User login
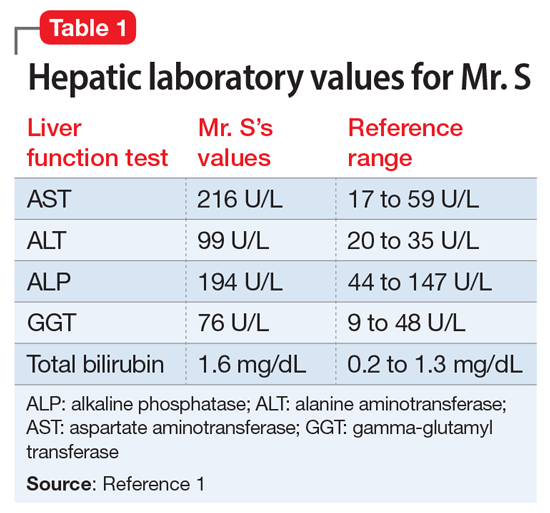
Mr. S, age 64, presents for an outpatient follow-up after a recent hospital discharge for alcohol detoxification. He reports a long history of alcohol use, which has resulted in numerous hospital admissions. He has recently been receiving care from a gastroenterologist because the results of laboratory testing suggested hepatic impairment (Table 1). Mr. S says that a friend of his was able to stop drinking by taking a medication, and he wonders if he can be prescribed a medication to help him as well.
A chart review shows that Mr. S recently underwent paracentesis, during which 6 liters of fluid were removed. Additionally, an abdominal ultrasound confirmed hepatic cirrhosis.
According to the World Health Organization, alcohol consumption contributes to 3 million deaths annually.2 The highest proportion of these deaths (21.3%) is due to alcohol-associated gastrointestinal complications, including alcoholic and infectious hepatitis, pancreatitis, and cirrhosis. Because the liver is the primary site of ethanol metabolism, it sustains the greatest degree of tissue injury with heavy alcohol consumption. Additionally, the association of harmful use of alcohol with risky sexual behavior may partially explain the higher prevalence of viral hepatitis among persons with alcohol use disorder (AUD) compared with the general population. Alcoholic liver disease (ALD) progresses through several stages, beginning with hepatic steatosis and progressing through alcohol-related hepatitis, fibrosis, cirrhosis, and potentially hepatocellular carcinoma.3
Liver markers of alcohol use
Although biological markers can be used in clinical practice to screen and monitor for alcohol abuse, making a diagnosis of ALD can be challenging. Typically, a history of heavy alcohol consumption in addition to certain physical signs and laboratory tests for liver disease are the best indicators of ALD. However, the clinical assessment can be confounded by patients who deny or minimize how much alcohol they have consumed. Furthermore, physical and laboratory findings may not be specific to ALD.
Liver enzymes, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyltransferase (GGT), have historically been used as the basis of diagnosing ALD. In addition to elevated bilirubin and evidence of macrocytic anemia, elevations in these enzymes may suggest heavy alcohol use, but these values alone are inadequate to establish ALD. Gamma-glutamyltransferase is found in cell membranes of several body tissues, including the liver and spleen, and therefore is not specific to liver damage. However, elevated GGT is the best indicator of excessive alcohol consumption because it has greater sensitivity than AST and ALT.1,3,4
