User login
Papulonecrotic Tuberculid Secondary to Mycobacterium avium Complex
To the Editor:
Papulonecrotic tuberculid (PNT) is a cutaneous hypersensitivity reaction to antigenic components of Mycobacterium species, most commonly Mycobacterium tuberculosis. According to a PubMed search of articles indexed for MEDLINE using the terms papulonecrotic tuberculid, Mycobacterium avium complex, and Mycobacterium, only 1 case of PNT secondary to infection with Mycobacterium avium complex (MAC) has been reported.1,2 Papulonecrotic tuberculid classically presents with symmetrical, dusky red papules with necrosis on the extremities.3 Patients may or may not have associated symptoms of fever and weight loss. It is diagnosed through skin biopsy as well as identification of a distant source of mycobacterial infection. Papulonecrotic tuberculid is considered a reactive process to a distant site of mycobacterial infection, and skin lesions contain few, if any, mycobacteria.4
A 65-year-old man was admitted to the hospital for expedited workup of chronic fevers, 20-lb weight loss, and night sweats of 8 months’ duration. He had a medical history of myelodysplastic syndrome and autoimmune hemolytic anemia. During hospitalization, positron emission tomography revealed multilevel vertebral lytic and sclerotic lesions. Subsequent T10 vertebral biopsy showed necrotizing granulomatous inflammation with extensive necrosis and acid-fast bacilli–positive organisms. The patient was empirically started on rifampicin, isoniazid, pyrazinamide, ethambutol, and pyridoxine for presumed M tuberculosis and placed on respiratory isolation.
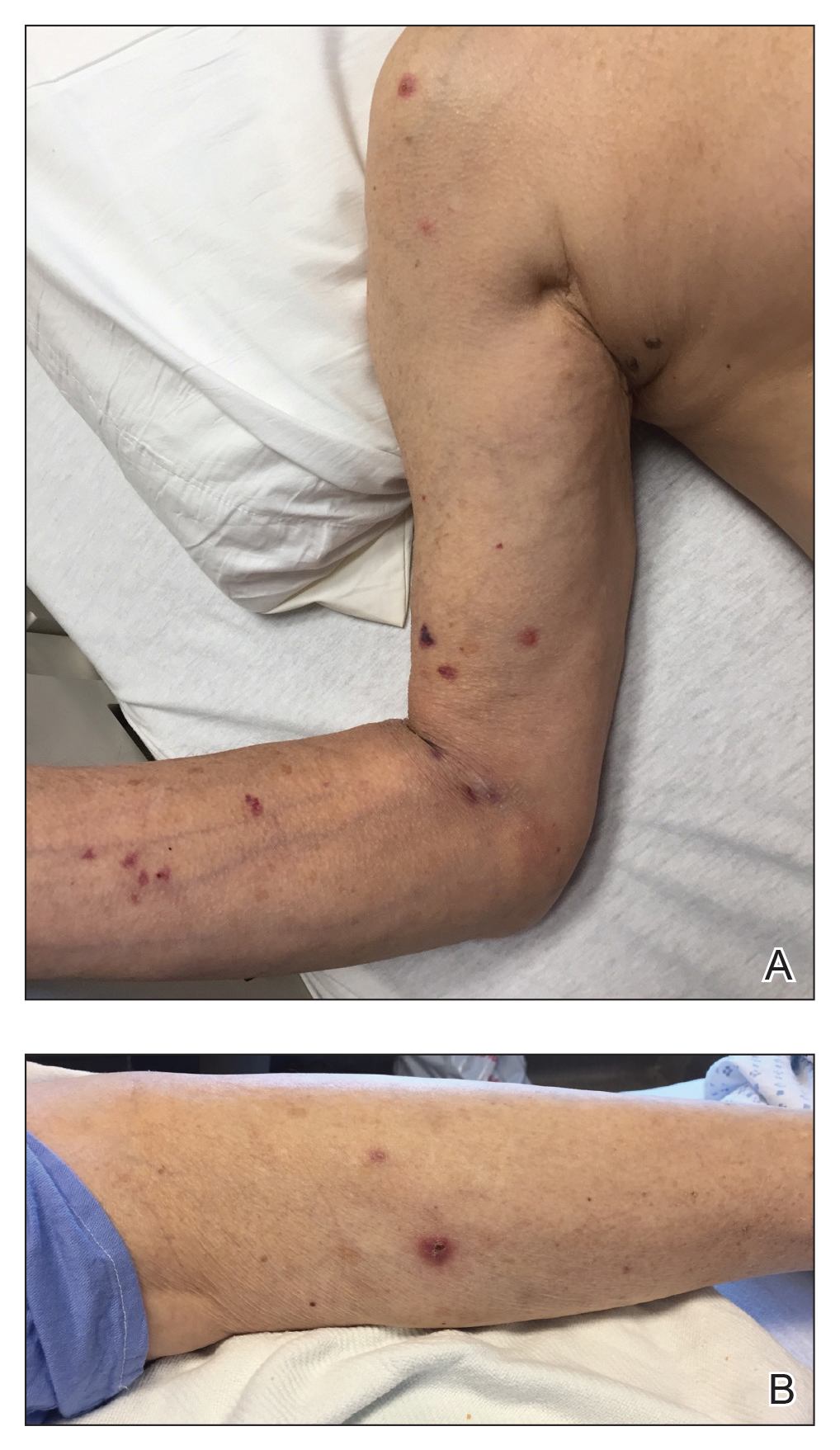
Dermatology was consulted for a recurrent tender rash on the bilateral upper and lower extremities of 5 years’ duration. Physical examination revealed numerous erythematous papulonecrotic lesions in various states of healing on the bilateral upper and lower extremities (Figure 1). Three years prior to the current presentation, 2 lesions were biopsied and demonstrated leukocytoclastic vasculitis with neutrophilic panniculitis and vasculopathy. A presumptive diagnosis of Sweet syndrome was made given the history of myelodysplastic syndrome, though an infectious etiology could not