User login
Scabies is caused by the mite Sarcoptes scabiei var hominis.1 It is in the arthropod class Arachnida, subclass Acari, and family Sarcoptidae.2 Historically, scabies was first described in the Old Testament and by Aristotle,2 but the causative organism was not identified until 1687 using a light microscope.3 Scabies affects all age groups, races, and social classes and is globally widespread. It is most prevalent in developing tropical countries.1 It is estimated that 300 million individuals worldwide are infested with scabies mites annually, with the highest burden in young children.4-7 In industrialized societies, infections often are seen in young adults and in institutional settings such as nursing homes.8 Scabies disproportionately impacts impoverished communities with crowded living conditions, poor hygiene and nutrition, and substandard housing.5,9 Controlling the spread of the disease in these communities presents challenges but is important because of the connection between scabies and chronic kidney disease.10 As such, scabies represents a major health problem in the developing world and has been the focus of major health initiatives.1,11
Identifying Characteristics
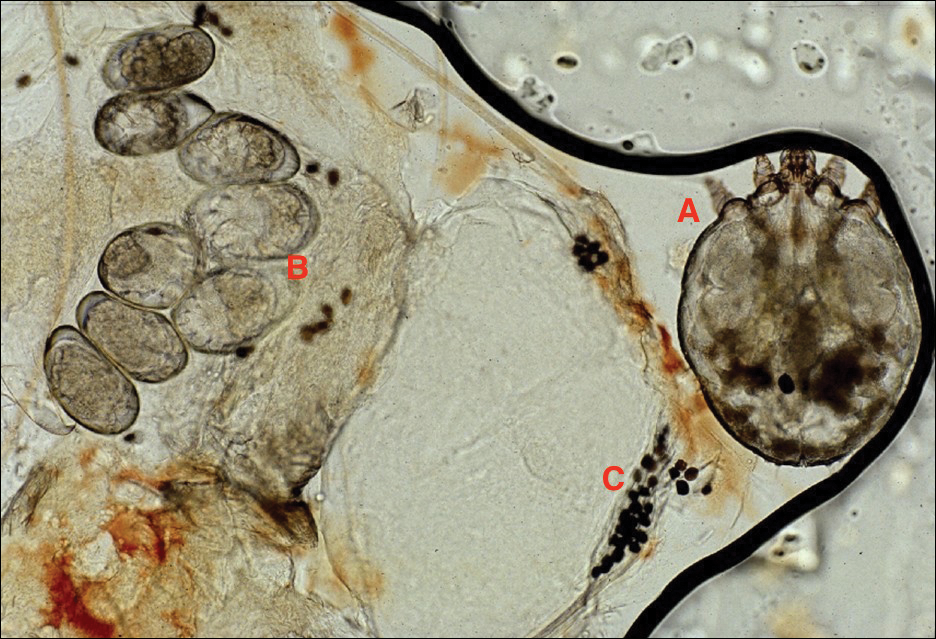
Adult females are 0.4-mm long and 0.3-mm wide, with males being smaller. Adult nymphs have 8 legs and larvae have 6 legs. Scabies mites are distinguishable from other arachnids by the position of a distinct gnathosoma and the lack of a division between the abdomen and cephalothorax.12 They are ovoid with a small anterior cephalic and caudal thoracoabdominal portion with hairlike projections coming off from the rudimentary legs. They can crawl as fast as 2.5 cm per minute on warm skin.2 The life cycle of the mite begins after mating: the male mite dies, and the female lays up to 3 eggs per day, which hatch in 3 to 4 days,2 in skin burrows within the stratum granulosum.12 Maturation from larva to adult takes 10 to 14 days.12 A female mite can live for 4 to 6 weeks and can produce up to 40 ova (Figure 1).
