User login
Distinguishing Generalized Bullous Fixed Drug Eruption From SJS/TEN: A Retrospective Study on Clinical and Demographic Features
To the Editor:
Generalized bullous fixed drug eruption (GBFDE) is a rare subtype of fixed drug eruption (FDE) that manifests as widespread blisters and erosions following exposure to a causative drug.1 Diagnostic criteria include involvement of at least 3 to 6 anatomic sites—head and neck, anterior trunk, posterior trunk, upper extremities, lower extremities, or genitalia—and more than 10% of the body surface area. It can be challenging to differentiate GBFDE from severe drug rashes such as Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) due to extensive body surface area involvement of blisters and erosions. Specific features distinguishing GBFDE from SJS/TEN include primary lesions consisting of larger erythematous to dusky, circular plaques that progress to bullae and coalesce into widespread erosions; history of FDE; lack of severe mucosal involvement; and better overall prognosis.2 Treatment typically involves discontinuation of the culprit medication and supportive care; evidence for systemic therapies is not well established.
Our study aimed to characterize the clinical and demographic features of GBFDE in our institution to highlight potential key differences between this diagnosis and SJS/TEN. An electronic medical record search was performed to identify patients who were clinically diagnosed with GBFDE at New York-Presbyterian/Weill Cornell Medical Center (New York, New York) in both outpatient and inpatient settings from January 2015 to December 2022. This retrospective study was approved by the Weill Cornell Medicine institutional review board (#22-05024777).
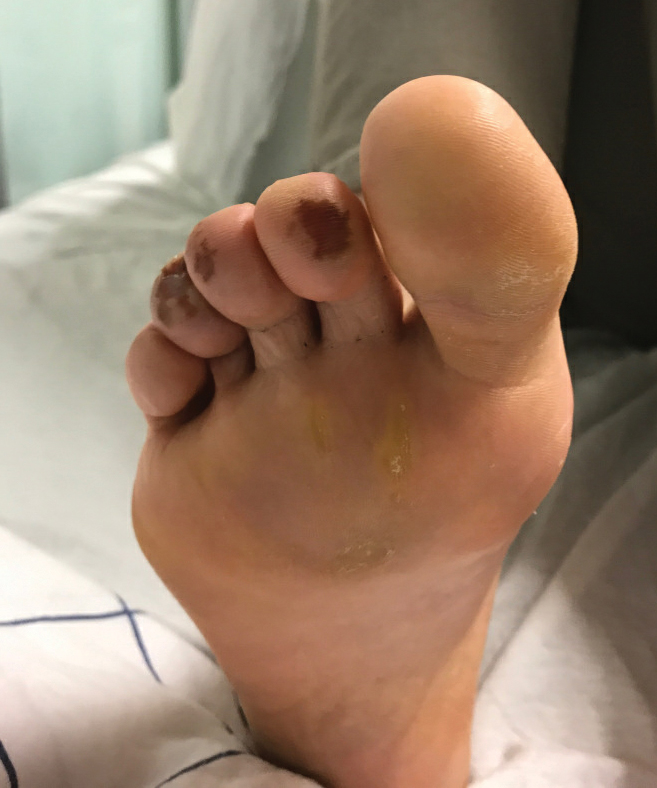
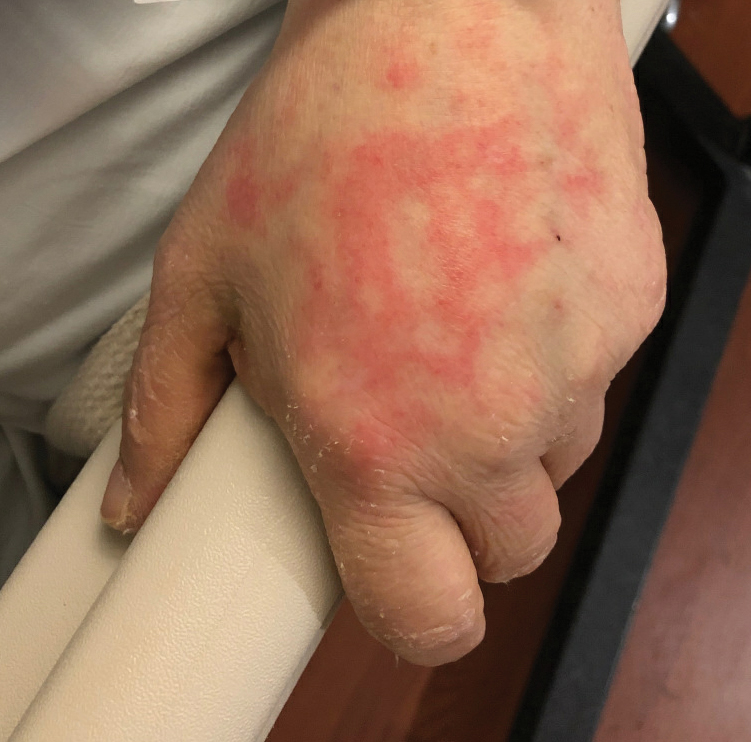
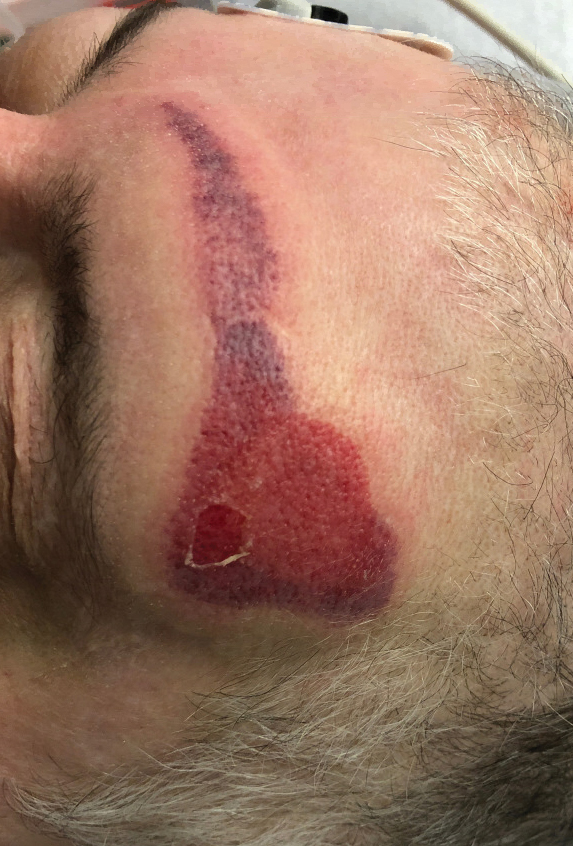
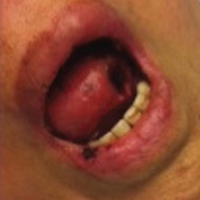
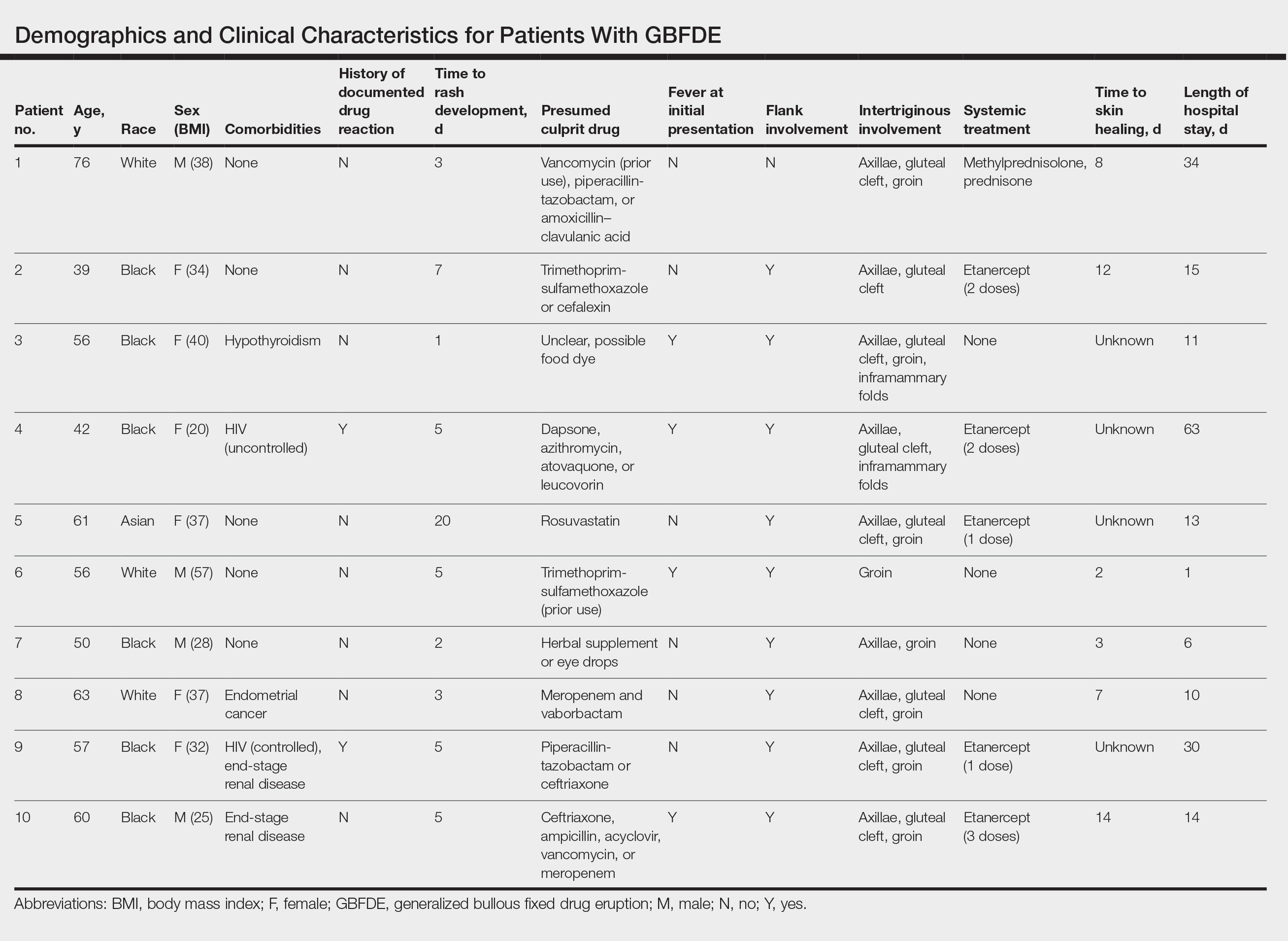
Ten patients were identified and included in the analysis (eTable). The mean age of the patients was 56 years (range, 39–76 years). Seven (70%) patients had skin of color (non-White) and 6 (60%) were female. The mean body mass index was 35 (range, 20–57), and 7 (70%) patients were clinically obese (body mass index >30). Only 2 (20%) patients had a history of a documented drug eruption (hives and erythema multiforme), and no patients had a history of FDE. Erythematous dusky patches followed by rapid development of blisters were noted within 3 days of drug initiation in 40% (4/10) and within 5 days in 80% (8/10) of patients. Antibiotics were identified as likely inciting agents in 8 (80%) patients. Biopsies were obtained in 3 (30%) patients and all 3 demonstrated cytotoxic CD8+ interface dermatitis with marked epithelial necrosis, neutrophilia, eosinophilia, and melanophage accumulation. Fever was present at initial presentation in only 4 (40%) patients, and only 1 (10%) patient had oral mucosal involvement. All 10 patients had intertriginous involvement (axillae, 90% [9/10]; gluteal cleft, 80% [8/10]; groin, 80% [8/10]; inframammary folds, 20% [2/10]), and there was considerable flank involvement in 9 (90%) patients. All 10 patients had initial erythematous to dusky, circular patches on the trunk and proximal extremities that then denuded most dramatically in the intertriginous areas (Figure). Six (60%) patients received systemic therapy, including 5 patients treated with a single dose of etanercept 50 mg. In patients with continued progression, 1 or 2 additional doses of etanercept 50 mg were administered at 48- to 72-hour intervals until blistering halted. Treatment with etanercept resulted in clinical improvement in all 5 patients, and there were no identifiable adverse events. The mean hospital stay was 19.7 days (range, 1–63 days).
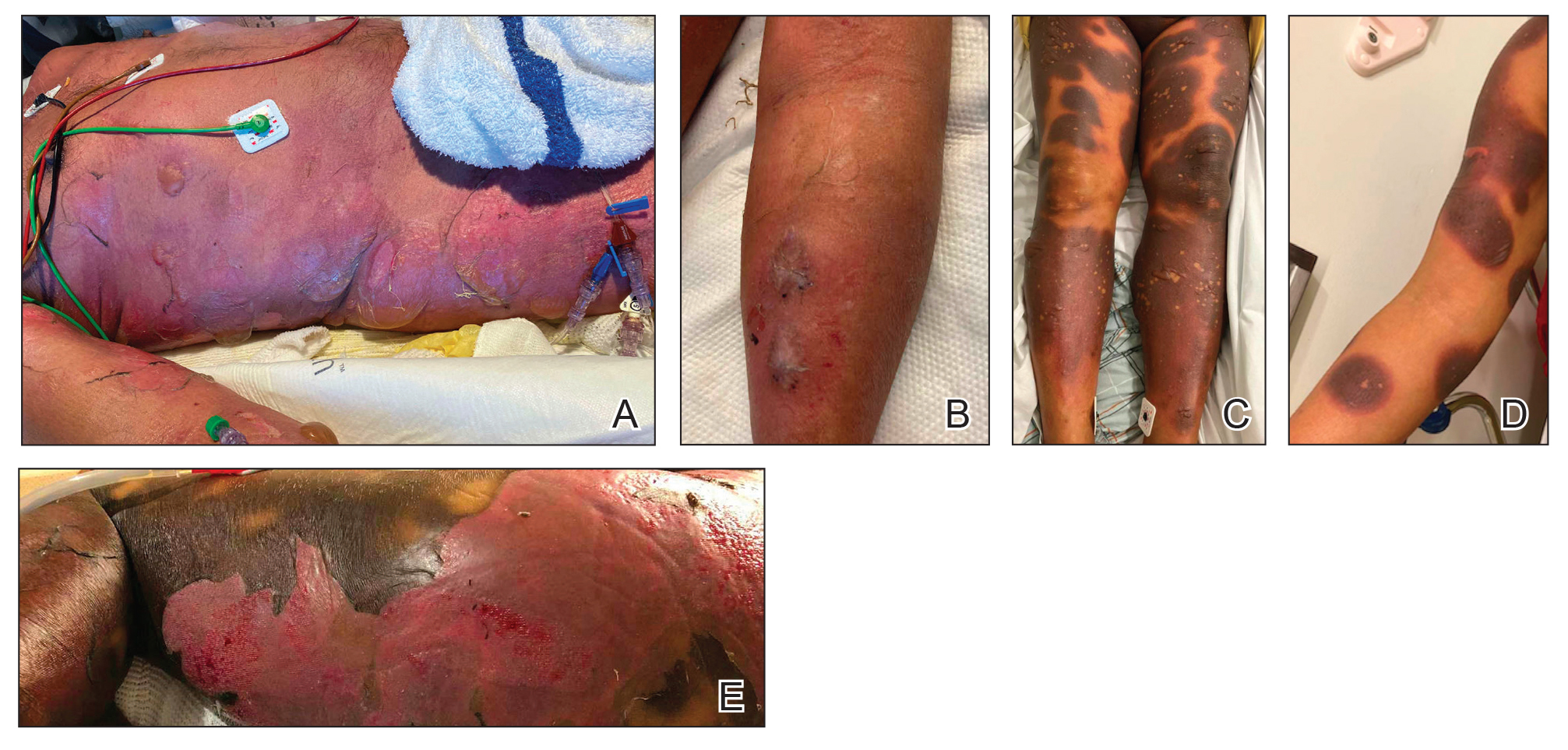
This study highlights notable demographic and clinical features of GBFDE that have not been widely described in the literature. Large erythematous and dusky patches with broad zones of blistering with particular localization to the neck, intertriginous areas, and flanks typically are not described in SJS/TEN and may be helpful in distinguishing these conditions from GBFDE. Mild or complete lack of mucosal and facial involvement as well as more rapid time from drug initiation to rash (as rapid as 1 day) were key factors that aided in distinguishing GBFDE from SJS/TEN in our patients. Although a history of FDE is considered a key characteristic in the diagnosis of GBFDE, none of our patients had a known history of FDE, suggesting GBFDE may be the initial manifestation of FDE in some patients. Histopathology showed similar findings consistent with FDE in the 3 patients in whom a biopsy was performed. The remaining patients were diagnosed clinically based on the presence of distinctive, perfectly circular, dusky plaques present at the periphery of larger denuded areas, which are characteristic of GBFDE. Lower levels of serum granulysin3 have been shown to help distinguish GBFDE from SJS/TEN, but this test is not readily available with time-sensitive results at most institutions, and exact diagnostic ranges for GBFDE vs SJS/TEN are not yet known.
Our study was limited by a small number of patients at a single institution. Another limitation was the retrospective design.
Interestingly, a high proportion of our patients were non-White and clinically obese, which are factors that should be considered for future research. Sixty percent (6/10) of the patients in our study were Black, which is a notable difference from our hospital’s general admission demographics with Black individuals constituting 12% of patients.4 Our study also highlighted the utility of etanercept, which has reported mortality benefits and decreased time to re-epithelialization in other severe blistering cutaneous drug reactions including SJS/TEN,5 as a potential therapeutic option in GBFDE.
It is imperative that clinicians recognize the differences between GBFDE and SJS/TEN, as correct diagnosis is crucial for identifying the most likely causative drug as well as providing accurate prognostic information and may have future therapeutic implications as we further understand the immunologic profiles of these severe blistering drug reactions.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399. doi:10.1007/s40257-020-00505-3
- Anderson HJ, Lee JB. A review of fixed drug eruption with a special focus on generalized bullous fixed drug eruption. Medicina (Kaunas). 2021;57:925. doi:10.3390/medicina57090925
- Cho YT, Lin JW, Chen YC, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539-548. doi:10.1016/j.jaad.2013.11.015
- Tran T, Shapiro A. New York-Presbyterian 2022 Health Equity Report. New York-Presbyterian; 2023. Accessed July 22, 2024. https://nyp.widen.net/s/jqfbrvrf9p/dalio-center-2022-health-equity-report
- Dreyer SD, Torres J, Stoddard M, et al. Efficacy of etanercept in the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis. Cutis. 2021;107:E22-E28. doi:10.12788/cutis.0288
To the Editor:
Generalized bullous fixed drug eruption (GBFDE) is a rare subtype of fixed drug eruption (FDE) that manifests as widespread blisters and erosions following exposure to a causative drug.1 Diagnostic criteria include involvement of at least 3 to 6 anatomic sites—head and neck, anterior trunk, posterior trunk, upper extremities, lower extremities, or genitalia—and more than 10% of the body surface area. It can be challenging to differentiate GBFDE from severe drug rashes such as Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) due to extensive body surface area involvement of blisters and erosions. Specific features distinguishing GBFDE from SJS/TEN include primary lesions consisting of larger erythematous to dusky, circular plaques that progress to bullae and coalesce into widespread erosions; history of FDE; lack of severe mucosal involvement; and better overall prognosis.2 Treatment typically involves discontinuation of the culprit medication and supportive care; evidence for systemic therapies is not well established.
Our study aimed to characterize the clinical and demographic features of GBFDE in our institution to highlight potential key differences between this diagnosis and SJS/TEN. An electronic medical record search was performed to identify patients who were clinically diagnosed with GBFDE at New York-Presbyterian/Weill Cornell Medical Center (New York, New York) in both outpatient and inpatient settings from January 2015 to December 2022. This retrospective study was approved by the Weill Cornell Medicine institutional review board (#22-05024777).
Ten patients were identified and included in the analysis (eTable). The mean age of the patients was 56 years (range, 39–76 years). Seven (70%) patients had skin of color (non-White) and 6 (60%) were female. The mean body mass index was 35 (range, 20–57), and 7 (70%) patients were clinically obese (body mass index >30). Only 2 (20%) patients had a history of a documented drug eruption (hives and erythema multiforme), and no patients had a history of FDE. Erythematous dusky patches followed by rapid development of blisters were noted within 3 days of drug initiation in 40% (4/10) and within 5 days in 80% (8/10) of patients. Antibiotics were identified as likely inciting agents in 8 (80%) patients. Biopsies were obtained in 3 (30%) patients and all 3 demonstrated cytotoxic CD8+ interface dermatitis with marked epithelial necrosis, neutrophilia, eosinophilia, and melanophage accumulation. Fever was present at initial presentation in only 4 (40%) patients, and only 1 (10%) patient had oral mucosal involvement. All 10 patients had intertriginous involvement (axillae, 90% [9/10]; gluteal cleft, 80% [8/10]; groin, 80% [8/10]; inframammary folds, 20% [2/10]), and there was considerable flank involvement in 9 (90%) patients. All 10 patients had initial erythematous to dusky, circular patches on the trunk and proximal extremities that then denuded most dramatically in the intertriginous areas (Figure). Six (60%) patients received systemic therapy, including 5 patients treated with a single dose of etanercept 50 mg. In patients with continued progression, 1 or 2 additional doses of etanercept 50 mg were administered at 48- to 72-hour intervals until blistering halted. Treatment with etanercept resulted in clinical improvement in all 5 patients, and there were no identifiable adverse events. The mean hospital stay was 19.7 days (range, 1–63 days).


This study highlights notable demographic and clinical features of GBFDE that have not been widely described in the literature. Large erythematous and dusky patches with broad zones of blistering with particular localization to the neck, intertriginous areas, and flanks typically are not described in SJS/TEN and may be helpful in distinguishing these conditions from GBFDE. Mild or complete lack of mucosal and facial involvement as well as more rapid time from drug initiation to rash (as rapid as 1 day) were key factors that aided in distinguishing GBFDE from SJS/TEN in our patients. Although a history of FDE is considered a key characteristic in the diagnosis of GBFDE, none of our patients had a known history of FDE, suggesting GBFDE may be the initial manifestation of FDE in some patients. Histopathology showed similar findings consistent with FDE in the 3 patients in whom a biopsy was performed. The remaining patients were diagnosed clinically based on the presence of distinctive, perfectly circular, dusky plaques present at the periphery of larger denuded areas, which are characteristic of GBFDE. Lower levels of serum granulysin3 have been shown to help distinguish GBFDE from SJS/TEN, but this test is not readily available with time-sensitive results at most institutions, and exact diagnostic ranges for GBFDE vs SJS/TEN are not yet known.
Our study was limited by a small number of patients at a single institution. Another limitation was the retrospective design.
Interestingly, a high proportion of our patients were non-White and clinically obese, which are factors that should be considered for future research. Sixty percent (6/10) of the patients in our study were Black, which is a notable difference from our hospital’s general admission demographics with Black individuals constituting 12% of patients.4 Our study also highlighted the utility of etanercept, which has reported mortality benefits and decreased time to re-epithelialization in other severe blistering cutaneous drug reactions including SJS/TEN,5 as a potential therapeutic option in GBFDE.
It is imperative that clinicians recognize the differences between GBFDE and SJS/TEN, as correct diagnosis is crucial for identifying the most likely causative drug as well as providing accurate prognostic information and may have future therapeutic implications as we further understand the immunologic profiles of these severe blistering drug reactions.
To the Editor:
Generalized bullous fixed drug eruption (GBFDE) is a rare subtype of fixed drug eruption (FDE) that manifests as widespread blisters and erosions following exposure to a causative drug.1 Diagnostic criteria include involvement of at least 3 to 6 anatomic sites—head and neck, anterior trunk, posterior trunk, upper extremities, lower extremities, or genitalia—and more than 10% of the body surface area. It can be challenging to differentiate GBFDE from severe drug rashes such as Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) due to extensive body surface area involvement of blisters and erosions. Specific features distinguishing GBFDE from SJS/TEN include primary lesions consisting of larger erythematous to dusky, circular plaques that progress to bullae and coalesce into widespread erosions; history of FDE; lack of severe mucosal involvement; and better overall prognosis.2 Treatment typically involves discontinuation of the culprit medication and supportive care; evidence for systemic therapies is not well established.
Our study aimed to characterize the clinical and demographic features of GBFDE in our institution to highlight potential key differences between this diagnosis and SJS/TEN. An electronic medical record search was performed to identify patients who were clinically diagnosed with GBFDE at New York-Presbyterian/Weill Cornell Medical Center (New York, New York) in both outpatient and inpatient settings from January 2015 to December 2022. This retrospective study was approved by the Weill Cornell Medicine institutional review board (#22-05024777).
