User login
Scarring Head Wound
The Diagnosis: Brunsting-Perry Cicatricial Pemphigoid
Physical examination and histopathology are paramount in diagnosing Brunsting-Perry cicatricial pemphigoid (BPCP). In our patient, histopathology showed subepidermal blistering with a mixed superficial dermal inflammatory cell infiltrate. Direct immunofluorescence was positive for linear IgG and C3 antibodies along the basement membrane. The scarring erosions on the scalp combined with the autoantibody findings on direct immunofluorescence were consistent with BPCP. He was started on dapsone 100 mg daily and demonstrated complete resolution of symptoms after 10 months, with the exception of persistent scarring hair loss (Figure).

Brunsting-Perry cicatricial pemphigoid is a rare dermatologic condition. It was first defined in 1957 when Brunsting and Perry1 examined 7 patients with cicatricial pemphigoid that predominantly affected the head and neck region, with occasional mucous membrane involvement but no mucosal scarring. Characteristically, BPCP manifests as scarring herpetiform plaques with varied blisters, erosions, crusts, and scarring.1 It primarily affects middle-aged men.2
Historically, BPCP has been considered a variant of cicatricial pemphigoid (now known as mucous membrane pemphigoid), bullous pemphigoid, or epidermolysis bullosa acquisita.3 The antigen target has not been established clearly; however, autoantibodies against laminin 332, collagen VII, and BP180 and BP230 have been proposed.2,4,5 Jacoby et al6 described BPCP on a spectrum with bullous pemphigoid and cicatricial pemphigoid, with primarily circulating autoantibodies on one end and tissue-fixed autoantibodies on the other.
The differential for BPCP also includes anti-p200 pemphigoid and anti–laminin 332 pemphigoid. Anti-p200 pemphigoid also is known as bullous pemphigoid with antibodies against the 200-kDa protein.7 It may clinically manifest similar to bullous pemphigoid and other subepidermal autoimmune blistering diseases; thus, immunopathologic differentiation can be helpful. Anti–laminin 332 pemphigoid (also known as anti–laminin gamma-1 pemphigoid) is characterized by autoantibodies targeting the laminin 332 protein in the basement membrane zone, resulting in blistering and erosions.8 Similar to BPCP and epidermolysis bullosa aquisita, anti–laminin 332 pemphigoid may affect cephalic regions and mucous membrane surfaces, resulting in scarring and cicatricial changes. Anti–laminin 332 pemphigoid also has been associated with internal malignancy.8 The use of the salt-split skin technique can be utilized to differentiate these entities based on their autoantibody-binding patterns in relation to the lamina densa.
Treatment options for mild BPCP include potent topical or intralesional steroids and dapsone, while more severe cases may require systemic therapy with rituximab, azathioprine, mycophenolate mofetil, or cyclophosphamide.4
This case highlights the importance of histopathologic examination of skin lesions with an unusual history or clinical presentation. Dermatologists should consider BPCP when presented with erosions, ulcerations, or blisters of the head and neck in middle-aged male patients.
- Brunsting LA, Perry HO. Benign pemphigoid? a report of seven cases with chronic, scarring, herpetiform plaques about the head and neck. AMA Arch Derm. 1957;75:489-501. doi:10.1001 /archderm.1957.01550160015002
- Jedlickova H, Neidermeier A, Zgažarová S, et al. Brunsting-Perry pemphigoid of the scalp with antibodies against laminin 332. Dermatology. 2011;222:193-195. doi:10.1159/000322842
- Eichhoff G. Brunsting-Perry pemphigoid as differential diagnosis of nonmelanoma skin cancer. Cureus. 2019;11:E5400. doi:10.7759/cureus.5400
- Asfour L, Chong H, Mee J, et al. Epidermolysis bullosa acquisita (Brunsting-Perry pemphigoid variant) localized to the face and diagnosed with antigen identification using skin deficient in type VII collagen. Am J Dermatopathol. 2017;39:e90-e96. doi:10.1097 /DAD.0000000000000829
- Zhou S, Zou Y, Pan M. Brunsting-Perry pemphigoid transitioning from previous bullous pemphigoid. JAAD Case Rep. 2020;6:192-194. doi:10.1016/j.jdcr.2019.12.018
- Jacoby WD Jr, Bartholome CW, Ramchand SC, et al. Cicatricial pemphigoid (Brunsting-Perry type). case report and immunofluorescence findings. Arch Dermatol. 1978;114:779-781. doi:10.1001/archderm.1978.01640170079018
- Kridin K, Ahmed AR. Anti-p200 pemphigoid: a systematic review. Front Immunol. 2019;10:2466. doi:10.3389/fimmu.2019.02466
- Shi L, Li X, Qian H. Anti-laminin 332-type mucous membrane pemphigoid. Biomolecules. 2022;12:1461. doi:10.3390/biom12101461
The Diagnosis: Brunsting-Perry Cicatricial Pemphigoid
Physical examination and histopathology are paramount in diagnosing Brunsting-Perry cicatricial pemphigoid (BPCP). In our patient, histopathology showed subepidermal blistering with a mixed superficial dermal inflammatory cell infiltrate. Direct immunofluorescence was positive for linear IgG and C3 antibodies along the basement membrane. The scarring erosions on the scalp combined with the autoantibody findings on direct immunofluorescence were consistent with BPCP. He was started on dapsone 100 mg daily and demonstrated complete resolution of symptoms after 10 months, with the exception of persistent scarring hair loss (Figure).

Brunsting-Perry cicatricial pemphigoid is a rare dermatologic condition. It was first defined in 1957 when Brunsting and Perry1 examined 7 patients with cicatricial pemphigoid that predominantly affected the head and neck region, with occasional mucous membrane involvement but no mucosal scarring. Characteristically, BPCP manifests as scarring herpetiform plaques with varied blisters, erosions, crusts, and scarring.1 It primarily affects middle-aged men.2
Historically, BPCP has been considered a variant of cicatricial pemphigoid (now known as mucous membrane pemphigoid), bullous pemphigoid, or epidermolysis bullosa acquisita.3 The antigen target has not been established clearly; however, autoantibodies against laminin 332, collagen VII, and BP180 and BP230 have been proposed.2,4,5 Jacoby et al6 described BPCP on a spectrum with bullous pemphigoid and cicatricial pemphigoid, with primarily circulating autoantibodies on one end and tissue-fixed autoantibodies on the other.
The differential for BPCP also includes anti-p200 pemphigoid and anti–laminin 332 pemphigoid. Anti-p200 pemphigoid also is known as bullous pemphigoid with antibodies against the 200-kDa protein.7 It may clinically manifest similar to bullous pemphigoid and other subepidermal autoimmune blistering diseases; thus, immunopathologic differentiation can be helpful. Anti–laminin 332 pemphigoid (also known as anti–laminin gamma-1 pemphigoid) is characterized by autoantibodies targeting the laminin 332 protein in the basement membrane zone, resulting in blistering and erosions.8 Similar to BPCP and epidermolysis bullosa aquisita, anti–laminin 332 pemphigoid may affect cephalic regions and mucous membrane surfaces, resulting in scarring and cicatricial changes. Anti–laminin 332 pemphigoid also has been associated with internal malignancy.8 The use of the salt-split skin technique can be utilized to differentiate these entities based on their autoantibody-binding patterns in relation to the lamina densa.
Treatment options for mild BPCP include potent topical or intralesional steroids and dapsone, while more severe cases may require systemic therapy with rituximab, azathioprine, mycophenolate mofetil, or cyclophosphamide.4
This case highlights the importance of histopathologic examination of skin lesions with an unusual history or clinical presentation. Dermatologists should consider BPCP when presented with erosions, ulcerations, or blisters of the head and neck in middle-aged male patients.
The Diagnosis: Brunsting-Perry Cicatricial Pemphigoid
Physical examination and histopathology are paramount in diagnosing Brunsting-Perry cicatricial pemphigoid (BPCP). In our patient, histopathology showed subepidermal blistering with a mixed superficial dermal inflammatory cell infiltrate. Direct immunofluorescence was positive for linear IgG and C3 antibodies along the basement membrane. The scarring erosions on the scalp combined with the autoantibody findings on direct immunofluorescence were consistent with BPCP. He was started on dapsone 100 mg daily and demonstrated complete resolution of symptoms after 10 months, with the exception of persistent scarring hair loss (Figure).

Brunsting-Perry cicatricial pemphigoid is a rare dermatologic condition. It was first defined in 1957 when Brunsting and Perry1 examined 7 patients with cicatricial pemphigoid that predominantly affected the head and neck region, with occasional mucous membrane involvement but no mucosal scarring. Characteristically, BPCP manifests as scarring herpetiform plaques with varied blisters, erosions, crusts, and scarring.1 It primarily affects middle-aged men.2
Historically, BPCP has been considered a variant of cicatricial pemphigoid (now known as mucous membrane pemphigoid), bullous pemphigoid, or epidermolysis bullosa acquisita.3 The antigen target has not been established clearly; however, autoantibodies against laminin 332, collagen VII, and BP180 and BP230 have been proposed.2,4,5 Jacoby et al6 described BPCP on a spectrum with bullous pemphigoid and cicatricial pemphigoid, with primarily circulating autoantibodies on one end and tissue-fixed autoantibodies on the other.
The differential for BPCP also includes anti-p200 pemphigoid and anti–laminin 332 pemphigoid. Anti-p200 pemphigoid also is known as bullous pemphigoid with antibodies against the 200-kDa protein.7 It may clinically manifest similar to bullous pemphigoid and other subepidermal autoimmune blistering diseases; thus, immunopathologic differentiation can be helpful. Anti–laminin 332 pemphigoid (also known as anti–laminin gamma-1 pemphigoid) is characterized by autoantibodies targeting the laminin 332 protein in the basement membrane zone, resulting in blistering and erosions.8 Similar to BPCP and epidermolysis bullosa aquisita, anti–laminin 332 pemphigoid may affect cephalic regions and mucous membrane surfaces, resulting in scarring and cicatricial changes. Anti–laminin 332 pemphigoid also has been associated with internal malignancy.8 The use of the salt-split skin technique can be utilized to differentiate these entities based on their autoantibody-binding patterns in relation to the lamina densa.