Disease Transmission
Without a host, mites are able to survive and remain capable of infestation for 24 to 36 hours at 21°C and 40% to 80% relative humidity. Lower temperatures and higher humidity prolong survival, but infectivity decreases the longer they are without a host.13
An adult human with ordinary scabies will have an average of 12 adult female mites on the body surface at a given time.14 However, hundreds of mites can be found in neglected children in underprivileged communities and millions in patients with crusted scabies.13 Transmission of typical scabies requires close direct skin-to-skin contact for 15 to 20 minutes.2,8 Transmission from clothing or fomites are an unlikely source of infestation with the exception of patients who are heavily infested such as in crusted scabies.12 In adults, sexual contact is an important method of transmission,12 and patients with scabies should be screened for other sexually transmitted diseases.8
Clinical Manifestations
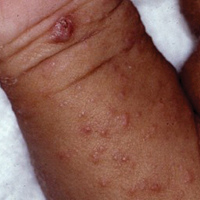
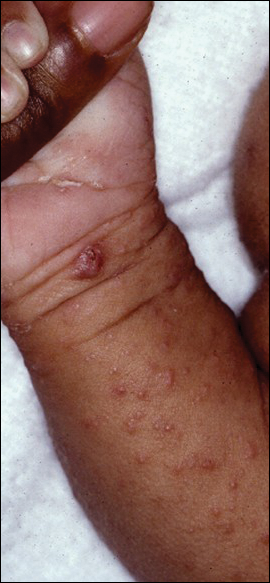
Signs of scabies on the skin include burrows, erythematous papules, and generalized pruritus (Figure 2).12 The scalp, face, and neck frequently are involved in infants and children,2 and the hands, wrists, elbows, genitalia, axillae, umbilicus, belt line, nipples, and buttocks commonly are involved in adults.12 Itching is characteristically worse at night.8 In tropical climates, patients with scabies are predisposed to secondary bacterial skin infections, particularly Streptococcus pyogenes (group A streptococci). The association between scabies and pyoderma caused by group A streptococci has been well established.15,16 Mika et al10 suggested that local complement inhibition plays an important role in the development of pyoderma in scabies-infested skin.
Prevention and Control in the Developing World
Low-cost diagnostic equipment can play a key role in the definitive diagnosis and management of scabies outbreaks in the developing world. Micali et al28 found that a $30 videomicroscope was as effective in scabies diagnosis as a $20,000 videodermatoscope. Because of the low cost of benzyl benzoate, it is commonly used as a first-line drug in many parts of the world,13 whereas permethrin cream 5% is the standard treatment in the developed world.29 Recognition of the role of scabies in patients with pyoderma is key, and one study indicated clinically apparent scabies went unnoticed by physicians in 52% of patients presenting with skin lesions.30 Drug shortages also can contribute to a high prevalence of scabies infestation in the community.31 Mass treatment with ivermectin has proven to be an effective means of reducing the prevalence of many parasitic diseases,1,32,33 and it shows great promise for crusted scabies, institutional outbreaks, and mass administration in highly endemic communites.8 However, there is evidence of ivermectin tolerance among mites, which could undermine the success of mass drug administration.34 Another important consideration is population mobility and the risk for rapid reintroduction of scabies infection across regions.35
Complicating disease control are the socioeconomic factors associated with scabies in the developing world. Families with scabies infestation typically do not own their homes, are less likely to have constant electricity, have a lower monthly income, and live in substandard housing.20 Families can spend a substantial part of their household income on treatment, impacting what they can spend on food.8,11 In addition to medication, control of scabies requires community education and involvement, along with access to primary care and attention to living conditions and environmental factors.34,36
- Romani L, Whitfeld MJ, Koroivueta J, et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med. 2015;373:2305-2313.
- Hicks MI, Elston DM. Scabies. Dermatol Ther. 2009;22:279-292.
- Ramos-e-Silva M. Giovan Cosimo Bonomo (1663-1696): discoverer of the etiology of scabies. Int J Dermatol. 1998;37:625-630.
- Chung SD, Wang KH, Huang CC, et al. Scabies increased the risk of chronic kidney disease: a 5-year follow-up study. J Eur Acad Dermatol Venereol. 2014;28:286-292.
- Wong SS, Poon RW, Chau S, et al. Development of conventional and real-time quantitative PCR assays for diagnosis and monitoring of scabies. J Clin Microbiol. 2015;53:2095-2102.
- Kearns TM, Speare R, Cheng AC, et al. Impact of an ivermectin mass drug administration on scabies prevalence in a remote Australian aboriginal community. PLoS Negl Trop Dis. 2015;9:e0004151.
- Gilmore SJ. Control strategies for endemic childhood scabies. PLoS One. 2011;6:e15990.
- Hay RJ, Steer AC, Engelman D, Walton S. Scabies in the developing world—its prevalence, complications, and management. Clin Microbiol Infect. 2012;18:313-323.