be ruled out at that time. Concurrently, the patient was diagnosed with autoimmune hemolytic anemia and was started on prednisone. Initially, the skin lesions improved with prednisone but never fully resolved; however, as the dosage of oral steroids decreased, the skin lesions worsened and presented in larger numbers with more frequency. The patient was titrated down to prednisone 5 mg daily with no additional treatment of the skin lesions at that time.
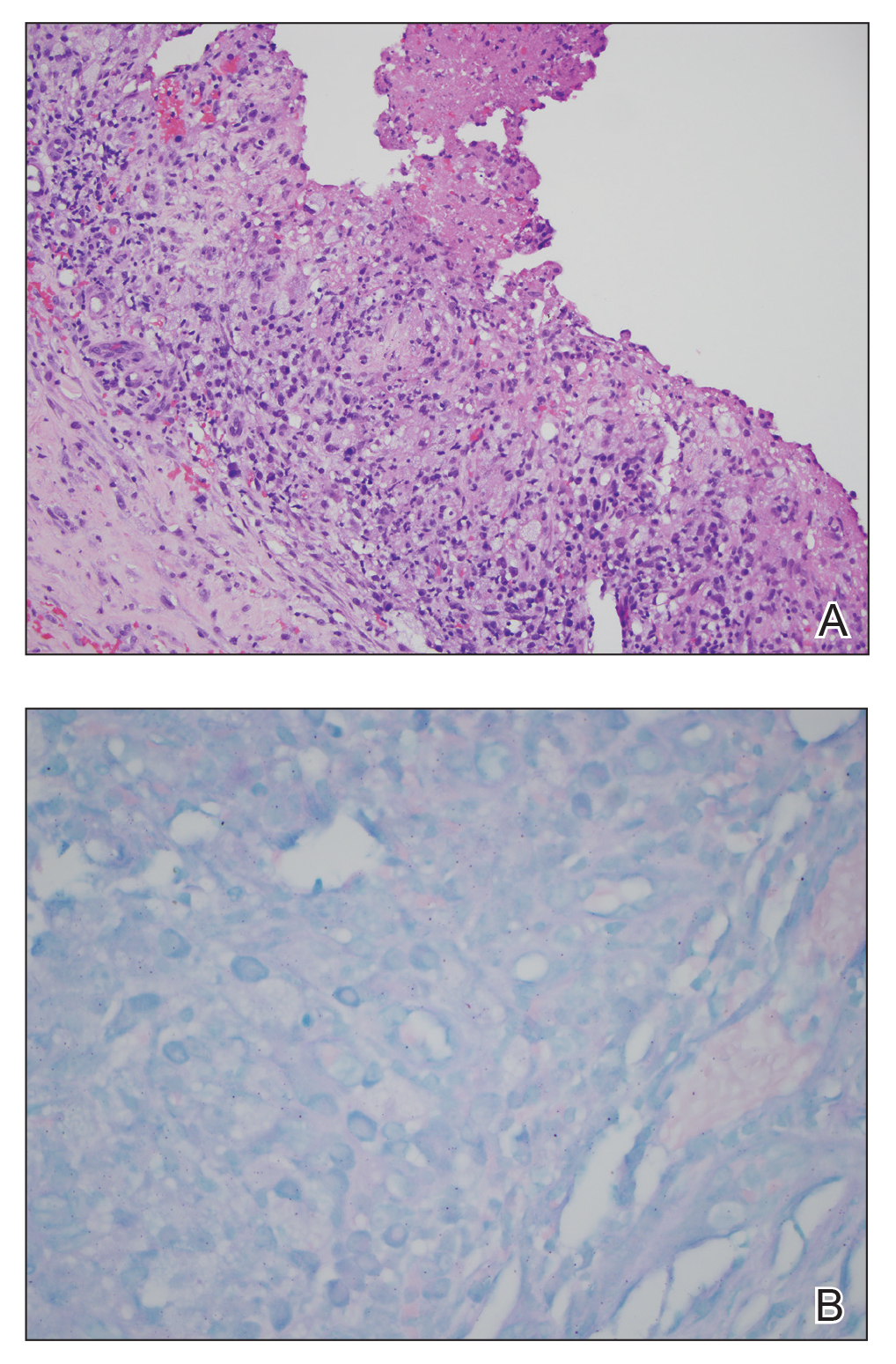
During the current hospitalization, 2 additional biopsies were taken from the arm for routine histopathology and tissue culture. Dermatopathology revealed robust neutrophilic and granulomatous inflammation as well as remarkable necrosis with a few mycobacteria identified on acid-fast and Fite stains (Figure 2). Tissue culture was negative. Additionally, the patient’s spinal biopsy was sent for polymerase chain reaction analysis for Mycobacterium typing, which confirmed MAC. The patient was diagnosed with Pott disease, a mycobacterial infection of the spine, as well as cutaneous papulonecrotic tuberculid secondary to MAC.
