User login
Pharmacotherapy for alcohol use disorder in patients with hepatic impairment
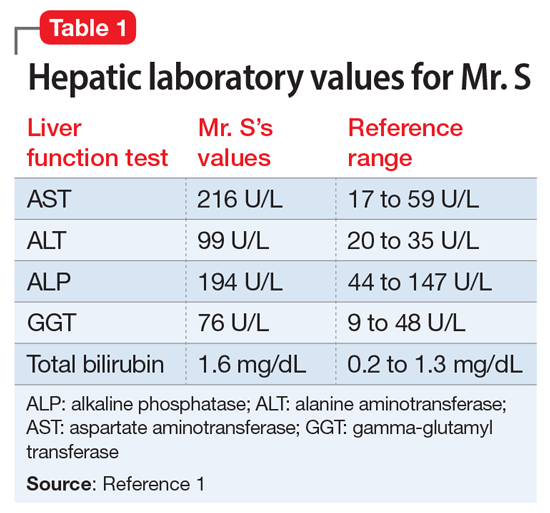
Mr. S, age 64, presents for an outpatient follow-up after a recent hospital discharge for alcohol detoxification. He reports a long history of alcohol use, which has resulted in numerous hospital admissions. He has recently been receiving care from a gastroenterologist because the results of laboratory testing suggested hepatic impairment (Table 1). Mr. S says that a friend of his was able to stop drinking by taking a medication, and he wonders if he can be prescribed a medication to help him as well.
A chart review shows that Mr. S recently underwent paracentesis, during which 6 liters of fluid were removed. Additionally, an abdominal ultrasound confirmed hepatic cirrhosis.
According to the World Health Organization, alcohol consumption contributes to 3 million deaths annually.2 The highest proportion of these deaths (21.3%) is due to alcohol-associated gastrointestinal complications, including alcoholic and infectious hepatitis, pancreatitis, and cirrhosis. Because the liver is the primary site of ethanol metabolism, it sustains the greatest degree of tissue injury with heavy alcohol consumption. Additionally, the association of harmful use of alcohol with risky sexual behavior may partially explain the higher prevalence of viral hepatitis among persons with alcohol use disorder (AUD) compared with the general population. Alcoholic liver disease (ALD) progresses through several stages, beginning with hepatic steatosis and progressing through alcohol-related hepatitis, fibrosis, cirrhosis, and potentially hepatocellular carcinoma.3
Liver markers of alcohol use
Although biological markers can be used in clinical practice to screen and monitor for alcohol abuse, making a diagnosis of ALD can be challenging. Typically, a history of heavy alcohol consumption in addition to certain physical signs and laboratory tests for liver disease are the best indicators of ALD. However, the clinical assessment can be confounded by patients who deny or minimize how much alcohol they have consumed. Furthermore, physical and laboratory findings may not be specific to ALD.
Liver enzymes, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyltransferase (GGT), have historically been used as the basis of diagnosing ALD. In addition to elevated bilirubin and evidence of macrocytic anemia, elevations in these enzymes may suggest heavy alcohol use, but these values alone are inadequate to establish ALD. Gamma-glutamyltransferase is found in cell membranes of several body tissues, including the liver and spleen, and therefore is not specific to liver damage. However, elevated GGT is the best indicator of excessive alcohol consumption because it has greater sensitivity than AST and ALT.1,3,4
Although these biomarkers are helpful in diagnosing ALD, they lose some of their utility in patients with advanced liver disease. Patients with severe liver dysfunction may not have elevated serum aminotransferase levels because the degree of liver enzyme elevation does not correlate well with the severity of ALD. For example, patients with advanced cirrhosis may have liver enzyme levels that appear normal. However, the pattern of elevation in transaminases can be helpful in making a diagnosis of liver dysfunction; using the ratio of AST to ALT may aid in diagnosing ALD, because AST is elevated more than twice that of ALT in >80% of patients with ALD.1,3,4
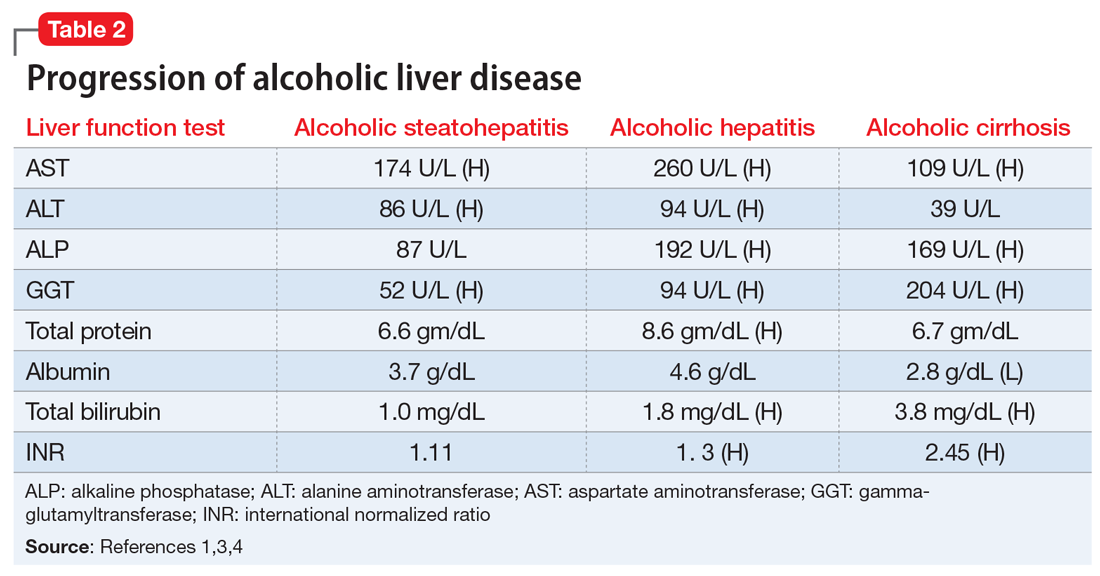
Table 21,3,4 shows the progression of ALD from steatohepatitis to alcoholic hepatitis to cirrhosis. In steatohepatitis, transaminitis is present but all other biomarkers normal. In alcoholic hepatitis, transaminitis is present along with elevated alkaline phosphatase, elevated bilirubin, and elevated international normalized ratio (INR). In alcoholic cirrhosis, the AST-to-ALT ratio is >2, and hypoalbuminemia, hyperbilirubinemia, and coagulopathy (evidenced by elevated INR) are present, consistent with long-term liver damage.1,3,4
Continue to: FDA-approved medications
FDA-approved medications
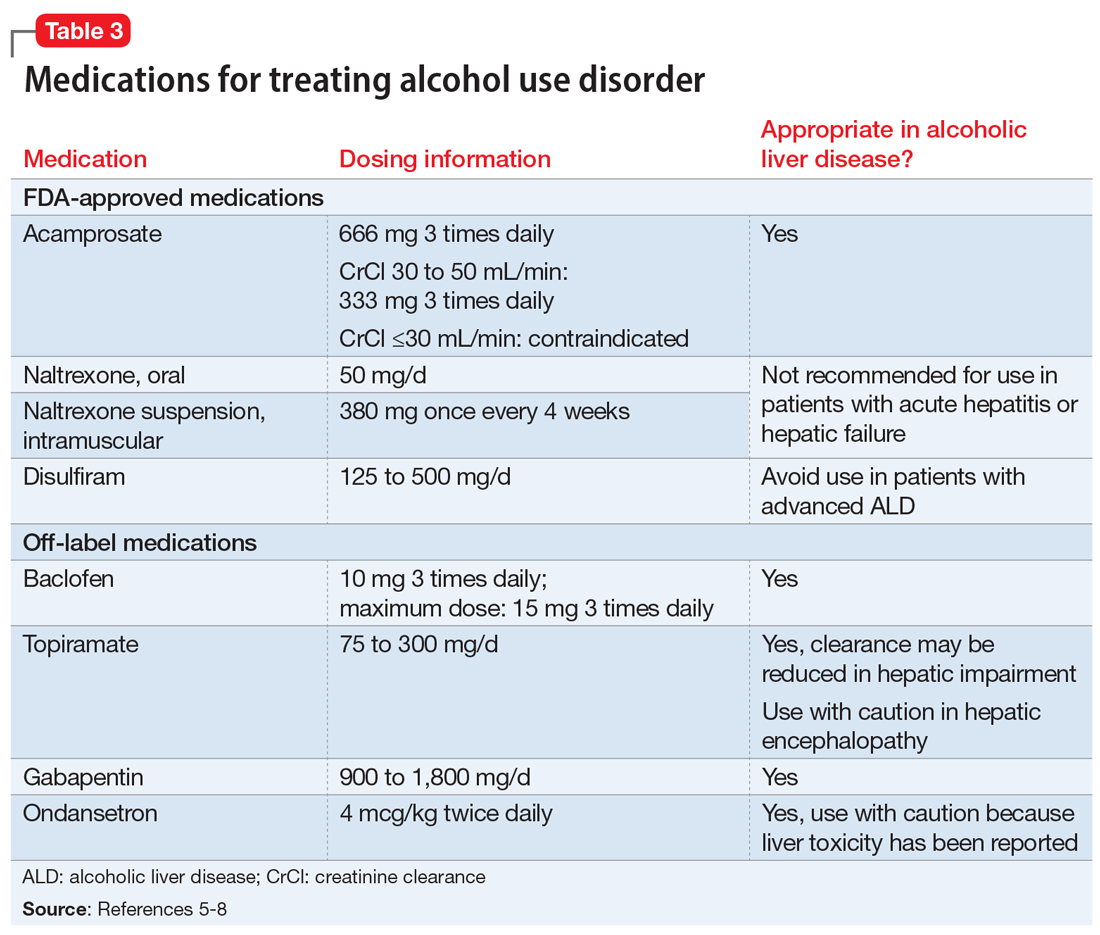
Three medications—acamprosate, naltrexone, and disulfiram—currently are FDA-approved for treating AUD.5,6 Additionally, several other medications have shown varying levels of efficacy in treating patients with AUD but are not FDA-approved for this indication (Table 3).5-8
Acamprosate is thought to create a balance of inhibitor and excitatory neurotransmitters by functioning as a glutamate antagonist and gamma-aminobutyric acid (GABA) agonist. This is speculated to aid in abstinence from alcohol. Data suggests that acamprosate may be more effective for maintaining abstinence than for inducing remission in individuals who have not yet detoxified from alcohol. Because of its renal excretion, acamprosate is the only FDA-approved medication for AUD that is not associated with liver toxicity. The most commonly reported adverse effect with acamprosate use is diarrhea.
Naltrexone, a mu-opioid receptor antagonist, is available in both tablet and long-acting IM injection formulations. Naltrexone blocks the binding of endorphins created by alcohol consumption to opioid receptors. This results in diminished dopamine release and is speculated to decrease reward and positive reinforcement with alcohol consumption, leading to fewer heavy drinking days. Due to hepatic metabolism, naltrexone use carries a risk of liver injury. Cases of hepatitis and clinically significant liver dysfunction as well as transient, asymptomatic, hepatic transaminase elevations have been observed in patients who receive naltrexone. Because of the absence of first-pass metabolism, long-acting IM naltrexone may produce less hepatotoxicity than the oral formulation. When the FDA approved both formulations of naltrexone, a “black-box” warning was issued concerning the risk of liver damage; however, these warnings have since been removed from their respective prescribing information.
Disulfiram inhibits acetaldehyde dehydrogenase, resulting in elevated acetaldehyde concentrations after consuming alcohol. In theory, this medication reduces a person’s desire to drink due to the negative physiological and physical effects associated with increased acetaldehyde, including hypotension, flushing, nausea, and vomiting. Although most of these reactions are short-lived, disulfiram can induce hepatotoxicity and liver failure that may prove fatal. Disulfiram should be avoided in patients with advanced ALD.
Off-label medications for AUD
Additional pharmacotherapeutic agents have been evaluated in patients with AUD. Baclofen, topiramate, gabapentin, and ondansetron have shown varying levels of efficacy and pose minimal concern in patients with ALD.
Continue to: Baclofen
Baclofen. Although findings are conflicting, baclofen is the only agent that has been specifically studied for treating AUD in patients with ALD. A GABA B receptor antagonist, baclofen is currently FDA-approved for treating spasticity. In a series of open-label and double-blind studies, baclofen has been shown to effectively reduce alcohol intake, promote abstinence, and prevent relapse.5,6 Further studies identified a possible dose-related response, noting that 20 mg taken 3 times daily may confer additional response over 10 mg taken 3 times daily.5,6 Conversely, the ALPADIR study failed to demonstrate superiority of baclofen vs placebo in the maintenance of abstinence from alcohol despite dosing at 180 mg/d.9 This study did, however, find a significant reduction in alcohol craving in favor of baclofen.9 Further, in a randomized controlled trial (RCT) conducted in veterans with chronic hepatitis C, baclofen 30 mg/d failed to show superiority over placebo with regard to increasing abstinence or reducing alcohol use
Topiramate. A recent meta-analysis found that topiramate use may result in fewer drinking days, heavy drinking days, and number of drinks per drinking day.7 Additionally, topiramate has demonstrated a statistically significant reduction in alcohol craving as well as the ability to decrease all liver function test values.5 This agent should be used with caution in patients with hepatic encephalopathy because the adverse cognitive effects associated with topiramate may confound the clinical course and treatment of such.
Gabapentin. The use of gabapentin to treat patients with AUD is supported by multiple RCTs. In studies that evaluated dose-related response, higher doses of gabapentin (up to 1,800 mg/d) showed greater efficacy than lower doses (ie, 900 mg/d).8 Because gabapentin does not undergo hepatic metabolism, its use in patients with ALD is considered safe. Although the abuse potential of gabapentin is less defined in patients with AUD, there have been reports of abuse in other high-risk populations (ie, those with opioid use disorder, incarcerated persons, and those who misuse prescriptions recreationally).8
Ondansetron is speculated to decrease the reward from alcohol via the down-regulation of dopaminergic neurons. Studies examining ondansetron for patients with AUD have found that it decreases alcohol cravings in those with early-onset alcoholism (initial onset at age ≤25), but not in late-onset alcoholism (initial onset at age >25).5 However, the ondansetron doses used in these trials were very low (4 mcg/kg), and those doses are not available commercially.5
CASE CONTINUED
Following a discussion of available pharmacotherapeutic options for AUD, Mr. S is started on baclofen, 10 mg 3 times daily, with plans for dose titration. At a 2-week follow-up appointment, Mr. S reports that he had not been taking baclofen as often as instructed; however, he denies further alcohol consumption and re-commits to baclofen treatment. Unfortunately, Mr. S is soon admitted to hospice care due to continued decompensation and is unable to attend any additional outpatient follow-up appointments. Three months after his initial outpatient contact, Mr. S dies due to alcoholic cirrhosis.
Related Resources
• Crabb DW, Im GY, Szabo G, et al. Diagnosis and treatment of alcohol-related liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71(1):306-333.
• Murail AR, Carey WD. Disease management. Liver test interpretation - approach to the patient with liver disease: a guide to commonly used liver tests. Cleveland Clinic Center for Continuing Education. Updated August 2017. www.clevelandclinicmeded. com/medicalpubs/diseasemanagement/hepatology/ guide-to-common-liver-tests/
Drug Brand Names
Acamprosate • Campral
Baclofen • Lioresal
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone • Revia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
1. Agrawal S, Dhiman RK, Limdi JK. Evaluation of abnormal liver function tests. Postgrad Med J. 2016;92(1086):223-234.
2. World Health Organization. Global status report on alcohol and health 2018. Published 2018. Accessed November 5, 2020. https://www.who.int/substance_abuse/publications/global_alcohol_report/gsr_2018/en/
3. Osna NA, Donohue TM, Kharbanda KK. Alcoholic liver disease: pathogenesis and current management. Alcohol Res. 2017;38(2):147-161.
4. Leggio L, Lee MR. Treatment of alcohol use disorder in patients with alcoholic liver disease. Am J Med. 2017;130(2):124-134.
5. Addolorato G, Mirijello A, Leggio L, et al. Management of alcohol dependence in patients with liver disease. CNS Drugs. 2013;27(4):287-299.
6. Vuittonet CL, Halse M, Leggio L, et al. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm. 2014;71(15):1265-1276.
7. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings. JAMA. 2014;311(18):1889-1900.
8. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
9. Reynaud M, Aubin HJ, Trinquet F, et al. A randomized, placebo-controlled study of high-dose baclofen in alcohol-dependent patients-the ALPADIR study. Alcohol Alcohol. 2017;52(4):439-446.
10. Hauser P, Fuller B, Ho S, et al. The safety and efficacy of baclofen to reduce alcohol use in veterans with chronic hepatitis C: a randomized controlled trial. Addiction. 2017;112(7):1173-1183.