- Hoy WE, White AV, Dowling A, et al. Post-streptococcal glomerulonephritis is a strong risk factor for chronic kidney disease in later life. Kidney Int. 2012;81:1026-1032.
- Mika A, Reynolds SL, Pickering D, et al. Complement inhibitors from scabies mites promote streptococcal growth—a novel mechanism in infected epidermis? PLoS Negl Trop Dis. 2012;6:e1563.
- McLean FE. The elimination of scabies: a task for our generation. Int J Dermatol. 2013;52:1215-1223.
- Hengge UR, Currie BJ, Jäger G, et al. Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis. 2006;6:769-779.
- Heukelbach J, Feldmeier H. Scabies. Lancet. 2006;367:1767-1774.
- Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ. 2005;331:619-622.
- Yeoh DK, Bowen AC, Carapetis JR. Impetigo and scabies—disease burden and modern treatment strategies [published online May 11, 2016]. J Infect. 2016;(72 suppl):S61-S67.
- Bowen AC, Mahé A, Hay RJ, et al. The global epidemiology of impetigo: a systematic review of the population prevalence of impetigo and pyoderma. PLoS One. 2015;10:e0136789.
- Bowen AC, Tong SY, Chatfield MD, et al. The microbiology of impetigo in indigenous children: associations between Streptococcus pyogenes, Staphylococcus aureus, scabies, and nasal carriage. BMC Infect Dis. 2014;14:727.
- Sesso R, Pinto SW. Five-year follow-up of patients with epidemic glomerulonephritis due to Streptococcus zooepidemicus. Nephrol Dial Transplant. 2005;20:1808-1812.
- Singh GR. Glomerulonephritis and managing the risks of chronic renal disease. Pediatr Clin North Am. 2009;56:1363-1382.
- La Vincente S, Kearns T, Connors C, et al. Community management of endemic scabies in remote aboriginal communities of northern Australia: low treatment uptake and high ongoing acquisition. PLoS Negl Trop Dis. 2009;3:e444.
- Clucas DB, Carville KS, Connors C, et al. Disease burden and health-care clinic attendances for young children in remote aboriginal communities of northern Australia. Bull World Health Organ. 2008;86:275-281.
- Stanton B, Khanam S, Nazrul H, et al. Scabies in urban Bangladesh. J Trop Med Hyg. 1987;90:219-226.
- Heukelbach J, de Oliveira FA, Feldmeier H. Ecoparasitoses and public health in Brazil: challenges for control [in Portuguese]. Cad Saude Publica. 2003;19:1535-1540.
- Edison L, Beaudoin A, Goh L, et al. Scabies and bacterial superinfection among American Samoan children, 2011-2012. PLoS One. 2015;10:e0139336.
- Steer AC, Jenney AW, Kado J, et al. High burden of impetigo and scabies in a tropical country. PLoS Negl Trop Dis. 2009;3:e467.
- Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960-967.
- Romani L, Koroivueta J, Steer AC, et al. Scabies and impetigo prevalence and risk factors in Fiji: a national survey. PLoS Negl Trop Dis. 2015;9:e0003452.
- Micali G, Lacarrubba F, Verzì AE, et al. Low-cost equipment for diagnosis and management of endemic scabies outbreaks in underserved populations. Clin Infect Dis. 2015;60:327-329.
- Pasay C, Walton S, Fischer K, et al. PCR-based assay to survey for knockdown resistance to pyrethroid acaricides in human scabies mites (Sarcoptes scabiei var hominis). Am J Trop Med Hyg. 2006;74:649-657.
- Heukelbach J, van Haeff E, Rump B, et al. Parasitic skin diseases: health care-seeking in a slum in north-east Brazil. Trop Med Int Health. 2003;8:368-373.
- Potter EV, Mayon-White R, Poon-King T, et al. Acute glomerulonephritis as a complication of scabies. In: Orkin M, Maibach HI, eds. Cutaneous Infestations and Insect Bites. New York, NY: Marcel Dekker; 1985.
- Mahé A. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689.
- Steer AC, Romani L, Kaldor JM. Mass drug administration for scabies control. N Engl J Med. 2016;374:1690.
