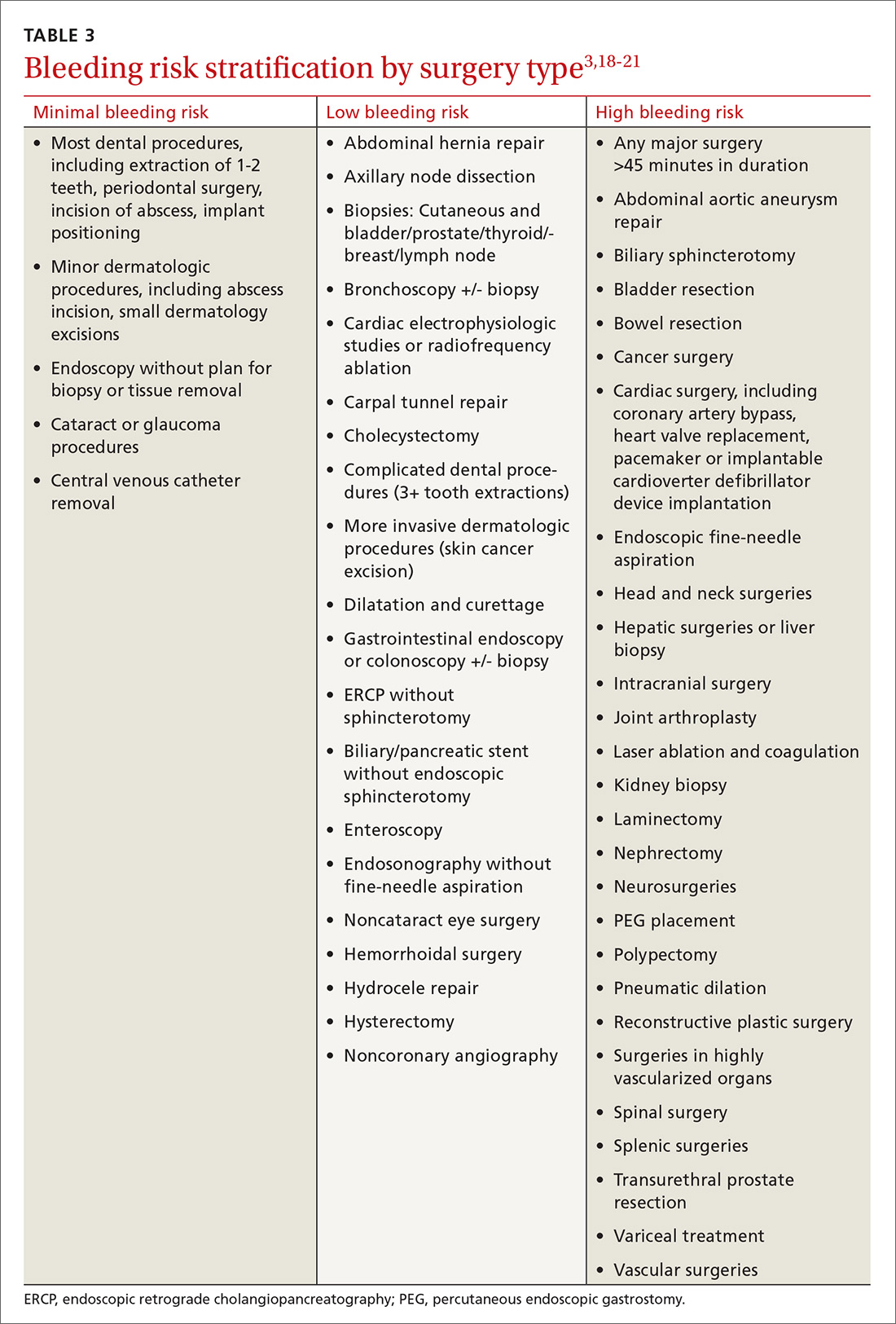
Establishing the bleeding risk associated with a procedure is imperative prior to urgent and elective surgeries to help determine when anticoagulation therapy should be discontinued and reinitiated, as well as whether bridging therapy is appropriate. The 2012 CHEST guidelines state that bleeding risk should be assessed based on timing of anticoagulation relative to surgery and whether the anticoagulation is being used as prophylaxis for, or treatment of, thromboembolism.3 Categorizing procedures as having a minimal, low, or high risk for bleeding can be helpful in making anticoagulation decisions (TABLE 3).3,18-21
In addition to the bleeding risk associated with procedures, patient-specific factors need to be considered. A bleeding event within the past 3 months, platelet abnormalities, a supratherapeutic INR at the time of surgery, a history of bleeding from previous bridging, a bleed history with a similar procedure, and a high HAS-BLED (Hypertension, Abnormal renal or liver function, Stroke, Bleeding history or predisposition, Labile INR, Elderly, Drugs/alcohol usage) score are all factors that elevate the risk for perioperative bleeding.10,11 Although validated only in patients taking warfarin, the HAS-BLED scoring system can be utilized in patients with AF to estimate annual risk for major bleeding (TABLE 210,11).10
With this risk information in mind, it’s time to move on to the 5 questions you’ll need to ask.
1. Should the patient’s oral anticoagulation be stopped prior to the upcoming procedure?
The answer, of course, hinges on the patient’s risk of bleeding.
Usually, it is not necessary to withhold any doses of oral anticoagulation if your patient is scheduled for a procedure with minimal risk for bleeding (TABLE 33,18-21).3 However, it may be reasonable to stop anticoagulation if your patient has additional features that predispose to high bleeding risk (eg, hemophilia, Von Willebrand disease, etc). The CHEST guidelines recommend adding an oral prohemostatic agent (eg, tranexamic acid) if anticoagulation will be continued during a dental procedure.3
If your patient is undergoing any other procedure that has a low to high risk for bleeding, oral anticoagulation should be withheld prior to the procedure in most instances,3,11 although there are exceptions. For example, cardiac procedures, such as AF catheter ablation and cardiac pacemaker placement, are often performed with uninterrupted oral anticoagulation despite their bleeding risk category.3
When in doubt, discuss the perceived bleeding and clotting risks directly with the specialist performing the procedure. In patients who have had a VTE or ischemic stroke within the past 3 months, consider postponing the invasive procedure until the patient is beyond this period of highest thrombotic risk.11
2. How far in advance of the procedure should the oral anticoagulant be withheld?
Warfarin may need to be stopped anywhere from 2 to 5 days prior to the procedure, depending on a number of variables.
Warfarin has a half-life of approximately 36 hours, so it can take 3 to 5 days for warfarin concentrations to drop to safe levels for procedures with low to moderate bleeding risk and 5 to 7 days for procedures with high bleeding risk.21 The 2012 CHEST guidelines recommend that warfarin therapy be discontinued 5 days prior to surgery to minimize the risk for bleeding.3 The Anticoagulation Forum, a leading expert panel that produced a set of useful anticoagulation guidelines in 2016, recommends stopping warfarin 4 to 5 days prior to a procedure.21 If the provider chooses to withhold warfarin before a procedure with minimal bleeding risk, it should be stopped 2 to 3 days prior.3
Consider checking INR values the week before. A 2017 consensus statement from the ACC recommends that the timing of warfarin discontinuation be based on an INR value taken 5 to 7 days prior to the surgical procedure.11 This allows for a more tailored approach to preparing the patient for surgery. If the INR is below goal range, warfarin may need to be withheld for only 3 to 4 days prior to a procedure. Conversely, INRs above goal range may require warfarin to be held 6 or more days, depending on the degree of INR elevation.
While not always feasible in clinical practice, the CHEST guidelines recommend obtaining an INR value the day prior to the procedure to determine if the INR value is low enough to proceed with surgery, or if a low dose of oral vitamin K needs to be administered to ensure that the INR is in a safe range the following day.3
DOACs
DOACs can be withheld for much shorter durations preoperatively than warfarin.