Mr. S, age 64, presents for an outpatient follow-up after a recent hospital discharge for alcohol detoxification. He reports a long history of alcohol use, which has resulted in numerous hospital admissions. He has recently been receiving care from a gastroenterologist because the results of laboratory testing suggested hepatic impairment (Table 1). Mr. S says that a friend of his was able to stop drinking by taking a medication, and he wonders if he can be prescribed a medication to help him as well.
A chart review shows that Mr. S recently underwent paracentesis, during which 6 liters of fluid were removed. Additionally, an abdominal ultrasound confirmed hepatic cirrhosis.
According to the World Health Organization, alcohol consumption contributes to 3 million deaths annually.2 The highest proportion of these deaths (21.3%) is due to alcohol-associated gastrointestinal complications, including alcoholic and infectious hepatitis, pancreatitis, and cirrhosis. Because the liver is the primary site of ethanol metabolism, it sustains the greatest degree of tissue injury with heavy alcohol consumption. Additionally, the association of harmful use of alcohol with risky sexual behavior may partially explain the higher prevalence of viral hepatitis among persons with alcohol use disorder (AUD) compared with the general population. Alcoholic liver disease (ALD) progresses through several stages, beginning with hepatic steatosis and progressing through alcohol-related hepatitis, fibrosis, cirrhosis, and potentially hepatocellular carcinoma.3
Liver markers of alcohol use
Although biological markers can be used in clinical practice to screen and monitor for alcohol abuse, making a diagnosis of ALD can be challenging. Typically, a history of heavy alcohol consumption in addition to certain physical signs and laboratory tests for liver disease are the best indicators of ALD. However, the clinical assessment can be confounded by patients who deny or minimize how much alcohol they have consumed. Furthermore, physical and laboratory findings may not be specific to ALD.
Liver enzymes, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyltransferase (GGT), have historically been used as the basis of diagnosing ALD. In addition to elevated bilirubin and evidence of macrocytic anemia, elevations in these enzymes may suggest heavy alcohol use, but these values alone are inadequate to establish ALD. Gamma-glutamyltransferase is found in cell membranes of several body tissues, including the liver and spleen, and therefore is not specific to liver damage. However, elevated GGT is the best indicator of excessive alcohol consumption because it has greater sensitivity than AST and ALT.1,3,4
Although these biomarkers are helpful in diagnosing ALD, they lose some of their utility in patients with advanced liver disease. Patients with severe liver dysfunction may not have elevated serum aminotransferase levels because the degree of liver enzyme elevation does not correlate well with the severity of ALD. For example, patients with advanced cirrhosis may have liver enzyme levels that appear normal. However, the pattern of elevation in transaminases can be helpful in making a diagnosis of liver dysfunction; using the ratio of AST to ALT may aid in diagnosing ALD, because AST is elevated more than twice that of ALT in >80% of patients with ALD.1,3,4
Table 21,3,4 shows the progression of ALD from steatohepatitis to alcoholic hepatitis to cirrhosis. In steatohepatitis, transaminitis is present but all other biomarkers normal. In alcoholic hepatitis, transaminitis is present along with elevated alkaline phosphatase, elevated bilirubin, and elevated international normalized ratio (INR). In alcoholic cirrhosis, the AST-to-ALT ratio is >2, and hypoalbuminemia, hyperbilirubinemia, and coagulopathy (evidenced by elevated INR) are present, consistent with long-term liver damage.1,3,4
Continue to: FDA-approved medications
FDA-approved medications
Three medications—acamprosate, naltrexone, and disulfiram—currently are FDA-approved for treating AUD.5,6 Additionally, several other medications have shown varying levels of efficacy in treating patients with AUD but are not FDA-approved for this indication (Table 3).5-8
Acamprosate is thought to create a balance of inhibitor and excitatory neurotransmitters by functioning as a glutamate antagonist and gamma-aminobutyric acid (GABA) agonist. This is speculated to aid in abstinence from alcohol. Data suggests that acamprosate may be more effective for maintaining abstinence than for inducing remission in individuals who have not yet detoxified from alcohol. Because of its renal excretion, acamprosate is the only FDA-approved medication for AUD that is not associated with liver toxicity. The most commonly reported adverse effect with acamprosate use is diarrhea.
Naltrexone, a mu-opioid receptor antagonist, is available in both tablet and long-acting IM injection formulations. Naltrexone blocks the binding of endorphins created by alcohol consumption to opioid receptors. This results in diminished dopamine release and is speculated to decrease reward and positive reinforcement with alcohol consumption, leading to fewer heavy drinking days. Due to hepatic metabolism, naltrexone use carries a risk of liver injury. Cases of hepatitis and clinically significant liver dysfunction as well as transient, asymptomatic, hepatic transaminase elevations have been observed in patients who receive naltrexone. Because of the absence of first-pass metabolism, long-acting IM naltrexone may produce less hepatotoxicity than the oral formulation. When the FDA approved both formulations of naltrexone, a “black-box” warning was issued concerning the risk of liver damage; however, these warnings have since been removed from their respective prescribing information.
Disulfiram inhibits acetaldehyde dehydrogenase, resulting in elevated acetaldehyde concentrations after consuming alcohol. In theory, this medication reduces a person’s desire to drink due to the negative physiological and physical effects associated with increased acetaldehyde, including hypotension, flushing, nausea, and vomiting. Although most of these reactions are short-lived, disulfiram can induce hepatotoxicity and liver failure that may prove fatal. Disulfiram should be avoided in patients with advanced ALD.
Off-label medications for AUD
Additional pharmacotherapeutic agents have been evaluated in patients with AUD. Baclofen, topiramate, gabapentin, and ondansetron have shown varying levels of efficacy and pose minimal concern in patients with ALD.
Continue to: Baclofen
Baclofen. Although findings are conflicting, baclofen is the only agent that has been specifically studied for treating AUD in patients with ALD. A GABA B receptor antagonist, baclofen is currently FDA-approved for treating spasticity. In a series of open-label and double-blind studies, baclofen has been shown to effectively reduce alcohol intake, promote abstinence, and prevent relapse.5,6 Further studies identified a possible dose-related response, noting that 20 mg taken 3 times daily may confer additional response over 10 mg taken 3 times daily.5,6 Conversely, the ALPADIR study failed to demonstrate superiority of baclofen vs placebo in the maintenance of abstinence from alcohol despite dosing at 180 mg/d.9 This study did, however, find a significant reduction in alcohol craving in favor of baclofen.9 Further, in a randomized controlled trial (RCT) conducted in veterans with chronic hepatitis C, baclofen 30 mg/d failed to show superiority over placebo with regard to increasing abstinence or reducing alcohol use
Topiramate. A recent meta-analysis found that topiramate use may result in fewer drinking days, heavy drinking days, and number of drinks per drinking day.7 Additionally, topiramate has demonstrated a statistically significant reduction in alcohol craving as well as the ability to decrease all liver function test values.5 This agent should be used with caution in patients with hepatic encephalopathy because the adverse cognitive effects associated with topiramate may confound the clinical course and treatment of such.
Gabapentin. The use of gabapentin to treat patients with AUD is supported by multiple RCTs. In studies that evaluated dose-related response, higher doses of gabapentin (up to 1,800 mg/d) showed greater efficacy than lower doses (ie, 900 mg/d).8 Because gabapentin does not undergo hepatic metabolism, its use in patients with ALD is considered safe. Although the abuse potential of gabapentin is less defined in patients with AUD, there have been reports of abuse in other high-risk populations (ie, those with opioid use disorder, incarcerated persons, and those who misuse prescriptions recreationally).8
Ondansetron is speculated to decrease the reward from alcohol via the down-regulation of dopaminergic neurons. Studies examining ondansetron for patients with AUD have found that it decreases alcohol cravings in those with early-onset alcoholism (initial onset at age ≤25), but not in late-onset alcoholism (initial onset at age >25).5 However, the ondansetron doses used in these trials were very low (4 mcg/kg), and those doses are not available commercially.5
CASE CONTINUED
Following a discussion of available pharmacotherapeutic options for AUD, Mr. S is started on baclofen, 10 mg 3 times daily, with plans for dose titration. At a 2-week follow-up appointment, Mr. S reports that he had not been taking baclofen as often as instructed; however, he denies further alcohol consumption and re-commits to baclofen treatment. Unfortunately, Mr. S is soon admitted to hospice care due to continued decompensation and is unable to attend any additional outpatient follow-up appointments. Three months after his initial outpatient contact, Mr. S dies due to alcoholic cirrhosis.
Related Resources
• Crabb DW, Im GY, Szabo G, et al. Diagnosis and treatment of alcohol-related liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71(1):306-333.
• Murail AR, Carey WD. Disease management. Liver test interpretation - approach to the patient with liver disease: a guide to commonly used liver tests. Cleveland Clinic Center for Continuing Education. Updated August 2017. www.clevelandclinicmeded. com/medicalpubs/diseasemanagement/hepatology/ guide-to-common-liver-tests/
Drug Brand Names
Acamprosate • Campral
Baclofen • Lioresal
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone • Revia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
Mr. S, age 64, presents for an outpatient follow-up after a recent hospital discharge for alcohol detoxification. He reports a long history of alcohol use, which has resulted in numerous hospital admissions. He has recently been receiving care from a gastroenterologist because the results of laboratory testing suggested hepatic impairment (Table 1). Mr. S says that a friend of his was able to stop drinking by taking a medication, and he wonders if he can be prescribed a medication to help him as well.
A chart review shows that Mr. S recently underwent paracentesis, during which 6 liters of fluid were removed. Additionally, an abdominal ultrasound confirmed hepatic cirrhosis.
According to the World Health Organization, alcohol consumption contributes to 3 million deaths annually.2 The highest proportion of these deaths (21.3%) is due to alcohol-associated gastrointestinal complications, including alcoholic and infectious hepatitis, pancreatitis, and cirrhosis. Because the liver is the primary site of ethanol metabolism, it sustains the greatest degree of tissue injury with heavy alcohol consumption. Additionally, the association of harmful use of alcohol with risky sexual behavior may partially explain the higher prevalence of viral hepatitis among persons with alcohol use disorder (AUD) compared with the general population. Alcoholic liver disease (ALD) progresses through several stages, beginning with hepatic steatosis and progressing through alcohol-related hepatitis, fibrosis, cirrhosis, and potentially hepatocellular carcinoma.3
Liver markers of alcohol use
Although biological markers can be used in clinical practice to screen and monitor for alcohol abuse, making a diagnosis of ALD can be challenging. Typically, a history of heavy alcohol consumption in addition to certain physical signs and laboratory tests for liver disease are the best indicators of ALD. However, the clinical assessment can be confounded by patients who deny or minimize how much alcohol they have consumed. Furthermore, physical and laboratory findings may not be specific to ALD.
Liver enzymes, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyltransferase (GGT), have historically been used as the basis of diagnosing ALD. In addition to elevated bilirubin and evidence of macrocytic anemia, elevations in these enzymes may suggest heavy alcohol use, but these values alone are inadequate to establish ALD. Gamma-glutamyltransferase is found in cell membranes of several body tissues, including the liver and spleen, and therefore is not specific to liver damage. However, elevated GGT is the best indicator of excessive alcohol consumption because it has greater sensitivity than AST and ALT.1,3,4
Although these biomarkers are helpful in diagnosing ALD, they lose some of their utility in patients with advanced liver disease. Patients with severe liver dysfunction may not have elevated serum aminotransferase levels because the degree of liver enzyme elevation does not correlate well with the severity of ALD. For example, patients with advanced cirrhosis may have liver enzyme levels that appear normal. However, the pattern of elevation in transaminases can be helpful in making a diagnosis of liver dysfunction; using the ratio of AST to ALT may aid in diagnosing ALD, because AST is elevated more than twice that of ALT in >80% of patients with ALD.1,3,4
Table 21,3,4 shows the progression of ALD from steatohepatitis to alcoholic hepatitis to cirrhosis. In steatohepatitis, transaminitis is present but all other biomarkers normal. In alcoholic hepatitis, transaminitis is present along with elevated alkaline phosphatase, elevated bilirubin, and elevated international normalized ratio (INR). In alcoholic cirrhosis, the AST-to-ALT ratio is >2, and hypoalbuminemia, hyperbilirubinemia, and coagulopathy (evidenced by elevated INR) are present, consistent with long-term liver damage.1,3,4
Continue to: FDA-approved medications
FDA-approved medications
Three medications—acamprosate, naltrexone, and disulfiram—currently are FDA-approved for treating AUD.5,6 Additionally, several other medications have shown varying levels of efficacy in treating patients with AUD but are not FDA-approved for this indication (Table 3).5-8
Acamprosate is thought to create a balance of inhibitor and excitatory neurotransmitters by functioning as a glutamate antagonist and gamma-aminobutyric acid (GABA) agonist. This is speculated to aid in abstinence from alcohol. Data suggests that acamprosate may be more effective for maintaining abstinence than for inducing remission in individuals who have not yet detoxified from alcohol. Because of its renal excretion, acamprosate is the only FDA-approved medication for AUD that is not associated with liver toxicity. The most commonly reported adverse effect with acamprosate use is diarrhea.
Naltrexone, a mu-opioid receptor antagonist, is available in both tablet and long-acting IM injection formulations. Naltrexone blocks the binding of endorphins created by alcohol consumption to opioid receptors. This results in diminished dopamine release and is speculated to decrease reward and positive reinforcement with alcohol consumption, leading to fewer heavy drinking days. Due to hepatic metabolism, naltrexone use carries a risk of liver injury. Cases of hepatitis and clinically significant liver dysfunction as well as transient, asymptomatic, hepatic transaminase elevations have been observed in patients who receive naltrexone. Because of the absence of first-pass metabolism, long-acting IM naltrexone may produce less hepatotoxicity than the oral formulation. When the FDA approved both formulations of naltrexone, a “black-box” warning was issued concerning the risk of liver damage; however, these warnings have since been removed from their respective prescribing information.
Disulfiram inhibits acetaldehyde dehydrogenase, resulting in elevated acetaldehyde concentrations after consuming alcohol. In theory, this medication reduces a person’s desire to drink due to the negative physiological and physical effects associated with increased acetaldehyde, including hypotension, flushing, nausea, and vomiting. Although most of these reactions are short-lived, disulfiram can induce hepatotoxicity and liver failure that may prove fatal. Disulfiram should be avoided in patients with advanced ALD.
Off-label medications for AUD
Additional pharmacotherapeutic agents have been evaluated in patients with AUD. Baclofen, topiramate, gabapentin, and ondansetron have shown varying levels of efficacy and pose minimal concern in patients with ALD.
Continue to: Baclofen
Baclofen. Although findings are conflicting, baclofen is the only agent that has been specifically studied for treating AUD in patients with ALD. A GABA B receptor antagonist, baclofen is currently FDA-approved for treating spasticity. In a series of open-label and double-blind studies, baclofen has been shown to effectively reduce alcohol intake, promote abstinence, and prevent relapse.5,6 Further studies identified a possible dose-related response, noting that 20 mg taken 3 times daily may confer additional response over 10 mg taken 3 times daily.5,6 Conversely, the ALPADIR study failed to demonstrate superiority of baclofen vs placebo in the maintenance of abstinence from alcohol despite dosing at 180 mg/d.9 This study did, however, find a significant reduction in alcohol craving in favor of baclofen.9 Further, in a randomized controlled trial (RCT) conducted in veterans with chronic hepatitis C, baclofen 30 mg/d failed to show superiority over placebo with regard to increasing abstinence or reducing alcohol use
Topiramate. A recent meta-analysis found that topiramate use may result in fewer drinking days, heavy drinking days, and number of drinks per drinking day.7 Additionally, topiramate has demonstrated a statistically significant reduction in alcohol craving as well as the ability to decrease all liver function test values.5 This agent should be used with caution in patients with hepatic encephalopathy because the adverse cognitive effects associated with topiramate may confound the clinical course and treatment of such.
Gabapentin. The use of gabapentin to treat patients with AUD is supported by multiple RCTs. In studies that evaluated dose-related response, higher doses of gabapentin (up to 1,800 mg/d) showed greater efficacy than lower doses (ie, 900 mg/d).8 Because gabapentin does not undergo hepatic metabolism, its use in patients with ALD is considered safe. Although the abuse potential of gabapentin is less defined in patients with AUD, there have been reports of abuse in other high-risk populations (ie, those with opioid use disorder, incarcerated persons, and those who misuse prescriptions recreationally).8
Ondansetron is speculated to decrease the reward from alcohol via the down-regulation of dopaminergic neurons. Studies examining ondansetron for patients with AUD have found that it decreases alcohol cravings in those with early-onset alcoholism (initial onset at age ≤25), but not in late-onset alcoholism (initial onset at age >25).5 However, the ondansetron doses used in these trials were very low (4 mcg/kg), and those doses are not available commercially.5
CASE CONTINUED
Following a discussion of available pharmacotherapeutic options for AUD, Mr. S is started on baclofen, 10 mg 3 times daily, with plans for dose titration. At a 2-week follow-up appointment, Mr. S reports that he had not been taking baclofen as often as instructed; however, he denies further alcohol consumption and re-commits to baclofen treatment. Unfortunately, Mr. S is soon admitted to hospice care due to continued decompensation and is unable to attend any additional outpatient follow-up appointments. Three months after his initial outpatient contact, Mr. S dies due to alcoholic cirrhosis.