- Mounsey KE, Holt DC, McCarthy JS, et al. Longitudinal evidence of increasing in vitro tolerance of scabies mites to ivermectin in scabies-endemic communities. Arch Dermatol. 2009;145:840-841.
- Currie BJ. Scabies and global control of neglected tropical diseases. N Engl J Med. 2015;373:2371-2372.
- O’Donnell V, Morris S, Ward J. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689-1690.
Scabies is caused by the mite Sarcoptes scabiei var hominis.1 It is in the arthropod class Arachnida, subclass Acari, and family Sarcoptidae.2 Historically, scabies was first described in the Old Testament and by Aristotle,2 but the causative organism was not identified until 1687 using a light microscope.3 Scabies affects all age groups, races, and social classes and is globally widespread. It is most prevalent in developing tropical countries.1 It is estimated that 300 million individuals worldwide are infested with scabies mites annually, with the highest burden in young children.4-7 In industrialized societies, infections often are seen in young adults and in institutional settings such as nursing homes.8 Scabies disproportionately impacts impoverished communities with crowded living conditions, poor hygiene and nutrition, and substandard housing.5,9 Controlling the spread of the disease in these communities presents challenges but is important because of the connection between scabies and chronic kidney disease.10 As such, scabies represents a major health problem in the developing world and has been the focus of major health initiatives.1,11
Identifying Characteristics
Adult females are 0.4-mm long and 0.3-mm wide, with males being smaller. Adult nymphs have 8 legs and larvae have 6 legs. Scabies mites are distinguishable from other arachnids by the position of a distinct gnathosoma and the lack of a division between the abdomen and cephalothorax.12 They are ovoid with a small anterior cephalic and caudal thoracoabdominal portion with hairlike projections coming off from the rudimentary legs. They can crawl as fast as 2.5 cm per minute on warm skin.2 The life cycle of the mite begins after mating: the male mite dies, and the female lays up to 3 eggs per day, which hatch in 3 to 4 days,2 in skin burrows within the stratum granulosum.12 Maturation from larva to adult takes 10 to 14 days.12 A female mite can live for 4 to 6 weeks and can produce up to 40 ova (Figure 1).

Disease Transmission
Without a host, mites are able to survive and remain capable of infestation for 24 to 36 hours at 21°C and 40% to 80% relative humidity. Lower temperatures and higher humidity prolong survival, but infectivity decreases the longer they are without a host.13
An adult human with ordinary scabies will have an average of 12 adult female mites on the body surface at a given time.14 However, hundreds of mites can be found in neglected children in underprivileged communities and millions in patients with crusted scabies.13 Transmission of typical scabies requires close direct skin-to-skin contact for 15 to 20 minutes.2,8 Transmission from clothing or fomites are an unlikely source of infestation with the exception of patients who are heavily infested such as in crusted scabies.12 In adults, sexual contact is an important method of transmission,12 and patients with scabies should be screened for other sexually transmitted diseases.8
Clinical Manifestations
Signs of scabies on the skin include burrows, erythematous papules, and generalized pruritus (Figure 2).12 The scalp, face, and neck frequently are involved in infants and children,2 and the hands, wrists, elbows, genitalia, axillae, umbilicus, belt line, nipples, and buttocks commonly are involved in adults.12 Itching is characteristically worse at night.8 In tropical climates, patients with scabies are predisposed to secondary bacterial skin infections, particularly Streptococcus pyogenes (group A streptococci). The association between scabies and pyoderma caused by group A streptococci has been well established.15,16 Mika et al10 suggested that local complement inhibition plays an important role in the development of pyoderma in scabies-infested skin.