When withholding anticoagulants, the goal is to have a low amount of anticoagulant effect (12%-25%) present during low-risk procedures and a nominal amount of anticoagulant effect (3%-6%) present for high-risk procedures.20 DOACs have much shorter half-lives than warfarin (7-19 hours vs 36-48 hours, respectively), so they can be withheld for much shorter durations preoperatively.20 For patients undergoing procedures that are considered to have a minimal risk for bleeding (such as minor dental and dermatologic procedures), DOACs do not generally need to be withheld; however, it may be ideal to time the procedure when the DOAC is at a trough concentration (before the next dose is due).3
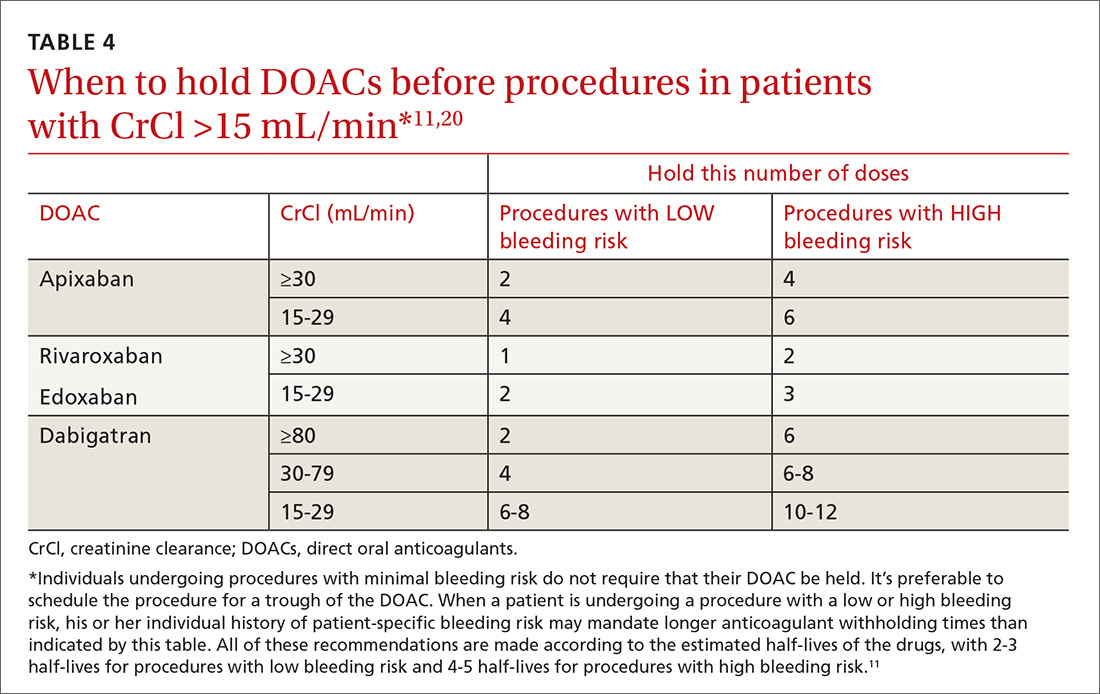
DOACs generally need to be withheld for only 1 to 3 days prior to major surgical procedures in patients with normal renal function (creatinine clearance [CrCl] >30 mL/min using the Cockcroft-Gault formula).20 The available oral direct factor Xa inhibitors (apixaban, rivaroxaban, and edoxaban) should generally be stopped 24 hours prior to a procedure that has a low bleeding risk, and 48 hours prior to procedures with high bleeding risk (TABLE 411,20).20 These medications may need to be withheld for an additional 1 to 2 days in patients with acute kidney injury or stage IV kidney disease.20
Dabigatran. About 80% of dabigatran is excreted renally, so its elimination is much more dependent on renal function than is that of the oral direct factor Xa inhibitors.20 Therefore, it generally needs to be withheld for at least 1 to 2 days longer than the oral factor Xa inhibitors unless CrCl >80 mL/min (TABLE 411,20).20
3. Is preoperative bridging with parenteral anticoagulation necessary?
In certain instances, patients who have a high thromboembolic risk and are discontinuing warfarin therapy may require bridging therapy with a low-molecular-weight heparin (LMWH) or unfractionated heparin (UFH). If a patient’s CrCl is <30 mL/min, then UFH is the preferred agent for perioperative bridging.21
But before any decision is made, it’s best to have a good understanding of what the guidelines—and the literature—have to say.
Key studies and guidelines
The 2012 CHEST guidelines recommend providing bridge therapy for any patient at high risk for thromboembolism (>10% annual risk) and consideration of bridge therapy in the setting of moderate clotting risk (5%-10% annual risk), depending on specific patient and procedural risk factors (TABLE 13,5,6,9-11).3
In 2015, a landmark clinical trial was published that significantly shaped how patients taking warfarin are managed periprocedurally.22 The Bridge (Bridging anticoagulation in patients who require temporary interruption of warfarin therapy for an elective invasive procedure or surgery) trial was the first prospective, randomized controlled trial to assess the efficacy and safety of parenteral bridging in patients with AF taking warfarin and undergoing an elective surgery.
Patients in the trial received either dalteparin at a therapeutic dose of 100 IU/kg or a matching placebo administered subcutaneously bid from 3 days before the procedure until 24 hours before the procedure, and then for 5 to 10 days after the procedure. The incidence of thromboembolic events was not significantly lower in the dalteparin group than in the placebo group (0.3% vs 0.4%, respectively; P=.73), while major bleeding rates were nearly 3-fold higher in the dalteparin group (3.2% vs 1.3%; P=.005). The trial concluded that placebo “was noninferior to perioperative bridging with LMWH for the prevention of arterial thromboembolism and decreased the risk of major bleeding.”22
Patients excluded from the trial included those with a mechanical heart valve, or a recent (within 3 months) embolism, stroke, or TIA, and only 3% of enrolled patients would have been classified as having a high bleeding risk according to CHEST guidelines.3,22
A prospective observational registry study produced similar findings and found that those patients who received bridging had more bleeding events and a higher incidence of myocardial infarction, stroke or systemic embolism, major bleeding, hospitalization, or death within 30 days than those who did not receive bridging.23 Other retrospective cohort studies comparing bridging to no bridging strategies in patients taking warfarin for VTE, mechanical heart valves, or AF have also failed to show a reduction in the incidence of thrombotic events with LMWH bridging.24,25
In 2016, the European Society of Cardiology suggested that “bridging does not seem to be beneficial, except in patients with mechanical heart valves.”26 Similarly, the 2016 Anticoagulation Forum guidelines state that “most patients with VTE can safely interrupt warfarin for invasive procedures without bridge therapy,” and that bridge therapy should be “reserved for those at highest recurrent VTE risk (eg, VTE within the previous month; prior history of recurrent VTE during anticoagulation therapy interruption; undergoing a procedure with high inherent risk for VTE, such as joint replacement surgery or major abdominal cancer resection).”21 They go on to state that even in these high-risk groups, the clinical decision to use bridging therapy needs to carefully weigh the benefits against the potential risks of bleeding.21
Controversy also surrounds the intensity of LMWH bridging. The Anticoagulation Forum guidelines state that the use of prophylactic rather than therapeutic dose LMWH may be considered, while the CHEST guidelines do not make a firm recommendation regarding LMWH dose while bridging.3,21 Ultimately, in patients who receive perioperative bridging with LMWH, the CHEST guidelines recommend that it should be stopped 24 hours prior to the procedure and resumed in accordance with the bleeding risk of the procedure (ie, prophylactic doses may be appropriate within 24 hours postprocedure, while full treatment doses may need to be delayed for 48 to 72 hours if surgical bleeding risk is high).3 UFH bridge therapy may be stopped 4 to 6 hours prior to surgery.3
DOACs. Given the rapid onset and relatively short half-lives of DOACs, use of a parenteral bridging agent is generally not necessary or recommended before or after an invasive procedure in patients taking a DOAC.20
4. When should oral anticoagulation be resumed postoperatively, and at what intensity?
Warfarin can generally be resumed the same day as the procedure (in the evening), assuming there are no active bleeding complications.3,11 Once fully reversed, it generally takes around 5 days for warfarin to become fully therapeutic, so it can be started soon after surgery without increasing the risk for early postoperative bleeding.20
DOACs. Consider the patient’s individual and procedural risks for bleeding when determining when to resume a DOAC postoperatively. That’s because unlike warfarin, which takes several days to take full effect, DOACs provide a nearly immediate anticoagulation effect.20,21 For procedures that have a low bleeding risk, it is recommended to resume therapeutic anticoagulation 24 hours after the procedure has ended.3,11,20 For procedures that have a high risk for bleeding, resumption of therapeutic anticoagulation should be delayed until 48 to 72 hours after the procedure has ended.3,11,20
5. Is postoperative bridging with parenteral anticoagulation necessary?
Warfarin. If a patient was deemed to be at sufficient VTE risk to be bridged preoperatively, then that patient likely also should be bridged postoperatively, particularly if the surgery itself is associated with a heightened thrombotic risk. While warfarin can generally be resumed postoperatively the same day as the procedure, full therapeutic doses of a LMWH should not be initiated sooner than 24 hours postoperatively, and initiation should be delayed for 48 to 72 hours for procedures with the highest bleeding risk (such as neurosurgery).3,11,21 Prophylactic doses of LMWH can generally be resumed as early as 12 hours postoperatively for procedures with high VTE risk (such as major orthopedic surgery).17
DOACs. In patients undergoing a procedure that carries both a high thromboembolic and high bleeding risk (such as major orthopedic surgery), initiation of a full-dose DOAC may need to be delayed for 2 to 3 days; however, more immediate VTE prophylaxis is usually necessary.3,17 Prophylaxis after such procedures can begin 12 hours after the procedure with a low-intensity LMWH, which should be continued until it is deemed safe to resume full-intensity DOAC therapy.3,17,18 If the patient is undergoing major orthopedic surgery, an FDA-approved prophylactic dose of a DOAC could be a temporary alternative to LMWH.27
Ms. P’s upcoming colonoscopy may require a biopsy and would be classified as a procedure with low bleeding risk (per TABLE 3), so warfarin should be withheld prior to her procedure. You could check her INR 5 to 7 days before her colonoscopy to guide how many doses need to be withheld; however, given the patient’s tight INR control over the previous 6 months, you can assume her INR will be in goal range at that check. As a result, you recommend that she avoid an extra INR check and stop taking her warfarin 5 days prior to the colonoscopy.
