User login
It’s time to rethink your approach to C diff infection
CASE 1
Beth O, a 63-year-old woman, presents to the emergency department (ED) with a 2-week history of diarrhea (6 very loose, watery stools per day) and lower abdominal pain. The patient denies any vomiting, sick contacts, or recent travel. Past medical history includes varicose veins. Her only active medication is loperamide, as needed, for the past 2 weeks. Ms. O also recently completed a 10-day course of clindamycin for an infected laceration on her finger.
Ms. O’s laboratory values are unremarkable, with a normal white blood cell (WBC) count and serum creatinine (SCr) level. Abdominal computed tomography (CT) reveals some abnormal bowel dilatation and a slight increase in colon wall thickness. There is a high suspicion for Clostridioides difficile (formerly Clostridium difficile) infection (CDI), and stool sent for polymerase chain reaction (PCR) testing comes back positive for C difficile toxin B. It is revealed to be a strain other than the BI/NAP1/027 epidemic strain (which has a higher mortality rate).
How should this patient be treated?
CASE 2
Sixty-eight-year-old Barbara Z presents to the ED from her skilled nursing facility with persistent diarrhea and abdominal cramping. She was diagnosed with CDI about 2 months ago and reports that her symptoms resolved within 4 to 5 days after starting a 14-day course of oral metronidazole.
Her past medical history is notable for multiple myeloma with bone metastasis, for which she is actively undergoing chemotherapy treatment. She also has chronic kidney disease (baseline SCr, 2.2 mg/dL), hypertension, and anemia of chronic disease. The patient’s medications include amlodipine and cholecalciferol. Her chemotherapy regimen consists of bortezomib, lenalidomide, and dexamethasone. CT of the abdomen shows diffuse colon wall thickening with surrounding inflammatory stranding—concerning for pancolitis. There is no evidence of toxic megacolon or ileus.
Ms. Z’s laboratory values are notable for a WBC count of 15,900 cells/mL and an SCr of 4.1 mg/dL. She is started on oral levofloxacin and metronidazole due to concern for an intra-abdominal infection. PCR testing is positive for C difficile, and an enzyme immunoassay (EIA) for C difficile toxin is positive.
What factors put Ms. Z at risk for C difficile, and how should she be treated?
Continue to: C difficile is one of the most...
C difficile is one of the most commonly reported pathogens in health care–associated infections and affects almost 1% of all hospitalized patients in the United States each year.1 From 2001 to 2010, the incidence of CDI doubled in patients discharged from hospitals,2 with an estimated cost of more than $5 billion annually.3 Furthermore, rates of community-associated CDI continue to increase and account for about 40% of cases.4
After colonization in the intestine, C difficile releases 2 toxins (TcdA and TcdB) that cause colitis.5 Patients may present with mild diarrhea that can progress to abdominal pain, cramping, fever, and leukocytosis. Fulminant CDI can lead to the formation of pseudomembranes in the colon, toxic megacolon, bowel perforation, shock, and death.2
Beginning in the early 2000s, hospitals reported increases in severe cases of CDI.6 A specific strain known as BI/NAP1/027 was identified and characterized by fluoroquinolone resistance, increased spore formation, and a higher mortality rate.6
Further complicating matters … Recurrent CDI occurs in up to 10% to 30% of patients,7 typically within 14 to 45 days of completion of antibiotic pharmacotherapy for CDI.8 Recurrence is characterized by new-onset diarrhea or abdominal symptoms after completion of treatment for CDI.5
It typically begins with an antibiotic
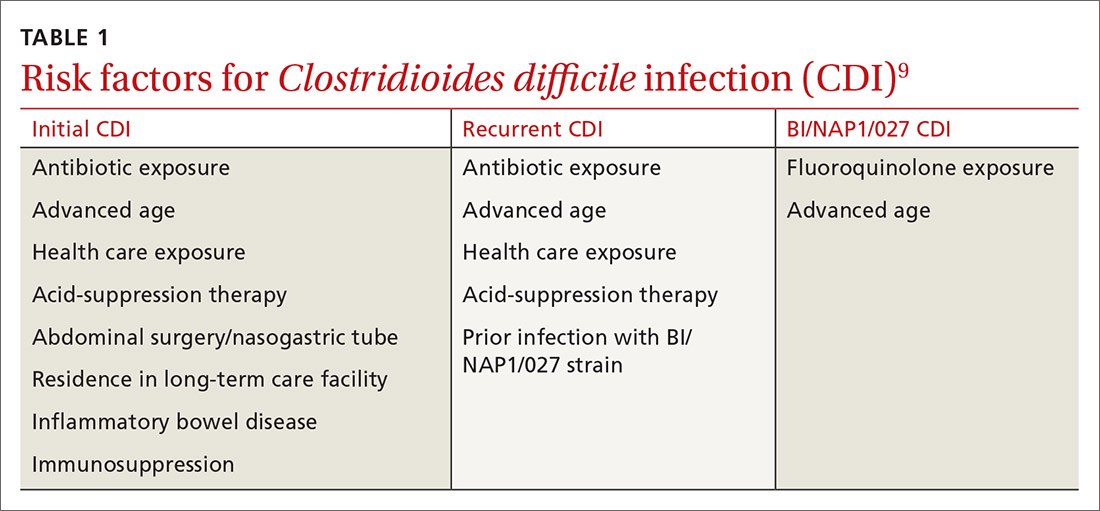
Risk factors for CDI are listed in TABLE 1.9 The most important modifiable risk factor for initial and recurrent CDI is recent use of antibiotics.10 Most antibiotics can disrupt normal intestinal flora, causing colonization of C difficile, but the strongest association seems to be with third- and fourth-generation cephalosporins, fluoroquinolones, carbapenems, and clindamycin.11 The risk for CDI occurs during antibiotic treatment, as well as up to 3 months after completion of antibiotic therapy.7 Exposure to multiple antibiotics and extended duration of antibacterial therapy can greatly increase the risk for CDI, so antimicrobial stewardship is key.11
Continue to: Continuing antibiotics while attempting...
Continuing antibiotics while attempting to treat CDI reduces the patient’s clinical response to CDI treatment, which can lead to recurrence.12 The Infectious Diseases Society of America (IDSA) guidelines include a strong recommendation to discontinue concurrent antibiotics as soon as possible in these scenarios.11
Acid-suppression therapy has also been associated with CDI. The mechanism is thought to be an interruption in the protection provided by stomach acid, and use over time may reduce the diversity of flora within the gut microbiome.13 The data demonstrating an association between acid-suppression therapy and CDI is conflicting, which may be a result of confounding factors such as the severity of CDI illness and diarrhea induced by use of proton pump inhibitors (PPIs).4 IDSA guidelines do not provide a recommendation regarding discontinuation of PPI therapy for the prevention of CDI, although inappropriate PPI therapy should always be discontinued.11
Advanced age is an important nonmodifiable risk factor for CDI. Older adults who live in long-term care facilities are at a higher risk for CDI, and these facilities have colonization rates as high as 50%.12
Community-associated risk. In an analysis of community-associated cases of CDI, 82% of patients reported some sort of health care exposure (ranging from physician office visit to surgery admission), 64% reported the receipt of antimicrobial therapy, and 31% reported the use of PPIs.14 Inflammatory bowel disease (IBD) may also put community dwellers at higher risk for CDI and its complications.15
CASES 1 & 2
Both CASE patients have risk factors for CDI. Ms. O (CASE 1) is likely at risk for CDI after completion of her recent course of clindamycin. Ms. Z (CASE 2) has several risk factors for recurrent CDI, including advanced age (≥ 65 years), residence in a long-term care facility, prior antibiotic exposure, and immunodeficiency because of chemotherapy/steroid use.
Continue to: Diagnosis
Diagnosis: Who and how to test
CDI should be both a clinical and laboratory-confirmed diagnosis. Patients should be tested for CDI if they have 3 or more episodes of unexplainable, new-onset unformed stools in 24 hours.11 Asymptomatic patients should not be tested to avoid unnecessary testing and treatment of those who are colonized but not infected.11 It is not recommended to routinely test patients who have taken laxatives within the previous 48 hours.11
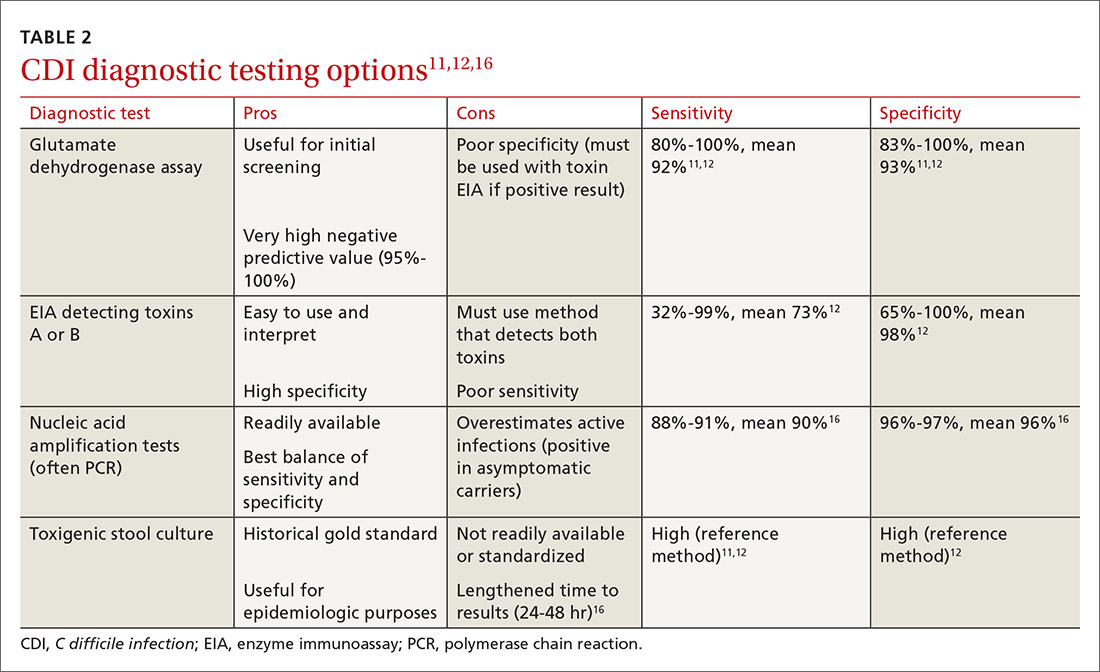
There are several stool-based laboratory test options for the diagnosis of CDI (TABLE 211,12,16) but no definitive recommendation for all institutions.11 Many institutions have now implemented PCR testing for the diagnosis of CDI. However, while the benefits of this test include reduced need for repeat testing and possible identification of carriers, it’s estimated that reports of CDI increase more than 50% when an institution switches to PCR testing.1 Nonetheless, a one-step, highly sensitive test such as PCR may be used if strict criteria are implemented and followed.
The increase in positive PCR tests has prompted evaluation of using another test in addition to or in place of PCR. Multistep testing options include a glutamate dehydrogenase assay (GDH) with a toxin EIA, GDH with a toxin EIA and final decision via PCR, or PCR with toxin EIA.11 Use of a multistep diagnostic algorithm may increase overall specificity up to 100%, which may improve determination of asymptomatic colonization vs active infection.16 (Patients who have negative toxin results with positive PCR likely have colonization but not infection and often do not require treatment.) IDSA guidelines recommend that the stool toxin test should be part of a multistep algorithm for diagnosis, rather than PCR alone, if strict criteria are not implemented for stool test submission.11
There is no need to perform a test of cure after a patient has been treated for CDI, and no repeat testing should be performed within 7 days of the previous test.11 After successful treatment, patients will continue to shed spores and test positively via PCR for weeks to months.11 When patients have a positive PCR test, there are several important infection control efforts that institutions should consider; see “IDSA weighs in on measures to combat C difficile.”
SIDEBAR
IDSA weighs in on measures to combat C difficile
The spores produced by Clostridioides difficile can survive for 5 months or longer on dry surfaces because of resistance to heat, acid, antibiotics, and many cleaning products.38 Unfortunately, spores transmitted from health care workers and the environment are the most likely cause of infection spreading in health care institutions. To prevent transmission of C difficile infection (CDI) throughout institutions, appropriate infection control measures are necessary.
Clinical practice guidelines from the Infectious Diseases Society of America (IDSA) recommend that patients with CDI be isolated to a private room with a dedicated toilet. Health care staff should wear gloves and gowns when entering the room of, or taking care of, a patient with CDI. For patients who are suspected of having CDI, contact precautions should be implemented while awaiting test results. When the diagnosis is confirmed, contact precautions should remain in place for at least 48 hours after resolution of diarrhea but may be continued until discharge.11
Practicing good hand hygiene is essential, especially in institutions with high rates of CDI or if fecal contamination is likely.11 Hand hygiene with soap and water is preferred, due to evidence of a higher spore removal rate, but alcohol-based alternatives may be used if necessary.11 In institutions with high rates of CDI, terminal (post-discharge) cleaning of rooms with a sporicidal agent should be considered.11
Asymptomatic carriers are also a concern for transmission of CDI in institutional settings. Screening and isolating patients who are carriers may prevent transmission, and some institutions have implemented this process to reduce the risk for CDI that originates in a health care facility.39 The IDSA guidelines do not make a recommendation regarding screening or isolation of asymptomatic carriers, so the decision is institution specific.11 These guidelines also recommend that patients presenting with similar infectious organisms be housed in the same room, if needed, to avoid cross-contamination to others or additional surfaces.11
For pediatric patients, testing recommendations vary by age. Testing is not generally recommended for neonates or infants ≤ 2 years of age with diarrhea because of the prevalence of colonization with C difficile.11 For children older than 2 years, testing for CDI is only recommended in the setting of prolonged or worsening diarrhea and if the patient has risk factors such as IBD, immunocompromised state, health care exposure, or recent antibiotic use.11 In addition, testing in this population should only be considered once other infectious and noninfectious causes of diarrhea have been excluded.11
Continue to: First-line treatment? Drug of choice has changed
First-line treatment? Drug of choice has changed
In 2018, the IDSA published new treatment guidelines that provide important updates from the 2010 guidelines.11 Chief among these was the elimination of metronidazole as a first-line therapy. Vancomycin or fidaxomicin are now recommended as first-line treatment options because of superior eradication of C difficile when compared with metronidazole.11 In the opinion of the authors, vancomycin should be considered the drug of choice because of cost. (See “The case for vancomycin.”)
SIDEBAR
The case for vancomycin
The majority of studies conducted prior to publication of the 2010 Infectious Diseases Society of America guidelines described numerically worse eradication rates of Clostridioides difficile infection (CDI) with metronidazole compared with vancomycin for all severities of infection, but statistical significance was not achieved. These studies also showed a nonsignificant increase in CDI recurrence with metronidazole.17,40,41
A 2005 systematic review demonstrated increased treatment failure rates with metronidazole.42 The rates of metronidazole discontinuation and transition to alternative options more than doubled in 2003-2004, to 25.7% of patients compared with 9.6% in earlier years.42 Metronidazole efficacy was further questioned in a prospective observational study conducted in 2005, in which only 50% of patients were cured after an initial course of treatment, while 28% had recurrence within 90 days.43
Vancomycin was found to be the superior treatment option to metronidazole and tolevamer in a 2014 randomized controlled trial.18 This study also demonstrated that vancomycin was the superior therapy when comparing treatment-naïve vs experienced patients and severity of CDI.18 A 2017 retrospective cohort study demonstrated decreased 30-day all-cause mortality for patients taking vancomycin vs metronidazole (adjusted relative risk = 0.86; 95% confidence interval, 0.74-0.98), although it should be noted that this difference was driven by those with severe CDI, and there was no statistically significant difference in mortality for patients with mild-to-moderate CDI.44
The results of these studies led to the recommendation of vancomycin over metronidazole as first-line pharmacotherapy for CDI in practice, despite the historical perspective that overutilization of oral vancomycin could potentially increase rates of vancomycinresistant Enterococcus.11
Metronidazole should only be used in the treatment of CDI as a lastresort medication because of cost or insurance coverage. Although the price of oral vancomycin is higher, favorable patient outcomes are substantially greater, and recent analyses have shown that vancomycin is actually more cost-effective than metronidazole as a result.24 Adverse effects for metronidazole include neurotoxicity, gastrointestinal discomfort, and disulfiram-like reaction.
Vancomycin does not harbor as many adverse effects because of extremely low systemic absorption when taken orally, but patients may experience gastrointestinal discomfort.45 While systemic exposure with oral administration of vancomycin is very low (< 1%), there have been case reports of nephrotoxicity and “red man syndrome” that are more typically seen with intravenous vancomycin.44
Given the low rate of systemic exposure, routine monitoring of renal function and serum drug levels is not usually necessary during oral vancomycin therapy. However, it may be appropriate to monitor renal function and serum levels of vancomycin in patients who have renal failure, have altered intestinal integrity, are age ≥ 65 years, or are receiving high doses of vancomycin.46
10-day vs 14-day treatment of CDI. Most studies for the treatment of CDI have used a 10-day regimen rather than increasing the duration to a 14-day regimen, and nearly all studies conducted have displayed high rates of symptom resolution at the end of 10 days of treatment.17,18 Thus, treatment duration beyond 10 days should only be considered for patients who continue to have symptoms or complications with CDI on Day 10 of treatment.
First recurrence. Metronidazole is no longer the recommended treatment for first recurrence of CDI treated initially with metronidazole; instead, a 10-day course of vancomycin should be used.11 For recurrent cases in patients initially treated with vancomycin, a tapered and pulsed regimen of vancomycin is recommended11:
- vancomycin PO 125 mg four times daily for 10 to 14 days followed by
- vancomycin PO 125 mg twice daily for 7 days, then
- vancomycin PO 125 mg once daily for 7 days, then
- vancomycin PO 125 mg every 2 to 3 days for 2 to 8 weeks.
Pediatric patients. The IDSA guidelines recommend use of metronidazole or vancomycin to treat an initial case or first recurrence of mild-to-moderate CDI in this population.11 Due to a lack of quality evidence, the drug of choice for initial treatment is inconclusive, so patient-specific factors and cost should be considered when choosing an agent.11 If not cost prohibitive, vancomycin should be the drug of choice for most cases of pediatric CDI, and for severe cases or multiple recurrences of CDI, vancomycin is clearly the drug of choice.
Recommended agents: A closer look
Oral vancomycin products. Vancocin, a capsule, and Firvanq, an oral solution, are 2 vancomycin products currently on the market for CDI. Although the capsules are a readily available treatment option, the cost of the full course of treatment can be a barrier for patients without insurance, or with high copays or deductibles (brand name, $4000; generic, $1252).19
Continue to: Historically, in an effort to keep costs down...
Historically, in an effort to keep costs down, an oral solution was often inexpensively compounded at hospitals or pharmacies.20
Fidaxomicin, an oral macrocyclic antibiotic with minimal systemic absorption, was first approved by the US Food and Drug Administration (FDA) for CDI in 2011.21 The IDSA guidelines recommend fidaxomicin for initial, and recurrent, cases of CDI as an alternative to vancomycin.11 This recommendation is based on 2 randomized double-blind trials comparing fidaxomicin to standard-dose oral vancomycin for initial or recurrent CDI.21,22
Pooled data from these 2 similar studies found that fidaxomicin was noninferior (10% noninferiority margin) to vancomycin for the primary outcome of clinical cure.23 Fidaxomicin was shown to be superior to vancomycin regarding rate of CDI recurrence (relative risk [RR] = 0.61; 95% confidence interval [CI], 0.43-0.87). These results were similar regardless of whether the CDI was an initial or recurrent case.23
Given the lack of systemic absorption, fidaxomicin is generally very well tolerated. The largest downside to fidaxomicin is its cost, which can be nearly $5000 for a standard 10-day course (vs as little as $165 for oral vancomycin).19 As a result, oral vancomycin solution is likely the most cost-effective therapy for initial cases of CDI.24 In patients with poor medication adherence, fidaxomicin offers the advantage of less-frequent dosing (twice daily vs 4 times daily with vancomycin).
