User login
Retiform Purpura on the Buttocks in 6 Critically Ill COVID-19 Patients
To the Editor:
There is emerging evidence of skin findings in patients with COVID-19, including perniolike changes of the toes as well as urticarial and vesicular eruptions.1 Magro et al2 reported 3 cases of livedoid and purpuric skin eruptions in critically ill COVID-19 patients with evidence of thrombotic vasculopathy on skin biopsy, including a 32-year-old man with striking buttocks retiform purpura. Histopathologic analysis revealed thrombotic vasculopathy and pressure-induced ischemic necrosis. Since that patient was first evaluated (March 2020), we identified 6 more cases of critically ill COVID-19 patients from a single academic hospital in New York City with essentially identical clinical findings. Herein, we report those 6 cases of critically ill and intubated patients with COVID-19 who developed retiform purpura on the buttocks only, approximately 11 to 21 days after onset of COVID-19 symptoms.
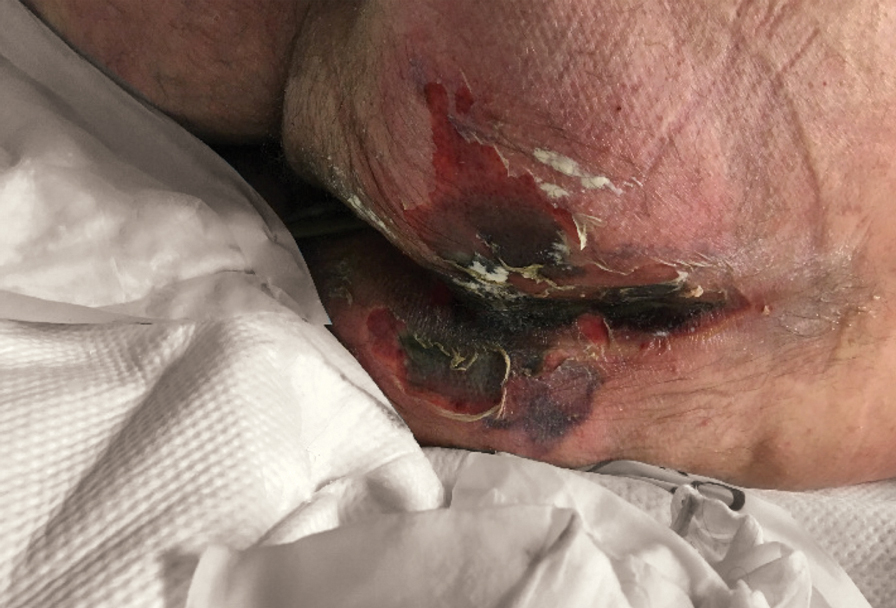
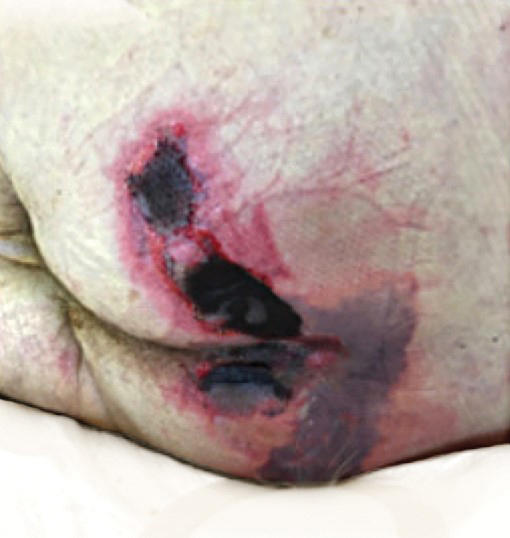
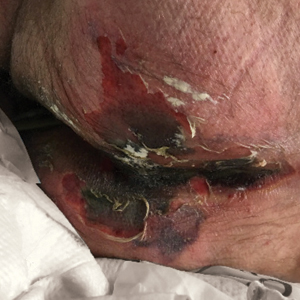
We provided consultation for 5 men and 1 woman (age range, 42–78 years) who were critically ill with COVID-19 and developed retiform purpura on the buttocks (Figures 1 and 2). All had an elevated D-dimer concentration: 2 patients, >700 ng/mL; 2 patients, >2000 ng/mL; 2 patients, >6000 ng/mL (reference, 229 ng/mL). Three patients experienced a peak D-dimer concentration on the day retiform purpura was reported.
Further evidence of coagulopathy in these patients included 1 patient with a newly diagnosed left popliteal deep vein thrombosis and 1 patient with a known history of protein C deficiency and deep vein thromboses. Five patients were receiving anticoagulation on the day the skin changes were documented; anticoagulation was contraindicated in the sixth patient because of oropharyngeal bleeding. Anticoagulation was continued at the treatment dosage (enoxaparin 80 mg twice daily) in 3 patients, and in 2 patients receiving a prophylactic dose (enoxaparin 40 mg daily), anticoagulation was escalated to treatment dose due to rising D-dimer levels and newly diagnosed retiform purpura. Skin biopsy was deferred for all patients due to positional and ventilatory restrictions. At that point in their care, 3 patients remained admitted on medicine floors, 2 were in the intensive care unit, and 1 had died.
Although the differential diagnosis for retiform purpura is broad and should be fully considered in any patient with this finding, based on the elevated D-dimer concentration, critical illness secondary to COVID-19, and striking similarity to earlier reported case of buttocks retiform purpura with thrombotic vasculopathy and pressure injury noted histopathologically,2 we suspect the buttocks retiform purpura in our 6 cases also represent a combination of cutaneous thrombosis and pressure injury. In addition to acral livedoid eruptions (also reported by Magro and colleagues2), we suspect that this cutaneous manifestation might be associated with a hypercoagulable state in some patients, especially in the setting of a rising D-dimer concentration. One study found that 31% of 184 patients with severe COVID-19 had thrombotic complications,3 a clinical picture that portends a poor prognosis.4
COVID-19 patients presenting with retiform purpura should be fully evaluated based on the broad differential for this morphology. We present 6 cases of buttocks retiform purpura in critically ill COVID-19 patients—all with strikingly similar morphologic findings, an elevated D-dimer concentration, and critical illness due to COVID-19—to alert clinicians to this constellation of findings and propose that this cutaneous manifestation could indicate an associated hypercoaguable state and should prompt a hematology consultation. Additionally, biopsy of this skin finding should be considered, especially if biopsy results might serve to guide management; however, obtaining a biopsy specimen can be technically difficult because of ventilatory requirements.
Given the magnitude of the COVID-19 pandemic and the propensity of these patients to experience thrombotic events, recognition of this skin finding in COVID-19 is important and might allow timely intervention.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212-e213. doi:10.1111/jdv.16387
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. doi:10.1016/j.thromres.2020.04.013
- Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-847. doi:10.1111/jth.14768
To the Editor:
There is emerging evidence of skin findings in patients with COVID-19, including perniolike changes of the toes as well as urticarial and vesicular eruptions.1 Magro et al2 reported 3 cases of livedoid and purpuric skin eruptions in critically ill COVID-19 patients with evidence of thrombotic vasculopathy on skin biopsy, including a 32-year-old man with striking buttocks retiform purpura. Histopathologic analysis revealed thrombotic vasculopathy and pressure-induced ischemic necrosis. Since that patient was first evaluated (March 2020), we identified 6 more cases of critically ill COVID-19 patients from a single academic hospital in New York City with essentially identical clinical findings. Herein, we report those 6 cases of critically ill and intubated patients with COVID-19 who developed retiform purpura on the buttocks only, approximately 11 to 21 days after onset of COVID-19 symptoms.
We provided consultation for 5 men and 1 woman (age range, 42–78 years) who were critically ill with COVID-19 and developed retiform purpura on the buttocks (Figures 1 and 2). All had an elevated D-dimer concentration: 2 patients, >700 ng/mL; 2 patients, >2000 ng/mL; 2 patients, >6000 ng/mL (reference, 229 ng/mL). Three patients experienced a peak D-dimer concentration on the day retiform purpura was reported.
Further evidence of coagulopathy in these patients included 1 patient with a newly diagnosed left popliteal deep vein thrombosis and 1 patient with a known history of protein C deficiency and deep vein thromboses. Five patients were receiving anticoagulation on the day the skin changes were documented; anticoagulation was contraindicated in the sixth patient because of oropharyngeal bleeding. Anticoagulation was continued at the treatment dosage (enoxaparin 80 mg twice daily) in 3 patients, and in 2 patients receiving a prophylactic dose (enoxaparin 40 mg daily), anticoagulation was escalated to treatment dose due to rising D-dimer levels and newly diagnosed retiform purpura. Skin biopsy was deferred for all patients due to positional and ventilatory restrictions. At that point in their care, 3 patients remained admitted on medicine floors, 2 were in the intensive care unit, and 1 had died.
Although the differential diagnosis for retiform purpura is broad and should be fully considered in any patient with this finding, based on the elevated D-dimer concentration, critical illness secondary to COVID-19, and striking similarity to earlier reported case of buttocks retiform purpura with thrombotic vasculopathy and pressure injury noted histopathologically,2 we suspect the buttocks retiform purpura in our 6 cases also represent a combination of cutaneous thrombosis and pressure injury. In addition to acral livedoid eruptions (also reported by Magro and colleagues2), we suspect that this cutaneous manifestation might be associated with a hypercoagulable state in some patients, especially in the setting of a rising D-dimer concentration. One study found that 31% of 184 patients with severe COVID-19 had thrombotic complications,3 a clinical picture that portends a poor prognosis.4
COVID-19 patients presenting with retiform purpura should be fully evaluated based on the broad differential for this morphology. We present 6 cases of buttocks retiform purpura in critically ill COVID-19 patients—all with strikingly similar morphologic findings, an elevated D-dimer concentration, and critical illness due to COVID-19—to alert clinicians to this constellation of findings and propose that this cutaneous manifestation could indicate an associated hypercoaguable state and should prompt a hematology consultation. Additionally, biopsy of this skin finding should be considered, especially if biopsy results might serve to guide management; however, obtaining a biopsy specimen can be technically difficult because of ventilatory requirements.
Given the magnitude of the COVID-19 pandemic and the propensity of these patients to experience thrombotic events, recognition of this skin finding in COVID-19 is important and might allow timely intervention.
To the Editor:
There is emerging evidence of skin findings in patients with COVID-19, including perniolike changes of the toes as well as urticarial and vesicular eruptions.1 Magro et al2 reported 3 cases of livedoid and purpuric skin eruptions in critically ill COVID-19 patients with evidence of thrombotic vasculopathy on skin biopsy, including a 32-year-old man with striking buttocks retiform purpura. Histopathologic analysis revealed thrombotic vasculopathy and pressure-induced ischemic necrosis. Since that patient was first evaluated (March 2020), we identified 6 more cases of critically ill COVID-19 patients from a single academic hospital in New York City with essentially identical clinical findings. Herein, we report those 6 cases of critically ill and intubated patients with COVID-19 who developed retiform purpura on the buttocks only, approximately 11 to 21 days after onset of COVID-19 symptoms.
We provided consultation for 5 men and 1 woman (age range, 42–78 years) who were critically ill with COVID-19 and developed retiform purpura on the buttocks (Figures 1 and 2). All had an elevated D-dimer concentration: 2 patients, >700 ng/mL; 2 patients, >2000 ng/mL; 2 patients, >6000 ng/mL (reference, 229 ng/mL). Three patients experienced a peak D-dimer concentration on the day retiform purpura was reported.
Further evidence of coagulopathy in these patients included 1 patient with a newly diagnosed left popliteal deep vein thrombosis and 1 patient with a known history of protein C deficiency and deep vein thromboses. Five patients were receiving anticoagulation on the day the skin changes were documented; anticoagulation was contraindicated in the sixth patient because of oropharyngeal bleeding. Anticoagulation was continued at the treatment dosage (enoxaparin 80 mg twice daily) in 3 patients, and in 2 patients receiving a prophylactic dose (enoxaparin 40 mg daily), anticoagulation was escalated to treatment dose due to rising D-dimer levels and newly diagnosed retiform purpura. Skin biopsy was deferred for all patients due to positional and ventilatory restrictions. At that point in their care, 3 patients remained admitted on medicine floors, 2 were in the intensive care unit, and 1 had died.