Ten patients were identified and included in the analysis (eTable). The mean age of the patients was 56 years (range, 39–76 years). Seven (70%) patients had skin of color (non-White) and 6 (60%) were female. The mean body mass index was 35 (range, 20–57), and 7 (70%) patients were clinically obese (body mass index >30). Only 2 (20%) patients had a history of a documented drug eruption (hives and erythema multiforme), and no patients had a history of FDE. Erythematous dusky patches followed by rapid development of blisters were noted within 3 days of drug initiation in 40% (4/10) and within 5 days in 80% (8/10) of patients. Antibiotics were identified as likely inciting agents in 8 (80%) patients. Biopsies were obtained in 3 (30%) patients and all 3 demonstrated cytotoxic CD8+ interface dermatitis with marked epithelial necrosis, neutrophilia, eosinophilia, and melanophage accumulation. Fever was present at initial presentation in only 4 (40%) patients, and only 1 (10%) patient had oral mucosal involvement. All 10 patients had intertriginous involvement (axillae, 90% [9/10]; gluteal cleft, 80% [8/10]; groin, 80% [8/10]; inframammary folds, 20% [2/10]), and there was considerable flank involvement in 9 (90%) patients. All 10 patients had initial erythematous to dusky, circular patches on the trunk and proximal extremities that then denuded most dramatically in the intertriginous areas (Figure). Six (60%) patients received systemic therapy, including 5 patients treated with a single dose of etanercept 50 mg. In patients with continued progression, 1 or 2 additional doses of etanercept 50 mg were administered at 48- to 72-hour intervals until blistering halted. Treatment with etanercept resulted in clinical improvement in all 5 patients, and there were no identifiable adverse events. The mean hospital stay was 19.7 days (range, 1–63 days).


This study highlights notable demographic and clinical features of GBFDE that have not been widely described in the literature. Large erythematous and dusky patches with broad zones of blistering with particular localization to the neck, intertriginous areas, and flanks typically are not described in SJS/TEN and may be helpful in distinguishing these conditions from GBFDE. Mild or complete lack of mucosal and facial involvement as well as more rapid time from drug initiation to rash (as rapid as 1 day) were key factors that aided in distinguishing GBFDE from SJS/TEN in our patients. Although a history of FDE is considered a key characteristic in the diagnosis of GBFDE, none of our patients had a known history of FDE, suggesting GBFDE may be the initial manifestation of FDE in some patients. Histopathology showed similar findings consistent with FDE in the 3 patients in whom a biopsy was performed. The remaining patients were diagnosed clinically based on the presence of distinctive, perfectly circular, dusky plaques present at the periphery of larger denuded areas, which are characteristic of GBFDE. Lower levels of serum granulysin3 have been shown to help distinguish GBFDE from SJS/TEN, but this test is not readily available with time-sensitive results at most institutions, and exact diagnostic ranges for GBFDE vs SJS/TEN are not yet known.
Our study was limited by a small number of patients at a single institution. Another limitation was the retrospective design.
Interestingly, a high proportion of our patients were non-White and clinically obese, which are factors that should be considered for future research. Sixty percent (6/10) of the patients in our study were Black, which is a notable difference from our hospital’s general admission demographics with Black individuals constituting 12% of patients.4 Our study also highlighted the utility of etanercept, which has reported mortality benefits and decreased time to re-epithelialization in other severe blistering cutaneous drug reactions including SJS/TEN,5 as a potential therapeutic option in GBFDE.
It is imperative that clinicians recognize the differences between GBFDE and SJS/TEN, as correct diagnosis is crucial for identifying the most likely causative drug as well as providing accurate prognostic information and may have future therapeutic implications as we further understand the immunologic profiles of these severe blistering drug reactions.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399. doi:10.1007/s40257-020-00505-3
- Anderson HJ, Lee JB. A review of fixed drug eruption with a special focus on generalized bullous fixed drug eruption. Medicina (Kaunas). 2021;57:925. doi:10.3390/medicina57090925
- Cho YT, Lin JW, Chen YC, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539-548. doi:10.1016/j.jaad.2013.11.015
- Tran T, Shapiro A. New York-Presbyterian 2022 Health Equity Report. New York-Presbyterian; 2023. Accessed July 22, 2024. https://nyp.widen.net/s/jqfbrvrf9p/dalio-center-2022-health-equity-report
- Dreyer SD, Torres J, Stoddard M, et al. Efficacy of etanercept in the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis. Cutis. 2021;107:E22-E28. doi:10.12788/cutis.0288
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399. doi:10.1007/s40257-020-00505-3
- Anderson HJ, Lee JB. A review of fixed drug eruption with a special focus on generalized bullous fixed drug eruption. Medicina (Kaunas). 2021;57:925. doi:10.3390/medicina57090925
- Cho YT, Lin JW, Chen YC, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539-548. doi:10.1016/j.jaad.2013.11.015
- Tran T, Shapiro A. New York-Presbyterian 2022 Health Equity Report. New York-Presbyterian; 2023. Accessed July 22, 2024. https://nyp.widen.net/s/jqfbrvrf9p/dalio-center-2022-health-equity-report
- Dreyer SD, Torres J, Stoddard M, et al. Efficacy of etanercept in the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis. Cutis. 2021;107:E22-E28. doi:10.12788/cutis.0288
PRACTICE POINTS
- Distinguishing features of generalized bullous fixed
drug eruption (GBFDE) may include truncal and proximal predilection with early intertriginous blistering. - Etanercept is a viable treatment option for GBFDE.
Retiform Purpura on the Buttocks in 6 Critically Ill COVID-19 Patients
To the Editor:
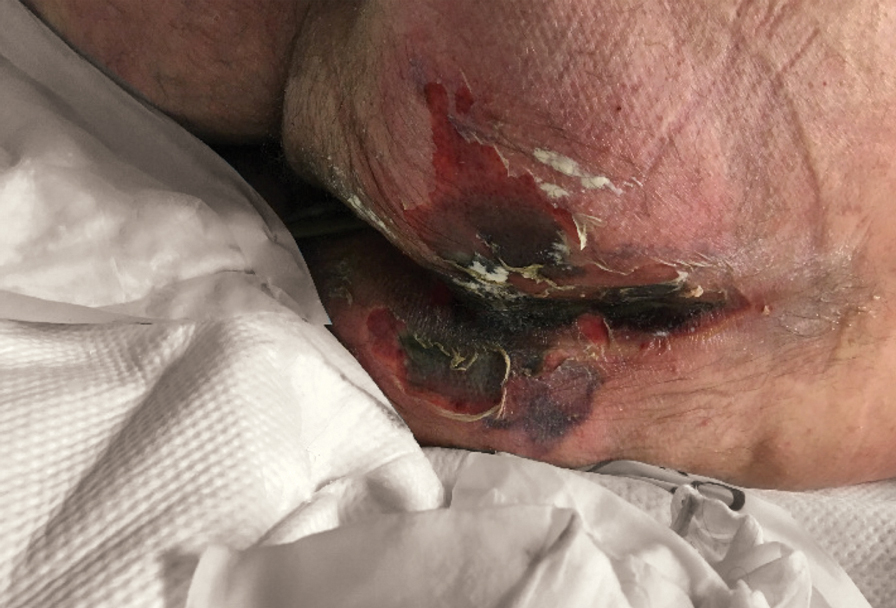
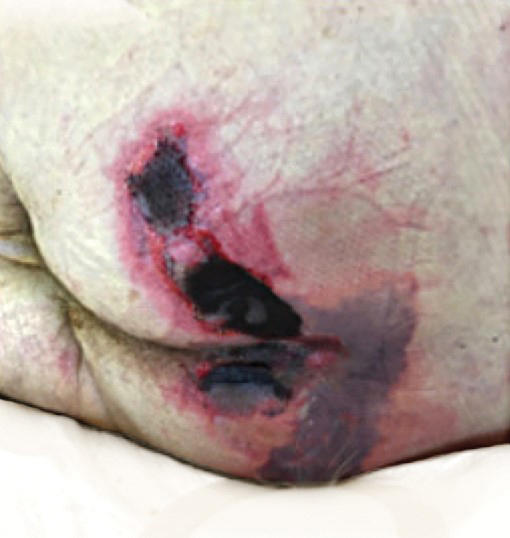
There is emerging evidence of skin findings in patients with COVID-19, including perniolike changes of the toes as well as urticarial and vesicular eruptions.1 Magro et al2 reported 3 cases of livedoid and purpuric skin eruptions in critically ill COVID-19 patients with evidence of thrombotic vasculopathy on skin biopsy, including a 32-year-old man with striking buttocks retiform purpura. Histopathologic analysis revealed thrombotic vasculopathy and pressure-induced ischemic necrosis. Since that patient was first evaluated (March 2020), we identified 6 more cases of critically ill COVID-19 patients from a single academic hospital in New York City with essentially identical clinical findings. Herein, we report those 6 cases of critically ill and intubated patients with COVID-19 who developed retiform purpura on the buttocks only, approximately 11 to 21 days after onset of COVID-19 symptoms.
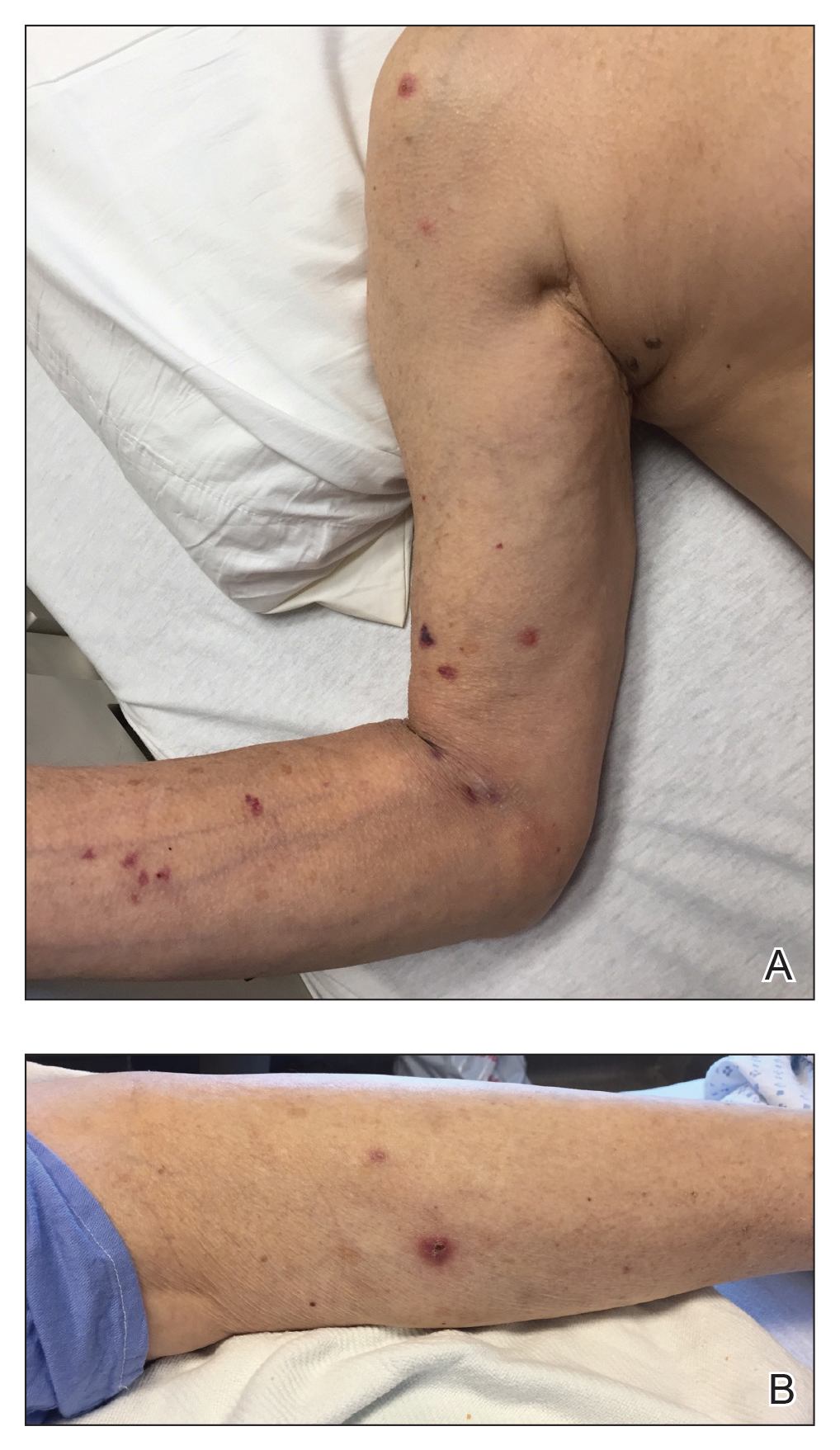
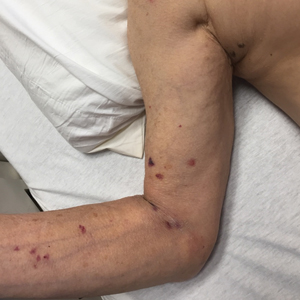
We provided consultation for 5 men and 1 woman (age range, 42–78 years) who were critically ill with COVID-19 and developed retiform purpura on the buttocks (Figures 1 and 2). All had an elevated D-dimer concentration: 2 patients, >700 ng/mL; 2 patients, >2000 ng/mL; 2 patients, >6000 ng/mL (reference, 229 ng/mL). Three patients experienced a peak D-dimer concentration on the day retiform purpura was reported.
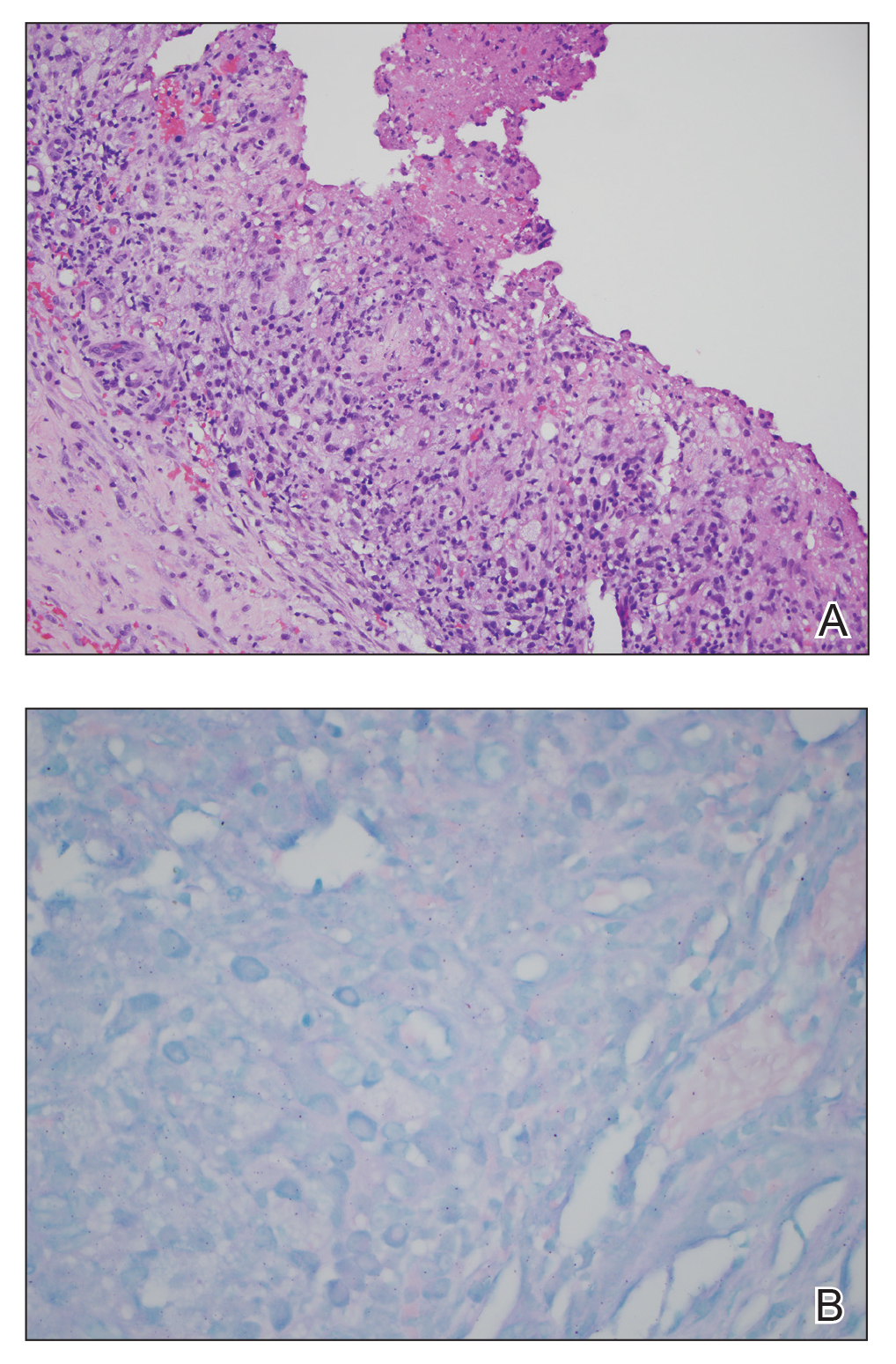
Further evidence of coagulopathy in these patients included 1 patient with a newly diagnosed left popliteal deep vein thrombosis and 1 patient with a known history of protein C deficiency and deep vein thromboses. Five patients were receiving anticoagulation on the day the skin changes were documented; anticoagulation was contraindicated in the sixth patient because of oropharyngeal bleeding. Anticoagulation was continued at the treatment dosage (enoxaparin 80 mg twice daily) in 3 patients, and in 2 patients receiving a prophylactic dose (enoxaparin 40 mg daily), anticoagulation was escalated to treatment dose due to rising D-dimer levels and newly diagnosed retiform purpura. Skin biopsy was deferred for all patients due to positional and ventilatory restrictions. At that point in their care, 3 patients remained admitted on medicine floors, 2 were in the intensive care unit, and 1 had died.
Although the differential diagnosis for retiform purpura is broad and should be fully considered in any patient with this finding, based on the elevated D-dimer concentration, critical illness secondary to COVID-19, and striking similarity to earlier reported case of buttocks retiform purpura with thrombotic vasculopathy and pressure injury noted histopathologically,2 we suspect the buttocks retiform purpura in our 6 cases also represent a combination of cutaneous thrombosis and pressure injury. In addition to acral livedoid eruptions (also reported by Magro and colleagues2), we suspect that this cutaneous manifestation might be associated with a hypercoagulable state in some patients, especially in the setting of a rising D-dimer concentration. One study found that 31% of 184 patients with severe COVID-19 had thrombotic complications,3 a clinical picture that portends a poor prognosis.4
COVID-19 patients presenting with retiform purpura should be fully evaluated based on the broad differential for this morphology. We present 6 cases of buttocks retiform purpura in critically ill COVID-19 patients—all with strikingly similar morphologic findings, an elevated D-dimer concentration, and critical illness due to COVID-19—to alert clinicians to this constellation of findings and propose that this cutaneous manifestation could indicate an associated hypercoaguable state and should prompt a hematology consultation. Additionally, biopsy of this skin finding should be considered, especially if biopsy results might serve to guide management; however, obtaining a biopsy specimen can be technically difficult because of ventilatory requirements.
Given the magnitude of the COVID-19 pandemic and the propensity of these patients to experience thrombotic events, recognition of this skin finding in COVID-19 is important and might allow timely intervention.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212-e213. doi:10.1111/jdv.16387
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. doi:10.1016/j.thromres.2020.04.013
- Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-847. doi:10.1111/jth.14768
To the Editor:
There is emerging evidence of skin findings in patients with COVID-19, including perniolike changes of the toes as well as urticarial and vesicular eruptions.1 Magro et al2 reported 3 cases of livedoid and purpuric skin eruptions in critically ill COVID-19 patients with evidence of thrombotic vasculopathy on skin biopsy, including a 32-year-old man with striking buttocks retiform purpura. Histopathologic analysis revealed thrombotic vasculopathy and pressure-induced ischemic necrosis. Since that patient was first evaluated (March 2020), we identified 6 more cases of critically ill COVID-19 patients from a single academic hospital in New York City with essentially identical clinical findings. Herein, we report those 6 cases of critically ill and intubated patients with COVID-19 who developed retiform purpura on the buttocks only, approximately 11 to 21 days after onset of COVID-19 symptoms.
We provided consultation for 5 men and 1 woman (age range, 42–78 years) who were critically ill with COVID-19 and developed retiform purpura on the buttocks (Figures 1 and 2). All had an elevated D-dimer concentration: 2 patients, >700 ng/mL; 2 patients, >2000 ng/mL; 2 patients, >6000 ng/mL (reference, 229 ng/mL). Three patients experienced a peak D-dimer concentration on the day retiform purpura was reported.
Further evidence of coagulopathy in these patients included 1 patient with a newly diagnosed left popliteal deep vein thrombosis and 1 patient with a known history of protein C deficiency and deep vein thromboses. Five patients were receiving anticoagulation on the day the skin changes were documented; anticoagulation was contraindicated in the sixth patient because of oropharyngeal bleeding. Anticoagulation was continued at the treatment dosage (enoxaparin 80 mg twice daily) in 3 patients, and in 2 patients receiving a prophylactic dose (enoxaparin 40 mg daily), anticoagulation was escalated to treatment dose due to rising D-dimer levels and newly diagnosed retiform purpura. Skin biopsy was deferred for all patients due to positional and ventilatory restrictions. At that point in their care, 3 patients remained admitted on medicine floors, 2 were in the intensive care unit, and 1 had died.
Although the differential diagnosis for retiform purpura is broad and should be fully considered in any patient with this finding, based on the elevated D-dimer concentration, critical illness secondary to COVID-19, and striking similarity to earlier reported case of buttocks retiform purpura with thrombotic vasculopathy and pressure injury noted histopathologically,2 we suspect the buttocks retiform purpura in our 6 cases also represent a combination of cutaneous thrombosis and pressure injury. In addition to acral livedoid eruptions (also reported by Magro and colleagues2), we suspect that this cutaneous manifestation might be associated with a hypercoagulable state in some patients, especially in the setting of a rising D-dimer concentration. One study found that 31% of 184 patients with severe COVID-19 had thrombotic complications,3 a clinical picture that portends a poor prognosis.4
COVID-19 patients presenting with retiform purpura should be fully evaluated based on the broad differential for this morphology. We present 6 cases of buttocks retiform purpura in critically ill COVID-19 patients—all with strikingly similar morphologic findings, an elevated D-dimer concentration, and critical illness due to COVID-19—to alert clinicians to this constellation of findings and propose that this cutaneous manifestation could indicate an associated hypercoaguable state and should prompt a hematology consultation. Additionally, biopsy of this skin finding should be considered, especially if biopsy results might serve to guide management; however, obtaining a biopsy specimen can be technically difficult because of ventilatory requirements.
Given the magnitude of the COVID-19 pandemic and the propensity of these patients to experience thrombotic events, recognition of this skin finding in COVID-19 is important and might allow timely intervention.
To the Editor:
There is emerging evidence of skin findings in patients with COVID-19, including perniolike changes of the toes as well as urticarial and vesicular eruptions.1 Magro et al2 reported 3 cases of livedoid and purpuric skin eruptions in critically ill COVID-19 patients with evidence of thrombotic vasculopathy on skin biopsy, including a 32-year-old man with striking buttocks retiform purpura. Histopathologic analysis revealed thrombotic vasculopathy and pressure-induced ischemic necrosis. Since that patient was first evaluated (March 2020), we identified 6 more cases of critically ill COVID-19 patients from a single academic hospital in New York City with essentially identical clinical findings. Herein, we report those 6 cases of critically ill and intubated patients with COVID-19 who developed retiform purpura on the buttocks only, approximately 11 to 21 days after onset of COVID-19 symptoms.
We provided consultation for 5 men and 1 woman (age range, 42–78 years) who were critically ill with COVID-19 and developed retiform purpura on the buttocks (Figures 1 and 2). All had an elevated D-dimer concentration: 2 patients, >700 ng/mL; 2 patients, >2000 ng/mL; 2 patients, >6000 ng/mL (reference, 229 ng/mL). Three patients experienced a peak D-dimer concentration on the day retiform purpura was reported.
Further evidence of coagulopathy in these patients included 1 patient with a newly diagnosed left popliteal deep vein thrombosis and 1 patient with a known history of protein C deficiency and deep vein thromboses. Five patients were receiving anticoagulation on the day the skin changes were documented; anticoagulation was contraindicated in the sixth patient because of oropharyngeal bleeding. Anticoagulation was continued at the treatment dosage (enoxaparin 80 mg twice daily) in 3 patients, and in 2 patients receiving a prophylactic dose (enoxaparin 40 mg daily), anticoagulation was escalated to treatment dose due to rising D-dimer levels and newly diagnosed retiform purpura. Skin biopsy was deferred for all patients due to positional and ventilatory restrictions. At that point in their care, 3 patients remained admitted on medicine floors, 2 were in the intensive care unit, and 1 had died.
Although the differential diagnosis for retiform purpura is broad and should be fully considered in any patient with this finding, based on the elevated D-dimer concentration, critical illness secondary to COVID-19, and striking similarity to earlier reported case of buttocks retiform purpura with thrombotic vasculopathy and pressure injury noted histopathologically,2 we suspect the buttocks retiform purpura in our 6 cases also represent a combination of cutaneous thrombosis and pressure injury. In addition to acral livedoid eruptions (also reported by Magro and colleagues2), we suspect that this cutaneous manifestation might be associated with a hypercoagulable state in some patients, especially in the setting of a rising D-dimer concentration. One study found that 31% of 184 patients with severe COVID-19 had thrombotic complications,3 a clinical picture that portends a poor prognosis.4
COVID-19 patients presenting with retiform purpura should be fully evaluated based on the broad differential for this morphology. We present 6 cases of buttocks retiform purpura in critically ill COVID-19 patients—all with strikingly similar morphologic findings, an elevated D-dimer concentration, and critical illness due to COVID-19—to alert clinicians to this constellation of findings and propose that this cutaneous manifestation could indicate an associated hypercoaguable state and should prompt a hematology consultation. Additionally, biopsy of this skin finding should be considered, especially if biopsy results might serve to guide management; however, obtaining a biopsy specimen can be technically difficult because of ventilatory requirements.