Ms. P has a CHA2DS2VASc score of 3, which puts her at a relatively low risk for acute ischemic stroke over the next 1 to 2 weeks. Given the results of the BRIDGE trial, you recommend no parenteral bridging agent before or after her procedure. You also recommend that the patient resume her usual dose of warfarin the same day as her procedure (in the evening) unless her gastroenterologist recommends otherwise. You schedule her for a follow-up INR 5 to 7 days after her colonoscopy.
Mr. Q’s total knee arthroplasty (TKA)—a procedure associated with a high risk of bleeding—requires an interruption in his apixaban therapy. Additionally, he is at high risk for recurrent thromboembolism, given his history of recurrent, unprovoked DVTs; however, he is past the highest risk period (VTE within the past 3 months; his last one was 9 months ago). He is otherwise healthy and has normal renal function, so his apixaban should be withheld for a total of 4 doses (48 hours) prior to his procedure. He should resume his full dose of apixaban 48 to 72 hours after his procedure to minimize the risk for bleeding.
However, given that a TKA is a procedure associated with a high rate of postoperative VTE, initiate prophylactic anticoagulation (such as enoxaparin 40 mg subcutaneously daily or apixaban 2.5 mg PO bid) about 12 hours after the procedure and continue it until full-dose apixaban is resumed.
CORRESPONDENCE
Jeremy Vandiver, PharmD, BCPS, University of Wyoming School of Pharmacy, 1000 E. University Ave., Dept. 3375, Laramie, WY 82071; jvandive@uwyo.edu.
1. Connelly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
2. Steinberg BA, Kim S, Piccini JP, et al. Use and associated risks of concomitant aspirin therapy with oral anticoagulation in patients with atrial fibrillation: insights from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Registry. Circulation. 2013;128:721-728.
3. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e326S-e350S.
4. Adam SS, McDuffie JR, Ortel TL, et al. Comparative effectiveness of warfarin and newer oral anticoagulants for the long-term prevention and treatment of arterial and venous thromboembolism. Department of Veteran Affairs Evidence-Based Synthesis Project #09-010; 2012. Available at: https://www.ncbi.nlm.nih.gov/books/NBK97541/. Accessed October 15, 2017.
5. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and Expert Panel Report. Chest. 2016;149:315-352.
6. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. J Am Coll Cardiol. 2014;64:2246-2280.
7. Centers for Disease Control and Prevention. Venous thromboembolism in adult hospitalizations — United States, 2007-2009. MMWR Morb Mortal Wkly Rep. 2012 June 8;61:401-404. Available at https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6122a1.htm. Accessed October 15, 2017.
8. Anderson FA, Wheeler HB, Goldberg HJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med. 1991;151:933-938.
9. Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012): The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2012;33:2451-2496.
10. Garwood CL, Korkis B, Grande D, et al. Anticoagulation bridge therapy in patients with atrial fibrillation: recent updates provide a rebalance of risk and benefit. Pharmacotherapy. 2017;37:712-714.
11. Doherty JU, Gluckman TJ, Hucker WJ, et al. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2017;69:871-898.
12. Douketis J, Tosetto A, Marcucci M, et al. Patient-level meta-analysis: effect of measurement timing, threshold, and patient age on ability of D-dimer testing to assess recurrence risk after unprovoked venous thromboembolism. Ann Intern Med. 2010;153:523-531.
13. Becattini C, Agnelli G, Schenone A, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med. 2012;366:1959-1967.
14. Tosetto A, Testa S, Martinelli I, et al. External validation of the DASH prediction rule: a retrospective cohort study. J Thromb Haemost. 2017;15:1963-1970.
15. Rodger MA, Le Gal G, Anderson DR, et al. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. BMJ. 2017;356:j1065.
16. Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism.
17. Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines, 8th ed. Chest. 2008;133:381S-453S.
18. Spyropoulos AC, Douketis JD. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood. 2012;120:2954-2962.
19. Eisen GM, Baron TH, Dominitz JA, et al. Guideline on the management of anticoagulation and antiplatelet therapy for endoscopic procedures. Gastrointest Endosc. 2002;55:775-779.
20. Burnett AE, Mahan CE, Vazquez SR. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis. 2016;41:206-232.
21. Witt DM, Clark NP, Kaatz S, et al. Guidance for the practical management of warfarin therapy in the treatment of venous thromboembolism. J Thromb Thrombolysis. 2016;41:187-205.
22. Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823-833.
23. Steinberg BA, Peterson ED, Kim S, et al. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circulation. 2015;131: 488-494.
24. Clark NP, Witt DM, Davies LE, et al. Bleeding, recurrent venous thromboembolism, and mortality risks during warfarin interruption for invasive procedures. JAMA Intern Med. 2015;175;1163-1168.
25. Sjögren V, Grzymala-Lubanski B, Renlund H, et al. Safety and efficacy of bridging with low-molecular-weight heparin during temporary interruptions of warfarin: a register-based cohort study. Clin Appl Thromb Hemost. 2017;23:961-966.
26. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893-2962.
27. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e278S-e325S.
Debra P is a 62-year-old African American woman who calls your office to report that she has an upcoming routine colonoscopy planned in 2 weeks. She has been taking warfarin for the past 2 years for ischemic stroke prevention secondary to atrial fibrillation (AF), and her gastroenterologist recommended that she contact her family physician (FP) to discuss periprocedural anticoagulation plans. Ms. P is currently taking warfarin 5 mg on Mondays, Wednesdays, and Fridays, and 2.5 mg all other days of the week. Her international normalized ratio (INR) was 2.3 when it was last checked 2 weeks ago, and it has been stable and within goal range for the past 6 months. Her medical history includes AF, well-controlled hypertension, and type 2 diabetes mellitus, as well as gout and stage 3 chronic kidney disease. Ms. P denies any history of stroke or transient ischemic attack (TIA). She is requesting instructions on how to manage her warfarin before and after her upcoming colonoscopy.
Jerry Q is a 68-year-old Caucasian man with longstanding osteoarthritis who is scheduled to undergo a total left knee arthroplasty in one week. His orthopedic surgeon recommended that he contact his FP for instructions regarding managing apixaban perioperatively. Jerry has been taking apixaban 5 mg bid for the past 9 months due to a history of recurrent deep vein thrombosis (DVT) and pulmonary embolism (PE) (both unprovoked). Mr. Q had been taking warfarin following his first DVT 4 years ago, but, after reporting that INR monitoring was a burden, he was started on apixaban. The patient has normal renal function and is relatively healthy otherwise. How should apixaban be managed before and after his upcoming surgery?
Each year, approximately 15% to 20% of patients taking an oral anticoagulant undergo a procedure that carries a heightened risk for bleeding.1,2 Stopping oral anticoagulation is often necessary before—and sometimes briefly after—many of these procedures in order to minimize the risk of bleeding.3 This means that countless decisions must be made by health care providers each year regarding if, when, and how to pause and resume oral anticoagulation. These decisions are not always straightforward, especially when you consider the risks for thrombosis and bleeding that are unique to the procedure and to the individual patient.
With these variables in mind, the health care provider must make decisions regarding anticoagulation during the periprocedural period based on the following 5 questions:
- Will this patient need to have his/her oral anticoagulant stopped prior to the procedure?
- If the patient’s oral anticoagulation needs to be held, when should it be stopped and for how long?
- Will periprocedural bridging with a parenteral anticoagulant be necessary prior to the procedure?
- When should the patient resume his or her oral anticoagulant after the procedure, and at what dosage?
- Will bridging with a parenteral anticoagulant be necessary after the procedure?
Before addressing these 5 questions, though, physicians must assess patients’ thrombotic and bleeding risks.4-6
Anticoagulant regimens and the risks of discontinuing them
The 2 most common indications for long-term oral anticoagulation are venous thromboembolism (VTE), which occurs in approximately one million Americans every year,7,8 and stroke prevention in the setting of AF (AF occurs in 3-6 million US adults per year).6
Warfarin is also often used in patients with mechanical heart valves for long-term stroke prevention; however, direct oral anticoagulants (DOACs) are not recommended for patients with mechanical heart valves because trials have not yet demonstrated their safety or efficacy in this population.4,5,9
Who’s at highest risk for an acute thromboembolic event?