Although the differential diagnosis for retiform purpura is broad and should be fully considered in any patient with this finding, based on the elevated D-dimer concentration, critical illness secondary to COVID-19, and striking similarity to earlier reported case of buttocks retiform purpura with thrombotic vasculopathy and pressure injury noted histopathologically,2 we suspect the buttocks retiform purpura in our 6 cases also represent a combination of cutaneous thrombosis and pressure injury. In addition to acral livedoid eruptions (also reported by Magro and colleagues2), we suspect that this cutaneous manifestation might be associated with a hypercoagulable state in some patients, especially in the setting of a rising D-dimer concentration. One study found that 31% of 184 patients with severe COVID-19 had thrombotic complications,3 a clinical picture that portends a poor prognosis.4
COVID-19 patients presenting with retiform purpura should be fully evaluated based on the broad differential for this morphology. We present 6 cases of buttocks retiform purpura in critically ill COVID-19 patients—all with strikingly similar morphologic findings, an elevated D-dimer concentration, and critical illness due to COVID-19—to alert clinicians to this constellation of findings and propose that this cutaneous manifestation could indicate an associated hypercoaguable state and should prompt a hematology consultation. Additionally, biopsy of this skin finding should be considered, especially if biopsy results might serve to guide management; however, obtaining a biopsy specimen can be technically difficult because of ventilatory requirements.
Given the magnitude of the COVID-19 pandemic and the propensity of these patients to experience thrombotic events, recognition of this skin finding in COVID-19 is important and might allow timely intervention.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212-e213. doi:10.1111/jdv.16387
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. doi:10.1016/j.thromres.2020.04.013
- Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-847. doi:10.1111/jth.14768
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212-e213. doi:10.1111/jdv.16387
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. doi:10.1016/j.thromres.2020.04.013
- Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-847. doi:10.1111/jth.14768
Practice Points
- Retiform purpura in a severely ill patient with COVID-19 and a markedly elevated D-dimer concentration might be a cutaneous sign of systemic coagulopathy.
- This constellation of findings should prompt consideration of skin biopsy and hematology consultation.
Lesions With a Distinct Black Pigment
The Diagnosis: Black-Spot Poison Ivy
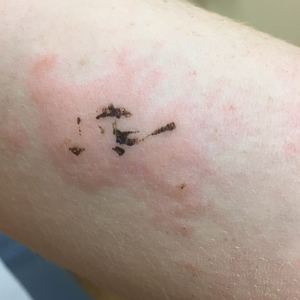
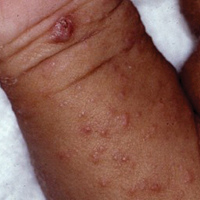
Due to the detailed account of the patient's history including acuity of current presentation, history of recent activities, travel history, and recent exposures, as well as a thorough skin examination, a diagnosis of black-spot poison ivy was made. In this case, the linear distribution of the lesions with overlying black pigment that could not be removed (Figures 1 and 2) provided important clues to diagnosis.
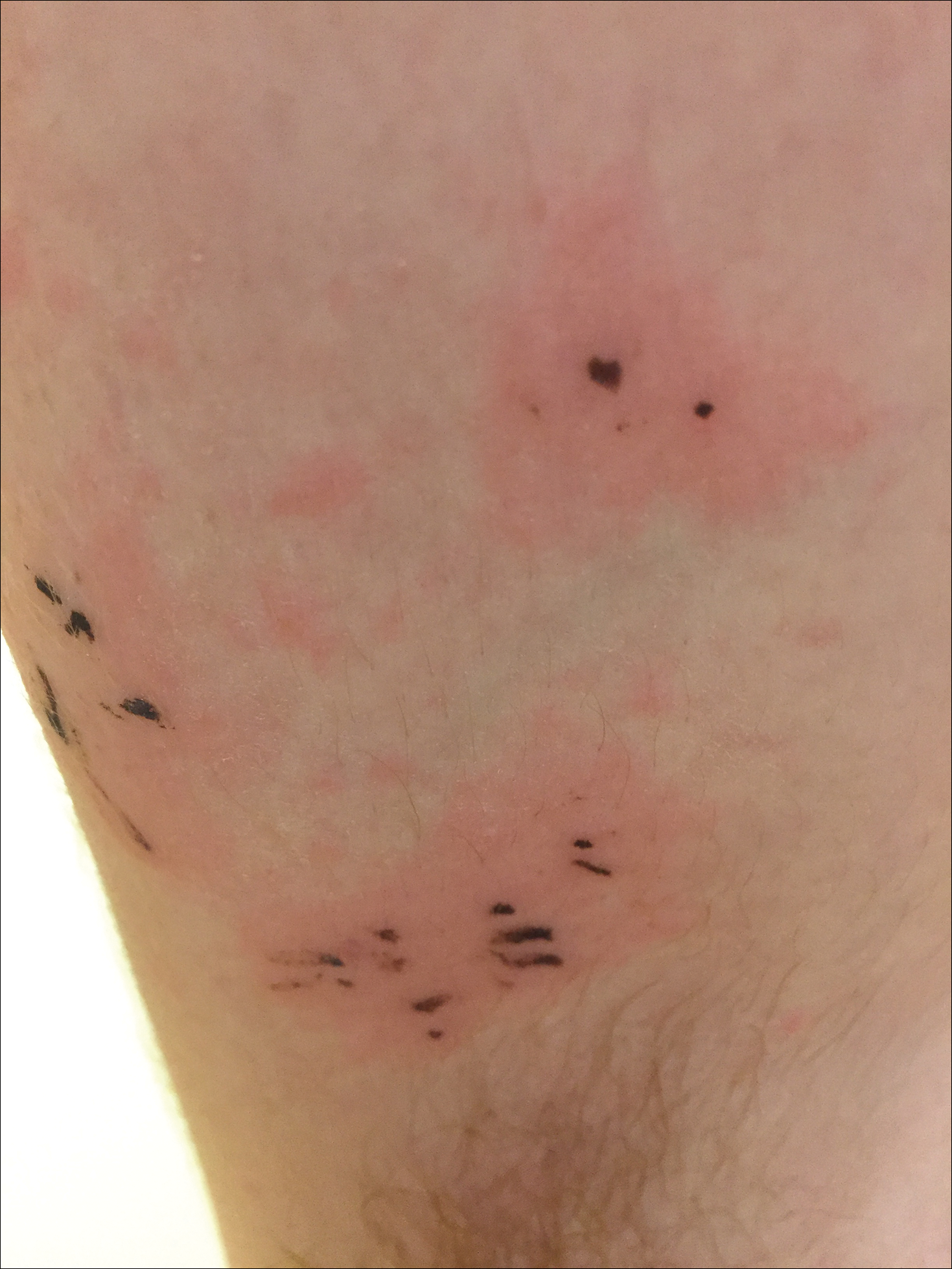
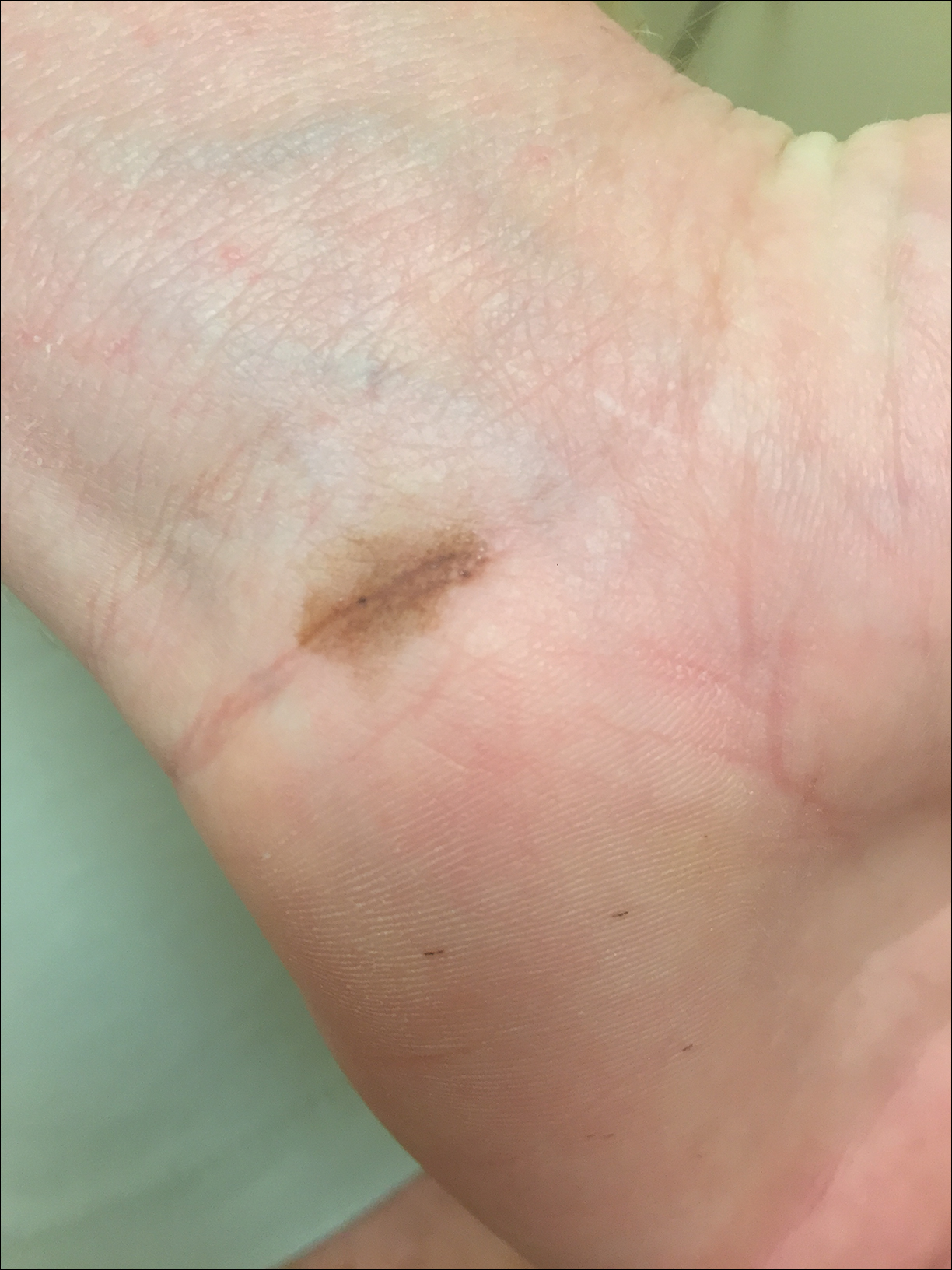
Poison ivy is an allergic contact dermatitis that affects an estimated 25 to 40 million Americans annually who are exposed to its resin. Poison ivy is a plant from the Toxicodendron genus, and an estimated 85% of the North American population report sensitivity to these plants, of which poison ivy (Toxicodendron radicans) is the most common.1 Other related plants include poison sumac and poison oak. Poison ivy and other Toxicodendron plants produce urushiol, the oleoresin responsible for one of the most common allergic contact dermatitides in the United States.2 Black-spot poison ivy is an uncommon presentation following exposure to urushiol or oleoresin,3 as sufficient concentration of urushiol on the skin rarely is achieved.3,4 This plant's resin oxidizes and turns coal black when exposed to air.5 Contact with enough of this oleoresin will produce black-spot poison ivy.6 Patients with sufficient concentrations of oleoresin on their skin to cause this black oxidation usually have similar black spots on their clothing.7 Interestingly, some Toxicodendron species, such as the Japanese lacquer tree, Toxicodendron vernicifluum, have a black lacquer sap that was historically used as ink.8 This ink was used on Chinese and Japanese jars and has caused contact dermatitis hundreds of years after they were created.7
Poison ivy is characterized by a generalized, pruritic, erythematous rash with vesicles and papules in a linear distribution.9 Black-spot poison ivy presents the same with the addition of black lacquer-like macules with surrounding erythema.10 The skin lesions usually appear on exposed areas 24 to 48 hours after contact.11 Histology of black-spot poison ivy lesions should reveal yellow material in the stratum corneum with epidermal necrosis, in addition to classic features of acute allergic contact dermatitis.3 Interestingly, because these lesions occur with the first exposure to poison ivy, a patient may not develop the typical itchy eczematous eruption characteristic of poison ivy dermatitis. Differential diagnosis includes superficial purpura; exogenous pigment such as marker, ink, or tattoo pigment; tinea nigra; purpuric allergic contact dermatitis to resins or dyes; arthropod assault; irritant contact dermatitis; and infectious and noninfectious vasculitis.11
Similar to poison ivy, treatment of black-spot poison ivy involves oral and topical steroids combined with antihistamines if the patient continues to experience pruritus.6,12 It was recommended to our patient to apply cool compresses with water or Burow solution to alleviate itching and promote drying of the lesions. Calamine lotion can provide similar outcomes.13 Once the oleoresin is oxidized and bound to skin, the black spots cannot be removed with soap, water, or alcohol. The black spots gradually desquamate 1 to 2 weeks after formation without scarring,11 and patients do not require further monitoring.1 Patients should clean or discard clothing and evaluate for possible sources of poison ivy exposure. Because this type of poison ivy dermatitis is rare, most health care workers likely have never seen black-spot poison ivy, and it is an important diagnosis to consider.13
- Baer RL. Poison ivy dermatitis. Cutis. 1990;46:34-36.
- Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
- Hurwitz RM, Rivera HP, Guin JD. Black-spot poison ivy dermatitis. an acute irritant contact dermatitis superimposed upon an allergic contact dermatitis. Am J Dermatopathol. 1984;6:319-322.
- Kurlan JG, Lucky AW. Black spot poison ivy: a report of 5 cases and a review of the literature. J Am Acad Dermatol. 2001;45:246-249.
- Guin JD. The black spot test for recognizing poison ivy and related species. J Am Acad Dermatol. 1980;2:332-333.
- Mallory SB, Hurwitz RM. Black-spot poison-ivy dermatitis. Clin Dermatol. 1986;4:149-151.
- Mallory SB, Miller OF, Tyler WB. Toxicodendron radicans dermatitis with black lacquer deposit on the skin. J Am Acad Dermatol. 1982;6:363-368.
- Rietschel R, Fowler J. Toxicodendron plants and species. Fisher's Contact Dermatitis. 4th ed. Baltimore, MD: Williams & Wilkins; 1995:469-472.
- Fisher AA. Poison ivy/oak dermatitis. part I: prevention--soap and water, topical barriers, hyposensitization. Cutis. 1996;57:384-386.
- McClanahan C, Asarch A, Swick BL. Black spot poison ivy. Int J Dermatol. 2014;53:752-753.
- Mu EW, Capell BC, Castelo-Soccio L. Black spots on a toddler's skin. Contemp Pediatr. 2013;30:31-32.
- Schram SE, Willey A, Lee PK, et al. Black-spot poison ivy. Dermatitis. 2008;19:48-51.
- Paniagua CT, Bean AS. Black-spot poison ivy: a rare phenomenon. J Am Acad Nurse Pract. 2011;23:275-277.
The Diagnosis: Black-Spot Poison Ivy
Due to the detailed account of the patient's history including acuity of current presentation, history of recent activities, travel history, and recent exposures, as well as a thorough skin examination, a diagnosis of black-spot poison ivy was made. In this case, the linear distribution of the lesions with overlying black pigment that could not be removed (Figures 1 and 2) provided important clues to diagnosis.


Poison ivy is an allergic contact dermatitis that affects an estimated 25 to 40 million Americans annually who are exposed to its resin. Poison ivy is a plant from the Toxicodendron genus, and an estimated 85% of the North American population report sensitivity to these plants, of which poison ivy (Toxicodendron radicans) is the most common.1 Other related plants include poison sumac and poison oak. Poison ivy and other Toxicodendron plants produce urushiol, the oleoresin responsible for one of the most common allergic contact dermatitides in the United States.2 Black-spot poison ivy is an uncommon presentation following exposure to urushiol or oleoresin,3 as sufficient concentration of urushiol on the skin rarely is achieved.3,4 This plant's resin oxidizes and turns coal black when exposed to air.5 Contact with enough of this oleoresin will produce black-spot poison ivy.6 Patients with sufficient concentrations of oleoresin on their skin to cause this black oxidation usually have similar black spots on their clothing.7 Interestingly, some Toxicodendron species, such as the Japanese lacquer tree, Toxicodendron vernicifluum, have a black lacquer sap that was historically used as ink.8 This ink was used on Chinese and Japanese jars and has caused contact dermatitis hundreds of years after they were created.7
Poison ivy is characterized by a generalized, pruritic, erythematous rash with vesicles and papules in a linear distribution.9 Black-spot poison ivy presents the same with the addition of black lacquer-like macules with surrounding erythema.10 The skin lesions usually appear on exposed areas 24 to 48 hours after contact.11 Histology of black-spot poison ivy lesions should reveal yellow material in the stratum corneum with epidermal necrosis, in addition to classic features of acute allergic contact dermatitis.3 Interestingly, because these lesions occur with the first exposure to poison ivy, a patient may not develop the typical itchy eczematous eruption characteristic of poison ivy dermatitis. Differential diagnosis includes superficial purpura; exogenous pigment such as marker, ink, or tattoo pigment; tinea nigra; purpuric allergic contact dermatitis to resins or dyes; arthropod assault; irritant contact dermatitis; and infectious and noninfectious vasculitis.11
Similar to poison ivy, treatment of black-spot poison ivy involves oral and topical steroids combined with antihistamines if the patient continues to experience pruritus.6,12 It was recommended to our patient to apply cool compresses with water or Burow solution to alleviate itching and promote drying of the lesions. Calamine lotion can provide similar outcomes.13 Once the oleoresin is oxidized and bound to skin, the black spots cannot be removed with soap, water, or alcohol. The black spots gradually desquamate 1 to 2 weeks after formation without scarring,11 and patients do not require further monitoring.1 Patients should clean or discard clothing and evaluate for possible sources of poison ivy exposure. Because this type of poison ivy dermatitis is rare, most health care workers likely have never seen black-spot poison ivy, and it is an important diagnosis to consider.13
The Diagnosis: Black-Spot Poison Ivy
Due to the detailed account of the patient's history including acuity of current presentation, history of recent activities, travel history, and recent exposures, as well as a thorough skin examination, a diagnosis of black-spot poison ivy was made. In this case, the linear distribution of the lesions with overlying black pigment that could not be removed (Figures 1 and 2) provided important clues to diagnosis.


Poison ivy is an allergic contact dermatitis that affects an estimated 25 to 40 million Americans annually who are exposed to its resin. Poison ivy is a plant from the Toxicodendron genus, and an estimated 85% of the North American population report sensitivity to these plants, of which poison ivy (Toxicodendron radicans) is the most common.1 Other related plants include poison sumac and poison oak. Poison ivy and other Toxicodendron plants produce urushiol, the oleoresin responsible for one of the most common allergic contact dermatitides in the United States.2 Black-spot poison ivy is an uncommon presentation following exposure to urushiol or oleoresin,3 as sufficient concentration of urushiol on the skin rarely is achieved.3,4 This plant's resin oxidizes and turns coal black when exposed to air.5 Contact with enough of this oleoresin will produce black-spot poison ivy.6 Patients with sufficient concentrations of oleoresin on their skin to cause this black oxidation usually have similar black spots on their clothing.7 Interestingly, some Toxicodendron species, such as the Japanese lacquer tree, Toxicodendron vernicifluum, have a black lacquer sap that was historically used as ink.8 This ink was used on Chinese and Japanese jars and has caused contact dermatitis hundreds of years after they were created.7
Poison ivy is characterized by a generalized, pruritic, erythematous rash with vesicles and papules in a linear distribution.9 Black-spot poison ivy presents the same with the addition of black lacquer-like macules with surrounding erythema.10 The skin lesions usually appear on exposed areas 24 to 48 hours after contact.11 Histology of black-spot poison ivy lesions should reveal yellow material in the stratum corneum with epidermal necrosis, in addition to classic features of acute allergic contact dermatitis.3 Interestingly, because these lesions occur with the first exposure to poison ivy, a patient may not develop the typical itchy eczematous eruption characteristic of poison ivy dermatitis. Differential diagnosis includes superficial purpura; exogenous pigment such as marker, ink, or tattoo pigment; tinea nigra; purpuric allergic contact dermatitis to resins or dyes; arthropod assault; irritant contact dermatitis; and infectious and noninfectious vasculitis.11
Similar to poison ivy, treatment of black-spot poison ivy involves oral and topical steroids combined with antihistamines if the patient continues to experience pruritus.6,12 It was recommended to our patient to apply cool compresses with water or Burow solution to alleviate itching and promote drying of the lesions. Calamine lotion can provide similar outcomes.13 Once the oleoresin is oxidized and bound to skin, the black spots cannot be removed with soap, water, or alcohol. The black spots gradually desquamate 1 to 2 weeks after formation without scarring,11 and patients do not require further monitoring.1 Patients should clean or discard clothing and evaluate for possible sources of poison ivy exposure. Because this type of poison ivy dermatitis is rare, most health care workers likely have never seen black-spot poison ivy, and it is an important diagnosis to consider.13
- Baer RL. Poison ivy dermatitis. Cutis. 1990;46:34-36.
- Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
- Hurwitz RM, Rivera HP, Guin JD. Black-spot poison ivy dermatitis. an acute irritant contact dermatitis superimposed upon an allergic contact dermatitis. Am J Dermatopathol. 1984;6:319-322.
- Kurlan JG, Lucky AW. Black spot poison ivy: a report of 5 cases and a review of the literature. J Am Acad Dermatol. 2001;45:246-249.
- Guin JD. The black spot test for recognizing poison ivy and related species. J Am Acad Dermatol. 1980;2:332-333.
- Mallory SB, Hurwitz RM. Black-spot poison-ivy dermatitis. Clin Dermatol. 1986;4:149-151.
- Mallory SB, Miller OF, Tyler WB. Toxicodendron radicans dermatitis with black lacquer deposit on the skin. J Am Acad Dermatol. 1982;6:363-368.
- Rietschel R, Fowler J. Toxicodendron plants and species. Fisher's Contact Dermatitis. 4th ed. Baltimore, MD: Williams & Wilkins; 1995:469-472.
- Fisher AA. Poison ivy/oak dermatitis. part I: prevention--soap and water, topical barriers, hyposensitization. Cutis. 1996;57:384-386.
- McClanahan C, Asarch A, Swick BL. Black spot poison ivy. Int J Dermatol. 2014;53:752-753.
- Mu EW, Capell BC, Castelo-Soccio L. Black spots on a toddler's skin. Contemp Pediatr. 2013;30:31-32.
- Schram SE, Willey A, Lee PK, et al. Black-spot poison ivy. Dermatitis. 2008;19:48-51.
- Paniagua CT, Bean AS. Black-spot poison ivy: a rare phenomenon. J Am Acad Nurse Pract. 2011;23:275-277.
- Baer RL. Poison ivy dermatitis. Cutis. 1990;46:34-36.
- Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
- Hurwitz RM, Rivera HP, Guin JD. Black-spot poison ivy dermatitis. an acute irritant contact dermatitis superimposed upon an allergic contact dermatitis. Am J Dermatopathol. 1984;6:319-322.
- Kurlan JG, Lucky AW. Black spot poison ivy: a report of 5 cases and a review of the literature. J Am Acad Dermatol. 2001;45:246-249.
- Guin JD. The black spot test for recognizing poison ivy and related species. J Am Acad Dermatol. 1980;2:332-333.
- Mallory SB, Hurwitz RM. Black-spot poison-ivy dermatitis. Clin Dermatol. 1986;4:149-151.
- Mallory SB, Miller OF, Tyler WB. Toxicodendron radicans dermatitis with black lacquer deposit on the skin. J Am Acad Dermatol. 1982;6:363-368.
- Rietschel R, Fowler J. Toxicodendron plants and species. Fisher's Contact Dermatitis. 4th ed. Baltimore, MD: Williams & Wilkins; 1995:469-472.
- Fisher AA. Poison ivy/oak dermatitis. part I: prevention--soap and water, topical barriers, hyposensitization. Cutis. 1996;57:384-386.
