User login
The Diagnosis: PASH (Pyoderma Gangrenosum, Acne, Hidradenitis Suppurativa) Syndrome
Obtaining our patient’s history of hidradenitis suppurativa (HS), a hallmark sterile neutrophilic dermatosis, was key to making the correct diagnosis of PASH (pyoderma gangrenosum, acne, HS) syndrome. In our patient, the history of HS increased the consideration of pyoderma gangrenosum (PG) due to the persistent breast and leg wounds. Additionally, it was important to consider a diagnosis of PG in lesions that were not responding to broad-spectrum antimicrobial treatment. In our patient, the concurrent presentation of draining abscesses in the axillae (Figure, A) and inflammatory nodulocystic facial acne (Figure, B) were additional diagnostic clues that suggested the triad of PASH syndrome.
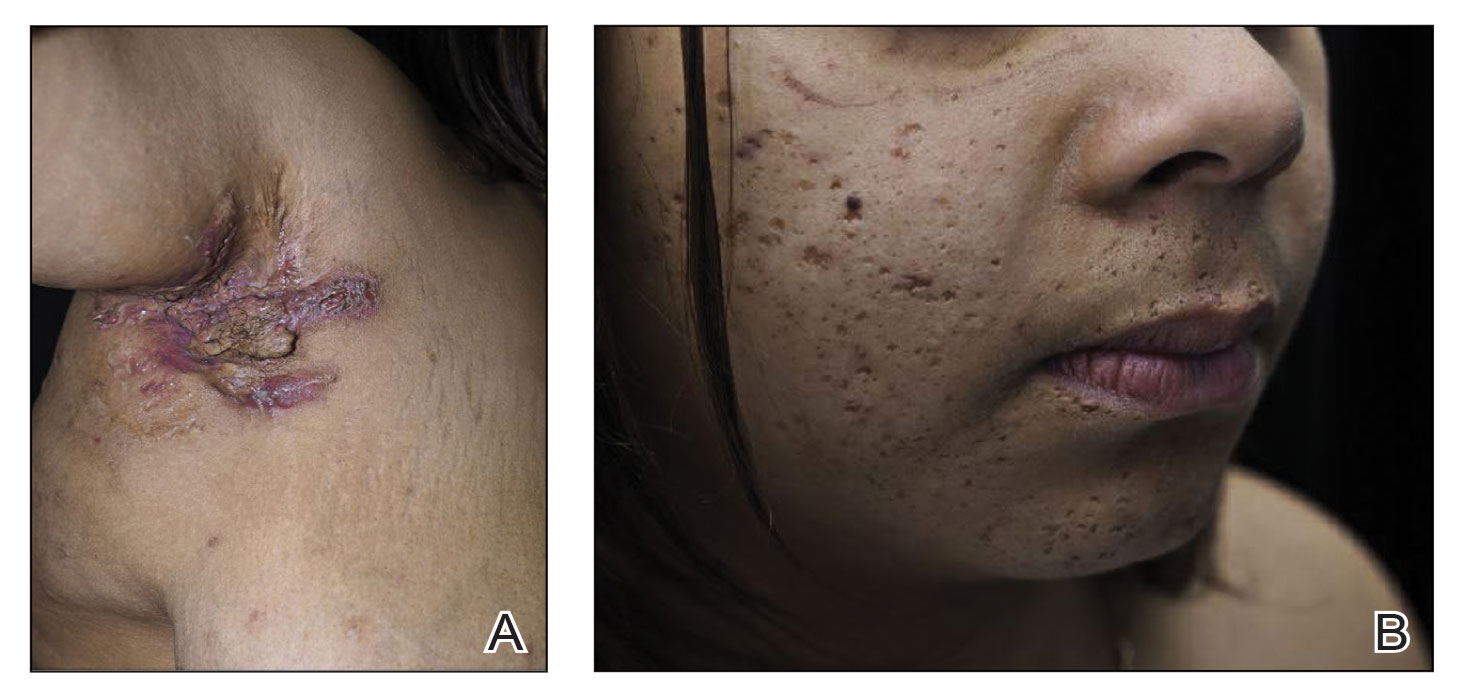
Although SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome also can present with cutaneous features of acne and HS, the lack of bone and joint involvement in our patient made this diagnosis less likely. Calciphylaxis can present as ulcerations on the lower extremities, but it usually presents with a livedolike pattern with overlying black eschar and is unlikely in the absence of underlying metabolic or renal disease. PAPA (pyogenic arthritis, PG, acne) syndrome is characterized by recurrent joint involvement and lacks features of HS. Lastly, our patient was immunocompetent with no risk factors for mycobacterial infection.