For cases of recurrent CDI, when treatment failure occurred with vancomycin, fidaxomicin should be considered as an efficacious alternative. If fidaxomicin is used, it is advisable to verify coverage with the patient’s insurance plan, since prior authorization is frequently required.
Continue to: When meds fail, consider a fecal microbiota transplant
When meds fail, consider a fecal microbiota transplant
Another important change in the IDSA guidelines for CDI management is the strong recommendation for fecal microbiota transplantation (FMT) in patients with multiple recurrences of CDI for whom appropriate antibiotic treatment courses have failed.11,25 The goal of FMT is to “normalize” an abnormal gut microbiome by transplanting donor stool into a recipient.26
FMT has been shown to be highly effective in 5 randomized clinical trials conducted since 2013, with CDI cure rates between 85% and 94%.11 This rate of cure is particularly impressive given that the studies only included patients with refractory CDI.
Patients with recurrent CDI who may be candidates for FMT should be referred to a center or specialist with experience in FMT. These transplants can be expensive because of the screening process involved in obtaining donor samples. (Historically, a single FMT has cost $3000-$5000, and it is seldom covered by insurance.27) The emergence of universal stool banks offers a streamlined solution to this process.26
Fresh or frozen stool is considered equally effective in treating refractory CDI.26 Oral capsule and freeze-dried stool formulations have been studied, but their use is considered investigational at this time.26
Delivery via colonoscopy to the right colon is the preferred route of infusion; however, delivery via enema or nasogastric, nasojejunal, or nasoduodenal infusion can be considered as well.26
Continue to: In preparing for stool transplantation...
In preparing for stool transplantation, patients should be treated with standard doses of oral vancomycin or fidaxomicin for 3 days before the procedure to suppress intestinal C difficile, and the last dose of antibiotics should be given 12 to 48 hours before the procedure.26 Bowel lavage with polyethylene glycol is recommended, regardless of whether stool is delivered via colonoscopy or upper GI route.
Short-term adverse events associated with FMT appear to be minimal; data is lacking for long-term safety outcomes.28 While only recommended currently for cases of recurrent CDI, there is promising data emerging for use of FMT for severe cases, even without recurrence.29
The role of probiotics remains unclear
Probiotics have been explored in numerous trials to determine if they are effective in preventing CDI in patients who have been prescribed antibiotics.11 While no randomized trials have conclusively shown benefit, several meta-analyses have shown that the use of probiotics may result in a 60% to 65% relative risk reduction in CDI incidence.30,31
One proviso to these meta-analyses is that the incorporated studies have typically included patients at very high risk for CDI, and subanalyses have only found a reduction in CDI incidence when patients are at a very high baseline risk. In addition, there are many differences in probiotic types, formulations, treatment durations, and follow-up. As a result, the IDSA guidelines state that there is “insufficient data at this time” to recommend routine administration of probiotics for either primary or secondary CDI prophylaxis.11
Due to insufficient high-quality data, the IDSA guidelines do not provide a recommendation regarding use as an adjunct treatment option for acute CDI.11 Probiotics should not be routinely used to prevent CDI; however, they may provide benefit if reserved for patients at the highest risk for CDI (eg, history of CDI, prolonged use of broad-spectrum antibiotics, high local incidence).
Continue to: What about surgical intervention?
What about surgical intervention?
In severe cases of CDI, surgery may be necessary and can reduce mortality.32 The surgical procedure with the strongest recommendation in the IDSA guidelines is the subtotal colectomy, though the diverting loop ileostomy is an alternative option.11 Patients who may benefit from surgery include those with a WBC count ≥ 25,000; lactate > 5 mmol/L11; altered mental status; megacolon; perforation of the colon; acute abdomen on physical examination; or septic shock due to CDI.33 Although surgery can be beneficial, the mortality rate remains high for those with CDI who undergo colectomy.33
Reserve bezlotoxumab for prevention of recurrence
Bezlotoxumab, a human monoclonal immunoglobulin GI/kappa antibody, was approved by the FDA in 2016 for the prevention of recurrent CDI. Its mechanism of action is to bind and neutralize C difficile toxin B. It was approved as a single infusion for adults who are receiving active antibiotic therapy for CDI and are considered to be at high risk for recurrence.34
This approval was based on 2 trials of more than 2500 patients, in which participants received bezlotoxumab or placebo while receiving treatment for primary or recurrent CDI. The primary outcome of these studies was recurrent infection within 12 weeks after infusion, which was significantly lower for bezlotoxumab in both studies: 17% vs 28% (P < 0.001) in one trial and 16% vs 26% (P < 0.001) in the other trial.35
Bezlotoxumab should only be used as an adjunct to prevent recurrence.32 There is no recommendation for or against bezlotoxumab in the IDSA guidelines because of the recent date of the drug’s approval. Its frequency of use will likely depend on the number of patients who meet criteria as high risk for recurrence and its estimated cost of $4560 per dose.34,36
CASES
CASE 1: In light of Ms. O’s recent completion of a course of clindamycin and unremarkable lab work, she should be treated for mild-to-moderate CDI. She has no comorbid conditions to warrant fidaxomicin, and thus vancomycin (capsules or oral solution) would be the best treatment option. Ms. O is started on vancomycin PO 125 mg qid for 10 days. She is also advised to discontinue loperamide as soon as possible, based on poor outcomes data seen with the use of antimotility agents in CDI.37
Continue to: CASE 2
CASE 2: Ms. Z has several risk factors for recurrent CDI and has an elevated WBC count and SCr level (WBC ≥ 15,000 and SCr > 1.5 mg/dL). Thus, she is classified as having severe, recurrent CDI. Oral levofloxacin and metronidazole should be discontinued, because they increase the risk for treatment failure and development of more virulent CDI strains, such as BI/NAP1/027. Since Ms. Z used metronidazole for treatment of her initial CDI, vancomycin or fidaxomicin should be used at this time. Either vancomycin PO 125 mg qid for 10 days or fidaxomicin 200 mg bid for 10 days would be an appropriate regimen; however, because of cost and unknown insurance coverage, vancomycin is the most appropriate regimen.
CORRESPONDENCE
Jeremy Vandiver, PharmD, BCPS, University of Wyoming School of Pharmacy, Saint Joseph Family Medicine Residency, 1000 E. University Avenue, Dept 3375, Laramie, WY 82071; jvandive@uwyo.edu
1. Polage CR, Gyorke CE, Kennedy MA, et al. Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern Med. 2015;175:1792-1801.
2. Reveles KR, Lee GC, Boyd NK, et al. The rise in Clostridium difficile infection incidence among hospitalized adults in the United States: 2001-2010. Am J Infect Control. 2014;42:1028-1032.
3. Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(suppl 2):S88-S92.
4. Tariq R, Singh S, Gupta A, et al. Association of gastric acid suppression with recurrent Clostridium difficile infection: a systematic review and meta-analysis. JAMA Intern Med. 2017;177:784-791.
5. Kachrimanidou M, Malisiovas N. Clostridium difficile infection: a comprehensive review. Crit Rev Microbiol. 2011;37:178-187.
6. O’Connor JR, Johnson S, Gerding DN. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology. 2009;136:1913-1924.
7. Kelly CP. A 76-year-old man with recurrent Clostridium difficile-associated diarrhea: review of C difficile infection. JAMA. 2009;301:954-962.
8. Cornely OA, Miller MA, Louie TJ, et al. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55(suppl 2):S154-S161.
9. Napolitano LM, Edmiston CE Jr. Clostridium difficile disease: diagnosis, pathogenesis, and treatment update. Surgery 2017;162:325-348.
10. Deshpande A, Pasupuleti V, Thota P, et al. Risk factors for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2015;36:452-460.
11. McDonald LC, Gerding DN, Johnson S, et al. Clinical Practice Guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66:e1-e48.
12. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498; quiz 499.
13. Seto CT, Jeraldo P, Orenstein R, et al. Prolonged use of a proton pump inhibitor reduces microbial diversity: implications for Clostridium difficile susceptibility. Microbiome. 2014;2:42.
14. Chitnis AS, Holzbauer SM, Belflower RM, et al. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med. 2013;173:1359-1367.
15. Negrón ME, Rezaie A, Barkema HW, et al. Ulcerative colitis patients with Clostridium difficile are at increased risk of death, colectomy, and postoperative complications: a population-based inception cohort study. Am J Gastroenterol. 2016;111:691-704.
16. Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA. 2015;313:398-408.
17. Zar FA, Bakkanagari SR, Moorthi KM, et al. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302-307.
18. Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59:345-354.
19. Vancomycin: product details. Redbook Online. www.micromedexsolutions.com. Published 2018. Accessed June 13, 2020.
20. Mergenhagen KA, Wojciechowski AL, Paladino JA. A review of the economics of treating Clostridium difficile infection. Pharmacoeconomics. 2014;32:639-650.
21. Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422-431.
22. Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12:281-289.
23. Crook DW, Walker AS, Kean Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clin Infect Dis. 2012;55 suppl 2:S93-103.
24. Ford DC, Schroeder MC, Ince D, et al. Cost-effectiveness analysis of initial treatment strategies for mild-to-moderate Clostridium difficile infection in hospitalized patients. Am J Health Syst Pharm. 2018;75:1110-1121.
25. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431-455.
26. Panchal P, Budree S, Scheeler A, et al. Scaling safe access to fecal microbiota transplantation: past, present, and future. Curr Gastroenterol Rep. 2018;20:14.
27. Arbel LT, Hsu E, McNally K. Cost-effectiveness of fecal microbiota transplantation in the treatment of recurrent Clostridium difficile infection: a literature review. Cureus. 2017;9:e1599.
28. Cammarota G, Ianiro G, Tilg H, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569-580.
29. Hocquart M, Lagier JC, Cassir N, et al. Early fecal microbiota transplantation improves survival in severe Clostridium difficile infections. Clin Infect Dis. 2018;66:645-650.
30. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
31. Johnston BC, Lytvyn L, Lo CK, et al. Microbial preparations (probiotics) for the prevention of Clostridium difficile infection in adults and children: an individual patient data meta-analysis of 6,851 participants. Infect Control Hosp Epidemiol. 2018:1-11.
32. Stewart DB, Hollenbeak CS, Wilson MZ. Is colectomy for fulminant Clostridium difficile colitis life saving? A systematic review. Colorectal Dis. 2013;15:798-804.
33. Julien M, Wild JL, Blansfield J, et al. Severe complicated Clostridium difficile infection: can the UPMC proposed scoring system predict the need for surgery? J Trauma Acute Care Surg. 2016;81:221-228.
34. Merck & Co, Inc. Sharp M. ZinplavaTM (bezlotoxumab [package insert] US Food and Drug Administration Web site. www.accessdata.fda.gov/drugsatfda_docs/label/2016/761046s000lbl.pdf. Revised October 2016. Accessed May 29, 2020.
35. Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017;376:305-317.
36. Chahine EB, Cho JC, Worley MV. Bezlotoxumab for the Prevention of Clostridium difficile recurrence. Consult Pharm. 2018;33:89-97.
37. Koo HL, Koo DC, Musher DM, et al. Antimotility agents for the treatment of Clostridium difficile diarrhea and colitis. Clin Infect Dis. 2009;48:598-605.
38. Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7:526-536.
39. Longtin Y, Paquet-Bolduc B, Gilca R, et al. Effect of detecting and isolating Clostridium difficile carriers at hospital admission on the incidence of C difficile infections: a quasi-experimental controlled study. JAMA Intern Med. 2016;176:796-804.
40. Teasley DG, Gerding DN, Olson MM, et al. Prospective randomised trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhoea and colitis. Lancet. 1983;2:1043-1046.
41. Wenisch C, Parschalk B, Hasenhündl M, et al. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis. 1996;22:813-818.
42. Pepin J, Alary ME, Valiquette L, et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40:1591-1597.
43. Musher DM, Aslam S, Logan N, et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005;40:1586-1590.
44. Stevens VW, Nelson RE, Schwab-Daugherty EM, et al. Comparative effectiveness of vancomycin and metronidazole for the prevention of recurrence and death in patients with Clostridium difficile infection. JAMA Intern Med. 2017;177:546-553.
45. CutisPharma. FirvanqTM (vancomycin hydrochloride) for oral solution [package insert]. US Food and Drug Administration Web site. www.accessdata.fda.gov/drugsatfda_docs/label/2018/208910s000lbl.pdf. Revised January 2018. Accessed May 29, 2020.
46.
CASE 1
Beth O, a 63-year-old woman, presents to the emergency department (ED) with a 2-week history of diarrhea (6 very loose, watery stools per day) and lower abdominal pain. The patient denies any vomiting, sick contacts, or recent travel. Past medical history includes varicose veins. Her only active medication is loperamide, as needed, for the past 2 weeks. Ms. O also recently completed a 10-day course of clindamycin for an infected laceration on her finger.
Ms. O’s laboratory values are unremarkable, with a normal white blood cell (WBC) count and serum creatinine (SCr) level. Abdominal computed tomography (CT) reveals some abnormal bowel dilatation and a slight increase in colon wall thickness. There is a high suspicion for Clostridioides difficile (formerly Clostridium difficile) infection (CDI), and stool sent for polymerase chain reaction (PCR) testing comes back positive for C difficile toxin B. It is revealed to be a strain other than the BI/NAP1/027 epidemic strain (which has a higher mortality rate).
How should this patient be treated?
CASE 2
Sixty-eight-year-old Barbara Z presents to the ED from her skilled nursing facility with persistent diarrhea and abdominal cramping. She was diagnosed with CDI about 2 months ago and reports that her symptoms resolved within 4 to 5 days after starting a 14-day course of oral metronidazole.
Her past medical history is notable for multiple myeloma with bone metastasis, for which she is actively undergoing chemotherapy treatment. She also has chronic kidney disease (baseline SCr, 2.2 mg/dL), hypertension, and anemia of chronic disease. The patient’s medications include amlodipine and cholecalciferol. Her chemotherapy regimen consists of bortezomib, lenalidomide, and dexamethasone. CT of the abdomen shows diffuse colon wall thickening with surrounding inflammatory stranding—concerning for pancolitis. There is no evidence of toxic megacolon or ileus.
Ms. Z’s laboratory values are notable for a WBC count of 15,900 cells/mL and an SCr of 4.1 mg/dL. She is started on oral levofloxacin and metronidazole due to concern for an intra-abdominal infection. PCR testing is positive for C difficile, and an enzyme immunoassay (EIA) for C difficile toxin is positive.
What factors put Ms. Z at risk for C difficile, and how should she be treated?
Continue to: C difficile is one of the most...
C difficile is one of the most commonly reported pathogens in health care–associated infections and affects almost 1% of all hospitalized patients in the United States each year.1 From 2001 to 2010, the incidence of CDI doubled in patients discharged from hospitals,2 with an estimated cost of more than $5 billion annually.3 Furthermore, rates of community-associated CDI continue to increase and account for about 40% of cases.4
After colonization in the intestine, C difficile releases 2 toxins (TcdA and TcdB) that cause colitis.5 Patients may present with mild diarrhea that can progress to abdominal pain, cramping, fever, and leukocytosis. Fulminant CDI can lead to the formation of pseudomembranes in the colon, toxic megacolon, bowel perforation, shock, and death.2
Beginning in the early 2000s, hospitals reported increases in severe cases of CDI.6 A specific strain known as BI/NAP1/027 was identified and characterized by fluoroquinolone resistance, increased spore formation, and a higher mortality rate.6
Further complicating matters … Recurrent CDI occurs in up to 10% to 30% of patients,7 typically within 14 to 45 days of completion of antibiotic pharmacotherapy for CDI.8 Recurrence is characterized by new-onset diarrhea or abdominal symptoms after completion of treatment for CDI.5
It typically begins with an antibiotic
Risk factors for CDI are listed in TABLE 1.9 The most important modifiable risk factor for initial and recurrent CDI is recent use of antibiotics.10 Most antibiotics can disrupt normal intestinal flora, causing colonization of C difficile, but the strongest association seems to be with third- and fourth-generation cephalosporins, fluoroquinolones, carbapenems, and clindamycin.11 The risk for CDI occurs during antibiotic treatment, as well as up to 3 months after completion of antibiotic therapy.7 Exposure to multiple antibiotics and extended duration of antibacterial therapy can greatly increase the risk for CDI, so antimicrobial stewardship is key.11
Continue to: Continuing antibiotics while attempting...
Continuing antibiotics while attempting to treat CDI reduces the patient’s clinical response to CDI treatment, which can lead to recurrence.12 The Infectious Diseases Society of America (IDSA) guidelines include a strong recommendation to discontinue concurrent antibiotics as soon as possible in these scenarios.11
Acid-suppression therapy has also been associated with CDI. The mechanism is thought to be an interruption in the protection provided by stomach acid, and use over time may reduce the diversity of flora within the gut microbiome.13 The data demonstrating an association between acid-suppression therapy and CDI is conflicting, which may be a result of confounding factors such as the severity of CDI illness and diarrhea induced by use of proton pump inhibitors (PPIs).4 IDSA guidelines do not provide a recommendation regarding discontinuation of PPI therapy for the prevention of CDI, although inappropriate PPI therapy should always be discontinued.11
Advanced age is an important nonmodifiable risk factor for CDI. Older adults who live in long-term care facilities are at a higher risk for CDI, and these facilities have colonization rates as high as 50%.12
Community-associated risk. In an analysis of community-associated cases of CDI, 82% of patients reported some sort of health care exposure (ranging from physician office visit to surgery admission), 64% reported the receipt of antimicrobial therapy, and 31% reported the use of PPIs.14 Inflammatory bowel disease (IBD) may also put community dwellers at higher risk for CDI and its complications.15
CASES 1 & 2
Both CASE patients have risk factors for CDI. Ms. O (CASE 1) is likely at risk for CDI after completion of her recent course of clindamycin. Ms. Z (CASE 2) has several risk factors for recurrent CDI, including advanced age (≥ 65 years), residence in a long-term care facility, prior antibiotic exposure, and immunodeficiency because of chemotherapy/steroid use.
Continue to: Diagnosis
Diagnosis: Who and how to test
CDI should be both a clinical and laboratory-confirmed diagnosis. Patients should be tested for CDI if they have 3 or more episodes of unexplainable, new-onset unformed stools in 24 hours.11 Asymptomatic patients should not be tested to avoid unnecessary testing and treatment of those who are colonized but not infected.11 It is not recommended to routinely test patients who have taken laxatives within the previous 48 hours.11
There are several stool-based laboratory test options for the diagnosis of CDI (TABLE 211,12,16) but no definitive recommendation for all institutions.11 Many institutions have now implemented PCR testing for the diagnosis of CDI. However, while the benefits of this test include reduced need for repeat testing and possible identification of carriers, it’s estimated that reports of CDI increase more than 50% when an institution switches to PCR testing.1 Nonetheless, a one-step, highly sensitive test such as PCR may be used if strict criteria are implemented and followed.
The increase in positive PCR tests has prompted evaluation of using another test in addition to or in place of PCR. Multistep testing options include a glutamate dehydrogenase assay (GDH) with a toxin EIA, GDH with a toxin EIA and final decision via PCR, or PCR with toxin EIA.11 Use of a multistep diagnostic algorithm may increase overall specificity up to 100%, which may improve determination of asymptomatic colonization vs active infection.16 (Patients who have negative toxin results with positive PCR likely have colonization but not infection and often do not require treatment.) IDSA guidelines recommend that the stool toxin test should be part of a multistep algorithm for diagnosis, rather than PCR alone, if strict criteria are not implemented for stool test submission.11
There is no need to perform a test of cure after a patient has been treated for CDI, and no repeat testing should be performed within 7 days of the previous test.11 After successful treatment, patients will continue to shed spores and test positively via PCR for weeks to months.11 When patients have a positive PCR test, there are several important infection control efforts that institutions should consider; see “IDSA weighs in on measures to combat C difficile.”