- McClanahan C, Asarch A, Swick BL. Black spot poison ivy. Int J Dermatol. 2014;53:752-753.
- Mu EW, Capell BC, Castelo-Soccio L. Black spots on a toddler's skin. Contemp Pediatr. 2013;30:31-32.
- Schram SE, Willey A, Lee PK, et al. Black-spot poison ivy. Dermatitis. 2008;19:48-51.
- Paniagua CT, Bean AS. Black-spot poison ivy: a rare phenomenon. J Am Acad Nurse Pract. 2011;23:275-277.
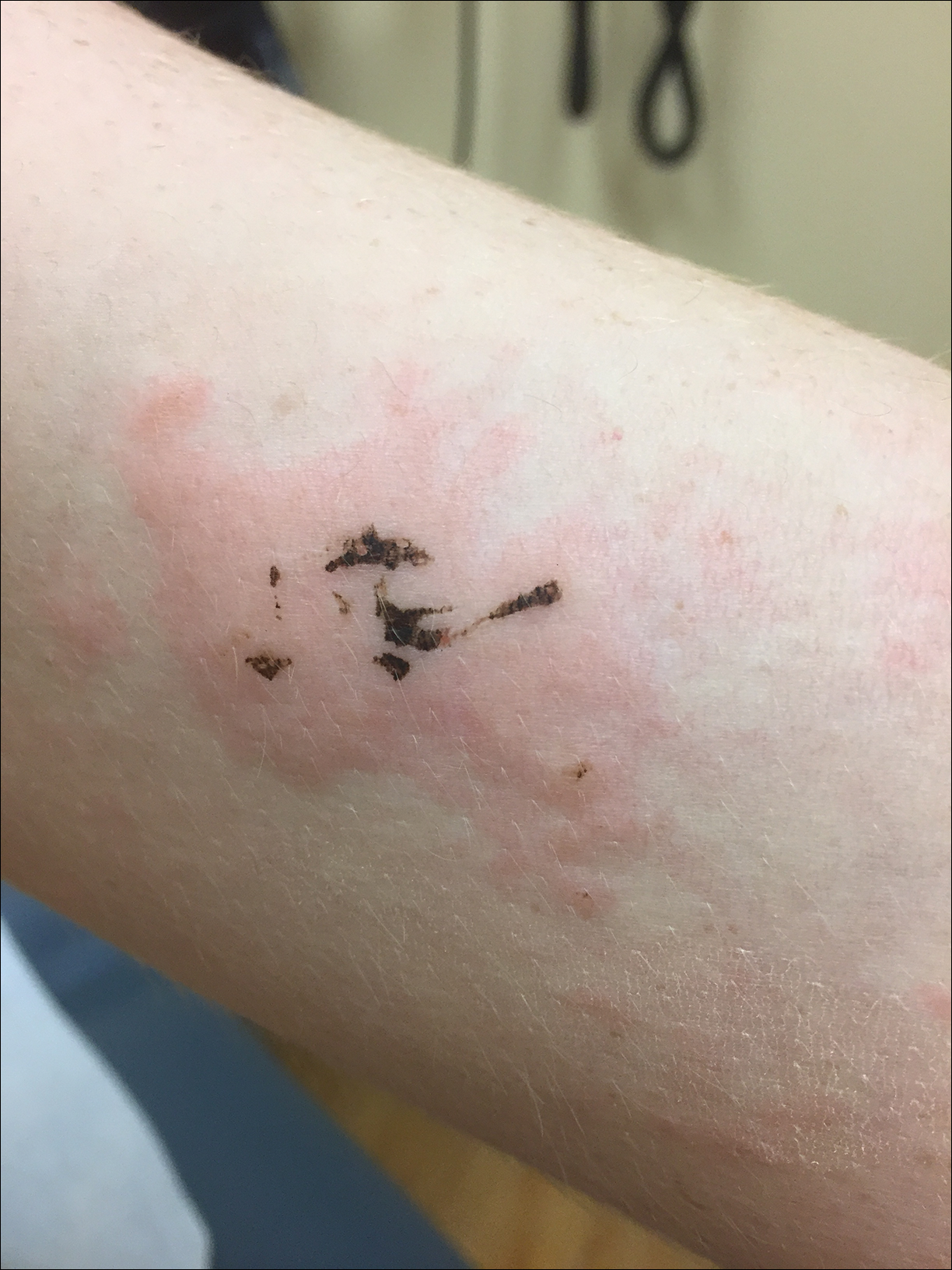
A 17-year-old adolescent boy presented to urgent care with a pruritic eruption on the bilateral arms of 1 day's duration. He was camping in the woods the night prior to presentation. On physical examination linear, erythematous, edematous plaques were observed bilaterally with overlying brown and black pigment on the arms. The pigment could not be removed with alcohol or vigorous scrubbing. The patient's condition improved with prednisone.
What’s Eating You? Scabies in the Developing World
Scabies is caused by the mite Sarcoptes scabiei var hominis.1 It is in the arthropod class Arachnida, subclass Acari, and family Sarcoptidae.2 Historically, scabies was first described in the Old Testament and by Aristotle,2 but the causative organism was not identified until 1687 using a light microscope.3 Scabies affects all age groups, races, and social classes and is globally widespread. It is most prevalent in developing tropical countries.1 It is estimated that 300 million individuals worldwide are infested with scabies mites annually, with the highest burden in young children.4-7 In industrialized societies, infections often are seen in young adults and in institutional settings such as nursing homes.8 Scabies disproportionately impacts impoverished communities with crowded living conditions, poor hygiene and nutrition, and substandard housing.5,9 Controlling the spread of the disease in these communities presents challenges but is important because of the connection between scabies and chronic kidney disease.10 As such, scabies represents a major health problem in the developing world and has been the focus of major health initiatives.1,11
Identifying Characteristics
Adult females are 0.4-mm long and 0.3-mm wide, with males being smaller. Adult nymphs have 8 legs and larvae have 6 legs. Scabies mites are distinguishable from other arachnids by the position of a distinct gnathosoma and the lack of a division between the abdomen and cephalothorax.12 They are ovoid with a small anterior cephalic and caudal thoracoabdominal portion with hairlike projections coming off from the rudimentary legs. They can crawl as fast as 2.5 cm per minute on warm skin.2 The life cycle of the mite begins after mating: the male mite dies, and the female lays up to 3 eggs per day, which hatch in 3 to 4 days,2 in skin burrows within the stratum granulosum.12 Maturation from larva to adult takes 10 to 14 days.12 A female mite can live for 4 to 6 weeks and can produce up to 40 ova (Figure 1).
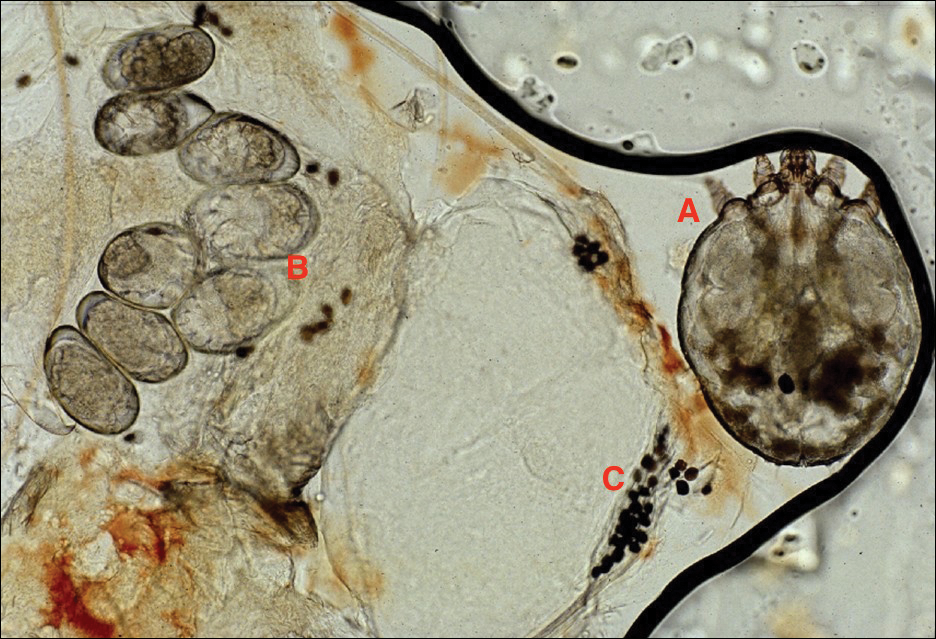
Disease Transmission
Without a host, mites are able to survive and remain capable of infestation for 24 to 36 hours at 21°C and 40% to 80% relative humidity. Lower temperatures and higher humidity prolong survival, but infectivity decreases the longer they are without a host.13
An adult human with ordinary scabies will have an average of 12 adult female mites on the body surface at a given time.14 However, hundreds of mites can be found in neglected children in underprivileged communities and millions in patients with crusted scabies.13 Transmission of typical scabies requires close direct skin-to-skin contact for 15 to 20 minutes.2,8 Transmission from clothing or fomites are an unlikely source of infestation with the exception of patients who are heavily infested such as in crusted scabies.12 In adults, sexual contact is an important method of transmission,12 and patients with scabies should be screened for other sexually transmitted diseases.8
Clinical Manifestations
Signs of scabies on the skin include burrows, erythematous papules, and generalized pruritus (Figure 2).12 The scalp, face, and neck frequently are involved in infants and children,2 and the hands, wrists, elbows, genitalia, axillae, umbilicus, belt line, nipples, and buttocks commonly are involved in adults.12 Itching is characteristically worse at night.8 In tropical climates, patients with scabies are predisposed to secondary bacterial skin infections, particularly Streptococcus pyogenes (group A streptococci). The association between scabies and pyoderma caused by group A streptococci has been well established.15,16 Mika et al10 suggested that local complement inhibition plays an important role in the development of pyoderma in scabies-infested skin.
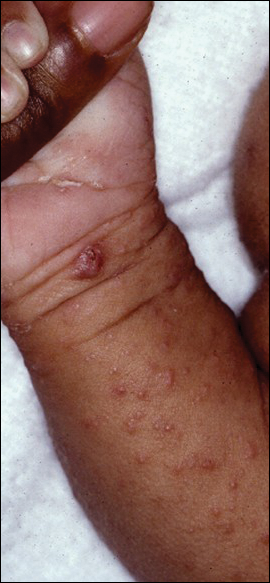
Prevention and Control in the Developing World
Low-cost diagnostic equipment can play a key role in the definitive diagnosis and management of scabies outbreaks in the developing world. Micali et al28 found that a $30 videomicroscope was as effective in scabies diagnosis as a $20,000 videodermatoscope. Because of the low cost of benzyl benzoate, it is commonly used as a first-line drug in many parts of the world,13 whereas permethrin cream 5% is the standard treatment in the developed world.29 Recognition of the role of scabies in patients with pyoderma is key, and one study indicated clinically apparent scabies went unnoticed by physicians in 52% of patients presenting with skin lesions.30 Drug shortages also can contribute to a high prevalence of scabies infestation in the community.31 Mass treatment with ivermectin has proven to be an effective means of reducing the prevalence of many parasitic diseases,1,32,33 and it shows great promise for crusted scabies, institutional outbreaks, and mass administration in highly endemic communites.8 However, there is evidence of ivermectin tolerance among mites, which could undermine the success of mass drug administration.34 Another important consideration is population mobility and the risk for rapid reintroduction of scabies infection across regions.35
Complicating disease control are the socioeconomic factors associated with scabies in the developing world. Families with scabies infestation typically do not own their homes, are less likely to have constant electricity, have a lower monthly income, and live in substandard housing.20 Families can spend a substantial part of their household income on treatment, impacting what they can spend on food.8,11 In addition to medication, control of scabies requires community education and involvement, along with access to primary care and attention to living conditions and environmental factors.34,36
- Romani L, Whitfeld MJ, Koroivueta J, et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med. 2015;373:2305-2313.
- Hicks MI, Elston DM. Scabies. Dermatol Ther. 2009;22:279-292.
- Ramos-e-Silva M. Giovan Cosimo Bonomo (1663-1696): discoverer of the etiology of scabies. Int J Dermatol. 1998;37:625-630.