Treatment options for mild BPCP include potent topical or intralesional steroids and dapsone, while more severe cases may require systemic therapy with rituximab, azathioprine, mycophenolate mofetil, or cyclophosphamide.4
This case highlights the importance of histopathologic examination of skin lesions with an unusual history or clinical presentation. Dermatologists should consider BPCP when presented with erosions, ulcerations, or blisters of the head and neck in middle-aged male patients.
- Brunsting LA, Perry HO. Benign pemphigoid? a report of seven cases with chronic, scarring, herpetiform plaques about the head and neck. AMA Arch Derm. 1957;75:489-501. doi:10.1001 /archderm.1957.01550160015002
- Jedlickova H, Neidermeier A, Zgažarová S, et al. Brunsting-Perry pemphigoid of the scalp with antibodies against laminin 332. Dermatology. 2011;222:193-195. doi:10.1159/000322842
- Eichhoff G. Brunsting-Perry pemphigoid as differential diagnosis of nonmelanoma skin cancer. Cureus. 2019;11:E5400. doi:10.7759/cureus.5400
- Asfour L, Chong H, Mee J, et al. Epidermolysis bullosa acquisita (Brunsting-Perry pemphigoid variant) localized to the face and diagnosed with antigen identification using skin deficient in type VII collagen. Am J Dermatopathol. 2017;39:e90-e96. doi:10.1097 /DAD.0000000000000829
- Zhou S, Zou Y, Pan M. Brunsting-Perry pemphigoid transitioning from previous bullous pemphigoid. JAAD Case Rep. 2020;6:192-194. doi:10.1016/j.jdcr.2019.12.018
- Jacoby WD Jr, Bartholome CW, Ramchand SC, et al. Cicatricial pemphigoid (Brunsting-Perry type). case report and immunofluorescence findings. Arch Dermatol. 1978;114:779-781. doi:10.1001/archderm.1978.01640170079018
- Kridin K, Ahmed AR. Anti-p200 pemphigoid: a systematic review. Front Immunol. 2019;10:2466. doi:10.3389/fimmu.2019.02466
- Shi L, Li X, Qian H. Anti-laminin 332-type mucous membrane pemphigoid. Biomolecules. 2022;12:1461. doi:10.3390/biom12101461
- Brunsting LA, Perry HO. Benign pemphigoid? a report of seven cases with chronic, scarring, herpetiform plaques about the head and neck. AMA Arch Derm. 1957;75:489-501. doi:10.1001 /archderm.1957.01550160015002
- Jedlickova H, Neidermeier A, Zgažarová S, et al. Brunsting-Perry pemphigoid of the scalp with antibodies against laminin 332. Dermatology. 2011;222:193-195. doi:10.1159/000322842
- Eichhoff G. Brunsting-Perry pemphigoid as differential diagnosis of nonmelanoma skin cancer. Cureus. 2019;11:E5400. doi:10.7759/cureus.5400
- Asfour L, Chong H, Mee J, et al. Epidermolysis bullosa acquisita (Brunsting-Perry pemphigoid variant) localized to the face and diagnosed with antigen identification using skin deficient in type VII collagen. Am J Dermatopathol. 2017;39:e90-e96. doi:10.1097 /DAD.0000000000000829
- Zhou S, Zou Y, Pan M. Brunsting-Perry pemphigoid transitioning from previous bullous pemphigoid. JAAD Case Rep. 2020;6:192-194. doi:10.1016/j.jdcr.2019.12.018
- Jacoby WD Jr, Bartholome CW, Ramchand SC, et al. Cicatricial pemphigoid (Brunsting-Perry type). case report and immunofluorescence findings. Arch Dermatol. 1978;114:779-781. doi:10.1001/archderm.1978.01640170079018
- Kridin K, Ahmed AR. Anti-p200 pemphigoid: a systematic review. Front Immunol. 2019;10:2466. doi:10.3389/fimmu.2019.02466
- Shi L, Li X, Qian H. Anti-laminin 332-type mucous membrane pemphigoid. Biomolecules. 2022;12:1461. doi:10.3390/biom12101461
A 60-year-old man presented to a dermatology clinic with a wound on the scalp that had persisted for 11 months. The lesion started as a small erosion that eventually progressed to involve the entire parietal scalp. He had a history of type 2 diabetes mellitus, hypertension, and Graves disease. Physical examination demonstrated a large scar over the vertex scalp with central erosion, overlying crust, peripheral scalp atrophy, hypopigmentation at the periphery, and exaggerated superficial vasculature. Some oral erosions also were observed. A review of systems was negative for any constitutional symptoms. A month prior, the patient had been started on dapsone 50 mg with a prednisone taper by an outside dermatologist and noticed some improvement.

Persistent Wounds Refractory to Broad-Spectrum Antibiotics
The Diagnosis: PASH (Pyoderma Gangrenosum, Acne, Hidradenitis Suppurativa) Syndrome
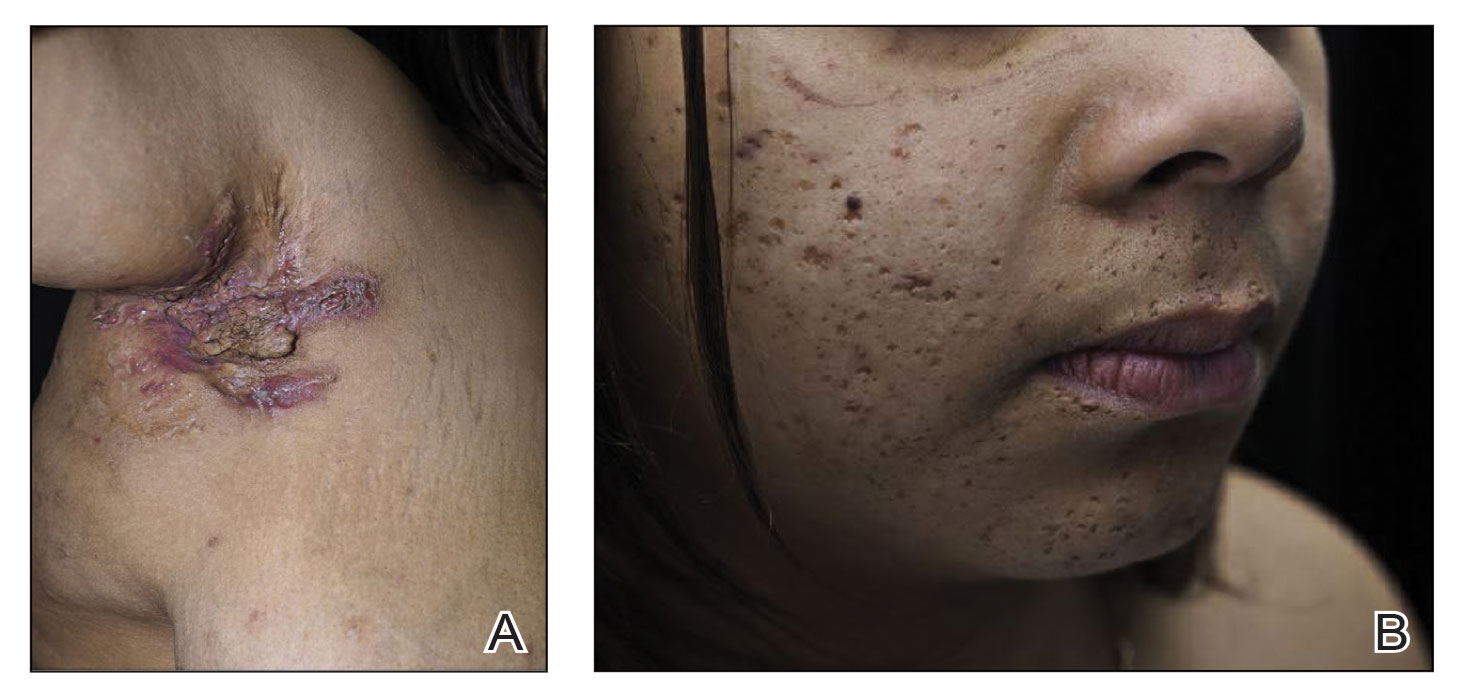
Obtaining our patient’s history of hidradenitis suppurativa (HS), a hallmark sterile neutrophilic dermatosis, was key to making the correct diagnosis of PASH (pyoderma gangrenosum, acne, HS) syndrome. In our patient, the history of HS increased the consideration of pyoderma gangrenosum (PG) due to the persistent breast and leg wounds. Additionally, it was important to consider a diagnosis of PG in lesions that were not responding to broad-spectrum antimicrobial treatment. In our patient, the concurrent presentation of draining abscesses in the axillae (Figure, A) and inflammatory nodulocystic facial acne (Figure, B) were additional diagnostic clues that suggested the triad of PASH syndrome.
Although SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome also can present with cutaneous features of acne and HS, the lack of bone and joint involvement in our patient made this diagnosis less likely. Calciphylaxis can present as ulcerations on the lower extremities, but it usually presents with a livedolike pattern with overlying black eschar and is unlikely in the absence of underlying metabolic or renal disease. PAPA (pyogenic arthritis, PG, acne) syndrome is characterized by recurrent joint involvement and lacks features of HS. Lastly, our patient was immunocompetent with no risk factors for mycobacterial infection.