Papulonecrotic tuberculid is the rarest form of cutaneous tuberculosis infection and rarely has been reported in connection to MAC.1 This condition is considered a hypersensitivity reaction that occurs in response to antigenic components of mycobacteria.4 Patients with PNT typically present with recurrent crops of painful papulonecrotic lesions distributed on the extremities. Histopathology in PNT classically reveals necrosis, notable inflammatory infiltrate, and lack of observed organisms.5 Diagnosis often is made through skin biopsy, though histopathology varies based on lesion maturity.4 Early lesions often reveal leukocytoclastic vasculitis, whereas late lesions usually demonstrate granulomatous inflammation.4 Mycobacterium avium complex is difficult to culture, as it is a slow-growing, fastidious bacterium and therefore polymerase chain reaction genotyping is useful for bacterial classification.6
Disseminated MAC infection also was on the differential for our patient; however, we felt it was less likely than PNT for several reasons. First, disseminated infection rarely presents with cutaneous involvement and is associated with pulmonary involvement in 90% of cases.7-9 Second, the granuloma formation noted on our patient’s skin biopsy was not typical for disseminated MAC but is well described in cases of PNT.4,8,9 Finally, in the rare cases in which cutaneous involvement has occurred with disseminated mycobacterial infections, skin biopsies typically revealed numerous Mycobacterium organisms.8,10 In contrast, skin lesions associated with PNT usually reveal few, if any, organisms, as was seen with our patient.2
The patient’s initial biopsies also supported a diagnosis of PNT, as early lesions of PNT typically show leukocytoclastic vasculitis. His response to low and high doses of prednisone also fit well with a PNT diagnosis. In fact, a case of PNT secondary to Mycobacterium bovis similarly showed an improvement in the rash with high-dose steroids but progression with lower doses.11 It is possible that our patient’s response to steroids complicated the diagnosis of his rash.
The treatment of PNT is clearance of the underlying infection. Macrolide antibiotics, such as clarithromycin and azithromycin, have the best efficacy against MAC, in combination with ethambutol and/or rifabutin.6,12 Treatment duration should be 1 year. Amikacin or streptomycin may be added to this regimen during early treatment.6 Mycobacterium avium complex is resistant to many antibiotics, including typical antituberculosis drugs, and sensitivities should be identified at the onset of treatment.11,12
Albeit rare, clinicians should be aware of PNT secondary to MAC or other mycobacterial infections. Because this condition is difficult to diagnose with varying histologic findings and often negative tissue cultures, a high index of suspicion is necessary when a patient presents with recurrent papulonecrotic lesions, especially in immunocompromised hosts and patients with exposure to mycobacteria.
- Williams JT, Pulitzer DR, DeVillez RL. Papulonecrotic tuberculid secondary to disseminated Mycobacterium avium complex. Int J Dermatol. 1994;33:109-112.
- Jordaan HF, Schneider JW. Papulonecrotic tuberculid. Int J Dermatol. 1995;34:217-219.
- Scollard DM, Dacso MM, Abad-Venida ML. Tuberculosis and leprosy: classical granulomatous diseases in the twenty-first century. Dermatol Clin. 2015;33:541-562.
- Kim GW, Park HJ, Kim HS, et al. Simultaneous occurrence of papulonecrotic tuberculid and erythema induratum in a patient with pulmonary tuberculosis. Pediatr Dermatol. 2013;30:256-259.
- Spelta K, Diniz LM. Cutaneous tuberculosis: a 26-year retrospective study in an endemic area. Rev Inst Med Trop Sao Paulo. 2016;58:49.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- Dyer J, Weiss J, Steiner WS, et al. Primary cutaneous Mycobacterium avium complex infection following squamous cell carcinoma excision. Cutis. 2016;98:E8-E11.
- Kollipara R, Richards K, Tschen J, et al. Disseminated Mycobacterium avium complex with cutaneous lesions. J Cutan Med Surg. 2016;20:272-274.
- Endly DC, Ackerman LS. Disseminated cutaneous Mycobacterium avium complex in a person with AIDS. Dermatol Online J. 2014;20:22616.
- Li JJ, Beresford R, Fyfe J, et al. Clinical and histopathological features of cutaneous nontuberculous mycobacterial infection: a review of 13 cases. J Cutan Pathol. 2017;44:433-443.
- Iden DL, Rogers RS 3rd, Schroeter AL. Papulonecrotic tuberculid secondary to Mycobacterium bovis. Arch Dermatol. 1978;114:564-566.
- Wong NM, Sun LK, Lau PY. Spinal infection caused by Mycobacterium avium complex in a patient with no acquired immune deficiency syndrome: a case report. J Orthop Surg (Hong Kong). 2008;16:359-363.