- Chung SD, Wang KH, Huang CC, et al. Scabies increased the risk of chronic kidney disease: a 5-year follow-up study. J Eur Acad Dermatol Venereol. 2014;28:286-292.
- Wong SS, Poon RW, Chau S, et al. Development of conventional and real-time quantitative PCR assays for diagnosis and monitoring of scabies. J Clin Microbiol. 2015;53:2095-2102.
- Kearns TM, Speare R, Cheng AC, et al. Impact of an ivermectin mass drug administration on scabies prevalence in a remote Australian aboriginal community. PLoS Negl Trop Dis. 2015;9:e0004151.
- Gilmore SJ. Control strategies for endemic childhood scabies. PLoS One. 2011;6:e15990.
- Hay RJ, Steer AC, Engelman D, Walton S. Scabies in the developing world—its prevalence, complications, and management. Clin Microbiol Infect. 2012;18:313-323.
- Hoy WE, White AV, Dowling A, et al. Post-streptococcal glomerulonephritis is a strong risk factor for chronic kidney disease in later life. Kidney Int. 2012;81:1026-1032.
- Mika A, Reynolds SL, Pickering D, et al. Complement inhibitors from scabies mites promote streptococcal growth—a novel mechanism in infected epidermis? PLoS Negl Trop Dis. 2012;6:e1563.
- McLean FE. The elimination of scabies: a task for our generation. Int J Dermatol. 2013;52:1215-1223.
- Hengge UR, Currie BJ, Jäger G, et al. Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis. 2006;6:769-779.
- Heukelbach J, Feldmeier H. Scabies. Lancet. 2006;367:1767-1774.
- Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ. 2005;331:619-622.
- Yeoh DK, Bowen AC, Carapetis JR. Impetigo and scabies—disease burden and modern treatment strategies [published online May 11, 2016]. J Infect. 2016;(72 suppl):S61-S67.
- Bowen AC, Mahé A, Hay RJ, et al. The global epidemiology of impetigo: a systematic review of the population prevalence of impetigo and pyoderma. PLoS One. 2015;10:e0136789.
- Bowen AC, Tong SY, Chatfield MD, et al. The microbiology of impetigo in indigenous children: associations between Streptococcus pyogenes, Staphylococcus aureus, scabies, and nasal carriage. BMC Infect Dis. 2014;14:727.
- Sesso R, Pinto SW. Five-year follow-up of patients with epidemic glomerulonephritis due to Streptococcus zooepidemicus. Nephrol Dial Transplant. 2005;20:1808-1812.
- Singh GR. Glomerulonephritis and managing the risks of chronic renal disease. Pediatr Clin North Am. 2009;56:1363-1382.
- La Vincente S, Kearns T, Connors C, et al. Community management of endemic scabies in remote aboriginal communities of northern Australia: low treatment uptake and high ongoing acquisition. PLoS Negl Trop Dis. 2009;3:e444.
- Clucas DB, Carville KS, Connors C, et al. Disease burden and health-care clinic attendances for young children in remote aboriginal communities of northern Australia. Bull World Health Organ. 2008;86:275-281.
- Stanton B, Khanam S, Nazrul H, et al. Scabies in urban Bangladesh. J Trop Med Hyg. 1987;90:219-226.
- Heukelbach J, de Oliveira FA, Feldmeier H. Ecoparasitoses and public health in Brazil: challenges for control [in Portuguese]. Cad Saude Publica. 2003;19:1535-1540.
- Edison L, Beaudoin A, Goh L, et al. Scabies and bacterial superinfection among American Samoan children, 2011-2012. PLoS One. 2015;10:e0139336.
- Steer AC, Jenney AW, Kado J, et al. High burden of impetigo and scabies in a tropical country. PLoS Negl Trop Dis. 2009;3:e467.
- Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960-967.
- Romani L, Koroivueta J, Steer AC, et al. Scabies and impetigo prevalence and risk factors in Fiji: a national survey. PLoS Negl Trop Dis. 2015;9:e0003452.
- Micali G, Lacarrubba F, Verzì AE, et al. Low-cost equipment for diagnosis and management of endemic scabies outbreaks in underserved populations. Clin Infect Dis. 2015;60:327-329.
- Pasay C, Walton S, Fischer K, et al. PCR-based assay to survey for knockdown resistance to pyrethroid acaricides in human scabies mites (Sarcoptes scabiei var hominis). Am J Trop Med Hyg. 2006;74:649-657.
- Heukelbach J, van Haeff E, Rump B, et al. Parasitic skin diseases: health care-seeking in a slum in north-east Brazil. Trop Med Int Health. 2003;8:368-373.
- Potter EV, Mayon-White R, Poon-King T, et al. Acute glomerulonephritis as a complication of scabies. In: Orkin M, Maibach HI, eds. Cutaneous Infestations and Insect Bites. New York, NY: Marcel Dekker; 1985.
- Mahé A. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689.
- Steer AC, Romani L, Kaldor JM. Mass drug administration for scabies control. N Engl J Med. 2016;374:1690.
- Mounsey KE, Holt DC, McCarthy JS, et al. Longitudinal evidence of increasing in vitro tolerance of scabies mites to ivermectin in scabies-endemic communities. Arch Dermatol. 2009;145:840-841.
- Currie BJ. Scabies and global control of neglected tropical diseases. N Engl J Med. 2015;373:2371-2372.
- O’Donnell V, Morris S, Ward J. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689-1690.
Scabies is caused by the mite Sarcoptes scabiei var hominis.1 It is in the arthropod class Arachnida, subclass Acari, and family Sarcoptidae.2 Historically, scabies was first described in the Old Testament and by Aristotle,2 but the causative organism was not identified until 1687 using a light microscope.3 Scabies affects all age groups, races, and social classes and is globally widespread. It is most prevalent in developing tropical countries.1 It is estimated that 300 million individuals worldwide are infested with scabies mites annually, with the highest burden in young children.4-7 In industrialized societies, infections often are seen in young adults and in institutional settings such as nursing homes.8 Scabies disproportionately impacts impoverished communities with crowded living conditions, poor hygiene and nutrition, and substandard housing.5,9 Controlling the spread of the disease in these communities presents challenges but is important because of the connection between scabies and chronic kidney disease.10 As such, scabies represents a major health problem in the developing world and has been the focus of major health initiatives.1,11
Identifying Characteristics
Adult females are 0.4-mm long and 0.3-mm wide, with males being smaller. Adult nymphs have 8 legs and larvae have 6 legs. Scabies mites are distinguishable from other arachnids by the position of a distinct gnathosoma and the lack of a division between the abdomen and cephalothorax.12 They are ovoid with a small anterior cephalic and caudal thoracoabdominal portion with hairlike projections coming off from the rudimentary legs. They can crawl as fast as 2.5 cm per minute on warm skin.2 The life cycle of the mite begins after mating: the male mite dies, and the female lays up to 3 eggs per day, which hatch in 3 to 4 days,2 in skin burrows within the stratum granulosum.12 Maturation from larva to adult takes 10 to 14 days.12 A female mite can live for 4 to 6 weeks and can produce up to 40 ova (Figure 1).

Disease Transmission
Without a host, mites are able to survive and remain capable of infestation for 24 to 36 hours at 21°C and 40% to 80% relative humidity. Lower temperatures and higher humidity prolong survival, but infectivity decreases the longer they are without a host.13
An adult human with ordinary scabies will have an average of 12 adult female mites on the body surface at a given time.14 However, hundreds of mites can be found in neglected children in underprivileged communities and millions in patients with crusted scabies.13 Transmission of typical scabies requires close direct skin-to-skin contact for 15 to 20 minutes.2,8 Transmission from clothing or fomites are an unlikely source of infestation with the exception of patients who are heavily infested such as in crusted scabies.12 In adults, sexual contact is an important method of transmission,12 and patients with scabies should be screened for other sexually transmitted diseases.8
Clinical Manifestations
Signs of scabies on the skin include burrows, erythematous papules, and generalized pruritus (Figure 2).12 The scalp, face, and neck frequently are involved in infants and children,2 and the hands, wrists, elbows, genitalia, axillae, umbilicus, belt line, nipples, and buttocks commonly are involved in adults.12 Itching is characteristically worse at night.8 In tropical climates, patients with scabies are predisposed to secondary bacterial skin infections, particularly Streptococcus pyogenes (group A streptococci). The association between scabies and pyoderma caused by group A streptococci has been well established.15,16 Mika et al10 suggested that local complement inhibition plays an important role in the development of pyoderma in scabies-infested skin.

Prevention and Control in the Developing World
Low-cost diagnostic equipment can play a key role in the definitive diagnosis and management of scabies outbreaks in the developing world. Micali et al28 found that a $30 videomicroscope was as effective in scabies diagnosis as a $20,000 videodermatoscope. Because of the low cost of benzyl benzoate, it is commonly used as a first-line drug in many parts of the world,13 whereas permethrin cream 5% is the standard treatment in the developed world.29 Recognition of the role of scabies in patients with pyoderma is key, and one study indicated clinically apparent scabies went unnoticed by physicians in 52% of patients presenting with skin lesions.30 Drug shortages also can contribute to a high prevalence of scabies infestation in the community.31 Mass treatment with ivermectin has proven to be an effective means of reducing the prevalence of many parasitic diseases,1,32,33 and it shows great promise for crusted scabies, institutional outbreaks, and mass administration in highly endemic communites.8 However, there is evidence of ivermectin tolerance among mites, which could undermine the success of mass drug administration.34 Another important consideration is population mobility and the risk for rapid reintroduction of scabies infection across regions.35
Complicating disease control are the socioeconomic factors associated with scabies in the developing world. Families with scabies infestation typically do not own their homes, are less likely to have constant electricity, have a lower monthly income, and live in substandard housing.20 Families can spend a substantial part of their household income on treatment, impacting what they can spend on food.8,11 In addition to medication, control of scabies requires community education and involvement, along with access to primary care and attention to living conditions and environmental factors.34,36
Scabies is caused by the mite Sarcoptes scabiei var hominis.1 It is in the arthropod class Arachnida, subclass Acari, and family Sarcoptidae.2 Historically, scabies was first described in the Old Testament and by Aristotle,2 but the causative organism was not identified until 1687 using a light microscope.3 Scabies affects all age groups, races, and social classes and is globally widespread. It is most prevalent in developing tropical countries.1 It is estimated that 300 million individuals worldwide are infested with scabies mites annually, with the highest burden in young children.4-7 In industrialized societies, infections often are seen in young adults and in institutional settings such as nursing homes.8 Scabies disproportionately impacts impoverished communities with crowded living conditions, poor hygiene and nutrition, and substandard housing.5,9 Controlling the spread of the disease in these communities presents challenges but is important because of the connection between scabies and chronic kidney disease.10 As such, scabies represents a major health problem in the developing world and has been the focus of major health initiatives.1,11
Identifying Characteristics
Adult females are 0.4-mm long and 0.3-mm wide, with males being smaller. Adult nymphs have 8 legs and larvae have 6 legs. Scabies mites are distinguishable from other arachnids by the position of a distinct gnathosoma and the lack of a division between the abdomen and cephalothorax.12 They are ovoid with a small anterior cephalic and caudal thoracoabdominal portion with hairlike projections coming off from the rudimentary legs. They can crawl as fast as 2.5 cm per minute on warm skin.2 The life cycle of the mite begins after mating: the male mite dies, and the female lays up to 3 eggs per day, which hatch in 3 to 4 days,2 in skin burrows within the stratum granulosum.12 Maturation from larva to adult takes 10 to 14 days.12 A female mite can live for 4 to 6 weeks and can produce up to 40 ova (Figure 1).