Although these biomarkers are helpful in diagnosing ALD, they lose some of their utility in patients with advanced liver disease. Patients with severe liver dysfunction may not have elevated serum aminotransferase levels because the degree of liver enzyme elevation does not correlate well with the severity of ALD. For example, patients with advanced cirrhosis may have liver enzyme levels that appear normal. However, the pattern of elevation in transaminases can be helpful in making a diagnosis of liver dysfunction; using the ratio of AST to ALT may aid in diagnosing ALD, because AST is elevated more than twice that of ALT in >80% of patients with ALD.1,3,4
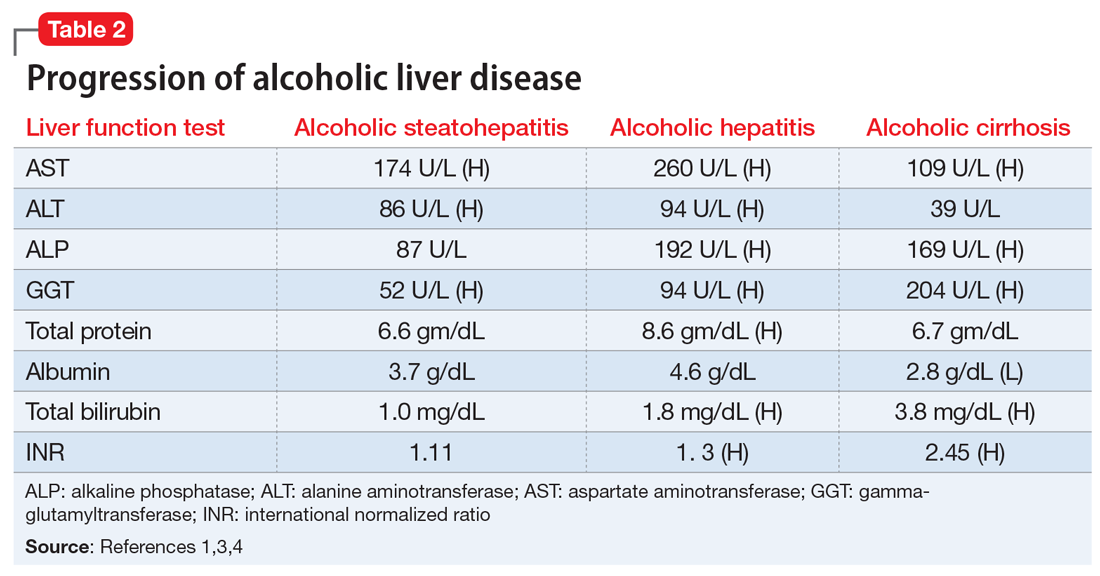
Table 21,3,4 shows the progression of ALD from steatohepatitis to alcoholic hepatitis to cirrhosis. In steatohepatitis, transaminitis is present but all other biomarkers normal. In alcoholic hepatitis, transaminitis is present along with elevated alkaline phosphatase, elevated bilirubin, and elevated international normalized ratio (INR). In alcoholic cirrhosis, the AST-to-ALT ratio is >2, and hypoalbuminemia, hyperbilirubinemia, and coagulopathy (evidenced by elevated INR) are present, consistent with long-term liver damage.1,3,4
Continue to: FDA-approved medications
FDA-approved medications
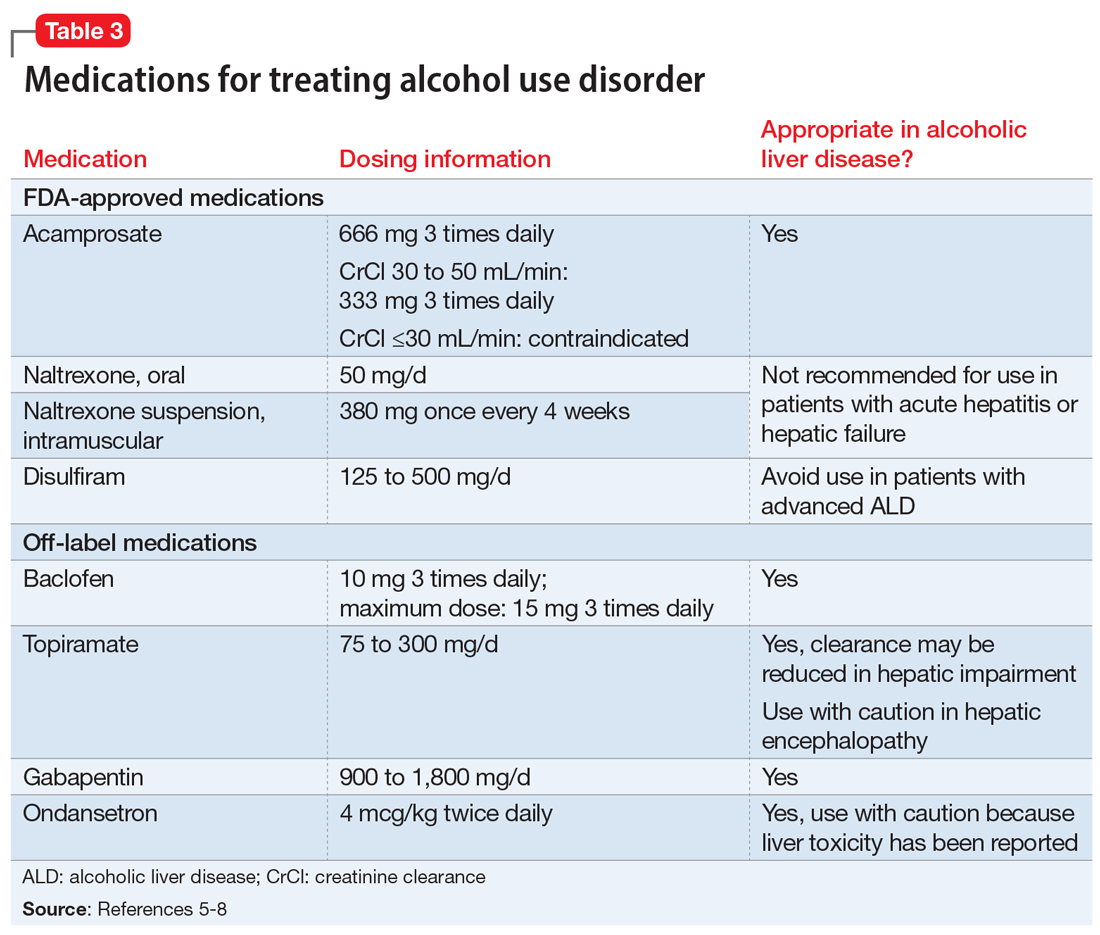
Three medications—acamprosate, naltrexone, and disulfiram—currently are FDA-approved for treating AUD.5,6 Additionally, several other medications have shown varying levels of efficacy in treating patients with AUD but are not FDA-approved for this indication (Table 3).5-8
Acamprosate is thought to create a balance of inhibitor and excitatory neurotransmitters by functioning as a glutamate antagonist and gamma-aminobutyric acid (GABA) agonist. This is speculated to aid in abstinence from alcohol. Data suggests that acamprosate may be more effective for maintaining abstinence than for inducing remission in individuals who have not yet detoxified from alcohol. Because of its renal excretion, acamprosate is the only FDA-approved medication for AUD that is not associated with liver toxicity. The most commonly reported adverse effect with acamprosate use is diarrhea.