To the Editor:
Papulonecrotic tuberculid (PNT) is a cutaneous hypersensitivity reaction to antigenic components of Mycobacterium species, most commonly Mycobacterium tuberculosis. According to a PubMed search of articles indexed for MEDLINE using the terms papulonecrotic tuberculid, Mycobacterium avium complex, and Mycobacterium, only 1 case of PNT secondary to infection with Mycobacterium avium complex (MAC) has been reported.1,2 Papulonecrotic tuberculid classically presents with symmetrical, dusky red papules with necrosis on the extremities.3 Patients may or may not have associated symptoms of fever and weight loss. It is diagnosed through skin biopsy as well as identification of a distant source of mycobacterial infection. Papulonecrotic tuberculid is considered a reactive process to a distant site of mycobacterial infection, and skin lesions contain few, if any, mycobacteria.4
A 65-year-old man was admitted to the hospital for expedited workup of chronic fevers, 20-lb weight loss, and night sweats of 8 months’ duration. He had a medical history of myelodysplastic syndrome and autoimmune hemolytic anemia. During hospitalization, positron emission tomography revealed multilevel vertebral lytic and sclerotic lesions. Subsequent T10 vertebral biopsy showed necrotizing granulomatous inflammation with extensive necrosis and acid-fast bacilli–positive organisms. The patient was empirically started on rifampicin, isoniazid, pyrazinamide, ethambutol, and pyridoxine for presumed M tuberculosis and placed on respiratory isolation.
Dermatology was consulted for a recurrent tender rash on the bilateral upper and lower extremities of 5 years’ duration. Physical examination revealed numerous erythematous papulonecrotic lesions in various states of healing on the bilateral upper and lower extremities (Figure 1). Three years prior to the current presentation, 2 lesions were biopsied and demonstrated leukocytoclastic vasculitis with neutrophilic panniculitis and vasculopathy. A presumptive diagnosis of Sweet syndrome was made given the history of myelodysplastic syndrome, though an infectious etiology could not be ruled out at that time. Concurrently, the patient was diagnosed with autoimmune hemolytic anemia and was started on prednisone. Initially, the skin lesions improved with prednisone but never fully resolved; however, as the dosage of oral steroids decreased, the skin lesions worsened and presented in larger numbers with more frequency. The patient was titrated down to prednisone 5 mg daily with no additional treatment of the skin lesions at that time.
During the current hospitalization, 2 additional biopsies were taken from the arm for routine histopathology and tissue culture. Dermatopathology revealed robust neutrophilic and granulomatous inflammation as well as remarkable necrosis with a few mycobacteria identified on acid-fast and Fite stains (Figure 2). Tissue culture was negative. Additionally, the patient’s spinal biopsy was sent for polymerase chain reaction analysis for Mycobacterium typing, which confirmed MAC. The patient was diagnosed with Pott disease, a mycobacterial infection of the spine, as well as cutaneous papulonecrotic tuberculid secondary to MAC.
Papulonecrotic tuberculid is the rarest form of cutaneous tuberculosis infection and rarely has been reported in connection to MAC.1 This condition is considered a hypersensitivity reaction that occurs in response to antigenic components of mycobacteria.4 Patients with PNT typically present with recurrent crops of painful papulonecrotic lesions distributed on the extremities. Histopathology in PNT classically reveals necrosis, notable inflammatory infiltrate, and lack of observed organisms.5 Diagnosis often is made through skin biopsy, though histopathology varies based on lesion maturity.4 Early lesions often reveal leukocytoclastic vasculitis, whereas late lesions usually demonstrate granulomatous inflammation.4 Mycobacterium avium complex is difficult to culture, as it is a slow-growing, fastidious bacterium and therefore polymerase chain reaction genotyping is useful for bacterial classification.6
Disseminated MAC infection also was on the differential for our patient; however, we felt it was less likely than PNT for several reasons. First, disseminated infection rarely presents with cutaneous involvement and is associated with pulmonary involvement in 90% of cases.7-9 Second, the granuloma formation noted on our patient’s skin biopsy was not typical for disseminated MAC but is well described in cases of PNT.4,8,9 Finally, in the rare cases in which cutaneous involvement has occurred with disseminated mycobacterial infections, skin biopsies typically revealed numerous Mycobacterium organisms.8,10 In contrast, skin lesions associated with PNT usually reveal few, if any, organisms, as was seen with our patient.2
The patient’s initial biopsies also supported a diagnosis of PNT, as early lesions of PNT typically show leukocytoclastic vasculitis. His response to low and high doses of prednisone also fit well with a PNT diagnosis. In fact, a case of PNT secondary to Mycobacterium bovis similarly showed an improvement in the rash with high-dose steroids but progression with lower doses.11 It is possible that our patient’s response to steroids complicated the diagnosis of his rash.