SIDEBAR
IDSA weighs in on measures to combat C difficile
The spores produced by Clostridioides difficile can survive for 5 months or longer on dry surfaces because of resistance to heat, acid, antibiotics, and many cleaning products.38 Unfortunately, spores transmitted from health care workers and the environment are the most likely cause of infection spreading in health care institutions. To prevent transmission of C difficile infection (CDI) throughout institutions, appropriate infection control measures are necessary.
Clinical practice guidelines from the Infectious Diseases Society of America (IDSA) recommend that patients with CDI be isolated to a private room with a dedicated toilet. Health care staff should wear gloves and gowns when entering the room of, or taking care of, a patient with CDI. For patients who are suspected of having CDI, contact precautions should be implemented while awaiting test results. When the diagnosis is confirmed, contact precautions should remain in place for at least 48 hours after resolution of diarrhea but may be continued until discharge.11
Practicing good hand hygiene is essential, especially in institutions with high rates of CDI or if fecal contamination is likely.11 Hand hygiene with soap and water is preferred, due to evidence of a higher spore removal rate, but alcohol-based alternatives may be used if necessary.11 In institutions with high rates of CDI, terminal (post-discharge) cleaning of rooms with a sporicidal agent should be considered.11
Asymptomatic carriers are also a concern for transmission of CDI in institutional settings. Screening and isolating patients who are carriers may prevent transmission, and some institutions have implemented this process to reduce the risk for CDI that originates in a health care facility.39 The IDSA guidelines do not make a recommendation regarding screening or isolation of asymptomatic carriers, so the decision is institution specific.11 These guidelines also recommend that patients presenting with similar infectious organisms be housed in the same room, if needed, to avoid cross-contamination to others or additional surfaces.11
For pediatric patients, testing recommendations vary by age. Testing is not generally recommended for neonates or infants ≤ 2 years of age with diarrhea because of the prevalence of colonization with C difficile.11 For children older than 2 years, testing for CDI is only recommended in the setting of prolonged or worsening diarrhea and if the patient has risk factors such as IBD, immunocompromised state, health care exposure, or recent antibiotic use.11 In addition, testing in this population should only be considered once other infectious and noninfectious causes of diarrhea have been excluded.11
Continue to: First-line treatment? Drug of choice has changed
First-line treatment? Drug of choice has changed
In 2018, the IDSA published new treatment guidelines that provide important updates from the 2010 guidelines.11 Chief among these was the elimination of metronidazole as a first-line therapy. Vancomycin or fidaxomicin are now recommended as first-line treatment options because of superior eradication of C difficile when compared with metronidazole.11 In the opinion of the authors, vancomycin should be considered the drug of choice because of cost. (See “The case for vancomycin.”)
SIDEBAR
The case for vancomycin
The majority of studies conducted prior to publication of the 2010 Infectious Diseases Society of America guidelines described numerically worse eradication rates of Clostridioides difficile infection (CDI) with metronidazole compared with vancomycin for all severities of infection, but statistical significance was not achieved. These studies also showed a nonsignificant increase in CDI recurrence with metronidazole.17,40,41
A 2005 systematic review demonstrated increased treatment failure rates with metronidazole.42 The rates of metronidazole discontinuation and transition to alternative options more than doubled in 2003-2004, to 25.7% of patients compared with 9.6% in earlier years.42 Metronidazole efficacy was further questioned in a prospective observational study conducted in 2005, in which only 50% of patients were cured after an initial course of treatment, while 28% had recurrence within 90 days.43
Vancomycin was found to be the superior treatment option to metronidazole and tolevamer in a 2014 randomized controlled trial.18 This study also demonstrated that vancomycin was the superior therapy when comparing treatment-naïve vs experienced patients and severity of CDI.18 A 2017 retrospective cohort study demonstrated decreased 30-day all-cause mortality for patients taking vancomycin vs metronidazole (adjusted relative risk = 0.86; 95% confidence interval, 0.74-0.98), although it should be noted that this difference was driven by those with severe CDI, and there was no statistically significant difference in mortality for patients with mild-to-moderate CDI.44
The results of these studies led to the recommendation of vancomycin over metronidazole as first-line pharmacotherapy for CDI in practice, despite the historical perspective that overutilization of oral vancomycin could potentially increase rates of vancomycinresistant Enterococcus.11
Metronidazole should only be used in the treatment of CDI as a lastresort medication because of cost or insurance coverage. Although the price of oral vancomycin is higher, favorable patient outcomes are substantially greater, and recent analyses have shown that vancomycin is actually more cost-effective than metronidazole as a result.24 Adverse effects for metronidazole include neurotoxicity, gastrointestinal discomfort, and disulfiram-like reaction.
Vancomycin does not harbor as many adverse effects because of extremely low systemic absorption when taken orally, but patients may experience gastrointestinal discomfort.45 While systemic exposure with oral administration of vancomycin is very low (< 1%), there have been case reports of nephrotoxicity and “red man syndrome” that are more typically seen with intravenous vancomycin.44
Given the low rate of systemic exposure, routine monitoring of renal function and serum drug levels is not usually necessary during oral vancomycin therapy. However, it may be appropriate to monitor renal function and serum levels of vancomycin in patients who have renal failure, have altered intestinal integrity, are age ≥ 65 years, or are receiving high doses of vancomycin.46
10-day vs 14-day treatment of CDI. Most studies for the treatment of CDI have used a 10-day regimen rather than increasing the duration to a 14-day regimen, and nearly all studies conducted have displayed high rates of symptom resolution at the end of 10 days of treatment.17,18 Thus, treatment duration beyond 10 days should only be considered for patients who continue to have symptoms or complications with CDI on Day 10 of treatment.
First recurrence. Metronidazole is no longer the recommended treatment for first recurrence of CDI treated initially with metronidazole; instead, a 10-day course of vancomycin should be used.11 For recurrent cases in patients initially treated with vancomycin, a tapered and pulsed regimen of vancomycin is recommended11:
- vancomycin PO 125 mg four times daily for 10 to 14 days followed by
- vancomycin PO 125 mg twice daily for 7 days, then
- vancomycin PO 125 mg once daily for 7 days, then
- vancomycin PO 125 mg every 2 to 3 days for 2 to 8 weeks.
Pediatric patients. The IDSA guidelines recommend use of metronidazole or vancomycin to treat an initial case or first recurrence of mild-to-moderate CDI in this population.11 Due to a lack of quality evidence, the drug of choice for initial treatment is inconclusive, so patient-specific factors and cost should be considered when choosing an agent.11 If not cost prohibitive, vancomycin should be the drug of choice for most cases of pediatric CDI, and for severe cases or multiple recurrences of CDI, vancomycin is clearly the drug of choice.
Recommended agents: A closer look
Oral vancomycin products. Vancocin, a capsule, and Firvanq, an oral solution, are 2 vancomycin products currently on the market for CDI. Although the capsules are a readily available treatment option, the cost of the full course of treatment can be a barrier for patients without insurance, or with high copays or deductibles (brand name, $4000; generic, $1252).19
Continue to: Historically, in an effort to keep costs down...
Historically, in an effort to keep costs down, an oral solution was often inexpensively compounded at hospitals or pharmacies.20
Fidaxomicin, an oral macrocyclic antibiotic with minimal systemic absorption, was first approved by the US Food and Drug Administration (FDA) for CDI in 2011.21 The IDSA guidelines recommend fidaxomicin for initial, and recurrent, cases of CDI as an alternative to vancomycin.11 This recommendation is based on 2 randomized double-blind trials comparing fidaxomicin to standard-dose oral vancomycin for initial or recurrent CDI.21,22
Pooled data from these 2 similar studies found that fidaxomicin was noninferior (10% noninferiority margin) to vancomycin for the primary outcome of clinical cure.23 Fidaxomicin was shown to be superior to vancomycin regarding rate of CDI recurrence (relative risk [RR] = 0.61; 95% confidence interval [CI], 0.43-0.87). These results were similar regardless of whether the CDI was an initial or recurrent case.23
Given the lack of systemic absorption, fidaxomicin is generally very well tolerated. The largest downside to fidaxomicin is its cost, which can be nearly $5000 for a standard 10-day course (vs as little as $165 for oral vancomycin).19 As a result, oral vancomycin solution is likely the most cost-effective therapy for initial cases of CDI.24 In patients with poor medication adherence, fidaxomicin offers the advantage of less-frequent dosing (twice daily vs 4 times daily with vancomycin).
For cases of recurrent CDI, when treatment failure occurred with vancomycin, fidaxomicin should be considered as an efficacious alternative. If fidaxomicin is used, it is advisable to verify coverage with the patient’s insurance plan, since prior authorization is frequently required.
Continue to: When meds fail, consider a fecal microbiota transplant
When meds fail, consider a fecal microbiota transplant
Another important change in the IDSA guidelines for CDI management is the strong recommendation for fecal microbiota transplantation (FMT) in patients with multiple recurrences of CDI for whom appropriate antibiotic treatment courses have failed.11,25 The goal of FMT is to “normalize” an abnormal gut microbiome by transplanting donor stool into a recipient.26
FMT has been shown to be highly effective in 5 randomized clinical trials conducted since 2013, with CDI cure rates between 85% and 94%.11 This rate of cure is particularly impressive given that the studies only included patients with refractory CDI.
Patients with recurrent CDI who may be candidates for FMT should be referred to a center or specialist with experience in FMT. These transplants can be expensive because of the screening process involved in obtaining donor samples. (Historically, a single FMT has cost $3000-$5000, and it is seldom covered by insurance.27) The emergence of universal stool banks offers a streamlined solution to this process.26
Fresh or frozen stool is considered equally effective in treating refractory CDI.26 Oral capsule and freeze-dried stool formulations have been studied, but their use is considered investigational at this time.26
Delivery via colonoscopy to the right colon is the preferred route of infusion; however, delivery via enema or nasogastric, nasojejunal, or nasoduodenal infusion can be considered as well.26
Continue to: In preparing for stool transplantation...
In preparing for stool transplantation, patients should be treated with standard doses of oral vancomycin or fidaxomicin for 3 days before the procedure to suppress intestinal C difficile, and the last dose of antibiotics should be given 12 to 48 hours before the procedure.26 Bowel lavage with polyethylene glycol is recommended, regardless of whether stool is delivered via colonoscopy or upper GI route.
Short-term adverse events associated with FMT appear to be minimal; data is lacking for long-term safety outcomes.28 While only recommended currently for cases of recurrent CDI, there is promising data emerging for use of FMT for severe cases, even without recurrence.29
The role of probiotics remains unclear
Probiotics have been explored in numerous trials to determine if they are effective in preventing CDI in patients who have been prescribed antibiotics.11 While no randomized trials have conclusively shown benefit, several meta-analyses have shown that the use of probiotics may result in a 60% to 65% relative risk reduction in CDI incidence.30,31
One proviso to these meta-analyses is that the incorporated studies have typically included patients at very high risk for CDI, and subanalyses have only found a reduction in CDI incidence when patients are at a very high baseline risk. In addition, there are many differences in probiotic types, formulations, treatment durations, and follow-up. As a result, the IDSA guidelines state that there is “insufficient data at this time” to recommend routine administration of probiotics for either primary or secondary CDI prophylaxis.11
Due to insufficient high-quality data, the IDSA guidelines do not provide a recommendation regarding use as an adjunct treatment option for acute CDI.11 Probiotics should not be routinely used to prevent CDI; however, they may provide benefit if reserved for patients at the highest risk for CDI (eg, history of CDI, prolonged use of broad-spectrum antibiotics, high local incidence).
Continue to: What about surgical intervention?
What about surgical intervention?
In severe cases of CDI, surgery may be necessary and can reduce mortality.32 The surgical procedure with the strongest recommendation in the IDSA guidelines is the subtotal colectomy, though the diverting loop ileostomy is an alternative option.11 Patients who may benefit from surgery include those with a WBC count ≥ 25,000; lactate > 5 mmol/L11; altered mental status; megacolon; perforation of the colon; acute abdomen on physical examination; or septic shock due to CDI.33 Although surgery can be beneficial, the mortality rate remains high for those with CDI who undergo colectomy.33
Reserve bezlotoxumab for prevention of recurrence
Bezlotoxumab, a human monoclonal immunoglobulin GI/kappa antibody, was approved by the FDA in 2016 for the prevention of recurrent CDI. Its mechanism of action is to bind and neutralize C difficile toxin B. It was approved as a single infusion for adults who are receiving active antibiotic therapy for CDI and are considered to be at high risk for recurrence.34
This approval was based on 2 trials of more than 2500 patients, in which participants received bezlotoxumab or placebo while receiving treatment for primary or recurrent CDI. The primary outcome of these studies was recurrent infection within 12 weeks after infusion, which was significantly lower for bezlotoxumab in both studies: 17% vs 28% (P < 0.001) in one trial and 16% vs 26% (P < 0.001) in the other trial.35
Bezlotoxumab should only be used as an adjunct to prevent recurrence.32 There is no recommendation for or against bezlotoxumab in the IDSA guidelines because of the recent date of the drug’s approval. Its frequency of use will likely depend on the number of patients who meet criteria as high risk for recurrence and its estimated cost of $4560 per dose.34,36
CASES
CASE 1: In light of Ms. O’s recent completion of a course of clindamycin and unremarkable lab work, she should be treated for mild-to-moderate CDI. She has no comorbid conditions to warrant fidaxomicin, and thus vancomycin (capsules or oral solution) would be the best treatment option. Ms. O is started on vancomycin PO 125 mg qid for 10 days. She is also advised to discontinue loperamide as soon as possible, based on poor outcomes data seen with the use of antimotility agents in CDI.37
Continue to: CASE 2
CASE 2: Ms. Z has several risk factors for recurrent CDI and has an elevated WBC count and SCr level (WBC ≥ 15,000 and SCr > 1.5 mg/dL). Thus, she is classified as having severe, recurrent CDI. Oral levofloxacin and metronidazole should be discontinued, because they increase the risk for treatment failure and development of more virulent CDI strains, such as BI/NAP1/027. Since Ms. Z used metronidazole for treatment of her initial CDI, vancomycin or fidaxomicin should be used at this time. Either vancomycin PO 125 mg qid for 10 days or fidaxomicin 200 mg bid for 10 days would be an appropriate regimen; however, because of cost and unknown insurance coverage, vancomycin is the most appropriate regimen.
CORRESPONDENCE
Jeremy Vandiver, PharmD, BCPS, University of Wyoming School of Pharmacy, Saint Joseph Family Medicine Residency, 1000 E. University Avenue, Dept 3375, Laramie, WY 82071; jvandive@uwyo.edu
CASE 1
Beth O, a 63-year-old woman, presents to the emergency department (ED) with a 2-week history of diarrhea (6 very loose, watery stools per day) and lower abdominal pain. The patient denies any vomiting, sick contacts, or recent travel. Past medical history includes varicose veins. Her only active medication is loperamide, as needed, for the past 2 weeks. Ms. O also recently completed a 10-day course of clindamycin for an infected laceration on her finger.
Ms. O’s laboratory values are unremarkable, with a normal white blood cell (WBC) count and serum creatinine (SCr) level. Abdominal computed tomography (CT) reveals some abnormal bowel dilatation and a slight increase in colon wall thickness. There is a high suspicion for Clostridioides difficile (formerly Clostridium difficile) infection (CDI), and stool sent for polymerase chain reaction (PCR) testing comes back positive for C difficile toxin B. It is revealed to be a strain other than the BI/NAP1/027 epidemic strain (which has a higher mortality rate).
How should this patient be treated?
CASE 2
Sixty-eight-year-old Barbara Z presents to the ED from her skilled nursing facility with persistent diarrhea and abdominal cramping. She was diagnosed with CDI about 2 months ago and reports that her symptoms resolved within 4 to 5 days after starting a 14-day course of oral metronidazole.
Her past medical history is notable for multiple myeloma with bone metastasis, for which she is actively undergoing chemotherapy treatment. She also has chronic kidney disease (baseline SCr, 2.2 mg/dL), hypertension, and anemia of chronic disease. The patient’s medications include amlodipine and cholecalciferol. Her chemotherapy regimen consists of bortezomib, lenalidomide, and dexamethasone. CT of the abdomen shows diffuse colon wall thickening with surrounding inflammatory stranding—concerning for pancolitis. There is no evidence of toxic megacolon or ileus.
Ms. Z’s laboratory values are notable for a WBC count of 15,900 cells/mL and an SCr of 4.1 mg/dL. She is started on oral levofloxacin and metronidazole due to concern for an intra-abdominal infection. PCR testing is positive for C difficile, and an enzyme immunoassay (EIA) for C difficile toxin is positive.
What factors put Ms. Z at risk for C difficile, and how should she be treated?
Continue to: C difficile is one of the most...
C difficile is one of the most commonly reported pathogens in health care–associated infections and affects almost 1% of all hospitalized patients in the United States each year.1 From 2001 to 2010, the incidence of CDI doubled in patients discharged from hospitals,2 with an estimated cost of more than $5 billion annually.3 Furthermore, rates of community-associated CDI continue to increase and account for about 40% of cases.4
After colonization in the intestine, C difficile releases 2 toxins (TcdA and TcdB) that cause colitis.5 Patients may present with mild diarrhea that can progress to abdominal pain, cramping, fever, and leukocytosis. Fulminant CDI can lead to the formation of pseudomembranes in the colon, toxic megacolon, bowel perforation, shock, and death.2
Beginning in the early 2000s, hospitals reported increases in severe cases of CDI.6 A specific strain known as BI/NAP1/027 was identified and characterized by fluoroquinolone resistance, increased spore formation, and a higher mortality rate.6
Further complicating matters … Recurrent CDI occurs in up to 10% to 30% of patients,7 typically within 14 to 45 days of completion of antibiotic pharmacotherapy for CDI.8 Recurrence is characterized by new-onset diarrhea or abdominal symptoms after completion of treatment for CDI.5
It typically begins with an antibiotic
Risk factors for CDI are listed in TABLE 1.9 The most important modifiable risk factor for initial and recurrent CDI is recent use of antibiotics.10 Most antibiotics can disrupt normal intestinal flora, causing colonization of C difficile, but the strongest association seems to be with third- and fourth-generation cephalosporins, fluoroquinolones, carbapenems, and clindamycin.11 The risk for CDI occurs during antibiotic treatment, as well as up to 3 months after completion of antibiotic therapy.7 Exposure to multiple antibiotics and extended duration of antibacterial therapy can greatly increase the risk for CDI, so antimicrobial stewardship is key.11
Continue to: Continuing antibiotics while attempting...