Given the magnitude of the COVID-19 pandemic and the propensity of these patients to experience thrombotic events, recognition of this skin finding in COVID-19 is important and might allow timely intervention.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212-e213. doi:10.1111/jdv.16387
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. doi:10.1016/j.thromres.2020.04.013
- Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-847. doi:10.1111/jth.14768
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212-e213. doi:10.1111/jdv.16387
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. doi:10.1016/j.thromres.2020.04.013
- Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-847. doi:10.1111/jth.14768
Practice Points
- Retiform purpura in a severely ill patient with COVID-19 and a markedly elevated D-dimer concentration might be a cutaneous sign of systemic coagulopathy.
- This constellation of findings should prompt consideration of skin biopsy and hematology consultation.
Cutaneous Manifestations of COVID-19: Characteristics, Pathogenesis, and the Role of Dermatology in the Pandemic
The virus that causes COVID-19—SARS-CoV-2—has infected more than 128 million individuals, resulting in more than 2.8 million deaths worldwide between December 2019 and April 2021. Disease mortality primarily is driven by hypoxemic respiratory failure and systemic hypercoagulability, resulting in multisystem organ failure.1 With more than 17 million Americans infected, the virus is estimated to have impacted someone within the social circle of nearly every American.2
The COVID-19 pandemic has highlighted resource limitations, delayed elective and preventive care, and rapidly increased the adoption of telemedicine, presenting a host of new challenges to providers in every medical specialty, including dermatology. Although COVID-19 primarily is a respiratory disease, clinical manifestations have been observed in nearly every organ, including the skin. The cutaneous manifestations of COVID-19 provide insight into disease diagnosis, prognosis, and pathophysiology. In this article, we review the cutaneous manifestations of COVID-19 and explore the state of knowledge regarding their pathophysiology and clinical significance. Finally, we discuss the role of dermatology consultants in the care of patients with COVID-19, and the impact of the pandemic on the field of dermatology.
Prevalence of Cutaneous Findings in COVID-19
Early reports characterizing the clinical presentation of patients hospitalized with COVID-19 suggested skin findings associated with the disease were rare. Cohort studies from Europe, China, and New York City in January through March 2020 reported a low prevalence or made no mention of rash.3-7 However, reports from dermatologists in Italy that emerged in May 2020 indicated a substantially higher proportion of cutaneous disease: 18 of 88 (20.4%) hospitalized patients were found to have cutaneous involvement, primarily consisting of erythematous rash, along with some cases of urticarial and vesicular lesions.8 In October 2020, a retrospective cohort study from Spain examining 2761 patients presenting to the emergency department or admitted to the hospital for COVID-19 found that 58 (2.1%) patients had skin lesions attributed to COVID-19.9
The wide range in reported prevalence of skin lesions may be due to variable involvement of dermatologic specialists in patient care, particularly in China.10 Some variation also may be due to variability in the timing of clinical examination, as well as demographic and clinical differences in patient populations. Of note, a multisystem inflammatory disease seen in US children subsequent to infection with COVID-19 has been associated with rash in as many as 74% of cases.11 Although COVID-19 disproportionately impacts people with skin of color, there are few reports of cutaneous manifestations in that population,12 highlighting the challenges of the dermatologic examination in individuals with darker skin and suggesting the prevalence of dermatologic disease in COVID-19 may be greater than reported.
Morphologic Patterns of Cutaneous Involvement in COVID-19
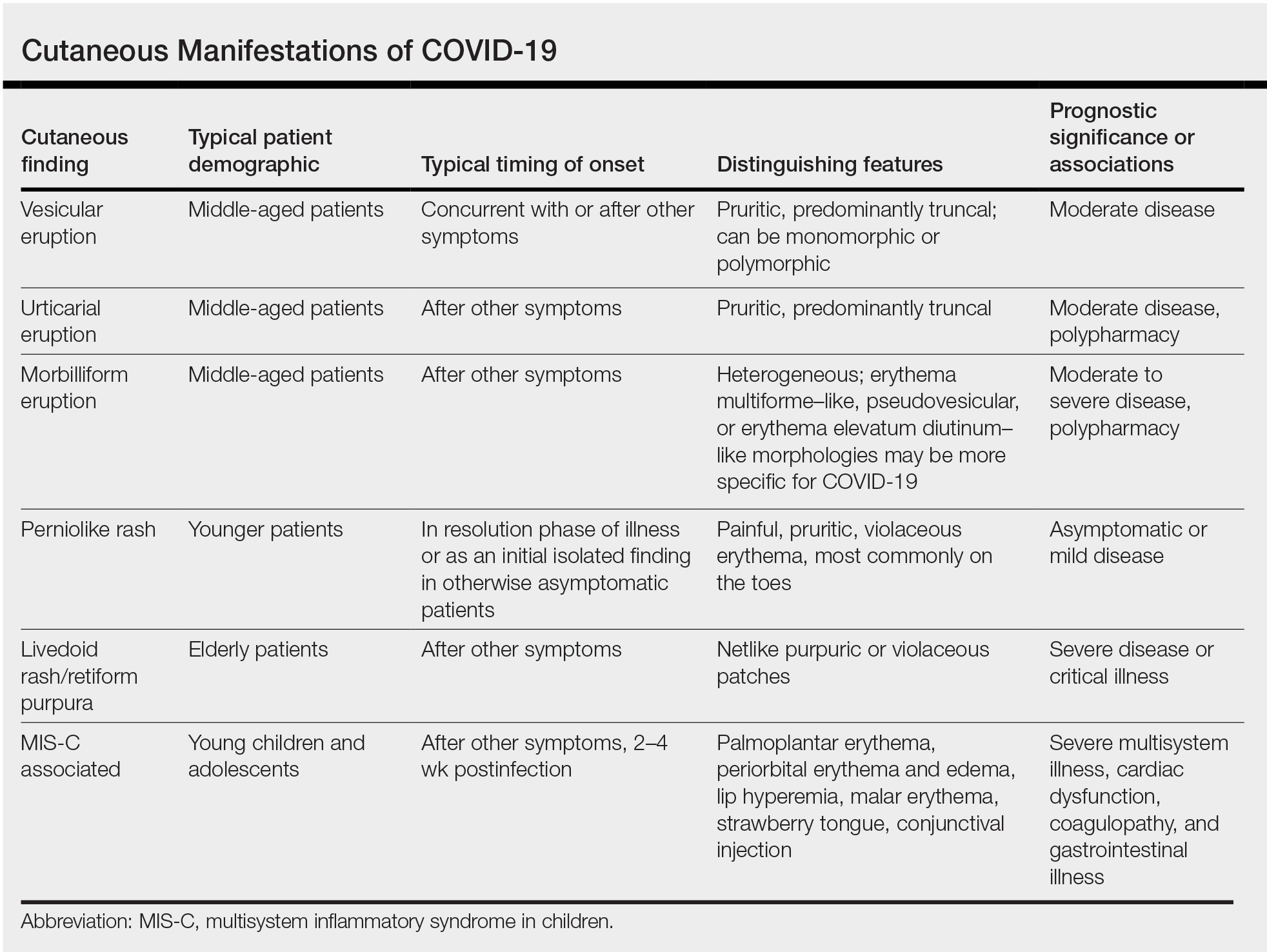
Researchers in Europe and the United States have attempted to classify the cutaneous manifestations of COVID-19. A registry established through the American Academy of Dermatology published a compilation of reports from 31 countries, totaling 716 patient profiles.13 A prospective Spanish study detailed the cutaneous involvement of 375 patients with suspected or confirmed COVID-19.14 Together, these efforts have revealed several distinct patterns of cutaneous involvement associated with COVID-19 (Table).9,15-18
Vesicular Rash
Vesicular rash associated with COVID-19 has been described in several studies and case series8,13,14 and is considered, along with the pseudopernio (or pseudochilblains) morphology, to be one of the more disease-specific patterns in COVID-19.14,18 Vesicular rash appears to comprise roughly one-tenth of all COVID-19–associated rashes.13,14 It usually is described as pruritic, with 72% to 83% of patients reporting itch.13,16
Small monomorphic or polymorphic vesicles predominantly on the trunk and to a lesser extent the extremities and head have been described by multiple authors.14,16 Vesicular rash is most common among middle-aged individuals, with studies reporting median and mean ages ranging from 40.5 to 55 years.9,13,14,16
Vesicular rash develops concurrent with or after other presenting symptoms of COVID-19; in 2 studies, vesicular rash preceded development of other symptoms in only 15% and 5.6% of cases, respectively.13,14 Prognostically, vesicular rash is associated with moderate disease severity.14,16 It may persist for an average of 8 to 10 days.14,16,18
Histopathologic examination reveals basal layer vacuolar degeneration, hyperchromatic keratinocytes, acantholysis, and dyskeratosis.9,16,18
Urticarial Rash
Urticarial lesions represent approximately 7% to 19% of reported COVID-19–associated rashes.9,13,14 Urticarial rashes in patients testing positive for SARS-CoV-2 primarily occur on the trunk.14 The urticaria, which typically last about 1 week,14 are seen most frequently in middle-aged patients (mean/median age, 42–48 years)13,14 and are associated with pruritus, which has been reported in 74% to 92% of patients.13,14 Urticarial lesions typically do not precede other symptoms of COVID-19 and are nonspecific, making them less useful diagnostically.14
Urticaria appears to be associated with more severe COVID-19 illness in several studies, but this finding may be confounded by several factors, including older age, increased tobacco use, and polypharmacy. Of 104 patients with reported urticarial rash and suspected or confirmed COVID-19 across 3 studies, only 1 death was reported.9,13,14
The histopathologic appearance is that of typical hives, demonstrating a perivascular infiltrate of lymphocytes and eosinophils with edema of the upper dermis.9,19
Morbilliform Eruption
Morbilliform eruption is a commonly reported morphology associated with COVID-19, accounting for 20% to 47% of rashes.9,13,14 This categorization may have limited utility from a diagnostic and prognostic perspective, given that morbilliform eruptions are common, nonspecific, and heterogenous and can arise from many causes.9,13,14 Onset of morbilliform eruption appears to coincide with14 or follow13,20,21 the development of other COVID-19–related symptoms, with 5% of patients reporting morbilliform rash as the initial manifestation of infection.13,14 Morbilliform eruptions have been observed to occur in patients with more severe disease.9,13,14
Certain morphologic subtypes, such as erythema multiforme–like, erythema elevatum diutinum–like, or pseudovesicular, may be more specific to COVID-19 infection.14 A small case series highlighted 4 patients with erythema multiforme–like eruptions, 3 of whom also were found to have petechial enanthem occurring after COVID-19 diagnosis; however, the investigators were unable to exclude drug reaction as a potential cause of rash in these patients.22 Another case series of 21 patients with COVID-19 and skin rash described a (primarily) petechial enanthem on the palate in 6 (28.5%) patients.23 It is unclear to what extent oral enanthem may be underrecognized given that some physicians may be disinclined to remove the masks of known COVID-19–positive patients to examine the oral cavity.
The histologic appearance of morbilliform rash seen in association with COVID-19 has been described as spongiotic with interface dermatitis with perivascular lymphocytic inflammation.9,21
COVID Toes, Pseudochilblains Rash, Perniolike Rash, and Acral Erythema/Edema
Of all the rashes associated with COVID-19, COVID toes, or pseudochilblains rash, has perhaps attracted the most attention. The characteristic violaceous erythema on the fingers and/or toes may be itchy or painful, presenting similar to idiopathic cases of pernio (Figure 1).14 The entity has been controversial because of an absence of a clear correlation with a positive SARS-CoV-2 polymerase chain reaction test or antibodies to the virus in a subset of reported cases.24,25 Onset of the rash late in the disease course, generally after symptom resolution in mild or asymptomatic cases, may explain the absence of viral DNA in the nasopharynx by the time of lesion appearance.14,26 Seronegative patients may have cleared SARS-CoV-2 infection before humoral immunity could occur via a strong type 1 interferon response.25
Across 3 studies, perniolike skin lesions constituted 18% to 29% of COVID-19–associated skin findings9,13,14 and persisted for an average of 12 to 14 days.13,14 Perniolike lesions portend a favorable outcome; patients with COVID toes rarely present with systemic symptoms or laboratory or imaging abnormalities9 and less commonly require hospitalization for severe illness. Perniolike lesions have been reported most frequently in younger patients, with a median or mean age of 32 to 35 years.13,14
Histology demonstrates lichenoid dermatitis with perivascular and periadnexal lymphocytic infiltrates.9 Notably, one study observed interface dermatitis of the intraepidermal portion of the acrosyringium, a rare finding in chilblain lupus, in 83% of patients (N=40).25 Direct immunofluorescence demonstrates a vasculopathic pattern, with some patients showing deposition of IgM or IgG, C3, and fibrinogen in dermal blood vessels. Vascular C9 deposits also have been demonstrated on immunohistochemistry.9 Biopsies of perniolike lesions in COVID-19 patients have demonstrated the presence of SARS-CoV-2 RNA,27 have identified SARS-CoV-2 spike protein in endothelial cells on immunohistochemistry, and have visualized intracytoplasmic viral particles in vascular endothelium on electron microscopy.28
Livedoid Rash/Retiform Purpura
Netlike purpuric or violaceous patches signifying vessel damage or occlusion have been seen in association with COVID-19, constituting approximately 6% of COVID-19–associated skin findings in 2 studies.13,14 Livedoid rash (Figure 2) and retiform purpura (Figure 3) are associated with older age and occur primarily in severely ill patients, including those requiring intensive care. In a registry of 716 patients with COVID-19, 100% of patients with retiform purpura were hospitalized, and 82% had acute respiratory distress syndrome.13 In another study, 33% (7/21) of patients with livedoid and necrotic lesions required intensive care, and 10% (2/21) died.14
Livedoid lesions and retiform purpura represent thrombotic disease in the skin due to vasculopathy/coagulopathy. Dermatopathology available through the American Academy of Dermatology registry revealed thrombotic vasculopathy.13 A case series of 4 patients with livedo racemosa and retiform purpura demonstrated pauci-inflammatory thrombogenic vasculopathy involving capillaries, venules, and arterioles with complement deposition.29 Livedoid and retiform lesions in the skin may be associated with a COVID-19–induced coagulopathy, a propensity for systemic clotting including pulmonary embolism, which mostly occurs in hospitalized patients with severe illness.30
Multisystem Inflammatory Disease in Children
A hyperinflammatory syndrome similar to Kawasaki disease and toxic shock syndrome associated with mucocutaneous, cardiac, and gastrointestinal manifestations has been reported following COVID-19 infection.31 This syndrome, known as multisystem inflammatory syndrome in children (MIS-C), predominantly affects adolescents and children older than 5 years,11 typically occurs 2 to 4 weeks after infection, and appears to be at least 100-times less common than COVID-19 infection among the same age group.31 Sixty percent31 to 74%11 of affected patients have mucocutaneous involvement, with the most common clinical findings being conjunctival injection, palmoplantar erythema, lip hyperemia, periorbital erythema and edema, strawberry tongue, and malar erythema, respectively.32
Because this condition appears to reflect an immune response to the virus, the majority of cases demonstrate negative SARS-CoV-2 polymerase chain reaction and positive antibody testing.33 Although cutaneous findings are similar to those seen in Kawasaki disease, certain findings have been noted in MIS-C that are not typical of Kawasaki disease, including heliotrope rash–like periorbital edema and erythema as well as erythema infectiosum–like malar erythema and reticulated erythematous eruptions.32
The course of MIS-C can be severe; in one case series of patients presenting with MIS-C, 80% (79/99) required intensive care unit admission, with 10% requiring mechanical ventilation and 2% of patients dying during admission.31 Cardiac dysfunction, coagulopathy, and gastrointestinal symptoms are common.11,31 It has been postulated that a superantigenlike region of the SARS-CoV-2 spike protein, similar to that of staphylococcal enterotoxin B, may underlie MIS-C and account for its similarities to toxic shock syndrome.34 Of note, a similar multisystem inflammatory syndrome associated with COVID-19 also has been described in adults, and it too may present with rash as a cardinal feature.35
Pathophysiology of COVID-19: What the Skin May Reveal About the Disease
The diverse range of cutaneous manifestations in COVID-19 reflects a spectrum of host immunologicresponses to SARS-CoV-2 and may inform the pathophysiology of the disease as well as potential treatment modalities.
Host Response to SARS-CoV-2
The body’s response to viral infection is 2-pronged, involving activation of cellular antiviral defenses mediated by type I and III interferons, as well as recruitment of leukocytes, mobilized by cytokines and chemokines.36,37 Infection with SARS-CoV-2 results in a unique inflammatory response characterized by suppression of interferons, juxtaposed with a rampant proinflammatory cytokine and chemokine response, reminiscent of a cytokine storm. Reflective of this imbalance, a study of 50 COVID-19 patients and 20 healthy controls found decreased natural killer cells and CD3+ T cells in COVID-19 patients, particularly severely or critically ill patients, with an increase in B cells and monocytes.38 This distinctive immune imbalance positions SARS-CoV-2 to thrive in the absence of inhibitory interferon activity while submitting the host to the deleterious effects of a cytokine surge.36
Type I Interferons
The perniolike lesions associated with mild COVID-19 disease14 may represent a robust immune response via effective stimulation of type I interferons (IFN-1). Similar perniolike lesions are observed in Aicardi-Goutières syndrome37 and familial chilblain lupus, hereditary interferonopathies associated with mutations in the TREX1 (three prime repair exonuclease 1) gene and characterized by inappropriate upregulation of IFN-1,39 resulting in chilblains. It has been suggested that perniolike lesions in COVID-19 result from IFN-1 activation—a robust effective immunologic response to the virus.14,26,40
On the other end of the spectrum, patients with severe COVID-19 may have a blunted IFN-1 response and reduced IFN-1–stimulated gene expression.36,38 Notably, low IFN-1 response preceded clinical deterioration and was associated with increased risk for evolution to critical illness.38 Severe disease from COVID-19 also is more commonly observed in older patients and those with comorbidities,1 both of which are known factors associated with depressed IFN-1 function.38,41 Reflective of this disparate IFN-1 response, biopsies of COVID-19 perniosis have demonstrated striking expression of myxovirus resistance protein A (MXA), a marker for IFN-1 signaling in tissue, whereas its expression is absent in COVID-19 livedo/retiform purpura.27
Familial chilblain lupus may be effectively treated by the Janus kinase inhibitor baricitinib,39 which inhibits IFN-1 signaling. Baricitinib recently received emergency use authorization by the US Food and Drug Administration for treatment of severe COVID-19 pneumonia,42,43 hinting to disordered IFN-1 signaling in the COVID-19 pathophysiology.
The impaired IFN-1 response in COVID-19 patients may be due to a unique characteristic of SARS-CoV-2: its ORF3b gene is a potent IFN-1 antagonist. In a series of experiments comparing SARS-CoV-2 to the related virus severe acute respiratory disease coronavirus (which was responsible for an epidemic in 2002), Konno et al44 found that SARS-CoV-2 is more effectively able to downregulate host IFN-1, likely due to premature stop codons on ORF3b that produce a truncated version of the gene with amplified anti–IFN-1 activity.
Cytokine Storm and Coagulation Cascade
This dulled interferon response is juxtaposed with a surge of inflammatory chemokines and cytokines, including IL-6, IL-8, IL-10, and tumor necrosis factor α, impairing innate immunity and leading to end-organ damage. This inflammatory response is associated with the influx of innate immune cells, specifically neutrophils and monocytes, which likely contribute to lung injury in COVID-19 acute respiratory distress syndrome.38 It also is thought to lead to downstream activation of coagulation, with a high incidence of thrombotic events observed in patients with severe COVID-19.1 In a retrospective study of 184 intensive care patients with COVID-19 receiving at least standard doses of thromboprophylaxis, venous thromboembolism occurred in 27% and arterial thrombotic events occurred in 3.7%.45
Livedo racemosa and retiform purpura are cutaneous markers of hypercoagulability, which indicate an increased risk for systemic clotting in COVID-19. A positive feedback loop between the complement and coagulation cascades appears to be important.13,14,29,46-48 In addition, a few studies have reported antiphospholipid antibody positivity in hospitalized COVID-19 patients.49,50
The high incidence of coagulopathy in severe COVID-19 has prompted many institutions to develop aggressive prophylactic anticoagulation protocols. Elevation of proinflammatory cytokines and observation of terminal complement activation in the skin and other organs has led to therapeutic trials of IL-6 inhibitors such as tocilizumab,51 complement inhibitors such as eculizumab, and Janus kinase inhibitors such as ruxolitinib and baricitinib.42,48
COVID Long-Haulers
The long-term effects of immune dysregulation in COVID-19 patients remain to be seen. Viral triggering of autoimmune disease is a well-established phenomenon, seen in DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome and other dermatologic diseases, raising the possibility that dermatologists will see a rising incidence of cutaneous autoimmune disease in the aftermath of the pandemic. Disordered interferon stimulation could lead to increased incidence of interferon-mediated disorders, such as sarcoidosis and other granulomatous diseases. Vasculitislike skin lesions could persist beyond the acute infectious period. Recent data from a registry of 990 COVID-19 cases from 39 countries suggest that COVID-19 perniolike lesions may persist as long as 150 days.52 In a time of many unknowns, these questions serve as a call to action for rigorous data collection, contribution to existing registries for dermatologic manifestations of COVID-19, and long-term follow-up of COVID-19 patients by the dermatology community.
Pandemic Dermatology
The pandemic has posed unprecedented challenges for patient care. The use of hydroxychloroquine as a popular but unproven treatment for COVID-19, 53 particularly early in the pandemic, has resulted in drug shortages for patients with lupus and other autoimmune skin diseases. Meanwhile, the need for patients with complex dermatologic conditions to receive systemic immunosuppression has had to be balanced against the associated risks during a global pandemic. To help dermatologists navigate this dilemma, various subspecialty groups have issued guidelines, including the COVID-19 Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists, which recommends a stepwise approach to shared decision-making with the goal of minimizing both the risk for disease flare and that of infection. The use of systemic steroids and rituximab, as well as the dose of immunosuppression—particularly broad-acting immunosuppression—should be limited where permitted. 54
Rapid adoption of telemedicine and remote monitoring strategies has enabled dermatologists to provide safe and timely care when in-person visits have not been possible, including for patients with confirmed or suspected COVID-19, as well as for hospitalized patients. 55-57 Use of telemedicine has facilitated preservation of personal protective equipment at a time when these important resources have been scarce. For patients with transportation or scheduling barriers, telemedicine has even expanded access to care.
However, this strategy cannot completely replace comprehensive in-person evaluation. Variability in video and photographic quality limits evaluation, while in-person physical examination can reveal subtle morphologic clues necessary for diagnosis. 5 8 Additionally, unequal access to technology may disadvantage some patients. For dermatologists to provide optimal care and continue to contribute accurate and insightful observations into COVID-19, it is essential to be physically present in the clinic and in the hospital when necessary, caring for patients in need of dermatologic expertise. Creative management strategies developed during this time will benefit patients and expand the reach of the specialty . 5 8
Final Thoughts
The COVID-19 pandemic has profoundly challenged the medical community and dermatology is no exception. By documenting and characterizing the diverse cutaneous manifestations of this novel disease, dermatologists have furthered understanding of its pathophysiology and management. By adapting quickly and developing creative ways to deliver care, dermatologists have found ways to contribute, both large and small. As we take stock at this juncture of the pandemic, it is clear there remains much to learn. We hope dermatologists will continue to take an active role in meeting the challenges of this time.