PASH syndrome is a rare inherited syndrome, but its constituent inflammatory conditions are ubiquitous. They share a common underlying mechanism consisting of overactivation of the innate immune systems driven by increased production of the inflammatory cytokines IL-1, IL-17, and tumor necrosis factor α, resulting in sterile neutrophilic dermatoses.1 The diagnosis is based on the clinical presentation, as laboratory investigations are nondiagnostic. Biopsies and cultures can be performed to rule out infectious etiologies. Additionally, PASH syndrome is considered part of a larger spectrum of syndromes including PAPA and PAPASH (pyogenic arthritis, acne, PG, HS) syndromes. The absence of pyogenic arthritis distinguishes PASH syndrome from PAPA and PAPASH syndromes.2 Clinically, PASH syndrome and the related sterile neutrophilic dermatoses share the characteristic of pronounced cutaneous involvement that substantially alters the patient’s quality of life. Cigarette smoking is an exacerbating factor and has a well-established association with HS.3 Therefore, smoking cessation should be encouraged in these patients to avoid exacerbation of the disease process.
Maintaining adequate immunosuppression is key to managing the underlying disease processes. Classic immunosuppressive agents such as systemic glucocorticoids and methotrexate may fail to satisfactorily control the disease.4 Treatment options currently are somewhat limited and are aimed at targeting the inflammatory cytokines that propagate the disease. The most consistent responses have been observed with anti–tumor necrosis factor α antagonists such as adalimumab, infliximab, and etanercept.5 Additionally, there is varied response to anakinra, suggesting the importance of selectively targeting IL-1β.6 Unfortunately, misdiagnosis for an infectious etiology is common, and antibiotics and debridement are of limited use for the underlying pathophysiology of PASH syndrome. Importantly, biopsy and debridement often are discouraged due to the risk of pathergy.7
Our case demonstrates the importance of maintaining a high clinical suspicion for immune-mediated lesions that are refractory to antimicrobial agents. Additionally, prior history of multiple neutrophilic dermatoses should prompt consideration for the PASH/PAPA/PAPASH disease spectrum. Early and accurate identification of neutrophilic dermatoses such as PG and HS are crucial to initiating proper cytokine-targeting treatment and achieving disease remission.
- Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562.
- Genovese G, Moltrasio C, Garcovich S, et al. PAPA spectrum disorders. G Ital Dermatol Venereol. 2020;155:542-550.
- König A, Lehmann C, Rompel R, et al. Cigarette smoking as a triggering factor of hidradenitis suppurativa. Dermatology. 1999;198:261-264.
- Ahn C, Negus D, Huang W. Pyoderma gangrenosum: a review of pathogenesis and treatment. Expert Rev Clin Immunol. 2018;14:225-233.
- Saint-Georges V, Peternel S, Kaštelan M, et al. Tumor necrosis factor antagonists in the treatment of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) syndrome. Acta Dermatovenerol Croat. 2018;26:173-178.
- Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
- Patel DK, Locke M, Jarrett P. Pyoderma gangrenosum with pathergy: a potentially significant complication following breast reconstruction. J Plast Reconstr Aesthet Surg. 2017;70:884-892.
The Diagnosis: PASH (Pyoderma Gangrenosum, Acne, Hidradenitis Suppurativa) Syndrome
Obtaining our patient’s history of hidradenitis suppurativa (HS), a hallmark sterile neutrophilic dermatosis, was key to making the correct diagnosis of PASH (pyoderma gangrenosum, acne, HS) syndrome. In our patient, the history of HS increased the consideration of pyoderma gangrenosum (PG) due to the persistent breast and leg wounds. Additionally, it was important to consider a diagnosis of PG in lesions that were not responding to broad-spectrum antimicrobial treatment. In our patient, the concurrent presentation of draining abscesses in the axillae (Figure, A) and inflammatory nodulocystic facial acne (Figure, B) were additional diagnostic clues that suggested the triad of PASH syndrome.

Although SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome also can present with cutaneous features of acne and HS, the lack of bone and joint involvement in our patient made this diagnosis less likely. Calciphylaxis can present as ulcerations on the lower extremities, but it usually presents with a livedolike pattern with overlying black eschar and is unlikely in the absence of underlying metabolic or renal disease. PAPA (pyogenic arthritis, PG, acne) syndrome is characterized by recurrent joint involvement and lacks features of HS. Lastly, our patient was immunocompetent with no risk factors for mycobacterial infection.
PASH syndrome is a rare inherited syndrome, but its constituent inflammatory conditions are ubiquitous. They share a common underlying mechanism consisting of overactivation of the innate immune systems driven by increased production of the inflammatory cytokines IL-1, IL-17, and tumor necrosis factor α, resulting in sterile neutrophilic dermatoses.1 The diagnosis is based on the clinical presentation, as laboratory investigations are nondiagnostic. Biopsies and cultures can be performed to rule out infectious etiologies. Additionally, PASH syndrome is considered part of a larger spectrum of syndromes including PAPA and PAPASH (pyogenic arthritis, acne, PG, HS) syndromes. The absence of pyogenic arthritis distinguishes PASH syndrome from PAPA and PAPASH syndromes.2 Clinically, PASH syndrome and the related sterile neutrophilic dermatoses share the characteristic of pronounced cutaneous involvement that substantially alters the patient’s quality of life. Cigarette smoking is an exacerbating factor and has a well-established association with HS.3 Therefore, smoking cessation should be encouraged in these patients to avoid exacerbation of the disease process.
Maintaining adequate immunosuppression is key to managing the underlying disease processes. Classic immunosuppressive agents such as systemic glucocorticoids and methotrexate may fail to satisfactorily control the disease.4 Treatment options currently are somewhat limited and are aimed at targeting the inflammatory cytokines that propagate the disease. The most consistent responses have been observed with anti–tumor necrosis factor α antagonists such as adalimumab, infliximab, and etanercept.5 Additionally, there is varied response to anakinra, suggesting the importance of selectively targeting IL-1β.6 Unfortunately, misdiagnosis for an infectious etiology is common, and antibiotics and debridement are of limited use for the underlying pathophysiology of PASH syndrome. Importantly, biopsy and debridement often are discouraged due to the risk of pathergy.7
Our case demonstrates the importance of maintaining a high clinical suspicion for immune-mediated lesions that are refractory to antimicrobial agents. Additionally, prior history of multiple neutrophilic dermatoses should prompt consideration for the PASH/PAPA/PAPASH disease spectrum. Early and accurate identification of neutrophilic dermatoses such as PG and HS are crucial to initiating proper cytokine-targeting treatment and achieving disease remission.
The Diagnosis: PASH (Pyoderma Gangrenosum, Acne, Hidradenitis Suppurativa) Syndrome
Obtaining our patient’s history of hidradenitis suppurativa (HS), a hallmark sterile neutrophilic dermatosis, was key to making the correct diagnosis of PASH (pyoderma gangrenosum, acne, HS) syndrome. In our patient, the history of HS increased the consideration of pyoderma gangrenosum (PG) due to the persistent breast and leg wounds. Additionally, it was important to consider a diagnosis of PG in lesions that were not responding to broad-spectrum antimicrobial treatment. In our patient, the concurrent presentation of draining abscesses in the axillae (Figure, A) and inflammatory nodulocystic facial acne (Figure, B) were additional diagnostic clues that suggested the triad of PASH syndrome.

Although SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome also can present with cutaneous features of acne and HS, the lack of bone and joint involvement in our patient made this diagnosis less likely. Calciphylaxis can present as ulcerations on the lower extremities, but it usually presents with a livedolike pattern with overlying black eschar and is unlikely in the absence of underlying metabolic or renal disease. PAPA (pyogenic arthritis, PG, acne) syndrome is characterized by recurrent joint involvement and lacks features of HS. Lastly, our patient was immunocompetent with no risk factors for mycobacterial infection.
PASH syndrome is a rare inherited syndrome, but its constituent inflammatory conditions are ubiquitous. They share a common underlying mechanism consisting of overactivation of the innate immune systems driven by increased production of the inflammatory cytokines IL-1, IL-17, and tumor necrosis factor α, resulting in sterile neutrophilic dermatoses.1 The diagnosis is based on the clinical presentation, as laboratory investigations are nondiagnostic. Biopsies and cultures can be performed to rule out infectious etiologies. Additionally, PASH syndrome is considered part of a larger spectrum of syndromes including PAPA and PAPASH (pyogenic arthritis, acne, PG, HS) syndromes. The absence of pyogenic arthritis distinguishes PASH syndrome from PAPA and PAPASH syndromes.2 Clinically, PASH syndrome and the related sterile neutrophilic dermatoses share the characteristic of pronounced cutaneous involvement that substantially alters the patient’s quality of life. Cigarette smoking is an exacerbating factor and has a well-established association with HS.3 Therefore, smoking cessation should be encouraged in these patients to avoid exacerbation of the disease process.
Maintaining adequate immunosuppression is key to managing the underlying disease processes. Classic immunosuppressive agents such as systemic glucocorticoids and methotrexate may fail to satisfactorily control the disease.4 Treatment options currently are somewhat limited and are aimed at targeting the inflammatory cytokines that propagate the disease. The most consistent responses have been observed with anti–tumor necrosis factor α antagonists such as adalimumab, infliximab, and etanercept.5 Additionally, there is varied response to anakinra, suggesting the importance of selectively targeting IL-1β.6 Unfortunately, misdiagnosis for an infectious etiology is common, and antibiotics and debridement are of limited use for the underlying pathophysiology of PASH syndrome. Importantly, biopsy and debridement often are discouraged due to the risk of pathergy.7
Our case demonstrates the importance of maintaining a high clinical suspicion for immune-mediated lesions that are refractory to antimicrobial agents. Additionally, prior history of multiple neutrophilic dermatoses should prompt consideration for the PASH/PAPA/PAPASH disease spectrum. Early and accurate identification of neutrophilic dermatoses such as PG and HS are crucial to initiating proper cytokine-targeting treatment and achieving disease remission.
- Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562.