Disease Transmission
Without a host, mites are able to survive and remain capable of infestation for 24 to 36 hours at 21°C and 40% to 80% relative humidity. Lower temperatures and higher humidity prolong survival, but infectivity decreases the longer they are without a host.13
An adult human with ordinary scabies will have an average of 12 adult female mites on the body surface at a given time.14 However, hundreds of mites can be found in neglected children in underprivileged communities and millions in patients with crusted scabies.13 Transmission of typical scabies requires close direct skin-to-skin contact for 15 to 20 minutes.2,8 Transmission from clothing or fomites are an unlikely source of infestation with the exception of patients who are heavily infested such as in crusted scabies.12 In adults, sexual contact is an important method of transmission,12 and patients with scabies should be screened for other sexually transmitted diseases.8
Clinical Manifestations
Signs of scabies on the skin include burrows, erythematous papules, and generalized pruritus (Figure 2).12 The scalp, face, and neck frequently are involved in infants and children,2 and the hands, wrists, elbows, genitalia, axillae, umbilicus, belt line, nipples, and buttocks commonly are involved in adults.12 Itching is characteristically worse at night.8 In tropical climates, patients with scabies are predisposed to secondary bacterial skin infections, particularly Streptococcus pyogenes (group A streptococci). The association between scabies and pyoderma caused by group A streptococci has been well established.15,16 Mika et al10 suggested that local complement inhibition plays an important role in the development of pyoderma in scabies-infested skin.

Prevention and Control in the Developing World
Low-cost diagnostic equipment can play a key role in the definitive diagnosis and management of scabies outbreaks in the developing world. Micali et al28 found that a $30 videomicroscope was as effective in scabies diagnosis as a $20,000 videodermatoscope. Because of the low cost of benzyl benzoate, it is commonly used as a first-line drug in many parts of the world,13 whereas permethrin cream 5% is the standard treatment in the developed world.29 Recognition of the role of scabies in patients with pyoderma is key, and one study indicated clinically apparent scabies went unnoticed by physicians in 52% of patients presenting with skin lesions.30 Drug shortages also can contribute to a high prevalence of scabies infestation in the community.31 Mass treatment with ivermectin has proven to be an effective means of reducing the prevalence of many parasitic diseases,1,32,33 and it shows great promise for crusted scabies, institutional outbreaks, and mass administration in highly endemic communites.8 However, there is evidence of ivermectin tolerance among mites, which could undermine the success of mass drug administration.34 Another important consideration is population mobility and the risk for rapid reintroduction of scabies infection across regions.35
Complicating disease control are the socioeconomic factors associated with scabies in the developing world. Families with scabies infestation typically do not own their homes, are less likely to have constant electricity, have a lower monthly income, and live in substandard housing.20 Families can spend a substantial part of their household income on treatment, impacting what they can spend on food.8,11 In addition to medication, control of scabies requires community education and involvement, along with access to primary care and attention to living conditions and environmental factors.34,36
- Romani L, Whitfeld MJ, Koroivueta J, et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med. 2015;373:2305-2313.
- Hicks MI, Elston DM. Scabies. Dermatol Ther. 2009;22:279-292.
- Ramos-e-Silva M. Giovan Cosimo Bonomo (1663-1696): discoverer of the etiology of scabies. Int J Dermatol. 1998;37:625-630.
- Chung SD, Wang KH, Huang CC, et al. Scabies increased the risk of chronic kidney disease: a 5-year follow-up study. J Eur Acad Dermatol Venereol. 2014;28:286-292.
- Wong SS, Poon RW, Chau S, et al. Development of conventional and real-time quantitative PCR assays for diagnosis and monitoring of scabies. J Clin Microbiol. 2015;53:2095-2102.
- Kearns TM, Speare R, Cheng AC, et al. Impact of an ivermectin mass drug administration on scabies prevalence in a remote Australian aboriginal community. PLoS Negl Trop Dis. 2015;9:e0004151.
- Gilmore SJ. Control strategies for endemic childhood scabies. PLoS One. 2011;6:e15990.
- Hay RJ, Steer AC, Engelman D, Walton S. Scabies in the developing world—its prevalence, complications, and management. Clin Microbiol Infect. 2012;18:313-323.
- Hoy WE, White AV, Dowling A, et al. Post-streptococcal glomerulonephritis is a strong risk factor for chronic kidney disease in later life. Kidney Int. 2012;81:1026-1032.
- Mika A, Reynolds SL, Pickering D, et al. Complement inhibitors from scabies mites promote streptococcal growth—a novel mechanism in infected epidermis? PLoS Negl Trop Dis. 2012;6:e1563.
- McLean FE. The elimination of scabies: a task for our generation. Int J Dermatol. 2013;52:1215-1223.
- Hengge UR, Currie BJ, Jäger G, et al. Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis. 2006;6:769-779.
- Heukelbach J, Feldmeier H. Scabies. Lancet. 2006;367:1767-1774.
- Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ. 2005;331:619-622.
- Yeoh DK, Bowen AC, Carapetis JR. Impetigo and scabies—disease burden and modern treatment strategies [published online May 11, 2016]. J Infect. 2016;(72 suppl):S61-S67.
- Bowen AC, Mahé A, Hay RJ, et al. The global epidemiology of impetigo: a systematic review of the population prevalence of impetigo and pyoderma. PLoS One. 2015;10:e0136789.
- Bowen AC, Tong SY, Chatfield MD, et al. The microbiology of impetigo in indigenous children: associations between Streptococcus pyogenes, Staphylococcus aureus, scabies, and nasal carriage. BMC Infect Dis. 2014;14:727.
- Sesso R, Pinto SW. Five-year follow-up of patients with epidemic glomerulonephritis due to Streptococcus zooepidemicus. Nephrol Dial Transplant. 2005;20:1808-1812.
- Singh GR. Glomerulonephritis and managing the risks of chronic renal disease. Pediatr Clin North Am. 2009;56:1363-1382.
- La Vincente S, Kearns T, Connors C, et al. Community management of endemic scabies in remote aboriginal communities of northern Australia: low treatment uptake and high ongoing acquisition. PLoS Negl Trop Dis. 2009;3:e444.
- Clucas DB, Carville KS, Connors C, et al. Disease burden and health-care clinic attendances for young children in remote aboriginal communities of northern Australia. Bull World Health Organ. 2008;86:275-281.
- Stanton B, Khanam S, Nazrul H, et al. Scabies in urban Bangladesh. J Trop Med Hyg. 1987;90:219-226.
- Heukelbach J, de Oliveira FA, Feldmeier H. Ecoparasitoses and public health in Brazil: challenges for control [in Portuguese]. Cad Saude Publica. 2003;19:1535-1540.
- Edison L, Beaudoin A, Goh L, et al. Scabies and bacterial superinfection among American Samoan children, 2011-2012. PLoS One. 2015;10:e0139336.
- Steer AC, Jenney AW, Kado J, et al. High burden of impetigo and scabies in a tropical country. PLoS Negl Trop Dis. 2009;3:e467.
- Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960-967.
- Romani L, Koroivueta J, Steer AC, et al. Scabies and impetigo prevalence and risk factors in Fiji: a national survey. PLoS Negl Trop Dis. 2015;9:e0003452.
- Micali G, Lacarrubba F, Verzì AE, et al. Low-cost equipment for diagnosis and management of endemic scabies outbreaks in underserved populations. Clin Infect Dis. 2015;60:327-329.
- Pasay C, Walton S, Fischer K, et al. PCR-based assay to survey for knockdown resistance to pyrethroid acaricides in human scabies mites (Sarcoptes scabiei var hominis). Am J Trop Med Hyg. 2006;74:649-657.
- Heukelbach J, van Haeff E, Rump B, et al. Parasitic skin diseases: health care-seeking in a slum in north-east Brazil. Trop Med Int Health. 2003;8:368-373.
- Potter EV, Mayon-White R, Poon-King T, et al. Acute glomerulonephritis as a complication of scabies. In: Orkin M, Maibach HI, eds. Cutaneous Infestations and Insect Bites. New York, NY: Marcel Dekker; 1985.
- Mahé A. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689.
- Steer AC, Romani L, Kaldor JM. Mass drug administration for scabies control. N Engl J Med. 2016;374:1690.
- Mounsey KE, Holt DC, McCarthy JS, et al. Longitudinal evidence of increasing in vitro tolerance of scabies mites to ivermectin in scabies-endemic communities. Arch Dermatol. 2009;145:840-841.
- Currie BJ. Scabies and global control of neglected tropical diseases. N Engl J Med. 2015;373:2371-2372.
- O’Donnell V, Morris S, Ward J. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689-1690.
- Romani L, Whitfeld MJ, Koroivueta J, et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med. 2015;373:2305-2313.
- Hicks MI, Elston DM. Scabies. Dermatol Ther. 2009;22:279-292.
- Ramos-e-Silva M. Giovan Cosimo Bonomo (1663-1696): discoverer of the etiology of scabies. Int J Dermatol. 1998;37:625-630.
- Chung SD, Wang KH, Huang CC, et al. Scabies increased the risk of chronic kidney disease: a 5-year follow-up study. J Eur Acad Dermatol Venereol. 2014;28:286-292.
- Wong SS, Poon RW, Chau S, et al. Development of conventional and real-time quantitative PCR assays for diagnosis and monitoring of scabies. J Clin Microbiol. 2015;53:2095-2102.
- Kearns TM, Speare R, Cheng AC, et al. Impact of an ivermectin mass drug administration on scabies prevalence in a remote Australian aboriginal community. PLoS Negl Trop Dis. 2015;9:e0004151.
- Gilmore SJ. Control strategies for endemic childhood scabies. PLoS One. 2011;6:e15990.
- Hay RJ, Steer AC, Engelman D, Walton S. Scabies in the developing world—its prevalence, complications, and management. Clin Microbiol Infect. 2012;18:313-323.
- Hoy WE, White AV, Dowling A, et al. Post-streptococcal glomerulonephritis is a strong risk factor for chronic kidney disease in later life. Kidney Int. 2012;81:1026-1032.
- Mika A, Reynolds SL, Pickering D, et al. Complement inhibitors from scabies mites promote streptococcal growth—a novel mechanism in infected epidermis? PLoS Negl Trop Dis. 2012;6:e1563.
- McLean FE. The elimination of scabies: a task for our generation. Int J Dermatol. 2013;52:1215-1223.
- Hengge UR, Currie BJ, Jäger G, et al. Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis. 2006;6:769-779.
- Heukelbach J, Feldmeier H. Scabies. Lancet. 2006;367:1767-1774.
- Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ. 2005;331:619-622.
- Yeoh DK, Bowen AC, Carapetis JR. Impetigo and scabies—disease burden and modern treatment strategies [published online May 11, 2016]. J Infect. 2016;(72 suppl):S61-S67.
- Bowen AC, Mahé A, Hay RJ, et al. The global epidemiology of impetigo: a systematic review of the population prevalence of impetigo and pyoderma. PLoS One. 2015;10:e0136789.
- Bowen AC, Tong SY, Chatfield MD, et al. The microbiology of impetigo in indigenous children: associations between Streptococcus pyogenes, Staphylococcus aureus, scabies, and nasal carriage. BMC Infect Dis. 2014;14:727.
- Sesso R, Pinto SW. Five-year follow-up of patients with epidemic glomerulonephritis due to Streptococcus zooepidemicus. Nephrol Dial Transplant. 2005;20:1808-1812.
- Singh GR. Glomerulonephritis and managing the risks of chronic renal disease. Pediatr Clin North Am. 2009;56:1363-1382.
- La Vincente S, Kearns T, Connors C, et al. Community management of endemic scabies in remote aboriginal communities of northern Australia: low treatment uptake and high ongoing acquisition. PLoS Negl Trop Dis. 2009;3:e444.
- Clucas DB, Carville KS, Connors C, et al. Disease burden and health-care clinic attendances for young children in remote aboriginal communities of northern Australia. Bull World Health Organ. 2008;86:275-281.
- Stanton B, Khanam S, Nazrul H, et al. Scabies in urban Bangladesh. J Trop Med Hyg. 1987;90:219-226.
- Heukelbach J, de Oliveira FA, Feldmeier H. Ecoparasitoses and public health in Brazil: challenges for control [in Portuguese]. Cad Saude Publica. 2003;19:1535-1540.
- Edison L, Beaudoin A, Goh L, et al. Scabies and bacterial superinfection among American Samoan children, 2011-2012. PLoS One. 2015;10:e0139336.
- Steer AC, Jenney AW, Kado J, et al. High burden of impetigo and scabies in a tropical country. PLoS Negl Trop Dis. 2009;3:e467.
- Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960-967.
- Romani L, Koroivueta J, Steer AC, et al. Scabies and impetigo prevalence and risk factors in Fiji: a national survey. PLoS Negl Trop Dis. 2015;9:e0003452.