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA . 2020;324:782-793. doi:10.1001/jama.2020.12839
- New York Times . Updated December 23, 2020. Accessed March 22, 2021. https://www.nytimes.com/2020/11/15/us/coronavirus-us-cases-deaths.html
- Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med . 2020;382:1708-1720. doi:10.1056/NEJMoa2002032
- Lechien JR, Chiesa-Estomba CM, Place S, et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med . 2020;288:335-344. doi:https://doi.org/10.1111/joim.13089
- Wu J, Liu J, Zhao X, et al. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID-19) in Jiangsu province: a multicenter descriptive study. Clin Infect Dis . 2020;71:706-712. doi:10.1093/cid/ciaa199
- Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of COVID-19 in New York City. N Engl J Med . 2020;382:2372-2374. doi:10.1056/NEJMc2010419
- Sun L, Shen L, Fan J, et al. Clinical features of patients with coronavirus disease 2019 from a designated hospital in Beijing, China. J Med Virol . 2020;92:2055-2066. https://doi.org/10.1002/jmv.25966
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatology Venereol . 2020;34:E212-E213. https://doi.org/10.1111/jdv.16387
- Giavedoni P, Podlipnik S, Pericàs JM, et al. Skin manifestations in COVID-19: prevalence and relationship with disease severity. J Clin Med . 2020;9:3261. doi:10.3390/jcm9103261
- Jimenez-Cauhe J, Ortega-Quijano D, Prieto-Barrios M, et al. Reply to “COVID-19 can present with a rash and be mistaken for dengue”: petechial rash in a patient with COVID-19 infection. J Am Acad Dermatol . 2020;83:E141-E142. doi:10.1016/j.jaad.2020.04.016
- Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med . 2020;383:334-346. doi:10.1056/NEJMoa2021680
- Shinkai K, Bruckner AL. Dermatology and COVID-19. JAMA . 2020;324:1133-1134. doi:10.1001/jama.2020.15276
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol . 2020;83:1118-1129. doi:10.1016/j.jaad.2020.06.1016
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol . 2020;183:71-77. https://doi.org/10.1111/bjd.19163
- Bouaziz JD, Duong TA, Jachiet M, et al. Vascular skin symptoms in COVID-19: a French observational study. J Eur Acad Dermatology Venereol . 2020;34:E451-E452. https://doi.org/10.1111/jdv.16544
- Fernandez-Nieto D, Ortega-Quijano D, Jimenez-Cauhe J, et al. Clinical and histological characterization of vesicular COVID-19 rashes: a prospective study in a tertiary care hospital. Clin Exp Dermatol . 2020;45:872-875. https://doi.org/10.1111/ced.14277
- Fernandez-Nieto D, Jimenez-Cauhe J, Suarez-Valle A, et al. Characterization of acute acral skin lesions in nonhospitalized patients: a case series of 132 patients during the COVID-19 outbreak. J Am Acad Dermatol . 2020;83:E61-E63. doi:10.1016/j.jaad.2020.04.093
- Marzano AV, Genovese G, Fabbrocini G, et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: Multicenter case series of 22 patients. J Am Acad Dermatol . 2020;83:280-285. doi:10.1016/j.jaad.2020.04.044
- Fernandez-Nieto D, Ortega-Quijano D, Segurado-Miravalles G, et al. Comment on: cutaneous manifestations in COVID-19: a first perspective. safety concerns of clinical images and skin biopsies. J Eur Acad Dermatol Venereol . 2020;34:E252-E254. https://doi.org/10.1111/jdv.16470
- Herrero-Moyano M, Capusan TM, Andreu-Barasoain M, et al. A clinicopathological study of eight patients with COVID-19 pneumonia and a late-onset exanthema. J Eur Acad Dermatol Venereol . 2020;34:E460-E464. https://doi.org/10.1111/jdv.16631
- Rubio-Muniz CA, Puerta-Peñ a M, Falkenhain-L ópez D, et al. The broad spectrum of dermatological manifestations in COVID-19: clinical and histopathological features learned from a series of 34 cases. J Eur Acad Dermatol Venereol . 2020;34:E574-E576. https://doi.org/10.1111/jdv.16734
- Jimenez-Cauhe J, Ortega-Quijano D, Carretero-Barrio I, et al. Erythema multiforme-like eruption in patients with COVID-19 infection: clinical and histological findings. Clin Exp Dermatol . 2020;45:892-895. https://doi.org/10.1111/ced.14281
- Jimenez-Cauhe J, Ortega-Quijano D, de Perosanz-Lobo D, et al. Enanthem in patients with COVID-19 and skin rash. JAMA Dermatol . 2020;156:1134-1136. doi:10.1001/jamadermatol.2020.2550
- Le Cleach L, Dousset L, Assier H, et al. Most chilblains observed during the COVID-19 outbreak occur in patients who are negative for COVID-19 on polymerase chain reaction and serology testing. Br J Dermatol . 2020;183:866-874. https://doi.org/10.1111/bjd.19377
- Hubiche T, Cardot-Leccia N, Le Duff F, et al. Clinical, laboratory, and interferon-alpha response characteristics of patients with chilblain-like lesions during the COVID-19 pandemic [published online November 25, 2020]. JAMA Dermatol . doi:10.1001/jamadermatol.2020.4324
- Freeman EE, McMahon DE, Lipoff JB, et al. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol . 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Magro CM, Mulvey JJ, Laurence J, et al. The differing pathophysiologies that underlie COVID-19-associated perniosis and thrombotic retiform purpura: a case series. Br J Dermatol . 2021;184:141-150. https://doi.org/10.1111/bjd.19415
- Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol . 2020;183:729-737. doi:10.1111/bjd.19327
- Droesch C, Do MH, DeSancho M, et al. Livedoid and purpuric skin eruptions associated with coagulopathy in severe COVID-19. JAMA Dermatol . 2020;156:1-3. doi:10.1001/jamadermatol.2020.2800
- Asakura H, Ogawa H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int J Hematol . 2021;113:45-57. doi:10.1007/s12185-020-03029-y
- Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med . 2020;383:347-358. doi:10.1056/NEJMoa2021756
- Young TK, Shaw KS, Shah JK, et al. Mucocutaneous manifestations of multisystem inflammatory syndrome in children during the COVID-19 pandemic. JAMA Dermatol . 2021;157:207-212. doi:10.1001/jamadermatol.2020.4779
- Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259-269. doi:10.1001/jama.2020.10369
- Cheng MH, Zhang S, Porritt RA, et al. Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation.
- Morris SB, Schwartz NG, Patel P, et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 Infection—United Kingdom and United States, March–August 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1450-1456. doi:10.15585/mmwr.mm6940e1
- Blanco-Melo D, Nilsson-Payant BE, Liu W-C, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036.e9-1045.e9. doi:10.1016/j.cell.2020.04.026
- Crow YJ, Manel N. Aicardi–Goutières syndrome and the type I interferonopathies. Nat Rev Immunol. 2015;15:429-440. doi:10.1038/nri3850
- Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718-724. doi:10.1126/science.abc6027
- Zimmermann N, Wolf C, Schwenke R, et al. Assessment of clinical response to janus kinase inhibition in patients with familial chilblain lupus and TREX1 mutation. JAMA Dermatol. 2019;155:342-346. doi:10.1001/jamadermatol.2018.5077
- Hubiche T, Le Duff F, Chiaverini C, et al. Negative SARS-CoV-2 PCR in patients with chilblain-like lesions. Lancet Infect Dis. 2021;21:315-316. doi:10.1016/S1473-3099(20)30518-1
- Agrawal A. Mechanisms and implications of age-associated impaired innate interferon secretion by dendritic cells: a mini-review. Gerontology. 2013;59:421-426. doi:10.1159/000350536
- Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384:795-807. doi:10.1056/NEJMoa2031994
- US Food and Drug Administration. Fact sheet for healthcare providers: emergency use authorization (EUA) of baricitinib. Accessed March 29, 2021. https://www.fda.gov/media/143823/download
- Konno Y, Kimura I, Uriu K, et al. SARS-CoV-2 ORF3b is a potent interferon antagonist whose activity is increased by a naturally occurring elongation variant. Cell Rep. 2020;32:108185. doi:10.1016/j.celrep.2020.108185
- Sacks D, Baxter B, Campbell BCV, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke: from the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS), and World Stroke Organization (WSO). J Vasc Interv Radiol. 2018;29:441-453. doi:10.1016/j.jvir.2017.11.026
- Lo MW, Kemper C, Woodruff TM. COVID-19: complement, coagulation, and collateral damage. J Immunol. 2020;205:1488-1495. doi:10.4049/jimmunol.2000644
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Yan B, Freiwald T, Chauss D, et al. SARS-CoV2 drives JAK1/2-dependent local and systemic complement hyper-activation [published online June 9, 2020]. Res Sq. doi:10.21203/rs.3.rs-33390/v1
- Marietta M, Coluccio V, Luppi M. COVID-19, coagulopathy and venous thromboembolism: more questions than answers. Intern Emerg Med. 2020;15:1375-1387. doi:10.1007/s11739-020-02432-x
- Zuo Y, Estes SK, Ali RA, et al. Prothrombotic antiphospholipid antibodies in COVID-19 [published online June 17, 2020]. medRxiv. doi:10.1101/2020.06.15.20131607
- Lan S-H, Lai C-C, Huang H-T, et al. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int J Antimicrob Agents. 2020;56:106103. doi:10.1016/j.ijantimicag.2020.106103
- McMahon D, Gallman A, Hruza G, et al. COVID-19 “long-haulers” in dermatology? duration of dermatologic symptoms in an international registry from 39 countries. Abstract presented at: 29th EADV Congress; October 29, 2020. Accessed March 29, 2020. https://eadvdistribute.m-anage.com/from.storage?image=PXQEdDtICIihN3sM_8nAmh7p_y9AFijhQlf2-_KjrtYgOsOXNVwGxDdti95GZ2Yh0
- Saag MS. Misguided use of hydroxychloroquine for COVID-19: the infusion of politics into science. JAMA. 2020;324:2161-2162. doi:10.1001/jama.2020.22389
- Zahedi Niaki O, Anadkat MJ, Chen ST, et al. Navigating immunosuppression in a pandemic: a guide for the dermatologist from the COVID Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists. J Am Acad Dermatol. 2020;83:1150-1159. doi:10.1016/j.jaad.2020.06.051
- Hammond MI, Sharma TR, Cooper KD, et al. Conducting inpatient dermatology consultations and maintaining resident education in the COVID-19 telemedicine era. J Am Acad Dermatol. 2020;83:E317-E318. doi:10.1016/j.jaad.2020.07.008
- Brunasso AMG, Massone C. Teledermatologic monitoring for chronic cutaneous autoimmune diseases with smartworking during COVID-19 emergency in a tertiary center in Italy. Dermatol Ther. 2020;33:E13495-E13495. doi:10.1111/dth.13695
- Trinidad J, Kroshinsky D, Kaffenberger BH, et al. Telemedicine for inpatient dermatology consultations in response to the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E69-E71. doi:10.1016/j.jaad.2020.04.096
- Madigan LM, Micheletti RG, Shinkai K. How dermatologists can learn and contribute at the leading edge of the COVID-19 global pandemic. JAMA Dermatology. 2020;156:733-734. doi:10.1001/jamadermatol.2020.1438
The virus that causes COVID-19—SARS-CoV-2—has infected more than 128 million individuals, resulting in more than 2.8 million deaths worldwide between December 2019 and April 2021. Disease mortality primarily is driven by hypoxemic respiratory failure and systemic hypercoagulability, resulting in multisystem organ failure.1 With more than 17 million Americans infected, the virus is estimated to have impacted someone within the social circle of nearly every American.2
The COVID-19 pandemic has highlighted resource limitations, delayed elective and preventive care, and rapidly increased the adoption of telemedicine, presenting a host of new challenges to providers in every medical specialty, including dermatology. Although COVID-19 primarily is a respiratory disease, clinical manifestations have been observed in nearly every organ, including the skin. The cutaneous manifestations of COVID-19 provide insight into disease diagnosis, prognosis, and pathophysiology. In this article, we review the cutaneous manifestations of COVID-19 and explore the state of knowledge regarding their pathophysiology and clinical significance. Finally, we discuss the role of dermatology consultants in the care of patients with COVID-19, and the impact of the pandemic on the field of dermatology.
Prevalence of Cutaneous Findings in COVID-19
Early reports characterizing the clinical presentation of patients hospitalized with COVID-19 suggested skin findings associated with the disease were rare. Cohort studies from Europe, China, and New York City in January through March 2020 reported a low prevalence or made no mention of rash.3-7 However, reports from dermatologists in Italy that emerged in May 2020 indicated a substantially higher proportion of cutaneous disease: 18 of 88 (20.4%) hospitalized patients were found to have cutaneous involvement, primarily consisting of erythematous rash, along with some cases of urticarial and vesicular lesions.8 In October 2020, a retrospective cohort study from Spain examining 2761 patients presenting to the emergency department or admitted to the hospital for COVID-19 found that 58 (2.1%) patients had skin lesions attributed to COVID-19.9
The wide range in reported prevalence of skin lesions may be due to variable involvement of dermatologic specialists in patient care, particularly in China.10 Some variation also may be due to variability in the timing of clinical examination, as well as demographic and clinical differences in patient populations. Of note, a multisystem inflammatory disease seen in US children subsequent to infection with COVID-19 has been associated with rash in as many as 74% of cases.11 Although COVID-19 disproportionately impacts people with skin of color, there are few reports of cutaneous manifestations in that population,12 highlighting the challenges of the dermatologic examination in individuals with darker skin and suggesting the prevalence of dermatologic disease in COVID-19 may be greater than reported.
Morphologic Patterns of Cutaneous Involvement in COVID-19
Researchers in Europe and the United States have attempted to classify the cutaneous manifestations of COVID-19. A registry established through the American Academy of Dermatology published a compilation of reports from 31 countries, totaling 716 patient profiles.13 A prospective Spanish study detailed the cutaneous involvement of 375 patients with suspected or confirmed COVID-19.14 Together, these efforts have revealed several distinct patterns of cutaneous involvement associated with COVID-19 (Table).9,15-18
Vesicular Rash
Vesicular rash associated with COVID-19 has been described in several studies and case series8,13,14 and is considered, along with the pseudopernio (or pseudochilblains) morphology, to be one of the more disease-specific patterns in COVID-19.14,18 Vesicular rash appears to comprise roughly one-tenth of all COVID-19–associated rashes.13,14 It usually is described as pruritic, with 72% to 83% of patients reporting itch.13,16
Small monomorphic or polymorphic vesicles predominantly on the trunk and to a lesser extent the extremities and head have been described by multiple authors.14,16 Vesicular rash is most common among middle-aged individuals, with studies reporting median and mean ages ranging from 40.5 to 55 years.9,13,14,16
Vesicular rash develops concurrent with or after other presenting symptoms of COVID-19; in 2 studies, vesicular rash preceded development of other symptoms in only 15% and 5.6% of cases, respectively.13,14 Prognostically, vesicular rash is associated with moderate disease severity.14,16 It may persist for an average of 8 to 10 days.14,16,18
Histopathologic examination reveals basal layer vacuolar degeneration, hyperchromatic keratinocytes, acantholysis, and dyskeratosis.9,16,18
Urticarial Rash
Urticarial lesions represent approximately 7% to 19% of reported COVID-19–associated rashes.9,13,14 Urticarial rashes in patients testing positive for SARS-CoV-2 primarily occur on the trunk.14 The urticaria, which typically last about 1 week,14 are seen most frequently in middle-aged patients (mean/median age, 42–48 years)13,14 and are associated with pruritus, which has been reported in 74% to 92% of patients.13,14 Urticarial lesions typically do not precede other symptoms of COVID-19 and are nonspecific, making them less useful diagnostically.14
Urticaria appears to be associated with more severe COVID-19 illness in several studies, but this finding may be confounded by several factors, including older age, increased tobacco use, and polypharmacy. Of 104 patients with reported urticarial rash and suspected or confirmed COVID-19 across 3 studies, only 1 death was reported.9,13,14
The histopathologic appearance is that of typical hives, demonstrating a perivascular infiltrate of lymphocytes and eosinophils with edema of the upper dermis.9,19
Morbilliform Eruption
Morbilliform eruption is a commonly reported morphology associated with COVID-19, accounting for 20% to 47% of rashes.9,13,14 This categorization may have limited utility from a diagnostic and prognostic perspective, given that morbilliform eruptions are common, nonspecific, and heterogenous and can arise from many causes.9,13,14 Onset of morbilliform eruption appears to coincide with14 or follow13,20,21 the development of other COVID-19–related symptoms, with 5% of patients reporting morbilliform rash as the initial manifestation of infection.13,14 Morbilliform eruptions have been observed to occur in patients with more severe disease.9,13,14
Certain morphologic subtypes, such as erythema multiforme–like, erythema elevatum diutinum–like, or pseudovesicular, may be more specific to COVID-19 infection.14 A small case series highlighted 4 patients with erythema multiforme–like eruptions, 3 of whom also were found to have petechial enanthem occurring after COVID-19 diagnosis; however, the investigators were unable to exclude drug reaction as a potential cause of rash in these patients.22 Another case series of 21 patients with COVID-19 and skin rash described a (primarily) petechial enanthem on the palate in 6 (28.5%) patients.23 It is unclear to what extent oral enanthem may be underrecognized given that some physicians may be disinclined to remove the masks of known COVID-19–positive patients to examine the oral cavity.
The histologic appearance of morbilliform rash seen in association with COVID-19 has been described as spongiotic with interface dermatitis with perivascular lymphocytic inflammation.9,21
COVID Toes, Pseudochilblains Rash, Perniolike Rash, and Acral Erythema/Edema
Of all the rashes associated with COVID-19, COVID toes, or pseudochilblains rash, has perhaps attracted the most attention. The characteristic violaceous erythema on the fingers and/or toes may be itchy or painful, presenting similar to idiopathic cases of pernio (Figure 1).14 The entity has been controversial because of an absence of a clear correlation with a positive SARS-CoV-2 polymerase chain reaction test or antibodies to the virus in a subset of reported cases.24,25 Onset of the rash late in the disease course, generally after symptom resolution in mild or asymptomatic cases, may explain the absence of viral DNA in the nasopharynx by the time of lesion appearance.14,26 Seronegative patients may have cleared SARS-CoV-2 infection before humoral immunity could occur via a strong type 1 interferon response.25
Across 3 studies, perniolike skin lesions constituted 18% to 29% of COVID-19–associated skin findings9,13,14 and persisted for an average of 12 to 14 days.13,14 Perniolike lesions portend a favorable outcome; patients with COVID toes rarely present with systemic symptoms or laboratory or imaging abnormalities9 and less commonly require hospitalization for severe illness. Perniolike lesions have been reported most frequently in younger patients, with a median or mean age of 32 to 35 years.13,14
Histology demonstrates lichenoid dermatitis with perivascular and periadnexal lymphocytic infiltrates.9 Notably, one study observed interface dermatitis of the intraepidermal portion of the acrosyringium, a rare finding in chilblain lupus, in 83% of patients (N=40).25 Direct immunofluorescence demonstrates a vasculopathic pattern, with some patients showing deposition of IgM or IgG, C3, and fibrinogen in dermal blood vessels. Vascular C9 deposits also have been demonstrated on immunohistochemistry.9 Biopsies of perniolike lesions in COVID-19 patients have demonstrated the presence of SARS-CoV-2 RNA,27 have identified SARS-CoV-2 spike protein in endothelial cells on immunohistochemistry, and have visualized intracytoplasmic viral particles in vascular endothelium on electron microscopy.28
Livedoid Rash/Retiform Purpura
Netlike purpuric or violaceous patches signifying vessel damage or occlusion have been seen in association with COVID-19, constituting approximately 6% of COVID-19–associated skin findings in 2 studies.13,14 Livedoid rash (Figure 2) and retiform purpura (Figure 3) are associated with older age and occur primarily in severely ill patients, including those requiring intensive care. In a registry of 716 patients with COVID-19, 100% of patients with retiform purpura were hospitalized, and 82% had acute respiratory distress syndrome.13 In another study, 33% (7/21) of patients with livedoid and necrotic lesions required intensive care, and 10% (2/21) died.14
Livedoid lesions and retiform purpura represent thrombotic disease in the skin due to vasculopathy/coagulopathy. Dermatopathology available through the American Academy of Dermatology registry revealed thrombotic vasculopathy.13 A case series of 4 patients with livedo racemosa and retiform purpura demonstrated pauci-inflammatory thrombogenic vasculopathy involving capillaries, venules, and arterioles with complement deposition.29 Livedoid and retiform lesions in the skin may be associated with a COVID-19–induced coagulopathy, a propensity for systemic clotting including pulmonary embolism, which mostly occurs in hospitalized patients with severe illness.30
Multisystem Inflammatory Disease in Children
A hyperinflammatory syndrome similar to Kawasaki disease and toxic shock syndrome associated with mucocutaneous, cardiac, and gastrointestinal manifestations has been reported following COVID-19 infection.31 This syndrome, known as multisystem inflammatory syndrome in children (MIS-C), predominantly affects adolescents and children older than 5 years,11 typically occurs 2 to 4 weeks after infection, and appears to be at least 100-times less common than COVID-19 infection among the same age group.31 Sixty percent31 to 74%11 of affected patients have mucocutaneous involvement, with the most common clinical findings being conjunctival injection, palmoplantar erythema, lip hyperemia, periorbital erythema and edema, strawberry tongue, and malar erythema, respectively.32
Because this condition appears to reflect an immune response to the virus, the majority of cases demonstrate negative SARS-CoV-2 polymerase chain reaction and positive antibody testing.33 Although cutaneous findings are similar to those seen in Kawasaki disease, certain findings have been noted in MIS-C that are not typical of Kawasaki disease, including heliotrope rash–like periorbital edema and erythema as well as erythema infectiosum–like malar erythema and reticulated erythematous eruptions.32
The course of MIS-C can be severe; in one case series of patients presenting with MIS-C, 80% (79/99) required intensive care unit admission, with 10% requiring mechanical ventilation and 2% of patients dying during admission.31 Cardiac dysfunction, coagulopathy, and gastrointestinal symptoms are common.11,31 It has been postulated that a superantigenlike region of the SARS-CoV-2 spike protein, similar to that of staphylococcal enterotoxin B, may underlie MIS-C and account for its similarities to toxic shock syndrome.34 Of note, a similar multisystem inflammatory syndrome associated with COVID-19 also has been described in adults, and it too may present with rash as a cardinal feature.35
Pathophysiology of COVID-19: What the Skin May Reveal About the Disease
The diverse range of cutaneous manifestations in COVID-19 reflects a spectrum of host immunologicresponses to SARS-CoV-2 and may inform the pathophysiology of the disease as well as potential treatment modalities.
Host Response to SARS-CoV-2
The body’s response to viral infection is 2-pronged, involving activation of cellular antiviral defenses mediated by type I and III interferons, as well as recruitment of leukocytes, mobilized by cytokines and chemokines.36,37 Infection with SARS-CoV-2 results in a unique inflammatory response characterized by suppression of interferons, juxtaposed with a rampant proinflammatory cytokine and chemokine response, reminiscent of a cytokine storm. Reflective of this imbalance, a study of 50 COVID-19 patients and 20 healthy controls found decreased natural killer cells and CD3+ T cells in COVID-19 patients, particularly severely or critically ill patients, with an increase in B cells and monocytes.38 This distinctive immune imbalance positions SARS-CoV-2 to thrive in the absence of inhibitory interferon activity while submitting the host to the deleterious effects of a cytokine surge.36
Type I Interferons
The perniolike lesions associated with mild COVID-19 disease14 may represent a robust immune response via effective stimulation of type I interferons (IFN-1). Similar perniolike lesions are observed in Aicardi-Goutières syndrome37 and familial chilblain lupus, hereditary interferonopathies associated with mutations in the TREX1 (three prime repair exonuclease 1) gene and characterized by inappropriate upregulation of IFN-1,39 resulting in chilblains. It has been suggested that perniolike lesions in COVID-19 result from IFN-1 activation—a robust effective immunologic response to the virus.14,26,40
On the other end of the spectrum, patients with severe COVID-19 may have a blunted IFN-1 response and reduced IFN-1–stimulated gene expression.36,38 Notably, low IFN-1 response preceded clinical deterioration and was associated with increased risk for evolution to critical illness.38 Severe disease from COVID-19 also is more commonly observed in older patients and those with comorbidities,1 both of which are known factors associated with depressed IFN-1 function.38,41 Reflective of this disparate IFN-1 response, biopsies of COVID-19 perniosis have demonstrated striking expression of myxovirus resistance protein A (MXA), a marker for IFN-1 signaling in tissue, whereas its expression is absent in COVID-19 livedo/retiform purpura.27
Familial chilblain lupus may be effectively treated by the Janus kinase inhibitor baricitinib,39 which inhibits IFN-1 signaling. Baricitinib recently received emergency use authorization by the US Food and Drug Administration for treatment of severe COVID-19 pneumonia,42,43 hinting to disordered IFN-1 signaling in the COVID-19 pathophysiology.
The impaired IFN-1 response in COVID-19 patients may be due to a unique characteristic of SARS-CoV-2: its ORF3b gene is a potent IFN-1 antagonist. In a series of experiments comparing SARS-CoV-2 to the related virus severe acute respiratory disease coronavirus (which was responsible for an epidemic in 2002), Konno et al44 found that SARS-CoV-2 is more effectively able to downregulate host IFN-1, likely due to premature stop codons on ORF3b that produce a truncated version of the gene with amplified anti–IFN-1 activity.
Cytokine Storm and Coagulation Cascade
This dulled interferon response is juxtaposed with a surge of inflammatory chemokines and cytokines, including IL-6, IL-8, IL-10, and tumor necrosis factor α, impairing innate immunity and leading to end-organ damage. This inflammatory response is associated with the influx of innate immune cells, specifically neutrophils and monocytes, which likely contribute to lung injury in COVID-19 acute respiratory distress syndrome.38 It also is thought to lead to downstream activation of coagulation, with a high incidence of thrombotic events observed in patients with severe COVID-19.1 In a retrospective study of 184 intensive care patients with COVID-19 receiving at least standard doses of thromboprophylaxis, venous thromboembolism occurred in 27% and arterial thrombotic events occurred in 3.7%.45
Livedo racemosa and retiform purpura are cutaneous markers of hypercoagulability, which indicate an increased risk for systemic clotting in COVID-19. A positive feedback loop between the complement and coagulation cascades appears to be important.13,14,29,46-48 In addition, a few studies have reported antiphospholipid antibody positivity in hospitalized COVID-19 patients.49,50
The high incidence of coagulopathy in severe COVID-19 has prompted many institutions to develop aggressive prophylactic anticoagulation protocols. Elevation of proinflammatory cytokines and observation of terminal complement activation in the skin and other organs has led to therapeutic trials of IL-6 inhibitors such as tocilizumab,51 complement inhibitors such as eculizumab, and Janus kinase inhibitors such as ruxolitinib and baricitinib.42,48
COVID Long-Haulers
The long-term effects of immune dysregulation in COVID-19 patients remain to be seen. Viral triggering of autoimmune disease is a well-established phenomenon, seen in DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome and other dermatologic diseases, raising the possibility that dermatologists will see a rising incidence of cutaneous autoimmune disease in the aftermath of the pandemic. Disordered interferon stimulation could lead to increased incidence of interferon-mediated disorders, such as sarcoidosis and other granulomatous diseases. Vasculitislike skin lesions could persist beyond the acute infectious period. Recent data from a registry of 990 COVID-19 cases from 39 countries suggest that COVID-19 perniolike lesions may persist as long as 150 days.52 In a time of many unknowns, these questions serve as a call to action for rigorous data collection, contribution to existing registries for dermatologic manifestations of COVID-19, and long-term follow-up of COVID-19 patients by the dermatology community.