- Genovese G, Moltrasio C, Garcovich S, et al. PAPA spectrum disorders. G Ital Dermatol Venereol. 2020;155:542-550.
- König A, Lehmann C, Rompel R, et al. Cigarette smoking as a triggering factor of hidradenitis suppurativa. Dermatology. 1999;198:261-264.
- Ahn C, Negus D, Huang W. Pyoderma gangrenosum: a review of pathogenesis and treatment. Expert Rev Clin Immunol. 2018;14:225-233.
- Saint-Georges V, Peternel S, Kaštelan M, et al. Tumor necrosis factor antagonists in the treatment of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) syndrome. Acta Dermatovenerol Croat. 2018;26:173-178.
- Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
- Patel DK, Locke M, Jarrett P. Pyoderma gangrenosum with pathergy: a potentially significant complication following breast reconstruction. J Plast Reconstr Aesthet Surg. 2017;70:884-892.
- Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562.
- Genovese G, Moltrasio C, Garcovich S, et al. PAPA spectrum disorders. G Ital Dermatol Venereol. 2020;155:542-550.
- König A, Lehmann C, Rompel R, et al. Cigarette smoking as a triggering factor of hidradenitis suppurativa. Dermatology. 1999;198:261-264.
- Ahn C, Negus D, Huang W. Pyoderma gangrenosum: a review of pathogenesis and treatment. Expert Rev Clin Immunol. 2018;14:225-233.
- Saint-Georges V, Peternel S, Kaštelan M, et al. Tumor necrosis factor antagonists in the treatment of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) syndrome. Acta Dermatovenerol Croat. 2018;26:173-178.
- Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
- Patel DK, Locke M, Jarrett P. Pyoderma gangrenosum with pathergy: a potentially significant complication following breast reconstruction. J Plast Reconstr Aesthet Surg. 2017;70:884-892.
A 28-year-old Black woman presented to the hospital for evaluation of worsening leg wounds as well as a similar eroding plaque on the left breast of 1 month’s duration. Broad-spectrum antibiotics prescribed during a prior emergency department visit resulted in no improvement. Her medical history was notable for hidradenitis suppurativa that previously was well controlled on adalimumab prior to discontinuation 1 year prior. A review of systems was negative for fever, chills, shortness of breath, chest pain, night sweats, and arthralgia. The patient had discontinued the antibiotics and was not taking any other medications at the time of presentation. She reported a history of smoking cigarettes (5 pack years). Physical examination revealed hyperkeratotic eroded plaques with violaceous borders circumferentially around the left breast (top) and legs with notable undermining (bottom). Inflammatory nodulocystic acne of the face as well as sinus tract formation with purulent drainage in the axillae also were present. Laboratory workup revealed an elevated erythrocyte sedimentation rate (116 mm/h [reference range, <20 mm/h]). Computed tomography of the leg wound was negative for soft-tissue infection. Aerobic and anaerobic tissue cultures demonstrated no growth.

Papulonecrotic Tuberculid Secondary to Mycobacterium avium Complex
To the Editor:
Papulonecrotic tuberculid (PNT) is a cutaneous hypersensitivity reaction to antigenic components of Mycobacterium species, most commonly Mycobacterium tuberculosis. According to a PubMed search of articles indexed for MEDLINE using the terms papulonecrotic tuberculid, Mycobacterium avium complex, and Mycobacterium, only 1 case of PNT secondary to infection with Mycobacterium avium complex (MAC) has been reported.1,2 Papulonecrotic tuberculid classically presents with symmetrical, dusky red papules with necrosis on the extremities.3 Patients may or may not have associated symptoms of fever and weight loss. It is diagnosed through skin biopsy as well as identification of a distant source of mycobacterial infection. Papulonecrotic tuberculid is considered a reactive process to a distant site of mycobacterial infection, and skin lesions contain few, if any, mycobacteria.4
A 65-year-old man was admitted to the hospital for expedited workup of chronic fevers, 20-lb weight loss, and night sweats of 8 months’ duration. He had a medical history of myelodysplastic syndrome and autoimmune hemolytic anemia. During hospitalization, positron emission tomography revealed multilevel vertebral lytic and sclerotic lesions. Subsequent T10 vertebral biopsy showed necrotizing granulomatous inflammation with extensive necrosis and acid-fast bacilli–positive organisms. The patient was empirically started on rifampicin, isoniazid, pyrazinamide, ethambutol, and pyridoxine for presumed M tuberculosis and placed on respiratory isolation.
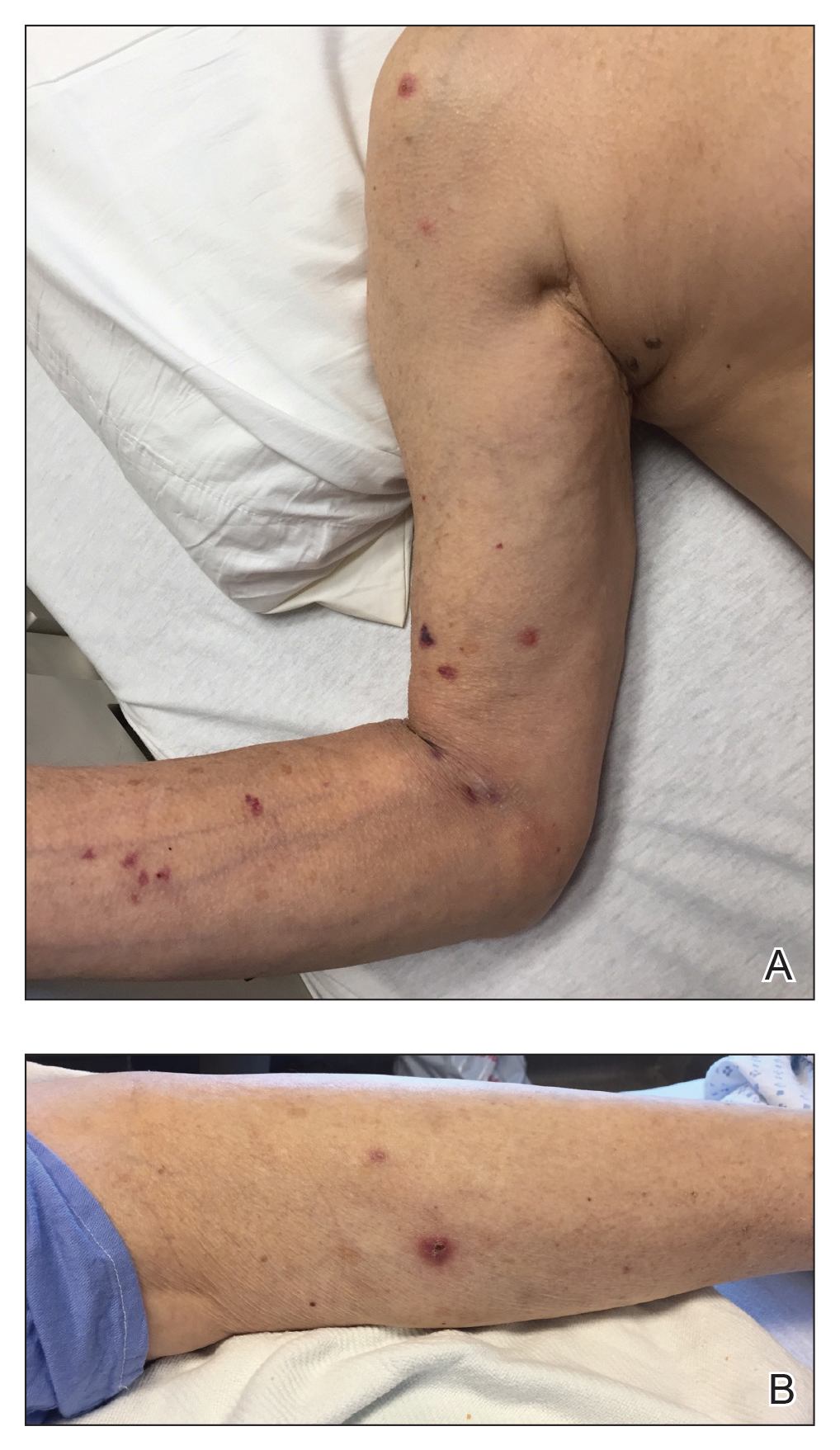
Dermatology was consulted for a recurrent tender rash on the bilateral upper and lower extremities of 5 years’ duration. Physical examination revealed numerous erythematous papulonecrotic lesions in various states of healing on the bilateral upper and lower extremities (Figure 1). Three years prior to the current presentation, 2 lesions were biopsied and demonstrated leukocytoclastic vasculitis with neutrophilic panniculitis and vasculopathy. A presumptive diagnosis of Sweet syndrome was made given the history of myelodysplastic syndrome, though an infectious etiology could not be ruled out at that time. Concurrently, the patient was diagnosed with autoimmune hemolytic anemia and was started on prednisone. Initially, the skin lesions improved with prednisone but never fully resolved; however, as the dosage of oral steroids decreased, the skin lesions worsened and presented in larger numbers with more frequency. The patient was titrated down to prednisone 5 mg daily with no additional treatment of the skin lesions at that time.