Related Resources
• Crabb DW, Im GY, Szabo G, et al. Diagnosis and treatment of alcohol-related liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71(1):306-333.
• Murail AR, Carey WD. Disease management. Liver test interpretation - approach to the patient with liver disease: a guide to commonly used liver tests. Cleveland Clinic Center for Continuing Education. Updated August 2017. www.clevelandclinicmeded. com/medicalpubs/diseasemanagement/hepatology/ guide-to-common-liver-tests/
Drug Brand Names
Acamprosate • Campral
Baclofen • Lioresal
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone • Revia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
1. Agrawal S, Dhiman RK, Limdi JK. Evaluation of abnormal liver function tests. Postgrad Med J. 2016;92(1086):223-234.
2. World Health Organization. Global status report on alcohol and health 2018. Published 2018. Accessed November 5, 2020. https://www.who.int/substance_abuse/publications/global_alcohol_report/gsr_2018/en/
3. Osna NA, Donohue TM, Kharbanda KK. Alcoholic liver disease: pathogenesis and current management. Alcohol Res. 2017;38(2):147-161.
4. Leggio L, Lee MR. Treatment of alcohol use disorder in patients with alcoholic liver disease. Am J Med. 2017;130(2):124-134.
5. Addolorato G, Mirijello A, Leggio L, et al. Management of alcohol dependence in patients with liver disease. CNS Drugs. 2013;27(4):287-299.
6. Vuittonet CL, Halse M, Leggio L, et al. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm. 2014;71(15):1265-1276.
7. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings. JAMA. 2014;311(18):1889-1900.
8. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
9. Reynaud M, Aubin HJ, Trinquet F, et al. A randomized, placebo-controlled study of high-dose baclofen in alcohol-dependent patients-the ALPADIR study. Alcohol Alcohol. 2017;52(4):439-446.
10. Hauser P, Fuller B, Ho S, et al. The safety and efficacy of baclofen to reduce alcohol use in veterans with chronic hepatitis C: a randomized controlled trial. Addiction. 2017;112(7):1173-1183.
1. Agrawal S, Dhiman RK, Limdi JK. Evaluation of abnormal liver function tests. Postgrad Med J. 2016;92(1086):223-234.
2. World Health Organization. Global status report on alcohol and health 2018. Published 2018. Accessed November 5, 2020. https://www.who.int/substance_abuse/publications/global_alcohol_report/gsr_2018/en/
3. Osna NA, Donohue TM, Kharbanda KK. Alcoholic liver disease: pathogenesis and current management. Alcohol Res. 2017;38(2):147-161.
4. Leggio L, Lee MR. Treatment of alcohol use disorder in patients with alcoholic liver disease. Am J Med. 2017;130(2):124-134.
5. Addolorato G, Mirijello A, Leggio L, et al. Management of alcohol dependence in patients with liver disease. CNS Drugs. 2013;27(4):287-299.
6. Vuittonet CL, Halse M, Leggio L, et al. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm. 2014;71(15):1265-1276.
7. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings. JAMA. 2014;311(18):1889-1900.
8. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
9. Reynaud M, Aubin HJ, Trinquet F, et al. A randomized, placebo-controlled study of high-dose baclofen in alcohol-dependent patients-the ALPADIR study. Alcohol Alcohol. 2017;52(4):439-446.
10. Hauser P, Fuller B, Ho S, et al. The safety and efficacy of baclofen to reduce alcohol use in veterans with chronic hepatitis C: a randomized controlled trial. Addiction. 2017;112(7):1173-1183.
Perceived Barriers and Facilitators of Clozapine Use: A National Survey of Veterans Affairs Prescribers (FULL)
Clozapine is an atypical antipsychotic that the US Food and Drug Administration (FDA) approved for use in schizophrenia and suicidality associated with schizophrenia or schizoaffective disorder. Clozapine has been shown to be superior to other antipsychotic treatment for treatment resistant schizophrenia (TRS), which is defined as failure of 2 adequate trials of antipsychotic therapy.1 Up to 30% of patients with schizophrenia are classified as treatment resistant.2
Clozapine is considered the drug of choice for patients with TRS in both the US Department of Veterans Affairs (VA) policies and other evidence-based guidelines and remains the only antipsychotic with FDA approval for TRS.2-5 Patients treated with clozapine have fewer psychiatric hospitalizations, fewer suicide attempts, lower rates of nonadherence, and less antipsychotic polypharmacy compared with patients who are treated with other antipsychotic therapy.6,7 A 2016 study by Gören and colleagues found that in addition to the clinical benefits, there is the potential for cost savings of $22,000 for each veteran switched to and treated with clozapine for 1 year even when accounting for the cost of monitoring and potential adverse event management.8 This translates to a total savings of > $80 million if current utilization were doubled and half of those patients continued treatment for 1 year within the Veterans Health Administration (VHA). However, despite evidence supporting use, < 10% of Medicaid-eligible patients and only 4% of patients with schizophrenia in the VHA are prescribed clozapine.8,9
Clozapine is underutilized for a variety of reasons, including intensive monitoring requirements, potential for severe adverse drug reactions, and concern for patient adherence.8 Common adverse effects (AEs) can range from mild to severe and include weight gain, constipation, sedation, orthostatic hypotension, and excessive salivation. Clozapine also carries a boxed warning for agranulocytosis, seizures, myocarditis, other cardiovascular and respiratory AEs (including orthostatic hypotension), and increased mortality in elderly patients with dementia.
Severe agranulocytosis occurs in between 0.05% and 0.86% of patients, which led the FDA to implement a Risk Evaluation and Mitigation Strategy (REMS) program for clozapine prescribing in 2015. Prior to the REMS program, each of the 6 clozapine manufacturers were required to maintain a registry to monitor for agranulocytosis. Per the REMS program requirements, health care providers (HCPs), dispensing pharmacies, and patients must be enrolled in the program and provide an updated absolute neutrophil count (ANC) prior to prescribing or dispensing clozapine. This is potentially time consuming, particularly during the first 6 months of treatment when the ANC must be monitored weekly and prescriptions are restricted to a 7-day supply. With recent changes to the REMS program, pharmacists are no longer permitted to enroll patients in the REMS system. This adds to the administrative burden on HCPs and may decrease further the likelihood of prescribing clozapine due to lack of time for these tasks. Within the VHA, a separate entity, the VA National Clozapine Coordinating Center (NCCC), reduces the administrative burden on HCPs by monitoring laboratory values, controlling dispensing, and communicating data electronically to the FDA REMS program.10
Despite the various administrative and clinical barriers and facilitators to prescribing that exist, previous studies have found that certain organizational characteristics also may influence clozapine prescribing rates. Gören and colleagues found that utilization at VHA facilities ranged from < 5% to about 20% of patients with schizophrenia. In this study, facilities with higher utilization of clozapine were more likely to have integrated nonphysician psychiatric providers in clinics and to have clear organizational structure and processes for the treatment of severe mental illness, while facilities with lower utilization rates were less likely to have a point person for clozapine management.11
Although many national efforts have been made to increase clozapine use in recent years, no study has examined HCP perception of barriers and facilitators of clozapine use in the VHA. The objective of this study is to identify barriers and facilitators of clozapine use within the VHA as perceived by HCPs so that these may be addressed to increase appropriate utilization of clozapine in veterans with TRS.
Methods
This study was conducted as a national survey of mental health providers within the VHA who had a scope of practice that allowed clozapine prescribing. Any HCP in a solely administrative role was excluded. The survey tool was reviewed by clinical pharmacy specialists at the Lexington VA Health Care System for content and ease of administration. Following appropriate institutional review board approval, the survey was submitted to the organizational assessment subcommittee and the 5 national VA unions for approval per VA policy. The survey tool was built and administered through REDCap (Nashville, Tennessee) software. An electronic link was sent out to the national VA psychiatric pharmacist and national psychiatry chief listservs for dissemination to the psychiatric providers at each facility with weekly reminders sent out during the 4-week study period to maximize participation. The 29-item survey was developed to assess demographic information, HCP characteristics, perceived barriers and facilitators of clozapine use, and general clozapine knowledge. Knowledge-based questions included appropriate indications, starting dose, baseline ANC requirement, ANC monitoring requirements, and possible AEs.
Primary outcomes assessed were perceived barriers to clozapine prescribing, opinions of potential interventions to facilitate clozapine prescribing, knowledge regarding clozapine, and the impact of medication management clinics on clozapine prescribing. For the purposes of this study, a clozapine clinic was defined as an interdisciplinary team dedicated to clozapine prescribing and monitoring.
Secondary outcomes included a comparison of clozapine prescribing rates among different subgroups of HCPs. Subgroups included HCP discipline, geographic region, presence of academic affiliation, level of comfort or familiarity with clozapine, and percentage of time spent in direct patient care. The regional Veterans Integrated Service Networks (VISN) were used to evaluate the effect of geographic region on prescribing practices.
Results of the survey were analyzed using descriptive statistics. The Mann-Whitney U test was utilized to compare ordinal data from questions that were scored on a Likert scale, and nominal data was compared utilizing the χ2 test. For all objectives, an α of < .05 was considered significant.
Results
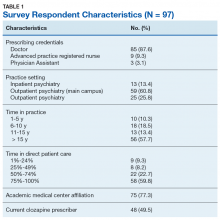
Ninety-eight HCPs from 17 VISNs responded during the 4-week survey period. One participant was excluded due to a solely administrative role. HCP characteristics and demographics are described in Table 1. The majority of respondents practice in an outpatient mental health setting either at the main VA campus or at a community-based outpatient clinic (CBOC).
Primary Outcomes
Perceived Barriers to Prescribing
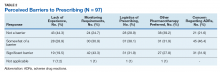
The majority of survey respondents rated all factors listed as at least somewhat of a barrier to prescribing. Table 2 describes the perception of these various factors as barriers to clozapine prescribing. Along with prespecified variables, a free text box was available to participants to identify other perceived barriers not listed. Among other concerns listed in this text box were patient buy-in (11.3%), process/coordination of prescribing (8.2%), time restrictions (7.2%), prescriber restrictions (7.2%), access (3.1%), credentialing problems (2.1%), and lack of clear education materials (1%).
Perceived Facilitators to Prescribing
When asked to consider the potential for increased prescribing with various interventions, most participants reported that all identified facilitators would be at least somewhat likely to increase their clozapine utilization. Table 3 describes the perception of these various factors as facilitators to clozapine prescribing. Other identified facilitators included nursing or pharmacy support for follow-ups (4.1%), advanced practice registered nurse credentialing for VHA prescribing (3.1%), utilization of national REMS program without the NCCC (3.1%), outside pharmacy use during titration phase (2.1%), prespecified coverage for HCPs while on leave (1%), and increased access to specialty consults for AEs (1%).
Clozapine Knowledge Assessment
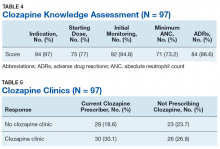
Overall, the average score on the clozapine knowledge assessment portion of the survey was 85.6%. The most commonly missed questions concerned the minimum ANC required to initiate clozapine and the appropriate starting dose for clozapine (Table 4). No significant difference was seen in clozapine utilization based on the clozapine knowledge assessment score when HCPs who scored≤ 60% were compared with those who scored ≥ 80% (P = .29).
Clozapine Clinic
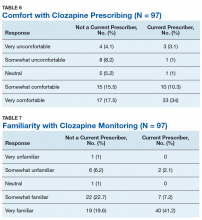
No statistically significant difference was found (P = .35) when rates of prescribing between facilities with or without a dedicated clozapine clinic were compared (Table 5). Additionally, the involvement of a pharmacist in clozapine management clinics did not lead to a statistically significant difference in utilization rates (P = .45).
Secondary Outcomes
Self-rated level of comfort with clozapine prescribing was significantly associated with rates of clozapine prescribing (P < .01). HCPs who rated themselves as somewhat or very comfortable were significantly more likely to prescribe clozapine (Table 6). Providers who rated themselves as very familiar with clozapine monitoring requirements (Table 7) were significantly more likely to prescribe clozapine (P < .01). This significance remained when comparing HCPs who rated themselves as very familiar to those who ranked themselves as somewhat familiar (P = .01). There was no statistically significant difference in clozapine prescribing based on academic medical center affiliation, time spent in direct patient care, or geographic location.
Discussion
This survey targeted VHA HCPs who were licensed to prescribe clozapine to identify barriers and facilitators of use, along with HCP characteristics that may impact clozapine utilization. The findings of this study indicate that even though HCPs may perceive many legitimate barriers to clozapine prescribing, such as the frequent laboratory monitoring requirements, some factors may increase their willingness to prescribe clozapine. Many of these facilitators involve addressing logistical concerns and the administrative burden that accompanies clozapine use. These findings echo previous studies done within and outside the VHA.8,9
While some identified barriers would require national policy changes to address, others could be addressed at VHA facilities. It may be prudent for each VA facility to identify a HCP who is familiar with clozapine to serve as a subject matter expert. This would be beneficial to those HCPs who feel their patients may benefit from clozapine, but who lack experience in prescribing, or for those with concerns about appropriateness of a specific patient. Additionally, this point of contact could be a valuable resource for concerns regarding administrative issues that may arise with the laboratory reporting system. In some facilities, it may be beneficial to set aside dedicated prescriber time in a clinic designed for clozapine management. Many HCPs in this survey identified the establishment of a clozapine clinic as an intervention that would increase their likelihood of prescribing clozapine. This type of clinic may alleviate some of the concerns regarding appointment availability for weekly or bimonthly appointments early in therapy by having additional staff and time dedicated to accommodating the need for frequent visits.
The majority of respondents to this survey were concerned about the logistics of clozapine monitoring and prescribing; however, this is largely dictated by FDA and VHA policies and regulations. Per national guidance, patients within the VHA should only receive prescriptions for clozapine from their local VA facility pharmacy. It takes many veterans ≥ 1 hour to travel to the closest VA hospital or CBOC. This is especially true for facilities with largely rural catchments. These patients often lack many resources that may be present in more urban areas, such as reliable public transportation. This creates challenges for both weekly laboratory monitoring and dispensing of weekly clozapine prescriptions early in therapy. The option to get clozapine from a local non-VA pharmacy and complete laboratory monitoring at a non-VA laboratory facility could make a clozapine trial more feasible for these veterans. Another consideration is increasing the availability of VA-funded transportation for these patients to assist them in getting to their appointments. Serious mental illness case workers or mental health intensive case management services also may prove useful in arranging for transportation for laboratory monitoring.
Providers with higher self-rated comfort and familiarity with monitoring requirements had a significantly increased likelihood of clozapine utilization. Lack of experience was commonly identified as a barrier to prescribing. Subsequently, the majority of respondents felt that educational sessions would increase their likelihood to prescribe clozapine. This could be addressed at both a facility and national level. As discussed above, a subject matter expert at each facility could provide some of this education and guidance for prescribers who have little or no experience with clozapine. Additionally, national educational presentations and academic detailing campaigns may be an efficient way to provide standardized education across the VHA. Dissemination of required education via the VA Talent Management System is another potential route that would ensure all providers received adequate training regarding the specific challenges of prescribing clozapine within the VA.
Strengths and Limitations
The strengths of this study lie in directly assessing HCP perceptions of barriers and facilitators. It is ultimately up to each individual HCP to decide to use clozapine. Addressing the concerns of these HCPs will be advantageous in efforts to increase clozapine utilization. Additionally, to the authors’ knowledge this is the first study to assess provider characteristics and knowledge of clozapine in relation to utilization rates.
The method of distribution was a major limitation of this study. This survey was distributed via national e-mail listservs; however, no listserv exists within the VA that targets all psychiatric providers. This study relied on the psychiatry chiefs and psychiatric pharmacists within each facility to further disseminate the survey, which could have led to lower response rates than what may be gathered via more direct contact methods. In addition, targeting psychiatric section chiefs and pharmacists may have introduced response bias. Another limitation to this study was the small number of responses. It is possible that this study was not adequately powered to detect significant differences in clozapine prescribing based on HCP characteristics or clozapine clinic availability. Further studies investigating the impact of provider characteristics on clozapine utilization are warranted.