When planning for interruptions in oral anticoagulation, it is important to identify patients at highest risk for an acute thromboembolic event. Patients with 10% or higher annual risk for VTE or ischemic stroke are generally placed into this high-risk category (TABLE 13,5,6,9-11).3 Keep in mind that the absolute risk for thromboembolism during a brief period of oral coagulation interruption is relatively low, even in those patients considered to be at high risk. Using a mathematical approach (although simplistic), a patient with a 10% annual risk for a thromboembolic event would have <0.3% chance for developing such an event in the acute phase, even if their anticoagulation was withheld for up to 10 days ([10%/365 days] × 10 days).
Patients with mechanical heart valves. Nearly all patients with a mechanical heart valve are at moderate to high risk for ischemic stroke.3
For patients with AF, the CHADS2 and CHA2DS2-VASc scoring tools can be used to estimate annual thrombosis risk based on the presence of risk factors (TABLE 210,11).6,9-11 It should be noted, however, that these scoring tools have not been validated specifically for periprocedural risk estimations. Nonetheless, the latest 2017 American College of Cardiology (ACC) guidelines recommend the use of the CHA2DS2-VASc scoring tool for making decisions regarding perioperative bridging in patients with AF.11
Patients with previous VTE. Multiple aspects of a patient’s past medical history need to be taken into account when estimating annual and acute risk for VTE. Patients at the highest risk for VTE recurrence (annual VTE risk ≥10%) include those with recent VTE (past 90 days), active malignancy, and/or severe thrombophilias (TABLE 13,5,6,9-11).3,5,6 Patients without any of these features can still be at moderate risk for recurrent VTE, as a single VTE without a clear provoking factor can confer a 5% to 10% annualized risk for recurrence.12,13 Previous proximal DVT and PE are associated with a higher risk for recurrence than a distal DVT, and males have a higher recurrence risk than females.5,12 There are scoring tools, such as DASH (D-dimer, Age, Sex, Hormones) and the “Men Continue and HERDOO2,” that can help estimate annualized risk for VTE recurrence; however, they are not validated (nor particularly useful) when making decisions in the perioperative period.14,15
Additional risk factors. Consider additional risk factors for thromboembolism, including estrogen/hormone replacement therapy, pregnancy, leg or hip fractures, immobility, trauma, spinal cord injury, central venous lines, congestive heart failure, thrombophilia, increased age, obesity, and varicose veins.5,16
In addition, some surgeries have a higher inherent risk for thrombosis. Major orthopedic surgery (knee and hip arthroplasty, hip fracture surgery) and surgeries for major trauma or spinal cord injuries are associated with an exceedingly high rate of VTE.17 Similarly, coronary artery bypass surgery, heart valve replacement, and carotid endarterectomies carry the highest risk for acute ischemic stroke.3
Who’s at highest risk for bleeding?
Establishing the bleeding risk associated with a procedure is imperative prior to urgent and elective surgeries to help determine when anticoagulation therapy should be discontinued and reinitiated, as well as whether bridging therapy is appropriate. The 2012 CHEST guidelines state that bleeding risk should be assessed based on timing of anticoagulation relative to surgery and whether the anticoagulation is being used as prophylaxis for, or treatment of, thromboembolism.3 Categorizing procedures as having a minimal, low, or high risk for bleeding can be helpful in making anticoagulation decisions (TABLE 3).3,18-21
In addition to the bleeding risk associated with procedures, patient-specific factors need to be considered. A bleeding event within the past 3 months, platelet abnormalities, a supratherapeutic INR at the time of surgery, a history of bleeding from previous bridging, a bleed history with a similar procedure, and a high HAS-BLED (Hypertension, Abnormal renal or liver function, Stroke, Bleeding history or predisposition, Labile INR, Elderly, Drugs/alcohol usage) score are all factors that elevate the risk for perioperative bleeding.10,11 Although validated only in patients taking warfarin, the HAS-BLED scoring system can be utilized in patients with AF to estimate annual risk for major bleeding (TABLE 210,11).10
With this risk information in mind, it’s time to move on to the 5 questions you’ll need to ask.
1. Should the patient’s oral anticoagulation be stopped prior to the upcoming procedure?
The answer, of course, hinges on the patient’s risk of bleeding.
Usually, it is not necessary to withhold any doses of oral anticoagulation if your patient is scheduled for a procedure with minimal risk for bleeding (TABLE 33,18-21).3 However, it may be reasonable to stop anticoagulation if your patient has additional features that predispose to high bleeding risk (eg, hemophilia, Von Willebrand disease, etc). The CHEST guidelines recommend adding an oral prohemostatic agent (eg, tranexamic acid) if anticoagulation will be continued during a dental procedure.3
If your patient is undergoing any other procedure that has a low to high risk for bleeding, oral anticoagulation should be withheld prior to the procedure in most instances,3,11 although there are exceptions. For example, cardiac procedures, such as AF catheter ablation and cardiac pacemaker placement, are often performed with uninterrupted oral anticoagulation despite their bleeding risk category.3
When in doubt, discuss the perceived bleeding and clotting risks directly with the specialist performing the procedure. In patients who have had a VTE or ischemic stroke within the past 3 months, consider postponing the invasive procedure until the patient is beyond this period of highest thrombotic risk.11
2. How far in advance of the procedure should the oral anticoagulant be withheld?
Warfarin may need to be stopped anywhere from 2 to 5 days prior to the procedure, depending on a number of variables.
Warfarin has a half-life of approximately 36 hours, so it can take 3 to 5 days for warfarin concentrations to drop to safe levels for procedures with low to moderate bleeding risk and 5 to 7 days for procedures with high bleeding risk.21 The 2012 CHEST guidelines recommend that warfarin therapy be discontinued 5 days prior to surgery to minimize the risk for bleeding.3 The Anticoagulation Forum, a leading expert panel that produced a set of useful anticoagulation guidelines in 2016, recommends stopping warfarin 4 to 5 days prior to a procedure.21 If the provider chooses to withhold warfarin before a procedure with minimal bleeding risk, it should be stopped 2 to 3 days prior.3
Consider checking INR values the week before. A 2017 consensus statement from the ACC recommends that the timing of warfarin discontinuation be based on an INR value taken 5 to 7 days prior to the surgical procedure.11 This allows for a more tailored approach to preparing the patient for surgery. If the INR is below goal range, warfarin may need to be withheld for only 3 to 4 days prior to a procedure. Conversely, INRs above goal range may require warfarin to be held 6 or more days, depending on the degree of INR elevation.
While not always feasible in clinical practice, the CHEST guidelines recommend obtaining an INR value the day prior to the procedure to determine if the INR value is low enough to proceed with surgery, or if a low dose of oral vitamin K needs to be administered to ensure that the INR is in a safe range the following day.3
DOACs
DOACs can be withheld for much shorter durations preoperatively than warfarin.
When withholding anticoagulants, the goal is to have a low amount of anticoagulant effect (12%-25%) present during low-risk procedures and a nominal amount of anticoagulant effect (3%-6%) present for high-risk procedures.20 DOACs have much shorter half-lives than warfarin (7-19 hours vs 36-48 hours, respectively), so they can be withheld for much shorter durations preoperatively.20 For patients undergoing procedures that are considered to have a minimal risk for bleeding (such as minor dental and dermatologic procedures), DOACs do not generally need to be withheld; however, it may be ideal to time the procedure when the DOAC is at a trough concentration (before the next dose is due).3
DOACs generally need to be withheld for only 1 to 3 days prior to major surgical procedures in patients with normal renal function (creatinine clearance [CrCl] >30 mL/min using the Cockcroft-Gault formula).20 The available oral direct factor Xa inhibitors (apixaban, rivaroxaban, and edoxaban) should generally be stopped 24 hours prior to a procedure that has a low bleeding risk, and 48 hours prior to procedures with high bleeding risk (TABLE 411,20).20 These medications may need to be withheld for an additional 1 to 2 days in patients with acute kidney injury or stage IV kidney disease.20
Dabigatran. About 80% of dabigatran is excreted renally, so its elimination is much more dependent on renal function than is that of the oral direct factor Xa inhibitors.20 Therefore, it generally needs to be withheld for at least 1 to 2 days longer than the oral factor Xa inhibitors unless CrCl >80 mL/min (TABLE 411,20).20
3. Is preoperative bridging with parenteral anticoagulation necessary?
In certain instances, patients who have a high thromboembolic risk and are discontinuing warfarin therapy may require bridging therapy with a low-molecular-weight heparin (LMWH) or unfractionated heparin (UFH). If a patient’s CrCl is <30 mL/min, then UFH is the preferred agent for perioperative bridging.21
But before any decision is made, it’s best to have a good understanding of what the guidelines—and the literature—have to say.