Continuing antibiotics while attempting to treat CDI reduces the patient’s clinical response to CDI treatment, which can lead to recurrence.12 The Infectious Diseases Society of America (IDSA) guidelines include a strong recommendation to discontinue concurrent antibiotics as soon as possible in these scenarios.11
Acid-suppression therapy has also been associated with CDI. The mechanism is thought to be an interruption in the protection provided by stomach acid, and use over time may reduce the diversity of flora within the gut microbiome.13 The data demonstrating an association between acid-suppression therapy and CDI is conflicting, which may be a result of confounding factors such as the severity of CDI illness and diarrhea induced by use of proton pump inhibitors (PPIs).4 IDSA guidelines do not provide a recommendation regarding discontinuation of PPI therapy for the prevention of CDI, although inappropriate PPI therapy should always be discontinued.11
Advanced age is an important nonmodifiable risk factor for CDI. Older adults who live in long-term care facilities are at a higher risk for CDI, and these facilities have colonization rates as high as 50%.12
Community-associated risk. In an analysis of community-associated cases of CDI, 82% of patients reported some sort of health care exposure (ranging from physician office visit to surgery admission), 64% reported the receipt of antimicrobial therapy, and 31% reported the use of PPIs.14 Inflammatory bowel disease (IBD) may also put community dwellers at higher risk for CDI and its complications.15
CASES 1 & 2
Both CASE patients have risk factors for CDI. Ms. O (CASE 1) is likely at risk for CDI after completion of her recent course of clindamycin. Ms. Z (CASE 2) has several risk factors for recurrent CDI, including advanced age (≥ 65 years), residence in a long-term care facility, prior antibiotic exposure, and immunodeficiency because of chemotherapy/steroid use.
Continue to: Diagnosis
Diagnosis: Who and how to test
CDI should be both a clinical and laboratory-confirmed diagnosis. Patients should be tested for CDI if they have 3 or more episodes of unexplainable, new-onset unformed stools in 24 hours.11 Asymptomatic patients should not be tested to avoid unnecessary testing and treatment of those who are colonized but not infected.11 It is not recommended to routinely test patients who have taken laxatives within the previous 48 hours.11
There are several stool-based laboratory test options for the diagnosis of CDI (TABLE 211,12,16) but no definitive recommendation for all institutions.11 Many institutions have now implemented PCR testing for the diagnosis of CDI. However, while the benefits of this test include reduced need for repeat testing and possible identification of carriers, it’s estimated that reports of CDI increase more than 50% when an institution switches to PCR testing.1 Nonetheless, a one-step, highly sensitive test such as PCR may be used if strict criteria are implemented and followed.
The increase in positive PCR tests has prompted evaluation of using another test in addition to or in place of PCR. Multistep testing options include a glutamate dehydrogenase assay (GDH) with a toxin EIA, GDH with a toxin EIA and final decision via PCR, or PCR with toxin EIA.11 Use of a multistep diagnostic algorithm may increase overall specificity up to 100%, which may improve determination of asymptomatic colonization vs active infection.16 (Patients who have negative toxin results with positive PCR likely have colonization but not infection and often do not require treatment.) IDSA guidelines recommend that the stool toxin test should be part of a multistep algorithm for diagnosis, rather than PCR alone, if strict criteria are not implemented for stool test submission.11
There is no need to perform a test of cure after a patient has been treated for CDI, and no repeat testing should be performed within 7 days of the previous test.11 After successful treatment, patients will continue to shed spores and test positively via PCR for weeks to months.11 When patients have a positive PCR test, there are several important infection control efforts that institutions should consider; see “IDSA weighs in on measures to combat C difficile.”
SIDEBAR
IDSA weighs in on measures to combat C difficile
The spores produced by Clostridioides difficile can survive for 5 months or longer on dry surfaces because of resistance to heat, acid, antibiotics, and many cleaning products.38 Unfortunately, spores transmitted from health care workers and the environment are the most likely cause of infection spreading in health care institutions. To prevent transmission of C difficile infection (CDI) throughout institutions, appropriate infection control measures are necessary.
Clinical practice guidelines from the Infectious Diseases Society of America (IDSA) recommend that patients with CDI be isolated to a private room with a dedicated toilet. Health care staff should wear gloves and gowns when entering the room of, or taking care of, a patient with CDI. For patients who are suspected of having CDI, contact precautions should be implemented while awaiting test results. When the diagnosis is confirmed, contact precautions should remain in place for at least 48 hours after resolution of diarrhea but may be continued until discharge.11
Practicing good hand hygiene is essential, especially in institutions with high rates of CDI or if fecal contamination is likely.11 Hand hygiene with soap and water is preferred, due to evidence of a higher spore removal rate, but alcohol-based alternatives may be used if necessary.11 In institutions with high rates of CDI, terminal (post-discharge) cleaning of rooms with a sporicidal agent should be considered.11
Asymptomatic carriers are also a concern for transmission of CDI in institutional settings. Screening and isolating patients who are carriers may prevent transmission, and some institutions have implemented this process to reduce the risk for CDI that originates in a health care facility.39 The IDSA guidelines do not make a recommendation regarding screening or isolation of asymptomatic carriers, so the decision is institution specific.11 These guidelines also recommend that patients presenting with similar infectious organisms be housed in the same room, if needed, to avoid cross-contamination to others or additional surfaces.11
For pediatric patients, testing recommendations vary by age. Testing is not generally recommended for neonates or infants ≤ 2 years of age with diarrhea because of the prevalence of colonization with C difficile.11 For children older than 2 years, testing for CDI is only recommended in the setting of prolonged or worsening diarrhea and if the patient has risk factors such as IBD, immunocompromised state, health care exposure, or recent antibiotic use.11 In addition, testing in this population should only be considered once other infectious and noninfectious causes of diarrhea have been excluded.11
Continue to: First-line treatment? Drug of choice has changed
First-line treatment? Drug of choice has changed
In 2018, the IDSA published new treatment guidelines that provide important updates from the 2010 guidelines.11 Chief among these was the elimination of metronidazole as a first-line therapy. Vancomycin or fidaxomicin are now recommended as first-line treatment options because of superior eradication of C difficile when compared with metronidazole.11 In the opinion of the authors, vancomycin should be considered the drug of choice because of cost. (See “The case for vancomycin.”)
SIDEBAR
The case for vancomycin
The majority of studies conducted prior to publication of the 2010 Infectious Diseases Society of America guidelines described numerically worse eradication rates of Clostridioides difficile infection (CDI) with metronidazole compared with vancomycin for all severities of infection, but statistical significance was not achieved. These studies also showed a nonsignificant increase in CDI recurrence with metronidazole.17,40,41
A 2005 systematic review demonstrated increased treatment failure rates with metronidazole.42 The rates of metronidazole discontinuation and transition to alternative options more than doubled in 2003-2004, to 25.7% of patients compared with 9.6% in earlier years.42 Metronidazole efficacy was further questioned in a prospective observational study conducted in 2005, in which only 50% of patients were cured after an initial course of treatment, while 28% had recurrence within 90 days.43
Vancomycin was found to be the superior treatment option to metronidazole and tolevamer in a 2014 randomized controlled trial.18 This study also demonstrated that vancomycin was the superior therapy when comparing treatment-naïve vs experienced patients and severity of CDI.18 A 2017 retrospective cohort study demonstrated decreased 30-day all-cause mortality for patients taking vancomycin vs metronidazole (adjusted relative risk = 0.86; 95% confidence interval, 0.74-0.98), although it should be noted that this difference was driven by those with severe CDI, and there was no statistically significant difference in mortality for patients with mild-to-moderate CDI.44
The results of these studies led to the recommendation of vancomycin over metronidazole as first-line pharmacotherapy for CDI in practice, despite the historical perspective that overutilization of oral vancomycin could potentially increase rates of vancomycinresistant Enterococcus.11
Metronidazole should only be used in the treatment of CDI as a lastresort medication because of cost or insurance coverage. Although the price of oral vancomycin is higher, favorable patient outcomes are substantially greater, and recent analyses have shown that vancomycin is actually more cost-effective than metronidazole as a result.24 Adverse effects for metronidazole include neurotoxicity, gastrointestinal discomfort, and disulfiram-like reaction.
Vancomycin does not harbor as many adverse effects because of extremely low systemic absorption when taken orally, but patients may experience gastrointestinal discomfort.45 While systemic exposure with oral administration of vancomycin is very low (< 1%), there have been case reports of nephrotoxicity and “red man syndrome” that are more typically seen with intravenous vancomycin.44
Given the low rate of systemic exposure, routine monitoring of renal function and serum drug levels is not usually necessary during oral vancomycin therapy. However, it may be appropriate to monitor renal function and serum levels of vancomycin in patients who have renal failure, have altered intestinal integrity, are age ≥ 65 years, or are receiving high doses of vancomycin.46
10-day vs 14-day treatment of CDI. Most studies for the treatment of CDI have used a 10-day regimen rather than increasing the duration to a 14-day regimen, and nearly all studies conducted have displayed high rates of symptom resolution at the end of 10 days of treatment.17,18 Thus, treatment duration beyond 10 days should only be considered for patients who continue to have symptoms or complications with CDI on Day 10 of treatment.
First recurrence. Metronidazole is no longer the recommended treatment for first recurrence of CDI treated initially with metronidazole; instead, a 10-day course of vancomycin should be used.11 For recurrent cases in patients initially treated with vancomycin, a tapered and pulsed regimen of vancomycin is recommended11:
- vancomycin PO 125 mg four times daily for 10 to 14 days followed by
- vancomycin PO 125 mg twice daily for 7 days, then
- vancomycin PO 125 mg once daily for 7 days, then
- vancomycin PO 125 mg every 2 to 3 days for 2 to 8 weeks.
Pediatric patients. The IDSA guidelines recommend use of metronidazole or vancomycin to treat an initial case or first recurrence of mild-to-moderate CDI in this population.11 Due to a lack of quality evidence, the drug of choice for initial treatment is inconclusive, so patient-specific factors and cost should be considered when choosing an agent.11 If not cost prohibitive, vancomycin should be the drug of choice for most cases of pediatric CDI, and for severe cases or multiple recurrences of CDI, vancomycin is clearly the drug of choice.
Recommended agents: A closer look
Oral vancomycin products. Vancocin, a capsule, and Firvanq, an oral solution, are 2 vancomycin products currently on the market for CDI. Although the capsules are a readily available treatment option, the cost of the full course of treatment can be a barrier for patients without insurance, or with high copays or deductibles (brand name, $4000; generic, $1252).19
Continue to: Historically, in an effort to keep costs down...
Historically, in an effort to keep costs down, an oral solution was often inexpensively compounded at hospitals or pharmacies.20
Fidaxomicin, an oral macrocyclic antibiotic with minimal systemic absorption, was first approved by the US Food and Drug Administration (FDA) for CDI in 2011.21 The IDSA guidelines recommend fidaxomicin for initial, and recurrent, cases of CDI as an alternative to vancomycin.11 This recommendation is based on 2 randomized double-blind trials comparing fidaxomicin to standard-dose oral vancomycin for initial or recurrent CDI.21,22
Pooled data from these 2 similar studies found that fidaxomicin was noninferior (10% noninferiority margin) to vancomycin for the primary outcome of clinical cure.23 Fidaxomicin was shown to be superior to vancomycin regarding rate of CDI recurrence (relative risk [RR] = 0.61; 95% confidence interval [CI], 0.43-0.87). These results were similar regardless of whether the CDI was an initial or recurrent case.23
Given the lack of systemic absorption, fidaxomicin is generally very well tolerated. The largest downside to fidaxomicin is its cost, which can be nearly $5000 for a standard 10-day course (vs as little as $165 for oral vancomycin).19 As a result, oral vancomycin solution is likely the most cost-effective therapy for initial cases of CDI.24 In patients with poor medication adherence, fidaxomicin offers the advantage of less-frequent dosing (twice daily vs 4 times daily with vancomycin).
For cases of recurrent CDI, when treatment failure occurred with vancomycin, fidaxomicin should be considered as an efficacious alternative. If fidaxomicin is used, it is advisable to verify coverage with the patient’s insurance plan, since prior authorization is frequently required.
Continue to: When meds fail, consider a fecal microbiota transplant
When meds fail, consider a fecal microbiota transplant
Another important change in the IDSA guidelines for CDI management is the strong recommendation for fecal microbiota transplantation (FMT) in patients with multiple recurrences of CDI for whom appropriate antibiotic treatment courses have failed.11,25 The goal of FMT is to “normalize” an abnormal gut microbiome by transplanting donor stool into a recipient.26
FMT has been shown to be highly effective in 5 randomized clinical trials conducted since 2013, with CDI cure rates between 85% and 94%.11 This rate of cure is particularly impressive given that the studies only included patients with refractory CDI.
Patients with recurrent CDI who may be candidates for FMT should be referred to a center or specialist with experience in FMT. These transplants can be expensive because of the screening process involved in obtaining donor samples. (Historically, a single FMT has cost $3000-$5000, and it is seldom covered by insurance.27) The emergence of universal stool banks offers a streamlined solution to this process.26
Fresh or frozen stool is considered equally effective in treating refractory CDI.26 Oral capsule and freeze-dried stool formulations have been studied, but their use is considered investigational at this time.26
Delivery via colonoscopy to the right colon is the preferred route of infusion; however, delivery via enema or nasogastric, nasojejunal, or nasoduodenal infusion can be considered as well.26
Continue to: In preparing for stool transplantation...
In preparing for stool transplantation, patients should be treated with standard doses of oral vancomycin or fidaxomicin for 3 days before the procedure to suppress intestinal C difficile, and the last dose of antibiotics should be given 12 to 48 hours before the procedure.26 Bowel lavage with polyethylene glycol is recommended, regardless of whether stool is delivered via colonoscopy or upper GI route.
Short-term adverse events associated with FMT appear to be minimal; data is lacking for long-term safety outcomes.28 While only recommended currently for cases of recurrent CDI, there is promising data emerging for use of FMT for severe cases, even without recurrence.29
The role of probiotics remains unclear
Probiotics have been explored in numerous trials to determine if they are effective in preventing CDI in patients who have been prescribed antibiotics.11 While no randomized trials have conclusively shown benefit, several meta-analyses have shown that the use of probiotics may result in a 60% to 65% relative risk reduction in CDI incidence.30,31
One proviso to these meta-analyses is that the incorporated studies have typically included patients at very high risk for CDI, and subanalyses have only found a reduction in CDI incidence when patients are at a very high baseline risk. In addition, there are many differences in probiotic types, formulations, treatment durations, and follow-up. As a result, the IDSA guidelines state that there is “insufficient data at this time” to recommend routine administration of probiotics for either primary or secondary CDI prophylaxis.11
Due to insufficient high-quality data, the IDSA guidelines do not provide a recommendation regarding use as an adjunct treatment option for acute CDI.11 Probiotics should not be routinely used to prevent CDI; however, they may provide benefit if reserved for patients at the highest risk for CDI (eg, history of CDI, prolonged use of broad-spectrum antibiotics, high local incidence).
Continue to: What about surgical intervention?
What about surgical intervention?
In severe cases of CDI, surgery may be necessary and can reduce mortality.32 The surgical procedure with the strongest recommendation in the IDSA guidelines is the subtotal colectomy, though the diverting loop ileostomy is an alternative option.11 Patients who may benefit from surgery include those with a WBC count ≥ 25,000; lactate > 5 mmol/L11; altered mental status; megacolon; perforation of the colon; acute abdomen on physical examination; or septic shock due to CDI.33 Although surgery can be beneficial, the mortality rate remains high for those with CDI who undergo colectomy.33
Reserve bezlotoxumab for prevention of recurrence
Bezlotoxumab, a human monoclonal immunoglobulin GI/kappa antibody, was approved by the FDA in 2016 for the prevention of recurrent CDI. Its mechanism of action is to bind and neutralize C difficile toxin B. It was approved as a single infusion for adults who are receiving active antibiotic therapy for CDI and are considered to be at high risk for recurrence.34
This approval was based on 2 trials of more than 2500 patients, in which participants received bezlotoxumab or placebo while receiving treatment for primary or recurrent CDI. The primary outcome of these studies was recurrent infection within 12 weeks after infusion, which was significantly lower for bezlotoxumab in both studies: 17% vs 28% (P < 0.001) in one trial and 16% vs 26% (P < 0.001) in the other trial.35
Bezlotoxumab should only be used as an adjunct to prevent recurrence.32 There is no recommendation for or against bezlotoxumab in the IDSA guidelines because of the recent date of the drug’s approval. Its frequency of use will likely depend on the number of patients who meet criteria as high risk for recurrence and its estimated cost of $4560 per dose.34,36
CASES
CASE 1: In light of Ms. O’s recent completion of a course of clindamycin and unremarkable lab work, she should be treated for mild-to-moderate CDI. She has no comorbid conditions to warrant fidaxomicin, and thus vancomycin (capsules or oral solution) would be the best treatment option. Ms. O is started on vancomycin PO 125 mg qid for 10 days. She is also advised to discontinue loperamide as soon as possible, based on poor outcomes data seen with the use of antimotility agents in CDI.37
Continue to: CASE 2
CASE 2: Ms. Z has several risk factors for recurrent CDI and has an elevated WBC count and SCr level (WBC ≥ 15,000 and SCr > 1.5 mg/dL). Thus, she is classified as having severe, recurrent CDI. Oral levofloxacin and metronidazole should be discontinued, because they increase the risk for treatment failure and development of more virulent CDI strains, such as BI/NAP1/027. Since Ms. Z used metronidazole for treatment of her initial CDI, vancomycin or fidaxomicin should be used at this time. Either vancomycin PO 125 mg qid for 10 days or fidaxomicin 200 mg bid for 10 days would be an appropriate regimen; however, because of cost and unknown insurance coverage, vancomycin is the most appropriate regimen.
CORRESPONDENCE
Jeremy Vandiver, PharmD, BCPS, University of Wyoming School of Pharmacy, Saint Joseph Family Medicine Residency, 1000 E. University Avenue, Dept 3375, Laramie, WY 82071; jvandive@uwyo.edu
1. Polage CR, Gyorke CE, Kennedy MA, et al. Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern Med. 2015;175:1792-1801.
2. Reveles KR, Lee GC, Boyd NK, et al. The rise in Clostridium difficile infection incidence among hospitalized adults in the United States: 2001-2010. Am J Infect Control. 2014;42:1028-1032.
3. Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(suppl 2):S88-S92.
4. Tariq R, Singh S, Gupta A, et al. Association of gastric acid suppression with recurrent Clostridium difficile infection: a systematic review and meta-analysis. JAMA Intern Med. 2017;177:784-791.
5. Kachrimanidou M, Malisiovas N. Clostridium difficile infection: a comprehensive review. Crit Rev Microbiol. 2011;37:178-187.
6. O’Connor JR, Johnson S, Gerding DN. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology. 2009;136:1913-1924.
7. Kelly CP. A 76-year-old man with recurrent Clostridium difficile-associated diarrhea: review of C difficile infection. JAMA. 2009;301:954-962.
8. Cornely OA, Miller MA, Louie TJ, et al. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55(suppl 2):S154-S161.
9. Napolitano LM, Edmiston CE Jr. Clostridium difficile disease: diagnosis, pathogenesis, and treatment update. Surgery 2017;162:325-348.
10. Deshpande A, Pasupuleti V, Thota P, et al. Risk factors for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2015;36:452-460.
11. McDonald LC, Gerding DN, Johnson S, et al. Clinical Practice Guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66:e1-e48.
12. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498; quiz 499.
13. Seto CT, Jeraldo P, Orenstein R, et al. Prolonged use of a proton pump inhibitor reduces microbial diversity: implications for Clostridium difficile susceptibility. Microbiome. 2014;2:42.
14. Chitnis AS, Holzbauer SM, Belflower RM, et al. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med. 2013;173:1359-1367.
15. Negrón ME, Rezaie A, Barkema HW, et al. Ulcerative colitis patients with Clostridium difficile are at increased risk of death, colectomy, and postoperative complications: a population-based inception cohort study. Am J Gastroenterol. 2016;111:691-704.
16. Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA. 2015;313:398-408.
17. Zar FA, Bakkanagari SR, Moorthi KM, et al. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302-307.
18. Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59:345-354.
19. Vancomycin: product details. Redbook Online. www.micromedexsolutions.com. Published 2018. Accessed June 13, 2020.
20. Mergenhagen KA, Wojciechowski AL, Paladino JA. A review of the economics of treating Clostridium difficile infection. Pharmacoeconomics. 2014;32:639-650.
21. Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422-431.
22. Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12:281-289.
23. Crook DW, Walker AS, Kean Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clin Infect Dis. 2012;55 suppl 2:S93-103.
24. Ford DC, Schroeder MC, Ince D, et al. Cost-effectiveness analysis of initial treatment strategies for mild-to-moderate Clostridium difficile infection in hospitalized patients. Am J Health Syst Pharm. 2018;75:1110-1121.
25. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431-455.
26. Panchal P, Budree S, Scheeler A, et al. Scaling safe access to fecal microbiota transplantation: past, present, and future. Curr Gastroenterol Rep. 2018;20:14.
27. Arbel LT, Hsu E, McNally K. Cost-effectiveness of fecal microbiota transplantation in the treatment of recurrent Clostridium difficile infection: a literature review. Cureus. 2017;9:e1599.
28. Cammarota G, Ianiro G, Tilg H, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569-580.
29. Hocquart M, Lagier JC, Cassir N, et al. Early fecal microbiota transplantation improves survival in severe Clostridium difficile infections. Clin Infect Dis. 2018;66:645-650.
30. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
31. Johnston BC, Lytvyn L, Lo CK, et al. Microbial preparations (probiotics) for the prevention of Clostridium difficile infection in adults and children: an individual patient data meta-analysis of 6,851 participants. Infect Control Hosp Epidemiol. 2018:1-11.
32. Stewart DB, Hollenbeak CS, Wilson MZ. Is colectomy for fulminant Clostridium difficile colitis life saving? A systematic review. Colorectal Dis. 2013;15:798-804.
33. Julien M, Wild JL, Blansfield J, et al. Severe complicated Clostridium difficile infection: can the UPMC proposed scoring system predict the need for surgery? J Trauma Acute Care Surg. 2016;81:221-228.
34. Merck & Co, Inc. Sharp M. ZinplavaTM (bezlotoxumab [package insert] US Food and Drug Administration Web site. www.accessdata.fda.gov/drugsatfda_docs/label/2016/761046s000lbl.pdf. Revised October 2016. Accessed May 29, 2020.
35. Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017;376:305-317.
36. Chahine EB, Cho JC, Worley MV. Bezlotoxumab for the Prevention of Clostridium difficile recurrence. Consult Pharm. 2018;33:89-97.
37. Koo HL, Koo DC, Musher DM, et al. Antimotility agents for the treatment of Clostridium difficile diarrhea and colitis. Clin Infect Dis. 2009;48:598-605.
38. Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7:526-536.
39. Longtin Y, Paquet-Bolduc B, Gilca R, et al. Effect of detecting and isolating Clostridium difficile carriers at hospital admission on the incidence of C difficile infections: a quasi-experimental controlled study. JAMA Intern Med. 2016;176:796-804.
40. Teasley DG, Gerding DN, Olson MM, et al. Prospective randomised trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhoea and colitis. Lancet. 1983;2:1043-1046.
41. Wenisch C, Parschalk B, Hasenhündl M, et al. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis. 1996;22:813-818.
42. Pepin J, Alary ME, Valiquette L, et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40:1591-1597.
43. Musher DM, Aslam S, Logan N, et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005;40:1586-1590.
44. Stevens VW, Nelson RE, Schwab-Daugherty EM, et al. Comparative effectiveness of vancomycin and metronidazole for the prevention of recurrence and death in patients with Clostridium difficile infection. JAMA Intern Med. 2017;177:546-553.
45. CutisPharma. FirvanqTM (vancomycin hydrochloride) for oral solution [package insert]. US Food and Drug Administration Web site. www.accessdata.fda.gov/drugsatfda_docs/label/2018/208910s000lbl.pdf. Revised January 2018. Accessed May 29, 2020.
46.
1. Polage CR, Gyorke CE, Kennedy MA, et al. Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern Med. 2015;175:1792-1801.
2. Reveles KR, Lee GC, Boyd NK, et al. The rise in Clostridium difficile infection incidence among hospitalized adults in the United States: 2001-2010. Am J Infect Control. 2014;42:1028-1032.
3. Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(suppl 2):S88-S92.
4. Tariq R, Singh S, Gupta A, et al. Association of gastric acid suppression with recurrent Clostridium difficile infection: a systematic review and meta-analysis. JAMA Intern Med. 2017;177:784-791.
5. Kachrimanidou M, Malisiovas N. Clostridium difficile infection: a comprehensive review. Crit Rev Microbiol. 2011;37:178-187.
6. O’Connor JR, Johnson S, Gerding DN. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology. 2009;136:1913-1924.
7. Kelly CP. A 76-year-old man with recurrent Clostridium difficile-associated diarrhea: review of C difficile infection. JAMA. 2009;301:954-962.
8. Cornely OA, Miller MA, Louie TJ, et al. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55(suppl 2):S154-S161.
9. Napolitano LM, Edmiston CE Jr. Clostridium difficile disease: diagnosis, pathogenesis, and treatment update. Surgery 2017;162:325-348.
10. Deshpande A, Pasupuleti V, Thota P, et al. Risk factors for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2015;36:452-460.
11. McDonald LC, Gerding DN, Johnson S, et al. Clinical Practice Guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66:e1-e48.
12. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498; quiz 499.
13. Seto CT, Jeraldo P, Orenstein R, et al. Prolonged use of a proton pump inhibitor reduces microbial diversity: implications for Clostridium difficile susceptibility. Microbiome. 2014;2:42.
14. Chitnis AS, Holzbauer SM, Belflower RM, et al. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med. 2013;173:1359-1367.
15. Negrón ME, Rezaie A, Barkema HW, et al. Ulcerative colitis patients with Clostridium difficile are at increased risk of death, colectomy, and postoperative complications: a population-based inception cohort study. Am J Gastroenterol. 2016;111:691-704.
16. Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA. 2015;313:398-408.
17. Zar FA, Bakkanagari SR, Moorthi KM, et al. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302-307.
18. Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59:345-354.
19. Vancomycin: product details. Redbook Online. www.micromedexsolutions.com. Published 2018. Accessed June 13, 2020.
20. Mergenhagen KA, Wojciechowski AL, Paladino JA. A review of the economics of treating Clostridium difficile infection. Pharmacoeconomics. 2014;32:639-650.
21. Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422-431.
22. Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12:281-289.
23. Crook DW, Walker AS, Kean Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clin Infect Dis. 2012;55 suppl 2:S93-103.
24. Ford DC, Schroeder MC, Ince D, et al. Cost-effectiveness analysis of initial treatment strategies for mild-to-moderate Clostridium difficile infection in hospitalized patients. Am J Health Syst Pharm. 2018;75:1110-1121.
25. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431-455.
26. Panchal P, Budree S, Scheeler A, et al. Scaling safe access to fecal microbiota transplantation: past, present, and future. Curr Gastroenterol Rep. 2018;20:14.
27. Arbel LT, Hsu E, McNally K. Cost-effectiveness of fecal microbiota transplantation in the treatment of recurrent Clostridium difficile infection: a literature review. Cureus. 2017;9:e1599.
28. Cammarota G, Ianiro G, Tilg H, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569-580.
29. Hocquart M, Lagier JC, Cassir N, et al. Early fecal microbiota transplantation improves survival in severe Clostridium difficile infections. Clin Infect Dis. 2018;66:645-650.
30. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
31. Johnston BC, Lytvyn L, Lo CK, et al. Microbial preparations (probiotics) for the prevention of Clostridium difficile infection in adults and children: an individual patient data meta-analysis of 6,851 participants. Infect Control Hosp Epidemiol. 2018:1-11.
32. Stewart DB, Hollenbeak CS, Wilson MZ. Is colectomy for fulminant Clostridium difficile colitis life saving? A systematic review. Colorectal Dis. 2013;15:798-804.
33. Julien M, Wild JL, Blansfield J, et al. Severe complicated Clostridium difficile infection: can the UPMC proposed scoring system predict the need for surgery? J Trauma Acute Care Surg. 2016;81:221-228.
34. Merck & Co, Inc. Sharp M. ZinplavaTM (bezlotoxumab [package insert] US Food and Drug Administration Web site. www.accessdata.fda.gov/drugsatfda_docs/label/2016/761046s000lbl.pdf. Revised October 2016. Accessed May 29, 2020.
35. Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017;376:305-317.
36. Chahine EB, Cho JC, Worley MV. Bezlotoxumab for the Prevention of Clostridium difficile recurrence. Consult Pharm. 2018;33:89-97.
37. Koo HL, Koo DC, Musher DM, et al. Antimotility agents for the treatment of Clostridium difficile diarrhea and colitis. Clin Infect Dis. 2009;48:598-605.
38. Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7:526-536.
39. Longtin Y, Paquet-Bolduc B, Gilca R, et al. Effect of detecting and isolating Clostridium difficile carriers at hospital admission on the incidence of C difficile infections: a quasi-experimental controlled study. JAMA Intern Med. 2016;176:796-804.
40. Teasley DG, Gerding DN, Olson MM, et al. Prospective randomised trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhoea and colitis. Lancet. 1983;2:1043-1046.
41. Wenisch C, Parschalk B, Hasenhündl M, et al. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis. 1996;22:813-818.
42. Pepin J, Alary ME, Valiquette L, et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40:1591-1597.
43. Musher DM, Aslam S, Logan N, et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005;40:1586-1590.
44. Stevens VW, Nelson RE, Schwab-Daugherty EM, et al. Comparative effectiveness of vancomycin and metronidazole for the prevention of recurrence and death in patients with Clostridium difficile infection. JAMA Intern Med. 2017;177:546-553.
45. CutisPharma. FirvanqTM (vancomycin hydrochloride) for oral solution [package insert]. US Food and Drug Administration Web site. www.accessdata.fda.gov/drugsatfda_docs/label/2018/208910s000lbl.pdf. Revised January 2018. Accessed May 29, 2020.
46.
PRACTICE RECOMMENDATIONS
› Keep in mind that previous exposure to antibiotics is the most important risk factor for initial and recurrent Clostridioides difficile infection (CDI). Thus, appropriate antimicrobial stewardship is key to prevention. C
› Begin with vancomycin or fidaxomicin (over metronidazole) for first-line treatment of CDI in adults. A
› Consider fecal microbiota transplantation in high-risk patients with recurrent CDI for whom antimicrobial therapy has failed. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Periprocedural management of oral anticoagulation: When and how to hit “pause”
Debra P is a 62-year-old African American woman who calls your office to report that she has an upcoming routine colonoscopy planned in 2 weeks. She has been taking warfarin for the past 2 years for ischemic stroke prevention secondary to atrial fibrillation (AF), and her gastroenterologist recommended that she contact her family physician (FP) to discuss periprocedural anticoagulation plans. Ms. P is currently taking warfarin 5 mg on Mondays, Wednesdays, and Fridays, and 2.5 mg all other days of the week. Her international normalized ratio (INR) was 2.3 when it was last checked 2 weeks ago, and it has been stable and within goal range for the past 6 months. Her medical history includes AF, well-controlled hypertension, and type 2 diabetes mellitus, as well as gout and stage 3 chronic kidney disease. Ms. P denies any history of stroke or transient ischemic attack (TIA). She is requesting instructions on how to manage her warfarin before and after her upcoming colonoscopy.
Jerry Q is a 68-year-old Caucasian man with longstanding osteoarthritis who is scheduled to undergo a total left knee arthroplasty in one week. His orthopedic surgeon recommended that he contact his FP for instructions regarding managing apixaban perioperatively. Jerry has been taking apixaban 5 mg bid for the past 9 months due to a history of recurrent deep vein thrombosis (DVT) and pulmonary embolism (PE) (both unprovoked). Mr. Q had been taking warfarin following his first DVT 4 years ago, but, after reporting that INR monitoring was a burden, he was started on apixaban. The patient has normal renal function and is relatively healthy otherwise. How should apixaban be managed before and after his upcoming surgery?
Each year, approximately 15% to 20% of patients taking an oral anticoagulant undergo a procedure that carries a heightened risk for bleeding.1,2 Stopping oral anticoagulation is often necessary before—and sometimes briefly after—many of these procedures in order to minimize the risk of bleeding.3 This means that countless decisions must be made by health care providers each year regarding if, when, and how to pause and resume oral anticoagulation. These decisions are not always straightforward, especially when you consider the risks for thrombosis and bleeding that are unique to the procedure and to the individual patient.
With these variables in mind, the health care provider must make decisions regarding anticoagulation during the periprocedural period based on the following 5 questions:
- Will this patient need to have his/her oral anticoagulant stopped prior to the procedure?
- If the patient’s oral anticoagulation needs to be held, when should it be stopped and for how long?
- Will periprocedural bridging with a parenteral anticoagulant be necessary prior to the procedure?
- When should the patient resume his or her oral anticoagulant after the procedure, and at what dosage?
- Will bridging with a parenteral anticoagulant be necessary after the procedure?
Before addressing these 5 questions, though, physicians must assess patients’ thrombotic and bleeding risks.4-6
Anticoagulant regimens and the risks of discontinuing them
The 2 most common indications for long-term oral anticoagulation are venous thromboembolism (VTE), which occurs in approximately one million Americans every year,7,8 and stroke prevention in the setting of AF (AF occurs in 3-6 million US adults per year).6
Warfarin is also often used in patients with mechanical heart valves for long-term stroke prevention; however, direct oral anticoagulants (DOACs) are not recommended for patients with mechanical heart valves because trials have not yet demonstrated their safety or efficacy in this population.4,5,9
Who’s at highest risk for an acute thromboembolic event?
When planning for interruptions in oral anticoagulation, it is important to identify patients at highest risk for an acute thromboembolic event. Patients with 10% or higher annual risk for VTE or ischemic stroke are generally placed into this high-risk category (TABLE 13,5,6,9-11).3 Keep in mind that the absolute risk for thromboembolism during a brief period of oral coagulation interruption is relatively low, even in those patients considered to be at high risk. Using a mathematical approach (although simplistic), a patient with a 10% annual risk for a thromboembolic event would have <0.3% chance for developing such an event in the acute phase, even if their anticoagulation was withheld for up to 10 days ([10%/365 days] × 10 days).
Patients with mechanical heart valves. Nearly all patients with a mechanical heart valve are at moderate to high risk for ischemic stroke.3
For patients with AF, the CHADS2 and CHA2DS2-VASc scoring tools can be used to estimate annual thrombosis risk based on the presence of risk factors (TABLE 210,11).6,9-11 It should be noted, however, that these scoring tools have not been validated specifically for periprocedural risk estimations. Nonetheless, the latest 2017 American College of Cardiology (ACC) guidelines recommend the use of the CHA2DS2-VASc scoring tool for making decisions regarding perioperative bridging in patients with AF.11
Patients with previous VTE. Multiple aspects of a patient’s past medical history need to be taken into account when estimating annual and acute risk for VTE. Patients at the highest risk for VTE recurrence (annual VTE risk ≥10%) include those with recent VTE (past 90 days), active malignancy, and/or severe thrombophilias (TABLE 13,5,6,9-11).3,5,6 Patients without any of these features can still be at moderate risk for recurrent VTE, as a single VTE without a clear provoking factor can confer a 5% to 10% annualized risk for recurrence.12,13 Previous proximal DVT and PE are associated with a higher risk for recurrence than a distal DVT, and males have a higher recurrence risk than females.5,12 There are scoring tools, such as DASH (D-dimer, Age, Sex, Hormones) and the “Men Continue and HERDOO2,” that can help estimate annualized risk for VTE recurrence; however, they are not validated (nor particularly useful) when making decisions in the perioperative period.14,15
Additional risk factors. Consider additional risk factors for thromboembolism, including estrogen/hormone replacement therapy, pregnancy, leg or hip fractures, immobility, trauma, spinal cord injury, central venous lines, congestive heart failure, thrombophilia, increased age, obesity, and varicose veins.5,16
In addition, some surgeries have a higher inherent risk for thrombosis. Major orthopedic surgery (knee and hip arthroplasty, hip fracture surgery) and surgeries for major trauma or spinal cord injuries are associated with an exceedingly high rate of VTE.17 Similarly, coronary artery bypass surgery, heart valve replacement, and carotid endarterectomies carry the highest risk for acute ischemic stroke.3
Who’s at highest risk for bleeding?
Establishing the bleeding risk associated with a procedure is imperative prior to urgent and elective surgeries to help determine when anticoagulation therapy should be discontinued and reinitiated, as well as whether bridging therapy is appropriate. The 2012 CHEST guidelines state that bleeding risk should be assessed based on timing of anticoagulation relative to surgery and whether the anticoagulation is being used as prophylaxis for, or treatment of, thromboembolism.3 Categorizing procedures as having a minimal, low, or high risk for bleeding can be helpful in making anticoagulation decisions (TABLE 3).3,18-21
In addition to the bleeding risk associated with procedures, patient-specific factors need to be considered. A bleeding event within the past 3 months, platelet abnormalities, a supratherapeutic INR at the time of surgery, a history of bleeding from previous bridging, a bleed history with a similar procedure, and a high HAS-BLED (Hypertension, Abnormal renal or liver function, Stroke, Bleeding history or predisposition, Labile INR, Elderly, Drugs/alcohol usage) score are all factors that elevate the risk for perioperative bleeding.10,11 Although validated only in patients taking warfarin, the HAS-BLED scoring system can be utilized in patients with AF to estimate annual risk for major bleeding (TABLE 210,11).10
With this risk information in mind, it’s time to move on to the 5 questions you’ll need to ask.
1. Should the patient’s oral anticoagulation be stopped prior to the upcoming procedure?
The answer, of course, hinges on the patient’s risk of bleeding.
Usually, it is not necessary to withhold any doses of oral anticoagulation if your patient is scheduled for a procedure with minimal risk for bleeding (TABLE 33,18-21).3 However, it may be reasonable to stop anticoagulation if your patient has additional features that predispose to high bleeding risk (eg, hemophilia, Von Willebrand disease, etc). The CHEST guidelines recommend adding an oral prohemostatic agent (eg, tranexamic acid) if anticoagulation will be continued during a dental procedure.3
If your patient is undergoing any other procedure that has a low to high risk for bleeding, oral anticoagulation should be withheld prior to the procedure in most instances,3,11 although there are exceptions. For example, cardiac procedures, such as AF catheter ablation and cardiac pacemaker placement, are often performed with uninterrupted oral anticoagulation despite their bleeding risk category.3
When in doubt, discuss the perceived bleeding and clotting risks directly with the specialist performing the procedure. In patients who have had a VTE or ischemic stroke within the past 3 months, consider postponing the invasive procedure until the patient is beyond this period of highest thrombotic risk.11
2. How far in advance of the procedure should the oral anticoagulant be withheld?
Warfarin may need to be stopped anywhere from 2 to 5 days prior to the procedure, depending on a number of variables.
Warfarin has a half-life of approximately 36 hours, so it can take 3 to 5 days for warfarin concentrations to drop to safe levels for procedures with low to moderate bleeding risk and 5 to 7 days for procedures with high bleeding risk.21 The 2012 CHEST guidelines recommend that warfarin therapy be discontinued 5 days prior to surgery to minimize the risk for bleeding.3 The Anticoagulation Forum, a leading expert panel that produced a set of useful anticoagulation guidelines in 2016, recommends stopping warfarin 4 to 5 days prior to a procedure.21 If the provider chooses to withhold warfarin before a procedure with minimal bleeding risk, it should be stopped 2 to 3 days prior.3
Consider checking INR values the week before. A 2017 consensus statement from the ACC recommends that the timing of warfarin discontinuation be based on an INR value taken 5 to 7 days prior to the surgical procedure.11 This allows for a more tailored approach to preparing the patient for surgery. If the INR is below goal range, warfarin may need to be withheld for only 3 to 4 days prior to a procedure. Conversely, INRs above goal range may require warfarin to be held 6 or more days, depending on the degree of INR elevation.