Pandemic Dermatology
The pandemic has posed unprecedented challenges for patient care. The use of hydroxychloroquine as a popular but unproven treatment for COVID-19, 53 particularly early in the pandemic, has resulted in drug shortages for patients with lupus and other autoimmune skin diseases. Meanwhile, the need for patients with complex dermatologic conditions to receive systemic immunosuppression has had to be balanced against the associated risks during a global pandemic. To help dermatologists navigate this dilemma, various subspecialty groups have issued guidelines, including the COVID-19 Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists, which recommends a stepwise approach to shared decision-making with the goal of minimizing both the risk for disease flare and that of infection. The use of systemic steroids and rituximab, as well as the dose of immunosuppression—particularly broad-acting immunosuppression—should be limited where permitted. 54
Rapid adoption of telemedicine and remote monitoring strategies has enabled dermatologists to provide safe and timely care when in-person visits have not been possible, including for patients with confirmed or suspected COVID-19, as well as for hospitalized patients. 55-57 Use of telemedicine has facilitated preservation of personal protective equipment at a time when these important resources have been scarce. For patients with transportation or scheduling barriers, telemedicine has even expanded access to care.
However, this strategy cannot completely replace comprehensive in-person evaluation. Variability in video and photographic quality limits evaluation, while in-person physical examination can reveal subtle morphologic clues necessary for diagnosis. 5 8 Additionally, unequal access to technology may disadvantage some patients. For dermatologists to provide optimal care and continue to contribute accurate and insightful observations into COVID-19, it is essential to be physically present in the clinic and in the hospital when necessary, caring for patients in need of dermatologic expertise. Creative management strategies developed during this time will benefit patients and expand the reach of the specialty . 5 8
Final Thoughts
The COVID-19 pandemic has profoundly challenged the medical community and dermatology is no exception. By documenting and characterizing the diverse cutaneous manifestations of this novel disease, dermatologists have furthered understanding of its pathophysiology and management. By adapting quickly and developing creative ways to deliver care, dermatologists have found ways to contribute, both large and small. As we take stock at this juncture of the pandemic, it is clear there remains much to learn. We hope dermatologists will continue to take an active role in meeting the challenges of this time.
The virus that causes COVID-19—SARS-CoV-2—has infected more than 128 million individuals, resulting in more than 2.8 million deaths worldwide between December 2019 and April 2021. Disease mortality primarily is driven by hypoxemic respiratory failure and systemic hypercoagulability, resulting in multisystem organ failure.1 With more than 17 million Americans infected, the virus is estimated to have impacted someone within the social circle of nearly every American.2
The COVID-19 pandemic has highlighted resource limitations, delayed elective and preventive care, and rapidly increased the adoption of telemedicine, presenting a host of new challenges to providers in every medical specialty, including dermatology. Although COVID-19 primarily is a respiratory disease, clinical manifestations have been observed in nearly every organ, including the skin. The cutaneous manifestations of COVID-19 provide insight into disease diagnosis, prognosis, and pathophysiology. In this article, we review the cutaneous manifestations of COVID-19 and explore the state of knowledge regarding their pathophysiology and clinical significance. Finally, we discuss the role of dermatology consultants in the care of patients with COVID-19, and the impact of the pandemic on the field of dermatology.
Prevalence of Cutaneous Findings in COVID-19
Early reports characterizing the clinical presentation of patients hospitalized with COVID-19 suggested skin findings associated with the disease were rare. Cohort studies from Europe, China, and New York City in January through March 2020 reported a low prevalence or made no mention of rash.3-7 However, reports from dermatologists in Italy that emerged in May 2020 indicated a substantially higher proportion of cutaneous disease: 18 of 88 (20.4%) hospitalized patients were found to have cutaneous involvement, primarily consisting of erythematous rash, along with some cases of urticarial and vesicular lesions.8 In October 2020, a retrospective cohort study from Spain examining 2761 patients presenting to the emergency department or admitted to the hospital for COVID-19 found that 58 (2.1%) patients had skin lesions attributed to COVID-19.9
The wide range in reported prevalence of skin lesions may be due to variable involvement of dermatologic specialists in patient care, particularly in China.10 Some variation also may be due to variability in the timing of clinical examination, as well as demographic and clinical differences in patient populations. Of note, a multisystem inflammatory disease seen in US children subsequent to infection with COVID-19 has been associated with rash in as many as 74% of cases.11 Although COVID-19 disproportionately impacts people with skin of color, there are few reports of cutaneous manifestations in that population,12 highlighting the challenges of the dermatologic examination in individuals with darker skin and suggesting the prevalence of dermatologic disease in COVID-19 may be greater than reported.
Morphologic Patterns of Cutaneous Involvement in COVID-19
Researchers in Europe and the United States have attempted to classify the cutaneous manifestations of COVID-19. A registry established through the American Academy of Dermatology published a compilation of reports from 31 countries, totaling 716 patient profiles.13 A prospective Spanish study detailed the cutaneous involvement of 375 patients with suspected or confirmed COVID-19.14 Together, these efforts have revealed several distinct patterns of cutaneous involvement associated with COVID-19 (Table).9,15-18
Vesicular Rash
Vesicular rash associated with COVID-19 has been described in several studies and case series8,13,14 and is considered, along with the pseudopernio (or pseudochilblains) morphology, to be one of the more disease-specific patterns in COVID-19.14,18 Vesicular rash appears to comprise roughly one-tenth of all COVID-19–associated rashes.13,14 It usually is described as pruritic, with 72% to 83% of patients reporting itch.13,16
Small monomorphic or polymorphic vesicles predominantly on the trunk and to a lesser extent the extremities and head have been described by multiple authors.14,16 Vesicular rash is most common among middle-aged individuals, with studies reporting median and mean ages ranging from 40.5 to 55 years.9,13,14,16
Vesicular rash develops concurrent with or after other presenting symptoms of COVID-19; in 2 studies, vesicular rash preceded development of other symptoms in only 15% and 5.6% of cases, respectively.13,14 Prognostically, vesicular rash is associated with moderate disease severity.14,16 It may persist for an average of 8 to 10 days.14,16,18
Histopathologic examination reveals basal layer vacuolar degeneration, hyperchromatic keratinocytes, acantholysis, and dyskeratosis.9,16,18
Urticarial Rash
Urticarial lesions represent approximately 7% to 19% of reported COVID-19–associated rashes.9,13,14 Urticarial rashes in patients testing positive for SARS-CoV-2 primarily occur on the trunk.14 The urticaria, which typically last about 1 week,14 are seen most frequently in middle-aged patients (mean/median age, 42–48 years)13,14 and are associated with pruritus, which has been reported in 74% to 92% of patients.13,14 Urticarial lesions typically do not precede other symptoms of COVID-19 and are nonspecific, making them less useful diagnostically.14
Urticaria appears to be associated with more severe COVID-19 illness in several studies, but this finding may be confounded by several factors, including older age, increased tobacco use, and polypharmacy. Of 104 patients with reported urticarial rash and suspected or confirmed COVID-19 across 3 studies, only 1 death was reported.9,13,14
The histopathologic appearance is that of typical hives, demonstrating a perivascular infiltrate of lymphocytes and eosinophils with edema of the upper dermis.9,19
Morbilliform Eruption
Morbilliform eruption is a commonly reported morphology associated with COVID-19, accounting for 20% to 47% of rashes.9,13,14 This categorization may have limited utility from a diagnostic and prognostic perspective, given that morbilliform eruptions are common, nonspecific, and heterogenous and can arise from many causes.9,13,14 Onset of morbilliform eruption appears to coincide with14 or follow13,20,21 the development of other COVID-19–related symptoms, with 5% of patients reporting morbilliform rash as the initial manifestation of infection.13,14 Morbilliform eruptions have been observed to occur in patients with more severe disease.9,13,14
Certain morphologic subtypes, such as erythema multiforme–like, erythema elevatum diutinum–like, or pseudovesicular, may be more specific to COVID-19 infection.14 A small case series highlighted 4 patients with erythema multiforme–like eruptions, 3 of whom also were found to have petechial enanthem occurring after COVID-19 diagnosis; however, the investigators were unable to exclude drug reaction as a potential cause of rash in these patients.22 Another case series of 21 patients with COVID-19 and skin rash described a (primarily) petechial enanthem on the palate in 6 (28.5%) patients.23 It is unclear to what extent oral enanthem may be underrecognized given that some physicians may be disinclined to remove the masks of known COVID-19–positive patients to examine the oral cavity.
The histologic appearance of morbilliform rash seen in association with COVID-19 has been described as spongiotic with interface dermatitis with perivascular lymphocytic inflammation.9,21
COVID Toes, Pseudochilblains Rash, Perniolike Rash, and Acral Erythema/Edema
Of all the rashes associated with COVID-19, COVID toes, or pseudochilblains rash, has perhaps attracted the most attention. The characteristic violaceous erythema on the fingers and/or toes may be itchy or painful, presenting similar to idiopathic cases of pernio (Figure 1).14 The entity has been controversial because of an absence of a clear correlation with a positive SARS-CoV-2 polymerase chain reaction test or antibodies to the virus in a subset of reported cases.24,25 Onset of the rash late in the disease course, generally after symptom resolution in mild or asymptomatic cases, may explain the absence of viral DNA in the nasopharynx by the time of lesion appearance.14,26 Seronegative patients may have cleared SARS-CoV-2 infection before humoral immunity could occur via a strong type 1 interferon response.25
Across 3 studies, perniolike skin lesions constituted 18% to 29% of COVID-19–associated skin findings9,13,14 and persisted for an average of 12 to 14 days.13,14 Perniolike lesions portend a favorable outcome; patients with COVID toes rarely present with systemic symptoms or laboratory or imaging abnormalities9 and less commonly require hospitalization for severe illness. Perniolike lesions have been reported most frequently in younger patients, with a median or mean age of 32 to 35 years.13,14
Histology demonstrates lichenoid dermatitis with perivascular and periadnexal lymphocytic infiltrates.9 Notably, one study observed interface dermatitis of the intraepidermal portion of the acrosyringium, a rare finding in chilblain lupus, in 83% of patients (N=40).25 Direct immunofluorescence demonstrates a vasculopathic pattern, with some patients showing deposition of IgM or IgG, C3, and fibrinogen in dermal blood vessels. Vascular C9 deposits also have been demonstrated on immunohistochemistry.9 Biopsies of perniolike lesions in COVID-19 patients have demonstrated the presence of SARS-CoV-2 RNA,27 have identified SARS-CoV-2 spike protein in endothelial cells on immunohistochemistry, and have visualized intracytoplasmic viral particles in vascular endothelium on electron microscopy.28
Livedoid Rash/Retiform Purpura
Netlike purpuric or violaceous patches signifying vessel damage or occlusion have been seen in association with COVID-19, constituting approximately 6% of COVID-19–associated skin findings in 2 studies.13,14 Livedoid rash (Figure 2) and retiform purpura (Figure 3) are associated with older age and occur primarily in severely ill patients, including those requiring intensive care. In a registry of 716 patients with COVID-19, 100% of patients with retiform purpura were hospitalized, and 82% had acute respiratory distress syndrome.13 In another study, 33% (7/21) of patients with livedoid and necrotic lesions required intensive care, and 10% (2/21) died.14
Livedoid lesions and retiform purpura represent thrombotic disease in the skin due to vasculopathy/coagulopathy. Dermatopathology available through the American Academy of Dermatology registry revealed thrombotic vasculopathy.13 A case series of 4 patients with livedo racemosa and retiform purpura demonstrated pauci-inflammatory thrombogenic vasculopathy involving capillaries, venules, and arterioles with complement deposition.29 Livedoid and retiform lesions in the skin may be associated with a COVID-19–induced coagulopathy, a propensity for systemic clotting including pulmonary embolism, which mostly occurs in hospitalized patients with severe illness.30
Multisystem Inflammatory Disease in Children
A hyperinflammatory syndrome similar to Kawasaki disease and toxic shock syndrome associated with mucocutaneous, cardiac, and gastrointestinal manifestations has been reported following COVID-19 infection.31 This syndrome, known as multisystem inflammatory syndrome in children (MIS-C), predominantly affects adolescents and children older than 5 years,11 typically occurs 2 to 4 weeks after infection, and appears to be at least 100-times less common than COVID-19 infection among the same age group.31 Sixty percent31 to 74%11 of affected patients have mucocutaneous involvement, with the most common clinical findings being conjunctival injection, palmoplantar erythema, lip hyperemia, periorbital erythema and edema, strawberry tongue, and malar erythema, respectively.32
Because this condition appears to reflect an immune response to the virus, the majority of cases demonstrate negative SARS-CoV-2 polymerase chain reaction and positive antibody testing.33 Although cutaneous findings are similar to those seen in Kawasaki disease, certain findings have been noted in MIS-C that are not typical of Kawasaki disease, including heliotrope rash–like periorbital edema and erythema as well as erythema infectiosum–like malar erythema and reticulated erythematous eruptions.32
The course of MIS-C can be severe; in one case series of patients presenting with MIS-C, 80% (79/99) required intensive care unit admission, with 10% requiring mechanical ventilation and 2% of patients dying during admission.31 Cardiac dysfunction, coagulopathy, and gastrointestinal symptoms are common.11,31 It has been postulated that a superantigenlike region of the SARS-CoV-2 spike protein, similar to that of staphylococcal enterotoxin B, may underlie MIS-C and account for its similarities to toxic shock syndrome.34 Of note, a similar multisystem inflammatory syndrome associated with COVID-19 also has been described in adults, and it too may present with rash as a cardinal feature.35
Pathophysiology of COVID-19: What the Skin May Reveal About the Disease
The diverse range of cutaneous manifestations in COVID-19 reflects a spectrum of host immunologicresponses to SARS-CoV-2 and may inform the pathophysiology of the disease as well as potential treatment modalities.
Host Response to SARS-CoV-2
The body’s response to viral infection is 2-pronged, involving activation of cellular antiviral defenses mediated by type I and III interferons, as well as recruitment of leukocytes, mobilized by cytokines and chemokines.36,37 Infection with SARS-CoV-2 results in a unique inflammatory response characterized by suppression of interferons, juxtaposed with a rampant proinflammatory cytokine and chemokine response, reminiscent of a cytokine storm. Reflective of this imbalance, a study of 50 COVID-19 patients and 20 healthy controls found decreased natural killer cells and CD3+ T cells in COVID-19 patients, particularly severely or critically ill patients, with an increase in B cells and monocytes.38 This distinctive immune imbalance positions SARS-CoV-2 to thrive in the absence of inhibitory interferon activity while submitting the host to the deleterious effects of a cytokine surge.36
Type I Interferons
The perniolike lesions associated with mild COVID-19 disease14 may represent a robust immune response via effective stimulation of type I interferons (IFN-1). Similar perniolike lesions are observed in Aicardi-Goutières syndrome37 and familial chilblain lupus, hereditary interferonopathies associated with mutations in the TREX1 (three prime repair exonuclease 1) gene and characterized by inappropriate upregulation of IFN-1,39 resulting in chilblains. It has been suggested that perniolike lesions in COVID-19 result from IFN-1 activation—a robust effective immunologic response to the virus.14,26,40
On the other end of the spectrum, patients with severe COVID-19 may have a blunted IFN-1 response and reduced IFN-1–stimulated gene expression.36,38 Notably, low IFN-1 response preceded clinical deterioration and was associated with increased risk for evolution to critical illness.38 Severe disease from COVID-19 also is more commonly observed in older patients and those with comorbidities,1 both of which are known factors associated with depressed IFN-1 function.38,41 Reflective of this disparate IFN-1 response, biopsies of COVID-19 perniosis have demonstrated striking expression of myxovirus resistance protein A (MXA), a marker for IFN-1 signaling in tissue, whereas its expression is absent in COVID-19 livedo/retiform purpura.27
Familial chilblain lupus may be effectively treated by the Janus kinase inhibitor baricitinib,39 which inhibits IFN-1 signaling. Baricitinib recently received emergency use authorization by the US Food and Drug Administration for treatment of severe COVID-19 pneumonia,42,43 hinting to disordered IFN-1 signaling in the COVID-19 pathophysiology.
The impaired IFN-1 response in COVID-19 patients may be due to a unique characteristic of SARS-CoV-2: its ORF3b gene is a potent IFN-1 antagonist. In a series of experiments comparing SARS-CoV-2 to the related virus severe acute respiratory disease coronavirus (which was responsible for an epidemic in 2002), Konno et al44 found that SARS-CoV-2 is more effectively able to downregulate host IFN-1, likely due to premature stop codons on ORF3b that produce a truncated version of the gene with amplified anti–IFN-1 activity.
Cytokine Storm and Coagulation Cascade
This dulled interferon response is juxtaposed with a surge of inflammatory chemokines and cytokines, including IL-6, IL-8, IL-10, and tumor necrosis factor α, impairing innate immunity and leading to end-organ damage. This inflammatory response is associated with the influx of innate immune cells, specifically neutrophils and monocytes, which likely contribute to lung injury in COVID-19 acute respiratory distress syndrome.38 It also is thought to lead to downstream activation of coagulation, with a high incidence of thrombotic events observed in patients with severe COVID-19.1 In a retrospective study of 184 intensive care patients with COVID-19 receiving at least standard doses of thromboprophylaxis, venous thromboembolism occurred in 27% and arterial thrombotic events occurred in 3.7%.45
Livedo racemosa and retiform purpura are cutaneous markers of hypercoagulability, which indicate an increased risk for systemic clotting in COVID-19. A positive feedback loop between the complement and coagulation cascades appears to be important.13,14,29,46-48 In addition, a few studies have reported antiphospholipid antibody positivity in hospitalized COVID-19 patients.49,50
The high incidence of coagulopathy in severe COVID-19 has prompted many institutions to develop aggressive prophylactic anticoagulation protocols. Elevation of proinflammatory cytokines and observation of terminal complement activation in the skin and other organs has led to therapeutic trials of IL-6 inhibitors such as tocilizumab,51 complement inhibitors such as eculizumab, and Janus kinase inhibitors such as ruxolitinib and baricitinib.42,48
COVID Long-Haulers
The long-term effects of immune dysregulation in COVID-19 patients remain to be seen. Viral triggering of autoimmune disease is a well-established phenomenon, seen in DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome and other dermatologic diseases, raising the possibility that dermatologists will see a rising incidence of cutaneous autoimmune disease in the aftermath of the pandemic. Disordered interferon stimulation could lead to increased incidence of interferon-mediated disorders, such as sarcoidosis and other granulomatous diseases. Vasculitislike skin lesions could persist beyond the acute infectious period. Recent data from a registry of 990 COVID-19 cases from 39 countries suggest that COVID-19 perniolike lesions may persist as long as 150 days.52 In a time of many unknowns, these questions serve as a call to action for rigorous data collection, contribution to existing registries for dermatologic manifestations of COVID-19, and long-term follow-up of COVID-19 patients by the dermatology community.
Pandemic Dermatology
The pandemic has posed unprecedented challenges for patient care. The use of hydroxychloroquine as a popular but unproven treatment for COVID-19, 53 particularly early in the pandemic, has resulted in drug shortages for patients with lupus and other autoimmune skin diseases. Meanwhile, the need for patients with complex dermatologic conditions to receive systemic immunosuppression has had to be balanced against the associated risks during a global pandemic. To help dermatologists navigate this dilemma, various subspecialty groups have issued guidelines, including the COVID-19 Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists, which recommends a stepwise approach to shared decision-making with the goal of minimizing both the risk for disease flare and that of infection. The use of systemic steroids and rituximab, as well as the dose of immunosuppression—particularly broad-acting immunosuppression—should be limited where permitted. 54
Rapid adoption of telemedicine and remote monitoring strategies has enabled dermatologists to provide safe and timely care when in-person visits have not been possible, including for patients with confirmed or suspected COVID-19, as well as for hospitalized patients. 55-57 Use of telemedicine has facilitated preservation of personal protective equipment at a time when these important resources have been scarce. For patients with transportation or scheduling barriers, telemedicine has even expanded access to care.
However, this strategy cannot completely replace comprehensive in-person evaluation. Variability in video and photographic quality limits evaluation, while in-person physical examination can reveal subtle morphologic clues necessary for diagnosis. 5 8 Additionally, unequal access to technology may disadvantage some patients. For dermatologists to provide optimal care and continue to contribute accurate and insightful observations into COVID-19, it is essential to be physically present in the clinic and in the hospital when necessary, caring for patients in need of dermatologic expertise. Creative management strategies developed during this time will benefit patients and expand the reach of the specialty . 5 8
Final Thoughts
The COVID-19 pandemic has profoundly challenged the medical community and dermatology is no exception. By documenting and characterizing the diverse cutaneous manifestations of this novel disease, dermatologists have furthered understanding of its pathophysiology and management. By adapting quickly and developing creative ways to deliver care, dermatologists have found ways to contribute, both large and small. As we take stock at this juncture of the pandemic, it is clear there remains much to learn. We hope dermatologists will continue to take an active role in meeting the challenges of this time.
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA . 2020;324:782-793. doi:10.1001/jama.2020.12839
- New York Times . Updated December 23, 2020. Accessed March 22, 2021. https://www.nytimes.com/2020/11/15/us/coronavirus-us-cases-deaths.html
- Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med . 2020;382:1708-1720. doi:10.1056/NEJMoa2002032
- Lechien JR, Chiesa-Estomba CM, Place S, et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med . 2020;288:335-344. doi:https://doi.org/10.1111/joim.13089
- Wu J, Liu J, Zhao X, et al. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID-19) in Jiangsu province: a multicenter descriptive study. Clin Infect Dis . 2020;71:706-712. doi:10.1093/cid/ciaa199
- Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of COVID-19 in New York City. N Engl J Med . 2020;382:2372-2374. doi:10.1056/NEJMc2010419
- Sun L, Shen L, Fan J, et al. Clinical features of patients with coronavirus disease 2019 from a designated hospital in Beijing, China. J Med Virol . 2020;92:2055-2066. https://doi.org/10.1002/jmv.25966
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatology Venereol . 2020;34:E212-E213. https://doi.org/10.1111/jdv.16387
- Giavedoni P, Podlipnik S, Pericàs JM, et al. Skin manifestations in COVID-19: prevalence and relationship with disease severity. J Clin Med . 2020;9:3261. doi:10.3390/jcm9103261
- Jimenez-Cauhe J, Ortega-Quijano D, Prieto-Barrios M, et al. Reply to “COVID-19 can present with a rash and be mistaken for dengue”: petechial rash in a patient with COVID-19 infection. J Am Acad Dermatol . 2020;83:E141-E142. doi:10.1016/j.jaad.2020.04.016
- Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med . 2020;383:334-346. doi:10.1056/NEJMoa2021680
- Shinkai K, Bruckner AL. Dermatology and COVID-19. JAMA . 2020;324:1133-1134. doi:10.1001/jama.2020.15276
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol . 2020;83:1118-1129. doi:10.1016/j.jaad.2020.06.1016
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol . 2020;183:71-77. https://doi.org/10.1111/bjd.19163
- Bouaziz JD, Duong TA, Jachiet M, et al. Vascular skin symptoms in COVID-19: a French observational study. J Eur Acad Dermatology Venereol . 2020;34:E451-E452. https://doi.org/10.1111/jdv.16544
- Fernandez-Nieto D, Ortega-Quijano D, Jimenez-Cauhe J, et al. Clinical and histological characterization of vesicular COVID-19 rashes: a prospective study in a tertiary care hospital. Clin Exp Dermatol . 2020;45:872-875. https://doi.org/10.1111/ced.14277
- Fernandez-Nieto D, Jimenez-Cauhe J, Suarez-Valle A, et al. Characterization of acute acral skin lesions in nonhospitalized patients: a case series of 132 patients during the COVID-19 outbreak. J Am Acad Dermatol . 2020;83:E61-E63. doi:10.1016/j.jaad.2020.04.093
- Marzano AV, Genovese G, Fabbrocini G, et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: Multicenter case series of 22 patients. J Am Acad Dermatol . 2020;83:280-285. doi:10.1016/j.jaad.2020.04.044
- Fernandez-Nieto D, Ortega-Quijano D, Segurado-Miravalles G, et al. Comment on: cutaneous manifestations in COVID-19: a first perspective. safety concerns of clinical images and skin biopsies. J Eur Acad Dermatol Venereol . 2020;34:E252-E254. https://doi.org/10.1111/jdv.16470
- Herrero-Moyano M, Capusan TM, Andreu-Barasoain M, et al. A clinicopathological study of eight patients with COVID-19 pneumonia and a late-onset exanthema. J Eur Acad Dermatol Venereol . 2020;34:E460-E464. https://doi.org/10.1111/jdv.16631
- Rubio-Muniz CA, Puerta-Peñ a M, Falkenhain-L ópez D, et al. The broad spectrum of dermatological manifestations in COVID-19: clinical and histopathological features learned from a series of 34 cases. J Eur Acad Dermatol Venereol . 2020;34:E574-E576. https://doi.org/10.1111/jdv.16734
- Jimenez-Cauhe J, Ortega-Quijano D, Carretero-Barrio I, et al. Erythema multiforme-like eruption in patients with COVID-19 infection: clinical and histological findings. Clin Exp Dermatol . 2020;45:892-895. https://doi.org/10.1111/ced.14281
- Jimenez-Cauhe J, Ortega-Quijano D, de Perosanz-Lobo D, et al. Enanthem in patients with COVID-19 and skin rash. JAMA Dermatol . 2020;156:1134-1136. doi:10.1001/jamadermatol.2020.2550
- Le Cleach L, Dousset L, Assier H, et al. Most chilblains observed during the COVID-19 outbreak occur in patients who are negative for COVID-19 on polymerase chain reaction and serology testing. Br J Dermatol . 2020;183:866-874. https://doi.org/10.1111/bjd.19377
- Hubiche T, Cardot-Leccia N, Le Duff F, et al. Clinical, laboratory, and interferon-alpha response characteristics of patients with chilblain-like lesions during the COVID-19 pandemic [published online November 25, 2020]. JAMA Dermatol . doi:10.1001/jamadermatol.2020.4324
- Freeman EE, McMahon DE, Lipoff JB, et al. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol . 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Magro CM, Mulvey JJ, Laurence J, et al. The differing pathophysiologies that underlie COVID-19-associated perniosis and thrombotic retiform purpura: a case series. Br J Dermatol . 2021;184:141-150. https://doi.org/10.1111/bjd.19415
- Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol . 2020;183:729-737. doi:10.1111/bjd.19327
- Droesch C, Do MH, DeSancho M, et al. Livedoid and purpuric skin eruptions associated with coagulopathy in severe COVID-19. JAMA Dermatol . 2020;156:1-3. doi:10.1001/jamadermatol.2020.2800
- Asakura H, Ogawa H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int J Hematol . 2021;113:45-57. doi:10.1007/s12185-020-03029-y
- Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med . 2020;383:347-358. doi:10.1056/NEJMoa2021756
- Young TK, Shaw KS, Shah JK, et al. Mucocutaneous manifestations of multisystem inflammatory syndrome in children during the COVID-19 pandemic. JAMA Dermatol . 2021;157:207-212. doi:10.1001/jamadermatol.2020.4779
- Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259-269. doi:10.1001/jama.2020.10369
- Cheng MH, Zhang S, Porritt RA, et al. Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation.