Conclusion
Even though clozapine is an effective medication for TRS, providers underutilize it for a variety of reasons. Commonly identified barriers to prescribing in this study included frequent monitoring requirements, logistics of prescribing (including the REMS program and transportation for laboratory monitoring), pharmacotherapy preferences, and concern about the potential AEs. Facilitators identified in this study included implementation of clozapine clinics, having a specified contact point within the facility to assist with administrative responsibility, educational sessions, and the ability to utilize outside laboratories.
While some of these barriers and facilitators cannot be fully addressed without national policy change, individual facilities should make every effort to identify institution-specific concerns and address these. Clozapine clinic implementation and educational sessions appear to be reasonable considerations. This study did not identify any HCP characteristics that significantly impacted the likelihood of prescribing clozapine aside from self-rated comfort and familiarity with clozapine. However, further studies are needed to fully assess the impact of provider characteristics on clozapine utilization.
1. Siskind D, Mccartney L, Goldschlager R, Kisely S. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209(5):385-392.
2. Lehman A, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guidelines for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(2 suppl):1-56.
3. US Department of Veterans Affairs. Recommendations for antipsychotic selection in schizophrenia and schizoaffective disorders. https://www.pbm.va.gov/PBM/clinicalguidance/clinicalrecommendations/AntipsychoticSelectionAlgorithmSchizophreniaJune2012.doc. Published June 2012. Accessed September 12, 2019.
4. Dixon L, Perkins D, Calmes C. Guidelines watch (September 2009): practice guidelines for the treatment of patients with schizophrenia. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/schizophrenia-watch.pdf. Published September 2009. Accessed September 12, 2019.
5. National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: prevention and management. https://www.nice.org.uk/guidance/cg178. Updated March 2014. Accessed September 12, 2019.
6. Meltzer HY, Alphs L, Green AI, et al; International Suicide Prevention Trial Study Group. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry. 2003;60(1):82-91.
7. Stroup TS, Gerhard T, Crystal S, Huang C, Olfson M. Comparative effectiveness of clozapine and standard antipsychotic treatment in adults with schizophrenia. Am J Psychiatry. 2016;173(2):166-173.
8. Gören JL, Rose AJ, Smith EG, Ney JP. The business case for expanded clozapine utilization. Psychiatr Serv. 2016;67(11):1197-1205.
9. Kelly DL, Freudenreich O, Sayer MA, Love RC. Addressing barriers to clozapine underutilization: a national effort. Psychiatr Serv. 2018;69(2):224-227.
10. US Department of Veterans Affairs. Clozapine patient management protocol (CPMP). https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=1818. Published December 23, 2008. Accessed September 12, 2019.
11. Gören JL, Rose AJ, Engle RL, et al. Organizational characteristics of Veterans Affairs clinics with high and low utilization of clozapine. Psychiatr Serv. 2016;67(11):1189-1196.
Clozapine is an atypical antipsychotic that the US Food and Drug Administration (FDA) approved for use in schizophrenia and suicidality associated with schizophrenia or schizoaffective disorder. Clozapine has been shown to be superior to other antipsychotic treatment for treatment resistant schizophrenia (TRS), which is defined as failure of 2 adequate trials of antipsychotic therapy.1 Up to 30% of patients with schizophrenia are classified as treatment resistant.2
Clozapine is considered the drug of choice for patients with TRS in both the US Department of Veterans Affairs (VA) policies and other evidence-based guidelines and remains the only antipsychotic with FDA approval for TRS.2-5 Patients treated with clozapine have fewer psychiatric hospitalizations, fewer suicide attempts, lower rates of nonadherence, and less antipsychotic polypharmacy compared with patients who are treated with other antipsychotic therapy.6,7 A 2016 study by Gören and colleagues found that in addition to the clinical benefits, there is the potential for cost savings of $22,000 for each veteran switched to and treated with clozapine for 1 year even when accounting for the cost of monitoring and potential adverse event management.8 This translates to a total savings of > $80 million if current utilization were doubled and half of those patients continued treatment for 1 year within the Veterans Health Administration (VHA). However, despite evidence supporting use, < 10% of Medicaid-eligible patients and only 4% of patients with schizophrenia in the VHA are prescribed clozapine.8,9
Clozapine is underutilized for a variety of reasons, including intensive monitoring requirements, potential for severe adverse drug reactions, and concern for patient adherence.8 Common adverse effects (AEs) can range from mild to severe and include weight gain, constipation, sedation, orthostatic hypotension, and excessive salivation. Clozapine also carries a boxed warning for agranulocytosis, seizures, myocarditis, other cardiovascular and respiratory AEs (including orthostatic hypotension), and increased mortality in elderly patients with dementia.
Severe agranulocytosis occurs in between 0.05% and 0.86% of patients, which led the FDA to implement a Risk Evaluation and Mitigation Strategy (REMS) program for clozapine prescribing in 2015. Prior to the REMS program, each of the 6 clozapine manufacturers were required to maintain a registry to monitor for agranulocytosis. Per the REMS program requirements, health care providers (HCPs), dispensing pharmacies, and patients must be enrolled in the program and provide an updated absolute neutrophil count (ANC) prior to prescribing or dispensing clozapine. This is potentially time consuming, particularly during the first 6 months of treatment when the ANC must be monitored weekly and prescriptions are restricted to a 7-day supply. With recent changes to the REMS program, pharmacists are no longer permitted to enroll patients in the REMS system. This adds to the administrative burden on HCPs and may decrease further the likelihood of prescribing clozapine due to lack of time for these tasks. Within the VHA, a separate entity, the VA National Clozapine Coordinating Center (NCCC), reduces the administrative burden on HCPs by monitoring laboratory values, controlling dispensing, and communicating data electronically to the FDA REMS program.10
Despite the various administrative and clinical barriers and facilitators to prescribing that exist, previous studies have found that certain organizational characteristics also may influence clozapine prescribing rates. Gören and colleagues found that utilization at VHA facilities ranged from < 5% to about 20% of patients with schizophrenia. In this study, facilities with higher utilization of clozapine were more likely to have integrated nonphysician psychiatric providers in clinics and to have clear organizational structure and processes for the treatment of severe mental illness, while facilities with lower utilization rates were less likely to have a point person for clozapine management.11
Although many national efforts have been made to increase clozapine use in recent years, no study has examined HCP perception of barriers and facilitators of clozapine use in the VHA. The objective of this study is to identify barriers and facilitators of clozapine use within the VHA as perceived by HCPs so that these may be addressed to increase appropriate utilization of clozapine in veterans with TRS.
Methods
This study was conducted as a national survey of mental health providers within the VHA who had a scope of practice that allowed clozapine prescribing. Any HCP in a solely administrative role was excluded. The survey tool was reviewed by clinical pharmacy specialists at the Lexington VA Health Care System for content and ease of administration. Following appropriate institutional review board approval, the survey was submitted to the organizational assessment subcommittee and the 5 national VA unions for approval per VA policy. The survey tool was built and administered through REDCap (Nashville, Tennessee) software. An electronic link was sent out to the national VA psychiatric pharmacist and national psychiatry chief listservs for dissemination to the psychiatric providers at each facility with weekly reminders sent out during the 4-week study period to maximize participation. The 29-item survey was developed to assess demographic information, HCP characteristics, perceived barriers and facilitators of clozapine use, and general clozapine knowledge. Knowledge-based questions included appropriate indications, starting dose, baseline ANC requirement, ANC monitoring requirements, and possible AEs.
Primary outcomes assessed were perceived barriers to clozapine prescribing, opinions of potential interventions to facilitate clozapine prescribing, knowledge regarding clozapine, and the impact of medication management clinics on clozapine prescribing. For the purposes of this study, a clozapine clinic was defined as an interdisciplinary team dedicated to clozapine prescribing and monitoring.
Secondary outcomes included a comparison of clozapine prescribing rates among different subgroups of HCPs. Subgroups included HCP discipline, geographic region, presence of academic affiliation, level of comfort or familiarity with clozapine, and percentage of time spent in direct patient care. The regional Veterans Integrated Service Networks (VISN) were used to evaluate the effect of geographic region on prescribing practices.
Results of the survey were analyzed using descriptive statistics. The Mann-Whitney U test was utilized to compare ordinal data from questions that were scored on a Likert scale, and nominal data was compared utilizing the χ2 test. For all objectives, an α of < .05 was considered significant.
Results
Ninety-eight HCPs from 17 VISNs responded during the 4-week survey period. One participant was excluded due to a solely administrative role. HCP characteristics and demographics are described in Table 1. The majority of respondents practice in an outpatient mental health setting either at the main VA campus or at a community-based outpatient clinic (CBOC).
Primary Outcomes
Perceived Barriers to Prescribing
The majority of survey respondents rated all factors listed as at least somewhat of a barrier to prescribing. Table 2 describes the perception of these various factors as barriers to clozapine prescribing. Along with prespecified variables, a free text box was available to participants to identify other perceived barriers not listed. Among other concerns listed in this text box were patient buy-in (11.3%), process/coordination of prescribing (8.2%), time restrictions (7.2%), prescriber restrictions (7.2%), access (3.1%), credentialing problems (2.1%), and lack of clear education materials (1%).
Perceived Facilitators to Prescribing
When asked to consider the potential for increased prescribing with various interventions, most participants reported that all identified facilitators would be at least somewhat likely to increase their clozapine utilization. Table 3 describes the perception of these various factors as facilitators to clozapine prescribing. Other identified facilitators included nursing or pharmacy support for follow-ups (4.1%), advanced practice registered nurse credentialing for VHA prescribing (3.1%), utilization of national REMS program without the NCCC (3.1%), outside pharmacy use during titration phase (2.1%), prespecified coverage for HCPs while on leave (1%), and increased access to specialty consults for AEs (1%).
Clozapine Knowledge Assessment
Overall, the average score on the clozapine knowledge assessment portion of the survey was 85.6%. The most commonly missed questions concerned the minimum ANC required to initiate clozapine and the appropriate starting dose for clozapine (Table 4). No significant difference was seen in clozapine utilization based on the clozapine knowledge assessment score when HCPs who scored≤ 60% were compared with those who scored ≥ 80% (P = .29).
Clozapine Clinic
No statistically significant difference was found (P = .35) when rates of prescribing between facilities with or without a dedicated clozapine clinic were compared (Table 5). Additionally, the involvement of a pharmacist in clozapine management clinics did not lead to a statistically significant difference in utilization rates (P = .45).
Secondary Outcomes
Self-rated level of comfort with clozapine prescribing was significantly associated with rates of clozapine prescribing (P < .01). HCPs who rated themselves as somewhat or very comfortable were significantly more likely to prescribe clozapine (Table 6). Providers who rated themselves as very familiar with clozapine monitoring requirements (Table 7) were significantly more likely to prescribe clozapine (P < .01). This significance remained when comparing HCPs who rated themselves as very familiar to those who ranked themselves as somewhat familiar (P = .01). There was no statistically significant difference in clozapine prescribing based on academic medical center affiliation, time spent in direct patient care, or geographic location.
Discussion
This survey targeted VHA HCPs who were licensed to prescribe clozapine to identify barriers and facilitators of use, along with HCP characteristics that may impact clozapine utilization. The findings of this study indicate that even though HCPs may perceive many legitimate barriers to clozapine prescribing, such as the frequent laboratory monitoring requirements, some factors may increase their willingness to prescribe clozapine. Many of these facilitators involve addressing logistical concerns and the administrative burden that accompanies clozapine use. These findings echo previous studies done within and outside the VHA.8,9
While some identified barriers would require national policy changes to address, others could be addressed at VHA facilities. It may be prudent for each VA facility to identify a HCP who is familiar with clozapine to serve as a subject matter expert. This would be beneficial to those HCPs who feel their patients may benefit from clozapine, but who lack experience in prescribing, or for those with concerns about appropriateness of a specific patient. Additionally, this point of contact could be a valuable resource for concerns regarding administrative issues that may arise with the laboratory reporting system. In some facilities, it may be beneficial to set aside dedicated prescriber time in a clinic designed for clozapine management. Many HCPs in this survey identified the establishment of a clozapine clinic as an intervention that would increase their likelihood of prescribing clozapine. This type of clinic may alleviate some of the concerns regarding appointment availability for weekly or bimonthly appointments early in therapy by having additional staff and time dedicated to accommodating the need for frequent visits.
The majority of respondents to this survey were concerned about the logistics of clozapine monitoring and prescribing; however, this is largely dictated by FDA and VHA policies and regulations. Per national guidance, patients within the VHA should only receive prescriptions for clozapine from their local VA facility pharmacy. It takes many veterans ≥ 1 hour to travel to the closest VA hospital or CBOC. This is especially true for facilities with largely rural catchments. These patients often lack many resources that may be present in more urban areas, such as reliable public transportation. This creates challenges for both weekly laboratory monitoring and dispensing of weekly clozapine prescriptions early in therapy. The option to get clozapine from a local non-VA pharmacy and complete laboratory monitoring at a non-VA laboratory facility could make a clozapine trial more feasible for these veterans. Another consideration is increasing the availability of VA-funded transportation for these patients to assist them in getting to their appointments. Serious mental illness case workers or mental health intensive case management services also may prove useful in arranging for transportation for laboratory monitoring.
Providers with higher self-rated comfort and familiarity with monitoring requirements had a significantly increased likelihood of clozapine utilization. Lack of experience was commonly identified as a barrier to prescribing. Subsequently, the majority of respondents felt that educational sessions would increase their likelihood to prescribe clozapine. This could be addressed at both a facility and national level. As discussed above, a subject matter expert at each facility could provide some of this education and guidance for prescribers who have little or no experience with clozapine. Additionally, national educational presentations and academic detailing campaigns may be an efficient way to provide standardized education across the VHA. Dissemination of required education via the VA Talent Management System is another potential route that would ensure all providers received adequate training regarding the specific challenges of prescribing clozapine within the VA.
Strengths and Limitations
The strengths of this study lie in directly assessing HCP perceptions of barriers and facilitators. It is ultimately up to each individual HCP to decide to use clozapine. Addressing the concerns of these HCPs will be advantageous in efforts to increase clozapine utilization. Additionally, to the authors’ knowledge this is the first study to assess provider characteristics and knowledge of clozapine in relation to utilization rates.
The method of distribution was a major limitation of this study. This survey was distributed via national e-mail listservs; however, no listserv exists within the VA that targets all psychiatric providers. This study relied on the psychiatry chiefs and psychiatric pharmacists within each facility to further disseminate the survey, which could have led to lower response rates than what may be gathered via more direct contact methods. In addition, targeting psychiatric section chiefs and pharmacists may have introduced response bias. Another limitation to this study was the small number of responses. It is possible that this study was not adequately powered to detect significant differences in clozapine prescribing based on HCP characteristics or clozapine clinic availability. Further studies investigating the impact of provider characteristics on clozapine utilization are warranted.
Conclusion
Even though clozapine is an effective medication for TRS, providers underutilize it for a variety of reasons. Commonly identified barriers to prescribing in this study included frequent monitoring requirements, logistics of prescribing (including the REMS program and transportation for laboratory monitoring), pharmacotherapy preferences, and concern about the potential AEs. Facilitators identified in this study included implementation of clozapine clinics, having a specified contact point within the facility to assist with administrative responsibility, educational sessions, and the ability to utilize outside laboratories.
While some of these barriers and facilitators cannot be fully addressed without national policy change, individual facilities should make every effort to identify institution-specific concerns and address these. Clozapine clinic implementation and educational sessions appear to be reasonable considerations. This study did not identify any HCP characteristics that significantly impacted the likelihood of prescribing clozapine aside from self-rated comfort and familiarity with clozapine. However, further studies are needed to fully assess the impact of provider characteristics on clozapine utilization.
Clozapine is an atypical antipsychotic that the US Food and Drug Administration (FDA) approved for use in schizophrenia and suicidality associated with schizophrenia or schizoaffective disorder. Clozapine has been shown to be superior to other antipsychotic treatment for treatment resistant schizophrenia (TRS), which is defined as failure of 2 adequate trials of antipsychotic therapy.1 Up to 30% of patients with schizophrenia are classified as treatment resistant.2
Clozapine is considered the drug of choice for patients with TRS in both the US Department of Veterans Affairs (VA) policies and other evidence-based guidelines and remains the only antipsychotic with FDA approval for TRS.2-5 Patients treated with clozapine have fewer psychiatric hospitalizations, fewer suicide attempts, lower rates of nonadherence, and less antipsychotic polypharmacy compared with patients who are treated with other antipsychotic therapy.6,7 A 2016 study by Gören and colleagues found that in addition to the clinical benefits, there is the potential for cost savings of $22,000 for each veteran switched to and treated with clozapine for 1 year even when accounting for the cost of monitoring and potential adverse event management.8 This translates to a total savings of > $80 million if current utilization were doubled and half of those patients continued treatment for 1 year within the Veterans Health Administration (VHA). However, despite evidence supporting use, < 10% of Medicaid-eligible patients and only 4% of patients with schizophrenia in the VHA are prescribed clozapine.8,9
Clozapine is underutilized for a variety of reasons, including intensive monitoring requirements, potential for severe adverse drug reactions, and concern for patient adherence.8 Common adverse effects (AEs) can range from mild to severe and include weight gain, constipation, sedation, orthostatic hypotension, and excessive salivation. Clozapine also carries a boxed warning for agranulocytosis, seizures, myocarditis, other cardiovascular and respiratory AEs (including orthostatic hypotension), and increased mortality in elderly patients with dementia.