Prevention and Control in the Developing World
Low-cost diagnostic equipment can play a key role in the definitive diagnosis and management of scabies outbreaks in the developing world. Micali et al28 found that a $30 videomicroscope was as effective in scabies diagnosis as a $20,000 videodermatoscope. Because of the low cost of benzyl benzoate, it is commonly used as a first-line drug in many parts of the world,13 whereas permethrin cream 5% is the standard treatment in the developed world.29 Recognition of the role of scabies in patients with pyoderma is key, and one study indicated clinically apparent scabies went unnoticed by physicians in 52% of patients presenting with skin lesions.30 Drug shortages also can contribute to a high prevalence of scabies infestation in the community.31 Mass treatment with ivermectin has proven to be an effective means of reducing the prevalence of many parasitic diseases,1,32,33 and it shows great promise for crusted scabies, institutional outbreaks, and mass administration in highly endemic communites.8 However, there is evidence of ivermectin tolerance among mites, which could undermine the success of mass drug administration.34 Another important consideration is population mobility and the risk for rapid reintroduction of scabies infection across regions.35
Complicating disease control are the socioeconomic factors associated with scabies in the developing world. Families with scabies infestation typically do not own their homes, are less likely to have constant electricity, have a lower monthly income, and live in substandard housing.20 Families can spend a substantial part of their household income on treatment, impacting what they can spend on food.8,11 In addition to medication, control of scabies requires community education and involvement, along with access to primary care and attention to living conditions and environmental factors.34,36
Scabies is caused by the mite Sarcoptes scabiei var hominis.1 It is in the arthropod class Arachnida, subclass Acari, and family Sarcoptidae.2 Historically, scabies was first described in the Old Testament and by Aristotle,2 but the causative organism was not identified until 1687 using a light microscope.3 Scabies affects all age groups, races, and social classes and is globally widespread. It is most prevalent in developing tropical countries.1 It is estimated that 300 million individuals worldwide are infested with scabies mites annually, with the highest burden in young children.4-7 In industrialized societies, infections often are seen in young adults and in institutional settings such as nursing homes.8 Scabies disproportionately impacts impoverished communities with crowded living conditions, poor hygiene and nutrition, and substandard housing.5,9 Controlling the spread of the disease in these communities presents challenges but is important because of the connection between scabies and chronic kidney disease.10 As such, scabies represents a major health problem in the developing world and has been the focus of major health initiatives.1,11
Identifying Characteristics
Adult females are 0.4-mm long and 0.3-mm wide, with males being smaller. Adult nymphs have 8 legs and larvae have 6 legs. Scabies mites are distinguishable from other arachnids by the position of a distinct gnathosoma and the lack of a division between the abdomen and cephalothorax.12 They are ovoid with a small anterior cephalic and caudal thoracoabdominal portion with hairlike projections coming off from the rudimentary legs. They can crawl as fast as 2.5 cm per minute on warm skin.2 The life cycle of the mite begins after mating: the male mite dies, and the female lays up to 3 eggs per day, which hatch in 3 to 4 days,2 in skin burrows within the stratum granulosum.12 Maturation from larva to adult takes 10 to 14 days.12 A female mite can live for 4 to 6 weeks and can produce up to 40 ova (Figure 1).

Disease Transmission
Without a host, mites are able to survive and remain capable of infestation for 24 to 36 hours at 21°C and 40% to 80% relative humidity. Lower temperatures and higher humidity prolong survival, but infectivity decreases the longer they are without a host.13
An adult human with ordinary scabies will have an average of 12 adult female mites on the body surface at a given time.14 However, hundreds of mites can be found in neglected children in underprivileged communities and millions in patients with crusted scabies.13 Transmission of typical scabies requires close direct skin-to-skin contact for 15 to 20 minutes.2,8 Transmission from clothing or fomites are an unlikely source of infestation with the exception of patients who are heavily infested such as in crusted scabies.12 In adults, sexual contact is an important method of transmission,12 and patients with scabies should be screened for other sexually transmitted diseases.8
Clinical Manifestations
Signs of scabies on the skin include burrows, erythematous papules, and generalized pruritus (Figure 2).12 The scalp, face, and neck frequently are involved in infants and children,2 and the hands, wrists, elbows, genitalia, axillae, umbilicus, belt line, nipples, and buttocks commonly are involved in adults.12 Itching is characteristically worse at night.8 In tropical climates, patients with scabies are predisposed to secondary bacterial skin infections, particularly Streptococcus pyogenes (group A streptococci). The association between scabies and pyoderma caused by group A streptococci has been well established.15,16 Mika et al10 suggested that local complement inhibition plays an important role in the development of pyoderma in scabies-infested skin.