Key studies and guidelines
The 2012 CHEST guidelines recommend providing bridge therapy for any patient at high risk for thromboembolism (>10% annual risk) and consideration of bridge therapy in the setting of moderate clotting risk (5%-10% annual risk), depending on specific patient and procedural risk factors (TABLE 13,5,6,9-11).3
In 2015, a landmark clinical trial was published that significantly shaped how patients taking warfarin are managed periprocedurally.22 The Bridge (Bridging anticoagulation in patients who require temporary interruption of warfarin therapy for an elective invasive procedure or surgery) trial was the first prospective, randomized controlled trial to assess the efficacy and safety of parenteral bridging in patients with AF taking warfarin and undergoing an elective surgery.
Patients in the trial received either dalteparin at a therapeutic dose of 100 IU/kg or a matching placebo administered subcutaneously bid from 3 days before the procedure until 24 hours before the procedure, and then for 5 to 10 days after the procedure. The incidence of thromboembolic events was not significantly lower in the dalteparin group than in the placebo group (0.3% vs 0.4%, respectively; P=.73), while major bleeding rates were nearly 3-fold higher in the dalteparin group (3.2% vs 1.3%; P=.005). The trial concluded that placebo “was noninferior to perioperative bridging with LMWH for the prevention of arterial thromboembolism and decreased the risk of major bleeding.”22
Patients excluded from the trial included those with a mechanical heart valve, or a recent (within 3 months) embolism, stroke, or TIA, and only 3% of enrolled patients would have been classified as having a high bleeding risk according to CHEST guidelines.3,22
A prospective observational registry study produced similar findings and found that those patients who received bridging had more bleeding events and a higher incidence of myocardial infarction, stroke or systemic embolism, major bleeding, hospitalization, or death within 30 days than those who did not receive bridging.23 Other retrospective cohort studies comparing bridging to no bridging strategies in patients taking warfarin for VTE, mechanical heart valves, or AF have also failed to show a reduction in the incidence of thrombotic events with LMWH bridging.24,25
In 2016, the European Society of Cardiology suggested that “bridging does not seem to be beneficial, except in patients with mechanical heart valves.”26 Similarly, the 2016 Anticoagulation Forum guidelines state that “most patients with VTE can safely interrupt warfarin for invasive procedures without bridge therapy,” and that bridge therapy should be “reserved for those at highest recurrent VTE risk (eg, VTE within the previous month; prior history of recurrent VTE during anticoagulation therapy interruption; undergoing a procedure with high inherent risk for VTE, such as joint replacement surgery or major abdominal cancer resection).”21 They go on to state that even in these high-risk groups, the clinical decision to use bridging therapy needs to carefully weigh the benefits against the potential risks of bleeding.21
Controversy also surrounds the intensity of LMWH bridging. The Anticoagulation Forum guidelines state that the use of prophylactic rather than therapeutic dose LMWH may be considered, while the CHEST guidelines do not make a firm recommendation regarding LMWH dose while bridging.3,21 Ultimately, in patients who receive perioperative bridging with LMWH, the CHEST guidelines recommend that it should be stopped 24 hours prior to the procedure and resumed in accordance with the bleeding risk of the procedure (ie, prophylactic doses may be appropriate within 24 hours postprocedure, while full treatment doses may need to be delayed for 48 to 72 hours if surgical bleeding risk is high).3 UFH bridge therapy may be stopped 4 to 6 hours prior to surgery.3
DOACs. Given the rapid onset and relatively short half-lives of DOACs, use of a parenteral bridging agent is generally not necessary or recommended before or after an invasive procedure in patients taking a DOAC.20
4. When should oral anticoagulation be resumed postoperatively, and at what intensity?
Warfarin can generally be resumed the same day as the procedure (in the evening), assuming there are no active bleeding complications.3,11 Once fully reversed, it generally takes around 5 days for warfarin to become fully therapeutic, so it can be started soon after surgery without increasing the risk for early postoperative bleeding.20
DOACs. Consider the patient’s individual and procedural risks for bleeding when determining when to resume a DOAC postoperatively. That’s because unlike warfarin, which takes several days to take full effect, DOACs provide a nearly immediate anticoagulation effect.20,21 For procedures that have a low bleeding risk, it is recommended to resume therapeutic anticoagulation 24 hours after the procedure has ended.3,11,20 For procedures that have a high risk for bleeding, resumption of therapeutic anticoagulation should be delayed until 48 to 72 hours after the procedure has ended.3,11,20
5. Is postoperative bridging with parenteral anticoagulation necessary?
Warfarin. If a patient was deemed to be at sufficient VTE risk to be bridged preoperatively, then that patient likely also should be bridged postoperatively, particularly if the surgery itself is associated with a heightened thrombotic risk. While warfarin can generally be resumed postoperatively the same day as the procedure, full therapeutic doses of a LMWH should not be initiated sooner than 24 hours postoperatively, and initiation should be delayed for 48 to 72 hours for procedures with the highest bleeding risk (such as neurosurgery).3,11,21 Prophylactic doses of LMWH can generally be resumed as early as 12 hours postoperatively for procedures with high VTE risk (such as major orthopedic surgery).17
DOACs. In patients undergoing a procedure that carries both a high thromboembolic and high bleeding risk (such as major orthopedic surgery), initiation of a full-dose DOAC may need to be delayed for 2 to 3 days; however, more immediate VTE prophylaxis is usually necessary.3,17 Prophylaxis after such procedures can begin 12 hours after the procedure with a low-intensity LMWH, which should be continued until it is deemed safe to resume full-intensity DOAC therapy.3,17,18 If the patient is undergoing major orthopedic surgery, an FDA-approved prophylactic dose of a DOAC could be a temporary alternative to LMWH.27
Ms. P’s upcoming colonoscopy may require a biopsy and would be classified as a procedure with low bleeding risk (per TABLE 3), so warfarin should be withheld prior to her procedure. You could check her INR 5 to 7 days before her colonoscopy to guide how many doses need to be withheld; however, given the patient’s tight INR control over the previous 6 months, you can assume her INR will be in goal range at that check. As a result, you recommend that she avoid an extra INR check and stop taking her warfarin 5 days prior to the colonoscopy.
Ms. P has a CHA2DS2VASc score of 3, which puts her at a relatively low risk for acute ischemic stroke over the next 1 to 2 weeks. Given the results of the BRIDGE trial, you recommend no parenteral bridging agent before or after her procedure. You also recommend that the patient resume her usual dose of warfarin the same day as her procedure (in the evening) unless her gastroenterologist recommends otherwise. You schedule her for a follow-up INR 5 to 7 days after her colonoscopy.
Mr. Q’s total knee arthroplasty (TKA)—a procedure associated with a high risk of bleeding—requires an interruption in his apixaban therapy. Additionally, he is at high risk for recurrent thromboembolism, given his history of recurrent, unprovoked DVTs; however, he is past the highest risk period (VTE within the past 3 months; his last one was 9 months ago). He is otherwise healthy and has normal renal function, so his apixaban should be withheld for a total of 4 doses (48 hours) prior to his procedure. He should resume his full dose of apixaban 48 to 72 hours after his procedure to minimize the risk for bleeding.
However, given that a TKA is a procedure associated with a high rate of postoperative VTE, initiate prophylactic anticoagulation (such as enoxaparin 40 mg subcutaneously daily or apixaban 2.5 mg PO bid) about 12 hours after the procedure and continue it until full-dose apixaban is resumed.
CORRESPONDENCE
Jeremy Vandiver, PharmD, BCPS, University of Wyoming School of Pharmacy, 1000 E. University Ave., Dept. 3375, Laramie, WY 82071; jvandive@uwyo.edu.
Debra P is a 62-year-old African American woman who calls your office to report that she has an upcoming routine colonoscopy planned in 2 weeks. She has been taking warfarin for the past 2 years for ischemic stroke prevention secondary to atrial fibrillation (AF), and her gastroenterologist recommended that she contact her family physician (FP) to discuss periprocedural anticoagulation plans. Ms. P is currently taking warfarin 5 mg on Mondays, Wednesdays, and Fridays, and 2.5 mg all other days of the week. Her international normalized ratio (INR) was 2.3 when it was last checked 2 weeks ago, and it has been stable and within goal range for the past 6 months. Her medical history includes AF, well-controlled hypertension, and type 2 diabetes mellitus, as well as gout and stage 3 chronic kidney disease. Ms. P denies any history of stroke or transient ischemic attack (TIA). She is requesting instructions on how to manage her warfarin before and after her upcoming colonoscopy.