While not always feasible in clinical practice, the CHEST guidelines recommend obtaining an INR value the day prior to the procedure to determine if the INR value is low enough to proceed with surgery, or if a low dose of oral vitamin K needs to be administered to ensure that the INR is in a safe range the following day.3
DOACs
DOACs can be withheld for much shorter durations preoperatively than warfarin.
When withholding anticoagulants, the goal is to have a low amount of anticoagulant effect (12%-25%) present during low-risk procedures and a nominal amount of anticoagulant effect (3%-6%) present for high-risk procedures.20 DOACs have much shorter half-lives than warfarin (7-19 hours vs 36-48 hours, respectively), so they can be withheld for much shorter durations preoperatively.20 For patients undergoing procedures that are considered to have a minimal risk for bleeding (such as minor dental and dermatologic procedures), DOACs do not generally need to be withheld; however, it may be ideal to time the procedure when the DOAC is at a trough concentration (before the next dose is due).3
DOACs generally need to be withheld for only 1 to 3 days prior to major surgical procedures in patients with normal renal function (creatinine clearance [CrCl] >30 mL/min using the Cockcroft-Gault formula).20 The available oral direct factor Xa inhibitors (apixaban, rivaroxaban, and edoxaban) should generally be stopped 24 hours prior to a procedure that has a low bleeding risk, and 48 hours prior to procedures with high bleeding risk (TABLE 411,20).20 These medications may need to be withheld for an additional 1 to 2 days in patients with acute kidney injury or stage IV kidney disease.20
Dabigatran. About 80% of dabigatran is excreted renally, so its elimination is much more dependent on renal function than is that of the oral direct factor Xa inhibitors.20 Therefore, it generally needs to be withheld for at least 1 to 2 days longer than the oral factor Xa inhibitors unless CrCl >80 mL/min (TABLE 411,20).20
3. Is preoperative bridging with parenteral anticoagulation necessary?
In certain instances, patients who have a high thromboembolic risk and are discontinuing warfarin therapy may require bridging therapy with a low-molecular-weight heparin (LMWH) or unfractionated heparin (UFH). If a patient’s CrCl is <30 mL/min, then UFH is the preferred agent for perioperative bridging.21
But before any decision is made, it’s best to have a good understanding of what the guidelines—and the literature—have to say.
Key studies and guidelines
The 2012 CHEST guidelines recommend providing bridge therapy for any patient at high risk for thromboembolism (>10% annual risk) and consideration of bridge therapy in the setting of moderate clotting risk (5%-10% annual risk), depending on specific patient and procedural risk factors (TABLE 13,5,6,9-11).3
In 2015, a landmark clinical trial was published that significantly shaped how patients taking warfarin are managed periprocedurally.22 The Bridge (Bridging anticoagulation in patients who require temporary interruption of warfarin therapy for an elective invasive procedure or surgery) trial was the first prospective, randomized controlled trial to assess the efficacy and safety of parenteral bridging in patients with AF taking warfarin and undergoing an elective surgery.
Patients in the trial received either dalteparin at a therapeutic dose of 100 IU/kg or a matching placebo administered subcutaneously bid from 3 days before the procedure until 24 hours before the procedure, and then for 5 to 10 days after the procedure. The incidence of thromboembolic events was not significantly lower in the dalteparin group than in the placebo group (0.3% vs 0.4%, respectively; P=.73), while major bleeding rates were nearly 3-fold higher in the dalteparin group (3.2% vs 1.3%; P=.005). The trial concluded that placebo “was noninferior to perioperative bridging with LMWH for the prevention of arterial thromboembolism and decreased the risk of major bleeding.”22
Patients excluded from the trial included those with a mechanical heart valve, or a recent (within 3 months) embolism, stroke, or TIA, and only 3% of enrolled patients would have been classified as having a high bleeding risk according to CHEST guidelines.3,22
A prospective observational registry study produced similar findings and found that those patients who received bridging had more bleeding events and a higher incidence of myocardial infarction, stroke or systemic embolism, major bleeding, hospitalization, or death within 30 days than those who did not receive bridging.23 Other retrospective cohort studies comparing bridging to no bridging strategies in patients taking warfarin for VTE, mechanical heart valves, or AF have also failed to show a reduction in the incidence of thrombotic events with LMWH bridging.24,25
In 2016, the European Society of Cardiology suggested that “bridging does not seem to be beneficial, except in patients with mechanical heart valves.”26 Similarly, the 2016 Anticoagulation Forum guidelines state that “most patients with VTE can safely interrupt warfarin for invasive procedures without bridge therapy,” and that bridge therapy should be “reserved for those at highest recurrent VTE risk (eg, VTE within the previous month; prior history of recurrent VTE during anticoagulation therapy interruption; undergoing a procedure with high inherent risk for VTE, such as joint replacement surgery or major abdominal cancer resection).”21 They go on to state that even in these high-risk groups, the clinical decision to use bridging therapy needs to carefully weigh the benefits against the potential risks of bleeding.21
Controversy also surrounds the intensity of LMWH bridging. The Anticoagulation Forum guidelines state that the use of prophylactic rather than therapeutic dose LMWH may be considered, while the CHEST guidelines do not make a firm recommendation regarding LMWH dose while bridging.3,21 Ultimately, in patients who receive perioperative bridging with LMWH, the CHEST guidelines recommend that it should be stopped 24 hours prior to the procedure and resumed in accordance with the bleeding risk of the procedure (ie, prophylactic doses may be appropriate within 24 hours postprocedure, while full treatment doses may need to be delayed for 48 to 72 hours if surgical bleeding risk is high).3 UFH bridge therapy may be stopped 4 to 6 hours prior to surgery.3
DOACs. Given the rapid onset and relatively short half-lives of DOACs, use of a parenteral bridging agent is generally not necessary or recommended before or after an invasive procedure in patients taking a DOAC.20
4. When should oral anticoagulation be resumed postoperatively, and at what intensity?
Warfarin can generally be resumed the same day as the procedure (in the evening), assuming there are no active bleeding complications.3,11 Once fully reversed, it generally takes around 5 days for warfarin to become fully therapeutic, so it can be started soon after surgery without increasing the risk for early postoperative bleeding.20
DOACs. Consider the patient’s individual and procedural risks for bleeding when determining when to resume a DOAC postoperatively. That’s because unlike warfarin, which takes several days to take full effect, DOACs provide a nearly immediate anticoagulation effect.20,21 For procedures that have a low bleeding risk, it is recommended to resume therapeutic anticoagulation 24 hours after the procedure has ended.3,11,20 For procedures that have a high risk for bleeding, resumption of therapeutic anticoagulation should be delayed until 48 to 72 hours after the procedure has ended.3,11,20
5. Is postoperative bridging with parenteral anticoagulation necessary?
Warfarin. If a patient was deemed to be at sufficient VTE risk to be bridged preoperatively, then that patient likely also should be bridged postoperatively, particularly if the surgery itself is associated with a heightened thrombotic risk. While warfarin can generally be resumed postoperatively the same day as the procedure, full therapeutic doses of a LMWH should not be initiated sooner than 24 hours postoperatively, and initiation should be delayed for 48 to 72 hours for procedures with the highest bleeding risk (such as neurosurgery).3,11,21 Prophylactic doses of LMWH can generally be resumed as early as 12 hours postoperatively for procedures with high VTE risk (such as major orthopedic surgery).17
DOACs. In patients undergoing a procedure that carries both a high thromboembolic and high bleeding risk (such as major orthopedic surgery), initiation of a full-dose DOAC may need to be delayed for 2 to 3 days; however, more immediate VTE prophylaxis is usually necessary.3,17 Prophylaxis after such procedures can begin 12 hours after the procedure with a low-intensity LMWH, which should be continued until it is deemed safe to resume full-intensity DOAC therapy.3,17,18 If the patient is undergoing major orthopedic surgery, an FDA-approved prophylactic dose of a DOAC could be a temporary alternative to LMWH.27
Ms. P’s upcoming colonoscopy may require a biopsy and would be classified as a procedure with low bleeding risk (per TABLE 3), so warfarin should be withheld prior to her procedure. You could check her INR 5 to 7 days before her colonoscopy to guide how many doses need to be withheld; however, given the patient’s tight INR control over the previous 6 months, you can assume her INR will be in goal range at that check. As a result, you recommend that she avoid an extra INR check and stop taking her warfarin 5 days prior to the colonoscopy.
Ms. P has a CHA2DS2VASc score of 3, which puts her at a relatively low risk for acute ischemic stroke over the next 1 to 2 weeks. Given the results of the BRIDGE trial, you recommend no parenteral bridging agent before or after her procedure. You also recommend that the patient resume her usual dose of warfarin the same day as her procedure (in the evening) unless her gastroenterologist recommends otherwise. You schedule her for a follow-up INR 5 to 7 days after her colonoscopy.
Mr. Q’s total knee arthroplasty (TKA)—a procedure associated with a high risk of bleeding—requires an interruption in his apixaban therapy. Additionally, he is at high risk for recurrent thromboembolism, given his history of recurrent, unprovoked DVTs; however, he is past the highest risk period (VTE within the past 3 months; his last one was 9 months ago). He is otherwise healthy and has normal renal function, so his apixaban should be withheld for a total of 4 doses (48 hours) prior to his procedure. He should resume his full dose of apixaban 48 to 72 hours after his procedure to minimize the risk for bleeding.
However, given that a TKA is a procedure associated with a high rate of postoperative VTE, initiate prophylactic anticoagulation (such as enoxaparin 40 mg subcutaneously daily or apixaban 2.5 mg PO bid) about 12 hours after the procedure and continue it until full-dose apixaban is resumed.
CORRESPONDENCE
Jeremy Vandiver, PharmD, BCPS, University of Wyoming School of Pharmacy, 1000 E. University Ave., Dept. 3375, Laramie, WY 82071; jvandive@uwyo.edu.
1. Connelly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
2. Steinberg BA, Kim S, Piccini JP, et al. Use and associated risks of concomitant aspirin therapy with oral anticoagulation in patients with atrial fibrillation: insights from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Registry. Circulation. 2013;128:721-728.
3. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e326S-e350S.
4. Adam SS, McDuffie JR, Ortel TL, et al. Comparative effectiveness of warfarin and newer oral anticoagulants for the long-term prevention and treatment of arterial and venous thromboembolism. Department of Veteran Affairs Evidence-Based Synthesis Project #09-010; 2012. Available at: https://www.ncbi.nlm.nih.gov/books/NBK97541/. Accessed October 15, 2017.
5. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and Expert Panel Report. Chest. 2016;149:315-352.
6. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. J Am Coll Cardiol. 2014;64:2246-2280.
7. Centers for Disease Control and Prevention. Venous thromboembolism in adult hospitalizations — United States, 2007-2009. MMWR Morb Mortal Wkly Rep. 2012 June 8;61:401-404. Available at https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6122a1.htm. Accessed October 15, 2017.
8. Anderson FA, Wheeler HB, Goldberg HJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med. 1991;151:933-938.
9. Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012): The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2012;33:2451-2496.
10. Garwood CL, Korkis B, Grande D, et al. Anticoagulation bridge therapy in patients with atrial fibrillation: recent updates provide a rebalance of risk and benefit. Pharmacotherapy. 2017;37:712-714.
11. Doherty JU, Gluckman TJ, Hucker WJ, et al. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2017;69:871-898.
12. Douketis J, Tosetto A, Marcucci M, et al. Patient-level meta-analysis: effect of measurement timing, threshold, and patient age on ability of D-dimer testing to assess recurrence risk after unprovoked venous thromboembolism. Ann Intern Med. 2010;153:523-531.
13. Becattini C, Agnelli G, Schenone A, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med. 2012;366:1959-1967.
14. Tosetto A, Testa S, Martinelli I, et al. External validation of the DASH prediction rule: a retrospective cohort study. J Thromb Haemost. 2017;15:1963-1970.
15. Rodger MA, Le Gal G, Anderson DR, et al. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. BMJ. 2017;356:j1065.
16. Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism.
17. Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines, 8th ed. Chest. 2008;133:381S-453S.
18. Spyropoulos AC, Douketis JD. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood. 2012;120:2954-2962.
19. Eisen GM, Baron TH, Dominitz JA, et al. Guideline on the management of anticoagulation and antiplatelet therapy for endoscopic procedures. Gastrointest Endosc. 2002;55:775-779.
20. Burnett AE, Mahan CE, Vazquez SR. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis. 2016;41:206-232.
21. Witt DM, Clark NP, Kaatz S, et al. Guidance for the practical management of warfarin therapy in the treatment of venous thromboembolism. J Thromb Thrombolysis. 2016;41:187-205.
22. Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823-833.
23. Steinberg BA, Peterson ED, Kim S, et al. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circulation. 2015;131: 488-494.
24. Clark NP, Witt DM, Davies LE, et al. Bleeding, recurrent venous thromboembolism, and mortality risks during warfarin interruption for invasive procedures. JAMA Intern Med. 2015;175;1163-1168.
25. Sjögren V, Grzymala-Lubanski B, Renlund H, et al. Safety and efficacy of bridging with low-molecular-weight heparin during temporary interruptions of warfarin: a register-based cohort study. Clin Appl Thromb Hemost. 2017;23:961-966.
26. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893-2962.
27. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e278S-e325S.
Debra P is a 62-year-old African American woman who calls your office to report that she has an upcoming routine colonoscopy planned in 2 weeks. She has been taking warfarin for the past 2 years for ischemic stroke prevention secondary to atrial fibrillation (AF), and her gastroenterologist recommended that she contact her family physician (FP) to discuss periprocedural anticoagulation plans. Ms. P is currently taking warfarin 5 mg on Mondays, Wednesdays, and Fridays, and 2.5 mg all other days of the week. Her international normalized ratio (INR) was 2.3 when it was last checked 2 weeks ago, and it has been stable and within goal range for the past 6 months. Her medical history includes AF, well-controlled hypertension, and type 2 diabetes mellitus, as well as gout and stage 3 chronic kidney disease. Ms. P denies any history of stroke or transient ischemic attack (TIA). She is requesting instructions on how to manage her warfarin before and after her upcoming colonoscopy.
Jerry Q is a 68-year-old Caucasian man with longstanding osteoarthritis who is scheduled to undergo a total left knee arthroplasty in one week. His orthopedic surgeon recommended that he contact his FP for instructions regarding managing apixaban perioperatively. Jerry has been taking apixaban 5 mg bid for the past 9 months due to a history of recurrent deep vein thrombosis (DVT) and pulmonary embolism (PE) (both unprovoked). Mr. Q had been taking warfarin following his first DVT 4 years ago, but, after reporting that INR monitoring was a burden, he was started on apixaban. The patient has normal renal function and is relatively healthy otherwise. How should apixaban be managed before and after his upcoming surgery?
Each year, approximately 15% to 20% of patients taking an oral anticoagulant undergo a procedure that carries a heightened risk for bleeding.1,2 Stopping oral anticoagulation is often necessary before—and sometimes briefly after—many of these procedures in order to minimize the risk of bleeding.3 This means that countless decisions must be made by health care providers each year regarding if, when, and how to pause and resume oral anticoagulation. These decisions are not always straightforward, especially when you consider the risks for thrombosis and bleeding that are unique to the procedure and to the individual patient.
With these variables in mind, the health care provider must make decisions regarding anticoagulation during the periprocedural period based on the following 5 questions:
- Will this patient need to have his/her oral anticoagulant stopped prior to the procedure?
- If the patient’s oral anticoagulation needs to be held, when should it be stopped and for how long?
- Will periprocedural bridging with a parenteral anticoagulant be necessary prior to the procedure?
- When should the patient resume his or her oral anticoagulant after the procedure, and at what dosage?
- Will bridging with a parenteral anticoagulant be necessary after the procedure?
Before addressing these 5 questions, though, physicians must assess patients’ thrombotic and bleeding risks.4-6
Anticoagulant regimens and the risks of discontinuing them
The 2 most common indications for long-term oral anticoagulation are venous thromboembolism (VTE), which occurs in approximately one million Americans every year,7,8 and stroke prevention in the setting of AF (AF occurs in 3-6 million US adults per year).6
Warfarin is also often used in patients with mechanical heart valves for long-term stroke prevention; however, direct oral anticoagulants (DOACs) are not recommended for patients with mechanical heart valves because trials have not yet demonstrated their safety or efficacy in this population.4,5,9
Who’s at highest risk for an acute thromboembolic event?
When planning for interruptions in oral anticoagulation, it is important to identify patients at highest risk for an acute thromboembolic event. Patients with 10% or higher annual risk for VTE or ischemic stroke are generally placed into this high-risk category (TABLE 13,5,6,9-11).3 Keep in mind that the absolute risk for thromboembolism during a brief period of oral coagulation interruption is relatively low, even in those patients considered to be at high risk. Using a mathematical approach (although simplistic), a patient with a 10% annual risk for a thromboembolic event would have <0.3% chance for developing such an event in the acute phase, even if their anticoagulation was withheld for up to 10 days ([10%/365 days] × 10 days).
Patients with mechanical heart valves. Nearly all patients with a mechanical heart valve are at moderate to high risk for ischemic stroke.3
For patients with AF, the CHADS2 and CHA2DS2-VASc scoring tools can be used to estimate annual thrombosis risk based on the presence of risk factors (TABLE 210,11).6,9-11 It should be noted, however, that these scoring tools have not been validated specifically for periprocedural risk estimations. Nonetheless, the latest 2017 American College of Cardiology (ACC) guidelines recommend the use of the CHA2DS2-VASc scoring tool for making decisions regarding perioperative bridging in patients with AF.11
Patients with previous VTE. Multiple aspects of a patient’s past medical history need to be taken into account when estimating annual and acute risk for VTE. Patients at the highest risk for VTE recurrence (annual VTE risk ≥10%) include those with recent VTE (past 90 days), active malignancy, and/or severe thrombophilias (TABLE 13,5,6,9-11).3,5,6 Patients without any of these features can still be at moderate risk for recurrent VTE, as a single VTE without a clear provoking factor can confer a 5% to 10% annualized risk for recurrence.12,13 Previous proximal DVT and PE are associated with a higher risk for recurrence than a distal DVT, and males have a higher recurrence risk than females.5,12 There are scoring tools, such as DASH (D-dimer, Age, Sex, Hormones) and the “Men Continue and HERDOO2,” that can help estimate annualized risk for VTE recurrence; however, they are not validated (nor particularly useful) when making decisions in the perioperative period.14,15
Additional risk factors. Consider additional risk factors for thromboembolism, including estrogen/hormone replacement therapy, pregnancy, leg or hip fractures, immobility, trauma, spinal cord injury, central venous lines, congestive heart failure, thrombophilia, increased age, obesity, and varicose veins.5,16
In addition, some surgeries have a higher inherent risk for thrombosis. Major orthopedic surgery (knee and hip arthroplasty, hip fracture surgery) and surgeries for major trauma or spinal cord injuries are associated with an exceedingly high rate of VTE.17 Similarly, coronary artery bypass surgery, heart valve replacement, and carotid endarterectomies carry the highest risk for acute ischemic stroke.3
Who’s at highest risk for bleeding?