Prevention and Control in the Developing World
Low-cost diagnostic equipment can play a key role in the definitive diagnosis and management of scabies outbreaks in the developing world. Micali et al28 found that a $30 videomicroscope was as effective in scabies diagnosis as a $20,000 videodermatoscope. Because of the low cost of benzyl benzoate, it is commonly used as a first-line drug in many parts of the world,13 whereas permethrin cream 5% is the standard treatment in the developed world.29 Recognition of the role of scabies in patients with pyoderma is key, and one study indicated clinically apparent scabies went unnoticed by physicians in 52% of patients presenting with skin lesions.30 Drug shortages also can contribute to a high prevalence of scabies infestation in the community.31 Mass treatment with ivermectin has proven to be an effective means of reducing the prevalence of many parasitic diseases,1,32,33 and it shows great promise for crusted scabies, institutional outbreaks, and mass administration in highly endemic communites.8 However, there is evidence of ivermectin tolerance among mites, which could undermine the success of mass drug administration.34 Another important consideration is population mobility and the risk for rapid reintroduction of scabies infection across regions.35
Complicating disease control are the socioeconomic factors associated with scabies in the developing world. Families with scabies infestation typically do not own their homes, are less likely to have constant electricity, have a lower monthly income, and live in substandard housing.20 Families can spend a substantial part of their household income on treatment, impacting what they can spend on food.8,11 In addition to medication, control of scabies requires community education and involvement, along with access to primary care and attention to living conditions and environmental factors.34,36
- Romani L, Whitfeld MJ, Koroivueta J, et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med. 2015;373:2305-2313.
- Hicks MI, Elston DM. Scabies. Dermatol Ther. 2009;22:279-292.
- Ramos-e-Silva M. Giovan Cosimo Bonomo (1663-1696): discoverer of the etiology of scabies. Int J Dermatol. 1998;37:625-630.
- Chung SD, Wang KH, Huang CC, et al. Scabies increased the risk of chronic kidney disease: a 5-year follow-up study. J Eur Acad Dermatol Venereol. 2014;28:286-292.
- Wong SS, Poon RW, Chau S, et al. Development of conventional and real-time quantitative PCR assays for diagnosis and monitoring of scabies. J Clin Microbiol. 2015;53:2095-2102.
- Kearns TM, Speare R, Cheng AC, et al. Impact of an ivermectin mass drug administration on scabies prevalence in a remote Australian aboriginal community. PLoS Negl Trop Dis. 2015;9:e0004151.
- Gilmore SJ. Control strategies for endemic childhood scabies. PLoS One. 2011;6:e15990.
- Hay RJ, Steer AC, Engelman D, Walton S. Scabies in the developing world—its prevalence, complications, and management. Clin Microbiol Infect. 2012;18:313-323.
- Hoy WE, White AV, Dowling A, et al. Post-streptococcal glomerulonephritis is a strong risk factor for chronic kidney disease in later life. Kidney Int. 2012;81:1026-1032.
- Mika A, Reynolds SL, Pickering D, et al. Complement inhibitors from scabies mites promote streptococcal growth—a novel mechanism in infected epidermis? PLoS Negl Trop Dis. 2012;6:e1563.
- McLean FE. The elimination of scabies: a task for our generation. Int J Dermatol. 2013;52:1215-1223.
- Hengge UR, Currie BJ, Jäger G, et al. Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis. 2006;6:769-779.
- Heukelbach J, Feldmeier H. Scabies. Lancet. 2006;367:1767-1774.
- Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ. 2005;331:619-622.
- Yeoh DK, Bowen AC, Carapetis JR. Impetigo and scabies—disease burden and modern treatment strategies [published online May 11, 2016]. J Infect. 2016;(72 suppl):S61-S67.
- Bowen AC, Mahé A, Hay RJ, et al. The global epidemiology of impetigo: a systematic review of the population prevalence of impetigo and pyoderma. PLoS One. 2015;10:e0136789.
- Bowen AC, Tong SY, Chatfield MD, et al. The microbiology of impetigo in indigenous children: associations between Streptococcus pyogenes, Staphylococcus aureus, scabies, and nasal carriage. BMC Infect Dis. 2014;14:727.
- Sesso R, Pinto SW. Five-year follow-up of patients with epidemic glomerulonephritis due to Streptococcus zooepidemicus. Nephrol Dial Transplant. 2005;20:1808-1812.
- Singh GR. Glomerulonephritis and managing the risks of chronic renal disease. Pediatr Clin North Am. 2009;56:1363-1382.
- La Vincente S, Kearns T, Connors C, et al. Community management of endemic scabies in remote aboriginal communities of northern Australia: low treatment uptake and high ongoing acquisition. PLoS Negl Trop Dis. 2009;3:e444.