The treatment of PNT is clearance of the underlying infection. Macrolide antibiotics, such as clarithromycin and azithromycin, have the best efficacy against MAC, in combination with ethambutol and/or rifabutin.6,12 Treatment duration should be 1 year. Amikacin or streptomycin may be added to this regimen during early treatment.6 Mycobacterium avium complex is resistant to many antibiotics, including typical antituberculosis drugs, and sensitivities should be identified at the onset of treatment.11,12
Albeit rare, clinicians should be aware of PNT secondary to MAC or other mycobacterial infections. Because this condition is difficult to diagnose with varying histologic findings and often negative tissue cultures, a high index of suspicion is necessary when a patient presents with recurrent papulonecrotic lesions, especially in immunocompromised hosts and patients with exposure to mycobacteria.
To the Editor:
Papulonecrotic tuberculid (PNT) is a cutaneous hypersensitivity reaction to antigenic components of Mycobacterium species, most commonly Mycobacterium tuberculosis. According to a PubMed search of articles indexed for MEDLINE using the terms papulonecrotic tuberculid, Mycobacterium avium complex, and Mycobacterium, only 1 case of PNT secondary to infection with Mycobacterium avium complex (MAC) has been reported.1,2 Papulonecrotic tuberculid classically presents with symmetrical, dusky red papules with necrosis on the extremities.3 Patients may or may not have associated symptoms of fever and weight loss. It is diagnosed through skin biopsy as well as identification of a distant source of mycobacterial infection. Papulonecrotic tuberculid is considered a reactive process to a distant site of mycobacterial infection, and skin lesions contain few, if any, mycobacteria.4
A 65-year-old man was admitted to the hospital for expedited workup of chronic fevers, 20-lb weight loss, and night sweats of 8 months’ duration. He had a medical history of myelodysplastic syndrome and autoimmune hemolytic anemia. During hospitalization, positron emission tomography revealed multilevel vertebral lytic and sclerotic lesions. Subsequent T10 vertebral biopsy showed necrotizing granulomatous inflammation with extensive necrosis and acid-fast bacilli–positive organisms. The patient was empirically started on rifampicin, isoniazid, pyrazinamide, ethambutol, and pyridoxine for presumed M tuberculosis and placed on respiratory isolation.
Dermatology was consulted for a recurrent tender rash on the bilateral upper and lower extremities of 5 years’ duration. Physical examination revealed numerous erythematous papulonecrotic lesions in various states of healing on the bilateral upper and lower extremities (Figure 1). Three years prior to the current presentation, 2 lesions were biopsied and demonstrated leukocytoclastic vasculitis with neutrophilic panniculitis and vasculopathy. A presumptive diagnosis of Sweet syndrome was made given the history of myelodysplastic syndrome, though an infectious etiology could not be ruled out at that time. Concurrently, the patient was diagnosed with autoimmune hemolytic anemia and was started on prednisone. Initially, the skin lesions improved with prednisone but never fully resolved; however, as the dosage of oral steroids decreased, the skin lesions worsened and presented in larger numbers with more frequency. The patient was titrated down to prednisone 5 mg daily with no additional treatment of the skin lesions at that time.