Establishing the bleeding risk associated with a procedure is imperative prior to urgent and elective surgeries to help determine when anticoagulation therapy should be discontinued and reinitiated, as well as whether bridging therapy is appropriate. The 2012 CHEST guidelines state that bleeding risk should be assessed based on timing of anticoagulation relative to surgery and whether the anticoagulation is being used as prophylaxis for, or treatment of, thromboembolism.3 Categorizing procedures as having a minimal, low, or high risk for bleeding can be helpful in making anticoagulation decisions (TABLE 3).3,18-21
In addition to the bleeding risk associated with procedures, patient-specific factors need to be considered. A bleeding event within the past 3 months, platelet abnormalities, a supratherapeutic INR at the time of surgery, a history of bleeding from previous bridging, a bleed history with a similar procedure, and a high HAS-BLED (Hypertension, Abnormal renal or liver function, Stroke, Bleeding history or predisposition, Labile INR, Elderly, Drugs/alcohol usage) score are all factors that elevate the risk for perioperative bleeding.10,11 Although validated only in patients taking warfarin, the HAS-BLED scoring system can be utilized in patients with AF to estimate annual risk for major bleeding (TABLE 210,11).10
With this risk information in mind, it’s time to move on to the 5 questions you’ll need to ask.
1. Should the patient’s oral anticoagulation be stopped prior to the upcoming procedure?
The answer, of course, hinges on the patient’s risk of bleeding.
Usually, it is not necessary to withhold any doses of oral anticoagulation if your patient is scheduled for a procedure with minimal risk for bleeding (TABLE 33,18-21).3 However, it may be reasonable to stop anticoagulation if your patient has additional features that predispose to high bleeding risk (eg, hemophilia, Von Willebrand disease, etc). The CHEST guidelines recommend adding an oral prohemostatic agent (eg, tranexamic acid) if anticoagulation will be continued during a dental procedure.3
If your patient is undergoing any other procedure that has a low to high risk for bleeding, oral anticoagulation should be withheld prior to the procedure in most instances,3,11 although there are exceptions. For example, cardiac procedures, such as AF catheter ablation and cardiac pacemaker placement, are often performed with uninterrupted oral anticoagulation despite their bleeding risk category.3
When in doubt, discuss the perceived bleeding and clotting risks directly with the specialist performing the procedure. In patients who have had a VTE or ischemic stroke within the past 3 months, consider postponing the invasive procedure until the patient is beyond this period of highest thrombotic risk.11
2. How far in advance of the procedure should the oral anticoagulant be withheld?
Warfarin may need to be stopped anywhere from 2 to 5 days prior to the procedure, depending on a number of variables.
Warfarin has a half-life of approximately 36 hours, so it can take 3 to 5 days for warfarin concentrations to drop to safe levels for procedures with low to moderate bleeding risk and 5 to 7 days for procedures with high bleeding risk.21 The 2012 CHEST guidelines recommend that warfarin therapy be discontinued 5 days prior to surgery to minimize the risk for bleeding.3 The Anticoagulation Forum, a leading expert panel that produced a set of useful anticoagulation guidelines in 2016, recommends stopping warfarin 4 to 5 days prior to a procedure.21 If the provider chooses to withhold warfarin before a procedure with minimal bleeding risk, it should be stopped 2 to 3 days prior.3
Consider checking INR values the week before. A 2017 consensus statement from the ACC recommends that the timing of warfarin discontinuation be based on an INR value taken 5 to 7 days prior to the surgical procedure.11 This allows for a more tailored approach to preparing the patient for surgery. If the INR is below goal range, warfarin may need to be withheld for only 3 to 4 days prior to a procedure. Conversely, INRs above goal range may require warfarin to be held 6 or more days, depending on the degree of INR elevation.
While not always feasible in clinical practice, the CHEST guidelines recommend obtaining an INR value the day prior to the procedure to determine if the INR value is low enough to proceed with surgery, or if a low dose of oral vitamin K needs to be administered to ensure that the INR is in a safe range the following day.3
DOACs
DOACs can be withheld for much shorter durations preoperatively than warfarin.
When withholding anticoagulants, the goal is to have a low amount of anticoagulant effect (12%-25%) present during low-risk procedures and a nominal amount of anticoagulant effect (3%-6%) present for high-risk procedures.20 DOACs have much shorter half-lives than warfarin (7-19 hours vs 36-48 hours, respectively), so they can be withheld for much shorter durations preoperatively.20 For patients undergoing procedures that are considered to have a minimal risk for bleeding (such as minor dental and dermatologic procedures), DOACs do not generally need to be withheld; however, it may be ideal to time the procedure when the DOAC is at a trough concentration (before the next dose is due).3
DOACs generally need to be withheld for only 1 to 3 days prior to major surgical procedures in patients with normal renal function (creatinine clearance [CrCl] >30 mL/min using the Cockcroft-Gault formula).20 The available oral direct factor Xa inhibitors (apixaban, rivaroxaban, and edoxaban) should generally be stopped 24 hours prior to a procedure that has a low bleeding risk, and 48 hours prior to procedures with high bleeding risk (TABLE 411,20).20 These medications may need to be withheld for an additional 1 to 2 days in patients with acute kidney injury or stage IV kidney disease.20
Dabigatran. About 80% of dabigatran is excreted renally, so its elimination is much more dependent on renal function than is that of the oral direct factor Xa inhibitors.20 Therefore, it generally needs to be withheld for at least 1 to 2 days longer than the oral factor Xa inhibitors unless CrCl >80 mL/min (TABLE 411,20).20
3. Is preoperative bridging with parenteral anticoagulation necessary?
In certain instances, patients who have a high thromboembolic risk and are discontinuing warfarin therapy may require bridging therapy with a low-molecular-weight heparin (LMWH) or unfractionated heparin (UFH). If a patient’s CrCl is <30 mL/min, then UFH is the preferred agent for perioperative bridging.21
But before any decision is made, it’s best to have a good understanding of what the guidelines—and the literature—have to say.
Key studies and guidelines
The 2012 CHEST guidelines recommend providing bridge therapy for any patient at high risk for thromboembolism (>10% annual risk) and consideration of bridge therapy in the setting of moderate clotting risk (5%-10% annual risk), depending on specific patient and procedural risk factors (TABLE 13,5,6,9-11).3
In 2015, a landmark clinical trial was published that significantly shaped how patients taking warfarin are managed periprocedurally.22 The Bridge (Bridging anticoagulation in patients who require temporary interruption of warfarin therapy for an elective invasive procedure or surgery) trial was the first prospective, randomized controlled trial to assess the efficacy and safety of parenteral bridging in patients with AF taking warfarin and undergoing an elective surgery.
Patients in the trial received either dalteparin at a therapeutic dose of 100 IU/kg or a matching placebo administered subcutaneously bid from 3 days before the procedure until 24 hours before the procedure, and then for 5 to 10 days after the procedure. The incidence of thromboembolic events was not significantly lower in the dalteparin group than in the placebo group (0.3% vs 0.4%, respectively; P=.73), while major bleeding rates were nearly 3-fold higher in the dalteparin group (3.2% vs 1.3%; P=.005). The trial concluded that placebo “was noninferior to perioperative bridging with LMWH for the prevention of arterial thromboembolism and decreased the risk of major bleeding.”22
Patients excluded from the trial included those with a mechanical heart valve, or a recent (within 3 months) embolism, stroke, or TIA, and only 3% of enrolled patients would have been classified as having a high bleeding risk according to CHEST guidelines.3,22
A prospective observational registry study produced similar findings and found that those patients who received bridging had more bleeding events and a higher incidence of myocardial infarction, stroke or systemic embolism, major bleeding, hospitalization, or death within 30 days than those who did not receive bridging.23 Other retrospective cohort studies comparing bridging to no bridging strategies in patients taking warfarin for VTE, mechanical heart valves, or AF have also failed to show a reduction in the incidence of thrombotic events with LMWH bridging.24,25
In 2016, the European Society of Cardiology suggested that “bridging does not seem to be beneficial, except in patients with mechanical heart valves.”26 Similarly, the 2016 Anticoagulation Forum guidelines state that “most patients with VTE can safely interrupt warfarin for invasive procedures without bridge therapy,” and that bridge therapy should be “reserved for those at highest recurrent VTE risk (eg, VTE within the previous month; prior history of recurrent VTE during anticoagulation therapy interruption; undergoing a procedure with high inherent risk for VTE, such as joint replacement surgery or major abdominal cancer resection).”21 They go on to state that even in these high-risk groups, the clinical decision to use bridging therapy needs to carefully weigh the benefits against the potential risks of bleeding.21
Controversy also surrounds the intensity of LMWH bridging. The Anticoagulation Forum guidelines state that the use of prophylactic rather than therapeutic dose LMWH may be considered, while the CHEST guidelines do not make a firm recommendation regarding LMWH dose while bridging.3,21 Ultimately, in patients who receive perioperative bridging with LMWH, the CHEST guidelines recommend that it should be stopped 24 hours prior to the procedure and resumed in accordance with the bleeding risk of the procedure (ie, prophylactic doses may be appropriate within 24 hours postprocedure, while full treatment doses may need to be delayed for 48 to 72 hours if surgical bleeding risk is high).3 UFH bridge therapy may be stopped 4 to 6 hours prior to surgery.3
DOACs. Given the rapid onset and relatively short half-lives of DOACs, use of a parenteral bridging agent is generally not necessary or recommended before or after an invasive procedure in patients taking a DOAC.20
4. When should oral anticoagulation be resumed postoperatively, and at what intensity?
Warfarin can generally be resumed the same day as the procedure (in the evening), assuming there are no active bleeding complications.3,11 Once fully reversed, it generally takes around 5 days for warfarin to become fully therapeutic, so it can be started soon after surgery without increasing the risk for early postoperative bleeding.20
DOACs. Consider the patient’s individual and procedural risks for bleeding when determining when to resume a DOAC postoperatively. That’s because unlike warfarin, which takes several days to take full effect, DOACs provide a nearly immediate anticoagulation effect.20,21 For procedures that have a low bleeding risk, it is recommended to resume therapeutic anticoagulation 24 hours after the procedure has ended.3,11,20 For procedures that have a high risk for bleeding, resumption of therapeutic anticoagulation should be delayed until 48 to 72 hours after the procedure has ended.3,11,20
5. Is postoperative bridging with parenteral anticoagulation necessary?
Warfarin. If a patient was deemed to be at sufficient VTE risk to be bridged preoperatively, then that patient likely also should be bridged postoperatively, particularly if the surgery itself is associated with a heightened thrombotic risk. While warfarin can generally be resumed postoperatively the same day as the procedure, full therapeutic doses of a LMWH should not be initiated sooner than 24 hours postoperatively, and initiation should be delayed for 48 to 72 hours for procedures with the highest bleeding risk (such as neurosurgery).3,11,21 Prophylactic doses of LMWH can generally be resumed as early as 12 hours postoperatively for procedures with high VTE risk (such as major orthopedic surgery).17
DOACs. In patients undergoing a procedure that carries both a high thromboembolic and high bleeding risk (such as major orthopedic surgery), initiation of a full-dose DOAC may need to be delayed for 2 to 3 days; however, more immediate VTE prophylaxis is usually necessary.3,17 Prophylaxis after such procedures can begin 12 hours after the procedure with a low-intensity LMWH, which should be continued until it is deemed safe to resume full-intensity DOAC therapy.3,17,18 If the patient is undergoing major orthopedic surgery, an FDA-approved prophylactic dose of a DOAC could be a temporary alternative to LMWH.27
Ms. P’s upcoming colonoscopy may require a biopsy and would be classified as a procedure with low bleeding risk (per TABLE 3), so warfarin should be withheld prior to her procedure. You could check her INR 5 to 7 days before her colonoscopy to guide how many doses need to be withheld; however, given the patient’s tight INR control over the previous 6 months, you can assume her INR will be in goal range at that check. As a result, you recommend that she avoid an extra INR check and stop taking her warfarin 5 days prior to the colonoscopy.
Ms. P has a CHA2DS2VASc score of 3, which puts her at a relatively low risk for acute ischemic stroke over the next 1 to 2 weeks. Given the results of the BRIDGE trial, you recommend no parenteral bridging agent before or after her procedure. You also recommend that the patient resume her usual dose of warfarin the same day as her procedure (in the evening) unless her gastroenterologist recommends otherwise. You schedule her for a follow-up INR 5 to 7 days after her colonoscopy.
Mr. Q’s total knee arthroplasty (TKA)—a procedure associated with a high risk of bleeding—requires an interruption in his apixaban therapy. Additionally, he is at high risk for recurrent thromboembolism, given his history of recurrent, unprovoked DVTs; however, he is past the highest risk period (VTE within the past 3 months; his last one was 9 months ago). He is otherwise healthy and has normal renal function, so his apixaban should be withheld for a total of 4 doses (48 hours) prior to his procedure. He should resume his full dose of apixaban 48 to 72 hours after his procedure to minimize the risk for bleeding.
However, given that a TKA is a procedure associated with a high rate of postoperative VTE, initiate prophylactic anticoagulation (such as enoxaparin 40 mg subcutaneously daily or apixaban 2.5 mg PO bid) about 12 hours after the procedure and continue it until full-dose apixaban is resumed.
CORRESPONDENCE
Jeremy Vandiver, PharmD, BCPS, University of Wyoming School of Pharmacy, 1000 E. University Ave., Dept. 3375, Laramie, WY 82071; jvandive@uwyo.edu.
Debra P is a 62-year-old African American woman who calls your office to report that she has an upcoming routine colonoscopy planned in 2 weeks. She has been taking warfarin for the past 2 years for ischemic stroke prevention secondary to atrial fibrillation (AF), and her gastroenterologist recommended that she contact her family physician (FP) to discuss periprocedural anticoagulation plans. Ms. P is currently taking warfarin 5 mg on Mondays, Wednesdays, and Fridays, and 2.5 mg all other days of the week. Her international normalized ratio (INR) was 2.3 when it was last checked 2 weeks ago, and it has been stable and within goal range for the past 6 months. Her medical history includes AF, well-controlled hypertension, and type 2 diabetes mellitus, as well as gout and stage 3 chronic kidney disease. Ms. P denies any history of stroke or transient ischemic attack (TIA). She is requesting instructions on how to manage her warfarin before and after her upcoming colonoscopy.
Jerry Q is a 68-year-old Caucasian man with longstanding osteoarthritis who is scheduled to undergo a total left knee arthroplasty in one week. His orthopedic surgeon recommended that he contact his FP for instructions regarding managing apixaban perioperatively. Jerry has been taking apixaban 5 mg bid for the past 9 months due to a history of recurrent deep vein thrombosis (DVT) and pulmonary embolism (PE) (both unprovoked). Mr. Q had been taking warfarin following his first DVT 4 years ago, but, after reporting that INR monitoring was a burden, he was started on apixaban. The patient has normal renal function and is relatively healthy otherwise. How should apixaban be managed before and after his upcoming surgery?
Each year, approximately 15% to 20% of patients taking an oral anticoagulant undergo a procedure that carries a heightened risk for bleeding.1,2 Stopping oral anticoagulation is often necessary before—and sometimes briefly after—many of these procedures in order to minimize the risk of bleeding.3 This means that countless decisions must be made by health care providers each year regarding if, when, and how to pause and resume oral anticoagulation. These decisions are not always straightforward, especially when you consider the risks for thrombosis and bleeding that are unique to the procedure and to the individual patient.
With these variables in mind, the health care provider must make decisions regarding anticoagulation during the periprocedural period based on the following 5 questions:
- Will this patient need to have his/her oral anticoagulant stopped prior to the procedure?
- If the patient’s oral anticoagulation needs to be held, when should it be stopped and for how long?
- Will periprocedural bridging with a parenteral anticoagulant be necessary prior to the procedure?
- When should the patient resume his or her oral anticoagulant after the procedure, and at what dosage?
- Will bridging with a parenteral anticoagulant be necessary after the procedure?
Before addressing these 5 questions, though, physicians must assess patients’ thrombotic and bleeding risks.4-6
Anticoagulant regimens and the risks of discontinuing them
The 2 most common indications for long-term oral anticoagulation are venous thromboembolism (VTE), which occurs in approximately one million Americans every year,7,8 and stroke prevention in the setting of AF (AF occurs in 3-6 million US adults per year).6
Warfarin is also often used in patients with mechanical heart valves for long-term stroke prevention; however, direct oral anticoagulants (DOACs) are not recommended for patients with mechanical heart valves because trials have not yet demonstrated their safety or efficacy in this population.4,5,9
Who’s at highest risk for an acute thromboembolic event?
When planning for interruptions in oral anticoagulation, it is important to identify patients at highest risk for an acute thromboembolic event. Patients with 10% or higher annual risk for VTE or ischemic stroke are generally placed into this high-risk category (TABLE 13,5,6,9-11).3 Keep in mind that the absolute risk for thromboembolism during a brief period of oral coagulation interruption is relatively low, even in those patients considered to be at high risk. Using a mathematical approach (although simplistic), a patient with a 10% annual risk for a thromboembolic event would have <0.3% chance for developing such an event in the acute phase, even if their anticoagulation was withheld for up to 10 days ([10%/365 days] × 10 days).
Patients with mechanical heart valves. Nearly all patients with a mechanical heart valve are at moderate to high risk for ischemic stroke.3
For patients with AF, the CHADS2 and CHA2DS2-VASc scoring tools can be used to estimate annual thrombosis risk based on the presence of risk factors (TABLE 210,11).6,9-11 It should be noted, however, that these scoring tools have not been validated specifically for periprocedural risk estimations. Nonetheless, the latest 2017 American College of Cardiology (ACC) guidelines recommend the use of the CHA2DS2-VASc scoring tool for making decisions regarding perioperative bridging in patients with AF.11
Patients with previous VTE. Multiple aspects of a patient’s past medical history need to be taken into account when estimating annual and acute risk for VTE. Patients at the highest risk for VTE recurrence (annual VTE risk ≥10%) include those with recent VTE (past 90 days), active malignancy, and/or severe thrombophilias (TABLE 13,5,6,9-11).3,5,6 Patients without any of these features can still be at moderate risk for recurrent VTE, as a single VTE without a clear provoking factor can confer a 5% to 10% annualized risk for recurrence.12,13 Previous proximal DVT and PE are associated with a higher risk for recurrence than a distal DVT, and males have a higher recurrence risk than females.5,12 There are scoring tools, such as DASH (D-dimer, Age, Sex, Hormones) and the “Men Continue and HERDOO2,” that can help estimate annualized risk for VTE recurrence; however, they are not validated (nor particularly useful) when making decisions in the perioperative period.14,15
Additional risk factors. Consider additional risk factors for thromboembolism, including estrogen/hormone replacement therapy, pregnancy, leg or hip fractures, immobility, trauma, spinal cord injury, central venous lines, congestive heart failure, thrombophilia, increased age, obesity, and varicose veins.5,16
In addition, some surgeries have a higher inherent risk for thrombosis. Major orthopedic surgery (knee and hip arthroplasty, hip fracture surgery) and surgeries for major trauma or spinal cord injuries are associated with an exceedingly high rate of VTE.17 Similarly, coronary artery bypass surgery, heart valve replacement, and carotid endarterectomies carry the highest risk for acute ischemic stroke.3
Who’s at highest risk for bleeding?
Establishing the bleeding risk associated with a procedure is imperative prior to urgent and elective surgeries to help determine when anticoagulation therapy should be discontinued and reinitiated, as well as whether bridging therapy is appropriate. The 2012 CHEST guidelines state that bleeding risk should be assessed based on timing of anticoagulation relative to surgery and whether the anticoagulation is being used as prophylaxis for, or treatment of, thromboembolism.3 Categorizing procedures as having a minimal, low, or high risk for bleeding can be helpful in making anticoagulation decisions (TABLE 3).3,18-21
In addition to the bleeding risk associated with procedures, patient-specific factors need to be considered. A bleeding event within the past 3 months, platelet abnormalities, a supratherapeutic INR at the time of surgery, a history of bleeding from previous bridging, a bleed history with a similar procedure, and a high HAS-BLED (Hypertension, Abnormal renal or liver function, Stroke, Bleeding history or predisposition, Labile INR, Elderly, Drugs/alcohol usage) score are all factors that elevate the risk for perioperative bleeding.10,11 Although validated only in patients taking warfarin, the HAS-BLED scoring system can be utilized in patients with AF to estimate annual risk for major bleeding (TABLE 210,11).10
With this risk information in mind, it’s time to move on to the 5 questions you’ll need to ask.