- Micali G, Lacarrubba F, Verzì AE, et al. Low-cost equipment for diagnosis and management of endemic scabies outbreaks in underserved populations. Clin Infect Dis. 2015;60:327-329.
- Pasay C, Walton S, Fischer K, et al. PCR-based assay to survey for knockdown resistance to pyrethroid acaricides in human scabies mites (Sarcoptes scabiei var hominis). Am J Trop Med Hyg. 2006;74:649-657.
- Heukelbach J, van Haeff E, Rump B, et al. Parasitic skin diseases: health care-seeking in a slum in north-east Brazil. Trop Med Int Health. 2003;8:368-373.
- Potter EV, Mayon-White R, Poon-King T, et al. Acute glomerulonephritis as a complication of scabies. In: Orkin M, Maibach HI, eds. Cutaneous Infestations and Insect Bites. New York, NY: Marcel Dekker; 1985.
- Mahé A. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689.
- Steer AC, Romani L, Kaldor JM. Mass drug administration for scabies control. N Engl J Med. 2016;374:1690.
- Mounsey KE, Holt DC, McCarthy JS, et al. Longitudinal evidence of increasing in vitro tolerance of scabies mites to ivermectin in scabies-endemic communities. Arch Dermatol. 2009;145:840-841.
- Currie BJ. Scabies and global control of neglected tropical diseases. N Engl J Med. 2015;373:2371-2372.
- O’Donnell V, Morris S, Ward J. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689-1690.
Practice Points
- Scabies infestation is one of the world’s leading causes of chronic kidney disease.
- Ivermectin can be used to treat mass infestations, and older topical therapies also are commonly used.
What's Eating You? Sticktight Flea Revisited
Identifying Characteristics
The sticktight flea (Echidnophaga gallinacea) earns its name by embedding its head in the host's skin using broad and serrated laciniae and can feed at one site for up to 19 days.1 It differs in morphology from dog (Ctenocephalides canis) and cat (Ctenocephalides felis) fleas, lacking genal (mustache area) and promotal (back of the head) ctenidia (combs), and is half the size of the cat flea. It has 2 pairs of setae (hairs) behind the antennae with an anteriorly flattened head (Figure).
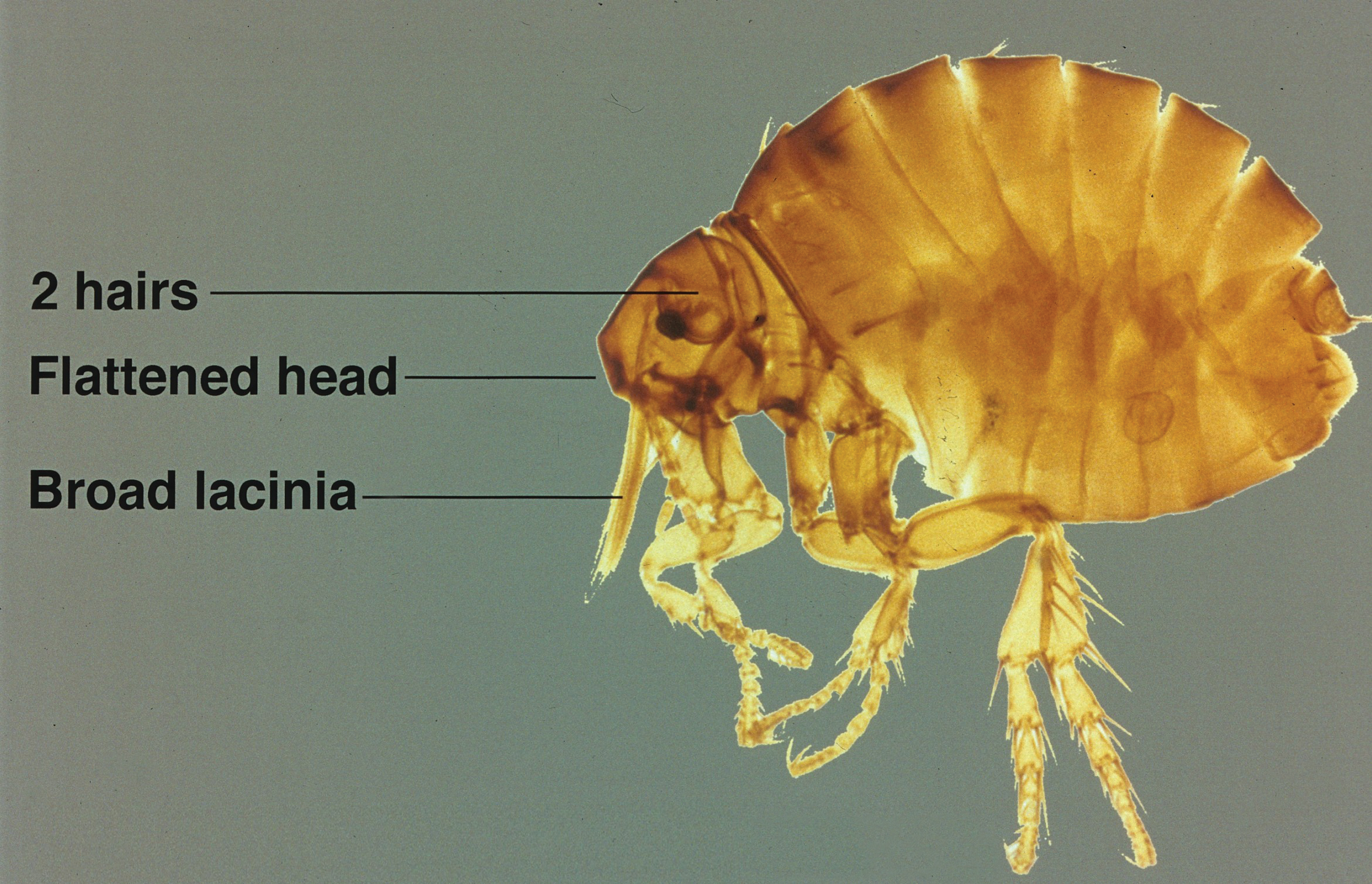
Disease Transmission
Although its primary host is poultry and it also is known as the stickfast or chicken flea, the sticktight flea has been found in many species of birds and mammals, including humans. It is becoming more common in dogs in many parts of the world, including the United States,2-5 and has been found to be the most common flea on dogs in areas of South Africa.6 Other noted hosts of E gallinacea are rodents, cottontail rabbits, cats, ground squirrels, and pigs.7-14 Human infestation occurs from exposure to affected animals.15 As blood feeders, fleas have long been known to serve as vectors for many diseases, including bubonic plague, typhus, and tularemia, as well as an intermediate host of the dog tapeworm (Dipylidium caninum).5Rickettsia felis, belonging to the spotted fever group, is an emerging infectious disease in humans commonly found in the cat flea (C felis) but also has been detected in E gallinacea.7Echidnophaga gallinacea is found worldwide in the tropics, subtropics, and temperate zones, and it is the only representative of the genus found in the United States.1 Given the wide range of wild and domestic animal hosts and wide geographic distribution for E gallinacea, it represents an increasing risk for humans.
Echidnophaga gallinacea favors feeding from fleshy areas without thick fur or plumage. In birds, the area around the eyes, comb, and wattles is included; in dogs, it can be the eyes, in between the toes, and in the genital area.1 Flea bites cause irritation and itching for hosts including humans, typically resulting in clusters of firm, pruritic, erythematous papules with a central punctum.15 Severe bites also may lead to bullous lesions. In birds, symptoms can be extreme, with infestation around the eyes leading to swelling and blindness, a decline in egg production, weight loss, and death in young birds.1 Similar to other fleas, E gallinacea is wingless and depends on jumping onto a host for transmission, which can be from the ground, carpeting and flooring, furniture, or another host. Fleas are champion jumpers (relative to body size) and can jump 100 times their length.16
Management
Treating sticktight fleas can be tricky, as they embed tightly into the host's skin. Animals should be treated by a qualified veterinarian. Removal of attached fleas in humans requires grasping the flea firmly with tweezers and pulling from the skin. If the infestation is considerable, malathion 5% liquid or gel can be applied. Patients can treat itching with topical steroids and antipruritic creams, and oral antihistamines can be used to relieve symptoms and reduce the likelihood of damaged skin as well as the potential for secondary infection. The flea-infested environment should be treated with insecticides. For treatment of hard surfaces, dichlorvos and propetamphos are effective. Organophosphates work well on fabric and carpeting. Domestic pets and livestock may be treated by a veterinarian with agents such as fipronil, selamectin, imidacloprid, metaflumizone, nitenpyram, lufenuron, methoprene, and pyriproxyfen.
- Gyimesi ZS, Hayden ER, Greiner EC. Sticktight flea (Echidnophaga gallinacea) infestation in a Victoria crowned pigeon (Goura victoria). J Zoo Wildl Med. 2007;38:594-596.
- Kalkofen UP, Greenberg J. Echidnophaga gallinacea infestation in dogs. J Am Vet Med Assoc. 1974;165:447-448.
- Harman DW, Halliwell RE, Greiner EC. Flea species from dogs and cats in north-central Florida. Vet Parasitol. 1987;23:135-140.
- Boughton RK, Atwell JW, Schoech SJ. An introduced generalist parasite, the sticktight flea (Echidnophaga gallinacea), and its pathology in the threatened Florida scrub-jay (Aphelocoma coerulescens). J Parasitol. 2006;92:941-948.
- Durden LA, Judy TN, Martin JE, et al. Fleas parasitizing domestic dogs in Georgia, USA: species composition and seasonal abundance. Vet Parasitol. 2005;130:157-162.
- Rautenbach GH, Boomker J, de Villiers IL. A descriptive study of the canine population in a rural town in southern Africa. J S Afr Vet Assoc. 1991;62:158-162.
- Leulmi H, Socolovschi C, Laudisoit A, et al. Detection of Rickettsia felis, Rickettsia typhi, Bartonella species and Yersinia pestis in fleas (Siphonaptera) from Africa. PLoS Negl Trop Dis. 2014;8:e3152.
- Guernier V, Lagadec E, LeMinter G, et al. Fleas of small mammals on Reunion Island: diversity, distribution and epidemiological consequences. PLoS Negl Trop Dis. 2014;8:e3129.
- Cantó GJ, Guerrero RI, Olvera-Ramírez AM, et al. Prevalence of fleas and gastrointestinal parasites in free-roaming cats in central Mexico [published online April 3, 2013]. PLoS One. 2013;8:e60744.
- Akucewich LH, Philman K, Clark A, et al. Prevalence of ectoparasites in a population of feral cats from north central Florida during the summer. Vet Parasitol. 2002;109:129-139.
- Linardi PM, Gomes AF, Botelho JR, et al. Some ectoparasites of commensal rodents from Huambo, Angola. J Med Entomol. 1994;31:754-756.
- Pfaffenberger GS, Valencia VB. Ectoparasites of sympatric cottontails (Sylvilagus audubonii Nelson) and jack rabbits (Lepus californicus Mearns) from the high plains of eastern New Mexico. J Parasitol. 1988;74:842-846.
- Hubbart JA, Jachowski DS, Eads DA. Seasonal and among-site variation in the occurrence and abundance of fleas on California ground squirrels (Otospermophilus beecheyi). J Vector Ecol. 2011;36:117-123.
- Braae UC, Ngowi HA, Johansen MV. Smallholder pig production: prevalence and risk factors of ectoparasites. Vet Parasitol. 2013;196:241-244.
- Carlson JC, Fox MS. A sticktight flea removed from the cheek of a two-year-old boy from Los Angeles. Dermatol Online J. 2009;15:4.
- Rothschild M, Schlein Y, Parker K, et al. The flying leap of the flea. Scientific American. 1973;229:92.
Identifying Characteristics
The sticktight flea (Echidnophaga gallinacea) earns its name by embedding its head in the host's skin using broad and serrated laciniae and can feed at one site for up to 19 days.1 It differs in morphology from dog (Ctenocephalides canis) and cat (Ctenocephalides felis) fleas, lacking genal (mustache area) and promotal (back of the head) ctenidia (combs), and is half the size of the cat flea. It has 2 pairs of setae (hairs) behind the antennae with an anteriorly flattened head (Figure).