- Morris SB, Schwartz NG, Patel P, et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 Infection—United Kingdom and United States, March–August 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1450-1456. doi:10.15585/mmwr.mm6940e1
- Blanco-Melo D, Nilsson-Payant BE, Liu W-C, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036.e9-1045.e9. doi:10.1016/j.cell.2020.04.026
- Crow YJ, Manel N. Aicardi–Goutières syndrome and the type I interferonopathies. Nat Rev Immunol. 2015;15:429-440. doi:10.1038/nri3850
- Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718-724. doi:10.1126/science.abc6027
- Zimmermann N, Wolf C, Schwenke R, et al. Assessment of clinical response to janus kinase inhibition in patients with familial chilblain lupus and TREX1 mutation. JAMA Dermatol. 2019;155:342-346. doi:10.1001/jamadermatol.2018.5077
- Hubiche T, Le Duff F, Chiaverini C, et al. Negative SARS-CoV-2 PCR in patients with chilblain-like lesions. Lancet Infect Dis. 2021;21:315-316. doi:10.1016/S1473-3099(20)30518-1
- Agrawal A. Mechanisms and implications of age-associated impaired innate interferon secretion by dendritic cells: a mini-review. Gerontology. 2013;59:421-426. doi:10.1159/000350536
- Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384:795-807. doi:10.1056/NEJMoa2031994
- US Food and Drug Administration. Fact sheet for healthcare providers: emergency use authorization (EUA) of baricitinib. Accessed March 29, 2021. https://www.fda.gov/media/143823/download
- Konno Y, Kimura I, Uriu K, et al. SARS-CoV-2 ORF3b is a potent interferon antagonist whose activity is increased by a naturally occurring elongation variant. Cell Rep. 2020;32:108185. doi:10.1016/j.celrep.2020.108185
- Sacks D, Baxter B, Campbell BCV, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke: from the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS), and World Stroke Organization (WSO). J Vasc Interv Radiol. 2018;29:441-453. doi:10.1016/j.jvir.2017.11.026
- Lo MW, Kemper C, Woodruff TM. COVID-19: complement, coagulation, and collateral damage. J Immunol. 2020;205:1488-1495. doi:10.4049/jimmunol.2000644
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Yan B, Freiwald T, Chauss D, et al. SARS-CoV2 drives JAK1/2-dependent local and systemic complement hyper-activation [published online June 9, 2020]. Res Sq. doi:10.21203/rs.3.rs-33390/v1
- Marietta M, Coluccio V, Luppi M. COVID-19, coagulopathy and venous thromboembolism: more questions than answers. Intern Emerg Med. 2020;15:1375-1387. doi:10.1007/s11739-020-02432-x
- Zuo Y, Estes SK, Ali RA, et al. Prothrombotic antiphospholipid antibodies in COVID-19 [published online June 17, 2020]. medRxiv. doi:10.1101/2020.06.15.20131607
- Lan S-H, Lai C-C, Huang H-T, et al. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int J Antimicrob Agents. 2020;56:106103. doi:10.1016/j.ijantimicag.2020.106103
- McMahon D, Gallman A, Hruza G, et al. COVID-19 “long-haulers” in dermatology? duration of dermatologic symptoms in an international registry from 39 countries. Abstract presented at: 29th EADV Congress; October 29, 2020. Accessed March 29, 2020. https://eadvdistribute.m-anage.com/from.storage?image=PXQEdDtICIihN3sM_8nAmh7p_y9AFijhQlf2-_KjrtYgOsOXNVwGxDdti95GZ2Yh0
- Saag MS. Misguided use of hydroxychloroquine for COVID-19: the infusion of politics into science. JAMA. 2020;324:2161-2162. doi:10.1001/jama.2020.22389
- Zahedi Niaki O, Anadkat MJ, Chen ST, et al. Navigating immunosuppression in a pandemic: a guide for the dermatologist from the COVID Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists. J Am Acad Dermatol. 2020;83:1150-1159. doi:10.1016/j.jaad.2020.06.051
- Hammond MI, Sharma TR, Cooper KD, et al. Conducting inpatient dermatology consultations and maintaining resident education in the COVID-19 telemedicine era. J Am Acad Dermatol. 2020;83:E317-E318. doi:10.1016/j.jaad.2020.07.008
- Brunasso AMG, Massone C. Teledermatologic monitoring for chronic cutaneous autoimmune diseases with smartworking during COVID-19 emergency in a tertiary center in Italy. Dermatol Ther. 2020;33:E13495-E13495. doi:10.1111/dth.13695
- Trinidad J, Kroshinsky D, Kaffenberger BH, et al. Telemedicine for inpatient dermatology consultations in response to the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E69-E71. doi:10.1016/j.jaad.2020.04.096
- Madigan LM, Micheletti RG, Shinkai K. How dermatologists can learn and contribute at the leading edge of the COVID-19 global pandemic. JAMA Dermatology. 2020;156:733-734. doi:10.1001/jamadermatol.2020.1438
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA . 2020;324:782-793. doi:10.1001/jama.2020.12839
- New York Times . Updated December 23, 2020. Accessed March 22, 2021. https://www.nytimes.com/2020/11/15/us/coronavirus-us-cases-deaths.html
- Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med . 2020;382:1708-1720. doi:10.1056/NEJMoa2002032
- Lechien JR, Chiesa-Estomba CM, Place S, et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med . 2020;288:335-344. doi:https://doi.org/10.1111/joim.13089
- Wu J, Liu J, Zhao X, et al. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID-19) in Jiangsu province: a multicenter descriptive study. Clin Infect Dis . 2020;71:706-712. doi:10.1093/cid/ciaa199
- Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of COVID-19 in New York City. N Engl J Med . 2020;382:2372-2374. doi:10.1056/NEJMc2010419
- Sun L, Shen L, Fan J, et al. Clinical features of patients with coronavirus disease 2019 from a designated hospital in Beijing, China. J Med Virol . 2020;92:2055-2066. https://doi.org/10.1002/jmv.25966
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatology Venereol . 2020;34:E212-E213. https://doi.org/10.1111/jdv.16387
- Giavedoni P, Podlipnik S, Pericàs JM, et al. Skin manifestations in COVID-19: prevalence and relationship with disease severity. J Clin Med . 2020;9:3261. doi:10.3390/jcm9103261
- Jimenez-Cauhe J, Ortega-Quijano D, Prieto-Barrios M, et al. Reply to “COVID-19 can present with a rash and be mistaken for dengue”: petechial rash in a patient with COVID-19 infection. J Am Acad Dermatol . 2020;83:E141-E142. doi:10.1016/j.jaad.2020.04.016
- Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med . 2020;383:334-346. doi:10.1056/NEJMoa2021680
- Shinkai K, Bruckner AL. Dermatology and COVID-19. JAMA . 2020;324:1133-1134. doi:10.1001/jama.2020.15276
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol . 2020;83:1118-1129. doi:10.1016/j.jaad.2020.06.1016
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol . 2020;183:71-77. https://doi.org/10.1111/bjd.19163
- Bouaziz JD, Duong TA, Jachiet M, et al. Vascular skin symptoms in COVID-19: a French observational study. J Eur Acad Dermatology Venereol . 2020;34:E451-E452. https://doi.org/10.1111/jdv.16544
- Fernandez-Nieto D, Ortega-Quijano D, Jimenez-Cauhe J, et al. Clinical and histological characterization of vesicular COVID-19 rashes: a prospective study in a tertiary care hospital. Clin Exp Dermatol . 2020;45:872-875. https://doi.org/10.1111/ced.14277
- Fernandez-Nieto D, Jimenez-Cauhe J, Suarez-Valle A, et al. Characterization of acute acral skin lesions in nonhospitalized patients: a case series of 132 patients during the COVID-19 outbreak. J Am Acad Dermatol . 2020;83:E61-E63. doi:10.1016/j.jaad.2020.04.093
- Marzano AV, Genovese G, Fabbrocini G, et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: Multicenter case series of 22 patients. J Am Acad Dermatol . 2020;83:280-285. doi:10.1016/j.jaad.2020.04.044
- Fernandez-Nieto D, Ortega-Quijano D, Segurado-Miravalles G, et al. Comment on: cutaneous manifestations in COVID-19: a first perspective. safety concerns of clinical images and skin biopsies. J Eur Acad Dermatol Venereol . 2020;34:E252-E254. https://doi.org/10.1111/jdv.16470
- Herrero-Moyano M, Capusan TM, Andreu-Barasoain M, et al. A clinicopathological study of eight patients with COVID-19 pneumonia and a late-onset exanthema. J Eur Acad Dermatol Venereol . 2020;34:E460-E464. https://doi.org/10.1111/jdv.16631
- Rubio-Muniz CA, Puerta-Peñ a M, Falkenhain-L ópez D, et al. The broad spectrum of dermatological manifestations in COVID-19: clinical and histopathological features learned from a series of 34 cases. J Eur Acad Dermatol Venereol . 2020;34:E574-E576. https://doi.org/10.1111/jdv.16734
- Jimenez-Cauhe J, Ortega-Quijano D, Carretero-Barrio I, et al. Erythema multiforme-like eruption in patients with COVID-19 infection: clinical and histological findings. Clin Exp Dermatol . 2020;45:892-895. https://doi.org/10.1111/ced.14281
- Jimenez-Cauhe J, Ortega-Quijano D, de Perosanz-Lobo D, et al. Enanthem in patients with COVID-19 and skin rash. JAMA Dermatol . 2020;156:1134-1136. doi:10.1001/jamadermatol.2020.2550
- Le Cleach L, Dousset L, Assier H, et al. Most chilblains observed during the COVID-19 outbreak occur in patients who are negative for COVID-19 on polymerase chain reaction and serology testing. Br J Dermatol . 2020;183:866-874. https://doi.org/10.1111/bjd.19377
- Hubiche T, Cardot-Leccia N, Le Duff F, et al. Clinical, laboratory, and interferon-alpha response characteristics of patients with chilblain-like lesions during the COVID-19 pandemic [published online November 25, 2020]. JAMA Dermatol . doi:10.1001/jamadermatol.2020.4324
- Freeman EE, McMahon DE, Lipoff JB, et al. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol . 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Magro CM, Mulvey JJ, Laurence J, et al. The differing pathophysiologies that underlie COVID-19-associated perniosis and thrombotic retiform purpura: a case series. Br J Dermatol . 2021;184:141-150. https://doi.org/10.1111/bjd.19415
- Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol . 2020;183:729-737. doi:10.1111/bjd.19327
- Droesch C, Do MH, DeSancho M, et al. Livedoid and purpuric skin eruptions associated with coagulopathy in severe COVID-19. JAMA Dermatol . 2020;156:1-3. doi:10.1001/jamadermatol.2020.2800
- Asakura H, Ogawa H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int J Hematol . 2021;113:45-57. doi:10.1007/s12185-020-03029-y
- Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med . 2020;383:347-358. doi:10.1056/NEJMoa2021756
- Young TK, Shaw KS, Shah JK, et al. Mucocutaneous manifestations of multisystem inflammatory syndrome in children during the COVID-19 pandemic. JAMA Dermatol . 2021;157:207-212. doi:10.1001/jamadermatol.2020.4779
- Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259-269. doi:10.1001/jama.2020.10369
- Cheng MH, Zhang S, Porritt RA, et al. Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation.
- Morris SB, Schwartz NG, Patel P, et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 Infection—United Kingdom and United States, March–August 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1450-1456. doi:10.15585/mmwr.mm6940e1
- Blanco-Melo D, Nilsson-Payant BE, Liu W-C, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036.e9-1045.e9. doi:10.1016/j.cell.2020.04.026
- Crow YJ, Manel N. Aicardi–Goutières syndrome and the type I interferonopathies. Nat Rev Immunol. 2015;15:429-440. doi:10.1038/nri3850
- Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718-724. doi:10.1126/science.abc6027
- Zimmermann N, Wolf C, Schwenke R, et al. Assessment of clinical response to janus kinase inhibition in patients with familial chilblain lupus and TREX1 mutation. JAMA Dermatol. 2019;155:342-346. doi:10.1001/jamadermatol.2018.5077
- Hubiche T, Le Duff F, Chiaverini C, et al. Negative SARS-CoV-2 PCR in patients with chilblain-like lesions. Lancet Infect Dis. 2021;21:315-316. doi:10.1016/S1473-3099(20)30518-1
- Agrawal A. Mechanisms and implications of age-associated impaired innate interferon secretion by dendritic cells: a mini-review. Gerontology. 2013;59:421-426. doi:10.1159/000350536
- Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384:795-807. doi:10.1056/NEJMoa2031994
- US Food and Drug Administration. Fact sheet for healthcare providers: emergency use authorization (EUA) of baricitinib. Accessed March 29, 2021. https://www.fda.gov/media/143823/download
- Konno Y, Kimura I, Uriu K, et al. SARS-CoV-2 ORF3b is a potent interferon antagonist whose activity is increased by a naturally occurring elongation variant. Cell Rep. 2020;32:108185. doi:10.1016/j.celrep.2020.108185
- Sacks D, Baxter B, Campbell BCV, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke: from the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS), and World Stroke Organization (WSO). J Vasc Interv Radiol. 2018;29:441-453. doi:10.1016/j.jvir.2017.11.026
- Lo MW, Kemper C, Woodruff TM. COVID-19: complement, coagulation, and collateral damage. J Immunol. 2020;205:1488-1495. doi:10.4049/jimmunol.2000644
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Yan B, Freiwald T, Chauss D, et al. SARS-CoV2 drives JAK1/2-dependent local and systemic complement hyper-activation [published online June 9, 2020]. Res Sq. doi:10.21203/rs.3.rs-33390/v1
- Marietta M, Coluccio V, Luppi M. COVID-19, coagulopathy and venous thromboembolism: more questions than answers. Intern Emerg Med. 2020;15:1375-1387. doi:10.1007/s11739-020-02432-x
- Zuo Y, Estes SK, Ali RA, et al. Prothrombotic antiphospholipid antibodies in COVID-19 [published online June 17, 2020]. medRxiv. doi:10.1101/2020.06.15.20131607
- Lan S-H, Lai C-C, Huang H-T, et al. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int J Antimicrob Agents. 2020;56:106103. doi:10.1016/j.ijantimicag.2020.106103
- McMahon D, Gallman A, Hruza G, et al. COVID-19 “long-haulers” in dermatology? duration of dermatologic symptoms in an international registry from 39 countries. Abstract presented at: 29th EADV Congress; October 29, 2020. Accessed March 29, 2020. https://eadvdistribute.m-anage.com/from.storage?image=PXQEdDtICIihN3sM_8nAmh7p_y9AFijhQlf2-_KjrtYgOsOXNVwGxDdti95GZ2Yh0
- Saag MS. Misguided use of hydroxychloroquine for COVID-19: the infusion of politics into science. JAMA. 2020;324:2161-2162. doi:10.1001/jama.2020.22389
- Zahedi Niaki O, Anadkat MJ, Chen ST, et al. Navigating immunosuppression in a pandemic: a guide for the dermatologist from the COVID Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists. J Am Acad Dermatol. 2020;83:1150-1159. doi:10.1016/j.jaad.2020.06.051
- Hammond MI, Sharma TR, Cooper KD, et al. Conducting inpatient dermatology consultations and maintaining resident education in the COVID-19 telemedicine era. J Am Acad Dermatol. 2020;83:E317-E318. doi:10.1016/j.jaad.2020.07.008
- Brunasso AMG, Massone C. Teledermatologic monitoring for chronic cutaneous autoimmune diseases with smartworking during COVID-19 emergency in a tertiary center in Italy. Dermatol Ther. 2020;33:E13495-E13495. doi:10.1111/dth.13695
- Trinidad J, Kroshinsky D, Kaffenberger BH, et al. Telemedicine for inpatient dermatology consultations in response to the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E69-E71. doi:10.1016/j.jaad.2020.04.096
- Madigan LM, Micheletti RG, Shinkai K. How dermatologists can learn and contribute at the leading edge of the COVID-19 global pandemic. JAMA Dermatology. 2020;156:733-734. doi:10.1001/jamadermatol.2020.1438
Practice Points
- Cutaneous manifestations of COVID-19 may reflect the range of host immunologic responses to SARS-CoV-2.
- Perniosis appears to be a late manifestation of COVID-19 associated with a comparatively benign disease course, whereas livedoid or other vasculopathic lesions portend poorer outcomes and may warrant further workup for occult thrombotic disease.
- Maculopapular, vesicular, and urticarial eruptions may be seen in association with COVID-19 but are nonspecific and necessitate a broad differential and workup.
- Challenges posed by the COVID-19 pandemic necessitate creative management strategies for immunosuppression and clinical assessment.
Papulonecrotic Tuberculid Secondary to Mycobacterium avium Complex
To the Editor:
Papulonecrotic tuberculid (PNT) is a cutaneous hypersensitivity reaction to antigenic components of Mycobacterium species, most commonly Mycobacterium tuberculosis. According to a PubMed search of articles indexed for MEDLINE using the terms papulonecrotic tuberculid, Mycobacterium avium complex, and Mycobacterium, only 1 case of PNT secondary to infection with Mycobacterium avium complex (MAC) has been reported.1,2 Papulonecrotic tuberculid classically presents with symmetrical, dusky red papules with necrosis on the extremities.3 Patients may or may not have associated symptoms of fever and weight loss. It is diagnosed through skin biopsy as well as identification of a distant source of mycobacterial infection. Papulonecrotic tuberculid is considered a reactive process to a distant site of mycobacterial infection, and skin lesions contain few, if any, mycobacteria.4
A 65-year-old man was admitted to the hospital for expedited workup of chronic fevers, 20-lb weight loss, and night sweats of 8 months’ duration. He had a medical history of myelodysplastic syndrome and autoimmune hemolytic anemia. During hospitalization, positron emission tomography revealed multilevel vertebral lytic and sclerotic lesions. Subsequent T10 vertebral biopsy showed necrotizing granulomatous inflammation with extensive necrosis and acid-fast bacilli–positive organisms. The patient was empirically started on rifampicin, isoniazid, pyrazinamide, ethambutol, and pyridoxine for presumed M tuberculosis and placed on respiratory isolation.
Dermatology was consulted for a recurrent tender rash on the bilateral upper and lower extremities of 5 years’ duration. Physical examination revealed numerous erythematous papulonecrotic lesions in various states of healing on the bilateral upper and lower extremities (Figure 1). Three years prior to the current presentation, 2 lesions were biopsied and demonstrated leukocytoclastic vasculitis with neutrophilic panniculitis and vasculopathy. A presumptive diagnosis of Sweet syndrome was made given the history of myelodysplastic syndrome, though an infectious etiology could not be ruled out at that time. Concurrently, the patient was diagnosed with autoimmune hemolytic anemia and was started on prednisone. Initially, the skin lesions improved with prednisone but never fully resolved; however, as the dosage of oral steroids decreased, the skin lesions worsened and presented in larger numbers with more frequency. The patient was titrated down to prednisone 5 mg daily with no additional treatment of the skin lesions at that time.
During the current hospitalization, 2 additional biopsies were taken from the arm for routine histopathology and tissue culture. Dermatopathology revealed robust neutrophilic and granulomatous inflammation as well as remarkable necrosis with a few mycobacteria identified on acid-fast and Fite stains (Figure 2). Tissue culture was negative. Additionally, the patient’s spinal biopsy was sent for polymerase chain reaction analysis for Mycobacterium typing, which confirmed MAC. The patient was diagnosed with Pott disease, a mycobacterial infection of the spine, as well as cutaneous papulonecrotic tuberculid secondary to MAC.
Papulonecrotic tuberculid is the rarest form of cutaneous tuberculosis infection and rarely has been reported in connection to MAC.1 This condition is considered a hypersensitivity reaction that occurs in response to antigenic components of mycobacteria.4 Patients with PNT typically present with recurrent crops of painful papulonecrotic lesions distributed on the extremities. Histopathology in PNT classically reveals necrosis, notable inflammatory infiltrate, and lack of observed organisms.5 Diagnosis often is made through skin biopsy, though histopathology varies based on lesion maturity.4 Early lesions often reveal leukocytoclastic vasculitis, whereas late lesions usually demonstrate granulomatous inflammation.4 Mycobacterium avium complex is difficult to culture, as it is a slow-growing, fastidious bacterium and therefore polymerase chain reaction genotyping is useful for bacterial classification.6
Disseminated MAC infection also was on the differential for our patient; however, we felt it was less likely than PNT for several reasons. First, disseminated infection rarely presents with cutaneous involvement and is associated with pulmonary involvement in 90% of cases.7-9 Second, the granuloma formation noted on our patient’s skin biopsy was not typical for disseminated MAC but is well described in cases of PNT.4,8,9 Finally, in the rare cases in which cutaneous involvement has occurred with disseminated mycobacterial infections, skin biopsies typically revealed numerous Mycobacterium organisms.8,10 In contrast, skin lesions associated with PNT usually reveal few, if any, organisms, as was seen with our patient.2
The patient’s initial biopsies also supported a diagnosis of PNT, as early lesions of PNT typically show leukocytoclastic vasculitis. His response to low and high doses of prednisone also fit well with a PNT diagnosis. In fact, a case of PNT secondary to Mycobacterium bovis similarly showed an improvement in the rash with high-dose steroids but progression with lower doses.11 It is possible that our patient’s response to steroids complicated the diagnosis of his rash.
The treatment of PNT is clearance of the underlying infection. Macrolide antibiotics, such as clarithromycin and azithromycin, have the best efficacy against MAC, in combination with ethambutol and/or rifabutin.6,12 Treatment duration should be 1 year. Amikacin or streptomycin may be added to this regimen during early treatment.6 Mycobacterium avium complex is resistant to many antibiotics, including typical antituberculosis drugs, and sensitivities should be identified at the onset of treatment.11,12
Albeit rare, clinicians should be aware of PNT secondary to MAC or other mycobacterial infections. Because this condition is difficult to diagnose with varying histologic findings and often negative tissue cultures, a high index of suspicion is necessary when a patient presents with recurrent papulonecrotic lesions, especially in immunocompromised hosts and patients with exposure to mycobacteria.
- Williams JT, Pulitzer DR, DeVillez RL. Papulonecrotic tuberculid secondary to disseminated Mycobacterium avium complex. Int J Dermatol. 1994;33:109-112.
- Jordaan HF, Schneider JW. Papulonecrotic tuberculid. Int J Dermatol. 1995;34:217-219.
- Scollard DM, Dacso MM, Abad-Venida ML. Tuberculosis and leprosy: classical granulomatous diseases in the twenty-first century. Dermatol Clin. 2015;33:541-562.
- Kim GW, Park HJ, Kim HS, et al. Simultaneous occurrence of papulonecrotic tuberculid and erythema induratum in a patient with pulmonary tuberculosis. Pediatr Dermatol. 2013;30:256-259.
- Spelta K, Diniz LM. Cutaneous tuberculosis: a 26-year retrospective study in an endemic area. Rev Inst Med Trop Sao Paulo. 2016;58:49.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- Dyer J, Weiss J, Steiner WS, et al. Primary cutaneous Mycobacterium avium complex infection following squamous cell carcinoma excision. Cutis. 2016;98:E8-E11.
- Kollipara R, Richards K, Tschen J, et al. Disseminated Mycobacterium avium complex with cutaneous lesions. J Cutan Med Surg. 2016;20:272-274.