Jerry Q is a 68-year-old Caucasian man with longstanding osteoarthritis who is scheduled to undergo a total left knee arthroplasty in one week. His orthopedic surgeon recommended that he contact his FP for instructions regarding managing apixaban perioperatively. Jerry has been taking apixaban 5 mg bid for the past 9 months due to a history of recurrent deep vein thrombosis (DVT) and pulmonary embolism (PE) (both unprovoked). Mr. Q had been taking warfarin following his first DVT 4 years ago, but, after reporting that INR monitoring was a burden, he was started on apixaban. The patient has normal renal function and is relatively healthy otherwise. How should apixaban be managed before and after his upcoming surgery?
Each year, approximately 15% to 20% of patients taking an oral anticoagulant undergo a procedure that carries a heightened risk for bleeding.1,2 Stopping oral anticoagulation is often necessary before—and sometimes briefly after—many of these procedures in order to minimize the risk of bleeding.3 This means that countless decisions must be made by health care providers each year regarding if, when, and how to pause and resume oral anticoagulation. These decisions are not always straightforward, especially when you consider the risks for thrombosis and bleeding that are unique to the procedure and to the individual patient.
With these variables in mind, the health care provider must make decisions regarding anticoagulation during the periprocedural period based on the following 5 questions:
- Will this patient need to have his/her oral anticoagulant stopped prior to the procedure?
- If the patient’s oral anticoagulation needs to be held, when should it be stopped and for how long?
- Will periprocedural bridging with a parenteral anticoagulant be necessary prior to the procedure?
- When should the patient resume his or her oral anticoagulant after the procedure, and at what dosage?
- Will bridging with a parenteral anticoagulant be necessary after the procedure?
Before addressing these 5 questions, though, physicians must assess patients’ thrombotic and bleeding risks.4-6
Anticoagulant regimens and the risks of discontinuing them
The 2 most common indications for long-term oral anticoagulation are venous thromboembolism (VTE), which occurs in approximately one million Americans every year,7,8 and stroke prevention in the setting of AF (AF occurs in 3-6 million US adults per year).6
Warfarin is also often used in patients with mechanical heart valves for long-term stroke prevention; however, direct oral anticoagulants (DOACs) are not recommended for patients with mechanical heart valves because trials have not yet demonstrated their safety or efficacy in this population.4,5,9
Who’s at highest risk for an acute thromboembolic event?
When planning for interruptions in oral anticoagulation, it is important to identify patients at highest risk for an acute thromboembolic event. Patients with 10% or higher annual risk for VTE or ischemic stroke are generally placed into this high-risk category (TABLE 13,5,6,9-11).3 Keep in mind that the absolute risk for thromboembolism during a brief period of oral coagulation interruption is relatively low, even in those patients considered to be at high risk. Using a mathematical approach (although simplistic), a patient with a 10% annual risk for a thromboembolic event would have <0.3% chance for developing such an event in the acute phase, even if their anticoagulation was withheld for up to 10 days ([10%/365 days] × 10 days).
Patients with mechanical heart valves. Nearly all patients with a mechanical heart valve are at moderate to high risk for ischemic stroke.3
For patients with AF, the CHADS2 and CHA2DS2-VASc scoring tools can be used to estimate annual thrombosis risk based on the presence of risk factors (TABLE 210,11).6,9-11 It should be noted, however, that these scoring tools have not been validated specifically for periprocedural risk estimations. Nonetheless, the latest 2017 American College of Cardiology (ACC) guidelines recommend the use of the CHA2DS2-VASc scoring tool for making decisions regarding perioperative bridging in patients with AF.11
Patients with previous VTE. Multiple aspects of a patient’s past medical history need to be taken into account when estimating annual and acute risk for VTE. Patients at the highest risk for VTE recurrence (annual VTE risk ≥10%) include those with recent VTE (past 90 days), active malignancy, and/or severe thrombophilias (TABLE 13,5,6,9-11).3,5,6 Patients without any of these features can still be at moderate risk for recurrent VTE, as a single VTE without a clear provoking factor can confer a 5% to 10% annualized risk for recurrence.12,13 Previous proximal DVT and PE are associated with a higher risk for recurrence than a distal DVT, and males have a higher recurrence risk than females.5,12 There are scoring tools, such as DASH (D-dimer, Age, Sex, Hormones) and the “Men Continue and HERDOO2,” that can help estimate annualized risk for VTE recurrence; however, they are not validated (nor particularly useful) when making decisions in the perioperative period.14,15
Additional risk factors. Consider additional risk factors for thromboembolism, including estrogen/hormone replacement therapy, pregnancy, leg or hip fractures, immobility, trauma, spinal cord injury, central venous lines, congestive heart failure, thrombophilia, increased age, obesity, and varicose veins.5,16
In addition, some surgeries have a higher inherent risk for thrombosis. Major orthopedic surgery (knee and hip arthroplasty, hip fracture surgery) and surgeries for major trauma or spinal cord injuries are associated with an exceedingly high rate of VTE.17 Similarly, coronary artery bypass surgery, heart valve replacement, and carotid endarterectomies carry the highest risk for acute ischemic stroke.3
Who’s at highest risk for bleeding?
Establishing the bleeding risk associated with a procedure is imperative prior to urgent and elective surgeries to help determine when anticoagulation therapy should be discontinued and reinitiated, as well as whether bridging therapy is appropriate. The 2012 CHEST guidelines state that bleeding risk should be assessed based on timing of anticoagulation relative to surgery and whether the anticoagulation is being used as prophylaxis for, or treatment of, thromboembolism.3 Categorizing procedures as having a minimal, low, or high risk for bleeding can be helpful in making anticoagulation decisions (TABLE 3).3,18-21
In addition to the bleeding risk associated with procedures, patient-specific factors need to be considered. A bleeding event within the past 3 months, platelet abnormalities, a supratherapeutic INR at the time of surgery, a history of bleeding from previous bridging, a bleed history with a similar procedure, and a high HAS-BLED (Hypertension, Abnormal renal or liver function, Stroke, Bleeding history or predisposition, Labile INR, Elderly, Drugs/alcohol usage) score are all factors that elevate the risk for perioperative bleeding.10,11 Although validated only in patients taking warfarin, the HAS-BLED scoring system can be utilized in patients with AF to estimate annual risk for major bleeding (TABLE 210,11).10
With this risk information in mind, it’s time to move on to the 5 questions you’ll need to ask.
1. Should the patient’s oral anticoagulation be stopped prior to the upcoming procedure?
The answer, of course, hinges on the patient’s risk of bleeding.
Usually, it is not necessary to withhold any doses of oral anticoagulation if your patient is scheduled for a procedure with minimal risk for bleeding (TABLE 33,18-21).3 However, it may be reasonable to stop anticoagulation if your patient has additional features that predispose to high bleeding risk (eg, hemophilia, Von Willebrand disease, etc). The CHEST guidelines recommend adding an oral prohemostatic agent (eg, tranexamic acid) if anticoagulation will be continued during a dental procedure.3
If your patient is undergoing any other procedure that has a low to high risk for bleeding, oral anticoagulation should be withheld prior to the procedure in most instances,3,11 although there are exceptions. For example, cardiac procedures, such as AF catheter ablation and cardiac pacemaker placement, are often performed with uninterrupted oral anticoagulation despite their bleeding risk category.3
When in doubt, discuss the perceived bleeding and clotting risks directly with the specialist performing the procedure. In patients who have had a VTE or ischemic stroke within the past 3 months, consider postponing the invasive procedure until the patient is beyond this period of highest thrombotic risk.11
2. How far in advance of the procedure should the oral anticoagulant be withheld?
Warfarin may need to be stopped anywhere from 2 to 5 days prior to the procedure, depending on a number of variables.
Warfarin has a half-life of approximately 36 hours, so it can take 3 to 5 days for warfarin concentrations to drop to safe levels for procedures with low to moderate bleeding risk and 5 to 7 days for procedures with high bleeding risk.21 The 2012 CHEST guidelines recommend that warfarin therapy be discontinued 5 days prior to surgery to minimize the risk for bleeding.3 The Anticoagulation Forum, a leading expert panel that produced a set of useful anticoagulation guidelines in 2016, recommends stopping warfarin 4 to 5 days prior to a procedure.21 If the provider chooses to withhold warfarin before a procedure with minimal bleeding risk, it should be stopped 2 to 3 days prior.3
Consider checking INR values the week before. A 2017 consensus statement from the ACC recommends that the timing of warfarin discontinuation be based on an INR value taken 5 to 7 days prior to the surgical procedure.11 This allows for a more tailored approach to preparing the patient for surgery. If the INR is below goal range, warfarin may need to be withheld for only 3 to 4 days prior to a procedure. Conversely, INRs above goal range may require warfarin to be held 6 or more days, depending on the degree of INR elevation.