Naltrexone, a mu-opioid receptor antagonist, is available in both tablet and long-acting IM injection formulations. Naltrexone blocks the binding of endorphins created by alcohol consumption to opioid receptors. This results in diminished dopamine release and is speculated to decrease reward and positive reinforcement with alcohol consumption, leading to fewer heavy drinking days. Due to hepatic metabolism, naltrexone use carries a risk of liver injury. Cases of hepatitis and clinically significant liver dysfunction as well as transient, asymptomatic, hepatic transaminase elevations have been observed in patients who receive naltrexone. Because of the absence of first-pass metabolism, long-acting IM naltrexone may produce less hepatotoxicity than the oral formulation. When the FDA approved both formulations of naltrexone, a “black-box” warning was issued concerning the risk of liver damage; however, these warnings have since been removed from their respective prescribing information.
Disulfiram inhibits acetaldehyde dehydrogenase, resulting in elevated acetaldehyde concentrations after consuming alcohol. In theory, this medication reduces a person’s desire to drink due to the negative physiological and physical effects associated with increased acetaldehyde, including hypotension, flushing, nausea, and vomiting. Although most of these reactions are short-lived, disulfiram can induce hepatotoxicity and liver failure that may prove fatal. Disulfiram should be avoided in patients with advanced ALD.
Off-label medications for AUD
Additional pharmacotherapeutic agents have been evaluated in patients with AUD. Baclofen, topiramate, gabapentin, and ondansetron have shown varying levels of efficacy and pose minimal concern in patients with ALD.
Continue to: Baclofen
Baclofen. Although findings are conflicting, baclofen is the only agent that has been specifically studied for treating AUD in patients with ALD. A GABA B receptor antagonist, baclofen is currently FDA-approved for treating spasticity. In a series of open-label and double-blind studies, baclofen has been shown to effectively reduce alcohol intake, promote abstinence, and prevent relapse.5,6 Further studies identified a possible dose-related response, noting that 20 mg taken 3 times daily may confer additional response over 10 mg taken 3 times daily.5,6 Conversely, the ALPADIR study failed to demonstrate superiority of baclofen vs placebo in the maintenance of abstinence from alcohol despite dosing at 180 mg/d.9 This study did, however, find a significant reduction in alcohol craving in favor of baclofen.9 Further, in a randomized controlled trial (RCT) conducted in veterans with chronic hepatitis C, baclofen 30 mg/d failed to show superiority over placebo with regard to increasing abstinence or reducing alcohol use
Topiramate. A recent meta-analysis found that topiramate use may result in fewer drinking days, heavy drinking days, and number of drinks per drinking day.7 Additionally, topiramate has demonstrated a statistically significant reduction in alcohol craving as well as the ability to decrease all liver function test values.5 This agent should be used with caution in patients with hepatic encephalopathy because the adverse cognitive effects associated with topiramate may confound the clinical course and treatment of such.
Gabapentin. The use of gabapentin to treat patients with AUD is supported by multiple RCTs. In studies that evaluated dose-related response, higher doses of gabapentin (up to 1,800 mg/d) showed greater efficacy than lower doses (ie, 900 mg/d).8 Because gabapentin does not undergo hepatic metabolism, its use in patients with ALD is considered safe. Although the abuse potential of gabapentin is less defined in patients with AUD, there have been reports of abuse in other high-risk populations (ie, those with opioid use disorder, incarcerated persons, and those who misuse prescriptions recreationally).8
Ondansetron is speculated to decrease the reward from alcohol via the down-regulation of dopaminergic neurons. Studies examining ondansetron for patients with AUD have found that it decreases alcohol cravings in those with early-onset alcoholism (initial onset at age ≤25), but not in late-onset alcoholism (initial onset at age >25).5 However, the ondansetron doses used in these trials were very low (4 mcg/kg), and those doses are not available commercially.5
CASE CONTINUED
Following a discussion of available pharmacotherapeutic options for AUD, Mr. S is started on baclofen, 10 mg 3 times daily, with plans for dose titration. At a 2-week follow-up appointment, Mr. S reports that he had not been taking baclofen as often as instructed; however, he denies further alcohol consumption and re-commits to baclofen treatment. Unfortunately, Mr. S is soon admitted to hospice care due to continued decompensation and is unable to attend any additional outpatient follow-up appointments. Three months after his initial outpatient contact, Mr. S dies due to alcoholic cirrhosis.