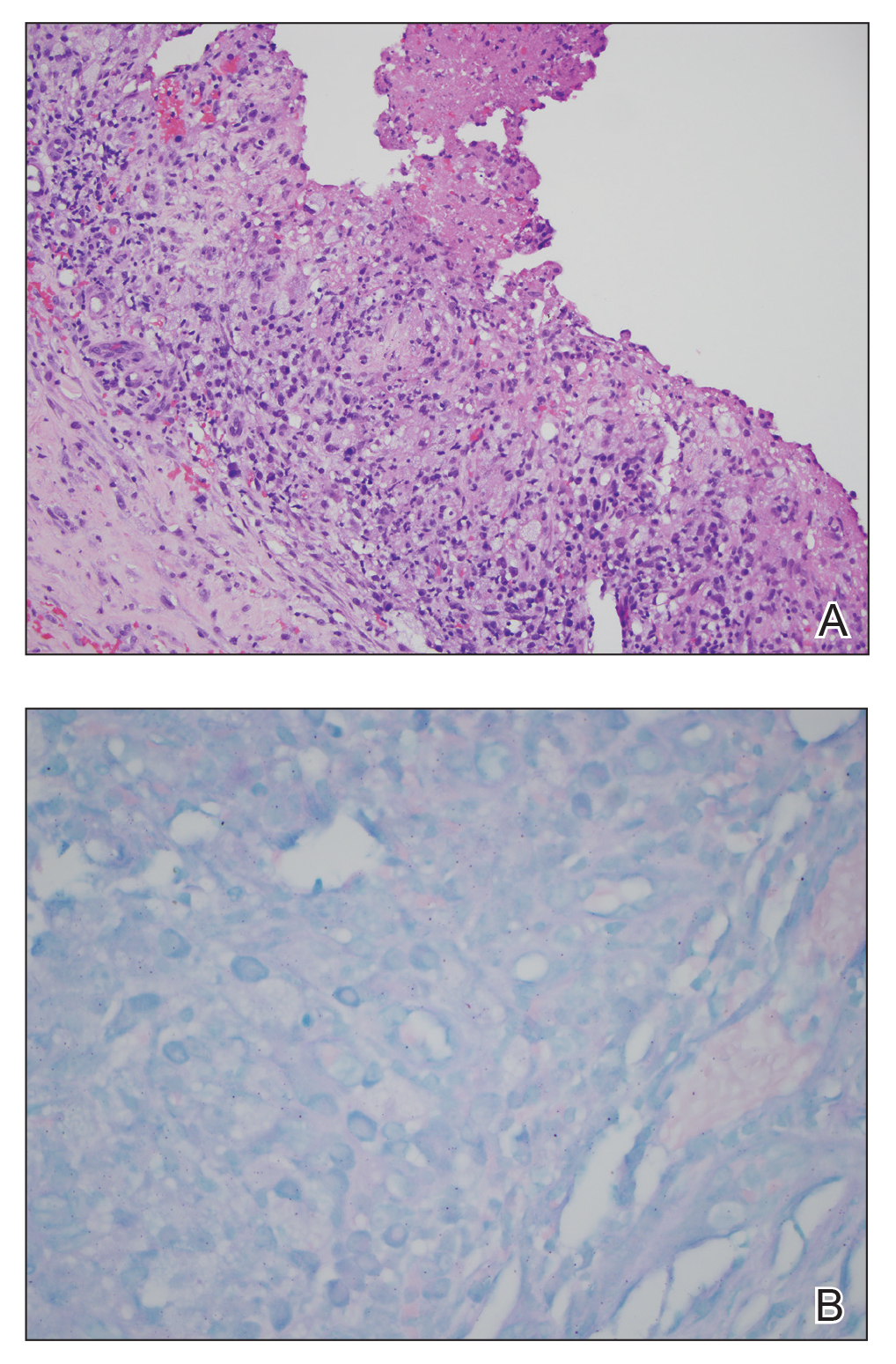
During the current hospitalization, 2 additional biopsies were taken from the arm for routine histopathology and tissue culture. Dermatopathology revealed robust neutrophilic and granulomatous inflammation as well as remarkable necrosis with a few mycobacteria identified on acid-fast and Fite stains (Figure 2). Tissue culture was negative. Additionally, the patient’s spinal biopsy was sent for polymerase chain reaction analysis for Mycobacterium typing, which confirmed MAC. The patient was diagnosed with Pott disease, a mycobacterial infection of the spine, as well as cutaneous papulonecrotic tuberculid secondary to MAC.
Papulonecrotic tuberculid is the rarest form of cutaneous tuberculosis infection and rarely has been reported in connection to MAC.1 This condition is considered a hypersensitivity reaction that occurs in response to antigenic components of mycobacteria.4 Patients with PNT typically present with recurrent crops of painful papulonecrotic lesions distributed on the extremities. Histopathology in PNT classically reveals necrosis, notable inflammatory infiltrate, and lack of observed organisms.5 Diagnosis often is made through skin biopsy, though histopathology varies based on lesion maturity.4 Early lesions often reveal leukocytoclastic vasculitis, whereas late lesions usually demonstrate granulomatous inflammation.4 Mycobacterium avium complex is difficult to culture, as it is a slow-growing, fastidious bacterium and therefore polymerase chain reaction genotyping is useful for bacterial classification.6
Disseminated MAC infection also was on the differential for our patient; however, we felt it was less likely than PNT for several reasons. First, disseminated infection rarely presents with cutaneous involvement and is associated with pulmonary involvement in 90% of cases.7-9 Second, the granuloma formation noted on our patient’s skin biopsy was not typical for disseminated MAC but is well described in cases of PNT.4,8,9 Finally, in the rare cases in which cutaneous involvement has occurred with disseminated mycobacterial infections, skin biopsies typically revealed numerous Mycobacterium organisms.8,10 In contrast, skin lesions associated with PNT usually reveal few, if any, organisms, as was seen with our patient.2
The patient’s initial biopsies also supported a diagnosis of PNT, as early lesions of PNT typically show leukocytoclastic vasculitis. His response to low and high doses of prednisone also fit well with a PNT diagnosis. In fact, a case of PNT secondary to Mycobacterium bovis similarly showed an improvement in the rash with high-dose steroids but progression with lower doses.11 It is possible that our patient’s response to steroids complicated the diagnosis of his rash.
The treatment of PNT is clearance of the underlying infection. Macrolide antibiotics, such as clarithromycin and azithromycin, have the best efficacy against MAC, in combination with ethambutol and/or rifabutin.6,12 Treatment duration should be 1 year. Amikacin or streptomycin may be added to this regimen during early treatment.6 Mycobacterium avium complex is resistant to many antibiotics, including typical antituberculosis drugs, and sensitivities should be identified at the onset of treatment.11,12
Albeit rare, clinicians should be aware of PNT secondary to MAC or other mycobacterial infections. Because this condition is difficult to diagnose with varying histologic findings and often negative tissue cultures, a high index of suspicion is necessary when a patient presents with recurrent papulonecrotic lesions, especially in immunocompromised hosts and patients with exposure to mycobacteria.
- Williams JT, Pulitzer DR, DeVillez RL. Papulonecrotic tuberculid secondary to disseminated Mycobacterium avium complex. Int J Dermatol. 1994;33:109-112.
- Jordaan HF, Schneider JW. Papulonecrotic tuberculid. Int J Dermatol. 1995;34:217-219.
- Scollard DM, Dacso MM, Abad-Venida ML. Tuberculosis and leprosy: classical granulomatous diseases in the twenty-first century. Dermatol Clin. 2015;33:541-562.
- Kim GW, Park HJ, Kim HS, et al. Simultaneous occurrence of papulonecrotic tuberculid and erythema induratum in a patient with pulmonary tuberculosis. Pediatr Dermatol. 2013;30:256-259.
- Spelta K, Diniz LM. Cutaneous tuberculosis: a 26-year retrospective study in an endemic area. Rev Inst Med Trop Sao Paulo. 2016;58:49.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- Dyer J, Weiss J, Steiner WS, et al. Primary cutaneous Mycobacterium avium complex infection following squamous cell carcinoma excision. Cutis. 2016;98:E8-E11.
- Kollipara R, Richards K, Tschen J, et al. Disseminated Mycobacterium avium complex with cutaneous lesions. J Cutan Med Surg. 2016;20:272-274.
- Endly DC, Ackerman LS. Disseminated cutaneous Mycobacterium avium complex in a person with AIDS. Dermatol Online J. 2014;20:22616.
- Li JJ, Beresford R, Fyfe J, et al. Clinical and histopathological features of cutaneous nontuberculous mycobacterial infection: a review of 13 cases. J Cutan Pathol. 2017;44:433-443.
- Iden DL, Rogers RS 3rd, Schroeter AL. Papulonecrotic tuberculid secondary to Mycobacterium bovis. Arch Dermatol. 1978;114:564-566.
- Wong NM, Sun LK, Lau PY. Spinal infection caused by Mycobacterium avium complex in a patient with no acquired immune deficiency syndrome: a case report. J Orthop Surg (Hong Kong). 2008;16:359-363.
To the Editor:
Papulonecrotic tuberculid (PNT) is a cutaneous hypersensitivity reaction to antigenic components of Mycobacterium species, most commonly Mycobacterium tuberculosis. According to a PubMed search of articles indexed for MEDLINE using the terms papulonecrotic tuberculid, Mycobacterium avium complex, and Mycobacterium, only 1 case of PNT secondary to infection with Mycobacterium avium complex (MAC) has been reported.1,2 Papulonecrotic tuberculid classically presents with symmetrical, dusky red papules with necrosis on the extremities.3 Patients may or may not have associated symptoms of fever and weight loss. It is diagnosed through skin biopsy as well as identification of a distant source of mycobacterial infection. Papulonecrotic tuberculid is considered a reactive process to a distant site of mycobacterial infection, and skin lesions contain few, if any, mycobacteria.4
A 65-year-old man was admitted to the hospital for expedited workup of chronic fevers, 20-lb weight loss, and night sweats of 8 months’ duration. He had a medical history of myelodysplastic syndrome and autoimmune hemolytic anemia. During hospitalization, positron emission tomography revealed multilevel vertebral lytic and sclerotic lesions. Subsequent T10 vertebral biopsy showed necrotizing granulomatous inflammation with extensive necrosis and acid-fast bacilli–positive organisms. The patient was empirically started on rifampicin, isoniazid, pyrazinamide, ethambutol, and pyridoxine for presumed M tuberculosis and placed on respiratory isolation.