Disease Transmission
Although its primary host is poultry and it also is known as the stickfast or chicken flea, the sticktight flea has been found in many species of birds and mammals, including humans. It is becoming more common in dogs in many parts of the world, including the United States,2-5 and has been found to be the most common flea on dogs in areas of South Africa.6 Other noted hosts of E gallinacea are rodents, cottontail rabbits, cats, ground squirrels, and pigs.7-14 Human infestation occurs from exposure to affected animals.15 As blood feeders, fleas have long been known to serve as vectors for many diseases, including bubonic plague, typhus, and tularemia, as well as an intermediate host of the dog tapeworm (Dipylidium caninum).5Rickettsia felis, belonging to the spotted fever group, is an emerging infectious disease in humans commonly found in the cat flea (C felis) but also has been detected in E gallinacea.7Echidnophaga gallinacea is found worldwide in the tropics, subtropics, and temperate zones, and it is the only representative of the genus found in the United States.1 Given the wide range of wild and domestic animal hosts and wide geographic distribution for E gallinacea, it represents an increasing risk for humans.
Echidnophaga gallinacea favors feeding from fleshy areas without thick fur or plumage. In birds, the area around the eyes, comb, and wattles is included; in dogs, it can be the eyes, in between the toes, and in the genital area.1 Flea bites cause irritation and itching for hosts including humans, typically resulting in clusters of firm, pruritic, erythematous papules with a central punctum.15 Severe bites also may lead to bullous lesions. In birds, symptoms can be extreme, with infestation around the eyes leading to swelling and blindness, a decline in egg production, weight loss, and death in young birds.1 Similar to other fleas, E gallinacea is wingless and depends on jumping onto a host for transmission, which can be from the ground, carpeting and flooring, furniture, or another host. Fleas are champion jumpers (relative to body size) and can jump 100 times their length.16
Management
Treating sticktight fleas can be tricky, as they embed tightly into the host's skin. Animals should be treated by a qualified veterinarian. Removal of attached fleas in humans requires grasping the flea firmly with tweezers and pulling from the skin. If the infestation is considerable, malathion 5% liquid or gel can be applied. Patients can treat itching with topical steroids and antipruritic creams, and oral antihistamines can be used to relieve symptoms and reduce the likelihood of damaged skin as well as the potential for secondary infection. The flea-infested environment should be treated with insecticides. For treatment of hard surfaces, dichlorvos and propetamphos are effective. Organophosphates work well on fabric and carpeting. Domestic pets and livestock may be treated by a veterinarian with agents such as fipronil, selamectin, imidacloprid, metaflumizone, nitenpyram, lufenuron, methoprene, and pyriproxyfen.
Identifying Characteristics
The sticktight flea (Echidnophaga gallinacea) earns its name by embedding its head in the host's skin using broad and serrated laciniae and can feed at one site for up to 19 days.1 It differs in morphology from dog (Ctenocephalides canis) and cat (Ctenocephalides felis) fleas, lacking genal (mustache area) and promotal (back of the head) ctenidia (combs), and is half the size of the cat flea. It has 2 pairs of setae (hairs) behind the antennae with an anteriorly flattened head (Figure).

Disease Transmission
Although its primary host is poultry and it also is known as the stickfast or chicken flea, the sticktight flea has been found in many species of birds and mammals, including humans. It is becoming more common in dogs in many parts of the world, including the United States,2-5 and has been found to be the most common flea on dogs in areas of South Africa.6 Other noted hosts of E gallinacea are rodents, cottontail rabbits, cats, ground squirrels, and pigs.7-14 Human infestation occurs from exposure to affected animals.15 As blood feeders, fleas have long been known to serve as vectors for many diseases, including bubonic plague, typhus, and tularemia, as well as an intermediate host of the dog tapeworm (Dipylidium caninum).5Rickettsia felis, belonging to the spotted fever group, is an emerging infectious disease in humans commonly found in the cat flea (C felis) but also has been detected in E gallinacea.7Echidnophaga gallinacea is found worldwide in the tropics, subtropics, and temperate zones, and it is the only representative of the genus found in the United States.1 Given the wide range of wild and domestic animal hosts and wide geographic distribution for E gallinacea, it represents an increasing risk for humans.
Echidnophaga gallinacea favors feeding from fleshy areas without thick fur or plumage. In birds, the area around the eyes, comb, and wattles is included; in dogs, it can be the eyes, in between the toes, and in the genital area.1 Flea bites cause irritation and itching for hosts including humans, typically resulting in clusters of firm, pruritic, erythematous papules with a central punctum.15 Severe bites also may lead to bullous lesions. In birds, symptoms can be extreme, with infestation around the eyes leading to swelling and blindness, a decline in egg production, weight loss, and death in young birds.1 Similar to other fleas, E gallinacea is wingless and depends on jumping onto a host for transmission, which can be from the ground, carpeting and flooring, furniture, or another host. Fleas are champion jumpers (relative to body size) and can jump 100 times their length.16
Management
Treating sticktight fleas can be tricky, as they embed tightly into the host's skin. Animals should be treated by a qualified veterinarian. Removal of attached fleas in humans requires grasping the flea firmly with tweezers and pulling from the skin. If the infestation is considerable, malathion 5% liquid or gel can be applied. Patients can treat itching with topical steroids and antipruritic creams, and oral antihistamines can be used to relieve symptoms and reduce the likelihood of damaged skin as well as the potential for secondary infection. The flea-infested environment should be treated with insecticides. For treatment of hard surfaces, dichlorvos and propetamphos are effective. Organophosphates work well on fabric and carpeting. Domestic pets and livestock may be treated by a veterinarian with agents such as fipronil, selamectin, imidacloprid, metaflumizone, nitenpyram, lufenuron, methoprene, and pyriproxyfen.
- Gyimesi ZS, Hayden ER, Greiner EC. Sticktight flea (Echidnophaga gallinacea) infestation in a Victoria crowned pigeon (Goura victoria). J Zoo Wildl Med. 2007;38:594-596.
- Kalkofen UP, Greenberg J. Echidnophaga gallinacea infestation in dogs. J Am Vet Med Assoc. 1974;165:447-448.
- Harman DW, Halliwell RE, Greiner EC. Flea species from dogs and cats in north-central Florida. Vet Parasitol. 1987;23:135-140.
- Boughton RK, Atwell JW, Schoech SJ. An introduced generalist parasite, the sticktight flea (Echidnophaga gallinacea), and its pathology in the threatened Florida scrub-jay (Aphelocoma coerulescens). J Parasitol. 2006;92:941-948.
- Durden LA, Judy TN, Martin JE, et al. Fleas parasitizing domestic dogs in Georgia, USA: species composition and seasonal abundance. Vet Parasitol. 2005;130:157-162.
- Rautenbach GH, Boomker J, de Villiers IL. A descriptive study of the canine population in a rural town in southern Africa. J S Afr Vet Assoc. 1991;62:158-162.
- Leulmi H, Socolovschi C, Laudisoit A, et al. Detection of Rickettsia felis, Rickettsia typhi, Bartonella species and Yersinia pestis in fleas (Siphonaptera) from Africa. PLoS Negl Trop Dis. 2014;8:e3152.
- Guernier V, Lagadec E, LeMinter G, et al. Fleas of small mammals on Reunion Island: diversity, distribution and epidemiological consequences. PLoS Negl Trop Dis. 2014;8:e3129.
- Cantó GJ, Guerrero RI, Olvera-Ramírez AM, et al. Prevalence of fleas and gastrointestinal parasites in free-roaming cats in central Mexico [published online April 3, 2013]. PLoS One. 2013;8:e60744.
- Akucewich LH, Philman K, Clark A, et al. Prevalence of ectoparasites in a population of feral cats from north central Florida during the summer. Vet Parasitol. 2002;109:129-139.
- Linardi PM, Gomes AF, Botelho JR, et al. Some ectoparasites of commensal rodents from Huambo, Angola. J Med Entomol. 1994;31:754-756.
- Pfaffenberger GS, Valencia VB. Ectoparasites of sympatric cottontails (Sylvilagus audubonii Nelson) and jack rabbits (Lepus californicus Mearns) from the high plains of eastern New Mexico. J Parasitol. 1988;74:842-846.
- Hubbart JA, Jachowski DS, Eads DA. Seasonal and among-site variation in the occurrence and abundance of fleas on California ground squirrels (Otospermophilus beecheyi). J Vector Ecol. 2011;36:117-123.
- Braae UC, Ngowi HA, Johansen MV. Smallholder pig production: prevalence and risk factors of ectoparasites. Vet Parasitol. 2013;196:241-244.
- Carlson JC, Fox MS. A sticktight flea removed from the cheek of a two-year-old boy from Los Angeles. Dermatol Online J. 2009;15:4.
- Rothschild M, Schlein Y, Parker K, et al. The flying leap of the flea. Scientific American. 1973;229:92.
- Gyimesi ZS, Hayden ER, Greiner EC. Sticktight flea (Echidnophaga gallinacea) infestation in a Victoria crowned pigeon (Goura victoria). J Zoo Wildl Med. 2007;38:594-596.
- Kalkofen UP, Greenberg J. Echidnophaga gallinacea infestation in dogs. J Am Vet Med Assoc. 1974;165:447-448.
- Harman DW, Halliwell RE, Greiner EC. Flea species from dogs and cats in north-central Florida. Vet Parasitol. 1987;23:135-140.
- Boughton RK, Atwell JW, Schoech SJ. An introduced generalist parasite, the sticktight flea (Echidnophaga gallinacea), and its pathology in the threatened Florida scrub-jay (Aphelocoma coerulescens). J Parasitol. 2006;92:941-948.
- Durden LA, Judy TN, Martin JE, et al. Fleas parasitizing domestic dogs in Georgia, USA: species composition and seasonal abundance. Vet Parasitol. 2005;130:157-162.
- Rautenbach GH, Boomker J, de Villiers IL. A descriptive study of the canine population in a rural town in southern Africa. J S Afr Vet Assoc. 1991;62:158-162.
- Leulmi H, Socolovschi C, Laudisoit A, et al. Detection of Rickettsia felis, Rickettsia typhi, Bartonella species and Yersinia pestis in fleas (Siphonaptera) from Africa. PLoS Negl Trop Dis. 2014;8:e3152.
- Guernier V, Lagadec E, LeMinter G, et al. Fleas of small mammals on Reunion Island: diversity, distribution and epidemiological consequences. PLoS Negl Trop Dis. 2014;8:e3129.
- Cantó GJ, Guerrero RI, Olvera-Ramírez AM, et al. Prevalence of fleas and gastrointestinal parasites in free-roaming cats in central Mexico [published online April 3, 2013]. PLoS One. 2013;8:e60744.
- Akucewich LH, Philman K, Clark A, et al. Prevalence of ectoparasites in a population of feral cats from north central Florida during the summer. Vet Parasitol. 2002;109:129-139.
- Linardi PM, Gomes AF, Botelho JR, et al. Some ectoparasites of commensal rodents from Huambo, Angola. J Med Entomol. 1994;31:754-756.
- Pfaffenberger GS, Valencia VB. Ectoparasites of sympatric cottontails (Sylvilagus audubonii Nelson) and jack rabbits (Lepus californicus Mearns) from the high plains of eastern New Mexico. J Parasitol. 1988;74:842-846.
- Hubbart JA, Jachowski DS, Eads DA. Seasonal and among-site variation in the occurrence and abundance of fleas on California ground squirrels (Otospermophilus beecheyi). J Vector Ecol. 2011;36:117-123.
- Braae UC, Ngowi HA, Johansen MV. Smallholder pig production: prevalence and risk factors of ectoparasites. Vet Parasitol. 2013;196:241-244.
- Carlson JC, Fox MS. A sticktight flea removed from the cheek of a two-year-old boy from Los Angeles. Dermatol Online J. 2009;15:4.
- Rothschild M, Schlein Y, Parker K, et al. The flying leap of the flea. Scientific American. 1973;229:92.
Practice Points
- Although the primary host of the sticktight flea is poultry, it has been found in many species of birds and mammals, including humans.
- Flea bites cause irritation and itching for hosts, typically resulting in clusters of firm, pruritic, erythematous papules with a central punctum.
- Removal of attached fleas in humans requires grasping the flea firmly with tweezers and pulling from the skin.