1. Should the patient’s oral anticoagulation be stopped prior to the upcoming procedure?
The answer, of course, hinges on the patient’s risk of bleeding.
Usually, it is not necessary to withhold any doses of oral anticoagulation if your patient is scheduled for a procedure with minimal risk for bleeding (TABLE 33,18-21).3 However, it may be reasonable to stop anticoagulation if your patient has additional features that predispose to high bleeding risk (eg, hemophilia, Von Willebrand disease, etc). The CHEST guidelines recommend adding an oral prohemostatic agent (eg, tranexamic acid) if anticoagulation will be continued during a dental procedure.3
If your patient is undergoing any other procedure that has a low to high risk for bleeding, oral anticoagulation should be withheld prior to the procedure in most instances,3,11 although there are exceptions. For example, cardiac procedures, such as AF catheter ablation and cardiac pacemaker placement, are often performed with uninterrupted oral anticoagulation despite their bleeding risk category.3
When in doubt, discuss the perceived bleeding and clotting risks directly with the specialist performing the procedure. In patients who have had a VTE or ischemic stroke within the past 3 months, consider postponing the invasive procedure until the patient is beyond this period of highest thrombotic risk.11
2. How far in advance of the procedure should the oral anticoagulant be withheld?
Warfarin may need to be stopped anywhere from 2 to 5 days prior to the procedure, depending on a number of variables.
Warfarin has a half-life of approximately 36 hours, so it can take 3 to 5 days for warfarin concentrations to drop to safe levels for procedures with low to moderate bleeding risk and 5 to 7 days for procedures with high bleeding risk.21 The 2012 CHEST guidelines recommend that warfarin therapy be discontinued 5 days prior to surgery to minimize the risk for bleeding.3 The Anticoagulation Forum, a leading expert panel that produced a set of useful anticoagulation guidelines in 2016, recommends stopping warfarin 4 to 5 days prior to a procedure.21 If the provider chooses to withhold warfarin before a procedure with minimal bleeding risk, it should be stopped 2 to 3 days prior.3
Consider checking INR values the week before. A 2017 consensus statement from the ACC recommends that the timing of warfarin discontinuation be based on an INR value taken 5 to 7 days prior to the surgical procedure.11 This allows for a more tailored approach to preparing the patient for surgery. If the INR is below goal range, warfarin may need to be withheld for only 3 to 4 days prior to a procedure. Conversely, INRs above goal range may require warfarin to be held 6 or more days, depending on the degree of INR elevation.
While not always feasible in clinical practice, the CHEST guidelines recommend obtaining an INR value the day prior to the procedure to determine if the INR value is low enough to proceed with surgery, or if a low dose of oral vitamin K needs to be administered to ensure that the INR is in a safe range the following day.3
DOACs
DOACs can be withheld for much shorter durations preoperatively than warfarin.
When withholding anticoagulants, the goal is to have a low amount of anticoagulant effect (12%-25%) present during low-risk procedures and a nominal amount of anticoagulant effect (3%-6%) present for high-risk procedures.20 DOACs have much shorter half-lives than warfarin (7-19 hours vs 36-48 hours, respectively), so they can be withheld for much shorter durations preoperatively.20 For patients undergoing procedures that are considered to have a minimal risk for bleeding (such as minor dental and dermatologic procedures), DOACs do not generally need to be withheld; however, it may be ideal to time the procedure when the DOAC is at a trough concentration (before the next dose is due).3
DOACs generally need to be withheld for only 1 to 3 days prior to major surgical procedures in patients with normal renal function (creatinine clearance [CrCl] >30 mL/min using the Cockcroft-Gault formula).20 The available oral direct factor Xa inhibitors (apixaban, rivaroxaban, and edoxaban) should generally be stopped 24 hours prior to a procedure that has a low bleeding risk, and 48 hours prior to procedures with high bleeding risk (TABLE 411,20).20 These medications may need to be withheld for an additional 1 to 2 days in patients with acute kidney injury or stage IV kidney disease.20
Dabigatran. About 80% of dabigatran is excreted renally, so its elimination is much more dependent on renal function than is that of the oral direct factor Xa inhibitors.20 Therefore, it generally needs to be withheld for at least 1 to 2 days longer than the oral factor Xa inhibitors unless CrCl >80 mL/min (TABLE 411,20).20
3. Is preoperative bridging with parenteral anticoagulation necessary?
In certain instances, patients who have a high thromboembolic risk and are discontinuing warfarin therapy may require bridging therapy with a low-molecular-weight heparin (LMWH) or unfractionated heparin (UFH). If a patient’s CrCl is <30 mL/min, then UFH is the preferred agent for perioperative bridging.21
But before any decision is made, it’s best to have a good understanding of what the guidelines—and the literature—have to say.
Key studies and guidelines
The 2012 CHEST guidelines recommend providing bridge therapy for any patient at high risk for thromboembolism (>10% annual risk) and consideration of bridge therapy in the setting of moderate clotting risk (5%-10% annual risk), depending on specific patient and procedural risk factors (TABLE 13,5,6,9-11).3
In 2015, a landmark clinical trial was published that significantly shaped how patients taking warfarin are managed periprocedurally.22 The Bridge (Bridging anticoagulation in patients who require temporary interruption of warfarin therapy for an elective invasive procedure or surgery) trial was the first prospective, randomized controlled trial to assess the efficacy and safety of parenteral bridging in patients with AF taking warfarin and undergoing an elective surgery.
Patients in the trial received either dalteparin at a therapeutic dose of 100 IU/kg or a matching placebo administered subcutaneously bid from 3 days before the procedure until 24 hours before the procedure, and then for 5 to 10 days after the procedure. The incidence of thromboembolic events was not significantly lower in the dalteparin group than in the placebo group (0.3% vs 0.4%, respectively; P=.73), while major bleeding rates were nearly 3-fold higher in the dalteparin group (3.2% vs 1.3%; P=.005). The trial concluded that placebo “was noninferior to perioperative bridging with LMWH for the prevention of arterial thromboembolism and decreased the risk of major bleeding.”22
Patients excluded from the trial included those with a mechanical heart valve, or a recent (within 3 months) embolism, stroke, or TIA, and only 3% of enrolled patients would have been classified as having a high bleeding risk according to CHEST guidelines.3,22
A prospective observational registry study produced similar findings and found that those patients who received bridging had more bleeding events and a higher incidence of myocardial infarction, stroke or systemic embolism, major bleeding, hospitalization, or death within 30 days than those who did not receive bridging.23 Other retrospective cohort studies comparing bridging to no bridging strategies in patients taking warfarin for VTE, mechanical heart valves, or AF have also failed to show a reduction in the incidence of thrombotic events with LMWH bridging.24,25
In 2016, the European Society of Cardiology suggested that “bridging does not seem to be beneficial, except in patients with mechanical heart valves.”26 Similarly, the 2016 Anticoagulation Forum guidelines state that “most patients with VTE can safely interrupt warfarin for invasive procedures without bridge therapy,” and that bridge therapy should be “reserved for those at highest recurrent VTE risk (eg, VTE within the previous month; prior history of recurrent VTE during anticoagulation therapy interruption; undergoing a procedure with high inherent risk for VTE, such as joint replacement surgery or major abdominal cancer resection).”21 They go on to state that even in these high-risk groups, the clinical decision to use bridging therapy needs to carefully weigh the benefits against the potential risks of bleeding.21
Controversy also surrounds the intensity of LMWH bridging. The Anticoagulation Forum guidelines state that the use of prophylactic rather than therapeutic dose LMWH may be considered, while the CHEST guidelines do not make a firm recommendation regarding LMWH dose while bridging.3,21 Ultimately, in patients who receive perioperative bridging with LMWH, the CHEST guidelines recommend that it should be stopped 24 hours prior to the procedure and resumed in accordance with the bleeding risk of the procedure (ie, prophylactic doses may be appropriate within 24 hours postprocedure, while full treatment doses may need to be delayed for 48 to 72 hours if surgical bleeding risk is high).3 UFH bridge therapy may be stopped 4 to 6 hours prior to surgery.3
DOACs. Given the rapid onset and relatively short half-lives of DOACs, use of a parenteral bridging agent is generally not necessary or recommended before or after an invasive procedure in patients taking a DOAC.20
4. When should oral anticoagulation be resumed postoperatively, and at what intensity?
Warfarin can generally be resumed the same day as the procedure (in the evening), assuming there are no active bleeding complications.3,11 Once fully reversed, it generally takes around 5 days for warfarin to become fully therapeutic, so it can be started soon after surgery without increasing the risk for early postoperative bleeding.20
DOACs. Consider the patient’s individual and procedural risks for bleeding when determining when to resume a DOAC postoperatively. That’s because unlike warfarin, which takes several days to take full effect, DOACs provide a nearly immediate anticoagulation effect.20,21 For procedures that have a low bleeding risk, it is recommended to resume therapeutic anticoagulation 24 hours after the procedure has ended.3,11,20 For procedures that have a high risk for bleeding, resumption of therapeutic anticoagulation should be delayed until 48 to 72 hours after the procedure has ended.3,11,20
5. Is postoperative bridging with parenteral anticoagulation necessary?
Warfarin. If a patient was deemed to be at sufficient VTE risk to be bridged preoperatively, then that patient likely also should be bridged postoperatively, particularly if the surgery itself is associated with a heightened thrombotic risk. While warfarin can generally be resumed postoperatively the same day as the procedure, full therapeutic doses of a LMWH should not be initiated sooner than 24 hours postoperatively, and initiation should be delayed for 48 to 72 hours for procedures with the highest bleeding risk (such as neurosurgery).3,11,21 Prophylactic doses of LMWH can generally be resumed as early as 12 hours postoperatively for procedures with high VTE risk (such as major orthopedic surgery).17
DOACs. In patients undergoing a procedure that carries both a high thromboembolic and high bleeding risk (such as major orthopedic surgery), initiation of a full-dose DOAC may need to be delayed for 2 to 3 days; however, more immediate VTE prophylaxis is usually necessary.3,17 Prophylaxis after such procedures can begin 12 hours after the procedure with a low-intensity LMWH, which should be continued until it is deemed safe to resume full-intensity DOAC therapy.3,17,18 If the patient is undergoing major orthopedic surgery, an FDA-approved prophylactic dose of a DOAC could be a temporary alternative to LMWH.27
Ms. P’s upcoming colonoscopy may require a biopsy and would be classified as a procedure with low bleeding risk (per TABLE 3), so warfarin should be withheld prior to her procedure. You could check her INR 5 to 7 days before her colonoscopy to guide how many doses need to be withheld; however, given the patient’s tight INR control over the previous 6 months, you can assume her INR will be in goal range at that check. As a result, you recommend that she avoid an extra INR check and stop taking her warfarin 5 days prior to the colonoscopy.
Ms. P has a CHA2DS2VASc score of 3, which puts her at a relatively low risk for acute ischemic stroke over the next 1 to 2 weeks. Given the results of the BRIDGE trial, you recommend no parenteral bridging agent before or after her procedure. You also recommend that the patient resume her usual dose of warfarin the same day as her procedure (in the evening) unless her gastroenterologist recommends otherwise. You schedule her for a follow-up INR 5 to 7 days after her colonoscopy.
Mr. Q’s total knee arthroplasty (TKA)—a procedure associated with a high risk of bleeding—requires an interruption in his apixaban therapy. Additionally, he is at high risk for recurrent thromboembolism, given his history of recurrent, unprovoked DVTs; however, he is past the highest risk period (VTE within the past 3 months; his last one was 9 months ago). He is otherwise healthy and has normal renal function, so his apixaban should be withheld for a total of 4 doses (48 hours) prior to his procedure. He should resume his full dose of apixaban 48 to 72 hours after his procedure to minimize the risk for bleeding.
However, given that a TKA is a procedure associated with a high rate of postoperative VTE, initiate prophylactic anticoagulation (such as enoxaparin 40 mg subcutaneously daily or apixaban 2.5 mg PO bid) about 12 hours after the procedure and continue it until full-dose apixaban is resumed.
CORRESPONDENCE
Jeremy Vandiver, PharmD, BCPS, University of Wyoming School of Pharmacy, 1000 E. University Ave., Dept. 3375, Laramie, WY 82071; jvandive@uwyo.edu.
1. Connelly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
2. Steinberg BA, Kim S, Piccini JP, et al. Use and associated risks of concomitant aspirin therapy with oral anticoagulation in patients with atrial fibrillation: insights from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Registry. Circulation. 2013;128:721-728.
3. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e326S-e350S.
4. Adam SS, McDuffie JR, Ortel TL, et al. Comparative effectiveness of warfarin and newer oral anticoagulants for the long-term prevention and treatment of arterial and venous thromboembolism. Department of Veteran Affairs Evidence-Based Synthesis Project #09-010; 2012. Available at: https://www.ncbi.nlm.nih.gov/books/NBK97541/. Accessed October 15, 2017.
5. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and Expert Panel Report. Chest. 2016;149:315-352.
6. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. J Am Coll Cardiol. 2014;64:2246-2280.
7. Centers for Disease Control and Prevention. Venous thromboembolism in adult hospitalizations — United States, 2007-2009. MMWR Morb Mortal Wkly Rep. 2012 June 8;61:401-404. Available at https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6122a1.htm. Accessed October 15, 2017.
8. Anderson FA, Wheeler HB, Goldberg HJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med. 1991;151:933-938.
9. Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012): The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2012;33:2451-2496.
10. Garwood CL, Korkis B, Grande D, et al. Anticoagulation bridge therapy in patients with atrial fibrillation: recent updates provide a rebalance of risk and benefit. Pharmacotherapy. 2017;37:712-714.
11. Doherty JU, Gluckman TJ, Hucker WJ, et al. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2017;69:871-898.
12. Douketis J, Tosetto A, Marcucci M, et al. Patient-level meta-analysis: effect of measurement timing, threshold, and patient age on ability of D-dimer testing to assess recurrence risk after unprovoked venous thromboembolism. Ann Intern Med. 2010;153:523-531.
13. Becattini C, Agnelli G, Schenone A, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med. 2012;366:1959-1967.
14. Tosetto A, Testa S, Martinelli I, et al. External validation of the DASH prediction rule: a retrospective cohort study. J Thromb Haemost. 2017;15:1963-1970.
15. Rodger MA, Le Gal G, Anderson DR, et al. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. BMJ. 2017;356:j1065.
16. Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism.
17. Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines, 8th ed. Chest. 2008;133:381S-453S.
18. Spyropoulos AC, Douketis JD. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood. 2012;120:2954-2962.
19. Eisen GM, Baron TH, Dominitz JA, et al. Guideline on the management of anticoagulation and antiplatelet therapy for endoscopic procedures. Gastrointest Endosc. 2002;55:775-779.
20. Burnett AE, Mahan CE, Vazquez SR. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis. 2016;41:206-232.
21. Witt DM, Clark NP, Kaatz S, et al. Guidance for the practical management of warfarin therapy in the treatment of venous thromboembolism. J Thromb Thrombolysis. 2016;41:187-205.
22. Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823-833.
23. Steinberg BA, Peterson ED, Kim S, et al. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circulation. 2015;131: 488-494.
24. Clark NP, Witt DM, Davies LE, et al. Bleeding, recurrent venous thromboembolism, and mortality risks during warfarin interruption for invasive procedures. JAMA Intern Med. 2015;175;1163-1168.
25. Sjögren V, Grzymala-Lubanski B, Renlund H, et al. Safety and efficacy of bridging with low-molecular-weight heparin during temporary interruptions of warfarin: a register-based cohort study. Clin Appl Thromb Hemost. 2017;23:961-966.
26. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893-2962.
27. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e278S-e325S.
1. Connelly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
2. Steinberg BA, Kim S, Piccini JP, et al. Use and associated risks of concomitant aspirin therapy with oral anticoagulation in patients with atrial fibrillation: insights from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Registry. Circulation. 2013;128:721-728.
3. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e326S-e350S.
4. Adam SS, McDuffie JR, Ortel TL, et al. Comparative effectiveness of warfarin and newer oral anticoagulants for the long-term prevention and treatment of arterial and venous thromboembolism. Department of Veteran Affairs Evidence-Based Synthesis Project #09-010; 2012. Available at: https://www.ncbi.nlm.nih.gov/books/NBK97541/. Accessed October 15, 2017.
5. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and Expert Panel Report. Chest. 2016;149:315-352.
6. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. J Am Coll Cardiol. 2014;64:2246-2280.
7. Centers for Disease Control and Prevention. Venous thromboembolism in adult hospitalizations — United States, 2007-2009. MMWR Morb Mortal Wkly Rep. 2012 June 8;61:401-404. Available at https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6122a1.htm. Accessed October 15, 2017.
8. Anderson FA, Wheeler HB, Goldberg HJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med. 1991;151:933-938.
9. Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012): The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2012;33:2451-2496.
10. Garwood CL, Korkis B, Grande D, et al. Anticoagulation bridge therapy in patients with atrial fibrillation: recent updates provide a rebalance of risk and benefit. Pharmacotherapy. 2017;37:712-714.
11. Doherty JU, Gluckman TJ, Hucker WJ, et al. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2017;69:871-898.
12. Douketis J, Tosetto A, Marcucci M, et al. Patient-level meta-analysis: effect of measurement timing, threshold, and patient age on ability of D-dimer testing to assess recurrence risk after unprovoked venous thromboembolism. Ann Intern Med. 2010;153:523-531.
13. Becattini C, Agnelli G, Schenone A, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med. 2012;366:1959-1967.
14. Tosetto A, Testa S, Martinelli I, et al. External validation of the DASH prediction rule: a retrospective cohort study. J Thromb Haemost. 2017;15:1963-1970.
15. Rodger MA, Le Gal G, Anderson DR, et al. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. BMJ. 2017;356:j1065.
16. Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism.
17. Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines, 8th ed. Chest. 2008;133:381S-453S.
18. Spyropoulos AC, Douketis JD. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood. 2012;120:2954-2962.
19. Eisen GM, Baron TH, Dominitz JA, et al. Guideline on the management of anticoagulation and antiplatelet therapy for endoscopic procedures. Gastrointest Endosc. 2002;55:775-779.
20. Burnett AE, Mahan CE, Vazquez SR. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis. 2016;41:206-232.
21. Witt DM, Clark NP, Kaatz S, et al. Guidance for the practical management of warfarin therapy in the treatment of venous thromboembolism. J Thromb Thrombolysis. 2016;41:187-205.
22. Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823-833.
23. Steinberg BA, Peterson ED, Kim S, et al. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circulation. 2015;131: 488-494.
24. Clark NP, Witt DM, Davies LE, et al. Bleeding, recurrent venous thromboembolism, and mortality risks during warfarin interruption for invasive procedures. JAMA Intern Med. 2015;175;1163-1168.
25. Sjögren V, Grzymala-Lubanski B, Renlund H, et al. Safety and efficacy of bridging with low-molecular-weight heparin during temporary interruptions of warfarin: a register-based cohort study. Clin Appl Thromb Hemost. 2017;23:961-966.
26. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893-2962.
27. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e278S-e325S.
PRACTICE RECOMMENDATIONS
› Don’t stop oral anticoagulation for procedures with minimal bleeding risk, such as minor dermatologic, dental, or ophthalmic procedures. C
› Reserve periprocedural bridging with a parenteral anticoagulant for those patients on warfarin who are at highest risk for thromboembolism (those with severe thrombophilia, active thrombosis, or mechanical heart valves). B
› Stop direct oral anticoagulants 24 to 48 hours prior to most invasive procedures, and do not employ periprocedural bridging. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series