Severe agranulocytosis occurs in between 0.05% and 0.86% of patients, which led the FDA to implement a Risk Evaluation and Mitigation Strategy (REMS) program for clozapine prescribing in 2015. Prior to the REMS program, each of the 6 clozapine manufacturers were required to maintain a registry to monitor for agranulocytosis. Per the REMS program requirements, health care providers (HCPs), dispensing pharmacies, and patients must be enrolled in the program and provide an updated absolute neutrophil count (ANC) prior to prescribing or dispensing clozapine. This is potentially time consuming, particularly during the first 6 months of treatment when the ANC must be monitored weekly and prescriptions are restricted to a 7-day supply. With recent changes to the REMS program, pharmacists are no longer permitted to enroll patients in the REMS system. This adds to the administrative burden on HCPs and may decrease further the likelihood of prescribing clozapine due to lack of time for these tasks. Within the VHA, a separate entity, the VA National Clozapine Coordinating Center (NCCC), reduces the administrative burden on HCPs by monitoring laboratory values, controlling dispensing, and communicating data electronically to the FDA REMS program.10
Despite the various administrative and clinical barriers and facilitators to prescribing that exist, previous studies have found that certain organizational characteristics also may influence clozapine prescribing rates. Gören and colleagues found that utilization at VHA facilities ranged from < 5% to about 20% of patients with schizophrenia. In this study, facilities with higher utilization of clozapine were more likely to have integrated nonphysician psychiatric providers in clinics and to have clear organizational structure and processes for the treatment of severe mental illness, while facilities with lower utilization rates were less likely to have a point person for clozapine management.11
Although many national efforts have been made to increase clozapine use in recent years, no study has examined HCP perception of barriers and facilitators of clozapine use in the VHA. The objective of this study is to identify barriers and facilitators of clozapine use within the VHA as perceived by HCPs so that these may be addressed to increase appropriate utilization of clozapine in veterans with TRS.
Methods
This study was conducted as a national survey of mental health providers within the VHA who had a scope of practice that allowed clozapine prescribing. Any HCP in a solely administrative role was excluded. The survey tool was reviewed by clinical pharmacy specialists at the Lexington VA Health Care System for content and ease of administration. Following appropriate institutional review board approval, the survey was submitted to the organizational assessment subcommittee and the 5 national VA unions for approval per VA policy. The survey tool was built and administered through REDCap (Nashville, Tennessee) software. An electronic link was sent out to the national VA psychiatric pharmacist and national psychiatry chief listservs for dissemination to the psychiatric providers at each facility with weekly reminders sent out during the 4-week study period to maximize participation. The 29-item survey was developed to assess demographic information, HCP characteristics, perceived barriers and facilitators of clozapine use, and general clozapine knowledge. Knowledge-based questions included appropriate indications, starting dose, baseline ANC requirement, ANC monitoring requirements, and possible AEs.
Primary outcomes assessed were perceived barriers to clozapine prescribing, opinions of potential interventions to facilitate clozapine prescribing, knowledge regarding clozapine, and the impact of medication management clinics on clozapine prescribing. For the purposes of this study, a clozapine clinic was defined as an interdisciplinary team dedicated to clozapine prescribing and monitoring.
Secondary outcomes included a comparison of clozapine prescribing rates among different subgroups of HCPs. Subgroups included HCP discipline, geographic region, presence of academic affiliation, level of comfort or familiarity with clozapine, and percentage of time spent in direct patient care. The regional Veterans Integrated Service Networks (VISN) were used to evaluate the effect of geographic region on prescribing practices.
Results of the survey were analyzed using descriptive statistics. The Mann-Whitney U test was utilized to compare ordinal data from questions that were scored on a Likert scale, and nominal data was compared utilizing the χ2 test. For all objectives, an α of < .05 was considered significant.
Results
Ninety-eight HCPs from 17 VISNs responded during the 4-week survey period. One participant was excluded due to a solely administrative role. HCP characteristics and demographics are described in Table 1. The majority of respondents practice in an outpatient mental health setting either at the main VA campus or at a community-based outpatient clinic (CBOC).
Primary Outcomes
Perceived Barriers to Prescribing
The majority of survey respondents rated all factors listed as at least somewhat of a barrier to prescribing. Table 2 describes the perception of these various factors as barriers to clozapine prescribing. Along with prespecified variables, a free text box was available to participants to identify other perceived barriers not listed. Among other concerns listed in this text box were patient buy-in (11.3%), process/coordination of prescribing (8.2%), time restrictions (7.2%), prescriber restrictions (7.2%), access (3.1%), credentialing problems (2.1%), and lack of clear education materials (1%).
Perceived Facilitators to Prescribing
When asked to consider the potential for increased prescribing with various interventions, most participants reported that all identified facilitators would be at least somewhat likely to increase their clozapine utilization. Table 3 describes the perception of these various factors as facilitators to clozapine prescribing. Other identified facilitators included nursing or pharmacy support for follow-ups (4.1%), advanced practice registered nurse credentialing for VHA prescribing (3.1%), utilization of national REMS program without the NCCC (3.1%), outside pharmacy use during titration phase (2.1%), prespecified coverage for HCPs while on leave (1%), and increased access to specialty consults for AEs (1%).
Clozapine Knowledge Assessment
Overall, the average score on the clozapine knowledge assessment portion of the survey was 85.6%. The most commonly missed questions concerned the minimum ANC required to initiate clozapine and the appropriate starting dose for clozapine (Table 4). No significant difference was seen in clozapine utilization based on the clozapine knowledge assessment score when HCPs who scored≤ 60% were compared with those who scored ≥ 80% (P = .29).
Clozapine Clinic
No statistically significant difference was found (P = .35) when rates of prescribing between facilities with or without a dedicated clozapine clinic were compared (Table 5). Additionally, the involvement of a pharmacist in clozapine management clinics did not lead to a statistically significant difference in utilization rates (P = .45).
Secondary Outcomes
Self-rated level of comfort with clozapine prescribing was significantly associated with rates of clozapine prescribing (P < .01). HCPs who rated themselves as somewhat or very comfortable were significantly more likely to prescribe clozapine (Table 6). Providers who rated themselves as very familiar with clozapine monitoring requirements (Table 7) were significantly more likely to prescribe clozapine (P < .01). This significance remained when comparing HCPs who rated themselves as very familiar to those who ranked themselves as somewhat familiar (P = .01). There was no statistically significant difference in clozapine prescribing based on academic medical center affiliation, time spent in direct patient care, or geographic location.
Discussion
This survey targeted VHA HCPs who were licensed to prescribe clozapine to identify barriers and facilitators of use, along with HCP characteristics that may impact clozapine utilization. The findings of this study indicate that even though HCPs may perceive many legitimate barriers to clozapine prescribing, such as the frequent laboratory monitoring requirements, some factors may increase their willingness to prescribe clozapine. Many of these facilitators involve addressing logistical concerns and the administrative burden that accompanies clozapine use. These findings echo previous studies done within and outside the VHA.8,9
While some identified barriers would require national policy changes to address, others could be addressed at VHA facilities. It may be prudent for each VA facility to identify a HCP who is familiar with clozapine to serve as a subject matter expert. This would be beneficial to those HCPs who feel their patients may benefit from clozapine, but who lack experience in prescribing, or for those with concerns about appropriateness of a specific patient. Additionally, this point of contact could be a valuable resource for concerns regarding administrative issues that may arise with the laboratory reporting system. In some facilities, it may be beneficial to set aside dedicated prescriber time in a clinic designed for clozapine management. Many HCPs in this survey identified the establishment of a clozapine clinic as an intervention that would increase their likelihood of prescribing clozapine. This type of clinic may alleviate some of the concerns regarding appointment availability for weekly or bimonthly appointments early in therapy by having additional staff and time dedicated to accommodating the need for frequent visits.
The majority of respondents to this survey were concerned about the logistics of clozapine monitoring and prescribing; however, this is largely dictated by FDA and VHA policies and regulations. Per national guidance, patients within the VHA should only receive prescriptions for clozapine from their local VA facility pharmacy. It takes many veterans ≥ 1 hour to travel to the closest VA hospital or CBOC. This is especially true for facilities with largely rural catchments. These patients often lack many resources that may be present in more urban areas, such as reliable public transportation. This creates challenges for both weekly laboratory monitoring and dispensing of weekly clozapine prescriptions early in therapy. The option to get clozapine from a local non-VA pharmacy and complete laboratory monitoring at a non-VA laboratory facility could make a clozapine trial more feasible for these veterans. Another consideration is increasing the availability of VA-funded transportation for these patients to assist them in getting to their appointments. Serious mental illness case workers or mental health intensive case management services also may prove useful in arranging for transportation for laboratory monitoring.
Providers with higher self-rated comfort and familiarity with monitoring requirements had a significantly increased likelihood of clozapine utilization. Lack of experience was commonly identified as a barrier to prescribing. Subsequently, the majority of respondents felt that educational sessions would increase their likelihood to prescribe clozapine. This could be addressed at both a facility and national level. As discussed above, a subject matter expert at each facility could provide some of this education and guidance for prescribers who have little or no experience with clozapine. Additionally, national educational presentations and academic detailing campaigns may be an efficient way to provide standardized education across the VHA. Dissemination of required education via the VA Talent Management System is another potential route that would ensure all providers received adequate training regarding the specific challenges of prescribing clozapine within the VA.
Strengths and Limitations
The strengths of this study lie in directly assessing HCP perceptions of barriers and facilitators. It is ultimately up to each individual HCP to decide to use clozapine. Addressing the concerns of these HCPs will be advantageous in efforts to increase clozapine utilization. Additionally, to the authors’ knowledge this is the first study to assess provider characteristics and knowledge of clozapine in relation to utilization rates.
The method of distribution was a major limitation of this study. This survey was distributed via national e-mail listservs; however, no listserv exists within the VA that targets all psychiatric providers. This study relied on the psychiatry chiefs and psychiatric pharmacists within each facility to further disseminate the survey, which could have led to lower response rates than what may be gathered via more direct contact methods. In addition, targeting psychiatric section chiefs and pharmacists may have introduced response bias. Another limitation to this study was the small number of responses. It is possible that this study was not adequately powered to detect significant differences in clozapine prescribing based on HCP characteristics or clozapine clinic availability. Further studies investigating the impact of provider characteristics on clozapine utilization are warranted.
Conclusion
Even though clozapine is an effective medication for TRS, providers underutilize it for a variety of reasons. Commonly identified barriers to prescribing in this study included frequent monitoring requirements, logistics of prescribing (including the REMS program and transportation for laboratory monitoring), pharmacotherapy preferences, and concern about the potential AEs. Facilitators identified in this study included implementation of clozapine clinics, having a specified contact point within the facility to assist with administrative responsibility, educational sessions, and the ability to utilize outside laboratories.
While some of these barriers and facilitators cannot be fully addressed without national policy change, individual facilities should make every effort to identify institution-specific concerns and address these. Clozapine clinic implementation and educational sessions appear to be reasonable considerations. This study did not identify any HCP characteristics that significantly impacted the likelihood of prescribing clozapine aside from self-rated comfort and familiarity with clozapine. However, further studies are needed to fully assess the impact of provider characteristics on clozapine utilization.
1. Siskind D, Mccartney L, Goldschlager R, Kisely S. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209(5):385-392.
2. Lehman A, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guidelines for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(2 suppl):1-56.
3. US Department of Veterans Affairs. Recommendations for antipsychotic selection in schizophrenia and schizoaffective disorders. https://www.pbm.va.gov/PBM/clinicalguidance/clinicalrecommendations/AntipsychoticSelectionAlgorithmSchizophreniaJune2012.doc. Published June 2012. Accessed September 12, 2019.
4. Dixon L, Perkins D, Calmes C. Guidelines watch (September 2009): practice guidelines for the treatment of patients with schizophrenia. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/schizophrenia-watch.pdf. Published September 2009. Accessed September 12, 2019.
5. National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: prevention and management. https://www.nice.org.uk/guidance/cg178. Updated March 2014. Accessed September 12, 2019.
6. Meltzer HY, Alphs L, Green AI, et al; International Suicide Prevention Trial Study Group. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry. 2003;60(1):82-91.
7. Stroup TS, Gerhard T, Crystal S, Huang C, Olfson M. Comparative effectiveness of clozapine and standard antipsychotic treatment in adults with schizophrenia. Am J Psychiatry. 2016;173(2):166-173.
8. Gören JL, Rose AJ, Smith EG, Ney JP. The business case for expanded clozapine utilization. Psychiatr Serv. 2016;67(11):1197-1205.
9. Kelly DL, Freudenreich O, Sayer MA, Love RC. Addressing barriers to clozapine underutilization: a national effort. Psychiatr Serv. 2018;69(2):224-227.
10. US Department of Veterans Affairs. Clozapine patient management protocol (CPMP). https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=1818. Published December 23, 2008. Accessed September 12, 2019.
11. Gören JL, Rose AJ, Engle RL, et al. Organizational characteristics of Veterans Affairs clinics with high and low utilization of clozapine. Psychiatr Serv. 2016;67(11):1189-1196.
1. Siskind D, Mccartney L, Goldschlager R, Kisely S. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209(5):385-392.
2. Lehman A, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guidelines for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(2 suppl):1-56.
3. US Department of Veterans Affairs. Recommendations for antipsychotic selection in schizophrenia and schizoaffective disorders. https://www.pbm.va.gov/PBM/clinicalguidance/clinicalrecommendations/AntipsychoticSelectionAlgorithmSchizophreniaJune2012.doc. Published June 2012. Accessed September 12, 2019.
4. Dixon L, Perkins D, Calmes C. Guidelines watch (September 2009): practice guidelines for the treatment of patients with schizophrenia. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/schizophrenia-watch.pdf. Published September 2009. Accessed September 12, 2019.
5. National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: prevention and management. https://www.nice.org.uk/guidance/cg178. Updated March 2014. Accessed September 12, 2019.
6. Meltzer HY, Alphs L, Green AI, et al; International Suicide Prevention Trial Study Group. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry. 2003;60(1):82-91.
7. Stroup TS, Gerhard T, Crystal S, Huang C, Olfson M. Comparative effectiveness of clozapine and standard antipsychotic treatment in adults with schizophrenia. Am J Psychiatry. 2016;173(2):166-173.
8. Gören JL, Rose AJ, Smith EG, Ney JP. The business case for expanded clozapine utilization. Psychiatr Serv. 2016;67(11):1197-1205.
9. Kelly DL, Freudenreich O, Sayer MA, Love RC. Addressing barriers to clozapine underutilization: a national effort. Psychiatr Serv. 2018;69(2):224-227.
10. US Department of Veterans Affairs. Clozapine patient management protocol (CPMP). https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=1818. Published December 23, 2008. Accessed September 12, 2019.
11. Gören JL, Rose AJ, Engle RL, et al. Organizational characteristics of Veterans Affairs clinics with high and low utilization of clozapine. Psychiatr Serv. 2016;67(11):1189-1196.
Impact of Psychotropic Medication Reviews on Prescribing Patterns
Due to the ever-expanding pool of psychopharmacologic agents available to treat mental health conditions, prescribers need to be vigilant about ensuring appropriate medication selection, evaluation, and monitoring. In response to this need, the Office of Mental Health Operations, Mental Health Services, and Pharmacy Benefits Management Services launched the Psychotropic Drug Safety Initiative (PDSI) to improve evidence-based psychotropic prescribing habits within the VA. Considering the large geriatric population in the VA, PDSI was particularly concerned with benzodiazepines and antipsychotic medications.
Background
The management of neuropsychiatric symptoms (NPS) for dementia is particularly burdensome to patients, caregivers, and prescribers. When nonpharmacologic interventions fail, patients are often prescribed antipsychotic medications to target these symptoms. The FDA has issued a black box warning regarding an increased risk of death associated with the use of both first- and second-generation antipsychotics for the treatment of dementia-related psychosis.1 Due to these risks, tapering or discontinuing these medications should be considered at regular intervals.