PASH syndrome is a rare inherited syndrome, but its constituent inflammatory conditions are ubiquitous. They share a common underlying mechanism consisting of overactivation of the innate immune systems driven by increased production of the inflammatory cytokines IL-1, IL-17, and tumor necrosis factor α, resulting in sterile neutrophilic dermatoses.1 The diagnosis is based on the clinical presentation, as laboratory investigations are nondiagnostic. Biopsies and cultures can be performed to rule out infectious etiologies. Additionally, PASH syndrome is considered part of a larger spectrum of syndromes including PAPA and PAPASH (pyogenic arthritis, acne, PG, HS) syndromes. The absence of pyogenic arthritis distinguishes PASH syndrome from PAPA and PAPASH syndromes.2 Clinically, PASH syndrome and the related sterile neutrophilic dermatoses share the characteristic of pronounced cutaneous involvement that substantially alters the patient’s quality of life. Cigarette smoking is an exacerbating factor and has a well-established association with HS.3 Therefore, smoking cessation should be encouraged in these patients to avoid exacerbation of the disease process.
Maintaining adequate immunosuppression is key to managing the underlying disease processes. Classic immunosuppressive agents such as systemic glucocorticoids and methotrexate may fail to satisfactorily control the disease.4 Treatment options currently are somewhat limited and are aimed at targeting the inflammatory cytokines that propagate the disease. The most consistent responses have been observed with anti–tumor necrosis factor α antagonists such as adalimumab, infliximab, and etanercept.5 Additionally, there is varied response to anakinra, suggesting the importance of selectively targeting IL-1β.6 Unfortunately, misdiagnosis for an infectious etiology is common, and antibiotics and debridement are of limited use for the underlying pathophysiology of PASH syndrome. Importantly, biopsy and debridement often are discouraged due to the risk of pathergy.7
Our case demonstrates the importance of maintaining a high clinical suspicion for immune-mediated lesions that are refractory to antimicrobial agents. Additionally, prior history of multiple neutrophilic dermatoses should prompt consideration for the PASH/PAPA/PAPASH disease spectrum. Early and accurate identification of neutrophilic dermatoses such as PG and HS are crucial to initiating proper cytokine-targeting treatment and achieving disease remission.
- Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562.
- Genovese G, Moltrasio C, Garcovich S, et al. PAPA spectrum disorders. G Ital Dermatol Venereol. 2020;155:542-550.
- König A, Lehmann C, Rompel R, et al. Cigarette smoking as a triggering factor of hidradenitis suppurativa. Dermatology. 1999;198:261-264.
- Ahn C, Negus D, Huang W. Pyoderma gangrenosum: a review of pathogenesis and treatment. Expert Rev Clin Immunol. 2018;14:225-233.
- Saint-Georges V, Peternel S, Kaštelan M, et al. Tumor necrosis factor antagonists in the treatment of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) syndrome. Acta Dermatovenerol Croat. 2018;26:173-178.
- Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
- Patel DK, Locke M, Jarrett P. Pyoderma gangrenosum with pathergy: a potentially significant complication following breast reconstruction. J Plast Reconstr Aesthet Surg. 2017;70:884-892.
The Diagnosis: PASH (Pyoderma Gangrenosum, Acne, Hidradenitis Suppurativa) Syndrome
Obtaining our patient’s history of hidradenitis suppurativa (HS), a hallmark sterile neutrophilic dermatosis, was key to making the correct diagnosis of PASH (pyoderma gangrenosum, acne, HS) syndrome. In our patient, the history of HS increased the consideration of pyoderma gangrenosum (PG) due to the persistent breast and leg wounds. Additionally, it was important to consider a diagnosis of PG in lesions that were not responding to broad-spectrum antimicrobial treatment. In our patient, the concurrent presentation of draining abscesses in the axillae (Figure, A) and inflammatory nodulocystic facial acne (Figure, B) were additional diagnostic clues that suggested the triad of PASH syndrome.

Although SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome also can present with cutaneous features of acne and HS, the lack of bone and joint involvement in our patient made this diagnosis less likely. Calciphylaxis can present as ulcerations on the lower extremities, but it usually presents with a livedolike pattern with overlying black eschar and is unlikely in the absence of underlying metabolic or renal disease. PAPA (pyogenic arthritis, PG, acne) syndrome is characterized by recurrent joint involvement and lacks features of HS. Lastly, our patient was immunocompetent with no risk factors for mycobacterial infection.