Dermatology was consulted for a recurrent tender rash on the bilateral upper and lower extremities of 5 years’ duration. Physical examination revealed numerous erythematous papulonecrotic lesions in various states of healing on the bilateral upper and lower extremities (Figure 1). Three years prior to the current presentation, 2 lesions were biopsied and demonstrated leukocytoclastic vasculitis with neutrophilic panniculitis and vasculopathy. A presumptive diagnosis of Sweet syndrome was made given the history of myelodysplastic syndrome, though an infectious etiology could not be ruled out at that time. Concurrently, the patient was diagnosed with autoimmune hemolytic anemia and was started on prednisone. Initially, the skin lesions improved with prednisone but never fully resolved; however, as the dosage of oral steroids decreased, the skin lesions worsened and presented in larger numbers with more frequency. The patient was titrated down to prednisone 5 mg daily with no additional treatment of the skin lesions at that time.
During the current hospitalization, 2 additional biopsies were taken from the arm for routine histopathology and tissue culture. Dermatopathology revealed robust neutrophilic and granulomatous inflammation as well as remarkable necrosis with a few mycobacteria identified on acid-fast and Fite stains (Figure 2). Tissue culture was negative. Additionally, the patient’s spinal biopsy was sent for polymerase chain reaction analysis for Mycobacterium typing, which confirmed MAC. The patient was diagnosed with Pott disease, a mycobacterial infection of the spine, as well as cutaneous papulonecrotic tuberculid secondary to MAC.
Papulonecrotic tuberculid is the rarest form of cutaneous tuberculosis infection and rarely has been reported in connection to MAC.1 This condition is considered a hypersensitivity reaction that occurs in response to antigenic components of mycobacteria.4 Patients with PNT typically present with recurrent crops of painful papulonecrotic lesions distributed on the extremities. Histopathology in PNT classically reveals necrosis, notable inflammatory infiltrate, and lack of observed organisms.5 Diagnosis often is made through skin biopsy, though histopathology varies based on lesion maturity.4 Early lesions often reveal leukocytoclastic vasculitis, whereas late lesions usually demonstrate granulomatous inflammation.4 Mycobacterium avium complex is difficult to culture, as it is a slow-growing, fastidious bacterium and therefore polymerase chain reaction genotyping is useful for bacterial classification.6
Disseminated MAC infection also was on the differential for our patient; however, we felt it was less likely than PNT for several reasons. First, disseminated infection rarely presents with cutaneous involvement and is associated with pulmonary involvement in 90% of cases.7-9 Second, the granuloma formation noted on our patient’s skin biopsy was not typical for disseminated MAC but is well described in cases of PNT.4,8,9 Finally, in the rare cases in which cutaneous involvement has occurred with disseminated mycobacterial infections, skin biopsies typically revealed numerous Mycobacterium organisms.8,10 In contrast, skin lesions associated with PNT usually reveal few, if any, organisms, as was seen with our patient.2
The patient’s initial biopsies also supported a diagnosis of PNT, as early lesions of PNT typically show leukocytoclastic vasculitis. His response to low and high doses of prednisone also fit well with a PNT diagnosis. In fact, a case of PNT secondary to Mycobacterium bovis similarly showed an improvement in the rash with high-dose steroids but progression with lower doses.11 It is possible that our patient’s response to steroids complicated the diagnosis of his rash.
The treatment of PNT is clearance of the underlying infection. Macrolide antibiotics, such as clarithromycin and azithromycin, have the best efficacy against MAC, in combination with ethambutol and/or rifabutin.6,12 Treatment duration should be 1 year. Amikacin or streptomycin may be added to this regimen during early treatment.6 Mycobacterium avium complex is resistant to many antibiotics, including typical antituberculosis drugs, and sensitivities should be identified at the onset of treatment.11,12
Albeit rare, clinicians should be aware of PNT secondary to MAC or other mycobacterial infections. Because this condition is difficult to diagnose with varying histologic findings and often negative tissue cultures, a high index of suspicion is necessary when a patient presents with recurrent papulonecrotic lesions, especially in immunocompromised hosts and patients with exposure to mycobacteria.
To the Editor:
Papulonecrotic tuberculid (PNT) is a cutaneous hypersensitivity reaction to antigenic components of Mycobacterium species, most commonly Mycobacterium tuberculosis. According to a PubMed search of articles indexed for MEDLINE using the terms papulonecrotic tuberculid, Mycobacterium avium complex, and Mycobacterium, only 1 case of PNT secondary to infection with Mycobacterium avium complex (MAC) has been reported.1,2 Papulonecrotic tuberculid classically presents with symmetrical, dusky red papules with necrosis on the extremities.3 Patients may or may not have associated symptoms of fever and weight loss. It is diagnosed through skin biopsy as well as identification of a distant source of mycobacterial infection. Papulonecrotic tuberculid is considered a reactive process to a distant site of mycobacterial infection, and skin lesions contain few, if any, mycobacteria.4
A 65-year-old man was admitted to the hospital for expedited workup of chronic fevers, 20-lb weight loss, and night sweats of 8 months’ duration. He had a medical history of myelodysplastic syndrome and autoimmune hemolytic anemia. During hospitalization, positron emission tomography revealed multilevel vertebral lytic and sclerotic lesions. Subsequent T10 vertebral biopsy showed necrotizing granulomatous inflammation with extensive necrosis and acid-fast bacilli–positive organisms. The patient was empirically started on rifampicin, isoniazid, pyrazinamide, ethambutol, and pyridoxine for presumed M tuberculosis and placed on respiratory isolation.
Dermatology was consulted for a recurrent tender rash on the bilateral upper and lower extremities of 5 years’ duration. Physical examination revealed numerous erythematous papulonecrotic lesions in various states of healing on the bilateral upper and lower extremities (Figure 1). Three years prior to the current presentation, 2 lesions were biopsied and demonstrated leukocytoclastic vasculitis with neutrophilic panniculitis and vasculopathy. A presumptive diagnosis of Sweet syndrome was made given the history of myelodysplastic syndrome, though an infectious etiology could not be ruled out at that time. Concurrently, the patient was diagnosed with autoimmune hemolytic anemia and was started on prednisone. Initially, the skin lesions improved with prednisone but never fully resolved; however, as the dosage of oral steroids decreased, the skin lesions worsened and presented in larger numbers with more frequency. The patient was titrated down to prednisone 5 mg daily with no additional treatment of the skin lesions at that time.
During the current hospitalization, 2 additional biopsies were taken from the arm for routine histopathology and tissue culture. Dermatopathology revealed robust neutrophilic and granulomatous inflammation as well as remarkable necrosis with a few mycobacteria identified on acid-fast and Fite stains (Figure 2). Tissue culture was negative. Additionally, the patient’s spinal biopsy was sent for polymerase chain reaction analysis for Mycobacterium typing, which confirmed MAC. The patient was diagnosed with Pott disease, a mycobacterial infection of the spine, as well as cutaneous papulonecrotic tuberculid secondary to MAC.
Papulonecrotic tuberculid is the rarest form of cutaneous tuberculosis infection and rarely has been reported in connection to MAC.1 This condition is considered a hypersensitivity reaction that occurs in response to antigenic components of mycobacteria.4 Patients with PNT typically present with recurrent crops of painful papulonecrotic lesions distributed on the extremities. Histopathology in PNT classically reveals necrosis, notable inflammatory infiltrate, and lack of observed organisms.5 Diagnosis often is made through skin biopsy, though histopathology varies based on lesion maturity.4 Early lesions often reveal leukocytoclastic vasculitis, whereas late lesions usually demonstrate granulomatous inflammation.4 Mycobacterium avium complex is difficult to culture, as it is a slow-growing, fastidious bacterium and therefore polymerase chain reaction genotyping is useful for bacterial classification.6
Disseminated MAC infection also was on the differential for our patient; however, we felt it was less likely than PNT for several reasons. First, disseminated infection rarely presents with cutaneous involvement and is associated with pulmonary involvement in 90% of cases.7-9 Second, the granuloma formation noted on our patient’s skin biopsy was not typical for disseminated MAC but is well described in cases of PNT.4,8,9 Finally, in the rare cases in which cutaneous involvement has occurred with disseminated mycobacterial infections, skin biopsies typically revealed numerous Mycobacterium organisms.8,10 In contrast, skin lesions associated with PNT usually reveal few, if any, organisms, as was seen with our patient.2
The patient’s initial biopsies also supported a diagnosis of PNT, as early lesions of PNT typically show leukocytoclastic vasculitis. His response to low and high doses of prednisone also fit well with a PNT diagnosis. In fact, a case of PNT secondary to Mycobacterium bovis similarly showed an improvement in the rash with high-dose steroids but progression with lower doses.11 It is possible that our patient’s response to steroids complicated the diagnosis of his rash.
The treatment of PNT is clearance of the underlying infection. Macrolide antibiotics, such as clarithromycin and azithromycin, have the best efficacy against MAC, in combination with ethambutol and/or rifabutin.6,12 Treatment duration should be 1 year. Amikacin or streptomycin may be added to this regimen during early treatment.6 Mycobacterium avium complex is resistant to many antibiotics, including typical antituberculosis drugs, and sensitivities should be identified at the onset of treatment.11,12
Albeit rare, clinicians should be aware of PNT secondary to MAC or other mycobacterial infections. Because this condition is difficult to diagnose with varying histologic findings and often negative tissue cultures, a high index of suspicion is necessary when a patient presents with recurrent papulonecrotic lesions, especially in immunocompromised hosts and patients with exposure to mycobacteria.
- Williams JT, Pulitzer DR, DeVillez RL. Papulonecrotic tuberculid secondary to disseminated Mycobacterium avium complex. Int J Dermatol. 1994;33:109-112.
- Jordaan HF, Schneider JW. Papulonecrotic tuberculid. Int J Dermatol. 1995;34:217-219.