Related Resources
• Crabb DW, Im GY, Szabo G, et al. Diagnosis and treatment of alcohol-related liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71(1):306-333.
• Murail AR, Carey WD. Disease management. Liver test interpretation - approach to the patient with liver disease: a guide to commonly used liver tests. Cleveland Clinic Center for Continuing Education. Updated August 2017. www.clevelandclinicmeded. com/medicalpubs/diseasemanagement/hepatology/ guide-to-common-liver-tests/
Drug Brand Names
Acamprosate • Campral
Baclofen • Lioresal
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone • Revia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
1. Agrawal S, Dhiman RK, Limdi JK. Evaluation of abnormal liver function tests. Postgrad Med J. 2016;92(1086):223-234.
2. World Health Organization. Global status report on alcohol and health 2018. Published 2018. Accessed November 5, 2020. https://www.who.int/substance_abuse/publications/global_alcohol_report/gsr_2018/en/
3. Osna NA, Donohue TM, Kharbanda KK. Alcoholic liver disease: pathogenesis and current management. Alcohol Res. 2017;38(2):147-161.
4. Leggio L, Lee MR. Treatment of alcohol use disorder in patients with alcoholic liver disease. Am J Med. 2017;130(2):124-134.
5. Addolorato G, Mirijello A, Leggio L, et al. Management of alcohol dependence in patients with liver disease. CNS Drugs. 2013;27(4):287-299.
6. Vuittonet CL, Halse M, Leggio L, et al. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm. 2014;71(15):1265-1276.
7. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings. JAMA. 2014;311(18):1889-1900.
8. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
9. Reynaud M, Aubin HJ, Trinquet F, et al. A randomized, placebo-controlled study of high-dose baclofen in alcohol-dependent patients-the ALPADIR study. Alcohol Alcohol. 2017;52(4):439-446.
10. Hauser P, Fuller B, Ho S, et al. The safety and efficacy of baclofen to reduce alcohol use in veterans with chronic hepatitis C: a randomized controlled trial. Addiction. 2017;112(7):1173-1183.
Mr. S, age 64, presents for an outpatient follow-up after a recent hospital discharge for alcohol detoxification. He reports a long history of alcohol use, which has resulted in numerous hospital admissions. He has recently been receiving care from a gastroenterologist because the results of laboratory testing suggested hepatic impairment (Table 1). Mr. S says that a friend of his was able to stop drinking by taking a medication, and he wonders if he can be prescribed a medication to help him as well.
A chart review shows that Mr. S recently underwent paracentesis, during which 6 liters of fluid were removed. Additionally, an abdominal ultrasound confirmed hepatic cirrhosis.
According to the World Health Organization, alcohol consumption contributes to 3 million deaths annually.2 The highest proportion of these deaths (21.3%) is due to alcohol-associated gastrointestinal complications, including alcoholic and infectious hepatitis, pancreatitis, and cirrhosis. Because the liver is the primary site of ethanol metabolism, it sustains the greatest degree of tissue injury with heavy alcohol consumption. Additionally, the association of harmful use of alcohol with risky sexual behavior may partially explain the higher prevalence of viral hepatitis among persons with alcohol use disorder (AUD) compared with the general population. Alcoholic liver disease (ALD) progresses through several stages, beginning with hepatic steatosis and progressing through alcohol-related hepatitis, fibrosis, cirrhosis, and potentially hepatocellular carcinoma.3
Liver markers of alcohol use
Although biological markers can be used in clinical practice to screen and monitor for alcohol abuse, making a diagnosis of ALD can be challenging. Typically, a history of heavy alcohol consumption in addition to certain physical signs and laboratory tests for liver disease are the best indicators of ALD. However, the clinical assessment can be confounded by patients who deny or minimize how much alcohol they have consumed. Furthermore, physical and laboratory findings may not be specific to ALD.