While not always feasible in clinical practice, the CHEST guidelines recommend obtaining an INR value the day prior to the procedure to determine if the INR value is low enough to proceed with surgery, or if a low dose of oral vitamin K needs to be administered to ensure that the INR is in a safe range the following day.3
DOACs
DOACs can be withheld for much shorter durations preoperatively than warfarin.
When withholding anticoagulants, the goal is to have a low amount of anticoagulant effect (12%-25%) present during low-risk procedures and a nominal amount of anticoagulant effect (3%-6%) present for high-risk procedures.20 DOACs have much shorter half-lives than warfarin (7-19 hours vs 36-48 hours, respectively), so they can be withheld for much shorter durations preoperatively.20 For patients undergoing procedures that are considered to have a minimal risk for bleeding (such as minor dental and dermatologic procedures), DOACs do not generally need to be withheld; however, it may be ideal to time the procedure when the DOAC is at a trough concentration (before the next dose is due).3
DOACs generally need to be withheld for only 1 to 3 days prior to major surgical procedures in patients with normal renal function (creatinine clearance [CrCl] >30 mL/min using the Cockcroft-Gault formula).20 The available oral direct factor Xa inhibitors (apixaban, rivaroxaban, and edoxaban) should generally be stopped 24 hours prior to a procedure that has a low bleeding risk, and 48 hours prior to procedures with high bleeding risk (TABLE 411,20).20 These medications may need to be withheld for an additional 1 to 2 days in patients with acute kidney injury or stage IV kidney disease.20
Dabigatran. About 80% of dabigatran is excreted renally, so its elimination is much more dependent on renal function than is that of the oral direct factor Xa inhibitors.20 Therefore, it generally needs to be withheld for at least 1 to 2 days longer than the oral factor Xa inhibitors unless CrCl >80 mL/min (TABLE 411,20).20
3. Is preoperative bridging with parenteral anticoagulation necessary?
In certain instances, patients who have a high thromboembolic risk and are discontinuing warfarin therapy may require bridging therapy with a low-molecular-weight heparin (LMWH) or unfractionated heparin (UFH). If a patient’s CrCl is <30 mL/min, then UFH is the preferred agent for perioperative bridging.21
But before any decision is made, it’s best to have a good understanding of what the guidelines—and the literature—have to say.
Key studies and guidelines
The 2012 CHEST guidelines recommend providing bridge therapy for any patient at high risk for thromboembolism (>10% annual risk) and consideration of bridge therapy in the setting of moderate clotting risk (5%-10% annual risk), depending on specific patient and procedural risk factors (TABLE 13,5,6,9-11).3
In 2015, a landmark clinical trial was published that significantly shaped how patients taking warfarin are managed periprocedurally.22 The Bridge (Bridging anticoagulation in patients who require temporary interruption of warfarin therapy for an elective invasive procedure or surgery) trial was the first prospective, randomized controlled trial to assess the efficacy and safety of parenteral bridging in patients with AF taking warfarin and undergoing an elective surgery.
Patients in the trial received either dalteparin at a therapeutic dose of 100 IU/kg or a matching placebo administered subcutaneously bid from 3 days before the procedure until 24 hours before the procedure, and then for 5 to 10 days after the procedure. The incidence of thromboembolic events was not significantly lower in the dalteparin group than in the placebo group (0.3% vs 0.4%, respectively; P=.73), while major bleeding rates were nearly 3-fold higher in the dalteparin group (3.2% vs 1.3%; P=.005). The trial concluded that placebo “was noninferior to perioperative bridging with LMWH for the prevention of arterial thromboembolism and decreased the risk of major bleeding.”22
Patients excluded from the trial included those with a mechanical heart valve, or a recent (within 3 months) embolism, stroke, or TIA, and only 3% of enrolled patients would have been classified as having a high bleeding risk according to CHEST guidelines.3,22
A prospective observational registry study produced similar findings and found that those patients who received bridging had more bleeding events and a higher incidence of myocardial infarction, stroke or systemic embolism, major bleeding, hospitalization, or death within 30 days than those who did not receive bridging.23 Other retrospective cohort studies comparing bridging to no bridging strategies in patients taking warfarin for VTE, mechanical heart valves, or AF have also failed to show a reduction in the incidence of thrombotic events with LMWH bridging.24,25
In 2016, the European Society of Cardiology suggested that “bridging does not seem to be beneficial, except in patients with mechanical heart valves.”26 Similarly, the 2016 Anticoagulation Forum guidelines state that “most patients with VTE can safely interrupt warfarin for invasive procedures without bridge therapy,” and that bridge therapy should be “reserved for those at highest recurrent VTE risk (eg, VTE within the previous month; prior history of recurrent VTE during anticoagulation therapy interruption; undergoing a procedure with high inherent risk for VTE, such as joint replacement surgery or major abdominal cancer resection).”21 They go on to state that even in these high-risk groups, the clinical decision to use bridging therapy needs to carefully weigh the benefits against the potential risks of bleeding.21
Controversy also surrounds the intensity of LMWH bridging. The Anticoagulation Forum guidelines state that the use of prophylactic rather than therapeutic dose LMWH may be considered, while the CHEST guidelines do not make a firm recommendation regarding LMWH dose while bridging.3,21 Ultimately, in patients who receive perioperative bridging with LMWH, the CHEST guidelines recommend that it should be stopped 24 hours prior to the procedure and resumed in accordance with the bleeding risk of the procedure (ie, prophylactic doses may be appropriate within 24 hours postprocedure, while full treatment doses may need to be delayed for 48 to 72 hours if surgical bleeding risk is high).3 UFH bridge therapy may be stopped 4 to 6 hours prior to surgery.3
DOACs. Given the rapid onset and relatively short half-lives of DOACs, use of a parenteral bridging agent is generally not necessary or recommended before or after an invasive procedure in patients taking a DOAC.20
4. When should oral anticoagulation be resumed postoperatively, and at what intensity?
Warfarin can generally be resumed the same day as the procedure (in the evening), assuming there are no active bleeding complications.3,11 Once fully reversed, it generally takes around 5 days for warfarin to become fully therapeutic, so it can be started soon after surgery without increasing the risk for early postoperative bleeding.20
DOACs. Consider the patient’s individual and procedural risks for bleeding when determining when to resume a DOAC postoperatively. That’s because unlike warfarin, which takes several days to take full effect, DOACs provide a nearly immediate anticoagulation effect.20,21 For procedures that have a low bleeding risk, it is recommended to resume therapeutic anticoagulation 24 hours after the procedure has ended.3,11,20 For procedures that have a high risk for bleeding, resumption of therapeutic anticoagulation should be delayed until 48 to 72 hours after the procedure has ended.3,11,20
5. Is postoperative bridging with parenteral anticoagulation necessary?
Warfarin. If a patient was deemed to be at sufficient VTE risk to be bridged preoperatively, then that patient likely also should be bridged postoperatively, particularly if the surgery itself is associated with a heightened thrombotic risk. While warfarin can generally be resumed postoperatively the same day as the procedure, full therapeutic doses of a LMWH should not be initiated sooner than 24 hours postoperatively, and initiation should be delayed for 48 to 72 hours for procedures with the highest bleeding risk (such as neurosurgery).3,11,21 Prophylactic doses of LMWH can generally be resumed as early as 12 hours postoperatively for procedures with high VTE risk (such as major orthopedic surgery).17
DOACs. In patients undergoing a procedure that carries both a high thromboembolic and high bleeding risk (such as major orthopedic surgery), initiation of a full-dose DOAC may need to be delayed for 2 to 3 days; however, more immediate VTE prophylaxis is usually necessary.3,17 Prophylaxis after such procedures can begin 12 hours after the procedure with a low-intensity LMWH, which should be continued until it is deemed safe to resume full-intensity DOAC therapy.3,17,18 If the patient is undergoing major orthopedic surgery, an FDA-approved prophylactic dose of a DOAC could be a temporary alternative to LMWH.27
Ms. P’s upcoming colonoscopy may require a biopsy and would be classified as a procedure with low bleeding risk (per TABLE 3), so warfarin should be withheld prior to her procedure. You could check her INR 5 to 7 days before her colonoscopy to guide how many doses need to be withheld; however, given the patient’s tight INR control over the previous 6 months, you can assume her INR will be in goal range at that check. As a result, you recommend that she avoid an extra INR check and stop taking her warfarin 5 days prior to the colonoscopy.
Ms. P has a CHA2DS2VASc score of 3, which puts her at a relatively low risk for acute ischemic stroke over the next 1 to 2 weeks. Given the results of the BRIDGE trial, you recommend no parenteral bridging agent before or after her procedure. You also recommend that the patient resume her usual dose of warfarin the same day as her procedure (in the evening) unless her gastroenterologist recommends otherwise. You schedule her for a follow-up INR 5 to 7 days after her colonoscopy.