PASH syndrome is a rare inherited syndrome, but its constituent inflammatory conditions are ubiquitous. They share a common underlying mechanism consisting of overactivation of the innate immune systems driven by increased production of the inflammatory cytokines IL-1, IL-17, and tumor necrosis factor α, resulting in sterile neutrophilic dermatoses.1 The diagnosis is based on the clinical presentation, as laboratory investigations are nondiagnostic. Biopsies and cultures can be performed to rule out infectious etiologies. Additionally, PASH syndrome is considered part of a larger spectrum of syndromes including PAPA and PAPASH (pyogenic arthritis, acne, PG, HS) syndromes. The absence of pyogenic arthritis distinguishes PASH syndrome from PAPA and PAPASH syndromes.2 Clinically, PASH syndrome and the related sterile neutrophilic dermatoses share the characteristic of pronounced cutaneous involvement that substantially alters the patient’s quality of life. Cigarette smoking is an exacerbating factor and has a well-established association with HS.3 Therefore, smoking cessation should be encouraged in these patients to avoid exacerbation of the disease process.
Maintaining adequate immunosuppression is key to managing the underlying disease processes. Classic immunosuppressive agents such as systemic glucocorticoids and methotrexate may fail to satisfactorily control the disease.4 Treatment options currently are somewhat limited and are aimed at targeting the inflammatory cytokines that propagate the disease. The most consistent responses have been observed with anti–tumor necrosis factor α antagonists such as adalimumab, infliximab, and etanercept.5 Additionally, there is varied response to anakinra, suggesting the importance of selectively targeting IL-1β.6 Unfortunately, misdiagnosis for an infectious etiology is common, and antibiotics and debridement are of limited use for the underlying pathophysiology of PASH syndrome. Importantly, biopsy and debridement often are discouraged due to the risk of pathergy.7
Our case demonstrates the importance of maintaining a high clinical suspicion for immune-mediated lesions that are refractory to antimicrobial agents. Additionally, prior history of multiple neutrophilic dermatoses should prompt consideration for the PASH/PAPA/PAPASH disease spectrum. Early and accurate identification of neutrophilic dermatoses such as PG and HS are crucial to initiating proper cytokine-targeting treatment and achieving disease remission.
The Diagnosis: PASH (Pyoderma Gangrenosum, Acne, Hidradenitis Suppurativa) Syndrome
Obtaining our patient’s history of hidradenitis suppurativa (HS), a hallmark sterile neutrophilic dermatosis, was key to making the correct diagnosis of PASH (pyoderma gangrenosum, acne, HS) syndrome. In our patient, the history of HS increased the consideration of pyoderma gangrenosum (PG) due to the persistent breast and leg wounds. Additionally, it was important to consider a diagnosis of PG in lesions that were not responding to broad-spectrum antimicrobial treatment. In our patient, the concurrent presentation of draining abscesses in the axillae (Figure, A) and inflammatory nodulocystic facial acne (Figure, B) were additional diagnostic clues that suggested the triad of PASH syndrome.

Although SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome also can present with cutaneous features of acne and HS, the lack of bone and joint involvement in our patient made this diagnosis less likely. Calciphylaxis can present as ulcerations on the lower extremities, but it usually presents with a livedolike pattern with overlying black eschar and is unlikely in the absence of underlying metabolic or renal disease. PAPA (pyogenic arthritis, PG, acne) syndrome is characterized by recurrent joint involvement and lacks features of HS. Lastly, our patient was immunocompetent with no risk factors for mycobacterial infection.
PASH syndrome is a rare inherited syndrome, but its constituent inflammatory conditions are ubiquitous. They share a common underlying mechanism consisting of overactivation of the innate immune systems driven by increased production of the inflammatory cytokines IL-1, IL-17, and tumor necrosis factor α, resulting in sterile neutrophilic dermatoses.1 The diagnosis is based on the clinical presentation, as laboratory investigations are nondiagnostic. Biopsies and cultures can be performed to rule out infectious etiologies. Additionally, PASH syndrome is considered part of a larger spectrum of syndromes including PAPA and PAPASH (pyogenic arthritis, acne, PG, HS) syndromes. The absence of pyogenic arthritis distinguishes PASH syndrome from PAPA and PAPASH syndromes.2 Clinically, PASH syndrome and the related sterile neutrophilic dermatoses share the characteristic of pronounced cutaneous involvement that substantially alters the patient’s quality of life. Cigarette smoking is an exacerbating factor and has a well-established association with HS.3 Therefore, smoking cessation should be encouraged in these patients to avoid exacerbation of the disease process.