Liver enzymes, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyltransferase (GGT), have historically been used as the basis of diagnosing ALD. In addition to elevated bilirubin and evidence of macrocytic anemia, elevations in these enzymes may suggest heavy alcohol use, but these values alone are inadequate to establish ALD. Gamma-glutamyltransferase is found in cell membranes of several body tissues, including the liver and spleen, and therefore is not specific to liver damage. However, elevated GGT is the best indicator of excessive alcohol consumption because it has greater sensitivity than AST and ALT.1,3,4
Although these biomarkers are helpful in diagnosing ALD, they lose some of their utility in patients with advanced liver disease. Patients with severe liver dysfunction may not have elevated serum aminotransferase levels because the degree of liver enzyme elevation does not correlate well with the severity of ALD. For example, patients with advanced cirrhosis may have liver enzyme levels that appear normal. However, the pattern of elevation in transaminases can be helpful in making a diagnosis of liver dysfunction; using the ratio of AST to ALT may aid in diagnosing ALD, because AST is elevated more than twice that of ALT in >80% of patients with ALD.1,3,4
Table 21,3,4 shows the progression of ALD from steatohepatitis to alcoholic hepatitis to cirrhosis. In steatohepatitis, transaminitis is present but all other biomarkers normal. In alcoholic hepatitis, transaminitis is present along with elevated alkaline phosphatase, elevated bilirubin, and elevated international normalized ratio (INR). In alcoholic cirrhosis, the AST-to-ALT ratio is >2, and hypoalbuminemia, hyperbilirubinemia, and coagulopathy (evidenced by elevated INR) are present, consistent with long-term liver damage.1,3,4
Continue to: FDA-approved medications
FDA-approved medications
Three medications—acamprosate, naltrexone, and disulfiram—currently are FDA-approved for treating AUD.5,6 Additionally, several other medications have shown varying levels of efficacy in treating patients with AUD but are not FDA-approved for this indication (Table 3).5-8
Acamprosate is thought to create a balance of inhibitor and excitatory neurotransmitters by functioning as a glutamate antagonist and gamma-aminobutyric acid (GABA) agonist. This is speculated to aid in abstinence from alcohol. Data suggests that acamprosate may be more effective for maintaining abstinence than for inducing remission in individuals who have not yet detoxified from alcohol. Because of its renal excretion, acamprosate is the only FDA-approved medication for AUD that is not associated with liver toxicity. The most commonly reported adverse effect with acamprosate use is diarrhea.
Naltrexone, a mu-opioid receptor antagonist, is available in both tablet and long-acting IM injection formulations. Naltrexone blocks the binding of endorphins created by alcohol consumption to opioid receptors. This results in diminished dopamine release and is speculated to decrease reward and positive reinforcement with alcohol consumption, leading to fewer heavy drinking days. Due to hepatic metabolism, naltrexone use carries a risk of liver injury. Cases of hepatitis and clinically significant liver dysfunction as well as transient, asymptomatic, hepatic transaminase elevations have been observed in patients who receive naltrexone. Because of the absence of first-pass metabolism, long-acting IM naltrexone may produce less hepatotoxicity than the oral formulation. When the FDA approved both formulations of naltrexone, a “black-box” warning was issued concerning the risk of liver damage; however, these warnings have since been removed from their respective prescribing information.
Disulfiram inhibits acetaldehyde dehydrogenase, resulting in elevated acetaldehyde concentrations after consuming alcohol. In theory, this medication reduces a person’s desire to drink due to the negative physiological and physical effects associated with increased acetaldehyde, including hypotension, flushing, nausea, and vomiting. Although most of these reactions are short-lived, disulfiram can induce hepatotoxicity and liver failure that may prove fatal. Disulfiram should be avoided in patients with advanced ALD.
Off-label medications for AUD
Additional pharmacotherapeutic agents have been evaluated in patients with AUD. Baclofen, topiramate, gabapentin, and ondansetron have shown varying levels of efficacy and pose minimal concern in patients with ALD.
Continue to: Baclofen
Baclofen. Although findings are conflicting, baclofen is the only agent that has been specifically studied for treating AUD in patients with ALD. A GABA B receptor antagonist, baclofen is currently FDA-approved for treating spasticity. In a series of open-label and double-blind studies, baclofen has been shown to effectively reduce alcohol intake, promote abstinence, and prevent relapse.5,6 Further studies identified a possible dose-related response, noting that 20 mg taken 3 times daily may confer additional response over 10 mg taken 3 times daily.5,6 Conversely, the ALPADIR study failed to demonstrate superiority of baclofen vs placebo in the maintenance of abstinence from alcohol despite dosing at 180 mg/d.9 This study did, however, find a significant reduction in alcohol craving in favor of baclofen.9 Further, in a randomized controlled trial (RCT) conducted in veterans with chronic hepatitis C, baclofen 30 mg/d failed to show superiority over placebo with regard to increasing abstinence or reducing alcohol use
Topiramate. A recent meta-analysis found that topiramate use may result in fewer drinking days, heavy drinking days, and number of drinks per drinking day.7 Additionally, topiramate has demonstrated a statistically significant reduction in alcohol craving as well as the ability to decrease all liver function test values.5 This agent should be used with caution in patients with hepatic encephalopathy because the adverse cognitive effects associated with topiramate may confound the clinical course and treatment of such.