- Endly DC, Ackerman LS. Disseminated cutaneous Mycobacterium avium complex in a person with AIDS. Dermatol Online J. 2014;20:22616.
- Li JJ, Beresford R, Fyfe J, et al. Clinical and histopathological features of cutaneous nontuberculous mycobacterial infection: a review of 13 cases. J Cutan Pathol. 2017;44:433-443.
- Iden DL, Rogers RS 3rd, Schroeter AL. Papulonecrotic tuberculid secondary to Mycobacterium bovis. Arch Dermatol. 1978;114:564-566.
- Wong NM, Sun LK, Lau PY. Spinal infection caused by Mycobacterium avium complex in a patient with no acquired immune deficiency syndrome: a case report. J Orthop Surg (Hong Kong). 2008;16:359-363.
To the Editor:
Papulonecrotic tuberculid (PNT) is a cutaneous hypersensitivity reaction to antigenic components of Mycobacterium species, most commonly Mycobacterium tuberculosis. According to a PubMed search of articles indexed for MEDLINE using the terms papulonecrotic tuberculid, Mycobacterium avium complex, and Mycobacterium, only 1 case of PNT secondary to infection with Mycobacterium avium complex (MAC) has been reported.1,2 Papulonecrotic tuberculid classically presents with symmetrical, dusky red papules with necrosis on the extremities.3 Patients may or may not have associated symptoms of fever and weight loss. It is diagnosed through skin biopsy as well as identification of a distant source of mycobacterial infection. Papulonecrotic tuberculid is considered a reactive process to a distant site of mycobacterial infection, and skin lesions contain few, if any, mycobacteria.4
A 65-year-old man was admitted to the hospital for expedited workup of chronic fevers, 20-lb weight loss, and night sweats of 8 months’ duration. He had a medical history of myelodysplastic syndrome and autoimmune hemolytic anemia. During hospitalization, positron emission tomography revealed multilevel vertebral lytic and sclerotic lesions. Subsequent T10 vertebral biopsy showed necrotizing granulomatous inflammation with extensive necrosis and acid-fast bacilli–positive organisms. The patient was empirically started on rifampicin, isoniazid, pyrazinamide, ethambutol, and pyridoxine for presumed M tuberculosis and placed on respiratory isolation.
Dermatology was consulted for a recurrent tender rash on the bilateral upper and lower extremities of 5 years’ duration. Physical examination revealed numerous erythematous papulonecrotic lesions in various states of healing on the bilateral upper and lower extremities (Figure 1). Three years prior to the current presentation, 2 lesions were biopsied and demonstrated leukocytoclastic vasculitis with neutrophilic panniculitis and vasculopathy. A presumptive diagnosis of Sweet syndrome was made given the history of myelodysplastic syndrome, though an infectious etiology could not be ruled out at that time. Concurrently, the patient was diagnosed with autoimmune hemolytic anemia and was started on prednisone. Initially, the skin lesions improved with prednisone but never fully resolved; however, as the dosage of oral steroids decreased, the skin lesions worsened and presented in larger numbers with more frequency. The patient was titrated down to prednisone 5 mg daily with no additional treatment of the skin lesions at that time.
During the current hospitalization, 2 additional biopsies were taken from the arm for routine histopathology and tissue culture. Dermatopathology revealed robust neutrophilic and granulomatous inflammation as well as remarkable necrosis with a few mycobacteria identified on acid-fast and Fite stains (Figure 2). Tissue culture was negative. Additionally, the patient’s spinal biopsy was sent for polymerase chain reaction analysis for Mycobacterium typing, which confirmed MAC. The patient was diagnosed with Pott disease, a mycobacterial infection of the spine, as well as cutaneous papulonecrotic tuberculid secondary to MAC.
Papulonecrotic tuberculid is the rarest form of cutaneous tuberculosis infection and rarely has been reported in connection to MAC.1 This condition is considered a hypersensitivity reaction that occurs in response to antigenic components of mycobacteria.4 Patients with PNT typically present with recurrent crops of painful papulonecrotic lesions distributed on the extremities. Histopathology in PNT classically reveals necrosis, notable inflammatory infiltrate, and lack of observed organisms.5 Diagnosis often is made through skin biopsy, though histopathology varies based on lesion maturity.4 Early lesions often reveal leukocytoclastic vasculitis, whereas late lesions usually demonstrate granulomatous inflammation.4 Mycobacterium avium complex is difficult to culture, as it is a slow-growing, fastidious bacterium and therefore polymerase chain reaction genotyping is useful for bacterial classification.6
Disseminated MAC infection also was on the differential for our patient; however, we felt it was less likely than PNT for several reasons. First, disseminated infection rarely presents with cutaneous involvement and is associated with pulmonary involvement in 90% of cases.7-9 Second, the granuloma formation noted on our patient’s skin biopsy was not typical for disseminated MAC but is well described in cases of PNT.4,8,9 Finally, in the rare cases in which cutaneous involvement has occurred with disseminated mycobacterial infections, skin biopsies typically revealed numerous Mycobacterium organisms.8,10 In contrast, skin lesions associated with PNT usually reveal few, if any, organisms, as was seen with our patient.2
The patient’s initial biopsies also supported a diagnosis of PNT, as early lesions of PNT typically show leukocytoclastic vasculitis. His response to low and high doses of prednisone also fit well with a PNT diagnosis. In fact, a case of PNT secondary to Mycobacterium bovis similarly showed an improvement in the rash with high-dose steroids but progression with lower doses.11 It is possible that our patient’s response to steroids complicated the diagnosis of his rash.
The treatment of PNT is clearance of the underlying infection. Macrolide antibiotics, such as clarithromycin and azithromycin, have the best efficacy against MAC, in combination with ethambutol and/or rifabutin.6,12 Treatment duration should be 1 year. Amikacin or streptomycin may be added to this regimen during early treatment.6 Mycobacterium avium complex is resistant to many antibiotics, including typical antituberculosis drugs, and sensitivities should be identified at the onset of treatment.11,12
Albeit rare, clinicians should be aware of PNT secondary to MAC or other mycobacterial infections. Because this condition is difficult to diagnose with varying histologic findings and often negative tissue cultures, a high index of suspicion is necessary when a patient presents with recurrent papulonecrotic lesions, especially in immunocompromised hosts and patients with exposure to mycobacteria.
To the Editor:
Papulonecrotic tuberculid (PNT) is a cutaneous hypersensitivity reaction to antigenic components of Mycobacterium species, most commonly Mycobacterium tuberculosis. According to a PubMed search of articles indexed for MEDLINE using the terms papulonecrotic tuberculid, Mycobacterium avium complex, and Mycobacterium, only 1 case of PNT secondary to infection with Mycobacterium avium complex (MAC) has been reported.1,2 Papulonecrotic tuberculid classically presents with symmetrical, dusky red papules with necrosis on the extremities.3 Patients may or may not have associated symptoms of fever and weight loss. It is diagnosed through skin biopsy as well as identification of a distant source of mycobacterial infection. Papulonecrotic tuberculid is considered a reactive process to a distant site of mycobacterial infection, and skin lesions contain few, if any, mycobacteria.4
A 65-year-old man was admitted to the hospital for expedited workup of chronic fevers, 20-lb weight loss, and night sweats of 8 months’ duration. He had a medical history of myelodysplastic syndrome and autoimmune hemolytic anemia. During hospitalization, positron emission tomography revealed multilevel vertebral lytic and sclerotic lesions. Subsequent T10 vertebral biopsy showed necrotizing granulomatous inflammation with extensive necrosis and acid-fast bacilli–positive organisms. The patient was empirically started on rifampicin, isoniazid, pyrazinamide, ethambutol, and pyridoxine for presumed M tuberculosis and placed on respiratory isolation.
Dermatology was consulted for a recurrent tender rash on the bilateral upper and lower extremities of 5 years’ duration. Physical examination revealed numerous erythematous papulonecrotic lesions in various states of healing on the bilateral upper and lower extremities (Figure 1). Three years prior to the current presentation, 2 lesions were biopsied and demonstrated leukocytoclastic vasculitis with neutrophilic panniculitis and vasculopathy. A presumptive diagnosis of Sweet syndrome was made given the history of myelodysplastic syndrome, though an infectious etiology could not be ruled out at that time. Concurrently, the patient was diagnosed with autoimmune hemolytic anemia and was started on prednisone. Initially, the skin lesions improved with prednisone but never fully resolved; however, as the dosage of oral steroids decreased, the skin lesions worsened and presented in larger numbers with more frequency. The patient was titrated down to prednisone 5 mg daily with no additional treatment of the skin lesions at that time.
During the current hospitalization, 2 additional biopsies were taken from the arm for routine histopathology and tissue culture. Dermatopathology revealed robust neutrophilic and granulomatous inflammation as well as remarkable necrosis with a few mycobacteria identified on acid-fast and Fite stains (Figure 2). Tissue culture was negative. Additionally, the patient’s spinal biopsy was sent for polymerase chain reaction analysis for Mycobacterium typing, which confirmed MAC. The patient was diagnosed with Pott disease, a mycobacterial infection of the spine, as well as cutaneous papulonecrotic tuberculid secondary to MAC.
Papulonecrotic tuberculid is the rarest form of cutaneous tuberculosis infection and rarely has been reported in connection to MAC.1 This condition is considered a hypersensitivity reaction that occurs in response to antigenic components of mycobacteria.4 Patients with PNT typically present with recurrent crops of painful papulonecrotic lesions distributed on the extremities. Histopathology in PNT classically reveals necrosis, notable inflammatory infiltrate, and lack of observed organisms.5 Diagnosis often is made through skin biopsy, though histopathology varies based on lesion maturity.4 Early lesions often reveal leukocytoclastic vasculitis, whereas late lesions usually demonstrate granulomatous inflammation.4 Mycobacterium avium complex is difficult to culture, as it is a slow-growing, fastidious bacterium and therefore polymerase chain reaction genotyping is useful for bacterial classification.6
Disseminated MAC infection also was on the differential for our patient; however, we felt it was less likely than PNT for several reasons. First, disseminated infection rarely presents with cutaneous involvement and is associated with pulmonary involvement in 90% of cases.7-9 Second, the granuloma formation noted on our patient’s skin biopsy was not typical for disseminated MAC but is well described in cases of PNT.4,8,9 Finally, in the rare cases in which cutaneous involvement has occurred with disseminated mycobacterial infections, skin biopsies typically revealed numerous Mycobacterium organisms.8,10 In contrast, skin lesions associated with PNT usually reveal few, if any, organisms, as was seen with our patient.2
The patient’s initial biopsies also supported a diagnosis of PNT, as early lesions of PNT typically show leukocytoclastic vasculitis. His response to low and high doses of prednisone also fit well with a PNT diagnosis. In fact, a case of PNT secondary to Mycobacterium bovis similarly showed an improvement in the rash with high-dose steroids but progression with lower doses.11 It is possible that our patient’s response to steroids complicated the diagnosis of his rash.
The treatment of PNT is clearance of the underlying infection. Macrolide antibiotics, such as clarithromycin and azithromycin, have the best efficacy against MAC, in combination with ethambutol and/or rifabutin.6,12 Treatment duration should be 1 year. Amikacin or streptomycin may be added to this regimen during early treatment.6 Mycobacterium avium complex is resistant to many antibiotics, including typical antituberculosis drugs, and sensitivities should be identified at the onset of treatment.11,12
Albeit rare, clinicians should be aware of PNT secondary to MAC or other mycobacterial infections. Because this condition is difficult to diagnose with varying histologic findings and often negative tissue cultures, a high index of suspicion is necessary when a patient presents with recurrent papulonecrotic lesions, especially in immunocompromised hosts and patients with exposure to mycobacteria.
- Williams JT, Pulitzer DR, DeVillez RL. Papulonecrotic tuberculid secondary to disseminated Mycobacterium avium complex. Int J Dermatol. 1994;33:109-112.
- Jordaan HF, Schneider JW. Papulonecrotic tuberculid. Int J Dermatol. 1995;34:217-219.
- Scollard DM, Dacso MM, Abad-Venida ML. Tuberculosis and leprosy: classical granulomatous diseases in the twenty-first century. Dermatol Clin. 2015;33:541-562.
- Kim GW, Park HJ, Kim HS, et al. Simultaneous occurrence of papulonecrotic tuberculid and erythema induratum in a patient with pulmonary tuberculosis. Pediatr Dermatol. 2013;30:256-259.
- Spelta K, Diniz LM. Cutaneous tuberculosis: a 26-year retrospective study in an endemic area. Rev Inst Med Trop Sao Paulo. 2016;58:49.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- Dyer J, Weiss J, Steiner WS, et al. Primary cutaneous Mycobacterium avium complex infection following squamous cell carcinoma excision. Cutis. 2016;98:E8-E11.
- Kollipara R, Richards K, Tschen J, et al. Disseminated Mycobacterium avium complex with cutaneous lesions. J Cutan Med Surg. 2016;20:272-274.
- Endly DC, Ackerman LS. Disseminated cutaneous Mycobacterium avium complex in a person with AIDS. Dermatol Online J. 2014;20:22616.
- Li JJ, Beresford R, Fyfe J, et al. Clinical and histopathological features of cutaneous nontuberculous mycobacterial infection: a review of 13 cases. J Cutan Pathol. 2017;44:433-443.
- Iden DL, Rogers RS 3rd, Schroeter AL. Papulonecrotic tuberculid secondary to Mycobacterium bovis. Arch Dermatol. 1978;114:564-566.
- Wong NM, Sun LK, Lau PY. Spinal infection caused by Mycobacterium avium complex in a patient with no acquired immune deficiency syndrome: a case report. J Orthop Surg (Hong Kong). 2008;16:359-363.
- Williams JT, Pulitzer DR, DeVillez RL. Papulonecrotic tuberculid secondary to disseminated Mycobacterium avium complex. Int J Dermatol. 1994;33:109-112.
- Jordaan HF, Schneider JW. Papulonecrotic tuberculid. Int J Dermatol. 1995;34:217-219.
- Scollard DM, Dacso MM, Abad-Venida ML. Tuberculosis and leprosy: classical granulomatous diseases in the twenty-first century. Dermatol Clin. 2015;33:541-562.
- Kim GW, Park HJ, Kim HS, et al. Simultaneous occurrence of papulonecrotic tuberculid and erythema induratum in a patient with pulmonary tuberculosis. Pediatr Dermatol. 2013;30:256-259.
- Spelta K, Diniz LM. Cutaneous tuberculosis: a 26-year retrospective study in an endemic area. Rev Inst Med Trop Sao Paulo. 2016;58:49.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- Dyer J, Weiss J, Steiner WS, et al. Primary cutaneous Mycobacterium avium complex infection following squamous cell carcinoma excision. Cutis. 2016;98:E8-E11.
- Kollipara R, Richards K, Tschen J, et al. Disseminated Mycobacterium avium complex with cutaneous lesions. J Cutan Med Surg. 2016;20:272-274.
- Endly DC, Ackerman LS. Disseminated cutaneous Mycobacterium avium complex in a person with AIDS. Dermatol Online J. 2014;20:22616.
- Li JJ, Beresford R, Fyfe J, et al. Clinical and histopathological features of cutaneous nontuberculous mycobacterial infection: a review of 13 cases. J Cutan Pathol. 2017;44:433-443.
- Iden DL, Rogers RS 3rd, Schroeter AL. Papulonecrotic tuberculid secondary to Mycobacterium bovis. Arch Dermatol. 1978;114:564-566.
- Wong NM, Sun LK, Lau PY. Spinal infection caused by Mycobacterium avium complex in a patient with no acquired immune deficiency syndrome: a case report. J Orthop Surg (Hong Kong). 2008;16:359-363.
Practice Points
- Papulonecrotic tuberculid (PNT) is a hypersensitivity reaction that presents with reddish papules with central necrosis on the extremities.
- Early PNT histopathology shows leukocytoclastic vasculitis. Later lesions demonstrate granulomatous inflammation on histopathology.
- Mycobacterium avium is difficult to culture; therefore, if you suspect it, we recommend polymerase chain reaction genotyping for bacterial classification.
Hydralazine-Associated Cutaneous Vasculitis Presenting With Aerodigestive Tract Involvement
Hydralazine-induced antineutrophil cytoplasmic antibody (ANCA)–positive vasculitis is a complex entity characterized by a distinctive clinical presentation comprising acral hemorrhagic vesiculopustules and necrotic ulcerations, at times with severe mucosal involvement. Although it is an established entity, a PubMed search of articles indexed for MEDLINE using the terms hydralazine vasculitis, ANCA positive vasculitis, and hydralazine associated vasculitis revealed a limited number of cases reported in the dermatologic literature (Table 1).1-6 We report a rare case of hydralazine-induced vasculitis associated with airway compromise and severe gastrointestinal tract bleeding.
RELATED ARTICLE: Sweet Syndrome Associated With Hydralazine-Induced Lupus Erythematosus
Case Report
A 71-year-old woman with a history of end-stage renal disease treated with hemodialysis, as well as hypertension, diabetes mellitus, and ischemic cardiomyopathy, presented to our emergency department with odynophagia, muscle weakness, shortness of breath, and a distinctive mucocutaneous eruption on the left eyelid, lips, and tongue of 2 days’ duration. Physical examination revealed an ill-appearing, afebrile, dyspneic woman with swelling of the left upper eyelid, conjunctival injection, ulcerations on the lips and tongue, and tense hemorrhagic vesicles, as well as vesiculopustules on the elbows, palms, fingers, lower legs, and toes (Figure 1). Given her dyspnea, flexible laryngoscopy was performed and revealed ulceration and edema involving the epiglottis, aryepiglottic folds, and arytenoids. The patient was intubated for airway protection and started on intravenous dexamethasone.
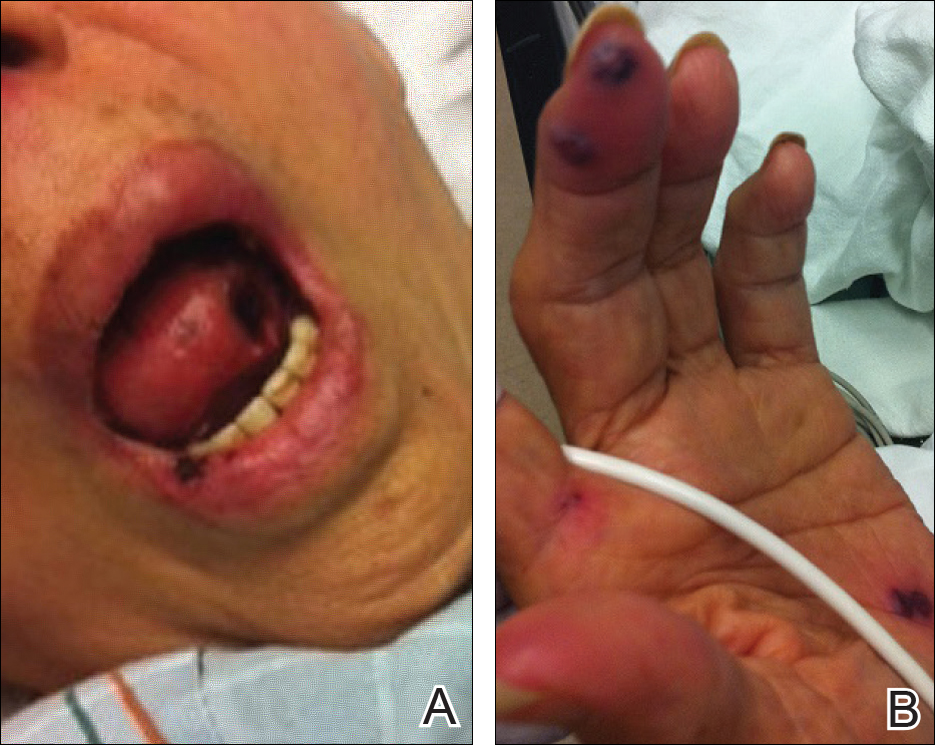
An extensive diagnostic workup commenced. Bacterial, viral, and fungal cultures of blood, skin tissue, and respiratory secretions, as well as human immunodeficiency virus screening, were all negative. Specifically, a tissue culture was performed on skin from the left thigh, viral culture and direct fluorescent antibody were performed on a vesicle on the right knee for herpes simplex virus and herpes zoster, and a superficial wound culture was taken from the left arm, all showing no growth. The patient’s home medications were reviewed and revealed she was currently taking hydralazine (100 mg 3 times daily), which was started approximately 2 years prior. Laboratory results revealed a positive antinuclear antibody titer of 1:320 (diffuse pattern), positive antihistone antibody, and positive ANCA with cytoplasmic and perinuclear accentuation (Table 2). Enzyme-linked immunosorbent assays showed IgG antibodies to myeloperoxidase (MPO) and proteinase 3 (Table 2). Skin biopsies from the right lower leg and right upper arm were compatible with necrotizing leukocytoclastic vasculitis characterized by mural and luminal fibrin deposition involving capillaries and venules of the superficial and deep dermis (Figure 2). The vessel walls were infiltrated by neutrophils with concomitant leukocytoclasia. Vessels in the mid dermis were occluded by cellular fibrin thrombi. Foci of neutrophilic interface dermatitis with subepidermal bulla formation were observed. Infectious stains were negative. On direct immunofluorescence, striking homogeneous mantles of staining of IgG were present within the cutaneous vasculature.
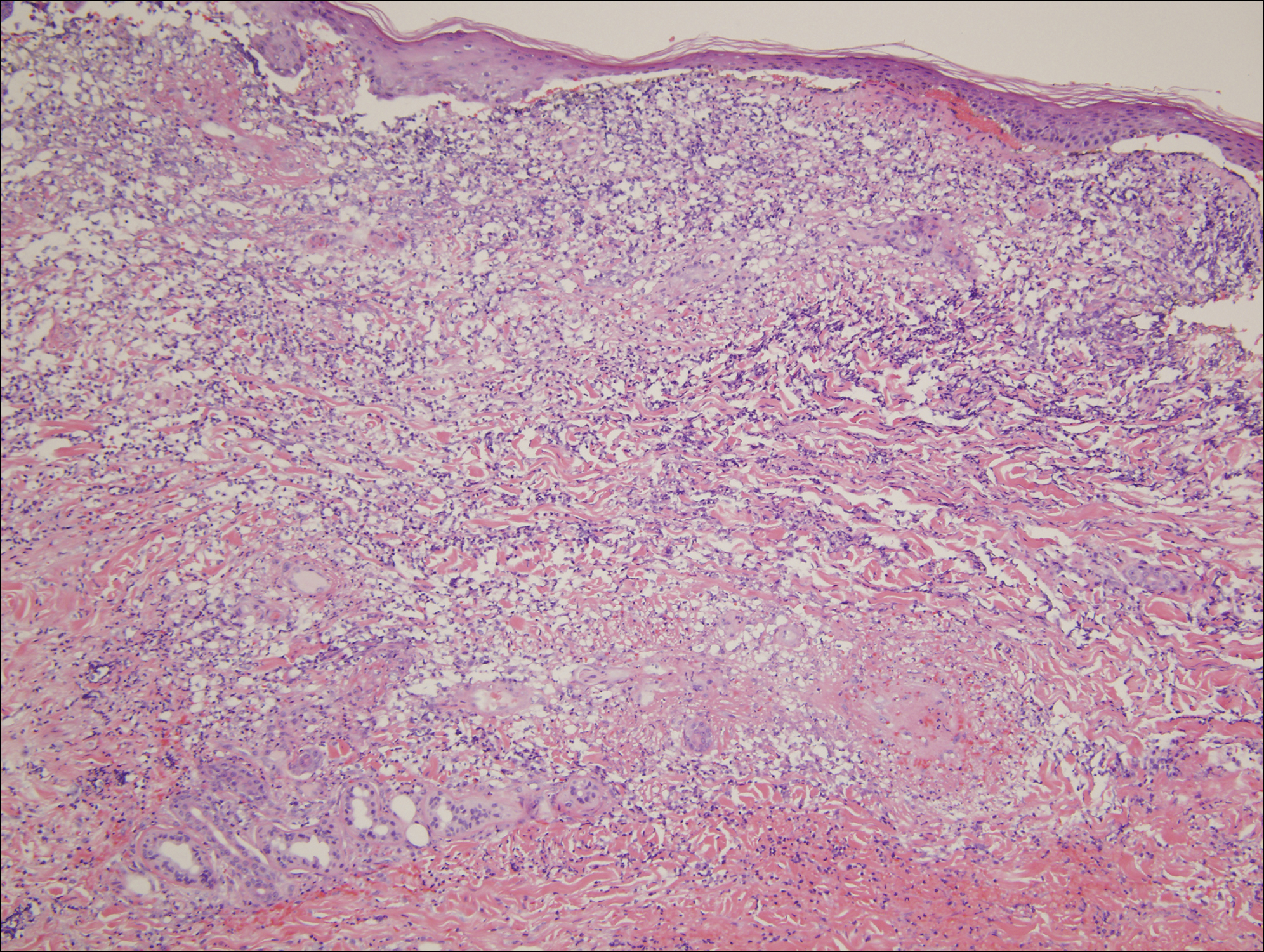
Because the infectious workup was negative and there was no other known instigating factor of vasculitis, concern for a drug-induced process prompted thorough review of the patient’s home medications and discontinuation of hydralazine. A diagnosis of hydralazine-associated cutaneous vasculitis was made when laboratory workup confirmed no underlying infectious process or rheumatologic condition and the medication known to cause her symptoms was on her medication list. The dexamethasone dose was increased, leading to rapid improvement of her mucocutaneous findings; however, on initiation of a steroid taper, she developed substantial gastrointestinal tract bleeding. An esophageal biopsy revealed a neutrophil-rich necrotizing process that essentially mirrored the cutaneous biopsy consistent with vasculitic involvement of the gastrointestinal tract. Steroids were again increased with resolution in gastrointestinal tract bleeding.