- Clucas DB, Carville KS, Connors C, et al. Disease burden and health-care clinic attendances for young children in remote aboriginal communities of northern Australia. Bull World Health Organ. 2008;86:275-281.
- Stanton B, Khanam S, Nazrul H, et al. Scabies in urban Bangladesh. J Trop Med Hyg. 1987;90:219-226.
- Heukelbach J, de Oliveira FA, Feldmeier H. Ecoparasitoses and public health in Brazil: challenges for control [in Portuguese]. Cad Saude Publica. 2003;19:1535-1540.
- Edison L, Beaudoin A, Goh L, et al. Scabies and bacterial superinfection among American Samoan children, 2011-2012. PLoS One. 2015;10:e0139336.
- Steer AC, Jenney AW, Kado J, et al. High burden of impetigo and scabies in a tropical country. PLoS Negl Trop Dis. 2009;3:e467.
- Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960-967.
- Romani L, Koroivueta J, Steer AC, et al. Scabies and impetigo prevalence and risk factors in Fiji: a national survey. PLoS Negl Trop Dis. 2015;9:e0003452.
- Micali G, Lacarrubba F, Verzì AE, et al. Low-cost equipment for diagnosis and management of endemic scabies outbreaks in underserved populations. Clin Infect Dis. 2015;60:327-329.
- Pasay C, Walton S, Fischer K, et al. PCR-based assay to survey for knockdown resistance to pyrethroid acaricides in human scabies mites (Sarcoptes scabiei var hominis). Am J Trop Med Hyg. 2006;74:649-657.
- Heukelbach J, van Haeff E, Rump B, et al. Parasitic skin diseases: health care-seeking in a slum in north-east Brazil. Trop Med Int Health. 2003;8:368-373.
- Potter EV, Mayon-White R, Poon-King T, et al. Acute glomerulonephritis as a complication of scabies. In: Orkin M, Maibach HI, eds. Cutaneous Infestations and Insect Bites. New York, NY: Marcel Dekker; 1985.
- Mahé A. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689.
- Steer AC, Romani L, Kaldor JM. Mass drug administration for scabies control. N Engl J Med. 2016;374:1690.
- Mounsey KE, Holt DC, McCarthy JS, et al. Longitudinal evidence of increasing in vitro tolerance of scabies mites to ivermectin in scabies-endemic communities. Arch Dermatol. 2009;145:840-841.
- Currie BJ. Scabies and global control of neglected tropical diseases. N Engl J Med. 2015;373:2371-2372.
- O’Donnell V, Morris S, Ward J. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689-1690.
- Romani L, Whitfeld MJ, Koroivueta J, et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med. 2015;373:2305-2313.
- Hicks MI, Elston DM. Scabies. Dermatol Ther. 2009;22:279-292.
- Ramos-e-Silva M. Giovan Cosimo Bonomo (1663-1696): discoverer of the etiology of scabies. Int J Dermatol. 1998;37:625-630.
- Chung SD, Wang KH, Huang CC, et al. Scabies increased the risk of chronic kidney disease: a 5-year follow-up study. J Eur Acad Dermatol Venereol. 2014;28:286-292.
- Wong SS, Poon RW, Chau S, et al. Development of conventional and real-time quantitative PCR assays for diagnosis and monitoring of scabies. J Clin Microbiol. 2015;53:2095-2102.
- Kearns TM, Speare R, Cheng AC, et al. Impact of an ivermectin mass drug administration on scabies prevalence in a remote Australian aboriginal community. PLoS Negl Trop Dis. 2015;9:e0004151.
- Gilmore SJ. Control strategies for endemic childhood scabies. PLoS One. 2011;6:e15990.
- Hay RJ, Steer AC, Engelman D, Walton S. Scabies in the developing world—its prevalence, complications, and management. Clin Microbiol Infect. 2012;18:313-323.
- Hoy WE, White AV, Dowling A, et al. Post-streptococcal glomerulonephritis is a strong risk factor for chronic kidney disease in later life. Kidney Int. 2012;81:1026-1032.
- Mika A, Reynolds SL, Pickering D, et al. Complement inhibitors from scabies mites promote streptococcal growth—a novel mechanism in infected epidermis? PLoS Negl Trop Dis. 2012;6:e1563.