Gabapentin. The use of gabapentin to treat patients with AUD is supported by multiple RCTs. In studies that evaluated dose-related response, higher doses of gabapentin (up to 1,800 mg/d) showed greater efficacy than lower doses (ie, 900 mg/d).8 Because gabapentin does not undergo hepatic metabolism, its use in patients with ALD is considered safe. Although the abuse potential of gabapentin is less defined in patients with AUD, there have been reports of abuse in other high-risk populations (ie, those with opioid use disorder, incarcerated persons, and those who misuse prescriptions recreationally).8
Ondansetron is speculated to decrease the reward from alcohol via the down-regulation of dopaminergic neurons. Studies examining ondansetron for patients with AUD have found that it decreases alcohol cravings in those with early-onset alcoholism (initial onset at age ≤25), but not in late-onset alcoholism (initial onset at age >25).5 However, the ondansetron doses used in these trials were very low (4 mcg/kg), and those doses are not available commercially.5
CASE CONTINUED
Following a discussion of available pharmacotherapeutic options for AUD, Mr. S is started on baclofen, 10 mg 3 times daily, with plans for dose titration. At a 2-week follow-up appointment, Mr. S reports that he had not been taking baclofen as often as instructed; however, he denies further alcohol consumption and re-commits to baclofen treatment. Unfortunately, Mr. S is soon admitted to hospice care due to continued decompensation and is unable to attend any additional outpatient follow-up appointments. Three months after his initial outpatient contact, Mr. S dies due to alcoholic cirrhosis.
Related Resources
• Crabb DW, Im GY, Szabo G, et al. Diagnosis and treatment of alcohol-related liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71(1):306-333.
• Murail AR, Carey WD. Disease management. Liver test interpretation - approach to the patient with liver disease: a guide to commonly used liver tests. Cleveland Clinic Center for Continuing Education. Updated August 2017. www.clevelandclinicmeded. com/medicalpubs/diseasemanagement/hepatology/ guide-to-common-liver-tests/
Drug Brand Names
Acamprosate • Campral
Baclofen • Lioresal
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone • Revia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
Mr. S, age 64, presents for an outpatient follow-up after a recent hospital discharge for alcohol detoxification. He reports a long history of alcohol use, which has resulted in numerous hospital admissions. He has recently been receiving care from a gastroenterologist because the results of laboratory testing suggested hepatic impairment (Table 1). Mr. S says that a friend of his was able to stop drinking by taking a medication, and he wonders if he can be prescribed a medication to help him as well.
A chart review shows that Mr. S recently underwent paracentesis, during which 6 liters of fluid were removed. Additionally, an abdominal ultrasound confirmed hepatic cirrhosis.
According to the World Health Organization, alcohol consumption contributes to 3 million deaths annually.2 The highest proportion of these deaths (21.3%) is due to alcohol-associated gastrointestinal complications, including alcoholic and infectious hepatitis, pancreatitis, and cirrhosis. Because the liver is the primary site of ethanol metabolism, it sustains the greatest degree of tissue injury with heavy alcohol consumption. Additionally, the association of harmful use of alcohol with risky sexual behavior may partially explain the higher prevalence of viral hepatitis among persons with alcohol use disorder (AUD) compared with the general population. Alcoholic liver disease (ALD) progresses through several stages, beginning with hepatic steatosis and progressing through alcohol-related hepatitis, fibrosis, cirrhosis, and potentially hepatocellular carcinoma.3
Liver markers of alcohol use
Although biological markers can be used in clinical practice to screen and monitor for alcohol abuse, making a diagnosis of ALD can be challenging. Typically, a history of heavy alcohol consumption in addition to certain physical signs and laboratory tests for liver disease are the best indicators of ALD. However, the clinical assessment can be confounded by patients who deny or minimize how much alcohol they have consumed. Furthermore, physical and laboratory findings may not be specific to ALD.