Comment
Our case highlights a distinct clinical presentation of hydralazine-induced ANCA-positive cutaneous vasculitis associated with severe involvement of the aerodigestive tract with gastrointestinal tract bleeding and airway compromise requiring intubation. Although discontinuation of hydralazine and in certain cases the addition of immunosuppressive agents may be adequate for resolution of symptoms, some cases progress despite treatment, leading to skin grafting, amputation, and death.3,4 Therefore, early recognition of hydralazine-induced cutaneous vasculitis and discontinuation of hydralazine are of paramount importance.
Reporting hydralazine-induced vasculitis is valuable because of its unique cutaneous, extracutaneous, and serologic findings. In our case, the cutaneous vasculitis presented clinically with acral hemorrhagic vesiculopustules and necrotic ulcerations resembling septic emboli, as opposed to classic lesions of palpable purpura typical of drug-induced leukocytoclastic vasculitis. Similar cutaneous findings have been described in other cases of hydralazine-induced vasculitis, indicating that this pattern of acral pseudoembolic vesiculopustules with necrosis and ulceration is characteristic of this entity.1,3,6 In addition, involvement of the oral cavity, larynx, and gastrointestinal tract have been reported in cases of hydralazine-induced vasculitis, indicating mucosal involvement is an important feature of this disease.3,6 Although involvement of the oral mucosa, larynx, and acral sites appears to be characteristic, the exact basis for this site localization remains elusive. A precedent has been established for a similar pattern of intraoral and laryngeal involvement in other ANCA-positive vasculitic syndromes, most notably Wegener granulomatosis.7 Similarly, there are certain occlusive vasculitic syndromes that show acral localization including chronic septic vasculitis and vasculitis of collagen vascular disease.
Serologic trends can aid in diagnosing hydralazine-induced vasculitis. In theory, the nonspecific cutaneous findings, often in association with joint pain and positive antinuclear antibodies, may lead clinicians to the misdiagnosis of a connective tissue disease, such as systemic lupus erythematosus (SLE). However, unlike SLE, hydralazine-induced vasculitis is associated with positive ANCAs, while antibodies against double-stranded DNA, a highly specific antibody for SLE, are uncommon.8,9 Our patient had both positive perinuclear ANCA with cytoplasmic ANCA as well as a positive antihistone antibodies, a combination highly suggestive of a drug-induced process.
Despite the often acute presentation of hydralazine-induced ANCA-positive vasculitis, afflicted patients have characteristically been on the drug for a long period of time. Our patient is exemplary of most reported cases, as the time from initiation of hydralazine to onset of vasculitis was 2 years.4
The mechanism by which hydralazine causes this reaction is still a matter of debate. It seems clear that there are certain at-risk populations, such as slow acetylators and patients with an underlying hypercoagulable state. There are several theories by which hydralazine induces autoantibody formation. The first involves hydralazine metabolization by MPO released from activated neutrophils to form reactive intermediate metabolites. Such metabolites can be cytotoxic and may cause abnormal degradation of chromatin in susceptible individuals, leading to an autoimmune response against histone-DNA complexes. Alternatively, hydralazine may act as a hapten and bind to MPO, inducing an immune response against the hydralazine-MPO complex, with resultant formation of anti-MPO antibodies in susceptible individuals.10
Conclusion
Hydralazine-induced ANCA-positive vasculitis is a syndromic complex characterized by a distinctive clinical presentation comprising acral hemorrhagic vesiculopustules and necrotic ulcerations, at times with severe mucosal involvement along with a characteristic ANCA-positive serologic profile. Drug withdrawal is the cornerstone of therapy, and depending on the severity of symptoms, additional immunosuppressive treatment such as corticosteroids may be necessary. Older age of onset, female gender, and underlying autoimmune diatheses likely define important risk factors. With more recognition and reporting of this disease, further trends in both clinical and serological presentation will emerge.
- Bernstein RM, Egerton-Vernon J, Webster J. Hydrallazine-induced cutaneous vasculitis. Br Med J. 1980;280:156-157.
- Finlay AY, Statham B, Knight AG. Hydrallazine-induced necrotising vasculitis. Br Med J (Clin Res Ed). 1981;282:1703-1704.
- Peacock A, Weatherall D. Hydralazine-induced necrotising vasculitis. Br Med J (Clin Res Ed). 1981;282:1121-1122.
- Yokogawa N, Vivino FB. Hydralazine-induced autoimmune disease: comparison to idiopathic lupus and ANCA-positive vasculitis. Mod Rheumatol. 2009;19:338-347.
- Sangala N, Lee RW, Horsfield C, et al. Combined ANCA-associated vasculitis and lupus syndrome following prolonged use of hydralazine: a timely reminder of an old foe. Int Urol Nephrol. 2010;42:503-506.
- Keasberry J, Frazier J, Isbel NM, et al. Hydralazine-induced anti-neutrophil cytoplasmic antibody-positive renal vasculitis presenting with a vasculitic syndrome, acute nephritis and a puzzling skin rash: a case report. J Med Case Rep. 2013;7:20.
- Wojciechowska J, Krajewski W, Krajewski P, et al. Granulomatosis with polyangiitis in otolaryngologist practice: a review of current knowledge. Clin Exp Otorhinolaryngol. 2016;9:8-13.
- Short AK, Lockwood CM. Antigen specificity in hydralazine associated ANCA positive systemic vasculitis. QJM. 1995;88:775-783.
- Nässberger L, Hultquist R, Sturfelt G. Occurrence of anti-lactoferrin antibodies in patients with systemic lupus erythematosus, hydralazine-induced lupus, and rheumatoid arthritis. Scand J Rheumatol. 1994;23:206-210.
- Cambridge G, Wallace H, Bernstein RM, et al. Autoantibodies to myeloperoxidase in idiopathic and drug-induced systemic lupus erythematosus and vasculitis. Br J Rheumatol. 1994;33:109-114.
Hydralazine-induced antineutrophil cytoplasmic antibody (ANCA)–positive vasculitis is a complex entity characterized by a distinctive clinical presentation comprising acral hemorrhagic vesiculopustules and necrotic ulcerations, at times with severe mucosal involvement. Although it is an established entity, a PubMed search of articles indexed for MEDLINE using the terms hydralazine vasculitis, ANCA positive vasculitis, and hydralazine associated vasculitis revealed a limited number of cases reported in the dermatologic literature (Table 1).1-6 We report a rare case of hydralazine-induced vasculitis associated with airway compromise and severe gastrointestinal tract bleeding.
RELATED ARTICLE: Sweet Syndrome Associated With Hydralazine-Induced Lupus Erythematosus
Case Report
A 71-year-old woman with a history of end-stage renal disease treated with hemodialysis, as well as hypertension, diabetes mellitus, and ischemic cardiomyopathy, presented to our emergency department with odynophagia, muscle weakness, shortness of breath, and a distinctive mucocutaneous eruption on the left eyelid, lips, and tongue of 2 days’ duration. Physical examination revealed an ill-appearing, afebrile, dyspneic woman with swelling of the left upper eyelid, conjunctival injection, ulcerations on the lips and tongue, and tense hemorrhagic vesicles, as well as vesiculopustules on the elbows, palms, fingers, lower legs, and toes (Figure 1). Given her dyspnea, flexible laryngoscopy was performed and revealed ulceration and edema involving the epiglottis, aryepiglottic folds, and arytenoids. The patient was intubated for airway protection and started on intravenous dexamethasone.

An extensive diagnostic workup commenced. Bacterial, viral, and fungal cultures of blood, skin tissue, and respiratory secretions, as well as human immunodeficiency virus screening, were all negative. Specifically, a tissue culture was performed on skin from the left thigh, viral culture and direct fluorescent antibody were performed on a vesicle on the right knee for herpes simplex virus and herpes zoster, and a superficial wound culture was taken from the left arm, all showing no growth. The patient’s home medications were reviewed and revealed she was currently taking hydralazine (100 mg 3 times daily), which was started approximately 2 years prior. Laboratory results revealed a positive antinuclear antibody titer of 1:320 (diffuse pattern), positive antihistone antibody, and positive ANCA with cytoplasmic and perinuclear accentuation (Table 2). Enzyme-linked immunosorbent assays showed IgG antibodies to myeloperoxidase (MPO) and proteinase 3 (Table 2). Skin biopsies from the right lower leg and right upper arm were compatible with necrotizing leukocytoclastic vasculitis characterized by mural and luminal fibrin deposition involving capillaries and venules of the superficial and deep dermis (Figure 2). The vessel walls were infiltrated by neutrophils with concomitant leukocytoclasia. Vessels in the mid dermis were occluded by cellular fibrin thrombi. Foci of neutrophilic interface dermatitis with subepidermal bulla formation were observed. Infectious stains were negative. On direct immunofluorescence, striking homogeneous mantles of staining of IgG were present within the cutaneous vasculature.

Because the infectious workup was negative and there was no other known instigating factor of vasculitis, concern for a drug-induced process prompted thorough review of the patient’s home medications and discontinuation of hydralazine. A diagnosis of hydralazine-associated cutaneous vasculitis was made when laboratory workup confirmed no underlying infectious process or rheumatologic condition and the medication known to cause her symptoms was on her medication list. The dexamethasone dose was increased, leading to rapid improvement of her mucocutaneous findings; however, on initiation of a steroid taper, she developed substantial gastrointestinal tract bleeding. An esophageal biopsy revealed a neutrophil-rich necrotizing process that essentially mirrored the cutaneous biopsy consistent with vasculitic involvement of the gastrointestinal tract. Steroids were again increased with resolution in gastrointestinal tract bleeding.
Comment
Our case highlights a distinct clinical presentation of hydralazine-induced ANCA-positive cutaneous vasculitis associated with severe involvement of the aerodigestive tract with gastrointestinal tract bleeding and airway compromise requiring intubation. Although discontinuation of hydralazine and in certain cases the addition of immunosuppressive agents may be adequate for resolution of symptoms, some cases progress despite treatment, leading to skin grafting, amputation, and death.3,4 Therefore, early recognition of hydralazine-induced cutaneous vasculitis and discontinuation of hydralazine are of paramount importance.
Reporting hydralazine-induced vasculitis is valuable because of its unique cutaneous, extracutaneous, and serologic findings. In our case, the cutaneous vasculitis presented clinically with acral hemorrhagic vesiculopustules and necrotic ulcerations resembling septic emboli, as opposed to classic lesions of palpable purpura typical of drug-induced leukocytoclastic vasculitis. Similar cutaneous findings have been described in other cases of hydralazine-induced vasculitis, indicating that this pattern of acral pseudoembolic vesiculopustules with necrosis and ulceration is characteristic of this entity.1,3,6 In addition, involvement of the oral cavity, larynx, and gastrointestinal tract have been reported in cases of hydralazine-induced vasculitis, indicating mucosal involvement is an important feature of this disease.3,6 Although involvement of the oral mucosa, larynx, and acral sites appears to be characteristic, the exact basis for this site localization remains elusive. A precedent has been established for a similar pattern of intraoral and laryngeal involvement in other ANCA-positive vasculitic syndromes, most notably Wegener granulomatosis.7 Similarly, there are certain occlusive vasculitic syndromes that show acral localization including chronic septic vasculitis and vasculitis of collagen vascular disease.
Serologic trends can aid in diagnosing hydralazine-induced vasculitis. In theory, the nonspecific cutaneous findings, often in association with joint pain and positive antinuclear antibodies, may lead clinicians to the misdiagnosis of a connective tissue disease, such as systemic lupus erythematosus (SLE). However, unlike SLE, hydralazine-induced vasculitis is associated with positive ANCAs, while antibodies against double-stranded DNA, a highly specific antibody for SLE, are uncommon.8,9 Our patient had both positive perinuclear ANCA with cytoplasmic ANCA as well as a positive antihistone antibodies, a combination highly suggestive of a drug-induced process.
Despite the often acute presentation of hydralazine-induced ANCA-positive vasculitis, afflicted patients have characteristically been on the drug for a long period of time. Our patient is exemplary of most reported cases, as the time from initiation of hydralazine to onset of vasculitis was 2 years.4
The mechanism by which hydralazine causes this reaction is still a matter of debate. It seems clear that there are certain at-risk populations, such as slow acetylators and patients with an underlying hypercoagulable state. There are several theories by which hydralazine induces autoantibody formation. The first involves hydralazine metabolization by MPO released from activated neutrophils to form reactive intermediate metabolites. Such metabolites can be cytotoxic and may cause abnormal degradation of chromatin in susceptible individuals, leading to an autoimmune response against histone-DNA complexes. Alternatively, hydralazine may act as a hapten and bind to MPO, inducing an immune response against the hydralazine-MPO complex, with resultant formation of anti-MPO antibodies in susceptible individuals.10
Conclusion
Hydralazine-induced ANCA-positive vasculitis is a syndromic complex characterized by a distinctive clinical presentation comprising acral hemorrhagic vesiculopustules and necrotic ulcerations, at times with severe mucosal involvement along with a characteristic ANCA-positive serologic profile. Drug withdrawal is the cornerstone of therapy, and depending on the severity of symptoms, additional immunosuppressive treatment such as corticosteroids may be necessary. Older age of onset, female gender, and underlying autoimmune diatheses likely define important risk factors. With more recognition and reporting of this disease, further trends in both clinical and serological presentation will emerge.
Hydralazine-induced antineutrophil cytoplasmic antibody (ANCA)–positive vasculitis is a complex entity characterized by a distinctive clinical presentation comprising acral hemorrhagic vesiculopustules and necrotic ulcerations, at times with severe mucosal involvement. Although it is an established entity, a PubMed search of articles indexed for MEDLINE using the terms hydralazine vasculitis, ANCA positive vasculitis, and hydralazine associated vasculitis revealed a limited number of cases reported in the dermatologic literature (Table 1).1-6 We report a rare case of hydralazine-induced vasculitis associated with airway compromise and severe gastrointestinal tract bleeding.
RELATED ARTICLE: Sweet Syndrome Associated With Hydralazine-Induced Lupus Erythematosus
Case Report
A 71-year-old woman with a history of end-stage renal disease treated with hemodialysis, as well as hypertension, diabetes mellitus, and ischemic cardiomyopathy, presented to our emergency department with odynophagia, muscle weakness, shortness of breath, and a distinctive mucocutaneous eruption on the left eyelid, lips, and tongue of 2 days’ duration. Physical examination revealed an ill-appearing, afebrile, dyspneic woman with swelling of the left upper eyelid, conjunctival injection, ulcerations on the lips and tongue, and tense hemorrhagic vesicles, as well as vesiculopustules on the elbows, palms, fingers, lower legs, and toes (Figure 1). Given her dyspnea, flexible laryngoscopy was performed and revealed ulceration and edema involving the epiglottis, aryepiglottic folds, and arytenoids. The patient was intubated for airway protection and started on intravenous dexamethasone.

An extensive diagnostic workup commenced. Bacterial, viral, and fungal cultures of blood, skin tissue, and respiratory secretions, as well as human immunodeficiency virus screening, were all negative. Specifically, a tissue culture was performed on skin from the left thigh, viral culture and direct fluorescent antibody were performed on a vesicle on the right knee for herpes simplex virus and herpes zoster, and a superficial wound culture was taken from the left arm, all showing no growth. The patient’s home medications were reviewed and revealed she was currently taking hydralazine (100 mg 3 times daily), which was started approximately 2 years prior. Laboratory results revealed a positive antinuclear antibody titer of 1:320 (diffuse pattern), positive antihistone antibody, and positive ANCA with cytoplasmic and perinuclear accentuation (Table 2). Enzyme-linked immunosorbent assays showed IgG antibodies to myeloperoxidase (MPO) and proteinase 3 (Table 2). Skin biopsies from the right lower leg and right upper arm were compatible with necrotizing leukocytoclastic vasculitis characterized by mural and luminal fibrin deposition involving capillaries and venules of the superficial and deep dermis (Figure 2). The vessel walls were infiltrated by neutrophils with concomitant leukocytoclasia. Vessels in the mid dermis were occluded by cellular fibrin thrombi. Foci of neutrophilic interface dermatitis with subepidermal bulla formation were observed. Infectious stains were negative. On direct immunofluorescence, striking homogeneous mantles of staining of IgG were present within the cutaneous vasculature.

Because the infectious workup was negative and there was no other known instigating factor of vasculitis, concern for a drug-induced process prompted thorough review of the patient’s home medications and discontinuation of hydralazine. A diagnosis of hydralazine-associated cutaneous vasculitis was made when laboratory workup confirmed no underlying infectious process or rheumatologic condition and the medication known to cause her symptoms was on her medication list. The dexamethasone dose was increased, leading to rapid improvement of her mucocutaneous findings; however, on initiation of a steroid taper, she developed substantial gastrointestinal tract bleeding. An esophageal biopsy revealed a neutrophil-rich necrotizing process that essentially mirrored the cutaneous biopsy consistent with vasculitic involvement of the gastrointestinal tract. Steroids were again increased with resolution in gastrointestinal tract bleeding.
Comment
Our case highlights a distinct clinical presentation of hydralazine-induced ANCA-positive cutaneous vasculitis associated with severe involvement of the aerodigestive tract with gastrointestinal tract bleeding and airway compromise requiring intubation. Although discontinuation of hydralazine and in certain cases the addition of immunosuppressive agents may be adequate for resolution of symptoms, some cases progress despite treatment, leading to skin grafting, amputation, and death.3,4 Therefore, early recognition of hydralazine-induced cutaneous vasculitis and discontinuation of hydralazine are of paramount importance.
Reporting hydralazine-induced vasculitis is valuable because of its unique cutaneous, extracutaneous, and serologic findings. In our case, the cutaneous vasculitis presented clinically with acral hemorrhagic vesiculopustules and necrotic ulcerations resembling septic emboli, as opposed to classic lesions of palpable purpura typical of drug-induced leukocytoclastic vasculitis. Similar cutaneous findings have been described in other cases of hydralazine-induced vasculitis, indicating that this pattern of acral pseudoembolic vesiculopustules with necrosis and ulceration is characteristic of this entity.1,3,6 In addition, involvement of the oral cavity, larynx, and gastrointestinal tract have been reported in cases of hydralazine-induced vasculitis, indicating mucosal involvement is an important feature of this disease.3,6 Although involvement of the oral mucosa, larynx, and acral sites appears to be characteristic, the exact basis for this site localization remains elusive. A precedent has been established for a similar pattern of intraoral and laryngeal involvement in other ANCA-positive vasculitic syndromes, most notably Wegener granulomatosis.7 Similarly, there are certain occlusive vasculitic syndromes that show acral localization including chronic septic vasculitis and vasculitis of collagen vascular disease.
Serologic trends can aid in diagnosing hydralazine-induced vasculitis. In theory, the nonspecific cutaneous findings, often in association with joint pain and positive antinuclear antibodies, may lead clinicians to the misdiagnosis of a connective tissue disease, such as systemic lupus erythematosus (SLE). However, unlike SLE, hydralazine-induced vasculitis is associated with positive ANCAs, while antibodies against double-stranded DNA, a highly specific antibody for SLE, are uncommon.8,9 Our patient had both positive perinuclear ANCA with cytoplasmic ANCA as well as a positive antihistone antibodies, a combination highly suggestive of a drug-induced process.
Despite the often acute presentation of hydralazine-induced ANCA-positive vasculitis, afflicted patients have characteristically been on the drug for a long period of time. Our patient is exemplary of most reported cases, as the time from initiation of hydralazine to onset of vasculitis was 2 years.4
The mechanism by which hydralazine causes this reaction is still a matter of debate. It seems clear that there are certain at-risk populations, such as slow acetylators and patients with an underlying hypercoagulable state. There are several theories by which hydralazine induces autoantibody formation. The first involves hydralazine metabolization by MPO released from activated neutrophils to form reactive intermediate metabolites. Such metabolites can be cytotoxic and may cause abnormal degradation of chromatin in susceptible individuals, leading to an autoimmune response against histone-DNA complexes. Alternatively, hydralazine may act as a hapten and bind to MPO, inducing an immune response against the hydralazine-MPO complex, with resultant formation of anti-MPO antibodies in susceptible individuals.10
Conclusion
Hydralazine-induced ANCA-positive vasculitis is a syndromic complex characterized by a distinctive clinical presentation comprising acral hemorrhagic vesiculopustules and necrotic ulcerations, at times with severe mucosal involvement along with a characteristic ANCA-positive serologic profile. Drug withdrawal is the cornerstone of therapy, and depending on the severity of symptoms, additional immunosuppressive treatment such as corticosteroids may be necessary. Older age of onset, female gender, and underlying autoimmune diatheses likely define important risk factors. With more recognition and reporting of this disease, further trends in both clinical and serological presentation will emerge.
- Bernstein RM, Egerton-Vernon J, Webster J. Hydrallazine-induced cutaneous vasculitis. Br Med J. 1980;280:156-157.
- Finlay AY, Statham B, Knight AG. Hydrallazine-induced necrotising vasculitis. Br Med J (Clin Res Ed). 1981;282:1703-1704.
- Peacock A, Weatherall D. Hydralazine-induced necrotising vasculitis. Br Med J (Clin Res Ed). 1981;282:1121-1122.
- Yokogawa N, Vivino FB. Hydralazine-induced autoimmune disease: comparison to idiopathic lupus and ANCA-positive vasculitis. Mod Rheumatol. 2009;19:338-347.
- Sangala N, Lee RW, Horsfield C, et al. Combined ANCA-associated vasculitis and lupus syndrome following prolonged use of hydralazine: a timely reminder of an old foe. Int Urol Nephrol. 2010;42:503-506.
- Keasberry J, Frazier J, Isbel NM, et al. Hydralazine-induced anti-neutrophil cytoplasmic antibody-positive renal vasculitis presenting with a vasculitic syndrome, acute nephritis and a puzzling skin rash: a case report. J Med Case Rep. 2013;7:20.
- Wojciechowska J, Krajewski W, Krajewski P, et al. Granulomatosis with polyangiitis in otolaryngologist practice: a review of current knowledge. Clin Exp Otorhinolaryngol. 2016;9:8-13.
- Short AK, Lockwood CM. Antigen specificity in hydralazine associated ANCA positive systemic vasculitis. QJM. 1995;88:775-783.
- Nässberger L, Hultquist R, Sturfelt G. Occurrence of anti-lactoferrin antibodies in patients with systemic lupus erythematosus, hydralazine-induced lupus, and rheumatoid arthritis. Scand J Rheumatol. 1994;23:206-210.
- Cambridge G, Wallace H, Bernstein RM, et al. Autoantibodies to myeloperoxidase in idiopathic and drug-induced systemic lupus erythematosus and vasculitis. Br J Rheumatol. 1994;33:109-114.
- Bernstein RM, Egerton-Vernon J, Webster J. Hydrallazine-induced cutaneous vasculitis. Br Med J. 1980;280:156-157.
- Finlay AY, Statham B, Knight AG. Hydrallazine-induced necrotising vasculitis. Br Med J (Clin Res Ed). 1981;282:1703-1704.
- Peacock A, Weatherall D. Hydralazine-induced necrotising vasculitis. Br Med J (Clin Res Ed). 1981;282:1121-1122.
- Yokogawa N, Vivino FB. Hydralazine-induced autoimmune disease: comparison to idiopathic lupus and ANCA-positive vasculitis. Mod Rheumatol. 2009;19:338-347.
- Sangala N, Lee RW, Horsfield C, et al. Combined ANCA-associated vasculitis and lupus syndrome following prolonged use of hydralazine: a timely reminder of an old foe. Int Urol Nephrol. 2010;42:503-506.
- Keasberry J, Frazier J, Isbel NM, et al. Hydralazine-induced anti-neutrophil cytoplasmic antibody-positive renal vasculitis presenting with a vasculitic syndrome, acute nephritis and a puzzling skin rash: a case report. J Med Case Rep. 2013;7:20.
- Wojciechowska J, Krajewski W, Krajewski P, et al. Granulomatosis with polyangiitis in otolaryngologist practice: a review of current knowledge. Clin Exp Otorhinolaryngol. 2016;9:8-13.
- Short AK, Lockwood CM. Antigen specificity in hydralazine associated ANCA positive systemic vasculitis. QJM. 1995;88:775-783.
- Nässberger L, Hultquist R, Sturfelt G. Occurrence of anti-lactoferrin antibodies in patients with systemic lupus erythematosus, hydralazine-induced lupus, and rheumatoid arthritis. Scand J Rheumatol. 1994;23:206-210.
- Cambridge G, Wallace H, Bernstein RM, et al. Autoantibodies to myeloperoxidase in idiopathic and drug-induced systemic lupus erythematosus and vasculitis. Br J Rheumatol. 1994;33:109-114.
Practice Points
- Hydralazine-induced small vessel vasculitis has a characteristic pattern of acral pseudoembolic vesiculopustules with necrosis and ulceration, along with involvement of the aerodigestive tract.
- Unlike systemic lupus erythematosus (SLE), hydralazine-induced vasculitis is associated with positive antineutrophil cytoplasmic antibodies, while antibodies against double-stranded DNA, a highly specific antibody for SLE, are uncommon.
- Increased recognition of the clinical and serological features of hydralazine-induced small vessel vasculitis may lead to earlier recognition of this disease and decreased time to discontinuation of hydralazine when appropriate.