Recently, the Centers for Medicare & Medicaid Services (CMS) expanded its goal to reduce antipsychotic use in nursing facilities by 25% by the end of 2015 and 30% by the end of 2016.2 A review investigated the impact of withdrawal vs continuation of antipsychotic medications in the setting of Alzheimer dementia (AD) and found that antipsychotic medications could be withdrawn without detrimental effects on patient behaviors.3 However, the study also noted that those patients with severe NPS at baseline or who had a history of positive response to an antipsychotic might be at increased risk of relapse or have a shorter time to relapse when antipsychotic medications are withdrawn.
Benzodiazepines as a class may be used for many indications, including anxiety disorders, seizure disorders, sleep disorders, or muscle spasms. However, due to known risks of cognitive impairments, sedation, falls, or dependence and addiction, these agents are typically recommended for only short-term treatment. These risks are potentially amplified in a geriatric population, because elderly patients have an increased sensitivity to the effects of these agents and may have impaired hepatic or renal function, leading to accumulation.
Due to these risks, the American Geriatrics Society (AGS) recommends against the use of any benzodiazepines for the treatment of insomnia, agitation, or delirium. Additionally, the AGS recommends that use of these agents for the treatment of behavioral problems related to dementia be reserved for those who have failed nonpharmacologic options and are at risk to themselves or others.4
Growing evidence suggests that benzodiazepine use may increase the risk of developing AD. A recent casecontrol study compare records of 1,796 patients with an AD diagnosis to 7,184 patients with no cognitive deficits. This study found that patients with a history of benzodiazepine use had a 51% increase in risk for AD. Additionally, use of long-acting benzodiazepines, such as diazepam and clonazepam, was strongly associated with the development of AD.5 These data further illustrate the importance of minimizing the use of benzodiazepines.
To ensure proper use of these 2 classes of medications, clinical pharmacy specialists (CPSs) at the Lexington VAMC (LVAMC) in Kentucky began conducting psychotropic medication reviews (PMRs). Each PMR contained a brief summary of evidence-based recommendations (both pharmacologic and nonpharmacologic) and a clinical review of the patient’s medication history, including an evaluation of the appropriateness of current therapy and supportive documentation. Patients were candidates for PMR if they met one of the following criteria: (1) use of a benzodiazepine in a patient with dementia; (2) use of a benzodiazepine in a patient aged > 75 years; and (3) use of an antipsychotic in a patient with dementia. These criteria were selected based on guidance set forth by the PDSI. The figure provides a sample of the PMR template in the electronic medical record (EMR).
The purpose of this study was to evaluate the impact of a pharmacist-conducted PMR based on prescriber response to written recommendations. Additionally, this study characterized any observed differences in prescriber response based on discipline. The results of this study will be used to evaluate the efficacy of LVAMC’s current method of clinical pharmacy intervention and identify areas for process improvement. This study was reviewed and approved by the LVAMC institutional review board and research and development committee.
Methods
Patients were included in this study if they had a PMR note entered in the EMR between September 2014 and January 2015. Baseline demographic information collected included patient age and gender. One study author manually reviewed all PMR notes and collected the type of pharmacy intervention (characterized as recommendation for medication adjustment, patient education, both medication adjustment and patient education, or no recommendation), provider discipline, provider response to intervention, and any changes to medication therapy that occurred as a result of pharmacist intervention.
When patients were not cognitively able to receive education, family members or caretakers were educated. The primary outcome of this study was prescriber response to pharmacist recommendations, which was characterized as acknowledged, ignored, accepted, or declined with justification. In instances where both medication adjustment and patient education was recommended, the recommendation was considered to be accepted only if both components of the recommendation were addressed. The secondary outcome sought to identify any difference in provider response based on discipline.
Results
Eighty-nine patients were included in the study. Due to the nature of LVAMC, the patient population was fairly homogeneous, with an average patient age of 80 years (range 52-95 years); > 97% of patients were male. Fifty patients were noted to have prescriptions for benzodiazepines and were aged > 75 years, 11 patients had prescriptions for benzodiazepines and a diagnosis of dementia, and 38 patients had prescriptions for antipsychotics and a diagnosis of dementia. Several patients fell into more than one of the categories (Table 1).
Specific written recommendations were made for 69 (78%) patients, with 20 patients having appropriate documentation in the chart for therapy continuation. The most common documented reasons for continuation of therapy were (a) patient/caregiver educated and consent to continue therapy already documented; (b) recent dose reduction or discontinuation attempt failed; (c) recent successful dose reduction; or (d) documented risk to patient or others if medication were to be discontinued.
Most recommendations were for medication adjustments (n = 54; 78.3%). Six (8.7%) recommendations were for patient education only, and 9 (13%) recommendations were for both medication adjustment and patient education. Overall, 33 (48%) of recommendations were accepted, 21 (30%) were not acknowledged, and 15 (22%) were declined. The most common outcome of accepted recommendations was a dose reduction with full taper planned by provider. The most common reasons for declined recommendations were (a) caregivers were educated and consented to continued treatment; (b) clinical presentation warranted continued treatment; (c) patient refused recommended changes; and (d) prescriber preferred to wait until next appointment to discuss with patient (Table 2).
Forty-nine recommendations were made to prescribers in the Psychiatry Department, 17 to prescribers in the Home-Based Primary Care (HBPC) Department, 15 to prescribers in the Primary Care Department, and 8 to prescribers in the Neurology Department. Prescribers in the Primary Care Department accepted recommendations at the highest rate (n = 13; 69%), while Neurology Department prescribers (n = 2; 33%) accepted recommendations at the lowest rate.
Discussion
This study illustrates the impact of including a psychiatric CPS as part of the interdisciplinary care team. Through the implementation of a PMR process, CPSs were able to provide specific, unbiased recommendations for the safe use of medications. It was felt that CPSs might have a greater impact by offering patient-specific recommendations rather than providing general information about the risks of these medications, which many providers are aware of already. Because nearly half of all recommendations were accepted, the authors feel that the PMR is an effective way to deliver provider education and improve safe prescribing practices.
There will be times when the use of these agents in at-risk populations is justified and appropriately documented, as was the case for 20 patients in this study. The goals of this study were not only to improve the use of evidence-based medications, but also the process of documenting justification for the continued use of these agents. The 22% of recommendations that were declined with justification from the provider were considered successful, because the PMR note prompted the provider to document in a note clear justification for the use of the agent in question.
The majority of recommendations were made to prescribers in the Mental Health Department, which was expected given the 2 classes of medications evaluated in the study. However, primary care prescribers accepted recommendations at the highest rate. There are several possible explanations. First, mental health prescribers are more likely to have complex, treatment-resistant psychiatric patients than do other disciplines. Additionally, these prescribers have an increased level of familiarity and comfort with second-generation antipsychotics and benzodiazepines and may have been more confident in documenting justifications to continue therapy.
Neurologists were the least likely to accept PMR recommendations. Unlike other services, prescribers in the Neurology Department spend a significant amount of their time providing care to patients at a university hospital and, therefore, are not present on the VA campus on a daily basis. This location disparity can lead to less frequent contact between prescriber and CPSs and may impact the professional relationship between these departments. Also, both the Neurology Department and the home-based Primary Care Department did not have staff actively involved in the PDSI, which may have decreased prescriber familiarity with the goals and intentions of PDSI and therefore decreased provider responsiveness to PMR notes.
Sometimes PMR notes were entered in the EMR when the patient did not have an upcoming appointment with the prescriber. As a result, there were instances when recommendations could not be implemented due to time and workload constraints. Many providers acknowledged the importance of shared medical decision making and preferred to wait to make medication adjustments until patients could be seen in the clinic.
Psychotropic medication review is a continually developing process, and these results illustrate provider response to the initial 5 months of a new service. During the time frame, PMR notes had been entered for all veterans identified as using antipsychotics or benzodiazepines in the setting of dementia but for only a fraction of those identified as using benzodiazepines who were aged > 75 years. It is reasonable to expect that as prescribers become more familiar with the PMR process and its intentions, they may be more likely to acknowledge recommendations and to respond with the appropriate documentation.
Psychotropic medication reviews were initiated as part of a PGY-2 psychiatric pharmacy residency project, and as such, the impact on the CPS workflow was not evaluated. Although this study suggests that the use of PMR was effective in improving evidence-based prescribing, it does not evaluate whether this process is sustainable in the long-term for the CPS.
Conclusions
The results of this study illustrate the value of a psychiatric CPS. Through the implementation of a simple PMR service, CPSs were able to impact evidence-based prescribing and related documentation. With nearly 50% of the recommendations accepted, the authors believe that use of the PMR is an effective way to deliver provider education and improve safe prescribing practices. Further review of the PMR process will be needed to evaluate the impact and sustainability on CPS workflow.
Click here to read the digital edition.
1. U.S. Food and Drug Administration. Information for healthcare professionals: conventional antipsychotics, 2013. U.S. Food and Drug Administration Website. http://www. fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124830.htm. Updated August 15, 2013. Accessed February 8, 2016.
2. Sprague K. CMS sets new goals for reducing use of antipsychotic medications. Consult Pharm. 2015;30(2):3.
3. Declercq T, Petrovic M, Azermai M, et al. Withdrawal versus continuation of chronic antipsychotic drugs for behavioural and psychological symptoms in older people with dementia. Cochrane Database Syst Rev. 2013;3:CD007726.
4. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
5. Billioti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ. 2014;349:g5205
Note: Page numbers differ between the print issue and digital edition.
Due to the ever-expanding pool of psychopharmacologic agents available to treat mental health conditions, prescribers need to be vigilant about ensuring appropriate medication selection, evaluation, and monitoring. In response to this need, the Office of Mental Health Operations, Mental Health Services, and Pharmacy Benefits Management Services launched the Psychotropic Drug Safety Initiative (PDSI) to improve evidence-based psychotropic prescribing habits within the VA. Considering the large geriatric population in the VA, PDSI was particularly concerned with benzodiazepines and antipsychotic medications.
Background
The management of neuropsychiatric symptoms (NPS) for dementia is particularly burdensome to patients, caregivers, and prescribers. When nonpharmacologic interventions fail, patients are often prescribed antipsychotic medications to target these symptoms. The FDA has issued a black box warning regarding an increased risk of death associated with the use of both first- and second-generation antipsychotics for the treatment of dementia-related psychosis.1 Due to these risks, tapering or discontinuing these medications should be considered at regular intervals.
Recently, the Centers for Medicare & Medicaid Services (CMS) expanded its goal to reduce antipsychotic use in nursing facilities by 25% by the end of 2015 and 30% by the end of 2016.2 A review investigated the impact of withdrawal vs continuation of antipsychotic medications in the setting of Alzheimer dementia (AD) and found that antipsychotic medications could be withdrawn without detrimental effects on patient behaviors.3 However, the study also noted that those patients with severe NPS at baseline or who had a history of positive response to an antipsychotic might be at increased risk of relapse or have a shorter time to relapse when antipsychotic medications are withdrawn.
Benzodiazepines as a class may be used for many indications, including anxiety disorders, seizure disorders, sleep disorders, or muscle spasms. However, due to known risks of cognitive impairments, sedation, falls, or dependence and addiction, these agents are typically recommended for only short-term treatment. These risks are potentially amplified in a geriatric population, because elderly patients have an increased sensitivity to the effects of these agents and may have impaired hepatic or renal function, leading to accumulation.
Due to these risks, the American Geriatrics Society (AGS) recommends against the use of any benzodiazepines for the treatment of insomnia, agitation, or delirium. Additionally, the AGS recommends that use of these agents for the treatment of behavioral problems related to dementia be reserved for those who have failed nonpharmacologic options and are at risk to themselves or others.4
Growing evidence suggests that benzodiazepine use may increase the risk of developing AD. A recent casecontrol study compare records of 1,796 patients with an AD diagnosis to 7,184 patients with no cognitive deficits. This study found that patients with a history of benzodiazepine use had a 51% increase in risk for AD. Additionally, use of long-acting benzodiazepines, such as diazepam and clonazepam, was strongly associated with the development of AD.5 These data further illustrate the importance of minimizing the use of benzodiazepines.
To ensure proper use of these 2 classes of medications, clinical pharmacy specialists (CPSs) at the Lexington VAMC (LVAMC) in Kentucky began conducting psychotropic medication reviews (PMRs). Each PMR contained a brief summary of evidence-based recommendations (both pharmacologic and nonpharmacologic) and a clinical review of the patient’s medication history, including an evaluation of the appropriateness of current therapy and supportive documentation. Patients were candidates for PMR if they met one of the following criteria: (1) use of a benzodiazepine in a patient with dementia; (2) use of a benzodiazepine in a patient aged > 75 years; and (3) use of an antipsychotic in a patient with dementia. These criteria were selected based on guidance set forth by the PDSI. The figure provides a sample of the PMR template in the electronic medical record (EMR).
The purpose of this study was to evaluate the impact of a pharmacist-conducted PMR based on prescriber response to written recommendations. Additionally, this study characterized any observed differences in prescriber response based on discipline. The results of this study will be used to evaluate the efficacy of LVAMC’s current method of clinical pharmacy intervention and identify areas for process improvement. This study was reviewed and approved by the LVAMC institutional review board and research and development committee.
Methods
Patients were included in this study if they had a PMR note entered in the EMR between September 2014 and January 2015. Baseline demographic information collected included patient age and gender. One study author manually reviewed all PMR notes and collected the type of pharmacy intervention (characterized as recommendation for medication adjustment, patient education, both medication adjustment and patient education, or no recommendation), provider discipline, provider response to intervention, and any changes to medication therapy that occurred as a result of pharmacist intervention.
When patients were not cognitively able to receive education, family members or caretakers were educated. The primary outcome of this study was prescriber response to pharmacist recommendations, which was characterized as acknowledged, ignored, accepted, or declined with justification. In instances where both medication adjustment and patient education was recommended, the recommendation was considered to be accepted only if both components of the recommendation were addressed. The secondary outcome sought to identify any difference in provider response based on discipline.
Results
Eighty-nine patients were included in the study. Due to the nature of LVAMC, the patient population was fairly homogeneous, with an average patient age of 80 years (range 52-95 years); > 97% of patients were male. Fifty patients were noted to have prescriptions for benzodiazepines and were aged > 75 years, 11 patients had prescriptions for benzodiazepines and a diagnosis of dementia, and 38 patients had prescriptions for antipsychotics and a diagnosis of dementia. Several patients fell into more than one of the categories (Table 1).
Specific written recommendations were made for 69 (78%) patients, with 20 patients having appropriate documentation in the chart for therapy continuation. The most common documented reasons for continuation of therapy were (a) patient/caregiver educated and consent to continue therapy already documented; (b) recent dose reduction or discontinuation attempt failed; (c) recent successful dose reduction; or (d) documented risk to patient or others if medication were to be discontinued.
Most recommendations were for medication adjustments (n = 54; 78.3%). Six (8.7%) recommendations were for patient education only, and 9 (13%) recommendations were for both medication adjustment and patient education. Overall, 33 (48%) of recommendations were accepted, 21 (30%) were not acknowledged, and 15 (22%) were declined. The most common outcome of accepted recommendations was a dose reduction with full taper planned by provider. The most common reasons for declined recommendations were (a) caregivers were educated and consented to continued treatment; (b) clinical presentation warranted continued treatment; (c) patient refused recommended changes; and (d) prescriber preferred to wait until next appointment to discuss with patient (Table 2).
Forty-nine recommendations were made to prescribers in the Psychiatry Department, 17 to prescribers in the Home-Based Primary Care (HBPC) Department, 15 to prescribers in the Primary Care Department, and 8 to prescribers in the Neurology Department. Prescribers in the Primary Care Department accepted recommendations at the highest rate (n = 13; 69%), while Neurology Department prescribers (n = 2; 33%) accepted recommendations at the lowest rate.
Discussion
This study illustrates the impact of including a psychiatric CPS as part of the interdisciplinary care team. Through the implementation of a PMR process, CPSs were able to provide specific, unbiased recommendations for the safe use of medications. It was felt that CPSs might have a greater impact by offering patient-specific recommendations rather than providing general information about the risks of these medications, which many providers are aware of already. Because nearly half of all recommendations were accepted, the authors feel that the PMR is an effective way to deliver provider education and improve safe prescribing practices.
There will be times when the use of these agents in at-risk populations is justified and appropriately documented, as was the case for 20 patients in this study. The goals of this study were not only to improve the use of evidence-based medications, but also the process of documenting justification for the continued use of these agents. The 22% of recommendations that were declined with justification from the provider were considered successful, because the PMR note prompted the provider to document in a note clear justification for the use of the agent in question.
The majority of recommendations were made to prescribers in the Mental Health Department, which was expected given the 2 classes of medications evaluated in the study. However, primary care prescribers accepted recommendations at the highest rate. There are several possible explanations. First, mental health prescribers are more likely to have complex, treatment-resistant psychiatric patients than do other disciplines. Additionally, these prescribers have an increased level of familiarity and comfort with second-generation antipsychotics and benzodiazepines and may have been more confident in documenting justifications to continue therapy.