Liver enzymes, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyltransferase (GGT), have historically been used as the basis of diagnosing ALD. In addition to elevated bilirubin and evidence of macrocytic anemia, elevations in these enzymes may suggest heavy alcohol use, but these values alone are inadequate to establish ALD. Gamma-glutamyltransferase is found in cell membranes of several body tissues, including the liver and spleen, and therefore is not specific to liver damage. However, elevated GGT is the best indicator of excessive alcohol consumption because it has greater sensitivity than AST and ALT.1,3,4
Although these biomarkers are helpful in diagnosing ALD, they lose some of their utility in patients with advanced liver disease. Patients with severe liver dysfunction may not have elevated serum aminotransferase levels because the degree of liver enzyme elevation does not correlate well with the severity of ALD. For example, patients with advanced cirrhosis may have liver enzyme levels that appear normal. However, the pattern of elevation in transaminases can be helpful in making a diagnosis of liver dysfunction; using the ratio of AST to ALT may aid in diagnosing ALD, because AST is elevated more than twice that of ALT in >80% of patients with ALD.1,3,4
Table 21,3,4 shows the progression of ALD from steatohepatitis to alcoholic hepatitis to cirrhosis. In steatohepatitis, transaminitis is present but all other biomarkers normal. In alcoholic hepatitis, transaminitis is present along with elevated alkaline phosphatase, elevated bilirubin, and elevated international normalized ratio (INR). In alcoholic cirrhosis, the AST-to-ALT ratio is >2, and hypoalbuminemia, hyperbilirubinemia, and coagulopathy (evidenced by elevated INR) are present, consistent with long-term liver damage.1,3,4
Continue to: FDA-approved medications
FDA-approved medications
Three medications—acamprosate, naltrexone, and disulfiram—currently are FDA-approved for treating AUD.5,6 Additionally, several other medications have shown varying levels of efficacy in treating patients with AUD but are not FDA-approved for this indication (Table 3).5-8
Acamprosate is thought to create a balance of inhibitor and excitatory neurotransmitters by functioning as a glutamate antagonist and gamma-aminobutyric acid (GABA) agonist. This is speculated to aid in abstinence from alcohol. Data suggests that acamprosate may be more effective for maintaining abstinence than for inducing remission in individuals who have not yet detoxified from alcohol. Because of its renal excretion, acamprosate is the only FDA-approved medication for AUD that is not associated with liver toxicity. The most commonly reported adverse effect with acamprosate use is diarrhea.
Naltrexone, a mu-opioid receptor antagonist, is available in both tablet and long-acting IM injection formulations. Naltrexone blocks the binding of endorphins created by alcohol consumption to opioid receptors. This results in diminished dopamine release and is speculated to decrease reward and positive reinforcement with alcohol consumption, leading to fewer heavy drinking days. Due to hepatic metabolism, naltrexone use carries a risk of liver injury. Cases of hepatitis and clinically significant liver dysfunction as well as transient, asymptomatic, hepatic transaminase elevations have been observed in patients who receive naltrexone. Because of the absence of first-pass metabolism, long-acting IM naltrexone may produce less hepatotoxicity than the oral formulation. When the FDA approved both formulations of naltrexone, a “black-box” warning was issued concerning the risk of liver damage; however, these warnings have since been removed from their respective prescribing information.
Disulfiram inhibits acetaldehyde dehydrogenase, resulting in elevated acetaldehyde concentrations after consuming alcohol. In theory, this medication reduces a person’s desire to drink due to the negative physiological and physical effects associated with increased acetaldehyde, including hypotension, flushing, nausea, and vomiting. Although most of these reactions are short-lived, disulfiram can induce hepatotoxicity and liver failure that may prove fatal. Disulfiram should be avoided in patients with advanced ALD.
Off-label medications for AUD
Additional pharmacotherapeutic agents have been evaluated in patients with AUD. Baclofen, topiramate, gabapentin, and ondansetron have shown varying levels of efficacy and pose minimal concern in patients with ALD.
Continue to: Baclofen
Baclofen. Although findings are conflicting, baclofen is the only agent that has been specifically studied for treating AUD in patients with ALD. A GABA B receptor antagonist, baclofen is currently FDA-approved for treating spasticity. In a series of open-label and double-blind studies, baclofen has been shown to effectively reduce alcohol intake, promote abstinence, and prevent relapse.5,6 Further studies identified a possible dose-related response, noting that 20 mg taken 3 times daily may confer additional response over 10 mg taken 3 times daily.5,6 Conversely, the ALPADIR study failed to demonstrate superiority of baclofen vs placebo in the maintenance of abstinence from alcohol despite dosing at 180 mg/d.9 This study did, however, find a significant reduction in alcohol craving in favor of baclofen.9 Further, in a randomized controlled trial (RCT) conducted in veterans with chronic hepatitis C, baclofen 30 mg/d failed to show superiority over placebo with regard to increasing abstinence or reducing alcohol use
Topiramate. A recent meta-analysis found that topiramate use may result in fewer drinking days, heavy drinking days, and number of drinks per drinking day.7 Additionally, topiramate has demonstrated a statistically significant reduction in alcohol craving as well as the ability to decrease all liver function test values.5 This agent should be used with caution in patients with hepatic encephalopathy because the adverse cognitive effects associated with topiramate may confound the clinical course and treatment of such.