Maintaining adequate immunosuppression is key to managing the underlying disease processes. Classic immunosuppressive agents such as systemic glucocorticoids and methotrexate may fail to satisfactorily control the disease.4 Treatment options currently are somewhat limited and are aimed at targeting the inflammatory cytokines that propagate the disease. The most consistent responses have been observed with anti–tumor necrosis factor α antagonists such as adalimumab, infliximab, and etanercept.5 Additionally, there is varied response to anakinra, suggesting the importance of selectively targeting IL-1β.6 Unfortunately, misdiagnosis for an infectious etiology is common, and antibiotics and debridement are of limited use for the underlying pathophysiology of PASH syndrome. Importantly, biopsy and debridement often are discouraged due to the risk of pathergy.7
Our case demonstrates the importance of maintaining a high clinical suspicion for immune-mediated lesions that are refractory to antimicrobial agents. Additionally, prior history of multiple neutrophilic dermatoses should prompt consideration for the PASH/PAPA/PAPASH disease spectrum. Early and accurate identification of neutrophilic dermatoses such as PG and HS are crucial to initiating proper cytokine-targeting treatment and achieving disease remission.
- Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562.
- Genovese G, Moltrasio C, Garcovich S, et al. PAPA spectrum disorders. G Ital Dermatol Venereol. 2020;155:542-550.
- König A, Lehmann C, Rompel R, et al. Cigarette smoking as a triggering factor of hidradenitis suppurativa. Dermatology. 1999;198:261-264.
- Ahn C, Negus D, Huang W. Pyoderma gangrenosum: a review of pathogenesis and treatment. Expert Rev Clin Immunol. 2018;14:225-233.
- Saint-Georges V, Peternel S, Kaštelan M, et al. Tumor necrosis factor antagonists in the treatment of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) syndrome. Acta Dermatovenerol Croat. 2018;26:173-178.
- Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
- Patel DK, Locke M, Jarrett P. Pyoderma gangrenosum with pathergy: a potentially significant complication following breast reconstruction. J Plast Reconstr Aesthet Surg. 2017;70:884-892.
- Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562.
- Genovese G, Moltrasio C, Garcovich S, et al. PAPA spectrum disorders. G Ital Dermatol Venereol. 2020;155:542-550.
- König A, Lehmann C, Rompel R, et al. Cigarette smoking as a triggering factor of hidradenitis suppurativa. Dermatology. 1999;198:261-264.
- Ahn C, Negus D, Huang W. Pyoderma gangrenosum: a review of pathogenesis and treatment. Expert Rev Clin Immunol. 2018;14:225-233.
- Saint-Georges V, Peternel S, Kaštelan M, et al. Tumor necrosis factor antagonists in the treatment of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) syndrome. Acta Dermatovenerol Croat. 2018;26:173-178.
- Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
- Patel DK, Locke M, Jarrett P. Pyoderma gangrenosum with pathergy: a potentially significant complication following breast reconstruction. J Plast Reconstr Aesthet Surg. 2017;70:884-892.
A 28-year-old Black woman presented to the hospital for evaluation of worsening leg wounds as well as a similar eroding plaque on the left breast of 1 month’s duration. Broad-spectrum antibiotics prescribed during a prior emergency department visit resulted in no improvement. Her medical history was notable for hidradenitis suppurativa that previously was well controlled on adalimumab prior to discontinuation 1 year prior. A review of systems was negative for fever, chills, shortness of breath, chest pain, night sweats, and arthralgia. The patient had discontinued the antibiotics and was not taking any other medications at the time of presentation. She reported a history of smoking cigarettes (5 pack years). Physical examination revealed hyperkeratotic eroded plaques with violaceous borders circumferentially around the left breast (top) and legs with notable undermining (bottom). Inflammatory nodulocystic acne of the face as well as sinus tract formation with purulent drainage in the axillae also were present. Laboratory workup revealed an elevated erythrocyte sedimentation rate (116 mm/h [reference range, <20 mm/h]). Computed tomography of the leg wound was negative for soft-tissue infection. Aerobic and anaerobic tissue cultures demonstrated no growth.