During the current hospitalization, 2 additional biopsies were taken from the arm for routine histopathology and tissue culture. Dermatopathology revealed robust neutrophilic and granulomatous inflammation as well as remarkable necrosis with a few mycobacteria identified on acid-fast and Fite stains (Figure 2). Tissue culture was negative. Additionally, the patient’s spinal biopsy was sent for polymerase chain reaction analysis for Mycobacterium typing, which confirmed MAC. The patient was diagnosed with Pott disease, a mycobacterial infection of the spine, as well as cutaneous papulonecrotic tuberculid secondary to MAC.
Papulonecrotic tuberculid is the rarest form of cutaneous tuberculosis infection and rarely has been reported in connection to MAC.1 This condition is considered a hypersensitivity reaction that occurs in response to antigenic components of mycobacteria.4 Patients with PNT typically present with recurrent crops of painful papulonecrotic lesions distributed on the extremities. Histopathology in PNT classically reveals necrosis, notable inflammatory infiltrate, and lack of observed organisms.5 Diagnosis often is made through skin biopsy, though histopathology varies based on lesion maturity.4 Early lesions often reveal leukocytoclastic vasculitis, whereas late lesions usually demonstrate granulomatous inflammation.4 Mycobacterium avium complex is difficult to culture, as it is a slow-growing, fastidious bacterium and therefore polymerase chain reaction genotyping is useful for bacterial classification.6
Disseminated MAC infection also was on the differential for our patient; however, we felt it was less likely than PNT for several reasons. First, disseminated infection rarely presents with cutaneous involvement and is associated with pulmonary involvement in 90% of cases.7-9 Second, the granuloma formation noted on our patient’s skin biopsy was not typical for disseminated MAC but is well described in cases of PNT.4,8,9 Finally, in the rare cases in which cutaneous involvement has occurred with disseminated mycobacterial infections, skin biopsies typically revealed numerous Mycobacterium organisms.8,10 In contrast, skin lesions associated with PNT usually reveal few, if any, organisms, as was seen with our patient.2
The patient’s initial biopsies also supported a diagnosis of PNT, as early lesions of PNT typically show leukocytoclastic vasculitis. His response to low and high doses of prednisone also fit well with a PNT diagnosis. In fact, a case of PNT secondary to Mycobacterium bovis similarly showed an improvement in the rash with high-dose steroids but progression with lower doses.11 It is possible that our patient’s response to steroids complicated the diagnosis of his rash.
The treatment of PNT is clearance of the underlying infection. Macrolide antibiotics, such as clarithromycin and azithromycin, have the best efficacy against MAC, in combination with ethambutol and/or rifabutin.6,12 Treatment duration should be 1 year. Amikacin or streptomycin may be added to this regimen during early treatment.6 Mycobacterium avium complex is resistant to many antibiotics, including typical antituberculosis drugs, and sensitivities should be identified at the onset of treatment.11,12
Albeit rare, clinicians should be aware of PNT secondary to MAC or other mycobacterial infections. Because this condition is difficult to diagnose with varying histologic findings and often negative tissue cultures, a high index of suspicion is necessary when a patient presents with recurrent papulonecrotic lesions, especially in immunocompromised hosts and patients with exposure to mycobacteria.
- Williams JT, Pulitzer DR, DeVillez RL. Papulonecrotic tuberculid secondary to disseminated Mycobacterium avium complex. Int J Dermatol. 1994;33:109-112.
- Jordaan HF, Schneider JW. Papulonecrotic tuberculid. Int J Dermatol. 1995;34:217-219.
- Scollard DM, Dacso MM, Abad-Venida ML. Tuberculosis and leprosy: classical granulomatous diseases in the twenty-first century. Dermatol Clin. 2015;33:541-562.
- Kim GW, Park HJ, Kim HS, et al. Simultaneous occurrence of papulonecrotic tuberculid and erythema induratum in a patient with pulmonary tuberculosis. Pediatr Dermatol. 2013;30:256-259.