- McLean FE. The elimination of scabies: a task for our generation. Int J Dermatol. 2013;52:1215-1223.
- Hengge UR, Currie BJ, Jäger G, et al. Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis. 2006;6:769-779.
- Heukelbach J, Feldmeier H. Scabies. Lancet. 2006;367:1767-1774.
- Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ. 2005;331:619-622.
- Yeoh DK, Bowen AC, Carapetis JR. Impetigo and scabies—disease burden and modern treatment strategies [published online May 11, 2016]. J Infect. 2016;(72 suppl):S61-S67.
- Bowen AC, Mahé A, Hay RJ, et al. The global epidemiology of impetigo: a systematic review of the population prevalence of impetigo and pyoderma. PLoS One. 2015;10:e0136789.
- Bowen AC, Tong SY, Chatfield MD, et al. The microbiology of impetigo in indigenous children: associations between Streptococcus pyogenes, Staphylococcus aureus, scabies, and nasal carriage. BMC Infect Dis. 2014;14:727.
- Sesso R, Pinto SW. Five-year follow-up of patients with epidemic glomerulonephritis due to Streptococcus zooepidemicus. Nephrol Dial Transplant. 2005;20:1808-1812.
- Singh GR. Glomerulonephritis and managing the risks of chronic renal disease. Pediatr Clin North Am. 2009;56:1363-1382.
- La Vincente S, Kearns T, Connors C, et al. Community management of endemic scabies in remote aboriginal communities of northern Australia: low treatment uptake and high ongoing acquisition. PLoS Negl Trop Dis. 2009;3:e444.
- Clucas DB, Carville KS, Connors C, et al. Disease burden and health-care clinic attendances for young children in remote aboriginal communities of northern Australia. Bull World Health Organ. 2008;86:275-281.
- Stanton B, Khanam S, Nazrul H, et al. Scabies in urban Bangladesh. J Trop Med Hyg. 1987;90:219-226.
- Heukelbach J, de Oliveira FA, Feldmeier H. Ecoparasitoses and public health in Brazil: challenges for control [in Portuguese]. Cad Saude Publica. 2003;19:1535-1540.
- Edison L, Beaudoin A, Goh L, et al. Scabies and bacterial superinfection among American Samoan children, 2011-2012. PLoS One. 2015;10:e0139336.
- Steer AC, Jenney AW, Kado J, et al. High burden of impetigo and scabies in a tropical country. PLoS Negl Trop Dis. 2009;3:e467.
- Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960-967.
- Romani L, Koroivueta J, Steer AC, et al. Scabies and impetigo prevalence and risk factors in Fiji: a national survey. PLoS Negl Trop Dis. 2015;9:e0003452.
- Micali G, Lacarrubba F, Verzì AE, et al. Low-cost equipment for diagnosis and management of endemic scabies outbreaks in underserved populations. Clin Infect Dis. 2015;60:327-329.
- Pasay C, Walton S, Fischer K, et al. PCR-based assay to survey for knockdown resistance to pyrethroid acaricides in human scabies mites (Sarcoptes scabiei var hominis). Am J Trop Med Hyg. 2006;74:649-657.
- Heukelbach J, van Haeff E, Rump B, et al. Parasitic skin diseases: health care-seeking in a slum in north-east Brazil. Trop Med Int Health. 2003;8:368-373.
- Potter EV, Mayon-White R, Poon-King T, et al. Acute glomerulonephritis as a complication of scabies. In: Orkin M, Maibach HI, eds. Cutaneous Infestations and Insect Bites. New York, NY: Marcel Dekker; 1985.
- Mahé A. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689.
- Steer AC, Romani L, Kaldor JM. Mass drug administration for scabies control. N Engl J Med. 2016;374:1690.
- Mounsey KE, Holt DC, McCarthy JS, et al. Longitudinal evidence of increasing in vitro tolerance of scabies mites to ivermectin in scabies-endemic communities. Arch Dermatol. 2009;145:840-841.
- Currie BJ. Scabies and global control of neglected tropical diseases. N Engl J Med. 2015;373:2371-2372.
- O’Donnell V, Morris S, Ward J. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689-1690.
Practice Points
- Scabies infestation is one of the world’s leading causes of chronic kidney disease.
- Ivermectin can be used to treat mass infestations, and older topical therapies also are commonly used.