Neurologists were the least likely to accept PMR recommendations. Unlike other services, prescribers in the Neurology Department spend a significant amount of their time providing care to patients at a university hospital and, therefore, are not present on the VA campus on a daily basis. This location disparity can lead to less frequent contact between prescriber and CPSs and may impact the professional relationship between these departments. Also, both the Neurology Department and the home-based Primary Care Department did not have staff actively involved in the PDSI, which may have decreased prescriber familiarity with the goals and intentions of PDSI and therefore decreased provider responsiveness to PMR notes.
Sometimes PMR notes were entered in the EMR when the patient did not have an upcoming appointment with the prescriber. As a result, there were instances when recommendations could not be implemented due to time and workload constraints. Many providers acknowledged the importance of shared medical decision making and preferred to wait to make medication adjustments until patients could be seen in the clinic.
Psychotropic medication review is a continually developing process, and these results illustrate provider response to the initial 5 months of a new service. During the time frame, PMR notes had been entered for all veterans identified as using antipsychotics or benzodiazepines in the setting of dementia but for only a fraction of those identified as using benzodiazepines who were aged > 75 years. It is reasonable to expect that as prescribers become more familiar with the PMR process and its intentions, they may be more likely to acknowledge recommendations and to respond with the appropriate documentation.
Psychotropic medication reviews were initiated as part of a PGY-2 psychiatric pharmacy residency project, and as such, the impact on the CPS workflow was not evaluated. Although this study suggests that the use of PMR was effective in improving evidence-based prescribing, it does not evaluate whether this process is sustainable in the long-term for the CPS.
Conclusions
The results of this study illustrate the value of a psychiatric CPS. Through the implementation of a simple PMR service, CPSs were able to impact evidence-based prescribing and related documentation. With nearly 50% of the recommendations accepted, the authors believe that use of the PMR is an effective way to deliver provider education and improve safe prescribing practices. Further review of the PMR process will be needed to evaluate the impact and sustainability on CPS workflow.
Click here to read the digital edition.
Due to the ever-expanding pool of psychopharmacologic agents available to treat mental health conditions, prescribers need to be vigilant about ensuring appropriate medication selection, evaluation, and monitoring. In response to this need, the Office of Mental Health Operations, Mental Health Services, and Pharmacy Benefits Management Services launched the Psychotropic Drug Safety Initiative (PDSI) to improve evidence-based psychotropic prescribing habits within the VA. Considering the large geriatric population in the VA, PDSI was particularly concerned with benzodiazepines and antipsychotic medications.
Background
The management of neuropsychiatric symptoms (NPS) for dementia is particularly burdensome to patients, caregivers, and prescribers. When nonpharmacologic interventions fail, patients are often prescribed antipsychotic medications to target these symptoms. The FDA has issued a black box warning regarding an increased risk of death associated with the use of both first- and second-generation antipsychotics for the treatment of dementia-related psychosis.1 Due to these risks, tapering or discontinuing these medications should be considered at regular intervals.
Recently, the Centers for Medicare & Medicaid Services (CMS) expanded its goal to reduce antipsychotic use in nursing facilities by 25% by the end of 2015 and 30% by the end of 2016.2 A review investigated the impact of withdrawal vs continuation of antipsychotic medications in the setting of Alzheimer dementia (AD) and found that antipsychotic medications could be withdrawn without detrimental effects on patient behaviors.3 However, the study also noted that those patients with severe NPS at baseline or who had a history of positive response to an antipsychotic might be at increased risk of relapse or have a shorter time to relapse when antipsychotic medications are withdrawn.
Benzodiazepines as a class may be used for many indications, including anxiety disorders, seizure disorders, sleep disorders, or muscle spasms. However, due to known risks of cognitive impairments, sedation, falls, or dependence and addiction, these agents are typically recommended for only short-term treatment. These risks are potentially amplified in a geriatric population, because elderly patients have an increased sensitivity to the effects of these agents and may have impaired hepatic or renal function, leading to accumulation.
Due to these risks, the American Geriatrics Society (AGS) recommends against the use of any benzodiazepines for the treatment of insomnia, agitation, or delirium. Additionally, the AGS recommends that use of these agents for the treatment of behavioral problems related to dementia be reserved for those who have failed nonpharmacologic options and are at risk to themselves or others.4
Growing evidence suggests that benzodiazepine use may increase the risk of developing AD. A recent casecontrol study compare records of 1,796 patients with an AD diagnosis to 7,184 patients with no cognitive deficits. This study found that patients with a history of benzodiazepine use had a 51% increase in risk for AD. Additionally, use of long-acting benzodiazepines, such as diazepam and clonazepam, was strongly associated with the development of AD.5 These data further illustrate the importance of minimizing the use of benzodiazepines.
To ensure proper use of these 2 classes of medications, clinical pharmacy specialists (CPSs) at the Lexington VAMC (LVAMC) in Kentucky began conducting psychotropic medication reviews (PMRs). Each PMR contained a brief summary of evidence-based recommendations (both pharmacologic and nonpharmacologic) and a clinical review of the patient’s medication history, including an evaluation of the appropriateness of current therapy and supportive documentation. Patients were candidates for PMR if they met one of the following criteria: (1) use of a benzodiazepine in a patient with dementia; (2) use of a benzodiazepine in a patient aged > 75 years; and (3) use of an antipsychotic in a patient with dementia. These criteria were selected based on guidance set forth by the PDSI. The figure provides a sample of the PMR template in the electronic medical record (EMR).
The purpose of this study was to evaluate the impact of a pharmacist-conducted PMR based on prescriber response to written recommendations. Additionally, this study characterized any observed differences in prescriber response based on discipline. The results of this study will be used to evaluate the efficacy of LVAMC’s current method of clinical pharmacy intervention and identify areas for process improvement. This study was reviewed and approved by the LVAMC institutional review board and research and development committee.
Methods
Patients were included in this study if they had a PMR note entered in the EMR between September 2014 and January 2015. Baseline demographic information collected included patient age and gender. One study author manually reviewed all PMR notes and collected the type of pharmacy intervention (characterized as recommendation for medication adjustment, patient education, both medication adjustment and patient education, or no recommendation), provider discipline, provider response to intervention, and any changes to medication therapy that occurred as a result of pharmacist intervention.
When patients were not cognitively able to receive education, family members or caretakers were educated. The primary outcome of this study was prescriber response to pharmacist recommendations, which was characterized as acknowledged, ignored, accepted, or declined with justification. In instances where both medication adjustment and patient education was recommended, the recommendation was considered to be accepted only if both components of the recommendation were addressed. The secondary outcome sought to identify any difference in provider response based on discipline.
Results
Eighty-nine patients were included in the study. Due to the nature of LVAMC, the patient population was fairly homogeneous, with an average patient age of 80 years (range 52-95 years); > 97% of patients were male. Fifty patients were noted to have prescriptions for benzodiazepines and were aged > 75 years, 11 patients had prescriptions for benzodiazepines and a diagnosis of dementia, and 38 patients had prescriptions for antipsychotics and a diagnosis of dementia. Several patients fell into more than one of the categories (Table 1).
Specific written recommendations were made for 69 (78%) patients, with 20 patients having appropriate documentation in the chart for therapy continuation. The most common documented reasons for continuation of therapy were (a) patient/caregiver educated and consent to continue therapy already documented; (b) recent dose reduction or discontinuation attempt failed; (c) recent successful dose reduction; or (d) documented risk to patient or others if medication were to be discontinued.
Most recommendations were for medication adjustments (n = 54; 78.3%). Six (8.7%) recommendations were for patient education only, and 9 (13%) recommendations were for both medication adjustment and patient education. Overall, 33 (48%) of recommendations were accepted, 21 (30%) were not acknowledged, and 15 (22%) were declined. The most common outcome of accepted recommendations was a dose reduction with full taper planned by provider. The most common reasons for declined recommendations were (a) caregivers were educated and consented to continued treatment; (b) clinical presentation warranted continued treatment; (c) patient refused recommended changes; and (d) prescriber preferred to wait until next appointment to discuss with patient (Table 2).
Forty-nine recommendations were made to prescribers in the Psychiatry Department, 17 to prescribers in the Home-Based Primary Care (HBPC) Department, 15 to prescribers in the Primary Care Department, and 8 to prescribers in the Neurology Department. Prescribers in the Primary Care Department accepted recommendations at the highest rate (n = 13; 69%), while Neurology Department prescribers (n = 2; 33%) accepted recommendations at the lowest rate.
Discussion
This study illustrates the impact of including a psychiatric CPS as part of the interdisciplinary care team. Through the implementation of a PMR process, CPSs were able to provide specific, unbiased recommendations for the safe use of medications. It was felt that CPSs might have a greater impact by offering patient-specific recommendations rather than providing general information about the risks of these medications, which many providers are aware of already. Because nearly half of all recommendations were accepted, the authors feel that the PMR is an effective way to deliver provider education and improve safe prescribing practices.
There will be times when the use of these agents in at-risk populations is justified and appropriately documented, as was the case for 20 patients in this study. The goals of this study were not only to improve the use of evidence-based medications, but also the process of documenting justification for the continued use of these agents. The 22% of recommendations that were declined with justification from the provider were considered successful, because the PMR note prompted the provider to document in a note clear justification for the use of the agent in question.
The majority of recommendations were made to prescribers in the Mental Health Department, which was expected given the 2 classes of medications evaluated in the study. However, primary care prescribers accepted recommendations at the highest rate. There are several possible explanations. First, mental health prescribers are more likely to have complex, treatment-resistant psychiatric patients than do other disciplines. Additionally, these prescribers have an increased level of familiarity and comfort with second-generation antipsychotics and benzodiazepines and may have been more confident in documenting justifications to continue therapy.
Neurologists were the least likely to accept PMR recommendations. Unlike other services, prescribers in the Neurology Department spend a significant amount of their time providing care to patients at a university hospital and, therefore, are not present on the VA campus on a daily basis. This location disparity can lead to less frequent contact between prescriber and CPSs and may impact the professional relationship between these departments. Also, both the Neurology Department and the home-based Primary Care Department did not have staff actively involved in the PDSI, which may have decreased prescriber familiarity with the goals and intentions of PDSI and therefore decreased provider responsiveness to PMR notes.
Sometimes PMR notes were entered in the EMR when the patient did not have an upcoming appointment with the prescriber. As a result, there were instances when recommendations could not be implemented due to time and workload constraints. Many providers acknowledged the importance of shared medical decision making and preferred to wait to make medication adjustments until patients could be seen in the clinic.
Psychotropic medication review is a continually developing process, and these results illustrate provider response to the initial 5 months of a new service. During the time frame, PMR notes had been entered for all veterans identified as using antipsychotics or benzodiazepines in the setting of dementia but for only a fraction of those identified as using benzodiazepines who were aged > 75 years. It is reasonable to expect that as prescribers become more familiar with the PMR process and its intentions, they may be more likely to acknowledge recommendations and to respond with the appropriate documentation.
Psychotropic medication reviews were initiated as part of a PGY-2 psychiatric pharmacy residency project, and as such, the impact on the CPS workflow was not evaluated. Although this study suggests that the use of PMR was effective in improving evidence-based prescribing, it does not evaluate whether this process is sustainable in the long-term for the CPS.
Conclusions
The results of this study illustrate the value of a psychiatric CPS. Through the implementation of a simple PMR service, CPSs were able to impact evidence-based prescribing and related documentation. With nearly 50% of the recommendations accepted, the authors believe that use of the PMR is an effective way to deliver provider education and improve safe prescribing practices. Further review of the PMR process will be needed to evaluate the impact and sustainability on CPS workflow.
Click here to read the digital edition.
1. U.S. Food and Drug Administration. Information for healthcare professionals: conventional antipsychotics, 2013. U.S. Food and Drug Administration Website. http://www. fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124830.htm. Updated August 15, 2013. Accessed February 8, 2016.
2. Sprague K. CMS sets new goals for reducing use of antipsychotic medications. Consult Pharm. 2015;30(2):3.
3. Declercq T, Petrovic M, Azermai M, et al. Withdrawal versus continuation of chronic antipsychotic drugs for behavioural and psychological symptoms in older people with dementia. Cochrane Database Syst Rev. 2013;3:CD007726.
4. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
5. Billioti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ. 2014;349:g5205
Note: Page numbers differ between the print issue and digital edition.
1. U.S. Food and Drug Administration. Information for healthcare professionals: conventional antipsychotics, 2013. U.S. Food and Drug Administration Website. http://www. fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124830.htm. Updated August 15, 2013. Accessed February 8, 2016.
2. Sprague K. CMS sets new goals for reducing use of antipsychotic medications. Consult Pharm. 2015;30(2):3.
3. Declercq T, Petrovic M, Azermai M, et al. Withdrawal versus continuation of chronic antipsychotic drugs for behavioural and psychological symptoms in older people with dementia. Cochrane Database Syst Rev. 2013;3:CD007726.
4. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
5. Billioti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ. 2014;349:g5205
Note: Page numbers differ between the print issue and digital edition.
Should you use an anticonvulsant to treat impulsivity and aggression?
Mr. V, age 29, is a US Army veteran who presents to the psychiatric emergency department because of increasing aggression. He recently returned from deployment overseas and lives with his parents. Mr. V’s mother reports that he has been increasingly “unstable” and describes an incident during which he punched a hole in his bedroom window after a temporary slow-down in the home’s Internet connection.
The workup and review of the history rules out substance abuse, posttraumatic stress disorder, bipolar disorder, seizure disorder, and personality disorders. He is currently taking only omeprazole, 40 mg/d, for acid reflux. The psychiatrist considers prescribing an antiepileptic medication to treat the agitation. Why this choice of agent?
According to DSM-5, patients who have repeated episodes of aggression can be given a diagnosis of intermittent explosive disorder, but such behavior can occur secondary to other psychiatric diagnoses (Table 1). No medications are FDA approved for aggression.1
Aggression and associated verbal and physical acts fall into 2 subtypes: impulsive type and premeditated (predatory) type. Impulsive aggression generally is described as an emotionally charged aggressive response characterized by a loss of behavioral control.
Premeditated aggression
Pharmacotherapy is directed primarily at treating impulsive aggression because this subtype is thought to be caused by neurologic deficits that can affect a person’s ability to process, and react appropriately to, external stimuli. Agitation can result from neuronal hyperactivity.2 Agents such as antiepileptic drugs (AEDs) have the potential to reduce the intensity and frequency of such behaviors.2
In this article, we focus on the use of AEDs for treating impulsive aggression in adults.
Reviewing the evidence for AEDs
The neurobiology of aggression involves multiple neurotransmitters, intracellular pathways, and ion channels.3 AEDs have several mechanisms of action, however; primary mechanisms include action on sodium and calcium channels and modulation of γ-aminobutyric acid (GABA), glutamate, and carbonic anhydrase.2,3 Agent-specific mechanisms of actions are listed in Table 2.
Phenytoin. Several double-blind, placebo-controlled trials have found a statistically significant difference between phenytoin and placebo for treating impulsive aggression, as measured by the Overt Aggression Scale (OAS)a or a modified version (MOAS/ OAS-M).1,2,4 Researchers found that phenytoin, 300 mg/d, but not 100 mg/d, decreased impulsive aggression.4
a Studies generally used the OAS, or one of its modifications, to evaluate aggressive behavior.2,4
Valproate. Trials of valproate for decreasing aggressive behaviors have produced mixed results with regard to primary outcome when used at standard dosages and within the therapeutic range measured by serum concentration.2,3 In a pooled analysis of studies that met stringent criteria (randomized, controlled trial, aggressive behavior as primary outcome, patients free of organic illness or neurologic illness), Jones and colleagues1 reported that valproate/divalproex did not produce statistically significant results compared with placebo for treating impulsive aggression.
Carbamazepine and oxcarbazepine. Double-blind, placebo-controlled trials and case studies of carbamazepine have shown mixed results. In contrast, oxcarbazepine has been found to significantly decrease aggressive behavior, measured by OAS/MOAS/ OAS-M scores.2,3 Total daily dosages of oxcarbazepine ranged from 1,500 to 2,400 mg.2-4 It has been speculated that oxcarbazepine might be a useful option for treating impulsive aggression because of its therapeutic value in temporal lobe seizures—a subtype of seizure disorder that involves the limbic system, which also modulates aggressiveness.5
Additionally, when compared with carbamazepine, oxcarbazepine has a lower risk of cardiotoxicity, neurotoxicity, and blood dyscrasia. Oxcarbazepine has fewer drug-drug interactions because of a lower degree of hepatic enzyme induction.