- Spelta K, Diniz LM. Cutaneous tuberculosis: a 26-year retrospective study in an endemic area. Rev Inst Med Trop Sao Paulo. 2016;58:49.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- Dyer J, Weiss J, Steiner WS, et al. Primary cutaneous Mycobacterium avium complex infection following squamous cell carcinoma excision. Cutis. 2016;98:E8-E11.
- Kollipara R, Richards K, Tschen J, et al. Disseminated Mycobacterium avium complex with cutaneous lesions. J Cutan Med Surg. 2016;20:272-274.
- Endly DC, Ackerman LS. Disseminated cutaneous Mycobacterium avium complex in a person with AIDS. Dermatol Online J. 2014;20:22616.
- Li JJ, Beresford R, Fyfe J, et al. Clinical and histopathological features of cutaneous nontuberculous mycobacterial infection: a review of 13 cases. J Cutan Pathol. 2017;44:433-443.
- Iden DL, Rogers RS 3rd, Schroeter AL. Papulonecrotic tuberculid secondary to Mycobacterium bovis. Arch Dermatol. 1978;114:564-566.
- Wong NM, Sun LK, Lau PY. Spinal infection caused by Mycobacterium avium complex in a patient with no acquired immune deficiency syndrome: a case report. J Orthop Surg (Hong Kong). 2008;16:359-363.
- Williams JT, Pulitzer DR, DeVillez RL. Papulonecrotic tuberculid secondary to disseminated Mycobacterium avium complex. Int J Dermatol. 1994;33:109-112.
- Jordaan HF, Schneider JW. Papulonecrotic tuberculid. Int J Dermatol. 1995;34:217-219.
- Scollard DM, Dacso MM, Abad-Venida ML. Tuberculosis and leprosy: classical granulomatous diseases in the twenty-first century. Dermatol Clin. 2015;33:541-562.
- Kim GW, Park HJ, Kim HS, et al. Simultaneous occurrence of papulonecrotic tuberculid and erythema induratum in a patient with pulmonary tuberculosis. Pediatr Dermatol. 2013;30:256-259.
- Spelta K, Diniz LM. Cutaneous tuberculosis: a 26-year retrospective study in an endemic area. Rev Inst Med Trop Sao Paulo. 2016;58:49.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- Dyer J, Weiss J, Steiner WS, et al. Primary cutaneous Mycobacterium avium complex infection following squamous cell carcinoma excision. Cutis. 2016;98:E8-E11.
- Kollipara R, Richards K, Tschen J, et al. Disseminated Mycobacterium avium complex with cutaneous lesions. J Cutan Med Surg. 2016;20:272-274.
- Endly DC, Ackerman LS. Disseminated cutaneous Mycobacterium avium complex in a person with AIDS. Dermatol Online J. 2014;20:22616.
- Li JJ, Beresford R, Fyfe J, et al. Clinical and histopathological features of cutaneous nontuberculous mycobacterial infection: a review of 13 cases. J Cutan Pathol. 2017;44:433-443.
- Iden DL, Rogers RS 3rd, Schroeter AL. Papulonecrotic tuberculid secondary to Mycobacterium bovis. Arch Dermatol. 1978;114:564-566.
- Wong NM, Sun LK, Lau PY. Spinal infection caused by Mycobacterium avium complex in a patient with no acquired immune deficiency syndrome: a case report. J Orthop Surg (Hong Kong). 2008;16:359-363.
Practice Points
- Papulonecrotic tuberculid (PNT) is a hypersensitivity reaction that presents with reddish papules with central necrosis on the extremities.
- Early PNT histopathology shows leukocytoclastic vasculitis. Later lesions demonstrate granulomatous inflammation on histopathology.
- Mycobacterium avium is difficult to culture; therefore, if you suspect it, we recommend polymerase chain reaction genotyping for bacterial classification.