- Scollard DM, Dacso MM, Abad-Venida ML. Tuberculosis and leprosy: classical granulomatous diseases in the twenty-first century. Dermatol Clin. 2015;33:541-562.
- Kim GW, Park HJ, Kim HS, et al. Simultaneous occurrence of papulonecrotic tuberculid and erythema induratum in a patient with pulmonary tuberculosis. Pediatr Dermatol. 2013;30:256-259.
- Spelta K, Diniz LM. Cutaneous tuberculosis: a 26-year retrospective study in an endemic area. Rev Inst Med Trop Sao Paulo. 2016;58:49.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- Dyer J, Weiss J, Steiner WS, et al. Primary cutaneous Mycobacterium avium complex infection following squamous cell carcinoma excision. Cutis. 2016;98:E8-E11.
- Kollipara R, Richards K, Tschen J, et al. Disseminated Mycobacterium avium complex with cutaneous lesions. J Cutan Med Surg. 2016;20:272-274.
- Endly DC, Ackerman LS. Disseminated cutaneous Mycobacterium avium complex in a person with AIDS. Dermatol Online J. 2014;20:22616.
- Li JJ, Beresford R, Fyfe J, et al. Clinical and histopathological features of cutaneous nontuberculous mycobacterial infection: a review of 13 cases. J Cutan Pathol. 2017;44:433-443.
- Iden DL, Rogers RS 3rd, Schroeter AL. Papulonecrotic tuberculid secondary to Mycobacterium bovis. Arch Dermatol. 1978;114:564-566.
- Wong NM, Sun LK, Lau PY. Spinal infection caused by Mycobacterium avium complex in a patient with no acquired immune deficiency syndrome: a case report. J Orthop Surg (Hong Kong). 2008;16:359-363.
- Williams JT, Pulitzer DR, DeVillez RL. Papulonecrotic tuberculid secondary to disseminated Mycobacterium avium complex. Int J Dermatol. 1994;33:109-112.
- Jordaan HF, Schneider JW. Papulonecrotic tuberculid. Int J Dermatol. 1995;34:217-219.
- Scollard DM, Dacso MM, Abad-Venida ML. Tuberculosis and leprosy: classical granulomatous diseases in the twenty-first century. Dermatol Clin. 2015;33:541-562.
- Kim GW, Park HJ, Kim HS, et al. Simultaneous occurrence of papulonecrotic tuberculid and erythema induratum in a patient with pulmonary tuberculosis. Pediatr Dermatol. 2013;30:256-259.
- Spelta K, Diniz LM. Cutaneous tuberculosis: a 26-year retrospective study in an endemic area. Rev Inst Med Trop Sao Paulo. 2016;58:49.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- Dyer J, Weiss J, Steiner WS, et al. Primary cutaneous Mycobacterium avium complex infection following squamous cell carcinoma excision. Cutis. 2016;98:E8-E11.
- Kollipara R, Richards K, Tschen J, et al. Disseminated Mycobacterium avium complex with cutaneous lesions. J Cutan Med Surg. 2016;20:272-274.
- Endly DC, Ackerman LS. Disseminated cutaneous Mycobacterium avium complex in a person with AIDS. Dermatol Online J. 2014;20:22616.
- Li JJ, Beresford R, Fyfe J, et al. Clinical and histopathological features of cutaneous nontuberculous mycobacterial infection: a review of 13 cases. J Cutan Pathol. 2017;44:433-443.
- Iden DL, Rogers RS 3rd, Schroeter AL. Papulonecrotic tuberculid secondary to Mycobacterium bovis. Arch Dermatol. 1978;114:564-566.
- Wong NM, Sun LK, Lau PY. Spinal infection caused by Mycobacterium avium complex in a patient with no acquired immune deficiency syndrome: a case report. J Orthop Surg (Hong Kong). 2008;16:359-363.
Practice Points
- Papulonecrotic tuberculid (PNT) is a hypersensitivity reaction that presents with reddish papules with central necrosis on the extremities.
- Early PNT histopathology shows leukocytoclastic vasculitis. Later lesions demonstrate granulomatous inflammation on histopathology.
- Mycobacterium avium is difficult to culture; therefore, if you suspect it, we recommend polymerase chain reaction genotyping for bacterial classification.
Comment on “Intraoperative Electrosurgical Smoke During Outpatient Surgery: A Survey of Dermatologic Surgeon and Staff Preferences”
To the Editor:
We read with great interest the recent Cutis article by Golda et al,1 “Intraoperative Electrosurgical Smoke During Outpatient Surgery: A Survey of Dermatologic Surgeon and Staff Preferences.” We applaud the growing interest in the topic of dermatologist safety, as there are currently no established guidelines for precautions while performing surgical procedures. In 2018 we conducted a comprehensive review2 to characterize the specific risks, hazard reduction strategies available, and current use of surgical smoke safety techniques during surgery among dermatologists, and ultimately recommend guidance based on the current available evidence. To conduct this review, we collected data from 45 manuscripts in the dermatology, surgery, infectious disease, obstetrics, and cancer biology literature. Herein, we summarize key findings.2
Dermatologic surgeons, residents, staff, and patients are exposed to many infectious, inhalational, chemical, and mutagenic hazards when performing procedures that liberate smoke and plume. These risks are commonplace; however, they are particularly notable during ablative laser and laser hair removal procedures, which produce a heavy plume (averaging >100,000 particles/cm3). Brief periods of heavy plume exposure also are commonplace during electrosurgery.
Infectious particles in surgical plume have been extensively studied, and viral transmission has been demonstrated in animal studies. Human papillomavirus transmission appears to be the most prevalent risk. Surgical smoke has been shown to cause acute and chronic inhalational injury in rat and sheep studies.3-6
Additionally, chemicals with carcinogenic potential are present in surgical smoke and have been described.7,8 Chemicals in the greatest quantity include hydrocarbons, nitriles, fatty acids, and phenols. Although there have been no human studies on smoke carcinogenesis to date, surgical smoke has been shown to have carcinogenic properties in vitro.
Given these risks—both evidence based and theoretical—we believe that diligent hazard reduction strategies should be employed whenever possible. Surgical masks and high-efficiency particulate air respirators, such as N95 respirator masks, have been well studied and do provide smoke protection. High-efficiency particulate air masks can be worn when possible, especially during procedures that produce heavy plume, though surgical masks are capable of filtering most of the noxious chemicals in surgical smoke. It should be noted that proper fit with minimal air leak is the most important aspect of overall performance.
Smoke evacuators provide another level of protection. The physician should consider the evacuator’s filtration efficiency, capture velocity, and suction strength when evaluating overall performance. Furthermore, the smoke collection tip should be within 2 in of the surgical field to maximize efficacy. Maintenance for smoke evacuation systems should include regular (as defined by manufacturer instructions) flushing of the smoke evacuator lines.
Despite the risks of surgical smoke and the available options of minimizing these risks, the hazards of surgical smoke and the importance of protection are likely underemphasized. Many dermatologic surgeons do not use surgical masks or smoke evacuators in routine practice, according to several survey studies.9-11
It is important for the dermatologic community to consider effective ways of spreading awareness. We propose that surgical smoke safety be taught early in residency training. Additionally, smoke safety can be implemented into certification examinations. Access to masks and smoke evacuation devices are an important part of dermatology training. Accreditation Council for Graduate Medical Education funds should be appropriated to provide for such resources.
Finally, and perhaps most importantly, continued awareness should be established in the dermatology community via standardized guidelines and periodic updates in the dermatology literature and lectures at local and national conferences. Not until these strategies are implemented will surgical smoke protection be viewed as a necessary and important component of routine practice when performing dermatologic surgical procedures.
- Golda N, Merrill B, Neill B. Intraoperative electrosurgical smoke during outpatient surgery: a survey of dermatologic surgeon and staff preferences. Cutis. 2019;104:120-124.
- Georgesen C, Lipner SR. Surgical smoke: risk assessment and mitigation strategies. J Am Acad Dermatol. 2018;79:746-755.
- Wenig BL, Stenson KM, Wenig BM, et al. Effects of plume produced by the Nd:YAG laser and electrocautery on the respiratory system. Lasers Surg Med. 1993;13:242-245.
- Baggish MS, Elbakry M. The effects of laser smoke on the lungs of rats. Am J Obstet Gynecol. 1987;156:1260-1265.
- Baggish MS, Baltoyannis P, Sze E. Protection of the rat lung from the harmful effects of laser smoke. Lasers Surg Med. 1988;8:248-253.
- Freitag L, Chapman GA, Sielczak M, et al. Laser smoke effect on the bronchial system. Lasers Surg Med. 1987;7:283-288.
- Barrett WL, Garber SM. Surgical smoke: a review of the literature. Is this just a lot of hot air? Surg Endosc. 2003;17:979-987.
- Hensman C, Baty D, Willis RG, et al. Chemical composition of smoke produced by high-frequency electrosurgery in a closed gaseous environment. Surg Endosc. 1998;12:1017-1019.
- Edwards BE, Reiman RE. Results of a survey on current surgical smoke control practices. AORN J. 2008;87:739-749.
- Oganesyan G, Eimpunth S, Kim SS, et al. Surgical smoke in dermatologic surgery. Dermatol Surg. 2014;40:1373-1377.
- Chapman LW, Korta DZ, Lee PK, et al. Awareness of surgical smoke risks and assessment of safety practices during electrosurgery among US dermatology residents. JAMA Dermatol. 2017;153:467-468.