Gabapentin. The use of gabapentin to treat patients with AUD is supported by multiple RCTs. In studies that evaluated dose-related response, higher doses of gabapentin (up to 1,800 mg/d) showed greater efficacy than lower doses (ie, 900 mg/d).8 Because gabapentin does not undergo hepatic metabolism, its use in patients with ALD is considered safe. Although the abuse potential of gabapentin is less defined in patients with AUD, there have been reports of abuse in other high-risk populations (ie, those with opioid use disorder, incarcerated persons, and those who misuse prescriptions recreationally).8
Ondansetron is speculated to decrease the reward from alcohol via the down-regulation of dopaminergic neurons. Studies examining ondansetron for patients with AUD have found that it decreases alcohol cravings in those with early-onset alcoholism (initial onset at age ≤25), but not in late-onset alcoholism (initial onset at age >25).5 However, the ondansetron doses used in these trials were very low (4 mcg/kg), and those doses are not available commercially.5
CASE CONTINUED
Following a discussion of available pharmacotherapeutic options for AUD, Mr. S is started on baclofen, 10 mg 3 times daily, with plans for dose titration. At a 2-week follow-up appointment, Mr. S reports that he had not been taking baclofen as often as instructed; however, he denies further alcohol consumption and re-commits to baclofen treatment. Unfortunately, Mr. S is soon admitted to hospice care due to continued decompensation and is unable to attend any additional outpatient follow-up appointments. Three months after his initial outpatient contact, Mr. S dies due to alcoholic cirrhosis.
Related Resources
• Crabb DW, Im GY, Szabo G, et al. Diagnosis and treatment of alcohol-related liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71(1):306-333.
• Murail AR, Carey WD. Disease management. Liver test interpretation - approach to the patient with liver disease: a guide to commonly used liver tests. Cleveland Clinic Center for Continuing Education. Updated August 2017. www.clevelandclinicmeded. com/medicalpubs/diseasemanagement/hepatology/ guide-to-common-liver-tests/
Drug Brand Names
Acamprosate • Campral
Baclofen • Lioresal
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone • Revia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
1. Agrawal S, Dhiman RK, Limdi JK. Evaluation of abnormal liver function tests. Postgrad Med J. 2016;92(1086):223-234.
2. World Health Organization. Global status report on alcohol and health 2018. Published 2018. Accessed November 5, 2020. https://www.who.int/substance_abuse/publications/global_alcohol_report/gsr_2018/en/
3. Osna NA, Donohue TM, Kharbanda KK. Alcoholic liver disease: pathogenesis and current management. Alcohol Res. 2017;38(2):147-161.
4. Leggio L, Lee MR. Treatment of alcohol use disorder in patients with alcoholic liver disease. Am J Med. 2017;130(2):124-134.
5. Addolorato G, Mirijello A, Leggio L, et al. Management of alcohol dependence in patients with liver disease. CNS Drugs. 2013;27(4):287-299.
6. Vuittonet CL, Halse M, Leggio L, et al. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm. 2014;71(15):1265-1276.
7. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings. JAMA. 2014;311(18):1889-1900.
8. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
9. Reynaud M, Aubin HJ, Trinquet F, et al. A randomized, placebo-controlled study of high-dose baclofen in alcohol-dependent patients-the ALPADIR study. Alcohol Alcohol. 2017;52(4):439-446.
10. Hauser P, Fuller B, Ho S, et al. The safety and efficacy of baclofen to reduce alcohol use in veterans with chronic hepatitis C: a randomized controlled trial. Addiction. 2017;112(7):1173-1183.
1. Agrawal S, Dhiman RK, Limdi JK. Evaluation of abnormal liver function tests. Postgrad Med J. 2016;92(1086):223-234.
2. World Health Organization. Global status report on alcohol and health 2018. Published 2018. Accessed November 5, 2020. https://www.who.int/substance_abuse/publications/global_alcohol_report/gsr_2018/en/
3. Osna NA, Donohue TM, Kharbanda KK. Alcoholic liver disease: pathogenesis and current management. Alcohol Res. 2017;38(2):147-161.
4. Leggio L, Lee MR. Treatment of alcohol use disorder in patients with alcoholic liver disease. Am J Med. 2017;130(2):124-134.
5. Addolorato G, Mirijello A, Leggio L, et al. Management of alcohol dependence in patients with liver disease. CNS Drugs. 2013;27(4):287-299.
6. Vuittonet CL, Halse M, Leggio L, et al. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm. 2014;71(15):1265-1276.
7. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings. JAMA. 2014;311(18):1889-1900.
8. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
9. Reynaud M, Aubin HJ, Trinquet F, et al. A randomized, placebo-controlled study of high-dose baclofen in alcohol-dependent patients-the ALPADIR study. Alcohol Alcohol. 2017;52(4):439-446.
10. Hauser P, Fuller B, Ho S, et al. The safety and efficacy of baclofen to reduce alcohol use in veterans with chronic hepatitis C: a randomized controlled trial. Addiction. 2017;112(7):1173-1183.