Topiramate. Several studies have confirmed the efficacy of topiramate for aggressive behavior.2,3 However, there have been reports that topiramate can induce or exacerbate aggression in some patients, an effect that might be dose-related. Aggression might respond better to a higher, short-term dosage (eg, 400 mg/d) than to lower (100 to 300 mg/d) dosages, which might exacerbate aggression.3
Gabapentin. Research on using gabapentin for aggression is limited. Speculation is that the combined activity of gabapentin on GABA and glutamate give the drug its antiaggressive effect.3 No randomized, double-blind, placebo-controlled trials are underway comparing gabapentin and placebo or other active medication for impulsive aggression.
Some case reports and small-scale, open-label studies report a decrease in aggression with gabapentin. As is the case with topiramate, a lower dosage (200 mg to 400 mg) has been reported to result in increased aggression—whereas a higher dosages (800 mg) decreases aggressive behavior.2,3
Lamotrigine. The results of several studies, including double-blind, placebo-controlled trials, support the use of lamotrigine for aggressive behavior. A number of these studies, however, used scales other than OAS (or its modifications) to determine this outcome. One trial showed increased aggression in several patients on lower-dosage lamotrigine (100 mg/d) that resolved when the dosage was increased.2,3
Treatment recommendations
Although all AEDs have some documented efficacy against aggression, choosing the appropriate agent depends on patient-specific variables. Avoiding divalproex in patients with liver dysfunction, for example, or carbamazepine in those with a preexisting cardiac conduction abnormality will improve outcomes by avoiding complications.
It is important to rule out all other causes of aggression before selecting a treatment. The presence of one or more of the diagnoses listed in Table 1 could lead to selection of an alternate class of medication. Nondrug therapies, such as cognitive-behavioral therapy, also should be considered.
Related Resources
• Coccaro EF. Aggression. Psychiatric assessment and treatment. Chicago, IL: Marcel Dekker, Inc.; 2003.
• Citrome LL. Aggression. http://emedicine.medscape.com/article/288689-overview. Updated June 18, 2012. Accessed February 28, 2014.
Drug Brand Names
Carbamazepine • Tegretol Phenytoin • Dilantin
Gabapentin • Neurontin Topiramate • Topamax
Lamotrigine • Lamictal Valproate/Divalproex
Omeprazole • Prilosec • Depakote
Oxcarbazepine • Trileptal
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jones RM, Arlidge J, Gilham R, et al. Efficacy of mood stabilizers in the treatment of impulsive or repetitive aggression: systemic review and meta-analysis. Br J Psychiatry. 2011;198(2):93-98.
2. Stanford MS, Anderson NE, Lake SL, et al. Pharmacologic treatment of impulsive aggression with antiepileptic drugs. Curr Treat Options Neurol. 2009;11(5):383-390.
3. Comai S, Tau M, Pavlovic Z, et al. The psychopharmacology of aggressive behavior: a translational approach: part 2: clinical studies using atypical antipsychotics, anticonvulsants, and lithium. J Clin Psychopharmacol. 2012;32(2):237-260.
4. Huband N, Ferriter M, Nathan R, et al. Antiepileptics for aggression and associated impulsivity. Cochrane Database Sys Rev. 2010;2:CD003499.
5. Mattes JA. Medications for aggressiveness in prison: focus on oxcarbazepine. J Am Acad Psychiatry Law. 2012;40(2):234-238.
Mr. V, age 29, is a US Army veteran who presents to the psychiatric emergency department because of increasing aggression. He recently returned from deployment overseas and lives with his parents. Mr. V’s mother reports that he has been increasingly “unstable” and describes an incident during which he punched a hole in his bedroom window after a temporary slow-down in the home’s Internet connection.
The workup and review of the history rules out substance abuse, posttraumatic stress disorder, bipolar disorder, seizure disorder, and personality disorders. He is currently taking only omeprazole, 40 mg/d, for acid reflux. The psychiatrist considers prescribing an antiepileptic medication to treat the agitation. Why this choice of agent?
According to DSM-5, patients who have repeated episodes of aggression can be given a diagnosis of intermittent explosive disorder, but such behavior can occur secondary to other psychiatric diagnoses (Table 1). No medications are FDA approved for aggression.1
Aggression and associated verbal and physical acts fall into 2 subtypes: impulsive type and premeditated (predatory) type. Impulsive aggression generally is described as an emotionally charged aggressive response characterized by a loss of behavioral control.
Premeditated aggression
Pharmacotherapy is directed primarily at treating impulsive aggression because this subtype is thought to be caused by neurologic deficits that can affect a person’s ability to process, and react appropriately to, external stimuli. Agitation can result from neuronal hyperactivity.2 Agents such as antiepileptic drugs (AEDs) have the potential to reduce the intensity and frequency of such behaviors.2
In this article, we focus on the use of AEDs for treating impulsive aggression in adults.
Reviewing the evidence for AEDs
The neurobiology of aggression involves multiple neurotransmitters, intracellular pathways, and ion channels.3 AEDs have several mechanisms of action, however; primary mechanisms include action on sodium and calcium channels and modulation of γ-aminobutyric acid (GABA), glutamate, and carbonic anhydrase.2,3 Agent-specific mechanisms of actions are listed in Table 2.
Phenytoin. Several double-blind, placebo-controlled trials have found a statistically significant difference between phenytoin and placebo for treating impulsive aggression, as measured by the Overt Aggression Scale (OAS)a or a modified version (MOAS/ OAS-M).1,2,4 Researchers found that phenytoin, 300 mg/d, but not 100 mg/d, decreased impulsive aggression.4
a Studies generally used the OAS, or one of its modifications, to evaluate aggressive behavior.2,4
Valproate. Trials of valproate for decreasing aggressive behaviors have produced mixed results with regard to primary outcome when used at standard dosages and within the therapeutic range measured by serum concentration.2,3 In a pooled analysis of studies that met stringent criteria (randomized, controlled trial, aggressive behavior as primary outcome, patients free of organic illness or neurologic illness), Jones and colleagues1 reported that valproate/divalproex did not produce statistically significant results compared with placebo for treating impulsive aggression.
Carbamazepine and oxcarbazepine. Double-blind, placebo-controlled trials and case studies of carbamazepine have shown mixed results. In contrast, oxcarbazepine has been found to significantly decrease aggressive behavior, measured by OAS/MOAS/ OAS-M scores.2,3 Total daily dosages of oxcarbazepine ranged from 1,500 to 2,400 mg.2-4 It has been speculated that oxcarbazepine might be a useful option for treating impulsive aggression because of its therapeutic value in temporal lobe seizures—a subtype of seizure disorder that involves the limbic system, which also modulates aggressiveness.5
Additionally, when compared with carbamazepine, oxcarbazepine has a lower risk of cardiotoxicity, neurotoxicity, and blood dyscrasia. Oxcarbazepine has fewer drug-drug interactions because of a lower degree of hepatic enzyme induction.
Topiramate. Several studies have confirmed the efficacy of topiramate for aggressive behavior.2,3 However, there have been reports that topiramate can induce or exacerbate aggression in some patients, an effect that might be dose-related. Aggression might respond better to a higher, short-term dosage (eg, 400 mg/d) than to lower (100 to 300 mg/d) dosages, which might exacerbate aggression.3
Gabapentin. Research on using gabapentin for aggression is limited. Speculation is that the combined activity of gabapentin on GABA and glutamate give the drug its antiaggressive effect.3 No randomized, double-blind, placebo-controlled trials are underway comparing gabapentin and placebo or other active medication for impulsive aggression.
Some case reports and small-scale, open-label studies report a decrease in aggression with gabapentin. As is the case with topiramate, a lower dosage (200 mg to 400 mg) has been reported to result in increased aggression—whereas a higher dosages (800 mg) decreases aggressive behavior.2,3
Lamotrigine. The results of several studies, including double-blind, placebo-controlled trials, support the use of lamotrigine for aggressive behavior. A number of these studies, however, used scales other than OAS (or its modifications) to determine this outcome. One trial showed increased aggression in several patients on lower-dosage lamotrigine (100 mg/d) that resolved when the dosage was increased.2,3
Treatment recommendations
Although all AEDs have some documented efficacy against aggression, choosing the appropriate agent depends on patient-specific variables. Avoiding divalproex in patients with liver dysfunction, for example, or carbamazepine in those with a preexisting cardiac conduction abnormality will improve outcomes by avoiding complications.
It is important to rule out all other causes of aggression before selecting a treatment. The presence of one or more of the diagnoses listed in Table 1 could lead to selection of an alternate class of medication. Nondrug therapies, such as cognitive-behavioral therapy, also should be considered.
Related Resources
• Coccaro EF. Aggression. Psychiatric assessment and treatment. Chicago, IL: Marcel Dekker, Inc.; 2003.
• Citrome LL. Aggression. http://emedicine.medscape.com/article/288689-overview. Updated June 18, 2012. Accessed February 28, 2014.
Drug Brand Names
Carbamazepine • Tegretol Phenytoin • Dilantin
Gabapentin • Neurontin Topiramate • Topamax
Lamotrigine • Lamictal Valproate/Divalproex
Omeprazole • Prilosec • Depakote
Oxcarbazepine • Trileptal
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Mr. V, age 29, is a US Army veteran who presents to the psychiatric emergency department because of increasing aggression. He recently returned from deployment overseas and lives with his parents. Mr. V’s mother reports that he has been increasingly “unstable” and describes an incident during which he punched a hole in his bedroom window after a temporary slow-down in the home’s Internet connection.
The workup and review of the history rules out substance abuse, posttraumatic stress disorder, bipolar disorder, seizure disorder, and personality disorders. He is currently taking only omeprazole, 40 mg/d, for acid reflux. The psychiatrist considers prescribing an antiepileptic medication to treat the agitation. Why this choice of agent?
According to DSM-5, patients who have repeated episodes of aggression can be given a diagnosis of intermittent explosive disorder, but such behavior can occur secondary to other psychiatric diagnoses (Table 1). No medications are FDA approved for aggression.1
Aggression and associated verbal and physical acts fall into 2 subtypes: impulsive type and premeditated (predatory) type. Impulsive aggression generally is described as an emotionally charged aggressive response characterized by a loss of behavioral control.
Premeditated aggression
Pharmacotherapy is directed primarily at treating impulsive aggression because this subtype is thought to be caused by neurologic deficits that can affect a person’s ability to process, and react appropriately to, external stimuli. Agitation can result from neuronal hyperactivity.2 Agents such as antiepileptic drugs (AEDs) have the potential to reduce the intensity and frequency of such behaviors.2
In this article, we focus on the use of AEDs for treating impulsive aggression in adults.
Reviewing the evidence for AEDs
The neurobiology of aggression involves multiple neurotransmitters, intracellular pathways, and ion channels.3 AEDs have several mechanisms of action, however; primary mechanisms include action on sodium and calcium channels and modulation of γ-aminobutyric acid (GABA), glutamate, and carbonic anhydrase.2,3 Agent-specific mechanisms of actions are listed in Table 2.
Phenytoin. Several double-blind, placebo-controlled trials have found a statistically significant difference between phenytoin and placebo for treating impulsive aggression, as measured by the Overt Aggression Scale (OAS)a or a modified version (MOAS/ OAS-M).1,2,4 Researchers found that phenytoin, 300 mg/d, but not 100 mg/d, decreased impulsive aggression.4
a Studies generally used the OAS, or one of its modifications, to evaluate aggressive behavior.2,4
Valproate. Trials of valproate for decreasing aggressive behaviors have produced mixed results with regard to primary outcome when used at standard dosages and within the therapeutic range measured by serum concentration.2,3 In a pooled analysis of studies that met stringent criteria (randomized, controlled trial, aggressive behavior as primary outcome, patients free of organic illness or neurologic illness), Jones and colleagues1 reported that valproate/divalproex did not produce statistically significant results compared with placebo for treating impulsive aggression.
Carbamazepine and oxcarbazepine. Double-blind, placebo-controlled trials and case studies of carbamazepine have shown mixed results. In contrast, oxcarbazepine has been found to significantly decrease aggressive behavior, measured by OAS/MOAS/ OAS-M scores.2,3 Total daily dosages of oxcarbazepine ranged from 1,500 to 2,400 mg.2-4 It has been speculated that oxcarbazepine might be a useful option for treating impulsive aggression because of its therapeutic value in temporal lobe seizures—a subtype of seizure disorder that involves the limbic system, which also modulates aggressiveness.5
Additionally, when compared with carbamazepine, oxcarbazepine has a lower risk of cardiotoxicity, neurotoxicity, and blood dyscrasia. Oxcarbazepine has fewer drug-drug interactions because of a lower degree of hepatic enzyme induction.
Topiramate. Several studies have confirmed the efficacy of topiramate for aggressive behavior.2,3 However, there have been reports that topiramate can induce or exacerbate aggression in some patients, an effect that might be dose-related. Aggression might respond better to a higher, short-term dosage (eg, 400 mg/d) than to lower (100 to 300 mg/d) dosages, which might exacerbate aggression.3
Gabapentin. Research on using gabapentin for aggression is limited. Speculation is that the combined activity of gabapentin on GABA and glutamate give the drug its antiaggressive effect.3 No randomized, double-blind, placebo-controlled trials are underway comparing gabapentin and placebo or other active medication for impulsive aggression.
Some case reports and small-scale, open-label studies report a decrease in aggression with gabapentin. As is the case with topiramate, a lower dosage (200 mg to 400 mg) has been reported to result in increased aggression—whereas a higher dosages (800 mg) decreases aggressive behavior.2,3
Lamotrigine. The results of several studies, including double-blind, placebo-controlled trials, support the use of lamotrigine for aggressive behavior. A number of these studies, however, used scales other than OAS (or its modifications) to determine this outcome. One trial showed increased aggression in several patients on lower-dosage lamotrigine (100 mg/d) that resolved when the dosage was increased.2,3
Treatment recommendations
Although all AEDs have some documented efficacy against aggression, choosing the appropriate agent depends on patient-specific variables. Avoiding divalproex in patients with liver dysfunction, for example, or carbamazepine in those with a preexisting cardiac conduction abnormality will improve outcomes by avoiding complications.
It is important to rule out all other causes of aggression before selecting a treatment. The presence of one or more of the diagnoses listed in Table 1 could lead to selection of an alternate class of medication. Nondrug therapies, such as cognitive-behavioral therapy, also should be considered.
Related Resources
• Coccaro EF. Aggression. Psychiatric assessment and treatment. Chicago, IL: Marcel Dekker, Inc.; 2003.
• Citrome LL. Aggression. http://emedicine.medscape.com/article/288689-overview. Updated June 18, 2012. Accessed February 28, 2014.
Drug Brand Names
Carbamazepine • Tegretol Phenytoin • Dilantin
Gabapentin • Neurontin Topiramate • Topamax
Lamotrigine • Lamictal Valproate/Divalproex
Omeprazole • Prilosec • Depakote
Oxcarbazepine • Trileptal
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jones RM, Arlidge J, Gilham R, et al. Efficacy of mood stabilizers in the treatment of impulsive or repetitive aggression: systemic review and meta-analysis. Br J Psychiatry. 2011;198(2):93-98.
2. Stanford MS, Anderson NE, Lake SL, et al. Pharmacologic treatment of impulsive aggression with antiepileptic drugs. Curr Treat Options Neurol. 2009;11(5):383-390.
3. Comai S, Tau M, Pavlovic Z, et al. The psychopharmacology of aggressive behavior: a translational approach: part 2: clinical studies using atypical antipsychotics, anticonvulsants, and lithium. J Clin Psychopharmacol. 2012;32(2):237-260.
4. Huband N, Ferriter M, Nathan R, et al. Antiepileptics for aggression and associated impulsivity. Cochrane Database Sys Rev. 2010;2:CD003499.
5. Mattes JA. Medications for aggressiveness in prison: focus on oxcarbazepine. J Am Acad Psychiatry Law. 2012;40(2):234-238.
1. Jones RM, Arlidge J, Gilham R, et al. Efficacy of mood stabilizers in the treatment of impulsive or repetitive aggression: systemic review and meta-analysis. Br J Psychiatry. 2011;198(2):93-98.
2. Stanford MS, Anderson NE, Lake SL, et al. Pharmacologic treatment of impulsive aggression with antiepileptic drugs. Curr Treat Options Neurol. 2009;11(5):383-390.
3. Comai S, Tau M, Pavlovic Z, et al. The psychopharmacology of aggressive behavior: a translational approach: part 2: clinical studies using atypical antipsychotics, anticonvulsants, and lithium. J Clin Psychopharmacol. 2012;32(2):237-260.
4. Huband N, Ferriter M, Nathan R, et al. Antiepileptics for aggression and associated impulsivity. Cochrane Database Sys Rev. 2010;2:CD003499.
5. Mattes JA. Medications for aggressiveness in prison: focus on oxcarbazepine. J Am Acad Psychiatry Law. 2012;40(2):234-238.