To the Editor:
We read with great interest the recent Cutis article by Golda et al,1 “Intraoperative Electrosurgical Smoke During Outpatient Surgery: A Survey of Dermatologic Surgeon and Staff Preferences.” We applaud the growing interest in the topic of dermatologist safety, as there are currently no established guidelines for precautions while performing surgical procedures. In 2018 we conducted a comprehensive review2 to characterize the specific risks, hazard reduction strategies available, and current use of surgical smoke safety techniques during surgery among dermatologists, and ultimately recommend guidance based on the current available evidence. To conduct this review, we collected data from 45 manuscripts in the dermatology, surgery, infectious disease, obstetrics, and cancer biology literature. Herein, we summarize key findings.2
Dermatologic surgeons, residents, staff, and patients are exposed to many infectious, inhalational, chemical, and mutagenic hazards when performing procedures that liberate smoke and plume. These risks are commonplace; however, they are particularly notable during ablative laser and laser hair removal procedures, which produce a heavy plume (averaging >100,000 particles/cm3). Brief periods of heavy plume exposure also are commonplace during electrosurgery.
Infectious particles in surgical plume have been extensively studied, and viral transmission has been demonstrated in animal studies. Human papillomavirus transmission appears to be the most prevalent risk. Surgical smoke has been shown to cause acute and chronic inhalational injury in rat and sheep studies.3-6
Additionally, chemicals with carcinogenic potential are present in surgical smoke and have been described.7,8 Chemicals in the greatest quantity include hydrocarbons, nitriles, fatty acids, and phenols. Although there have been no human studies on smoke carcinogenesis to date, surgical smoke has been shown to have carcinogenic properties in vitro.
Given these risks—both evidence based and theoretical—we believe that diligent hazard reduction strategies should be employed whenever possible. Surgical masks and high-efficiency particulate air respirators, such as N95 respirator masks, have been well studied and do provide smoke protection. High-efficiency particulate air masks can be worn when possible, especially during procedures that produce heavy plume, though surgical masks are capable of filtering most of the noxious chemicals in surgical smoke. It should be noted that proper fit with minimal air leak is the most important aspect of overall performance.
Smoke evacuators provide another level of protection. The physician should consider the evacuator’s filtration efficiency, capture velocity, and suction strength when evaluating overall performance. Furthermore, the smoke collection tip should be within 2 in of the surgical field to maximize efficacy. Maintenance for smoke evacuation systems should include regular (as defined by manufacturer instructions) flushing of the smoke evacuator lines.
Despite the risks of surgical smoke and the available options of minimizing these risks, the hazards of surgical smoke and the importance of protection are likely underemphasized. Many dermatologic surgeons do not use surgical masks or smoke evacuators in routine practice, according to several survey studies.9-11
It is important for the dermatologic community to consider effective ways of spreading awareness. We propose that surgical smoke safety be taught early in residency training. Additionally, smoke safety can be implemented into certification examinations. Access to masks and smoke evacuation devices are an important part of dermatology training. Accreditation Council for Graduate Medical Education funds should be appropriated to provide for such resources.
Finally, and perhaps most importantly, continued awareness should be established in the dermatology community via standardized guidelines and periodic updates in the dermatology literature and lectures at local and national conferences. Not until these strategies are implemented will surgical smoke protection be viewed as a necessary and important component of routine practice when performing dermatologic surgical procedures.
To the Editor:
We read with great interest the recent Cutis article by Golda et al,1 “Intraoperative Electrosurgical Smoke During Outpatient Surgery: A Survey of Dermatologic Surgeon and Staff Preferences.” We applaud the growing interest in the topic of dermatologist safety, as there are currently no established guidelines for precautions while performing surgical procedures. In 2018 we conducted a comprehensive review2 to characterize the specific risks, hazard reduction strategies available, and current use of surgical smoke safety techniques during surgery among dermatologists, and ultimately recommend guidance based on the current available evidence. To conduct this review, we collected data from 45 manuscripts in the dermatology, surgery, infectious disease, obstetrics, and cancer biology literature. Herein, we summarize key findings.2
Dermatologic surgeons, residents, staff, and patients are exposed to many infectious, inhalational, chemical, and mutagenic hazards when performing procedures that liberate smoke and plume. These risks are commonplace; however, they are particularly notable during ablative laser and laser hair removal procedures, which produce a heavy plume (averaging >100,000 particles/cm3). Brief periods of heavy plume exposure also are commonplace during electrosurgery.
Infectious particles in surgical plume have been extensively studied, and viral transmission has been demonstrated in animal studies. Human papillomavirus transmission appears to be the most prevalent risk. Surgical smoke has been shown to cause acute and chronic inhalational injury in rat and sheep studies.3-6
Additionally, chemicals with carcinogenic potential are present in surgical smoke and have been described.7,8 Chemicals in the greatest quantity include hydrocarbons, nitriles, fatty acids, and phenols. Although there have been no human studies on smoke carcinogenesis to date, surgical smoke has been shown to have carcinogenic properties in vitro.
Given these risks—both evidence based and theoretical—we believe that diligent hazard reduction strategies should be employed whenever possible. Surgical masks and high-efficiency particulate air respirators, such as N95 respirator masks, have been well studied and do provide smoke protection. High-efficiency particulate air masks can be worn when possible, especially during procedures that produce heavy plume, though surgical masks are capable of filtering most of the noxious chemicals in surgical smoke. It should be noted that proper fit with minimal air leak is the most important aspect of overall performance.
Smoke evacuators provide another level of protection. The physician should consider the evacuator’s filtration efficiency, capture velocity, and suction strength when evaluating overall performance. Furthermore, the smoke collection tip should be within 2 in of the surgical field to maximize efficacy. Maintenance for smoke evacuation systems should include regular (as defined by manufacturer instructions) flushing of the smoke evacuator lines.
Despite the risks of surgical smoke and the available options of minimizing these risks, the hazards of surgical smoke and the importance of protection are likely underemphasized. Many dermatologic surgeons do not use surgical masks or smoke evacuators in routine practice, according to several survey studies.9-11
It is important for the dermatologic community to consider effective ways of spreading awareness. We propose that surgical smoke safety be taught early in residency training. Additionally, smoke safety can be implemented into certification examinations. Access to masks and smoke evacuation devices are an important part of dermatology training. Accreditation Council for Graduate Medical Education funds should be appropriated to provide for such resources.
Finally, and perhaps most importantly, continued awareness should be established in the dermatology community via standardized guidelines and periodic updates in the dermatology literature and lectures at local and national conferences. Not until these strategies are implemented will surgical smoke protection be viewed as a necessary and important component of routine practice when performing dermatologic surgical procedures.
- Golda N, Merrill B, Neill B. Intraoperative electrosurgical smoke during outpatient surgery: a survey of dermatologic surgeon and staff preferences. Cutis. 2019;104:120-124.
- Georgesen C, Lipner SR. Surgical smoke: risk assessment and mitigation strategies. J Am Acad Dermatol. 2018;79:746-755.
- Wenig BL, Stenson KM, Wenig BM, et al. Effects of plume produced by the Nd:YAG laser and electrocautery on the respiratory system. Lasers Surg Med. 1993;13:242-245.
- Baggish MS, Elbakry M. The effects of laser smoke on the lungs of rats. Am J Obstet Gynecol. 1987;156:1260-1265.
- Baggish MS, Baltoyannis P, Sze E. Protection of the rat lung from the harmful effects of laser smoke. Lasers Surg Med. 1988;8:248-253.
- Freitag L, Chapman GA, Sielczak M, et al. Laser smoke effect on the bronchial system. Lasers Surg Med. 1987;7:283-288.
- Barrett WL, Garber SM. Surgical smoke: a review of the literature. Is this just a lot of hot air? Surg Endosc. 2003;17:979-987.
- Hensman C, Baty D, Willis RG, et al. Chemical composition of smoke produced by high-frequency electrosurgery in a closed gaseous environment. Surg Endosc. 1998;12:1017-1019.
- Edwards BE, Reiman RE. Results of a survey on current surgical smoke control practices. AORN J. 2008;87:739-749.
- Oganesyan G, Eimpunth S, Kim SS, et al. Surgical smoke in dermatologic surgery. Dermatol Surg. 2014;40:1373-1377.
- Chapman LW, Korta DZ, Lee PK, et al. Awareness of surgical smoke risks and assessment of safety practices during electrosurgery among US dermatology residents. JAMA Dermatol. 2017;153:467-468.
- Golda N, Merrill B, Neill B. Intraoperative electrosurgical smoke during outpatient surgery: a survey of dermatologic surgeon and staff preferences. Cutis. 2019;104:120-124.
- Georgesen C, Lipner SR. Surgical smoke: risk assessment and mitigation strategies. J Am Acad Dermatol. 2018;79:746-755.
- Wenig BL, Stenson KM, Wenig BM, et al. Effects of plume produced by the Nd:YAG laser and electrocautery on the respiratory system. Lasers Surg Med. 1993;13:242-245.
- Baggish MS, Elbakry M. The effects of laser smoke on the lungs of rats. Am J Obstet Gynecol. 1987;156:1260-1265.
- Baggish MS, Baltoyannis P, Sze E. Protection of the rat lung from the harmful effects of laser smoke. Lasers Surg Med. 1988;8:248-253.
- Freitag L, Chapman GA, Sielczak M, et al. Laser smoke effect on the bronchial system. Lasers Surg Med. 1987;7:283-288.
- Barrett WL, Garber SM. Surgical smoke: a review of the literature. Is this just a lot of hot air? Surg Endosc. 2003;17:979-987.
- Hensman C, Baty D, Willis RG, et al. Chemical composition of smoke produced by high-frequency electrosurgery in a closed gaseous environment. Surg Endosc. 1998;12:1017-1019.
- Edwards BE, Reiman RE. Results of a survey on current surgical smoke control practices. AORN J. 2008;87:739-749.
- Oganesyan G, Eimpunth S, Kim SS, et al. Surgical smoke in dermatologic surgery. Dermatol Surg. 2014;40:1373-1377.
- Chapman LW, Korta DZ, Lee PK, et al. Awareness of surgical smoke risks and assessment of safety practices during electrosurgery among US dermatology residents. JAMA Dermatol. 2017;153:467-468.