Mr. Q’s total knee arthroplasty (TKA)—a procedure associated with a high risk of bleeding—requires an interruption in his apixaban therapy. Additionally, he is at high risk for recurrent thromboembolism, given his history of recurrent, unprovoked DVTs; however, he is past the highest risk period (VTE within the past 3 months; his last one was 9 months ago). He is otherwise healthy and has normal renal function, so his apixaban should be withheld for a total of 4 doses (48 hours) prior to his procedure. He should resume his full dose of apixaban 48 to 72 hours after his procedure to minimize the risk for bleeding.
However, given that a TKA is a procedure associated with a high rate of postoperative VTE, initiate prophylactic anticoagulation (such as enoxaparin 40 mg subcutaneously daily or apixaban 2.5 mg PO bid) about 12 hours after the procedure and continue it until full-dose apixaban is resumed.
CORRESPONDENCE
Jeremy Vandiver, PharmD, BCPS, University of Wyoming School of Pharmacy, 1000 E. University Ave., Dept. 3375, Laramie, WY 82071; jvandive@uwyo.edu.
1. Connelly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
2. Steinberg BA, Kim S, Piccini JP, et al. Use and associated risks of concomitant aspirin therapy with oral anticoagulation in patients with atrial fibrillation: insights from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Registry. Circulation. 2013;128:721-728.
3. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e326S-e350S.
4. Adam SS, McDuffie JR, Ortel TL, et al. Comparative effectiveness of warfarin and newer oral anticoagulants for the long-term prevention and treatment of arterial and venous thromboembolism. Department of Veteran Affairs Evidence-Based Synthesis Project #09-010; 2012. Available at: https://www.ncbi.nlm.nih.gov/books/NBK97541/. Accessed October 15, 2017.
5. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and Expert Panel Report. Chest. 2016;149:315-352.
6. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. J Am Coll Cardiol. 2014;64:2246-2280.
7. Centers for Disease Control and Prevention. Venous thromboembolism in adult hospitalizations — United States, 2007-2009. MMWR Morb Mortal Wkly Rep. 2012 June 8;61:401-404. Available at https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6122a1.htm. Accessed October 15, 2017.
8. Anderson FA, Wheeler HB, Goldberg HJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med. 1991;151:933-938.
9. Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012): The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2012;33:2451-2496.
10. Garwood CL, Korkis B, Grande D, et al. Anticoagulation bridge therapy in patients with atrial fibrillation: recent updates provide a rebalance of risk and benefit. Pharmacotherapy. 2017;37:712-714.
11. Doherty JU, Gluckman TJ, Hucker WJ, et al. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2017;69:871-898.
12. Douketis J, Tosetto A, Marcucci M, et al. Patient-level meta-analysis: effect of measurement timing, threshold, and patient age on ability of D-dimer testing to assess recurrence risk after unprovoked venous thromboembolism. Ann Intern Med. 2010;153:523-531.
13. Becattini C, Agnelli G, Schenone A, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med. 2012;366:1959-1967.
14. Tosetto A, Testa S, Martinelli I, et al. External validation of the DASH prediction rule: a retrospective cohort study. J Thromb Haemost. 2017;15:1963-1970.
15. Rodger MA, Le Gal G, Anderson DR, et al. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. BMJ. 2017;356:j1065.
16. Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism.
17. Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines, 8th ed. Chest. 2008;133:381S-453S.
18. Spyropoulos AC, Douketis JD. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood. 2012;120:2954-2962.
19. Eisen GM, Baron TH, Dominitz JA, et al. Guideline on the management of anticoagulation and antiplatelet therapy for endoscopic procedures. Gastrointest Endosc. 2002;55:775-779.
20. Burnett AE, Mahan CE, Vazquez SR. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis. 2016;41:206-232.
21. Witt DM, Clark NP, Kaatz S, et al. Guidance for the practical management of warfarin therapy in the treatment of venous thromboembolism. J Thromb Thrombolysis. 2016;41:187-205.
22. Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823-833.
23. Steinberg BA, Peterson ED, Kim S, et al. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circulation. 2015;131: 488-494.
24. Clark NP, Witt DM, Davies LE, et al. Bleeding, recurrent venous thromboembolism, and mortality risks during warfarin interruption for invasive procedures. JAMA Intern Med. 2015;175;1163-1168.
25. Sjögren V, Grzymala-Lubanski B, Renlund H, et al. Safety and efficacy of bridging with low-molecular-weight heparin during temporary interruptions of warfarin: a register-based cohort study. Clin Appl Thromb Hemost. 2017;23:961-966.
26. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893-2962.
27. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e278S-e325S.
1. Connelly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
2. Steinberg BA, Kim S, Piccini JP, et al. Use and associated risks of concomitant aspirin therapy with oral anticoagulation in patients with atrial fibrillation: insights from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Registry. Circulation. 2013;128:721-728.
3. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e326S-e350S.
4. Adam SS, McDuffie JR, Ortel TL, et al. Comparative effectiveness of warfarin and newer oral anticoagulants for the long-term prevention and treatment of arterial and venous thromboembolism. Department of Veteran Affairs Evidence-Based Synthesis Project #09-010; 2012. Available at: https://www.ncbi.nlm.nih.gov/books/NBK97541/. Accessed October 15, 2017.
5. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and Expert Panel Report. Chest. 2016;149:315-352.
6. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. J Am Coll Cardiol. 2014;64:2246-2280.
7. Centers for Disease Control and Prevention. Venous thromboembolism in adult hospitalizations — United States, 2007-2009. MMWR Morb Mortal Wkly Rep. 2012 June 8;61:401-404. Available at https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6122a1.htm. Accessed October 15, 2017.
8. Anderson FA, Wheeler HB, Goldberg HJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med. 1991;151:933-938.
9. Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012): The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2012;33:2451-2496.
10. Garwood CL, Korkis B, Grande D, et al. Anticoagulation bridge therapy in patients with atrial fibrillation: recent updates provide a rebalance of risk and benefit. Pharmacotherapy. 2017;37:712-714.
11. Doherty JU, Gluckman TJ, Hucker WJ, et al. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2017;69:871-898.
12. Douketis J, Tosetto A, Marcucci M, et al. Patient-level meta-analysis: effect of measurement timing, threshold, and patient age on ability of D-dimer testing to assess recurrence risk after unprovoked venous thromboembolism. Ann Intern Med. 2010;153:523-531.
13. Becattini C, Agnelli G, Schenone A, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med. 2012;366:1959-1967.
14. Tosetto A, Testa S, Martinelli I, et al. External validation of the DASH prediction rule: a retrospective cohort study. J Thromb Haemost. 2017;15:1963-1970.
15. Rodger MA, Le Gal G, Anderson DR, et al. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. BMJ. 2017;356:j1065.
16. Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism.
17. Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines, 8th ed. Chest. 2008;133:381S-453S.
18. Spyropoulos AC, Douketis JD. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood. 2012;120:2954-2962.
19. Eisen GM, Baron TH, Dominitz JA, et al. Guideline on the management of anticoagulation and antiplatelet therapy for endoscopic procedures. Gastrointest Endosc. 2002;55:775-779.
20. Burnett AE, Mahan CE, Vazquez SR. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis. 2016;41:206-232.
21. Witt DM, Clark NP, Kaatz S, et al. Guidance for the practical management of warfarin therapy in the treatment of venous thromboembolism. J Thromb Thrombolysis. 2016;41:187-205.
22. Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823-833.
23. Steinberg BA, Peterson ED, Kim S, et al. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circulation. 2015;131: 488-494.
24. Clark NP, Witt DM, Davies LE, et al. Bleeding, recurrent venous thromboembolism, and mortality risks during warfarin interruption for invasive procedures. JAMA Intern Med. 2015;175;1163-1168.
25. Sjögren V, Grzymala-Lubanski B, Renlund H, et al. Safety and efficacy of bridging with low-molecular-weight heparin during temporary interruptions of warfarin: a register-based cohort study. Clin Appl Thromb Hemost. 2017;23:961-966.
26. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893-2962.
27. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e278S-e325S.
PRACTICE RECOMMENDATIONS
› Don’t stop oral anticoagulation for procedures with minimal bleeding risk, such as minor dermatologic, dental, or ophthalmic procedures. C
› Reserve periprocedural bridging with a parenteral anticoagulant for those patients on warfarin who are at highest risk for thromboembolism (those with severe thrombophilia, active thrombosis, or mechanical heart valves). B
› Stop direct oral anticoagulants 24 to 48 hours prior to most invasive procedures, and do not employ periprocedural bridging. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series